Abstract
Tetracyclines are a group of broad‐spectrum antibiotics largely employed in infectious, dermatological and surgical fields. Some adverse events may occur during treatment, including photosensitivity reactions, which are divided in phototoxic or photoallergic. We performed a systematic search on Pubmed, Cochrane and Embase from database inception to August 9, 2020 aim to summarize all available papers on photosensitive reactions related to tetracyclines in all clinical settings where they are used on human being. On the basis of our inclusion criteria, we selected only randomized controlled trials, open comparative trials and prospective cohort studies performed on both volunteers and patients, moreover we included a pharmacovigilance register. Thirty‐eight articles met inclusion criteria, describing photo‐sensitive effects due to doxycycline, minocycline, tetracycline, lymecycline, sarecycline, demethylchlortetracycline, chlortetracycline and metacycline, across six diagnoses (acne, Lyme disease, Gulf Veteran Illness, adbominal aortic aneurysms, traveler's diarrhea and pterygium) and several volunteers who were deliberately exposed to natural or artificial light sources. Not all drugs belonging to tetracyclines class are available to date, moreover the studies included lacked a homogeneous design and most of them involved a scarce number of patients, including reactions induced in volunteers during photo‐testing. Available data on incidence, severity and clinical relevance of tetracyclines‐related photo‐sensitive reactions are scarce, heterogeneous and weak. What we can extrapolate is that some tetracyclines are more often related to phototoxic skin reactions than others and some of those seem to have a very low risk of phototoxicity.
Keywords: narrative review, photosensitive skin reactions, photosensitivity, sun‐burn like reactions, tetracycline
1. INTRODUCTION
Different natural and artificial chemical substances, including drugs, are known to induce photosensitive skin reactions. Tetracyclines (TCs) are a group of broad‐spectrum antibiotics discovered in the 1940s and exhibited activity against a range of microorganisms including gram‐positive and gram‐negative bacteria (e.g., chlamydiae, mycoplasmas, rickettsiae), and protozoa; they are largely prescribed in dermatology and infectious diseases, both for the anti‐bacterial and anti‐inflammatory actions.1 TCs are considered generally well tolerated, although some adverse events may occur during treatment, one of them is photosensitivity.2 From the photobiological point of view, the photosensitivity reactions are a consequence of the absorption of light energy by a photosensitizing substance (e.g., a drug) and of the subsequent photochemical reactions determining changes in living tissues. They are usually divided in phototoxic or photoallergic, depending on the different mechanism involved.3 TCs have been reported as phototoxic, but not photoallergic drugs.
The correct evaluation of the presence of photosensitivity is theoretically based on the confirm with the challenge–rechallenge test or with photo‐testing. These evaluations are often not performed, for: ethic reason, risks due to the re‐challenge, and the absence of specific setting where perform it.
The studies evaluating the incidence of photo‐reactions due to TCs are few and heterogeneous. The aim of this review is to offer an overview about the TCs that cause photosensitive reactions, in order to better manage their use. The reactions reported have been observed both in patients during treatment and in volunteers exposed to artificial light sources.
2. METHODS
2.1. Search strategy and selection criteria
We performed a search in Pubmed, Embase and Cochrane Central Register of Controlled Trials databases from database inception until August 9, 2020.
Our search criteria were as follows: ((phototoxicity) OR (photosensitivity)) AND (TC). Moreover, we manually searched additional articles that met our inclusion criteria from the reference list. We conduct this systematic review according with the preferred reporting items for systematic reviews and meta‐analyses and consolidated standards of reporting trials reporting guidelines, to investigate the incidence of photosensitivity in patients treated with TCs.4, 5
We included only results in English, on human subjects, with full text available. We selected the papers who specifically assessed photosensitivity reactions that were considered as a side effect of a therapy with TCs and described by a physician. We excluded studies that did not contain original data (i.e., reviews and commentaries), and studies performed in vitro or in animal models. The search was restricted to randomized controlled trials, open label cohort comparative and prospective cohort studies. We also included a pharmacovigilance register who met our inclusion criteria.
The characteristics of study (type of publication, year and study design), the number of patients enrolled, the number of photosensitive reactions observed, the drug taken and its indication, the reaction occurred, were evaluated during data extraction by G. O., who performed the search. V. B. was consulted to review the characteristics of eligibility of studies candidate, and, in the case of discrepancies, G. M. was consulted.
In case of missing information, only complete data were considered for statistical analysis. Standard descriptive statistics were used to summarize the data. Descriptive data, expressed in mean values or percentages, were generated by pooling patients from eligible studies.
3. RESULTS
We identified 821 results from key‐words research, and 28 additional papers from reference list. After duplicate removal, a total of 520 articles were screened for title and abstract, of which 38 met the inclusion criteria for review (Figure 1).
FIGURE 1.
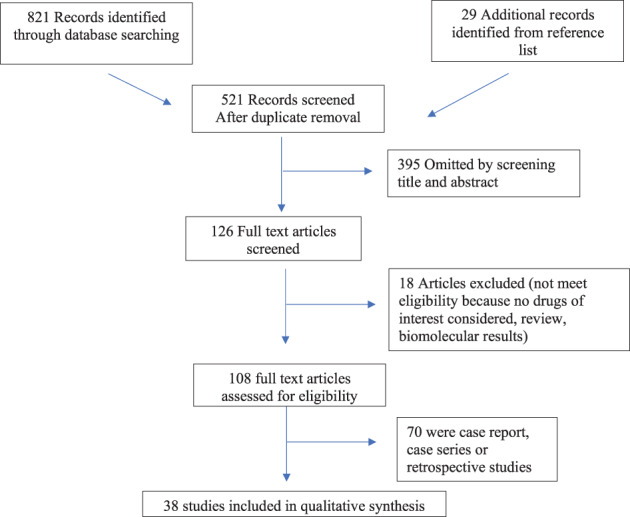
Study flowchart
Due to the heterogeneity and poor quality of studies, we not performed a quantitative synthesis of data. As a result of the generally high risk of bias across the include studies, we could present only a narrative synthesis of the evidence and it was not possible to perform a meta‐analysis. The generally poor quality of the evidence base implies that caution is needed in the interpretation of our findings since there is significant uncertainty regarding the included papers (Tables 1 and 2).
TABLE 1.
Studies included photo‐sensitive reactions occurred in patients
| Authors | Type of study | Year | No. of patients | Drug | Dose | Reaction |
|---|---|---|---|---|---|---|
| Acne | ||||||
| Pariser DM, Green LJ | RCT | 2019 | 1/483 | Sarecycline | 1.5 mg/kg/day | Sunburn |
|
Moore A, Green LJ, et al. |
RCT | 2018 | 1/513 | Sarecycline | 1.5 mg/kg/day | Photosensitivity |
|
Leyden JJ, Sniukiene V, Berk DR, Kaoukhov A |
RCT | 2018 | 10/212 | Sarecycline |
0.75 mg/kg/day 1.5 mg/kg/day 3 mg/kg/day |
Photosensitivity |
|
Kus S, Yucelten D, et al. |
RCT | 2005 | 2/26 | Doxycycline | 200 mg/day first month, 100 mg/day the following 2 months | Photosensitivity |
|
Sanchez J, Somolinos AL, et al. |
RCT |
2005 |
0/20 | Doxycycline | 20 mg | Photosensitivity |
|
Gruber F, Grubišić‐Greblo H, |
Open, non‐ randomized and comparative study | 1998 | 0/34 | Minocycline | 100 mg | Photosensitivity |
|
Goulden V, Glass D, et al. |
Prospective cohort study | 1996 | 7/700 | Minocycline | 100 daily, 100/200 on alternate days and 200 daily | Photosensitivity |
|
Layton AM, Cunlife WJ |
Prospective cohort study | 1993 |
38 /106 |
Doxycycline | 150–200 mg | Phototoxicity |
|
Hubbell CG, Hobbs ER, et al. |
RCT |
1982 |
0/25 | Minocycline |
100 mg |
Phototoxicity |
|
Hubbell CG, Hobbs ER, et al. |
RCT |
1982 |
0/24 | Tetracycline | 500 mg | Phototoxicity |
|
Cullen SI |
RCT | 1976 | 0/42 | Minocycline | 100 mg | Phototoxic |
|
Cullen SI |
RCT | 1976 | 0/40 | Tetracycline | 500 mg | Phototoxic |
|
Orentreich N, Harber L et al. |
Prospective cohort study | 1961 | 27/108 | Demethylchlortetracycline |
600 mg Furunculosis and folliculitis |
Phototoxic |
|
Fuhrman DL, Drowns B et al. |
Prospective cohort study | 1960 | 7/70 | Demethylchlortetracycline. |
600 mg/day Acne, sycosis barbae and other pyogenic infections |
Photosensitivity, polymorphous light eruption |
| Lyme disease | ||||||
|
Velušček M, Bajrović FF, et al. |
Prospective cohort study | 2018 |
16/858 |
Doxycycline | 200 mg/day | Photosensitivity |
|
Ogrinc K, Logar M, et al. |
Prospective cohort study | 2006 | 7/23 | Doxycycline | 200 mg/day | Photosensibility |
|
Strle F, Maraspin V, et al. |
Prospective cohort study | 1996 | 5/42 | Doxycycline | 200 mg/day |
Photosensitivity |
|
Luger SW, Paparone P, et al. |
RCT | 1995 | 7/113 | Doxycycline | 300 mg/day | Photosensitivity |
|
Nowakowski J, Nadelman RB, et al. |
RCT | 1995 | 4/38 | Doxycycline |
300 mg/day |
Photosensitivity |
|
Nadelman RB, Luger SW, et al. |
RCT | 1992 | 9/60 | Doxycycline | 300 mg/day | Photosensitivity |
| Gulf vet | ||||||
|
Donta ST, Engel CC Jr, et al. |
RCT | 2004 | 36/245 | Doxycycline | 200 mg/day | Photosensitivity |
| Traveler's diarrhea | ||||||
|
Hipskind JE |
Prospective cohort study | 1993 | 0/42 |
Doxycycline |
Not specified | Photosensitivity |
| Abdominal aortic aneurysms | ||||||
|
Baxter BT, Pearce WH, et al. |
Prospective cohort study | 2002 | 11/36 |
Doxycycline |
200 mg | Photosensitivity |
| Pterygium | ||||||
|
Rua O, Larrayoz IM, |
RCT | 2014 | 0/49 | Doxycycline | 200 mg | Phototoxicity |
| Register | ||||||
|
Lebrun‐Vignes B, Kreft‐Jais C. |
Pharmacovigilance register | 2012 |
69 18 1 1 |
Doxycycline Minociclyne Lymecycline Metacycline |
Not specified | Photosensitivity |
TABLE 2.
Studies included photo‐sensitive reactions occurred in volunteers deliberately exposed to sunlight or artificial light sources
| Studies performed on volunteers | ||||||
|---|---|---|---|---|---|---|
| Author | Type of study | Year | No patients involved | Drug | Dose | Reaction occurred and source of light |
|
Pariser DM, Green LJ |
RCT | 2019 |
12/18 at 24 h 2/18 at 48 h |
Sarecycline | 240 mg | Phototoxicity assessed with irradiation with 16 J/cm2 of UVA, then another area was irradiated with UVA/UVB at 50% of the subject's minimal erythema dose. |
|
Bjellerup M, Ljunggren B |
Double‐blind cross‐over | 1994 | 15 |
Doxycycline vs. lymecycline |
200 mg 1200 mg |
Phototesting performed with UVA radiation |
|
Bjellerup M, Ljunggren B |
Double blind cross‐over | 1987 |
4/8 0/8 |
Doxycycline vs. lymecycline |
200 mg 1200 mg |
Photosensitive reaction with fluorescent tube |
|
Rosén K, Swanbeck G |
Interventional cohort | 1982 | 1/10 |
Doxycycline |
200 mg | Phototoxic Reactions after exposition to High‐Intensity UVA Lamp |
| Maibach HI, Epstein J, et al. | Observational cohort | 1974 | 3/200 |
Demethylchlortetracycline |
600 mg |
Phototoxic dermatitis, then Photosensitive lichenoid eruption in 10–20 days during sun exposure and application of a Mylan plastic |
|
Frost P, Weinstein GD, et al. |
RCT | 1972 |
6/15 0/17 |
Doxycycline vs. minocycline |
200 mg | Sunburn reaction during sun exposure |
|
Frost P, Weinstein GD, et al. |
Interventional cohort | 1971 |
12/13 1/14 |
Demethylchlortetracycline Methacycline |
600 mg 600 mg |
Abnormal reactions to sun during a boat trip |
| Dahlen RF, Shapiro SI, et al. | Interventional cohort | 1970 | 20 | Demethylchlortetracycline | 300 mg | Phototoxic reaction due to sunlight, assessing the efficacy of a sunscreen |
|
Blank H, Cullen SI, et al. |
RCT | 1968 |
9/10 2/10 |
Demethylchlortetracycline or doxycycline |
600 mg 200 mg |
Phototoxic reaction during sun exposition on a boat |
|
Willis I, Kligman |
Interventional cohort | 1968 | 10/10 |
Demethylchlortetracycline |
Skin contact | Photocontact allergy on stripped skin with 1600 W high pressure xenon arc lamp |
|
Willis I, Kligman |
Interventional cohort | 1968 | 10/12 |
Demethylchlortetracycline |
1200 mg | Photosensitivity with 1600 W high pressure xenon arc lamp |
|
Kligman AM, Breit R |
Interventional cohort | 1968 | See text for details |
Demethylchlortetracycline, tetracycline HCl, chlortetracycline |
Various doses | Phototoxic reaction induced by oral administration, dermal injection and topical application |
|
Schorr WF, Monash S |
Interventional cohort | 1963 |
5 sunlight 1 hot quartz lamp |
Tetracycline and demethylchlortetracycline |
0.2 ml at various concentrations intradermal injection | Minimal primary irritant effect occurred which disappeared in 48–72 h with sunlight or hot quartz lamp irradiation |
|
Harber LC, Tromovitch TA, Baer RL |
RCT | 1961 |
10 10 15 carbon arc radiation |
Demethylchlortetracycline vs. tetracycline |
600 mg 1000 mg 1400 mg |
Photosensitivity with sunlight or carbon arc radiation |
| Orentreich N, Harber L et al. | Interventional cohort | 1961 | 27/108 | Demethylchlortetracycline |
600 mg |
Phototoxic reaction (of whom 7/108 with photo‐onycholysis) |
|
Cahn M, Levy EJ, et al. |
Interventional cohort | 1961 |
15/74 15/64 3/18 |
Demethylchlortetracycline |
600 mg 600 or 450 or 300 mg |
Photosensitivity with artificial light source |
|
Cahn M, Levy EJ, et al. |
Interventional cohort | 1961 |
2/50 0/76 |
Demethylchlortetracycline |
450 mg 300 mg |
Photosensitivity with sunlight exposition + UV lamp |
3.1. Study on volunteers
In this section we included studies performed on subjects exposed to the sun or to the artificial light source deliberately. Patients were both affected by diseases or healthy. Between the studies discussed below, not all authors performed double blind studies assessing the phototoxicity induced by the drug examined. Most of the studies described either missed a specific design and included a mix of different treatment approaches, administration route, and light source (Table 2).
Fifteen papers were aimed to provoke a phototoxic reaction by the administration of TCs.
One hundred eighty‐two photosensitivity reactions have been observed in association with eight TCs: demethylchlortetracycline (DMC) (10 studies), doxycycline (4 studies), lymecycline (2 studies), TC (3 study), chlortetracycline (1 study), minocycline (1 study), metacycline (1 study) and sarecycline (1 study).
Blanck et al. performed a double‐blind study in which he prescribed DMC, doxycycline and placebo for 1 week and then exposed the subjects to sunlight on a boat for 5 h in the south‐east of Florida. They observed 9 phototoxic reactions in 10 patients taking DMC 600 mg/day, 2 out of 10 taking doxycycline 200 mg and 1 doubtful reaction in a patient taking placebo.6
Frost et al., in a double‐blind study including also placebo administration, described 6 sunburn reactions after sun exposure in 15 volunteers taking doxycycline 200 mg and 0 in 15 who took minocycline 200 mg for 1 week (1972).7
In another double‐blind study performed, 12 reactions in 13 patients taking DMC 600 mg and 1 reaction in 14 patients treated with methacycline 600 mg took place during a trip boat in the south of Florida (1971).8
Harber et al. described the decrease of the minimal erythema dose in patients treated with DMC or with TC or with lactose capsules and then exposed to sunlight or with arc carbon radiation. He noted that the photosensitivity related to DMC manifested as an exaggerated sunburn‐like reaction.9
Bjellerup et al. performed a double‐blind cross‐over phototoxicity study and observed that, taking only stronger reactions and stinging sensations into account, doxycycline produced highly significant phototoxic reactions (in 4/8 patients) compared to lymecycline and DMC (0/8), that exhibited only weak phototoxicity in patients tested with fluorescent tube exposition (1987).10
In a different double‐blind cross‐over study, they tested 15 patients with UVA photo‐exposition, and observed that doxycycline 200 mg produced highly significant phototoxic reaction compared to lymecycline 1200 mg, that caused only weak reactions, and to placebo.11
In a very old study without an established design, performed in 1961, 184 men affected with acne were treated with DMC 600 mg/day and 46 with placebo; 80 were exposed to artificial light sources and 104 to sunlight. In the first group, 15/74 who completed the 30 days therapy showed a 4+ erythema. In the group exposed to sunlight, 15/64 showed a 4+ erythema. The author also observed erythema 4+ in 3/18 men treated for 6 days and in 2/50 patients suffering from acne treated with 450 mg/day.12
Klingman et al. assessed the phototoxicity caused by three TCs in relation to different administration routes, including oral, topical and intradermal injection, after the exposition at several light source.
A phototoxic reaction occurred in 4 out of 26 subjects treated with DMC 600 mg/daily orally and in 13/31 treated with 1200 mg /daily exposed to Xenon‐Mylar radiation >310 nm, in 0/11 and 0/14 subjects respectively treated with the two regimen of DMC and exposed to UVB 290–310 nm, in 3/18 and in 2/14 respectively of subjects irradiated with Xenon solar radiation, finally in 0/7 and 0/11 respectively of them irradiated with black light (320–400 nm). The same experiment revealed no phototoxic reactions in subjects treated with Chlortetracycline or TC HCl. They then assessed the minimum phototoxic concentration of DMC after dermal injection, and also its reaction related to the topical application on both normal and stripped skin. They finally assessed the different wavelength in photoactivating sites injected with 0.125% concentration of DMC. The authors concluded that local testing does not correspond to the results obtained with systemic administration, since they demonstrated a phototoxic potentiality, not confirmed in the clinical practice and the experimental observations permitted to affirm that not all TCs have the same phototoxic potentiality.13
Orentreich et al. performed a study where patients affected by acne, furunculosis and folliculitis were treated with DMC 600 mg/day and were then exposed to sunlight, 4 were irradiated with carbon arc lamp. They observed 27/108 sunburns events, all resolved 1 week after therapy cessation. They also described photo‐onicolysis in seven of these patients.14
Dahlen et al. assessed erythema due to sunlight in 20 patients treated with DMC while evaluating the ability of a sunscreen to protect against longwave UV radiations.15
Maibach et al. observed 3 lichenoid eruption occurred in a group of 200 patients treated with DMC 600 mg for 4 days and exposed to sunlight. The author suggested that the lichenoid reaction occurred when wavelengths greater than 3.100 Angstrom crossed the epidermis, after he applied a Mylan plastic on the exposed area.16
Schorr et al. investigated the intradermal injection of DMC and TC in 6 subjects. After exposition to sunlight (5/6) or hot quartz lamp (1 or 2/6), they concluded that 315 μm UV wavelength induced a reaction.17
Willis et al. exposed to a 1600 W high pressure xenon arc lamp subjects treated with DMC 1200 mg given orally and applied by contact on normal and striped skin. They observed an erythema ++ in 10/12 patients who underwent stripping of epidermal horny layer, and only mild (+) erythema in 3/12 patients who did not underwent stripping.18
A study performed in Sweden assess a phototoxic reaction in 1/10 patients exposed to a high intensity sun lamp UVA SUN 2000 after administration on doxycycline 200 mg.19
A recent phase I phototoxicity study assessed the photo‐tests reactions to sarecycline: 12/18 volunteers developed mild erythema at 24 h after UV exposure, while 2/18 48 h after UV exposure.20
3.2. Studies performed on patients
Twenty‐three papers were included: 10 of them are RCT, 9 prospective cohort studies, 3 comparative studies, and 1 a pharmacovigilance register. Most patients were prescribed medications with appropriate standard dosing. The majority of reactions were reported by medical personnel and did not required a specific medical treatment, except than the discontinuation of the therapy. The duration of exposure to the drug and the latency between the drug and the photo‐reaction were not always evaluated.
Two‐hundred‐four reactions have been observed in 1727 patients treated with doxycycline; 25/794 with minocycline, 12/1208 treated with sarecycline, 0/64 treated with TC, 34/178 treated with DMC, 1 with lymecycline and 1 with metacycline (the last two were assessed in the pharmacovigilance register).
The majority of studies included patients treated for acne, which considered 2403 patients and 84 photo‐ reactions (65 phototoxic, 27 described as photosensitive and 1 as a sunburn),14, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30 followed by infectious diseases, including: Lyme disease,31, 32, 33, 34, 35, 36 traveler's diarrhea,37 Gulf Veteran illness.38
Between them, 84 photosensitive reactions were observed in 1421 patients. One study has been performed of patients suffering from pterygium, a condition of common, benign, tumor‐like growth of the cornea, (no reactions in 49 patients)39 and 1 study on abdominal aortic aneurysms (11 photosensitive reactions observed in 36 patients).40
Culprit drugs were: doxycycline, minocycline, TC, sarecycline, DMC and oxytetracycline (Table 1).
The total of reactions reported included data published from a pharmacovigilance database, recorded between 1985 and the end of 2007 in France. It included 69 photosensitivity reactions related to doxycycline, 18 to Minocycline, 1 to Lymecycline and 1 to metacycline. All these reactions were described as photosensitive.41
4. DISCUSSION
The photo‐sensitive reactions due to TCs are heterogeneous and depend on several factors such as: concentration of the drug into the skin, skin type and degree of pigmentation, thickness of the horny layer, immunological and inflammatory status of the patient, irradiance (W/m2) and dose (J/m2) of the activating radiation, wavelength and penetration of the light. TCs are usually associated with phototoxic, but not photoallergic reaction, the former does not depend on an immunological response therefore it can also appear at the first exposure to the drug, but, being dose dependent, it occurs when an adequate amount of the drug and an adequate amounts of non‐ionizing electromagnetic radiations impact the skin. UVA (320–400 nm) is the part of the sun spectrum most frequently associated with phototoxicity, nevertheless UVB (290–320 nm) and visible light (400–760 nm) may contribute to the development of this kind of reaction.42 From a clinical point of view, a phototoxic reaction resembles an acute exaggerated sunburn characterized by erythema, oedema, sometimes vesciculation, burning sensation followed by desquamation and, depending on the skin type, pigmentation, limited to the sun‐exposed skin and appears within minutes or hours after light exposure.
Concerning the mechanism of photosensitization of a drug, there are four main pathways: (1) energy is transferred from the excited photosensitizer (triplet state) to the molecular oxygen generating the excited singlet oxygen which induces oxidation of lipids and proteins of cellular membranes or DNA damage in the skin; (2) an electron or hydrogen transfer to the photosensitizer may generate free‐radical species able of directly damaging the skin biomolecules; moreover the free‐radicals, in the presence of oxygen, could form peroxyl radicals or hydroxyl radical, an intermediate in the oxidative damage of macromolecules such as DNA, lipids and proteins in the skin; (3) a covalent photo‐binding reaction occurring between the photosensitizing substance and cutaneous molecules that can induce cell damages and (4) the photosensitizer generate a photoproduct acting either as toxic substance or as a new photosensitizer.43, 44
Persistence in drug ingestion and sunlight exposure does not result in a decrease in the incidence of photoxicity, since the development of tolerance is not associated with this type of reaction.12
TCs are generally used for medium‐long period treatment, with different indications: infectious (e.g., for both treatment and prevention of brucellosis and malaria), skin diseases (e.g., for acne, because of both antibiotic and anti‐inflammatory properties) or other conditions.
Performing this review, we included also studies evaluating volunteers who were deliberately exposed to light sources, artificial or natural. This kind of trials are theoretically the best way to assess the incidence of phototoxic reaction, in a well‐established setting, on patients without comorbidities or other treatments ongoing. These observations concluded that not all TCs have same phototoxic potential, and that different way of administration are related to different severity reaction; these studies given a basis to the theory that what is observed experimentally in a controlled setting is often not correspondent to the real life evidences. The major contributor of these results was provided by Kligman and Bjellerup, who first assessed the severity of phototoxic reactions comparing different TCs administered by different ways.
Onycholysis is a peculiar clinical sign often described, not always coexistent with the photosensitivity. Photo‐onycholysis is a rare phototoxic reaction caused by prolonged and intense ultraviolet exposure. It can involve both hand and feet, sparing or not some fingers. Usually, it involves some fingers of the hands, in fact the sparing of the photo‐protected toenails strongly supported the diagnosis of photo‐induced onycolysis.45
Lymecycline is not available in North America (but widely used in Europe) and, excluding the drugs currently not anymore available (DMC, oxytetracycline), the number of TCs related phototoxic reactions, considering the wide use if these drugs, is relatively low. It can be the consequence either of actual low incidence or misdiagnosis or scarce tendency to report adverse events usually mild in severity. Prescriptions of TCs during summer are reduced as a number of dermatologists tend to suspend their use, afraid of phototoxic reactions. The older and no more available TCs such as DMC, were largely more prone to induce skin reactions. The TCs family encompasses a number of molecules and not all of them induce significant phototoxic reactions. To date, the use of TCs during summer is still a debated topic, and obviously more data would be welcome to better clarify how phototoxic events are related to different TCs. As a matter of fact, a not relevant number of events are described in relation to minocycline and lymecycline, while most of the reports are related to Doxycycline and DMC. There is a tendency to uniform the entire family of TCs under the definition of “photosensitive drugs,” without discriminating among the different molecules. Some precautions have to be taken with regards to patients who are forced to assume a TCs with a well‐known photosensitivity potential, like doxycycline. For example, in a sunlight setting, the patient must be informed to limit sun exposure, to apply sunscreens and to wear protective clothes, but for other molecules the risk is relatively low and the therapy should be administered independently from the season.
Our review has some limitations. There is a high heterogeneity in the design, populations and objectives of the included studies, moreover, most of them involved a small number of patients. The photo‐reactions occurred are often described as “photosensitive,” without a distinction between phototoxic and photoallergic. Another fact that considerably limits the results reported in the literature, is that some drugs are not yet available in all countries (i.e., sarecycline) or are no longer available (DMC, oxytetracycline). Moreover, a limitation of the studies included concerns the difficulty of carrying out photo‐tests to confirm the cause and effect relationship with the drug taken. Some authors have performed these specific evaluations on volunteers and patients, with both artificial light sources and sunlight. Since these works were aimed to provoke the photo‐reactions, we separately describe them. No reports of challenge/re‐challenge test were found.
In conclusion, more attention in needed in recognize and report the occurrence of photosensitive reactions, clarifying if phototoxic or photo‐allergy reactions occurred, in order to distinguish the real risk of phototoxicity related to each drug. To discriminate between true drug‐related skin reactions and mere excess of sun exposure can help in identifying the real risk of phototoxicity related to each drug. We also recommend keep in mind that not all TCs are related to the same risk and to suggest to the patients who need the therapy to prevent sun exposure and use sunscreens and protective textiles if long sun exposition is performed.
CONFLICT OF INTEREST
The authors confirm that there is no conflict of interest to disclose.
ACKNOWLEDGMENTS
No grants or other forms of payment were given to any of the authors of this manuscript. All the authors made substantive intellectual contributions to the published study and have approved the final version.
Odorici G, Monfrecola G, Bettoli V. Tetracyclines and photosensitive skin reactions: A narrative review. Dermatologic Therapy. 2021;34(4):e14978. 10.1111/dth.14978
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1.Nelson ML, Levy SB. The history of the tetracyclines. Ann N Y Acad Sci. 2011;1241:17‐32. [DOI] [PubMed] [Google Scholar]
- 2.Kim WB, Shelley AJ, Novice K, Joo J, Lim HW, Glassman SJ. Drug‐induced phototoxicity: a systematic review. J Am Acad Dermatol. 2018;79(6):1069‐1075. [DOI] [PubMed] [Google Scholar]
- 3.Phototoxic GM. Photoallergic reactions. In: Johansen JD, Frosch PJ, Lepoittevin J‐P, eds. Contact Dermatitis. Berlin Heidelberg: Springer; 2011:361‐376. [Google Scholar]
- 4.PRISMA, http://www.prisma-statement.org
- 5.CONSORT, http://www.consort-statement.org/
- 6.Blank H, Cullen SI, Catalano PM. Photosensitivity studies with demethylchlortetracycline and doxycycline. Arch Dermatol. 1968;97(1):1‐2. [PubMed] [Google Scholar]
- 7.Frost P, Weinstein GD, Gomez EC. Phototoxic potential of minocycline and doxycycline. Arch Dermatol. 1972;105(5):681‐683. [PubMed] [Google Scholar]
- 8.Frost P, Weinstein GD, Gomez EC. Methacycline and demeclocycline in relation to sunlight. JAMA. 1971;216(2):326‐329. [PubMed] [Google Scholar]
- 9.Harber LC, Tromovitch TA, Baer RL. Studies on photosensitivity due to demethylchloretetracycline. J Invest Dermatol. 1961;37:189‐193. [PubMed] [Google Scholar]
- 10.Bjellerup M, Ljunggren B. Double blind cross‐over studies on phototoxicity to three tetracycline derivatives in human volunteers. Photodermatol. 1987;4(6):281‐287. [PubMed] [Google Scholar]
- 11.Bjellerup M, Ljunggren B. Differences in phototoxic potency should be considered when tetracyclines are prescribed during summer‐time. A study on doxycycline and lymecycline in human volunteers, using an objective method for recording erythema. Br J Dermatol. 1994;130(3):356‐360. [DOI] [PubMed] [Google Scholar]
- 12.Cahn MM, Levy EJ, McMillen JA. Nature and incidence of photosensitivity reactions to demethylchlortetracycline. Arch Dermatol. 1961;84:485‐489. [DOI] [PubMed] [Google Scholar]
- 13.Kligman AM, Breit R. The identification of phototoxic drugs by human assay. J Invest Dermatol. 1968;51(2):90‐99. [DOI] [PubMed] [Google Scholar]
- 14.Orentreich N, Harber L, Tromovitch TA. Photosensitivity and photo‐onycholysis due to demethylchlortetracycline. Arch Dermatol. 1961;83:730‐737. [DOI] [PubMed] [Google Scholar]
- 15.Dahlen RF, Shapiro SI, Berry CZ, Schreiber MM. A method for evaluating sunscreen protection from longwave ultraviolet. J Invest Dermatol. 1970;55(3):165‐169. [DOI] [PubMed] [Google Scholar]
- 16.Maibach HI, Epstein J, Sams M. Letter: photosensitive lichenoid eruption associated with demeclocycline. Arch Dermatol. 1974;109(1):97‐98. [PubMed] [Google Scholar]
- 17.Schorr WF, Monash S. Photo‐irradiation studies of two tetracyclines tetracycline and demethylchlortetracycline. Arch Dermatol. 1963;88(4):440‐444. [DOI] [PubMed] [Google Scholar]
- 18.Willis I, Kligman AM. Diagnosis of photosensitization reactions by the Scotch Tape provocative patch test. J Invest Dermatol. 1968;51(2):116‐119. [DOI] [PubMed] [Google Scholar]
- 19.Rosén K, Swanbeck G. Phototoxic reactions from some common drugs provoked by a high‐intensity UVA lamp. Acta Derm Venereol. 1982;62(3):246‐248. [PubMed] [Google Scholar]
- 20.Pariser DM, Green LJ, Lain EL, et al. Safety and tolerability of sarecycline for the treatment of acne vulgaris: results from a phase III, multicenter, open‐label study and a phase I phototoxicity study. J Clin Aesthet Dermatol. 2019;12(11):E53‐E62. [PMC free article] [PubMed] [Google Scholar]
- 21.Moore AY, Charles JEM, Moore S. Sarecycline: a narrow spectrum tetracycline for the treatment of moderate‐to‐severe acne vulgaris. Future Microbiol. 2019;14(14):1235‐1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leyden JJ, Sniukiene V, Berk DR, Kaoukhov A. Efficacy and safety of sarecycline, a novel, once‐daily, narrow spectrum antibiotic for the treatment of moderate to severe facial acne vulgaris. J Drugs Dermatol. 2018;17(3):333‐338. [PubMed] [Google Scholar]
- 23.Kus S, Yucelten D, Aytug A. Comparison of efficacy of azithromycin vs. doxycycline in the treatment of acne vulgaris. Clin Exp Dermatol. 2005;30(3):215‐220. [DOI] [PubMed] [Google Scholar]
- 24.Sanchez J, Somolinos AL, Almodóvar PI, Webster G, Bradshaw M, Powala C. A randomized, double‐blind, placebo‐controlled trial of the combined effect of doxycycline hyclate 20‐mg tablets and metronidazole 0.75% topical lotion in the treatment of rosacea. J Am Acad Dermatol. 2005;53(5):791‐797. [DOI] [PubMed] [Google Scholar]
- 25.Gruber F, Grubisić‐Greblo H, Kastelan M, Brajac I, Lenković M, Zamolo G. Azithromycin compared with minocycline in the treatment of acne comedonica and papulo‐pustulosa. J Chemother. 1998;10(6):469‐473. [DOI] [PubMed] [Google Scholar]
- 26.Goulden V, Glass D, Cunliffe WJ. Safety of long‐term high‐dose minocycline in the treatment of acne. Br J Dermatol. 1996;134(4):693‐695. [DOI] [PubMed] [Google Scholar]
- 27.Layton AM, Cunliffe WJ. Phototoxic eruptions due to doxycycline—a dose‐related phenomenon. Exp Dermatol. 1993;18(5):425‐427. [DOI] [PubMed] [Google Scholar]
- 28.Hubbell CG, Hobbs ER, Rist T, White JW Jr. Efficacy of minocycline compared with tetracycline in treatment of acne vulgaris. Arch Dermatol. 1982;118(12):989‐992. [PubMed] [Google Scholar]
- 29.Cullen SI, Cohan RH. Minocycline therapy in acne vulgaris. Cutis. 1976;17(6):1208‐1214. [PubMed] [Google Scholar]
- 30.Fuhrman DL, Drowns BV. Demethylchlortetracycline. The clinical aspect of its use in dermatology. Arch Dermatol. 1960;82:244‐246. [DOI] [PubMed] [Google Scholar]
- 31.Velušček M, Bajrović FF, Strle F, Stupica D. Doxycycline‐induced photosensitivity in patients treated for erythema migrans. BMC Infect Dis. 2018;18(1):365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ogrinc K, Logar M, Lotric‐Furlan S, Cerar D, Ruzić‐Sabljić E, Strle F. Doxycycline versus ceftriaxone for the treatment of patients with chronic Lyme borreliosis. Wien Klin Wochenschr. 2006;118(21–22):696‐701. [DOI] [PubMed] [Google Scholar]
- 33.Strle F, Maraspin V, Lotric‐Furlan S, Ruzić‐Sabljić E, Cimperman J. Azithromycin and doxycycline for treatment of Borrelia culture‐positive erythema migrans. Infection. 1996;24(1):64‐68. [DOI] [PubMed] [Google Scholar]
- 34.Luger SW, Paparone P, Wormser GP, et al. Comparison of cefuroxime axetil and doxycycline in treatment of patients with early Lyme disease associated with erythema migrans. Antimicrob Agents Chemother. 1995;39(3):661‐667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nowakowski J, Nadelman RB, Forseter G, McKenna D, Wormser GP. Doxycycline versus tetracycline therapy for Lyme disease associated with erythema migrans. J Am Acad Dermatol. 1995;32(2 Pt 1):223‐227. [DOI] [PubMed] [Google Scholar]
- 36.Nadelman RB, Luger SW, Frank E, Wisniewski M, Collins JJ, Wormser GP. Comparison of cefuroxime axetil and doxycycline in the treatment of early Lyme disease. Ann Intern Med. 1992;117(4):273‐280. [DOI] [PubMed] [Google Scholar]
- 37.Hipskind JE. A prophylactic program to prevent traveler's diarrhea in United States Naval personnel comparing doxycycline and trimethoprim‐sulfamethoxazole. Mil Med. 1993;158(3):141‐144. [PubMed] [Google Scholar]
- 38.Donta ST, Engel CCJ, Collins JF, Baseman JB. Benefits and harms of doxycycline treatment for Gulf War veterans' illnesses: a randomized, double‐blind, placebo‐controlled trial. Ann Intern med. 2004;141(2):85‐94. [DOI] [PubMed] [Google Scholar]
- 39.Rúa O, Larráyoz IM, Barajas MT, Velilla S, Martínez A. Oral doxycycline reduces pterygium lesions; results from a double blind, randomized, placebo controlled clinical trial. PLoS One. 2012;7(12):e52696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baxter BT, Pearce WH, Waltke EA, et al. Prolonged administration of doxycycline in patients with small asymptomatic abdominal aortic aneurysms: report of a prospective (Phase II) multicenter study. J Vasc Surg. 2002;36(1):1‐12. [DOI] [PubMed] [Google Scholar]
- 41.Lebrun‐Vignes B, Kreft‐Jais C, Castot A, Chosidow O. French Network of Regional Centers of Pharmacovigilance. Comparative analysis of adverse drug reactions to tetracyclines: results of a French national survey and review of the literature. Br J Dermatol. 2012;166(6):1333‐1341. [DOI] [PubMed] [Google Scholar]
- 42.Lee A, Thomson J. Drug‐induced skin reactions. In: Lee A, ed. Adverse Drug Reactions. 2nd ed.London: Pharmaceutical Press; 2006:125‐156. [Google Scholar]
- 43.Zaheer MR, Gupta A, Iqbal J, et al. Molecular mechanisms of drug photodegradation and photosensitization. Curr Pharm Des. 2016;22:768‐782. [DOI] [PubMed] [Google Scholar]
- 44.Hasan T, Kochevar IE, McAuliffe DJ, et al. Mechanism of tetracycline phototoxicity. J Invest Dermatol. 1984;83:179‐183. [DOI] [PubMed] [Google Scholar]
- 45.Elmas ÖF, Akdeniz N. A case of doxycyclin‐induced photo‐onycholysis with dermoscopic features. Balkan Med J. 2020;37(2):113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. The data are not publicly available due to privacy or ethical restrictions.


