Abstract
Drought is a major abiotic factor limiting plant growth and crop production. There is limited information on effect of interaction between biochar and Arbuscular mycorrhizal fungi (AMF) on okra growth, root morphological traits and soil enzyme activities under drought stress. We studied the influence of biochar and AMF on the growth of Okra (Abelmoschus esculentus) in pot experiments in a net house under drought condition. The results showed that the biochar treatment significantly increased plant growth (the plant height by 14.2%, root dry weight by 30.0%) and root morphological traits (projected area by 22.3% and root diameter by 22.7%) under drought stress. In drought stress, biochar treatment significantly enhanced the chlorophyll ‘a’ content by 32.7%, the AMF spore number by 22.8% and the microbial biomass as compared to the control. Plant growth parameters such as plant height, shoot and root dry weights significantly increased by AMF alone, by 16.6%, 21.0% and 40.0% respectively under drought condition. Other plant biometrics viz: the total root length, the root volume, the projected area and root diameter improved significantly with the application of AMF alone by 38.3%, 60.0%,16.8% and 15.9% respectively as compared with control. Compared to the control, AMF treatment alone significantly enhanced the total chlorophyll content by 36.6%, the AMF spore number by 39.0% and the microbial biomass by 29.0% under drought condition. However, the highest values of plant growth parameters (plant height, shoot dry weight, root dry weight) and root morphological traits (the total root length, root volume, projected area, root surface area) were observed in the combined treatment of biochar and AMF treatment viz: 31.9%, 34.2%, 60.0% and 68.6%, 66.6%, 45.5%, 41.8%, respectively compared to the control under drought stress. The nitrogen content, total chlorophyll content and microbial biomass increased over un-inoculated control. The soil enzymes; alkaline phosphatase, dehydrogenase and fluorescein diacetate enzyme activities significantly increased in the combined treatment by 55.8%, 68.7% and 69.5%, respectively as compared to the control under drought stress. We conclude that biochar and AMF together is potentially beneficial for cultivation of okra in drought stress conditions.
Keyword: Okra, Drought stress, Biochar, AMF, Plant growth, Root morphological traits, Chlorophyll content, Soil enzymes, Microbial biomass
1. Introduction
Drought stress is a major environmental stress, causing reduction of biological function in many crops (Pereira et al., 2006, Golldack et al., 2014, Hussain et al., 2018, Shehzad et al., 2021). Drought stress negatively impacts seed germination (Kaya et al., 2006, Farooq et al., 2009). Under drought condition the germination rate was reduced in chickpea (Awari and Mate 2015). Several studies have observed the negative effects of drought stress on plant growth of maize (Anjum et al., 2017, Dar et al., 2021), chickpea (Samarah et al., 2009, Pushpavalli et al., 2015), rice (Hussain et al., 2016, Wang et al., 2016), tomato (Starck et al., 2000) and soybean (Kobraee et al., 2011, Li et al., 2013, Maleki et al., 2013, Sheteiwy et al., 2021). Maes et al. (2009) reported that Jatropha curcas under different water regimes identified anatomical and morphological changes with an increase in adaxial stomatal ratio in response to drought. Drought negatively affects yield in different crops such as okra (Mueller et al., 2019, Chaturvedi et al., 2019, Ranawake et al., 2012, Haider et al., 2021), faba bean (Li et al., 2018) and chickpea (Pushpavalli et al., 2015). The impact of drought stress was studied on plant growth and yield of okra plant by Abdulrahman and Nadir (2018).
Physiological and biochemical functions of plants such as turgor (Chowdhury et al. 2016), mesophyll conductance (Zhou et al., 2013, Zhou et al., 2014), photosynthesis ( Christophe et al., 2011, Mak et al., 2014, Osakabe et al., 2014, Lyu et al., 2016, Asha et al., 2021), transpiration rate (Asha et al., 2021), and relative water content (Saccardy et al., 1998, Sanchez-Blanco et al., 2006), reduced under drought stress. Soltys-Kalina et al. (2016) showed that the 3-week drought treatment decreased the leaf water content of potato cultivars. Drought stress significantly reduced chlorophyll a, b and total pigments in wheat and pumpkin (Al-Ayed, 1998, Sawhney and Singh, 2002). Farooq et al. (2009) reported drought stress reduced leaf water potential and turgor pressure, stomatal closure, and decreased cell growth. Water stress reduced the amounts of starch and total sugar (Sawhney and Singh 2002). Begum et al. (2019) indicated that drought stress decreased plant height, chlorophyll and carotenoid content in Maize (Zea mays). Chaturvedi et al. (2019) observed that the significant reduction in relative water content and membrane stability index along with reduced leaf photosynthetic rate explains the possible membrane damage in okra affecting photosynthetic efficiency in drought condition. Several studies have reported that drought stress decreases the protein concentration of plants (Schwanz et al., 1996, Heckathorn et al., 1997).
Studies have shown that drought stress reduced uptake of plant nutrients (Razi and Sen, 1996, Christophe et al., 2011, Sardans and Peñuelas, 2012, Rouphael et al., 2012, Heckathorn et al., 2014). soil nutrients (Cramer et al., 2009, Waraich et al., 2011, Fierer and Schimel, 2002) and soil enzyme activities (Sanaullah et al., 2011). Bista et al (2018) indicated that drought reduced N and P content, indicating that it reduced nutrient acquisition. Drought stress decreases the concentration of nitrogen and phosphorus in plant tissue. Ge et al. (2012) demonstrated that drought stress induced sharp decreases in total K and P uptake of maize organs. Sardans and Peñuelas (2005) reported that water stress decreased urease, protease activity, phosphatase activity and β-glycosidase activity in soil. Geng et al. (2015) reported that drought strongly affect soil respiration, soil microbial activity and fungal properties.
Biochar is a carbon-enriched biomaterial prepared through a process called pyrolysis (McGlashan et al. 2012). Lehmann et al. (2006) studied biochar benefits on reducing emissions and sequestering of greenhouse gases, impacting soil quality. Many have reported application of biochar, enhancing soil fertility, carbon sequestration and bio-energy production (Fiaz et al., 2014, Ok et al., 2015, Rizwan et al., 2016, Jabborova et al., 2020a). Biochar application to soils increased crop production due to the improvement of soil physicochemical and biological properties (Ahmad et al. 2014). Biochar enhanced soil structure, water holding capacity and surface area under drought condition (Andrenelli et al., 2016, Bamminger et al., 2016, Lim et al., 2016; (Yaseen, 2021)). Biochar positive effect on the plant growth (Kammann et al., 2011, Artiola et al., 2012, Mulcahy et al., 2013), yield (Akhtar et al. 2014) plant nutrients (Usman et al., 2016) and plant physiological properties (Haider et al., 2015, Lyu et al., 2016, Xiao et al., 2016) were studied in different plants under drought stress. Several studies have reported that biochar application increased plant biomass and nutrient uptake under water stress (Kammann et al., 2011, Akhtar et al., 2014, Haider et al., 2015, Kubar et al., 2021). The biochar application increased leaf quality rate and growth of tomato over the control (Githinji, 2014, Vaccari et al., 2015). Addition of biochar significantly enhanced the photosynthetic rate, chlorophyll contents, stomatal conductance, relative water contents and water use efficiency in tomato leaves under drought stress (Akhtar et al., 2014). Batool et al. (2015) demonstrated that biochar increased the water use efficiency and photosynthesis of okra in drought stress condition.
Arbuscular mycorrhizal fungi (AMF) are important groups of soil microbes in symbiotic relationship with plant roots (Brundrett and Tedersoo, 2018). AMF are major component of rhizosphere microflora in natural ecosystems and play a significant role in ecosystems through nutrient cycling (Heflish et al., 2021) . AMF helps to improve higher branching of plant root system, plant growth and productivity of several field crops (Cavagnaro et al., 2006, Nunes et al., 2010, Alizadeh et al., 2011, Abd El-Aal et al., 2021). Abdel Latef (2011) reported that the plants inoculated with AMF increased plant photosynthesis, plant enzyme activities such as superoxide dismutase, catalase, peroxidase and ascorbate peroxidase. Several studies have reported that AMF improve the growth, plant nutrient and water uptake of host plants under drought stress (Gholamhoseini et al., 2013, Baum et al., 2015, Zhao et al., 2015a, Zhao et al., 2015b, Bowles et al., 2018). Augé (2001) reported that AMF enhance plant performance, change the plant–water relationship, and improve plant productivity in drought condition. The AMF increased water use efficiency and stomatal conductance (Birhane et al., 2012, Ruiz-Lozano and Aroca, 2010). Augé et al. (2015) demonstrated that AMF improved water use efficiency and stomatal conductance in drought stress. Subramanian and Charest (1999) reported that the AMF enhanced the nitrogen availability of host plant in drought stress. Pedranzani et al. (2016) observed that AMF improved plant physiological properties such as antioxidant enzyme activity and jasmonate synthesis of Digitaria eriantha under drought stress. Biochar amendment and AMF inoculation improved plant growth, plant nutrition, photosynthetic rate and stomatal morphology under drought stress (Hashem et al., 2019).
Medicinal plants have been used in most parts of the world and has become of increasing interest in recent times for the use of various plants as sources of molecules having medicinal properties (Egamberdieva and Jabborova, 2018, Jabborova et al., 2019, Jabborova et al., 2020b, Mamarasulov et al., 2020, Jabborova et al., 2021a, Jabborova et al., 2021b, Jabborova et al., 2021c). Okra (Abelmoschus esculentus L.) is a vegetable and herbal crop; possess nutraceutical and therapeutic properties, owing to the presence of various important bioactive compounds and their associated bioactivities (Elkhalifa et al., 2021). A little is known about the combined effect of biochar and AMF on plant growth and physiological properties in drought stresses. The present study was conducted to evaluate the prospective effect of biochar and AMF application on okra plant growth, root morphological traits, physiological properties, microbial biomass, the number of AMF spores and soil enzymatic activities under drought condition.
2. Materials and methods
2.1. Soil, biochar, AMF and seed
Field soil collected from Indian Agricultural Research Institute was used for the experiment. The biochar used in the study was produced at 400–500 °C from woody biomass (Amazon online shop, New Delhi, India), with a particle size of less than 2 mm. Variety Pusa A-4 seed was procured from Division of Vegetable Science, and AMF from the Division of Microbiology, IARI, New Delhi, India respectively.
2.2. Experimental design
The impact of biochar and AFM on the growth of Okra (Abelmoschus esculentus) was studied in pot experiments in a net house at Division of Microbiology, IARI, New Delhi, India. All the experiments were carried out in a randomized block design with three replications. Experimental treatments included: T1 control (soil without biochar), T2 biochar alone, T3 AFM alone and T4 combined biochar + AMF. Seed was cultivated into plastic pots (20 cm diameter, 20 cm depth) containing 5.0 kg of soil. During the 40 days of plant growth, drought conditions (50% of the field capacity) were maintained. After forty days plants were harvest and plant height, shoot and dry root weights were measured.
2.3. Measurement of root morphological traits of okra
The roots of okra were washed carefully with water. The whole root system was spread out and analyzed using a scanning system (Expression 4990, Epson, CA) with a blue board as a background. Digital images of the root system were analyzed using Win RHIZO software (Régent Instruments, Québec, Canada). The total root length, the root surface area, the root volume, the projected area and the root diameter were evaluated.
2.4. Organic elemental analysis
C and N were determined by Elemental Analyzer (CHNS) Eurovictor. For this purpose, 0.5 mg of each sample was placed in tin capsules and completely oxidized, at 950℃, to their elemental gases. The resultant combustion products were mechanically homogenized in a gas control zone and separated in a gas chromatographic column. Finally, eluted gases were conveyed to a thermal conductivity detector and amounts of N and C obtained.
2.5. Physiological parameter measurement
SPAD values were analyzed using the leaves from the okra plants using a SPAD-502 m (Konica-Minolta, Japan). SPAD measurements were made as estimates of chlorophyll content.
2.6. Analysis of AMF spores from soil
The AMF spores were extracted from 10 g soil samples using wet sieving and decanting method. Soil sample was put over a series of soil sieves arranged in descending order of sieve sizes. The clean spores were mesh sieved and washed several times with distilled water before being transferred into water in a clean Petri-dish. The AMF spores were counted under a stereomicroscope (Dare et al. 2013).
2.7. Analysis of soil microbial biomass determination
The method to measure biomass C was as given by Vance et al. (1987). Three of six 17.5 g replicates of each soil sample were fumigated with purified CHCl3, for 24 h. After removal of the CHCl3, the C was extracted from fumigated and unfumigated samples with 0.5 M K2SO4, for 1 h on an end-over-end shaker. The fumigated and unfumigated samples were filtered sequentially through filter paper (Whatman). The obtained supernatant liquid was measured at 280 nm.
2.8. Analysis of soil enzymes
The alkaline phosphatase activity was assayed by the method given by Tabatabai and Bremner (1969). For each soil, two sets of 1 g soil were placed in conical flasks. One set was used as the control. Then 0.2 mL toluene and 4 mL of MUB (modified universal buffer) (pH 11) were added and 1 mL of p-nitrophenyl phosphate solution was added to the other set of samples. These were incubated at 37 OC for 1 hr. Calcium chloride (1 mL of 0.5 M) and 4 mL of 0.5 M NaOH were added after incubation. Flasks were swirled for a few seconds and 1 mL of p-nitrophenyl phosphate solution was added to the remaining set of samples. All suspensions were filtered through Whatman No. 1 filter paper quickly and the yellow colour intensity was measured at 440 nm wavelength.
The fluorescein diacetate hydrolytic activity was determined following the method of Green et al. (2006). 0.5 mg soil was incubated with 25 mL of sodium phosphate (0.06 M; pH 7.6). 0.25 mL of 4.9 mM FDA substrate solution was added to all assay vials. All vials were mixed and incubated in a water bath at 37 °C for 2 h. Then soil suspension was centrifuged at 8000 rpm for 5 min. The clear supernatant was measured at 490 nm against a reagent blank solution in a spectrophotometer.
Dehydrogenase activity was determined using the method described by Casida et al. (1964). Fresh homogenized soil samples (5 g) were placed in test tubes with 5 mL substrate (3% v/w TTC). The tubes were incubated at 25 °C for 24 h. A blank sample was similarly prepared with 1 mL of a 3% TTC solution. After incubation, the samples were centrifuged at 4500 rpm for 10 min. The supernatant liquid was discarded. The TPF formed was extracted with methanol. 5 mL of methanol was added to each of the tubes and vigorously shaken for a few minutes. The operation was repeated twice (10 mL of methanol was used for extraction). Again the tubes were centrifuged. The obtained supernatant liquid was poured into a clean tube, and the absorbance of the solution was measured at 485 nm.
2.9. Statistical analyses
Experimental data were analyzed with the StatView Software using ANOVA. The significance of the effect of treatment was determined by the magnitude of the F value (P < 0.05 < 0.001).
3. Results
The result presented in Table 1 indicated the biochar treatment significantly increased the plant height by 14.2% and root dry weight by 30.0% as compared to control under drought stress. The okra plant height, shoot dry weight and root dry weight significantly enhanced under the treatment involving the AMF alone (Table 1). AMF treatment significantly increased the plant height by 16.6%, shoot dry weight by 21.0% and root dry weight by 40.0% compared to the control. In drought condition, when the combination of biochar and AMF treatment were applied, the plant height improved by 31.9%, shoot dry weight by 34.2% and root dry weight by 60.0% compared to the control respectively.
Table 1.
Effect of drought stress on plant height, shoot dry weight and root dry weight in okra.
| Treatments | Plant height(cm) | Shoot dry weight (g) | Root dry weight (g) |
|---|---|---|---|
| Control | 21.00 ± 0.80 | 0.76 ± 0.01 | 0.10 ± 0.01 |
| Biochar | 24.00 ± 0.85* | 0.85 ± 0.01 | 0.13 ± 0.01* |
| AMF | 24.50 ± 0.50* | 0.92 ± 0.01* | 0.14 ± 0.01* |
| Biochar + AMF | 27.76 ± 0.15* | 1.02 ± 0.01* | 0.16 ± 0.01* |
Data are means of three replicates (n = 3), * asterisk differed significantly at P < 0.05*
In Table 2, mean data regarding the root morphological traits as affected by the biochar and AMF application in drought condition is presented. The root morphological traits indicated that root parameters significantly increased the total root length, the root surface area, the projected area, the root diameter and the root volume by biochar alone, AMF alone and combined with biochar with AMF treatment under drought stress. Compared to the control, biochar alone treatment significantly enhanced the projected area by 22.3% and the root diameter by 22.7% under drought stress. The total root length and root volume sharply increased by biochar alone, which significantly increased by 47.3% and 40.0% respectively than the control. The projected area and root diameter was improved with the application of AMF by 16.8% and 15.9% as compared with control, respectively. Under drought stress, the AMF alone significantly enhanced the total root length by 38.1% and the root volume by 60.0%. The highest values of total root length (68.6%) and root volume (66.6%) were observed in the treatment of biochar and AMF combination as compared to control and individuals under drought stress. Similarly, significant increase in the projected area (45.5%), root surface area (41.8%), and root diameter (27.2%) were also observed.
Table 2.
Effect of drought stress on root morphological traits in okra.
| Treatments | Total root length (cm) | Projected area (cm2) | Root surface area (cm2) | Root volume (cm3) | Root diameter (mm) |
|---|---|---|---|---|---|
| Control | 69.58 ± 4.13 | 12.99 ± 0.76 | 5.38 ± 0.57 | 0.15 ± 0.01 | 0.44 ± 0.01 |
| Biochar | 102.39 ± 1.80* | 15.78 ± 0.90* | 6.11 ± 0.60 | 0.21 ± 0.01** | 0.54 ± 0.01* |
| AMF | 96.12 ± 4.11* | 15.07 ± 0.36 | 5.94 ± 0.09 | 0.24 ± 0.01* | 0.51 ± 0.01* |
| Biochar + AMF | 117.28 ± 5.49** | 18.78 ± 2.13** | 7.52 ± 1.17** | 0.25 ± 0.01** | 0.56 ± 0.02** |
Data are means of three replicates (n = 3), * asterisk differed significantly at P < 0.05*, P < 0.01**
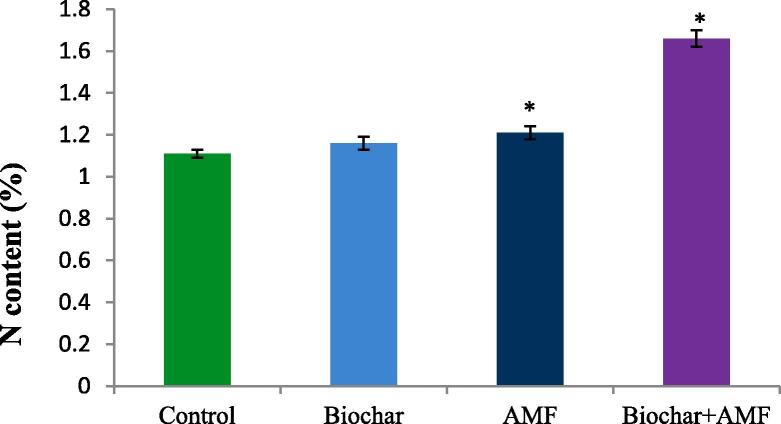
As shown in Fig. 1, the biochar alone and AMF alone treatments marginally increased the nitrogen content in okra leaf under drought condition. The AMF alone application increased the nitrogen content by 9.0% compared to the control in drought stress. The nitrogen content was highest in the combined treatment of biochar and AMF. Under drought stress, combination of biochar and AMF treatment significantly enhanced the nitrogen content by 49.5% over the control.
Fig. 1.
Effect of drought stress on the nitrogen content of leaf in okra. Data are means of three replicates (n = 3), * asterisk differed significantly at P < 0.05*.
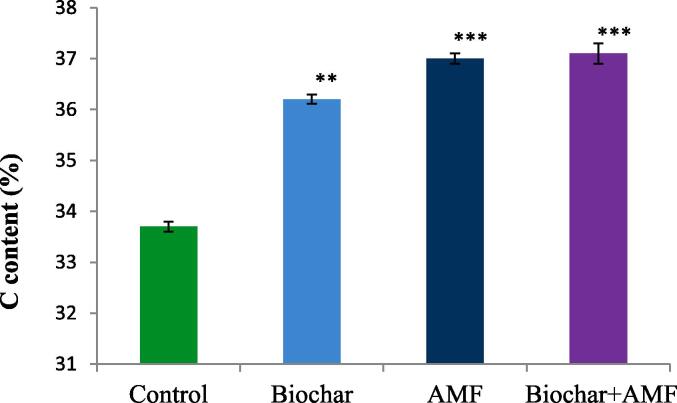
Results showed that combination of biochar and AMF resulted in the highest carbon content compared to all treatment (Fig. 2). Under drought condition, biochar alone and AMF alone treatments gradually increased the carbon content as compared to control. Compared to the control, combination of biochar and AMF treatment significantly increased the carbon content by 10.4% under drought stress.
Fig. 2.
Effect of drought stress on the carbon content of leaf in okra. Data are means of three replicates (n = 3), * asterisk differed significantly at P < 0.05*,.
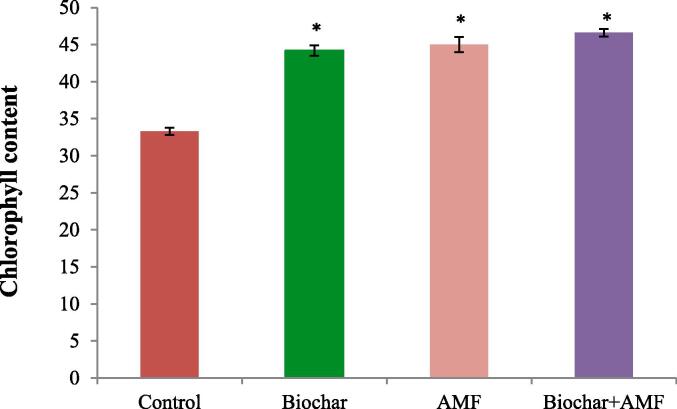
Data in Fig. 3 indicated that under drought stress, biochar alone and AMF treatments significantly increased total chlorophyll a content compared to the control. In drought stress, biochar treatment significantly enhanced the chlorophyll a content by 32.7% than the control and AMF alone significantly enhanced total chlorophyll content by 36.6% in drought condition. The highest values of total chlorophyll content was observed in the combined treatment of biochar and AMF recording a significant increase of 39.9% compared to the control under drought stress.
Fig. 3.
Effect of drought stress on the chlorophyll content of leaf in okra. Data are means of three replicates (n = 3), * asterisk differed significantly at P < 0.05*.
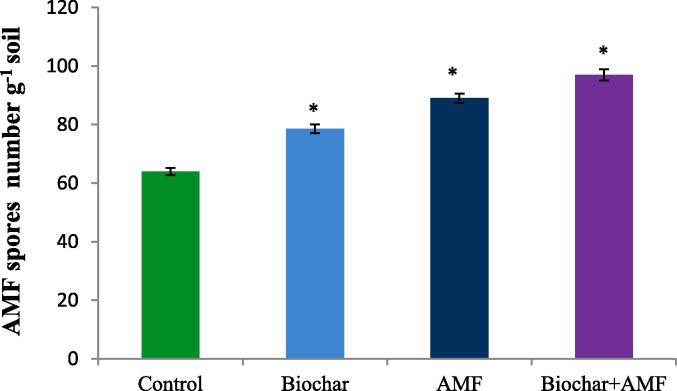
AMF spore number (per g of soil) increased in the treatment of biochar alone (Fig. 4). Biochar alone treatment significantly increased the AMF spores number by 22.8%. Under drought stress, AMF alone and combined with biochar and AMF treatments were more effective in increasing the AMF spores number in soil (Fig. 4). Compared to the control, the AMF spores in soil increased by 39.0% in AMF alone treatment. However, in the treatment where biochar and AMF were combined it significantly enhanced the AMF spores number by 51.5% compared to the control under water stress.
Fig. 4.
Effect of drought stress on the AMF spore numbers in soil. Data are means of three replicates (n = 3), * asterisk differed significantly at P < 0.05*
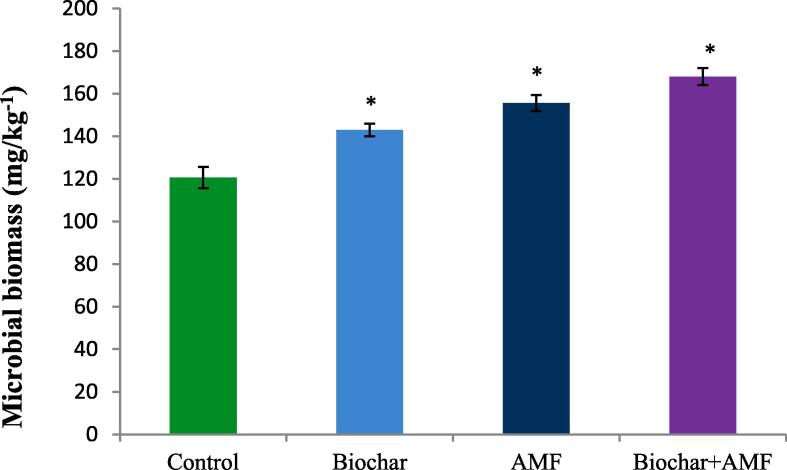
In water stress condition, biochar alone and AMF had significant impacts on the microbial biomass in soil increased most under 18.5% and 29.0% as compared the control (Fig. 5). Under drought stress, the microbial biomass reached a maximum in biochar and AMF combined treatment compared with all treatments. This treatment significantly increased the microbial biomass by 39.3% in soil than the control under drought stress.
Fig. 5.
Effect of drought stress on the microbial biomass in soil. Data are means of three replicates (n = 3), * asterisk differed significantly at P < 0.05*
The effect of biochar alone, the AMF alone and combined with biochar and AMF treatments on soil enzymes activities are given in Table 3. Compared to the control, biochar alone significantly influenced the alkaline phosphatase, the dehydrogenase and fluorescein diacetate enzyme activities in soil under drought condition. The dehydrogenase and fluorescein diacetate activities increased by 36.4% and 42.7% respectively when soil was amended by biochar alone as compared to the control in drought stress. Similarly, the alkaline phosphatase activity of the biochar alone and the AMF alone treatments significantly enhanced by 35.8% and 42.0% than the control. Under water stress, the dehydrogenase and fluorescein diacetate enzyme activity increased by 43.7% and 46.5% in AMF alone treatment as compared to the control. In drought condition, interaction between biochar and AMF significantly increased the alkaline phosphatase, the dehydrogenase and fluorescein diacetate enzyme activities in soil and were found much greater as compared to all other treatments. The increase was 55.8% (phosphatase), 68.7% (dehydrogenase enzyme) and 69.5% (fluorescein diacetate) in soil as compared to the control.
Table 3.
Effect of drought stress on soil enzymes activities.
| Treatments | Alkaline phosphatase (μg g−1h−1) | Dehydrogenase activity (μg g−1h−1) | Fluorescein diacetate activity (µg g−1h−1) |
|---|---|---|---|
| Control | 41.73 ± 0.64 | 32.00 ± 1.00 | 34.83 ± 0.76 |
| Biochar | 56.67 ± 1.15* | 43.67 ± 2.21* | 49.67 ± 1.53* |
| AMF | 58.67 ± 1.53* | 46.00 ± 1.00* | 51.00 ± 1.00* |
| Biochar + AMF | 65.00 ± 1.00* | 54.00 ± 2.05** | 59.00 ± 1.07** |
Data are means of three replicates (n = 3), * asterisk differed significantly at P < 0.05*
4. Discussion
We have studied the influence of biochar and AFM on the growth of okra (Abelmoschus esculentus) under drought stress condition. Drought stress reduces plant height, shoot dry weight and root dry weight. Drought stress and salinity stress decreased the germination rate and plant growth in various crops (Farooq et al., 2009, Awari and Mate, 2015, Hussain et al., 2016, Egamberdieva and Jabborova, 2013; Egamberdieva et al., 2016, Egamberdieva et al., 2017, Jabborova et al., 2020c, Sheteiwy et al., 2021, Ijaz et al., 2021). Similarly, Mueller et al. (2019) reported that okra growth and the total dry weight of okra was reduced by drought stress. Water stress reduced the plant growth parameter such as shoot fresh weight, dry weight, leaf number, leaf area, plant height and stem diameter in okra (Kusvuran 2012). Similarly, drought stress reducing plant height of Brassica napus has been reported by Zhao et al. (2006). Raza et al. (2012) observed a decrease in plant height in wheat by water stress.
In the present study, biochar significantly increased plant height, root dry weight, total root length, projected area and the root diameter as compared to control under drought stress. Numerous scientists reported that biochar improved plant growth, development and yield in various plants under stress (Kammann et al., 2011, Artiola et al., 2012, Mulcahy et al., 2013, Jabborova et al., 2021d). Similarly, Hashem et al. (2019) reported that biochar enhanced shoot length, root length, leaf area, number of primary branches, plant number of secondary branches in chickpea under drought stress.
Haider et al. (2015) observed a positive effect of biochar amendment on stem and leaf dry weight in maize. This finding is consistent with the report of de MeloCarvalho et al. (2013) who observed biochar amendment increased the leaf area index, biomass and yield in rice under water stress.
According to Batool et al. (2015) biochar application increased the leaf area, plant height in okra (Abelmoschus esculentus L.). Olmo et al. (2014) reported that biochar increased biomass of field grown wheat under semiarid Mediterranean conditions.
Studies have shown that the AMF significantly increased improved plant height, shoot dry weight, total root length, root volume and root diameter under drought stress. Numerous studies have reported that AMF improve the growth and water uptake of host plants under drought stress (Gholamhoseini et al., 2013, Baum et al., 2015, Benhiba et al., 2015, Zhao et al., 2015a, Zhao et al., 2015b, Chitarra et al., 2016, Quiroga et al., 2017, Bowles et al., 2018). This finding is consistent with the report of Hashem et al. (2018) who observed an increase in chickpea shoot length, root length, leaf area, number of primary branches, plant number of secondary branches by AMF compared to the control under drought stress. Similarly, Begum et al. (2019) reported that AMF-inoculated maize plants showed significant increase in height (36.32%) and dry weight (75.73%) over the control under drought stress. Drought stressed AM plants exhibited increased performance in terms of growth and biomass production, water and nutrient acquisition, and oxidative stress alleviation compared to control plants was reported by Essahibi et al., (2018).
In our study, combining biochar and AMF treatments improved plant height, shoot dry weight, root dry weight, the total root length, root volume, projected area, root surface area compared to the control and other treatments under drought stress condition. Our results were similar with a previous study which found that the combination of biochar and AMF increased plant growth (Hashem et al., 2019). Combination of biochar and AMF increased shoot and root biomass, leaf area meter, root surface area and root length in soursop (Annona muricata L.) seedlings (Harun et al. 2021). Similar results were reported by Budi and Setyaningsih (2013). They observed biochar and AMF significantly increased shoot dry weight, and root dry weight.
The present study demonstrated that drought stress reduced the nitrogen, carbon and chlorophyll content in the plants. Many previous studies found that drought stress decreased plant nutrients (Christophe et al., 2011, Rouphael et al., 2012, Heckathorn et al., 2014, Khalofah et al., 2021). Similarly, He et al. (2014) observed reduction in the concentration of nitrogen and phosphorus in plant tissue under drought stress. Total K and P uptake of maize organs showed a sharp decrease under drought stress (Ge et al., 2012). Drought reduced nitrogen and P content (Bista et al., 2018). Similarly, drought reduced chlorophyll content and leaf photosynthesis (Zhang et al., 2011, Hazrati et al., 2016, Bashri et al., 2021). This finding confirms earlier studies of Sawhney and Singh (2002) who observed that drought stress reduced chlorophyll a, b and total pigments in pumpkin. In drought stress reduced chlorophyll and carotenoid content in maize were demonstrated by Begum et al. (2019).
In the present study, the biochar addition significantly increased the carbon content and the chlorophyll a content in okra compared to control under drought stress. Numerous researchers have reported that biochar application increased nutrient uptake (Kammann et al., 2011, Akhtar et al., 2014, Haider et al., 2015) and plant physiological properties (Haider et al., 2015, Lyu et al., 2016, Xiao et al., 2016) in various plants under water stress. Similar results were reported by Akhtar et al. (2014). Biochar significantly enhanced the photosynthetic rate, chlorophyll contents, stomatal conductance, relative water contents and water use efficiency in tomato leaves under drought stress condition. In another study, biochar (Lantana camara, 450 °C) increased the photosynthesis, the WUE, and Gs of okra (Abelmoschus esculentus L. Moench) under drought stress as compared to the control (Batool et al. 2015). Similarly, biochar improved photosynthesis and the water use efficiency of okra in drought stress.
The present study demonstrates that AMF treatment significantly enhanced the nitrogen and carbon content. Numerous researchers noticed that AMF inoculation improved plant nutrient and water uptake in various plants under drought stress (Gholamhoseini et al., 2013, Baum et al., 2015, Zhao et al., 2015a, Zhao et al., 2015b, Bowles et al., 2018). A similar positive effect was reported with AMF treated maize showing enhanced nitrogen uptake under drought stress (Subramanian and Charest 1999). Wang et al. (2008) reported that AMF-inoculation increased uptake of minerals such as N, Mg and K in cucumber under water stress. Zhao et al., 2015a, Zhao et al., 2015b noted that AMF inoculation significantly increased P concentration in maize plants under drought condition. Similar results were obtained with AMF treated plants showing an increase in the plant physiological properties such as antioxidant enzyme activity and jasmonate synthesis of Digitaria eriantha under drought stress (Pedranzani et al., 2016). This finding confirms the observations of Abdel-Salam et al. (2018) who reported that AMF inoculation increased chlorophyll content and rate of photosynthesis in damask rose under drought stress condition. Gong et al. (2013) demonstrated that mycorrhizal seedlings had greater shoot dry weight, root dry weight, plant height, root length, instantaneous water use efficiency, net photosynthetic rate, stomatal conductance and photochemical quenching values when compared with non-mycorrhizal seedlings under water stress.
Combination of biochar and AMF resulted in significant enhancement in the nitrogen content, the carbon content and total chlorophyll content under drought stress. Similar findings were also noticed by Hashem et al. (2019), who showed that the combined application of AMF significantly increased total nitrogen content and total phosphorus content of shoot and root in chickpea under drought stress. Li and Cai (2021) reported that dual biochar and AMF increase the phosphorus content in maize under drought stress. Similarly, Hashem et al. (2019) has documented that the combined application of AMF and biochar significantly increased photosynthetic rate, relative water content chlorophyll a, chlorophyll b and total chlorophylls in chickpea under drought stress. Similar results have been reported by Li and Cai (2021) significantly enhanced the chlorophyll content and photosynthetic rate in maize under water stress.
This research demonstrated that drought stress decreased AMF spore number, microbial biomass and soil enzyme activities. Similar findings were reported by Geng et al., 2015, Mariotte et al., 2015 drought strongly decreased soil microbial activity and fungal properties. Water stress reduced enzyme activities such as urease, protease activity, phosphatase activity and β-glycosidase activity in soil as reported by Sardans and Peñuelas (2005). Li and Sarah (2003) observed that drought stress decreased enzyme activities with increasing activity along a climatic transect in Israel.
The experiment demonstrated that biochar significantly improved the AMF spores number, the microbial biomass, the alkaline phosphatase, the dehydrogenase and fluorescein diacetate enzyme activities in soil under drought stress. Hashem et al. (2019) reported biochar treatment protected AMF from the deleterious effects of drought by improving the number of spores (36.73%), mycelium (79.68%), vesicles (28.65%) and arbuscules (28.55%) over drought stressed plants. Similar findings were also noticed by Li and Cai (2021). Biochar application significantly increased microbial biomass by 38.0% and 65.9% under drought condition. Jabborova et al. (2020a) reported that biochar addition improved protease, acid phosphomonoesterase and alkaline phosphomonoesterase activities in soil. Similar result was confirmed by Ahmad et al. (2014). He found a stronger positive effect of biochar amendment on microbial biomass activity.
In a previous study, under drought stress AMF alone significantly increased the AMF spores number, the microbial biomass, the alkaline phosphomonoestrase, the dehydrogenase and fluorescein diacetate enzyme activities in soil. Similarly, Hashem et al. (2019) improved the number of spores of Arbuscular mycorrhizal fungi in chickpea under drought stress. Similar findings were also noticed by Li and Cai (2021) AMF inoculation improved soil microbial biomass. Qin et al. (2020) indicate that AMF can enhance the release of soil nutrients required for plant growth in response to increased soil enzyme activity.
However, combination of AMF and biochar strongly enhanced the AMF spores number, the microbial biomass, alkaline phosphatase, the dehydrogenase and fluorescein diacetate enzyme activities in soil compared to control and other treatments. Similar resulted were observed by Hashem et al., 2019, Li and Cai, 2021. Dual biochar and AMF inoculation significantly improved soil microbial activity, phosphatase activity by 40% in the maize rhizosphere under drought stress.
5. Conclusion
This study investigated the impact of biochar and AMF inoculation in mitigating the drought stress on okra. Under drought stress, biochar application could enhance root morphological traits viz: the total root length, the projected area, the root diameter and the root volume and soil enzyme activities. In drought stress, AMF could form a good symbiotic relationship with okra seedlings, and AMF symbiosis indeed improved root morphology, chlorophyll content, AMF spores number, microbial biomass, and improved the uptake of N. Combined inoculation with biochar and AMF clearly showed best results compared to the biochar alone and AMF alone treatments under drought condition. Dual application was more effective in enhancing plant growth, root morphological traits and chlorophyll content compared to other treatments. The AMF had synergetic effect with biochar for improving microbial biomass, AMF spores and enzymes activities in soil under drought stress. This finding reveals the prospective and potential use of okra combined with biochar and AMF for the successful crop cultivation under drought stress.
Funding
This work has been financed by the Department of Biotechnology, Government of India (DBT), and TWAS.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank colleagues at Division of Microbiology, ICAR-Indian Agricultural Research Institute, Pusa, New Delhi, India for providing necessary support laboratory and net house facilities. This project was supported by Researchers Supporting Project Number (RSP-2021/5) King Saud University, Riyadh, Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Dilfuza Jabborova, Email: dilfuzajabborova@yahoo.com.
Kannepalli Annapurna, Email: annapurna96@gmail.com.
Ali Tan Kee Zuan, Email: tkz@upm.edu.my.
References
- Abd El-Aal E.M., Shahen M., Sayed S., Kesba H., Ansari M.J., El-Ashry R.M., Aioub A.A.A., Salma A.S.A., Eldeeb A.M. In vivo and In vitro management of Meloidogyne incognita (Tylenchida: Heteroderidae) using Rhizosphere Bacteria. Pseudomonas spp. and Serratia spp. compared with oxamyl. Saudi J. Biol. Sci. 2021 doi: 10.1016/j.sjbs.2021.06.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel Latef A.A. Influence of arbuscular mycorrhizal fungi and copper on growth, ac- cumulation of osmolyte, mineral nutrition and antioxidant enzyme activity of pepper (Capsicum annuum L.) Mycorrhiza. 2011;21:495–503. doi: 10.1007/s00572-010-0360-0. [DOI] [PubMed] [Google Scholar]
- Abdel-Salam E., Alatar A., El-Sheikh M.A. Inoculation with arbuscular mycorrhizal fungi alleviates harmful effects of drought stress on damask rose. Saudi J. Biol. Sci. 2018;25:1772–1780. doi: 10.1016/j.sjbs.2017.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdulrahman F.A., Nadir H.A. Effect of water stress on okra yield at vegetative stage. Agric. 2018;30(2):111–116. [Google Scholar]
- Ahmad Mahtab, Rajapaksha Anushka Upamali, Lim Jung Eun, Zhang Ming, Bolan Nanthi, Mohan Dinesh, Vithanage Meththika, Lee Sang Soo, Ok Yong Sik. Biochar as a sorbent for contaminant management in soil and water: a review. Chemosphere. 2014;99:19–33. doi: 10.1016/j.chemosphere.2013.10.071. [DOI] [PubMed] [Google Scholar]
- Akhtar Saqib Saleem, Li Guitong, Andersen Mathias Neumann, Liu Fulai. Biochar enhances yield and quality of tomato under reduced irrigation. AgriWaterManag. 2014;138:37–44. [Google Scholar]
- Al-Ayed M.S. Growth and some metabolic changes in Cucurbita pepo under water stress and ultra–violet-B radiation. Saudi J. Bio. Sci. 1998;5:45–55. [Google Scholar]
- Alizadeh O., Zare M., Nasr A.H. Evaluation effect of Mycorrhiza inoculate under drought stress condition on grain yield of sorghum (Sorghum bicolor) Adv Environ Biol. 2011;5:2361–2364. [Google Scholar]
- Andrenelli M.C., Maienza A., Genesio L., Miglietta F., Pellegrini S., Vaccari F.P., Vignozzi N. Field application of pelletized biochar: short term effect on the hydrological properties of a silty clay loam soil. Agric. Water Manag. 2016;163:190–196. [Google Scholar]
- Anjum S.A., Ashraf U., Tanveer M., Khan I., Hussain S., Zohaib A., Abbas F., Saleem M.F., Wang L. Drought tolerance in three maize cultivars is related to differential osmolyte accumulation, antioxidant defense system, and oxidative damage. Front. Plant Sci. 2017;8:1–12. doi: 10.3389/fpls.2017.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artiola J.F., Rasmussen C., Freitas R. Effects of a biochar-amended alkaline soil on the growth of romaine lettuce and bermudagrass. Soil Sci. 2012;177:561–570. [Google Scholar]
- Asha A.D., Nivetha N., Krishna G.K., Thakur J.K., Rathi M.S., Manjunatha B.S., Chinnusamy V., Paul S. Amelioration of short-term drought stress during different growth stages in Brassica juncea by rhizobacteria mediated maintenance of ROS homeostasis. Physiol. Plant. 2021 doi: 10.1111/ppl.13399. [DOI] [PubMed] [Google Scholar]
- Augé Robert M. Water relations, drought and vesicular-arbuscular mycorrhizal symbiosis. Mycorrhiza. 2001;11(1):3–42. [Google Scholar]
- Augé, R.M., Toler, H.D., Saxton, A.M., 2015. Arbuscular mycorrhizal symbiosis alters stomatal conductance of host plants more under drought than under amply watered conditions: a meta-analysis. [DOI] [PubMed]
- Awari V.R., Mate S.N. Effect of drought stress on early seedling growth of chickpea (Cicer arietinum L.) genotypes. Life Sci. Int. Res. J. 2015;2:356–361. [Google Scholar]
- Bamminger Chris, Poll Christian, Sixt Christina, Högy Petra, Wüst Dominik, Kandeler Ellen, Marhan Sven. Short-term response of soil microorganisms to biochar addition in a temperate agroecosystem under soil warming. Agri. Ecosys. Environ. 2016;233:308–317. [Google Scholar]
- Bashri G., Singh S., Prasad S.M., Ansari M.J., Usmani S., Alfarraj S., Alharbi S.A., Brestic M., Farooq S. Kinetin mitigates Cd-induced damages to growth, photosynthesis and PS II photochemistry of Trigonella seedlings by up-regulating ascorbate-glutathione cycle. PLoS ONE. 2021;16(6):e0249230. doi: 10.1371/journal.pone.0249230. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Batool A., Taj S., Rashid A., Khalid A., Qadeer S., Saleem A.R., Ghufran M.A. Potential of soil amendments (biochar and gypsum) in increasing water use efficiency of Abelmoschus esculentus L. Moench. Front. Plant Sci. 2015;6:1–13. doi: 10.3389/fpls.2015.00733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum, C., El-Tohamy, W., Gruda, N., 2015. Increasing the productivity and product quality of vegetable crops using arbuscular mycorrhizal fungi: a review.
- Begum N., Ahanger M.A., Su Y., Lei Y., Mustafa N.S., Ahmad P., Zhang L. Improved drought tolerance by AMF inoculation in maize (Zea mays) involves physiological and biochemical implications. Plants. 2019;8(12):579. doi: 10.3390/plants8120579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benhiba L., Fouad M.O., Essahibi A., Ghoulam C., Qaddoury A. Arbuscular mycorrhizal symbiosis enhanced growth and antioxidant metabolism in date palm subjected to long-term drought. Trees Struct. Funct. 2015;29:1725–1733. [Google Scholar]
- Birhane Emiru, Sterck Frank J., Fetene Masresha, Bongers Frans, Kuyper Thomas W. Arbuscular mycorrhizal fungi en- hance photosynthesis, water use efficiency, and growth of frankincense seedlings under pulsed water availability conditions. Oecologia. 2012;169(4):895–904. doi: 10.1007/s00442-012-2258-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bista D.R., Heckathorn S.A., Jayawardena D.M., Mishra S., Boldt J.K. Effects of drought on nutrient uptake and the levels of nutrient-uptake proteins in roots of drought-sensitive and-tolerant grasses. Plants. 2018;7(2):28. doi: 10.3390/plants7020028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles T.M., Jackson L.E., Cavagnaro T.R. Mycorrhizal fungi enhance plant nutrient acquisition and modulate nitrogen loss with variable water regimes. Glob. Chang Biol. 2018;24:e171–e182. doi: 10.1111/gcb.13884. [DOI] [PubMed] [Google Scholar]
- Brundrett Mark C., Tedersoo Leho. Evolutionary history of mycorrhizal symbioses and global host plant diversity. New Phytol. 2018;220(4):1108–1115. doi: 10.1111/nph.14976. [DOI] [PubMed] [Google Scholar]
- Budi S.W., Setyaningsih L. Arbuscular mycorrhizal fungi and biochar improved early growth of neem (Melia azedarach Linn.) seedling under greenhouse conditions. Jurnal Manajemen Hutan Tropika. 2013;19(2):103–110. [Google Scholar]
- Casida L.E., Klein D.A., Santoro Thomas. Soil dehydrogenase activity. Soil Sci. 1964;98(6):371–376. [Google Scholar]
- Cavagnaro T.R., Jackson L.E., Six J., Ferris H., Goyal S., Asami D. Arbuscular mycorrhizas, microbial communities, nutrient availability, and soil aggregates in organic tomato production. Plant Soil. 2006;282:209–225. [Google Scholar]
- Chaturvedi A.K., Surendran U., Gopinath G., Chandran K.M., Anjali N.K., Ct M.F. Elucidation of stage specific physiological sensitivity of okra to drought stress through leaf gas exchange, spectral indices, growth and yield parameters. Agric. Water Manag. 2019;1(222):92–104. [Google Scholar]
- Chitarra W., Pagliarani C., Maserti B., Lumini E., Siciliano I., Cascone P., Schubert A., Gambino G., Balestrini R., Guerrieri E. Insights on the impact of arbuscular mycorrhizal symbiosis on tomato tolerance to water stress. Plant Physiol. 2016;171:1009–1023. doi: 10.1104/pp.16.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury J.A., Karim M.A., Khaliq Q.A., Ahmed A.U., Khan M.S.A. Effect of drought stress on gas exchange characteristics of four soybean genotypes. Bangladesh J. Agr. Res. 2016;41:195–205. [Google Scholar]
- Christophe S., Jean-Christophe A., Annabelle L., Alain O., Marion P., Anne-Sophie V. In: Abiotic Stress in Plants—Mechanisms and Adaptations. Shanker A., Venkateswarlu B., editors. InTech; Rijeka, Crotia: 2011. Plant N fluxes and modulation by nitrogen, heat and water stresses: A review based on comparison of legumes and non-legume plants; pp. 79–118. [Google Scholar]
- Cramer M.D., Hawkins H.J., Verboom G.A. The importance of nutritional regulation of plant water flux. Oecologia. 2009;161:15–24. doi: 10.1007/s00442-009-1364-3. [DOI] [PubMed] [Google Scholar]
- Dar Z.A., Dar S.A., Khan J.A., Lone A.A., Langyan S., Lone B.A., Kanth R.H., Iqbal A., Rane J., Wani S.H., Alfarraj S., Alharbi S.A., Brestic M., Ansari M.J. Identification for surrogate drought tolerance in maize inbred lines utilizing high-throughput phenomics approach. PLoS ONE. 2021;16(7):e0254318. doi: 10.1371/journal.pone.0254318. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Dare M.O., Abaidoo R., Fagbola O., Asideu R. Diversity of AMF in soils of yam (Diosocera spp) cropping systems in four agroecologies of Nigeria. Achieves of Agronomy and Soil Science. 2013;59(4):521–531. [Google Scholar]
- de MeloCarvalho M.T., Madari B.E., Bastiaans L., van Oort P.A.J., Heinemann A.B., da Silva M.A.S., Maia A.H.N., Meinke H. Biochar improves fertility of a clay soil in the Brazilian Savannah: short term effects and impact on rice yield. J. Agri. Rural Devel. Trop. Subtrop. 2013;114(2):101–107. http://nbn-resolving.de/urn: nbn:de:hebis:34-2013081343330 [Google Scholar]
- Egamberdieva D., Jabborova D. Improvement of cotton production in arid saline soils by beneficial microbes. InCrop Yields: Production, Management Practices and Impact of. Clim. Change. 2013:109–122. [Google Scholar]
- Egamberdieva D., Jabborova D., Berg G. Synergistic interactions between Bradyrhizobium japonicum and the endophyte Stenotrophomonas rhizophila and their effects on growth, and nodulation of soybean under salt stress. Plant Soil. 2016;405(1):35–45. [Google Scholar]
- Egamberdieva D., Jabborova D. In: Vegetation of Central Asia and Environs. Egamberdieva D., Öztürk M., editors. Springer Nature Switzerland AG; 2018. Medicinal plants of Uzbekistan and their traditional uses; pp. 211–237. [Google Scholar]
- Egamberdieva D., Wirth S., Jabborova D., Räsänen L.A., Liao H. Coordination between Bradyrhizobium and Pseudomonas alleviates salt stress in soybean through altering root system architecture. J. Plant Interact. 2017;12(1):100–107. [Google Scholar]
- Elkhalifa A.E., Alshammari E., Adnan M., Alcantara J.C., Awadelkareem A.M., Eltoum N.E., Mehmood K., Panda B.P., Ashraf S.A. Okra (Abelmoschus Esculentus) as a Potential Dietary Medicine with Nutraceutical Importance for Sustainable Health Applications. Molecules. 2021;26(3):696. doi: 10.3390/molecules26030696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essahibi A., Benhiba L., Babram M.A., Ghoulam C., Qaddoury A. Influence of arbuscular mycorrhizal fungi on the functional mechanisms associated with drought tolerance in carob (Ceratonia siliqua L.) Trees Struct. Funct. 2018;32:87–97. [Google Scholar]
- Farooq M., Wahid A., Kobayashi N., Fujita D., Basra S.M.A. Plant drought stress: effects, mechanisms and management. Agron. Sustain. Dev. 2009;29:185–212. [Google Scholar]
- Fiaz K., Danish S., Younis U., Malik S.A., Raza Shah M.H., Niaz S. Drought impact on Pb/Cd toxicity remediated by biochar in Brassica campestris. J Soil Sci Plant Nutri. 2014;14:845–854. [Google Scholar]
- Fierer N., Schimel J.P. Effects of drying–rewetting frequency on soil carbon and nitrogen transformations. Soil Biol. Biochem. 2002;34:777–787. [Google Scholar]
- Ge T.D., Sun N.B., Bai L.P., Tong C.L., Sui F.G. Effects of drought stress on phosphorus and potassium uptake dynamics in summer maize (Zea mays) throughout the growth cycle. Acta Physiol. Plant. 2012;34:2179–2186. [Google Scholar]
- Geng S.M., Yan D.H., Zhang T.X., Weng B.S., Zhang Z.B., Qin T.L. Effects of drought stress on agriculture soil. Nat. Hazards. 2015;75:1997–2011. [Google Scholar]
- Gholamhoseini M., Ghalavand A., Dolatabadian A., Jamshidi E., Khodaei-Joghan A. Effects of arbuscular mycorrhizal inoculation on growth, yield, nutrient uptake and irrigation water productivity of sunflowers grown under drought stress. Agric. Water Manag. 2013;117:106–114. [Google Scholar]
- Githinji Leonard. Effect of biochar application rate on soil physical and hydraulic properties of a sandy loam. Arch. Agron. Soil Sci. 2014;60(4):457–470. [Google Scholar]
- Golldack D., Li C., Mohan H., Probst N. Tolerance to drought and salt stress in plants: Unraveling the signaling networks. Front. Plant Sci. 2014;5:151. doi: 10.3389/fpls.2014.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong M., Tang M., Chen H., Zhang Q., Feng X. Effects of two Glomus species on the growth and physiological performance of Sophora davidii seedlings under water stress. New For. 2013;44:399–408. [Google Scholar]
- Haider M.U., Hussain M., Farooq M., Ul-Allah S., Ansari M.J., Alwahibi M.S., Farooq S., Ali S. Zinc biofortification potential of diverse mungbean [Vigna radiata (L.) Wilczek] genotypes under field conditions. PLoS ONE. 2021;16(6):e0253085. doi: 10.1371/journal.pone.0253085. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Haider Ghulam, Koyro Hans-Werner, Azam Farooqe, Steffens Diedrich, Müller Christoph, Kammann Claudia. Biochar but not humic acid product amendment affected maize yields via improving plant-soil moisture relations. Plant Soil. 2015;395(1-2):141–157. [Google Scholar]
- Harun N.S., Jaafar N.M., Sakimin S.Z. The effects of rice husk biochar rate on arbuscular mycorrhizal fungi and growth of soursop (Annona muricata L.) seedlings. Sustainability. 2021;13(4):1817. [Google Scholar]
- Hashem A., Kumar A., Al-Dbass A.M., Alqarawi A.A., Al-Arjani A.B.F., Singh G., Farooq M., Abd-Allah E.F. Arbuscular mycorrhizal fungi and biochar improves drought tolerance in chickpea. Saudi J. Biol. Sci. 2019;26:614–624. doi: 10.1016/j.sjbs.2018.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazrati S., Tahmasebi-Sarvestani Z., Modarres-Sanavy S.A.M., Mokhtassi-Bidgoli A., Nicola S. Effects of water stress and light intensity on chlorophyll fluorescence parameters and pigments of Aloe vera L. Plant Physiol. Biochem. 2016;106:141–148. doi: 10.1016/j.plaphy.2016.04.046. [DOI] [PubMed] [Google Scholar]
- Heckathorn S.A., DeLucia E.H., Zielinski R.E. The contribution of drought-related decreases in foliar nitrogen concentration to decreases in photosynthetic capacity during and after drought in prairie grasses. Physiol. Plant. 1997;101:173–182. [Google Scholar]
- Heckathorn S.A., Giri A., Mishra S., Bista D. In: Climate Change and Plant Abiotic Stress Tolerance. Tuteja N., Gill S.S., editors. Wiley-VCH Verlag Gmb H & Co. KGaA; Weinheim, Germany: 2014. Heat Stress and Roots; pp. 109–136. [Google Scholar]
- Heflish A.A., Hanfy A.E., Ansari M.J., Dessoky E.S., Attia A.O., Elshaer M.M., Gaber M.K., Kordy A., Doma A.S., Abdelkhalek A., Behiry S.I. Green Biosynthesized Silver Nanoparticles using Acalypha wilkesiana Extract control root-knot nematode. Journal of King Saud University –. Science. 2021;33(6):101516. doi: 10.1016/j.jksus.2021.101516. [DOI] [Google Scholar]
- Hussain M., Farooq S., Hasan W., Ul-allah S., Tanveer M. Drought stress in sunflower: Physiological effects and its management through breeding and agronomic alternatives. Agric. Water Manag. J. 2018;201:152–166. [Google Scholar]
- Hussain S., Khan F., Cao W., Wu L., Geng M. Seed priming alters the production and detoxification of reactive oxygen intermediates in rice seedlings grown under sub-optimal temperature and nutrient supply. Front. Plant Sci. 2016;7:439. doi: 10.3389/fpls.2016.00439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ijaz M., Nawaz A., Ul-Allah S., Sher A., Sattar A., Sarwar M., Hussain I., Ur Rehman A., Wahid M.A., Ansari M.J., Hessini K., Farooq S. Optimizing sowing date for peanut genotypes in arid and semi-arid subtropical regions. PLoS ONE. 2021;16(6):e0252393. doi: 10.1371/journal.pone.0252393. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Jabborova D., Davranov K., Egamberdieva D. In: Medically Important Plant Biomes: Source of Secondary Metabolites. Egamberdieva D., Tiezzi A., editors. Springer Nature Singapore Pte Ltd; 2019. Antibacterial, antifungal, and antiviral properties of medicinal plants; pp. 51–65. [Google Scholar]
- Jabborova D., Wirth S., Kannepalli A., Narimanov A., Desouky S., Davranov K., Sayyed R.Z., Enshasy H., Abd Malek R., Syed A., Bahkali A.H. Co-inoculation of rhizobacteria and biochar application improves growth and nutrients in soybean and enriches soil nutrients and enzymes. Agronomy. 2020;10:1142. doi: 10.3390/agronomy10081142. [DOI] [Google Scholar]
- Jabborova D., Annapurna K., Fayzullaeva M., Sulaymonov K., Kadirova D., Jabbarov Z., Sayyed R.Z. Isolation and characterization of endophytic bacteria from ginger (Zingiber officinale Rosc.) Ann. Phytomed. 2020;9:116–121. [Google Scholar]
- Jabborova D., Enakiev Y., Sulaymanov K., Kadirova D., Ali A., Annapurna K. Plant growth-promoting bacteria Bacillus subtilis promote growth and physiological parameters of Zingiber officinale Roscoe. Plant Sci. Today. 2021;8:66–71. [Google Scholar]
- Jabborova D., Sayyed R.Z., Azimov A., Jabbarov Z., Matchanov A., Enakiev Y., Baazeem A., Sabagh A.E., Danish S., Datta R. Impact of mineral fertilizers on mineral nutrients in the ginger rhizome and on soil enzymes activities and soil properties. Saudi Journal of Biological Sciences. 2021 doi: 10.1016/j.sjbs.2021.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabborova D., Choudhary R., Karunakaran R., Ercisli S., Ahlawat J., Sulaymanov K., Azimov A., Jabbarov Z. The Chemical Element Composition of Turmeric Grown in Soil-Climate Conditions of Tashkent Region, Uzbekistan. Plants. 2021;10(7):1426. doi: 10.3390/plants10071426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabborova D., Annapurna K., Paul S., Kumar S., Ibrahim M., Elkelish A.A. Beneficial features of biochar and AMF for improving spinach plant growth, root morphological traits, physiological properties and soil enzymatic activities. J. Fungi. 2021;2021(7):571. doi: 10.3390/jof7070571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabborova D.P., Narimanov A.A., Enakiev Y.I., Davranov K.D. Effect of Bacillus subtilis 1 strain on the growth and development of wheat (Triticum aestivum L.) under saline condition. Bulgar. J. Agric. Sci. 2020;26(4):744–747. [Google Scholar]
- Kammann Claudia Irene, Linsel Sebastian, Gößling Johannes W., Koyro Hans-Werner. Influence of biochar on drought tolerance of Chenopodium quinoa Willd and on soil–plant relations. Plant Soil. 2011;345(1-2):195–210. [Google Scholar]
- Kaya M.D., Okçu G., Atak M., Çıkılı Y., Kolsarıcı Ö. Seed treatments to overcome salt and drought stress during germination in sunflower (Helianthus annuus L.) Eur. J. Agron. 2006;24(4):291–295. [Google Scholar]
- Khalofah A., Khan M.I., Arif M., Hussain A., Ullah R., Irfan M., Mahpara S., Shah R.U., Ansari M.J., Kintl A., Brtnicky M., Danish S., Datta R. Deep placement of nitrogen fertilizer improves yield, nitrogen use efficiency and economic returns of transplanted fine rice. PLoS ONE. 2021;16(2):e0247529. doi: 10.1371/journal.pone.0247529. [International, referred & indexed Journal. ISSN- 1932-6203, NAAS Rating-8.77, JrnID-P004, UGC-CARE Listed as Group A Journal] [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kobraee S., Shamsi K., Rasekhi B. Soybean production under water deficit conditions. Sch. Res. Libr. 2011;2:423–434. [Google Scholar]
- Kubar M.S., Shar A.H., Kubar K.A., Rind N.A., Ullah H., Kalhoro S.A., Wang C., Feng M., Gujar A., Sun H., Yang W., El Enshasy H., Brestic M., Zivcak M., Ondrisik P., Aljuaid B.S., El-Shehawi A.M., Ansari M.J. Optimizing nitrogen supply promotes biomass, physiological characteristics and yield components of soybean (Glycine max L. Merr.) Saudi J. Biol. Sci. 2021 doi: 10.1016/j.sjbs.2021.06.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusvuran S. Influence of drought stress on growth, ion accumulation and antioxidative enzymes in okra genotypes. Int. J. Agric. Biol. 2012;14(3) [Google Scholar]
- Lehmann J., Gaunt J., Rondon M. Bio-char sequestration in terrestrial ecosystems—a review. Mitig. Adapt. Strateg. Global Change. 2006;11(2):403–427. [Google Scholar]
- Li M., Cai L. Biochar and Arbuscular Mycorrhizal Fungi Play Different Roles in Enabling Maize to Uptake Phosphorus. Sustainability. 2021;13:3244. [Google Scholar]
- Li D., Liu H., Qiao Y., Wang Y., Cai Z., Dong B., Shi C., Liu Y., Li X., Liu M. Effects of elevated CO2 on the growth, seed yield, and water use efficiency of soybean (Glycine max. L.) under drought stress. Agric. Water Manag. 2013;129:105–112. [Google Scholar]
- Li X.Z., Sarah P. Enzyme activities along a climatic transect in the Judean Desert. Catena. 2003;53:349–363. [Google Scholar]
- Li P., Zhang Y., Wu X., Liu Y. Drought stress impact on leaf proteome variations of faba bean (Vicia faba L.) in the Qinghai-Tibet Plateau of China. 3. Biotech. 2018;8:110. doi: 10.1007/s13205-018-1088-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim T.J., Spokas K.A., Feyereisen G., Novak J.M. Predicting the impact of biochar additions on soil hydraulic properties. Chemosphere. 2016;142:136–144. doi: 10.1016/j.chemosphere.2015.06.069. [DOI] [PubMed] [Google Scholar]
- Lyu S., Du G., Liu Z., Zhao L., Lyu D. Effects of biochar on photosystem function and activities of protective enzymes in Pyrus ussuriensis Maxim. under drought stress. Acta Physiol Plant. 2016;38:1–10. [Google Scholar]
- Maes W.H., Achten W.M., Reubens B., Raes D., Samson R., Muys B. Plant–water relationships and growth strategies of Jatropha curcas L. seedlings under different levels of drought stress. J. Arid Environ. 2009;73:877–884. [Google Scholar]
- Mak M., Babla M., Xu S., Carrigan A.O., Liu X., Gong Y., Holford P., Chen Z. Leaf mesophyll K+, H+ and Ca2+ fluxes are involved in drought-induced decrease in photosynthesis and stomatal closure in soybean. Environ. Exp. Bot. 2014;98:1–12. [Google Scholar]
- Maleki A., Naderi A., Naseri R., Fathi A., Bahamin S., Maleki R. Physiological performance of soybean cultivars under drought stress. Bull. Environ. Pharmacol. Life Sci. 2013;2:38–44. [Google Scholar]
- Mamarasulov B., Davranov K., Jabborova D. Phytochemical, pharmacological, and biological properties of Ajuga turkestanica (Rgl.) Brig (Lamiaceae) Annals of Phytomedicine. 2020;9:44–57. [Google Scholar]
- Mariotte P., Robroek B.J.M., Jassey V.E.J., Buttler A. Subordinate plants mitigate drought effects on soil ecosystem processes by stimulating fungi. Funct. Ecol. 2015;29:1578–1586. [Google Scholar]
- McGlashan N., Shah N., Caldecott B., Workman M. High-level techno-economic assessment of negative emissions technologies. Process Saf. Environ. Prot. 2012;90(6):501–510. [Google Scholar]
- Mueller A., Eltigani A., George E. The abundance of arbuscular mycorrhizal fungal species in symbiosis with okra plants is affected by induced drought conditions in a calcareous substrate. Rhizosphere. 2019;1(10) [Google Scholar]
- Mulcahy D.N., Mulcahy D.L., Dietz D. Biochar soil amendment increases tomato seedling resistance to drought in sandy soils. J. Arid Environ. 2013;88:222–225. [Google Scholar]
- Nunes J.L.D., de Souza P.V.D., Marodin G.A.B., Fachinello J.C. Effect of arbuscular mycorrhizal fungi and indole butyric acid interaction on vegetative growth of ‘Aldrighi’ peach rootstock seedlings. Cienc. Agrotecnol. 2010;34:80–86. [Google Scholar]
- Ok Yong Sik, Chang Scott X., Gao Bin, Chung Hyun-Joong. SMART biochar technology-a shifting paradigm towards advanced materials and healthcare research. Environ Technol Innov. 2015;4:206–209. [Google Scholar]
- Olmo Manuel, Alburquerque José Antonio, Barrón Vidal, del Campillo María Carmen, Gallardo Antonio, Fuentes Mariano, Villar Rafael. Wheat growth and yield responses to biochar addition under Mediterranean climate conditions. Biol Fert Soil. 2014;50(8):1177–1187. [Google Scholar]
- Osakabe Y., Osakabe K., Shinozaki K., Tran L.P. Response of plants to water stress. Front. Plant Sci. 2014;5:1–19. doi: 10.3389/fpls.2014.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedranzani H., Rodríguezrivera M., Gutiérrez M., Porcel R., Hause B., Ruizlozano J.M. Arbuscular mycorrhizal symbiosis regulates physiology and performance of Digitaria eriantha plants subjected to abiotic stresses by modulating antioxidant and jasmonate levels. Mycorrhiza. 2016;26:141–152. doi: 10.1007/s00572-015-0653-4. [DOI] [PubMed] [Google Scholar]
- Pereira J., Chaves M.M., Caldeira M.C., Correia A.V. In: Plant Growth and Climate Change. Morison J.I.L., Morecroft M.D., editors. Blackwell Publishing Ltd.; Oxford, UK: 2006. Water availability and productivity; pp. 118–145. [Google Scholar]
- Pushpavalli R., Zaman-allah M., Turner N.C., Baddam R., Rao M.V., Vadez V. Higher flower and seed number leads to higher yield under water stress conditions imposed during reproduction in chickpea. Funct. Plant Biol. 2015;42:162–174. doi: 10.1071/FP14135. [DOI] [PubMed] [Google Scholar]
- Qin M., Zhang Q., Pan J., Jiang S., Liu Y., Bahadur A., Peng Z., Yang Y., Feng H. Effect of arbuscular mycorrhizal fungi on soil enzyme activity is coupled with increased plant biomass. Eur. J. Soil Sci. 2020;71(1):84–92. [Google Scholar]
- Quiroga G., Erice G., Aroca R., Chaumont F., Ruiz-Lozano J.M. Enhanced drought stress tolerance by the arbuscular mycorrhizal symbiosis in a drought-sensitive maize cultivar is related to a broader and differential regulation of host plant aquaporins than in a drought-tolerant cultivar. Front. Plant Sci. 2017;8:1–15. doi: 10.3389/fpls.2017.01056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranawake A., Dahanayaka N., Amarasingha U., Rodrigo W., Rodrigo U. Effect of Water Stress on Growth and Yield of Mung Bean (Vigna radiata L) Trop. Agric. Res. Extens. 2012;14(4) doi: 10.4038/tare.v14i4.4851. [DOI] [Google Scholar]
- Raza M.A.S., Saleem M.F., Anjum S.A., Khaliq T., Wahid M.A. Foliar application of potassium under water deficit conditions improved the growth and yield of wheat (Triticum aestivum L.) J. Animal Plant Sci. 2012;22:431–437. [Google Scholar]
- Razi S.S., Sen S.P. Amelioration of water stress effects on wetland rice by urea-N plant growth regulations and foliar spray of a diazotrophic Bacteruim klebsiella sp. Original paper. Biol Fertil Soils. 1996 [Google Scholar]
- Rizwan M., Ali S., Qayyum M.F., Ibrahim M., Rehman M.Z., Abbas T., Ok Y.S. Mechanisms of biochar-mediated alleviation of toxicity of trace elements in plants: a critical review. Environ. Sci. Pollut. Res. 2016;23:2230–2248. doi: 10.1007/s11356-015-5697-7. [DOI] [PubMed] [Google Scholar]
- Rouphael Y., Cardarelli M., Schwarz D., Franken P., Colla G. In: Plant Responses to Drought Stress. Aroca R., editor. Springer; Berlin, Germany: 2012. Effects of drought on nutrient uptake and assimilation in vegetable crops; pp. 171–195. [Google Scholar]
- Ruiz-Lozano J.M., Aroca R. In: Arbuscular mycorrhizas: physiology and function. Koltai H., Kapulnik Y., editors. Springer; Netherlands: 2010. Host response to osmotic stresses: stomatal behaviour and water use efficiency of arbuscular mycorrhizal plants; pp. 239–256. [Google Scholar]
- Saccardy K., Pineau B., Roche O., Cornic G. Photo chemical efficiency of photosystem and xanthophyll cycle components in Zea mays leaves exposed to water stress and high light. Photosy. Res. 1998;56:57–66. [Google Scholar]
- Samarah N.H., Haddad N., Alqudah A.M. Yield potential evaluation in chickpea genotypes under late terminal drought in relation to the length of reproductive stage. Ital. J. Agron. 2009;3:111–117. [Google Scholar]
- Sanaullah M., Blagodatskaya E., Chabbi A., Rumpel C., Kuzyakov Y. Drought effects on microbial biomass and enzyme activities in the rhizosphere of grasses depend on plant community composition. Appl. Soil Ecol. 2011;48(1):38–44. [Google Scholar]
- Sanchez-Blanco J., Fernandez T., Morales A., Morte A., Alarcon J.J. Variation in water stress, gas exchange, and growth in Rasmanrins officinalis plants infected with Glamus deserticola under drought conditions.J. Plant Physiol. 2006;161:675–682. doi: 10.1078/0176-1617-01191. [DOI] [PubMed] [Google Scholar]
- Sardans J., Peñuelas J. Drought decreases soil enzyme activity in a Mediterranean Quercus ilex L. forest. Soil Biol. Biochem. 2005;37(3):455–461. [Google Scholar]
- Sardans J., Peñuelas J. The role of plants in the effects of global change on nutrient availability and stoichiometry in the plant-soil system. Plant Physiol. 2012;160:1741–1761. doi: 10.1104/pp.112.208785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawhney V., Singh D.P. Effect of chemical desiccation at the post-anthesis stage on some physiological and biochemical changes in the flag leaf of contrasting wheat genotypes. Field Crops Res. 2002;77:1–6. [Google Scholar]
- Schwanz P., Picon C., Vivin P., Dreyer E., Guehl J., Polle A. Responses of antioxidative systems to drought stress in pendunculate oak and maritime pine as modulated by elevated CO2. Plant Physiol. 1996;110:393–402. doi: 10.1104/pp.110.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehzad M., Zhou Z., Ditta A., Khan M., Cai X., Xu Y., Maqbool A., Khalofah A., Shaban M., Naeem M., Ansari M.J., Wang K., Liu F., Farooq S. Identification and characterization of genes related to salt stress tolerance within segregation distortion regions of genetic map in F2 Population of upland cotton. PLoS ONE. 2021;16(3):e0247593. doi: 10.1371/journal.pone.0247593. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Sheteiwy M.S., Ali D.F., Xiong Y.C., Brestic M., Skalicky M., Hamoud Y.A., Ulhassan Z., Shaghaleh H., AbdElgawad H., Farooq M., Sharma A. Physiological and biochemical responses of soybean plants inoculated with Arbuscular mycorrhizal fungi and Bradyrhizobium under drought stress. BMC Plant Biol. 2021;21(1):1–21. doi: 10.1186/s12870-021-02949-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltys-Kalina Dorota, Plich Jarosław, Strzelczyk-Żyta Danuta, Śliwka Jadwiga, Marczewski Waldemar. The effect of drought stress on the leaf relative water content and tuber yield of a half-sib family of ‘Katahdin’-derived potato cultivars. Breeding science. 2016;66(2):328–331. doi: 10.1270/jsbbs.66.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starck Z., Niemyska B., Bogdan J., Tawalbeh R.N.A. Response of tomato plants to chilling stress in association with nutrient or phosphorus starvation. Plant Soil. 2000;226:99–106. doi: 10.1023/A:1026497104077. [DOI] [Google Scholar]
- Subramanian K.S., Charest C. Acquisition of N by axternal hyphae of an arbuscular mycor- rhizal fungus and its impact on physiological responses in maize under drought-stressed and well watered conditions. Mycorrhiza. 1999;9:69–75. [Google Scholar]
- Tabatabai M.A., Bremner J.M. Use of p-nitrophenol phosphate for the assay of soil phosphatase activity.Soil Biol. Biochem. 1969;1:301–307. doi: 10.1186/1756-0500-7-221. [DOI] [Google Scholar]
- Usman Adel Rabie A., Al-Wabel Mohammad I., Ok Yong S., Al-Harbi Abdulaziz, Wahb-Allah Mahmoud, El-Naggar Ahmed Hamdy, Ahmad Mahtab, Al-Faraj Abdulelah, Al-Omran Abdulrasoul. Conocarpus biochar induces changes in soil nutrient availability and tomato growth under saline irrigation. Pedosphere. 2016;26(1):27–38. [Google Scholar]
- Vaccari F.P, Maienza A., Miglietta F., Baronti S., Di Lonardo S., Giagnoni L., Lagomarsino A., Pozzi A., Pusceddu E., Ranieri R., Valboa G., Genesio L. Biochar stimulates plant growth but not fruit yield of processing tomato in a fertile soil. Agri Ecosys Environ. 2015;207:163–170. [Google Scholar]
- Vance E.D., Brookes P.C., Jenkinson D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987;19(6):703–707. [Google Scholar]
- Wang W., Chen Q., Hussain S., Mei J., Dong H., Peng S., Huang J., Cui K., Nie L. Pre-sowing seed treatments in direct-seeded early rice: consequences for emergence, seedling growth and associated metabolic events under chilling stress. Sci. Rep. 2016;6(1) doi: 10.1038/srep19637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Li X., Zhou J., Wang G., Dong Y. Effects of arbuscular mycorrhizal fungi on growth and yield of cucumber plants. Commun. Soil Sci. Plant Anal. 2008;39:499–509. [Google Scholar]
- Waraich E.A., Rashid A., Ashraf M.Y. Role of mineral nutrition in alleviation of drought stress in plants. Aust. J. Crop Sci. 2011;5(764–777):20. [Google Scholar]
- Xiao Qian, Zhu Li-xia, Shen Yu-fang, Li Shi-qing. Sensitivity of soil water retention and availability to biochar addition in rainfed semi-arid farmland during a three-year field experiment. Field Crops Res. 2016;196:284–293. [Google Scholar]
- Yaseen S., Amjad S.F., Mansoora N., Kausar S., Shahid H., Alamri S.A.M., Alrumman S.A., Eid E.M., Ansari M.J., Danish S., Datta R. Supplemental Effects of Biochar and Foliar Application of Ascorbic Acid on Physio-Biochemical Attributes of Barley (Hordeum vulgare L.) under Cadmium-Contaminated Soil. Sustainability. 2021;13(16):9126. doi: 10.3390/su13169128. [DOI] [Google Scholar]
- Zhang Y.J., Xie Z.K., Wang Y.J., Su P.X., An L.P., Gao H. Effect of water stress on leaf photosynthesis, chlorophyll content, and growth of oriental lily. Russ. J. Plant Physiol. 2011;58:844–850. [Google Scholar]
- Zhao, R., Guo, W., Bi, N., Guo, J., Wang, L., Zhao, J., et al., 2015. Arbuscular mycorrhizal fungi affect the growth, nutrient uptake and water status of maize (Zea mays L). grown in two types of coal mine spoils under drought stress.
- Zhao R., Guo W., Bi N., Guo J., Wang L., Zhao J., Zhang J. Arbuscular mycorrhizal fungi affect the growth, nutrient uptake and water status of maize (Zea mays L.) grown in two types of coal mine spoils under drought stress. Appl. Soil Ecol. 2015;88:41–49. [Google Scholar]
- Zhao Tong-Jin, Sun Shan, Liu Yang, Liu Jing-Mei, Liu Qiang, Yan Yong-Bin, Zhou Hai-Meng. Regulating the drought- responsive element (DRE)- mediated signaling pathway by synergic functions of transactive and transinactive DRE binding factors in Brassica napus. The Journal of Biological Chemistry. 2006;281(16):10752–10759. doi: 10.1074/jbc.M510535200. [DOI] [PubMed] [Google Scholar]
- Zhou S., Duursma R.A., Medlyn B.E., Kelly J.W., Prentice I.C. How should we model plant responses to drought? An analysis of stomatal and non-stomatal responses to water stress. Agric. For. Meteorol. 2013;182:204–214. [Google Scholar]
- Zhou S., Medlyn B., Sabate S., Sperlich D., Prentice I.C. Short-term water stress impacts on stomatal, mesophyll and biochemical limitations to photosynthesis differ consistently among tree species from contrasting climates. Tree Physiol. 2014;34:1035–1046. doi: 10.1093/treephys/tpu072. [DOI] [PubMed] [Google Scholar]