Abstract
Improvement in salinity tolerance of plants is of immense significance as salt stress particularly threatens the productivity of agricultural crops. This study was designed to assess the tolerance level of six Brassica napus varieties (Super, Sandal, Faisal, CON-111, AC Excel and Punjab) under different levels of salinity (0, 50, 100, 150 & 200 mM) with three replications under CRD. Salt induced osmotic stress curtailed the plant growth attributes, photosynthetic pigments and disturbed ionic homeostasis (K+, Na+, Ca2+, Cl-) but least disturbance as compared to control was found in Super and Sandal cultivars. Punjab canola and AC Excel canola cultivars were least tolerant to salinity because these displayed greater decline in all growth and biochemical attributes. Plants subjected to NaCl induced stress exhibited considerable decline in all attributes under study with proline as exception. Antioxidants (CAT, SOD & POD) showed an obvious change in Canola plants under stress, but greatest decline was displayed at 200 mM NaCl level in all six cultivars. Over all these attributes presented a comparatively stable trend in super and sandal cultivars. This shows presence of physiological resilience and metabolic capacity in these two cultivars to tackle salinity. Similarly, all yield attributes displayed adverse behavior under 150 mM & 200 mM salinity stress. Our results demonstrated that Super and Sandal cultivars of Brassica napus exhibit good performance in salinity tolerance and can be good option for cultivation in salt affected areas.
Keyword: Canola, NaCl induced salt stress, Proline, Flavonoids, Antioxidants, Yield
1. Introduction
Canola (Brassica napus) a member of family Brassicaceae, is valued as food and fodder crop across the world cultivated on about 42.2 m hectors area (Rahman et al., 2016) In provision of foodstuff, canola family generally quoted at level 3rd after cereals and pulses. Seeds are crushed for abstraction of oil, meal residue obtained after the crushing is utilized as major animal forage resource. Canola is considered as an important crop because of its oil contents comprising small amount of erucic acid hence supplying healthier quality of cooking oil, but its entire production is reduced due to the presence of several stresses in addition to salt stress (Belouchrani et al., 2021, Schillinger and Paulitz, 2018). Oil seed crops specifically grown for oil are supposed to be tolerant to some extent at various levels of salinity. Rape seed varieties reveals differential capability to sustain their metabolic activities under saline conditions, but germination and certain stages of growth are more susceptible to different concentrations of salinity (Schillinger and Paulitz, 2018, Rasel et al., 2020).
Salinity stress is one of the main restrictions to crop harvest in arid and semiarid regions of the world. As it is growing about 10% annually, it is assumed that 50% arable land will be wasted due to salinity up to the mid of 21st century (Akhter et al., 2021a, Al-Dakheel and Hussain, 2016, Moghadam et al., 2020, Sabagh et al., 2019, Ijaz et al., 2021). Presently, substantial agricultural production is disturbed by elevated soil salinity worldwide (Bybordi et al., 2010, Moghadam et al., 2020, Zhang et al., 2001). Over 50% of standard yield of different crops is considerably reduced in infertile and partially fertile lands exhibiting salt stress (Bray, 2000). Salinity can cause the reduction in crop yield by means of disturbing nutritional and water equilibrium of the plants (Belouchrani et al., 2021, Khan et al., 2010). Salt tolerance in plants is a complex phenomenon, and this is centered on multiple associated factors including on morphological, physiological and biochemical processes. Na+ and Cl+ ions toxicity generates high osmotic potential that results in insufficient supply of water and nutrients to plant roots (Aqeel et al., 2021, Bybordi et al., 2010, Sharif et al., 2018). The ultimate general antagonistic effects of salt stress on the Brassica napus are the reduction in plant height, size, and yield, osmotic stress and all of these collectively compromise plant growth, development and survival (Kumar, 1995, Moghadam et al., 2020, Naveed et al., 2020, Noman et al., 2018a), uptake, and homeostasis in plant body (Hasanuzzaman et al., 2018, Manivannan et al., 2016, Wu et al., 2019). Mineral imbalances such as increment of sodium ions up to toxic levels may cause a disturbance in normal metabolic processes of plant body. Such imbalances result in increased production of Reactive Oxygen Species (ROS). In response to ROS production, plant switch on its various defense mechanism like the production of antioxidant both enzymatic and non-enzymatic (Apel and Hirt, 2004, Mahmood et al., 2016, Naveed et al., 2020, Noman et al., 2018b, Shehzad et al., 2021). Salt stress caused enhancement in activities of ROS scavenging enzymes i.e. peroxidase in plants and leads to higher tissue lignification which in turn confines plant growth (Akhter et al., 2021a, Sabagh et al., 2019, Sharif et al., 2018).
Due to continuous increase in salt affected areas across the globe, it is imperative to adopt strategies for extending canola cultivation in salt affected areas both by conventional breeding as well as development of genetically engineered plants (Sabagh et al., 2019, Zhang et al., 2001, Ilyas et al., 2020). Therefore, we hypothesized that newly established varieties of canola may have different tolerance levels against various salinity regimes. Objective of our study was to evaluate physiological and biochemical modulations in five Canola varieties in relation to growth under different levels of salinity. Obvious and differential changes in physio-biochemical responses were noticed that helped us to categorized salt tolerant and sensitive canola varieties.
2. Materials and methods
2.1. Experimental layout
A pot experiment was executed in experimental area of Government College Women University Faisalabad, to evaluate the effect of NaCl stress on six varieties of canola during 2019–2020. Stone free, clean soil was fertilized to ensure healthy growth of seeds and seedlings. Seeds of six canola varieties/ accessions namely Super canola, Sandal canola, CON-111, Punjab canola, Faisal canola and AC Excel were obtained from Ayyub Agriculture Research Institute (AARI) Faisalabad. Obtained seeds were first sterilized by distilled water for approximately 10 min and then treated with 10% hypochlorite solution for 11–15 min. After proper sterilization seeds were dried with the help of filter paper. Pots were arranged as control and according to salinity levels. Seeds were sown in fifty-eight plastic pots each containing 3Kg of soil. Already fertilized soil pots were watered in alternative days. Seeds of all six varieties were subjected to 5 NaCl stress levels (0, 50, 100,150 & 200 mM). The experiment was designed in CRD with three replications. The NaCl stress was applied after two weeks of germination and retained in uninterrupted periods till crop maturity.
2.2. Plant sampling
Three plants from each pot were selected for recording morphological attributes, Root/ Shoot length, Root/Shoot fresh and dry weights, No. of leaves, leaf length and width and Plant height. The length (cm) of root, shoot, leaves (length & width) and plant height was measured by using meter rod, No. of leaves were counted manually whilst shoot/root fresh and dry weights (g) and seeds weight (g) were determined with the help of rechargeable balance.
2.3. Plant pigments contents
Chlorophyll analysis was done on fresh leaves. The method for estimation of pigments was used that of Arnon (1949). Fresh leaf sample (500 mg) was grinded in Ten mL of 80% acetone and chlorophyll was extracted. The absorbance of the extract was deciphered in a spectrophotometer at 645, 680 and 663 nm against 80% acetone blank. Chlorophyll determination formula
Chl.a (mg g-1 f.wt.) = [12.7 (OD663) − 2.69 (OD645)] × V/1000 × W
Chl.b (mg g-1 f.wt.) = [22.9 (OD645) − 4.68 (OD663)] × V/1000 × W
Total chl. (mg g-1 f.wt.) = [20.2(OD645) + 8.02 (OD663)] × V/1000 × W
Carotenoids (mg g-1 f.wt.) = [(OD480) + 0.114 (OD663) – 0.638(OD645)] / 2500
and some of these leaves were stored immediately at −20 °C to determine the enzymatic antioxidants activity. Yield parameters were measured at crop maturity.
2.4. Biochemical parameters
2.4.1. Enzymatic antioxidants activities
Antioxidant enzymes extraction was done by grinding 0.5 g fresh leaves in 5 mL amount of 50 mM chilled phosphate buffer (pH 7.8) in tissue grinder. The homogenate extract was centrifuged at 15000 rpm for 18 min at 4 °C. The extracted supernatant was treated for enzyme activities assays for catalase following the protocols developed by Britton and Maehly (1955). For catalase activity the reaction mixture was composed of 100uL of plant extract, 100 mm of Na phosphate buffer at pH of 7.0 and 30 mm of Hydrogen peroxide. The absorbance was taken at 240 nm of wavelength of spectrometer which was decreased with the degradation of hydrogen peroxide. The per unit activity was defined as the quantity of catalase required a 0.001 / min change in absorbance.
2.4.2. Peroxidase
For peroxidase (mg g-1FW) activity measure the reaction mixture comprising of 500 mL of plant extract, 100 mL of Na phosphate buffer at the pH of 7.0, 5 mM of 4-methylcatechols and 5 mm of hydrogen per oxide in a volume of 3 mL was put in spectro photometre and increase in absorbance was read at 420 nm wavelength as a result of oxidation of 4-methylcatechol by hydrogen peroxide. Per unity activity of the POD was defined as the 0.001 alteration in absorbance/min (Britton and Maehly, 1955).
2.4.3. SOD
The activity of SOD (mg g-1FW) was evaluated by assessing the nitroblue tetrazolium (NBT) photoreduction inhibition by super oxide dismutase enzyme. For the reaction the required chemicals were 50 mM Na2CO3, 50 mM Na Phosphate buffer with a pH of 7.6, 0.1 mM EDTA, 12 mM L- Methionine, 10uM ribo Flavin, 50uM NBT & 100 mL 0f plant extract .The final volume of reaction mixture was 3 mL. The reaction mixture was incubated at room temperature for fifteen minutes under the white light and afterwards the absorbance reading was recorded at the wavelength of 560nmThe amount of enzyme required to inhibit the photo-chemical reduction of NBT was defined as one unit of SOD activity
2.4.4. Proline
Proline (mg g-1FW) contents were calculated by homogenizing 0.25 g of garden-fresh leaf by taking 30% sulpho-sylicylic acid (5 mL) (Bates et al., 1973). The reaction mixture consisting of equal amounts of (2 mL) crude extract, ninhydrin reagent and glacial acetic was incubated in a water bath at 100 °C. After cooling with ice Toulene was added in 4 mL volume with vigorous shaking for 10–20 s. The absorbance was read at 520 nm wavelength of spectrophotometer. Standard curves were drawn to determine proline contents
2.4.5. Phenolics and flavonoids
For the estimation of phenolics fresh leaf sample (0.25 g) of canola plant and 80% acetone (2 mL) were grinded together for extraction sample. Then for centrifugation purpose this sample was added in falcon tubes having the capacity of 15 mL. All samples were centrifuged for 15 min then 100 µl sample extract was transferred in test tubes following the addition of 1 mL distilled water in each test tube. The sample was then subjected to the protocol devised by (Ribarova and Atanassova, 2005), The flavonoids content were calculated using the technique of AlCl3 colorimetric (Ribarova and Atanassova, 2005).
2.4.6. Mineral nutrients
Leaves of B.napus (0.1 g) were oven dried and mixed with 5 mL conc. H2SO4 in digestion flasks individually (Wolf, 1982). This mixture was kept for 12 h at room temperature. Digestion flasks were heated and 0.5 mL of 35% H2O2 was added at 100○C and heated (till 380○C) until disappearance of fumes. Then digested material was cooled down and H2O2 was added befors heating at 380○C again until the digestive solution became colorless. The volume of the digested material was maintained to 50 mL and, filtered before further use. The amount of potassium (K+), calcium (Ca2+) and sodium (Na+) ions were measured with flame photometer, whereas, chloride (Cl-) ions determined with a chloride meter (Model 926, Sherwood Scientific Ltd., Cambridge, UK) (Tavakkoli et al. 2010).
2.5. Statistical analysis
Recorded Data of two factors with CRD was analyzed statistically by running Co-stat software with 5% probability level to compare treatment means(Steel et al., 1997). Statistical program “R (v 4.0.1)” was used for estimating Pearson's correlation co-efficient and for principal component analysis among various measured variables (R Development Core Team, 2020).
3. Results
3.1. Morphological parameters
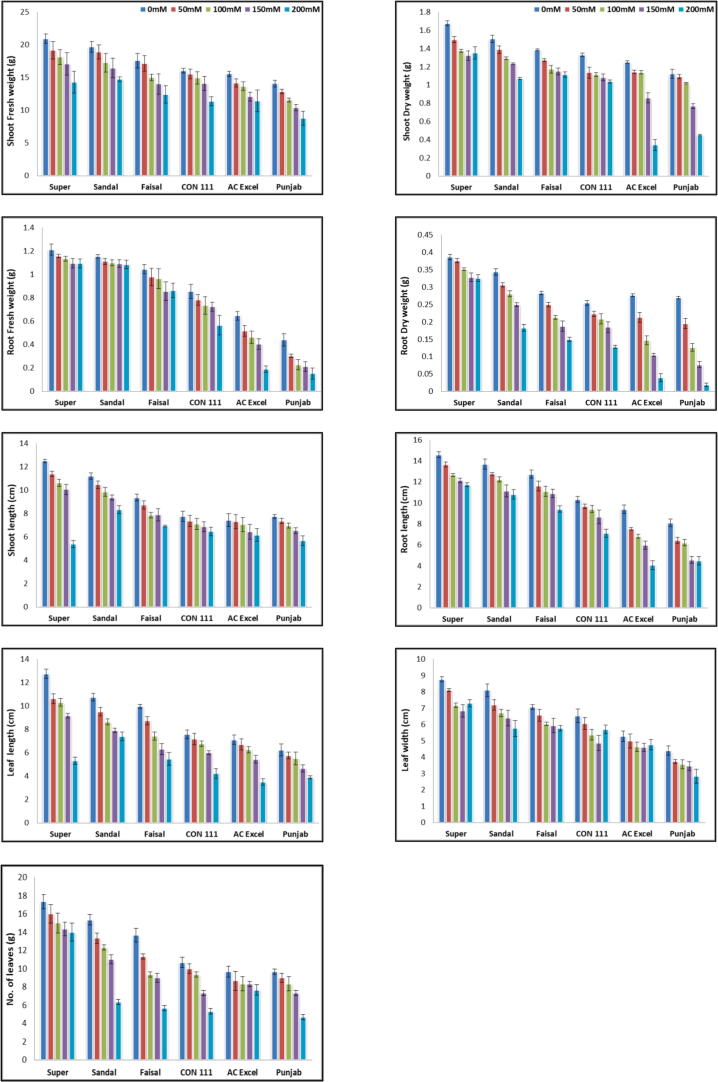
NaCl imposed salt stress critically affected all the six cultivars of Brassica napus i.e., Super canola, Sandal canola, CON-111, Punjab canola, Faisal canola and AC Excel. Salinity clearly reduced the) Shoot fresh weight, Shoot dry weight, Root Fresh weight, root dry weight, shoot / root length(cm), leave length & width (cm) and number of leaves(Fig. 1: a-i). The maximum reduction of 26% in root length was documented in AC Excel, Punjab. The Super and Sandal ecotypes behave as tolerant with minimum reduction of 18% in root length. Similar behavior was observed for the shoot length where the maximum decrease of 34% was recorded in AC Excel and Punjab while minimum reduction (27%) was observed in Super and Sandal. For root and shoot fresh weight 51% and 39% decrease was observed in Super and Sandal while the reduction %age for the AC Excel and Punjab remained 59% & 44% for RFW & SFW respectively. The RDW & SDW values also proved to be significantly different. The %age decrease for these attributes wer recorded to be 49% & 32% for the susceptible AC Excel and Punjab. The Decrease in value of RDW & SDW for Super and Sandal remained49% and 29%. The data recorded for Leaf length, leaf width and number of leaves (Fig. 1: i) revealed a significant (P ≤ 0.05) decrease with the increasing amount of salt stress. The behavior of Super and Sandal showed a tolerance towards salt stress as compared to other varieties .All these parameters showed maximum significant (P ≤ 0.05) reduction at 200 mM salt stress level. The Super canola and Sandal canola are more tolerant varieties to salt stress as compared to AC Excel & Punjab. Super canola variety showed highest growth under controlled environment. Maximum decrease in plant height (62%) was displayed by AC Excel and Punjab, while Sandal and Super displayed minimum decline (58%). However, the behavior of varieties under control environment was considerably better than under NaCl induced stress environment.
Fig. 1.
(a) Shoot fresh weight (g), (b) Shoot dry weight (g), (c) Root Fresh weight(g),(d) root dry weight(g) (e) shoot length(cm), (f) root length(cm), (g) leave length (cm) (h) leave width (cm) (i) number of leaves of six Brassica napus cultivars treated with 0, 50, 100, 150 and 200 mM NaCl stress. Vertical Bars indicate Mean ± SE (n = 3), significant P ≤ 0.05.
3.2. Photosynthetic pigments
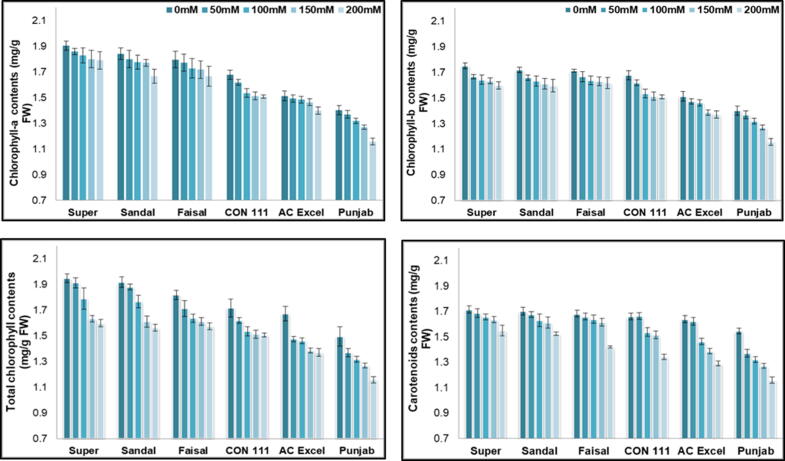
Evaluation of physiological characteristics such as photosynthetic pigments are essential in respect to enhance plant productivity under NaCl salinity in various crop cultivars. Total amount and activity of photosynthetic pigments played a vital role in plant growth. In present study, treatment of 50 mM, 100 mM, 150 mM & 200 mM salt stress noticeably (P ≤ 0.05) lowered the chlorophyll pigments i.e., chlorophyll ‘a’, chlorophyll ‘carotenoids and total chlorophyll contents in all canola cultivars (Fig. 2: a-d). For photosynthetic pigments the behavior of all the six verities were observed to be of same pattern as in their morphological traits. The values for chlorophyll s were maximum in Super, Sandal and Faisal under controlled conditions. These verities also behaved better under salt stress. The CON 111 showed an intermediate behavior while the response of AC Excel and Punjab (31% decrease) observed to be poor when subjected to salt stress. Increasing salt concentration caused a gradual decrease in chlorophyll a contents. Similar results were obtained for chlorophyll b and total chlorophyll contents. The carotenoids contents were observed to be least in Punjab whereas the Super and Sandal showed a minimum decline (25% decrease) in carotenoids contents under 200 mmol salt stress.
Fig. 2.
(a) Chlorophyll a contents (mg/g), (b) Chlorophyll b contents (mg/g), (c) Total chlorophyll contents (mg/g), (d) Carotenoid contents (mg/g) of six Brassica napus cultivars treated with 0, 50, 100, 150 and 200 mM NaCl stress. Vertical Bars indicate Mean ± SE (n = 3), significant P ≤ 0.05.
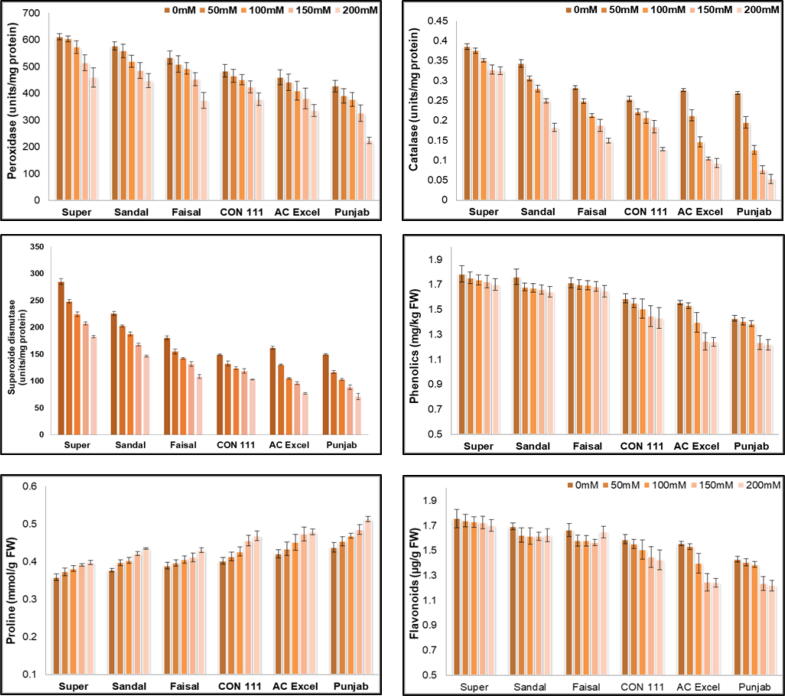
3.3. Antioxidants
Antioxidants play a vital role against ROS to combat the biotic or abiotic stresses. The less amount of these mean a more susceptible behavior. In this research study the amount of antioxidants was observed to be maximum in Super cultivar when grown under controlled conditions (Fig. 3 a, b, c). The reduction of 11% in PerOxidase (POD) activity was observed under 200 mmol of sodium chloride stress followed by Sandal, Faisal and CON 11. The maxim (CAT) and Superoxide dismutase (SOD) the similar trends were observed with a reduction in activity from 42 % in Punjab to 11 % in Super for catalase and 41 % in Punjab to 25 % in Super for SOD activities.
Fig. 3.
(a) peroxidase (units/mg protein), (b) Catalase (units/mg protein), (c) Superoxide dismutase (units/mg protein), (d) phenolic (mg/kg FW), (e) Proline (mmol/g FW), (f) Flavonoids (µg/g FW) of six Brassica napus cultivars treated with 0, 50, 100, 150 and 200 mM NaCl stress. Vertical Bars indicate Mean ± SE (n = 3), significant P ≤ 0.05.
3.3.1. Phenolics & flavonoids
The results of secondary metabolites showed a like phenolics and flavonoids showed non-significant differences when grown under controlled or stressed conditions for Super cultivar (Fig. 3 d & f). Faisal cultivar showed a decrease in flavonoids contents but an increase was observed when it was exposed to 200 mmol of salt stressed. For Phenolics the AC Excel and Punjab showed maximum decrease. The behavior of AC Excel and Punjab was almost similar for their flavonoids contents when exposed to 200 mmol salt stress.
3.3.2. Proline
It was examined that proline contents and salt stress are directly proportional to each other, as salt stress increases proline value increases, Proline contents were lesser in Brassica napus cultivars treated with 50 mL or 100 mL salt stress than in 200 mL salt stress cultivars (Fig. 4: e). It is considered as basic reaction of canola plants under NaCl stress to save compartmental injury. The maximum increase (34.165 %) in proline contents was observed in Punjab cultivar.
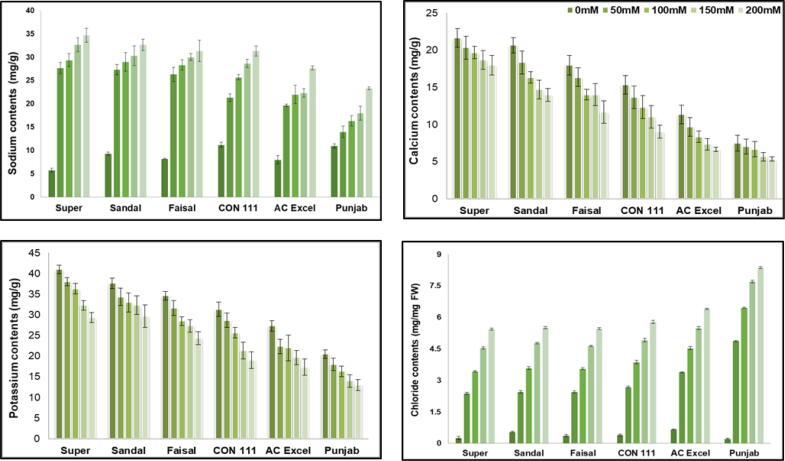
Fig. 4.
(a) Sodium contents (mg/g), (b) calcium contents (mg/g), (c) potassium contents of six Brassica napus cultivars treated with 0, 50, 100, 150 and 200 mM NaCl stress. Vertical Bars indicate Mean ± SE (n = 3), significant P ≤ 0.05.
3.4. Mineral nutrients
The data analysis regarding the ion concentration revealed that the salt stress has an augmenting effect on sodium and chloride ions. The maximum increase in these two ions was visible in Super cultivar. For sodium ions the maximum (27%) sodium increase was observed in Super cultivar when exposed to 200 mM salt stress. The affects were almost similar for Sandal, Faisal and CON 111 cultivars. The minimum increase (9 %) in sodium ions concentration was observed in Punjab cultivar (Fig. 4 a).
The maximum increase (32%) in chloride ions was observed in Punjab cultivar while the minimum increase (11%) was observed in Super cultivar followed by Sandal Faisal and CON 111. Under controlled conditions the minimum values of chloride ions were measured in Super and Punjab cultivars (Fig. 4 d). The potassium contents showed a minimum decline of 11% in Super cultivar and maximum decline of 27% in Punjab cultivar. The maximum decrease (23%) for calcium ions was observed in Punjab cultivar whereas the minimum reduction (9%) for Calcium contents was observed in Super Cultivar when plants were exposed to salt stress (Fig. 4b & c).
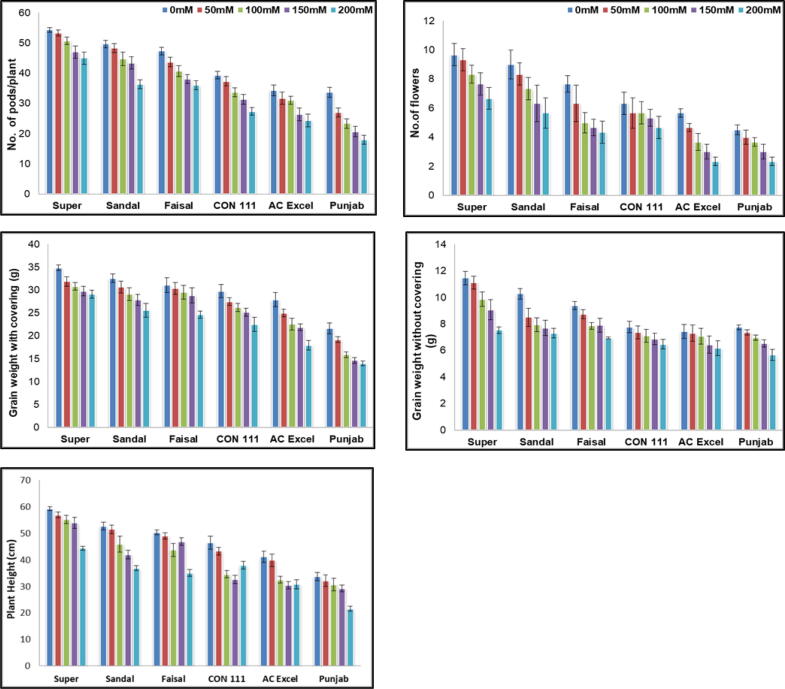
3.5. Yield
The six ecotypes of canola when treated with 0, 50, 100 and 150 mM of sodium chloride showed significant decrease in number of pods per plant. The data revealed that 50 mM concentration of salt did not affect the number of pods per plant significantly in Super and Sandal cultivars but the effects were obvious in other ecotypes. The concentration of 150 mM and 200 mM salt showed a sharp decline in number of pods per plant. Maximum reduction was observed in Punjab cultivar.
For number of flowers the behavior of Super and Sandal remained best under controlled conditions. 50 mM salt stress dud not showed any significant differences in Super ecotype but it was effective in all the other cultivars. In AC Excel and Punjab cultivars the maximum reduction in number of flowers was observed while minimum reduction was observed in Super cultivar when treated with 200 mM salt stress. Grain weight with covering (g) was observed to be least effected in Super and Sandal cultivars and maximum decrease was documented in Punjab cultivar when treated with salt stress.
The behavior of Super cultivar remained less effected for 50 mM salt stress but the increase in salt concentration adversely affected the grain weight without covering. The maximum reduction in wt of grains (g) without covering was observed in Punjab cultivar. The effects were non-significant in CON 111 and AC Excel. For plant height data collected revealed that the effects of 0 mM, 50 mM and 100 mM remained nonsignificant in Super cultivar. In Sandal, Faisal and AC Excel the effect of 50 mM remained non-significant. The minimum differences were observed in Punjab under 0 mM, 100 mM and 150 mM salt stress but the effect of 200 mM salt stress was more obvious. In AC Excel the effect of 100 mmol, 150 mmol and 200 mmol were almost similar.
3.6. Pearson correlation and principal component analysis
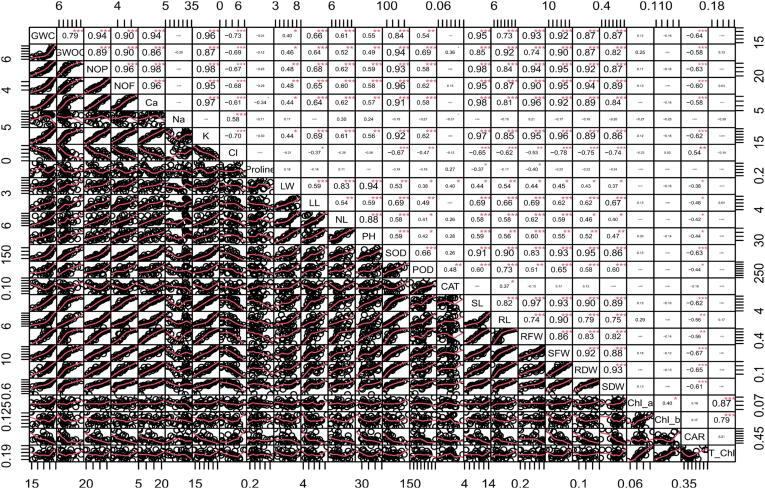
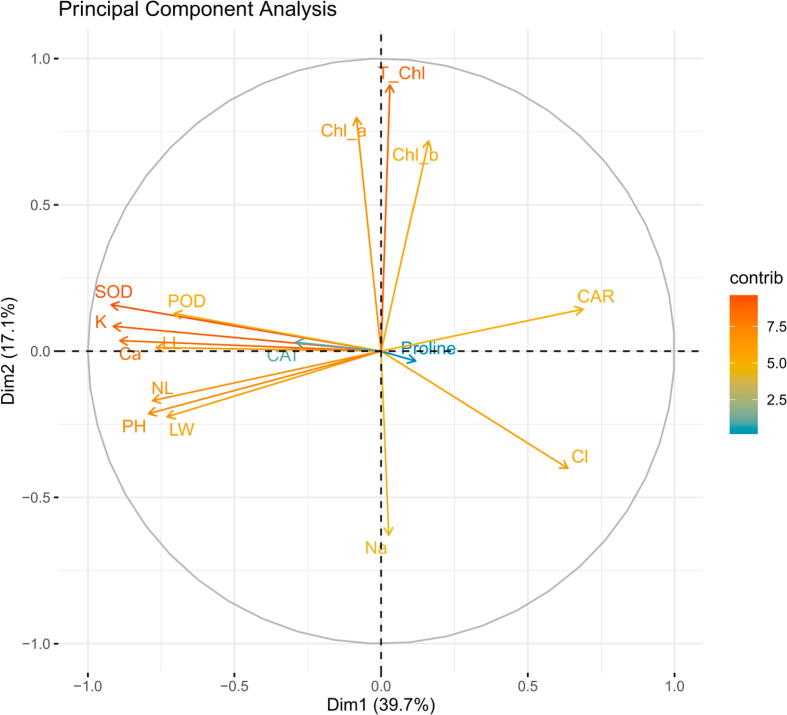
To check relationship among different attributes and salinity tolerance in Canola verities, we performed Pearson correlation and principal component analysis (PCA). Pearson correlation i.e. positive and negative was recorded between various attributes in B. napus varities under salt stress. It was noticed that salinity exerted significant changes in physio-biochemical parameters antioxidative metabolism, and nutrient balance that were positively related with growth and yield. (Fig. 5). A negative correlation was observed among Cl- and all other attributes studied in this experiment except proline. Mineral ions were correlated positively with growth except Cl-. Shoot fresh and dry biomass, plant height, number of flowers, number of pods were correlated with each other positively. SOD presented very positive correlation with growth attributes while correlation of POD, Ca and K with all these was also positive. Cl and Na exhibited a positive correlation with each other. This correlation analysis showed an obvious relationship between varieties of canola and plant characteristics (Fig. 6). This correlational analysis between studied plant characteristics of six B. napus varieties is verified by PCA-Biplot. Fig. 7 shows PCA-Biplot of different attributes of B. napus varieties under salt stress. This PCA biplot examined the degree of relationship between among the treatments and attributes. Both PC1 and PC2 jointly presented 56.8 % of variability in data. PC1 elaborates a clear distinction of canola attributes after exposure to salinity levels., PC1 shared 39.7 % of the observed variation as compared to the shar of PC2 i.e. 17.1%. In the dataset, the distribution of components points out that salt toxicity in the rooting media significantly influenced all the varieties with reference to their studied parameters Figure, 7). It clearly demonstrates that variables in control plants did not significantly match with the same variables in salinity treated Canola plants.
Fig. 5.
(a) No. of pods, (b) No. of flowers (c) Grain weight with covering (g) (d) grain weight without covering (g) , (e) Plant height (cm) of six Brassica napus cultivars treated with 0, 50, 100, 150 and 200 mM NaCl stress. Vertical Bars indicate Mean ± SE (n = 3), significant P ≤ 0.05.
Fig. 6.
Correlation matrix of different attributes in six Brassica napus cultivars treated with 0, 50, 100, 150 and 200 mM NaCl stress. Vertical Bars indicate Mean ± SE (n = 3), significant P ≤ 0.05.
Fig. 7.
PCA-Biplot of different attributes of six Brassica napus cultivars treated with 0, 50, 100, 150 and 200 mM NaCl stress. Vertical Bars indicate Mean ± SE (n = 3), significant P ≤ 0.05.
4. Discussion
NaCl imposed salt stress critically affected all the six cultivars of Brassica napus i.e., Super canola, Sandal canola, CON-111, Punjab canola, Faisal canola and AC Excel. Salinity clearly reduced the shoot/root length, shoot/root fresh and dry weights, leaf length and width, number of leaves and plant height. All these parameters showed maximum significant (P ≤ 0.05) reduction at 200 mM salt stress level. These results are in accordance with the past research of (Munns and Tester, 2008, Shahbazi et al., 2011, Umar et al., 2011). This declination takes place due to disruption in metabolism of ion exchange, lower vital nutrients concentration or by the accumulation of toxic substances in plants under stress (Vital et al., 2008). Greater amount of ROS production under high osmotic stress mainly causes the decrease in all growth attributes (Akhter et al., 2021b, Hasanuzzaman et al., 2013, Bashri et al., 2021). Displaying of lower yield and growth by crop plants under NaCl induced salt stress is caused by the alteration in biochemical mechanisms (Waqas et al., 2019). The Super canola and Sandal canola are more tolerant varieties to salt stress as compared to AC Excel & Punjab. Super canola variety showed highest growth under controlled environment.
Evaluation of biochemical characteristics such as photosynthetic pigments are essential in respect to enhance plant productivity under NaCl salinity in various crop cultivars (Akhter et al., 2021b, Hasanuzzaman et al., 2013). It was observed and hence proved in many studies that the decline in photosynthetic attributes consequently decreased the morphological parameters, overall growth, hence yield of many crops. Various metabolic disorders developed in Brassica napus seedlings when exposed to relative salt (Ayaz et al., 2000, Hasanuzzaman et al., 2018, Kumar, 1995). Application of NaCl stress at early stages of Brassica napus cultivars have negative impact on some biochemical attributes, caused chlorophyll contents to decline (Su et al., 2013). Total amount and activity of photosynthetic pigments played a vital role in plant growth. In present study, treatment of 50 mM, 100 mM, 150 mM & 200 mM salt stress noticeably (P ≤ 0.05) lowered the chlorophyll pigments i.e., chlorophyll ‘a’, chlorophyll ‘b’ and total chlorophyll contents in all canola cultivars. Production of reactive oxygen species (ROS) under osmotic stress caused by salt stress initiates the breakdown of chloroplast that ultimately decreases its total amount as studied in maize (Ahmad et al., 2020), quinoa (Abdallah et al., 2020) and tomato (Ahanger et al., 2019). Increase in Na+ & Cl- ions in leaves also caused declination of Chl-b, Chl-a and total chlorophyll contents, because in the presence of these ions different enzymes function gets disturbed that are participating in chlorophyll production (Ahanger et al., 2019, Ahmad et al., 2020). The osmolyte proline that contains antioxidant properties also aids in signal transformation metabolism (Nahar et al., 2013, Dar et al., 2021). In current research high proline value was observed in NaCl stressed plants than the control ones. It was examined that proline contents and salt stress are directly proportional to each other, as salt stress increases proline value increases, these results are comparable with previous studies (Nounjan and Theerakulpisut, 2012, Javeed et al., 2021). Hence, observed that most compatible osmo-protectant is proline under NaCl affected cultivars. Proline contents were lesser in Brassica napus cultivars treated with 50 mL or 100 mL salt stress than in 200 mL salt stress cultivars. It is considered as basic reaction of canola plants under NaCl stress to save compartmental injury. Terpenes, alkaloids, phenolics and flavonoids etc. are considered as secondary metabolites. These act as toxic substances during abiotic stress tolerance but are not directly linked with plant development and growth (Taiz and Zeiger, 2006, Khan et al., 2020). Whereas phenolics and terpenes from secondary metabolites are exception due to their structural properties as these perform important functions in abiotic stress tolerance (Ruiz and Romero, 2001). All canola cultivars examined in present experiment displayed enhanced total phenolic contents under salt stress environment. Enzymatic antioxidants play a defensive role that plants really needs for its survival under NaCl stress. These enzymes act as protective sheath against ROS formation under stress environments (Alves, R.d.C., 2020, Hajihashemi et al., 2020). Ion analysis studies of current research elaborate that increased salt stress caused reduction in Na+ and Cl- ions. Similarly, the yield parameters i.e., total number of flowers, number of pods, grains weight (with and without covering) displayed declined values under NaCl stress as compared to control. These results are in accordance with past studies in rapeseed (Rameeh, 2012), in canola (Bandehagh et al., 2021), and in spring canola cultivars (Tahmasebpour et al., 2018).
5. Conclusion
NaCl induced salt stress adversely affected all six cultivars of canola (Brassica napus). Salinity decreased all morphological and biochemical parameters. However, among the six cultivars, Super and Sandal proved to be more tolerant under NaCl salinity stress, these cultivars showed lesser declination in all parameters, whereas Punjab canola and AC Excel canola cultivars were least tolerant to salinity because these displayed greater decline in all growth and biochemical attributes. However, proline contents tend to increase under salt stress in all six cultivars. 50 mM and 100 mM salt stress concentrations were proved to be less harmful for all six cultivars. Whereas 200 mM and 150 mM concentrations were very harmful for cultivars. All six varieties showed adverse symptoms and growth under 200 mM NaCl salt stress. Super canola performed better under control conditions as well as under salinity environment among all six varieties of Brassica napus.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
We are thankful to Department of Botany, GCWUF for providing lab facilities to conduct this research. Authors would like to acknowledge Taif University Researchers Supporting Project number (TURSP-2020/94), Taif University, Taif, Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Muhammad Aqeel, Email: aqeelbutt99@gmail.com.
Ali Noman, Email: alinoman@gcuf.edu.pk.
References
- Abdallah M.-M.-S., El Sebai T.N., Ramadan A.-A.-E.-M., El-Bassiouny H.M.S. Physiological and biochemical role of proline, trehalose, and compost on enhancing salinity tolerance of quinoa plant. Bulletin of the National Research Centre. 2020;44:1–13. [Google Scholar]
- Ahanger, M.A., Qin, C., Maodong, Q., Dong, X.X., Ahmad, P., Abd_Allah, E.F., Zhang, L., 2019. Spermine application alleviates salinity induced growth and photosynthetic inhibition in Solanum lycopersicum by modulating osmolyte and secondary metabolite accumulation and differentially regulating antioxidant metabolism. Plant Physiology and Biochemistry 144, 1-13. [DOI] [PubMed]
- Ahmad S., Cui W., Kamran M., Ahmad I., Meng X., Wu X., Su W., Javed T., El-Serehy H.A., Jia Z. Exogenous application of melatonin induces tolerance to salt stress by improving the photosynthetic efficiency and antioxidant defense system of maize seedling. J. Plant Growth Regul. 2020:1–14. [Google Scholar]
- Akhter N., Aqeel M., Hameed M., Alhaithloul H.A.S., Alghanem S.M., Shahnaz M.M., Hashem M., Alamri S., Khalid N., Al-Zoubi O.M. Foliar architecture and physio-biochemical plasticity determines survival of Typha domingensis pers. Ecotypes in nickel and salt affected soil. Environ. Pollut. 2021;286 doi: 10.1016/j.envpol.2021.117316. [DOI] [PubMed] [Google Scholar]
- Akhter N., Aqeel M., Shahnaz M.M., Alnusairi G.S., Alghanem S.M., Kousar A., Hashem M., Kanwal H., Alamri S., Ilyas A. Physiological homeostasis for ecological success of Typha (Typha domingensis Pers.) populations in saline soils. Physiol. Mol. Biol. Plants. 2021;27:687–701. doi: 10.1007/s12298-021-00963-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Dakheel A.J., Hussain M.I. Genotypic Variation for Salinity Tolerance in Cenchrus ciliaris L. Front. Plant Sci. 2016;7:1090. doi: 10.3389/fpls.2016.01090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves, R.d.C., Nicolau, M.C.M., Checchio, M.V., Sousa, G.d.S., Oliveira, F.d.A.d., Prado, R.M., Gratão, P.L., 2020. Salt stress alleviation by seed priming with silicon in lettuce seedlings: an approach based on enhancing antioxidant responses. Bragantia 79, 19-29.
- Apel K., Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- Aqeel M., Khalid N., Tufail A., Ahmad R.Z., Akhter M.S., Luqman M., Javed M.T., Irshad M.K., Alamri S., Hashem M., Noman A. varieties. Environmental Science and Pollution Research; 2021. Elucidating the distinct interactive impact of cadmium and nickel on growth, photosynthesis, metal-homeostasis, and yield responses of mung bean (Vigna radiata L.) pp. 1–15. [DOI] [PubMed] [Google Scholar]
- Arnon D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant physiology. 1949;24:1. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayaz F., Kadioglu A., Turgut R. Water stress effects on the content of low molecular weight carbohydrates and phenolic acids in Ctenanthe setosa (Rosc.) Eichler. Can. J. Plant Sci. 2000;80:373–378. [Google Scholar]
- Bandehagh A., Dehghanian Z., Henry R., Hossain M.A. Brassica Breeding and Biotechnology; IntechOpen: 2021. Salinity Tolerance in Canola: Insights from Proteomic Studies. [Google Scholar]
- Bashri G., Singh S., Prasad S.M., Ansari M.J., Usmani S., Alfarraj S., Alharbi S.A., Brestic M. Kinetin mitigates Cd-induced damages to growth, photosynthesis and PS II photochemistry of Trigonella seedlings by up-regulating ascorbate-glutathione cycle. PLoS ONE. 2021;16(6) doi: 10.1371/journal.pone.0249230. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Bates L.S., Waldren R.P., Teare I. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39:205–207. [Google Scholar]
- Belouchrani A.S., Bouderbala A., Drouiche N., Lounici H. The interaction effect to fertilization on the mineral nutrition of canola under different salinity levels. J. Plant Growth Regul. 2021;40:848–854. [Google Scholar]
- Bray E.A. Response to abiotic stress. Biochemistry and molecular biology of plants. 2000:1158–1203. [Google Scholar]
- Britton C., Maehly A. Assay of catalases and peroxidases. Methods Enzymol. 1955;2:764–775. [Google Scholar]
- Bybordi A., Tabatabaei S.J., Ahmadev A. Effect of salinity on the growth and peroxidase and IAA oxidase activities in canola. J. Food Agric. Environ. 2010;8:109–112. [Google Scholar]
- Dar, Z.A., Dar, S.A., Khan, J.A., Lone, A.A., langyan, S., Lone, B A, Kanth, R H, Iqbal, A., Rane,J., Wani, S.H., Alfarraj, S., Alharbi, S.A., Brestic,M., Ansari, M.J. (2021) Identification for surrogate drought tolerance in maize inbred lines utilizing high-throughput phenomics approach. PLoS ONE 16(7): e0254318 https://doi.org/10.1371/journal.pone.0254318 [DOI] [PMC free article] [PubMed] [Retracted]
- Hasanuzzaman M., Nahar K., Fujita M. Plant response to salt stress and role of exogenous protectants to mitigate salt-induced damages, Ecophysiology and responses of plants under salt stress. Springer. 2013:25–87. [Google Scholar]
- Hasanuzzaman M., Nahar K., Rohman M., Anee T., Huang Y., Fujita M. Exogenous silicon protects Brassica napus plants from salinity-induced oxidative stress through the modulation of AsA-GSH pathway, thiol-dependent antioxidant enzymes and glyoxalase systems. Gesunde Pflanzen. 2018;70:185–194. [Google Scholar]
- Hajihashemi S., Skalicky M., Brestic M., Vachová P. Cross-talk between nitric oxide, hydrogen peroxide and calcium in salt-stressed. Chenopodium quinoa Willd. at seed germination stage. Plant Physiol. Biochem. 2020;154(2020):657–664. doi: 10.1016/j.plaphy.2020.07.022. [DOI] [PubMed] [Google Scholar]
- Ijaz M., Nawaz A., Ul-Allah S., Sher A., Sattar A., Sarwar M., Hussain I., Ur-Rehman A., Wahid M.A., Ansari M.J., Hessini K. Optimizing sowing date for peanut genotypes in arid and semi-arid subtropical regions. PLoS ONE. 2021;16(6) doi: 10.1371/journal.pone.0252393. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ilyas, N., Amjid, M.W., Saleem, M.S., Khan, W., Masoud, F., Rana, R.M., Maqsood, R.H., Zahid, A., Shah, G.A., Anwar, A., Ahmad, M.Q., Shaheen, M., Riaz, H., Ansari, M.J. (2020) Quantitative trait loci (QTL) mapping for physiological and biochemical attributes in a Pasban90/Frontana recombinant inbred lines (RILs) population of wheat (Triticum aestivum) under salt stress condition. Saudi Journal of Biological Sciences, Vol. 27: 341-351https://doi.org/10.1016/j.sjbs.2019.10.003 [DOI] [PMC free article] [PubMed]
- Javeed, H.M.R.; Ali, M.; Skalicky, M.; Nawaz, F.; Qamar, R.; Rehman, A.u.; Faheem, M.; Mubeen, M.; Iqbal, M.M.; Rahman, M.H.u.; Vachova P.; Brestic M.; Baazeem A., El Sabagh A.: Lipoic Acid Combined with Melatonin Mitigates Oxidative Stress and Promotes Root Formation and Growth in Salt-Stressed Canola Seedlings (Brassica napus L.). Molecules 2021, 26, 3147. https:// doi.org/10.3390/molecules26113147 [DOI] [PMC free article] [PubMed]
- Khan, N., Syeed, S., Masood, A., Nazar, R., Iqbal, N., 2010. Application of salicylic acid increases contents of nutrients and antioxidative metabolism in mungbean and alleviates adverse effects of salinity stress. International Journal of Plant Biology 1, e1-e1.
- Khan, I., Raza, M. A., Awan, S. A., Shah, G. A., Rizwan, M., Ali, B., Tariq R., Brestic M., Zhang X., Huang L., Ali S.: Amelioration of salt induced toxicity in pearl millet by seed priming with silver nanoparticles (AgNPs): The oxidative damage, antioxidant enzymes and ions uptake are major determinants of salt tolerant capacity. Plant Physiology and Biochemistry, 156, 221–232. doi:10.1016/j.plaphy.2020.09.018 [DOI] [PubMed]
- Kumar, D., 1995. Salt tolerance in oilseed brassicas-present status and future prospects. Plant Breeding Abstracts (United Kingdom).
- Mahmood S., Daur I., Al-Solaimani S.G., Ahmad S., Madkour M.H., Yasir M., Hirt H., Ali S., Ali Z. Plant growth promoting rhizobacteria and silicon synergistically enhance salinity tolerance of mung bean. Front. Plant Sci. 2016;7:876. doi: 10.3389/fpls.2016.00876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manivannan, A., Soundararajan, P., Muneer, S., Ko, C.H., Jeong, B.R., 2016. Silicon mitigates salinity stress by regulating the physiology, antioxidant enzyme activities, and protein expression in Capsicum annuum ‘Bugwang’. BioMed Research International 2016. [DOI] [PMC free article] [PubMed]
- Moghadam N.K., Motesharezadeh B., Maali-Amiri R., Lajayer B.A., Astatkie T. Effects of potassium and zinc on physiology and chlorophyll fluorescence of two cultivars of canola grown under salinity stress. Arabian J. Geosci. 2020;13:1–8. [Google Scholar]
- Munns R., Tester M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008;59:651–681. doi: 10.1146/annurev.arplant.59.032607.092911. [DOI] [PubMed] [Google Scholar]
- Nahar, K., Hasanuzzaman, M., Alam, M., Fujita, M., 2013. Exogenous glutathione-induced drought stress tolerance in Vigna radiata seedlings through enhanced antioxidant defense and methylglyoxal system, Interdrought IV Conference September.
- Naveed M., Sajid H., Mustafa A., Niamat B., Ahmad Z., Yaseen M., Kamran M., Rafique M., Ahmar S., Chen J.-T. Alleviation of salinity-induced oxidative stress, improvement in growth, physiology and mineral nutrition of canola (Brassica napus L.) through calcium-fortified composted animal manure. Sustainability. 2020;12:846. [Google Scholar]
- Noman A., Ali Q., Maqsood J., Iqbal N., Javed M.T., Rasool N., Naseem J. Deciphering physio-biochemical, yield, and nutritional quality attributes of water-stressed radish (Raphanus sativus L.) plants grown from Zn-Lys primed seeds. Chemosphere. 2018;195:175–189. doi: 10.1016/j.chemosphere.2017.12.059. [DOI] [PubMed] [Google Scholar]
- Noman A., Ali Q., Naseem J., Javed M.T., Kanwal H., Islam W., Aqeel M., Khalid N., Zafar S., Tayyeb M. Sugar beet extract acts as a natural bio-stimulant for physio-biochemical attributes in water stressed wheat (Triticum aestivum L.) Acta Physiologiae Plantarum. 2018;40:110. [Google Scholar]
- Nounjan N., Theerakulpisut P. Effects of exogenous proline and trehalose on physiological responses in rice seedlings during salt-stress and after recovery. Plant, Soil and Environment. 2012;58:309–315. [Google Scholar]
- R Development Core Team, 2020. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, http://www.R-project.org. Accessed 15 March, 2021.
- Rahman A., Nahar K., Hasanuzzaman M., Fujita M. Calcium supplementation improves Na+/K+ ratio, antioxidant defense and glyoxalase systems in salt-stressed rice seedlings. Front. Plant Sci. 2016;7:609. doi: 10.3389/fpls.2016.00609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rameeh V. Ions uptake, yield and yield attributes of rapeseed exposed to salinity stress. Journal of soil science and plant nutrition. 2012;12:851–861. [Google Scholar]
- Rasel, M., Tahjib-Ul-Arif, M., Hossain, M.A., Hassan L., Farzana S. Brestic M.: Screening of Salt-Tolerant Rice Landraces by Seedling Stage Phenotyping and Dissecting Biochemical Determinants of Tolerance Mechanism multidimensional roles in salt-stressed plants. Journal of Plant Growth Regulation, 2020, https ://doi.org/10.1007/s0034 4-020-10235 -9
- Ribarova F., Atanassova M. Total phenolics and flavonoids in Bulgarian fruits and vegetables. Journal of the university of chemical technology and metallurgy. 2005;40:255–260. [Google Scholar]
- Ruiz J.M., Romero L. Bioactivity of the phenolic compounds in higher plants. Stud. Nat. Prod. Chem. 2001;25:651–681. [Google Scholar]
- Sabagh A.E., Hossain A., Barutçular C., Islam M.S., Ratnasekera D., Kumar N., Meena R.S., Gharib H.S., Saneoka H., da Silva J.A.T. Drought and salinity stress management for higher and sustainable canola ('Brassica napus' L.) production: A critical review. Aust. J. Crop Sci. 2019;13:88–96. [Google Scholar]
- Shehzad M., Zhou Z., Ditta A., Khan M., Cai X., Xu Y., Maqbool A., Khalofah A., Shaban M., Naeem M., Ansari M.J., Wang K., Liu F. Identification and characterization of genes related to salt stress tolerance within segregation distortion regions of genetic map in F2 Population of upland cotton. PLoS ONE. 2021;16(3) doi: 10.1371/journal.pone.0247593. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Schillinger W.F., Paulitz T.C. Canola versus wheat rotation effects on subsequent wheat yield. Field Crops Research. 2018;223:26–32. [Google Scholar]
- Shahbazi E., Arzani A., Saeidi G. Effects of NaCl treatments on seed germination and antioxidant activity of canola (Brassica napus L.) cultivars. Bangladesh Journal of Botany. 2011;40:67–73. [Google Scholar]
- Sharif P., Seyedsalehi M., Paladino O., Van Damme P., Sillanpää M., Sharifi A. Effect of drought and salinity stresses on morphological and physiological characteristics of canola. Int. J. Environ. Sci. Technol. 2018;15:1859–1866. [Google Scholar]
- Steel R., Torrie J., Dicke D. McGraw-Hill; New York: 1997. Principles and procedures of statistics: A biochemical approach. [Google Scholar]
- Su J., Wu S., Xu Z., Qiu S., Luo T., Yang Y., Chen Q., Xia Y., Zou S., Huang B.-L. Comparison of salt tolerance in Brassicas and some related species. American Journal of Plant Sciences. 2013;4:1911. [Google Scholar]
- Tahmasebpour B., Sabzi Nojadeh M., Esmaeilpour M. Salt stress tolerance of spring canola (Brassica napus L.) cultivars. International Journal of Plant Biology & Research. 2018 [Google Scholar]
- Taiz, L., Zeiger, E., 2006. Plant physiology/by Lincoln Taiz and Eduardo Zeiger.
- Umar S., Diva I., Anjum N.A., Iqbal M., Ahmad I., Pereira E. Potassium-induced alleviation of salinity stress in Brassica campestris L. Central European Journal of Biology. 2011;6:1054–1063. [Google Scholar]
- Vital S.A., Fowler R.W., Virgen A., Gossett D.R., Banks S.W., Rodriguez J. Opposing roles for superoxide and nitric oxide in the NaCl stress-induced upregulation of antioxidant enzyme activity in cotton callus tissue. Environ. Exp. Bot. 2008;62:60–68. [Google Scholar]
- Waqas, M., Yaning, C., Iqbal, H., Shareef, M., ur Rehman, H., Iqbal, S., Mahmood, S., 2019. Soil drenching of paclobutrazol: An efficient way to improve quinoa performance under salinity. Physiologia plantarum 165, 219-231. [DOI] [PubMed]
- Wu H., Guo J., Wang C., Li K., Zhang X., Yang Z., Li M., Wang B. An effective screening method and a reliable screening trait for salt tolerance of Brassica napus at the germination stage. Front. Plant Sci. 2019;10:530. doi: 10.3389/fpls.2019.00530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H.-X., Hodson J.N., Williams J.P., Blumwald E. Engineering salt-tolerant Brassica plants: characterization of yield and seed oil quality in transgenic plants with increased vacuolar sodium accumulation. Proc. Natl. Acad. Sci. 2001;98:12832–12836. doi: 10.1073/pnas.231476498. [DOI] [PMC free article] [PubMed] [Google Scholar]