Abstract
Rationale and Objective:
The Prostate Imaging Reporting and Data System version 2 (PI-RADSv2) published a set of minimum technical standards (MTS) to improve image quality and reduce variability in multiparametric prostate MRI. The effect of PIRADSv2 MTS on image quality has not been validated. We aimed to determine whether adherence to PI-RADSv2 MTS improves study adequacy and perceived quality.
Materials and Methods:
Sixty-two prostate MRI examinations including T2 weighted (T2W) and diffusion weighted image (DWI) consecutively referred to our center from 62 different institutions within a 12-month period (September 2017 to September 2018) were included. Six readers assessed images as adequate or inadequate for use in PCa detection and a numerical image quality ranking was given using a 1–5 scale. The PI-RADSv2 MTS were synthesized into sets of seven and 10 rules for T2W and DWI, respectively. Image adherence was assessed using Digital Imaging and Communications in Medicine (DICOM) metadata. Statistical analysis of survey results and image adherence was performed based on reader quality scoring (Kendall Rank tau-b) and reader adequate scoring (Wilcoxon test for association) for T2 and DWI quality assessment.
Results:
Out of 62 images, 52 (83%) T2W and 38 (61%) DWIs were rated to be adequate by a majority of readers. Reader adequacy scores showed no significant association with adherence to PI-RADSv2. There was a weak (tau-b = 0.22) but significant (p value = 0.01) correlation between adherence to PIRADSv2 MTS and image quality for T2W. Studies following all PI-RADSv2 T2W rules achieved a higher median average quality score (3.58 for 7/7 vs. 3.0 for <7/7, p = 0.012). No statistical relationship with PI-RADSv2 MTS adherence and DWI quality was found.
Conclusion:
Among 62 sites performing prostate MRI, few were considered of high quality, but the majority were considered adequate. DWI showed considerably lower rates of adequate studies in the sample. Adherence to PI-RADSv2 MTS did not increase the likelihood of having a qualitatively adequate T2W or DWI.
Keywords: Prostate, Quality control, MRI, PI-RADS, Diffusion weighted imaging
INTRODUCTION
Approximately, 175,000 people will be diagnosed with prostate cancer (PCa) in the United States in 2019 making it the most common solid organ malignancy in men. However, only approximately 31,600 will die from PCa in the same period (1). Trying to distinguish clinically significant PCa (CSPCa) from indolent PCa is a central challenge in PCa management. Traditional screening methods which rely on prostate-specific antigen screening and transrectal ultrasound (TRUS) guided biopsy results in overdiagnosis and overtreatment (2). The introduction of prostate multiparametric MRI (mpMRI) improves the detection of clinically significant cancers while reducing the diagnosis of indolent cancer. The creation of the Prostate Imaging Reporting and Data System Version 1 was an effort to standardize the interpretation of mpMRI. Prostate Imaging Reporting and Data System version 2 (PI-RADSv2) simplified the original system, reducing the role of dynamic contrast enhanced MRI and creating a single composite score for each lesion. Importantly, PI-RADSv2 also suggested standards for the acquisition of the individual MRI sequences based on the expert opinion of the PI-RADSv2 committee members (3,4).
PI-RADSv2 has consistently been shown to have a high negative predictive value allowing physicians to triage patients and avoid biopsies in patients unlikely to have PCa (5,6). Similarly, other studies have shown that PI-RADSv2 in the context of MRI guided biopsies has increased sensitivity for clinically significant PCa over traditional TRUS guided biopsy methods.(7–10)
However, the problem of variable image quality among centers is a major limitation of mpMRI. Low quality prostate MRIs could easily limit lesion localization lowering diagnostic yield in MRI guided biopsies resulting in no patient benefit over standard ultrasound guidance. As mpMRI disseminates to a wider user-base, scans that are of limited diagnostic value may become more common. Image quality is notoriously difficult to assess but clearly could impact the results of recent large studies that showed little difference between MRI-guided and TRUS-guided biopsies (6,11).
The PI-RADSv2 Minimum Technical Standards (MTS) were created with the goal of standardizing imaging protocols and reducing image quality variance (4). However, Esses et al. found that adherence to the guidelines varied widely across different institutions with some standards only achieving 17% adherence (12). However, it is reasonable to ask whether adherence to the standards solves the quality problem. It is possible that the sites not adhering to the standards may be doing so because they find that the MTS do not work well in their setting.
Therefore, this study was designed to evaluate variability in quality of prostate mpMRI and relate quality to the degree of adherence to the PI-RADSv2 MTS for T2 and DWI sequences, the two major components of mpMRI.
MATERIALS AND METHODS
Study Population
We identified a cross-sectional cohort of 62 prostate MRI examinations performed at outside imaging facilities which had been consecutively referred to our center for secondary interpretation within a 12-month period (September 2017 to September 2018) including both T2 weighted (T2W) and diffusion weighted image (DWI) sequences. All patients enrolled at the National Cancer Institute Center for Cancer Research under an Institutional Review Board approved trial (National Clinical Trials identifier NCT02594202) and signed an informed consent form. After anonymization, Digital Imaging and Communications in Medicine (DICOM) files for T2W and raw DWI sequences were uploaded to the local picture archiving and communication system. The age of patients ranged from 48 to 80 with a mean age of 66 (SD ± 8). The distribution of MRI vendors for acquisition was Siemens 58%, General Electric 35%, and Philips 6%. Ninety percent of exams were performed using a 3 Tesla scanner (Table 1).
TABLE 1.
Summary of the Range of Prostate MRI Acquisition Parameters in this Study Population
| T2 Parameters | Mean ± SD | Median | Range |
|---|---|---|---|
| Slice thickness (ST) | 3.0 ± 0.5 | 3 | 1.1–5 |
| Field of view (FOV) | 187 ± 25 | 180 | 120–250 |
| In-plane resolution (phase) | 0.5 ± 0.2 | 0.4 | 0.2–1.1 |
| In-plane resolution (frequency) | 0.5 ± 0.2 | 0.4 | 0.2–1.1 |
| Slice gap (SG) | Mean ± SD | No. w/ gap | Mean gap* |
| 0.2 ± 0.3 | 24 | 0.60 | |
| Phase encoding direction | # Vertical | # Horizontal | |
| 15 | 47 | ||
| DWI parameters | Mean ± SD | Median | Range |
| High B value | 1222 ± 427 | 1400 | 500–2000 |
| # of B values | 2.7 ± 1.0 | 2 | 2–7 |
| TE | 80.4 ± 15.6 | 83 | 47–110 |
| TR | 6438 ± 2637 | 5622 | 2800–13500 |
| Slice thickness (ST) | 3.4 ± 0.6 | 3.25 | 2.7–6.0 |
| Field of view (FOV) | 232 ± 63 | 250 | 90–380 |
| In-plane resolution (phase) | 1.4 ± 0.5 | 1.3 | 0.5–2.6 |
| In-plane resolution (frequency) | 1.4 ± 0.5 | 1.3 | 0.5–2.6 |
| Slice gap (SG) | Mean ± SD | No. w/ gap | Mean gap* |
| 0.1 ± 0.3 | 13 | 0.58 | |
| Phase encoding direction | # Vertical | # Horizontal | |
| 47 | 15 | ||
| Machine attributes | |||
| Magnet strength | #of 1.5T | # of 3T | |
| 6 | 56 | ||
| Vendor | GE | Siemens | Philips |
| 22 | 36 | 4 |
DWI, diffusion weighted image; TE, echo time; TR, repetition time.
Mean gap calculation excludes all sequences with no gap
Assessment of PIRADSv2 MTS Adherence
DICOM files and metadata were used to assess adherence to PI-RADSv2 MTS for T2W and DWI sequences. This assessment was aided by an in-house developed automated assessment tool which assessed adherence to PI-RADSv2 MTS. In addition to the PI-RADSv2 MTS, we recorded the phase encoding direction for each image as we hypothesized that the phase encoding direction might have an effect on image quality and severity of distortion on DWI sequences with horizontal phase encoding direction (right–>left) performing better than vertical phase encoding direction (anterior –> posterior).
Survey Design
Three experienced (TB, JM, YML) and three novice readers (MC, ET, MM) from five different institutions (noncontributors to the test cases) were recruited to assess each image and sequence on an array of subjective image quality characteristics. Readers assessed images as either “adequate” or “inadequate” for use in PCa detection. Readers’ assessment of image quality was ranked on a 1–5 Likert scale with 5 being the best and 1 being impossible to interpret. Readers were trained on a different set of reference images before being given the test set. For training one expert radiologist (BT) selected cases considered to represent the spectrum of image quality (1–4). Since scores of “5” were unusual, readers were instructed to give a score of 5 when the image quality exceeded the reference image for the quality score of 4. Readers also assessed images according to visual characteristics and for the presence and severity of perceived artifacts. The visual characteristics chosen were blurriness and contrast (13,14). The perceived artifacts chosen were noise, motion, geometric distortion, and aliasing/ghosting. The visual characteristics and perceived artifacts were graded on a 1–5 scale with a score of 1 indicating that the specific artifact was so severe that the image was impossible to interpret and 5 indicating the absence of the artifact. Readers were not told whether an image was compliant with the PI-RADSv2 MTS. However, readers were aware of the number of b-values obtained and the b-value of the highest b-value image.
Statistical Analysis
Association between PI-RADSv2 adherence, image adequacy, and image quality were assessed by Kendall’s tau-b rank correlation, Wilcoxon rank sum test, or chi-square test accounting for inter-reader correlation arising from multiple readers reviewing the same cases (15,16). Reader agreement on image quality was determined by Fleiss’ kappa. Standard errors and 95% confidence intervals of kappa statistics were estimated from 2000 bootstrap samples by random sampling on the case level. p values < 0.05 were considered statistically significant.
RESULTS
Adequacy and Quality of Prostate mpMRI
Of the 62 studies, 52 (84%) T2W and 38 (61%) DWI were rated to be diagnostically adequate. However, for only 35 (56%) of the studies, both sequences were rated as adequate by a majority. For T2W, only 10 (16%) were scored as high quality (score >3) by a majority of readers. For DWI, only 6 (9%) were scored as high quality by a majority of readers.
Reader Agreement
Reader agreement on image adequacy was fair for T2W (κ = 0.40) and DWI (κ = 0.39). Reader agreement on image quality was poor in T2W (κ = 0.17) and fair in DWI (κ = 0.21). Reader agreement on image blurriness and contrast was poor in both T2 and DWI (Table 2) suggesting there is considerable disagreement regarding image quality.
TABLE 2.
Reader Agreement on Subjective Image Quality Survey
| Agreement Metric | Adequate | Quality | Blurriness | Contrast | |
|---|---|---|---|---|---|
| T2 | Complete agreement* | 58% | 2% | 0% | 3% |
| Majority agreement* | 84% | 47% | 50% | 50% | |
| Fleiss’ kappa (SE) | 0.40 (0.08) | 0.17 (0.03) | 0.17 (0.03) | 0.19 (0.03) | |
| Conclusion† | Fair | Slight | Slight | Slight | |
| DWI | Complete agreement* | 29% | 5% | 0% | 0% |
| Majority agreement* | 61% | 47% | 50% | 39% | |
| Fleiss’ Kappa (SE) | 0.39 (0.06) | 0.21 (0.04) | 0.18 (0.03) | 0.14 (0.02) | |
| Conclusion† | Fair | Fair | Slight | Slight |
Complete Agreement defined as % of cases where all readers agreed on score, Majority agreement defined as % of cases where the majority of readers agreed on score.
Conclusion determined by Kappa score: moderate ≥ 0.41, fair ≥ 0.21, and slight ≤ 0.20.
Adherence Rates
Adherence to PI-RADSv2 MTS varied widely. Eighteen studies met all seven T2 standards and six studies met all 10 DWI standards. The T2W imaging standards with the lowest rates of adherence were in-plane resolution phase ≤ 0.4 mm and no interslice gap, with adherence rates of 52% and 61%, respectively. For DWI, the standards with the lowest rates of adherence were field-of-view (FOV) ≤ 220 mm and high-b value ≥ 1400 s/mm2 with adherence rates of 35% and 50%, respectively (Table 3).
TABLE 3.
Summary of Overall Rates of Adherence to PIRADSv2 Minimum Technical Standards (MTS)
| T2W Technical Standards | % Adherence |
|---|---|
| Magnet strength ≥ 1.5T | 100% |
| Slice thickness (ST) ≤ 3 mm | 92% |
| No interslice gap | 61% |
| Field of view lower limit (FOV LL) ≥ 120 mm | 100% |
| Field of view upper limit (FOV UL) ≤ 200 mm | 82% |
| In-plane resolution phase (IPRP) ≤ 0.7 mm | 92% |
| In-plane resolution frequency (IPRF) ≤ 0.4 mm | 52% |
| DWI technical standards | % Adherence |
| Magnet strength ≥ 1.5 T | 100% |
| High b-value images ≥ 1400 s/mm2 | 50% |
| TR ≥ 3000 ms | 94% |
| TE ≤ 90 ms | 73% |
| Slice thickness ≤ 4 mm | 97% |
| No interslice gap | 79% |
| Field-of-view lower limit (FOV LL) ≥ 160 mm | 87% |
| Field-of-view upper limit (FOV UL) ≤ 220 mm | 35% |
| In-plane resolution phase (IPRP) ≤ 2.5 mm | 98% |
| In-plane resolution frequency (IPRF) ≤ 2.5 mm | 98% |
DWI, diffusion weighted image; PIRADSv2, Prostate Imaging Reporting and Data System version 2; TR, repetition time.
Adherence and Diagnostic Adequacy
There was no significant association between adherence to PI-RADSv2 MTS and image adequacy for either T2 (p value = 0.227) or DWI (p value = 0.304) indicating that following the technical standards had no effect on the likelihood of an image being scored as diagnostically adequate (Fig 1, Table 4).
Figure 1.
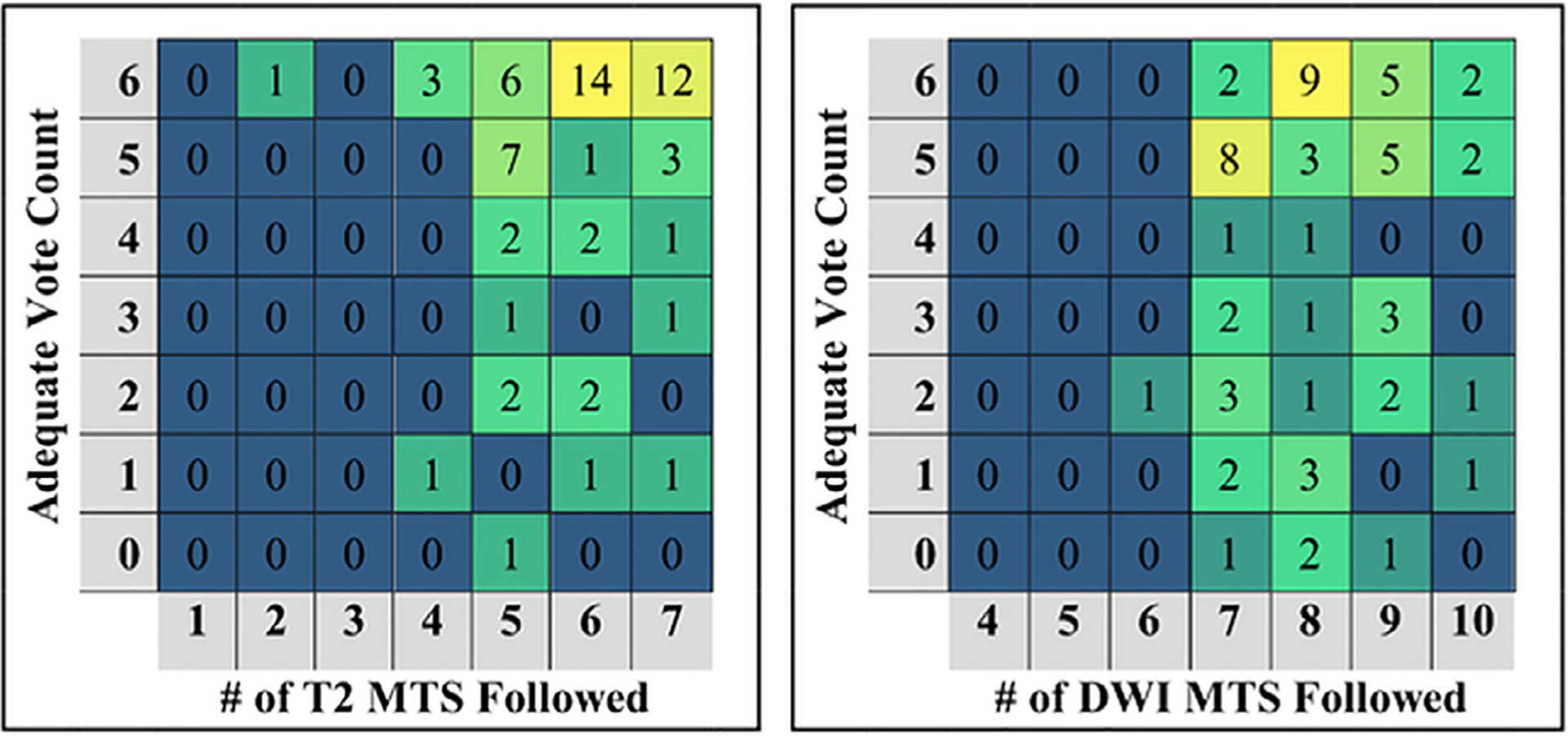
The distribution of T2 (left) and DWI (right) imaging studies organized by the number of adequate votes received and by the number of PIRADSv2 minimum technical standards (MTS) followed. The numbers within the grid indicate the number of studies following the number of MTS indicated (column) and received the number of votes indicated (row). DWI, diffusion weighted image; PIRADSv2, Prostate Imaging Reporting and Data System version 2.
TABLE 4.
Summary of Results of Statistical Analysis of PIRADSv2 Minimum Technical Standards Adherence Association with Image Adequacy Using Wilcoxon Rank Sum Test and with Image Quality and Perceived Artifacts Using Kendall Rank Correlation
| Perceived Artifacts | Visual Characteristics | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Statistic | Adequacy | Quality | Noise | Motion | Distortion | Aliasing | Blurriness | Contrast | ||
| Adherence | T2 | tau-b (SE) | - | 0.22 (0.08)† | 0.19 (0.07) | 0.1 (0.07) | 0.17 (0.06) | 0.14 (0.06) | 0.23 (0.09)† | 0.22 (0.08)† |
| p value | 0.227 | 0.010* | 0.012* | 0.133 | 0.005* | 0.017* | 0.008* | 0.009* | ||
| DWI | tau-b (SE) | 0.01 (0.08) | 0.06 (0.08) | 0.02 (0.05) | 0.04 (0.08) | 0.02 (0.06) | 0.04 (0.08) | 0.04 (0.09) | ||
| p value | 0.304 | 0.943 | 0.457 | 0.659 | 0.616 | 0.754 | 0.634 | 0.685 | ||
PIRADSv2, Prostate Imaging Reporting and Data System version 2.
Significant results with p-value ≤ 0.05.
Kendall Rank tau-b ≥ 0.2 indicates presence of weak correlation.
Adherence and Quality
There was a significant association between adherence to PI-RADSv2 technical standards and image quality for T2W (p value = 0.010) but not for DWI (p value = 0.943) indicating that T2W images were more likely to receive a higher quality score if they followed the PIRADSv2 MTS while DWI images were not. However, the strength of the correlation (as determined by the tau-b) between T2 image quality and adherence was only 0.22 (standard error (SE) 0.08), indicating a weak correlation (Fig 2, Table 4). Significant associations were also found between the perceived quality features and perceived artifact metrics of Noise, Distortion, Aliasing, Blurriness, and Contrast for T2 images (Table 4).
Figure 2.
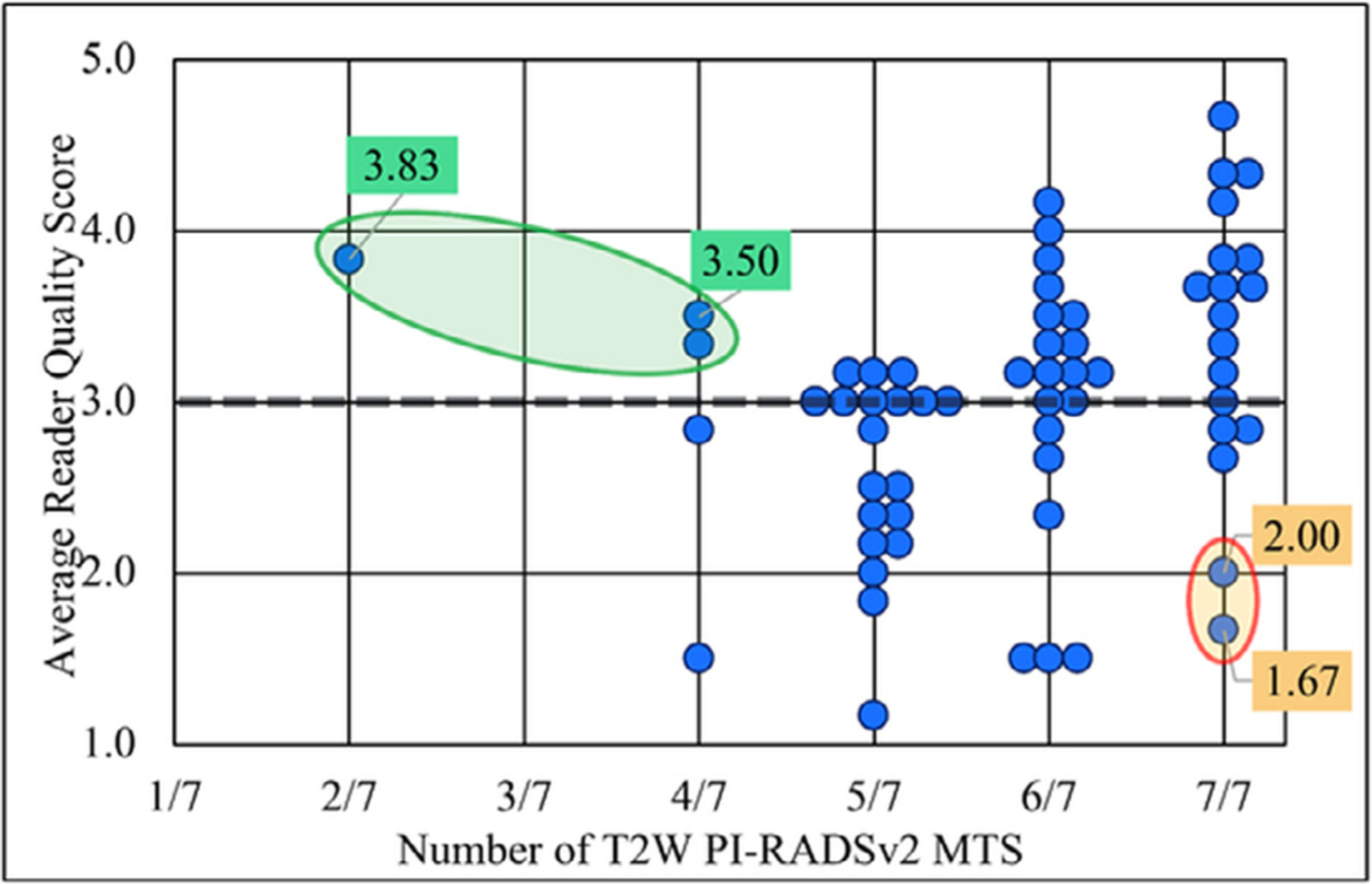
Relationship between adherence to T2W PI-RADSv2 minimum technical standards (MTS) and average reader quality score. Select outlier images have been given color coded labels containing their average reader quality score. Green labels: Non-compliant but high quality images. Orange labels: Compliant but low quality images. PIRADSv2, Prostate Imaging Reporting and Data System version 2; T2W, T2 weighted.
For DWI, not only was there no significant association between adherence to PI-RADSv2 technical standards and image quality, but the tau-b degree of correlation between the two measures was −0.01 (SE 0.08) signifying a near complete absence of correlation (Table 4). No significant associations were found for any of the DWI quality/artifact metrics and adherence (Fig 3, Table 4).
Figure 3.
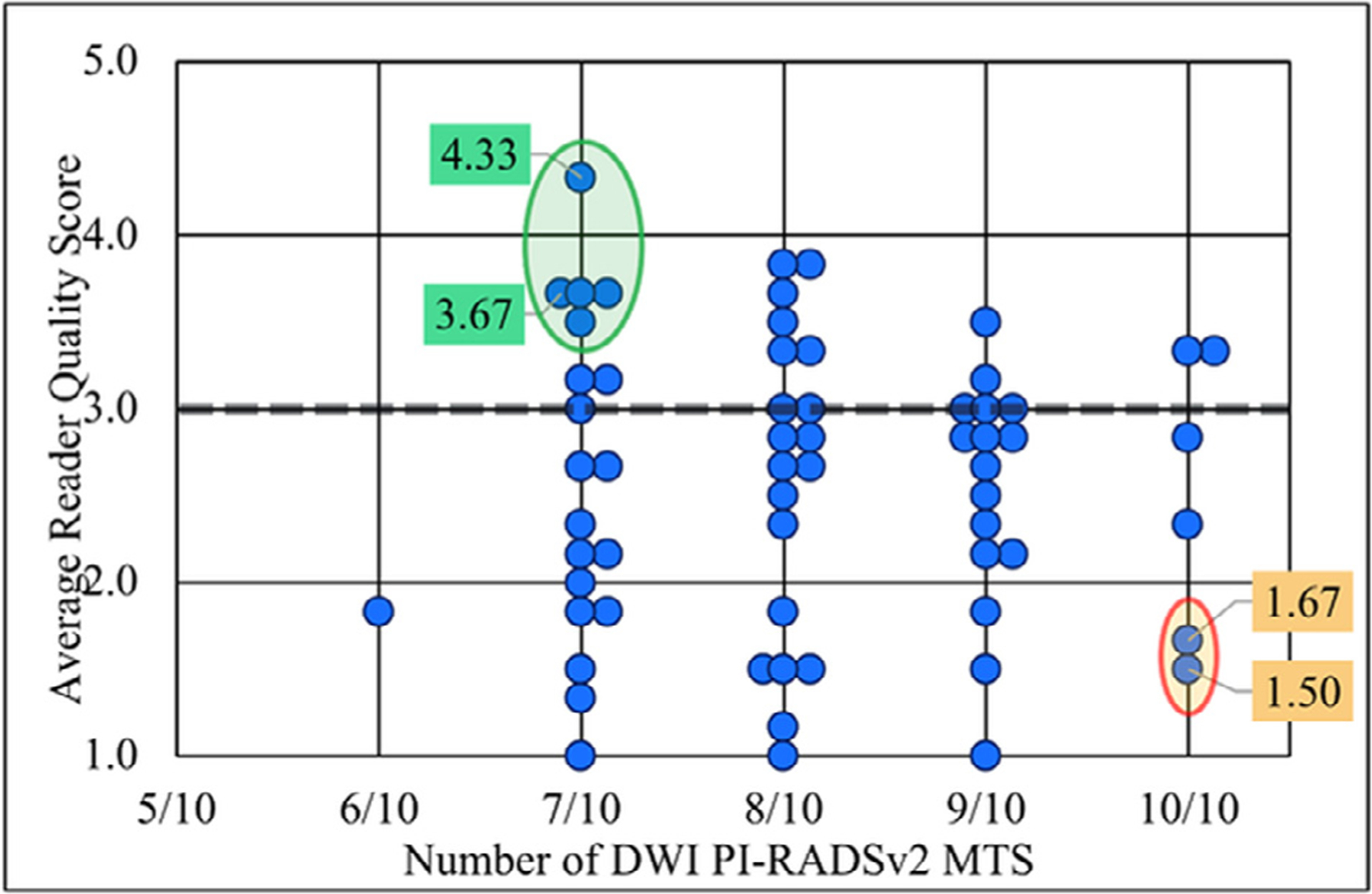
Relationship between adherence to DWI PI-RADSv2 minimum technical standards (MTS) and average reader quality score. Select outlier images have been given color coded labels containing their average reader quality score. Green labels: Non-compliant but high quality images. Orange labels: Compliant but low quality images. DWI, diffusion weighted image; PIRADSv2, Prostate Imaging Reporting and Data System version 2.
Performance of Individual PIRADSv2 Standards
There was no significant association between any specific PI-RADSv2 MTS and image adequacy or image quality (Table 5).
TABLE 5.
Summary of Association of Adherence to Individual PIRADSv2 Minimum Technical Standards and Phase Encoding Direction with Diagnostic Adequacy, Quality and Distortion in Prostate MRI as Measured by the Chi-Square and Wilcoxon Rank Sum Tests for Association
| T2W Technical Standards | Adequacy† | Quality†† | Distortion†† |
|---|---|---|---|
| p value | p value | p value | |
| Slice thickness | 0.436 | 0.276 | 0.310 |
| Slice gap | 0.416 | 0.123 | 0.366 |
| FOV upper limit | 0.596 | 0.258 | *0.003 |
| IPRP | 0.970 | 0.885 | 0.528 |
| IPRF | 0.996 | 0.184 | 0.256 |
| Phase encoding dir. | 0.069 | *0.032 | *0.004 |
| DWI technical standards | p value | p value | p value |
| High B value | 0.610 | 0.078 | 0.479 |
| TE | 0.123 | 0.628 | 0.866 |
| TR | 0.162 | 0.074 | *0.033 |
| Slice thickness | 0.982 | 0.643 | 0.924 |
| Slice gap | 0.930 | 0.347 | 0.473 |
| FOV lower limit | 0.777 | 0.741 | 0.982 |
| FOV upper limit | 0.864 | 0.354 | 0.446 |
| IPRP | 0.313 | 0.335 | 0.123 |
| IPRF | 0.313 | 0.335 | 0.123 |
| Phase encoding Dir. | 0.576 | 0.350 | 0.779 |
FOV LL, field-of-view lower limit; FOV UL, field-of-view upper limit; IPRF, in-plane resolution frequency; IPRP, in-plane resolution phase; PIRADSv2, Prostate Imaging Reporting and Data System version 2; TE, echo time; TR, repetition time.
significant findings with p value ≤ 0.5.
Chi-square test.
Wilcoxon Rank Sum test.
Individual PI-RADSv2 standards were also analyzed for their effect on image distortion. There was a significant association in T2W imaging with adherence to the FOV upper limit ≤200 mm standard (p value = 0.003). In DWI, adherence to the repetition time ≥ 3000 ms standard (p value = 0.033) was associated with less distorted images. There was no significant association between adherence to any other specific standard and distortion for either T2W or DWI (Table 5).
Analysis of the use of horizontal phase encoding direction found a significant association with T2W image quality (p value = 0.032) where images acquired with a horizontal phase encoding direction performed better than those with a vertical phase encoding direction. This same association also existed for distortion in T2W sequences (p value = 0.004) where images were less likely to be distorted if phase encoding direction was horizontal. However, this association did not exist for DWI (Table 5).
Perceived Artifact and Impact on Diagnostic Adequacy and Image Quality
All perceived artifacts (noise, motion, geometric distortion, and aliasing) had a significant association (p values ≤ 0.01) for both image adequacy and quality. For T2W images, the perceived artifact with the greatest effect on image quality was noise. The tau-b correlation coefficient for noise and image quality was 0.58 (SE 0.09). For DWI, both noise and distortion had particularly strong correlations with image quality with tau-b correlation coefficients of 0.61 (SE 0.09) and 0.54 (SE 0.09), respectively (Table 6).
TABLE 6.
Kendall Rank tau-b Correlation Statistics for Visual Characteristics with Perceived Artifacts and Frequency of Reader Scores <3 (Interpretation Difficult or Impossible) for Each Type of Artifact
| Perceived Artifact | ||||||
|---|---|---|---|---|---|---|
| tau-b (SE) | Noise | Motion | Distortion | Aliasing | ||
| Visual characteristics | T2 | Quality | 0.58 (0.09) | 0.38 (0.07) | 0.31 (0.07) | 0.30 (0.07) |
| Blurriness | 0.58 (0.09) | 0.34 (0.07) | 0.25 (0.07) | 0.24 (0.06) | ||
| Contrast | 0.57 (0.09) | 0.30 (0.07) | 0.26 (0.07) | 0.25 (0.06) | ||
| % Reader Scores < 3 | 19% | 13% | 6% | 8% | ||
| DWI | Quality | 0.61 (0.09) | 0.31 (0.06) | 0.54 (0.09) | 0.40 (0.07) | |
| Blurriness | 0.61 (0.09) | 0.29 (0.06) | 0.50 (0.08) | 0.34 (0.06) | ||
| Contrast | 0.60 (0.09) | 0.25 (0.05) | 0.46 (0.08) | 0.28 (0.06) | ||
| % Reader Scores < 3 | 30% | 10% | 27% | 26% | ||
The frequency with which readers scored perceived artifacts (noise, motion, geometric distortion, and aliasing) as making image interpretation difficult or impossible (reader score < 3) was also assessed for both T2 and DWI. For T2, the frequency at which readers gave scores <3 (interpretation difficult or impossible) was 19%, 13%, 6%, and 8% for noise, motion, geometric distortion, and aliasing, respectively. For DWI, 30%, 10%, 27%, and 26% of reader scores were <3 (interpretation difficult or impossible) for noise, motion, geometric distortion, and aliasing respectively (Fig 4).
Figure 4.
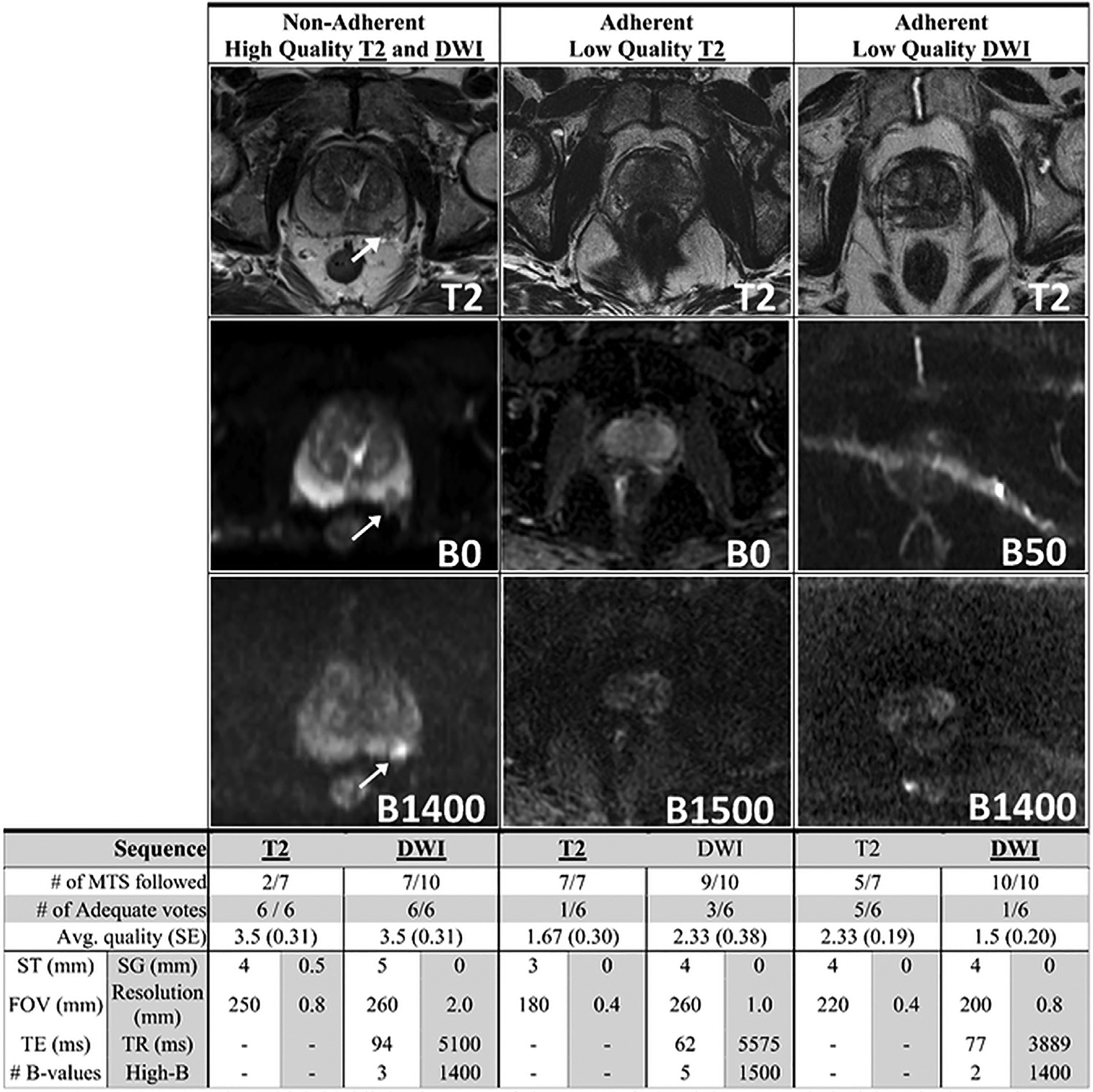
Examples which highlight the gaps of the PIRADSv2 minimum technical standards (MTS) all acquired at 3 Teslas. (Left Column) An example of an image that is non-adherent to the PIRADSv2 MTS yet still achieved a high-quality image with a clearly visible and easily defined lesion. (Middle Column) 100% adherent to the PIRADSv2 MTS for T2 sequences yet the T2 sequence appears noisy and the separation between the peripheral zone and transition zone cannot be easily appreciated. (Right Column) 100% adherent to the PIRADSv2 MTS for DWI sequences yet the low b-value sequence has significant artifacts and the prostate is difficult to visualize in the high b-value sequence. DWI, diffusion weighted image; PIRADSv2, Prostate Imaging Reporting and Data System version 2.
DISCUSSION
Performance of PIRADSv2 Minimum Technical Standards
This study shows a disheartening rate of inadequate exams among mpMRI studies of the prostate. Adherence to PI-RADSv2 MTS did not appear to play a role in improving image quality. Many centers found a way to produce diagnostic images while essentially overlooking the PI-RADSv2 standards while many who demonstrated strict adherence to the standards had poor results. While our study findings indicated that T2W imaging adherence to the PI-RADSv2 MTS did increase the likelihood of being scored as higher quality, the actual effect was very slight. Furthermore, adherence to the technical standards did not significantly increase the probability that an image would be seen as diagnostically adequate. For DWI, the results were even more striking with adherence to the PI-RADSv2 MTS having no significant correlation with either adequacy or quality scores.
The analysis of each PIRADSv2 MTS found no significant relationship with quality or adequacy for any current individual standard. This suggests a “recipe-like” standard may not be adequate, but rather a standard based on objective imaging characteristics may be needed. Despite this, we did find a significant improvement in quality and distortion for T2W sequences using horizontal phase encoding direction suggesting this feature should be included in future PIRADS versions.
Variable Quality and Adequacy of prostate imaging
As expected, many centers struggle to achieve adequate image quality for prostate MRI. This problem is particularly acute for DWI sequences with only 61% (as opposed to 83% for T2) scored as adequate by a majority of our study readers. Additionally, among those images which are adequate for use relatively few were considered by readers to be high quality. The wide variability in image quality indicates a need for quality assurance and protocols to standardize image quality in prostate mpMRI. We suggest greater involvement of MRI vendors and MRI physicists in the improvement of mpMRI. This has long been discussed in the prostate mpMRI community (4,17). However, the problem has never been clearly defined.
Adherence to PIRADSv2 Minimum Technical Standards
Several of the PI-RADS standards had particularly low adherence rates suggesting they may be difficult to implement on some scanners. In T2W sequences, the in-plane resolution frequency ≤ 0.4 mm had an adherence rate of only 52% and, in DWI, only 35% of images analyzed were adherent to upper limit for FOV ≤ 220 mm. This is the same pattern of adherence rates found in the previous study to assess rates of adherence to the PIRADSv2 Technical Standards by Esses et al. (12). Our results from 2018 only found improvements in adherence for resolution and slice thickness. There has been no improvement for the other standards since the 2015–2016 data collection by Esses et al.
Notably, there were T2W images that followed only a few of the standards and were still seen as adequate by all 6 readers and scored as high quality. The presence of nonadherent high-quality images indicates that the current T2 standards do not represent a true minimum standard. However, the problem of nonadherent high-quality outliers is more pronounced in the DWI dataset with many nonadherent images achieving high quality and very few adherent images achieving high quality.
Future Directions
Looking forward, it is apparent the general quality of prostate mpMRI should be improved to deliver better diagnosis in PCa care. Although providing formal recommendations for quality prostate MRI is beyond the scope of this paper, there are several observations that may be useful in improving mpMRI. Image noise was most frequently implicated in making interpretation difficult for both T2W and DWI sequences. Image noise is particularly important at high b-values in DWI. Noise is likely to become a greater factor if distortion corrections are employed as these methods typically come at a cost of decreased signal to noise ratio either intrinsically or from decreased signal averaging to accommodate additional scans. Techniques to reduce noise during postprocessing should also be considered (18).
Image distortion particularly for DWI was the second most frequently implicated perceived artifact. One of the most prominent sources of distortion in prostate imaging is the inhomogeneous B0 field in the abdominal area (19,20). Most biological tissues are slightly diamagnetic with the exception of gas filled cavities. If the rectum is filled with gas during a scan, the difference in effective B0 fields at the rectum/prostate interface creates a magnetic field gradient which shifts intensity from one location to another creating geometric distortion with signal loss near the interface due to spin dephasing (21). While recognized in the literature, corrections for susceptibility artifacts are not yet incorporated into the PIRADS standard.
Susceptibility artifact is important during DWI acquisition due to ubiquitous use of the echo planar imaging sequence which magnifies magnetic inhomogeneity (22). Susceptibility artifact can be reduced by eliminating rectal gas by bowel preparation, (23) or by the administration of a spasmolytic agent (e.g. buscopan) (24). During acquisition, we have shown that the effect of the susceptibility artifact can be mitigated by altering the phase encoding direction from vertical (anterior-posterior) to horizontal (left-right). Although not seen in our cohort, flipping the phase encoding direction can theoretically move the distortion to the lateral axis and away from the prostate. Limiting spin evolution during readout by reducing echo times (19), restricting the FOV (25), or by parallel imaging has the potential to diminish susceptibility effects. Using B0 field maps to adjust the signal phase in postprocessing or combining scans of opposite phase encoding polarity to cancel improper phase evolution have also been shown to be effective in minimizing distortion artifacts (26). These and other candidate strategies need further exploration and investigation in larger studies.
Limitations
This study has several limitations. First, we evaluated the impact of adherence to each standard in a binary fashion (compliant or noncompliant) on adequacy and quality; however, we did not evaluate the effect of the actual values of image acquisition settings on quality. Second, we approached the quality evaluation for image acquisition parameters in a binary fashion using strict adherence criteria of MTS in the PI-RADS documents instead of quantifying the degree of difference from the MTS defined in PI-RADS. We agree that this may underestimate the importance of PI-RADS MTS. However, there is an inherent value judgment regarding how closely an MTS standard was followed and to avoid this subjectivity we opted for a more absolute criterion of compliance. Third, readers evaluated the adequacy of images for use in diagnosis, but we did not assess the actual effect on diagnostic accuracy. To do both of the previously mentioned would require a vast sample size and a prospective design to avoid ascertainment bias. Moreover, we did not evaluate the quality of apparent diffusion coefficient (ADC) maps directly. Since PI-RADSv2 has not specified standard methodology for the postprocessing calculation of ADC maps, we decided to evaluate only image sequences that could be acquired without postprocessing. Additionally, we did not get a chance to evaluate the experience level of the technologists who conducted the image acquisition since it was not possible to reach out all the 62 different centers. Finally, reader agreement on image quality evaluated by Fleiss’ kappa score was only slight or fair indicating a lack of consensus on image quality, highlighting a need for more objective quality control.
CONCLUSION
In conclusion, the results indicate image quality in prostate mpMRI is highly variable. Adherence to PI-RADSv2 MTS has a minimal effect in reducing this variability for T2 imaging sequences and no effect for DWI sequence. Noise and distortion are the most obvious sources of problems in most prostate mpMRIs. These results suggest that simply prescribing a set of sequence specifications is unlikely to solve the problem of image quality. Image quality also depends on patient factors such as the presence of rectal gas, total-hip replacement, amount of body fat, postbiopsy hemorrhage, and abstinence from ejaculation (27–30). It may be difficult to devise new purely technical standards to account for these factors. However, there are other quality assurance methods that can be devised such as standards for patient factors or perhaps methods which use machine learning techniques such as texture analysis or convolutional neural networks (31,32).
Image quality remains a significant barrier to improving prostate mpMRI. Meanwhile, scan settings should be individually tailored to each scanner and institution with PI-RADS MTS used as a possible starting point for optimization and not boundaries for optimization. There is an urgent need for new quality assurance tools, involvement of MRI manufacturers, the MR physics community and individual efforts on the part of radiologists to improve mpMRI.
ACKNOWLEDGMENTS
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
GRANT SUPPORT
This research was made possible through the NIH Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and contributions to the Foundation for the NIH from the Doris Duke Charitable Foundation (DDCF Grant #2014194), the American Association for Dental Research, the Colgate-Palmolive Company, Genentech, Elsevier, and other private donors.
Abbreviations:
- PCa
Prostate Cancer
- CSPCa
Clinically significant prostate cancer
- TRUS
Transrectal ultrasound
- mpMRI
multi-parametric MRI
- PI-RADS
Prostate Imaging Reporting and Data System
- MTS
Minimum Technical Standards
- FOV
field-of-view
- IPRP
in-plane resolution phase
- IPRF
in-plane resolution frequency
- ST
Slice Thickness
- SG
Slice Gap
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019; 69:7–34. [DOI] [PubMed] [Google Scholar]
- 2.Shen X, Kumar P. Trade-off between treatment of early prostate cancer and incidence of advanced prostate cancer in the prostate screening era. J Urol 2016; 195:1397–1402. [DOI] [PubMed] [Google Scholar]
- 3.Weinreb JC, Barentsz JO, Choyke PL, et al. PI-RADS prostate imaging—reporting and data system: 2015, version 2. Eur Urol 2016; 69:16–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Padhani AR, Weinreb J, Rosenkrantz AB, et al. Prostate imaging-reporting and data system steering committee: PI-RADS v2 status update and future directions. Eur Urol 2019; 75:385–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu C, Liu S-L, Wang Z-X, et al. Using the prostate imaging reporting and data system version 2 (PI-RIDS v2) to detect prostate cancer can prevent unnecessary biopsies and invasive treatment. Asian J Androl 2018; 20:459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Leest M, Cornel E, Israël B, et al. Head-to-head comparison of transrectal ultrasound-guided prostate biopsy versus multiparametric prostate resonance imaging with subsequent magnetic resonance-guided biopsy in biopsy-naïve men with elevated prostate-specific antigen: a large prospective multicenter clinical study. Eur Urol 2019; 75:570–578. [DOI] [PubMed] [Google Scholar]
- 7.Mehralivand S, Bednarova S, Shih JH, et al. Prospective evaluation of PI-RADS version 2 using the International Society of Urological Pathology Prostate Cancer Grade Group System. J Urol 2017; 198:583–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim SH, Choi MS, Kim MJ, et al. Validation of prostate imaging reporting and data system version 2 using an MRI-ultrasound fusion biopsy in prostate cancer diagnosis. Am J Roentgenol 2017; 209:800–805. [DOI] [PubMed] [Google Scholar]
- 9.Ahmed HU, El-Shater Bosaily A, Brown LC, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 2017; 389:815–822. [DOI] [PubMed] [Google Scholar]
- 10.Kasivisvanathan V, Rannikko AS, Borghi M, et al. MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med 2018; 378:1767–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rouvière O, Puech P, Renard-Penna R, et al. Use of prostate systematic and targeted biopsy on the basis of multiparametric MRI in biopsy-naive patients (MRI-FIRST): a prospective, multicentre, paired diagnostic study. Lancet Oncol 2019; 20:100–109. [DOI] [PubMed] [Google Scholar]
- 12.Esses SJ, Taneja SS, Rosenkrantz AB. Imaging facilities’ adherence to PI-RADS v2 minimum technical standards for the performance of prostate MRI. Acad Radiol 2018; 25:188–195. [DOI] [PubMed] [Google Scholar]
- 13.Ullrich T, Quentin M, Oelers C, et al. Magnetic resonance imaging of the prostate at 1.5 versus 3.0 T: a prospective comparison study of image quality. Eur J Radiol 2017; 90:192–197. [DOI] [PubMed] [Google Scholar]
- 14.Loening AM, Litwiller DV, Saranathan M, et al. Increased speed and image quality for pelvic single-shot fast spin-echo imaging with variable refocusing flip angles and full-fourier acquisition. Radiology 2017; 282:561–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosner B, Glynn RJ, Ting Lee M. Incorporation of clustering effects for the Wilcoxon Rank Sum test: a large–sample approach. Biometrics 2003; 59:1089–1098. [DOI] [PubMed] [Google Scholar]
- 16.Shih JH, Fay MP. Pearson’s chi-square test and rank correlation inferences for clustered data. Biometrics 2017; 73:822–834. [DOI] [PubMed] [Google Scholar]
- 17.Rosenkrantz AB, Oto A, Turkbey B, et al. Prostate imaging reporting and data system (PI-RADS), version 2: a critical look. AJR Am J Roentgenol 2016; 206:1179–1183. [DOI] [PubMed] [Google Scholar]
- 18.Zhang X, Peng J, Xu M, et al. Denoise diffusion-weighted images using higher-order singular value decomposition. Neuroimage 2017; 156:128–145. [DOI] [PubMed] [Google Scholar]
- 19.Nketiah G, Selnæs KM, Sandsmark E, et al. Geometric distortion correction in prostate diffusion-weighted MRI and its effect on quantitative apparent diffusion coefficient analysis. Magn Reson Med 2018; 79:2524–2532. [DOI] [PubMed] [Google Scholar]
- 20.Rakow-Penner RA, White NS, Margolis DJA, et al. Prostate diffusion imaging with distortion correction. Magn Reson Imaging 2015; 33:1178–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Port JD, Pomper MG. Quantification and minimization of magnetic susceptibility artifacts on GRE images. J Comput Assist Tomogr 2000; 24:958–964. [DOI] [PubMed] [Google Scholar]
- 22.Advanced MR Neuroimaging: From Theory to Clinical Practice. In: Subjects - Bioscience, Engineering & Technology, Physical Sciences Chapter, SPIE; 2017. doi: 10.1201/9781351216548. eBook ISBN - 9781351216548 https://www.taylorfrancis.com/books/9781351216548. [DOI] [Google Scholar]
- 23.Caglic I, Hansen NL, Slough RA, et al. Evaluating the effect of rectal distension on prostate multiparametric MRI image quality. Eur J Radiol 2017; 90:174–180. [DOI] [PubMed] [Google Scholar]
- 24.Ullrich T, Quentin M, Schmaltz AK, et al. Hyoscine butylbromide significantly decreases motion artefacts and allows better delineation of anatomic structures in mp-MRI of the prostate. Eur Radiol 2018; 28:17–23. [DOI] [PubMed] [Google Scholar]
- 25.Korn N, Kurhanewicz J, Banerjee S, et al. Reduced-FOV excitation decreases susceptibility artifact in diffusion-weighted MRI with endorectal coil for prostate cancer detection. Magn Reson Imaging 2015; 33: 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hong X, To XV, Teh I, et al. Evaluation of EPI distortion correction methods for quantitative MRI of the brain at high magnetic field. Magn Reson Imaging 2015; 33:1098–1105. [DOI] [PubMed] [Google Scholar]
- 27.Sharif-Afshar A-R, Feng T, Koopman S, et al. Impact of post prostate biopsy hemorrhage on multiparametric magnetic resonance imaging. Can J Urol 2015; 22:7698–7702. [PubMed] [Google Scholar]
- 28.Medved M, Sammet S, Yousuf A, et al. MR imaging of the prostate and adjacent anatomic structures before, during, and after ejaculation: qualitative and quantitative evaluation. Radiology 2014; 271:452–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenkrantz AB, Kopec M, Kong X, et al. Prostate cancer vs. postbiopsy hemorrhage: diagnosis with T2- and diffusion-weighted imaging. J Magn Reson Imaging 2010; 31:1387–1394. [DOI] [PubMed] [Google Scholar]
- 30.Kabakus IM, Borofsky S, Mertan FV, et al. Does abstinence from ejaculation before prostate MRI improve evaluation of the seminal vesicles. Am J Roentgenol 2016; 207:1205–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Esses SJ, Lu X, Zhao T, et al. Automated image quality evaluation of T 2-weighted liver MRI utilizing deep learning architecture. J Magn Reson Imaging 2018; 47:723–728. [DOI] [PubMed] [Google Scholar]
- 32.Küstner T, Gatidis S, Liebgott A, et al. A machine-learning framework for automatic reference-free quality assessment in MRI. Magn Reson Imaging 2018; 53:134–147. [DOI] [PubMed] [Google Scholar]


