Abstract
Background
Most Hepatocellular Carcinomas (HCCs) are diagnosed at an advanced stage. However, HCC early diagnosis is complicated by the coexistence of inflammation and cirrhosis. The unsatisfactory sensitivity and specificity of Alpha-fetoprotien (AFP) for screening of early-stage HCC paved the way for new novel biomarkers to complement AFP such as AFP-L3. The aim of this study was the Evaluation of alpha fetoprotein-L3 (AFP-L3) as earlier marker in diagnosis of hepatocellular carcinoma in Egyptian patients. This study was conducted on 80 patients categorized into 2 groups; group 2 (40 patients with chronic active hepatitis) and group 3 (40 patients with HCC). HCC diagnosis was done by clinical, triphasic CT and positive US for focal lesion, in addition to 20 healthy individuals as controls (group 1).
Results
The median range of AFP and AFP-L3 were highly statistically significant difference between HCC group and other groups [p < 0.001]. In this study ALT, AST, Total & direct bilirubin and albumin results showed highly significant differences between HCC group and other groups. Serum AFP-L3 shows sensitivity 100%, specificity 100%, positive predictive value 100% and negative predictive value 100% with AUC = 1 in HCC cases.
Conclusion
Serum AFP-L3 may serve as a diagnostic biomarker for the detection of early stage of HCC and show higher sensitivity than AFP.
Keywords: AFP-L3, HCC, Biomarkers
Abbreviations: AFP, α- fetoprotein; AFP-L3, α- fetoprotein L3; HCC, Hepatocellular carcinoma; CAH, chronic active hepatitis; HCV, Hepatitis C virus
1. Introduction
HCC is represents the sixth common cancer and the fourth leading cause of cancer mortality worldwide. Geographical distribution of HCC varies throughout the world (Bray et al., 2018).
In Egypt it represents the fourth common cancer (Rashed et al., 2020) ;it is the most cancer occur in men and the second in women (Elshamy, 2016).
The large majority of HCC cases occur in the setting of chronic liver disease, with cirrhosis (e.g. HCV-infected cirrhotic patients and HBV-infected cirrhotic patients) being the primary risk factor for HCC(Singal et al., 2020).
Nevertheless, the majority of HCC patients are diagnosed at later stages, restricting therapeutic options to a mild treatment that leads to medium survival of < 1 year (Altekruse et al., 2009). In this regard, across the abdominal Ultrasound is presently the most widely used instrument For HCC detection and monitoring, mainly due to Cost-effectiveness.
Recently, AFP is the most widely serum biomarker for HCC patients diagnosis. However, the diagnostic specificity and sensitivity of AFP biomarker was far below satisfaction in the early stage (Zhang et al., 2019). Thus, it is imperative to identify novel biomarkers for HCC diagnosis.
AFP has one sugar chain per molecule, and it can be divided into the L. culinaris agglutinin non-binding fraction (L1), the weakly binding fraction (L2), and associate effectively binding fraction (L3). AFP-L3 is detected mainly in patients with HCC (Sauzay et al., 2016).
AFP L3 is produced by malignant hepatocytes, even if HCC is at its early Stages (Elsawabi et al., 2019, Li et al., 2001). It can be detected in about 35% of patients with small mass of HCC (<3 cm).Therefore, AFP-L3 might be used as a reliable early HCC biomarker (Yen et al., 2018). The present study was aim to evaluate AFP-L3 as earlier marker in the diagnosis of HCC in Egyptian patients.
2. Material and methods
This study was conducted on 100 subjects. They were selected from out patient's clinics and Tropical medicine Department in New Damietta hospital, Al-Azhar University, Egypt between October 2018 and October 2019. Blood samples were gathered from all subjects that were classified into three groups:
Group 1 (control group): includes 20 apparently healthy individual.
Group 2: includes 40 patients subdivided into two groups
-
I-
(20 Patients) suffering from Chronic active hepatitis (CAH) without complications.
-
II-
(20 Patients) suffering from chronic active hepatitis (CAH) with complications (end stage liver cirrhosis).
Group 3: includes 40 patients with HCC on top of CAH.
-
a-
(10 patients) with early HCC: tumor size between (0.6to 2.5 cm).
-
b-
(30 patients) with late stage HCC: tumor size>5 cm.
2.1. Inclusion criteria
Inclusion criteria of group1 [control]: the subjects in the control group were apparently healthy with normal all laboratory tests and ultrasound (U/S) and did not have any complaints.
Inclusion criteria of group 2: included patients with CAH post virus C or virus B.
Inclusion criteria of group 3 [HCC]: included patients with a compatible clinical picture of HCC, with triphasic CT and positive U/S for focal lesion.
2.2. Exclusion criteria
1- CAH & HCC due to other causes.
2- Chronic systemic diseases e.g. Diabetes mellitus, renal disorders, hypertension, autoimmune diseases and other malignancies.
2.3. Methods
All included patients were subjected to the following:
-
1-
Detailed history taking: including personal history with complete physical examination.
-
2-
Laboratory investigations: Complete blood count (CBC) by coulter counter (automated haematology analyzer, MEK-631 K, NIHON KOHDEN Corporation Japan 1999) system., liver function tests (ALT,AST, direct bilirubin, serum albumin and total bilirubin), kidney function tests (creatinine) by automatic analyzer (Hitachi 801).
-
3-
AFP was measured using (Chimiluminescence Immunoassay, SIEMENS)
-
4-
AFP-L3 was determined using ELISA kit designed for human Alpha-Fetoprotien Lens Culinaris Agglutiin 3 (AFP-L3) in serum. (ELSIA, Shanghai Korain Biotech Co., LTD)
2.4. 2.4. Statistical Analysis
The collected data were analyzed and processed using the SPSS (Statistical Package for Social Sciences) version 22. Basic descriptive statistics including means and standard deviation were performed. Comparison of qualitative data between groups was calculated by Chi-square test. ANOVA (Analysis of variance) test and Kruskal Wallis test (KW) were used to compare quantitative data: means and standard deviations between groups. Differences were considered statistically significant if p-value < 0.05.
3. Results
In Table 1, the median range of AFP and AFP-L3 between HCC group and other groups were highly statistically significantly different (p < 0.001).
Table 1.
Comparison of the (AFP and AFP-L3) within the studied groups.
| Variable | Group 1 (control group) N = 20 | Group 2 |
Group 3 (CAH + |
Test of sig. | ||
|---|---|---|---|---|---|---|
| CAH without complications N = 20 | CAH with complications N = 20 | Early HCC N = 10 | Late HCC N = 30 | |||
| AFP | ||||||
| Median (range) | 3.85 (2–6.3) | 7.21 (3.9–12) | 10.8 (4.1–17.2) | 23.4 (10.7 – 40.1) | 1120.5 (0.96 – 20203) | KW = 78.751P < 0.001* |
| P1 | 0.284 | 0.042* | 0.005* | < 0.001* | ||
| P2 | 0.257 | 0.039* | < 0.001* | |||
| P3 | 0.063 | < 0.001* | ||||
| P4 | < 0.001* | |||||
| AFB-L3 | ||||||
| Median (range) | 0.36 (0.15–0.61) | 0.825 (0.55–1.6) | 2.21 (1.1 – 4.8) | 128.5 (23– 156) | 187.86 (68.52–685.14) | KW = 91.590P < 0.001* |
| P1 | 0.049* | 0.015* | < 0.001* | < 0.001* | ||
| P2 | 0.028* | < 0.001* | < 0.001* | |||
| P3 | < 0.001* | < 0.001* | ||||
| P4 | 0.047* | |||||
KW: Kruskal Wallis test
P = Inter group significance
P1 = significance in relation to control group
P2 = significance in relation to CAH without complications group
P3 = significance in relation to CAH with complications group
P4 = significance in relation to early HCC group
Statistically significant if P < 0.05
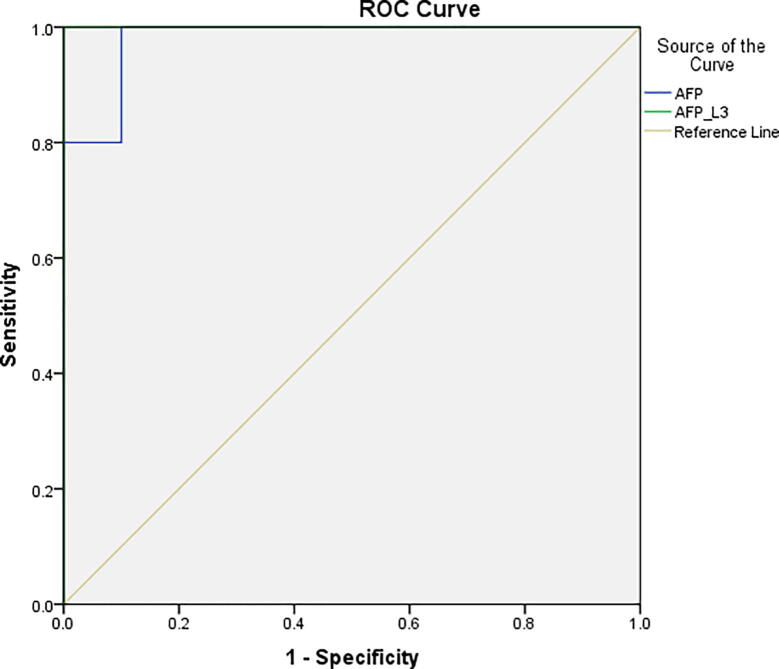
In Table 2 and Fig. 1, AFP-L3 shows more Sensitivity100% and Specificity100% than AFP. Also, AFP-L3 shows PPV, NPV and Accuracy 100%. However, AFP shows Sensitivity 60%, Specificity 85%, PPV 65%, NPV 80% and Accuracy 75%.
Table 2.
Prediction of ability of AFP and AFP_L3 to differentiate between CAH without complications & early HCC cases.
| AFP | AFP-L3 | |
|---|---|---|
| AUC | 0.980 | 1 |
| Cut off point | >4.3 | >12.3 |
| Sensitivity | 60% | 100% |
| Specificity | 85% | 100% |
| PPV | 65% | 100% |
| NPV | 80% | 100% |
| Accuracy | 75% | 100% |
| P | 0.003* | < 0.001* |
AUC: area under the curve.
P: probability.
NPV: Negative predictive value.
PPV: Positive predictive value.
significant p value (<0.05).
Fig. 1.
Shows Sensitivity and Specificity of AFP and AFP-L3 in CAH without complication &early HCC cases.
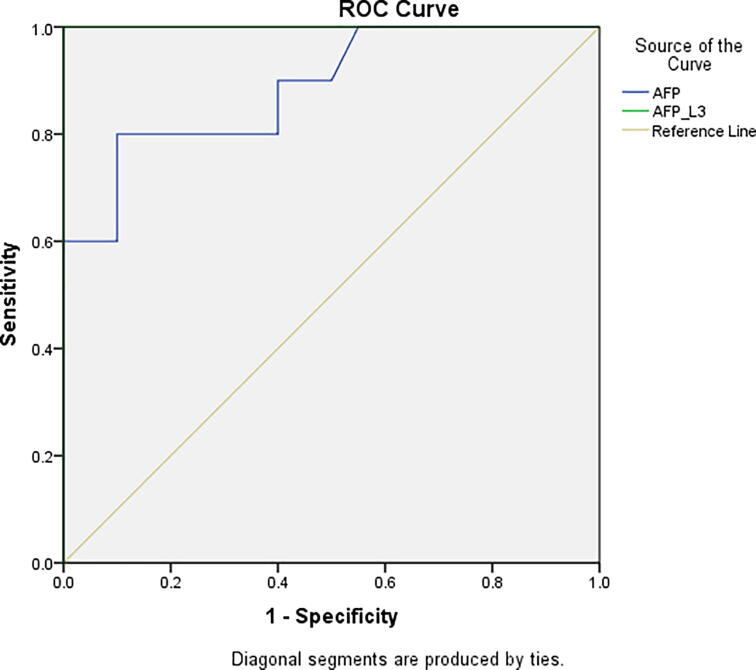
Table 3 and Fig. 2 show AFP as predictor of HCC that gave sensitivity (80%), specificity (90%), PPV (90%), NPV (80%) and Accuracy (80%). However, AFP-L3 as predictor of HCC had (sensitivity 100%, specificity 100%, PPV 100%, NPV 100% and Accuracy 100%).
Table 3.
Prediction of ability of AFP and AFP_L3 to differentiate CAH cases with complications and early HCC cases.
| AFP | AFP-L3 | |
|---|---|---|
| AUC | 0.888 | 1 |
| Cut off point | >16.15 | >13.9 |
| Sensitivity | 80% | 100% |
| Specificity | 90% | 100% |
| PPV | 90% | 100% |
| NPV | 80% | 100% |
| Accuracy | 80% | 100% |
| P | 0.001* | < 0.001* |
AUC: area under the curve.
P: probability.
NPV: Negative predictive value.
PPV: Positive predictive value.
significant p value (<0.05).
Fig. 2.
Shows Sensitivity and Specificity of AFP and AFP-L3 in CAH cases with complication &early HCC cases.
4. Discussion
HCC is represents the sixth common cancer and the fourth leading cause of cancer mortality worldwide. Geographical distribution of HCC varies throughout the world (Bray et al., 2018).
Recently, AFP is the most widely serum biomarker for HCC patients diagnosis. However, the diagnostic specificity and sensitivity of AFP biomarker was far below satisfaction in the early stage (Zhang et al., 2019). Thus, it is imperative to identify novel biomarkers for HCC diagnosis.
AFP L3 is produced by malignant hepatocytes, even if HCC is at its early Stages (Elsawabi et al., 2019, Li et al., 2001). It can be detected in about 35% of patients with small mass of HCC (<3 cm).
In the current study, there was a significant increase in AFP level in the HCC group when compared to control and CAH groups (p value = 0.001). This result is in agreement with other studies (Mehinovic et al., 2018), (Shalably et al., 2019), (Ibrahim et al., 2018). The increase in secretion of AFP during the development of HCC to inhibit immune response of liver cancer cells caused by increasing in selective transcriptional activation in AFP gene in the malignant hepatocytes showed by (Wu et al., 2006).
Moreover, we found in this context that the diagnostic performance of AFP as a marker for differentiating HCC from benign liver diseases and healthy candidates revealed that the diagnostic sensitivity was 80%, the specificity was 90%, PPV was 90% and NPV was 80% and accuracy was 80%, as shown in (Table 3) that is not far from the results of (Ibrahim et al., 2018)who reported that sensitivity of HCC is (75%) and specificity was (100%). Another study by (Mehinovic et al., 2018) reported that AFP has sensitivity, and specificity ranges from 39% to 97%, and 76% to 95%, respectively.
The problem with AFP as a biomarker for HCC is that in up to 40% of HCC patients, AFP levels are normal, especially in the early stage of the disease (Qi et al., 2019). Addition to the fact that several patients with benign liver diseases, such as gastrointestinal cancer, chronic hepatitis and liver cirrhosis also have elevated serum AFP (Tsuchiya et al., 2015, Omata et al., 2010) found that a significant percentage of small HCCs < 3 cm do not secrete AFP to reach a diagnostic level. Moreover, the level of AFP is elevated in patients with both chronic liver disease and HCC. Consequently, there is a wide overlap between the two groups.
Furthermore, (Stefaniuk et al., 2010) reported significant higher AFP levels accompanying many liver diseases (viral hepatitis, liver cirrhosis, liver tumors: primarily HCC and hepatoblastoma) and other neoplasms (cancers of the digestive tract: pancreas, stomach, gallbladder and large intestine).
In this study, we found that there was a statistically highly significant elevation (P < 0.001) in AFP-L3 level among HCC cases when compared with cases with no HCC. This is in agreement with other studies (Hu et al., 2019), (El-Halawany et al., 2020), (Park et al., 2017)in which the median value of serum AFP-L3 was significantly higher in HCC cases when compared with cases without HCC.
In the current study, we found that AFP-L3 showed sensitivity 100%, specificity 100%, PPV 100% and NPV 100%. Therefore, it was more specific and sensitive than AFP as shown in Table (3). This result is close to a study by (Ibrahim et al., 2018) in which AFP-L3 showed sensitivity of 97.5% and specificity of 100%. Our result were even higher than those reported by (Cerban et al., 2019) , where they assessed the diagnostic performance of AFP-L3 in the diagnosis of HCC. The sensitivity of AFP-L3 was 84.75% and specificity was 75.76%. Also, the study of (El-Halawany et al., 2020) reported that the sensitivity and specificity of diagnostic value of AFP-L3 were 80% which approach with our result. The sensitivity of AFP-L3 was found to be associated with HCC stage. In small HCC (HCC < 2 cm in diameter), AFP-L3 had a sensitivity of only 45%. The sensitivity increased with increase in size of the HCC, and reached 90% when HCC was 5 cm in diameter or greater (Stefaniuk et al., 2010).
This study had certain limitations. The data were obtained in a single center; the study population was small, in the future the study will be carried out on a large scale and in various stages of HCC.
5. Conclusion
In the current study we found serum AFP-L3 may serve as a novel diagnostic biomarker for the detection of early stage of HCC with higher sensitivity than AFP.
6. Ethics declaration
6.1. Ethics approval and consent to publication
This study was reviewed and approved by the Research Ethics Committee of the Faculty of Science, Mansoura University. Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the research committee and informed consent was obtained from every subject.
7. Availability of data and material
All data and materials are available.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Hassnaa M. Ibrahim, Email: nanasam883@gmail.com.
Magdy Z. Elghannam, Email: elghannam2017@gmail.com.
Om Ali Y. Elkhawaga, Email: dr.omaliyousef@gmail.com.
Ahmed M.A. El-Sokkary, Email: aelsokkary@mans.edu.eg.
References
- Altekruse S.F., McGlynn K.A., Reichman M.E. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J. Clin. Oncol. 2009;27(9):1485. doi: 10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray F. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- Cerban R. Alpha-Fetoprotein, Alpha-Fetoprotein-L3, Protein Induced by Vitamin K Absence, Glypican 3 and Its Combinations for Diagnosis of Hepatocellular Carcinoma. Surg. Gastroenterol. 2019;24(1):37–44. [Google Scholar]
- El-Halawany F., Mahmoud S., Oun M.R. Detection of Serum Biomarkers In Hepatocellular Carcinoma Patients. MAGHREB-MACHREK. 2020;2(2):01–12. [Google Scholar]
- Elsawabi A.S. α-Fetoprotein (AFP)-L3% and transforming growth factor B1 (TGFB1) in prognosis of hepatocellular carcinoma after radiofrequency. Egypt. Liver J. 2019;9(1):1–7. [Google Scholar]
- Elshamy K. Springer; 2016. Challenges and future trends for cancer care in Egypt Cancer Care in Countries and Societies in Transition; pp. 117–146. [Google Scholar]
- R. Hu S. Zhao B. Shen G. Guo Hu, R., Zhao, S., Shen, B., & Guo, G. (2019). Diagnostic value of serum alpha-fetoprotein, alpha-fetoprotein variant and abnormal prothrombin in primary hepatocellular carcinoma. Zhonghua gan zang bing za zhi= Zhonghua ganzangbing zazhi= Chinese journal of hepatology, 27(8), 634-637 [DOI] [PubMed]
- Ibrahim A.A.A. Diagnostic accuracy of lectin-reactive α-fetoprotein (AFP-L3) in the diagnosis of hepatitis C virus-related hepatocellular carcinoma. Benha Med. J. 2018;35(3):312. [Google Scholar]
- Li D., Mallory T., Satomura S. AFP-L3: a new generation of tumor marker for hepatocellular carcinoma. Clin. Chim. Acta. 2001;313(1–2):15–19. doi: 10.1016/s0009-8981(01)00644-1. [DOI] [PubMed] [Google Scholar]
- Omata M., Lesmana L.A., Tateishi R., Chen P.-J., Lin S.-M., Yoshida H. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol. Int. 2010;4(2):439–474. doi: 10.1007/s12072-010-9165-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehinovic L. Evaluation of Diagnostic Efficiency of Alpha-Fetoprotein in Patients with Liver Cirrhosis and Hepatocellular Carcinoma: Single-Center Experience. Open Access Macedonian J. Med. Sci. 2018;6(9):1668. doi: 10.3889/oamjms.2018.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.J. Usefulness of AFP, AFP-L3, and PIVKA-II, and their combinations in diagnosing hepatocellular carcinoma. Medicine. 2017;96(11) doi: 10.1097/MD.0000000000005811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi F. The diagnostic value of PIVKA-II, AFP, AFP-L3, CEA, and their combinations in primary and metastatic hepatocellular carcinoma. J. Clin. Lab. Anal. 2019;e23158 doi: 10.1002/jcla.23158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashed W.M., Kandeil M.A.M., Mahmoud M.O., Ezzat S. Hepatocellular Carcinoma (HCC) in Egypt: A comprehensive overview. J. Egypt. National Cancer Ins. 2020;32(1):1–11. doi: 10.1186/s43046-020-0016-x. [DOI] [PubMed] [Google Scholar]
- Sauzay C. Alpha-foetoprotein (AFP): A multi-purpose marker in hepatocellular carcinoma. Clin. Chim. Acta. 2016;463:39–44. doi: 10.1016/j.cca.2016.10.006. [DOI] [PubMed] [Google Scholar]
- Shalably N.M. Evaluation of Fucosylated Haptoglobin as a Diagnostic Biomarker for Hepatocellular Carcinoma in Egypt. The Open Biomarkers J. 2019;9(1) [Google Scholar]
- Singal A.G., Lampertico P., Nahon P. Epidemiology and surveillance for hepatocellular carcinoma: New trends. J. Hepatol. 2020;72(2):250–261. doi: 10.1016/j.jhep.2019.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefaniuk P., Cianciara J., Wiercinska-Drapalo A. Present and future possibilities for early diagnosis of hepatocellular carcinoma. World J. Gastroenterol.: WJG. 2010;16(4):418. doi: 10.3748/wjg.v16.i4.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya N. Biomarkers for the early diagnosis of hepatocellular carcinoma. World J. Gastroenterol.: WJG. 2015;21(37):10573. doi: 10.3748/wjg.v21.i37.10573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W. Combined serum hepatoma-specific alpha-fetoprotein and circulating alpha-fetoprotein-mRNA in diagnosis of hepatocellular carcinoma. Hepatobiliary Pancreatic Dis. Int.: HBPD INT. 2006;5(4):538–544. [PubMed] [Google Scholar]
- Yen C.-W. Did AFP-L3 save ultrasonography in community screening? Kaohsiung J. Med. Sci. 2018;34(10):583–587. doi: 10.1016/j.kjms.2018.05.005. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Wang Y., Liu H., Li B. Six genes as potential diagnosis and prognosis biomarkers for hepatocellular carcinoma through data mining. J. Cell. Physiol. 2019;234(6):9787–9792. doi: 10.1002/jcp.27664. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and materials are available.