Abstract
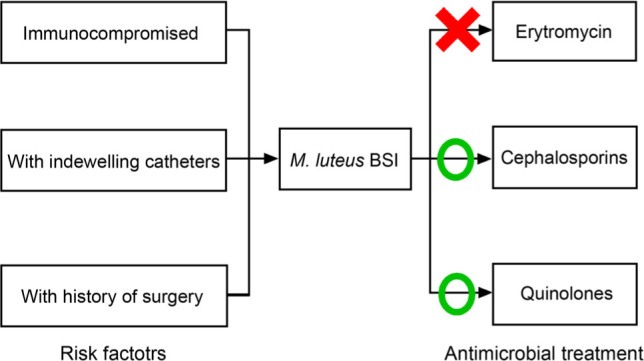
Few pieces of research have focused on Micrococcus luteus bloodstream infection (BSI) because of its low incidence; hence data is needed to illustrate this uncommon infection. This study aimed to explore the clinical characteristics of patients with M. luteus BSI. From January 2010 to December 2019, inpatients that met the criteria for M. luteus BSI were included in this study. Data was collected by reviewing electronic records. Ninety-seven patients were enrolled in this study. Sixty-three percent of the patients have a higher neutrophil percentage (NEUT%). The average blood C-reactive protein (CRP) concentration was 5.5 ± 6.4 mg/dl. 48.5% of the patients had malignancy, and 40.2% underwent invasive surgeries. Linezolid was found to have the largest average diameter of the inhibition zone (36 mm), while erythromycin was found to have the smallest average zone diameter (15 mm). However, some M. luteus strains had a potentially broad antimicrobial resistance spectrum. Cephalosporins (59.2%) and quinolones (21.4%) were the most commonly used antibiotics for empirical therapies. In conclusion, M. luteus BSI mainly happens in immunocompromised patients or those with former invasive surgeries or indwelling catheters. M. luteus strains are less responsive to erythromycin. Cephalosporins and quinolones are effective empirical antibiotics for M. luteus BSI; however, vancomycin and teicoplanin should be considered for potentially broadly drug-resistant M. luteus strains.
Keywords: antimicrobial resistance, bloodstream infection, clinical characteristics, Micrococcus luteus
Introduction
Micrococcus luteus, a member of the Micrococcus family, is a kind of catalase-, oxidase-, and Gram-positive cocci broadly found in natural environments such as soil and water resources and it is usually considered a normal inhabitant of human skin and oropharynx mucosa (Erbasan 2018). In 1922, Alexander Fleming, discoverer of penicillin, first found M. luteus in the nasal secretion of a patient. In recent years, M. luteus has been reported to possibly cause infections such as hepatic and brain abscess, native valve endocarditis, bacteremia, and septic arthritis in immunosuppressive patients (Wharton et al. 1986; Peces et al. 1997; Andreopoulos et al. 2000; Erbasan 2018; Ianniello et al. 2019). It indicates that M. luteus should be considered as a clinically potential opportunistic pathogen.
However, few studies have focused on M. luteus infection, especially in bloodstream infections (BSI), because of its low pathogenicity and incidence. A Medline search revealed only three case reports on M. luteus BSI, indicating that the clinical features of the patients remained uncertain. Hence, we described the clinical characteristics of patients with M. luteus BSI in a tertiary-care hospital in China, hoping to provide more information for this infrequent infection.
Experimental
Materials and Methods
Setting and design of the study. This retrospective study was performed at the First Medical Center of Chinese People’s Liberation Army General Hospital (FMC-PLAGH) in Beijing, China. FMC-PLAGH is one of the largest tertiary-care hospitals in China, with more than 2,400 beds and 150 thousand inpatients per year.
From January 2010 to December 2019, inpatients with at least two consecutive blood culture results positive for M. luteus were included in this study. Data of the enrolled patients was collected by reviewing the electronic records of the Infection Management and Disease Control Department of FMC-PLAGH. The included information was as followed: age, gender, body mass index (BMI), hospitalization department, risk factors, laboratory examination results, admission time, hospitalization time, Sequential Organ Failure Assessment (SOFA) score, type of antibiotics used, results of antimicrobial susceptibility test, and outcome.
Definitions. The diagnosis of M. luteus BSI met the following criteria: 1) isolation of at least two consecutive blood culture samples positive for M. luteus; 2) having one of the following symptoms: fever, chill, or hypotension; and 3) exclusion of contamination during the process of sample collection and blood culture (CDC 2021). Incidence of M. luteus BSI was calculated as cases divided by the admission number of the year. BSI was considered as a community-acquired when the positive blood culture was obtained within 48 h after admission. In comparison it was considered as hospital-acquired when it happened beyond 48 h after admission. Antimicrobial therapies are considered empirical when they are used before the susceptibility test results are available, and they are considered definitive after the results of susceptibility tests return.
Blood culture, identification, and antimicrobial susceptibility test. Blood culture, identification, and antimicrobial susceptibility test were performed in the Microbiology Center of FMC-PLAGH. BacT/ALERT 3D system (Becton-Dickinson, Sparks, MD, USA) was used for blood culture. Species identification was performed using VITEK 2 system (BioMérieux, Marcy l’Étoile, France) and confirmed using a MALDI-TOF technology via VITEK-MS system (BioMérieux, Marcy l’Étoile, France). An antimicrobial susceptibility test was performed by either a Kirby-Bauer Disk Diffusion method (Oxoid, UK) or the VITEK 2 system. Due to the lack of recommended standards for defining the susceptibility of M. luteus to the antimicrobial agents used in this study, only diameters of the inhibition zone or minimum inhibitory concentrations (MIC) were reported.
Results
Epidemiological trends of M. luteus BSI in FMC-PLAGH. As shown in Fig. 1, during the nine-years, the incidence of M. luteus BSI ranged from 0 to 12.6 per 100,000, and the proportion among all BSIs ranged between 0 and 20.3 per 1,000. The overall incidence and proportion among all BSIs for M. luteus BSI was 6.7 per 100,000 and 9.5 per 1,000, respectively. The results indicated that M. luteus was still an uncommon pathogen for BSI; however, the odds seemed to be rising from 2010 to 2018.
Fig. 1.
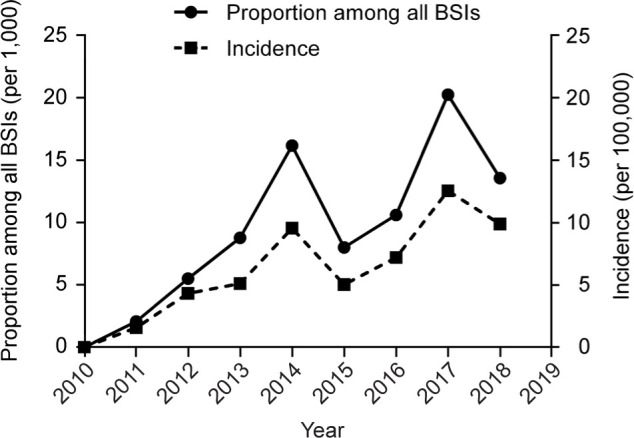
Epidemiological trends of Micrococcus luteus bloodstream infection (BSI) in the First Medical Center of the Chinese People’s Liberation Army General Hospital. Incidence was calculated as cases divided by the admission number of the year. Data for 2019 was unavailable due to a lack of hospital admission number.
Characteristics of the patients with M. luteus BSI. The clinical characteristics of the patients with M. luteus BSI were listed in Table I. A total of 97 patients with M. luteus BSI were identified during the 2010– 2019 period, among which three died during their hospital stay. The average age was 51.9 ± 22.0 years old, and 62.9% of the patients were male. The average Body Mass Index (BMI) was within the normal range, and the SOFA score of the patients was 1.4 ± 1.8. Most of the BSIs were acquired in the hospital (72.2%). Moreover, polymicrobial BSI was not commonly seen in M. luteus BSIs (5.2%). Nearly 90% of the patients suffered from fever, followed by chill (18.6%) and fatigue (12.4%). The average maximal body temperature of patients with M. luteus BSI was 38.7 ± 0.9°C (normal range 36.3–37.2°C, axillary temperature). As for laboratory examination results, only one-third of the patients had abnormal white blood cell (WBC) count results; however, around two-thirds were having a higher neutrophil percentage (NEUT%). The average C-reactive protein (CRP) concentration was 5.5 ± 6.4 mg/dl, which was more elevated than the upper limit of the normal range (0.8 mg/dl). 48.5% of the patients had malignancy, followed by immune diseases (15.5%), and infectious diseases (13.4%). In addition, 40.2% of the patients underwent invasive surgeries, while 38.1% had an indwelling catheter.
Table I.
Characteristics of the patients with M. luteus BSI.
| Variable | Value |
|---|---|
| Age (year), mean ± SD (range) | 51.9 ± 22.0 (2-92) |
| Age ≤ 12 | 8 (8.2) |
| Age ≥ 60 | 38 (39.2) |
| Male | 61 (62.9) |
| BMI, mean ± SD (range) | 22.6 ± 4.5 (13.4-35.4) |
| SOFA Score, mean ± SD (range) | 1.4 ± 1.8 (0-8) |
| Infection type | |
| Hospital-acquired | 70 (72.2) |
| Community-acquired | 27 (27.8) |
| Hospitalization time (day), mean ± SD (range) | 32.2 ± 36.4 (6-267) |
| Polymicrobial bloodstream infection | 5 (5.2) |
| Symptom | |
| Fever | 87 (89.7) |
| Chill | 18 (18.6) |
| Fatigue | 12 (12.4) |
| Maximal body temperature (T, °C), mean ± SD (range) | 38.7 ± 0.9 (36.5-41.0) |
| 37.2 < T ≤ 38 | 8 (8.2) |
| 38 < T ≤ 39 | 43 (44.3) |
| 39<T<41 | 36 (37.1) |
| WBC count (× 109/l), mean ± SD (range) | 7.9 ± 4.3 (0.6-21.8) |
| WBC count above 10 × 109/l (n = 95) | 31 (32.6) |
| NEUT%, mean ± SD (range) | 72.2 ± 18.3 (4-97.6) |
| NEUT% above 70% (n = 92) | 58 (63.0) |
| CRP concentration (mg/dl), mean ± SD (range) | 5.5 ± 6.4 (0.09-33.5) |
| CRP above 0.8 mg/dl (n = 79) | 60 (75.9) |
| Underlying diseases | |
| Malignancy | 47 (48.5) |
| Immune diseases | 15 (15.5) |
| Infectious diseases | 13 (13.4) |
| Risk factors | |
| Underwent invasive surgery | 39 (40.2) |
| With indwelling catheter | 37 (38.1) |
| ICU stay | 15 (15.5) |
| Any of the above | 65 (67.0) |
| Outcome | |
| Survive | 94 (96.9) |
| Died | 3 (3.1) |
| Causes of death | |
| Lung infection, renal failure | 1 (33.3) |
| Pancreatic cancer, hepatic encephalopathy | 1 (33.3) |
| Acute leukemia | 1 (33.3) |
BMI – body mass index, SOFA – sequential organ failure assessment,
WBC – white blood cell, NEUT% – neutrophil percentage, CRP – C-reactive protein,
ICU – intensive care unit
Antimicrobial susceptibility test. As shown in %Table II and Table SI, data of antimicrobial susceptibility tests for 90 patients were collected and analyzed. Of the 14 antibiotics tested, linezolid was found to have the largest diameter of the inhibition zone (36 mm), followed by cefoxitin (35 mm), rifampin (33 mm), cefazolin (32 mm), and penicillin (32 mm), while erythromycin was found to have the most minor average diameter of the inhibition zone (15 mm). Overall, except for erythromycin, all the other antimicrobial agents tested were considered to have a strong effect against M. luteus. However, some M. luteus strains were found to be comparatively less responsive to gentamycin, cephalosporins, levofloxacin, and carbapenems comparing to other strains (Table SI, strains nos. 5, 15, 51, 69, and 92).
Table II.
Results of antimicrobial susceptibility tests
| Antimicrobial agent | Number of samples | Average diameter of inhibition zone (mm) |
|---|---|---|
| Linezolid | 90 | 36 |
| Cefoxitin | 88 | 35 |
| Rifampin | 87 | 33 |
| Cefazolin | 89 | 32 |
| Penicillin | 90 | 32 |
| Ampicillin-sulbactam | 89 | 31 |
| Cefuroxime | 88 | 31 |
| Trimethoprim-sulfamethoxazole | 90 | 29 |
| Ertapenem | 90 | 27 |
| Gentamycin | 90 | 26 |
| Vancomycin | 88 | 25 |
| Levofloxacin | 90 | 25 |
| Clindamycin | 90 | 25 |
| Erythromycin | 89 | 15 |
Antimicrobial treatment for patients with M. luteus BSI. All the patients with M. luteus BSI received antimicrobial treatment.
Cephalosporins (59.2%) and quinolones (21.4%) were the most commonly used antibiotics for empirical therapies. After the results of susceptibility tests returned, the antibiotic usage shifted to cephalosporins (35.2%), glycopeptides (26.7%), and carbapenems (18.1%). The results were shown in Table SII.
Discussion
According to the College of American Pathologists, Micrococcus species used to be considered one of the most common blood contaminants (Dargère et al. 2018). However, some subsets, such as M. luteus, have been proven to cause infections under certain circumstances (Fosse et al. 1985; Hirata et al. 2009; Ianniello et al. 2019). At present, it is still challenging to distinguish between M. luteus infection and contamination. Clinical judgment, positive blood culture bottles, short time to growth period (time of bacterial inoculation to detection), and new microbiologic technologies are considered possible methods to avoid blood culture contamination. In our study, all the patients had at least two consecutive positive blood culture results. They had BSI-related clinical symptoms, with a comparatively short time to growth period, and in most cases (94.8%) M. luteus was the only pathogen identified. These showed that the patients were more likely to suffer from M. luteus BSI than blood culture contamination.
From 2010 to 2019, 97 patients with M. luteus BSI were included in this study, making it the study with the largest sample size to date. According to our results, M. luteus is an uncommon BSI pathogen, with incidence lower than 13 per 100,000. A favorable prognosis was expected in patients with M. luteus BSI, as most patients had low SOFA scores and survived through their hospitalization. Those who died were suffering from other severe diseases rather than M. luteus BSI (Table I), indicating that M. luteus was of low virulence. The most common features of patients with M. luteus BSI are abrupt fever, high blood NEUT%, and CRP concentration, and comparatively normal WBC count results. Immunocompromised patients, especially those who undergo invasive operations or with indwelling catheters, are more likely to suffer from M. luteus BSI, according to former research (Miltiadous and Elisaf 2011).
As only a few cases of M. luteus BSI was confirmed during recent decades, the detailed antibiotic susceptibility of M. luteus remained unclear. Here we presented data of different isolates from 90 patients (%Table II and Table SI). Due to the lack of evidence of proper breakpoints for zone diameters and MICs for M. luteus, we were unlikely to judge if the isolates were susceptible or resistant to a specific antibiotic precisely. Hence, we only reported the average diameters of inhibition zones in this study. Overall, the average inhibition zone diameters of most of the antibiotics tested were large, ranging from 25 mm to 36 mm. Erythromycin, however, was found to have a smaller average diameter of inhibition zone (15 mm), which might be attributed to the existence of certain linear plasmids. In 2002 and 2010, Wolfgang Liebl and Julian R. Dib discovered two novel plasmids designated pMEC2 and pLMA1, which conferred resistance to erythromycin in M. luteus (Liebl et al. 2002; Dib et al. 2010). Hence macrolides such as erythromycin are not recommended as antimicrobial agents for M. luteus BSIs. Although most M. luteus strains were responsive to almost every antibiotic tested, some isolates were found to be less responsive to gentamycin, most cephalosporins, levofloxacin, and even carbapenems (Table SI, No. 5, 15, 51, 69, and 92). For BSI induced by these M. luteus strains, glycopeptides should be considered for antimicrobial treatment.
Empirical treatment for M. luteus infection has not yet reached any consensus. In the past, patients with native valve infective endocarditis and brain abscess due to M. luteus had been treated with vancomycin, aminoglycosides, rifampin, and beta-lactam antibiotics, and all yielded good responses (Miltiadous and Elisaf 2011; Erbasan 2018; Ianniello et al. 2019; Khan et al. 2019). In FMC-PLAGH, cephalosporins and quinolones are frequently used empirically for they have a broad antibacterial spectrum and fewer adverse events. This strategy was also proven to be useful as most M. luteus strains were responsive to these antibiotics. For M. luteus strains that were less responsive to multiple antibiotics, vancomycin and teicoplanin are proper candidates as they showed good inhibiting ability against these strains (Table SI). Nevertheless, the frequent use of glycopeptides (26.7%) in this study was considered unnecessary and should be avoided, for it might give rise to the appearance of multidrug-resistant bacteria. Instead, for most M. luteus BSIs, cephalosporins, quinolones, or other antibiotics other than macrolides will be enough for antimicrobial treatment.
However, this study has several limitations. The incidence of M. luteus BSI could vary among different countries, regions, or even hospitals, giving rise to significant bias. Moreover, all the data in this study was collected retrospectively so that some details on patients’ symptoms could be lost. Therefore, further researches are still needed to discover more detailed features of M. luteus BSI.
Conclusions
As an unusual infection, M. luteus BSI mainly happens in immunocompromised patients or those with former invasive surgeries or indwelling catheters. High NEUT% and CRP levels were commonly seen in M. luteus BSI patients. M. luteus strains were mostly responsive to linezolid, cefoxitin, and rifampin, but were less responsive to erythromycin. Cephalosporins and quinolones are effective empirical antibiotics for M. luteus BSI; however, vancomycin and teicoplanin should be considered for potentially broadly drug-resistant M. luteus strains.
Supplementary Material
Acknowledgments
We thank Dr. Gang Chen and Dr. Cheng Ye for their valuable advice.
Ethical statement
Approval was obtained from the ethics committee of FMC-PLAGH. The ethics committee board of FMC-PLAGH waived the consent to participate form of this retrospective study.
Funding
This work was supported by the National Key R&D Program of China (grant number 2016YFC1304700) and the Military Medical Innovation Program of China (grant number 16CXZ041).
Conflict of interest
The authors do not report any financial or personal connections with other persons or organizations, which might negatively affect the contents of this publication and/or claim authorship rights to this publication.
Literature
- Andreopoulos T, Papanikolaou G, Politou M, Konstantopoulos K, Stefanou J, Loukopoulos D. Micrococcus luteus: a putative cause of hepatic abscess? Panminerva Med. 2000September;42(3):231–232. [PubMed] [Google Scholar]
- CDC . National Healthcare Safety Network (NHSN). Bloodstream infection event (Central line-associated bloodstream infection and non-central line associated bloodstream infection). Atlanta (USA): Centers for Disease Control and Prevention; 2021. [cited 2021 Jun 20]. Available from https://www.cdc.gov/nhsn/pdfs/pscmanual/4psc_clabscurrent.pdf [Google Scholar]
- Dargère S, Cormier H, Verdon R. Contaminants in blood cultures: importance, implications, interpretation and prevention. Clin Microbiol Infect. 2018September;24(9):964–969. 10.1016/j.cmi.2018.03.030 [DOI] [PubMed] [Google Scholar]
- Dib JR, Wagenknecht M, Hill RT, Farías ME, Meinhardt F. First report of linear megaplasmids in the genus Micrococcus. Plasmid. 2010January;63(1):40–45. 10.1016/j.plasmid.2009.10.001 [DOI] [PubMed] [Google Scholar]
- Erbasan F. Brain abscess caused by Micrococcus luteus in a patient with systemic lupus erythematosus: case-based review. Rheumatol Int. 2018December;38(12):2323–2328. 10.1007/s00296-018-4182-2 [DOI] [PubMed] [Google Scholar]
- Fosse T, Toga B, Peloux Y, Granthil C, Bertrando J, Sethian M. Meningitis due to Micrococcus luteus. Infection. 1985November;13(6): 280–281. 10.1007/BF01645439 [DOI] [PubMed] [Google Scholar]
- Hirata Y, Sata M, Makiuchi Y, Morikane K, Wada A, Okabe N, Tomoike H. Comparative analysis of Micrococcus luteus isolates from blood cultures of patients with pulmonary hypertension receiving epoprostenol continuous infusion. J Infect Chemother. 2009; 15(6):424–425. 10.1007/s10156-009-0720-X [DOI] [PubMed] [Google Scholar]
- Ianniello NM, Andrade DC, Ivancic S, Eckardt PA, Lemos Ramirez JC. Native valve infective endocarditis due to Micrococcus luteus in a non-Hodgkin’s lymphoma patient. IDCases. 2019;18: e00657. 10.1016/j.idcr.2019.e00657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A, Aung TT, Chaudhuri D. The first case of native mitral valve endocarditis due to Micrococcus luteus and review of the literature. Case Rep Cardiol. 2019December04;2019:1–3. 10.1155/2019/5907319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebl W, Kloos WE, Ludwig W. Plasmid-borne macrolide resistance in Micrococcus luteus. Microbiology. 2002August01;148(8):2479–2487. 10.1099/00221287-148-8-2479 [DOI] [PubMed] [Google Scholar]
- Miltiadous G, Elisaf M. Native valve endocarditis due to Micrococcus luteus: a case report and review of the literature. J Med Case Reports. 2011December;5(1):251. 10.1186/1752-1947-5-251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peces R, Gago E, Tejada F, Laures AS, Alvarez-Grande J. Relapsing bacteraemia due to Micrococcus luteus in a haemodialysis patient with a Perm-Cath catheter. Nephrol Dial Transplant. 1997November1; 12(11):2428–2429. 10.1093/ndt/12.11.2428 [DOI] [PubMed] [Google Scholar]
- Wharton M, Rice JR, McCallum R, Gallis HA. Septic arthritis due to Micrococcus luteus. J Rheumatol. 1986June;13(3):659–660. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


