Abstract
Spodoptera frugiperda is a highly polyphagous migratory lepidopteran pest species. It causes infestation in crops leading to the severe crop losses. Being a new invasive parasite, its susceptibility to insecticides needs to be explored; and therefore, there is an urgent need to develop the potent insecticides for the effective control of this insect pest. To attain the crop sustainability, the antifeedant, toxicity and nutritional effects on larvae of Spodoptera frugiperda were studied with six mono- and eight bis- substituted chalcones. The antifeedant activity was calculated when 50% of the larvae control ate 50% of the diet through the FR factor. Toxicity was assessed through larval, pupal mortality and the emergence of adults and nutritional effects with consumption rates (IC), growth (GR) and consumption efficiency (EIC). The bis-chalcones 6b, 6e, 6f and 6h caused lethal effect on S. frugiperda in the first larval stages, being 6b the most toxic (85%). Adults who survived showed malformations and decreased size, which led to death. The larvae fed with aggregate in the bis-chalcones diet: 6b, 6e and 6f had the highest percentage of intake and the poorest conversion of nutrient absorption (ECI), which suggests that the larva metabolizes food for energy and results in a decrease of growth and death in early stages. Bis-chalcones showed more toxicity than mono-chalcones and 6b causes the most toxic and dietary change.
Keywords: Mono- and bis-chalcones, Spodoptera frugiperda, Antifeedant, Toxicity, Nutritional effects
1. Introduction
Spodoptera frugiperda also known as the fall armyworm is a highly polyphagous migratory lepidopteran pest species. It infects a wide range of crops including wheat, soybean, millets, peanut, sorghum, sugarcane and corn. It causes infestation in crops leading to the severe crop losses (Assefa and Ayalew, 2019). It is a destructive insect pest responsible for major problems in agricultural crop production, especially maize (Maruthadurai and Ramesh, 2020). The abilities of this insect pest to breed rapidly, migrate, and feed on a wide range of host plants, makes it very difficult to control (Maruthadurai and Ramesh, 2020, Deshmukh et al., 2020). Selection of proper insecticide is one of the important requirements for the crop sustainability, which ensure the less toxicity to humans and more specific to key pests. The presently used pesticides for agricultural work to control insects and mealy bugs are highly toxic in nature for the human life. To achieve the eco-friendly, egalitarian and ethical pest management, there is a need of a mechanism which provides pest specific, less toxic and cost-effective procedure.
Chalcones are commonly known as α, β-unsaturated ketone consisting of two aromatic rings which are joined by three carbon chain. Chalcones are considered as one of the most significant type of natural products found in various plant species. Chalcones are very reactive species due to the presence of a conjugated enone system (Ameta et al., 2011). The chemistry of chalcones is always going be a point of attraction for researchers due its large number of derivatives, derived from the good number of replaceable hydrogens present on the aromatic rings.
Chalcones constitute the central core of biologically active heterocyclic compounds. Chalcones provide a skeletal framework for a variety of novel biologically active heterocycles of high therapeutic potential and good medicinal profile (Zhuang et al., 2017). The electrophilic nature of the α,β-unsaturated carbonyl system is the key factor which makes it capable to form irreversible bonds with other bioactive macromolecules, resulting different types of bioactivity. Such type of reactivity may be affected by the presence of substituents on aromatic rings or by α-X-substitution of the enone system (Amslinger et al., 2013).
Chalcones belong to the class flavonoid and are used as the main precursor of flavonoids and isoflavonoids. Flavonoids are the well-known functional secondary metabolites, widely spread in the various plant species (Panche et al., 2016). Chalcones, flavones and chromones have been studied as insect antifeedant activities (Morimoto et al., 2003, Janaki et al., 2016, Susurluk et al., 2007).
There are many methods reported for the synthesis of chalcones but are usually prepared by the Claisen-Schmidt condensation reaction of a substituted acetophenone and a substituted aromatic aldehyde in presence of aqueous alkali (Prasad et al., 2007, Rao et al., 2004). Chalcones provides a skeletal framework for the synthesis of various bioactive heterocyclic compounds through condensation and ring closure reactions. Chalcone has always been a favorite molecule of researchers because it is a bioactive molecule and is used for deriving many other bioactive molecules and it’s various pharmacological applications such as antitumor (Iftikhar et al., 2017), antimicrobial (Prasad et al., 2006), anticancer (Mirzaei et al., 2020, Burmaoglu et al., 2019), inhibitor topoisomerase- I (Yoon et al., 2007), antiviral (Dong et al., 2017), antihyperglycemic (Satyanarayana et al., 2004), tubulin inhibitor (Bueno et al., 2018), antioxidant (Díaz-Carillo et al., 2018) antidiabetic (Shukla et al., 2017). Moreover, chalcones have also been reported for insect antifeedant ((Nalwar et al., 2009), nematicidal (Awasthi et al., 2009) and larvicidal (Das et al., 2005, Begum et al., 2011, Gautam and Chourasia, 2010) activities. Therefore, chalcone moiety is considered as an important synthon and is widely applicable for the design and synthesis of novel biologically active derivatives which are particularly important for the future drug candidates.
Being a new invasive parasite, there is no information available on its susceptibility to insecticide; and therefore, there is an urgent need to develop the potent insecticides for the effective control of this insect pest. Hence the present study was aimed to search and develop novel candidates from the existing drug space for the pest control.
2. Materials and methods
All the synthetics utilized for the production of chalcones were procured from Merck and Sigma-Aldrich, USA and utilized without further purifications. Melting points were estimated in open capillaries on melting point equipment and were uncorrected.
2.1. General method for the synthesis of chalcones
2.1.1. Method for the synthesis of mono-chalcones (3a-f)
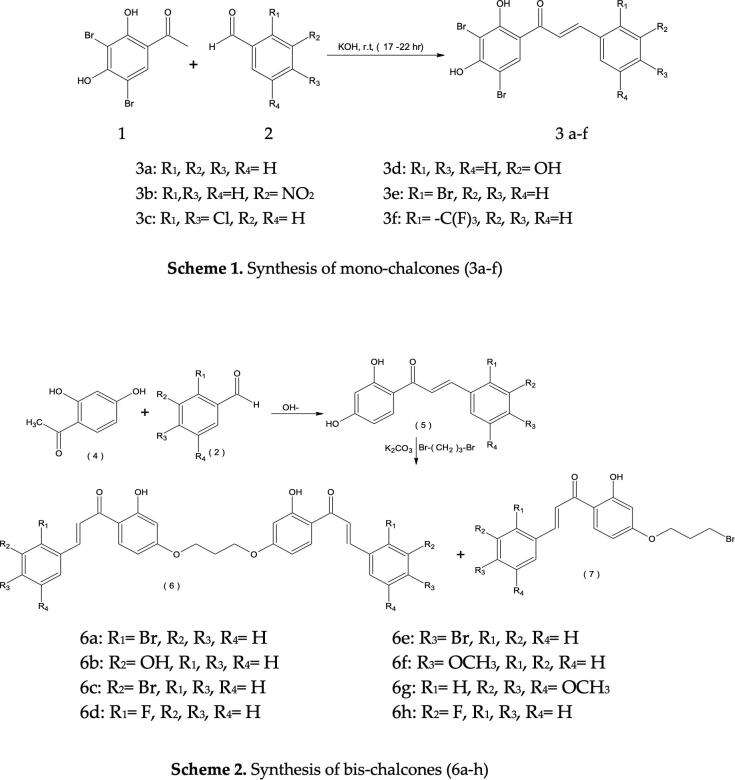
A combination of 3,5-dibromo-2, 4-dihydroxyacetophenone (0.01 mol) and substituted benzaldehydes (0.01 mol) was dissolved in ethanol (30 mL) and afterward 40% aqueous KOH solution was poured in it. The reaction mixture was stirred for the time being at room temperature and poured into squashed ice followed by acidification with dil. HCl. The solid obtained was filtered and recrystallized using ethanol to give mono-chalcones (3a-f) (Fig. 1: Scheme 1). The characterization of the synthesized compounds was carried out by their elementary analysis and spectral studies.
Fig. 1.
Scheme 1 {Synthesis of mono-chalcones (3a-f)} & Scheme 2 {Synthesis of bis-substituted chalcones (6a-h)}
3a. (2E)-1-(3,5-dibromo-2,4-dihydroxyphenyl)-3-phenylprop-2-en-1-one. Yield: 82%, m.p. 128–130 °C. 1H NMR (500 MHz, CDCl3) δ: 9.13 (s, 2H, 2xOH), 7.88 (s, 1H, —CH—), 7.60 (d, β H, J = 15.57), 7.54 (d, α H, J = 15.57), 7.51 (d, 2H, Ar), 7.51 (dd, 3H, Ar). 13C NMR (100 MHz, CDCl3) δ: 97.80, 101.42, 120.90 (Cα), 128.67, 130.26, 134.96, 143.65 (Cβ), 160.82. 190.65 (C O) ppm. MS: m/z 398 (M+). Calcd. for C15H10Br2O3; Found: C: 45.36%, H: 2.53%, Br: 40.15%, O: 12.06%.
3b. (2E)-1-(3,5-dibromo-2,4-dihydroxyphenyl)-3-(3-nitrophenyl)prop-2-en-1-one. Yield: 77%, m.p. 158–160 °C. 1H NMR (500 MHz, CDCl3) δ: 9.13 (s, 2H, 2xOH), 8.45 (s, 1 h, Ar), 8.18–7.83 (d, 2H, Ar), 7.88 (s, 1H, —CH—), 7.78 (d, β H, J = 15.57), 7.63 (dd, 1H, Ar), 7.50 (d, α H, J = 15.57). 13C NMR (100 MHz, CDCl3) δ: 97.80, 101.42, 120.06 (Cα), 122.22, 124.68, 130.01, 135.27, 141.57 (Cβ), 149.51, 160.82, 162.08, 190.65 (C O) ppm. MS: m/z 443 (M+). Calcd. for C15H9Br2NO5; Found: C: 40.66%, H: 2.05%, Br: 36.07%, N = 3.16, O: 18.06%.
3c. (2E)-1-(3,5-dibromo-2,4-dihydroxyphenyl)-3-(2,4-dichlorophenyl)prop-2-en-1-one. Yield: 75%, m.p. 190–192 °C. 1H NMR (500 MHz, CDCl3) δ: 9.13 (s, 2H, 2xOH), 7.88 (s, 1H, —CH—), 7.71 (d, β H, J = 15.57), 7.60 (d, 1H, Ar), 7.55 (d, α H, J = 15.57), 7.52 (s, 1H, Ar), 7.35 (d, 1H, Ar). 13C NMR (100 MHz, CDCl3) δ: 97.80, 101.42, 123.24 (Cα), 127.71, 129.80, 135.27, 137.49 (Cβ), 160.82, 162.08, 190.65 (C O) ppm. MS: m/z 466 (M+). Calcd. for C15H8Br2Cl2O3; Found: C: 38.58%, H: 1.73%, Br: 34.23%, Cl: 15.18%, O: 10.28%.
3d. (2E)-1-(3,5-dibromo-2,4-dihydroxyphenyl)-3-(3-hydroxyphenyl)prop-2-en-1-one. Yield: 70%, m.p. 188–190 °C. 1H NMR (500 MHz, CDCl3) δ: 9.25 (s, 3H, 3xOH), 7.88 (s, 1H, —CH—), 7.77 (d, β H, J = 15.57), 7.55 (d, α H, J = 15.57), 7.32 (dd, 1H, Ar), 7.12 (d. 1H, Ar), 6.88 (d, 1H, Ar), 6.82 (s, 1H, Ar). 13C NMR (100 MHz, CDCl3) δ: 97.80, 101.42, 115.09, 118.18, 120.77 (Cα), 129.79, 135.27, 144.50 (Cβ), 157.70, 160.82, 162.08, 190.65 (C O) ppm. MS: m/z 414 (M+). Calcd. for C15H10Br2O4; Found: C: 43.51%, H: 2.43%, Br: 38.60%, O: 15.46%.
3e. (2E)-3-(2-bromophenyl)-1-(3,5-dibromo-2,4-dihydroxyphenyl)prop-2-en-1-one. Yield: 80%, m.p. 179–181 °C. 1H NMR (500 MHz, CDCl3) δ: 9.13 (s, 2H, 2xOH), 7.98 (d, β H, J = 15.58), 7.88 (s, 1H, —CH—), 7.77–7.63 (d, 2H, Ar), 7.50 (d, α H, J = 15.58), 7.43–7.33 (dd, 2H, Ar). 13C NMR (100 MHz, CDCl3) δ: 97.80, 101.42, 121.89, 125.22 (Cα), 130.79, 132.54, 143.66 (Cβ), 160.82, 162.08, 190.65 (C O) ppm. MS: m/z 476 (M+). Calcd. for C15H9Br3O3; Found: C: 37.77%, H: 1.90%, Br: 50.26%, O: 10.06%.
3f. (2E)-1-(3,5-dibromo-2,4-dihydroxyphenyl)-3-[2-(trifluoromethyl)phenyl]prop-2-en-1-one. Yield: 70%, m.p. 163–165 °C. 1H NMR (500 MHz, CDCl3) δ: 9.13 (s, 2H, 2xOH), 8.81 (d, β H, J = 15.58), 7.99–7.86 (d, 2H, Ar),7.88 (s, 1H, —CH—), 7.72–7.60 (dd, 2H, Ar), 7.26 (d, α H, J = 15.58). 13C NMR (100 MHz, CDCl3) δ: 97.80, 101.42, 113.00, 120.13, 122.35 (Cα), 129.56, 131.05, 135.27, 140,20 (Cβ), 160.2, 62.08, 190.65 (C O) ppm. MS: m/z 466 (M+). Calcd. for C16H9Br2F3O3; Found: C: 41.23%, H: 1.95%, Br: 34.29%, F: 12.23%, O: 10.30%.
2.1.2. Method for the synthesis of bis-substituted chalcones (6a-h)
A suitable 2,4-dihydroxy chalcone 3 (0.01 mol), 1,3-dibromopropane (0.01 mol) and anhydrous potassium carbonate (0.02 mol) in dry acetone (100 mL) was refluxed for 10–15 h. The negative test with alcoholic FeCl3 was performed to check the completion of the reaction. Acetone was filtered off after the completion of reaction. With continuous stirring, the residue left inside the flask was treated with ice. Separated solid was filtered and washed with aqueous NaOH (5%) and lastly with water. The solid obtained was dried and recrystallized from ethanol. The ethanol insoluble solid residue resulted bis-chalcones (6) while filtrate (ethanol dissolvable) after concentration gave a product which on crystallization resulted compounds (7) (Fig. 1: Scheme 2).
2.2. Characterization of synthesize materials
The progress of the reaction was monitored by thin layer chromatography (TLC) using 8:2 hexanes: ethyl acetate. Melting points were observed in open capillaries on melting point equipment and are uncorrected. 1H NMR and 13C NMR spectra were recorded using CDCl3 as solvent on FT-NMR spectrometer Bruker AV III, 500 MHz and 100 MHz respectively. GC-MS were recorded on JEOL GC Mate spectrometer and elemental analysis was carried out on a Carlo Erba 1108 analyzer.
2.3. Antifeedant, toxicity and nutritional effect of synthesized mono- and bis-chalcones on second instar larval diet of Spodoptera frugiperda
2.3.1. Test insects
Spodoptera frugiperda larvae were obtained from Instituto de Química Orgánica, Facultad de Bioquímica, Química y Farmacia, UNT, Tucumán, (4000), Argentina. The larval diet comprised of a combination of yeast (3 g), bean boil and milled (250 g), wheat germ (12.5 g), methyl p-hydroxybenzoate (1.5 g), ascorbic corrosive (1.5 g), formaldehyde (4 mL of a 38% water arrangement), agar (12.5 g), and water (500 mL).
2.3.2. No choice test
Bits of larval diet were completely blended with the solution of synthetic chalcones to leave 100 µg of compound for every gm of diet (treated), solvent were left to evaporate at room temperature (Di Toto et al., 2010) Another part was impregnated with acetone and utilized as control diet. Traces of solvent were taken out in a desiccator. Cotton stopped test tubes containing second instar larvae (1 for every tube) were added with one or the other control or treated eating routine and kept at 25 °C and 60 ± 15% relative humidity. After endless supply of half of control diet, remaining diets were eliminated and weighed precisely and proportion was determined as FR50 = T/C (Cole, 1994) (T and C address weights of diets devoured in the treated and control tests, individually).
2.3.3. Toxicity test
Control and treated diets were set in cotton stopped test tubes (20 replicates for every treated and 20 for control experiments) with second instar larvae (1 for every tube) and put away in a growth chamber (25 °C and 60 ± 15% relative humidity) until the first era of grown-ups arose. Larval formative periods and death rates were recorded to treat with all the synthesized chalcones (100 μg/mL) and control tests (Villafañe et al., 2011)
2.3.4. Nutritional indices
Homogeneous size 2nd instar larvae were kept in a test tube (1 for every tube, 20 replicates) and weight of the larvae was estimated occasionally with one or the other test or control diets and kept at 25 °C. Larval and diet weights were precisely enrolled. Average diet consumption (CI), growth rate (GR) and efficiency in the consumption index (ECI) were determined during a 10th day time frame (Hidalgo et al., 2016). Rates communicated as treatment-control proportion and qualities communicated as (GRT/GRC) 100% (CIT/CIC) 100% and (ECIT/ECIC) 100% in the tables (Villafañe et al., 2011). Control is considered as 100%.
3. Results
3.1. Synthesis of mono- and bis- substituted chalcones
We have successfully synthesized six mono- chalcones 3a-f (Fig. 1: Scheme 1) and eight bis-chalcones 6a-h (Fig. 1: Scheme 2) via Claisen Schimdt condensation followed by purification and characterization.
3.2. Synthesized chalcones: No choice test
The consolidation of mono- and bis-chalcones into artificial diet of Spodoptera frugiperda at the portion of 100 μg/g; created a gentle antifeedant impact on mono-chalcone A (FR50 = 0.83 ± 0.16); while antifeedant impact of the others mono and bis-chalcones did not uncover any impacts (FR50), as demonstrated in Table 1.
Table 1.
Antifeedant and toxic impacts of synthesized mono and bis-chalcones on second instar larval diet of Spodoptera frugiperda.
| Compounds | FR50a | Larval mortality (%) | Pupal mortality (%) | Malformed adults (%) |
|---|---|---|---|---|
| 3a | 0,83 ± 0,16c | 35 | 0 | 65 |
| 3b | 0,93 ± 0,24b,c | 50 | 0 | 50 |
| 3c | 0,96 ± 0,15b | 10 | 15 | 75 |
| 3d | 0,93 ± 0,15b | 20 | 10 | 70 |
| 3e | 0,98 ± 0,17b | 5 | 0 | 95 |
| 3f | 0,91 ± 0,20b,c | 25 | 0 | 75 |
| 6a | 1,05 ± 0,13a | 20 | 15 | 75 |
| 6b | 0,93 ± 0,20a,b | 85 | 5 | 10 |
| 6c | 0,91 ± 0,18b | 40 | 20 | 40 |
| 6d | 1,06 ± 0,10a | 15 | 15 | 70 |
| 6e | 0,98 ± 0,13a,b | 60 | 10 | 30 |
| 6f | 0,93 ± 0,20a,b | 60 | 5 | 35 |
| 6 g | 0,89 ± 0,16a,b | 55 | 20 | 25 |
| 6h | 0,91 ± 0,18a,b | 60 | 20 | 20 |
Note: aMean ± SD. Means followed by a similar letter are not significantly different (P > 0.05, Tukey various range test).
3.3. Characterization of synthesize materials
All the synthesized chalcones were characterized by the FTIR, NMR, Mass and elemental analysis (Supplementary Information). All the characterization data are given in the Supplementary Information file of the article which justifies the structures of the synthesized compounds.
4. Discussion
The present study describes the synthesis, purification, characterization and antifeedant studied of some synthesized bis- and mono-substituted chalcones and it reveals that some chalcones could be new candidates as antifeedant agents based on this study. The results acquired in the trial of toxicity for every single one of the synthesized chalcones reveal that mono-chalcone 3b and bis-chalcones 6b, 6e, 6f and 6h, caused deadly impact on S. frugiperda in the primary larval stages, being 6b the most toxic (85%). Grown-ups who endure showed malformations and diminished size (Fig. 3), which prompted to death prior to laying eggs. The bis-chalcones appeared more toxic than mono-chalcones (Table 1).
Fig. 3.

Insect of S. frugiperda. (A) View of untreated insect. (B) Treated insect (6b) showing decrease in growth.
To assess the mechanism of activity that prompts the mortality delivered by the therapeutics, we noticed the nutritional impacts created by integration of chalcones to second instar larval diet of Spodoptera frugiperda. In light of the outcomes on food utilization, it was discovered that mono and bis-chalcones 3a, 3d, 3f, 6b, 6c, 6e, 6f and 6g (100 µg/g) can modify GR and ECI values (Table 2).
Table 2.
Nutritional impacts of synthesized mono and bis-chalcones on second instar larvval diet of Spodoptera frugiperda.
| Compounds | CIT/CICa (%) | GRT/GRCb (%) | ECIT/ECICc (%) |
|---|---|---|---|
| 3a | 88 ± 13a,b | 40 ± 27d | 45 ± 25e |
| 3b | 98 ± 12a | 77 ± 30a,b | 79 ± 23b,c,d |
| 3c | 80 ± 8d,e | 69 ± 14a,b | 86 ± 12b |
| 3d | 74 ± 5a | 43 ± 16c,d | 58 ± 20c,d |
| 3e | 80 ± 4c,d | 54 ± 16a,b,c | 67 ± 19b,c |
| 3f | 77 ± 5d,e | 47 ± 17b,c | 62 ± 20b,c,d |
| 6a | 98 ± 3a | 97 ± 15a | 99 ± 15a |
| 6b | 47 ± 7e | 15 ± 10d,e | 31 ± 17b,c |
| 6c | 53 ± 9d,e | 21 ± 15d,e | 37 ± 25b,c |
| 6d | 100 ± 3a | 94 ± 11a,b | 94 ± 10a |
| 6e | 66 ± 11c | 38 ± 24c | 54 ± 27b |
| 6f | 65 ± 9c | 31 ± 16c,d | 46 ± 20b |
| 6 g | 59 ± 9c,d | 23 ± 14c,d,e | 38 ± 18b,c |
| 6h | 45 ± 10e | 10 ± 9e | 21 ± 14c |
Note: aNumbers in sections address mean ± SD. bMeans inside a segment followed by a similar letter are not fundamentally unique (P > 0.05, Tukey different reach test). aCIT/CIC (Consumption Index); bGRT/GRC (Growth Rate); cECIT/ECIC (Efficiency in the Consumption Index). For examination purposes, rates of nutritional indices are communicated as a connection among treatment and control. 6d.
A fall in ECI shows that more food is processed for energy and less is transformed to body substance (for example growth), bringing about diminished larval growth and expanded larval mortality in the beginning phases of their life cycle and perhaps uncovering the existence of toxic compounds (Fig. 2). Bis-chalcones 6b, 6e and 6f had the most noteworthy level of intake and the least fortunate change of supplement absorption (ECI), which suggests that the larva uses nourishment for energy and results in a diminishing of growth and death in beginning phases (Table 2).
Fig. 2.

Larvae of S. frugiperda. (A) View of untreated larva. (B) Treated larva (6b) showing decrease in growth.
The use of chalcones as eco-accommodating plant development controllers and plant creation is of extraordinary pertinence. Because of the various biological activities of chalcones, there are numerous prospects for their utilization in farming and crop sustainability. Concerning pest deterrent and weed control, the most intriguing biological activities of chalcones are the insecticidal, bactericidal, antifeedant and phytotoxic activities (Diaz-Tielas et al., 2016).
In continuation of earlier endeavor (Hidalgo et al., 2019), the current investigation is the second report on the antifeedant, toxicity and nutritional impacts of synthesized chalcones on Spodoptera frugiperda larvae. The utilization of synthesized chalcones is promising in the area of nematicidal activity (Caboni et al., 2016) and insecticidal, since the pests cause extraordinary agronomic loss. Numerous synthetic chalcones are profoundly dynamic against Phenacoccus solanopsis that causes significant cotton crop loss by forming colonies on leaves and stems progressing into white waxy dense masses resulting in sucking of huge amount of essential nutrients from plants (Nalwar et al., 2009). Similarly, some synthesized chalcones also responded actively against Achaea janta L. which causes genuine harm to castor (Ricinus communis) bringing about huge financial losses (Ganesamoorthy and Ganesan 2014). Earlier, some synthesized chalcones were found to be larvicidal against Aedes albopictus, which is an insect that communicates different kinds of sicknesses, including dengue fever, zika infection and chikungunya fever (Lee et al., 2018).
Spodoptera frugiperda is a polyphagous lepidopteran, which is a significant pest in corn fields, that benefits from ears and corn leaves (Marenco et al., 1992). The most serious harm is caused during its initial larval stages (Murúa and Virla 2004). Hence, the chosen compounds for control of this pest ought to ideally create larval mortality. As demonstrated in Table 1, bis-chalcone 6b executed 85% and 6e, 6f, 6h killed 60% of Spodoptera frugiperda larvae at the dose tested, while the remaining synthesized mono- and bis-chalcones had less impact. The main toxic activity was seen in the beginning phases larvae. The expansion of mono and bis-chalcones 3b, 6a and 6d to the larval diet did not altogether adjust neither utilization nor larval growth or effectiveness in changing the consumed supplements into biomass. Adults who survived showed malformations and decreased size, which led to death (Fig. 2).
5. Conclusions
We have ssyntheszed some mono- and bis- substituted chalcones which worked effectively as an antifeedant agent on S. frugiperda. From the current investigation, we have concluded that among the synthesized chalcones, bis-chalcones 6b, 6e, 6f and 6h showed comparable utilizations and comparative decrease in the size and biomass transformation.. All mono-chalcones created high contorted grown-up crisis. The expansion of bis-chalcones to the larval diet showed more noteworthy toxicity than mono-chalcones and 6b being the most elevated toxicity and nutritional alteration. FR values from Table 1 helped to calculate the antifeedant activity when 50% of the larvae control ate 50% of the diet. Larvae which survived showed malformations and decrease in growth which led to death. The anti-larval activity against S. frugiperda shown from the data of some of the newly synthesized chalcones opens the door for future exploitation of these promising chalcones molecules in controlling S. frugiperda pest for sustainable agriculture/ crop industry.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors (KLA, JRH and AN) are thankful to DST New Delhi, India and MINCYT, Argentina for sanctioning international cooperation Indo-Argentina research grant. The authors (APD & KLA) are also thankful to Dean, SLAS, Mody University of Science and Technology for providing necessary laboratory facilities. The authors thank Taif University Researchers Supporting Project number (TURSP-2020/119), Taif University, Taif, Saudi Arabia for providing financial support.
Funding
This research was funded by Taif University Researches Supporting Project number (TURSP-2020/119), Taif University, Taif, Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
R.Z. Sayyed, Email: sayyedrz@gmail.com.
Keshav Lalit Ameta, Email: klameta77@hotmail.com.
References
- Ameta K.L., Kumar B., Singh R.N. Microwave Induced Improved Synthesis of Some Novel Substituted 1,3-Diarylpropenones and their Antimicrobial Activity. E- J. Chem. 2011;8:665–670. [Google Scholar]
- Amslinger S., Al-Rifai N., Winter K., Wörmann K., Scholz R., Baumeister P., Wild M. Reactivity assessment of chalcones by a kinetic thiol assay. Org. Biomol. Chem. 2013;11:549–554. doi: 10.1039/C2OB27163J. [DOI] [PubMed] [Google Scholar]
- Assefa F., Ayalew D. Status and control measures of fall armyworm (Spodoptera frugiperda) infestations in maize fields in Ethiopia: A review. Cogent Food Agric. 2019;5:1641902–1641917. doi: 10.1080/23311932.2019.1641902. [DOI] [Google Scholar]
- Awasthi S.K., Mishra N., Dixit S.K., Singh A., Yadav M., Yadav S.S., Rathaur S. Antifilarial activity of 1,3-diarylpropen-1-one: effect on glutathione-S-transferase, a phase II detoxification enzyme. Am. J Trop. Med. Hyg. 2009;80:764–768. [PubMed] [Google Scholar]
- Begum N.A., Roy N., Laskar R.A., Roy K. Mosquito larvicidal studies of some chalcone analogues and their derived products: structure–activity relationship analysis. Med Chem. Res. 2011;20:184–191. [Google Scholar]
- Bueno O., Tobajas G., Quesada E., Estevez-Gallego J., Noppen S., Camarasa M.J., Díaz J.F., Liekens S., Priego E.-M., Perez-Perez M.-J. Conformational mimetics of the a-methyl chalcone TUB091 binding tubulin: design, synthesis and antiproliferative activity. Eur. J. Med. Chem. 2018;148:337–348. doi: 10.1016/j.ejmech.2018.02.019. [DOI] [PubMed] [Google Scholar]
- Burmaoglu S., Ozcan S., Balcioglu S., Gencel M., Noma S., Essiz S., Ates B., Algul O. Synthesis, biological evaluation and molecular docking studies of bis-chalcone derivatives as xanthine oxidase inhibitors and anticancer agents. Bioorg. Chem. 2019;91:103149–103156. doi: 10.1016/j.bioorg.2019.103149. [DOI] [PubMed] [Google Scholar]
- Caboni P., Aissani N., Demurtas M., Ntalli N., Onnis V. Nematicidal activity of acetophenones and chalcones against Meloidogyne incognita and structure-activity considerations. Pest Manag. Sci. 2016;72:125–130. doi: 10.1002/ps.3978. [DOI] [PubMed] [Google Scholar]
- Cole M.D. Key antifungal, antibacterial and anti-insect assays—a critical review. Biochem. Syst. Ecol. 1994;22:837–856. [Google Scholar]
- Das B.P., Begum N.A., Choudhury D.N., Banerji J. Larvicidal studies of chalcones and their derivatives. J. Indian Chem. Soc. 2005;82:161–164. [Google Scholar]
- Deshmukh S., Pavithra H.B., Kalleshwaraswamy C.M., Shivanna B.K., Maruthi M.S., Mota-Sanchez D. Field Efficacy of Insecticides for Management of Invasive Fall Armyworm, Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae) on Maize in India. Fla. Entomol. 2020;103:221–227. [Google Scholar]
- Di Toto B.L., Álvarez O., Popich C.S., Neske A., Bardón A. Antifeedant and toxic effects of acetogenins from Annona montana on Spodoptera frugiperda. J. Pest Sci. 2010;83:307–310. doi: 10.1007/s10340-010-0299-0. [DOI] [Google Scholar]
- Díaz-Carillo J.T., Díaz-Camacho S.P., Delgado-Vargas F., Rivero I.A., López-Angulo G., Sarmiento-Sánchez J.I., Montes-Avila J. Synthesis of leading chalcones with high antiparasitic, against Hymenolepis nana, and antioxidant activities. Braz. J Pharm. Sci. 2018;54:1–13. [Google Scholar]
- Diaz-Tielas C., Grana E., Reigosa M.J., Sanchez-Moreiras A.M. Biological activities and novel applications of chalcones. Planta Daninha. 2016;34:607–616. [Google Scholar]
- Dong L.R., Hu D.Y., Wu Z.X. Study of the Synthesis, Antiviral Bioactivity and Interaction Mechanisms of Novel Chalcone Derivatives that Contain the 1,1-Dichloropropene Moiety. Chinese Chem. Lett. 2017;28:1566–1570. [Google Scholar]
- Ganesamoorthy T., Ganesan V. Synthesis, spectral studies, antimicrobial and insect antifeedant activities of some substituted styryl 4′-fluorophenyl ketones. Arabian J. Chem. 2014;7:1055–1064. [Google Scholar]
- Gautam N., Chourasia O.P. Synthesis, antimicrobial and insecticidal activity of some new cinnoline based chalcones and cinnoline based pyrazoline derivatives. Indian J. Chem. 2010;49:830–835. [Google Scholar]
- Hidalgo J.R., Santillán M., Parellada E.A., Neske A., Khyaliya P., Ameta K.L. Synthetic bis- and mono-chalcones with insecticide effects on Spodoptera frugiperda (Lepidoptera: Noctuidae) Int. J. Pest Manag. 2019;66 doi: 10.1080/09670874.2019.1575487. [DOI] [Google Scholar]
- Hidalgo J.R., Parellada E.A., Blessing L.D.T., Bardón A., Ameta K.L., Vera N., Neske A. Natural and Derivatized Acetogenins Promising for the Control of Spodoptera frugiperda. J. Agric. Chem. Environ. 2016;5:200–210. doi: 10.4236/jacen.2016.54021. [DOI] [Google Scholar]
- Iftikhar S., Khan S., Bilal A., Manzoor S., Abdullah M., Emwas A.H., Sioud S., Gao X., Chotana G.A., Faisal A., Saleem R.S.Z. Synthesis and evaluation of modified chalcone based p53 stabilizing agents. Bioorg. Med. Chem. Lett. 2017;27:4101–4106. doi: 10.1016/j.bmcl.2017.07.042. [DOI] [PubMed] [Google Scholar]
- Janaki P., Sekar K.G., Thirunarayanan G. Synthesis, spectral correlation and insect antifeedant activities of some 2-benzimidazole chalcones. J. Saudi Chem. Soc. 2016;20:58–68. [Google Scholar]
- Lee S.-H., Choi J.Y., Lee B.R., Fang Y., Kim J.H., Park D.H., Park M.G., Woo R.M., Kim W.J., Je Y.H. Insect growth regulatory and larvicidal activity of chalcones against Aedes albopictus. Entomol. Res. 2018;48:55–59. doi: 10.1111/1748-5967.12288. [DOI] [Google Scholar]
- Marenco R.J., Foster R.E., Sanchez C.A. Sweet Corn Response to Fall Armyworm (Lepidoptera: Noctuidae) Damage During Vegetative Growth. J. Econ. Entomol. 1992;85:1285–1292. doi: 10.1093/jee/85.4.1285. [DOI] [Google Scholar]
- Maruthadurai R., Ramesh R. Occurrence, damage pattern and biology of fall armyworm, Spodoptera frugiperda (J.E. smith) (Lepidoptera: Noctuidae) on fodder crops and green amaranth in Goa, India. Phytoparasitica. 2020;48:15–23. doi: 10.1007/s12600-019-00771-w. [DOI] [Google Scholar]
- Mirzaei S., Hadizadeh F., Eisvand F., Mosaffa F., Ghodsi R. Synthesis, structure-activity relationship and molecular docking studies of novel quinoline-chalcone hybrids as potential anticancer agents and tubulin inhibitors. J. Mol. Struct. 2020;1202:127310–127322. [Google Scholar]
- Morimoto M.K., Nakano T.S., Ozaki T., Nakano A., Komai K. Insect antifeedant activity of flavones and chromones against Spodoptera litura. J. Agric. Food Chem. 2003;51:389–393. doi: 10.1021/jf025627a. [DOI] [PubMed] [Google Scholar]
- Murúa G., Virla E. Population Parameters of Spodoptera Frugiperda (Smith) (LEP.: Noctuidae) Fed on Corn and Two Predominant Grasess in Tucuman (Argentina) Acta Zoológica Mex. 2004;20:199–2010. [Google Scholar]
- Nalwar Y., Sayyed M., Mokle S., Zanwar P., Vibhute Y. Synthesis and Insect Antifeedant Activity of Some New Chalcones Against Phenacoccus solanopsis. World J. Chem. 2009;4:123–126. [Google Scholar]
- Panche A.N., Diwan A.D., Chandra S.R. Flavonoids: an overview. J. Nutr. Sci. 2016;5 doi: 10.1017/jns.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad Y.R., Kumar P.R., Deepti C.A., Ramana M.V. Synthesis and Antimicrobial Activity of Some Novel Chalcones of 2-Hydroxy -1-Acetonapthone and 3-Acetyl Coumarin. Eur. J. Chem. 2006;3:236–241. [Google Scholar]
- Prasad Y.R., Rao A.L., Rambabu R., Kumar P.R. Synthesis and biological evaluation of some novel chalcone derivatives. Oriental J. Chem. 2007;23:927–937. [Google Scholar]
- Rao S.M., Kotesh J., Marukulla R., Duddeck H. Synthesis and spectroscopic characterization of some chromanochalcones and their dihydro derivatives. Arkivoc. 2004;xiv:96–102. [Google Scholar]
- Satyanarayana M., Tiwari P., Tripathi B.K., Srivastava A.K., Pratap R. Synthesis and antihyperglycemic activity of chalcone based aryloxypropanolamines. Bioorg. Med. Chem. 2004;12:883–889. doi: 10.1016/j.bmc.2003.12.026. [DOI] [PubMed] [Google Scholar]
- Shukla P., Satyanarayana M., Verma P.C., Tiwari J., Dwivedi A.P., Srivastava R., Rehuja N., Tamrakar A.K., Dwivedi A.K., Kushwaha H.N., Gautam N., Singh S., Srivastava M., Nath C., Raghubir R., Srivastava A.K., Pratap R. Chalcone-based aryloxypropanolamine as a potential antidiabetic and antidyslipidaemic agent. Curr. Sci. 2017;112:1675–1689. [Google Scholar]
- Susurluk H., Caliskan Z., Gurkan U., Kirmizigul S., Goreu N. Antifeedant activity of some Tanacetum species and bioassay guided isolation of the secondary metabolites of Tanacetum cadmium ssp. Cadmium (Compositae) Ind. Crops Prod. 2007;26:220–228. [Google Scholar]
- Villafañe E., Tolosa D., Bardón A., Neske A. Toxic effects of Citrus aurantium and C. limon essential oils on Spodoptera frugiperda (Lepidoptera: Noctuidae) Nat. Prod. Commun. 2011;6:1389–1392. [PubMed] [Google Scholar]
- Yoon G., Kang B.Y., Cheon S.H. Topoisomerase inhibition and cytotoxicity of licochalcones A and E from Glycyrrhiza inflate. Arch. Pharm Res. 2007;30:313–316. doi: 10.1007/BF02977611. [DOI] [PubMed] [Google Scholar]
- Zhuang C., Zhang W., Sheng C., Zhang W., Xing C., Miao Z. Chalcone: A privileged structure in medicinal chemistry. Chem. Rev. 2017;117:7762–7810. doi: 10.1021/acs.chemrev.7b00020. [DOI] [PMC free article] [PubMed] [Google Scholar]