Abstract
The evolution of NDM genes (blaNDM) in E. coli is accounted for expansive multidrug resistance (MDR), causing severe infections and morbidities in the pediatric population. This study aimed to analyze the phylogeny and mutations in NDM variants of E. coli recovered from the pediatric population. Carbapenem-resistant clinical strains of E. coli were identified using microbiological phenotypic techniques. PCR technique used to amplify the blaNDM genes, identified on agarose gel, and analyzed by DNA sequencing. The amino acid substitutions were examined for mutations after aligning with wild types. Mutational and phylogenetic analysis was performed using Lasergene, NCBI blastn, Clustal Omega, and MEGA software, whereas PHYRE2 software was used for the protein structure predictions. PCR amplification of the blaNDM genes detected 113 clinical strains of E. coli with the contribution of blaNDM-1 (46%), blaNDM-4 (3.5%), and blaNDM-5 (50%) variants. DNA sequencing of blaNDM variants showed homology to the previously described blaNDM-1, blaNDM-4, and blaNDM-5 genes available at GenBank and NCBI database. In addition, the mutational analysis revealed in frame substitutions of Pro60Ala and Pro59Ala in blaNDM-4 and blaNDM-5, respectively. The blaNDM-1 was ortholog with related sequences of E. coli available at GenBank. The phylogenetic analysis indicated that the NDM gene variants resemble other microbes reported globally with some new mutational sites.
Keywords: E. coli, Phylogeny, NDM, Resistant genes, Multidrug resistance, Carbapenemases
1. Introduction
Multidrug-resistant (MDR) Escherichia coli is a substantial threat to the healthcare system and is associated with adverse outcomes. E. coli contain several virulence factors leading to various diseases, including urinary tract infection, diarrhea, and neonatal meningitis. Infections with MDR E. coli account for much higher mortality rates in the pediatric population (WHO, 2010). Genetic mutations are pivotal to MDR development (Cherry et al., 2017). Carbapenems were the mainstay for a long time to treat the infections with extended-spectrum β-lactamases (ESBLs) and AmpC β-lactamases producing E. coli. However, new carbapenemases like New Delhi metallo-β-lactamase (NDM) have created a potential threat for their utility (Paterson and Doi, 2007).
The NDM was first identified in New Delhi from an Indian-returned Swedish patient, and subsequently, several clones have been reported from diversified bacterial strains (Rolain et al., 2010). Nearly 24 variants of blaNDM genes were reported among bacteria based on different substitutions of amino acids in their protein structure. Out of these, the NDM-1 to 7 variants, being widely prevailed globally, has become a dominant concern (Wu et al., 2019). The information obtained from the latest bioinformatics analysis of the sequenced DNA of NDM producing E. coli, their protein 3D format, and phylogenetics has become imperative for evolving breakthrough antibacterial agents because such structural aspects and genetic features influence the mechanisms of antibiotic-hydrolysis (Gromiha et al., 2019). Understanding structural and genetic features of NDM variants that affect the hydrolysis of antibiotics can assist in designing the new and distinct NDM inhibiting agents (Rolain et al., 2010). A few local studies have been carried out in Pakistan in this context. However, they bear a different focus point, divergence in their scopes such as involving adult patients only (Fakhuruddin et al., 2012, Nahid et al., 2013), specific bacteria (Amin et al., 2013), limited coverage from wards (Javed et al., 2016), or specimen types (Anis-ur-Rehman et al., 2008).
The majority of such in-depth genetic studies have been conducted on the local adult population and with various limitations in few local studies, including children. This study aimed to report the currently prevalent NDM variants of E. coli, infecting children, identifying their mutation sites, and studying their phylogenetics. This will aid in targeted antibiotic therapy through precise and timely identification of culprit MDR mutated strain of bacteria to reduce morbidity and mortality among children.
2. Materials and methods
2.1. Study design and specimen collection
From April 2017 to March 2019, all in- and out-patient specimens were obtained from The Children's Hospital and The Institute of Child Health, Lahore, Pakistan, after taking ethical approval by the Institutional Review Board (Ref No. 09/Ch/ICH) and from the University of Punjab, Lahore (Ref No. D/6001-ACAD).
2.2. Isolation and phenotypic characterization of MBL producing E. coli
The specimens analyzed during the study include blood, urine, cerebrospinal fluid (CSF), pus swabs, peritoneal dialysis (PD) catheter, central venous pressure (CVP) line, endotracheal tube (ETT), wound swab, ear tip, and tracheal secretions. The specimens were cultured on standardized blood, chocolate, CLED, and MacConkey agar plates and incubated at 37 °C overnight (Qamar et al., 2017). E. coli were screened out through Gram’s staining, colony morphology, biochemical and API 20 E (bioMerieux, France) strip tests. Carbapenem resistance was detected by Kirby Baur disc diffusion test. Modified Hodge test was employed to identify carbapenemase-producing (CP) E. coli. The combined disc (CDT) and double-disc synergy test (DDST) were performed for detection of MBL production (Wayne, 2018). Their antibiogram was also established as detailed in our previous study (Nosheen et al., 2020), which presented the MDR in the bacteria under study.
2.3. Molecular analysis of the blaNDM in E. coli
Freshly grown E. coli colonies suspended in 250 µl TE buffer, vortexed well, and kept for 10 min in a boiling water bath. Later, the suspension was centrifuged for five minutes at 14,000 rpm to obtain the DNA template from the supernatant stored at −70 °C until subsequent use (Ejaz et al., 2020). The target gene (815 bp) was amplified by PCR using a maximum of 0.5 µM each of the forward (ATGGAATTGCCCAATATTATG) and the reverse (TCAGCGCAGCTTGTCGGCC) primers (Nordmann et al., 2011). The thermal cycle was optimized and programmed for an initial denaturation step of 10 min at 94 °C, followed by 35 cycles of amplification, each of which comprised 30 sec at 94 °C, 40 sec at 57 °C, and 45 sec at 72 °C with a final extension at 72 °C for 5 min. Gel stain was used to analyze PCR products after electrophoresis using 1% agarose gels in TAE buffer at 95 V for 45 min.
2.4. DNA sequencing and bioinformatics analyses
Amplified blaNDM products were sequenced in Singapore on 96-capillary 3730xl Genetic Analyzer. The amino acid substitutions were examined for mutations after aligning with wild types. DNA Star, Lasergene molecular biology software version 16.2.0.130 was used for the pairwise sequence alignments. The nucleotide studies were performed using the NCBI blastn suite program, DNA translation, sequence manipulation suite, and multiple nucleotide alignment and Clustal Omega (Sievers et al., 2011).
2.5. Phylogenetic studies and prediction of the 3D protein structure of blaNDM genes of E. coli
The phylogenetic tree of 113 nucleotide sequences of E. coli was established with sequenced genetic data of different NDM producing E. coli, available at GenBank, through MEGA version 10.0.5. The initial tree for heuristic search was obtained automatically by applying Neighbor-Join (NJ) and Bio-NJ algorithms to a matrix of pairwise distances estimated by the maximum composite likelihood approach. The topology with a superior log likelihood value was selected, and the codon positions comprised were first till third and non-coding (Kumar et al., 2018). The complete dataset included 826 positions. Following the node in the phylogenetic tree, each descendant represented a taxonomic unit, expressing the most recent ancestor, while the length of the edges indicated time estimation. The PHYRE2 software was used to predict the three-dimensional structure of proteins.
3. Results
Of 6468 isolated bacterial strains, 1522 (24%) were E. coli, out of which 113 (7.4%) were carbapenamase producers.
3.1. Molecular characteristics of blaNDM genes in E. coli
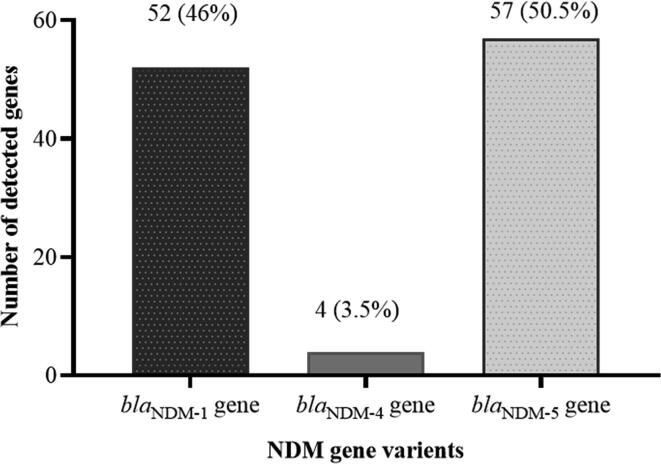
Agarose gel electrophoresis of the amplified PCR gene products confirmed the presence of blaNDM genes among all of the 113 E. coli strains (Fig. 1). The detected blaNDM genes variants include 52 (46%) blaNDM-1, 4 (3.5%) to be blaNDM-4 and 57 (50.5%) to be blaNDM-5 (Fig. 2).
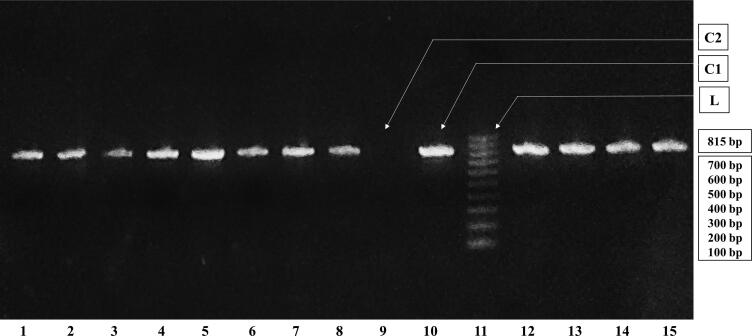
Fig. 1.
Agarose gel electrophoresis of amplified PCR products. Each batch of the gel contained a 100 bp leader (L) to estimate the gene size, a positive control (C1) showing the detection of blaNDM, a negative control (C2) showing the absence of blaNDM.
Fig. 2.
Distribution blaNDM variants in E. coli (n = 113).
3.2. DNA sequence analysis
The chromatograms of blaNDM-1, -4, and -5 genes did not show undesirable ambiguities in contig and no overlapping dirty peaks. Multiple nucleotide sequence alignments of DNA sequencing data of blaNDM-1, -4, and -5 genes of E. coli were observed using several bioinformatics software. The amino acids in each column were analyzed to identify their homology with various associated sequences which share a common evolutionary history, available at the GenBank, NCBI database. The isolated blaNDM genes were identical to previously described blaNDM-1, blaNDM-4, and blaNDM-5 E. coli plasmids present in the GenBank database.
3.3. E. coli blaNDM gene mutations
The sequence of the blaNDM-1 gene was found ortholog with several related E. coli sequences at GenBank (Fig. 3a). However, as shown in Fig. 3b, the blaNDM-4 sequence substituted amino acid proline (P) from the GenBank sequence for alanine (A) in the sequence under study, in frame 60. In contrast, blaNDM-5 presented substitution in frame 59, A from P of sequence from GenBank (Fig. 3c). These mutations found in E. coli genes responsible for producing specific variants of NDM in bacteria affect their ability to recognize substrates and catalyze the antibiotics.
Fig. 3.
Multiple sequence alignment compared the amino acids of proteins in NCBI database. a) NDM-1 gene encoding protein by blaNDM-1 gene. b) NDM-4 gene encoding protein by blaNDM-4 gene. c) NDM-5 gene encoding protein by blaNDM-5 gene.
3.4. Phylogenetic studies of blaNDM genes
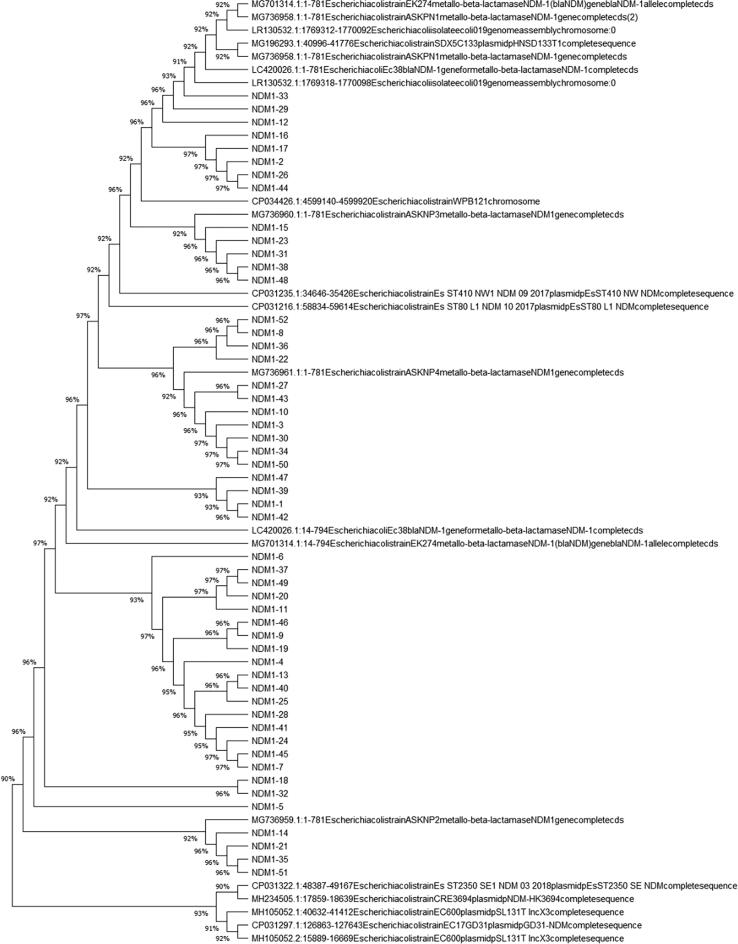
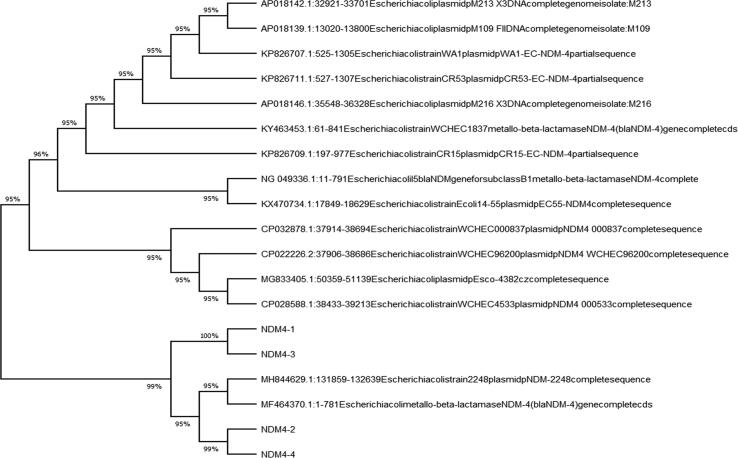
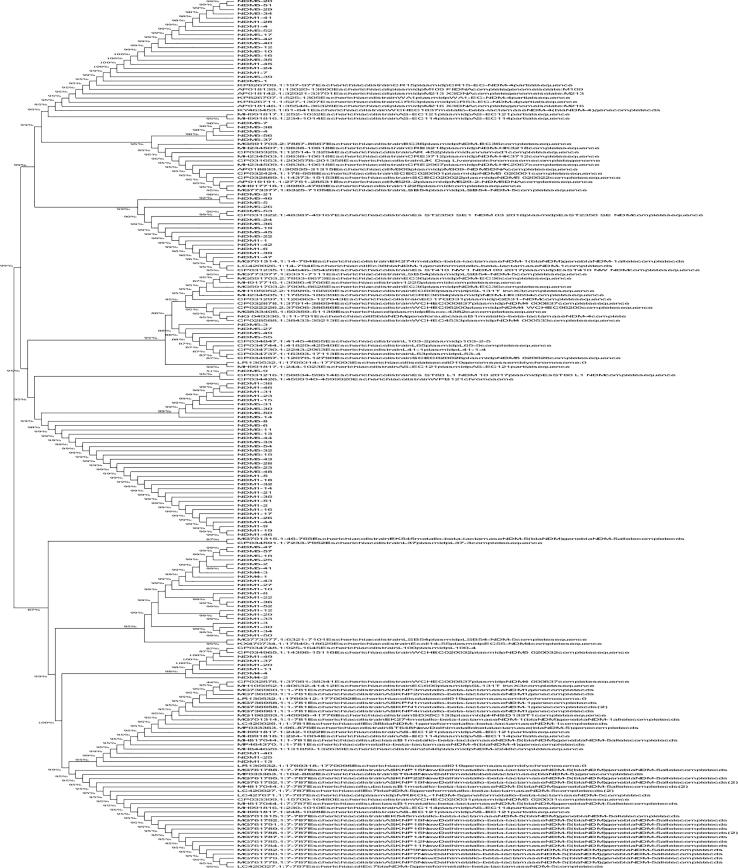
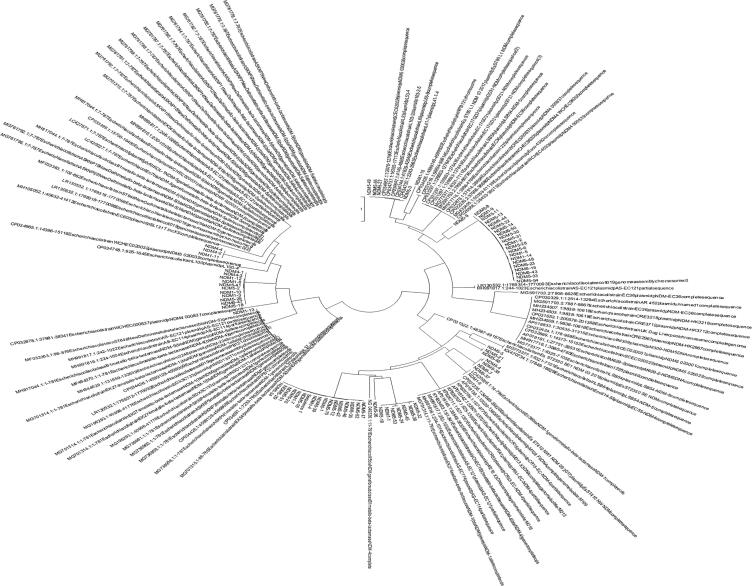
Fig. 4a, Fig. 4b, Fig. 4c depict the phylogenetic tree, which represented the evolutionary history of E. coli carrying the blaNDM-1, -4, and -5 genes individually. All the variants of blaNDM-1, -4, and -5, along with the same genes carried by other E. coli isolated from different areas worldwide and available at GenBank (Fig. 5). The phylogenetic analysis showed that the blaNDM-1, -4, and -5 genes exhibited minimal variation compared to other E. coli strains isolated from different countries that possessed the same genes.
Fig. 4a.
Phylogenetic tree of blaNDM-1 genes isolated from clinical strains of E. coli.
Fig. 4b.
Phylogenetic tree of blaNDM-4 genes isolated from clinical strains of E. coli.
Fig. 4c.
Phylogenetic tree of blaNDM-5 genes isolated from clinical strains of E. coli.
Fig. 5.
Phylogenetic tree (circular) of blaNDM-1, blaNDM-4, and blaNDM-5 genes isolated from clinical strains of E. coli.
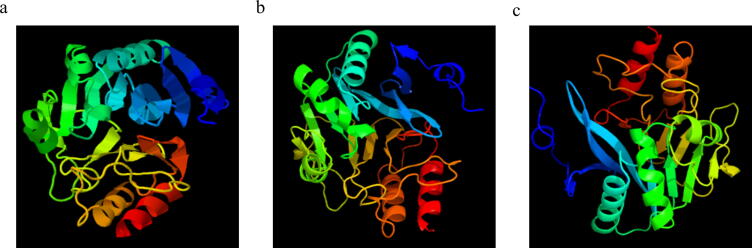
3.5. Prediction of the three-dimensional structure of the blaNDM genes
The three-dimensional crystal structure model of the E. coli blaNDM-1, -4, and -5 genes is shown in Fig. 6, where α-helices are colored blue, β-strands green, loop regions cyan. In contrast, active sites were represented in red color, which presents the amino acid substitution or mutation sites in comparison to other E. coli NDM variants previously mentioned.
Fig. 6.
3D protein structure of NDM isolated from clinical strains of E. coli. a) blaNDM-1, (b) blaNDM-4, and (c) blaNDM-5 genes.
4. Discussion
Antibiotics misuse has exerted extensive selection pressure on the health care system, as there are limited therapeutic options for MDR infections. This threat is being foreseen to worsen since the first reported case of NDM-1, an incessant upsurge in MDR of bacteria carrying the NDM gene. This study has expanded the E. coli characterization from phenotypic to molecular level for identifying NDM-1, −4, and −5 variants, from all in- and out-patients and including all specimen types among children, along with its bioinformatics and phylogenetic analysis. This information is vital as it exerts a direct impact on the selection of targeted antibiotic regimens.
E. coli burden in this study (24%) was comparable with the US (Dahle et al., 2012), higher than Russian (Edelstein et al., 2003), while lesser than reported from Bangladesh (Islam et al., 2016). The variation in incidence could be due to different levels of public hygiene, climatic variation, and health education. The carbapenem resistance of E. coli in the current study was in line with Georgian research (Gupta et al., 2011). The present study determined that 7.4% of E. coli were carbapenemase producers based on MHT. Three local studies, conducted at Rawalpindi and Lahore, showed 38%, 33.1%, and 10.6% E. coli to be CP, respectively (Abbas et al., 2019, Amjad et al., 2011, Javed et al., 2016), while a greater frequency was identified in Greece (Falagas et al., 2010). The greater frequency of carbapenem-resistant E. coli infection in uprising countries might be due to poor socio-economic conditions and insufficient compliance towards antibiotic protocols.
In this study, the PCR identified E. coli gene variant NDM-1 (46%), NDM-4 (3.5%), and NDM-5 (50%). However, the above findings varied from others in Egypt (Soliman et al., 2020) and India (Ranjan et al., 2016), presenting different variants of NDM in multiple geographical areas. The current prevalence of the NDM-5 variant among pediatrics was excessive than in Algeria (Sassi et al., 2014). The prevalence of NDM-4 in this study was comparable to that in a Chinese study. (Bi et al., 2018). Furthermore, no other local research has expressed the frequency of specified NDM variants in E. coli among Pakistani children. However, it has been reported in a pediatric study on Klebsiella (Heinz et al., 2019).
The multiple sequence alignments of blaNDM-1, -4, and -5 genes of E. coli in the present study exhibited no amino acid substitution in blaNDM-1 gene structure, while in blaNDM-4, proline60alanine, and blaNDM-5, alanine59proline substitution from the sequence of GenBank was noted. An Algerian research reported amino acid substitutions in genes of three NDM-5 producing E. coli that were similar to those found in India (Sassi et al., 2014). A research study from the UK revealed that their E. coli gene differed from NDM-1 by two amino-acid substitutions (Hornsey et al., 2011). Variability in these substitutions could be because of perpetual mutations in the genetic material of E. coli, leading to magnified MDR.
In this research, the phylogenetic tree showed that blaNDM-1, -4, and -5 genes of E. coli carry resemblance with various bacteria, reported worldwide, submitted online on GenBank exhibiting fewer gene variations. Phylogenetic trees of E. coli have been established at various parts of the world, including Rome (Garcia-Fernandez et al., 2020), China (Zheng et al., 2016), and locally as well (Shahzad et al., 2016), whose focus remained converged on uropathogenic strains of adult males only. In this study, the phylogenetic tree highlights the potential of E. coli to disseminate NDM among various bacteria worldwide (Mushtaq et al., 2011), although the protein crystal structure models of blaNDM-1, -4, and -5 genes, support the knowledge of genomics and functionality of proteins, adding to the formerly present data which, in turn would be advantageous in formation of better MBL inhibitors.
The precise identification of bacteria and its resistance mechanism has become imperative because it directly influences choosing a targeted regimen. There is a grave necessity for comprehensive studies to provide statistics of the diversity of blaNDM gene variants in children and efficient monitoring in hospitals to control MDR and support the antibiotic policy optimization. The present study is limited with NDM variants; however, NDM lineages suggest working on the phylogenetic analysis of other carbapenamase genes. Some new mutated sites found in NDM-1, −4, and −5 proteins suggest further need to design antibacterial drugs targeting these active sites to combat NDM harboring bacterial strain. The dissemination of MDR E. coli can be alleviated by the proficient screening of such bacteria, targeted therapy, and stringent strategies for rational use of antibiotics and infection control.
5. Conclusion
The study of substitution of amino acid showed orthology of blaNDM-1 with related sequences in GenBank. The blaNDM-4 and blaNDM-5 exhibited substitutions, presenting mutation sites. E. coli strains harbored more NDM-5 than NDM-1 and NDM-4 variants. The phylogenetic analysis revealed that the blaNDM-1, blaNDM-4, and blaNDM-5 genes from strains circulating among the pediatric population exhibited minimal variation to the NDM gene structure of E. coli strains, isolated from various countries around the globe. The variations in NDM genes may have occurred as a result of horizontal gene transfer from diverse geographical extents and environmental reservoirs, providing deep insight into phylogenetics and biological bioinformatics domains. The new protein mutations presented in this study have not been explored from clinical strains isolated from pediatric patients in this region of the world. The 3D crystal structure of blaNDM presents a structural comparison with other MBLs subclasses, which might help to design mechanism-based inhibitors, bridge research, and develop new chemicals and modified medicines.
6. Consent for publication
All authors consent to publish this manuscript in Saudi journal of Biological Science.
7. Availability of data and material
The data used and analyzed during the current study available from the corresponding author.
Author contributions
Sumbal Nosheen, Nadeem Irfan Bukhari, and Hasan Ejaz data curation conceived and designed the study. Sumbal Nosheen, Kashaf Junaid, Naeem Anwar, and Sonia Younas helped with specimen processing, performed formal analysis, optimized methodology, and wrote the original draft. Fahad Ahmad and Sumbal Nosheen performed software analysis. Nadeem Irfan Bukhari and Hasan Ejaz supervised, administered, and provided resources for the project. Hasan Ejaz, Nadeem Irfan Bukhari and Naeem Anwar performed final editing. All authors critically reviewed the manuscript and approved the final version.
Funding
The current study did not receive any funding.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Authors are thankful to The Children’s Hospital and The Institute of Child Health, Lahore, and the University of the Punjab, Lahore, Pakistan, for the facilitation.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abbas S., Ejaz H., Jahan S., Younas S., Alzahrani B., Farraj D.A.A., Alkufeidy R.M. Molecular Detection of blaIMP Genes in Metallo-Beta-Lactamase Producing Clinical Gram-Negative Isolates. Clin. Lab. 2019;65(8) doi: 10.7754/Clin.Lab.2019.190202. [DOI] [PubMed] [Google Scholar]
- Amin H., Zafar A., Ejaz H., Jameel N.-U.-A. Phenotypic characterization of ESBL producing Enterobacter cloacae among children. Pak. J. Med. Sci. 2013;29(1):144–147. doi: 10.12669/pjms.291.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amjad A., Mirza I., Abbasi S., Farwa U., Malik N., Zia F. Modified Hodge test: A simple and effective test for detection of carbapenemase production. Iran. J. Microbiol. 2011;3(4):189–193. [PMC free article] [PubMed] [Google Scholar]
- Anis-ur-Rehman M.J., Siddiqui T.S., Idris M. Frequency and clinical presentation of UTI among children of Hazara Division. Pakistan. J. Ayub Med. Coll. Abbottabad. 2008;20(1):63–65. [PubMed] [Google Scholar]
- Bi R., Kong Z., Qian H., Jiang F., Kang H., Gu B., Ma P. High prevalence of blaNDM variants among carbapenem-resistant Escherichia coli in Northern Jiangsu Province. China. Front. Microbiol. 2018;9(2):2704–2709. doi: 10.3389/fmicb.2018.02704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry, J., Demmler-Harrison, G. J., Kaplan, S. L., Steinbach, W. J., Hotez, P. J. (2017). Feigin and Cherry's Textbook of Pediatric Infectious Diseases E-Book (8th ed. Vol. 2): Elsevier Health Sciences.
- Dahle K.W., Korgenski E.K., Hersh A.L., Srivastava R., Gesteland P.H. Clinical value of an ambulatory-based antibiogram for uropathogens in children. J. Pediatr. Infect. Dis. 2012;1(4):333–336. doi: 10.1093/jpids/pis055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelstein M., Pimkin M., Palagin I., Edelstein I., Stratchounski L. Prevalence and molecular epidemiology of CTX-M extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae in Russian hospitals. Antimicrob. Agents. Chemother. 2003;47(12):3724–3732. doi: 10.1128/AAC.47.12.3724-3732.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejaz H., Alzahrani B., Hamad M.F.S., Abosalif K.O.A., Junaid K., Abdalla A.E., Elamir M.Y.M., Aljaber N.J., Hamam S.S.M., Younas S. Molecular Analysis of the Antibiotic Resistant NDM-1 Gene in Clinical Isolates of Enterobacteriaceae. Clin. Lab. 2020;66(3) doi: 10.7754/Clin.Lab.2019.190727. [DOI] [PubMed] [Google Scholar]
- Fakhuruddin M., Asif D., Raziuddin A. Emerging carbapenem resistance in Enterobacteriaceae. Pak. J. Med. Sci. 2012;6:834–838. [Google Scholar]
- Falagas M.E., Maraki S., Karageorgopoulos D.E., Kastoris A.C., Mavromanolakis E., Samonis G. Antimicrobial susceptibility of multidrug-resistant (MDR) and extensively drug-resistant (XDR) Enterobacteriaceae isolates to fosfomycin. Int. J. Antimicrob. Agents. 2010;35(3):240–243. doi: 10.1016/j.ijantimicag.2009.10.019. [DOI] [PubMed] [Google Scholar]
- Garcia-Fernandez, A., Villa, L., Bibbolino, G., Bressan, A., Trancassini, M., Pietropaolo, V., Venditti, M., Antonelli, G., Carattoli, A., 2020. Novel Insights and Features of the NDM-5-Producing Escherichia coli Sequence Type 167 High-Risk Clone. mSphere 5(2). [DOI] [PMC free article] [PubMed]
- Gromiha M.M., Nagarajan R., Selvaraj S. Protein Structural Bioinformatics: An Overview. 2019;445–459 [Google Scholar]
- Gupta N., Limbago B.M., Patel J.B., Kallen A.J. Carbapenem-resistant Enterobacteriaceae: epidemiology and prevention. Clin. Infec. Dis. 2011;53(1):60–67. doi: 10.1093/cid/cir202. [DOI] [PubMed] [Google Scholar]
- Heinz E., Ejaz H., Bartholdson Scott J., Wang N., Gujaran S., Pickard D., Wilksch J., Cao H., Haq I.U., Dougan G., Strugnell R.A. Resistance mechanisms and population structure of highly drug resistant Klebsiella in Pakistan during the introduction of the carbapenemase NDM-1. Sci Rep. 2019;9(1):2392. doi: 10.1038/s41598-019-38943-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornsey M., Phee L., Wareham D.W. A novel variant, NDM-5, of the New Delhi metallo-β-lactamase in a multidrug-resistant Escherichia coli ST648 isolate recovered from a patient in the United Kingdom. Antimicrob. Agents. Chemother. 2011;55(12):5952–5954. doi: 10.1128/AAC.05108-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam M., Ahamed S., Arafat M., Hasan I., Rahman M., Nazir K. Molecular detection and antibiogram of shiga toxin producing Escherichia coli (STEC) isolated from diarrheic children. Bangladesh J. Vet. Med. 2016;14(2):289–295. [Google Scholar]
- Javed, H., Ejaz, H., Zafar, A., Rathore, A. W., ul Haq, I., 2016. Metallo-beta-lactamase producing Escherichia coli and Klebsiella pneumoniae: A rising threat for hospitalized children. J. Pak. Med. Assoc. 66(9), 1068-1072. [PubMed]
- Kumar S., Stecher G., Li M., Knyaz C., Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018;35(6):1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mushtaq S., Irfan S., Sarma J., Doumith M., Pike R., Pitout J., Livermore D., Woodford N. Phylogenetic diversity of Escherichia coli strains producing NDM-type carbapenemases. J. Antimicrob. Chemother. 2011;66(9):2002–2005. doi: 10.1093/jac/dkr226. [DOI] [PubMed] [Google Scholar]
- Nahid F., Khan A.A., Rehman S., Zahra R. Prevalence of metallo-β-lactamase NDM-1-producing multi-drug resistant bacteria at two Pakistani hospitals and implications for public health. J. Infect. Public Health. 2013;6(6):487–493. doi: 10.1016/j.jiph.2013.06.006. [DOI] [PubMed] [Google Scholar]
- Nordmann P., Poirel L., Carrër A., Toleman M.A., Walsh T.R. How to detect NDM-1 producers. J. Clin. Microbiol. 2011;49(2):718–721. doi: 10.1128/JCM.01773-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosheen S., Bukhari N.I., Ejaz H., Abbas N. Antibiogram and recent incidence of multi-drug resistant carbapenemase producing Escherichia coli isolated from paediatric patients. Pak. J. Med. Sci. 2020;36(2):246–250. doi: 10.12669/pjms.36.2.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson D.L., Doi Y. Editorial commentary: a step closer to extreme drug resistance (XDR) in gram-negative bacilli. Clin. Infec. Dis. 2007;45(9):1179–1181. doi: 10.1086/522287. [DOI] [PubMed] [Google Scholar]
- Qamar M.U., Saleem S., Arshad U., Rasheed M.F., Ejaz H., Shahzad N., Shah J. Antibacterial efficacy of Manuka honey against New Delhi Metallo-β-Lactamase producing Gram negative bacteria isolated from blood cultures. Pak. J. Zool. 2017;49(6):1997–2003. [Google Scholar]
- Ranjan A., Shaik S., Mondal A., Nandanwar N., Hussain A., Semmler T., Kumar N., Tiwari S.K., Jadhav S., Wieler L.H. Molecular epidemiology and genome dynamics of New Delhi metallo-β-lactamase-producing extraintestinal pathogenic Escherichia coli strains from India. Antimicrob. Agents Chemother. 2016;60(11):6795–6805. doi: 10.1128/AAC.01345-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolain J., Parola P., Cornaglia G. New Delhi metallo-beta-lactamase (NDM-1): towards a new pandemia? Clin. Microbiol. Infect. 2010;16(12):1699–1701. doi: 10.1111/j.1469-0691.2010.03385.x. [DOI] [PubMed] [Google Scholar]
- Sassi A., Loucif L., Gupta S.K., Dekhil M., Chettibi H., Rolain J.-M. NDM-5 carbapenemase-encoding gene in multidrug-resistant clinical isolates of Escherichia coli from Algeria. Antimicrob. Agents. Chemother. 2014;58(9):5606–5608. doi: 10.1128/AAC.02818-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahzad N., Aslam B., Hussain I., Ijaz M., Rasool M.H., Tasneem F., Hamid T., Tayyeb A., Hussain T. Distribution and phylogenetic analysis of bacterial isolates from urinary tract infection patients of Pakistan. Pak. J. Zool. 2016;48(6):246–250. [Google Scholar]
- Sievers F., Wilm A., Dineen D., Gibson T.J., Karplus K., Li W., Lopez R., McWilliam H., Remmert M., Söding J. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011;7(1):539–543. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman A.M., Zarad H.O., Nariya H., Shimamoto T., Shimamoto T. Genetic analysis of carbapenemase-producing Gram-negative bacteria isolated from a university teaching hospital in Egypt. Infect. Genet. Evol. 2020;77 doi: 10.1016/j.meegid.2019.104065. [DOI] [PubMed] [Google Scholar]
- Wayne, P. (2018). Performance Standards for Antimicrobial Susceptibility Testing (30 ed.). USA: Clinical and Laboratory Standards Institute.
- WHO Multidrug and extensively drug-resistant TB (M/XDR-TB): 2010 Global report on surveillance and response. World Health Organization. 2010;5(57):180–191. [Google Scholar]
- Wu W., Feng Y., Tang G., Qiao F., McNally A., Zong Z. NDM metallo-β-lactamases and their bacterial producers in health care settings. Clin. Microbiol. Rev. 2019;32(2):e00115–e118. doi: 10.1128/CMR.00115-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B., Dong H., Xu H., Lv J., Zhang J., Jiang X., Du Y., Xiao Y., Li L. Coexistence of MCR-1 and NDM-1 in clinical Escherichia coli isolates. Clin. Infect. Dis. 2016;63(10):1393–1395. doi: 10.1093/cid/ciw553. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used and analyzed during the current study available from the corresponding author.