Abstract
The present study focused on extracting green larvicides from extracts of the combination of Foeniculum vulgare and Matricaria chamomilla using different solvents of increasing polarity in a Soxhlet extractor and evaluating their ovicidal, larvicidal, and cytotoxic activities. The most promising among all tested extracts was hexane extract. The ovicidal activity of the hexane PH2 extract resulted in a significant (p < 0.05) decrease in egg hatchability from 95.00 ± 6.16% to 15 ± 9.04% at doses ranging from 62.5 to 500 µg/mL. The larval mortality with the hexane extract ranged from 13.33 ± 3.3% to 93.33 ± 3.3% at doses ranging from 31.25 to 250 µg/mL, respectively. The LC50 and LC90 values of the larvicidal activity of the hexane extract were estimated to be 148.3 and 242.17 µg/mL, respectively, after 24 h of exposure. Similarly, the LC50 values after 48 and 72 h of exposure were 124.93 and 100.3 µg/mL, respectively, against the third instar of Cx. pipiens. PH2 treatment of larvae resulted in histopathological changes such as degenerated epithelial cells and destruction of microvilli on the epithelial cells. The PH2 extract achieved a dose-dependent decrease in the rate of cell survival. The IC50 value of PH2-treated HUVECs was 192.07 µg/mL after 24 h of incubation. The cells showed changes in cellular and nuclear morphology. In conclusion, the hexane extract of PH2 could be used in mosquito management programs.
Keywords: Larvicide, Plant extract, Vector mosquito, Culex pipiens, Extract, Non-target organism
1. Introduction
Mosquito-borne diseases are increasing in incidence and geographical distribution, emerging in new areas (Paixão et al., 2018, Stanaway et al., 2016, Bhatt et al., 2013). Around 500 million cases and 2.7 million deaths due to mosquito-borne diseases are recorded every year (Bustamante, 2020). There is currently no specific treatment for dengue, chikungunya, Japanese encephalitis, West Nile virus, Ross river fever, St. Louis encephalitis, and Zika fever. Patients are hospitalized to receive only supportive treatment. In addition, in some diseases like malaria, the available drugs have become useless due to the development of resistance (White, 2004).
Although vector control is the best tactic to reduce disease transmission, this approach mainly uses synthetic insecticides, which cause many problems to human health and non-target organisms, as well as constituting environmental pollution, resulting in ecological disruption without solving the problem of insecticide resistance and the appearance of new species (Devine and Furlong, 2007, Sarwar et al., 2009).
Thus, researchers’ attention has been directed to other alternatives to reduce the risks associated with using synthetic insecticides. Such alternatives include natural products, particularly botanical extracts, since they are rich in secondary metabolites. These have been proven to have promising different biological activities (JUCÁ, Mércia Marques et al., 2020, Ntie-Kang et al., 2016), including insecticidal and insect-repelling activities (Mishra et al., 2020). Natural products have attracted substantial interest as insecticidal agents because they are target-specific, less toxic, and biodegradable and can overcome insecticidal resistance (Younoussa et al., 2020, Mishra et al., 2020, Said-Al Ahl et al., 2017).
Foeniculum vulgare Mill (Apiaceae) and Matricaria chamomilla L (Asteraceae) are plants that are widely used in traditional healers (Diaz-Maroto et al., 2006, Miraj and Alesaeidi, 2016). The activities of these two plants have been documented in many review articles regarding their antibacterial, antifungal, antioxidant, antithrombotic, anti-inflammatory, estrogenic, hepatoprotective, antidiabetic, antihirsutism, antidepressive, antiosteoarthritis, and antidiarrheal effects (Rather et al., 2016, Badgujar et al., 2014, Miraj and Alesaeidi, 2016).
In insect control research, the use of combinations of insecticides is recommended not only to improve the efficiency of insecticides but also to overcome insect resistance and preserve the efficacy of insecticides for many years (Nelson and Kursar, 1999, Younoussa et al., 2020). Combinations of plant extracts are widely reported in the literature, and believed to increase their effects, achieving synergistic or balanced effects (Cheesman et al., 2017) and thus potentially preventing issues regarding insect resistance (Isman et al., 2006). Synergism can result from crude plant extract or the combination of different plant extracts. Herbalists always recommend the use of the crude extract over the use of isolated compounds (Williamson et al., 2009).
In the current investigation, we evaluated the effects of the combination of two plant extracts against the third instar of Cx. pipiens in laboratory conditions.
2. Materials and methods
2.1. Test materials
Plants were purchased from Riyadh Herb Market, Riyadh Province, Saudi Arabia, and the voucher specimens of Foeniculum vulgare family Apiaceae seeds (KSU-031) and Matricaria chamomilla family Asteraceae fruits (KSU-032) were deposited at herbarium of Bioproduct Research Chair, King Saud University. The extracts of F. vulgare seeds and M. chamomilla fruits were ground to a powder, mixed at a 1:1 ratio, and extracted using a Soxhlet extractor. Four different solvents, namely, hexane, chloroform, ethyl acetate, and methanol, were used. Similarly, F. vulgare was extracted individually using the same solvents. All extracts were prepared in DMSO.
2.2. Total phenolic content (TPC)
The TPC of PH2 extract was analyzed using FC reagent (Al-Zharani et al., 2019). Different concentrations of 2 µl of standard gallic acid (5–90 g/ mL) were mixed with 20 µl of FC reagent in a 96-well plate. After mixing, the samples were incubated for 10 min (25 °C); then, 80 µl of 7.5% sodium carbonate was added and incubated in the dark for 2 h at 25 °C. Finally, plate was read at 765 nm and the concentration was calculated. The TPC was calculated as mg/g gallic acid equivalent (GAE/g).
2.3. Total flavonoid content (TFC)
The aluminum chloride method was employed to calculate TFC (Al-Zharani et al., 2019). Quercetin was used for the preparation of a standard curve by dissolving quercetin (1.0 mg) in of DMSO (1.0 mL) then, different concentrations were prepared using DMSO (5–90 μg/mL). Two microliters of standard quercetin solutions or PH2 extract was added to methanol (60 µl), 4 µl of 10% aluminum chloride, 1 M potassium acetate (4 µl), and 112 µl of distilled water in a 96-well plate. After mixing, the plates were kept at 25 °C for 60 min and the absorbance (368 nm) was read (Thermo Scientific Multiskan, China). The concentration was calculated as mg quercetin equivalent (QE)/g. All of the experiments were performed in triplicate.
2.4. Mosquito larvicidal assays
Mosquito colonies of Culex pipiens were maintained as previously described (Al-Mekhlafi, 2018). For each test, 8 mL of water containing 20 larvae (third instar) was placed into a 6-well plate and aliquots of the PH2 methanol, ethyl acetate, and hexane extracts solubilized in DMSO were then added. The DMSO (negative control) was used for comparison. Mortality was reported after 24 and 48 h of treatment. The assays were carried out at 25 ± 2 °C in triplicate with different concentrations of methanol, ethyl acetate (625, 500, 375, and 250 μg/mL), and hexane (250, 125, 62.50, and 31.25 μg/mL). The data was analyzed and LC50 and LC90 values were calculated using SPSS 16 software.
2.5. Ovicidal bioassay
The eggs were kept in a six-well plate containing 8 mL of tap water. Different concentrations of PH2 extract (500, 250, 125, 62.5 and 0 µg/mL) were prepared for the assay. The experiment was carried out in triplicate at 25 ± 2 °C for 24 h and then each sample was transferred to a well containing tap water to assess hatching. DMSO was used as a negative control
2.6. Histopathological changes
The dead treated and control larvae were immersed in formalin (pH 7.2) and processed as reported previously (Al-Mekhlafi, 2018). In brief, each larva was porcesssed in ethanol, embedded in paraffin, and cut into thin sections using a microtome and stained with hematoxylin and eosin and the midgut region was observed under a microscope (Olympus, Japan).
2.7. Cell lines
Human umbilical vein endothelial cells (HUVECs) were purchased from the American Type Cell Culture Collection (ATCC, USA) and grown in Dulbecco’s Modified Eagle Medium (DMEM). DMEM was supplemented with L-glutamine , sodium pyruvate , 10% fetal bovine serum (Gibco, USA) and penicillin–streptomycin 1% (UFC, Saudi Arabia).
2.8. MTT assay
MTT assay was performed as reported previously (Abutaha et al., 2021). Briefly, HUVECs (5 × 105 cells/well) were seeded for 24 h in a 24-well plate. The following day, the cells were treated with PH2 extract and incubated for 24 h. Later, MTT (5 mg/mL) (Merck, USA) solution was removed after incubation (2 h) and DMSO (100 µl) was added. The absorbance was read at 550 nm using a microtiter plate reader (Multiskan™ FC, USA). The percentage survival was calculated and a graph was plotted using Origin software.
2.9. Morphological assessment
Cells (1 × 105 cells/ mL) were seeded for 24 h in a 24-well plate and allowed to adhere overnight. After 24 h of treatment with PH2 extract (200 μg/ mL), the morphological features were observed and imaged under phase-contrast microscope. The cells in the same plate were fixed with ice-cold methanol and then stained with Hoechst 33,342 for 20 min. The wells were washed with PBS and imaged under a fluorescence microscope.
2.10. Fourier transform infrared spectroscopy (FTIR)
PH2 extract was mixed with potassium bromide and compacted into a disk. The samples were scanned by FTIR (NICOLET 6700, USA). The spectrum was recorded within the region of 4000–400 cm−1.
2.11. GC–MS analysis
Hexane extract of PH2 was analyzed in GC–MS using Perkin Elmer Clarus 600 (PerkinElmer, USA) linked to HP-88 column (0.25 μm film × 0.25 mm i.d. × 30 m length). One microliter of the PH2 extract was injected. Helium (99.9%) at a flow rate of 1 mL/min was used. The column oven temperature was set at 40 °C, increased by 10 °C per min to reach 200 °C (held for 3 min), and then held at 300 °C. The phyto-compounds were identified using mass spectral database libraries (Adams and WILEY).
2.12. Statistical analysis
The results are expressed as mean and standard deviation using SPSS program (version 21.0 for Windows; SPSS, Chicago, IL, USA). The significance of differences (p ≤ 0.001) was evaluated using ANOVA followed by Tukey’s test. The LC50 values were assessed using OriginPro 8.5.
3. Results
3.1. Total phenol and flavonoid contents
The total phenol and flavonoid contents of PH2 extract were quantified as 5.82 GAE/mg and 2.18 QE/g, respectively.
3.2. Larvicidal bioassay
The larvicidal potential of the methanol extract ranged from 3.33 ± 3.33% to 66.67 ± 6.67%. The LC50 value was estimated to be 590 µg/mL. The larval mortality of the ethyl acetate extract against the third instar of Cx. pipiens was 13.33 ± 3.33% and 53.33 ± 3.33% at 500 and 625 µg/mL, respectively. The LC50 value was estimated to be 677 µg/mL after 24 h of exposure (Table 1). Meanwhile, the mortality of larvae treated with PH2 hexane extract ranged from 13.33 ± 3.3% to 93.33 ± 3.3% at doses ranging from 31.25 to 250 µg/mL, respectively. The LC50 and LC90 values were estimated to be 148.3 and 242.17 µg/mL, respectively, after 24 h of exposure. Similarly, the LC50 values after 48 and 72 h of exposure were 124.93 and 100.3 µg/mL, respectively (Table 2). No mortality was observed in the negative control (0.1% DMSO). All of the extracts tested showed dose- and time-dependent activity against the third instar of Cx. pipiens.
Table 1.
Larvicidal activity of PH2 methanol and ethyl acetate extracts against Culex pipiens.
| F | df | LC90 (μg/mL) | LC50 (μg/mL) | Mortality (%) | Time | Extract type | |||
|---|---|---|---|---|---|---|---|---|---|
| Concentration (μg/ml) | |||||||||
| 625 | 500 | 375 | 250 | ||||||
| 44.79 | 4 | 0.00 | 590.65 | 66.67 ± 6.67a | 46.67 ± 3.33b | 3.33 ± 3.33c | 0.00 ± 0c | 24 | Methanol |
| 52.18 | 4 | 671.98 | 484.45 | 80.00 ± 5.77 a | 60.00 ± 5.77b | 13.33 ± 6.67c | 6.67 ± 3.33c | 48 | |
| 73.33 | 4 | 627.55 | 301.55 | 93.33 ± 3.33 a | 70.00 ± 5.77 ab | 56.67 ± 3.33 ab | 46.67 ± 8.82b | 72 | |
| 236.17 | 4 | 0.00 | 677.72 | 53.33 ± 3.33 a | 13.33 ± 3.33b | 0.00 ± 00b | 0.00 ± 0b | 24 | Ethyl acetate |
| 89.33 | 4 | 665.92 | 442.08 | 80.00 ± 5.77 a | 56.67 ± 3.33 a | 53.33 ± 3.33 a | 6.67 ± 3.33b | 48 | |
| 12o | 4 | 595.79 | 390.34 | 86.67 ± 3.33 a | 76.67 ± 3.33 ab | 63.33 ± 3.33b | 10.00 ± 00c | 72 | |
Table 2.
Larvicidal activity of PH2 hexane extract against Culex pipiens.
| F | df | LC90 (μg/mL) | LC50 (μg/mL) | Mortality (%) | Time | Extract type | |||
|---|---|---|---|---|---|---|---|---|---|
| Concentration (μg/ml) | |||||||||
| 250 | 125 | 62.50 | 31.25 | ||||||
| 227.87 | 4 | 242.17 | 148.43 | 93.33 ± 3.33 a | 40.00 ± 5.77b | 13.33 ± 3.33c | 0.00 ± 0c | 24 | Hexane |
| 803.5 | 4 | 217.86 | 124.93 | 100.00 ± 0 a | 56.67 ± 3.33b | 30.00 ± 0c | 0.00 ± 0 d | 48 | |
| 139.20 | 4 | 201.1461 | 100.3904 | 100.00 ± 0 a | 76.67 ± 3.33b | 50.00 ± 5.77c | 0.00 ± 0 d | 72 | |
3.3. Ovicidal activity
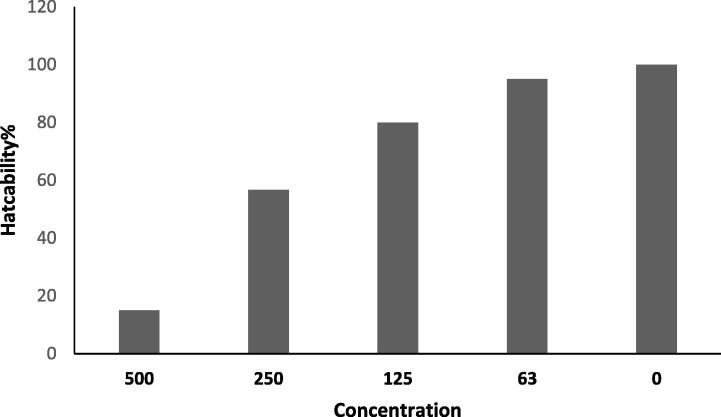
The ovicidal activity of the PH2 hexane extract resulted in a significant (p < 0.001) decrease in egg hatchability from 95.00 ± 6.16% to 15 ± 9.04% at doses ranging from 62.5 to 500 µg/mL PH2 (Fig. 1). No failure in hatching was observed in the control.
Fig. 1.
Percentage hatchability of the PH2 hexane extract against Culex pipiens.
3.4. Histopathological study
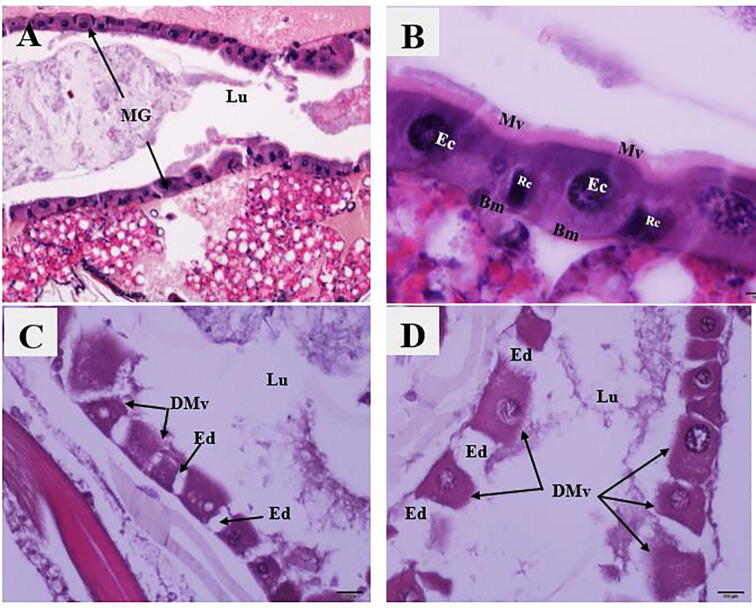
The damage caused by PH2 extract to third instar Cx. pipiens was assessed histologically at 148.3 µg/mL. The histopathological observation revealed some changes such as edema between the degenerated epithelial cells and destruction of microvilli on the apical surface of the epithelial cells (Fig. 2C, D). Histopathological evaluation of the negative control showed no damage to the midgut tissues (Fig. 2A, B).
Fig. 2.
Photomicrographs of midguts of Cx. pipiens larvae demonstrating: {A, B} Longitudinal sections in the midguts (MG) of control larvae with normal and healthy epithelial cells (Ec), microvilli (Mv), nuclei (n), and regenerative cells (Rc). Note the absence of lesions. H&E stain. {C, D}: Longitudinal sections in midguts of PH2-treated larvae, with edema (Ed) between the degenerated epithelial cells and degraded microvilli (DMv). H&E stain.
3.5. Cytotoxicity
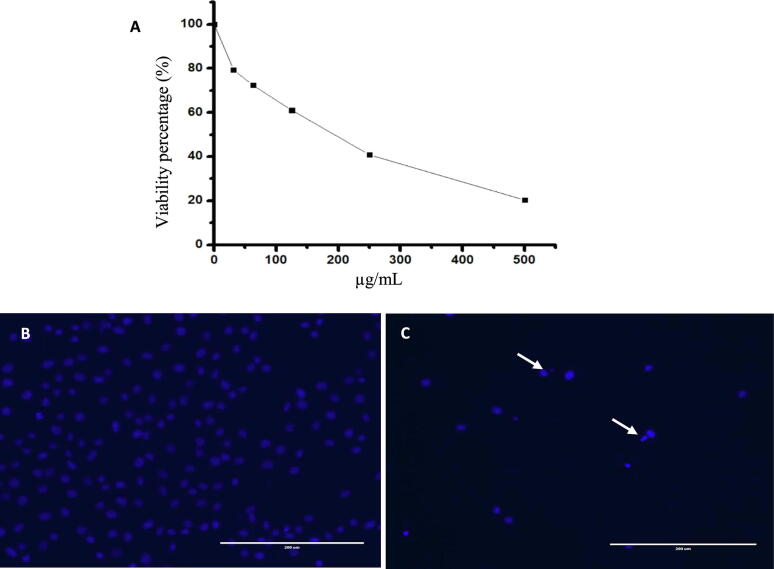
Upon evaluation of the toxicity of PH2 extract by MTT assay using HUVECs, a concentration-dependent decrease in the rate of cell survival was identified, with an IC50 value of 192.07 µg/mL for a 24 h incubation period. To assess the morphological changes, cells were observed under a microscope. As illustrated in Fig. 3, the cells treated with PH2 extract showed changes in the cellular and nuclear morphology. The cells were detached, round in shape, and revealed DNA fragmentation.
Fig. 3.
MTT assay (A) for relative cell viabilities of normal HUVECs incubated with PH2 extract for 24 h. Microscopic images of HUVECs treated (B) with and without PH2 extract (C). The arrows indicate the fragmented nucleus.
3.6. Fourier transform infrared (FTIR) spectrometry
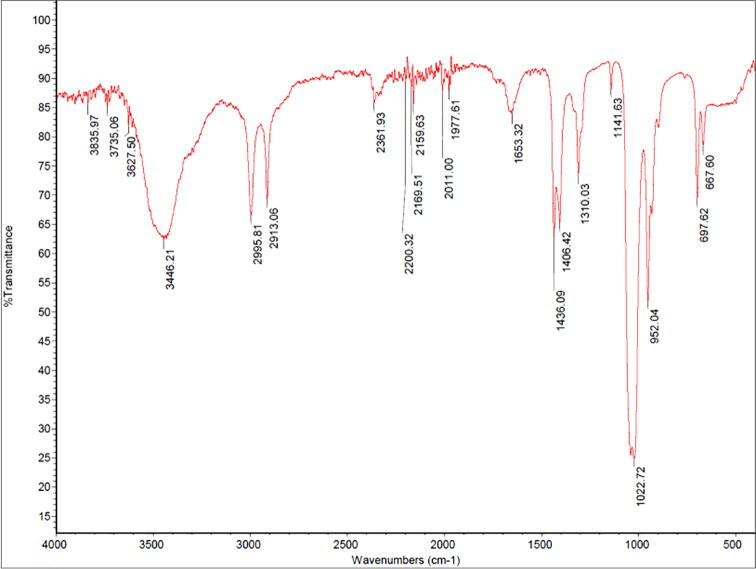
The FTIR spectrum is shown in Fig. 4. A broad peak at 3449 cm−1 represents to O–H stretching phenol groups. Peaks at 2913 and 2995 cm−1 represent C–H of alkyne groups. The absorbance at 2361 cm−1 corresponds to nitro compounds (N–O). The band at 1022 cm−1 represents the C = C of aromatic rings. The absorbance at 1436 cm−1 represents carbonyl groups. The peaks at 1141 to 1436 cm−1 are related to the bending of O–H, C–H, and C–N–C, and stretching attributed to carboxyls, alkanes, and amines. Fig. 5.
Fig. 4.
FTIR spectrum from hexane extract of PH2.
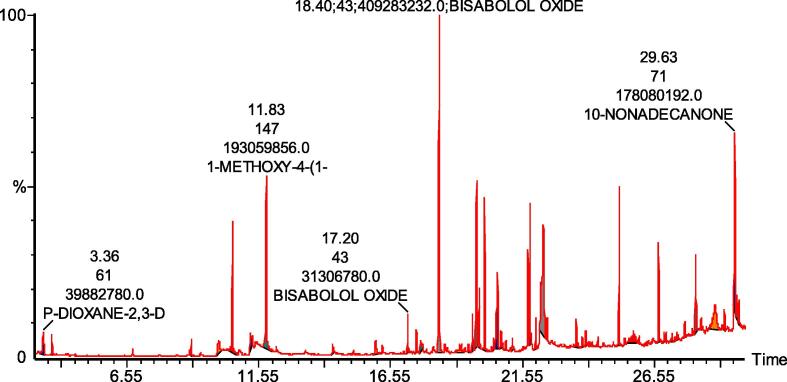
Fig. 5.
GC–MS chromatogram of hexane extract of PH2.
3.7. GC–MS analysis
The major compounds of the PH2 extract were bisabolol oxide A (17.1%), 1-methoxy-4-(1-z-propenyl) benzene (10.5%), methyl ester of hexadecanoic acid (8.3%), and 10-nonadecanone (7.03%) (Table 3).
Table 3.
Compounds identified in the hexane extract of PH2 using GC–MS.
| No. | Name | RT | Area % |
|---|---|---|---|
| 1 | HYDRAZINECARBOTHIOAMIDE | 3.69 | 3.460 |
| 3 | ESTRAGOLE | 10.54 | 6.100 |
| 4 | 1-METHOXY-4-(1-Z-PROPENYL) BENZENE | 11.83 | 10.560 |
| 5 | 7-METHYL-1-NAPHTHOL | 14.35 | 0.860 |
| 6 | 2-NONENAL | 15.98 | 0.610 |
| 7 | BISABOLOL OXIDE B | 17.20 | 0.900 |
| 8 | BISABOLOL OXIDE A | 18.40 | 17.110 |
| 9 | 2-DECEN-1-OL (CAS) | 19.17 | 0.170 |
| 11 | BISABOLOL OXIDE A (PYRAN OXIDE) | 19.66 | 2.290 |
| 12 | 1,6-DIOXASPIRO[4.4]NON-3-ENE | 19.81 | 5.860 |
| 13 | METHYL ESTER OF HEXADECANOIC ACID | 20.12 | 8.380 |
| 14 | (-)-SELINA-4.ALPHA.,11-DIOL | 20.46 | 0.330 |
| 15 | HEXADECANOIC ACID | 20.61 | 4.700 |
| 16 | 3,4-SECODAMMAR-4(28)-EN-3-OIC | 20.74 | 1.400 |
| 17 | METHYL ESTER OF 6-OCTADECENOIC ACID | 21.84 | 3.190 |
| 18 | METHYL ESTER OF OCTADECANOIC ACID | 22.06 | 1.880 |
| 19 | (Z,Z)-6,9-CIS-3,4-EPOXY-NONADECANE | 22.35 | 4.270 |
| 20 | OCTADECANOIC ACID | 22.49 | 1.080 |
| 21 | 11-DECYL-DOCOSANE | 23.60 | 1.810 |
| 22 | METHYL ESTER OF EICOSANOIC ACID | 23.82 | 0.640 |
| 23 | TETRACOSANE | 25.23 | 6.900 |
| 24 | VITAMIN E | 25.77 | 0.510 |
| 25 | METHYL ESTER OF TETRACOSANOIC ACID | 26.93 | 0.470 |
| 26 | OCTADECANE | 27.43 | 0.830 |
| 27 | DODECANAL | 27.71 | 0.480 |
| 28 | SQUALENE | 28.13 | 5.570 |
| 29 | 1-DOCOSANOL | 28.34 | 0.520 |
| 30 | 2-UNDECEN-1-OL | 29.21 | 0.580 |
| 31 | 10-NONADECANONE | 29.63 | 7.030 |
4. Discussion
Mosquitoes in the aquatic stage (eggs and larvae) are an attractive target for mosquito control because, in stagnant water, they are easily managed. However, introducing synthetic mosquitocides into waterbodies may pose risks to humans, animals, and the environment (Subramanian et al., 2012, Bagavan and Rahuman, 2011). Larvicides derived from natural sources, mainly plants, are a promising alternative for managing mosquitoes (Warikoo et al., 2012, Hemalatha et al., 2015). H. forskaolii is used in Tanzania to control insect vector-borne diseases (Asnake et al., 2016). It was shown that H. forskaolii root chloroform extract was toxic to C. quinquefasciatus, A. gambiae, and A. aegypti with LC50 values of 6.0, 2.0, and 3.8 μg/mL, respectively (Sillo et al., 2019). Similarly, in Portugal, extract of the aerial parts of F. vulgare resulted in LC90 and LC99 values of 37.1 and 52.4 µl/L, respectively, against Ae. aegypti larvae (Rocha et al., 2015). In our recent study (Al‐Mekhlafi et al., 2020), the ethyl acetate extract of M. chamomilla showed an LC50 value of 287 μg/mL after 24 h of exposure and reduced the hatchability of the eggs of Cx. pipiens. In the present investigation, the mixture of H. forskaolii and M. chamomilla showed dose- and time-dependent toxicity against Cx. pipiens with an LC50 value of 148.4 μg/mL. The toxicity of the mixed plants helped to reduce the LC50 value of ethyl acetate extract of M. chamomilla from 287.1 μg/mL as reported in our previous study (Al‐Mekhlafi et al., 2020) to 148.4 μg/mL after extracting H. forskaolii and M. chamomilla together.
Based on the LC50, the activity of the plant extracts can be classified into three categories. Extracts showing LC50 of < 50 μg/mL are classified as very active extracts, while those with LC50 higher than 750 μg/mL are classified as inactive (Komalamisra et al., 2005, Magalhães et al., 2010, Dias and Moraes, 2014). The LC50 value reported in the present study was 148.4 μg/mL, indicating that the plant extract is considered active.
The combination of insecticidal agents is encouraged to optimize the efficacy of the insecticides, solve the problem of insect resistance, and preserve the efficiency of the insecticidal agents for years. In the current investigation, H. forskaolii solvent extracts tested individually were inactive against mosquito larvae, while its combination with M. chamomilla extract showed synergistic properties. The combination of Callitris glaucophylla and Khaya senegalensis extract at a ratio of 1:1 exerted synergistic effects on A. aegypti (Shaalan et al., 2005). Synergistic efficacy was also observed in the combination of E. camaldulensis with C. rigidus extracts at a 3:1 ratio against A. gambiae larvae (Ríos et al., 2017). Similarly, combinations of Canarium schweinfurthii, Aucoumea klaineana, and Dacryodes edulis led to the improvement of their efficiency and exhibited significant activity against A. gambiae (Obame et al., 2016). The combination of thymol and carvacrol at a 1:4 ratio exhibited significant synergistic activity against Cx. pipiens larvae, compared with the compounds tested individually (Youssefi et al., 2019).
Histopathological assessment of the third instar of Cx. pipiens exposed to PH2 extract showed a change in the midgut architecture, mainly in the basal membrane, epithelial cells, and microvilli. Such damage was due to secondary metabolites in PH2 extract. Secondary metabolites lower the surface tension of the mucosal membrane and as a result damage the digestive system. Damage in the midgut region changes various functions, such as osmoregulation, digestion, nutrition absorption, and ion transport (Sina and Shukri, 2016). However, as in other insects, the mosquito’s stomach does not function only in digestion, but also in mechanical and chemical defense against invaders (Terra, 2001). Damage to the digestive tract due to larvicidal agents generally affects the digestion and absorption of food in this region (Rohmah et al., 2020). In addition, damage to the regenerative cells impairs the development of larvae and hence metamorphosis (Procópio et al., 2015). Extracts of different parts of A. bilimbi including leaf, flower, and fruit were reported to damage the villi, epithelial cells, and peritrophic membrane of Anopheles barbirostris and Ae. aegypti larvae (Das et al., 2011, Suluvoy and Grace, 2017, (Rohmah et al., 2020).
A higher LC50 indicates that it would take more of the extract to induce a toxic response, while a small LD50 value could be deleterious to human health (Okeleye et al., 2013). The American National Cancer Institute considered that an IC50 of<30 µg/mL is toxic (Hasibuan et al., 2014). When applying this information to our data, PH2 extract had some toxicity against HUVECs, as determined using MTT assay and fluorescence microscopy. However, the results cannot be generalized to human health and the risk should be assessed using different models.
The efficacy of the extract against mosquito larvae may be related to the presence of phenols, flavonoids, terpenoids, saponins, tannins, andalkaloids , which have been reported for their insecticidal activities (Shaalan et al., 2005). These toxic secondary metabolites are consumed orally or absorbed by the cuticle and affect the physiology of insects, causing their death (Rattan, 2010). Many reports have revealed that the main constituents of the extract are responsible for bio-activity, as it contains the main fraction of the extract (Gul, 1994, Vani et al., 2009, Glolade and Lockwood, 2008). Therefore, we considered four major compounds from PH2 extract. From the GC–MS results, bisabolol oxide A, 1-methoxy-4-(1-z-propenyl) benzene, methyl ester of hexadecanoic acid, and 10-nonadecanone were found to be the major compounds of PH2 extract. An oil rich in bisabolol oxide A from different plant extracts showed antimicrobial (Tolouee et al., 2010, Alireza, 2012, Sashidhara et al., 2006, Roby et al., 2013) and larvicidal activities against Aedes aegypti, with a CL50 of 15.9 mg/mL (Furtado et al., 2005). It has also been reported that 1-methoxy-4-(1-z-propenyl) benzene possesses insecticidal activity (CHEBI, 2021). Estragole exhibited strong larvicidal activity with LC50 of 41.67 and 38.56 µg/mL for An. sinensis and An. anthropophagus, respectively (He et al., 2018). Estragole was shown to possess contact toxicity against Lasioderma serricorne adults (LD50: 15.58 mg/adult) (Wang et al., 2014).
5. Conclusions
PH2 hexane extract showed larvicidal activity again Cx. pipiens as well as structural change of the midgut region. Caution is required in the use of the PH2 extract as a larvicideagainst Cx. pipiens due to its toxic effects on normal HUVECs, as determined using MTT assay.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
This work was funded by the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University, through the Research Groups Program Grant no. (RGP-1442 -0037)
Footnotes
Peer review under responsibility of King Saud University.
References
- Abutaha N.M., Farooq M.F., Mohammed A.Z., Alotaibi A., Cordero M.A.W., Bepari A. Cytotoxic Activity and Toxicity Study of HF8, A Poly-Herbal Formulation. Journal of King Saud University-Science. 2021;101377 [Google Scholar]
- Alireza M. Antimicrobial activity and chemical composition of essential oils of chamomile from Neyshabur. Iran. Journal of Medicinal Plants Research. 2012;6:820–824. [Google Scholar]
- Al-Mekhlafi F.A., Abutaha N., Al-Malki A.M., Al-Wadaan M. Inhibition of the growth and development of mosquito larvae of Culex pipiens L. (Diptera: Culicidae) treated with extract from flower of Matricaria chamomilla (Asteraceae) Entomological Research. 2020;50(3):138–145. [Google Scholar]
- Al-Mekhlafi Fa.hd.A. Larvicidal, ovicidal activities and histopathological alterations induced by Carum copticum (Apiaceae) extract against Culex pipiens (Diptera: Culicidae) Saudi journal of biological sciences. 2018;25(1):52–56. doi: 10.1016/j.sjbs.2017.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Zharani M., Nasr F.A., Abutaha N., Alqahtani A.S., Noman O.M., Mubarak M., Wadaan M.A. Apoptotic induction and anti-migratory effects of Rhazya stricta fruit extracts on a human breast cancer cell line. Molecules. 2019;24(21):3968. doi: 10.3390/molecules24213968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asnake S., Teklehaymanot T., Hymete A., Erko B., Giday M. Survey of medicinal plants used to treat malaria by Sidama people of Boricha district, Sidama zone, south region of Ethiopia. Evid Based Complement Alternat Med. 2016;2016:9690164. doi: 10.1155/2016/9690164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badgujar, S. B., Patel, V. V., & Bandivdekar, A. H. (2014). Foeniculum vulgare Mill: a review of its botany, phytochemistry, pharmacology, contemporary application, and toxicology. BioMed research international, 2014. [DOI] [PMC free article] [PubMed]
- Bagavan A., Rahuman A.A. Evaluation of larvicidal activity of medicinal plant extracts against three mosquito vectors. Asian Pac J Trop Med. 2011;4:29–34. doi: 10.1016/S1995-7645(11)60027-8. [DOI] [PubMed] [Google Scholar]
- Bhatt S., Gething P.W., Brady O.J., Messina J.P., Farlow A.W., Moyes C.L., Myers M.F. The global distribution and burden of dengue. Nature. 2013;496(7446):504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustamante, Jaleesa. “Mosquito Deaths & Mosquito Borne Disease Statistics [2020].” MosquitoReviews, 18 Dec. 2019, mosquitoreviews.com/learn/disease-death-statistics.
- CHEBI, Chemical Entities of Biological Interest (2021)
- Cheesman M.J., Ilanko A., Blonk B., Cock I.E. Developing new antimicrobial therapies: are synergistic combinations of plant extracts/compounds with conventional antibiotics the solution? Pharmacognosy reviews. 2017;11(22):57. doi: 10.4103/phrev.phrev_21_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S.C., Sultana S., Roy S., Hasan S.S. Antibacterial and cytotoxic activities of methanolic extracts of leaf and fruit parts of the plant Averrhoa bilimbi (Oxalidaceae) American Journal of Scientific and Industrial Research. 2011;2(4):531–536. [Google Scholar]
- Devine G.J., Furlong M.J. Insecticide use: Contexts and ecological consequences. Agriculture and Human values. 2007;24(3):281–306. [Google Scholar]
- Dias CN, Moraes DFC. Essential oils and their compounds as Aedes aegypti L. (Diptera: Culicidae) larvicides: review. Parasitol Res. 2014; 113 (2): 565–92. pmid:24265058 [DOI] [PubMed]
- Diaz-Maroto M.C., Pérez-Coello M.S., Esteban J., Sanz J. Comparison of the volatile composition of wild fennel samples (Foeniculum vulgare Mill.) from Central Spain. Journal of agricultural and food chemistry. 2006;54(18):6814–6818. doi: 10.1021/jf0609532. [DOI] [PubMed] [Google Scholar]
- Furtado R.F., De Lima M.G.A., Neto M.A., Bezerra J.N.S., Silva E.M.G. Atividade larvicida de óleos essenciais Contra Aedes aegypti L. (Diptera: Culicidae) Neotrop Entomol. 2005;34:843–847. [Google Scholar]
- Glolade A.A., Lockwood G.B. Toxicity of Ocimum sanctum L. essential oil to Aedes aegypti larvae and its chemical composition. Journal of essential oil bearing plants. 2008;11(2):148–153. [Google Scholar]
- Gul P. Seasonal variation of oil and menthol content in Mentha arvensis Linn. Pakistan J. For. 1994;44:16–20. [Google Scholar]
- Hasibuan R.P.A.Z. Cytotoxic effect of n-hexane, ethyl acetate and ethanol extracts of Plectranthus amboinicus, (Lour.) Spreng.) on HeLa and Vero cells lines. Int. J. PharmTech Res. 2014;6:1806–1809. [Google Scholar]
- He Q., Wang W., Zhu L. Larvicidal activity of Zanthoxylum acanthopodium essential oil against the malaria mosquitoes, Anopheles anthropophagus and Anopheles sinensis. Malaria journal. 2018;17(1):1–7. doi: 10.1186/s12936-018-2341-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemalatha P., Elumalai D., Janaki A. Larvicidal activity of Lantana camara aculeate against three important mosquito species. J Entomol Zool Stud. 2015;3(1):174–181. https://www.ebi.ac.uk/chebi/searchId.do?chebiId=24852. Accessed 15-3-2021. [Google Scholar]
- Isman M.B. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annual Review of Entomology. 2006;51(1):45–66. doi: 10.1146/annurev.ento.51.110104.151146. [DOI] [PubMed] [Google Scholar]
- JUCÁ, Mércia Marques, et al. Flavonoids: biological activities and therapeutic potential. Natural product research, 2020, 34.5: 692-705. [DOI] [PubMed]
- Komalamisra N., Trongtokit Y., Rongsriyam Y., Apiwathnasorn C. Screening for larvicidal activity in some Thai plants against four mosquito vector species. Southeast Asian J Trop Med Public Health. 2005;36(6):1412–1422. pmid:16610643. [PubMed] [Google Scholar]
- Magalhães L.A.M., Lima M.P., Marques M.O.M., Facanali R., Pinto A.C.S., Tadei W.P. Chemical composition and larvicidal activity against Aedes aegypti larvae of essential oils from four Guarea species. Molecules. 2010;15(8):5734–5741. doi: 10.3390/molecules15085734. pmid:20724962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miraj S., Alesaeidi S. A systematic review study of therapeutic effects of Matricaria recuitta chamomile (chamomile) Electronic physician. 2016;8(9):3024. doi: 10.19082/3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra P., Tripathi A., Dikshit A., Pandey A. Natural Bioactive Products in Sustainable Agriculture. Springer; Singapore: 2020. Insecticides Derived from Natural Products: Diversity and Potential Applications; pp. 83–99. [Google Scholar]
- Nelson A.C., Kursar T.A. Interactions among plant defense compounds: a method for analysis. Chemoecology. 1999;9(2):81–92. [Google Scholar]
- Ntie-Kang F., Njume L.E., Malange Y.I., Günther S., Sippl W., Yong J.N. The chemistry and biological activities of natural products from northern african plant families: From taccaceae to Zygophyllaceae. Natural products and bioprospecting. 2016;6(2):63–96. doi: 10.1007/s13659-016-0091-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obame L.C., Ondo J.P., Padzys G.S. Ovicidal and larvicidal activities against Anopheles gambiae, antioxidant and antibacterial proprieties of Aucoumea klaineana Pierre, Canarium schweinfurthii Engl and Dacryodes edulis (G. Don) Lam essential oils from Gabon. International Journal of Pharmacology Research. 2016;6(1):68–75. [Google Scholar]
- Okeleye, B. I., Mkwetshana, N. T., & Ndip, R. N. (2013). Evaluation of the antibacterial and antifungal potential of Peltophorum africanum: toxicological effect on human chang liver cell line. The Scientific World Journal, 2013. [DOI] [PMC free article] [PubMed]
- Paixão E.S., Teixeira M.G., Rodrigues L.C. Zika, chikungunya and dengue: the causes and threats of new and re-emerging arboviral diseases. BMJ global health. 2018;3(Suppl 1) doi: 10.1136/bmjgh-2017-000530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Procópio T.F., Fernandes K.M., Pontual E.V. Schinus terebinthifolius leaf extract causes midgut damage, interfering with survival and development of Aedes aegypti larvae. PLoS One. 2015;10(5) doi: 10.1371/journal.pone.0126612. article e0126612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rather M.A., Dar B.A., Sofi S.N., Bhat B.A., Qurishi M.A. Foeniculum vulgare: A comprehensive review of its traditional use, phytochemistry, pharmacology, and safety. Arabian Journal of Chemistry. 2016;9:S1574–S1583. [Google Scholar]
- Rattan R. S., “Mechanism of action of insecticidal secondary metabolites of plant origin,” Crop Protection, vol. 29, no. 9, pp. 913–920, 2010.View at: Publisher Site | Google Scholar
- Ríos N., Stashenko E.E., Duque J.E. Evaluation of the insecticidal activity of essential oils and their mixtures against Aedes aegypti (Diptera: Culicidae) Revista Brasileira de Entomologia. 2017;61(4):307–311. [Google Scholar]
- Roby M.H.H., Sarhan M.A., Selim K.A.H., Khalel K.I. Antioxidant and antimicrobial activities of essential oil and extracts of fennel (Foeniculum vulgare L.) and chamomile (Matricaria chamomilla L.) Industrial Crops and Products. 2013;44:437–445. [Google Scholar]
- Rocha D.K., Matos O., Novo M.T., Figueiredo A.C., Delgado M., Moiteiro C. Larvicidal activity against Aedes aegypti of Foeniculum vulgare essential oils from Portugal and Cape Verde. Natural product communications. 2015;10(4) 1934578X1501000438. [PubMed] [Google Scholar]
- Rohmah, E. A., Subekti, S., & Rudyanto, M. (2020). Larvicidal Activity and Histopathological Effect of Averrhoa bilimbi Fruit Extract on Aedes aegypti from Surabaya, Indonesia. Journal of Parasitology Research, 2020. [DOI] [PMC free article] [PubMed]
- Said-Al Ahl H.A., Hikal W.M., Tkachenko K.G. Essential oils with potential as insecticidal agents: A review. Int. J. Environ. Plan. Manag. 2017;3:23–33. [Google Scholar]
- Sarwar M., Ahmad N., Toufiq M. Host plant resistance relationshiphs in chickpea (cicer arietinum l.) against gram pod borer (helicoverpa armigera hubner. Pakistan Journal of Botany. 2009;41(6):3047–3052. [Google Scholar]
- Sashidhara K.V., Verma R.S., Ram P. Essential oil composition of Matricaria recutita L. from the lower region of the Himalayas. Flavour and Fragrance Journal. 2006;21:274–276. [Google Scholar]
- Shaalan E.A., Canyon D.V., Younes M.W.F., Abdel-Wahab H., Mansour A.H. Synergistic efficacy of botanical blends with and without synthetic insecticides against Aedes aegypti and Culex annulirostris mosquitoes. Journal of Vector Ecology. 2005;30(2):284–288. [PubMed] [Google Scholar]
- Sillo A.J., Makirita W.E., Swai H., Chacha M. Larvicidal activity of Hypoestes forskaolii (Vahl) R. Br root extracts against Anopheles gambiae Giless. s, Aedes aegypti L, and Culex quinquefasciatus Say. Journal of experimental pharmacology. 2019;11:23. doi: 10.2147/JEP.S187837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sina I.Z., Shukri M.S.M. Larvicidal activities of extract flower Averrhoa bilimbi L. towards important species mosquito, Anopheles barbirostris (Diptera: Culicidae) International Journal of Zoological Research. 2016;12(1):25–31. [Google Scholar]
- Stanaway J.D., Shepard D.S., Undurraga E.A., Halasa Y.A., Coffeng L.E., Brady O.J., Chuang T.W. The global burden of dengue: an analysis from the Global Burden of Disease Study 2013. The Lancet infectious diseases. 2016;16(6):712–723. doi: 10.1016/S1473-3099(16)00026-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian J., Kovendan K., Kumar P.M., Murugan K., Walton W. Mosquito larvicidal activity of Aloe vera (Family: liliaceae) leaf extract and Bacillus sphaericus, against Chikungunya vector. Aedes aegypti. Saudi J Biol Sci. 2012;19:503–509. doi: 10.1016/j.sjbs.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suluvoy J.K., Grace V.M.B. “Phytochemical profile and free radical nitric oxide (NO) scavenging activity of Averrhoa bilimbi L. fruit extract”, 3. Biotech. 2017;7(1):85. doi: 10.1007/s13205-017-0678-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terra W.R. The origin and functions of the insect peritrophic membrane and peritrophic gel. Archives of Insect Biochemistry and Physiology. 2001;47(2):47–61. doi: 10.1002/arch.1036. [DOI] [PubMed] [Google Scholar]
- Tolouee M., Alinezhad S., Saberi R., Eslamifar A., Zad S.J., Jaimand K., Taeb J., Rezaee M.B., Kawachi M., Shams-Ghahfarokhi M., Razzaghi-Abyaneh M. Effect of Matricaria chamomilla L. flower essential oil on the growth and ultrastructure of Aspergillus niger van Tieghem. International Journal of Food Microbiology. 2010;139:127–133. doi: 10.1016/j.ijfoodmicro.2010.03.032. [DOI] [PubMed] [Google Scholar]
- Vani S.R., Cheng S.F., Chuah C.H. Comparative Study of Volatile Compounds from Genus Ocimum. American Journal of Applied Sciences. 2009;6(3):523–528. [Google Scholar]
- Wang Y., You C.X., Wang C.F., Yang K., Chen R., Zhang W.J. Chemical constituents and insecticidal activities of the essential oil from Amomum tsaoko against two stored-product insects. J Oleo Sci. 2014;63:1019–1026. doi: 10.5650/jos.ess14087. [DOI] [PubMed] [Google Scholar]
- Warikoo R., Ray A., Sandhu J.K., Samal R., Wahab N., Kumar S. Larvicidal and irritant activities of hexane leaf extracts of tropical biomedicine. Asian Pac J Trop Biomed. 2012;2(2):152–155. doi: 10.1016/S2221-1691(11)60211-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White N.J. Antimalarial drug resistance. The Journal of clinical investigation. 2004;113(8):1084–1092. doi: 10.1172/JCI21682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson E.M. Trease and Evans Pharmacognosy. 16th edition, Elsevier; Amsterdam, Netherlands: 2009. Synergy and other interactions in phytomedicines; pp. 53–61. [Google Scholar]
- Younoussa, L., Kenmoe, F., Oumarou, M. K., Batti, A. C. S., Tamesse, J. L., & Nukenine, E. N. (2020). Combined Effect of Methanol Extracts and Essential Oils of Callistemon rigidus (Myrtaceae) and Eucalyptus camaldulensis (Myrtaceae) against Anopheles gambiae Giles larvae (Diptera: Culicidae). International Journal of Zoology, 2020.
- Youssefi M.R., Tabari M.A., Esfandiari A. Efficacy of two monoterpenoids, carvacrol and thymol, and their combinations against eggs and larvae of the west nile vector Culex pipiens. Molecules. 2019;24(10):1867. doi: 10.3390/molecules24101867. [DOI] [PMC free article] [PubMed] [Google Scholar]
Further Reading
- Nibret E., Ashour M.L., Rubanza C.D., Wink M. Screening of some Tanzanian medicinal plants for their trypanocidal and cytotoxic activities. Phytother Res. 2009;24:945–947. doi: 10.1002/ptr.3066. [DOI] [PubMed] [Google Scholar]
- Obame-Engonga L.C., Sima-Obiang C., Ngoua-Meye-Misso R.L. Larvicidal and ovicidal properties against Anopheles gambiae, antioxidant and antibacterial activities of the combination of essential oils Eucalyptus citriodora, Cymbopogon giganteus and Cymbopogon nardus from Gabon. Journal of Multidisciplinary Engineering Science and Technology. 2017;4(8):7887–7894. [Google Scholar]