Abstract
There is only limited literature studies on the activities of inflammation and matrix accumulation in the renal tissues of rats induced with diabetes through Streptozotocin. The present the investigation involves the examination of the protective actions of Myrcene (MYN), a monoterpene on the oxidative stress, inflammation, and matrix accumulation. For this purpose an experimental setup was created which involves injecting MYN 50 mg/kg for about 45 days in the STZ diabetic rats. Modifications in the enzymes, collagens, growth factor B1 and Kappa factor P65 were identified and tracked. The levels of the inflammatory markers like TF-α1, ICAM-1, VCAM-1, MCP-1 were tracked and noted. The current experimental results showed an alteration in the glucose metabolism and enhanced condition. Also an increased level of TGF-β-1 and Nuclear factor-kB expression was seen in the renal tissues. MYN was found to reduce glucose oxidative stress and exhibit an anti-inflammatory effect via inhibiting NF-kB signalling. The conclusion of the current study reveals that MYN regulates the inflammatory activities and matrix accumulation by inhibiting the activities of inflammatory cytokine, pro-inflammatory signalling.
Keywords: Anti-inflammation, Myrcene, Matrix accumulation, Oxidative stress, Diabetic rats
1. Introduction
In recent times, many extensive studies and numerous researches has revealed that major factors and causative agents for many dreadful diseases were infections, obesity, radiation, and diet. These factors stimulate chronic diseases by activating inflammation (Duraipandiyan et al., 2020, Pang et al., Ajaikumar et al., 2018, Guru et al., 2021). These factors alter and regulate the mechanism of signalling pathways of inflammation, resulting in dreadful diseases. (Prasad et al., 2012, Pawelec et al., 2014, Nasef et al., 2017, Issac et al., 2020). A tremendous evidence suggests that many plant-based active molecule has the ability to arrest several diseases. Neuroceutical derived bio compounds have remarkably prevented chronic diseases by influencing inflammatory pathways (Kannappan et al., 2011) were reported. The injuries and the irritants are responded by swelling of the tissues. Thus swelling is used to maintain proper tissue homeostasis during stress (Medzhitov, 2010). In earlier studies anti-inflammatory potentials of plant-based bioactive molecules used to prevent inflammation in lungs and in the skins of the animal when used in-vitro was established. Inflammation is a defense mechanism of organism to discard the stimuli expressed by accelerating blood flow, enhanced metabolic process, dilation of blood vessels, discharging of mediators and accumulation of cellular influx (Ferrero-Miliani et al., 2007).
Myrcene (MYN) is found in larger composition in the essential oils like hops, lemongrass and bay leaf (NTP, 2010, Index, 2013, Kuppusamya et al., 2018). They are being widely utilized in cosmetics, soaps and fragrant products and in the beverages industry (Okaru and Lachenmeier, 2017). Inflammation in renal tissues caused diabetic nephropathy(DN) which leads to chronic disorder for diabetic patients. Therefore, the prime objective of the present study is to test the efficacy of MYN on the inflammation, accumulation of matrix in the renal tissues of rats induced with diabetes using STZ.
2. Materials and methods
2.1. Reagents and chemicals
Drugs like Myrcene (MYN) and Streptozotocin (STZ) were procured from Sigma Aldrich chemicals Pvt. Ltd. USA. The equipments used for the experimental analysis were bought from Apex Diagnostics, Mumbai, India. Bovine Serum Albumin (BSA) was procured from Merck Pvt. Ltd. Mumbai, India. Antibodies against TGF-β1 (Rabit polyclonal) was bought from Aldrich Zigma USA.
2.2. Experimental sections
Adult Wistar rats of average weight of 150 gms was obtained from the Department of experimental and Research Unit. These rats were shipped at the temperature of 22–25 °C with alternating light and dark cycle for about 12 h each. Alsoa standard pellet diet and water lipitum were supplied to the rats.
2.3. Inducing diabetic for experimental animals
Rats were stimulated with diabetes using STZ injection of 45 mg/kg (IP) dissolved in 0.01 M citric buffer solution of pH 4.5. The animals were grouped into four groups and maintained. Fasting blood glucose level (FBG) was measured 72 h after injection. A limit of Glucose level, about 200 mg/dL was considered as normal and the rats with glucose level above this limitwas taken for the experimental investigation.
2.4. Experimental setup and grouping of animals
The whole experimental rats were grouped into 4 groups and the experiment lasted for the duration of about 45 days. Group I (CON) rats were characterized as the control group. Group-2 constitutes (DIA) –Diabetic rats that received STZ dose of 45 mg/kg dissolved in 0.1 M citrate buffer solution of pH 4.5. Group-3 includes (DIA + MYN) – Diabetic rats treated with MYN dose of 25 mg/kg of Body Weight per day dissolved in Carboxymethyl Cellulose. Intake of food, body weight and consumption of fluid were measured at routine periods. All the rats were maintained in a clean metallic cages and the test samples (Urine) were collected systematically. The samples were taken and preserved in 0.2 ml of 10 N HCL until the experimental analysis.
2.5. Glucose tolerance analysis
On 44th day the animals were not given any food over night. Blood samples were collected at 2 intervals of 60 mins after injecting 2gm/kg glucose. The OGTT curve was drawn by plotting blood glucose using Graph pad prism version 5.1. Animals were executed with an IM injection of ketamine hydrochloride (35 mg/kg). Immediately kidney was taken out, cleaned, dried and preserved in 0.1 M Tris HCL buffer and the collected supernatant was used for subsequent analysis.
2.6. Biochemical testing
The content of glucose and level of insulin were quantified and the glycatedhemoglobin and Fructosamine were estimated. Determination of the level of TNF-α, IL-6 was carried out by ELISA based on manufacturer’s instructions.
2.7. Western plotting methods for TGF--β1 in the kidney tissue
The kidneys taken out from the executed rats were assimilated in an buffer solution of 20 mM Tris HCl, of pH 7.4 mixed with 150mN of NaCl2 INM Ethylene diamine, tetraacetic acid (EDTA), 0.5% Triton X-100, 0.1% dodecyl sulphate SDS, 1 mM phenyl methane sulfonyl fluoride and 10 UL of protease Inhibitor cocktail centrifuged at the rate of 10,000g for 15 min, at 4 °C. The obtained 50µmg of solubilized protein was electrophoresed by SDS-PAGE. After an hour, the animal membrane was kept in a blocking buffer containing 0.1% Tween 20 followed by probing of primary antibody rabbit polyclonal anti-TGF-β1 1: 1000 diluted in blocking buffer over night at the temperature of about 4 °C. The membrane was then washed with 0.1% TBST for 5 min and subsequently incubated with HRP conjugated anti-rabbit antibody 1:1500 dilution for two hours. B actin was used as a control 1:1000 dilution. The bands were identified by colour reactions with H2O2 and then scanned. Intensity was determined through AlfaSasa FC software.
2.8. RTPCR. Analysis of TGF--β1, TNF-α, and NF-kB
Extraction of RNA was done using Trizol by the Guanidium thiocyanate method (Chomczynski and Sacchi,1987). Then 2 Ug of RNA was assimilated with Oligo DT primer (0.5 µg/µgL) dNTP mix and RNase free water and then heated at 65 °C for 5mins. An attempt of Reverse transcription was done with 5X Maloney-Murine Leukemia virus, and inhibitor solutions, incubated at 42 °C for 90 min and the reaction was continued at 85 Deg. C. for 5 min. PCR amplification was carried out in a mixture of 2.5 µL of buffersolution, 3.5 Ub of RNA free water, 1 UL of primers, 1 UL dNTPs, and 5UL reverse-transcribed template solution.
Denaturing of TGF-β1 thermocyclic conditions was done at 75 °C for 30 Sec followed by annealing at 53 °C for 30 secs and at 72 °C for 40 Secs with primer extension of 30 cycles. Similarly denaturization of PCR conditions of TNF-α was done at 94 °C for 30 sec followed by annealing at 52 °C for 30 sec and at 72 °C for 30 sec with primer extension. The estimation of NF-kB was done by denaturizing at 95 °C for 3 min, followed with annealing at 51 °C for 30 Secs and at 40 °C with primer extension of 30 cycles. Five UL of PCR products were mixed with 1.5% agarose Gel and electrophoresed for 20 Min, visualized and photographed. All reactions were triplicated. The quantity was indicated as the ratio of band intensities of TNF-α, TGF-β1 and NF-kB and compared with β-actin as control.
2.9. Statistical analysis
The indicated values are represented as Mean and SD values, and ANOVA analysis was done for in-vitro investigations.
3. Results
As illustrated in Fig. 1a, OGTT in diabetic (Group-2) rats exhibited hyperglycemia and marked enhancement of glucose at 60 mm and 120 mm (P < 0.05) after loading glucose orally. Meanwhile, both MYN treated control and Diabetic rats gave normal response. The level of glucose and AUC glucose (mmol/L/Min) for the experimental animal group are as follows. Control group was found to have the level of 9.41 ± 0.4, whereas Group 2 (Diabetic) rats showed the value of 22.13 ± 2.0, Group-3 (Dia _ MYN) rats had the level of 9.63 ± 0.71 and Group-4 control + MYN exhibited the level of 9.21 ± 0.41, which is more or less equal to the Group-1. AUC glucose was found to be significantly high in Diabetic rats compared to control group. Thus it is evident that MYN supplements have significantly reduced the AUC glucose values. This is clearly shown in the graph as illustrated in Fig. 1b.
Fig. 1.
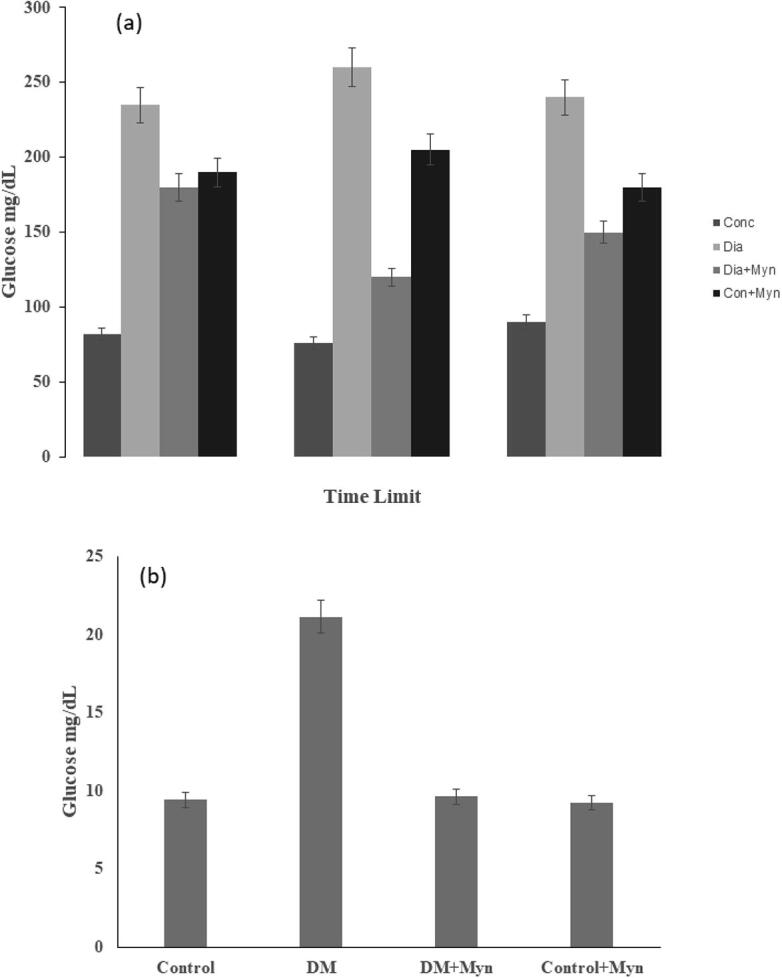
(a) Effects of MYN on oral Glucose tolerance (OGT) (b) AUC Glucose Level graph in all the groups.
3.1. Effects of MYN on glucose, insulin, glycated hemoglobin, Fructosamine, and cytokine levels estimation
Table 1, shows the levels of glucose Insulin, glycated hemoglobin, Fructosamine, and cytokine in MYN treated rats. Results showed that Group-2 (Diabetic) rats exhibited a significant increase (p < 0.05) in the level of Blood glucose, glycated hemoglobin, fructosamine, TNF-α and IL-6, with a substantial decline in the level of Insulin. MYN contributed and brought both levels to be equal when compared to MYN unsupplemented diabetes rats.
Table 1.
Estimation of control factors in Myn treated and control animals.
| Parameter | Control | Dia | Dia + Myn | Con + Myn |
|---|---|---|---|---|
| Glucose (nmol/L) | 6.21 ± 0.3 | 17.85 ± 1.6 | 6.22 ± 03 | 6.29 ± 0.21 |
| Insulin (pmol/L) | 91.52 ± 2.41 | 34.01 ± 3.2 | 83.80 ± 0.03 | 97.18 ± 6.21 |
| FAM(nmol/L) | 0.94 ± 0.03 | 2.21 ± 0.01 | 0.91 ± 0.05 | 0.91 ± 0.06 |
| GHb | 0.004 ± 0.002 | 0.007 ± 0.003 | 0.005 ± 0.001 | 0.004 ± 0.001 |
| TNF-α(fmol/mL) | 864 ± 85.32 | 2315.02 ± 17.6 | 1281 ± 13.5 | 893.14 ± 3.71 |
| IL-6 (fmol/mL) | 3862.15 ± 21.2 | 4972.6 ± 43.2 | 3912.67 ± 32 | 3834.2 ± 13.16 |
3.2. TNF-α, TGF-β1, and NF-kB
Densitometry data on the expression of TGF-β1 in the Kidney tissueswere shown in the Fig-2a respectively. Expression of TGF-β1 was found to be high in diabetic rat where MYN treated inhibited at a significant level. Moreover, control and control + MYN exhibited no significant change in expression level.
Increased mRNA levels of TGF-β1, TNF-α and NF-kB were recorded in Diabetic rats and compared with control. Results showed that a detectable and significant reduction (p < 0.05) in the expression level of TGF-β1, TNF- α and NF-kB was obtained, when injecting MYN. Insignificant difference was observed in control and control + MYN when treated with MYN. These are clearly illustrated in the graphs shown in the Fig. 2b, Fig. 3a &3b respectively. The levels of MMP-2, TMP-1 and TGF-β1 had played a crucial role in regulating the production of the collagen and degradation, and is illustrated in the Fig. 4a &b.
Fig. 2.
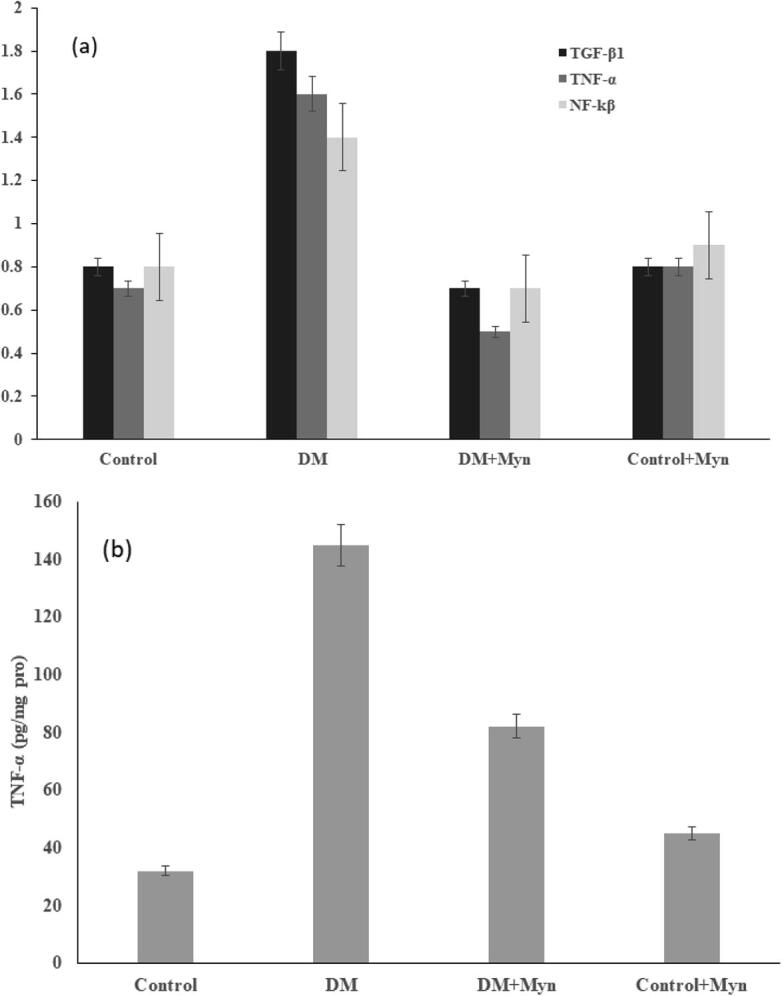
(a) Level and Relative value of TGF- β1, TNF-α, NF-kβ (b) Levels of TNF-α Content.
Fig. 3.
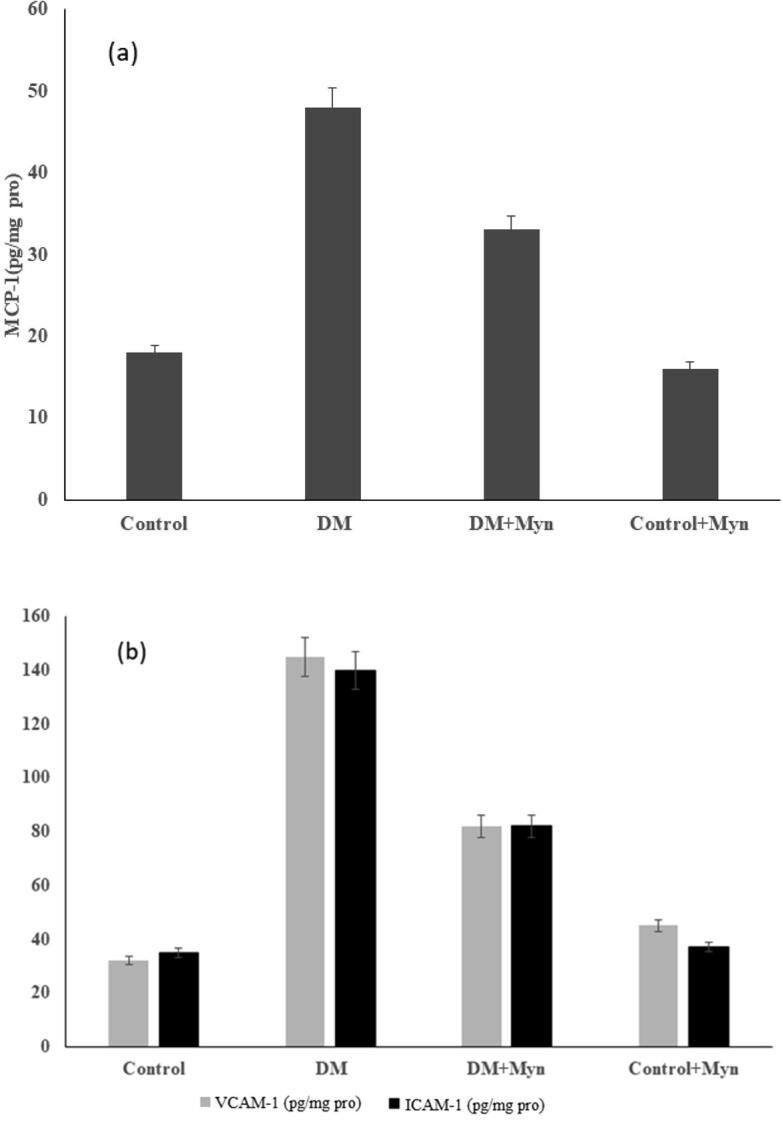
(a) Levels of MCP-1(b) Levels of ICAM-1, VCAM-1 (pg/mg pro).
Fig. 4.
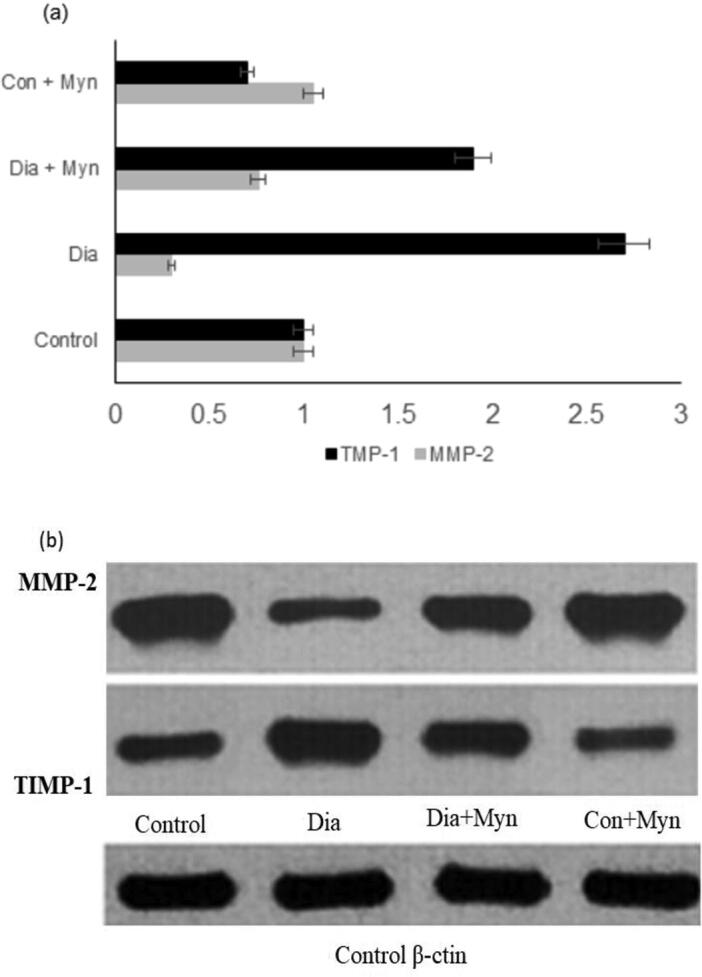
(a) Densilometric analysis of matrix metaloproteinease (b) Protein expression in Western plotting system.
This was clearly established in Western plotting. The levels of TIMP-1 and RTGF β-1 were increased whereas MMP-2 expression was inhibited in the Diabetic group of rats. While in control group expression of TIMP-1 and TGF-β1 were controlled and MMP-2 expression was augmented in control + MYN treated rats.
3.3. Oxidative stress
The level of MDA, SOD and GSH-Px was assessed and ROS was generated in kidney tissue. There was a significant increase in ROS and MDA levels and decrease in SOD and GSH-PX activities in MYN treated diabetic group as shown in Fig. 5a respectively. On the contrary, in control and control + MYN treated showed a decline in ROS and MDA level with increased SOD and GSH-PX levels as shown in Fig. 5b respectively.
Fig. 5.
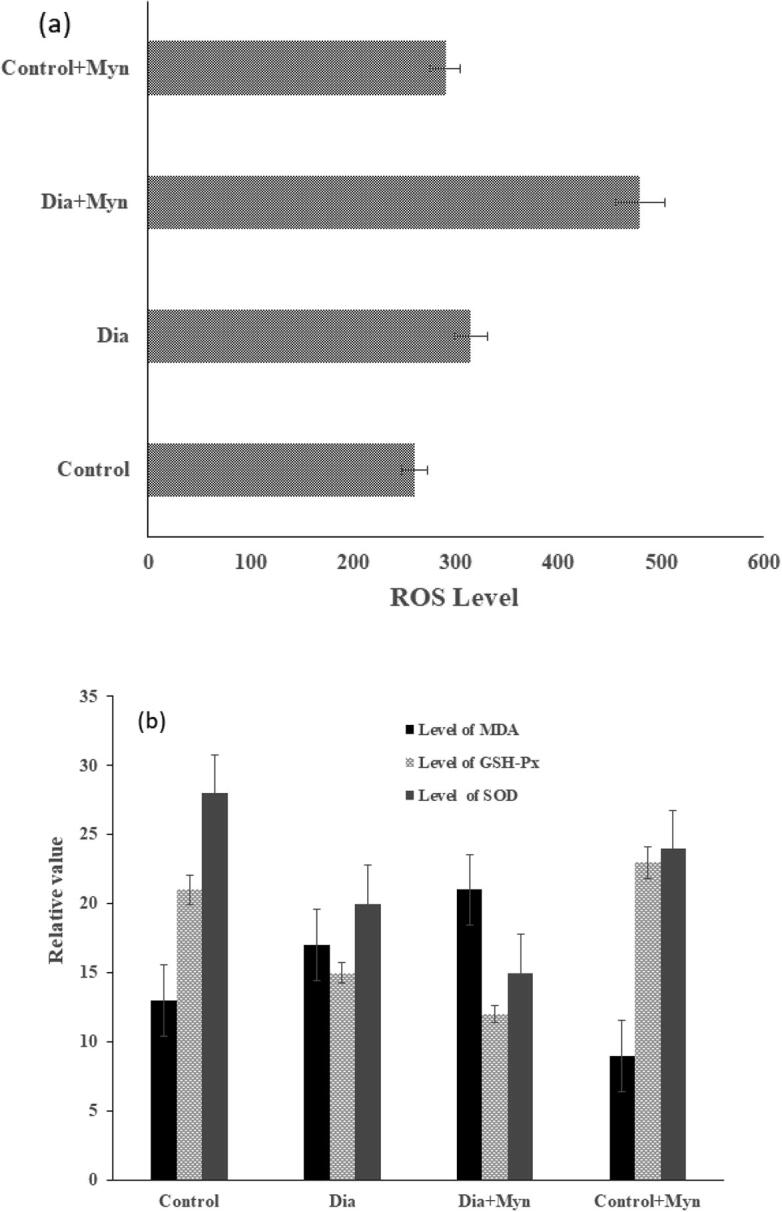
(a) Generation of ROS in Renal Tissue(b) Levels of MDA, GSH-Px, Level of SOD.
4. Discussion
In this experimental procedures, the investigation of protective effects of Myrcene on the swelling and Matrix accumulation in the renal tissues of rats induced with diabetes rats by Streptozotocin was carried out. The biochemical and histopathological findings evidenced the advantages of MYN in the inhibition of Diabetic nephropathy and MYN could improve kidney performance and decline the matrix accumulation and fibrosis in diabetic rats. There are large number of evidences proving that the swelling of the tissues and the oxidative stress are interlinked. Both participated in the evolution of diabetic nephropathy (Kashihara et al., 2010, Stanton, 2011, Wada and Makino, 2013, Navarro-González et al., 2011, Ilavenil et al., 2017, Park et al., 2017). ROS generated signaling such as glycolysis, Xanthine oxidase, NAD PH Oxidase, advanced glycation are known to be a potential, driven contributor to the progression of DN. Moreover, mitochondrial production of ROS in hyperglycemic condition was identified to be the key elements or inhibitor for those pathways (Forbes et al., 2008). In hyperglycemic conditions, immune complexes can stimulate renal cells by triggering proteinsignaling, which leads to the discharge of chemokines and upregulation of adhesive molecules (Ilavenil et al., 2016a, Ilavenil et al., 2016b). This process catalyzes the renal incursion of monocyte, lymphocyte, they enable secrete pro-inflammatory cytokines. The activity or the role of Leukocytes is to boost the swelling response, to increase the injury and fibrosis (Lim and Tesch, 2012). In the present investigation, MYN could reduce these risk factors in the renal tissue of diabetic rats. NF-kB belongs to pleiotropic transcription factor that could integrate a network of extra or intracellular signaling. Moreover, NF-kB activation has been known to be a close association with kidney inflammation (Sanz et al., 2010). The pathway of NF-kB dimmers then translocate into the nucleus of the cell, wherein they attach NF-kB bound JKb. In currentinvestigation, the binding activity of NF-kB in DNA was markedly rised in the kidney tissues of rats induced with diabetes by STZ. Moreover, MYN has shown to be attenuated the swelling response in kidney of a diabetic viz., positive control of NF-kB signalling.
Renin-angiotensin system (RASO) has played a key role in the progression of DBN (Gurley and Coffman, 2007, Carey and Siragy, 2003). Studies reported that Ang. II can be conferred and induce damagein the kidney by endured inflammation, cell growth and fibrosis (Remuzzi et al., 2005). In the present study, MYN has played a beneficial role by inhibiting the activity of rASO, resulting from reduction in the progression of DN.
Also an augmented levels of TNF-α is found in the rat's kidney. These results were consistent with other studies (Navarro et al., 2006, DiPetrillo et al., 2003). MYN treatment has attenuated an overexpression in TNF-α levels. These then binds with its receptors and stimulate the swelling response by targeting the expression of transcription factors and inflammatory mediators (King, 2008). Similarly, MYN attenuated TGF-β level is compared to diabetic rats. Further beneficial effects of MYN have shown to be related to inhibit TGF-β signaling, this may be due to its potential to attenuate hyperglycemia. The enhanced level of TGF-β1 mRNA in diabetic induced rat kidney could be catalyzed by extended hyperglycemia, while glucose-stimulated TGF-β1 production in cultured mesangial cells was reported (Wang et al., 2005). Moreover, TNF-α could accelerate transcription of TGF-β1, TGF-β1 binds to its receptor type II that induce gene encoding several matrix molecules for inflammation (Riser et al., 2000).
5. Conclusion
In this article, investigation of myrcene effects on inflammation and accumulation of matrix in renal tissues of streptozotocin-induced diabetes rats wasconducted and explored that myrcene has the ability to inhibits the proinflammatory cytokine (IL-6) and TNFa by stimulating NF-kB. Simultaneously, it is obvious that myrcene might have increased expression of anti-inflammatory cytokine and in turn exert the strongest inhibitory effectors of the expression of pro-inflammatory cytokines and NF-kB activation signalling. Consequently, Myrcene inhibits the accumulation of the matrix and genesis of inflammation. The current study strongly suggest that myrcene is novel compound and emerging therapeutic option in clinical practices.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
References
- Ajaikumar, B. Kunnumakkara, Sailo, Bethsebie L., Banik, Kishore, Harsha, Choudhary, Prasad, Sahdeo, Gupta, Subash Chandra, Bharti, Alok Chandra, Aggarwal, Bharat B., 2018. Chronic diseases, infammation, and spices: how are they linked? J. Transl. Med. 16, 14. [DOI] [PMC free article] [PubMed]
- Carey R.M., Siragy H.M. The intrarenal renin-angiotensin system and diabetic nephropathy. Trends Endocrinol. Metab. 2003;14:274–281. doi: 10.1016/s1043-2760(03)00111-5. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanatephenol-chloroform extraction. Anal. Biochem. 1987;62:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- DiPetrillo K., Coutermarsh B., Gesek F.A. Urinary tumor necrosis factor contributes to sodium retention and renal hypertrophy during diabetes. Am. J. Physiol. Renal. Physiol. 2003;284:113–121. doi: 10.1152/ajprenal.00026.2002. [DOI] [PubMed] [Google Scholar]
- Duraipandiyan V., Balamurugan R., Al-Dhabi Naif Abdullah, William Raja T., Ganesan P., Ahilan B., Valan Arasu M., Ignacimuthu S. Galal Ali Esmail. The down regulation of PTP1B expression and attenuation of disturbed glucose and lipid metabolism using Borassus flabellifer (L) fruit methanol extract in high fat diet and streptozotocin induced diabetic rats. Saudi J. Biol. Sci. 2020;27(1):433–440. doi: 10.1016/j.sjbs.2019.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrero-Miliani L., Nielsen O.H., Andersen P.S., Girardin S.E. Chronic inflammation: importance of NOD2 and NALP3 in interleukin-1beta generation. Clin. Exp. Immunol. 2007;147:227–235. doi: 10.1111/j.1365-2249.2006.03261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes J.M., Coughlan M.T., Cooper M.E. Oxidative stress as a major culprit in kidney disease in diabetes. Diabetes. 2008;57:1446–1454. doi: 10.2337/db08-0057. [DOI] [PubMed] [Google Scholar]
- Gurley S.B., Coffman T.M. The renin-angiotensin system and diabetic nephropathy. SeminNephrol. 2007;27:144–152. doi: 10.1016/j.semnephrol.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Guru, A., Lite, C., Freddy, Allen J., Issac, Praveen Kumar, Pasupuleti, Mukesh, Saraswathi, N.T., Arasu, Mariadhas Valan, Al-Dhabi, Naif Abdullah, Arshad, Aziz, Arockiaraj, Jesu, 2021. Intracellular ROS scavenging and antioxidant regulation of WL15 from cysteine and glycine-rich protein 2 demonstrated in zebrafish in vivo model. Develop. Comp. Immunol. [DOI] [PubMed]
- Ilavenil, S., Kim, Da Hye, Vijayakumar, Mayakrishnan, Srigopalram, Srisesharam, Roh, Sang Gun, Arasu, Mariadhas Valan, Lee, Jong Suk, Choi, Ki Choon, 2016a. Potential role of marine algae extract on 3T3‑L1 cell proliferation and differentiation: an in vitro approach. Biol. Res. 49, 38. [DOI] [PMC free article] [PubMed]
- Ilavenil, S., Kim, Da Hye, Srigopalram, Srisesharam, Arasu, Mariadhas Valan, Lee, Kyung Dong, Lee, Jeong Chae, Lee, Jong Suk, Renganathan, Senthil, Choi, Ki Choon, 2016b. Potential application of p-coumaric acid on differentiation of C2C12 skeletal muscle and 3T3-L1 preadipocytes—an in vitro and in silico approach. Molecules 21, 997. [DOI] [PMC free article] [PubMed]
- Ilavenil S., Kim Da Hye, Srigopalram Srisesharam, Kuppusamy Palaniselvam, Arasu M.V., Lee Kyung Dong, Lee Jung Chae, Song Yeon Hee, Jeong Young-Il, Choi Ki Choon. Ferulic acid in Lolium multiflorum inhibits adipogenesis in 3T3-L1 cells and reduced high-fat-diet-induced obesity in Swiss albino mice via regulating p38MAPK and p44/42 signal pathways. J. Funct. Foods. 2017;37:293–302. [Google Scholar]
- Issac, P.K., Guru, Ajay, Chandrakumar, Sri Snehaa, Lite, Christy, Saraswathi, N.T., Arasu, Mariadhas Valan, Al-Dhabi, Naif Abdullah, Arshad, Aziz, Arockiaraj, Jesu, 2020. Molecular process of glucose uptake and glycogen storage due to hamamelitannin via insulin signalling cascade in glucose metabolism. Mol. Biol. Rep. 1–14. [DOI] [PubMed]
- Kannappan R., Gupta S.C., Kim J.H., Reuter S., Aggarwal B.B. Neuroprotection by spice-derived nutraceuticals: you are what you eat! Mol. Neurobiol. 2011;44(2):142–159. doi: 10.1007/s12035-011-8168-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashihara N., Haruna Y., Kondeti V.K., Kanwar Y.S. Oxidative stress in diabetic nephropathy. Curr. Med. Chem. 2010;17:4256–4269. doi: 10.2174/092986710793348581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King G.L. The role of inflammatory cytokines in diabetes and its complications. J. Periodontol. 2008;79:1527–1534. doi: 10.1902/jop.2008.080246. [DOI] [PubMed] [Google Scholar]
- Kuppusamya P., Lee Kyung Dong, Song Chae Eun, Ilavenil Soundharrajan, Srigopalram Srisesharam, Arasu Mariadhas Valan, Choi Ki Choon. Quantification of major phenolic and flavonoid markers in forage crop Lolium multiflorum using HPLC-DAD. Revista Brasileira de. Farmacognosia. 2018;28:282–288. [Google Scholar]
- Lim A.K., Tesch G.H. Inflammation in diabetic nephropathy. Mediators Inflamm. 2012;2012 doi: 10.1155/2012/146154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R. Infammation 2010: new adventures of an old fame. Cell. 2010;140(6):771–776. doi: 10.1016/j.cell.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Index Merck. Merck & Co., Inc; Whitehouse Station (NJ), USA: 2013. The Merck index: an encyclopedia of chemicals, drugs, and biologicals. [Google Scholar]
- Nasef N.A., Mehta S., Ferguson L.R. Susceptibility to chronic infammation: an update. Arch. Toxicol. 2017;91(3):1131–1141. doi: 10.1007/s00204-016-1914-5. [DOI] [PubMed] [Google Scholar]
- Navarro J.F., Milena F.J., Mora C., Leon C., Garcia J. Renal proinflammatory cytokine gene expression in diabetic nephropathy: effects of angiotensinconverting enzyme inhibition and pentoxifylline administration. Am. J. Nephrol. 2006;26:562–570. doi: 10.1159/000098004. [DOI] [PubMed] [Google Scholar]
- Navarro-González J.F., Mora-Fernández C., Muros de Fuentes M., García-Pérez J. Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy. Nat. Rev. Nephrol. 2011;7:327–340. doi: 10.1038/nrneph.2011.51. [DOI] [PubMed] [Google Scholar]
- NTP NTP technical report on the toxicology and carcinogenesis studies of β-myrcene (CAS No.123-35-3) in F344/N rats and B6C3F1 mice (gavage studies) Natl Toxicol Program Tech Rep Ser. 2010;557(557):1–163. PMID:21415873. [PubMed] [Google Scholar]
- Okaru A.O., Lachenmeier D.W. The food and beverage occurrence of furfuryl alcohol and myrcene – two emerging potential human carcinogens? Toxics. 2017;5(1):9. doi: 10.3390/toxics5010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang L., Antonisamy P., Esmail G.A., Alzeer A.F., Al-Dhabi N.A., Arasu M.V., Ponmurugan K., Kim YO, Kim H. Nephroprotective effect of pigmented violacein isolated from Chromobacterium violaceum in wistar rats. Saudi Journal of Biological Sciences. Oct 2020;1527(12):3307–3312. doi: 10.1016/j.sjbs.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H.K., Kim Su Kang, Kweon Hae Yong, Lee Kwan Gill, Arasu Mariadhas Valan, Kim Young Ock. Promoter polymorphism (590, T/C) of interleukin 4 (IL4) gene is associated with rheumatoid arthritis: an updated meta-analysis. Saudi J. Biol. Sci. 2017;24:444–449. doi: 10.1016/j.sjbs.2016.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawelec G., Goldeck D., Derhovanessian E. Infammation, ageing and chronic disease. Curr. Opin. Immunol. 2014;29:23–28. doi: 10.1016/j.coi.2014.03.007. [DOI] [PubMed] [Google Scholar]
- Prasad S., Sung B., Aggarwal B.B. Age-associated chronic diseases require age-old medicine: role of chronic infammation. Prev. Med. 2012;54(Suppl):S29–S37. doi: 10.1016/j.ypmed.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remuzzi G., Perico N., Macia M., Ruggenenti P. The role of renin-angiotensin-aldosterone system in the progression of chronic kidney disease. Kidney Int. Suppl. 2005;99:S57–S65. doi: 10.1111/j.1523-1755.2005.09911.x. [DOI] [PubMed] [Google Scholar]
- Riser B.L., Denichilo M., Cortes P., Baker C., Grondin J.M., Yee J., Narins R.G. Regulation of connective tissue growth factor activity in cultured rat mesangial cells and its expression in experimental diabetic glomerulosclerosis. J. Am. Soc. Nephrol. 2000;11:25–38. doi: 10.1681/ASN.V11125. [DOI] [PubMed] [Google Scholar]
- Sanz A.B., Sanchez-Niño M.D., Ramos A.M., Moreno J.A., Santamaria B., Ruiz-Ortega M., Egido J., Ortiz A. NF-kappaB in renal inflammation. J. Am. Soc. Nephrol. 2010;21:1254–1262. doi: 10.1681/ASN.2010020218. [DOI] [PubMed] [Google Scholar]
- Stanton R.C. Oxidative stress and diabetic kidney disease. Curr. Diab. Rep. 2011;11:330–336. doi: 10.1007/s11892-011-0196-9. [DOI] [PubMed] [Google Scholar]
- Wada J., Makino H. Inflammation and the pathogenesis of diabetic nephropathy. Clin. Sci. (Lond) 2013;124:139–152. doi: 10.1042/CS20120198. [DOI] [PubMed] [Google Scholar]
- Wang W., Koka V., Lan H.Y. Transforming growth factor-beta and Smadsignaling in kidney diseases. Nephrology (Carlton) 2005;10:48–56. doi: 10.1111/j.1440-1797.2005.00334.x. [DOI] [PubMed] [Google Scholar]


