Abstract
Some anthropogenic substances in drinking water are known or suspected endocrine disrupting compounds (EDCs), but EDCs are not routinely measured. We conducted a pilot study of 10 public drinking water utilities in Iowa, where common contaminants (e.g., pesticides) are suspected EDCs. Raw (untreated) and finished (treated) drinking water samples were collected in spring and fall and concentrated using solid phase extraction. We assessed multiple endocrine disrupting activities using novel mammalian cell-based assays that express nuclear steroid receptors (aryl hydrocarbon [AhR], androgenic [AR], thyroid [TR], estrogenic [ER] and glucocorticoid [GR]). We quantified each receptor’s activation relative to negative controls and compared activity by season and utility/sample characteristics. Among 62 samples, 69% had AhR, 52% AR, 3% TR, 2% ER, and 0% GR activity. AhR and AR activities were detected more frequently in spring (p = 0 .002 and < 0.001, respectively). AR activity was more common in samples of raw water (p = 0 .02) and from surface water utilities (p = 0 .05), especially in fall (p = 0 .03). Multivariable analyses suggested spring season, surface water, and nitrate and disinfection byproduct concentrations as determinants of bioactivity. Our results demonstrate that AR and AhR activities are commonly found in Iowa drinking water, and that their detection varies by season and utility/sample characteristics. Screening EDCs with cell-based bioassays holds promise for characterizing population exposure to diverse EDCs mixtures.
Keywords: Endocrine disrupting compounds, Public water supplies, Drinking water, Biological activity, Hormones
Graphical Abstract
1. Introduction
The presence of endocrine disrupting compounds (EDCs) in drinking water sources is a topic of ecological and human health concern (Guillette Jr. et al., 1995; UNEP/WHO, 2012). Most EDCs in the environment arise from anthropogenic sources, including personal care products, pharmaceuticals, pesticides, and industrial chemicals (e.g., plasticizers, flame retardants) (Frye et al., 2012; National Institute of Environmental Health Sciences (NIEHS), 2018). Other sources include livestock waste runoff (Yost et al., 2013), effluent from human wastewater (Belfroid et al., 1999; Sun et al., 2013), pulp and paper mills (Jenkins et al., 2001; Jenkins et al., 2003), and crop production in intensive agricultural areas (crops are sources of naturally-occurring phytoestrogens) (Kolpin et al., 2010). Disruption of the endocrine system by environmental EDCs is an established phenomenon in wildlife and plants (Adeel et al., 2017; Murray et al., 2017; UNEP/WHO, 2012). Evidence of adverse effects on human reproductive and thyroid systems is also growing (Annamalai and Namasivayam, 2015; UNEP/WHO, 2012), and EDCs are hypothesized risk factors for some hormone-related malignancies (Adeel et al., 2017; Street et al., 2018). Human exposure to these compounds in the environment may occur from consumption of fish and/or drinking water contaminated with EDCs (Benotti et al., 2009).
Only a small number of known EDCs (e.g., pesticides) are regulated in public drinking water in the U.S., and conventional water treatments vary in their ability to remove these compounds (Deblonde et al., 2011; Snyder et al., 2003). Typically, an a priori selection of specific EDCs of interest are measured in water resources, such as rivers, lakes and streams (Aris et al., 2014; Kabir et al., 2015), wastewater (Jackson and Sutton, 2008), and drinking water (Benotti et al., 2009; Padhye et al., 2014). Most studies employ chemical approaches such as chromatography and spectrometry to measure these contaminants (Campbell et al., 2006). However, biological and chemical reactivity in the environment can induce structural changes to chemicals that may influence their bioactivity, and water is a complex mixture that may include numerous compounds with unpredictable endocrine disrupting potential. Measuring a selection of predetermined EDCs may miss identification of other EDCs that are present and important to bioactivity (Campbell et al., 2006). This may be especially problematic in agricultural areas where EDC mixtures in drinking water can arise from several sources including pesticides, phytoestrogens, and hormones and pharmaceuticals from animal waste (Frye et al., 2012).
In vitro and in vivo bioassays to assess EDCs typically measure different types of bioactivity in standardized organisms, such as yeast (Westlund and Yargeau, 2017). Advantages of bioassay methods over single chemical analyte approaches include greater sensitivity and improved efficiency to measure the combined bioactivity of a mixture (Cespedes et al., 2004; Daniels et al., 2018; Leusch et al., 2017), a measure potentially useful for assessing associated impacts on human health. However, yeast-based assays are limited in the expression of mammalian proteins and are less sensitive than mammalian assays (Campana et al., 2015; Leusch et al., 2017), therefore mammalian cell lines may be preferable for this purpose (Daniels et al., 2018; Snyder et al., 2001). Studies evaluating endocrine activity in drinking waters in the U.S. are limited and have typically not quantified bioactivity beyond estrogenic activity (Conley et al., 2017). Bioactivity in drinking waters outside the U.S. are largely described for urban and industrial areas (Escher et al., 2014; Leusch et al., 2018; Neale and Escher, 2019; Rosenmai et al., 2018; Shi et al., 2018). In this pilot study, we applied several recently developed mammalian-cell based bioassays (Stavreva et al., 2012; Stavreva et al., 2016) to quantify EDC activity in untreated and treated water samples from 10 public water utilities across Iowa, a region impacted by intensive agricultural activity and prone to environmental contamination by EDCs. Our objective was to determine bioactivity across five EDCs classes (androgenic, estrogenic, aryl hydrocarbon, thyroid, and glucocorticoid) and to evaluate determinants of activity.
2. Materials and methods
2.1. Study setting and selection of public water supplies (PWS)
Contamination by agricultural chemicals and their byproducts and naturally-occurring compounds (phytoestrogens) from crop production is common in Iowa’s water resources, especially in surface water and shallow ground water (Hladik et al., 2014; Kolpin et al., 2010; Kross et al., 1993; Weyer et al., 2006; Zirkle et al., 2016). Roughly half (55%) of the state’s population on public water supplies (PWS) get their drinking water from PWS sourced from groundwater and the remainder from surface (36%) or influenced groundwater (9%) (Iowa Department of Natural Resources (IDNR), 2017).
We selected 10 PWS that captured a range of utility sizes and maximized variation in characteristics that plausibly influence EDCs occurrence. These characteristics comprised treatment methods (e.g., activated carbon [AC] use or not), water source (ground or surface), average levels of regulated contaminants (e.g., nitrate-nitrogen [NO3-N]; total trihalomethanes [TTHM], a marker for total chlorinated DBPs; and atrazine, a triazine herbicide), and intensity of local agricultural inputs (e.g., number of animal feeding operations [AFOs] within the utility service area’s county) (Ciparis et al., 2012; Esplugas et al., 2007; Guillette Jr. and Edwards, 2005; Hatfield and Follett, 2008; Iowa Department of Natural Resources (IDNR), 2016; Liu et al., 2009; Pereira et al., 2011; Wu et al., 2009). We selected utilities served by surface water, alluvial groundwater, and deeper groundwater sources, with varying usage of AC and levels of regulated contaminants. This information was based on 2014 data from a University of Iowa Center for Health Effects of Environmental Contamination (CHEEC) database (Center for Health Effects of Environmental Contamination (CHEEC), 2016). Participation for water sample collection was solicited from utility managers by email and a follow-up telephone contact; all agreed to provide samples and no additional recruitment was required.
2.2. Sample collection and processing
We collected paired raw (i.e., untreated) and finished (i.e., treated) water samples on the same date from each PWS and conducted the water sample collection in two seasons of high agricultural activity (spring [May], fall [November]) in 2016. Each utility provided at least one raw and one finished water sample per season, and 5 facilities provided multiples of each due to having multiple inlets for raw or finished water. In total, 32 raw and 30 finished water samples were available for analysis. Samples were collected according to a standard U.S. Geological Survey protocol (Ciparis et al., 2012; Stavreva et al., 2012) in 1 L chemically cleaned amber glass bottles with Teflon-lined caps and shipped on ice to the Iowa State Hygienic Laboratory (SHL). Upon receipt, samples were acidified to pH ~3 with 6 N hydrochloric acid and stored at 4 °C. Samples were filtered through a 0.6–0.8 μm filter using a solvent-rinsed glass apparatus within one week of collection. Filtered samples and blanks were subjected to solid phase extraction using OASIS HLB disks (Waters Corporation, Milford, MA) and an automated SPE-DEX (Horizon Technology, Salem, NH) system (Stavreva et al., 2012). Analytes were eluted with 100% methanol and then reduced to dryness under ultra-high purity N2. All extracts were held at −20 °C until all samples were processed, then shipped overnight to the National Cancer Institute’s Center for Cancer Research Laboratory of Receptor Biology and Gene Expression.
2.3. Laboratory methods and bioactivity characterization
We employed a series of recently developed assays using mammalian cell lines that express green fluorescent protein (GFP)-tagged nuclear steroid receptor constructs to elucidate EDC activity (Fig. 1). Assay methods and cell line development for thyroid [TR], androgenic [AR], and glucocorticoid [GR] receptors have been described previously (Stavreva et al., 2012; Stavreva et al., 2016). Development of GFP assays for aryl hydrocarbon (AhR) and estrogenic (ER) receptors are briefly described herein. Generation of the GFP-GR-ERα chimeric receptor followed the approach used by Martinez et al. (2005), except that we used human GR instead of rat GR (Martinez et al., 2005). GFP-GR-ERα was introduced into HT1080 cells to create a cell line stably expressing the chimera in a Tet-Off manner and capable of translocation to the nucleus in response to estrogen (E2) stimulation. Nuclei were stained with DRAQ5™ or 4′,6-diamidino-2-phenylindole (DAPI) and we examined the translocation efficiency in response to E2 (Supplemental Fig. 1). We employed a previously described GFP-AhR-expressing cell line using murine hepatoma cells Tao BpRc1, deficient in endogenous AhR and stably expressing a green-fluorescent-protein (GFP)-labeled AhR (Elbi et al., 2002) under tetracycline control (Mai et al., 2011). Supplemental Fig. 2 demonstrates the GFP-AhR translocation in response to the AhR ligand (CAY10465) and the concentration- and time-dependent translocation curves for the GFP-AhR bioassay.
Fig. 1.
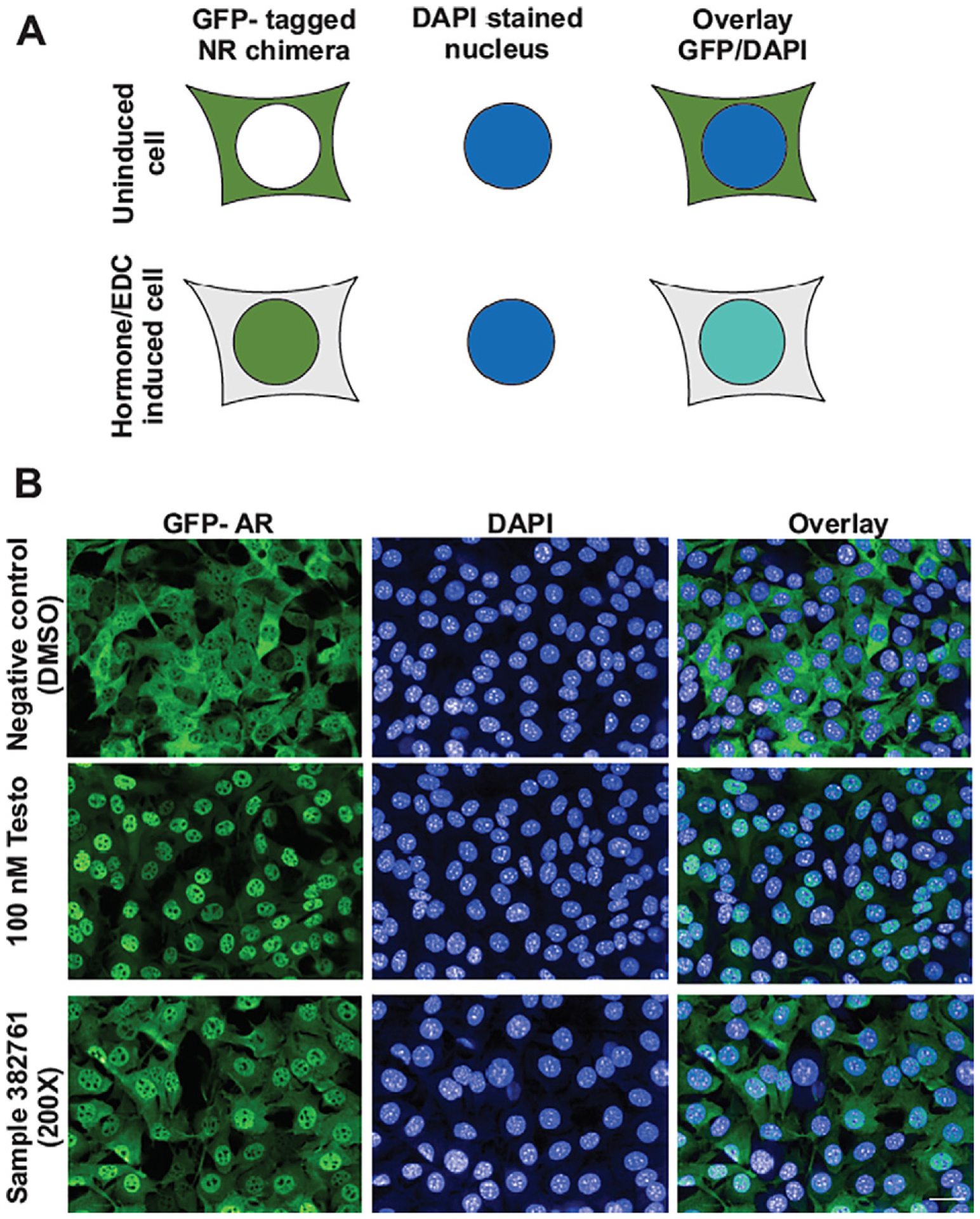
Translocation assay. A) Schematic of the screening approach. The GFP-tagged nuclear receptor (NR) chimera (colored in green) is localized in the cytoplasm before induction but in the presence of ligand or EDC interacting with the NR translocates to the nucleus (colored in blue) in a concentration-dependent manner. B) Representative micrographs of the GFP-AR (green), DAPI stained nucleus (blue), and combined channels of cells treated with vehicle (DMSO), 100 nM testosterone and 200× concentrated water sample. Scale bar is 20 μm.
Concentrated samples were added to cells for 30 min when screening for glucocorticoids and androgens, and for 3 h when screening for aryl hydrocarbon, estrogenic and thyroid activity. Blanks, solvent controls, and blind duplicates were included in each batch. We completed screening for four replicates of each sample at 100× and 200× concentrations, respectively. Sample data were analyzed using SigmaPlot v. 11 (SPSS Inc., Chicago, IL) as previously described (Stavreva et al., 2012; Stavreva et al., 2016). Briefly, the mean was computed across four replicates of each sample, and one-way ANOVAs compared activity to negative controls following Holm-Sidak correction for multiple comparisons.
2.3.1. Cell treatment
Prior to imaging, cells were grown for 24 h in DMEM (Dulbecco’s Modified Eagle’s Medium, Gibco) containing 10% charcoal stripped serum (Hyclone, Logan, UT) without tetracycline (to allow the expression of the GFP-tagged constructs) in 96 well plates (Matrical, Catalog Number MGB096–1-2-LG-L) at a density 30,000 cells per well, or in 384 well plates (Matrical, Catalog Number MGB101–1-2-LG-L) at a density of 7000 cells per well (again in DMEM medium containing 10% charcoal stripped serum without tetracycline). Water sample extracts were applied at final concentrations of 100× and 200× and incubated at 37 °C for 30 min when screening for glucocorticoids and androgens or for 3 h when screening for aryl hydrocarbon, estrogenic and thyroid activity. Negative controls containing the vehicle (dimethyl sulfoxide [DMSO]) as well as blank samples were included on the same plate. Upon treatment, cells were fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) for 10 min and washed 3× with PBS. Cells were further stained with DAPI or DRAQ5™ for 10 min and after 3 final washes with PBS were imaged either immediately on the Perkin Elmer Opera Image Screening System or kept in PBS at 4 °C for later imaging.
2.3.2. Automated imaging and analysis by Perkin Elmer Opera Image Screening System
A PerkinElmer (Waltham, MA) Opera High-Content Screening platform was used for fully automated confocal collection of images. This system employed a 40× water immersion objective lens, laser illuminated Nipkow disk, and cooled charge-coupled device cameras to digitally capture high-resolution confocal fluorescence micrographs (300 nm pixel size with 2 × 2 camera pixel binning). An image analysis pipeline was customized using the Columbus software (Perkin Elmer) to automatically segment the nucleus using the DAPI or the DRAQ5™ channel and then construct a ring region (cytoplasm) around the nucleus mask for each cell in the digital micrographs. The pipeline automatically calculated the mean GFP intensity in both compartments using the GFP channel and translocation was calculated as a ratio of these intensities. Each value was further normalized to the value for the control (DMSO) sample on the same plate.
2.4. Statistical analysis
We evaluated the prevalence of detection of global EDC activities overall and by sampling season using SAS (v 9.4). Using the CHEEC monitoring data for 2014, we determined annual mean concentrations of NO3-N and TTHM for each of the 10 utilities and the overall median of these values across all utilities (NO3-N: 3.19 mg/L, TTHM: 40.6 μg/L). We then compared bioactivity detection prevalence by season, raw versus finished water, source (ground, surface water), NO3-N and TTHM levels (< or ≥ the overall median), and whether AC was used as a tertiary treatment using univariate multilevel models to determine significant differences (α = 0.05) while accounting for correlations between samples from the same utility. We evaluated predictors of EDC activity with multivariable multilevel logistic regression models including all potential predictors as covariates, estimating prevalence odds ratios (POR) and 95% confidence intervals (CI).
3. Results
The characteristics of the 10 utilities reflected our selection criteria that maximized variability across population served, source water, and other characteristics (Table 1). Annual average concentrations of NO3-N and TTHM in 2014 were below their Environmental Protection Agency maximum contaminant levels (10 mg/L and 80 μg/L, respectively) for all utilities. Atrazine levels were unavailable for most utilities, which was expected as monitoring of this herbicide is required only in certain Iowa PWS based on history of local atrazine use and prior exceedances of regulatory limits. Only two utilities (Council Bluffs and Iowa City) used AC for tertiary treatment. We excluded AFO counts from further analyses due to limited variability in this metric across the sampled utilities.
Table 1.
Characteristics of 10 Iowa public water utilities and type of samples collected in a pilot study of endocrine disrupting chemical activity in Iowa.
| Utility | Population servedb | Source | N AFOs in Countyc | Atrazine (μg/L)d | NO3-N (mg/L)d | TTHM (μg/L)d | ACe | N Raw samplesf | N Finished samplesf |
|---|---|---|---|---|---|---|---|---|---|
| Des Moinesa | 233,020 | Surface | 24 | 0.10 | 3.19 | 67.2 | No | 3 | 3 |
| Iowa City | 63,265 | Shallow alluvial aquifer | 50 | – | 2.98 | 41.1 | Yes | 2 | 1 |
| Cedar Rapids | 128,201 | Shallow alluvial aquifer | 36 | 0.17 | 3.69 | 0.4 | No | 2 | 2 |
| Dubuque | 57,637 | Ground | 51 | – | <DL | 40.0 | No | 1 | 1 |
| Ottumwa | 25,023 | Surface | 29 | 0.93 | 4.65 | 58.4 | No | 1 | 1 |
| Waterloo | 70,070 | Ground | 68 | – | 5.59 | 5.3 | No | 1 | 1 |
| Sioux City | 82,759 | Ground | 104 | – | <DL | 53.1 | No | 2 | 2 |
| Mason City | 28,079 | Ground | 38 | – | <DL | 3.4 | No | 1 | 1 |
| Council Bluffs | 63,795 | Ground | 95 | – | <DL | 31.5 | Yes | 2 | 2 |
| Keokuka | 10,780 | Surface | 24 | – | 2.45 | 62.2 | No | 1 | 1 |
Abbreviations: NO3-N: nitrate-nitrogen; TTHM: total trihalomethanes; AC: activated carbon: AFOs: animal feeding operations.
Uses AC only for taste/odor, not tertiary treatment.
Source: Environmental Working Group’s Tap Water Database (2018).
Source: Iowa Department of Natural Resources database of permitted animal feeding operations (2016).
Annual mean concentrations for finished water, 2014. Source: University of Iowa Center for Health Effects of Environmental Contamination (2014). – indicates not measured.
AC used as tertiary treatment.
Number of samples collected in sampling efforts (both fall and spring).
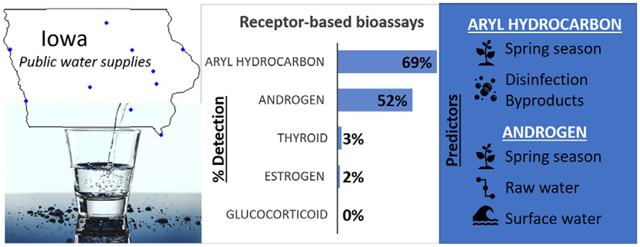
We observed no statistically significant bioactivity in samples concentrated to 100×, therefore present results only for samples concentrated 200×. Overall, all 10 utilities had at least one water sample with significant EDC activity of any type. Further, 9 utilities (90%) had significant EDC activity in at least one raw and one finished water sample. Most activity was attributed to AhR and AR; two samples yielded significant TR activity, one (a raw water sample) had ER activity, and no samples had GR activity (Table 2). The observed estrogenic and thyroid hormone activity were not found in the same samples. The low prevalence of detection of ER, TR, and GR activity precluded additional comparisons for these classes.
Table 2.
Overall detection of EDC activity, by assay and water sample type.
| Aryl hydrocarbon (AhR) | Androgen (AR) | Estrogen (ER) | Thyroid (Ty) | Glucocorticoid (GR) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N samples | N detectsa | % | N detects | % | N detects | % | N detects | % | N detects | % | |
| Total | 62 | 43 | 69.4 | 32 | 51.6 | 1 | 1.6 | 2 | 3.2 | 0 | 0 |
| Raw | 32 | 23 | 71.9 | 20 | 62.5 | 1 | 3.1 | 1 | 3.1 | 0 | 0 |
| Finished | 30 | 20 | 66.7 | 12 | 40.0 | 0 | 0 | 1 | 3.3 | 0 | 0 |
Statistically significant (p < 0.05) detections.
Overall, significant AhR activity was detected in 69% and AR activity in 52% of samples (Table 2). AhR activity was also detected in 63% of the AR positive samples, and this concordance was higher among spring (77%) than fall samples (41%). AhR and AR activities were each more common in the spring compared to the fall (60.5% vs. 39.5%; p = 0.002 and 71.9% vs. 28.1%; p < 0.001, respectively; Table 3). AR activity was higher in utilities with surface versus groundwater sources (p = 0.05) and in raw water versus finished water (p = 0.02). Levels of NO3-N and TTHM and AC treatment were unrelated to detection of either AhR or AR activity. In season-stratified comparisons, AR activity was detected more frequently in the spring in raw water samples compared with finished water samples (p = 0.04) but there were no significant differences in the fall (p = 0.14). Conversely, AR activity was more common in in the fall in utilities with surface water compared to ground water (p = 0.03) but not there were no significant differences in the spring (p = 0.16). We found no significant within-season contrasts across strata of treatment and regulated contaminants, although AhR activity was higher in spring samples from utilities with NO3-N ≥ median (p = 0.08).
Table 3.
Detections of aryl hydrocarbon (AhR) and androgen receptor (AR) activity, by sampling season, and Iowa public water utility characteristics.
| Aryl hydrocarbon (AhR) | Androgen (AR) | |||||||
|---|---|---|---|---|---|---|---|---|
| Utility characteristic | N samples | N detects1 | % | p-Value | N detects | % | p-Value | |
| Overall | Total | 62 | 43 | 69.4 | 32 | 51.6 | ||
| Spring | 31 | 26 | 60.5 | 23 | 71.9 | |||
| Fall | 31 | 17 | 39.5 | 0.002 | 9 | 28.1 | <0.001 | |
| Raw water | 32 | 23 | 71.9 | 20 | 62.5 | |||
| Finished water | 30 | 20 | 66.7 | 0.45 | 12 | 40.0 | 0.02 | |
| Surface water | 28 | 19 | 67.9 | 19 | 67.9 | |||
| Ground water | 34 | 24 | 70.6 | 0.99 | 13 | 38.2 | 0.05 | |
| NO3-N < median2 | 26 | 16 | 61.5 | 13 | 50.0 | |||
| NO3-N ≥ median | 36 | 27 | 75.0 | 0.37 | 19 | 52.8 | 0.85 | |
| TTHM <median2 | 28 | 17 | 60.7 | 13 | 46.4 | |||
| TTHM ≥median | 34 | 26 | 76.5 | 0.25 | 19 | 55.9 | 0.38 | |
| AC treatment3 | 6 | 4 | 66.7 | 3 | 50.0 | |||
| No AC treatment | 24 | 16 | 33.3 | 0.59 | 9 | 37.5 | 0.73 | |
| Spring | Raw water | 16 | 14 | 87.5 | 14 | 87.5 | ||
| Finished water | 15 | 12 | 80.0 | 0.51 | 9 | 60.0 | 0.04 | |
| Surface water | 14 | 12 | 85.7 | 12 | 85.7 | |||
| Ground water | 17 | 14 | 82.4 | 0.86 | 11 | 64.7 | 0.16 | |
| NO3-N < median | 13 | 9 | 69.2 | 10 | 76.9 | |||
| NO3-N ≥ median | 18 | 17 | 94.4 | 0.08 | 13 | 72.2 | 0.83 | |
| TTHM <median | 14 | 11 | 78.6 | 9 | 64.3 | |||
| TTHM ≥median | 17 | 15 | 88.2 | 0.52 | 14 | 82.4 | 0.24 | |
| AC treatment | 3 | 3 | 100.0 | 2 | 66.7 | |||
| No AC treatment | 12 | 9 | 75.0 | 0.59 | 7 | 58.3 | 0.73 | |
| Fall | Raw water | 16 | 9 | 56.3 | 6 | 37.5 | ||
| Finished water | 15 | 8 | 53.3 | 0.86 | 3 | 20.0 | 0.14 | |
| Surface water | 14 | 7 | 50.0 | 7 | 50.0 | |||
| Ground water | 17 | 10 | 58.8 | 0.76 | 2 | 11.8 | 0.03 | |
| NO3-N < median | 13 | 7 | 53.8 | 3 | 23.1 | |||
| NO3-N ≥ median | 18 | 10 | 55.6 | 0.90 | 6 | 33.3 | 0.56 | |
| TTHM <median | 14 | 6 | 42.9 | 4 | 28.6 | |||
| TTHM ≥median | 17 | 11 | 64.7 | 0.22 | 5 | 29.4 | 0.72 | |
| AC treatment | 3 | 1 | 33.3 | 1 | 33.3 | |||
| No AC treatment | 12 | 7 | 58.3 | 0.59 | 2 | 16.7 | 0.73 | |
Abbreviations: NO3-N: nitrate-nitrogen; TTHM: total trihalomethanes; AC: activated carbon.
Statistically significant (p < 0.05) detections.
Annual average levels across all utilities were split at the median.
AC = activated carbon. Raw water samples excluded from this comparison.
In multivariable analyses, we observed greater odds of AhR activity for utilities with NO3-N and TTHM at or above their medians compared to levels bmedian (TTHM POR = 6.7, CI: 1.4–33.2; NO3-N POR = 8.9, CI: 1.2–69.0) after adjustment for other characteristics (Table 4). Odds of AhR activity were higher in spring samples compared to those collected in the fall (OR = 5.9, CI = 2.4–14.0). The odds of AR activity were higher in samples from surface water sources (POR = 40.4; CI: 1.9–874.9), in raw water samples (POR = 4.61; CI: 1.44–14.73), and in spring samples (POR = 14.3; CI: 3.8–53.5). Tertiary AC treatment was not a significant predictor.
Table 4.
Determinants of aryl hydrocarbon (AhR) and androgen (AR) detections in samples from 10 Iowa public drinking water supplies from multilevel multivariable logistic regression models.
| AhR | AR | |
|---|---|---|
| Covariate | PORa (95% CI) | POR (95% CI) |
| Spring vs. fall | 5.9 (2.4–14.0) | 14.3 (3.8–53.5) |
| Raw vs. finished | 1.3 (0.7–2.6) | 4.6 (1.4–14.7) |
| Surface vs. ground | 0.12 (0.01–1.41) | 40.4 (1.9–874.9) |
| TTHM ≥ median (vs. <) | 6.7 (1.4–33.2) | 0.71 (0.06–8.06) |
| NO3-N ≥ median (vs. <) | 8.9 (1.2–69.0) | 0.22 (0.01–3.80) |
| AC treatment vs. none | 1.7 (0.2–13.6) | 0.32 (0.03–3.53) |
Abbreviations: NO3-N: nitrate-nitrogen; TTHM: total trihalomethanes; AC: activated carbon
Prevalence odds ratio.
4. Discussion
In this study, we detected significant activity of AhR and AR, but infrequent thyroid or estrogen activities and no glucocorticoid activity, in concentrated samples of both untreated and treated drinking water from public water utilities in Iowa. Our analyses suggest that AhR and AR activity in drinking water may be partially explained by season-specific determinants, source waters, and levels of common regulated contaminants.
While both AhR and AR activities were frequently detected in our samples, we observed variation in their detection depending on season of sampling and the source water serving the utility. We designed the sampling periods to coincide with seasons of agricultural activity in Iowa (e.g., most fertilizer nitrogen is applied in the spring, and some additional applications in the fall) (Iowa State University, 2015; Malone et al., 2010), when drinking water resources are most vulnerable to contamination from agricultural EDCs. Our findings support season-specific EDC determinants, as both AhR and AR were more commonly detected in the spring even when accounting for source, levels of regulated contaminants, and AC treatment. We also hypothesized greater EDCs detections in raw versus finished water, and in samples from utilities served by surface waters, which are more vulnerable to contamination from exogenous sources. However, we observed such differences only for AR activity. These different patterns of activity are likely explained by different sources, fate, and transport of compounds with affinity for these receptors. The androgen receptor has a strong affinity (in the low nanomolar range) for its physiological ligands, including dihydro-testosterone, a more biologically active form of testosterone (Davey and Grossmann, 2016). The AR activity detected in our study could be explained by the common use of steroidal growth hormones such as trenbolone acetate and testosterone in animal agriculture (Graham and Nachman, 2010). The AhR is a key transcriptional factor involved in gene regulation in response to toxins that also possesses physiological functions independent of exogenous chemical exposure (Mandal, 2005). AhR ligands include toxic and carcinogenic compounds such as polychlorinated dibenzo-p-dioxins and dibenzofurans, polychlorinated biphenyls, some polycyclic aromatic hydrocarbons, and their polybrominated forms (Hilscherova et al., 2000). Many other compounds including heterocyclic amines, carotenoids, pesticides and pharmaceuticals (Hilscherova et al., 2000) also bind AhR but with weaker affinity and may degrade more easily. The AhR response extends beyond xenobiotic metabolism; its role in endocrine activity and immunologic function has also been recognized (Barouki et al., 2007; Medlock Kakaley et al., 2019; Stockinger et al., 2014; Zhu et al., 2019). The ubiquity of substances with affinity for the AhR may explain the high prevalence of activity detected in many of our samples. We found a positive association between AhR activity and TTHM and NO3-N above the median. Studies evaluating the impact of nitrate and DBPs on endocrine disrupting activities in drinking water have mostly evaluated estrogenic activity. Schmidt et al. (2017) found that estrogens declined in the presence of nitrate (Schmidt et al., 2017). There is limited evidence that some DBPs exhibit estrogenic and androgenic activity, but most interactions with hormone receptors were weak (Kim et al., 2019). While chlorination of wastewater has been shown to decrease estrogenic activity and increase antiestrogenic activity (Schiliro et al., 2009; Wu et al., 2009), treatment of source drinking water using several methods did not result in notable changes in endocrine activity (Leusch et al., 2019). Future work is needed to disentangle relationships between EDC activity and contaminants in drinking water to identify potential predictors of EDC activity.
There are limited U.S. studies that used in vitro bioassays to examine EDC activity in public water systems with which to compare our findings (Conley et al., 2017). Further, studies of EDC activity in drinking water outside the U.S. have targeted urban and industrial areas (Neale and Escher, 2019). Detection of androgenic activity in urban and industrial areas is uncommon (Escher et al., 2014; Leusch et al., 2018; Rosenmai et al., 2018; Shi et al., 2018), contrary to our study findings of frequent AR detection in Iowa, an agriculturally-intensive area with low population density. Interestingly, surface streams near cattle operations in Iowa tested positive for 17β-trenbolone (TRB), a synthetic androgen (Cavallin et al., 2014). Further research is needed to evaluate sources of AR activity in Iowa’s water resources.
In agreement with our findings for AhR activity, treated drinking waters in Uppsala, Sweden and metropolitan Australia also showed elevated AhR activity, highlighting the persistence of AhR after treatment (Escher et al., 2014; Rosenmai et al., 2018). Specific estrogens, including estrone, have been previously identified in Iowa’s streams (Kolpin et al., 2010). Two U.S. studies of undisclosed public water utilities identified estrogens in source waters but found no detections in finished drinking water, indicating their likely removal through treatment (Benotti et al., 2009; Conley et al., 2017). We found limited evidence of estrogenic activity in our study, in a single raw water sample. We also observed very low detection rates for thyroid hormone activity, and no glucocorticoid activity. Similar to our findings, thyroid hormone receptor activity was not detected via in vitro assays in source water (Hu et al., 2013; Shi et al., 2012) or in tap water samples (Shi et al., 2012) in China, nor in samples from wastewater treatment plants in France (Jugan et al., 2009). We acknowledge that the TR assay used in this study is limited in detecting interactions with thyroid receptor alpha (TRα1) (Stavreva et al., 2016), which could also explain low thyroid hormone activity and may require further investigation. Glucocorticoid activity was detected via CALUX assays in effluent from sewage treatment plants in Japan (Suzuki et al., 2015) and in raw, wastewater and surface water extracts but not in drinking water in the Netherlands (Van der Linden et al., 2008). Our findings differ from those of a U.S. study by Stavreva et al. (Stavreva et al., 2012; Stavreva et al., 2016), which screened over 100 samples from source waters in 14 states (not including Iowa) using the same bioassays and observed glucocorticoid activity in over 28% of samples, androgen activity in 37%, and thyroid activity in 53%. One plausible explanation for these contrasting results is that the utility characteristics we evaluated as determinants are not major contributors to the activity of these compounds in Iowa’s drinking water. Notably, samples in Stavreva et al. were also not from drinking water and came predominantly from surface waters in the eastern and northeastern areas of the U.S., with potentially higher population densities. If population density-related factors are a predominant source of EDCs in the ambient environment, this may explain the low levels of bioactivity observed in our study, as Iowa is among the lowest U.S. states for population density (ranked 38th) (Bureau, U.S.C, 2010). The distance between wastewater effluent discharge points and drinking water intakes might also impact the extent of dilution and degradation and consequently the likelihood of EDCs entering a drinking water source (National Research Council (NRC), 1998). Further, if bioactivity changes throughout a PWS distribution system, we might expect different detection rates depending on where in the system the samples were taken. Although half of the utilities in our study provided multiple samples of raw and finished water per season, small sample sizes limited our ability to evaluate differences in bioactivity between samples collected at different points within a system. Measurements of bioactivity from samples collected at multiple points in a distribution system, in raw water samples taken closer to upstream sources, and in private wells (which may have higher levels of EDCs and limited or no disinfection treatments) all may be informative in future studies.
Although bioassays have been used to screen for EDC activity in wastewater and surface waters, few investigations have employed such assays to detect biologic activity in treated drinking waters in the U.S. Further, areas with high agricultural activity and low population density are understudied and likely to have several sources of EDC activity (e.g., pesticides, phytoestrogens, and hormones and pharmaceuticals from animal waste). Traditional chemical assays limit the scope of measurement to known and well-characterized compounds, while bioassays enable screening more broadly for biologically relevant hormonal activity. This may be valuable to investigations of health risks associated with EDCs in drinking water, a heterogeneous mixture of compounds. In vitro bioassays may also complement targeted chemical analyses in such studies (Conley et al., 2017). For example, in a study of 25 drinking water treatment plants across the U.S., a complementary T47D-KBluc bioassay suggested bioactivity in drinking water samples from 16 plants where no specific natural or synthetic estrogens were quantified using LC-FTMS (Conley et al., 2017). In addition to the improved characterization of mixtures for exposure assessment in epidemiologic studies, the techniques employed in our pilot study have other potential applications, such as bioassay-directed fractionation to enable identification of novel EDCs in the environment. Functional assays may also be used to screen source water and wastewater for watershed protection and related efforts (Escher et al., 2014).
We did not measure concentrations of equivalent EDC activity for specific compounds in this pilot study, as was done previously with these bioassays (Stavreva et al., 2012; Stavreva et al., 2016) but such comparisons would be a useful addition to future work. We note that our study objective was specifically focused on screening, i.e., detection of activity, rather than on demonstrating dose-response, and that we did not observe much activity at 100×. However, in a post hoc analysis we attempted to evaluate concentrations >200× and determined that at high concentrations (333×), we start to see inhibitory effects and large variability from field-to-field not observed at lower levels, and that the cells showed signs of stress; controls did not demonstrate these signs. This stress makes cells less capable of translocating, and therefore the translocation values for many of the samples were diminished at these high concentrations. Also of note, the solid phase extraction method used in our study only captures organic compounds. Higher detection rates of AR activity during the spring season in raw water, and in PWS sourced by surface water support our hypotheses relating these characteristics to greater vulnerability to EDCs contamination. These patterns were not as consistent for AhR activity, which was only higher in the spring but in multivariable analyses was associated with higher levels of both NO3-N and TTHM, two routinely monitored contaminants with known health relevance. Predictive models relating global EDCs activities to established determinants may enable population-level exposure assessment in epidemiologic studies of EDCs in drinking water, where measurements from repeated sampling are not available or feasible to collect. To extend our work and enable such approaches, future exposure studies would ideally capture a range of climactic and other conditions that might influence EDCs contamination of drinking water resources. We collected samples only at two time points, and there may be greater temporal variability and other determinants than those we evaluated in our study. Moreover, we collected only grab samples from the utilities without consideration of retention time or other factors that might also influence the likelihood of detecting EDC activity. Additionally, although certain EDCs are potent at very low levels (Sumpter, 2014), the biological relevance of the global EDCs activities observed in our concentrated samples is unknown. Lastly, interpretations are limited by the complexity of EDCs, and the potential for reactivity with other components in the environment that may influence specific bioactivity in drinking water mixtures.
Our findings contribute to the limited published data on detection of bioactivity in public drinking water in agriculturally-intensive areas. Additional strengths of this study include our sampling from PWS across Iowa, which are served by varied source waters and provide drinking water to a large proportion of the state’s population on PWS. Our study provides important information regarding monitoring of EDCs in drinking water and would ideally be reproduced by other methods and in other locations.
5. Conclusions
The results from this pilot study indicate that activity of specific types of EDCs are detectable in concentrated treated public drinking water and suggest potential avenues for future research to identify the predictors of EDC activity. Expanded studies of determinants of bioactivity in drinking water may enable opportunities to characterize EDCs exposures for epidemiologic study populations. An important next step will be to evaluate global EDC activity in water samples from private wells, in which specific EDCs such as pesticides have been previously identified.
Supplementary Material
HIGHLIGHTS.
Raw and treated drinking water samples from 10 public utilities in Iowa were collected in two seasons.
Mammalian cell-based assays were used to assess 5 classes of bioactivity.
Aryl hydrocarbon and androgenic activity was common; thyroid, estrogen, and glucocorticoid activities were not.
Season, water source, and regulated water contaminants were potential determinants of bioactivity.
Bioassays may enable characterization of exposure to a mixture of endocrine disrupting compounds.
Acknowledgements
We thank Arbor Quist and Meredith Cervi for analytic support on this study. We also thank the 10 public drinking water utilities in Iowa for their participation.
Funding sources
This work was supported by the Intramural Research Program of the National Institutes of Health. The authors declare no conflicts of interest.
Footnotes
Declaration of competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.scitotenv.2019.136317.
References
- Adeel M, et al. , 2017. Environmental impact of estrogens on human, animal and plant life: a critical review. Environ. Int 99, 107–119. [DOI] [PubMed] [Google Scholar]
- Annamalai J, Namasivayam V, 2015. Endocrine disrupting chemicals in the atmosphere: their effects on humans and wildlife. Environ. Int 76, 78–97. [DOI] [PubMed] [Google Scholar]
- Aris AZ, Shamsuddin AS, Praveena SM, 2014. Occurrence of 17alpha-ethynylestradiol (EE2) in the environment and effect on exposed biota: a review. Environ. Int 69, 104–119. [DOI] [PubMed] [Google Scholar]
- Barouki R, Coumoul X, Fernandez-Salguero PM, 2007. The aryl hydrocarbon receptor, more than a xenobiotic-interacting protein. FEBS Lett. 581 (19), 3608–3615. [DOI] [PubMed] [Google Scholar]
- Belfroid AC, et al. , 1999. Analysis and occurrence of estrogenic hormones and their glucuronides in surface water and waste water in the Netherlands. Sci. Total Environ 225 (1–2), 101–108. [DOI] [PubMed] [Google Scholar]
- Benotti MJ, et al. , 2009. Pharmaceuticals and endocrine disrupting compounds in U.S. drinking water. Environ. Sci. Technol 43 (3), 597–603. [DOI] [PubMed] [Google Scholar]
- Bureau, U.S.C, 2010. 2010Census: Population Density Data. December12, 2018]; Available from.https://www.census.gov/data/tables/2010/dec/density-data-text.html.
- Campana C, Pezzi V, Rainey WE, 2015. Cell-based assays for screening androgen receptor ligands. Semin. Reprod. Med 33 (3), 225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell CG, et al. , 2006. Biologically directed environmental monitoring, fate, and transport of estrogenic endocrine disrupting compounds in water: a review. Chemosphere 65 (8), 1265–1280. [DOI] [PubMed] [Google Scholar]
- Cavallin JE, et al. , 2014. Integrated assessment of runoff from livestock farming operations: analytical chemistry, in vitro bioassays, and in vivo fish exposures. Environ. Toxicol. Chem 33 (8), 1849–1857. [DOI] [PubMed] [Google Scholar]
- Center for Health Effects of Environmental Contamination (CHEEC), 2016. Iowa Environmental Databases. Available from.https://cheec.uiowa.edu/data/environmental-databases.
- Cespedes R, et al. , 2004. Integrated procedure for determination of endocrine-disrupting activity in surface waters and sediments by use of the biological technique recombinant yeast assay and chemical analysis by LC-ESI-MS. Anal. Bioanal. Chem 378 (3), 697–708. [DOI] [PubMed] [Google Scholar]
- Ciparis S, Iwanowicz LR, Voshell JR, 2012. Effects of watershed densities of animal feeding operations on nutrient concentrations and estrogenic activity in agricultural streams. Sci. Total Environ 414, 268–276. [DOI] [PubMed] [Google Scholar]
- Conley JM, et al. , 2017. Comparison of in vitro estrogenic activity and estrogen concentrations in source and treated waters from 25 U.S. drinking water treatment plants. Sci. Total Environ 579, 1610–1617. [DOI] [PubMed] [Google Scholar]
- Daniels KD, et al. , 2018. Downstream trends of in vitro bioassay responses in a wastewater effluent-dominated river. Chemosphere 212, 182–192. [DOI] [PubMed] [Google Scholar]
- Davey RA, Grossmann M, 2016. Androgen receptor structure, function and biology: from bench to bedside. Clin. Biochem. Rev 37 (1), 3–15. [PMC free article] [PubMed] [Google Scholar]
- Deblonde T, Cossu-Leguille C, Hartemann P, 2011. Emerging pollutants in wastewater: a review of the literature. Int. J. Hyg. Environ. Health 214 (6), 442–448. [DOI] [PubMed] [Google Scholar]
- Elbi C, Misteli T, Hager GL, 2002. Recruitment of dioxin receptor to active transcription sites. Mol. Biol. Cell 13 (6), 2001–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escher BI, et al. , 2014. Benchmarking organic micropollutants in wastewater, recycled water and drinking water with in vitro bioassays. Environ. Sci. Technol 48 (3), 1940–1956. [DOI] [PubMed] [Google Scholar]
- Esplugas S, et al. , 2007. Ozonation and advanced oxidation technologies to remove endocrine disrupting chemicals (EDCs) and pharmaceuticals and personal care products (PPCPs) in water effluents. J. Hazard. Mater 149 (3), 631–642. [DOI] [PubMed] [Google Scholar]
- Frye CA, et al. , 2012. Endocrine disrupters: a review of some sources, effects, and mechanisms of actions on behaviour and neuroendocrine systems. J. Neuroendocrinol 24 (1), 144–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham JP, Nachman KE, 2010. Managing waste from confined animal feeding operations in the United States: the need for sanitary reform. J. Water Health 8 (4), 646–670. [DOI] [PubMed] [Google Scholar]
- Guillette LJ Jr., Edwards TM, 2005. Is nitrate an ecologically relevant endocrine disruptor in vertebrates? Integr. Comp. Biol 45 (1), 19–27. [DOI] [PubMed] [Google Scholar]
- Guillette LJ Jr., et al. , 1995. Organization versus activation: the role of endocrine-disrupting contaminants (EDCs) during embryonic development in wildlife. Environ. Health Perspect 103 (Suppl. 7), 157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield J, Follett R, 2008. Nitrogen in the Environment: Sources, Problems, and Management. Elsevier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilscherova K, et al. , 2000. Cell bioassays for detection of aryl hydrocarbon (AhR) and estrogen receptor (ER) mediated activity in environmental samples. Environ. Sci. Pollut. Res. Int 7 (3), 159–171. [DOI] [PubMed] [Google Scholar]
- Hladik ML, Kolpin DW, Kuivila KM, 2014. Widespread occurrence of neonicotinoid insecticides in streams in a high corn and soybean producing region, USA. Environ. Pollut 193, 189–196. [DOI] [PubMed] [Google Scholar]
- Hu X, et al. , 2013. In vitro assessment of thyroid hormone disrupting activities in drinking water sources along the Yangtze River. Environ. Pollut 173, 210–215. [DOI] [PubMed] [Google Scholar]
- Iowa Department of Natural Resources (IDNR), 2016January2016. Animal Feeding Operation Database. Available from.https://programs.iowadnr.gov/animalfeedingoperations/.
- Iowa Department of Natural Resources (IDNR), 2017. Iowa Drinking Water Annual Compliance Report.
- Iowa State University, 2015. Fertilizer Nitrogen Application this Fall. [cited2019 December 10]; Available from.https://crops.extension.iastate.edu/cropnews/2015/10/fertilizer-nitrogen-application-fall.
- Jackson J, Sutton R, 2008. Sources of endocrine-disrupting chemicals in urban wastewater, Oakland, CA. Sci. Total Environ 405 (1), 153–160. [DOI] [PubMed] [Google Scholar]
- Jenkins R, et al. , 2001. Identification of androstenedione in a river containing paper mill effluent. Environ. Toxicol. Chem 20 (6), 1325–1331. [DOI] [PubMed] [Google Scholar]
- Jenkins RL, et al. , 2003. Androstenedione and progesterone in the sediment of a river receiving paper mill effluent. Toxicol. Sci 73 (1), 53–59. [DOI] [PubMed] [Google Scholar]
- Jugan ML, et al. , 2009. In vitro assessment of thyroid and estrogenic endocrine disruptors in wastewater treatment plants, rivers and drinking water supplies in the greater Paris area (France). Sci. Total Environ 407 (11), 3579–3587. [DOI] [PubMed] [Google Scholar]
- Kabir ER, Rahman MS, Rahman I, 2015. A review on endocrine disruptors and their possible impacts on human health. Environ. Toxicol. Pharmacol 40 (1), 241–258. [DOI] [PubMed] [Google Scholar]
- Kim DH, Park CG, Kim YJ, 2019. Characterizing the potential estrogenic and androgenic activities of two disinfection byproducts, mono-haloacetic acids and haloacetamides, using in vitro bioassays. Chemosphere 242, 125198. [DOI] [PubMed] [Google Scholar]
- Kolpin DW, et al. , 2010. Phytoestrogens and mycotoxins in Iowa streams: an examination of underinvestigated compounds in agricultural basins. J. Environ. Qual 39 (6), 2089–2099. [DOI] [PubMed] [Google Scholar]
- Kross BC, et al. , 1993. The nitrate contamination of private well water in Iowa. Am. J. Public Health 83 (2), 270–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leusch FD, et al. , 2017. Analysis of the sensitivity of in vitro bioassays for androgenic, progestagenic, glucocorticoid, thyroid and estrogenic activity: suitability for drinking and environmental waters. Environ. Int 99, 120–130. [DOI] [PubMed] [Google Scholar]
- Leusch FDL, et al. , 2018. Analysis of endocrine activity in drinking water, surface water and treated wastewater from six countries. Water Res. 139, 10–18. [DOI] [PubMed] [Google Scholar]
- Leusch FDL, et al. , 2019. Transformation of endocrine disrupting chemicals, pharmaceutical and personal care products during drinking water disinfection. Sci. Total Environ 657, 1480–1490. [DOI] [PubMed] [Google Scholar]
- Liu ZH, Kanjo Y, Mizutani S, 2009. Removal mechanisms for endocrine disrupting compounds (EDCs) in wastewater treatment - physical means, biodegradation, and chemical advanced oxidation: a review. Sci. Total Environ 407 (2), 731–748. [DOI] [PubMed] [Google Scholar]
- Mai J, et al. , 2011. Are assumptions about the model type necessary in reaction-diffusion modeling? A FRAP application. Biophys. J 100 (5), 1178–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone RW, et al. , 2010. Soil-test N recommendations augmented with PEST-optimized RZWQM simulations. J. Environ. Qual 39 (5), 1711–1723. [DOI] [PubMed] [Google Scholar]
- Mandal PK, 2005. Dioxin: a review of its environmental effects and its aryl hydrocarbon receptor biology. J. Comp. Physiol. B 175 (4), 221–230. [DOI] [PubMed] [Google Scholar]
- Martinez ED, et al. , 2005. An estrogen receptor chimera senses ligands by nuclear translocation. J. Steroid Biochem. Mol. Biol 97 (4), 307–321. [DOI] [PubMed] [Google Scholar]
- Medlock Kakaley EK, et al. , 2019. De facto water reuse: bioassay suite approach delivers depth and breadth in endocrine active compound detection. Sci. Total Environ 699, 134297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray CM, et al. , 2017. Detection of a synthetic sex steroid in the American crocodile (Crocodylus acutus): evidence for a novel environmental androgen. Chemosphere 180, 125–129. [DOI] [PubMed] [Google Scholar]
- National Institute of Environmental Health Sciences (NIEHS), 2018. Endocrine Disruptors. December6, 2018]; Available from.https://www.niehs.nih.gov/health/topics/agents/endocrine/index.cfm.
- National Research Council (NRC), 1998. Issues in Potable Reuse: The Viability of Augmenting Drinking Water Supplies With Reclaimed Water. National Academy of Sciences. [PubMed] [Google Scholar]
- Neale PA, Escher BI, 2019. In vitro bioassays to assess drinking water quality. Curr. Opin. Environ. Sci. Health 7, 1–7. [Google Scholar]
- Padhye LP, et al. , 2014. Year-long evaluation on the occurrence and fate of pharmaceuticals, personal care products, and endocrine disrupting chemicals in an urban drinking water treatment plant. Water Res. 51, 266–276. [DOI] [PubMed] [Google Scholar]
- Pereira RO, et al. , 2011. Removal of estrogens through water disinfection processes and formation of by-products. Chemosphere 82 (6), 789–799. [DOI] [PubMed] [Google Scholar]
- Rosenmai AK, et al. , 2018. In vitro bioanalysis of drinking water from source to tap. Water Res. 139, 272–280. [DOI] [PubMed] [Google Scholar]
- Schiliro T, et al. , 2009. The endocrine disrupting activity of surface waters and of wastewater treatment plant effluents in relation to chlorination. Chemosphere 75 (3), 335–340. [DOI] [PubMed] [Google Scholar]
- Schmidt N, Page D, Tiehm A, 2017. Biodegradation of pharmaceuticals and endocrine disruptors with oxygen, nitrate, manganese (IV), iron (III) and sulfate as electron acceptors. J. Contam. Hydrol 203, 62–69. [DOI] [PubMed] [Google Scholar]
- Shi P, et al. , 2018. Toxicological and chemical insights into representative source and drinking water in eastern China. Environ. Pollut 233, 35–44. [DOI] [PubMed] [Google Scholar]
- Shi W, et al. , 2012. Occurrence of thyroid hormone activities in drinking water from eastern China: contributions of phthalate esters. Environ. Sci. Technol 46 (3), 1811–1818. [DOI] [PubMed] [Google Scholar]
- Snyder SA, et al. , 2001. Identification and quantification of estrogen receptor agonists in wastewater effluents. Environ. Sci. Technol 35 (18), 3620–3625. [DOI] [PubMed] [Google Scholar]
- Snyder SA, et al. , 2003. Pharmaceuticals, personal care products, and endocrine disruptors in water: implications for the water industry. Environ. Eng. Sci 20 (5). [Google Scholar]
- Stavreva DA, et al. , 2012. Prevalent glucocorticoid and androgen activity in US water sources. Sci. Rep 2, 937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavreva DA, et al. , 2016. Novel cell-based assay for detection of thyroid receptor beta-interacting environmental contaminants. Toxicology 368–369, 69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockinger B, et al. , 2014. The aryl hydrocarbon receptor: multitasking in the immune system. Annu. Rev. Immunol 32, 403–432. [DOI] [PubMed] [Google Scholar]
- Street ME, et al. , 2018. Current knowledge on endocrine disrupting chemicals (EDCs) from animal biology to humans, from pregnancy to adulthood: highlights from a National Italian Meeting. Int. J. Mol. Sci 19 (6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumpter JP, 2014. The challenge: do pharmaceuticals present a risk to the environment, and what needs to be done to answer the question? Environ. Toxicol. Chem 33 (9), 1915. [DOI] [PubMed] [Google Scholar]
- Sun Y, et al. , 2013. Ecological risk of estrogenic endocrine disrupting chemicals in sewage plant effluent and reclaimed water. Environ. Pollut 180, 339–344. [DOI] [PubMed] [Google Scholar]
- Suzuki G, et al. , 2015. Detection of glucocorticoid receptor agonists in effluents from sewage treatment plants in Japan. Sci. Total Environ 527–528, 328–334. [DOI] [PubMed] [Google Scholar]
- UNEP/WHO, 2012. State of the Science of Endocrine Disrupting Chemicals — 2012. Switzerland United Nations Environment Programme/World Health Organization, Geneva. [Google Scholar]
- Van der Linden SC, et al. , 2008. Detection of multiple hormonal activities in wastewater effluents and surface water, using a panel of steroid receptor CALUX bioassays. Environ. Sci. Technol 42 (15), 5814–5820. [DOI] [PubMed] [Google Scholar]
- Westlund P, Yargeau V, 2017. Investigation of the presence and endocrine activities of pesticides found in wastewater effluent using yeast-based bioassays. Sci. Total Environ 607–608, 744–751. [DOI] [PubMed] [Google Scholar]
- Weyer PJ, et al. , 2006. Comparison of nitrate levels in raw water and finished water from historical monitoring data on Iowa municipal drinking water supplies. Environ. Monit. Assess 116 (1–3), 81–90. [DOI] [PubMed] [Google Scholar]
- Wu QY, et al. , 2009. Effect of chlorination on the estrogenic/antiestrogenic activities of biologically treated wastewater. Environ. Sci. Technol 43 (13), 4940–4945. [DOI] [PubMed] [Google Scholar]
- Yost EE, et al. , 2013. Comprehensive assessment of hormones, phytoestrogens, and estrogenic activity in an anaerobic swine waste lagoon. Environ. Sci. Technol 47 (23), 13781–13790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu K, et al. , 2019. Aryl hydrocarbon receptor pathway: role, regulation and intervention in atherosclerosis therapy (review). Mol. Med. Rep 20 (6), 4763–4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zirkle KW, et al. , 2016. Assessing the relationship between groundwater nitrate and animal feeding operations in Iowa (USA). Sci. Total Environ 566–567, 1062–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


