Abstract
Mycobacterium chimaera is the newly described species belonging to Mycobacterium avium complex (MAC), with morphology and growth characteristics closely related to Mycobacterium intracellulare. The aim of this retrospective study was to analyze the frequency and clinical significance of M. chimaera identification in the population of patients with previous positive respiratory cultures for M. intracellulare or MAC. 200 strains of M. intracellulare or MAC, isolated from respiratory specimens of patients hospitalized in pulmonary wards, between 2011 and 2020, were retrospectively analyzed with GenoType NTM-DR test. 88 (44%) of strains were re-classified to M. chimaera species. Analysis of clinical data in 30 patients with positive M. chimaera isolates revealed that they were diagnosed with chronic obstructive pulmonary disease (COPD) – 27%, past tuberculosis – 20%, or interstitial lung diseases – 17%, respectively. Non-tuberculous mycobacterial lung disease (NTMLD) caused by M. chimaera has been recognized in 53% of patients, most often in those presenting with post-tuberculous lung lesions. M. chimaera was almost exclusively isolated from respiratory specimens of patients with underlying lung diseases, especially those with COPD and/or past tuberculosis. NTMLD due to M. chimaera was diagnosed predominantly in patients with past tuberculosis.
Keywords: Mycobacterium chimaera, chronic obstructive lung disease, cystic fibrosis, mycobacteriosis, tuberculosis
Introduction
Non-tuberculosis mycobacteria (NTM), also called opportunistic or atypical mycobacteria, are widespread as part of environmental microbiota (Griffith et al. 2007). Most atypical mycobacteria are natural inhabitants of various ecological niches, common for humans and animals, including natural waters, drinking water supply systems, and soil. As a result of their widespread expansion, humans are exposed to constant contact with these opportunistic microorganisms.
Non-tuberculosis mycobacteria are less pathogenic than Mycobacterium tuberculosis; however, they have become a cause of respiratory colonization increasingly. Several species can induce a non-tuberculous mycobacterial lung disease (NTMLD), especially in patients with chronic lung diseases, such as cystic fibrosis (Adjemian et al. 2018; Gardner et al. 2019), chronic obstructive pulmonary disease (COPD) (Andrejak et al. 2013; Hoefsloot et al. 2013; Diel et al. 2017), past tuberculosis (Adzic-Vukicevic et al. 2018; Bakuła et al. 2018; Szturmowicz et al. 2018), or chronic thromboembolic pulmonary hypertension (Wilińska et al. 2014).
To date, more than 190 species of atypical mycobacteria have been described. A steady increase in the number of newly identified species is observed as a result of the continuous development of molecular methods enabling the identification of mycobacteria (Tortoli 2014).
One of the most frequently isolated species was those belonging to M. avium complex (MAC). Traditionally, MAC has been thought to consist of M. avium and M. intracellulare. Presently, owing to the application of molecular biology techniques, MAC includes eight species (M. avium, M. intracellulare, M. marseillaise, M. timonense, M. bouchedurhonense, M. colombiense, M. vulneris, and M. chimaera) and four subspecies (M. avium subsp. avium, M. avium subsp. hominissium, M. avium subsp. silvaticum, and M. avium subsp. paratuberculosis) (Wallace et al. 2013).
M. chimaera was first characterized in 2004 by Tortoli, who described 12 isolates of slow-growing bacteria, closely related to M. intracellulare, isolated from the respiratory tract (Tortoli et al. 2004). Subsequent isolates came from Germany, the United States, and Zambia (Schweickert et al. 2008; Bills et al. 2009; Malama et al. 2014). The natural reservoir of M. chimaera is unknown; however, Sax et al. (2015) showed that it is commonly detected in aquatic environments and biofilms.
The increased interest in M. chimaera has been associated with several cases of invasive cardiovascular infection in patients undergoing cardiac surgery in the extracorporeal circulation (Archermann et al. 2013; Kohler et al. 2015; Sax et al. 2015). Environmental studies of the hospital’s water distribution system showed that M. chimaera was found in water samples collected from devices used for extracorporeal blood circulation. Due to the favorable conditions for growth, mycobacteria multiplied in water tanks and formed a biofilm. They were further dispersed in the air during the operation of the device. Positive cultures were obtained from the air samples only when the apparatus was in operation. Brand new devices were colonized with M. chimaera after only three months of operation, despite maintaining the equipment following the instruction (Sax et al. 2015).
Since 2011 several patients have been identified who underwent cardiac surgery and were diagnosed with M. chimaera infection. The time between the procedure and the appearance of NTMLD ranged from one to four years. Patients reported non-specific clinical symptoms, and the infection was identified in different organs, which made it difficult to correctly diagnose mycobacteriosis (ECDC 2015; Kohler et al. 2015). Pulmonary infections due to M. chimaera have been reported sporadically (Alhanna et al. 2012; Hasan et al. 2017).
Thus, the present retrospective study aimed to analyze the frequency and clinical significance of M. chimaera isolation in the population of patients, who had previously obtained positive respiratory cultures for M. intracellulare or MAC.
Experimental
Materials and Methods
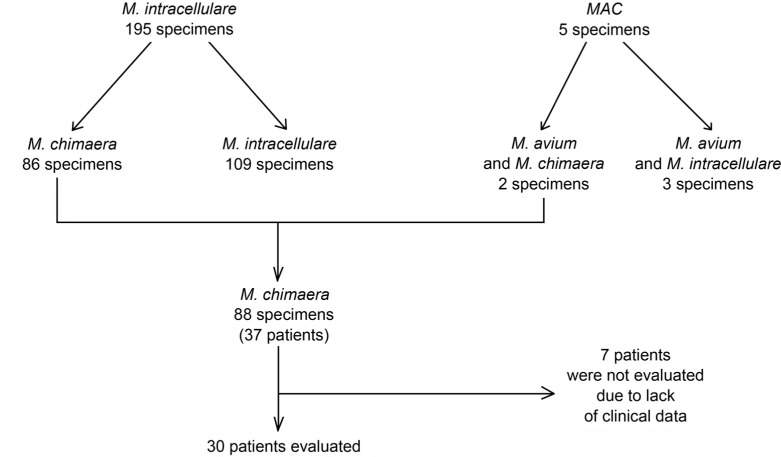
Respiratory strains of either M. intracellulare or MAC, identified from 2011 through 2020 by the Geno-Type CM test (Hain Lifescience) in various respiratory wards, have been re-evaluated with GenoType NTM-DR test (Hain Lifescience). This method identifies various MAC species via the DNA-STRIP method, analyzing the 23S rRNA gene polymorphism. The data concerning the number of specimens tested and the number of patients are presented in Fig. 1.
Fig. 1.
Results of M. chimaera identification in 200 respiratory samples.
The strain identification process consisted of three stages: DNA isolation, amplification by biotin-labelled primers, and reverse hybridization. The last step involved the following: chemical denaturation of amplification products, hybridization of biotin-labelled single-stranded amplicons on the probe-covered membrane, washing, addition of streptavidin conjugate/alkaline phosphatase, and the staining reaction using alkaline phosphatase (Zabost et al. 2015).
Clinical data concerning age, sex, and concomitant diseases were collected from the doctors who took care of the patients after obtaining the patients’ agreement. According to the guidelines (Griffith et al. 2007; Daley et al. 2020), NTMLD was recognized in patients with new clinical symptoms, new radiological signs (infiltrations or nodular-bronchiectatic form of disease), and in whom M. chimaera was cultured once from bronchial washings or twice from separate sputum specimens.
Statistical analysis. All analyses were performed with R – a software environment for statistical computing and graphics (https://www.r-project.org/) (R core team 2016). Continuous variables (age) have been presented as means and standard deviations, categorical ones – as absolute numbers and percentages of the entire population. Comparison of categorical parameters between two groups has been performed with a chi-square test. Comparison of continuous parameters has been performed with the t-Student test or the U Mann-Whitney test. P < 0.05 was considered significant.
Results
200 respiratory strains of either M. intracellulare or MAC were re-evaluated. Among 195 strains initially classified as M. intracellulare, 86 (43.0%) were re-classified as M. chimaera. Among five cases initially identified as caused by a mixture of M. avium and M. intracellulare, there were two mixed infections of M. avium and M. chimaera. No resistance to aminoglycosides and macrolides was found in the analyzed group of strains.
Therefore, a total of 88 out of 200 strains (44%) isolated from 37 patients were re-classified as M. chimaera. Further analysis included 30 patients from whom the clinical information has been obtained. There were 14 women and 16 men aged 26–92 years (median age – 60 years).
Diseases concomitant with M. chimaera infection were presented in Table I. The three most frequent comorbidities were COPD, past tuberculosis, and interstitial lung diseases. In 23% of patients, two or more coexisting diseases have been recognized.
Table I.
Clinical characteristics of patients with positive M. chimaera isolates.
| Concomitant diseases | Number (%) of patients with a given diagnosis | ||
|---|---|---|---|
| Men n = 16 |
Women n = 14 |
Total 30 |
|
| COPD | 7 (44) | 1 (7) | 8 (27) |
| Previous tuberculosis | 5 (31) | 1 (7) | 6 (20) |
| Interstitial lung diseasea | 5 (31) | 0 | 5 (17) |
| Cystic fibrosis | 0 | 2 (14) | 2 (7) |
| Post-inflammatory lung fibrosis | 1 (6) | 2 (14) | 3 (10) |
| Bronchiectasis | 1 (6) | 3 (21) | 4 (13) |
| Neoplastic diseaseb | 2 (13) | 0 | 2 (7) |
| Diabetes mellitus | 1 (6) | 1 (7) | 2 (7) |
| Othersc | 0 | 4 (21) | 4 (13) |
| At least 2 diagnoses | 5 (31) | 2 (14) | 7 (23) |
| No concomitant disease | 1 (6) | 1 (7) | 2 (7) |
a – idiopathic pulmonary fibrosis (2 pts), sarcoidosis (2), proteinosis (1)
b – mielofibrosis, lung cancer
c – bronchial asthma (1), hypothyreosis (1), mediastinal cyst (1), rheumatoid arthritis (1)
The patterns of most frequent respiratory co-morbidities in patients with positive M. chimaera cultures were different in males compared to females (Table I). COPD, past tuberculosis, and interstitial lung diseases were diagnosed more often in men, cystic fibrosis and bronchiectasis – in women.
Criteria of NTMLD, according to ATS/IDSA (Griffith et al. 2007), as well as to the recent international guidelines (Daley et al. 2020), were fulfilled in 16 patients (53%), whereas colonization was recognized in 14 patients (47%). The pathogenicity of isolated M. chimaera strains was not related to sex and age of patients (%Table II). NTMLD was observed more frequently among patients with past tuberculosis, bronchiectasis, and cystic fibrosis than the remaining ones.
Table II.
Pathogenicity of isolated M. chimaera strains depending on sex, age, and concomitant pulmonary disease.
| NTMLD n = 16 |
Colonization n = 14 |
Total | p | |
|---|---|---|---|---|
| Sex | ||||
| Men | 8 (50) | 8 (50) | 16(100) | |
| Women | 8 (57) | 6(43) | 14(100) | 0,86 |
| Age | ||||
| Years (mean+/-SD) | 58,3 (16,77) | 62,1 (12,86) | 60,1 (15,19) | 0,72 |
| Diagnosis | ||||
| Previous tuberculosis | 5 (83) | 1 (17) | 6 (100) | |
| COPD | 3 (38) | 5 (62) | 8 (100) | |
| Cystic fibrosis | 2(100) | 0 | 2 (100) | |
| Interstitial lung diseasesa | 2 (40) | 3 (60) | 5 (100) | |
| Post-inflammatory lung fibrosis | 0 | 3 (100) | 3 (100) | |
| Bronchiectasis | 4(100) | 0 | 4(100) | |
| Neoplastic diseasesb | 1 (50) | 1 (50) | 2 (100) | |
| Diabetes mellitus | 1 (50) | 1 (50) | 2 (100) | |
| Othersc | 0 | 4 (100) | 4 (100) | |
| No concomitant disease | 2(100) | 0 | 2 (100) | |
a – idiopathic pulmonary fibrosis (2 pts), sarcoidosis (2), proteinosis (1)
b – mielofibrosis, lung cancer
c – bronchial asthma (1), hypothyreosis (1), mediastinal cyst (1), rheumatoid arthritis (1)
Discussion
M. chimaera has been underdiagnosed in the past due to the lack of appropriate methods of identification. The growth time of M. chimaera species and its morphology are identical to those of M. intracellulare. This led to its initial classification in the M. avium complex MAC-A (Tortoli et al. 2004). The identification methods used at that time (the Accu Probe and Lipav 1 tests) did not allow for the identification of M. chimaera within MAC species. Based on the 16S rRNA gene analysis, the species has been classified as M. intracellulare (Bills et al. 2009). The ITS region (16S-23S rRNA) analysis, introduced in 2004, enabled the identification of M. chimaera with great confidence (Tortoli et al. 2004; Turenne et al. 2007).
In the present study, the analysis of the 23S rRNA gene polymorphism with the GenoType NTM-DR test enabled to identify M. chimaera in 44% of strains recognized previously with the GenoType CM as M. intracellulare. Schweickert et al. (2008), analyzed 166 clinical isolates of M. intracellulare; however, after their sequencing, 143 strains (86%) were re-classified to M. chimaera.
Wallace et al. (2013) investigated water samples obtained from the households of patients suffering from NTM infections caused by MAC. Genotyping of M. intracellulare strains isolated from the water samples showed that 73% of them were M. chimaera. However, the analysis of strains isolated from clinical specimens of patients resulted in recognition of M. chimaera in only four out of 54 patients (Wallace et al. 2013).
In the group of patients studied in this work, M. chimaera has been isolated most frequently from the patients diagnosed with COPD, past pulmonary tuberculosis, and fibrotic interstitial lung diseases. NTMLD has been recognized in 53% of patients, mostly in those with a history of past tuberculosis, with bronchiectasis, or cystic fibrosis.
Analyses carried out in the United States (Boyle et al. 2015) and Germany (Schweickert et al. 2008) suggested that M. chimaera was less pathogenic than other MAC species, such as M. intracellulare and M. avium. Schweickert et al. (2008) identified 143 strains of M. chimaera, but only three isolates were from patients (3.3%) with NTMLD. Boyle et al. (2015), reported 43% of cases diagnosed with NTMLD among 126 patients with M. chimaera isolates. The same authors reported NTMLD in 61% to 70% of patients infected with M. avium or M. intracellulare (Schweickert et al. 2008; Boyle et al. 2015).
In the present study, the most interesting observation concerned M. chimaera infections that developed in patients with past tuberculosis, especially in the male population. A similar observation was made by Moon et al. (2016), who found past lung tuberculosis in 64% of patients with M. chimaera infection. The other NTM type often responsible for NTMLD in patients with post-tuberculous lung lesions is Mycobacterium kansasii (Augustynowicz-Kopeć et al. 2019). It is not known whether the higher incidence of NTMLD in those NTM-infected patients, who had a history of past tuberculosis, is related to the favorable conditions for their colonization in the altered lung parenchyma, or a defect in the intracellular killing of NTM in these patients.
In our study group, M. chimaera infections were diagnosed in all the patients with previously recognized bronchiectasis. This predisposition is difficult to comment on because bronchiectasis may be an early radiologic sign of NTMLD or one of the lung diseases predisposing to NTMLD. An analysis of the radiological appearance of NTMLD, performed by our group in COPD patients, revealed that 50% of patients with bronchiectasis were diagnosed with NTMLD, and the remaining had NTM colonization confirmed (Szturmowicz et al. 2020).
Another disease predisposing to the development of NTMLD is cystic fibrosis (Adjemian et al. 2018; Gardner et al. 2019). In our study, two patients with cystic fibrosis developed pulmonary infection with M. chimaera as an etiological factor. Larcher et al. (2019) described patients with cystic fibrosis, in whom recognition and treatment of NTMLD due to M. chimaera resulted in the improvement of general condition and lung function.
Conclusions
Almost half of M. intracellulare isolates, have been reclassified due to implementing the molecular GenoType NTM-DR test to M. chimaera, in the present study. M. chimaera isolation concerned patients diagnosed with COPD, past tuberculosis, and various fibrotic interstitial lung diseases. Analysis of clinical data documented NTMLD in 53% of patients infected with M. chimaera. Patients diagnosed with past tuberculosis and with cystic fibrosis, may be a risk group for M. chimaera NTMLD, nevertheless, this observation must be confirmed in a larger population.
Ethical statement
The study has been approved by the Bioethical Committee of the National Tuberculosis and Lung Diseases Research Institute (KB-9/2015).
Funding
Financial resources: The statutory activity of National Tuberculosis and Lung Diseases Research Institute, Task No 1.22.
Conflict of interest
The authors do not report any financial or personal connections with other persons or organizations, which might negatively affect the contents of this publication and/or claim authorship rights to this publication.
Literature
- Adjemian J, Olivier KN, Prevots DR. Epidemiology of pulmonary nontuberculous mycobacterial sputum positivity in patients with cystic fibrosis in the United States, 2010–2014. Ann Am Thorac Soc. 2018;15(7):817–826. 10.1513/AnnalsATS.201709-727OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adzic-Vukicevic T, Barac A, Blanka-Protic A, Laban-Lazovic M, Lukovic B, Skodric-Trifunovic V, Rubino S. Clinical features of infection caused by non-tuberculous mycobacteria: 7 years’ experience. Infection. 2018;46(3):357–363. 10.1007/s15010-018-1128-2 [DOI] [PubMed] [Google Scholar]
- Alhanna J, Purucker M, Steppert C, Grigull-Daborn A, Schiffel G, Gruber H, Borgmann S. Mycobacterium chimaera causes tuberculosis-like infection in a male patient with anorexia nervosa. Int J Eat Disord. 2012;45:450–452. 10.1002/eat.20942 [DOI] [PubMed] [Google Scholar]
- Andrejak C, Nielsen R, Thomson R, Dahaut P, Sorensen HT, Thomsen RW. Chronic respiratory disease, inhaled corticosteroids and risk of non-tuberculosis mycobacteriosis. Thorax. 2013; 68(3):256–262. 10.1136/thoraxjnl-2012-201772 [DOI] [PubMed] [Google Scholar]
- Archermann Y, Rössle M, Hoffmann M, Deggim V, Kuster S, Zimmermann DR, Bloemberg G, Hombach M, Hasse B. Prosthetic valve endocarditis and bloodstream infection due to Mycobacterium chimaera. J Clin Microbiol. 2013;51(6):1769–1773. 10.1128/JCM.00435-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustynowicz-Kopeć E, Siemion-Szcześniak I, Zabost A, Wyrostkiewicz D, Filipczak D, Oniszh K, Gawryluk D, Radzikowska E, Korzybski D, Szturmowicz M. Interferon gamma release assays in patients with respiratory isolates of non-tuberculous mycobacteria – preliminary study. Pol J Microbiol. 2019;68(1):15–19. 10.21307/pjm-2019-002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakuła Z, Kościuch J, Safianowska A, Proboszcz M, Bielecki J, van Ingen J, Krenke R, Jagielski T. Clinical, radiological and molecular features of Mycobacterium kansasii pulmonary disease. Respir Med. 2018;139:91–100. 10.1016/j.rmed.2018.05.007 [DOI] [PubMed] [Google Scholar]
- Bills ND, Hinrichs SH, Aden TA, Wickert RS, Iwen PC. Molecular identification of Mycobacterium chimaera as a cause of infection in a patient with chronic obstructive pulmonary disease. Diagn Micr Infec Dis. 2009;63:292–295. 10.1016/j.diagmicrobio.2008.12.002 [DOI] [PubMed] [Google Scholar]
- Boyle DP, Zembower TR, Reddy S, Qi Ch. Comparison of clinical features, virulence, and relapse among Mycobacterium avium complex species Am J Respir Crit Care Med. 2015;191(11):1310–1317. 10.1164/rccm.201501-0067OC [DOI] [PubMed] [Google Scholar]
- Daley ChL, Iaccarino JM, Lange Ch, Cambau E, Wallace RJ, Andrejak C, Böttger EC, Brozek J, Griffith DE, Guglielmetti L, et al. Treatment of nontuberculous mycobacterial pulmonary disease: An official ATS/ERS/ESCMID/IDSA clinical practice guideline. Clin Infect Dis. 2020;71(4):e1–e36. 10.1093/cid/ciaa241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diel R, Jacob J, Lampenius N, Loebinger M, Nienhaus A, Rab KF, Ringshausen FC. Burden of non-tuberculous mycobacterial pulmonary disease in Germany. Eur Respir J. 2017;49(4):1602109. 10.1183/13993003.02109-2016 [DOI] [PubMed] [Google Scholar]
- ECDC . ECDC Technical Document. EU protocol for case detection, laboratory diagnosis and environmental testing of Mycobacterium chimaera infections potentially associated with heater-cooler units: case definition and environmental testing methodology. Stockholm (Sweden): European Centre for Disease Prevention and Control; 2015[cited 2021 Feb 26]. Available from https://www.ecdc.europa.eu/sites/default/files/media/en/publications/Publications/EU-protocol-for-M-chimaera.pdf [Google Scholar]
- Gardner AI, McClenaghan E, Saint G, McNamara PS, Brodile M, Thomas MF. Epidemiology of nontuberculous mycobacteria infection in children and young people with cystic fibrosis: analysis of UK Cystic Fibrosis registry. Clin Infect Dis. 2019February15;68(5):731–737. 10.1093/cid/ciy531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley Ch, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, et al. ; ATS Mycobacterial Diseases Subcommittee . An official ATS/IDSA statement: diagnosis, treatment and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175(4):367–416. 10.1164/rccm.200604-571ST [DOI] [PubMed] [Google Scholar]
- Hasan NA, Warren RL, Epperson LE, Malecha A, Alexander DC, Turenne ChY, MacMillan D, Birol I, Pleasance S, Coope R, et al. Complete genome sequence of Mycobacterium chimaera SJ42, a nonoutbreak strain from an immunocompromised patient with pulmonary disease. Genome Announc. 2017September;5(37):e00963-17. https://doi.org/10.1128%2FgenomeA.00963-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoefsloot W, van Ingen J, Magis-Escurra C. Prevalence of nontuberculous mycobacteria in COPD patients with exacerbations. J Infect. 2013;66(6):542–545. 10.1016/j.jinf.2012.12.011 [DOI] [PubMed] [Google Scholar]
- Kohler P, Kuster SP, Bloemberg G, Schulthess B, Frank M, Tanner FC, Rössle M, Böni Ch, Falk V, Wilhelm MJ, et al. Healthcare-associated prosthetic heart valve, aortic vascular graft, and disseminated Mycobacterium chimaera infections subsequent to open heart surgery. Eur Heart J. 2015;36(40):2745–2753. 10.1093/eurheartj/ehv342 [DOI] [PubMed] [Google Scholar]
- Larcher R, Lounnas M, Dumont Y, Michon AL, Bonzon L, Chiron R, Carriere Ch, Klouche K, Godreuil S. Mycobacterium chimaera pulmonary disease in cystic fibrosis patients, France, 2010–2017. Emerg Infect Dis. 2019;25(3):611–613. https://dx.doi.org/10.3201%2Feid2503.181590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malama S, Munyeme M, Mwanza S, Muma JB. Isolation and characterization of non-tuberculous mycobacteria from humans and animals in Namwala District of Zambia. BMC Res Notes. 2014;7:622. https://dx.doi.org/10.1186%2F1756-0500-7-622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon SM, Kim SY, Jhun BW, Lee H, Park HY, Jeon K, Huh HJ, Ki Ch-S, Lee NY, Shin SJ, et al. Clinical characteristics and treatment outcomes of pulmonary disease caused by Mycobacterium chimaera. Diagn Micr Infec Dis. 2016;86:382–384. 10.1016/j.diagmicrobio.2016.09.016 [DOI] [PubMed] [Google Scholar]
- R core team . R: A language and environment for statistical computing. Vienna (Austria): R Foundation for Statistical Computing; 2016. Available from https://www.R-project.org [Google Scholar]
- Sax H, Bloemberg G, Hasse B, Sommerstein R, Kohler P, Achermann Y, Rössle M, Falk V, Kuster SP, Böttger EC, et al. Prolonged outbreak of Mycobacterium chimaera infection after open-chest heart surgery. Clin Infect Dis. 2015;61(1):67–75. 10.1093/cid/civ198 [DOI] [PubMed] [Google Scholar]
- Schweickert B, Goldenberg O, Richter E, Göbel UB, Petrich A, Buchholz P, Moter A. Occurrence and clinical relevance of Mycobacterium chimaera sp. nov., Germany. Emerg Infect Dis. 2008; 14(9):1443–1446. 10.3201/eid1409.071032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szturmowicz M, Oniszh K, Wyrostkiewicz D, Radwan-Rohrenschef P, Filipczak D, Zabost A. Non-tuberculous mycobacteria in respiratory specimens of patients with obstructive lung diseases – colonization or disease? Antibiotics. 2020July20;9(7):424. 10.3390/antibiotics9070424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szturmowicz M, Siemion-Szcześniak I, Wyrostkiewicz D, Klatt M, Brzezińska S, Zabost A, Lewandowska A, Filipczak D, Oniszh K, Skoczylas A, et al. Factors predisposing to non-tuberculous mycobacterial lung disease in the patients with respiratory isolates of non-tuberculous mycobacteria. Adv Respir Med 2018;86:261–267. 10.5603/ARM.a2018.0043 [DOI] [PubMed] [Google Scholar]
- Tortoli E, Rindi L, Garcia MJ, Chiaradonna P, Dei R, Garzelli C, Kroppenstedt RM, Lari N, Mattei R, Mariottini A, et al. Proposal to elevate the genetic variant MAC-A, included in the Mycobacterium avium complex, to species rank as Mycobacterium chimaera sp. nov. Int J Syst Evol Microbiol. 2004;54(Pt4):1277–1285. 10.1099/ijs.0.02777-0 [DOI] [PubMed] [Google Scholar]
- Tortoli E. Microbiological features and clinical relevance of new species of the genus Mycobacterium. Clin Microbiol Rev 2014; 27(4): 727–752. 10.1128/cmr.00035-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turenne CY, Wallace R Jr, Behr MA. Mycobacterium avium in the postgenomic era. Clin Microbiol Rev. 2007;20(2):205–229. 10.1128/CMR.00036-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace RJ Jr, Iakhiaeva E, Williams MD, Brown-Elliott BA, Vasireddy S, Vasireddy R, Lande L, Peterson DD, Sawicki J, Kwait R, et al. Absence of Mycobacterium intracellulare and presence of Mycobacterium chimaera in household water and biofilm samples of patients in the United States with Mycobacterium avium complex respiratory disease. J Clin Microbiol. 2013;51(6):1747–1752. 10.1128/JCM.00186-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilińska E, Oniszh K, Augustynowicz-Kopeć E, Zabost A, Fijałkowska A, Kurzyna M, Wieteska M, Torbicki A, Kuś J, Szturmowicz M. Non-tuberculous mycobacterial lung disease (NTMLD) in patients with chronic thromboembolic pulmonary hypertension and idiopathic pulmonary arterial hypertension. Pneumonol Alergol Pol. 2014;82(6):495–502. 10.5603/PiAP.2014.0066 [DOI] [PubMed] [Google Scholar]
- Zabost A, Augustynowicz-Kopeć E. Use of GenoType MTBDR plus assay for the detection of mycobacteria molecular rifampicin and isoniazid resistance. Post Nauk Med. 2015;4:249–254. [Google Scholar]