Pericytes, the key components of the neurovascular unit (NVU), are responsible for the formation, regulation and maintenance of the blood‐brain barrier (BBB). Cerebral capillaries are uniquely enriched in pericytes and degeneration of these cells have major contributions to the brain pathophysiology of several neurological diseases, including stroke. Despite their pivotal role in the brain architecture, it remained unclear whether transplantation of pericytes may reconstitute the BBB and ultimately contribute to recovery after stroke. The major limitations have been the heterogeneous nature of pericytes and their scarcity as a cell source.
In a recent study, Sun and colleagues 1 generated pericyte‐like cells from human‐induced pluripotent stem cells (iPSCs). They recapitulated the developmental differentiation of pericytes from cranial neural crest cells (CNC) and optimized previously existing protocols. This shortened differentiation time and the use of stable and chemically defined protocols is an important prerequisite for the scalability and good manufacturing practice (GMP) requirements to advance from in vitro to preclinical and clinical in vivo cell‐based studies. Since pericytes are contractile cells surrounding the blood vessels and regulate blood circulation upon neuronal activity, Sun and colleagues determined the contractile properties of the iPSC‐derived pericytes by in vitro experiments. They confirmed the expression of major contractile proteins (eg vimentin, tropomyosin and myosin) and the pericyte's ability to compress a matrigel in a contraction assay similar to primary human brain vascular pericytes and aortic smooth muscle cells, which were used as a positive control. Next, the generated pericytes were transplanted through intravenous injections in a mouse model of transient middle cerebral artery occlusion (tMCAo) 6 hours after stroke induction. The transplants grafted at the site of injury, reduced brain damage and restored functional deficits at 3‐ and 7‐day post‐injury. Histological analysis of the brain tissue revealed that transplanted pericytes were associated with reduced brain swelling, reconstruction of major protein components of the BBB and reduced neuronal apoptosis. Overall, this study provides the first evidence that transplanted iPSC‐derived pericytes may significantly contribute to BBB reconstruction and promote functional recovery.
Since most current cell therapy strategies focus on mesenchymal stem cells or iPSC‐derived neuronal cells, these findings expand the spectrum of potentially suitable cell sources in stroke. Major uncertainties in the field still remain in preclinical and clinical cell therapies about the route of administration. Although the authors report a relatively low efficiency of engraftment following systemic transplantation (only 30% of endothelial cells were surrounded by the transplanted pericytes at the injury site at 7 days post‐injury), these cell numbers were sufficient to restore major components of the damaged BBB. From a therapeutic perspective, on the other hand, delayed transplantation of pericytes might be favourable to determine the translational viability of these preclinical findings. Since acute transplantation of pericytes will require an allogeneic transplantation from a healthy donor, important aspects need further investigation concerning the immunogenicity of pericytes. Fortunately, current advancements in genetic engineering towards universal and hypoimmunogenic iPSC may overcome this limitation in the near future.2 Moreover, it remains unclear whether the observed beneficial effects after stroke are permanent due to the relatively short‐time course. Many regenerative processes, for example, neo‐angiogenesis have been reported to have only transient effects following stroke and be less relevant for long‐term recovery.3 Since the functional tests have been performed at relatively early time points, these findings most likely rather indicate protective than regenerative effects of pericytes; and their long‐term benefit remains to be elucidated. Recent studies have also described a dual role of pericytes in stroke recovery; similar as previously observed for astrocytes and microglia.4, 5 Apart from their beneficial role to maintain BBB integrity, subclasses of pericytes can contribute to scar formation and inhibit axonal regeneration in major brain injuries including stroke.6, 7 A determinantal role of pericytes has also been observed acutely after ischaemia. Pericytes remained contracted causing capillary constriction and reduced blood flow to the injured brain.8 Future research might dissect the heterogeneous nature of the transplanted iPSC‐derived pericyte‐like cells and their contribution to stroke recovery.
Apart from stroke, these findings are also highly relevant to other major neurological disorders with a vascular component such as Alzheimer's disease (AD), vascular dementia (VD), spinal cord injury (SCI) or traumatic brain injury (TBI).9 It has become apparent that pericyte loss is an important contributor of vascular dysfunction and leads to worsening of disease pathophysiology and neurodegeneration in AD patients.10, 11 Recent findings further highlighted that degeneration of pericytes has a direct influence on the cognition, independent of the main pathological hallmarks of the AD.12 Still lacking a disease‐modifying therapy, the AD field requires better characterization of the disease with the advancements in diagnostics and innovative solutions to tackle the complex underlying pathology. The findings by Sun and colleagues encourage future research to investigate the therapeutic effects of pericyte‐based cell therapy in mouse models of AD, which may become especially powerful and clinically viable with the current advancements of CSF and blood biomarkers for BBB damage.12 However, it is important to note that translating the results from an acute vascular dysfunction model such as stroke to a chronic impairment as in the case of AD would require further optimization especially for the delivery route, the cell number and time point of transplantation (Figure 1).
FIGURE 1.
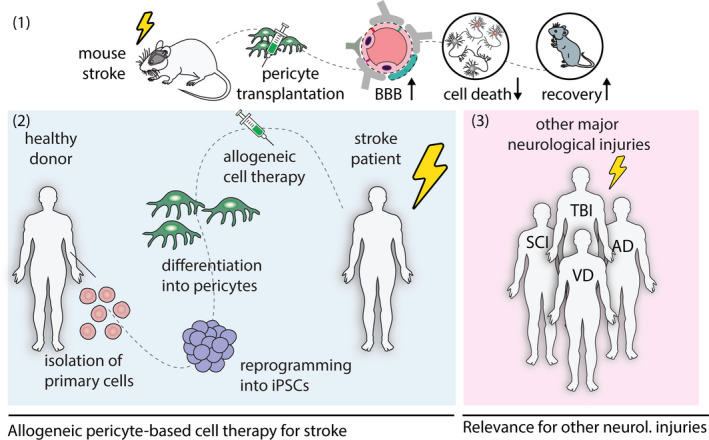
Pericyte‐based cell therapy following brain injury. (1) Transplantation of iPSC‐derived pericyte‐like cells restores blood‐brain barrier (BBB), reduces neuronal cell death and improves recovery in a mouse model of stroke. (2) A potential translational approach will consist of the generation of pericyte‐like cells from a donor's reprogrammed primary blood cells. These cells will be injected systemically to stroke patients that exhibit severe damage of the BBB. (3) Patients with major neurological disease include acute injuries, for example spinal cord injury, traumatic brain injury or a chronic injury, for example Alzheimer's disease and vascular dementia. These patients may also benefit from pericyte‐based cell therapy since all of these injuries also exhibit BBB disruption and pericyte degeneration as an important hallmark
Despite the need for further optimization and future confirmatory studies, regeneration of the neurovascular unit with cell‐based therapies has shown to have a great potential to restore neuronal integrity and functionality. The promising results from the stroke model illustrate that the efficacy of the vascular repair could be tested with further preclinical studies in other neurological disorders. Overall, therapeutic strategies aiming at restoring cerebrovascular health could pave a new way forward to help millions of patients suffering from neurological diseases.
CONFLICT OF INTEREST
The authors declare no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Kirabali T, Rust R. iPS‐derived pericytes for neurovascular regeneration. Eur J Clin Invest. 2021;51:e13601. 10.1111/eci.13601
REFERENCES
- 1.Sun J, Huang Y, Gong J, et al. Transplantation of hPSC‐derived pericyte‐like cells promotes functional recovery in ischemic stroke mice. Nat Commun. 2020;11:5196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deuse T, Hu X, Gravina A, et al. Hypoimmunogenic derivatives of induced pluripotent stem cells evade immune rejection in fully immunocompetent allogeneic recipients. Nat Biotechnol. 2019;37:252‐258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu SW, Friedman B, Cheng Q, Lyden PD. Stroke‐evoked angiogenesis results in a transient population of microvessels. J Cereb Blood Flow Metab. 2007;27:755‐763. [DOI] [PubMed] [Google Scholar]
- 4.Haruwaka K, Ikegami A, Tachibana Y, et al. Dual microglia effects on blood brain barrier permeability induced by systemic inflammation. Nat Commun. 2019;10:5816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson MA, Burda JE, Ren Y, et al. Astrocyte scar formation aids central nervous system axon regeneration. Nature. 2016;532:195‐200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dias DO, Kim H, Holl D, et al. Reducing pericyte‐derived scarring promotes recovery after spinal cord injury. Cell. 2018;173:153‐165.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dias DO, Kalkitsas J, Kelahmetoglu Y, et al. Pericyte‐derived fibrotic scarring is conserved across diverse central nervous system lesions. bioRxiv. 2020. 10.1101/2020.04.30.068965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yemisci M, Gursoy‐Ozdemir Y, Vural A, Can A, Topalkara K, Dalkara T. Pericyte contraction induced by oxidative‐nitrative stress impairs capillary reflow despite successful opening of an occluded cerebral artery. Nat Med. 2009;15:1031‐1037. [DOI] [PubMed] [Google Scholar]
- 9.Sweeney MD, Zhao Z, Montagne A, Nelson AR, Zlokovic BV. Blood‐brain barrier: from physiology to disease and back. Physiol Rev. 2019;99:21‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montagne A, Nikolakopoulou AM, Zhao Z, et al. Pericyte degeneration causes white matter dysfunction in the mouse central nervous system. Nat Med. 2018;24:326‐337. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Nikolakopoulou AM, Montagne A, Kisler K, et al. Pericyte loss leads to circulatory failure and pleiotrophin depletion causing neuron loss. Nat Neurosci. 2019;22:1089‐1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nation DA, Sweeney MD, Montagne A, et al. Blood–brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat Med. 2019;25:270. [DOI] [PMC free article] [PubMed] [Google Scholar]


