Abstract
Background
This study aimed to evaluate the differences in outcome arising from the use of semi‐compliant (SCB) versus non‐compliant balloon (NCB) systems for predilatation during self‐expanding transcatheter aortic valve replacement (TAVR).
Methods
251 TAVR procedures with the implantation of self‐expanding valves after predilatation were analyzed. SCB systems were used in 166 and NCB systems in 85 patients. The primary endpoint was defined as device success, a composite endpoint comprising the absence of procedural mortality, correct valve positioning, adequate valve performance and the absence of more than a mild paravalvular leak. The secondary endpoints were chosen in accordance with the valve academic research consortium (VARC‐2) endpoint definitions.
Results
No significant differences were observed with regard to procedural device success between the SCB‐ and NCB cohort (SCB: 142 [85.5%%] vs. NCB: 77 [90.6%]; P = .257). There was a notable difference between the rates of conversion to open surgery and the postdilatation rate, both of which were higher for the NCB group (SCB: 1 [0.6%] vs. NCB: 4 [5.1%]; P = .042; SCB: 30 [18.1%] vs. NCB: 34 [40%]; P < .001). In a multivariate logistic regression analysis, the use of semi‐compliant balloon systems for predilatation was associated with a lower risk for postdilatation (OR: 0.296; 95% CI: 0.149‐0.588) and conversion to open surgery (OR: 0.205; 95% CI: 0.085‐0.493; P = .001) but not for device success.
Conclusion
While the balloon compliance did not affect the procedural mortality, device success or the rate of paravalvular leakage, the use of semi‐compliant balloons for predilatation during TAVR should be investigated in larger randomized trials in the light of the lower rates of postdilatation and conversion to open surgery compared to their non‐compliant counterparts.
Keywords: balloon, compliant, non‐compliant, predilatation, self‐expanding, TAVI, TAVR, transcatheter, valve
1. INTRODUCTION
The purpose of using balloon aortic valvuloplasty (BAV) during transcatheter aortic valve replacement (TAVR) is to ensure optimal positioning and expansion of the transcatheter heart valve (THV), leading to a reduction in possible complications and paravalvular leakage (PVL). Various balloons systems have been in use for predilatation until now.
The compliance of a balloon device is defined by the diameter increase that results from a predetermined increase in inflation pressure.1 Semi‐compliant balloon (SCB) systems respond to an increase in inflation pressure by initially increasing in diameter in areas that lie more proximally or distally to the area of the highest resistance, thus taking on a characteristic dumbbell shape during the gradual pressure increase. Non‐compliant balloon systems (NCB), on the other hand, expand uniformly over their longitudinal axis and generally cannot be expanded past a predetermined maximum diameter. Therefore, NCB systems offer the advantage of deforming in a more predictable and stable manner during inflation than their semi‐compliant competitors (Figure 1).2, 3 The radial force exhibited by NCBs in the stenotic target area is higher than the one exhibited by SCB systems, a finding reported within the context of percutaneous coronary interventions.4 It is therefore believed that SCB systems are only partially able to overcome the often strong lesion resistance.5 Another concern with SCB systems is that their overexpansion in areas that lie proximally and distally to the actual stenotic target site could produce a higher degree of intimal injury or potential disruption in healthy surrounding areas, a hypothesis investigated by several studies that has ultimately not been proven definitively.5, 6, 7, 8 A similar analysis regarding the impact of balloon compliance during predilatation on the outcome of patients undergoing TAVR has not yet been undertaken, and the available data on the development and change of vessel stress gradients during SCB use is scarce. Other significant outcome and safety concerns that need to be investigated in greater detail include the potential of insufficient THV expansion due to incomplete valve dilatation due to the lower radial force exerted by SCBs during predilatation.9, 10 Moreover, higher degrees of radial force or overexpansion in the neighbouring non‐target areas might consequently increase the risk of conduction disturbances or even aortic rupture.11
FIGURE 1.
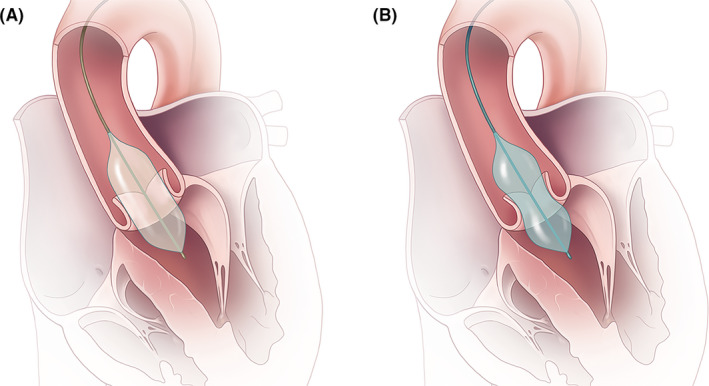
The different expansion properties of non‐compliant and semi‐compliant balloon systems. A, Non‐compliant balloons expand uniformly over their longitudinal axis and can generally not be expanded past their nominal diameter. B, Semi‐compliant balloons initially increase in areas proximally and distally to the highest resistance area, thus taking on a characteristic dumbbell shape during the gradual pressure increase. Only at the late phase of expansion and with markedly increased inflation pressures a cylindrical shape is achieved
In conclusion, the compliance of BAV devices defines the products' behaviour during its application, with potential implications for procedural results and postprocedural outcomes in patients undergoing TAVR. However, these differences have not been sufficiently explored. Our study aimed to evaluate the impact of BAV device compliance on adverse events and short‐term outcomes in patients undergoing self‐expanding TAVR.
2. METHODS
2.1. Patient selection
Between June 2009 and December 2016, 532 patients with severe aortic stenosis underwent TAVR at the Department of Cardiovascular Surgery and Cardiology at the Heart Center Hietzing (Vienna, Austria) and were enrolled in the VIenna CardioThOracic Aortic Valve RegistrY (VICTORY). To minimize a potential selection bias, enhance the cohorts' comparability and limit the influence of different THV device‐related outcome parameters, only patients who received a self‐expandable transcatheter heart valve via the transfemoral access site and underwent predilatation (n = 251) were selected for analysis. 166 of the 251 patients were treated with SCB systems, whereas 85 patients underwent predilatation with NCBs. Figure 2 displays a flow chart depicting the overall TAVR cohort, inclusion and exclusion of patients, and the respective treatment cohorts' assignment.
FIGURE 2.
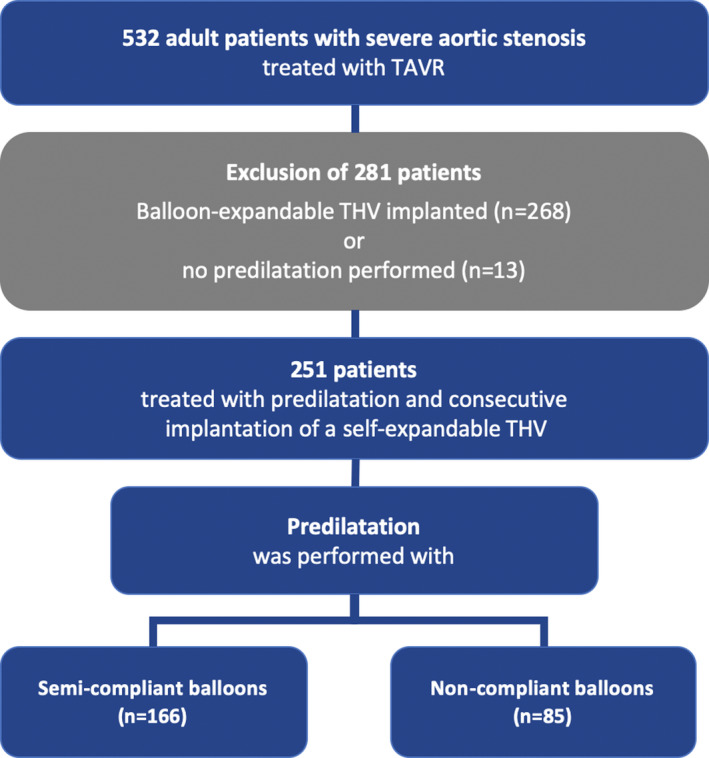
532 patients with severe aortic stenosis underwent TAVR and were enrolled in the VIenna CardioThOracic Aortic Valve RegistrY (VICTORY). To ensure adequate comparability of the cohorts and limit different THV devices' influence on outcome parameters, only patients who received a self‐expandable transcatheter heart valve via the transfemoral access site were selected for analysis. Out of 251 patients, 166 were treated with semi‐compliant balloon systems for predilatation, whereas 85 patients underwent predilatation with non‐compliant balloons
The native aortic valve annulus dimensions were assessed via multi‐slice computed tomography to determine the compatible size and type of transcatheter valve. The predilatation of the native aortic valve was conducted under rapid pacing; the self‐expandable valve implantation was performed in a standard fashion by the institution's heart team and has been described before.
2.2. Materials
The choice of the respective predilatation balloon used during TAVR was at the operator's discretion but followed a few basic, institutional principles. Depending on the extent and the distribution of the aortic valve calcification, the chosen balloon size was either matched to the minimal diameter of the aortic annulus in case of a low calcific burden or had a diameter 2mm shorter than the minimum diameter in case of severe asymmetric calcification, to minimize the risk of aortic root rupture. TRUE Dilatation devices (Bard Peripheral Vascular Inc, Arizona, USA) were used as the representative device for the NCB cohort. The semi‐compliant expansion devices included: NuCLEUS‐X, Z‐MED and‐MED II (Braun Interventional Systems Inc, Bethlehem, PA), VACS II and VACS III (Osypka, Rheinfelden, Baden, Germany). NuCLEUS‐X, Z‐Med and Z‐MED II can be overexpanded by up to 10 per cent of the nominal diameter until the rated burst pressure (RBP) is reached. In addition, the NuCLEUS‐X balloon incorporates a radiopaque labelled waist at its midportion, which inflates only up to 90% of the balloon's nominal diameter, thus resulting in a higher degree of overall balloon compliance. Different ratios were calculated retrospectively to assess the relative over‐ or undersizing of balloon dilatation systems using their nominal dimensions in a fully inflated state and included:
2.3. Study endpoints and clinical outcomes
Clinical outcomes were defined according to the Valve Academic Research Consortium (VARC)‐2 criteria.12 The mortality analysis was conducted using electronic hospital records and was supplemented by a query to the federal institute for statistics – Statistics Austria. The primary endpoint of the analysis is specified as device success, a composite endpoint as suggested by the VARC‐2, composed of the following components: the absence of procedural mortality, correct positioning of a single THV device in proper anatomical position, and an intended valve performance with threshold values for the mean gradient of <20 mmHg or a peak velocity of <3 m/s, and no moderate or severe paravalvular leak (PVL). The secondary endpoints were adverse events as defined by the VARC‐2. The study was approved by the Ethics Committee of the City of Vienna (EK18‐028‐VK); informed consent was waived due to the analysis's retrospective nature.
The analytic methods, materials and data that support the findings of this study are available from the corresponding author on reasonable request.
2.4. Statistical analysis
Continuous variables were expressed as the median and interquartile range (IQR) or the mean and standard deviation (±SD) and compared using the Mann‐Whitney U test or the independent‐samples t test, respectively. Categorical data were expressed as absolute numbers and percentages and compared using the chi‐square test or Fisher's exact test. The calculated balloon‐specific indices and ratios are listed in Table 2.
TABLE 2.
Procedural characteristics of patients treated with TAVR using self‐expanding transcatheter heart valves
| Semi‐compliant balloon systems (n = 166) | Non‐compliant balloon systems (n = 85) | P value | |
|---|---|---|---|
| Procedural characteristics | |||
| Prothesis, n (%) | .021 | ||
| Symetis Accurate | 26 (15.7) | 27 (31.8) | |
| Core Valve | 102 (61.4) | 46 (54.1) | |
| Core Valve Evolut R | 38 (22.9) | 12 (14.1) | |
| Prothesis size (mm), median (IQR) | 26 (3) | 29 (3) | <.001 |
| Balloon size (mm), median (IQR) | 20 (2) | 22 (4) | .140 |
| Balloon cover index, median (IQR) | ‐0.2 (0.3) | ‐0.2 (0.3) | .192 |
| Balloon sealing index, median (IQR) | ‐0.1 (0.1) | ‐0.1 (0.1) | .217 |
| Annular overexpansion index, median (IQR) | 0.1 (0.2) | 0.1 (0.2) | .559 |
| Skin to skin time (min), median (IQR) | 85 (39) | 84 (30) | .939 |
| Fluoro time (min), median (IQR) | 16.3 (9.4) | 17.1 (10.4) | .475 |
| Contrast (cc), median (IQR) | 226 (110) | 235 (114.3) | .853 |
| Radiation (cGy), median (IQR) | 7335 (8972) | 9969 (11 786) | .037 |
| Postinterventional Characteristics | |||
| Mean gradient (mmHg), median (IQR) | 10 (9) | 9 (6) | .057 |
| Maximum gradient (mmHg), median (IQR) | 18 (15) | 14 (8) | .058 |
| Peak velocity (m/s), median (IQR) | 2 (1) | 1.9 (0) | .039 |
| Total hours ventilated, median (IQR) | 1 (7) | 0 (6) | .691 |
| Total hours in ICU, median (IQR) | 48 (75) | 48 (76) | .885 |
| Maximum serum creatinine in 72 h (mg/dL), median (IQR) | 0.9 (0.7) | 1.1 (0.6) | .840 |
| Red blood cell units total, mean (SD) | 0.5 (1.2) | 1.0 (2.5) | .375 |
| Days to discharge, median (IQR) | 11 (7) | 10 (8) | .830 |
| Length of stay after TAVR, median (IQR) | 11 (7) | 10 (8) | .830 |
Abbreviations: ICU, intensive care unit; IQR, interquartile range; NCB, Noncompliant balloon devices; post‐op, postoperative; SCB, semi‐compliant balloon devices; TA, transapical; TF, transfemoral; further abbreviations as listed in Table 1.
Risk factors for the composite primary endpoint, as well as the secondary endpoints postdilatation and conversion to open surgery, were assessed using a univariate and multivariate logistic regression model to estimate the odds ratios and their associated 95% confidence intervals (CIs). Only factors found to have P‐values of <.1 in the univariate model were included in the multivariate analysis and assessed in a backward selection of factors based on their likelihood ratio. The results of the multivariate analyses were graphically displayed using forest plots. The statistical analysis was conducted using SPSS (version 24.0, IBM Corp). All reported P‐values are two‐sided, with an alpha level set at <.05 for statistical significance.
3. RESULTS
3.1. Baseline characteristics
The baseline patient characteristics are presented in Table 1. The average patient age in the SCB cohort was 83 and 82 years in the NCB cohort. The surgical risk for procedural mortality, according to the EuroSCORE II, was 5.3% for SCB and 4.2% for NCB patients. Notably, SCB systems were used more often in female patients (SCB: 126 [75.9%] vs. NCB: 49 [57.6%]; P = .003). SCB use was significantly lower in patients with diabetes mellitus (SCB: 17 [10.2%] vs. NCB: 19 [22.4%]; P = .010) and prior percutaneous coronary intervention (SCB: 44 [26.5%] vs. NCB: 36 [42.4%]; P = .011), yet higher in patients with atrial fibrillation (SCB: 63 [33.3%] vs. NCB: 15 [17.9%]; P = .011). With regard to the preoperative echocardiographic evaluation, the median aortic valve area was higher in the NCB patient cohort (0.66 [±0.3] cm2 vs. 0.7 [±0.3] cm2; P = .038). The mean and minimum annulo‐aortic diameters, as assessed by the preoperative multi‐slice CT were also higher for the NCB treatment group (SCB: 24 [±3.5] mm vs. NCB: 24.8 [±3] mm; P = .010; SCB: 22 [±3] mm vs. NCB: 23 [±4] mm; P = .020, respectively).
TABLE 1.
Baseline clinical characteristics of patients treated with TAVR using self‐expanding transcatheter heart valves
| Semi‐compliant balloon systems (n = 166) | Non‐compliant balloon systems (n = 85) | P value | |
|---|---|---|---|
| Demographics | |||
| Female, n (%) | 126 (75.9) | 49 (57.6) | .003 |
| Age in years, median (IQR) | 83 (9) | 82 (10) | .544 |
| Body mass index, median (IQR) | 25.2 (7.59) | 25.9 (8.2) | .144 |
| Risk profile | |||
| Logistic EuroSCORE, median (IQR) | 19.9 (21.5) | 15.92 (18.6) | .133 |
| EuroSCORE II, median (IQR) | 5.3 (7.3) | 4.2 (5.3) | .106 |
| STS score, median (IQR) | 4.9 (3.8) | 3.9 (2.9) | .011 |
| Incremental risk score, median (IQR) | 9 (15) | 9 (15.8) | .497 |
| Chronic health conditions and risk factors | |||
| Hypertension, n (%) | 148 (89.7) | 70 (82.4) | .100 |
| Dyslipidaemia, n (%) | 89 (53.6) | 44 (51.8) | .781 |
| Diabetes mellitus (IDDM), n (%) | 17 (10.2) | 19 (22.4) | .010 |
| Chronic renal insufficiency, n (%) | 15 (9) | 5 (5.9) | .383 |
| Dialysis, n (%) | 1 (0.6) | 1 (1.2) | 1.000 |
| Atrial fibrillation, n (%) | 53 (33.3) | 15 (17.9) | .011 |
| Permanent pacemaker, n (%) | 35 (21.1) | 99 (10.6) | .038 |
| Chronic obstructive pulmonary disease, n (%) | 15 (9.3) | 7 (2.9) | .852 |
| Previous Coronary artery Bypass, n (%) | 16 (9.6) | 13 (15.3) | .185 |
| Previous valve surgery, n (%) | 17 (10.2) | 4 (4.7) | .134 |
| Previous percutaneous coronary intervention, n (%) | 44 (26.5) | 36 (42.4) | .011 |
| Peripheral vascular disease, n (%) | 22 (13.8) | 14 (16.5) | .567 |
| Cerebrovascular disease, n (%) | 22 (13.3) | 12 (14.1) | .850 |
| Cerebrovascular accident, n (%) | 20 (12.3) | 15 (17.6) | .248 |
| Coronary artery disease present, n (%) | 82 (49.4) | 44 (51.8) | .723 |
| Previous myocardial infection, n (%) | 28 (16.9) | 10 (11.8) | .286 |
| Creatinine (mg/dL), median (IQR) | 1.1 (0.7) | 1.1 (0.5) | .952 |
| Preoperative echocardiographic data | |||
| Aortic valve area (cm2), median (IQR) | 0.7 (0.3) | 0.7 (0.3) | .038 |
| Indexed aortic valve area (cm2/m2), median (IQR) | 0.4 (0.2) | 0.4 (0.2) | .399 |
| Mean pressure gradient (mmHg), median (IQR) | 45 (20) | 43 (18) | .953 |
| Maximum pressure gradient (mmHg), median (IQR) | 67 (27) | 67 (27) | .980 |
| Peak velocity (m/s), median (IQR) | 4.1 (1) | 4.2 (0.7) | .540 |
| Left ventricular ejection fraction (%), median (IQR) | 55 (20) | 55 (15) | .542 |
| sPAP (mmHg), median (IQR) | 37 (50) | 39 (50) | .761 |
| CT measurements | |||
| Mean diameter (mm), median (IQR) | 24 (3.5) | 24.8 (3) | .010 |
| Minimum diameter (mm), median (IQR) | 22 (3) | 23 (4) | .020 |
| Maximum diameter (mm), median (IQR) | 26 (4) | 27 (3) | .144 |
| Area (mm2), mean (±SD) | 425 (74.9) | 481 (107.9) | .001 |
| Perimeter (mm), mean (±SD) | 74.8 (6.3) | 79.7 (9.2) | .001 |
| Aortic ovalarity index, median (IQR) | 0.2 (0.2) | 0.1 (0.1) | .168 |
| Calcium Score (aortic valve)a, median (IQR) | 975.2 (784) | 1434.6 (623) | .302 |
Abbreviations: EuroSCORE, European System of Operative Risk Evaluation; IQR, interquartile range, SD, standard deviation, sPAP, systolic pulmonary artery pressure, STS score, Society of Thoracic Surgeons Predictive Risk of Mortality.
Assessing non‐contrast‐enhanced CT images in 3Mensio™ with a threshold for detection of 450 Hounsfield units [13].
3.2. Procedural characteristics
There was a significant difference in the prosthesis size, with larger THV devices being used predominantly in the NCB cohort (SCB: 26 [±3] mm vs. NCB: 29 [±3] mm; P < .001). The indices used to assess over‐ or undersizing of balloon systems for BAV did not reveal any significant difference between the SCB and NCB cohort. The postinterventional echocardiographic evaluation demonstrated that the median postprocedural gradient (SCB: 10 [±9] mmHg vs. NCB: 9 [±6] mmHg; P = .057), as well as the maximum gradient (SCB: 18 [±15] mmHg vs. NCB: 14 [±8] mmHg; P = .058) and the maximum flow velocity over the prosthetic aortic valve (SCB: 2 [±1] m/s vs. NCB: 1.9 [±0] m/s; P = .039) were all higher for SCB patients. Procedural characteristics are listed in Table 2.
3.3. Primary study endpoint and VARC‐2 adverse events
The mortality analysis revealed that the 30‐day all‐cause mortality did not differ between the SCB and NCB cohort (SCB: 11 [6.6%] vs. NCB: 5 [7.1%]; P = .897). Out of the measured VARC‐2 adverse outcomes, only the rate of postdilatation (SCB: 30 [18.1%] vs. NCB: 34 [40%]; P < .001) and the rate of conversion to open surgery (SCB: 1 [0.6%] vs. NCB: 4 [5.1%]; P = .042) were different for the two groups ‐ they both occurred more frequently in the NCB cohort. The remaining events, including the rate of paravalvular leakage or the onset of new bundle branch or atrioventricular blocks, occurred with equal frequency in both groups. Acute kidney injury, neurological adverse events and the overall device success were not significantly different between the cohorts. Secondary endpoints are presented in detail in Table 3.
TABLE 3.
Adverse events (VARC II Criteria) of patients treated with TAVR using self‐expanding transcatheter heart valves
| Semi‐compliant balloon systems (n = 166) | Non‐compliant balloon systems (n = 85) | P value | |
|---|---|---|---|
| VARC‐2 adverse events | |||
| Neurological events, n (%) | 5 (3) | 5 (5.9) | .313 |
| Any bleeding, n (%) | 16 (9.6) | 8 (9.4) | .954 |
| Minor bleeding, n (%) | 29 (17.9) | 8 (10.4) | .134 |
| Major bleeding, n (%) | 16 (9.6) | 8 (9.4) | .954 |
| Acute kidney injury, n (%) | 27 (16.3) | 16 (18.8) | .611 |
| Dialysis, n (%) | 3 (1.8) | 2 (2.4) | 1.000 |
| Major access related complication, n (%) | 4 (2.4) | 3 (3.5) | .692 |
| Myocardial infarction, n (%) | 4 (1.7) | 3 (3.8) | 1.000 |
| New AV‐block, n (%) | 20 (12.3) | 11 (13.9) | .861 |
| New bundle‐branch‐block, n (%) | 57 (14.8) | 12 (3.1) | .589 |
| New atrial fibrillation, n (%) | 14 (8.6) | 10 (12.5) | .345 |
| New pacemaker, n (%) | 32 (19.9) | 15 (19) | .871 |
| Conversion to open surgery, n (%) | 1 (0.6) | 4 (5.1) | .042 |
| Unplanned valve in valve implantation, n (%) | 1 (0.6) | 3 (3.8) | .106 |
| PVL more than trace n (%) | 15 (9.6) | 9 (11.4) | .671 |
| PVL—moderate or severe, n (%) | 6 (2.6) | 0 | .100 |
| Postdilatation necessary, n (%) | 30 (18.1) | 34 (40) | <.001 |
| Reoperation for bleeding or tamponade, n (%) | 12 (7.4) | 6 (7.1) | .930 |
| Reoperation for valvular dysfunction, n (%) | 0 | 1 (1.2) | .331 |
| Reoperation for other cardiac reason, n (%) | 25 (15.4) | 6 (7.5) | .820 |
| Reoperation for non‐cardiac reason, n (%) | 10 (6.2) | 4 (17) | 1.000 |
| Device success, n (%) | 142 (85.5) | 77 (90.6) | .257 |
| Procedural mortality, n (%) | 5 (3) | 1 (1) | .725 |
| 30‐day mortality, n (%) | 11 (6.6) | 6 (7.1) | .897 |
In a multivariate logistic regression analysis of predictive factors for the primary endpoint, the balloon‐type did not affect the outcome (univariate OR: 0.813; 95% CI: 0.355‐1.971; P = .647, Table S4, Figure 3). However, analyzing the secondary endpoints of postdilatation and conversion to open surgery in a uni‐ and multivariate logistic regression analysis, the use of compliant balloons systems during predilatation was independently associated with lower rates in both endpoints (postdilatation OR: 0.296; 95% CI: 0.149‐0.588; P = .001; conversion to open surgery: OR: 0.205; 95% CI: 0.085‐0.493; P = .001; Tables S5 and S6, Figure 3).
FIGURE 3.
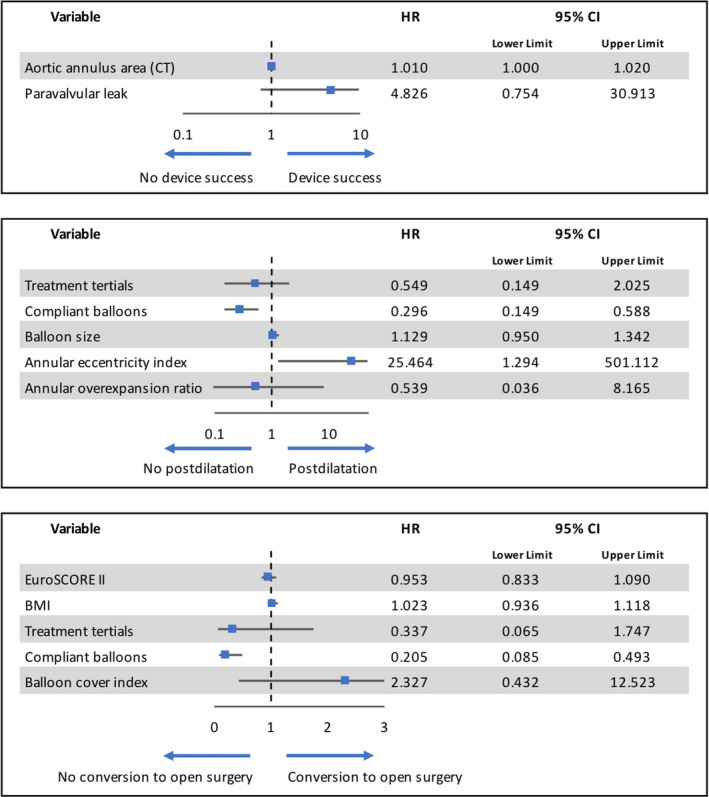
Results of the multivariate logistic regression analyses using a stepwise backward selection of factors based on their likelihood ratio for the primary endpoint VARC‐2 defined device success as well as the secondary endpoints of postdilatation and conversion to open surgery
4. DISCUSSION
Our study is the first clinical analysis that aimed to explore the impact of predilatation during TAVR with balloons of varying degrees of compliance. Although the results seem to slightly favour the use of semi‐compliant balloon systems due to an increase in the postdilatation rates and the frequency of conversion to open surgery in the NCB cohort, there was neither a difference in the THV device success nor the procedural and 30‐day all‐cause mortality of the two patient collectives.
This analysis's primary endpoint, the procedural device success, did not differ between the two patient collectives, despite the somewhat higher need for conversion to open‐heart surgery in the SCB cohort. This finding is in line with similar results reported for studies investigating TAVR without or moderate predilatation.9, 14 In the multivariate logistic regression analysis for the primary endpoint, the only factor associated with higher device success rates was the aortic annulus area measured in CT, indicating that larger annuli displayed higher device success rates in this particular cohort. This finding might be related to generally lower gradients measured during transthoracic echocardiography after implantation of larger THV devices; however, it has to be noted that the implantation of self‐expandable valves in patients with particularly large or elliptical annuli tends to demonstrate generally lower rates of device success compared to balloon‐expandable valves.15, 16, 17
The principal advantage of NCBs is their ability to exert a more focused effect on highly calcified areas effectively without impacting the surrounding healthy areas. They are thus well‐suited to treating severely and diffusely calcified valves, a fact evidenced by the higher median calcium score of NCB patients. This benefit is dampened by a somewhat increased need for postdilatation that might be related to the slightly stronger preoperative calcification of the native aortic valve, especially in patients with very oval aortic annuli. The increased postdilatation rate could be one possible explanation for the significantly better postprocedural hemodynamic parameters in the NCB cohort, despite baseline hemodynamic parameters being comparable between the two groups. However, it has to be noted that the number of inflations in stand‐alone BAV was associated with increased procedural mortality in some studies and that larger THV devices tend to yield lower transvalvular gradients.17, 18, 19 Another possible explanation for this finding would be the overall larger device size of NCB balloons used in this study. This hypothesis is supported by the findings of several recent studies that indicate that the use of larger BAV balloons results in improved postinterventional hemodynamics. Smaller balloons do not seem to reduce procedural device success and require fewer postprocedural pacemaker implantations.9, 20, 21 Our study also demonstrated that device success and postprocedural pacemaker implantation rates were not affected by the balloon's compliance.
Aggressive predilatation and displacement of native aortic valve calcification might worsen the valve frame's embedding in the calcified landing zone. As a result, valve anchoring is hampered, potentially causing valve migration or increased rates of paravalvular leakage, as demonstrated in a recent metanalysis.22 With regard to balloon compliance during predilatation, however, neither treatment cohort demonstrated an increase in the degree of postinterventional paravalvular leakage, and no cases of valve embolization were reported either.
The sites deemed most susceptible to aortic rupture during TAVR are the aortic annulus and the sub‐annular left ventricular outflow tract. Pronounced calcification is the primary predictor of such an event, especially in patients treated with balloon‐expandable or aggressively oversized THVs.23, 24 In this analysis, one of the only adverse effects significantly related to the use of NCBs was the increased occurrence of aortic rupture, which is also directly related to the second significantly different outcome between the two patient collectives—the rate of conversion to open surgery. However, it is important to state that the overall absolute incidence of cases was low. As a result, the significance of this finding has to be interpreted with due caution, especially since the ruptures themselves seemed not to occur after predilatation but THV implantation. Since the ruptures tended to occur in areas with the highest annular calcification load and the NCB group displaying a higher mean calcification score, they could have initially been at a higher risk of rupture. The radial force exhibited by NCBs on the target site is higher, however, the pressure on the surrounding tissue located in the supra‐annular and the LVOT region is higher for SCBs. Furthermore, unlike NCBs, SCBs can be overinflated beyond the nominal diameter, leading to higher inflation peak pressures in areas surrounding the resistance.6 Consequently, NCBs might prove useful in patients with sub‐annular calcification, as SCBs primarily exert their mechanical pressure on lower resistance areas in the supra‐annular and sub‐annular region before reaching their nominal diameter at the annulus level in the late phase of the expansion. Newer generation SCB balloons such as NuCLEUS (NuMed) and NuCLEUS‐X (NuMed) were designed to improve balloon positioning while enabling consistency of device shape during inflation.2 NCBs, on the other hand, offer a high degree of predictability and safety as they cannot be overinflated. However, ample undersizing seems necessary, especially in patients with severe eccentric calcification patterns due to the rapid and vigorous expansion of these balloon systems.25, 26 Other individual and procedural aspects that need to be considered and could have played a role in the occurrence of the rupture events include a low balloon placement and unfavourable anatomical properties.
As far as the devices' mechanical performance is concerned, the anticipated rupture of SCB balloons due to their thin material caused by severe leaflet calcification carries the added risk of device fragment embolization. At the same time, it does not affect procedural mortality.27, 28 However, because the SCB patient collective had a lower calcification score, the potential risk of aortic rupture might not be reflected accurately in our analysis.
A particularly noteworthy finding of our study is that the neurological adverse event rates did not differ between the two cohorts (P = .313). Similar results have been reported in a comparison of moderate versus no predilatation in patients treated with balloon‐expandable valves, but the impact of balloon compliance and device expansion force on heavily stenotic aortic valves and calcium deformation requires further research.9
It is also conceivable that the impact of balloon compliance on periprocedural hemodynamics may affect the patient outcome after TAVR. Prolonged rapid pacing sequences are associated with a longer duration of a low cardiac output state while also creating a prothrombotic environment, increasing the risk of stroke and acute kidney injury (AKI).29, 30 Unfortunately, the rapid pacing times were not measured and can therefore not be reported due to our analysis's retrospective nature. Thus, their contribution to the hemodynamic outcome cannot be assessed in detail. NCB use may have increased rapid pacing times due to the pronounced postdilatation rates. On the other hand, SCB use could result in long rapid pacing times due to difficulties resulting from a reduced efficiency in overcoming severe aortic stenosis. SCBs may thus require longer inflation times or even multiple inflations, a phenomenon already observed in PCI.4, 5 The design of the True Flow balloon catheter (Bard Peripheral Vascular Inc, Arizona, USA), and the hollow balloon system developed by Strait Access Technologies (SAT, Cape Town, South Africa) allows them to circumvent the risks as mentioned above. Its cylindrical hollow shape ensures a continuous blood flow during the balloon expansion and entirely eliminates the need for rapid pacing.31 This might lead to improved perioperative hemodynamic stability and a better outcome after TAVR.
Longer rapid pacing times and higher expansion forces have also been described as risk factors for conduction disorders during TAVR.14, 29, 32 However, our analysis did not demonstrate an effect of balloon compliance on new‐onset atrial fibrillation, bundle branch blocks or postprocedural pacemaker implantation rates. Although our results indicate that the increased radial force and potentially longer cumulative rapid pacing times associated with NCBs do not cause conduction abnormalities, our findings must be validated by further research.
4.1. Limitations and future perspectives
The principal intrinsic limitation of the study is its retrospective character. Furthermore, the ability to infer conclusions based on group comparisons is somewhat limited because of a potential selection bias regarding the individual interventionalist's choice of a specific balloon‐type for predilatation. In addition, a large proportion of patients had to be excluded from the analysis. The reason for exclusion was either due to the lack of predilatation or the use of balloon‐expandable THV devices. Excluding these patients from our analysis was intended to ensure comparability since balloon‐expandable THV devices exhibit greater radial expansion forces, thus frequently eliminating the need for predilatation on the one hand while also displaying significantly lower rates of paravalvular leaks compared to self‐expandable THV devices.33, 34 However, the study does pave the way for further studies regarding the impact of balloon compliance on TAVR outcomes, as well as more detailed studies on the outcome impact of predilatation itself.
The outcome parameters could have been skewed by the larger annulo‐aortic dimensions in the NCB cohort, which lead to the implantation of THV models with slightly larger orifice areas. Moreover, the lack of documented rapid pacing times leaves a lot of room for speculation regarding the cause of the improved hemodynamic outcomes for the NCB group.
Further improvements in future iterations of this study and similar trials could include documenting a low or unfavourable balloon placement, recording the pacing time and excluding patients with morphologically unfavourable LVOT and aorta configurations. Future investigations should also include hollow balloon devices and adjust for the aforementioned parameters' relative contribution to the outcome, possibly matching the patient collectives more precisely regarding these outcomes.
5. CONCLUSION
Although the choice of balloon systems with different mechanical properties and compliance for BAV during TAVR does not affect device success or procedural mortality, future research is needed to validate our findings regarding the increased rates of postdilatation and conversion to open surgery in patients treated with non‐compliant balloon systems.
CONFLICTS OF INTEREST
Dr Mach has received a research grant from Edwards Lifesciences, JenaValve and Symetis. Dr. Andreas is a proctor, speaker and consultant for Edwards Lifesciences, Abbott, Medtronic and received institutional grants from Edwards Lifesciences, Abbott, Medtronic and LSI. All other authors have reported that they have no relationships relevant to the content.
AUTHOR CONTRIBUTIONS
MM, GDK and MG were responsible for the conceptualization and design of the study and the overall supervision. MA and BW participated in the design of the study. TP, WH and PS performed the data acquisition. MM conducted the data analysis. MM, EH and PS wrote the manuscript. WH, TS, AS, CA, MA, BW, GD and MG assisted in the writing and the critical revision of the manuscript. All authors have read and contributed to the submitted manuscript, have participated in the review process and have approved this version of the manuscript for submission.
Supporting information
Table S4‐S6
Mach M, Szalkiewicz P, Poschner T, et al. The use of semi‐compliant versus non‐compliant balloon systems for predilatation during the implantation of self‐expandable transcatheter aortic valves. Eur J Clin Invest. 2021;51:e13570. 10.1111/eci.13570
REFERENCES
- 1.Safian RD, Hoffmann MA, Almany S, et al. Comparison of coronary angioplasty with compliant and non‐compliant balloons (the angioplasty compliance trial). Am J Cardiol. 1995;76(7):518‐520. [DOI] [PubMed] [Google Scholar]
- 2.Keeble TR, Khokhar A, Akhtar MM, Mathur A, Weerackody R, Kennon S. Percutaneous balloon aortic valvuloplasty in the era of transcatheter aortic valve implantation: a narrative review. Open Heart. 2016;3(2):e000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sathananthan J, Hensey M, Sellers S, et al. Performance of the TRUE dilatation balloon valvuloplasty catheter beyond rated burst pressure: A bench study. Catheter Cardiovasc Interv. 2020;96(2):E187‐E195. [DOI] [PubMed] [Google Scholar]
- 4.Özel E, Taştan A, Öztürk A, Özcan EE, Uyar S, Şenarslan Ö. What is better for predilatation in bioresorbable vascular scaffold implantation: A non‐compliant or a compliant balloon? Anatol J Cardiol. 2016;16(4):244‐249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seyithanoglu BY, Zaki Masud AR, Ergene O, et al. Compliant vs non‐compliant balloons. A prospective randomized study. Jpn Heart J. 1998;39(1):45–54. [DOI] [PubMed] [Google Scholar]
- 6.Goel P, Agarwal R, Kaul U, Wasir HS. Effects of balloon compliance on angiographic and clinical outcomes after PTCA. Int J Cardiol. 1995;51(1):1‐4. [DOI] [PubMed] [Google Scholar]
- 7.Bach RG, Kern MJ, Aguirre FV, Donohue TJ, Bell C, Penick D. Effects of percutaneous transluminal coronary angioplasty balloon compliance on angiographic and clinical outcomes. Am J Cardiol. 1993;72(12):904‐907. [DOI] [PubMed] [Google Scholar]
- 8.Mooney MR, Mooney JF, Longe TF, Brandenburg RO. Effect of balloon material on coronary angioplasty. Am J Cardiol. 1992;69(17):1481‐1482. [DOI] [PubMed] [Google Scholar]
- 9.Abramowitz Y, Jilaihawi H, Chakravarty T, et al. Feasibility and safety of balloon‐expandable transcatheter aortic valve implantation with moderate or without predilatation. EuroIntervention. 2016;11(10):1132‐1139. [DOI] [PubMed] [Google Scholar]
- 10.Kim WK, Blumenstein J, Liebetrau C, et al. Comparison of outcomes using balloon‐expandable versus self‐expanding transcatheter prostheses according to the extent of aortic valve calcification. Clin Res Cardiol. 2017;106(12):995‐1004. [DOI] [PubMed] [Google Scholar]
- 11.Coughlan JJ, Kiernan T, Mylotte D, Arnous S. Annular rupture during transcatheter aortic valve implantation: predictors, management and outcomes. Interv Cardiol Rev. 2018;13(3):140‐144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kappetein AP, Head SJ, Généreux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium‐2 consensus document (VARC‐2). Eur J Cardio‐thoracic Surg. 2012;42(5):S45‐S60. [DOI] [PubMed] [Google Scholar]
- 13.Jilaihawi H, Makkar RR, Kashif M, et al. A revised methodology for aortic‐valvar complex calcium quantification for transcatheter aortic valve implantation. Eur Heart J Cardiovasc Imaging. 2014;15(12):1324‐1332. [DOI] [PubMed] [Google Scholar]
- 14.Nombela‐Franco L, Rodés‐Cabau J, Delarochellière R, et al. Predictive factors, efficacy, and safety of balloon post‐dilation after transcatheter aortic valve implantation with a balloon‐expandable valve. JACC Cardiovasc Interv. 2012;5(5):499‐512. [DOI] [PubMed] [Google Scholar]
- 15.Armijo G, Tang GHL, Kooistra N, et al. Third‐generation balloon and self‐expandable valves for aortic stenosis in large and extra‐large aortic annuli from the TAVR‐LARGE registry. Circ Cardiovasc Interv. 2020;13(8):e009047. [DOI] [PubMed] [Google Scholar]
- 16.Maeno Y, Abramowitz Y, Yoon SH, et al. Transcatheter aortic valve replacement with different valve types in elliptic aortic annuli. Circ J. 2017;81(7):1036‐1042. [DOI] [PubMed] [Google Scholar]
- 17.Hahn RT, Leipsic J, Douglas PS, et al. Comprehensive echocardiographic assessment of normal transcatheter valve function. JACC Cardiovasc Imaging. 2019;12(1):25‐34. [DOI] [PubMed] [Google Scholar]
- 18.Agarwal A, Kini AS, Attanti S, et al. Results of repeat balloon valvuloplasty for treatment of aortic stenosis in patients aged 59 to 104 years. Am J Cardiol. 2005;95(1):43‐47. [DOI] [PubMed] [Google Scholar]
- 19.O'Neill W. The mansfield scientific aortic valvuloplasty registry investigators. Predictors of long‐term survival after percutaneous aortic valvuloplasty: report of the mansfield scientific balloon aortic valvuloplasty registry. J Am Coll Cardiol. 1991;17(1):193‐198. [DOI] [PubMed] [Google Scholar]
- 20.Abramowitz Y, Jilaihawi H, Chakravarty T, et al. Sapien 3 Transcatheter Aortic Valve Implantation with Moderate or Without Predilation. J Invasive Cardiol. 2016;28(10):421‐426. [PubMed] [Google Scholar]
- 21.Lange P, Greif M, Vogel A, et al. Reduction of pacemaker implantation rates after CoreValve® implantation by moderate predilatation. EuroIntervention. 2014;9(10):1151‐1157. [DOI] [PubMed] [Google Scholar]
- 22.Auffret V, Regueiro A, Campelo‐Parada F, et al. Feasibility, safety, and efficacy of transcatheter aortic valve replacement without balloon predilation: a systematic review and meta‐analysis. Catheter Cardiovasc Interv. 2017;90(5):839‐850. [DOI] [PubMed] [Google Scholar]
- 23.Barbanti M, Yang TH, Rodès Cabau J, et al. Anatomical and procedural features associated with aortic root rupture during balloon‐expandable transcatheter aortic valve replacement. Circulation. 2013;128(3):244‐253. [DOI] [PubMed] [Google Scholar]
- 24.Girdauskas E, Owais T, Fey B, et al. Subannular perforation of left ventricular outflow tract associated with transcatheter valve implantation: pathophysiological background and clinical implications. Eur J Cardiothorac Surg. 2017;51(1):91‐96. [DOI] [PubMed] [Google Scholar]
- 25.McKay RG. The mansfield scientific aortic valvuloplasty registry: Overview of acute hemodynamic results and procedural complications. J Am Coll Cardiol. 1991;17(2):485‐491. [DOI] [PubMed] [Google Scholar]
- 26.Percutaneous balloon aortic valvuloplasty . Acute and 30‐day follow‐up results in 674 patients from the NHLBI balloon valvuloplasty registry. Circulation. 1991;84(6):2383‐2397. [DOI] [PubMed] [Google Scholar]
- 27.Seropian IM, Romeo FJ, Falconi M, Agatiello CR, Berrocal DH. Balloon rupture during aortic valvuloplasty with compliant balloon: predictors and outcomes. Cardiovasc Interv Ther. 2020;35(3):291‐299. [DOI] [PubMed] [Google Scholar]
- 28.Kubo S, Fuku Y, Shimamoto T, et al. Vascular injury caused by retrieval of ruptured and detached balloon valvuloplasty catheter during transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2017;10(15):1593‐1595. [DOI] [PubMed] [Google Scholar]
- 29.Fefer P, Bogdan A, Grossman Y, et al. Impact of rapid ventricular pacing on outcome after transcatheter aortic valve replacement. J Am Heart Assoc. 2018;7(14):e009038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Witzke C, Don CW, Cubeddu RJ, et al. Impact of rapid ventricular pacing during percutaneous balloon aortic valvuloplasty in patients with critical aortic stenosis: Should we be using it? Catheter Cardiovasc Interv. 2010;75(3):444‐452. [DOI] [PubMed] [Google Scholar]
- 31.Scherman J, Ofoegbu C, Myburgh A, et al. Preclinical evaluation of a transcatheter aortic valve replacement system for patients with rheumatic heart disease. EuroIntervention. 2019;15(11):E975‐E982. [DOI] [PubMed] [Google Scholar]
- 32.Ferrera C, Nombela‐Franco L, Garcia E, et al. Clinical and hemodynamic results after direct transcatheter aortic valve replacement versus pre‐implantation balloon aortic valvuloplasty: a case‐matched analysis. Catheter Cardiovasc Interv. 2017;90(5):809‐816. [DOI] [PubMed] [Google Scholar]
- 33.Egron S, Fujita B, Gullón L, et al. Radial force: an underestimated parameter in oversizing transcatheter aortic valve replacement prostheses. in vitro analysis with five commercialized valves. Asaio j. 2018;64(4):536‐543. [DOI] [PubMed] [Google Scholar]
- 34.Abdel‐Wahab M, Mehilli J, Frerker C, et al. CHOICE investigators. Comparison of balloon‐expandable vs self‐expandable valves in patients undergoing transcatheter aortic valve replacement: the CHOICE randomized clinical trial. JAMA. 2014;311(15):1503‐1514. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S4‐S6


