Abstract
Cyclic nucleotide phosphodiesterase (PDE) is an important regulator of the cellular concentrations of the second messengers cyclic AMP (cAMP) and cGMP. Insulin activates the 3B isoform of PDE in adipocytes in a phosphoinositide 3-kinase-dependent manner; however, downstream effectors that mediate signaling to PDE3B remain unknown. Insulin-induced phosphorylation and activation of endogenous or recombinant PDE3B in 3T3-L1 adipocytes have now been shown to be inhibited by a dominant-negative mutant of the serine-threonine kinase Akt, suggesting that Akt is necessary for insulin-induced phosphorylation and activation of PDE3B. Serine-273 of mouse PDE3B is located within a motif (RXRXXS) that is preferentially phosphorylated by Akt. A mutant PDE3B in which serine-273 was replaced by alanine was not phosphorylated either in response to insulin in intact cells or by purified Akt in vitro. In contrast, PDE3B mutants in which alanine was substituted for either serine-296 or serine-421, each of which lies within a sequence (RRXS) preferentially phosphorylated by cAMP-dependent protein kinase, were phosphorylated by Akt in vitro or in response to insulin in intact cells. Moreover, the serine-273 mutant of PDE3B was not activated by insulin when expressed in adipocytes. These results suggest that PDE3B is a physiological substrate of Akt and that Akt-mediated phosphorylation of PDE3B on serine-273 is important for insulin-induced activation of PDE3B.
Akt is a protein serine-threonine kinase that contains a pleckstrin homology domain and whose kinase domain has structural similarity with those of protein kinase C (PKC) isozymes and cyclic AMP (cAMP)-dependent protein kinase (PKA) (9, 21). Thus, Akt has also been termed protein kinase B. Akt was originally shown to be activated by growth factors such as platelet-derived growth factor and insulin, but later the enzyme was also found to be activated by cytokines and ligands for G protein-coupled receptors (21, 33, 34). Moreover, expression of polyomavirus middle T antigen as well as cellular stresses such as hyperosmolarity, heat shock, and fluid shear stress also induces activation of Akt (17, 27, 42). However, the mechanisms by which Akt is activated by these diverse stimuli are not fully understood. The activation of Akt by growth factors or cytokines is blocked by pharmacological or molecular biological inhibitors of phosphoinositide (PI) 3-kinase (7, 19, 24), indicating that PI 3-kinase is an upstream regulator of Akt, although PI 3-kinase-independent stimuli that induce activation of Akt also appear to exist (27, 33, 38).
Akt is a general mediator of cell survival and protection from apoptosis (9, 21). It has also been suggested to participate in meiosis in oocytes (3), in endocytosis elicited by RAS (5), in differentiation of adipocytes (25), and in various metabolic actions of insulin (23, 25, 44, 45). In spite of the potential importance of Akt in such diverse biological activities, only a few proteins have been identified as physiological substrates of this enzyme. The first identified substrate of Akt was glycogen synthase kinase 3β (GSK3β). Akt phosphorylates GSK3β on Ser9 and thereby inactivates it both in vitro and in intact cells (11, 46), an action that likely results in stimulation of glycogen synthase activity (10). BAD, a member of the BCL2 family of proteins, is another cellular substrate of Akt. Akt phosphorylates BAD on Ser136, resulting in an increase in its affinity for 14-3-3 proteins and consequent suppression of apoptosis and promotion of cell survival (12).
The cyclic nucleotides cAMP and cGMP are important second messengers that mediate a variety of biological activities. Cyclic nucleotide phosphodiesterase (PDE), which catalyzes the hydrolysis of cAMP and cGMP, contributes to regulation of the cellular concentrations of these nucleotides (6, 30). A large family of structurally related PDE enzymes, encoded by at least 17 different genes, has been identified (6, 14, 30). Several hormones, including insulin and leptin, activate the PDE3B isoform (also known as cGMP-inhibited PDE) of this family of enzymes in adipocytes or pancreatic β cells (13, 48), subsequently resulting in prevention of lipolysis or in inhibition of insulin secretion (18, 48). Although the mechanism by which the activity of PDE3B is regulated by these hormones remains unclear, the observation that wortmannin, a relatively specific blocker of PI 3-kinase (40), inhibits activation of PDE3B (35, 48) suggests that PDE3B activity is regulated by this lipid kinase.
Phosphorylation of PDE3B is also implicated in its activation (13, 41). PDE3B is phosphorylated in response to exposure of cells to insulin, and wortmannin prevents this effect (35). The correlation between the extents of phosphorylation and activation of PDE3B supports the hypothesis that the activity of this enzyme is stimulated directly by a serine-threonine kinase that acts downstream of PI 3-kinase. Although mitogen-activated protein kinase and p70 S6 kinase are downstream elements of PI 3-kinase, these protein kinases appear not to contribute to the regulation of PDE3B (47). We therefore designed the present study to determine (i) whether signaling downstream of Akt is responsible for phosphorylation and activation of PDE3B, (ii) whether Akt is necessary for insulin-induced phosphorylation and activation of PDE3B, and (iii) whether phosphorylation of PDE3B is indeed important in regulation of its enzymatic activity. For these investigations, we used a constitutively active and a dominant-negative mutant of Akt as well as various mutants of PDE3B containing substitutions at putative phosphorylation sites.
MATERIALS AND METHODS
Cloning of mouse PDE3B cDNA and construction of PDE3B mutants.
We amplified an ∼490-bp cDNA fragment by PCR with sense and antisense primers corresponding to nucleotides 826 to 849 and 1308 to 1329 of rat PDE3B cDNA (43), respectively, and cDNA synthesized from RNA extracted from mouse 3T3-L1 adipocytes as a template. We then used the resulting PCR product as a probe to screen a mouse fat cell cDNA library (Clontech) and obtained a clone that contained a full-length (3.6-kb) mouse PDE3B cDNA, including a 3,300-bp open reading frame encoding a protein of 1,100 amino acids. The deduced amino acid sequence of the encoded protein revealed that mouse PDE3B has 95.3 and 80.1% overall identity with rat (43) and human (31) PDE3B, respectively. Mouse PDE3B was tagged with the hemagglutinin (HA) epitope (YPYDVPDYA) at its NH2 terminus with the use of PCR. Ser273, Ser274, Ser296, or Ser421 of HA-tagged mouse PDE3B (PDE3B-WT) was replaced by alanine with the use of a Quick Change site-directed mutagenesis kit (Stratagene), and the resultant mutants were termed PDE3B-S273A, PDE3B-S274A, PDE3B-S296A, and PDE3B-S421A, respectively.
Cells and antibodies.
3T3-L1 preadipocytes were maintained and induced to differentiate into adipocytes, as described previously (39). To establish CHO-IR cells that stably express PDE3B-WT, PDE3B-S273A, PDE3B-S274A, PDE3B-S296A, or PDE3B-S421A, we transfected CHO-IR cells with pSV40-hgh, which confers resistance to hygromycin, and an SRα vector encoding HA epitope-tagged wild-type or mutant mouse PDE3B enzymes. Transfected cells were selected and cloned as described previously (23), and the resultant cell lines were termed CHO-IR/PDE3B-WT, CHO-IR/PDE3B-S273A, CHO-IR/PDE3B-S274A, CHO-IR/PDE3B-S296A, and CHO-IR/PDE3B-S421A.
Monoclonal antibodies to the HA epitope tag were obtained from Boehringer Mannheim. Monoclonal antibodies to PKCλ were obtained from Transduction Laboratories. Polyclonal antibodies to Akt were as described previously (23). Polyclonal antibodies to PDE3B were generated against a peptide corresponding to amino acids 424 to 440 (RRSSGASGLLTSEHHSR) of rat PDE3B. These polyclonal antibodies both precipitate mouse PDE3B and recognize the protein on immunoblot analysis.
Construction of adenovirus vectors.
Adenovirus vectors encoding a dominant-negative mutant of the p85 subunit of PI 3-kinase (AxCAΔp85) (39), HA epitope-tagged wild-type Akt (AxCAAkt-WT), a mutant Akt in which Thr308 and Ser473 are replaced by alanine (AxCAAkt-AA), or a mutant Akt in which Lys179 in the kinase domain was replaced by asparatate (AxCAAkt-K179D) (23) or dominant-negative (AxCAλΔNKD) or constitutively active (AxCAλΔPD) mutants of PKCλ (29) were described previously. An adenovirus vector encoding β-galactosidase (AxCALacZ) was kindly provided by I. Saito. The SRC myristoylation signal sequence (GSSKSKPKDPSQR) was added to the NH2 terminus of rat Akt1 or bovine p110α, a catalytic subunit of PI 3-kinase, with the use of PCR; the resultant myristoylated Akt and myristoylated p110 were termed Myr-Akt and Myr-p110, respectively. Complementary DNAs encoding PDE3B-WT, PDE3B-S273A, PDE3B-S274A, PDE3B-S296A, PDE3B-S421A, Myr-Akt, or Myr-p110 were subcloned into pAxCAwt (32), and adenovirus vectors containing these cDNAs were generated with an adenovirus expression kit (Takara, Tokyo, Japan) as described previously (23, 39). The resulting adenoviruses were termed AxCAPDE3B-WT, AxCAPDE3B-S273A, AxCAPDE3B-S274A, AxCAPDE3B-S296A, AxCAPDE3B-S421A, AxCAMyr-Akt, and AxCAMyr-p110, respectively. 3T3-L1 adipocytes were infected with adenovirus vectors at the indicated multiplicity of infection (MOI), as described previously (23, 39). The cells were subjected to experiments at 24 to 48 h after infection.
In vitro phosphorylation of PDE3B by Akt.
A baculovirus that encodes the catalytic subunit of PI 3-kinase (p110α) (22) was kindly provided by M. D. Waterfield. To construct a baculovirus encoding a fusion protein of Akt with glutathione S-transferase (GST), we transfected Sf9 cells with a full-length rat Akt1 cDNA (26) subcloned into a pAcGHLT vector (Pharmingen) with the use of a Bacvector 2000 transfection kit (Novagen). Sf9 cells coinfected with baculoviruses encoding the Akt-GST fusion protein and p110α were lysed, and the lysates were incubated with glutathione-conjugated beads; the beads were then washed, and the activated Akt-GST was eluted from the beads with reduced glutathione.
PDE3B-WT, PDE3B-S273A, PDE3B-S274A, and PDE3B-S296A proteins were immunoprecipitated with antibodies to HA from the corresponding transfected CHO-IR cells, and the immunoprecipitates were incubated for 5 min at 30°C with or without 1 μg of activated Akt in 30 μl of a reaction mixture containing 20 mM Tris-HCl (pH 7.5), 10 mM MgCl2, 25 μM unlabeled ATP, 1 μM protein kinase inhibitor, and 3 μCi of [γ-32P]ATP. The reaction was terminated by washing the immunoprecipitates with ice-cold HEPES-buffered saline (pH 7.5), and they were then subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE); the extent of 32P incorporation into PDE3B proteins was assessed with an image analyzer.
In vivo labeling of PDE3B with 32P.
3T3-L1 adipocytes or CHO-IR cells were deprived of serum for 16 h, washed twice with KRH buffer (25 mM HEPES-NaOH [pH 7.4], 119 mM NaCl, 4.95 mM KCl, 2.54 mM CaCl2, 0.3 mM potassium phosphate, 1.19 mM MgSO4) containing 1% bovine serum albumin (BSA), and then incubated for 2 h at 37°C with KRH buffer containing 3% BSA and [32P]orthophosphate (0.5 mCi/ml). After subsequent incubation in the absence or presence of 100 nM insulin or 1 μM isoproterenol for 15 min, the cells were washed twice with KRH buffer containing 1% BSA and lysed in a solution containing 50 mM Tris-HCl (pH 7.5), 5 mM MgCl2, 1 mM EDTA, 0.2 mM EGTA, 1% Thesit, 3 mM benzamidine, 10 mM sodium pyrophosphate, 50 mM NaF, 1 mM phenylmethylsulfonyl fluoride (PMSF), 1 mM dithiothreitol, leupeptin (10 μg/ml), and pepstatin A (10 μg/ml). The lysate was centrifuged (15,000 × g for 20 min), and the resulting supernatant was subjected to immunoprecipitation with polyclonal antibodies to PDE3B or with monoclonal antibodies to HA. The immunoprecipitates were washed five times with 0.1 M sodium phosphate buffer (pH 7.5) containing 1% N-lauroylsarcosine, boiled in SDS sample buffer, and subjected to SDS-PAGE on a 7% gel; incorporation of radioactivity into PDE3B was visualized with a Fuji BAS2000 image analyzer.
Assay of PDE3B.
The activity of endogenous PDE3B in the membrane fraction of cells was measured with the PDE3B assay described by Rahn et al. (35), with modifications. 3T3-L1 adipocytes cultured in 35-mm plates were deprived of serum for 16 h, incubated first for 30 min in KRH buffer containing 5 mM glucose and bacitracin (0.5 mg/ml) and then for 15 min in the presence or absence of insulin (100 nM) or isoproterenol (1 μM), and then immediately frozen with liquid nitrogen. The frozen cells were homogenized in 500 μl of homogenization buffer {50 mM TES [N-tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid] [pH 7.0], 250 mM sucrose, 1 mM EDTA, 0.1 mM EGTA, 3 mM benzamidine, 10 mM sodium pyrophosphate, 50 mM NaF, 1 mM sodium orthovanadate, 1 mM PMSF, leupeptin [10 μg/ml], pepstatin A [10 μg/ml]} by passing them 15 times through a 22-gauge needle attached to a syringe. The homogenate was centrifuged at 100,000 × g for 45 min, and the resultant pellet (membrane fraction) was resuspended in 500 μl of a solubilization buffer (50 mM Tris-HCl [pH 7.6], 5 mM MgCl2, 1 mM EDTA, 0.1 mM EGTA, 100 mM NaBr, 1% Thesit, 3 mM benzamidine, 10 mM sodium pyrophosphate, 50 mM NaF, 1 mM sodium orthovanadate, 1 mM PMSF, leupeptin [10 μg/ml], pepstatin A [10 μg/ml]) and maintained on ice for 30 min to complete extraction of PDE3B. The extract was centrifuged at 15,000 × g for 30 min, and 100 μl of the resulting supernatant was mixed with 100 μl of a reaction mixture containing 50 mM Tris-HCl (pH 8.0), 5 mM MgCl2, 2 mM EGTA, BSA (0.1 mg/ml), and 0.4 μM [8-3H]cAMP. After incubation for 10 min at 30°C in the absence or presence of 0.5 μM cilostamide, the reaction was terminated by boiling the mixture for 5 min. Twenty micrograms of snake venom (V7000; Sigma) was added, and the mixture was incubated for 10 min at 37°C, after which 1 ml of distilled water was added and the sample was applied to a column of cation-exchange resin (AG 50W-W8 prepacked column; Bio-Rad). After extensive washing of the column with distilled water, [3H]adenosine was eluted with 1.5 ml of 3 M ammonium hydroxide and the amount of radioactivity in the eluate was determined with a liquid scintillation counter.
Assay of cAMP.
3T3-L1 adipocytes cultured in 35-mm plates were deprived of serum for 16 h and incubated first for 20 min with or without 5 μM forskolin. The medium was then aspirated, and the cells were incubated for an additional 10 min in the presence or absence of 100 nM insulin, following which the cells were immediately frozen with liquid nitrogen. The frozen cells were homogenized in 500 μl of ice-cold 6% (wt/vol) trichloroacetic acid, and the homogenate was centrifuged at 2,000 × g for 15 min at 4°C to remove insoluble materials. After extraction of trichloroacetic acid with water-saturated diethylether, the supernatant was dried at 60°C and dissolved in 100 μl of a solution containing 50 mM acetate (pH 5.8) and 0.02% BSA and then assayed for cAMP concentration with the use of a cAMP enzyme immunoassay kit (Amersham).
Nucleotide sequence accession number.
The nucleotide sequence data determined in this study have been submitted to the EMBL database under accession no. AJ132271.
RESULTS
Role of PI 3-kinase in insulin-induced phosphorylation of endogenous and recombinant PDE3B in 3T3-L1 adipocytes.
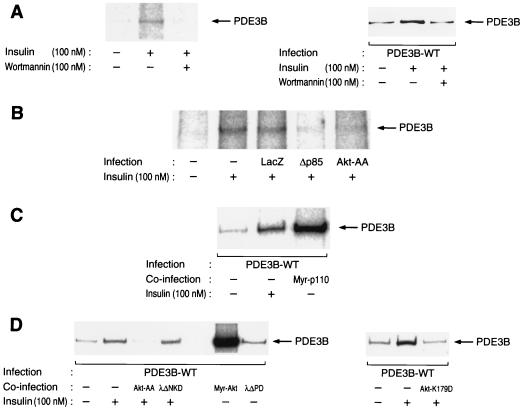
We labeled 3T3-L1 adipocytes with [32P]orthophosphate, incubated them in the absence or presence of insulin, and subjected cell lysates to immunoprecipitation with antibodies to PDE3B. The immunoprecipitates were then subjected to SDS-PAGE and autoradiography. Insulin induced a twofold increase in the extent of 32P incorporation into PDE3B (Fig. 1A). We confirmed the effect of insulin on phosphorylation of PDE3B in 3T3-L1 adipocytes expressing HA-tagged PDE3B. Fully differentiated 3T3-L1 adipocytes were thus infected with an adenovirus encoding HA-tagged wild-type PDE3B (AxCAPDE3B-WT), labeled with [32P]orthophosphate, incubated in the absence or presence of insulin, lysed, and subjected to immunoprecipitation with antibodies to HA. The resulting precipitates were then subjected to SDS-PAGE and autoradiography. Insulin induced an approximately threefold increase in the extent of phosphorylation of recombinant PDE3B in the adipocytes (Fig. 1A). Insulin-induced phosphorylation of both endogenous and recombinant PDE3B was abolished by pretreatment of cells with wortmannin (Fig. 1A), a relatively specific inhibitor of PI 3-kinase, suggesting that this effect of insulin is mediated by PI 3-kinase. We further examined this hypothesis with the use of a dominant-negative mutant of the p85 regulatory subunit of PI 3-kinase. We have previously shown that infection of 3T3-L1 adipocytes with an adenovirus vector encoding this mutant protein (AxCAΔp85) resulted in inhibition of the insulin-induced increase in the amount of PI 3-kinase activity associated with immunoprecipitates prepared with antibodies to phosphotyrosine (39), as well as in inhibition of various biological actions of insulin, including stimulation of glucose transport (39) and activation of Akt (23) or of PKCλ (29). Infection of 3T3-L1 adipocytes with AxCAΔp85 at an MOI of 30 PFU per cell, a virus dose sufficient to inhibit almost completely the insulin-induced increase in Akt activity (23), abolished the effect of insulin on phosphorylation of PDE3B (Fig. 1B). In contrast, infection of the cells with a control virus that encodes β-galactosidase (AxCALacZ) had no effect on insulin-induced phosphorylation of PDE3B. To further investigate the role of PI 3-kinase in the regulation of PDE3B phosphorylation, we examined the effect of a constitutively active mutant of this enzyme. Myr-p110, a chimeric protein consisting of the catalytic subunit of PI 3-kinase ligated to a myristoylation signal sequence at its NH2 terminus, was expressed in 3T3-L1 adipocytes with the use of an adenovirus vector (AxCAMyr-p110). Infection of the cells with AxCAMyr-p110 resulted in a marked increase in the phosphorylation of PDE3B (Fig. 1C). These results with Δp85 and Myr-p110 indicate that PI 3-kinase is necessary and sufficient for insulin-induced phosphorylation of PDE3B in 3T3-L1 adipocytes.
FIG. 1.
Effects of wortmannin and various mutant signaling proteins on insulin-induced phosphorylation of PDE3B in 3T3-L1 adipocytes. (A) Effect of wortmannin on insulin-induced phosphorylation of PDE3B. Cells infected (right panel) or not infected (left panel) with AxCAPDE3B-WT at an MOI of 30 PFU/cell were labeled with [32P]orthophosphate, incubated in the absence or presence of 100 nM wortmannin for 30 min, and then stimulated (or not) with 100 nM insulin for 15 min. The cells were lysed and subjected to immunoprecipitation with polyclonal antibodies to PDE3B for endogenous PDE3B (left panel) or with monoclonal antibodies to HA for recombinant PDE3B (right panel). The immunoprecipitates were then subjected to SDS-PAGE, and 32P-labeled PDE3B was visualized by autoradiography. (B) Effects of Δp85 and Akt-AA on insulin-induced phosphorylation of endogenous PDE3B. Cells that had been infected (or not) with AxCALacZ, AxCAΔp85, or AxCAAkt-AA at MOIs of 200, 20, and 200 PFU/cell, respectively, were labeled with [32P]orthophosphate, and the effect of insulin on phosphorylation of endogenous PDE3B was assayed as for panel A. (C and D) Effects of Myr-p110, mutant Akt, and mutant PKCλ proteins on insulin-induced phosphorylation of recombinant PDE3B. Cells were infected with AxCAPDE3B-WT at an MOI of 30 PFU/cell, and, after 12 h, they were infected again (or not) with AxCAMyr-p110, AxCAAkt-AA, AxCAAkt-K179D, AxCAMyr-Akt, AxCAλΔNKD, or AxCAλΔPD at MOIs of 20, 200, 200, 20, 150, and 150 PFU/cell, respectively. The cells were labeled with [32P]orthophosphate, incubated in the absence or presence of 100 nM insulin for 15 min, lysed, and subjected to immunoprecipitation with monoclonal antibodies to HA. The immunoprecipitates were then subjected to SDS-PAGE and autoradiography. Data are representative of two (C) or three (D) independent experiments.
Role of Akt in insulin-induced phosphorylation of PDE3B.
Because Akt is a downstream effector of PI 3-kinase, we next examined whether Akt contributes to insulin-induced phosphorylation of PDE3B. A mutant Akt containing a myristoylation signal sequence showed a kinase activity that was at least 15 times that of wild-type Akt precipitated from quiescent cells (data not shown). Coinfection of 3T3-L1 adipocytes with AxCAPDE3B-WT and an adenovirus vector encoding myristoylated Akt (AxCAMyr-Akt) resulted in marked phosphorylation of HA-tagged wild-type PDE3B in quiescent cells (Fig. 1D), suggesting that signaling through Akt is sufficient for phosphorylation of PDE3B.
To investigate further the role of Akt in phosphorylation of PDE3B, we examined the effects of a dominant-negative mutant (Akt-AA) of Akt (23, 29). Infection of adipocytes with AxCAAkt-AA at an MOI of 200 PFU/cell, a virus dose sufficient to prevent insulin activation of Akt (23), almost completely prevented insulin-induced phosphorylation of both endogenous (Fig. 1B) and recombinant (Fig. 1D) PDE3B. Moreover, infection of the cells with an adenovirus encoding Akt-K179D (AxCAAkt-K179D), a kinase-defective mutant of Akt in which Lys179 in the kinase domain is replaced by alanine, also inhibited insulin-induced phosphorylation of PDE3B (Fig. 1D), whereas expression of Akt-WT at a level similar to that of Akt-AA or Akt-K179D did not inhibit insulin-induced phosphorylation of PDE3B (data not shown). These results suggest that Akt is required for insulin-induced phosphorylation of PDE3B.
Role of PKCλ in insulin-induced phosphorylation of PDE3B.
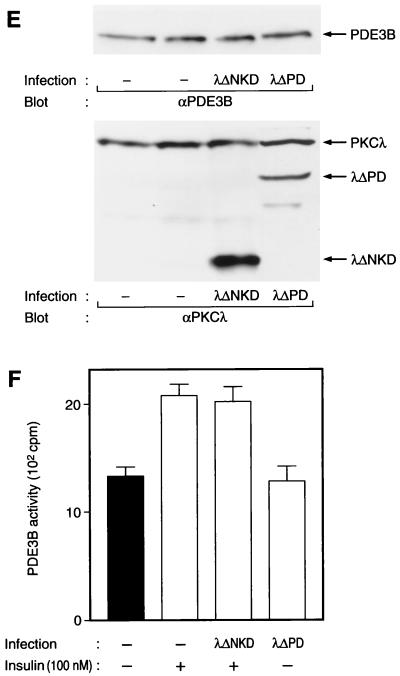
Atypical PKC, which comprises PKCλ and PKCζ isoforms, also acts as a downstream effector of PI 3-kinase (1, 29). We have recently shown that PKCλ, but not PKCζ, is expressed in 3T3-L1 adipocytes and that PKCλ is activated by insulin in these cells in a PI 3-kinase-dependent manner (29). We thus investigated whether PKCλ participates in insulin-induced phosphorylation of PDE3B. Infection of 3T3-L1 adipocytes with AxCAλΔPD, an adenovirus that encodes a constitutively active mutant of PKCλ (29), induced an ∼1.5-fold increase in the extent of phosphorylation of PDE3B (Fig. 1D). However, infection of the cells with AxCAλΔNKD, an adenovirus that encodes a dominant-negative mutant of PKCλ, did not inhibit insulin-induced phosphorylation of PDE3B even at an MOI of 150 PFU/cell (Fig. 1D), a virus dose sufficient to prevent insulin activation of PKCλ and to inhibit markedly insulin stimulation of glucose uptake in 3T3-L1 adipocytes (29). These results suggest that PKCλ is not required for insulin-induced phosphorylation of PDE3B, although PKCλ appears to be able to transmit signals that result in phosphorylation of PDE3B under certain conditions.
Phosphorylation of PDE3B on Ser273 by Akt both in vivo and in vitro.
Akt preferentially phosphorylates substrates that conform to the sequence RXRXXS (2). Because Ser273 of mouse PDE3B is present within such a motif, we examined whether this serine residue is phosphorylated by Akt. We constructed a mutant PDE3B in which Ser273 was replaced by alanine (PDE3B-S273A) and, as controls, mutants in which Ser296 or Ser421 was replaced by alanine (PDE3B-S296A and PDE3B-S421A, respectively). The latter two serine residues are each located within a general consensus motif (RRXS) preferentially phosphorylated by PKA.
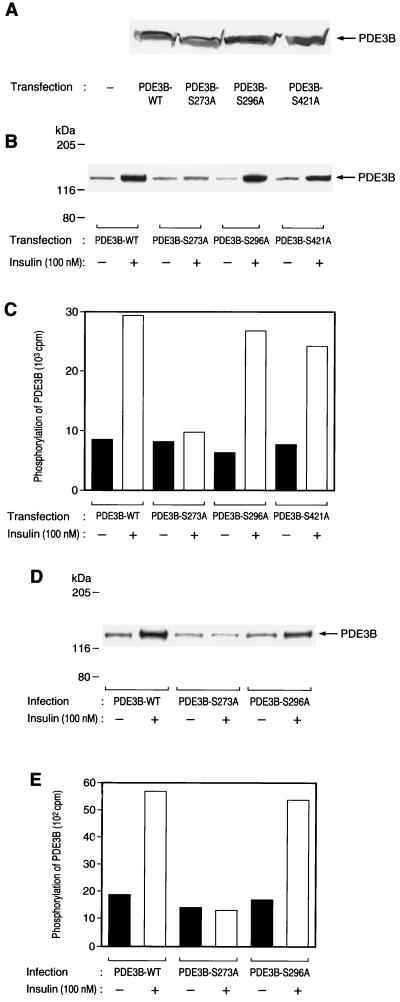
CHO-IR cells that stably express HA-tagged wild-type or mutant PDE3B proteins were labeled with [32P]orthophosphate, incubated in the absence or presence of insulin, lysed, and subjected to immunoprecipitation with antibodies to HA. The resulting immunoprecipitates were then examined for PDE3B phosphorylation by SDS-PAGE and autoradiography. The extents of expression of wild-type and mutant PDE3B proteins were virtually identical, as assessed by immunoblot analysis (Fig. 2A). Insulin induced a three- to fourfold increase in the extent of phosphorylation of PDE3B-WT, PDE3B-S296A, and PDE3B-S421A. In contrast, the extent of insulin-induced phosphorylation of PDE3B-S273A was minimal (Fig. 2B and C). We performed similar experiments with 3T3-L1 adipocytes. The adipocytes were infected with adenovirus vectors encoding wild-type or mutant PDE3B proteins, and the effects of insulin on the phosphorylation of these proteins were assayed by in vivo labeling with [32P]orthophosphate. Insulin induced an approximately threefold increase in the extent of phosphorylation of PDE3B-WT or PDE3B-S296A in 3T3-L1 adipocytes, but it had no effect on 32P incorporation into PDE3B-S273A in these cells (Fig. 2D and E). These results suggest that Ser273 of PDE3B is a phosphorylation site targeted by insulin in intact cells.
FIG. 2.
Effects of insulin on phosphorylation of various mutant PDE3B proteins in CHO-IR cells and 3T3-L1 adipocytes. (A) Expression of mutant PDE3B proteins in CHO-IR cells. CHO-IR cells stably expressing wild-type or various mutant PDE3B proteins were lysed and subjected to immunoblot analysis with antibodies to HA. (B and C) Effects of insulin on phosphorylation of mutant PDE3B proteins in CHO-IR cells. CHO-IR/PDE3B-WT, CHO-IR/PDE3B-S273A, CHO-IR/PDE3B-S296A, and CHO-IR/PDE3B-S421A cells were labeled with [32P]orthophosphate, incubated in the absence or presence of 100 nM insulin for 15 min, and lysed. The lysates were subjected to immunoprecipitation with antibodies to HA, the resulting precipitates were subjected to SDS-PAGE, and 32P incorporation into PDE3B was visualized (B) or quantitated (C) with an image analyzer. (D and E) Effects of insulin on phosphorylation of mutant PDE3B proteins in 3T3-L1 adipocytes. Adipocytes that had been infected with AxCAPDE3B-WT, AxCAPDE3B-S273A, or AxCAPDE3B-S296A at an MOI of 20 PFU/cell were labeled with [32P]orthophosphate, incubated in the absence or presence of 100 nM insulin for 15 min, and lysed. The lysates were subjected to immunoprecipitation with antibodies to HA, the precipitates were subjected to SDS-PAGE, and 32P incorporation into PDE3B was visualized (D) or quantitated (E) with an image analyzer. All data are representative of three independent experiments.
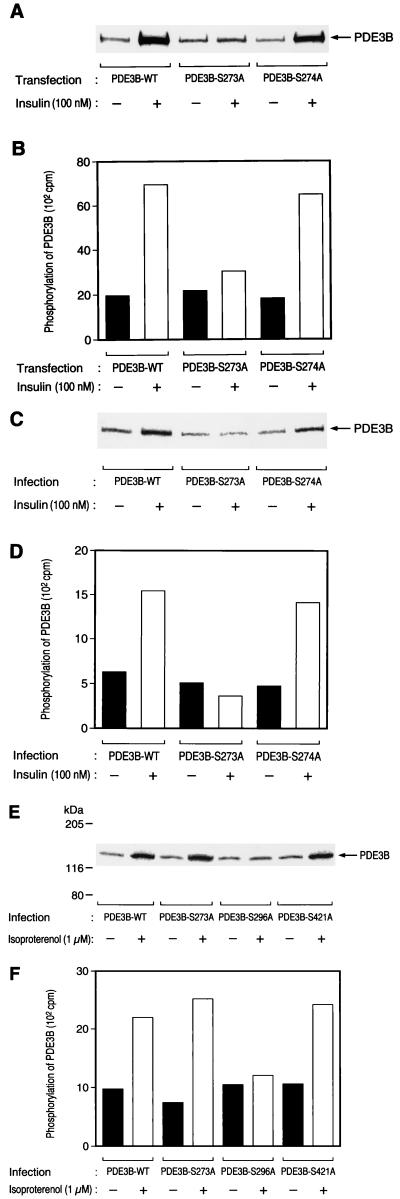
Ser273 of mouse PDE3B is present within the sequence RPRRRS273S274. It was thus possible that Ser274 is phosphorylated in response to exposure of cells to insulin and that substitution at Ser273 disrupts the native conformation of PDE3B and prevents phosphorylation of Ser274. To exclude this possibility, we constructed a mutant PDE3B in which Ser274 was replaced by alanine (PDE3B-S274A). Insulin increased 32P incorporation into PDE3B-S274A to an extent similar to that observed with PDE3B-WT both in CHO-IR cells (Fig. 3A and B) and in 3T3-L1 adipocytes (Fig. 3C and D), suggesting that Ser274 of PDE3B is not phosphorylated in response to insulin in intact cells.
FIG. 3.
Effects of insulin and isoproterenol on the phosphorylation of various mutant PDE3B proteins. CHO-IR/PDE3B-WT, CHO-IR/PDE3B-S273A, and CHO-IR/PDE3B-S274A cells (A and B) and 3T3-L1 adipocytes that had been infected with AxCAPDE3B-WT, AxCAPDE3B-S273A, or AxCAPDE3B-S274A at an MOI of 20 PFU/cell (C and D) or with AxCAPDE3B-WT, AxCAPDE3B-S273A, AxCAPDE3B-S296A, or AxCAPDE3B-S421A at an MOI of 20 PFU/cell (E and F) were labeled with [32P]orthophosphate, incubated (or not) with 100 nM insulin (A to D) or with 1 μM isoproterenol (E and F) for 15 min, and lysed. The lysates were subjected to immunoprecipitation with antibodies to HA, the immunoprecipitates were subjected to SDS-PAGE, and 32P incorporation into PDE3B was visualized (A, C, and E) or quantitated (B, D, and F) with an image analyzer. Data are representative of three independent experiments.
β-Adrenergic agonists were also known to stimulate phosphorylation of PDE3B in adipocytes (13). Isoproterenol induced an approximately two- to threefold increase in the extent of phosphorylation of PDE3B-WT, PDE3B-S273A, and PDE3B-421 in 3T3-L1 adipocytes (Fig. 3E and F). In contrast, isoproterenol-induced 32P incorporation into PDE3B-S296A was minimal (Fig. 3E and F), suggesting that Ser296 of PDE3B is a phosphorylation site targeted by isoproterenol in intact cells.
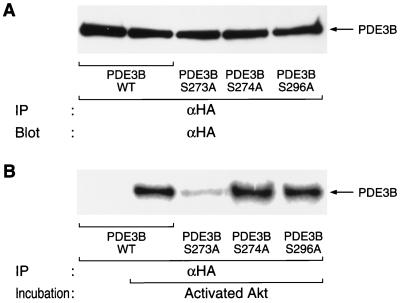
We next investigated whether Akt directly phosphorylates PDE3B. Wild-type or various mutants of PDE3B were immunoprecipitated from quiescent CHO-IR cells stably expressing the corresponding protein and were then incubated in the absence or presence of activated recombinant Akt isolated from Sf9 cells that had been coinfected with baculoviruses encoding a catalytic subunit of PI 3-kinase and wild-type Akt. Incorporation of 32P from [γ-32P]ATP into wild-type PDE3B was observed on incubation in the presence of Akt but not in the absence of Akt (Fig. 4), indicating that PDE3B is phosphorylated by Akt. Substitution of Ser273 by alanine markedly reduced the extent of Akt-mediated incorporation of 32P into PDE3B. In contrast, PDE3B-S274A, PDE3B-S296A (Fig. 4), and PDE3B-S421A (data not shown) were phosphorylated in vitro by Akt to extents similar to that observed with the wild-type protein. Thus, Akt appears to catalyze the phosphorylation of PDE3B at Ser273 both in vitro and in intact cells.
FIG. 4.
Direct phosphorylation of PDE3B at Ser273 by Akt in vitro. PDE3B-WT, PDE3B-S273A, PDE3B-S274A, and PDE3B-S296A were immunoprecipitated (IP) with antibodies to HA from CHO-IR cells stably expressing the corresponding protein and were then either subjected to immunoblot analysis with antibodies to HA (A) or incubated for 5 min at 30°C with or without 1 μg of activated Akt prepared as described in Materials and Methods (B). The immunoprecipitates from panel B were then subjected to SDS-PAGE, and 32P incorporation into PDE3B proteins was visualized by autoradiography. Data are representative of three independent experiments.
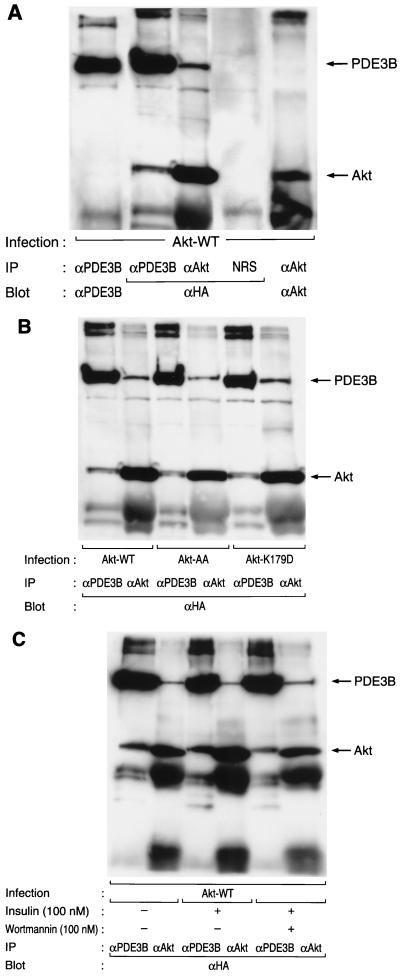
Association of Akt with PDE3B in intact cells.
Both BAD and GSK3β, putative in vivo substrates of Akt, have been shown to associate with Akt in intact cells (12, 46). To investigate whether PDE3B associates with Akt, we lysed CHO-IR cells expressing PDE3B-WT (HA-tagged wild-type PDE3B) that had been infected with AxCAAkt-WT, which encodes HA-tagged wild-type Akt, and subjected the lysate to immunoprecipitation with polyclonal antibodies to Akt, polyclonal antibodies to PDE3B, or control serum. The immunoprecipitates were then subjected to immunoblot analysis with antibodies to HA. A 140-kDa protein that reacted with antibodies to HA was immunoprecipitated with antibodies to Akt but not with control serum (Fig. 5A). Conversely, an ∼60-kDa protein that reacted with antibodies to HA was apparent in the immunoprecipitate prepared with antibodies to PDE3B but not in that prepared with control serum (Fig. 5A). These results indicate that Akt associates with PDE3B in intact cells. Furthermore, PDE3B appeared to associate with Akt-AA and with Akt-K179D (Fig. 5B). Treatment of cells with insulin or wortmannin did not affect the association of Akt with PDE3B (Fig. 5C).
FIG. 5.
Association of Akt with PDE3B in intact cells. (A and B) CHO-IR/PDE3B-WT cells that had been infected with AxCAAkt-WT, AxCAAkt-AA, or AxCAAkt-K179D at an MOI of 20 PFU/cell were lysed and subjected to immunoprecipitation (IP) with polyclonal antibodies to Akt (αAkt), polyclonal antibodies to PDE3B (αPDE3B), or control serum (NRS). The immunoprecipitates were then subjected to immunoblot analysis with antibodies to HA (αHA), to Akt, or to PDE3B, as indicated. (C) CHO-IR/PDE3B-WT cells that had been infected with AxCAAkt-WT were deprived of serum for 16 h, incubated in the absence or presence of 100 nM wortmannin for 30 min, and then stimulated (or not) with 100 nM insulin for 15 min; then, association of Akt with PDE3B was assayed as in panels A and B. Data are representative of three independent experiments.
Effects of various mutant proteins on insulin-induced activation of PDE3B.
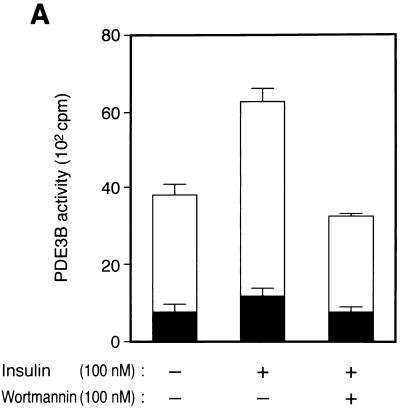
We next investigated whether insulin-induced activation of PDE3B is mediated by Akt. Membrane fractions were prepared from 3T3-L1 adipocytes that had been incubated in the absence or presence of insulin, and solubilized extracts of these fractions were assayed for PDE activity. PDE activity in the extracts prepared from both insulin-treated and nontreated cells was inhibited by ∼80% by 0.5 μM cilostamide (Fig. 6A), a specific inhibitor of PDE3A and PDE3B (30). Because PDE3A is not expressed in 3T3-L1 adipocytes (43), these data suggest that ∼80% of PDE activity in the membrane fraction of these cells is attributable to PDE3B. Insulin induced an ∼1.5-fold increase in cilostamide-sensitive PDE activity, and this effect was completely inhibited by 100 nM wortmannin (Fig. 6A), suggesting that insulin-induced activation of PDE3B is mediated by PI 3-kinase.
FIG. 6.
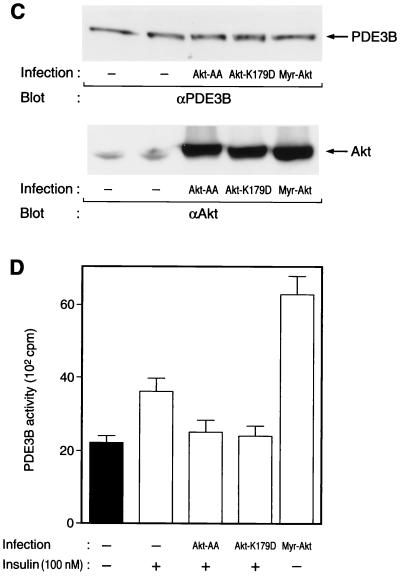
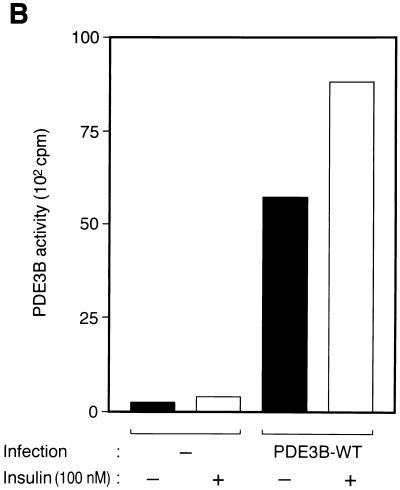
Effects of wortmannin and various mutant proteins on insulin-induced activation of PDE3B in 3T3-L1 adipocytes. (A) Effect of wortmannin on insulin activation of PDE3B. Cells were incubated in the absence or presence of 100 nM wortmannin for 30 min and then with or without 100 nM insulin for 15 min. PDE activity in solubilized membrane fractions was assayed in the absence or presence of cilostamide as described in Materials and Methods. Open columns show cilostamide-sensitive PDE activity, and closed columns show cilostamide-insensitive PDE activity. (B) Comparison of the activities of endogenous and recombinant PDE3B. Cells that had been infected (or not) with AxCAPDE3B-WT at an MOI of 30 PFU/cell were incubated in the absence or presence of insulin, after which solubilized membrane fractions were prepared as described in Materials and Methods and diluted 1:50 with solubilization buffer. The diluted samples were then assayed for PDE3B activity. (C to F) Effects of various mutant proteins on insulin activation of endogenous PDE3B. Cells that had been infected with AxCAAkt-AA (200 PFU/cell), AxCAAkt-K179D (200 PFU/cell), or AxCAMyr-Akt (30 PFU/cell) (C and D), or with AxCAλΔNKD (150 PFU/cell) or AxCAλΔPD (150 PFU/cell) (E and F), were incubated in the absence or presence of 100 nM insulin for 15 min, after which total cell lysates (C and E) or solubilized membrane fractions (D and F) were prepared and then subjected to immunoblot analysis (C and E) or assayed for cilostamide-sensitive PDE activity (D and F). Quantitative data are means ± standard errors from three independent experiments.
To evaluate directly the effect of insulin on PDE3B activity, we infected 3T3-L1 adipocytes with AxCAPDE3B-WT and then assayed PDE activity in the solubilized membrane fraction prepared from these cells. The amount of PDE activity in the membrane fraction prepared from the cells infected with AxCAPDE3B-WT was at least 25 times that in the corresponding fraction from uninfected cells (Fig. 6B), indicating that most of the PDE activity in the membrane fraction of the infected cells was attributable to recombinant PDE3B. The activity of the recombinant PDE3B showed an ∼1.5-fold increase in response to insulin (Fig. 6B).
We next examined the effects of various mutant proteins on insulin-induced activation of endogenous PDE3B. Even in the absence of insulin, PDE3B activity in cells expressing Myr-Akt was about twice that in control cells treated with insulin (Fig. 6D), indicating that Myr-Akt was able to activate PDE3B. Moreover, infection of cells with AxCAAkt-AA at an MOI of 200 PFU/cell, a virus dose sufficient to prevent insulin-induced phosphorylation of PDE3B (Fig. 1B and C), almost completely blocked insulin activation of PDE3B (Fig. 6D). In addition, infection of cells with AxCAAkt-K179D, a virus that also inhibited insulin-induced phosphorylation of PDE3B (Fig. 1C), also prevented activation of PDE3B by insulin (Fig. 6D). Expression of mutant Akt proteins did not affect the amount of PDE3B proteins in the cells (Fig. 6C). These results suggest that insulin-induced activation of PDE3B is mediated by Akt. In contrast, expression of λΔPD, a constitutively active mutant of PKCλ, did not increase PDE3B activity (Fig. 6F) whereas this mutant stimulated phosphorylation of PDE3B (Fig. 1C). Furthermore, a dominant-negative mutant of PKCλ, λΔNKD, did not interfere with the effect of insulin on PDE3B activity (Fig. 6F). Expression of neither λΔPD nor λΔNKD affected the amount of PDE3B protein (Fig. 6E). These results suggest that PKCλ does not contribute to insulin-induced activation of PDE3B.
Lack of effect of insulin on the activity of PDE3B-S273A.
We examined whether Akt-mediated phosphorylation of PDE3B is indeed important for activation of PDE3B. 3T3-L1 adipocytes that had been infected with AxCAPDE3B-WT, AxCAPDE3B-S273A, AxCAPDE3B-S274A, or AxCAPDE3B-S296A were incubated in the absence or presence of insulin, after which PDE activity was assayed in solubilized membrane fractions. Although insulin induced an ∼1.5-fold increase in the activity of PDE3B-WT, it had no effect on PDE activity in the membrane fraction prepared from the adipocytes expressing PDE3B-S273A (Table 1). In contrast, insulin increased PDE activity in the membrane fractions prepared from the cells expressing either PDE3B-S274A or PDE3B-S296A to an extent similar to that observed with PDE3B-WT (Table 1). Furthermore, isoproterenol also induced an ∼1.5-fold increase in the activity of PDE3B-WT, PDE3B-273A, and PDE3B-421A, whereas the isoproterenol-induced increase in the activity of PDE3B-S296A was minimal. These results suggest that phosphorylation of PDE3B at Ser273 and Ser296 is important for activation of PDE3B induced by insulin and isoproterenol, respectively (Table 1).
TABLE 1.
Effects of insulin and isoproterenol on the activities of various PDE3B mutants in 3T3-L1 adipocytesa
| Infection | PDE3B activityb
|
|
|---|---|---|
| Insulin (100 nM) | Isoproterenol (1 μM) | |
| PDE3B-WT | 154 ± 14 | 146 ± 7 |
| PDE3B-S273A | 104 ± 7 | 141 ± 6 |
| PDE3B-S274A | 144 ± 11 | ND |
| PDE3B-S296A | 150 ± 11 | 102 ± 3 |
| PDE3B-S421A | ND | 162 ± 18 |
Cells that had been infected with AxCAPDE3B-WT, AxCAPDE3B-S273A, AxCAPDE3B-S274A, AxCAPDE3B-S296A, or AxCAPDE3B-S421A at an MOI of 30 PFU/cell were incubated in the absence or presence of insulin or isoproterenol, after which PDE activity was assayed in solubilized membrane fractions as described in the legend to Fig. 6B.
Data are expressed as percentages of the corresponding basal activity (in the absence of stimuli) and are means ± standard errors from three independent experiments. ND, not done.
Effect of a dominant-negative mutant of Akt on the insulin-induced decrease in cAMP.
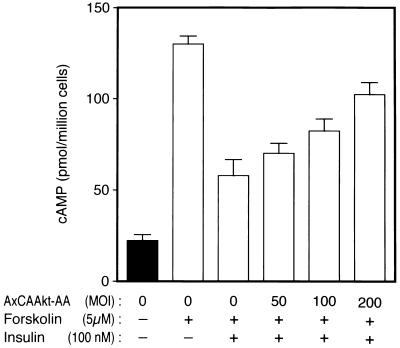
Finally, we investigated the effect of a dominant-negative mutant of Akt (Akt-AA) on insulin-induced lowering of the level of cAMP. Insulin was known to lower the cellular cAMP level that had been elevated by β-adrenergic agonists (8, 28), of which receptors are coupled to adenylate cyclase. Treatment of 3T3-L1 adipocytes with forskolin, a direct activator of adenylate cyclase, caused an approximately sixfold increase in the level of cAMP, and incubation of the cells with insulin resulted in an ∼60% decrease in cAMP within 10 min (Fig. 7). Infection of the cells with AxCAAkt-AA inhibited the effect of insulin in a dose-dependent manner (Fig. 7), suggesting that Akt is required for the insulin-induced decrease in cAMP.
FIG. 7.
Effect of Akt-AA on the insulin-induced decrease in cAMP in 3T3-L1 adipocytes. Cells that had been infected (or not) with AxCAAkt-AA at the indicated MOI were incubated for 20 min with or without forskolin and for an additional 10 min in the presence or absence of 100 nM insulin, after which cell extracts were prepared and assayed for cAMP as described in Materials and Methods. Data are means ± standard errors from three independent experiments.
DISCUSSION
With the use of immunoprecipitation of endogenous or epitope-tagged recombinant proteins, we have shown that insulin induces phosphorylation of PDE3B in 3T3-L1 adipocytes. Insulin-induced phosphorylation of PDE3B was prevented by expression of a dominant-negative mutant of the regulatory subunit of PI 3-kinase (Δp85), and expression of a constitutively active mutant of PI 3-kinase (Myr-p110) stimulated phosphorylation of PDE3B in the absence of insulin. Furthermore, a mutant Akt in which the sites of ligand-induced phosphorylation were replaced by alanine (Akt-AA), which prevents insulin activation of Akt (23), abolished insulin-induced phosphorylation of PDE3B, and expression of a constitutively active mutant of Akt (Myr-Akt) resulted in phosphorylation of PDE3B in quiescent 3T3-L1 adipocytes. These data suggest that the PI 3-kinase–Akt pathway is necessary and sufficient for insulin-induced phosphorylation of PDE3B.
Akt-K179D or similar kinase-deficient mutants of Akt that contain a substitution at Lys179 in the kinase domain have little effect on growth factor-induced activation of Akt (23, 46). However, such mutants block certain biological effects that are likely mediated by Akt, including growth factor-induced phosphorylation of PHAS1 (4E-BP1) (20), growth factor-induced phosphorylation of BAD and consequent protection of cells from apoptosis (12), and insulin-induced activation of glycogen synthase (44). We have now shown that Akt-K179D inhibited insulin-induced phosphorylation of PDE3B. One possible explanation for these observations is that Akt-K179D and similar kinase-deficient mutants of Akt compete with endogenous Akt for cellular substrates of the enzyme and thereby exert dominant-negative effects on various biological activities.
We have shown that Akt associates with PDE3B when these proteins are overexpressed in CHO cells. It is noteworthy that not only wild-type but kinase-deficient mutants of Akt form a complex with PDE3B, indicating that the interaction is independent of the kinase activity of Akt. This result is consistent with the finding that both wild-type and a kinase-inactive mutant of Akt make a complex with BAD (12). Moreover, treatment of the cells with insulin or wortmannin did not affect the interaction. We do not know why Akt and PDE3B form a constitutive complex in cells whereas PDE3B primarily resides in the membrane fraction and Akt translocates from cytosol to plasma membrane in response to insulin (4). One possibility is that overexpression of these proteins may result in “overflow” from their authentic intracellular compartments and that this overflow may cause constitutive complex formation. It remains to be investigated under what conditions endogenous PDE3B and Akt interact in intact cells.
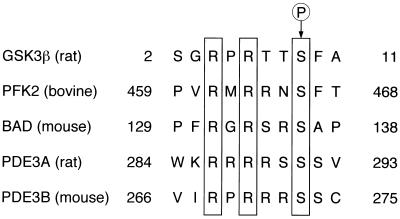
Several proteins have been shown to be phosphorylated by Akt in vitro or in intact cells. These proteins include GSK3β, BAD, and phosphofructose-2-kinase (Fig. 8) (11, 12, 15, 16). On the basis of studies with peptides derived from these proteins, it has been suggested that Akt preferentially phosphorylates substrates that conform to the sequence RXRXXS. Substitution of alanine for Ser273 of mouse PDE3B, a serine residue that resides in such a consensus motif, almost completely prevented the effect of insulin on the phosphorylation of this protein in intact cells, supporting the hypothesis that PDE3B is a physiological substrate of Akt. We also showed that PDE3B underwent phosphorylation on incubation with Akt in vitro and that, again, replacement of Ser273, but not of other serine residues, with alanine markedly reduced the extent of Akt-mediated phosphorylation of PDE3B. These results suggest that Akt directly phosphorylates PDE3B on Ser273.
FIG. 8.
Alignment of amino acid sequences conforming to a motif preferentially phosphorylated by Akt in GSK3β, phosphofructose-2-kinase (PFK2), BAD, PDE3A, and PDE3B. Conserved arginine residues at positions −3 and −5 relative to the phosphorylated serine (arrow) are boxed. Residue numbers are indicated at each end of the sequences.
Rahn et al. (36) showed that insulin increased incorporation of 32P into a specific phosphopeptide generated by tryptic digestion of PDE3B that had been immunoprecipitated from 32P-labeled rat adipocytes. They also showed that the mobility of this peptide on two-dimensional gel electrophoresis was similar to that of a tryptic peptide generated from recombinant PDE3B that had been phosphorylated by PKA in vitro. Because the latter peptide contained only one serine residue (Ser302, which corresponds to Ser296 of the mouse enzyme), these investigators concluded that the site of insulin-induced phosphorylation in PDE3B is identical to that targeted by PKA in vitro. However, we have now shown that substitution of Ser296 of mouse PDE3B does not affect insulin-induced phosphorylation of the enzyme. In contrast, phosphorylation of PDE3B induced by isoproterenol, a reagent that increases cellular cAMP concentration and subsequently activates PKA, was markedly attenuated by substitution of Ser296. These results suggest that Ser296 of mouse PDE3B is phosphorylated in response to isoproterenol but not insulin.
Previous studies have suggested that phosphorylation of PDE3B correlates with its catalytic activity (13, 41). Indeed, we have now shown that insulin activation of PDE3B was inhibited by wortmannin or by Akt-AA, both of which also prevented insulin-induced phosphorylation of PDE3B. Furthermore, the importance of phosphorylation of PDE3B for its enzymatic activity was directly evaluated with the use of various mutants of PDE3B containing substitutions for various serine residues. When expressed in 3T3-L1 adipocytes, PDE3B-S273A was not activated in response to insulin, whereas mutant PDE3B proteins containing substitutions at Ser274 or Ser296 were activated normally. These results indicate that phosphorylation of PDE3B on Ser273 is required for insulin-induced activation of the enzyme. The activity of PDE3B-S273A in quiescent cells was similar to that of the wild-type enzyme (data not shown), indicating that phosphorylation of Ser273 of PDE3B is not important for basal catalytic activity.
Because PKCλ, an atypical isoform of PKC, acts as a downstream effector of PI 3-kinase (1, 29), we examined the possible role of this enzyme in the phosphorylation and activation of PDE3B. We have recently shown that expression of λΔPD promotes glucose transport in quiescent 3T3-L1 adipocytes and that λΔNKD inhibits insulin stimulation of glucose transport (29). Although AxCAλΔPD induced phosphorylation of PDE3B, infection with this virus did not increase the activity of PDE3B in 3T3-L1 adipocytes. Moreover, infection of the cells with AxCAλΔNKD at an MOI of 150 PFU/cell, a virus dose sufficient to inhibit almost completely insulin-induced activation of PKCλ in 3T3-L1 adipocytes (29), affected neither phosphorylation nor activation of PDE3B induced by insulin. It is thus likely that λΔPD mediates the phosphorylation of PDE3B at residues that are not important in the regulation of its activity and that PKCλ does not contribute to the physiological signaling cascade that leads to activation of PDE3B.
Because PDE3B catalyzes the hydrolysis of cAMP, activation of this enzyme likely leads to lowering of the cellular level of cAMP. Indeed, it has been reported that insulin decreases the level of cAMP that had been elevated by cathecolamine (8, 28). We have now shown that insulin decreased the level of cAMP in the cells that had been treated with forskolin and that Akt-AA markedly inhibited the insulin-induced decrease in cAMP, suggesting that Akt is involved in the effect of insulin on cellular cAMP concentration.
In summary, we have identified PDE3B as a physiological substrate of Akt and demonstrated that Akt-mediated phosphorylation of Ser273 is important for insulin activation of PDE3B. Of all known PDEs, only PDE3B and PDE3A possess a serine residue located within an RXRXXS motif (Fig. 8). It is thus also possible that Akt contributes to the regulation of PDE3A, which is preferentially expressed in heart and vascular smooth muscle cells and implicated in cardiac contractility and vasodilatation (6, 37).
ACKNOWLEDGMENTS
We thank I. Saito and M. D. Waterfield for pAxCALacZ and a baculovirus that encodes p110α, respectively.
This work was supported by a grant-in-aid for the Research for the Future Program from the Japan Society for the Promotion of Science (to M.K.); by Health Sciences Research Grants (Research on Human Genome and Gene Therapy) from the Ministry of Health and Welfare (to M.K.); by grants from the Ministry of Education, Science, Sports, and Culture of Japan (to M.K. and W.O.); by a grant from the Uehara Memorial Foundation (to M.K.); by a grant for studies on the pathophysiology and complications of diabetes from Tsumura Pharma Ltd. (to M.K.); by a grant from Takeda Science Foundation; and by a grant from ONO Medical Research Foundation (to W.O.). T.K. is a Japan Health Sciences Foundation (JHSF) Fellow.
REFERENCES
- 1.Akimoto K, Takahashi R, Moriya S, Nishioka N, Takayanagi J, Kimura K, Fukui Y, Osada S, Mizuno K, Hirai S-I, Kazlauskas A, Ohno S. EGF or PDGF receptors activate atypical PKCλ through phosphatidylinositol 3-kinase. EMBO J. 1996;15:788–798. [PMC free article] [PubMed] [Google Scholar]
- 2.Alessi D R, Caudwell F B, Andjelkovic M, Hemmings B A, Cohen P. Molecular basis for the substrate specificity of protein kinase B; comparison with MAPKAP kinase-1 and p70 S6 kinase. FEBS Lett. 1996;399:333–338. doi: 10.1016/s0014-5793(96)01370-1. [DOI] [PubMed] [Google Scholar]
- 3.Andersen C B, Roth R A, Conti M. Protein kinase B/Akt induces resumption of meiosis in Xenopus oocytes. J Biol Chem. 1998;273:18705–18708. doi: 10.1074/jbc.273.30.18705. [DOI] [PubMed] [Google Scholar]
- 4.Andjelkovic M, Alessi D R, Meier R, Fernandez A, Lamb N J, Frech M, Cron P, Cohen P, Lucocq J M, Hemmings B A. Role of translocation in the activation and function of protein kinase B. J Biol Chem. 1997;272:31515–31524. doi: 10.1074/jbc.272.50.31515. [DOI] [PubMed] [Google Scholar]
- 5.Barbieri M A, Kohn A D, Roth R A, Stahl P D. Protein kinase B/Akt and Rab5 mediate Ras activation of endocytosis. J Biol Chem. 1998;273:19367–19370. doi: 10.1074/jbc.273.31.19367. [DOI] [PubMed] [Google Scholar]
- 6.Beavo J A, Conti M, Heaslip R J. Multiple cyclic nucleotide phosphodiesterases. Mol Pharmacol. 1994;46:399–405. [PubMed] [Google Scholar]
- 7.Burgering B M T, Coffer P J. Protein-kinase-B (c-AKT) in phosphatidylinositol-3-OH kinase signal-transduction. Nature. 1995;376:599–602. doi: 10.1038/376599a0. [DOI] [PubMed] [Google Scholar]
- 8.Butcher R W, Sneyd J G T, Park C R, Sutherland E W. Effects of insulin on adenosine 3′,5′-monophosphate in the rat epididymal fat pad. J Biol Chem. 1966;241:1651–1653. [PubMed] [Google Scholar]
- 9.Coffer P J, Jin J, Woodgett J R. Protein kinase B (c-Akt): a multifunctional mediator of phosphatidylinositol 3-kinase activation. Biochem J. 1998;335:1–13. doi: 10.1042/bj3350001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen P. The role of protein phosphorylation in neural and hormonal control of cellular activity. Nature. 1982;296:613–620. doi: 10.1038/296613a0. [DOI] [PubMed] [Google Scholar]
- 11.Cross D A E, Alessi D R, Cohen P, Andjelkovich M, Hemmings B A. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 12.Datta S R, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg M E. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 13.Degerman E, Smith C J, Tornqvist H, Vasta V, Belfrage P, Manganiello V C. Evidence that insulin and isoprenaline activate the cGMP-inhibited low-Km cAMP phosphodiesterase in rat fat cells by phosphorylation. Proc Natl Acad Sci USA. 1990;87:533–537. doi: 10.1073/pnas.87.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Degerman E, Belhrage P, Manganiello V C. Structure, localization, and regulation of cGMP-inhibited phosphodiesterase (PDE3B) J Biol Chem. 1997;272:6823–6826. doi: 10.1074/jbc.272.11.6823. [DOI] [PubMed] [Google Scholar]
- 15.del Peso L, Gonzalez-Garcia M, Page C, Herrera R, Nunez G. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science. 1997;278:687–689. doi: 10.1126/science.278.5338.687. [DOI] [PubMed] [Google Scholar]
- 16.Deprez J, Vertommen D, Alessi D R, Hue L, Rider M K. Phosphorylation and activation of heart 6-phosphofructo-2-kinase by protein kinase B and other protein kinases of the insulin signaling cascades. J Biol Chem. 1997;272:17269–17275. doi: 10.1074/jbc.272.28.17269. [DOI] [PubMed] [Google Scholar]
- 17.Dimmeler S, Assmus B, Hermann C, Haendeler J, Zeiher A M. Fluid shear stress stimulates phosphorylation of Akt in human endothelial cells—involvement in suppression of apoptosis. Circ Res. 1998;83:334–341. doi: 10.1161/01.res.83.3.334. [DOI] [PubMed] [Google Scholar]
- 18.Eriksson H, Ridderstrale M, Degerman E, Ekholm D, Smith C J, Manganiello V C, Belfrage P, Tornqvist H. Evidence for the key role of the adipocyte cGMP-inhibited cAMP phosphodiesterase in the antilipolytic action of insulin. Biochim Biophys Acta. 1995;1266:101–107. doi: 10.1016/0167-4889(94)00237-9. [DOI] [PubMed] [Google Scholar]
- 19.Franke T F, Yang S I, Chan T O, Datta K, Kazluskas A, Morrison D K, Kaplan D R, Tsichlis P N. The protein kinase encoded by the AKT protooncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell. 1995;81:727–736. doi: 10.1016/0092-8674(95)90534-0. [DOI] [PubMed] [Google Scholar]
- 20.Gingras A C, Kennedy S G, O’Leary M A, Sonenberg N, Hay N. 4E-BP1, a repressor of mRNA translation, is phosphorylated and inactivated by the Akt (PKB) signaling pathway. Genes Dev. 1998;12:502–513. doi: 10.1101/gad.12.4.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hemmings B A. Akt signaling: linking membrane events to life and death decisions. Science. 1997;275:628–630. doi: 10.1126/science.275.5300.628. [DOI] [PubMed] [Google Scholar]
- 22.Hiles I D, Otsu M, Volinia S, Fry M J, Gout I, Dhand R, Panayotou G, Ruiz-Larrea F, Thompson A, Totty N F, Hsuan J J, Courtneidge S A, Parker P, Waterfield M D. Phosphatidylinositol 3-kinase: structure and expression of the 110 kD catalytic subunit. Cell. 1992;70:419–429. doi: 10.1016/0092-8674(92)90166-a. [DOI] [PubMed] [Google Scholar]
- 23.Kitamura T, Ogawa W, Sakaue H, Hino Y, Kuroda S, Takata M, Matsumoto M, Maeda T, Konishi H, Kikkawa U, Kasuga M. Requirement for activation of the serine-threonine kinase Akt (protein kinase B) in insulin stimulation of protein synthesis but not of glucose transport. Mol Cell Biol. 1998;18:3708–3717. doi: 10.1128/mcb.18.7.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kohn A D, Kovacina K S, Roth R A. Insulin stimulates the kinase-activity of RAC-PK, a pleckstrin homology domain-containing Ser/Thr kinase. EMBO J. 1995;14:4288–4295. doi: 10.1002/j.1460-2075.1995.tb00103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kohn A D, Summers S A, Birnbaum M J, Roth R A. Expression of constitutively active Akt Ser/Thr kinase in 3T3-L1 adipocytes stimulates glucose uptake and glucose transporter 4 translocation. J Biol Chem. 1996;271:31372–31378. doi: 10.1074/jbc.271.49.31372. [DOI] [PubMed] [Google Scholar]
- 26.Konishi H, Shinomura T, Kuroda S-I, Ono Y, Kikkawa U. Molecular cloning of rat RAC protein kinase α and β and their association with protein kinase Cζ. Biochem Biophys Res Commun. 1994;205:817–825. doi: 10.1006/bbrc.1994.2738. [DOI] [PubMed] [Google Scholar]
- 27.Konishi H, Matsuzaki H, Tanaka M, Ono Y, Tokunaga C, Kuroda S, Kikkawa U. Activation of RAC-protein kinase by heat shock and hyperosmolarity stress through a pathway independent of phosphatidylinositol 3-kinase. Proc Natl Acad Sci USA. 1996;93:7639–7643. doi: 10.1073/pnas.93.15.7639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kono T, Barham F W. Effects of insulin on the levels of adenosine 3′:5′-monophosphate and lipolysis in isolated rat epididymal fat cells. J Biol Chem. 1973;248:7417–7426. [PubMed] [Google Scholar]
- 29.Kotani K, Ogawa W, Matsumoto M, Kitamura T, Sakaue H, Hino Y, Miyake K, Sano W, Akimoto K, Ohno S, Kasuga M. Requirement of atypical PKCλ for insulin stimulation of glucose uptake but not for Akt activation in 3T3-L1 adipocytes. Mol Cell Biol. 1998;18:6971–6982. doi: 10.1128/mcb.18.12.6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manganiello V C, Murata T, Taira M, Belfrage P, Degerman E. Diversity in cyclic nucleotide phosphodiesterase isoenzyme families. Arch Biochem Biophys. 1995;322:1–13. doi: 10.1006/abbi.1995.1429. [DOI] [PubMed] [Google Scholar]
- 31.Miki T, Taira M, Hockman S, Shimada F, Lieman J, Napolitano M, Ward D, Taira M, Makino H, Manganiello V C. Characterization of the cDNA and gene encoding human PDE3B, the cGIP1 isoform of the human cyclic GMP-inhibited cyclic nucleotide phosphodiesterase family. Genomics. 1996;36:476–485. doi: 10.1006/geno.1996.0493. [DOI] [PubMed] [Google Scholar]
- 32.Miyake S, Makimura M, Kanegae Y, Harada S, Sato Y, Takamori K, Tokuda C, Saito I. Efficient generation of recombinant adenoviruses using adenovirus DNA terminal protein complex and a cosmid bearing the full length virus genome. Proc Natl Acad Sci USA. 1996;93:1320–1324. doi: 10.1073/pnas.93.3.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moule S K, Welsh G I, Edgell N J, Foulstone E J, Proud C G, Denton R M. Regulation of protein kinase B and glycogen synthase kinase-3 by insulin and beta-adrenergic agonists in rat epididymal fat cells. Activation of protein kinase B by wortmannin-sensitive and -insensitive mechanisms. J Biol Chem. 1997;272:7713–7719. doi: 10.1074/jbc.272.12.7713. [DOI] [PubMed] [Google Scholar]
- 34.Murga C, Laguinge L, Wetzker R, Cuadrado A, Gutkind J S. Activation of Akt/protein kinase B by G protein-coupled receptors. J Biol Chem. 1998;273:19080–19085. doi: 10.1074/jbc.273.30.19080. [DOI] [PubMed] [Google Scholar]
- 35.Rahn T, Ridderstrale M, Tornqvist H, Manganiello V C, Fredrikson G, Belfrage P, Degerman E. Essential role of phosphatidylinositol 3-kinase in insulin-induced activation and phosphorylation of the cGMP-inhibited cAMP phosphodiesterase in rat adipocytes. Studies using the selective inhibitor wortmannin. FEBS Lett. 1994;350:314–318. doi: 10.1016/0014-5793(94)00797-7. [DOI] [PubMed] [Google Scholar]
- 36.Rahn T, Ronnstrand L, Leroy M J, Wernstedt C, Tornqvist H, Manganiello V C, Belfrage P, Degerman E. Identification of the site in the cGMP-inhibited phosphodiesterase phosphorylated in adipocytes in response to insulin and isoproterenol. J Biol Chem. 1996;271:11575–11580. doi: 10.1074/jbc.271.19.11575. [DOI] [PubMed] [Google Scholar]
- 37.Remme W J. Inodilator therapy for heart failure. Early, late, or not at all? Circulation. 1993;87(Suppl. 5):IV97–IV107. [PubMed] [Google Scholar]
- 38.Sable C L, Filippa N, Hemmings B, Van Obberghen E. cAMP stimulates protein kinase B in a wortmannin-insensitive manner. FEBS Lett. 1997;409:253–257. doi: 10.1016/s0014-5793(97)00518-8. [DOI] [PubMed] [Google Scholar]
- 39.Sakaue H, Ogawa W, Takata M, Kuroda S, Kotani K, Matsumoto M, Sakaue M, Nishio S, Ueno H, Kasuga M. Phosphoinositide 3-kinase is required for insulin-induced but not for growth hormone- or hyperosmolarity-induced glucose uptake in 3T3-L1 adipocytes. Mol Endocrinol. 1997;11:1552–1562. doi: 10.1210/mend.11.10.9986. [DOI] [PubMed] [Google Scholar]
- 40.Shepherd P R, Withers D J, Siddle K. Phosphoinositide 3-kinase: the key switch mechanism in insulin signalling. Biochem J. 1998;333:471–490. doi: 10.1042/bj3330471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shibata H, Robinson F R, Soderling T R, Kono T. Effects of okadaic acid on insulin-sensitive cAMP phosphodiesterase in rat adipocytes. Evidence that insulin may stimulate enzyme by phosphorylation. J Biol Chem. 1991;266:17948–17953. [PubMed] [Google Scholar]
- 42.Summers S A, Lipfert L, Birnbaum M J. Polyoma middle T antigen activates the Ser/Thr kinase Akt in a PI 3-kinase-dependent manner. Biochem Biophys Res Commun. 1998;246:76–81. doi: 10.1006/bbrc.1998.8575. [DOI] [PubMed] [Google Scholar]
- 43.Taira M, Hockman S C, Calvo J C, Taira M, Belfrage P, Manganiello V C. Molecular cloning of the rat adipocyte hormone-sensitive cyclic GMP-inhibited cyclic nucleotide phosphodiesterase. J Biol Chem. 1993;268:18573–18579. [PubMed] [Google Scholar]
- 44.Takata M, Ogawa W, Kitamura T, Hino Y, Kuroda S, Kotani K, Klip A, Gingras A-C, Sonenberg N, Kasuga M. Requirement for Akt (protein kinase B) in insulin-induced activation of glycogen synthase and phosphorylation of 4E-BP1 (PHAS-1) J Biol Chem. 1999;274:20611–20618. doi: 10.1074/jbc.274.29.20611. [DOI] [PubMed] [Google Scholar]
- 45.Ueki K, Yamamoto-Hond R, Kaburagi Y, Yamauchi T, Tobe K, Burgering B M T, Coffer P J, Komuro I, Akanuma Y, Yazaki Y, Kadowaki T. Potential role of protein kinase B in insulin-induced glucose transport, glycogen synthesis, and protein synthesis. J Biol Chem. 1998;273:5315–5322. doi: 10.1074/jbc.273.9.5315. [DOI] [PubMed] [Google Scholar]
- 46.van Weeren P C, de Bruyn K M, de Vries-Smits A M, van Lint J, Burgering B M T. Essential role for protein kinase B (PKB) in insulin-induced glycogen synthase kinase 3 inactivation. J Biol Chem. 1998;273:13150–13156. doi: 10.1074/jbc.273.21.13150. [DOI] [PubMed] [Google Scholar]
- 47.Wijkander J, Landstrom T R, Manganiello V, Belfrage P, Degerman E. Insulin-induced phosphorylation and activation of phosphodiesterase 3B in rat adipocytes: possible role for protein kinase B but not mitogen-activated protein kinase or p70 S6 kinase. Endocrinology. 1998;139:219–227. doi: 10.1210/endo.139.1.5693. [DOI] [PubMed] [Google Scholar]
- 48.Zhao A Z, Bornfeldt K E, Beavo J A. Leptin inhibits insulin secretion by activation of phosphodiesterase 3B. J Clin Investig. 1998;102:869–873. doi: 10.1172/JCI3920. [DOI] [PMC free article] [PubMed] [Google Scholar]