Graphical abstract
Keywords: Fruit peels, Polyphenols, Silver nanoparticles, Antioxidant, Antimicrobial, Larvicidal
Abstract
The agricultural wastes adversely affect the environment; however, they are rich in polyphenols; therefore, this study aimed to employ polyphenol-enriched waste extracts for silver nanoparticles synthesis, and study the larvicidal activity of silver nanoparticles fabricated by pomegranate and watermelon peels extracts (PPAgNPs and WPAgNPs) against all larval instars of Spodoptera littoralis. The polyphenol profile of pomegranate and watermelon peel extracts (PP and WP) and silver nanoparticles was detected by HPLC. The antioxidant activity was estimated by DPPH, and FARP assays and the antimicrobial activity was evaluated by disc assay. The Larvicidal activity of AgNPs against Egyptian leaf worm was performed by dipping technique. The obtained AgNPs were spherical with size ranged 15–85 nm and capped with proteins and polyphenols. The phenolic compounds in silver nanoparticles increased about extracts; therefore, they have the best performance in antioxidant/reducing activity, and inhibit the growth of tested bacteria and yeast. The PPAgNPs were the most effective against the first instar larvae instar (LC50 = 68.32 µg/ml), followed by pomegranate extract with (LC50 = 2852 µg/ml). The results indicated that obvious increase in polyphenols content in silver nanoparticles enhance their larvicidal effect and increasing mortality of 1st larval of S. littoralis Egyptian leafworms causing additive effect and synergism. We recommend recycling phenolic enriched agricultural wastes in producing green silver nanoprticles to control cotton leafworm that causes economic loses to crops.
1. Introduction
African cotton leafworm (Spodoptera littoralis) is a lepidopteran insect. It has economic importance because the wide range of host plants of different families besides feeding on the plant with a high population can cause leaves skeletonization, losing crops, consumed shoot system, and causing economic losses. Chemical controlled caused development resistance to several groups of insecticides and adverse effects on the beneficial animals and the environment (Bakr and Al Yousef, 2015). Therefore, the researchers try to find new promising approach to suppress the population of S. littoralis by using agricultural wastes in fabricating nanoparticles. The peels of fruit and vegetables have considerable polyphenols content (Saad et al., 2021). Pomegranate and watermelon peels contain extensive amounts of phenolic compounds (Jung, 2015, Rolim et al., 2018, Yuan and Fang, 2018). The polyphenols are secondary metabolites that act as first defense line to protect plants from various stress conditions and bacterial and fungal infections (Dahham et al., 2010, Devi et al., 2011). Besides, they may act as potential insecticides (Ateyyat et al., 2012, Ghaly et al., 2014). Flavonoids are known to modulate the feeding and egg laying behavior of insects (Goławska and Łukasik, 2012). Therefore, the extracts of these peels contain significant content of phenolic compounds, which can act as reducing and protective agents for nanoparticles preparation. Synthesis of nanoparticles using physical and chemical methods is costly, has adverse impacts on the environment and needs high energy (El-Saadony et al., 2018, El-Saadony et al., 2019, El-Saadony et al., 2020). On the other hand, biosynthesis of nanoparticles is considered a clean and an economic alternative route when fabricated using bacteria (Reda et al., 2020, Sheiha et al., 2020, El-Saadony et al., 2021a, Reda et al., 2021), fungi (El-Saadony et al., 2021b) and plants is cheaper and favors large-scale production, as it requires simpler downstream processing. The application of botanicals for the synthesis of nanostructured materials offers numerous benefits than the use of safe chemicals in the synthesis (Borase et al., 2014). Moreover, these green synthesized nanoparticles are reported to be more effective insecticides (El-Saadony et al., 2020), less expensive, biodegradable, and safe for humankind and the environment than synthetic counterparts (Sabbour et al., 2015, Murugan et al., 2016). In search of environmentally friendly and effective pesticides, nanoparticles could be a new and good strategy. Nanoparticles are a particle with a size range between 1 and 100 nm, exhibit completely new or improved properties as compared to the original materials that differ in specific characteristics such as size, distribution, and morphology (El-Saadony et al., 2021a, El-Saadony et al., 2021c). Saranya et al. (2020) stated that nanosized particles with a wide range of use in insecticides with different mode of actions (Benelli, 2018). Newly transformed nanoparticles acquired a new property e.g. large specific surface area, thermal stability, biodegradability, and high affinity with the target site, hence; penetrate rapidly enhancing the biocidal activities (Benelli, 2018). Silver nanoparticles (AgNPs) have been used in many medical, pharmaceutical and agricultural applications as a pure free metal or a compound forms because it possesses broad-spectrum antioxidant and antimicrobial activities, and they are non-toxic to humans (Tian et al., 2018, Jacob et al., 2019). Recently, nanomaterials received great attention showed antimicrobial and biocidal activities (Bhattacharyya et al., 2010, Rai and Ingle, 2012, Bodaiah et al., 2016). The literature survey confirms scarce studies focusing watermelon and pomegranate peels phenolic compounds to use in fabrication of silver nanoparticles and the usage of these nanoparticles as insecticidal. Due to the vast potentiality of plants, the study aimed to employ phenolic compounds isolated from watermelon and pomegranate peels aquas extracts for silver nanoparticles synthesis. Estimate the antioxidant, antimicrobial of polyphenol extracts and silver nanoparticles. Besides the larvicidal activity of watermelon and pomegranate extracts, and silver nanoparticles were tested against all larval instars of S. littoralis. In addition, the study assessed the effects of these materials on the antifeedant of the 6th larval instar.
2. Materials and methods
The peels of pomegranate (Punica granatum L.) and watermelon (Citrullus lanatus) were obtained from a juice factory in 10th Ramadan City, Sharkia, Egypt. All chemicals and cultures used in this study were of analytical grade. Egyptian cotton leafworm rearing: A laboratory colony of the Egyptian cotton leafworm, Spodoptera littoralis (Boisd.), (Lepidoptera: Noctuidae) was obtained from Plant Protection Institute, Agriculture Research Center, Zagazig, Sharkia Government, Egypt. The colony was reared according to Eldefrawi et al. (1964). The resulting larvae were fed on castor leaves.
2.1. Extraction of phenolic compounds from pomegranate and watermelon aquas extract
The extraction method were followed Saad et al., 2020. The pomegranate and watermelon peels were washed, dried in an oven at 105 °C for 3 h, and ground in a mixer, then sieved through 20-mesh screen. Ten grams of powdered peels were homogenized in 150 ml of distalled water and stirred for 4 h at 40 °C in a conical flask. The liquid extract was vacuum filtrated through Whatman No. 1 filter paper. The filtrates evaporated by a rotary evaporator then lyophilized and weighted to analyze the total extract yield (%) as following equation (1)
| (1) |
where (pp) is pomegranate peel powder, and (wp) is watermelon peel powder
2.2. Green synthesis of silver nanoparticles
100 mg of phenolic extracts of pomegranate and watermelon peels was homogenized in 100 ml of silver nitrate solution (1 mM), and stirred overnight at room temperature. Then, silver nanoparticle solutions were centrifuged at 13,000 rpm for 15 min (Sigma 3–30 k, Germany). The supernatant was discarded and the silver nanoparticles pellets were rinsed several times with deionized distilled water, and then centrifuged again and the best samples were selected based on their appearance properties, such as intensity of darkness and applied for further experiments.
2.3. Characterization of green silver nanoparticles
Five advanced instruments were characterized the pomegranate silver nanoparticles (PPAgNPs) and watermelon silver nanoparticles (WPAgNPs). UV–VIS spectroscopy analysis to estimate the optical property of PPAgNPs and WPAgNPs mixture by Laxco™ dual-beam spectrophotometer in the range of 200–700 nm. The size and shape of silver nanoparticles were estimated using JEOL 1010 TEM (Japan). FTIR analysis was used to recognize the various groups of active compounds in pomegranate and watermelon peels extracts involved in the biotransformation and stabilization of AgNPs. The obtained PPAgNPs and WPAgNPs were analyzed by FT-IR spectroscopy (“Bruker Tensor 37, Kaller”, Germany) in the range of 4000 cm−1 to 400 cm−1 using KBr pellets. DLS analysis were carried out by using Nano “Z2 Malven, Malvern Hills, UK. The size was estimated based on the Brownian motion of the PPAgNPs and WPAgNPs in suspension. The zeta potential analysis was carried out to determine the surface charge of PPAgNPs and WPAgNPs, that ensure the stability of synthesized nanoparticles.
2.4. Total phenolic compounds in PPAgNPs, WPAgNPs and their substrate
The total polyphenols (TP) was evaluated according to Chen et al. (2015) with some modifications. In brief, 50 µL of each solution at (500 µg/mL) was mixed with 50 µL of diluted Folin-Ciocalteu reagent and 50 µL Na2CO3 (7.5%) in microtiter plate wells, and incubated at room temperature for 60 min, then the absorbance was measured at 760 nm using microtiter plate reader (BioTek Elx808, USA). The total polyphenols content was presented as mg gallic acid equivalent/mL of solutions.
Total flavonoids content (TF) was estimated as per Chen et al. (2017) with some modifications. 20 µL of each solution at (500 µg/mL) was mixed with 20 µL of sodium nitrite (5%) in microtiter plate wells and 20 µL of ethanolic AlCl3 (10%) was added. The plate was incubated at room temperature for 10 min. 50 µL of NaOH was added to stop the reaction. the absorbance was measured at 450 nm. The TF was expressed as mg quercetin equivalent per mL of each solution.
2.5. Phenolic compounds profile in PP, PW, PPAgNPs, and WPAgNPs
Phenolic profile was assesed by HPLC Shimadzu series (Shimadzu-prominence-20A, Japan) with separation column (Gemini, C18, 4.6 × 150 mm × 5um) with 2 ml/min flow rate, and the mobile phase was 0.01% acetic acid in water (A) acetonitrile (B). The HPLC pumps, autosampler, column, oven, and diode array system were monitored and controlled, and the chromatographic data were collected using Class VP software (Shimadzu 5.0). The phenolics of PP, PW, PPAgNPs, and WPAgNPs were performed by HPLC at 280 nm (Goupy et al., 1999, Hassanin et al., 2020). Flavonoid compounds in samples were estimated at 370 nm (Mattila et al., 2000).
2.6. Biological activity of PPAgNPs, WPAgNPs and their substrate
2.6.1. Antioxidant activity
2.6.1.1. DPPH radical scavenging assay
The radical scavenging activity of PP, WP, PPAgNPs and WPAgNPs at concentration of (500 µg/mL) was estimated according to Bhakya et al. (2016) with some modifications. 100 µL of each solution was mixed with 1 µL of ethanolic DPPH was placed in the wells of microtiter plate and incubated at the roomfperature in the dark for 30 min. the absorbance was measured at 517 nm using microtiter plate reader (BioTek Elx808, USA) then applied in the equation (2)
| (2) |
2.6.1.2. Ferric reducing/antioxidant power assay
The antioxidant activity of PP, WP, PPAgNPs, and WPAgNPs was measured by Fe+3 reduction ability to Fe+2 according to Benzie and Strain (1996) with some modifications. 100 µL of PP, WP, PPAgNPs and WPAgNPs (500 μg/ml) was mixed with 100 µL of the fresh prepared FRAP reagent (25 ml phosphate buffer, 2.5 ml PFC solution, and 2.5 ml FeCl3 solution) in microtiter plate. The plate was incubated in the dark at 37 °C for 30 min. Then absorbance of blue colored complex was determined at 595 nm against distilled water blank using microtiter plate reader (BioTek Elx808, USA). FeSO4.7H2O was used for calibration. Ascorbic acid was used as positive control. Results were expressed as mM Fe2+/ mg sample.
2.6.2. Antimicrobial activity
Antimicrobial activity of PP, WP, PPAgNPs and WPAgNPs (500 μg/ml) were carried out against different pathogenic bacteria; [Gram-positive bacteria (G+) (Bacillus cereus, Listeria monocytogenes and Staphylococcus aureus), Gram-negative bacteria (G-) (Escherichia coli, Pseudomonas aeruginosa and Klebseila penumonia)], and yeasts (Candida gelberta, Candida albicans, and Candida apis). The bacterial cultures were grown over night in Muller Hinton broth (MHB) under shaking incubator at 37°C until (1x108 CFU/mL) concentration. The antimicrobial activity of PP, WP, PPAgNPs and WPAgNPs was performed by disc diffusion method (Akl et al., 2020, Ashour et al., 2020). The Muller Hinton plates were inoculated with 100 μL of activated bacterial and Candida isoletes by the spread plate method. Previously saturated paper discs (5 mm) with PP, WP, PPAgNPs and WPAgNPs (500 μg/ml) were placed on the surface of MHA plates. The plates were incubated at 37 °C for 24 h. The inhibition zones around the discs were recorded by ruler (mm). The positive control was Levofloxacin antibiotic.
2.7. Larvicidal activity of plant extracts, silver nitrate and their combinations.
The 1st larval instar of Egyptian leaf worm was fed for 24 h on castor bean leaves treated with leaf dipping technique in solutions of PP and WP (1000, 2000 and 4000 µg/ml), PPAgNPs and WPAgNPs (8.5, 17 and 34 µg/ml) after air drying, whereas control treatments fed on castor bean leaves immerged in water. Each treatment was replicated three times (20 larvae/replicate). After 24 h exposure, the survival larvae were transferred to clean containers, fed on untreated leaves, and kept under observation till the end of each larval stage under constant conditions. The larval mortality was recorded and corrected according to Abbott (1925). The second experimental phase included the treatment of 1st larval instars with combinations of silver nitrate and plant extracts. The PPAgNPs or WPAgNPs (8.5 µg/ml) + 1000 µg/ml (pomegranate and watermelon extract), PPAgNPs or WPAgNPs (17 µg/ml) + 2000 µg/ml (pomegranate and watermelon extract) and PPAgNPs and WPAgNPs (34 µg/ml) + 4000 µg/ml (pomegranate and Watermelon extract) at 1:1 mixing ratio were freshly prepared. The larval mortality values were determined as mentioned before. The joint action was determined according to the equation of the co-toxicity factor given by Mansour et al. (1966) as follows in equation (3):
| (3) |
A positive factor of 20 or more is considered synergism, a factor of −20 or less means antagonism, and a value between –20 and + 20 is an additive effect expected.
Benz equation (Benz, 1971) was used to calculate the expected mortality in the combined treatment using the observed mortality in the separate treatments and then compared to the observed percentage mortality in the combined treatment.
WG = Corrected mortality and A&B = Treatments.
2.8. Statistical analysis
Regression toxicity lines were established for the pesticides and the slope, LC25, and LC50 values were determined through probit analysis (Finney, 1952). Data statistical analysis was performed using the Biostat 2009 software [version5.8.4.3, 2010]. Data of the biological studies were analyzed using (ANOVA) test and the means were analyzed using LSD test at (p ≤ 0.05) (Steel and Torrie, 1960).
3. Results
3.1. Green synthesis of PPAgNPs and WPAgNPs from phenolic extracts
Pomegranate and watermelon peels extracts were pale yellow or green but with the addition of silver nitrate solution, their color converted to dark brown within 24 h. The establishment of dark brown color after mixing the extracts with silver ions was a clear indicator of the metal ions reduction and the formation of silver nanoparticles in the mixture. The addition of phenolic extracts to silver nitrate solution at a ratio of (1:100 v,v) achive the best yield of nanoparticles. The transformation of silver nanoparticles without any precipitations indicate that synthesized nanoparticles have a very small particle size. During our experiments no precipitations was observed in nanoparticle solutions of pomegranate and watermelon peels extracts.
3.2. Characterization of PPAgNPs and WPAgNPs
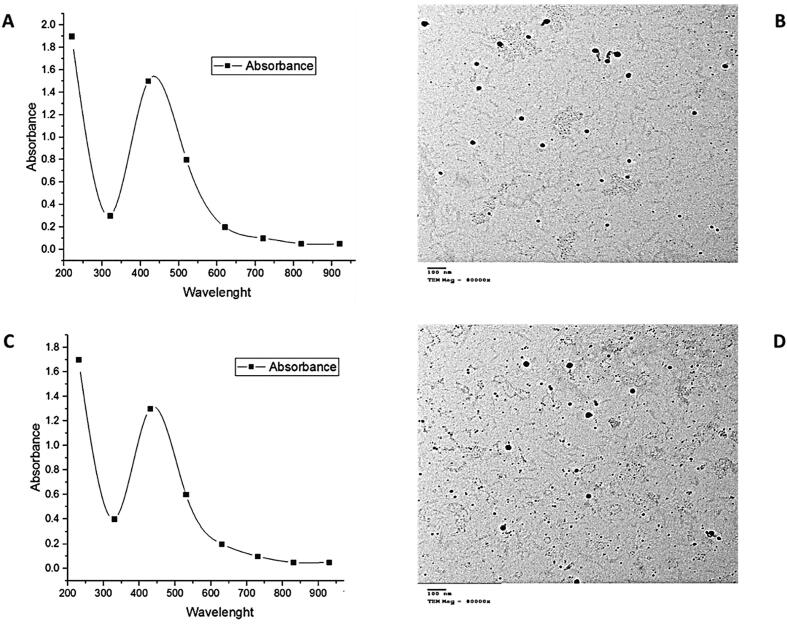
To confirm the formation and proper stability of silver nanoparticles, their absorption spectrum was read after 24 h in the range of 200–700 nm using a Laxco™ dual-beam spectrophotometer. Results of UV–Vis and TEM analyses presented in (Fig. 1) showed that the aqueous pomegranate and watermelon peels were produced different concentrations of silver nanoparticles. The highest absorbance at 420 nm was 1.5 a.u (absorbance unit) and recorded for PPAgNPs (Fig. 1A), which were synthesized using pomegranate peels extract, absorptions of 1.2 a.u observed at 430 nm for WPAgNPs synthesized by watermelon peels extract (Fig. 1C).
Fig. 1.
(A, B) UV spectrum and TEM of silver nanoparticles synthesized by pomegranate peels extract; (C, D) UV spectrum and TEM of silver nanoparticles synthesized by watermelon peels extract.
Fig. 1 (B, D) showed the morphological features of PPAgNPs and WPAgNPs, they were investigated via transmission electron microscope. The size of PPAgNPs was ranged from 15 to 70 nm; however, WPAgNPs size was in the range of 20–85 nm. The both silver nanoparticles were spherical. The difference in size mainly depended on the phenolic compounds content in extracts that employed to reduce silver ions, silver nitrate concentration, and temperature conditions.
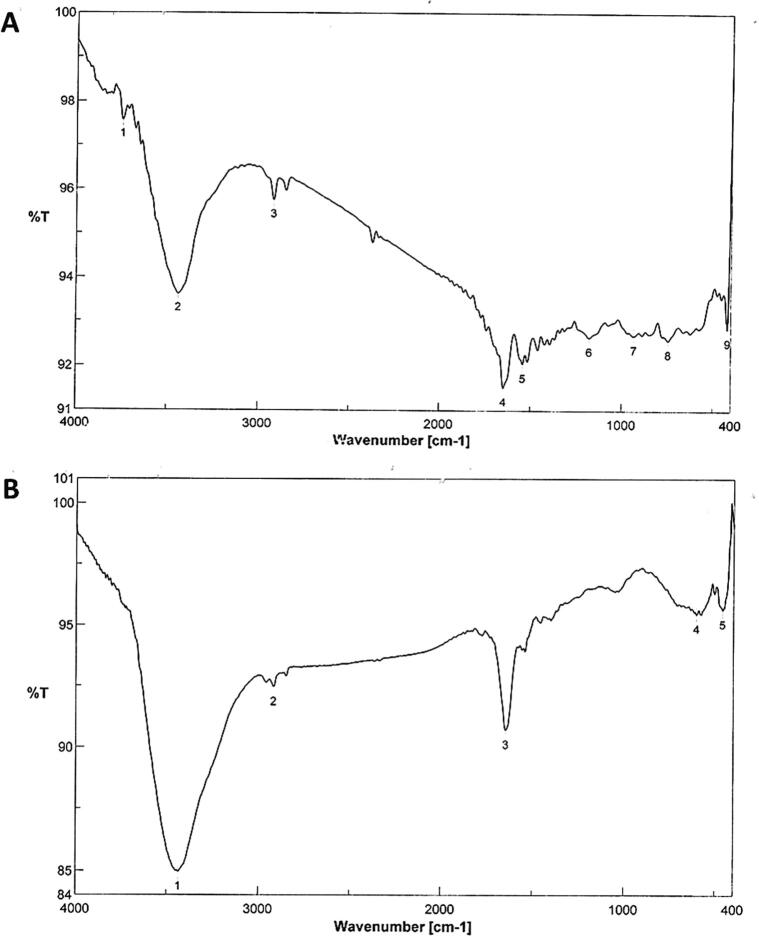
FTIR spectrum of PPAgNPs and WPAgNPs was shown in Fig. 2 (A,B). The spectrum revealed that, the tested AgNPs were capped with reducing polyphenols in pomegranate and watermelon peels extracts. Eight bands (3436, 2918, 1646, 1542, 1179, 933, 744, and 419 cm−1) were assigned to active groups in PPAgNPs suspension, however five bands (3436, 2918, 1646,603, and 460 cm−1) found in WPAgNPs FTIR spectrum. The active groups were NH of amide A overlapped with OH and CO of amide I at 3436, 1646 cm−1, respectively. In addition, aliphatic CH appeared at about 2918-cm_1 Furthermore, the CCO appeared at 1179 cm_1. These data strongly revealed the presence of phenolic compounds and protein covering AgNPs which may play a role in the reduction and stabilization of biosynthesized AgNPs.
Fig. 2.
(A) FTIR spectrum of silver nanoparticles synthesized by pomegranate peels extract; (B) FTIR spectrum of silver nanoparticles synthesized by watermelon peels extract. Fourier Transform Infrared Spectroscopy (FTIR).
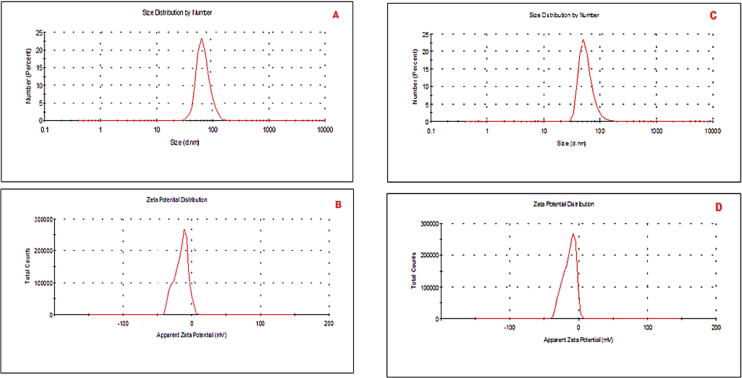
The DLS results of PPAgNPs were showed single peak in Fig. 3 (A, B). The average size was 61 nm with net negative charge of – 25.46 mV; however, Fig. 3 (C, D) showed the average size of WPAgNPs i.e., 75 and net negative charge −21.33 Results indicated that PPAgNPs, which were synthesized using pomegranate peels extract, had smaller size than WPAgNPs.
Fig. 3.
DLS analysis of green AgNPs fabricated by PPWE, and WPWE; A: WPAgNPs size, C: PP AgNPs size; B: PPAgNPs zeta potential, D: WPAgNPs zeta potential; dynamic light scattering (DLS). Pomegrante peels water extrat (PPWE); watermelon peels water extrat (WPWE); silver nanoparticles fabricated by pomegrnate peels extract (PPAgNPs); silver nanoparticles fabricated by watermelon peels extract (WPAgNPs).
3.3. Phenolic compounds content in PP, WP, PPAgNPs, and WPAgNPs
Table 1 showed the total phenolic and flavonoids content in pomegranate and watermelon peels extracts and silver nanoparticles. FTIR profile indicate the presense of phenolic compounds in nanoparticles suspension. Generally, the phenolic compounds significantly increased in silver nanoparticles about extracts where the total phenolic content in WPAgNPs and PPAgNPs increased with about 20% about PP and WP extract, and 15% increase in flavonoids content. The increase in polyphenols in nanoparticles because the quinoid compound produced due to the oxidation of the phenol group in phenolics can be adsorbed on the surface of nanoparticles increasing the phenolic content and accounting for their suspension stabilization. On the other hand, Table 2 showed the phenolic profile in PP, WP extracts, PPAgNPs, and WPAgNPs. The results in Table 2 were correlated with results in Table 1 where the phenolic content in silver nanoparticles significantly increased with about 10–75% in PPAgNPs, and WPAgNPs as compared to PP and WP extract. Quercetin, Gallic acid, Catechein, Cyanidin-3-o-glu, Punicalagin, Ellagic acid were the highest phenolic compounds content in PP extract i.e. (1.10, 5.70, 3.60, 1.50, 2.40, and 0.90 mg/g), and i.e (1.45, 6.22, 4.10, 2.15, 2.98, and 1.74 mg/g) in PPAgNPs. However, Genistein, Ferulic acid, and Kampeferol in medium content and other phenolic compounds in lower content.
Table 1.
Total phenolic and flavonoids in extracts and AgNPs (mean ± SD).
| Sample | Total flavonoids mg/g | Total phenolic mg/g |
|---|---|---|
| PPWE | 360 ± 0.9b | 580 ± 1.2b |
| WPWE | 286 ± 0.8d | 350 ± 1.1d |
| PPAgNPs | 420 ± 0.5a | 701 ± 0.9a |
| WPAgNPs | 325 ± 0.9c | 415 ± 0.5c |
Pomegrante peels water extrat (PPWE); watermelon peels water extrat (WPWE); silver nanoparticles fabricated by pomegrnate peels extract (PPAgNPs); silver nanoparticles fabricated by watermelon peels extract (WPAgNPs). Data are means ± SD;Means with different lowercase letters in the same column are significantly different (p ≤ 0.05).
Table 2.
Phenolic compounds content in extracts and silver nanoparticles (mg/g).
| Phenolic compound | PPWE | WPWE | PPAgNPs | WPAgNPs |
|---|---|---|---|---|
| Quercetin | 1.10b | 0.05d | 1.45a | 0.14c |
| Gallic acid | 5.70b | 2.60c | 6.22a | 2.88c |
| Benzoic acid | 0.09b | 0.03c | 0.34a | 0.12b |
| Genistein | 0.47b | 0.02d | 0.72a | 0.11c |
| Daidezein | 0.31b | 0.05d | 0.56a | 0.14c |
| Glycitein | 0.20b | 0.04c | 0.45a | 0.13b |
| Ferulic acid | 0.52b | 0.15d | 0.77a | 0.24c |
| Catechein | 3.60b | 1.50c | 4.10a | 1.77c |
| Vanillic | 0.04c | 0.03c | 0.29a | 0.12b |
| 3,4,5-methoxy-cinnamic | 0.45b | 0.17d | 0.70a | 0.26c |
| Rutin | 0.30b | 0.08d | 0.55a | 0.17c |
| Cyanidin-3-o-glu | 1.50b | nd | 2.15a | 0.01c |
| Punicalagin | 2.40 | nd | 2.98a | 0.03 |
| Luteolin-7-glucose | 0.13b | 0.08c | 0.38a | 0.17b |
| Apignin-7-glucose | 0.16b | 0.05c | 0.41a | 0.14b |
| P-oh- benzoic | 0.09b | 0.06c | 0.34a | 0.15b |
| Chlorogenic | 0.15b | 0.09c | 0.40a | 0.18b |
| Syringic | 0.09b | nd | 0.34a | 0.01c |
| Ellagic acid | 0.90b | 0.75c | 1.74a | 0.94b |
| Kampeferol | 0.60b | 0.56b | 0.85a | 0.75a |
Pomegrante peels water extrat (PPWE); watermelon peels water extrat (WPWE); silver nanoparticles fabricated by pomegrnate peels extract (PPAgNPs); silver nanoparticles fabricated by watermelon peels extract (WPAgNPs).Means with different lowercase letters in the same raw are significantly different (p ≤ 0.05).
3.4. Antioxidant activity of PP, WP, PPAgNPs, and WPAgNPs
Fig. 4A showed that PP extract scavenged 65% of DPPḢ; however, WP extract scavenged 57% of DPPḢ radical. The scavenging activity of DPPḢ was significantly increased with 20% in PPAgNPs and WPAgNPs as compared to TBHQ. The same route in Fig. 4B where ferric reducing power increased in silver nanoparticles than extracts with about 25%.
Fig. 4.
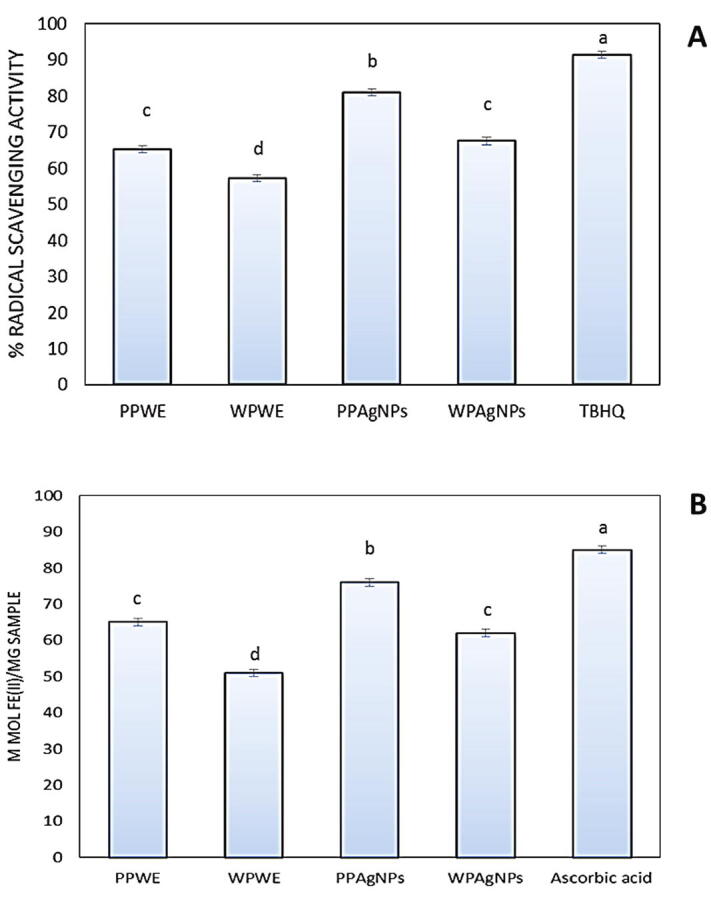
(A) DPPH scavenging activity of extracts and AgNPs after 30 min, (B) reducing power of extracts and AgNPs; 2,2-diphenyl-1-picrylhydrazyl (DPPH). Pomegrante peels water extrat (PPWE); watermelon peels water extrat (WPWE); silver nanoparticles fabricated by pomegrnate peels extract (PPAgNPs); silver nanoparticles fabricated by watermelon peels extract (WPAgNPs). Data are means ± SE means with different letters indicate significant differences (p < 0.05).
3.5. Antimicrobial activity of PP, WP, PPAgNPs, and WPAgNPs
Table 3 showed the antimicrobial activity of the polyphenolic extraxts (PP and WP) and green silver nanoparticles (PPAgNPs and WPAgNPs). The polyphenolic extracts and silver nanoparticles at 500 µg/mL significantly inhibited the bacterial and Candida growth with inhibition zones diameters in the range of (8–21 mm) as compared to levofloxacin 500 µg/mL with a range of 18–28 mm. The most sensitive G+ and G- bacteria were S. aureus, and K. penumonia; however, L. monocytogensis, and P. aeruginosa were the resistant ones to pomegranate and watermelon peels extracts and silver nanoparticles concentration. On the other hand, C. gelbrta was the most resistant yeast to polyphenolic extracts and silver nanoparticles. The PP extract has the best antimicrobial activity, consequently PPAgNPs because the highest phenolic content.
Table 3.
Antimicrobial activity of PPWE, WPWE, and green AgNPs (mean ± SD).
| Sample |
Microorganisms |
||||||||
|---|---|---|---|---|---|---|---|---|---|
|
Bacteria |
Fungi |
||||||||
|
G+ |
G- |
||||||||
| B. cereus | S. aureus | L. monocytogensis | E. coli | K. penumonia | P. aeruginosa | C.g elbrta | C. apis | C. albicans | |
| PPWE | 15 ± 0.1d | 18 ± 0.2d | 14 ± 0.6d | 9 ± 0.8d | 11 ± 0.7d | 8 ± 0.2d | 8 ± 0.2d | 10 ± 0.1d | 12 ± 0.2d |
| WPWE | 12 ± 0.2e | 15 ± 0.3e | 11 ± 0.1e | – | 8 ± 0.1e | – | – | – | 9 ± 0.0e |
| PPAgNPs | 22 ± 0.5b | 25 ± 0.7b | 21 ± 0.2b | 17 ± 0.3b | 18 ± 0.2b | 15 ± 0.2b | 16 ± 0.5b | 17 ± 0.5b | 19 ± 0.1b |
| WPAgNPs | 18 ± 0.6c | 21 ± 0.1c | 17 ± 0.9c | 13 ± 0.5c | 14 ± 0.3c | 11 ± 0.3c | 12 ± 0.2c | 13 ± 0.4c | 15 ± 0.9c |
| Levofloxacin | 25 ± 0.1a | 28 ± 0.5a | 24 ± 0.1a | 20 ± 0.7a | 21 ± 0.4a | 18 ± 0.5a | 19 ± 0.5a | 20 ± 0.2a | 22 ± 0.3a |
Pomegrante peels water extrat (PPWE); watermelon peels water extrat (WPWE); silver nanoparticles fabricated by pomegrnate peels extract (PPAgNPs); silver nanoparticles fabricated by watermelon peels extract (WPAgNPs). Data are means ± SD; Means with different small letters in the same column are significantly different (p < 0.05)
3.6. Insecticidal application of polyphenolic extracts and green silver nanoparticles
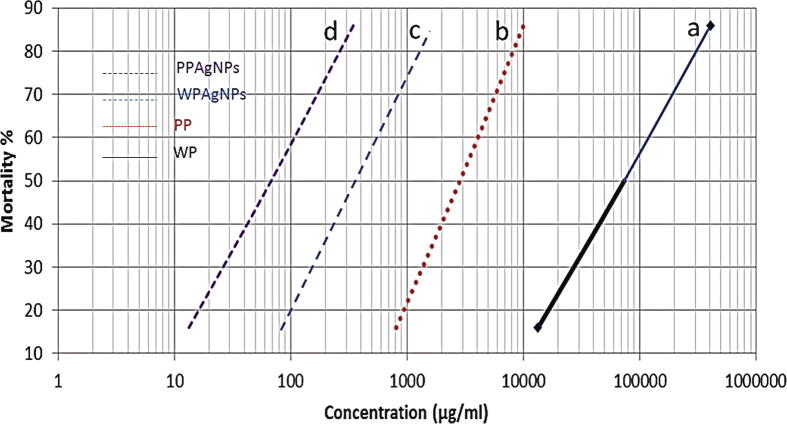
Exposure of the 1st larval instar of Spodoptera littoralis to PP, WP, PPAgNPs and WPAgNPs showed in Table 4. The larvicidal activities of tested substances against 1st instar larvae are presented in terms of mortality, LC25, LC50, LC90, and slope values in Fig. 5. In all treatments, the mortality rates significantly increased with increasing concentrations. The obtained resulted revealed that PPAgNPs was the most effective substance against the 1st instar larvae instar (LC50 = 68.32 µg/ml), followed by WPAgNPs with LC50 = 130.45 µg/ml whereas watermelon peel extract was the least effective substance (LC50 = 73681 µg/ml) however, pomegranate peel extract occupied the middle situation among the compounds (LC50 = 2852 µg/ml).
Table 4.
Larval toxicity of silver nitrate and plant extracts (Pomegranate and Watermelon) against Egyptian cotton leafworm.
| Mortality rate | PP | WP | PPAgNPs | WPAgNPs |
|---|---|---|---|---|
| LC25 | 1211b | 23156a | 22.69d | 60.51c |
| LC50 | 2852b | 73681a | 68.32d | 130.45c |
| LC90 | 14524b | 665319a | 555.32d | 1000c |
| Slope | 1.813 | 1.3412 | 1.4085 | 1.3941 |
Lethal concentration killing 25% of insects (LC25); Lethal concentration killing 50% of insects (LC50); Lethal concentration killing 90% of insects (LC90). Means with different lowercases at the same raw indicate significant differences (p < 0.05)
Fig. 5.
Toxicity lines of silver nitrate and plant extracts (Pomegranate and Watermelon) against Egyptian cotton leafworm, Pomegrante peels water extrat (PP); watermelon peels water extrat (WP); silver nanoparticles fabricated by pomegrnate peels extract (PPAgNPs); silver nanoparticles fabricated by watermelon peels extract (WPAgNPs). Means with different lowercases indicate significant differences.
The combined effect of PPAgNPs + PP and WPAgNPs + WP enhance their larvicidal activity and increasing mortality causing additive effect and synergism. The synergistic effect showed clearly with the lower combined concentration of PPAgNPs + PP and WPAgNPs + WP while increasing the combined concentration reduced their larvicidal effect and reduced mortality against 1st larval of S. littoralis Egyptian leafworms (Table 5).
Table 5.
The joint action of silver nanoparticles with pomegranate and watermelon peels extracts. (mean ± SD).
| Treatments | Combined concentration (µg/ml) | Observed mortality (%) | Expected mortality (%) | co-toxicity factor | Interaction |
|---|---|---|---|---|---|
| PPAgNPs + PPWE | 8.5 + 1000 | 20.00e | 10.00e | + 100.00 ± 0.0a | synergism |
| 17 + 2000 | 56.67c | 52.00b | + 8.98 ± 0.1d | additive | |
| 34 + 4000 | 90.00a | 78.33a | + 15.38 ± 0.3c | additive | |
| WPAgNP + WPWE | 8.5 + 1000 | 18.00e | 12.00e | + 50.86 ± 0.9b | synergism |
| 17 + 2000 | 33.33d | 22.66d | + 47.06 ± 0.8b | synergism | |
| 34 + 4000 | 62.00b | 45.55c | + 12.52 ± 0.4c | additive |
Pomegrante peels water extrat (PPWE); watermelon peels water extrat (WPWE); silver nanoparticles fabricated by pomegrnate peels extract (PPAgNPs); silver nanoparticles fabricated by watermelon peels extract (WPAgNPs).Data are preseted means ± SD. Means with different lowercases at the same column indicate significant differences
4. Discussion
Fruit and vegatables peels and wastes are a valuble source of phytochemicals, and their extracts exhibit considrable antioxidant and antimicrobial activity. Al-Sayed and Ahmed (2013) identified four phenolic compounds in sharlyn melon peels extract namely, 4-hydroxybenzoic acid, vanillin, chlorgenic acid, and coumaric acid. Also, the phenolic compounds, 3 hydroxybenzoic, chlorogenic, neochlorogenic, and isovanillic acids, also, apigenin7αglucoside, luteolin‐7‐o‐glucoside, and quercetin‐3‐galactoside in three melon flesh samples (Ganji et al., 2019), but, Major compounds in pomegranate peels extract were tannins and flavonoids such as; illogic acid, gallic acids, punicalin, and punicalagin (Al-Rawahi et al., 2014). Therefore, these phenolic extratcts were employed to synthsize various nanoparticles. Marchiol et al. (2014) used extracts of different organs (leaves, stems and roots) of various plants including Brassica juncea, Festuca rubra and Medicago sativa for synthesis of silver nanoparticles. Extracts of different plant organs indicated different performances during synthesis of nanoparticles. In Brassica juncea and Festuca rubra, root extracts showed a better performance, while, in Medicagosativa, leaf extracts were the best. In this study, the fabrication mechanism of AgNPs by polyphenols in pomegrnate and watermelon peels extracts briefed by the higher total phenolics content in pomegranate and watermelon peels extracts that facilitate the reduction of silver ions to nanoscale-sized silver particles. The electron donating ability of the phenolic compounds as they are strong antioxidants with high reducing capacity (Martínez-Castañon et al., 2008). The phyiscal and chemical properties of obtained AgNPs in this study are in agreement with Pirtarighat et al. (2019) who found that silver nanoparticles synthesized by Salvia spinosa extract exhibited surface plasmon resonance found at 450 nm confirmed the formation of AgNPs. Also, Padalia et al. (2015) produced silver nanoparticles by Calendula officinalis extract with size between 10 and 90 nm and they had spherical, hexagonal, and irregular shapes. In addition, Chidambaram et al. (2014) prepared silver nanoparticles from petal extracts of Calendula officinalis with 2–20 nm size ranges. Fruthermore, Baghizadeh et al. (2015), reported the synthesis of silver nanoparticles form marigold seed extracts with spherical shape and diameter of 5 to 25 nm. The active groups in AgNPs in our study similar to the same conclusion reported by Balakumaran et al., 2016, El-Bendary et al., 2020. The negative charge on the nanoparticles size ensure the the stability of the colloidal solutions. The samples of highly positive or negative values (+30 mV or −30 mV) remain stable without aggregation tendency (El-Saadony et al., 2019, Kratošová et al., 2019).
Abdel-Aziz et al. (2014) stated that the total phenolic compounds and total flavonides were higher in AgNPs-containing Chenopodium murale leaf extract compared to the plant extract. The highest phenolic content in AgNPs and waste extracts is attributed to their antioxidant and reducing activity. Adoni et al. (2020) found that green AgNPs synthesized by Artemisia annua leaf extract scavenged 84.99% of DPPH however; the scavenging activity of Artemisia annua extract was detected to be 73.04% at 500 µg/mL.
Similar results obtained by Adoni et al. (2020) who found that silver nanoparticles synthesized by Artemisia annua extract showed moderate inhibitory activity against E. coli, B. subtilis, S. aureous, and K. pneumoniae as compared to levofloxacin. Also, Phongtongpasuk et al. (2016) reported that eco-friendly AgNPs fabricated by dragon fruit peels extract have antibacterial activity against Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus. El-Bendary et al. (2020) found that silver nanoparticles have good performance against Candida species.
Watermelon rind extracts has mosquitocidal and antimicrobial properties. Watermelon extract showed potency against Culex quinquefasciatus (LC50 = 51.31 µg/ml) and lower effective on larvae of Aedes agypti (LC50 = 73681 µg/ml) (Aruna et al., 2014). Watermelon outer peel consists of various bioactive components, including citrulline and carotenoids (Quek et al., 2007, Mort et al., 2008). Many parts of pomegranate e.g. bark, leaves, fruit, and fruit peel have antimicrobial and antifungal (Al-Zoreky, 2009), molluscicidal (Tripathi and Singh, 2000) and insecticidal effect (Gandhi and Pillai, 2011). The insecticidal effect against larvae of the red flour beetle, Tribolium castaneum treated with aqueous pomegranate fruit peel extracts using topical application and ingestion treatment (10 and 26% mortality) with anti-feeding effects (53.26%) (Hamouda et al., 2014). The bioactivity of pomegranate peels extracts related to considerable levels of phenolic compounds (Jung, 2015) which are produced during cell metabolism as defense and signaling compounds as well as protecting the plant from various distress conditions besides their role relieve stresses against bacterial and fungal diseases (Devi et al., 2011). In addition, they may act as a potential insecticide (Ghaly et al., 2014). Flavonoids are also known to modulate the feeding and oviposition behavior of insects (Goławska and Łukasik, 2012). Flavanone naringine and the two flavanols, quercetin dehydrate and rutin hydrate prove aphicidal effect increase nymph’s mortality with raising the concentrations (Ateyyat et al., 2012, Gupta et al., 2017). Also, peel extracts pomegranate disrupting development and causing deformities involved in vital activities like feeding, walking, or flying, making the insect vulnerable to several sorts of mortality agents and preventing them from causing damage to the crop (Hamouda et al., 2014). The phytochemical constituents present in petroleum ether extract of Punica granatum peel were phenols and saponins caused mortality against third instar larvae of Culex pipiens (LC50 = 95.6632 µg/ml) (Farag et al., 2018) beside tannins (Koide et al., 1998).
The synergism mechanism may be due to plant aqueous extract synthesized AgNPs with insecticidal potentiation (Gul et al., 2016). AgNPs are more reactive because of their increased surface to volume ratio and excess of the phenolic compounds that attached to nanoparticles surface (Vani and Brindhaa, 2013, Di Ilio and Cristofaro, 2021). Besides they can absorbed easily into the cuticular lipids causing physical damage (Barik et al., 2008) and causing partial lysis of the midgut epithelial cells; vesicles and damaged membranes at the apical side of epithelial cells (Foldbjerg et al., 2015, Sultana et al., 2018). While the permeable free silver ions can induce the oxidative stress and detoxification genes (Nair et al., 2013). Another proposed mechanism for AgNPs could be due to the denaturation of proteins containing the sulfur or phosphorous compounds like DNA that drove to the denaturation of enzymes and organelles (Choi et al., 2008, Sondi and Salopek-Sondi, 2004) causing stop ATP synthesis.
5. Conclusions
Agricultural wastes are rich in polyphenols. In this study pomegranate and watermelon peels extracts were employed to synthesize green silver nanoparticles with considerable antioxidant and antimicrobial activity because of higher content of phenolic compounds. The obtained PPAgNPs proved the insecticidal effectiveness against the first instar of Egyptian cotton leafworm with synergistic effect when combined with the tested extracts (pomegranate and watermelon) especially at lower concentration. Increasing the concentrations of combination lower potency causing additive effect.
Author contributions:
A.M.S.; M.T.E-S.; M. M.R.; A.M.E.T and T.F.T. designed the study plan. A.M.S.; M.T.E-S.; M. M.R.; and T.F.T. helped in conducting the experiment and collected literature. A.M.S.; M.T.E-S.; S.S; A.E.T; and M.A.M.M. analyzed the data and drafted the manuscript. S.E.E.F., A.A.N, S.O.A, F.M.A., and M.E.S. provided technical help in writing the manuscript. All the authors read and approved the final version of the manuscript.
Funding
The current work was funded by Taif University Researchers Supporting Program (Project number: TURSP-2020/92), Taif University, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ackknowledgement
The authors thank Taif University, Saudi Arabia, for financial support through its Researchers Supporting Project (TURSP-2020–105).
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Ahmed M. Saad, Email: ahmedm4187@gmail.com.
Samy Sayed, Email: samy_mahmoud@hotmail.com.
Moataz A.M. Moustafa, Email: moataz.moustafa79@gmail.com.
References
- Abbott W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925;18:265–267. [Google Scholar]
- Abdel-Aziz M.S., Shaheen M.S., El-Nekeety A.A., Abdel-Wahhab M.A. Antioxidant and antibacterial activity of silver nanoparticles biosynthesized using Chenopodium murale leaf extract. J. Saudi Chem. 2014;18:356–363. [Google Scholar]
- Adoni M., Yadam M., Gaddam S.A., Rayalacheruvu U., Kotakadi V.S. Antimicrobial, antioxidant, and dye degradation properties of biosynthesized silver nanoparticles from Artemisia annua L. Lett. Appl. NanoBioSci. 2020;10:1981–1992. [Google Scholar]
- Akl B., Nader M.M., El-Saadony M. Biosynthesis of silver nanoparticles by Serratia marcescens ssp sakuensis and its antibacterial application against some pathogenic bacteria. J. Agri. Chem. Biotechnol. 2020;11:1–8. [Google Scholar]
- Al-Rawahi A.S., Edwards G., Al-Sibani M., Al-Thani G., Al-Harrasi A.S., Rahman M.S. Phenolic constituents of pomegranate peels (Punica granatum L.) cultivated in Oman. Euro. J. Med. 2014;Plants:315–331. [Google Scholar]
- Al-Sayed H.M., Ahmed A.R. Utilization of watermelon rinds and sharlyn melon peels as a natural source of dietary fiber and antioxidants in cake. Annals Agric. Sci. 2013;58:83–95. [Google Scholar]
- Al-Zoreky N. Antimicrobial activity of pomegranate (Punica granatum L.) fruit peels. Int. J. Food Microbiol. 2009;134:244–248. doi: 10.1016/j.ijfoodmicro.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Aruna A., Vijayalakshmi K., Karthikeyan V. Larvicidal activity of Methanolic Extract of the leaves of Citrullus lanatus. Int. J. Advanc. Pharm. Biol. Chem. 2014;3:717–722. [Google Scholar]
- Ashour E.A., Abd El-Hack M.E., Shafi M.E., Alghamdi W.Y., Taha A.E., Swelum A.A., Tufarelli V., Mulla Z.S., El-Ghareeb W.R., El-Saadony M.T. Impacts of green coffee powder supplementation on growth performance, carcass characteristics, blood indices, meat quality and gut microbial load in broilers. Agriculture. 2020;10:457. [Google Scholar]
- Ateyyat M., Abu-Romman S., Abu-Darwish M., Ghabeish I. Impact of flavonoids against woolly apple aphid, Eriosoma lanigerum (Hausmann) and its sole parasitoid, Aphelinus mali (Hald.) J. Agric. Sci. 2012;4:227. [Google Scholar]
- Baghizadeh A., Ranjbar S., Gupta V.K., Asif M., Pourseyedi S., Karimi M.J., Mohammadinejad R. Green synthesis of silver nanoparticles using seed extract of Calendula officinalis in liquid phase. J. Mol. Liq. 2015;207:159–163. [Google Scholar]
- Bakr R.F., Al Yousef A.B.F. Toxicological effect of the botanical extract castor oil seeds Ricinus communis and their biochemical activity on the cotton leafworm, Spodoptera littoralis (Boisd.)(Lepidoptera: Noctuidae). Egypt. Acad. J. Biol. Sci. C, Physiol. Mol. Biol. 2015;7:99–111. [Google Scholar]
- Balakumaran M., Ramachandran R., Balashanmugam P., Mukeshkumar D., Kalaichelvan P. Mycosynthesis of silver and gold nanoparticles: optimization, characterization and antimicrobial activity against human pathogens. Microbiol. Res. 2016;182:8–20. doi: 10.1016/j.micres.2015.09.009. [DOI] [PubMed] [Google Scholar]
- Barik T., Sahu B., Swain V. Nanosilica—from medicine to pest control. Parasitol. Res. 2008;103:253–258. doi: 10.1007/s00436-008-0975-7. [DOI] [PubMed] [Google Scholar]
- Benelli G. Mode of action of nanoparticles against insects. Envir. Sci. Pol. Res. 2018;25:12329–12341. doi: 10.1007/s11356-018-1850-4. [DOI] [PubMed] [Google Scholar]
- Benz, G., 1971. Synergism of micro-organisms and chemical insecticides. Burges, HD Microbial control of insects & mites.
- Benzie I.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal. Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Bhakya S., Muthukrishnan S., Sukumaran M., Muthukumar M. Biogenic synthesis of silver nanoparticles and their antioxidant and antibacterial activity. Appli. Nanosci. 2016;6:755–766. [Google Scholar]
- Bhattacharyya A., Bhaumik A., Rani P.U., Mandal S., Epidi T.T. Nano-particles-A recent approach to insect pest control. African J. Biotechnol. 2010;9:3489–3493. [Google Scholar]
- Bodaiah B., Kiranmayi M., Sudhakar P., Varma A., Bhushanam K. Insecticidal activity of green synthesized silver nanoparticles. Int. J. Recent Sci. Res. 2016;7:10652–10656. [Google Scholar]
- Borase H.P., Salunke B.K., Salunkhe R.B., Patil C.D., Hallsworth J.E., Kim B.S., Patil S.V. Plant extract: a promising biomatrix for ecofriendly, controlled synthesis of silver nanoparticles. Appl. Biochem. Biotechnol. 2014;173:1–29. doi: 10.1007/s12010-014-0831-4. [DOI] [PubMed] [Google Scholar]
- Chen Y., Chen G., Fu X., Liu R.-H. Phytochemical profiles and antioxidant activity of different varieties of Adinandra tea (Adinandra jack) J. Agric Food Chem. 2015;63:169–176. doi: 10.1021/jf503700v. [DOI] [PubMed] [Google Scholar]
- Chen Y., Ma X., Fu X., Yan R. Phytochemical content, cellular antioxidant activity and antiproliferative activity of Adinandra nitida tea (Shiyacha) infusion subjected to in vitro gastrointestinal digestion. RSC Adv. 2017;7:50430–50440. [Google Scholar]
- Chidambaram J., Saritha K., Maheswari R., Muzammil M.S. Efficacy of green synthesis of silver nanoparticles using flowers of Calendula officinalis. Chem. Sci. Trans. 2014;3:773–777. [Google Scholar]
- Choi O., Deng K.K., Kim N.-J., Ross L., Jr, Surampalli R.Y., Hu Z. The inhibitory effects of silver nanoparticles, silver ions, and silver chloride colloids on microbial growth. Water Res. 2008;42:3066–3074. doi: 10.1016/j.watres.2008.02.021. [DOI] [PubMed] [Google Scholar]
- Dahham S.S., Ali M.N., Tabassum H., Khan M. Studies on antibacterial and antifungal activity of pomegranate (Punica granatum L.) Am. Eurasian J. Agric. Environ. Sci. 2010;9:273–281. [Google Scholar]
- Devi A., Singh V., Bhatt A. In vitro antibacterial activity of pomegranate and Daru (wild pomegranate) against dental plaque bacteria. Int. J. Pharm. Pharm. Sci. 2011;3:182–184. [Google Scholar]
- Di Ilio V., Cristofaro M. Polyphenolic extracts from the olive mill wastewater as a source of biopesticides and their effects on the life cycle of the Mediterranean fruit fly Ceratitis capitata (Diptera, Tephriditae) I. J. Tropical Insect Sci. 2021;41:359–366. [Google Scholar]
- El-Bendary M.A., Moharam M.E., Abdelraof M., Allam M.A., Roshdy A.M., Shaheen M.N., Elmahdy E.M., Elkomy G.M. Multi-bioactive silver nanoparticles synthesized using mosquitocidal Bacilli and their characterization. Arch. Microbiol. 2020;202:63–75. doi: 10.1007/s00203-019-01718-9. [DOI] [PubMed] [Google Scholar]
- El-Saadony M.T., El-Wafai N.A., El-Fattah A., Mahgoub S. Biosynthesis, optimization and characterization of silver nanoparticles biosynthesized by Bacillus subtilis ssp spizizenii MT5 isolated from heavy metals polluted soil. Zagazig J. Agric. Res. 2018;45(6):2439–2454. [Google Scholar]
- El-Saadony M.T., El-Wafai N.A., El-Fattah H.I.A., Mahgoub S.A. Biosynthesis, optimization and characterization of silver nanoparticles using a soil isolate of Bacillus pseudomycoides MT32 and their antifungal activity against some pathogenic fungi. Adv. Anim. Vet. Sci. 2019;7:238–249. [Google Scholar]
- El-Saadony M.T., El-Hack A., Mohamed E., Taha A.E., Fouda M.M., Ajarem J.S., Maodaa NS., Allam A.A., Elshaer N. Ecofriendly synthesis and insecticidal application of copper nanoparticles against the storage pest Tribolium castaneum. Nanomaterials. 2020;10:587. doi: 10.3390/nano10030587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., Sitohy M.Z., Ramadan M.F., Saad A.M. Green nanotechnology for preserving and enriching yogurt with biologically available iron (II) Innovative Food Sci. Emerging Technol. 2021;69(1) [Google Scholar]
- El-Saadony M.T., Desoky E.-S.M., Saad A.M., Eid R.S., Selem E., Elrys A.S. Biological silicon nanoparticles improve Phaseolus vulgaris L. yield and minimize its contaminant contents on a heavy metals-contaminated saline soil. J. Enviro. Sci. 2021;106:1–14. doi: 10.1016/j.jes.2021.01.012. [DOI] [PubMed] [Google Scholar]
- El-Saadony, M. T., Saad, A. M., Najjar, A. A., Alzahrani, S. O., Alkhatib, F. M., Selem, E., Desoky, S. M., Fouda, S. S., El-Tahan, A. M., Hassan, M. A.A. (2021c). The use of biological selenium nanoparticles in controlling Triticum aestivum L. crown root and rot diseases induced by Fusarium species and improve yield under drought and heat stress. Saudi J. Biol. Sci. In press. [DOI] [PMC free article] [PubMed]
- Eldefrawi M., Toppozada A., Mansour N., Zeid M. Toxicological studies on the Egyptian cotton leafworm, Prodenia litura. I. Susceptibility of different larval instars of Prodenia to insecticides. J. Economic Entomol. 1964;57:591–593. [Google Scholar]
- Farag S., Adel Hussein M., Hafez S.E., Khaled A.S., Kamel O.M.H.M., Zyaan O.H. Ultra-structural studies on the midgut of culex pipiens larvae treated with pomegranate peel extract, Punica granatum. J. Egy. Soc. Parasitology. 2018;48:77–84. [Google Scholar]
- Finney D.J. Cambridge University Press; Cambridge: 1952. Probit analysis: a statistical treatment of the sigmoid response curve. [Google Scholar]
- Foldbjerg R., Jiang X., Miclăuş T., Chen C., Autrup H., Beer C. Silver nanoparticles–wolves in sheep's clothing? Toxicol. Res. 2015;4:563–575. [Google Scholar]
- Gandhi N., Pillai S. Control of Rhyzopertha dominica (Coleoptera: Bostrichidae) by pulverized leaves of Punica granatum (Lythraceae) and Murraya koenigii (Rutaceae) I J. Agric. Biol. 2011;13 [Google Scholar]
- Ganji S.M., Singh H., Friedman M. Phenolic content and antioxidant activity of extracts of 12 melon (Cucumis melo) peel powders prepared from commercial melons. J. Food Sci. 2019;84:1943–1948. doi: 10.1111/1750-3841.14666. [DOI] [PubMed] [Google Scholar]
- Ghaly N., Mina S., Abdel-Aziz N., Sammour E. Insecticidal activity of the main flavonoids from the leaves of Kalanchoe beharensis and Kalanchoe longiflora. J. Nat. Prod. 2014;7:196–202. [Google Scholar]
- Goławska S., Łukasik I. Antifeedant activity of luteolin and genistein against the pea aphid Acyrthosiphon pisum. J. Pest. Sci. 2012;85:443–450. doi: 10.1007/s10340-012-0452-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goupy P., Hugues M., Boivin P., Amiot M.J. Antioxidant composition and activity of barley (Hordeum vulgare) and malt extracts and of isolated phenolic compounds. J. Sci. Food Agri. 1999;79:1625–1634. [Google Scholar]
- Gul S., Ismail M., Khan M.I., Khan S.B., Asiri A.M., Rahman I.U., Khan M.A., Kamboh M.A. Novel synthesis of silver nanoparticles using melon aqueous extract and evaluation of their feeding deterrent activity against housefly Musca domestica. Asian Pacific J. Tropical Dis. 2016;6:311–316. [Google Scholar]
- Gupta G., Dharma K., Kumar N.R. Insecticidal effects of aqueous extracts of wild pomegranate peel and seed (Punica granatum L.) against rose aphids. Macrosiphum Rosaeformis. J. Appli Natural Sci. 2017;9:1397–1405. [Google Scholar]
- Hamouda A.B., Mechi A., Zarred K., Chaieb I., Laarif A. Insecticidal Activities of Fruit Peel Extracts of Pomegranate (Punica granatum) against the red flour beetle Tribolium castaneum. Tunis. Tunisian J. Plant Prot. 2014;9:91–100. [Google Scholar]
- Hassanin A.A., Saad A.M., Bardisi E.A., Salama A., Sitohy M.Z. Transfer of anthocyanin accumulating Delila and Rosea1 genes from the transgenic tomato Micro-Tom cultivar to Moneymaker cultivar by conventional breeding. J. Agric Food Chem. 2020;68(39):10741–10749. doi: 10.1021/acs.jafc.0c03307. [DOI] [PubMed] [Google Scholar]
- Jacob J.M., John M.S., Jacob A., Abitha P., Kumar S.S., Rajan R., Natarajan S., Pugazhendhi A. Bactericidal coating of paper towels via sustainable biosynthesis of silver nanoparticles using Ocimum sanctum leaf extract. Mater. Res. Express. 2019;6 [Google Scholar]
- Jung J.-S. Insecticidal effects from ethanol extracts of root peel, stem peel, and fruit peel of pomegranate (Punica granatum L.) on house dust mite. Int. J. Bio-Sci. Bio-Technol. 2015;7:25–36. [Google Scholar]
- Koide T., Nose M., Inoue M., Ogihara Y., Yabu Y., Ohta N. Trypanocidal effects of gallic acid and related compounds. Planta Med. 1998;64:27–30. doi: 10.1055/s-2006-957360. [DOI] [PubMed] [Google Scholar]
- Kratošová G., Holišová V., Konvičková Z., Ingle A.P., Gaikwad S., Škrlová K., Prokop A., Rai M., Plachá D. From biotechnology principles to functional and low-cost metallic bionanocatalysts. Biotechnol. Adv. 2019;37:154–176. doi: 10.1016/j.biotechadv.2018.11.012. [DOI] [PubMed] [Google Scholar]
- Mansour N., Eldefrawi M., Toppozada A., Zeid M. Toxicological studies on the Egyptian cotton leaf worm, Prodenia litura. VI. Potentiation and antagonism of organophosphorus and carbamate insecticides. J. Econ. Entomol. 1966;59:307–311. [Google Scholar]
- Marchiol L., Mattiello A., Pošćić F., Giordano C., Musetti R. In vivo synthesis of nanomaterials in plants: location of silver nanoparticles and plant metabolism. Nanoscale Res. Lett. 2014;9:1–11. doi: 10.1186/1556-276X-9-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Castañon G.-A., Nino-Martinez N., Martinez-Gutierrez F., Martinez-Mendoza J., Ruiz F. Synthesis and antibacterial activity of silver nanoparticles with different sizes. J. Nanoparticle Res. 2008;10:1343–1348. [Google Scholar]
- Mattila P., Astola J., Kumpulainen J. Determination of flavonoids in plant material by HPLC with diode-array and electro-array detections. J. Agric Food Chem. 2000;48:5834–5841. doi: 10.1021/jf000661f. [DOI] [PubMed] [Google Scholar]
- Mort A., Zheng Y., Qiu F., Nimtz M., Bell-Eunice G. Structure of xylogalacturonan fragments from watermelon cell-wall pectin. Endopolygalacturonase can accommodate a xylosyl residue on the galacturonic acid just following the hydrolysis site. Carbo Res. 2008;343:1212–1221. doi: 10.1016/j.carres.2008.03.021. [DOI] [PubMed] [Google Scholar]
- Murugan K., Nataraj D., Madhiyazhagan P., Sujitha V., Chandramohan B., Panneerselvam C., Dinesh D., Chandirasekar R., Kovendan K., Suresh U. Carbon and silver nanoparticles in the fight against the filariasis vector Culex quinquefasciatus: genotoxicity and impact on behavioral traits of non-target aquatic organisms. Parasitol Res. 2016;115:1071–1083. doi: 10.1007/s00436-015-4837-9. [DOI] [PubMed] [Google Scholar]
- Nair P.M.G., Park S.Y., Choi J. Evaluation of the effect of silver nanoparticles and silver ions using stress responsive gene expression in Chironomus riparius. Chemosphere. 2013;92:592–599. doi: 10.1016/j.chemosphere.2013.03.060. [DOI] [PubMed] [Google Scholar]
- Padalia H., Moteriya P., Chanda S. Green synthesis of silver nanoparticles from marigold flower and its synergistic antimicrobial potential. Arabian J. Chem. 2015;8:732–741. [Google Scholar]
- Phongtongpasuk S., Poadang S., Yongvanich N. Environmental-friendly method for synthesis of silver nanoparticles from dragon fruit peel extract and their antibacterial activities. Energy Procedia. 2016;89:239–247. [Google Scholar]
- Pirtarighat S., Ghannadnia M., Baghshahi S. Green synthesis of silver nanoparticles using the plant extract of Salvia spinosa grown in vitro and their antibacterial activity assessment. J. Nanostructure Chem. 2019;9:1–9. [Google Scholar]
- Quek S.Y., Chok N.K., Swedlund P. The physicochemical properties of spray-dried watermelon powders. Chem. Eng. Process. Process Intensif. 2007;46:386–392. [Google Scholar]
- Rai M., Ingle A. Role of nanotechnology in agriculture with special reference to management of insect pests. Appli. Microbial. Biotechnol. 2012;94:287–293. doi: 10.1007/s00253-012-3969-4. [DOI] [PubMed] [Google Scholar]
- Reda F.M., El-Saadony M.T., El-Rayes T.K., Attia A.I., El-Sayed S.A., Ahmed S.Y., Madkour M., Alagawany M. Use of biological nano zinc as a feed additive in quail nutrition: biosynthesis, antimicrobial activity and its effect on growth, feed utilisation, blood metabolites and intestinal microbiota. Ital. J. Animal Sci. 2021;20:324–335. [Google Scholar]
- Reda F.M., El-Saadony M.T., Elnesr S.S., Alagawany M., Tufarelli V. Effect of dietary supplementation of biological curcumin nanoparticles on growth and carcass traits, antioxidant status, immunity and caecal microbiota of Japanese quails. Animals. 2020;10:754. doi: 10.3390/ani10050754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolim P., Fidelis G., Padilha C., Santos E., Rocha H., Macedo G. Phenolic profile and antioxidant activity from peels and seeds of melon (Cucumis melo L. var. reticulatus) and their antiproliferative effect in cancer cells. Brazilian J. Med. Biol. Res. 2018;51 doi: 10.1590/1414-431X20176069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad A.M., Mohamed A.S., Ramadan M.F. Storage and heat processing affect flavors of cucumber juice enriched with plant extracts. Int. J. Veg. Sci. 2020;27:277–287. [Google Scholar]
- Sabbour M.M., Abd El-Aziz S.E., Shadia E. Efficacy of nano-diatomaceous earth against red flour beetle, Tribolium castaneum and confused flour beetle, Tribolium confusum (Coleoptera: Tenebrionidae) under laboratory and storage conditions. Bull. Environ. Pharmacol. Life Sci. 2015;4:54–59. [Google Scholar]
- Saad, A.M., El‐Saadony, M.T., Mohamed, A.S., Ahmed, A.I., Sitohy, M.Z., 2021. Impact of cucumber pomace fortification on the nutritional, sensorial and technological quality of soft wheat flour‐based noodles. Int. J. Food Sci. Technol. In press.
- Saranya, S., Selvi, A., Babujanarthanam, R., Rajasekar, A., Madhavan, J., 2020. Insecticidal Activity of Nanoparticles and Mechanism of Action, Model Organisms to Study Biological Activities and Toxicity of Nanoparticles. Springer, pp. 243–266.
- Sheiha A.M., Abdelnour S.A., El-Hack A., Mohamed E., Khafaga A.F., Metwally K.A., Ajarem J.S., Maodaa S.N., Allam A.A., El-Saadony M.T. Effects of dietary biological or chemical-synthesized nano-selenium supplementation on growing rabbits exposed to thermal stress. Animals. 2020;10:430. doi: 10.3390/ani10030430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sondi I., Salopek-Sondi B. Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interf. Sci. 2004;275:177–182. doi: 10.1016/j.jcis.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Steel, R.G.D., Torrie, J.H., 1960. Principles and procedures of statistics. Principles and procedures of statistics.
- Sultana N., Raul P.K., Goswami D., Das B., Gogoi H.K., Raju P.S. Nanoweapon: control of mosquito breeding using carbon-dot-silver nanohybrid as a biolarvicide. Enviro. Chem. Lett. 2018;16:1017–1023. [Google Scholar]
- Tian X., Jiang X., Welch C., Croley T.R., Wong T.-Y., Chen C., Fan S., Chong Y., Li R., Ge C. Bactericidal effects of silver nanoparticles on lactobacilli and the underlying mechanism. ACS Appl. Mater. Interfaces. 2018;10:8443–8450. doi: 10.1021/acsami.7b17274. [DOI] [PubMed] [Google Scholar]
- Tripathi S., Singh D. Molluscicidal activity of Punica granatum bark and Canna indica root. Brazilian J. Med. Biol. Res. 2000;33:1351–1355. doi: 10.1590/s0100-879x2000001100014. [DOI] [PubMed] [Google Scholar]
- Vani C., Brindhaa U. Silica nanoparticles as nanocides against Corcyra cephalonica (S.), the stored grain pest. Int. J. Pharma Bio Sciences. 2013;4 [Google Scholar]
- Yuan, Z., Fang, Y., 2018. Flavonol s and F lavone s C hanges in P omegranate (Punica granatum L.) Fruit peel during F ruit D evelopment.