Abstract
Reducing toxic effects of pesticide residues in agricultural soils through organic amendments is an eco-friendly technique. Cypermethrin (CYP) and Chlorpyrifos (CPP) are widely used pesticides in peach growing orchards in Swat valley of Pakistan. The aim of the current study was to investigate the degradation behavior of CYP and CPP in soil by the application of different combination of organic/inorganic amendments. A total of 36 soil samples were used in the current incubation study which was collected from 4 peach orchards in district Swat, Khyber Pakhtunkhwa (KPK), Pakistan. Different amendments including urea, farm yard manure (FYM) and saprofil were applied alone and in various combinations. The initial concentrations of CYP and CPP in the tested soil was range from 0.94 to 4.8 mg kg−1 and 0.024 to 4.12 to mg kg−1. Soil samples were taken at 5, 15, 30 and 45 days after exposure to different treatments. The extraction of pesticides from soils was done through quick, easy, cheap, effective, rugged, and safe (QuEChERS) extraction method. Soils amended with urea, FYM and saprofil individually and in combinations significantly reduced the concentrations of CYP and CPP. However, the concentration of CYP (24.6) and CPP (27.0) in soil showed higher reduction through the application of FYM. While the concentrations of CYP and CPP were declined with the 5, 15, 30 and 45 days intervals, however, reduction at day 30 and 45 was faster for CYP (16.7 to 8.46) than CPP (20.2 to 12.3). At day 5 and 15, the CYP (42.5 to 30.7) was slightly lower than CPP (42.9 to 32.7).The highest half-life value (t ½) of CYP was in control treatment (32 days) and the shortest was soil amended with FYM (18.6 days). While the longest half-life value (t ½) of CPP was maximum in control treatment (42 days) and the minimum was in FYM (22 days). Based on our findings, it was concluded that soil application of FYM is recommended for the degradation of CYP and CPP.
Keywords: Removal, Soil contaminant, Insecticide, Organic bioresources, QuEChERS method
1. Introduction
In Pakistan, Swat valley located in Khyber Pakhtunkhwa (KPK) province is highly favoring for peach cultivation due to considerably cooler and wetter climate. Swat is famous for peach production mostly grown in the valley bottom plains marketed in the national markets with a brand name of “Swat Peaches”. The enormous growth in world’s population exerts burden over the food production that leads to application of pesticides in order to maximize the yield of agriculture crops. Although, application of pesticides contribute in maximization of food production by controlling insect and pest but on the other side contaminate the food item due to its accumulation effect in food chain (Popp et al., 2013). Different techniques like phytoremediation, soil flushing, immobilization, land filling etc. are being used to remediate/degrade the pesticides residues in soil and other matrix of the plant (Tang et al. 2013). The bioremediation through bio-stimulation develops as one of the most appropriate approach for remediation of soil contaminated through pesticides application (Niti et al., 2013). Organic manures are familiar as a valuable source of nutrients to improve soil structure and contribute to crop yields (Basak et al., 2020, Singh et al., 2017). Additionally, bio-processed materials like mushroom spent compost (Meng et al. 2017), farmyard manure, vermin-compost and biogas slurry have also been recently recognized as a good source of bio stimulation process through a large number of pesticide degradation in soil/plant nutrients(Kadian et al. 2008).
In Pakistan, pesticides such as Cypermethrin (CYP) and Chlorpyrifos (CPP) are mainly used for the control of insect/pest on agricultural crops, fruits and vegetables on large scale (Ahmad and Arif 2010). The pesticide residues are degredated naturally by physical, chemical and biochemical means, but because of its high stability and water solubility, the pesticide residues persist in the environment (Odukkathil and Vasudevan 2013). Organic amendments play a very important role in enhancing the soil fertility and soil microbial activity (Brtnicky et al., 2019, Caban et al., 2018). These amendments enrich the matrix with a diverse micro flora, nutrients and hence act as efficient bio-stimulating agent.
Hence, a number of researchers has investigated the potential of compost, farmyard manure, urban waste and public green compost to accelerate the pesticide dissipation in different bio-beds and soil (Kadian et al., 2008, Vischetti et al., 2004). Recently CPP biodegradation using different types of composts and various agro-residues such as coconut husk, peat moss, peanut shell, rice husk (Romyen et al. 2007) has been reported (Coppola et al. 2007). Hazardous effects of CPP can be minimized with the application of organic amendments (Lasat, 2000, Tejada et al., 2011, Liu et al., 2018). The CYP persistence also differs significantly among soils with different modes of fertilization. The CYP dissipates faster in soils fertilized with phosphorus and potassium application. The slowest dissipation usually observed in nitrogen and potassium application. No significant differences in dissipation is observed between long-term fertilization with inorganic N, P, K and fertilization with organic manure (Wenjun et al., 2007, Xie and Zhou, 2008). In case of Pakistan, organic sources are cheap and easily available to the farmers in rural areas to apply for getting high yield but on the other hand the organic sources had a potential as bio-stimulant for reducing the residual effect of chemicals as well (Ponni and Arumugam, 2007, Tejada et al., 2014). In previous studies, different organic amendments such as farm yard manure, corn straw, and green manure were used to reduce the residual effect of the pesticides (Wang et al., 2006, Zhao et al., 2013). It reflect that organic amendments had a potential for reducing the residual effect of pesticides in soil and environment (Kadian et al., 2012, Marín-Benito et al., 2013).
However, systematic investigations on CPP and CYP contaminated soils remediation using organic amendments particularly in horticultural crops and peach orchards are not studies yet. Therefore, the current study has been designed to evaluate the effects of organic sources as bio-stimulant to reduce the residues of CYP and CPP in peach production Swat district, Pakistan. Out of total peach production (6796818 tones), cultivated area in the country with 15,409 ha, Swat district only contributes 52,800 tones production with 7150 ha cultivated area (Crop Statistics Khyber Pakhtunkwa 2018–19). In this study we have investigated the capability of urea, farmyard manure (FYM) and saprofil alone and in-combinations on the reduction of CYP and CPP residues in soils of peach orchard in the studied area.
2. Material and methods
2.1. Soil sample collection
Soil samples were collected from four sub-locations of district Swat (35.2227° N, 72.4258° E with 980 m elevation) i.e., Matta, Khwazakhaila, Kabal, and Barikot. A total of 9 soil samples were collected from peach orchardslocations in each subdivision (36 samples in total). The samples were collected from 0 to 30 cm soil depth with steel auger for pesticide soil residues analysis. A true representative soil sample was made via collecting about 10 sub-samples and mixed them well. Each composite sample weighing one-kilogram soil was taken in a polypropylene bucket and stored in a labeled plastic bag. Soil samples were brought to the soil and water testing laboratory at Agricultural Research Institute Tarnab Peshawar, Khyber Pakhtunkhwa, Pakistan for analysis. Dried and sieved soil samples were stored in plastic bottles at − 20 °C for further analyses.
2.2. Experimental strategy and procedure
This incubation study was organized by using complete randomized design (CRD) having three replicates. The air dried soil was grinded, sieved and filled in 2 kg soil in poly propylene pots. Before experimentation, a representative soil sample was analyzed for its physicochemical properties as well as the concentration of CYP and CPP.
Treatments include; T1: control soil (without amendment), T2: soil amended with urea (46% N), T3: soil amended with farm yard manure (20 t ha−1), T4: soil amended with 5 t ha-1saprofil, T5: soil amended with half urea (45 N kg ha−1) + half FYM (10 t ha−1), T6: soil amended with half urea (45 N kg ha−1) + half saprofil (2.5 t ha−1); T7: soil amended with half FYM (10 t ha−1) + half saprofil (2.5 t ha−1), T8: ¼ urea (22.5 N kg ha−1 + ¼ FYM (5 t ha−1 + ¼ Saprofil (1.25 t ha−1). The quantities of all amendments were calculated on soil weight basis and their percentage of N. The urea was purchased from Sigma Chemical Pvt. Ltd. with AR grade, well rotten FYM was purchased from a local farm at Tarnab, Peshawar, Pakistan, saprofil fertilizer was purchased from Organeco Pakistan PVT Ltd. Total N and organic matter from FYM and saprofil was determined in Laboratory at Agriculture Research Institute, Tarnab Peshawar.
Based on the percentages of N, doses of FYM and saprofil were arranged. The amendments were ground passed through 4 mm mesh sieve prior to use. Soil samples from each pot were taken on day 5, 15, 30 and day-45 and were exposed to extraction for pesticide residue analysis of CYP and CPP. All the treatments were maintained at 13% field capacity moisture regime and incubated for 48 h at room temperature (25 °C). The pots were arranged in randomized way. A total of 96 pots were included, each treatment had 12 pots for four times sampling with three replications. Soil samples were taken after exposure to above mentioned different treatments for 5, 15, 30 and 45 days. Subsequently, 10 g soil was analyzed for CYP and CPP residues in the laboratory for pesticides residues analysis at Agricultural Research Institute Tarnab Peshawar.
2.3. Extraction of pesticide residues from soil samples
Extraction of pesticide residues from soil was carried out using solid phase extraction QuEChERS (quick, easy, cheap, effective, rugged and safe) method (Lehotay et al., 2005). In brief, 10 g of each soil type were placed into a 50-mL centrifuge tube, and then 10 mL acetonitrile with 1% acetic acid was added. The samples were shaken vigorously for one min, and then 6 g MgSO4, 1.5 g NaCl, and one g sodium citrate tribasic dehydrate (Na3 citrate) were added. Each tube was directly shaken after adding the salt. They were shaken vigorously for one min and then centrifuged for five min at 4000 U min−1. A 1 mL aliquot of supernatant was transferred to a dispersive clean-up tube containing MgSO4, graphitized carbonblack (GCB), C18 and primary secondary amine (PSA). These tubes were shaken for 30 s and centrifuged for five min at 4000 U min−1.
The cleaned extract was analyzed on a GC-2014C (Shimadzu) containing a capillary column using a Ni63 electroncapture detector (ECD) for the detection of CYP and CPP. These paration of pesticides residues were performed in a 30 m × 0.25 mminternal diameter, 0.25 μm thickness film submerged ina 5% diphenyl, 95% methylpolysiloxane HP-5MS column. Nitrogen gas was used as a carrier at 9.6 psi pressure and 2 mL min−1 flow. The injector was used at a constant temperature of 280 °C, whereas the detector temperature was 300 °C. In addition, the initial temperature of the oven was 110 °C (for 3 min isothermal) to 275 °C (for 15 min isothermal). The injection volume was 1 μL.The GC method performance was estimated by evaluating some quality parameters, such as the recovery values,limits of quantification (LOQ) and limits of detection (LOD). The LOQ and LOD were evaluated according to the following equations described by Thompson (2015).
2.4. Pesticides and reagents
The reference standard of CYP and CPP (99% purity) was purchased from Sigma Chemicals Pvt. Ltd. The physical and chemical properties of CYP and CPP (California Department of Pesticide Regulation 2006) are listed in Table1. CYP and CPP were dissolved in acetone (0.5 mL) to attain a concentration of 50 mg kg−1. CYP and CPP at this concentration were added to soil. Acetonitrile (HPLC grade), anhydrous magnesium sulphate (99.5%), glacial acetic acid, anhydrous sodium acetate (99.5%), primary-secondary amine (PSA) and sodium chloride were obtained from Sigma-Aldrich (Germany). All chemicals used in the study were of analytical grade.
Table 1.
Average pesticides recoveries from peach fruit growing sites (mg kg−1).
| CYP | CPP | Propacholr | Carbofuran | Metalchlor | Endosulfan | Cyhalothrin | Difenaconazole | Acetamaprid |
|---|---|---|---|---|---|---|---|---|
| 31.98 | 28.91 | 12.7 | 6.77 | 18.66 | 8.89 | 22.25 | 10.66 | 26.36 |
CYP = Cypermethrin, CPP = Chlorpyrifos
2.5. Qa/Qc
For quality control and assurance field samples were accompanied by field blanks (4), transportation blanks (4) and laboratory blanks (6) that were subjected to the same spiking, extraction, and cleanup procedures as field samples. Pesticides were not detected in any type of blanks, indicating no contamination during transport and analysis. Instrument stability was checked and at least three replicates of soils samples were analyzed on three consecutive days and relative standard deviation (RSD) ranged from 8% for CYP to 13% for CPP. Dynamic range for linearity ranged from quantitation limit (IQL) (typically 0.5 pg µL−1) to 200 ng µL−1, resulting in six-point calibration variance (R2) of at least 0.99. Instrument detection limits (IDL) and instrument quantification limits (IQL) were established when the response for characteristic ions exceeded the background noise by ratios of 3:1 and 10:1, respectively. Analytical method detection limits (MDLs) were obtained from seven replicate analyses of all samples spiked with recovery standards. In order to ensure the optimum recovery of the compounds of interest at given analytical conditions, soil samples were spiked at various concentration levels, the spiking concentration was kept as those of the analytes’ concentration in pure solvent. Both the pesticide mixtures in pure solvent as well as the spiked samples at the same levels were analyzed simultaneously.
2.6. Statistical analysis
In this study, data were analyzed through Microsoft Excel 2017 and Statistix 8.1. One-way ANOVA test was performed to compare different treatments. We performed the one-way ANOVA test by post hoc test using Tukey HSD test (Abdi and Williams 2010) to make multiple comparisons between averages of different treatments.
3. Results
3.1. Physio-chemical properties of soil
Results of the physico-chemical characteristics of experimental soil showed that pH of soil was 7.4 having 5.2% CaCO3 and moderately calcareous having 1.12% organic matter indicating that the organic matter was marginal and having 0.056 m mol L-1 electrical conductivity revealed that soil was non saline. The phosphorus concentration was 6.2 mg kg−1 while K was 122 mg kg−1. Total nitrogen concentration was 0.048 mg kg−1 indicating the deficiency of nitrogen.
The levels and identity of pesticide residues found in analysed samples are presented in Table 1. Nine pesticides i.e. CYP, CPP, Propachlor, Carbofuran, Metachlore, Endosulfan, Cyhalothrine, Difenaconazole and Acetamaprid were found at levels between 6.77 µg kg−1 to 31.98 µg kg−1. Different concentration of pesticides in peach orchards soil in District Swat show the use of pesticides applied for control of insect pest. The correlation coefficient matrix for the pesticide residues and soil physico-chemical properties is presented in Table 2. The data showed a positive correlation of CYP, CPP and endosulfan with organic matter which are 0.38, 0.25 and 0.27 respectively. Soil pH was significantly correlated with metachlore and endosulfan.
Table 2.
Correlation between soil physico-chemical properties and pesticides residues.
| Physico-chemical properties/pesticides | pH | EC | MC (%) | OM (%) | CEC |
|---|---|---|---|---|---|
| CYP | 0.168 | 0.116 | −0.133 | 0.38 | 0.014 |
| CPP | 0.135 | 0.055 | −0.106 | 0.25 | 0.209 |
Organic matter (OM), Cation exchange capacity (CEC), Moisture Content (MC), Electrical Conductivity (EC)
3.2. Concentration of CPP and CYP
The CPP was found in all peach orchards soil which may attribute to frequent use by farmers on their orchards. The CPP used on peach orchards by farmers when plants had no leaves, dropped from trees on surface of soil and accumulated in the soils which have persistence property in soil.
3.3. Organic-inorganic amendments for the degradation of pesticide residues
3.3.1. Degradation of CYP
The degradation of CYP in soil was assessed at three different sources of fertilizers amendment (Urea, farm yard manure and saprofil) and their combinations under controlled environmental conditions. The residues of CYP were evaluated from 5th day to 45thday in all the treatments (Table 3).
Table 3.
Effect of organic and inorganic amendments to reduce residual CYP and CPP levels in soil CPP.
| Days/ Treatments | 5 | 15 | 30 | 45 | Mean |
|---|---|---|---|---|---|
| CYP | |||||
| Control | 44.28 ± 1.3a | 36.12 ± 2.8a | 26.24 ± 1.2a | 16.24 ± 2.4a | 30.72 |
| Urea | 39.18 ± 3.2b | 32.48 ± 1.6bc | 24.48 ± 1.6ab | 12.62 ± 1.2b | 27.19 |
| FYM | 42.48 ± 2.8ab | 30.68 ± 1.4c | 16.68 ± 2.2e | 8.46 ± 2.4c | 24.58 |
| Saporfil | 40.24 ± 1.6ab | 34.18 ± 2.6ab | 22.46 ± 1.8bc | 10.28 ± 2.8bc | 26.79 |
| urea*FYM | 40.26 ± 4.8ab | 32.31 ± 2.8bc | 21.49 ± 3.2c | 9.75 ± 1.4bc | 25.95 |
| Urea* Saporfil | 39.18 ± 3.6b | 31.26 ± 1.6bc | 22.24 ± 1.8bc | 10.68 ± 2.8bc | 25.84 |
| FYM* Saporfil | 40.28 ± 2.8ab | 32.14 ± 1.4bc | 20.42 ± 1.2 cd | 12.24 ± 1.6b | 26.27 |
| Urea*FYM*Saporfil | 39.12 ± 1.8b | 29.55 ± 3.6c | 18.37 ± 3.6e | 10.25 ± 3.6bc | 24.88 |
| Control | 45.42 ± 1.24a | 38.62 ± 1.22a | 30.46 ± 1.82a | 22.33 ± 1.22a | 34.21 |
| Urea | 41.62 ± 2.32b | 34.48 ± 2.41b | 24.68 ± 2.60b | 18.68 ± 2.42b | 29.87 |
| FYM | 42.86 ± 1.36ab | 32.68 ± 2.21b | 20.21 ± 2.58c | 12.32 ± 1.60d | 27.00 |
| Saprofil | 40.24 ± 1.60b | 34.64 ± 1.80b | 22.62 ± 2.80bc | 16.48 ± 2.62bc | 28.50 |
| urea*FYM | 42.56 ± 2.81ab | 32.28 ± 2.62b | 21.32 ± 2.20c | 15.62 ± 2.82c | 27.95 |
| Urea*Saprofil | 39.16 ± 2.40b | 33.42 ± 1.42b | 24.62 ± 2.78b | 16.82 ± 2.38bc | 28.51 |
| FYM*saprofil | 42.24 ± 1.62b | 32.16 ± 2.60b | 22.68 ± 3.22bc | 14.24 ± 2.40 cd | 27.83 |
| Urea*FYM*Saprofil | 41.36 ± 1.80b | 32.24 ± 1.18b | 21.07 ± 1.60c | 12.68 ± 3.62d | 26.84 |
Signifcant diferences were tested according to Tukey’s HSD test at p < 0.05 using Statistics 8.1. Within column, values followed by the similar small English letter (s) are not signifcantly diferent at p < 0.05. Cypermethrin(CYP), Chlorpyrifos (CPP), Farmyard Manure (FYM)
After 5 days, treatments (Urea, Urea*Saprofil and Urea*FYM*Saprofil) significantly reduced concentration of CYP as compared to other treatments. After 15 days, effect of different treatments on reduction of CYP in soil showed that Urea, Urea*saprofil and Urea*FYM*saprofil treatments significantly reduced residual level of CYP in soil as compared to control. The treatments (FYM, Saprofil, Urea*FYM showed non-significant results to reduce level of CYP in soil. However after 30 days of CYP application, maximum reduction was recorded in soil amended with FYM as compared to the other treatments. The addition of FYM at initially promotes process of immobilization process which affect microbial activities in soil and CYP degradation. Similarly, at 45th day all tested treatments significantly reduced CYP concentration in all soils except the soil amended with urea at 30th day after treatment. The presents study suggests that organic amendment certainly enhanced degradation of soil applied CYP. These results have implications in managing the persistent residues of CYP in soil.
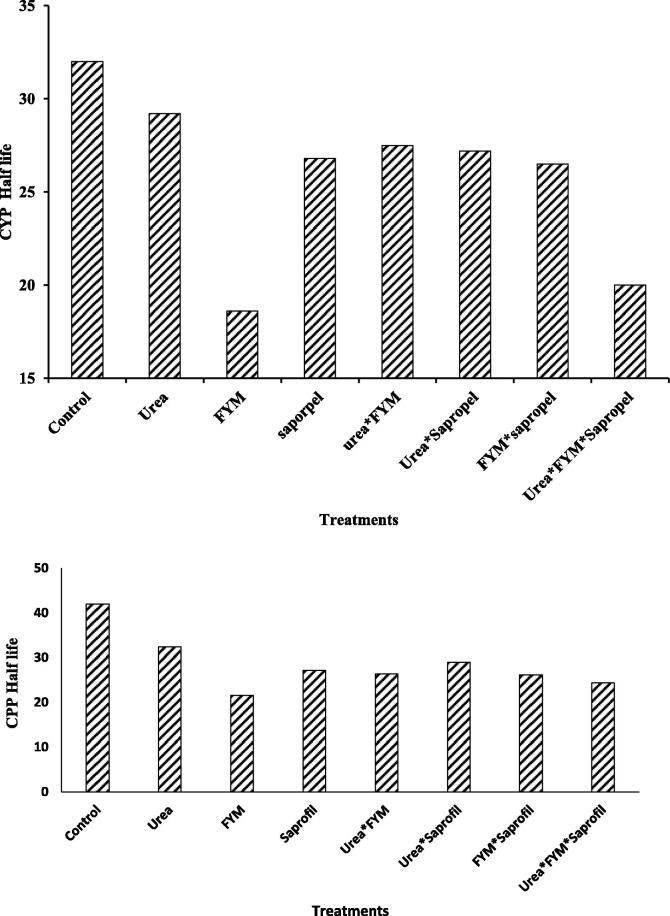
The CYP residue data was statistically interpreted for computation of regression equation and half-life values. Half-life values in different treatments were derived by applying first order kinetics to residual CYP and CPP data (Fig. 1; Table 4). The degradation of the pestiides followed the first order quadratic equations for CYP (r2 > 0.995) for all the treatments (Fig. 2). The degradation of CYP followed by the first order quadratic equation in all treatments with r2 values of 0.9997 (Control), 0.9992 (urea), 0.9953 (FYM), 0.9974 (Saprofil), urea*FYM 0.9977, Urea*FYM 0.9998, FYM*Saprofil 0.9945 and Urea*FYM*Saprofil 0.9977.
Fig. 1.
Percent reduction of CYP in soil amended with different organic and inorganic sources.
Table 4.
Regression equations and half-life correlation coefficient for CYP.
| Days/ Treatments | Y | Half Life | R2 |
|---|---|---|---|
| ontrol | −0.46x2 − 7.1x + 51.92 | 32 | 0.9997 |
| Urea | −1.29x2 − 2.318x + 42.66 | 29.2 | 0.9992 |
| FYM | 0.895x2 − 16.081x + 58.065 | 18.6 | 0.9953 |
| Saporfil | −1.53x2 − 2.51x + 44.54 | 26.8 | 0.9974 |
| Urea*FYM | 0.3617x2 − 11.586x + 50.578 | 27.5 | 0.9977 |
| Urea*Saprofil | −0.91x2 − 4.902x + 44.92 | 27.2 | 0.9998 |
| FYM*saprofil | −0.01x2 − 9.534x + 50.18 | 26.5 | 0.9945 |
| Urea*FYM*Saprofil | 0.3617x2 − 11.586x + 50.578 | 20 | 0.9977 |
Cypermethrin(CYP), Farmyard Manure (FYM)
Fig. 2.
Half life of Cypermethrin in soil amended with different organic and inorganic sources.
It is clear from the results that reduction in residual level of CYP was significantly more in amended soil as compared to control. The data presented in Table 4 revealed that, control treatment showed maximum half-life of CYP at 32nd day as compared to other treatments. Minimum half-life was recorded for soil amended with FYM with 19 days followed by combination of treatment (Urea*FYM*Saprofil) with 20 days.
3.3.2. Degradation of CPP
The same process was adopted to assess the degradation of CPP in soil at three different sources of fertilizers amendment (Urea, FYM and saprofil) and their combinations under controlled environmental conditions. The concentration of CPP in soil at different treatments was measured to determine ability of organic amendments to decrease concentration of CPP. Significant reduction in concentration of CPP was observed by treatments (Urea, Urea*Saprofil and Urea*FYM*saprofil) after 5 days as compared to other treatments. Concentration of CPP significantly reduced residual level of CPP in soil after 15 day the effect of different treatments Urea, Urea* saprofil and Urea*FYM*saprofil treatments as compared to control. While combination of treatments (FYM, saprofil, Urea*FYM showed non-significant results. However, soil amended with FYM reduced maximum concentration as compared to rest of treatments after 30 days.
The first order quadratic equation with r2values for the degradation of CPP in the all treatments were observed as 0.9982 (Control), 0.9923 (urea), 0.9933 (FYM), 0.9785 (saprofil), urea*FYM 0.9836, Urea*FYM 0.9935, FYM*saprofil 0.9948 and Urea*FYM*Saprofil 0.9973 (Table 3). The findings reveal that amended soil reduce more residues of CP as compared to control. The data presented in Table 5 revealed that, control treatment showed maximum half-life of CP at day 32. Minimum half-life was recorded for soil amended with FYM 18.6 days followed by combination of treatment (Urea*FYM*saprofil) with 20 days. From half-life data, it is clear that farmyard manure is most suitable for degradation of CPP as compared to other organic treatments which showed somewhat longer half-life.
Table 5.
Regression equations and half-life correlation coefficient for CPP.
| Treatments | Y | Half life | R2 |
|---|---|---|---|
| Control | −7.744x + 53.567 | 42 | 0.9982 |
| Urea | −7.862x + 49.52 | 32.4 | 0.9923 |
| FYM | −10.409x + 53.04 | 21.6 | 0.9933 |
| Saprofil | −8.33x + 49.32 | 27.2 | 0.9785 |
| urea*FYM | −9.178x + 50.89 | 26.4 | 0.9836 |
| Urea*Saprofil | −7.582x + 47.46 | 29 | 0.9935 |
| FYM*saprofil | −9.348x + 51.2 | 26.2 | 0.9984 |
| Urea*FYM*Saprofil | −9.7207x + 51.14 | 24.4 | 0.9973 |
Chlorpyrifos (CPP), Farmyard Manure (FYM)
4. Discussion
Pesticides bound to soil organic matter after application. This retention of pesticide residues in soil governed by various factors such as composition of organic matter, soil texture, and land use patterns (Sultana et al. 2014). As soil pH increases , metachlore rapidly metabolizes with a half-life of 2 days in alkaline aerobic conditions and 3 days in alkaline anaerobic conditions (Tomlin, 2000). There was no significant relationship between CYP and CPP with soil pH that leads to low degradation of pesticides. The CEC of soil showed a positive correlation with CPP, and a negative correlation was observed with CYP. Mineralization of maleamide semialdehyde (product of CPP degradation) yields water, CO2 and ammonium ion. Positive coreraltion between CPP and CEC might be due to adsorption of ammonium ions on clay particles (Tejoprakash & Khanna, 2010). The weak correlation between soil properties and pesticides suggest that multiple factors including land use, clay type, and organic matter composition contribute towards retention of pesticides in soil. The results were found to consistent with Mishra et al. (2014). Tahir et al. (2011) reported that CPP ranged between 0.03 and 2.46 mg kg−1 for different fruits orchards soil at Nawab Shah District Sindh. Latif, (2011) found concentration level 0.05–0.96 mg kg−1 at Hyderabad, Sindh. Rafique (2016) reported residues of CPP range 0.18–1.99 mg kg−1 from Okara District, Punjab. The residual level of CYP was in range of 0.024–4.12 mg kg−1 with a mean value of 1.764 mg kg−1 for peach orchard soil (Table 3). Highcontent of CYP in soil might be due to extensive application of CYPfor pest control. Presence of CYP residues in fruit contaminated soil also reported 14.4–44.6 mg kg−1 for Swat valley (Nafees et al. 2009). Anwar et al. (2011) investigated residues (0.031 mg kg−1) in peach fruits in Nawab Shah, Sindh, and Shah et al. (2011) reported concentration of CYP (1.86 mg kg−1) in alkaline soils. Various organic and inorganic amendments were applied to biodegrade different group’s pesticides for various fruits crops (Mohapatra and Phale, 2021, Aioub et al., 2019, Mukherjee, 2009). Different studies showed that combined application of organic and inorganic amendments were effective to reduce residual effect of CYP. Using different treatments, variations in concentration of CYP in soil were measured to determine ability of organic sources to reduce CYP level from soil (Rafique et al. 2016). Results demonstrated that soil amended with FYM was best organic source to reduce residual level of CYP (Farooq et al. 2019). Although, addition of organic manures has been an integral part of sustainable agriculture practices; present findings give a new dimension of its utilization for removal of persistent pesticides (Kadian et al. 2008). Study conducted by Mukherjee, (2009) on biological degradation showed that 20%–45% pesticides in soil were reduced in forage treatment compared with control. Half-life (t ½) is the time required for any pesticide to undergo degradation to half of its initial concentration were determined by first-order kinetic model (Lartiges and Garrigues, 1995). Present results validate the general observation that CYP degradation in soil is often considered as first order quadratic equations (Chen et al. 2012). From half-life data, it is clear that FYM is most suitable for degradation of CYP as compared to other organic treatments which showed somewhat longer half-life (Rani and Dhania 2014). The addition of FYM initially promotes process of immobilization which affects microbial activities in soil and degradation of CPP (Briceño et al. 2007). Significant reduction of CPP concentration in soil was observed after 45 days of all tested treatments except soil amended with urea. Various organic and inorganic amendments were successfully applied to biodegrade different groups (organochlorine and organophosphate) pesticides in soil (Mohapatra and Phale, 2021, Aioub et al., 2019, Mukherjee, 2009). Combined application of organic and inorganic amendments were remarkably useful to decrease residual consequence of CPP. Our findings verified that soil amended with FYM was finest organic resource to diminish residual level of CPP. The current results of our investigated study are a supporting baseline for utilization of organic amendments for removal of persistent pesticides (Kadian et al. 2008). Mukherjee, (2009) showed that 20%–45% pesticides in soil were reduced in forage treatment compared with control.
5. Conclusion
Based on the present investigations, it can be concluded that addition of inorganic (urea) and organic amendments (Saprofil and FYM) alone and in combinations have potential to accelerate the degradation of CYP and CPP. However, FYM is the most effective organic amendment for the biodegradation of CYP and CPP. The degradation of CYP and CPP increases with the increasing in time interval. The higher biodegradation of CYP and CPP with the application of FYM specify that the soil microorganisms have better capability to degrade pesticides residue in orchard soil. It is further concluded that application of FYM to orchard soils is an eco-friendly approach to improve soil conditions and minimize the residual level of insecticide from soil environment.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
References
- Ahmad M., Arif M.I. Resistance of beet armyworm Spodoptera exigua (Lepidoptera: Noctuidae) to endosulfan, organophosphorus and pyrethroid insecticides in Pakistan. Crop Prot. 2010;29(12):1428–1433. [Google Scholar]
- Aioub A.A., Li Y., Qie X., Zhang X., Hu Z. Reduction of soil contamination by cypermethrin residues using phytoremediation with Plantago major and some surfactants. Environ. Sci. Eur. 2019;31(1):1–12. [Google Scholar]
- Rafique N., Tariq S.R., Ahmed D. Monitoring and distribution patterns of pesticide residues in soil from cotton/wheat fields of Pakistan. Environ. Monit. Assess. 2016;188(12):695. doi: 10.1007/s10661-016-5668-6. [DOI] [PubMed] [Google Scholar]
- Chen S., Luo J., Hu M., Lai K., Geng P., Huang H. Enhancement of cypermethrin degradation by a coculture of Bacillus cereus ZH-3 and Streptomyces aureus HP-S-01. Bioresour. Technol. 2012;110:97–104. doi: 10.1016/j.biortech.2012.01.106. [DOI] [PubMed] [Google Scholar]
- Rani K., Dhania G. Bioremediation and biodegradation of pesticide from contaminated soil and water—a noval approach. Int. J. Curr. Microbiol. App. Sci. 2014;3(10):23–33. [Google Scholar]
- Briceño G., Palma G., Durán N. Influence of organic amendment on the biodegradation and movement of pesticides. Critical Rev. Environ. sci. Technol. 2007;37(3):233–271. [Google Scholar]
- Farooq M.A., Arif M.J., Gogi M.D., Atta B., Nawaz A. Impact of different integrated pest management modules on pest infestation, pesticide residue and yield in mango fruits. J. Innovative Sci. 2019;5(2):72–82. [Google Scholar]
- Lartiges S.B., Garrigues P.P. Degradation kinetics of organophosphorus and organonitrogen pesticides in different waters under various environmental conditions. Environ. Sci. Technol. 1995;29(5):1246–1254. doi: 10.1021/es00005a016. [DOI] [PubMed] [Google Scholar]
- Shah M.M., Hassan A., Mahmood Q., Akbar K., Khan M.S. Assessment of pesticide residues on selected vegetables of Pakistan. J. Chemical Society of Pak. 2011;33(6):816. [Google Scholar]
- Anwar T.A.H.I.R., Ahmad I., Tahir S.E.E.M.A. Determination of pesticide residues in fruits of Nawabshah district, Sindh Pakistan. Pak. J. Bot. 2011;43(2):1133–1139. [Google Scholar]
- Tejada M., Gómez I., del Toro M. Use of organic amendments as a bioremediation strategy to reduce the bioavailability of chlorpyrifos insecticide in soils. Effects on soil biology. Ecotoxicol. Environ. Saf. 2011;74(7):2075–2081. doi: 10.1016/j.ecoenv.2011.07.005. [DOI] [PubMed] [Google Scholar]
- Thompson C.J. Princeton University Press; 2015. Mathematical statistical mechanics. [Google Scholar]
- Liu Y., Lonappan L., Brar S.K., Yang S. Impact of biochar amendment in agricultural soils on the sorption, desorption, and degradation of pesticides: a review. Sci. Total Environ. 2018;645:60–70. doi: 10.1016/j.scitotenv.2018.07.099. [DOI] [PubMed] [Google Scholar]
- Latif Y., Sherazi S.T.H., Bhanger M.I. Assessment of pesticide residues in commonly used vegetables in Hyderabad Pakistan. Ecotoxicol. Environ. Saf. 2011;74(8):2299–2303. doi: 10.1016/j.ecoenv.2011.07.030. [DOI] [PubMed] [Google Scholar]
- Lasat, M. M. (2000). The use of plants for the removal of toxic metals from contaminated soils. US Environmental Protection Agency.
- California Department of Pesticide Regulation, 2006. Pesticide use reporting 432 (PRU). CDPR, Sacramento, CA. http://www.cdpr.ca.gov/docs/pur. htm. Accessed 09 Jan 2006.
- Odukkathil G., Vasudevan N. Toxicity and bioremediation of pesticides in agricultural soil. Rev. Environ. Sci. Bio/Technol. 2013;12(4):421–444. [Google Scholar]
- Tang J., Zhu W., Kookana R., Katayama A. Characteristics of biochar and its application in remediation of contaminated soil. J. Biosci. Bioeng. 2013;116(6):653–659. doi: 10.1016/j.jbiosc.2013.05.035. [DOI] [PubMed] [Google Scholar]
- Popp J., Pető K., Nagy J. Pesticide productivity and food security. A review. Agronomy for sustainable development. 2013;33(1):243–255. [Google Scholar]
- Mishra N., Mahapatra P., Mohanty S., Pradhan M. Effect of soil amelioration, inorganic, organic and bio-fertilizer application on yield, quality and economics of snow pea (Pisum sativum L. var. macrocarpon) J. Crop Weed. 2014;10(1):48–52. [Google Scholar]
- Mukherjee I. Effect of organic amendments on degradation of atrazine. Bulletin of Environ. Contam. Toxicol. 2009;83(6):832–835. doi: 10.1007/s00128-009-9849-7. [DOI] [PubMed] [Google Scholar]
- Nafees M., Jan M.R. Residues of cypermethrin and endosulfan in soils of Swat valley. Soil Environ. 2009;28(11):113–118. [Google Scholar]
- Niti C., Sunita S., Kamlesh K., Rakesh K. Bioremediation: An emerging technology for remediation of pesticides. Res. J. Chem. Environ. 2013;17:4. [Google Scholar]
- Abdi H, Williams LJ (2010) Newman-Keuls Test and Tukey Test. Encyclopedia of Research Design. Thousand Oaks CA: Sage 1-5.
- Basak B.B., Saha A., Gajbhiye N.A., Manivel P. Potential of Organic Nutrient Sources for Improving Yield and Bioactive Principle of Ashwagandha (Withania Somnifera) through Enhanced Soil Fertility and Biological Functions. Commun. Soil Sci. Plant Anal. 2020;51(6):779–793. doi: 10.1080/00103624.2020.1729368. [DOI] [Google Scholar]
- Brtnicky M., Dokulilova T., Holatko J., Pecina V., Kintl A., Latal O., Vyhnanek T., Prichystalova J., Datta R. Long-term effects of biochar-based organic amendments on soil microbial parameters. Agronomy. 2019;9(11):747. [Google Scholar]
- Caban J.R., Kuppusamy S., Kim J.H., Yoon Y.-E., Kim S.Y., Lee Y.B. Green manure amendment enhances microbial activity and diversity in antibiotic-contaminated soil. Appl. Soil Ecol. 2018;129:72–76. [Google Scholar]
- Coppola L., Castillo M.d.P., Monaci E., Vischetti C. Adaptation of the biobed composition for chlorpyrifos degradation to southern Europe conditions. J. Agri. Food Chem. 2007;55(2):396–401. doi: 10.1021/jf062744n. [DOI] [PubMed] [Google Scholar]
- Kadian N., Gupta A., Satya S., Mehta R.K., Malik A. Biodegradation of herbicide (atrazine) in contaminated soil using various bioprocessed materials. Bioresour. Technol. 2008;99(11):4642–4647. doi: 10.1016/j.biortech.2007.06.064. [DOI] [PubMed] [Google Scholar]
- Kadian N., Malik A., Satya S., Dureja P. Effect of organic amendments on microbial activity in chlorpyrifos contaminated soil. J. Environ. Manage. 2012;95:S199–S202. doi: 10.1016/j.jenvman.2010.10.023. [DOI] [PubMed] [Google Scholar]
- Lehotay S.J., de Kok A., Hiemstra M., Van Bodegraven P. Validation of a fast and easy method for the determination of residues from 229pesticides in fruits and vegetables using gas and liquid chromatography and mass spectrometric detection. J AOAC Int. 2005;88:595–614. [PubMed] [Google Scholar]
- Marín-Benito J., Brown C., Herrero-Hernández E., Arienzo M., Sánchez-Martín M., Rodríguez-Cruz M. Use of raw or incubated organic wastes as amendments in reducing pesticide leaching through soil columns. Sci. Total Environ. 2013;463:589–599. doi: 10.1016/j.scitotenv.2013.06.051. [DOI] [PubMed] [Google Scholar]
- Meng L., Li W., Zhang S., Wu C., Lv L. Feasibility of co-composting of sewage sludge, spent mushroom substrate and wheat straw. Bioresour. Technol. 2017;226:39–45. doi: 10.1016/j.biortech.2016.11.054. [DOI] [PubMed] [Google Scholar]
- Mohapatra B., Phale P.S. Microbial degradation of naphthalene and substituted naphthalenes: Metabolic diversity and genomic insight for bioremediation. Front. Bioeng. Biotechnol. 2021;9:144. doi: 10.3389/fbioe.2021.602445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponni C., Arumugam S. Effect of certain organic manures and biostimulants on growth and yield of Phyllanthus niruri. Asian J. Horticulture. 2007;2(2):148–150. [Google Scholar]
- Romyen S., Luepromchai E., Hawker D., Karnchanasest B. Potential of agricultural by-product in reducing chlorpyrifos leaching through soil. JApSc. 2007;7(18):2686–2690. [Google Scholar]
- Singh R.J., Ghosh B.N., Sharma N.K., Patra S., Dadhwal K.S., Meena V.S., Deshwal J.S., Mishra P.K. Effect of seven years of nutrient supplementation through organic and inorganic sources on productivity, soil and water conservation, and soil fertility changes of maize-wheat rotation in north-western Indian Himalayas. Agric. Ecosyst. Environ. 2017;249:177–186. doi: 10.1016/j.agee.2017.08.024. [DOI] [Google Scholar]
- Sultana J., Syed J.H., Mahmood A., Ali U., Rehman M.Y.A., Malik R.N.…Zhang G. Investigation of organochlorine pesticides from the Indus Basin, Pakistan: Sources, air–soil exchange fluxes and risk assessment. Sci. Total Environ. 2014;497:113–122. doi: 10.1016/j.scitotenv.2014.07.066. [DOI] [PubMed] [Google Scholar]
- Tejoprakash N., Khanna S.G. Molecular Approaches for Chlorpyrifos Degradation (Doctoral dissertation) 2010 [Google Scholar]
- Tejada M., Rodríguez-Morgado B., Gómez I., Parrado J. Degradation of chlorpyrifos using different biostimulants/biofertilizers: effects on soil biochemical properties and microbial community. Appl. Soil Ecol. 2014;84:158–165. [Google Scholar]
- Vischetti C., Capri E., Trevisan M., Casucci C., Perucci P. Biomassbed: a biological system to reduce pesticide point contamination at farm level. Chemosphere. 2004;55(6):823–828. doi: 10.1016/j.chemosphere.2003.11.042. [DOI] [PubMed] [Google Scholar]
- Wang, F., Bian, Y.-R., Jiang, X., Gao, H.-J., Yu, G.-F., & Deng, J.-C. (2006). Residual Characteristics of Organochlorine Pesticides in Lou Soils with Different Fertilization Modes1 1Project supported by the National Natural Science Funds for Distinguished Young Scholar (No. 40325001), the National Key Basic Research and Development Program of China (No. 2002CB410805), and the Natural Science Foundation of Jiangsu Province (No. BK2005220). Pedosphere, 16(2), 161-168. https://doi.org/https://doi.org/10.1016/S1002-0160(06)60039-8
- Wenjun X., Jianmin Z., Xiaoqin C., Huoyan W. Effect of Long-Term Fertilization on the Persistence of Cypermethrin in Soil. Better Crops. 2007;91(4):10–11. [Google Scholar]
- Xie W., Zhou J. Cypermethrin persistence and soil properties as affected by long-term fertilizer management. Acta Agriculturae Scandinavica Section B-Soil and Plant Sci. 2008;58(4):314–321. [Google Scholar]
- Zhao L., Dong Y.-H., Wang H. Residues of organochlorine pesticides and polycyclic aromatic hydrocarbons in farm-raised livestock feeds and manures in Jiangsu, China. Sci. Total Environ. 2013;450:348–355. doi: 10.1016/j.scitotenv.2012.09.017. [DOI] [PubMed] [Google Scholar]
Further Reading
- Harris M.L., Wilson L.K., Elliott J.E., Bishop C.A., Tomlin A.D., Henning K.V. Transfer of DDT and metabolites from fruit orchard soils to American robins (Turdus migratorius) twenty years after agricultural use of DDT in Canada. Arch. Environ. Contam. Toxicol. 2000;39(2):205–220. doi: 10.1007/s002440010098. [DOI] [PubMed] [Google Scholar]
- Bhandari G., Zomer P., Atreya K., Mol H.G.J., Yang X., Geissen V. Pesticide residues in Nepalese vegetables and potential health risks. Environ. Res. 2019;172:511–521. doi: 10.1016/j.envres.2019.03.002. [DOI] [PubMed] [Google Scholar]
- Kumari D., John S. Health risk assessment of pesticide residues in fruits and vegetables from farms and markets of Western Indian Himalayan region. Chemosphere. 2019;224:162–167. doi: 10.1016/j.chemosphere.2019.02.091. [DOI] [PubMed] [Google Scholar]
- Li J., Wang P., Shi S., Xue J. Background biomonitoring of residue levels of 137 pesticides in the blood plasma of the general population in Beijing. Environ. Monit. Assess. 2018;190(5):315. doi: 10.1007/s10661-018-6694-3. [DOI] [PubMed] [Google Scholar]
- Müller M.H.B., Polder A., Brynildsrud O.B., Karimi M., Lie E., Manyilizu W.B., Mdegela R.H., Mokiti F., Murtadha M., Nonga H.E., Skaare J.U., Lyche J.L. Organochlorine pesticides (OCPs) and polychlorinated biphenyls (PCBs) in human breast milk and associated health risks to nursing infants in Northern Tanzania. Environ. Res. 2017;154:425–434. doi: 10.1016/j.envres.2017.01.031. [DOI] [PubMed] [Google Scholar]
- Ahmad M., Sultana S., Fazl-i-Hadi S., Ben Hadda T., Rashid S., Zafar M., Yaseen G. An Ethnobotanical study of Medicinal Plants in high mountainous region of Chail valley (District Swat-Pakistan) J. Ethnobiol. Ethnomed. 2014;10(1):1–18. doi: 10.1186/1746-4269-10-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankhla M.S., Kumari M., Sharma K., Kushwah R.S., Kumar R. Water contamination through pesticide & their toxic effect on human health. Int. J. Res. App. Sci. Eng. Technol. (IJRASET) 2018;6(1):967–970. [Google Scholar]
- Silva V., Mol H.G.J., Zomer P., Tienstra M., Ritsema C.J., Geissen V. Pesticide residues in European agricultural soils – A hidden reality unfolded. Sci. Total Environ. 2019;653:1532–1545. doi: 10.1016/j.scitotenv.2018.10.441. [DOI] [PubMed] [Google Scholar]
- Van Toan P., Sebesvari Z., Bläsing M., Rosendahl I., Renaud F.G. Pesticide management and their residues in sediments and surface and drinking water in the Mekong Delta. Vietnam Sci. the Total Environ. 2013;452:28–39. doi: 10.1016/j.scitotenv.2013.02.026. [DOI] [PubMed] [Google Scholar]