Abstract
A Newcastle disease virus (NDV) oncolysate has been established as a unique and effective immune-stimulatory root for tumor treatment. Thus, the aim of the current study was to investigate the effects of intratumoral administration of NDV oncolysate on immune response and tumor regression of C57BL/6 mouse model of human papillomavirus (HPV) related transplanted with TC-1 syngeneic cancer cells. To further investigate the mechanism underlying the antitumor response, cytolytic and lymphocyte proliferation responses in splenocytes were measured using lactate dehydrogenase (LDH) release and MTT assays, respectively. In this regard, levels of IL-10, IFN-γ, and IL-4 were measured using ELISA after re-stimulation. The immune responses efficacy was evaluated by in vivo tumor regression assay. The results showed that immunization with the different titers of NDV lysate significantly reduced tumor volume in comparison with a combination of virus lysate and tumor cell lysate. Also, virus lysate could significantly enhance cytotoxic T lymphocyte production and lymphocyte proliferation rates versus tumor cell lysate. Also, our major findings are that the peritumorally injection of NDV oncolysate effectively induces antitumor immune responses through increased levels of IL-4, IFN-γ, and reduction of IL-10. These results indicate that this treatment is a specific, active immune mechanism stimulator, and may prove to be a useful therapeutic for a treatment against cervical cancers and merits further investigation.
Keywords: Oncolytic, Oncolysate, Newcastle disease virus, Human papillomavirus, Tumor microenvironment
Abbreviations: NDV, Newcastle disease virus; HPV, Human papillomavirus; HB1, Hitchner B1; OVs, Oncolytic viruses; VO, Viral oncolysate; LDH, Lactate dehydrogenase; PBS, Phosphate-buffered saline; ELISA, Enzyme-Linked Immunosorbent Assay; T-Vec, Talimogene laherparepvec; FDA, Food and drug administration; DAMP, Danger-associated molecular pattern; RPMI, Roswell park memorial institute; FBS, Fetal bovine serum; MOI, Multiplicity of infection; UVB, Ultraviolet B
1. Introduction
Although chemotherapy and its role in the treatment of cancer has a long history, the use of this method is still unclear due to its side effects. Therefore, scientists always looking for new procedures to overcome the problems with chemotherapy (Arruebo et al., 2011, Falzone et al., 2018). Oncolytic viruses (OVs) as a nascent medicament for cancer therapy has been identified for almost a century but only over the past ten years have clinical investigations recorded a therapy profit in the cases with cancer (Vähä-Koskela et al., 2007, Goldufsky et al., 2013, Mozaffari Nejad et al., 2021). In this therapy, viruses can selectively propagate in and kill cancer cells while no effect on normal cells. The effectiveness of OVs is performed in two forms: 1) straight tumor lysis; and 2) induction of an antitumor immune response in humans or animals (Jiang et al., 2015). Talimogene laherparepvec (T-Vec) and Oncorine (H101) are two genetically engineered OVs for cancer therapy in global marketing as drugs (Cao et al., 2020, Mozaffari Nejad et al., 2020). Among the different oncolytic viruses that have been experimented with, several belong to the Paramyxo Viridae family such as measles, respiratory syncytial virus (RSV), morbillivirus, and Newcastle disease virus (NDV) that cause diseases between both human and animals (Keshavarz et al., 2019, Keshavarz et al., 2020a, Mozaffari Nejad et al., 2020).
Oncolysate has been used as an auxiliary method for immunotherapy. Lysed tumor cells turn the suppressive tumor microenvironment in favor of immune cells to recognize the hidden antigens of tumors (Batliwalla et al., 1998). There are a variety of ways to allocate oncolysates such as; hypochlorous acid oxidizes, ultraviolet B (UVB) radiation, freeze and thaw, and hyperthermia. All of the mentioned procedures yield necrotic antigens from the tumor cells (Chiang et al., 2010, Vandenberk et al., 2016). In addition to these mechano-chemical procedures, there is a way that harnesses viruses to develop oncolysates. Viral oncolysate (VO) was the first introduced into clinical trials as a treatment adjunct to chemotherapy and nonspecific immunomodulators (Ioannides et al., 1989). The first application of the VO returns to the late 1970 s when (Murray et al., 1977) monitor the effect of viral oncolysate obtained by NDV on melanoma patients. Viral oncolysates are homogenates of a tumor and virus-infected cells. VO-based method shields animals against transplanted autologous tumors (Chiang et al., 2010, Fournier and Schirrmacher, 2013). In the tumor milieu, the neoplastic cells unleash suppressive signals to hamper the responses of the immune cells.
Therefore, in this study, we explain the effect of NDV oncolysate on the stimulation of immune responses against tumors, evidence of cytotoxic T cells activation against tumor cells after VO administration, as well as the main balance of cytokines involved in tumor immunity.
2. Materials and methods
2.1. Cell culture
For our model research studies, we apply the murine lung cancer cell line (TC-1), and the murine T-lymphoma cell line EL4 [carcinogen-induced lymphoma of C57BL/6 mice (H-2b) origin] prepared from the Cell Bank of Institute Pasture of Tehran, Iran which was provided in RPMI (Roswell Park Memorial Institute) 1640 medium completed with 10% (v/v) fetal bovine serum (FBS) and finally the cells were kept at 37 ˚C under 5% (v/v) CO2 for experiments.
2.2. Newcastle disease virus purification
The avirulent, nonlytic Hitchner B1 strain of NDV (HB1 NDV) was used for virus infection. Briefly; the HB1 NDV was prepared from Razi Institute of Serum and Vaccine Research Center, Karaj, Iran. It was inoculated in the allantoic cavity of 9–11 days-old specified pathogens free (SPF) embryonated chicken eggs and incubated at 37 ˚C for 72 h. The allantoic fluid was harvested aseptically and centrifuged at 30000 × g for 3 h at 4 ◦C (Mozaffari Nejad et al., 2020).
The re-suspension of purified virus was carried out in PBS (pH 7.4), followed by titration via haemagglutination test. Finally, obtained virus was stored at −80 ˚C for further analysis.
2.3. Animals
Female C57BL/6 (H2b) mice, six to eight weeks-old, were procured from the Institute Pasture of Tehran, Iran for this research confirmed by the Ethical Committee guidelines for the care and use of laboratory animal’s protocol of Hamadan University of Medical Sciences, Hamadan, Iran (IR.UMSHA. REC.1396.403).
2.4. Preparation of Viral oncolysate
According to a previous study (Mozaffari Nejad et al., 2020), the TC-1 Cells 24 h after the seeding was inoculated with HB1 NDV of the multiplicity of infections (MOIs) of 10, and 15 and maintained at 37 ◦C, with 5% CO2 until the cytopathic effect (CPE) appears. Subsequently, cells were lysed with 3 freeze and thaw lysing cycles using liquid nitrogen and a 37 ˚C water bath. The cell suspension was centrifuged for 10 min at 1000 g/min. The supernatant were harvested and was further centrifuged at 20000 rpm for 1 h and the pellet was harvested and finally, was homogenized and suspended in phosphate-buffered saline (PBS) (Wallack 1980). One ml aliquots were frozen at −80 °C for use later.
2.5. Tumor induction and therapy
For tumor induction, 6 groups of female C57BL/6 mice with ten animals in each group were used by subcutaneous administration in the left flank of the mice on day 0 with 3 × 105 TC-1 tumor cells/mouse combined with 100 μl PBS (Mohebbi et al., 2019). After 14 days of tumor cells injection, all animals were treated peritumorally with various formulations (Table 1).
Table 1.
Different mice groups for in vivo study: The concentration and routes of administration are for each group.
| Groups | Treated with | Concentration |
|---|---|---|
| VTCL 15 | Viral tumor cell lysate with MOI 15 | 1 × 107 cells/mL |
| VTCL 10 | Viral tumor cell lysate with MOI 10 | 1 × 107 cells/mL |
| TCL | TC-1 tumor cell lysate 5 cycles of freezing and thawing (liquid nitrogen and a 37 ˚C water bath) | 1 × 107 cells/mL |
| TCL/ VTCL 15 | TC-1 tumor cell lysate 5 cycles of freezing and thawing combined with viral tumor cell lysate with MOI 15 (50/50) | 5 × 106 + 5 × 106 cells/mL |
| TCL/ VTCL 10 | TC-1 tumor cell lysate 5 cycles of freezing and thawing combined with viral tumor cell lysate with MOI 10 (50/50) | 5 × 106 + 5 × 106 cells/mL |
| PBS | Control group administration just PBS | 100 µl |
VTCL 15: Viral Tumor Cell Lysate MOI 15; VTCL 10: Viral Tumor Cell Lysate MOI 10; TCL: Tumor Cell Lysate; PBS: Phosphate-Buffered Saline.
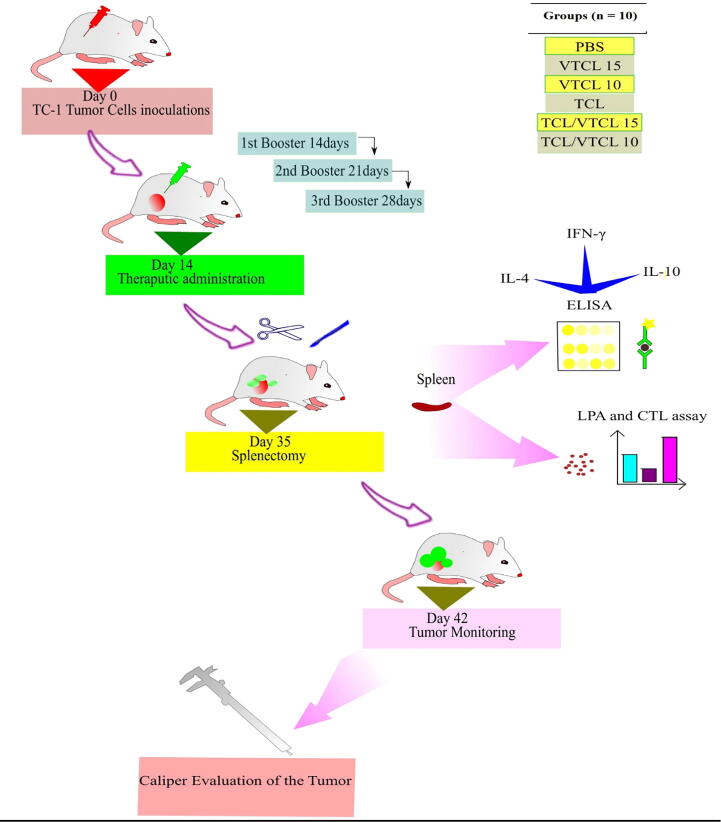
We repeat the same process of using the same amount of treatment in the second and third weeks (Thrice at one-week intervals). After one week of TC-1 tumor cell administration, every three days’ interval a lab technician blindly and based on the tumor codes checked the tumors 'size using a digital caliper. A simple mathematical formula was used to check the volume of the tumors, (length × width2)/2. The length indicated the tumor diameter and the width denote the vertical growth of the tumor (Keshavarz et al., 2020). We checked the tumor growth for six weeks. Besides, after the last treatment, the spleens of three mice from each groups were sacrificed and used for immunological studies. The schematic picture of all tentative procedures is presented in Fig. 1.
Fig. 1.
Schematic overview of all experimental procedures.
2.6. Cytokine assay
After 7 days of final treatment, the animals were sacrificed (3 animals of each group) and their spleens were segregated and the next red blood cells (RBCs) were cleaned using incubation in lysis buffer (0.15 M NH4Cl, 1 mM KHCO3, 0.1 mM Na2EDTA, pH 7.2) at 37 °C for 5 min. We seeded mononuclear cells at a density of 2 × 105 cells/well in 24-well plates (TPP, Switzerland) for 72 h in RPMI 1640 completed with L-glutamine (1% v/v), 2.5 mM 2-mercaptoethanol, HEPES (1% v/v), FBS (10% v/v) and E7-specific H-2Db CTL epitope at a concentration of 1 μg/ml (Biomatik, Canada) and finally incubated at 37 °C in 5% CO2 for 48 h. The supernatants of the cells were collected and then IL-10, IL-4, and IFN- γ cytokines were measured by commercial sandwich-based ELISA kits. For analysis, we used Il-10 (eBioscience, Inc. USA), IFN- γ, and IL-4 (R&D Systems Inc., USA) according to the protocol for the user. We confirmed all analysis three times. Finally, the optical density (OD) of each plates were read at 450 nm wavelengths (Abdolalipour et al., 2020, Keshavarz et al., 2020a).
2.7. Lymphocyte proliferation assay (LPA)
We acquire white blood cells 7 days after the mice received the oncolysate for the third times. The cells were cultured in MDEM in combination with 1 μg per ml of the E7-specific H 2Db CLT epitope at 37 °C. We used 5 μg per ml T cell mitogen phytohemagglutinin (PHA) to positively control the process. After 72 h, we added to each well 5 μg per ml of MTT salt [3-(4, 5 dimethylthiazal-2-yl) − 2,5-diphenyl tetrazolium bromide] and incubated at 37 °C under 5% (v/v) CO2 for 5 h. Afterward, we added 100 DMSO μl to the dissolving of crystals of the formazan. Finally, the optical density (OD) of the cultured cells was measured with an OD meter at 540 nm and the results were evaluated as a stimulation index (SI). The SI ratio was calculated as follows: OD values of stimulated cells minus relative cell numbers of unstimulated cells divided by relative OD values of unstimulated cells (Mohebbi et al., 2019, Keshavarz et al., 2020a). All experiments were assayed three times (n = 3) for each mouse.
2.8. Cytotoxicity assay
After 7 days of final treatment, the animals were sacrificed (3 animals of each group) and their spleens were isolated. Mono-cell suspensions of single nuclear cells were applied as the effector cells (splenocytes cells). 4 × 104 EL4 cells in a volume of 100 μl (as a target cells) were co-cultured with the effector cells (100 μl) at 50:1 effector-to-target cell (E/T) ratio for 8 h in phenol-red-free RPMI 1640. For the preparation of target cells, EL4 cells were stimulated with a synthetic E7-specific cytotoxic T lymphocytes (CTL) epitope at a concentration of 1 μg/ml (Biomatik, Ontario, Canada,>99% purity) (specific antigen) and then incubated for 4 h. After centrifugation at 250 g/min for 10 min, 50 μl/well the supernatants were removed and transferred to new 96-well plates, and finally, the CTL activity was surveyed according to detection kit protocol (Takara Biochemicals, Japan) (Mohebbi et al., 2019, Keshavarz et al., 2020a).
The ‘high control’ was used for measuring total LDH release from the target cells (all EL4 cells were lysed with a medium containing 1% Triton X-100). The ‘low control’ was used for measuring the natural release of LDH from the target cells (which was obtained by adding EL4 cells only to the test medium). For all samples, including the controls, the experiment was done in thrice. The LDH-mediated alteration of tetrazolium salt into a red formazan product was measured at 490 nm after incubation at room temperature for 30 min. The percentage of specific cytolysis was determined by the following formula (Jahromi et al., 2014):
Cytotoxicity = [(experimental value − effector cell control) − low control / high control − low control] × 100.
2.9. Statistical analysis
All the experiments have done thrice and we employed GraphPad software, version 5.01 (GraphPad Software, CA, USA) to analyze the data. One-way ANOVA statistical test was used to juxtapose the means in each group. Tukey’s post hoc test was implemented to confirm the differences in the means. The meaningfulness of the difference indicated when the P-value was: P < 0.05, P < 0.01, and P < 0.001.
3. Results
3.1. Cytokine assay
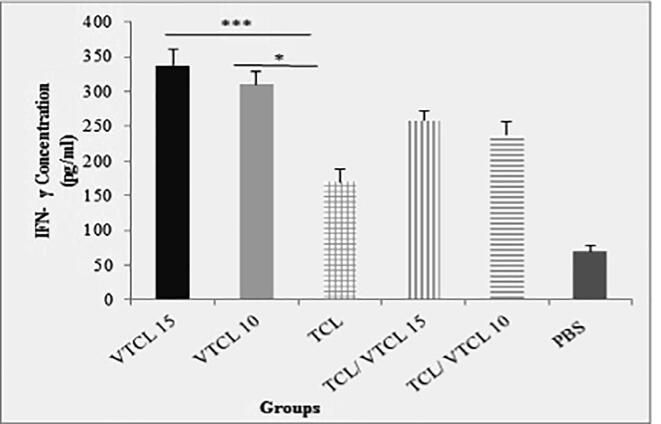
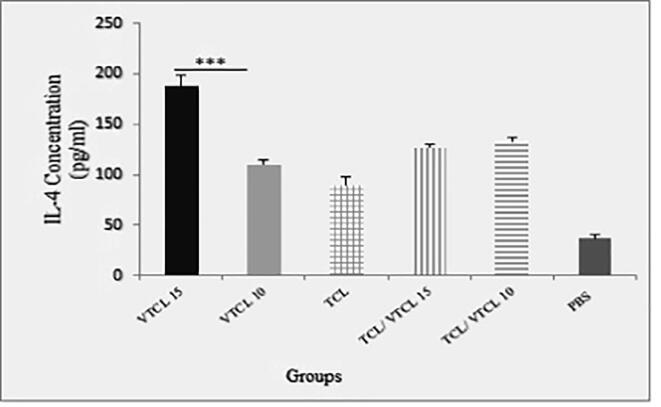
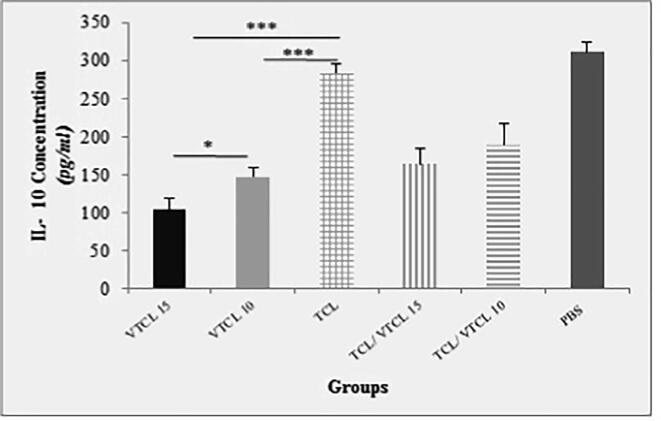
To determine the specific balance of the cellular and humoral immunity following inoculation of oncolysate, the levels of cytokines were measured in different groups of mice. After 7 days of the last treatment, the spleen of the mice was eradicated and after stimulation with a specific antigen (E7-specific H-2Db CTL epitope), the levels of IFN-γ, IL-4, and IL-10 were assessed. As shown in (Fig. 2a) the spleens of the VTCL15 and VTCL10 treated mice secrets significant levels of IFN-γ (P < 0.001) in comparison to TCL, TCL/ VTCL15, TCL/ VTCL10, and PBS groups. Moreover, the levels of IL-4 were increased when the VTCL15 was conducted compared to VTCL10, TCL, TCL/ VTCL15, TCL/ VTCL10, and PBS groups (P < 0.001) (Fig. 2b). On the other hand, the secretion of IL-10 in the VTCL15 group showed a significant decrease compared to VTCL10, TCL, TCL/ VTCL15, TCL/ VTCL10, and PBS groups (P < 0.001) (Fig. 2c). The results of cytokines evaluation showed that the VTCL15 group caused a balance of cellular and humoral responses and on the other hand, by reducing anti-inflammatory responses may play a critical role in reinforcing antitumor immunity.
Fig. 2a.
The concentration of IFN-γ in supernatants following stimulation of splenocytes with E7 antigen. All treated groups > PBS groups *** (P < 0.001) indicates statistically significant difference between treated groups compared with control group. * (P < 0.05) indicates statistically significant difference between VTCL 10 compared with other groups.
Fig. 2b.
The concentration of IL-4 in supernatants following stimulation of splenocytes with E7 antigen. All treated groups > PBS groups *** (P < 0.001) indicates statistically significant difference between treated groups compared with control group; VTCL 15 > other treated groups *** (P < 0.001).
Fig. 2c.
The concentration of IL-10 in supernatants following stimulation of splenocytes with E7 antigen. All treated groups < PBS groups *** (P < 0.001) indicates statistically significant difference between treated groups compared with control group; VTCL 15 < TCL/VTCL10 *** (P < 0.001); No significance difference between VTCL 15 and VTCL 10 group * (P > 0.05).
3.2. Lymphocyte proliferation and cytotoxicity assay
To verify the impact of the VTCL on lymphocyte propagation we employed lymphocyte proliferation assay (LPA). The mice that were administered with VTCL 15, and 10 display a meaningful increase in the lymphocytes in comparison to the mice that attained TCL/VTCL 10 and 15 (P < 0.001). Also, we could not see any differences between the TCL/VTCL 10 and 15 groups. Moreover, those treated with VTCL15 and VTCL10 showed a significant lymphocyte proliferation response when compared with the TCL group (P < 0.001) (Fig. 3a). These results suggest that treatment with VTCL 15 and VTCL 10 can significantly stimulate an E7-specific T-cell response.
Fig. 3a.
Lymphocyte proliferation levels after in vitro stimulation with E7 antigen. All treated groups > PBS groups *** (P < 0.001); VTCL 15 > other treated groups *** (P < 0.001); VTCL 10 > other treated groups *** (P < 0.001); No significancy between other groups (P > 0.05).
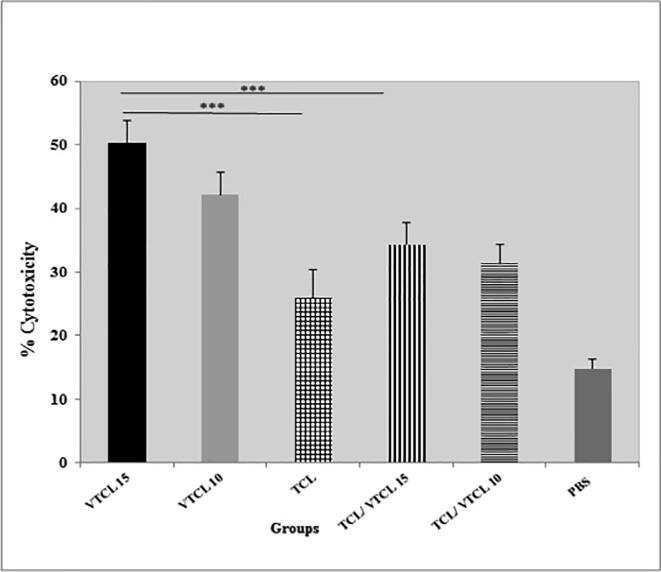
To further obtain a clear picture of the VTCL activity, we performed the LDH cytotoxicity release assay. The CTLs primary encounter with HPV-16 antigens such as E7. Then, the cytotoxicity of the CTLs on the tumor cells analyzed using LDH release assay. The best cytotoxicity effect was found to be at the ratio of 100 CTLs: 1 tumor cells. The ratio was the foundation of measuring all the in vitro cytotoxicity effects. As shown in (Fig. 3b), treated mice with VTCL15 and VTCL10 groups can significantly induce higher antigen-specific CTL responses compared to TCL and PBS groups (P < 0.001). Moreover, treated with VTCL 15 and VTCL 10 showed a significant E7-specific lytic response when compared with the TCL/VTCL 15 and 10 groups (P < 0.001). The cytotoxicity analysis signified that the group of mice that received VTCL 15 express higher lytic activity as compared to other groups. Cytotoxicity effects have not been seen in the mice that were injected with PBS and TCL. Overall, the result demonstrated that VTCL 15 and VTCL 10 groups could increase the specific cytolytic immune responses against tumor cells in the syngeneic model of papillomavirus.
Fig. 3b.
CTL-mediated tumor-specific cytotoxicity in vitro. All treated groups > PBS groups *** (P < 0.001); VTCL 15 > other treated groups *** (P < 0.001); VTCL 10 > other treated groups *** (P < 0.001); No significancy between other groups (P > 0.05).
3.3. Virus tumor cell lysate inhibits tumor growth
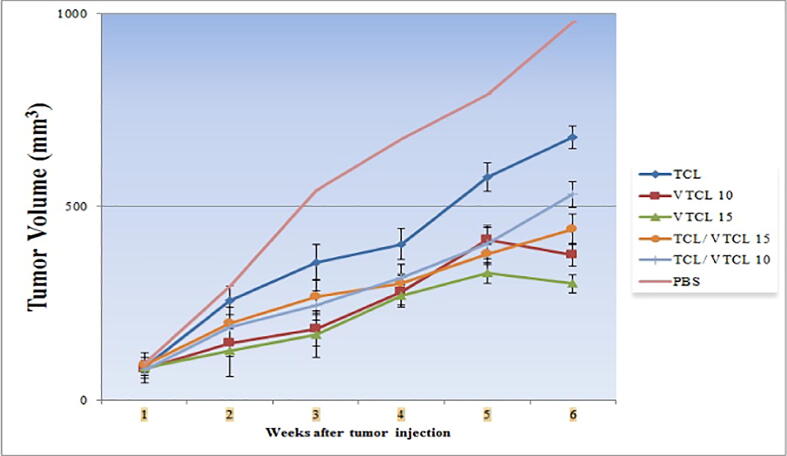
Two weeks after TC-1 injection and tumor appearance, mice were accidentally divided into 6 groups and treated three times at one-week intervals with VTCL 15, VTCL 10, the combination of TCL/VTCL 15, TCL/VTCL 10, and TCL. For the control group, an equal volume of PBS was injected. After the treatment, the groups were followed for 42 days and the response of VTCL on tumor size changes was measured with a digital caliper. As expected, tumor volume decreased significantly in the VTCL 15 and VTCL 10 administered groups when compared to the other groups (P < 0.001) (Fig. 4). In this context, tumor volume decreased significantly in the TCL/VTCL 15 and TCL/VTCL 10 treated groups when contrasted to the TCL and PBS groups (P < 0.001). Moreover, no significant difference was found between the VTCL 15/TCL and VTCL 10/TCL groups (P > 0.05). Overall, our results showed that treatment with VTCL could potentially increase antitumor responses and inhibit tumor growth. Notably, the largest decrease was observed in the VTCL 15 group compared to other groups (P < 0.001).
Fig. 4.
In vivo anti-tumoral response experiment in NDV-loaded VTCL mice. All treated groups < PBS groups (P < 0.001); VTCL 15 < VTCL 10 (P < 0.01); VTCL 15 < TCL, TCL/VTCL15 and TCL/VTCL10) (P < 0.001); VTCL 10 < TCL and TCL/VTCL10 (P < 0.01).
4. Discussion
Cervical cancer is the fourth most common cancer in women after breast, colorectal, and lung cancer, and the most common cause of deaths worldwide in 2020 (Sung et al., 2021). Human papillomavirus genotypes 16 and 18 are the most important causes of cervical cancer (Sabet et al., 2021). The use of chemotherapy and radiation therapy regimens in patients with metastatic cervical cancer does not improve survival in most cases (Yang et al., 2019). Hence, it is important to develop a cancer therapy with high efficacy selectivity killing malignant cells. Virotherapy using oncolytic viruses had been proposed as a potent cancer therapeutic. Oncolytic viruses replicate selectively in tumor cells and exert anti-tumor cytotoxic activity (Keshavarz et al., 2020a, Mozaffari Nejad et al., 2020). Thus, the focus of this study relates to evaluate the antitumor immunity of NDV oncolysate in mice model.
To evaluate the response of immune system cells to a specific antigen, the proliferation of these cells can be measured after their proximity to the antigen. Therefore, in this study, the proliferation of spleen cells in mice treated in response to their stimulation with E7 protein (tumor cell-specific antigen) was evaluated. The results of the lymphocyte stimulation test showed that mice in VTCL 15 and VTCL 10 groups had higher stimulation index than other study groups. These results showed that these two groups have a great ability to elicit cytotoxic responses against tumor cells. There was no significant difference between TCL, TCL / VTCL 15, and TCL / VTCL 10 groups with a control group (PBS).
Specific cytotoxic responses of lymphocytes to virus-infected cells and tumor cells are one of the most important and effective mechanisms in killing these cells. In our study, the spleen cells of mice injected with oncolysate were adjacent to the EL4 cell line as the target cell and the lysis of EL4 cells was used as the effector cell by measuring the enzyme lactate dehydrogenase due to the function of the spleen cells and comparisons with a control group were checked. In a previous study, CD8+ T cells antitumor responses were measured after injection of oncolytic vesicular stomatitis virus (VSV) into a melanoma model. The results indicated that VSV virus as a booster vector can cause rapid proliferation of CD8+ T cells and cytotoxic responses against tumor cells (Bridle et al., 2013). Also, in another clinical study in patients with pancreatic cancer, herpes oncolytic virus (HF10) was used as a treatment. The results after injecting the virus into the pancreatic tumor showed that the amount of CD4+ and CD8+ T cells and macrophages inside the tumor increased significantly in patients compared to the control samples, which indicates the anti-tumor responses induced by the oncolytic virus (Nakao et al., 2011). In a related study, Zamarin and colleagues preformed a modified Newcastle virus that expresses the NS1 influenza protein to evaluate antitumor responses. The results of this study demonstrated that the level of CTL (CD8+) cells in mice treated with this virus was significantly higher than in the control group and this method can be used as a treatment for cancer (Zamarin et al., 2009). The findings of our study in this regard too confirmed that VTCL 15 and VTCL 10 groups potentially increase the amount of CD4+ and CD8+ T cells in the spleen and also increase anti-tumor responses.
Interferons (IFNs), interleukins (ILs), and tumor necrosis factors (TNFs) as biological response modifiers are played an important role in cancer immunotherapies to increase the host cellular immunity (Bisht et al., 2010, Ekmekcioglu and Grimm, 2016). Given the importance of the cytokine response in the antiviral and antitumor immune response, if the cytokine response of type Th1 cells can be induced in treated mice, the cellular immune system will be able to kill the virus-infected tumor cells using various mechanisms. IFN‐γ is the major macrophage-activating cytokine and plays an important role in innate and cellular immunity. The IFN‐γ is a subtype of Th1 cells and is produced from Th1 and CD8 + T cells (Keshavarz et al., 2020). In the present study, the cytokine response of IFN‐γ spleen cells of treated mice was evaluated in comparison with a control group. All groups (TCL, TCL / VTCL 15, TCL / VTCL 10, VTCL 10, and VTCL 15) were significantly different from the PBS control group.
Interleukins 4 and 10 and transforming growth factor-β (TGF-β) induce the conversion to Th2 lymphocytes, which will definitely play a role in balancing cellular and humoral immunity. According to the above, quantitative measurement of the expression of these key interleukins can show a good interpretation of the viral oncolysate performance in the environment used in both in vitro and in vivo (Mohebbi et al., 2019, Keshavarz et al., 2020a). IL-10 is an anti-inflammatory cytokine with anti-tumor and anti-metastatic activities that exerts its activity by inhibiting the synthesis of vascular endothelial growth factor (VEGF) and other angiogenic cytokines (Huang et al., 1999). Our findings revealed that a significant decrease of IL-10 concentration in all groups compared to the control group (PBS) (P < 0.001). Moreover, the group (VTCL 15) showed the lowest secretion of IL-10 compared to the TCL / VTCL 10 group (P < 0.001). There was no significant difference between the other groups (P > 0.05). The previous report by (Li et al., 2007) applied the herpes oncolytic virus (FusOn-H2) for the treatment of breast cancer. Evaluations of the anti-tumor response of this virus on the tumor model showed that after virus injection, the secretion of Th1 cytokines (IFN-γ and IL-2) increased on average 3 to 5 times compared to the control group. On the other hand, there was a slight increase in the secretion of Th2 cytokines (IL-4 and IL-5) in the splenocytes prepared from mice treated with oncological herpes virus, while IL-10 levels were the same among different treatment groups. A prior survey conducted in an animal model confirmed that the NDV can increase the level of IFN‐γ and reduces the accumulation of myeloid suppressor cells in the tumor medium (Koks et al., 2015).
However, as predicted, we showed that there was a significant increase in IL-4 and IFN-γ with all oncolysate formulations compared to TCL and PBS. The increase in IFN-γ concentration was much higher than that of IL-4, which indicates a switch from a Th2 to Th1 dominated profile. Besides, we observed the level of IL-10 a significant difference compared with that in the control group. IL-10 is a critically important cytokine for attenuating the cellular immune response after removing a danger signal (Iyer and Cheng, 2012, Ng et al., 2013).
The mechanisms of oncolytic NDV-mediated damage of the tumor cells have been evaluated in some investigates. They have described that NDV can suppress the growth of tumor cells through apoptosis via the mitochondrial intrinsic pathway (Elankumaran et al., 2006, Ravindra et al., 2008).
Consistent with the above studies, the results of this research also showed that after monitoring the tumor growth rate in the studied mice after 6 weeks of oncolysate injection, in both VTCL15 and VTCL10 groups, the decrease of tumor volume was more than the other groups, which was statistically significant. In addition, all groups showed a significant decrease in tumor volume compared to the control group (PBS)
5. Conclusion
The results showed that the proximity of oncolysate of NDV HB1 strain with tumor tissue could increase the release of lactate dehydrogenase, which indicates the activity of the immune cytotoxicity index. Also, increasing the induction of cytokines (IFN-γ, IL-4) and decreasing the IL-10 slowed tumor growth in the mouse model. In this study, we successfully demonstrated that the NDV oncolysate is an immune system stimulator both at the cellular and humoral aspects. Therefore, this system of immunotherapy with the trigger of the virus may tremendously impact cancer immunotherapy in the future.
6. Declarations
Ethics approval: The ethics committee of the Hamadan University of Medical Sciences approved the study protocol (Ethical approval code: IR.UMSHA. REC.1396.403).
Consent to participate: Not Applicable.
Consent for publication: All authors consent to publish this manuscript in Saudi Journal of Biological Science.
Availability of data and material: Data will be available on request to corresponding or first author.
Author contributions
MYA, ASMN and PM drafted the experimental design and ASMN performed the experiments. MYA, FF and PM helped in data collection, data analysis and initial draft of manuscript text. All authors read and approved the final manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper
Acknowledgements
Authors are thankful to the Influenza and Respiratory Viruses Department, Pasteur Institute of Iran, Tehran, Iran for providing laboratory to conduct the experiment. This study was financed by Vice-Chancellor of Research and Technology of Hamadan University of Medical Sciences, Hamadan, Iran, supporting project number (project number: 9606073585), and supported by the Biotechnology Development Council of the Islamic Republic of Iran (961102).
Footnotes
Peer review under responsibility of King Saud University.
References
- Abdolalipour E., Mahooti M., Salehzadeh A., Torabi A., Mohebbi S.R., Gorji A., Ghaemi A. Evaluation of the antitumor immune responses of probiotic Bifidobacterium bifidum in human papillomavirus-induced tumor model. Microbial Pathog. 2020;145 doi: 10.1016/j.micpath.2020.104207. [DOI] [PubMed] [Google Scholar]
- Arruebo M., Vilaboa N., Sáez-Gutierrez B., Lambea J., Tres A., Valladares M., González-Fernández Á. Assessment of the evolution of cancer treatment therapies. Cancers. 2011;3:3279–3330. doi: 10.3390/cancers3033279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batliwalla F.M., Bateman B.A., Serrano D., Murray D., Macphail S., Maino V.C., Ansel J.C., Gregersen P.K., Armstrong C.A. A 15-year follow-up of AJCC stage III malignant melanoma patients treated postsurgically with Newcastle disease virus (NDV) oncolysate and determination of alterations in the CD8 T cell repertoire. Mol Med. 1998;4:783–794. [PMC free article] [PubMed] [Google Scholar]
- Bisht M., Bist S., Dhasmana D. Biological response modifiers: Current use and future prospects in cancer therapy. Indian J. Cancer. 2010;47:443. doi: 10.4103/0019-509X.73559. [DOI] [PubMed] [Google Scholar]
- Bridle B.W., Clouthier D., Zhang L., Pol J., Chen L., Lichty B.D., Bramson J.L., Wan Y. Oncolytic vesicular stomatitis virus quantitatively and qualitatively improves primary CD8+ T-cell responses to anticancer vaccines. Oncoimmunology. 2013;2 doi: 10.4161/onci.26013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao G.-D., He X.-B., Sun Q., Chen S., Wan K., Xu X., Feng X., Li P.-P., Chen B., Xiong M.-M. Front; Onco: 2020. The oncolytic virus in cancer diagnosis and treatment; p. 10. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Chiang C.L.-L., Benencia F., Coukos G. Whole tumor antigen vaccines. Seminars in immunology (Elsevier) 2010:132–143. doi: 10.1016/j.smim.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekmekcioglu S., Grimm E.A. Cytokines, interferons, and hematopoietic growth factors. Holland-Frei Cancer Medicine. 2016:1–17. [Google Scholar]
- Elankumaran S., Rockemann D., Samal S.K. Newcastle disease virus exerts oncolysis by both intrinsic and extrinsic caspase-dependent pathways of cell death. J. Virol. 2006;80:7522–7534. doi: 10.1128/JVI.00241-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falzone L., Salomone S., Libra M. Evolution of cancer pharmacological treatments at the turn of the third millennium. Front. Pharmacol. 2018;9:1300. doi: 10.3389/fphar.2018.01300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier P., Schirrmacher V. Oncolytic Newcastle disease virus as cutting edge between tumor and host. Biology. 2013;2:936–975. doi: 10.3390/biology2030936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldufsky J., Sivendran S., Harcharik S., Pan M., Bernardo S., Stern R.H., Friedlander P., Ruby C.E., Saenger Y., Kaufman H.L. Oncolytic virus therapy for cancer. Oncolytic Virother. 2013;2:31. doi: 10.2147/OV.S38901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S., Ullrich S.E., Bar-Eli M. Regulation of tumor growth and metastasis by interleukin-10: the melanoma experience. J. Interferon Cytokine Res. 1999;19:697–703. doi: 10.1089/107999099313532. [DOI] [PubMed] [Google Scholar]
- Ioannides C., Platsoucas C., O'Brian C., Patenia R., Bowen J., Wharton J., Freedman R. Viral oncolysates in cancer treatment: immunological mechanisms of action. Anticancer Res. 1989;9:535–544. [PubMed] [Google Scholar]
- Iyer S.S., Cheng G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Critical Reviews™ in Immunology. 2012;32 doi: 10.1615/critrevimmunol.v32.i1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahromi S.R., Arrefhosseini S.R., Ghaemi A., Alizadeh A., Sabetghadam F., Togha M. Effect of oral genistein administration in early and late phases of allergic encephalomyelitis. Iran. J. Basic Med. Sci. 2014;17:509. [PMC free article] [PubMed] [Google Scholar]
- Jiang H., Gomez-Manzano C., Rivera-Molina Y., Lang F.F., Conrad C.A., Fueyo J. Oncolytic adenovirus research evolution: from cell-cycle checkpoints to immune checkpoints. Curr. Opin. Virol. 2015;13:33–39. doi: 10.1016/j.coviro.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavarz M., Ebrahimzadeh M.S., Miri S.M., Dianat-Moghadam H., Ghorbanhosseini S.S., Mohebbi S.R., Keyvani H., Ghaemi A. Oncolytic Newcastle disease virus delivered by Mesenchymal stem cells-engineered system enhances the therapeutic effects altering tumor microenvironment. Virol. J. 2020;17:1–13. doi: 10.1186/s12985-020-01326-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavarz M., Nejad A.S.M., Esghaei M., Bokharaei-Salim F., Dianat-Moghadam H., Keyvani H., Ghaemi A. Oncolytic Newcastle disease virus reduces growth of cervical cancer cell by inducing apoptosis. Saudi J. Biol. Sci. 2020;27:47–52. doi: 10.1016/j.sjbs.2019.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavarz M., Solaymani-Mohammadi F., Miri S.M., Ghaemi A. Oncolytic paramyxoviruses-induced autophagy; a prudent weapon for cancer therapy. J. Biomed. Sci. 2019;26:1–11. doi: 10.1186/s12929-019-0542-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koks C.A., Garg A.D., Ehrhardt M., Riva M., Vandenberk L., Boon L., Vleeschouwer S.D., Agostinis P., Graf N., Van Gool S.W. Newcastle disease virotherapy induces long-term survival and tumor-specific immune memory in orthotopic glioma through the induction of immunogenic cell death. Int. J. Cancer. 2015;136:E313–E325. doi: 10.1002/ijc.29202. [DOI] [PubMed] [Google Scholar]
- Li H., Dutuor A., Fu X., Zhang X. Induction of strong antitumor immunity by an HSV-2-based oncolytic virus in a murine mammary tumor model. J. Gene Med. 2007;9:161–169. doi: 10.1002/jgm.1005. [DOI] [PubMed] [Google Scholar]
- Mohebbi A., Ebrahimzadeh M.S., Rahimi S.B., Saeidi M., Tabarraei A., Mohebbi S.R., Shirian S., Gorji A., Ghaemi A. Non-replicating Newcastle disease virus as an adjuvant for DNA vaccine enhances antitumor efficacy through the induction of TRAIL and granzyme B expression. Virus Res. 2019;261:72–80. doi: 10.1016/j.virusres.2018.12.014. [DOI] [PubMed] [Google Scholar]
- Murray D.R., Cassel W.A., Torbin A.H., Olkowski Z.L., Moore M.E. Viral oncolysate in the management of malignant melanoma. II. Clinical studies. Cancer. 1977;40:680–686. doi: 10.1002/1097-0142(197708)40:2<680::aid-cncr2820400214>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Nakao A., Kasuya H., Sahin T., Nomura N., Kanzaki A., Misawa M., Shirota T., Yamada S., Fujii T., Sugimoto H. A phase I dose-escalation clinical trial of intraoperative direct intratumoral injection of HF10 oncolytic virus in non-resectable patients with advanced pancreatic cancer. Cancer Gene Ther. 2011;18:167–175. doi: 10.1038/cgt.2010.65. [DOI] [PubMed] [Google Scholar]
- Mozaffari Nejad A.S., Fotouhi F., Mehrbod P., Keshavarz M., Alikhani M.Y., Ghaemi A. Oncolytic effects of Hitchner B1 strain of newcastle disease virus against cervical cancer cell proliferation is mediated by the increased expression of cytochrome C, autophagy and apoptotic pathways. Microbial Pathog. 2020;147 doi: 10.1016/j.micpath.2020.104438. [DOI] [PubMed] [Google Scholar]
- Mozaffari Nejad A.S., Noor T., Munim Z.H., Alikhani M.Y., Ghaemi A. A bibliometric review of oncolytic virus research as a novel approach for cancer therapy. Virol. J. 2021;18:1–14. doi: 10.1186/s12985-021-01571-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng T., Britton G.J., Hill E.V., Verhagen J., Burton B.R., Wraith D.C. Regulation of adaptive immunity; the role of interleukin-10. Front. Immunol. 2013;4:129. doi: 10.3389/fimmu.2013.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindra P., Tiwari A.K., Sharma B., Rajawat Y.S., Ratta B., Palia S., Sundaresan N., Chaturvedi U., Kumar G.A., Chindera K. HN protein of Newcastle disease virus causes apoptosis in chicken embryo fibroblast cells. Arch. Virol. 2008;153:749–754. doi: 10.1007/s00705-008-0057-2. [DOI] [PubMed] [Google Scholar]
- Sabet F., Mosavat A., Ghezeldasht S.A., Basharkhah S., Shamsian S.A.A., Abbasnia S., Shamsian K., Rezaee S.A. Prevalence, genotypes and phylogenetic analysis of human papillomaviruses (HPV) in northeast Iran. Int. J. Infect. Dis. 2021;103:480–488. doi: 10.1016/j.ijid.2020.12.015. [DOI] [PubMed] [Google Scholar]
- Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer. J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- Vähä-Koskela M.J., Heikkilä J.E., Hinkkanen A.E. Oncolytic viruses in cancer therapy. CancerLett. 2007;254:178–216. doi: 10.1016/j.canlet.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberk L., Belmans J., Van Woensel M., Riva M., Van Gool S.W. Exploiting the immunogenic potential of cancer cells for improved dendritic cell vaccines. Front. Immunol. 2016;6:663. doi: 10.3389/fimmu.2015.00663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallack M.K. A new approach in the immunotherapy of carcinoma of the colon and rectum. J. Surg. Oncol. 1980;13:29–34. doi: 10.1002/jso.2930130105. [DOI] [PubMed] [Google Scholar]
- Yang J., Cai H., Xiao Z.-X., Wang H., Yang P. Effect of radiotherapy on the survival of cervical cancer patients: an analysis based on SEER database. Medicine. 2019;98 doi: 10.1097/MD.0000000000016421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamarin D., Martínez-Sobrido L., Kelly K., Mansour M., Sheng G., Vigil A., García-Sastre A., Palese P., Fong Y. Enhancement of oncolytic properties of recombinant newcastle disease virus through antagonism of cellular innate immune responses. Mol. Ther. 2009;17:697–706. doi: 10.1038/mt.2008.286. [DOI] [PMC free article] [PubMed] [Google Scholar]