Abstract
Investigation of genetic variability and population relationship of 50 accessions of the apricot (Prunus armeniaca L.) was carried out using ISSR markers. The results revealed that the number of alleles per locus varied from 4 to 8 with a mean value of 6.75, and the mean effective number of alleles (Ne) per locus was 1.54. Similarly, the polymorphic information content (PIC) values ranged from 0.464 to 0.424, with a mean value of 0.424. The mean heterozygosity, marker index, resolving power, and effective multiplex ratio (EMR) ranged from 0.001 to 0.002, 0.01–0.06, 1.76–3.84, and 1–4.12. The dendrogram clustered genotypes into two main clades based on their origins. The population structure revealed two sub-populations with some admixtures. The average expected heterozygosity and population differentiation within two sub-populations was 0.1428 and 0.216, respectively. The results outcome reveals that the four ISSR markers comprehensively separated the indigenous germplasm from the exotic germplasm. The genetic divergence within indigenous genotypes and exotic genotypes could allow for future insights into apricot breeding programs.
Keywords: Diversity, Apricot, Polymorphism, Genotype, Structure, Population
1. Introduction
Apricot (Prunus armeniaca L.), originating from the Rosaceae family, is a remarkably beneficial stone fruit, which is extensively dispersed throughout the globe (Decroocq et al., 2003). It first grew in the Tien-Shan Mountains, in Central Asia, dispersing Eastern and Western regions (Zhebentyayeva et al., 2012). In Asia, a majorly apricot grows in Northwestern Himalayan regions such as Tibet, Southern regions of China, and few temperate areas of Northern areas of India viz., Himachal Pradesh, Jammu and Kashmir, and Uttarakhand. Among the stone fruits, apricot reveals the highest genetic divergence, showing robust relation between the cultivars and the regions of their production. It has been established that genetic diversity analysis during gross vegetative development necessitates a longer and extensive interpretation. To ensure long-term changes to the unpredictable ecological environment, genetic diversity is essential. (Sreekanth et al., 2012). Rich plant genetic diversity can present immense records of genetic history for crop genetics and breeding experimentations. Various investigations have demarcated that genetic drift and artificial selection have depleted an immense amount of genetic variation in crops, thereby decreasing the germplasm potential of crops in the current agriculture setup (Laido et al., 2013). The evaluation of genetic variability of crop germplasm is essential for selecting superior genotypes and conservation of those resources at a high risk of getting extinct (Ouborg et al., 2006, Shah et al., 2021). Conventional methods based on phenotypic studies used to determine, distinguish cultivars, and ancestry of fruit tree species are unprogressive and have a high environmental impact. Therefore, different approaches must be adopted to overcome these issues. Genetic markers are currently imperative for the analysis of diversity analysis and classification of plant germplasms with no environmental influence showing a high rate of reproducibility and efficiency. For evaluation of variability and genetic characterization of apricot and other stone fruits, numerous kinds of genetic markers have been employed, such as SSR, RAPD, AFLP, and RFLP (Hormaza, 2002, Hurtado et al., 2002, Yilmaz et al., 2012, Ballester and de-Vicente, 1998, Wang et al., 2011; Shah et al., 2020). The low reproducibility of RAPD, high cost of AFLP and SSR primer’s development the sequence of flanking regions must be known are some limitations of these primers. ISSR’s are best to overcome these limitations (Reddy et al., 2002). These markers have been employed in certain fruit crops, which include plum (Lisek et al., 2007), pistachio (Kafkas et al., 2006), olive (Terzopoulos et al., 2005), mulberry (Vijayan and Chatterjee, 2003, Vijayan et al., 2006a, Vijayan et al., 2006b) and citrus (Shahsavar et al., 2007, Abouzari et al., 2020) for the intend of variety determination, cultivar characterization, assessment of natural population variability, evaluation of phylogenetic relationship, innovative breeding, and genetic linkage mapping. ISSR primers were also used in genus Prunus (Yilmaz et al., 2012, Goulao et al., 2001, Li et al., 2009, Liu and Liu., D., Zhang, A., Feng, C., Yang, J., Yoon, J., Li. S., , 2007, Shahi-Gharahlar et al., 2011, Ganopoulos et al., 2011) and revealed superior levels of reproducibility and a higher rate of polymorphism as compared to AFLP (Goulao et al., 2001, Hassanpour et al., 2013, Yılmaz et al., 2009, Yilmaz et al., 2009). ISSR markers are one of the most efficient markers because of their capacity to reveal several informative bands from a single amplification (Farajpour et al., 2011). Therefore, the current investigation was carried on the 50 genotypes using 4 ISSR markers to examine the genetic diversity between the apricot accessions grown in J&K and reveal the core structure between and within populations.
2. Material and methods
2.1. Plant material
The current investigation was done during 2019–2020 in the CITH, Srinagar. A total of 50 exclusive apricot genotypes collected from various geographical areas of Jammu and Kashmir were used for the investigation. The information on coordinates and habitat characteristics are presented in (Table 1).
Table 1.
Apricot accessions utilized in this investigation.
| S.NO | Cultivar Name | Location | District | Latitude | Longitude | Origin |
|---|---|---|---|---|---|---|
| 1 | Harcot | CITH | Budgam | 33.9749°N | 74.7895°E | Exotic |
| 2 | Hartlay | CITH | Budgam | 33.9741°N | 74.7889°E | Exotic |
| 3 | Irani | CITH | Budgam | 33.9739°N | 74.7884°E | Exotic |
| 4 | Communis-Holi | CITH | Budgam | 33.9725°N | 74.7882°E | Exotic |
| 5 | Tilton | CITH | Budgam | 33.9719°N | 74.7875°E | Exotic |
| 6 | Rival | CITH | Budgam | 33.9716°N | 74.7872°E | Exotic |
| 7 | Tokpopa nimu | CITH | Budgam | 33.9711°N | 74.7863°E | Exotic |
| 8 | Fair medister | CITH | Budgam | 33.9706°N | 74.7858°E | Exotic |
| 9 | Viva Gold | CITH | Budgam | 33.9701°N | 74.7850°E | Exotic |
| 10 | Cummins | CITH | Budgam | 33.9721°N | 74.7877°E | Exotic |
| 11 | Turkey | CITH | Budgam | 33.9714°N | 74.7867°E | Exotic |
| 12 | New- Castle | CITH | Budgam | 33.9731°N | 74.7881°E | Exotic |
| 13 | Chinese Apricot | CITH | Budgam | 33.9752°N | 74.7497°E | Exotic |
| 14 | Unknown | Hardas | Ladakh | 34.6061°N | 76.0981°E | Indigenous |
| 15 | Unknown | Ushkara | Baramulla | 34.2504°N | 74.3788°E | Indigenous |
| 16 | Unknown | Chardari | Baramulla | 34.1852°N | 74.3634°E | Indigenous |
| 17 | Unknown | Kantibag | Baramulla | 34.2406°N | 74.3674°E | Indigenous |
| 18 | Unknown | Uri | Baramulla | 34.0831°N | 74.0543°E | Indigenous |
| 19 | Unknown | Rangwar | Baramulla | 34.2343°N | 74.3676°E | Indigenous |
| 20 | Unknown | Beerwah | Budgam | 34.0128°N | 74.5956°E | Indigenous |
| 21 | Unknown | Katiyawali | Baramulla | 34.1754°N | 74.3531°E | Indigenous |
| 22 | unknown | Gatha Baderwah | Doda | 32.9973°N | 75.7007°E | Indigenous |
| 23 | unknown | Khanpora | Baramulla | 34.2086°N | 74.3275°E | Indigenous |
| 24 | unknown | Brazllo | Kulgam | 33.6467°N | 75.0589°E | Indigenous |
| 25 | unknown | Shiva | Baramulla | 34.3521°N | 74.4748°E | Indigenous |
| 26 | unknown | Dogar | Baramulla | 33.1829°N | 74.3619°E | Indigenous |
| 27 | unknown | Narapora | Shopian | 34.7611°N | 74.8019°E | Indigenous |
| 28 | unknown | Buniyar | Baramulla | 34.1009°N | 74.2004°E | Indigenous |
| 29 | unknown | Gozahama | Ganderbal | 34.1934°N | 74.6755°E | Indigenous |
| 30 | unknown | Kokarnag | Anantnag | 33.6801°N | 75.3895°E | Indigenous |
| 31 | unknown | Malpora | Baramulla | 34.3528°N | 74.4732°E | Indigenous |
| 32 | unknown | Dangerpora | Pulwama | 33.8756°N | 74.9793°E | Indigenous |
| 33 | unknown | Sopore | Baramulla | 34.2604°N | 74.4681°E | Indigenous |
| 34 | unknown | Duroo | Baramulla | 34.3516°N | 74.4633°E | Indigenous |
| 35 | unknown | Pazelpora | Baramulla | 34.3587°N | 74.4831°E | Indigenous |
| 36 | unknown | Kanispora | Baramulla | 34.2184°N | 74.3998°E | Indigenous |
| 37 | unknown | Darpora | Baramulla | 34.3570°N | 74.4323°E | Indigenous |
| 38 | unknown | Goripora | Baramulla | 34.3465°N | 74.4212°E | Indigenous |
| 39 | unknown | Mundji | Baramulla | 34.3607°N | 74.4738°E | Indigenous |
| 40 | unknown | Handwara | Kupwara | 34.4043°N | 74.2831°E | Indigenous |
| 41 | unknown | Brath Kalan | Baramulla | 34.3446°N | 74.4065°°E | Indigenous |
| 42 | unknown | Wadura | Baramulla | 34.3528°N | 74.4018°E | Indigenous |
| 43 | unknown | Badwenchak | Qazigund | 33.5927°N | 75.1658°E | Indigenous |
| 44 | unknown | Sheeri | Baramulla | 34.1107°N | 74.1837°E | Indigenous |
| 45 | unknown | Krawah | Banihal | 33.2518°N | 75.1048°E | Indigenous |
| 46 | unknown | Chadoora | Budgam | 33.9453°N | 75.7967°E | Indigenous |
| 47 | unknown | Kralpora | Budgam | 34.4997°N | 74.1177°E | Indigenous |
| 48 | unknown | Bhangra | Doda | 32.9831°N | 75.7116°E | Indigenous |
| 49 | unknown | Kapra Baderwah | Doda | 32.9833°N | 75.7112°E | Indigenous |
| 50 | unknown | Rawalpora | Srinagar | 34.0042°N | 74.4676°E | Indigenous |
2.2. DNA extraction
The genomic DNA was extracted from young leaves of each apricot accession by using the CTAB method (Doyle and Doyle, 1987). The extracted DNA was purified and dissolved in 1X TE buffer (Tris-EDTA). The DNA concentration of each sample was normalized to 20–60 ng µL−1 after quantifying the DNA on Nanodrop (Thermo scientific USA). The quantity and quality of the DNA were checked by 0.8 per cent agarose gel electrophoresis.
2.3. Primer selection
4 ISSR (Inter simple sequence repeat) polymorphic primers were selected after the literature survey as listed in (Table 2) along with annealing temperature, primer sequence, and band size to reveal the genetic variability of germplasms under investigation. The primers were diluted to a working concentration of 10 pico moles using autoclaved deionized water before PCR reaction.
Table 2.
List of selected ISSR primers along with their primer sequence.
| ISSR primer | Annealing temp (C) | Total bands (no.) | Sequence (5́ –3́) | Band size | Refrence |
|---|---|---|---|---|---|
| BC817 | 49 | 8 | (CA) 8 A | 180–800 | Li et al., 2013 |
| BC818 | 52 | 7 | (CA) 8 G | 400–850 | Li et al., 2013 |
| BC825 | 52 | 4 | (AC) 8 T | 250–1200 | Li et al., 2013 |
| BC835 | 54 | 8 | (AG) 8 CTC | 280–1200 | Li et al., 2013 |
| Mean | 6.75 | ||||
2.4. PCR amplification with ISSR primers
PCR reactions were operated in 25 µL reaction mixture containing, 0.2 mM dNTPs, 2.5 mM MgCl2,10 Pm primer,1X buffer (20 Mm Tris-Cl pH 8.4, 50 Mm KCl), 1 unit of Taq polymerase and 25–60 ng/ µL DNA. The cycling program was as, pre-denaturation for 5 min at 94 °C, 35 cycles of denaturation for 60 sec at 94 °C, Annealing for 60 sec at 37 °C, extension for 2 min at 72 °C, and followed by final extension for 7 min at 72 °C. The products were analyzed based on band sizes, which were revealed on running the products on electrophoresis unit on a 2% agarose gel with 0.5XTBE buffer, using EtBr as staining agent and visualization under UV light. To check the preferential power of the tested primers, polymorphic information content (PIC), average heterozygosity (H.av), effective multiplex ratio (EMR), marker index (MI), and resolving power (RP) was calculated (Powell et al., 1996).
2.5. Data analysis
The PCR products were separated on 2% agarose gel stained with EtBr by using an electrophoresis system. The bands were visualized under a gel documentation system with the help of UV light. Exclusive DNA bands that prevailed clear, as (1) present or (0) absent, were scored for all examined accessions. MS Excel was utilized to analyze the polymorphic band, the total number of bands, and rate of polymorphism among the species, within and across the different sub-groups from each genotype taken into consideration. On applying the formula, the PIC = 2 × F (1-F) PIC value of consecutive primers was enumerated. Jaccard’s similarity coefficient was estimated by employing NTSYS-pc version 2.02e (Applied Biostatistics, Inc., Setauket, NY, USA) and UPGMA (unweighted pair-group method with arithmetic averages) was allowed to design a dendrogram to reveal the similarity matrix through cluster analysis. The assumed population (K) value was fixed from 1 to 5, and the inspection was reproduced two times. We set the Iterations from 1, 00,000, and 2, 00,000 and adopted a model having admixture and correlated allele frequencies. The accurate value of (K) was enumerated by determining the 1 K value to calculate the most probable number of groups (Evanno et al., 2005). Then STRUCTURE HARVESTER v.0.6.1 software was used to reveal the most prominent K value between the groups (Earl and von-Holdt, 2011). Germplasms that showed affiliation likelihood greater than 80% were specified as individual groups, and those with an affiliation probability of less than 80% were assigned as admixture. Also, the STRUCTURE program was utilized to reveal the exp heterozygosity and (Fst) among individuals in a sub-population.
3. Results
3.1. Marker parameters
The investigation carried using ISSR primers revealed that the size variation of amplified bands ranged from 180 to 1200 bp for all the primers. The total genetic variability parameters such as PIC, RP, and MI across all the fifty accessions are shown separately in Table 2. PIC is a characteristic feature of a primer, and accordingly, for all four primers, PIC values were determined. The average value of PIC was 0.424, and a higher PIC value (0.46) was calculated for the marker BC835, and a lower value (0.38) was observed for the marker BC818. Marker index was also calculated for all primers as it is also a feature of a primer. The marker index values varied from 0.001 to 0.006 with a mean value of 0.04. The maximum value (0.06) was scored with the marker BC818 and the minimum value (0.001) for the primer BC835 (Table 2). The H.av ranged from 0.001 to 0.002. For a primer, the RP specifies its discriminatory power, and its values varied from 1.76 to 3.84 with a mean value of 2.88. Similarly, the EMR value varied from 1.00 to 5.760 with an average value of 3.250.
3.2. Genetic divergence
The four selected primer pairs developed a total of 27 bands and a mean value of 6.75 bands per primer (Table 3). Primer BC817 (8bands) showed the highest polymorphism between the ISSR loci, and Primer BC825 (6bands) revealed the least polymorphism. The highest percentage of polymorphic bands (PPB) was 87.50% in primer BC817, and the lowest PPB, 83.33%, was observed in primer BC825. Table 5 shows Nei’s gene diversity (h) in detail which ranged from 0.3411 (BC817) to 0.2316 (BC825) with a mean value of 0.2847 and Shannon’s information index (I) average value was 0.4187. The average Na was 1.8408, and Ne was 1.5458.
Table 3.
Genetic characterization of 4 ISSR loci for 50 apricot accessions.
| Primers | PIC | MI | H.av | RP | EMR |
|---|---|---|---|---|---|
| BC817 | 0.396 | 0.003 | 0.002 | 3.840 | 2.120 |
| BC818 | 0.383 | 0.006 | 0.001 | 3.440 | 4.120 |
| BC825 | 0.458 | 0.005 | 0.001 | 2.480 | 5.760 |
| BC835 | 0.461 | 0.001 | 0.001 | 1.760 | 1.000 |
| Mean | 0.424 | 0.004 | 0.001 | 2.880 | 3.250 |
Table 5.
Assignment of sub-populations (K) to the individuals based on probability.
| Code | Genotype | K1 | K2 | Sub-population |
|---|---|---|---|---|
| 1 | GeN01 | 0.976 | 0.024 | 1 |
| 2 | GeN02 | 0.977 | 0.023 | 1 |
| 3 | GeN03 | 0.888 | 0.112 | 1 |
| 4 | GeN04 | 0.973 | 0.027 | 1 |
| 5 | GeN05 | 0.959 | 0.041 | 1 |
| 6 | GeN06 | 0.877 | 0.123 | 1 |
| 7 | GeN07 | 0.921 | 0.079 | 1 |
| 8 | GeN08 | 0.865 | 0.135 | Admixture of 1and2 |
| 9 | GeN09 | 0.765 | 0.235 | Admixture of 1and2 |
| 10 | GeN10 | 0.917 | 0.083 | 1 |
| 11 | GeN11 | 0.783 | 0.217 | Admixture of 1and2 |
| 12 | GeN12 | 0.517 | 0.483 | Admixture of 1and2 |
| 13 | GeN13 | 0.721 | 0.279 | Admixture of 1and2 |
| 14 | GeN14 | 0.045 | 0.955 | 2 |
| 15 | GeN15 | 0.167 | 0.833 | 2 |
| 16 | GeN16 | 0.050 | 0.950 | 2 |
| 17 | GeN17 | 0.073 | 0.927 | 2 |
| 18 | GeN18 | 0.564 | 0.436 | Admixture of 1and2 |
| 19 | GeN19 | 0.068 | 0.932 | 2 |
| 20 | GeN20 | 0.109 | 0.891 | 2 |
| 21 | GeN21 | 0.056 | 0.944 | 2 |
| 22 | GeN22 | 0.044 | 0.956 | 2 |
| 23 | GeN23 | 0.060 | 0.940 | 2 |
| 24 | GeN24 | 0.052 | 0.948 | 2 |
| 25 | GeN25 | 0.066 | 0.934 | 2 |
| 26 | GeN26 | 0.372 | 0.628 | Admixture of 1and 2 |
| 27 | GeN27 | 0.068 | 0.932 | 2 |
| 28 | GeN28 | 0.121 | 0.879 | 2 |
| 29 | GeN29 | 0.068 | 0.932 | 2 |
| 30 | GeN30 | 0.071 | 0.929 | 2 |
| 31 | GeN31 | 0.398 | 0.602 | Admixture of 1and 2 |
| 32 | GeN32 | 0.149 | 0.851 | 2 |
| 33 | GeN33 | 0.749 | 0.251 | Admixture of 1and 2 |
| 34 | GeN34 | 0.925 | 0.075 | 1 |
| 35 | GeN35 | 0.543 | 0.457 | Admixture of 1and 2 |
| 36 | GeN36 | 0.835 | 0.165 | 1 |
| 37 | GeN37 | 0.117 | 0.883 | 2 |
| 38 | GeN38 | 0.138 | 0.862 | 2 |
| 39 | GeN39 | 0.534 | 0.466 | Admixture of 1and 2 |
| 40 | GeN40 | 0.735 | 0.265 | Admixture of 1and 2 |
| 41 | GeN41 | 0.711 | 0.289 | Admixture of 1and 2 |
| 42 | GeN42 | 0.535 | 0.465 | Admixture of 1and 2 |
| 43 | GeN43 | 0.324 | 0.676 | Admixture of 1and 2 |
| 44 | GeN44 | 0.751 | 0.249 | Admixture of 1and 2 |
| 45 | GeN45 | 0.931 | 0.069 | 1 |
| 46 | GeN46 | 0.529 | 0.471 | Admixture of 1and 2 |
| 47 | GeN47 | 0.917 | 0.083 | 1 |
| 48 | GeN48 | 0.442 | 0.558 | Admixture of 1and 2 |
| 49 | GeN49 | 0.851 | 0.149 | 1 |
| 50 | GeN50 | 0894 | 0.106 | 1 |
3.3. Cluster analysis
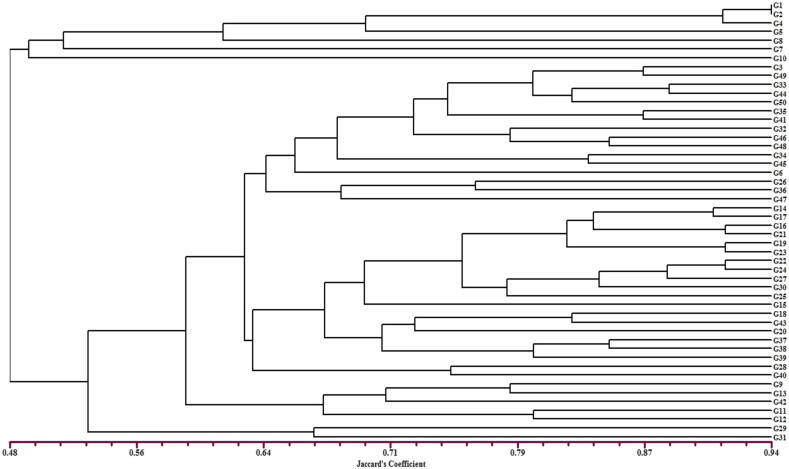
Understanding the crop’s allelic diversity and population differentiation is essential for efficient utilization and conservation of available genetic resources for breeding (Laido et al., 2013). The 50 genotypes formed two main clusters (I and II) based on UPGMA analysis with Jaccard’s similarity coefficient varying from 0.48 to 0.94 (Fig. 1). Cluster I contained seven genotypes G1, G2, G4, G5, G7, G8 and G10, all exotic genotypes established at CITH Srinagar. Cluster II contained forty-three genotypes, most of which have an indigenous origin with some exotic accessions. The Jaccard’s similarity coefficient (JSC) revealed the maximum similarity was marked among cultivars 1 (Harcot) and 2 (Hartly) (0.944) and the least among two genotypes 11(exotic) and 31(indigenous) (0.235) (Fig. 1).
Fig 1.
UPGMA clustering of 50 genotypes of apricot based on ISSR markers.
3.4. Structure analysis
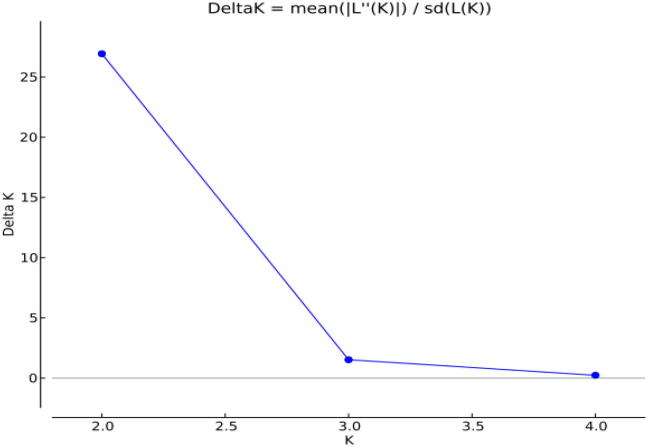
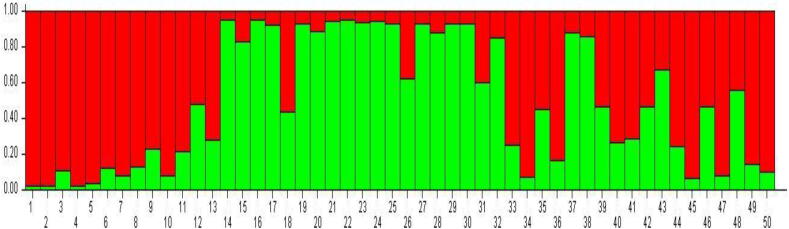
Model-based cluster analysis assembled 50 apricot accessions into two genetically diverse subpopulations (K2) and showed the maximum natural log of a probability (−468.8), equivalent to the posterior probability. The log-probability value L (K) averaged over two replicates almost increased linearly from K = 1(—508.9) up to K = 2 (−468.8) and then at K = 3 decreased for the model with admixture and correlated frequency. The method of (Evanno et al., 2005) considerably backed K = 2 as the possible number of clusters (Fig. 2) since the maximum change of the probability of the data (ΔK) was observed at K = 2. Apart from this, some degree of admixtures was additionally recognized among some individuals to a population (Table 3). Among 50 individuals, 14 individuals were recorded in population one respectively, mostly exotic accessions, and the rest of the 18 individuals were placed in population 2. There were some genotypes, viz 18, with the varied proportion of admixtures (Fig. 3). All genotypes in both populations showing affiliation likelihood more significant than 80% were not displayed in admixtures, and those genotypes that revealed affiliation probability less than 80% were counted as admixtures. The percentage of individual populations was 43.75 and 56.25% for populations 1 and 2, respectively. The expected heterozygosity between two populations at a specified locus was observed as 0.0760 in the Ist sub-population and 0.2097 in the second sub-population with an average of 0.1428. Similarly, 0.0001 was the Fst (population differentiation measurement) between two sub-populations was observed as 0.0001 (in the first sub-population) to 0.4325 (in the second subpopulation), with a mean value of 0.216 (Table 4). Estimating allele frequency divergence between populations utilizing point estimates of p revealed a distance of 0.0760 between populations one and two.
Fig 2.
Second order of change of the log likelihood of the data (Delta K) as a function of K, calculated over two replications.
Fig 3.
Graphical representation of population structure in the apricot selections/genotypes. Each individual is represented by a vertical line and different colors in the same line indicate the individual's estimated.
Table 4.
Genetic diversity parameters for the accessions studied.
| Locus | Na | Ne | h | I |
|---|---|---|---|---|
| BC817 | 2.0000 | 1.6951 | 0.3411 | 0.523 |
| BC818 | 1.9210 | 1.5642 | 0.3241 | 0.4821 |
| BC825 | 1.6800 | 1.4286 | 0.2316 | 0.3167 |
| BC835 | 1.7623 | 1.4955 | 0.2421 | 0.3532 |
| Mean | 1.8408 | 1.5458 | 0.2847 | 0.4187 |
Na = Observed number of alleles, Ne = Effective number of alleles, h = Nei's gene diversity, I = Shannon's Information index.
4. Discussion
The size of Amplified bands in ISSR genotyping ranged from 180 to 1200 bp for all the primers. A wide range in size (200 – 2,500 bp) was observed (Kumar et al., 2009). This may be due to the screening of a high number of ISSR on a large number of genotypes. PIC value ranged from 0.38 to 0.46, and the mean value was 0.424. The PIC value ranged from 0.5 to 0.25, considered an informative marker (Saki et al., 2016). Thus all markers in our study are informative markers. The MI values varied from 0.001 to 0.006 with a mean value of 0.04. The H.av ranged from 0.001 to 0.002. RP values varied from 1.76 to 3.84, with an average value of 2.88. Similarly, the EMR value ranged from 1.00 to 5.760 with a mean value of 3.250. The values of markers parameters results of our study are less than observed by (Kumar et al., 2009) while studying the genetic diversity of apricot of Leh and Nubra valley through ISSR markers. The high values of PIC, RP, MI, EMR of a specific primer indicate their high discriminating power within the species, and these primers are also useful for further studies. Previous studies on genetic diversity studies with ISSR marker system revealed the suitability of these primers to reveal the genetic diversity of woody plants and fruit-bearing species such as apple, strawberry, citrus, apricot (Raji and Siril, 2021, Silva et al., 2016)
The average number of bands 6.75 per primer in our study is higher than reported by Kumar et al. (2009) (5.27) but less than observed by (Liu and Liu., D., Zhang, A., Feng, C., Yang, J., Yoon, J., Li. S., , 2007, Li et al., 2009, Yılmaz et al., 2009, Yilmaz et al., 2009, Yilmaz et al., 2012, Ganopoulos et al., 2011) in prunus and other stone fruit species. Primer BC817 (8bands) showed the highest polymorphism between the ISSR loci, and Primer BC825 (6bands) revealed the least polymorphism. Liu et al. (2015) obtained 9 and 6 highest and lowest bands, respectively, with a mean value of 7.4 bands per locus in apricot germplasm. The higher percentage of polymorphic bands (PPB) was 87.50% in primer BC817, and the lowest PPB, 83.33%, was observed in primer BC825. The % of PPB in our result is less than noticed by (Feng et al. 2005, PPB = 90%); (Liu et al., 2010, PPB = 78.06%); (Yilmaz et al. 2012, PPB = 88%); (Li et al., 2009, PPB = 84%). Previous research also supports the capability of ISSRs to reveal polymorphisms compared to other random primers (Zietkiewicz et al., 1994). The number of bands produced per primer varied significantly due to the number of annealing sites within the genome and primer structure (Bishoyi et al., 2014). Fruit trees showing a high percentage of polymorphism are expected to be rich sources of genes for fulfilling the breeding objectives. Table 5 showed the details about Nei’s gene diversity (h) which varied from 0.3411 (BC817) to 0.2316 (BC825) with an average of 0.2847 and the average Shannon’s information index (I) was detected as 0.4187. These values show there is abundant genetic diversity within the genotypes. In our study, the average Nei’s gene diversity (h) is almost similar to 0.2757, but Shannon’s information index (I) is higher than the average of 0.3720 reported by Liu et al., (2019). The average observed number of alleles and an effective number of alleles was observed as 1.8408 and 1.5458, respectively. Measurement of genetic variability requires an effective number of alleles and is more suitable than an observed number of alleles (Raji and Siril, 2021).
Preservation and efficient utilization of available genetic resources for breeding purposes require an understanding of the crop’s genetic variation and the population structure (Laido et al., 2013). The 50 genotypes clustered into two main groups, with Jaccard’s similarity coefficient varying from 0.48 to 0.94. Kumar et al. (2009) reported Leh and Nubra valley apricot genotypes form different clades through ISSR genotyping with a similarity coefficient of 0.05 to 0.39. Cluster I contained seven genotypes that were all exotic. This may be due to genetic closeness among the exotic cultivars, which can be justified by the great extent of relatedness in their genetic makeup. The same kind of association has been revealed in black gram (Gaffor et al., 2001). Cluster II contained forty-three genotypes and most of which have an indigenous origin. The above grouping of genotypes based on their geographic locations and ancestry agrees with the results of Romero et al., 2003, Zhebentyayeva et al., 2003. The Jaccard’s similarity coefficient (JSC) revealed the maximum similarity was marked among cultivars 1 (Harcot) and 2 (Hartly) (0.944) and the least among genotype 11(exotic) and 31(indigenous) (0.235). The similarity in the genomic and amplified Harcot and Hartley cultivars region is probably related to their close genetic diversity. The most distinct genotypes 11 and 31 can be utilized for breeding purposes for the development of new varieties. The most similar genotypes, cultivars 1 (Harcot) and 2 (Hartly) may vary with less difference in morphological difference. This reveals that in comparison to other cultivars, genotype one and genotype two are genetically adjacent and similar in genetic makeup associated with their amplified region and similarity to the genomic regions. The cluster dendrogram separates the indigenous collection from exotic collections except for some cultivars. Similar results were obtained in groundnut (Dwivedi et al., 2001) and azuki bean (Fernandez et al., 2002).
Structure analysis and cluster analysis indicated two groups of the apricot in the provinces of Jammu and Kashmir. The results reveal a significant correlation between genetic divergence and geographical distance (He et al., 2007, Shah et al., 2020). Two subpopulations revealed that sub-population-I had mostly exotic accessions, and sub-population-II contained indigenous genotypes with some of the genotypes found admixtures of these two subpopulations. The average expected heterozygosity and population differentiation measurement (Fst) ranged was 0.1428, 0.216. Population structure information estimation obtained in apricot accessions will be enhanced in the future for performing association mapping in apricot for heterogeneity of traits (Table 6).
Table 6.
Heterozygosity and Fst value calculated for 2 apricot sub-populations.
| S. No. | Sub-population | Expected heterozygosity | Fst value |
|---|---|---|---|
| 1 | 1 | 0.0760 | 0.0001 |
| 2 | 2 | 0.2097 | 0.4325 |
| Average | 0.1428 | 0.216 | |
5. Conclusion
The results outcome reveals that the four ISSR markers comprehensively separated the indigenous germplasm from the exotic germplasm. A better understanding of the diversity and distribution of germplasm in this region may be understood by screening a good number of ISSR markers and increasing the area of sampling. The genetic divergence within indigenous genotypes and exotic genotypes could allow for future insights into apricot breeding programs. Furthermore, the conservation of critical genetic resources will be essential for future breeding programs in apricot.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The author would like to extend their sincere appreciation to the Researchers Supporting Project Number (RSP-2020/24), King Saud University, Riyadh, Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abouzari A., Solouki M., Golein B., Fakheri B.A., Sabouri A. Screening of molecular markers associated to cold tolerance-related traits in Citrus. Scientia Horticulturae. 2020;263 [Google Scholar]
- Ballester J., de-Vicente M.C. Determination of F-1 hybrid seed purity in pepper using PCR-based markers. Euphytica. 1998;103(2):223–226. doi: 10.1023/a:1018372523343. [DOI] [Google Scholar]
- Bishoyi A.K., Pillai V.V., Geetha K.A., Maiti S. Assessment of genetic diversity in Clitoria ternatea populations from different parts of India by RAPD and ISSR markers. Genet. Resour. Crop Evol. 2014;61:1597–1609. doi: 10.1007/s10722-014-0145-y. [DOI] [Google Scholar]
- Decroocq V., Fave M.G., Hagen L., Bordenave L., Decroocq S. Development and transferability of apricot and grape EST microsatellite markers across taxa. Theor. Appl. Gen. 2003;106:912–922. doi: 10.1007/s00122-002-1158-z. [DOI] [PubMed] [Google Scholar]
- Doyle, J.J., Doyle J.L., 1987. A rapid isolation procedure for small quantities of fresh leaf tissue.
- Dwivedi S.L., Gurtu S., Chandra S., Yuejin W., Nigam S.N. Assessment of genetic diversity among selected groundnut germplasm through RAPD analysis. Plant Breed. 2001;120:345–349. [Google Scholar]
- Earl D., von-Holdt, B. STRUCTURE HARVESTER: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conservation Genetics. Resources. 2011;4(2):359–361. doi: 10.1007/s12686-011-9548-7. [DOI] [Google Scholar]
- Evanno, G., Regnaut, S., Goudet, J., 2005. Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol. 14(8):2611–20. https://doi.org 10.1111/j.1365-294x.2005.02553.x [DOI] [PubMed]
- Farajpour M., Ebrahimi M., Amiri R., Noori S.A.S., Sanjari S., Golzari R. Study of genetic variation in yarrow using inter-simple sequence repeat (ISSR) and random amplified polymorphic DNA (RAPD) markers. Afr J Biotechnol. 2011;10:73–86. [Google Scholar]
- Feng C.J., Zhang Y.H., Xu X.Y., Shi G.H. Genetic diversity revealed by ISSR marker in apricot. J. Agric. Univ. Hebei. 2005;28:52–56. [Google Scholar]
- Fernandez M.E., Figueiras A.M., Benito C. The use of ISSR and RAPD marker for detecting DNA polymorphism, genotype identification and genetic diversity among barley cultivars with known origin. Theor. Appl. Genet. 2002;104:845–851. doi: 10.1007/s00122-001-0848-2. [DOI] [PubMed] [Google Scholar]
- Gaffor A., Sharif A., Ahmad Z., Zahid M.A., Rabbani M.A. Genetic diversity in blackgram (vigna mungo L.Hepper) Field Crop Res. 2001;69:183–190. [Google Scholar]
- Ganopoulos I.V., Kazantzis K., Chatzicharisis I., Karayiannis I., Tsaftaris A.S. Genetic diversity, structure and fruit trait associations in Greek sweet cherry cultivars using microsatellite based (SSR/ISSR) and morpho-physiological markers. Euphytica. 2011;181(2):237–251. [Google Scholar]
- Goulao L., Monte-Corvo L., Oliveira C.M. ISSR Analysis of Cultivars of Pear and Suitability of Molecular Markers for Clone Discrimination. J. Amer. Soc. Hort. Sci. 2001;126(5):517–522. [Google Scholar]
- Hassanpour, H., Hamidoghli, Y., Samizadeh, H., 2013. Estimation of genetic diversity in some Iranian cornelian cherries (Cornus mas L.) accessions using ISSR markers, Biochem. Syst. Ecol. 48 (2013) 257–262, DOI: 10.1016/j. bse.2013.01.002.
- He T.M., Chen X.S., Zheng X., Gao J.S., Lin P.J., Wen L., Liang Q., Yan., W., Using SSR markers to determine the population genetic structure of apricot (Prunus armeniaca L.) in the Ily Valley of West China. Genet. Resour. Crop Evol. 2007;54:563–572. [Google Scholar]
- Hormaza J.I. Molecular characterization and similarity relationships among apricot genotypes using simple sequence repeats. Theoretical and Applied Genetics. 2002;104:321–328. doi: 10.1007/s001220100684. [DOI] [PubMed] [Google Scholar]
- Hurtado M.A., Romero C., Vilanova S., Abbott A.G., Llácer, G., Badenes, M.L., Genetic diversity in apricot cultivars based on AFLP markers. Euphytica. 2002;127:297–301. [Google Scholar]
- Kafkas S., Ozkan H., Ak B.E., Acar I., Atli H.S., Koyuncu S. Detecting DNA polymorphism and genetic diversity in a wide pistachio germplasm: Comparison of AFLP, ISSR and RAPD markers. J. Amer. Soc. Hort. Sci. 2006;131:522–529. [Google Scholar]
- Kumar M., Mishra G.P., Singh R., Kumar J., Naik P.K., Singh S.B. Correspondence of ISSR and RAPD markers for comparative analysis of genetic diversity among different apricot genotypes from cold arid deserts of trans-Himalayas. Physiology and Molecular Biology of Plants. 2009;15(3):225–236. doi: 10.1007/s12298-009-0026-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laido, G., Mangini, G., Taranto, F., Gadaleta, A., Blanco, A., Cattivelli, L., Marone, D., Mastrangelo, A., Papa, R., De Vita, P., 2013. Genetic diversity and population structure of tetraploid wheats (Triticum turgidum L.) estimated by SSR, DArT and pedigree data. PLoS ONE 8:e67280. DOI: 10.1371/journal.pone.0067280 [DOI] [PMC free article] [PubMed]
- Li M., Zhao Z., Miao X.J. Genetic variability of wild apricot (Prunus armeniaca L.) populations in the Ili Valley as revealed by ISSR markers. Genetic Resources and Crop Evolution. 2013;60(8):2293–2302. doi: 10.1007/s10722-013-9996-x. [DOI] [Google Scholar]
- Li M.M., Cai Y.L., Qian Z.Q., Zhao G.F. Genetic diversity and differentiation in Chinese sour cherry Prunus pseudocerasus Lindl., and its implications for conservation. Genet Resour Crop Evol. 2009;56(4):455–464. doi: 10.1007/s10722-008-9378-y. [DOI] [Google Scholar]
- Lisek A., Korbin M., Rozpara E., Zueawicz E. Plum cultivar DNA polymorphism generated with RAPD and ISSR markers. Acta Hort. 2007;734:281–285. [Google Scholar]
- Liu B.Y., Li Y.Y., Tang Y.C., Wang L.Y. Assessment of genetic diversity and relationship of tea germplasm in yunnan as revealed by ISSR markers. Acta Agronom. Sin. 2010;36:391–1340. [Google Scholar]
- Liu, M.P., Du, H.Y., Zhu, G.P., Fu, D.L., Tana, W.Y., 2015. Genetic diversity analysis of sweet kernel apricot in China based on SSR and ISSR markers. Genet. Mol. Res. 14 (3): 9722-9729. DOI http://dx.doi.org/10.4238/2015.August.19.4 [DOI] [PubMed]
- Liu S., Cornille A., Decroocq S., Tricon D., Chague A., Eyquard J., Decroocq V. The complex evolutionary history of apricots: species divergence, gene flow and multiple domestication events. Molecular Ecology. 2019;28:5299–5314. doi: 10.1111/mec.15296. [DOI] [PubMed] [Google Scholar]
- Liu W., Liu., D., Zhang, A., Feng, C., Yang, J., Yoon, J., Li. S., Genetic diversity and phylogenetic relationships among plum germplasm resources in China assessed with inter-simple sequence repeat markers. J. Amer. Soc. Hort. Sci. 2007;132:619–628. [Google Scholar]
- Ouborg N.J., Vergeer P., Mix C. The rough edges of the conservation genetics paradigm for plants. J. Ecol. 2006;94:1233–1248. doi: 10.1111/j.1365-2745.2006.01167.x. [DOI] [Google Scholar]
- Powell, W., Morgante, M., Andre, C., Hanafey, M., Vogel, J., Tingey, S., 1996. The comparison of RFLP, RAPD, AFLP and SSR (microsatellite) markers for germplasm analysis. Mol Breed. 2(3):225–38. https://doi.org 10.1007/bf00564200
- Raji R., Siril E.A. Genetic diversity analysis of promising Ceylon olive (Elaeocarpus serratus L.) genotypes using morphological traits and ISSR markers. Current. Plant Biology. 2021;26 doi: 10.1016/j.cpb.2021.100201. [DOI] [Google Scholar]
- Reddy M.P., Sarla N., Siddiq E.A. Inter simple sequence repeat (ISSR) polymorphism and its application in plant breeding. Euphytica. 2002;128(1):9–17. [Google Scholar]
- Romero, C., Pedryc, A., Munoz, V., Llacer, G., Badenes, M.L., 2003. Genetic diversity of different apricot geographical groups determined by SSR markers. Genome. 46(2):244–52. https://doi.org 10.1139/g02-128 [DOI] [PubMed]
- Saki S., Bagheri H., Deljou A., Zeinalabedini M. Evaluation of genetic diversity amongst Descurainia sophia L. genotypes by inter-simple sequence repeat (ISSR) marker. Physiol. Mol. Biol. Plants. 2016;22:97–105. doi: 10.1007/s12298-015-0330-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah, R. A., Bakshi, P., Sharma, N., Jasrotia, A., Itoo, H., Gupta R., Singh, A., 2021. Diversity Assessment and Selection of Superior Persian Walnut (Juglans regia L.) Trees of Seedling Origin from North-Western Himalayan Region. Resources, Environment and Sustainability. 3 (2021) 100015. DOI: 10.1016/j.resenv.2021.100015
- Shah R., Baksi P., Jasrotia A., Bhat D., Gupta R., Bakshi M. Genetic diversity of walnut (Juglans regia L.) seedlings through SSR markers in north-western Himalayan region of Jammu. Bangladesh. Journal of Botany. 2020;49(4):1003–1012. doi: 10.3329/bjb.v49i4.52517. [DOI] [Google Scholar]
- Shahi-Gharahlar A., Zamani Z., Fatahi R., Bouzari N. Estimation of genetic diversity in some Iranian wild Prunus subgenus Cerasus accessions using inter-simple sequence repeat (ISSR) markers. Biochem Syst Ecol. 2011;39(4–6):826–833. doi: 10.1016/j.bse.2011.07.018. [DOI] [Google Scholar]
- Shahsavar A.R., Izadpanah K., Tafazoli E., Tabatabaei B.E.S. Characterization of Citrus germplasm including unknown variants by inter-simple sequence repeat (ISSR) markers. Sci. Hort. 2007;112:310–314. [Google Scholar]
- Silva, A.V.C., Nascimento, A.L.S., Vitoria, M.F., Rabbani, A.R.C., Soares, A.N.R., Ledo, A.S. 2016. Diversity and genetic stability in banana genotypes in a breeding program using inter simple sequence repeats (ISSR) markers. Genet Mol Res 16 (1): gmr16019402 [DOI] [PubMed]
- Sreekanth P., Balasundaran M., Nazeem P., Suma T. Genetic diversity of nine natural Tectona grandis L. populations of the Western Ghats in Southern India. Conserv. Gen. 2012;13:1409–1419. doi: 10.1007/s10592-012-0383-5. [DOI] [Google Scholar]
- Terzopoulos P.J., Kolano B., Bebeli P.J., Kaltsikes P.J., Metzidakis I. Identification of Olea europaea L. cultivars using intersimple sequence repeat markers. Sci. Hort. 2005;105:45–51. doi: 10.1016/j.scienta.2005.01.011. [DOI] [Google Scholar]
- Vijayan K., Chatterjee S.N. ISSR profiling of Indian cultivars of mulberry (Morus spp.) and its relevance to breeding programs. Euphytica. 2003;131:53–63. [Google Scholar]
- Vijayan K., Srivastava P.P., Nair C.V., Tikader A., Awasthi A.K., Urs Raje, S. Molecular characterization and identification of markers associated with leaf yield traits in mulberry using ISSR markers. Plant Breed. 2006;125:298–301. [Google Scholar]
- Vijayan K., Tikader A., Kar P.K., Srivastava P.P., Awasthi A.K., Thangavelu K., Saratchandra B. Assessment of genetic relationships between wild and cultivated mulberry (Morus) species using PCR based markers. Genetic Res. Crop Evol. 2006;53:873–882. [Google Scholar]
- Wang Y., Zhang J., Sun H., Ning N., Yang L. Construction and evaluation of a primary core collection of apricot germplasm in China. Sci. Hortic. 2011;128:311–319. [Google Scholar]
- Yılmaz K.U., Ercisli S., Asma B.M., Dogan Y., Kafkas S. Genetic relatedness in Prunus genus revealed by inter-simple sequence repeat markers. Hort Science. 2009;44(2):2. [Google Scholar]
- Yilmaz, K.U., Paydas-Kargi, S., Dogan, Y., Kafkas, S., 2012. Genetic diversity analysis based on ISSR, RAPD and SSR among Turkish Apricot Germplasms in Iran Caucasian eco-geographical group. Sci Hortic (Amsterdam).138:138–43. https://doi.org 10.1016/j.scienta.2012.02.017
- Yilmaz K.U., Zengin Y., Ercisli S., Orhan E., Yalcinkaya E., Taner O., Erdogan A. Biodiversity, ex situ conservation and characterization of cornelian cherry (Cornus mas L.) genotypes in Turkey. Biotechnol. Biotechnol. Equip. 2009;23:1143–1149. doi: 10.1080/13102818.2009.10817629. [DOI] [Google Scholar]
- Zhebentyayeva, T., Ledbetter, C., Burgos, L., and Llacer, G., 2012. Apricot. In Fruit Breeding. Handbook of Plant Breeding, Vol. 8, M.L. Badenes, and D.H. Byrne, eds. (New York: Springer), p. 415–457. DOI: 10.1007/978-1-4419-0763-912.
- Zhebentyayeva T.N., Reighard G.L., Gorina V.M., Abbott A.G. Microsatellite (SSR) analysis for assessment of genetic variability in apricot. Theor. Appl. Genet. 2003;106:435–444. doi: 10.1007/s00122-002-1069-z. [DOI] [PubMed] [Google Scholar]
- Zietkiewicz E., Rafalski A., Labuda D. Genome fingerprinting by simple sequence repeat (SSR)-anchored polymerase chain reaction amplification. Genomics. 1994;20:176–183. doi: 10.1006/geno.1994.1151. [DOI] [PubMed] [Google Scholar]