Abstract
Objective
This study investigated whether arterial stiffening is a determinant of subtle retinal microvascular changes that precede diabetic retinopathy.
Research design and methods
This study used cross‐sectional data from the Maastricht Study, a type 2 diabetes‐enriched population‐based cohort study. We used multivariable linear regression analysis to investigate, in individuals without and with type 2 diabetes, the associations of carotid distensibility coefficient and carotid‐femoral pulse wave velocity with retinal microvascular diameters and flicker light‐induced dilation and adjusted for cardiovascular and lifestyle risk factors.
Results
The retinal microvascular diameter study population consisted of N = 2434 participants (51.4% men, mean ± SD age 59.8 ± 8.1 years, and 28.1% type 2 diabetes). No measures of arterial stiffness were significantly associated with microvascular diameters. Greater carotid distensibility coefficient (i.e., lower carotid stiffness) was significantly associated with greater retinal arteriolar flicker light‐induced dilation (per standard deviation, standardized beta [95% CI] 0.06 [0.00; 0.12]) and non‐significantly, but directionally similarly, associated with greater retinal venular flicker light‐induced dilation (0.04 [−0.02; 0.10]). Carotid‐femoral pulse wave velocity (i.e., aortic stiffness) was not associated with retinal microvascular flicker light‐induced dilation. The associations between carotid distensibility coefficient and retinal arteriolar and venular flicker light‐induced dilation were two‐ to threefold stronger in individuals with type 2 diabetes than in those without.
Conclusion
In this population‐based study greater carotid, but not aortic, stiffness was associated with worse retinal flicker light‐induced dilation and this association was stronger in individuals with type 2 diabetes. Hence, carotid stiffness may be a determinant of retinal microvascular dysfunction.
Keywords: aortic stiffness, arterial stiffness, carotid stiffness, diabetic retinopathy, microvascular dysfunction, retinal microvascular diameter, type 2 diabetes mellitus
1. INTRODUCTION
Clinical diabetic retinopathy is preceded by subtle retinal microvascular changes that include initial widening and later narrowing of retinal microvascular diameters, and impaired retinal microvascular flicker light‐induced dilation.1, 2 These changes are thought to reflect impaired retinal autoregulation, which predisposes to enhanced retinal capillary pressure and consequent capillary dilation, leakage, rupture, and nonperfusion, which are hallmark features of non‐proliferative diabetic retinopathy.2 Widening and narrowing of retinal microvascular diameters are thought to reflect impairment of autoregulation and microvascular remodeling, respectively.2, 3 In turn, lower retinal microvascular flicker light‐induced dilation is thought to reflect dysfunction of the neurovascular coupling unit (i.e., dysfunction of both neuronal and endothelial cells).4 Because hyperglycemia is associated with neurodegeneration5 and endothelial cell dysfunction2, 6, 7 individuals with type 2 diabetes may be especially at risk for lower retinal microvascular flicker light‐induced dilation. Indeed, we have observed lower retinal microvascular flicker light‐induced dilation in individuals with prediabetes as well as in individuals with type 2 diabetes.8
Arterial stiffening may be a potentially reversible determinant of adverse changes in retinal vascular structure and function.9 Mechanistically, arterial stiffening is thought to enhance the propagation of arterial pressure and flow waves.3 This increases transmission of detrimental pulsatile energy into the retinal microcirculation, which is especially vulnerable to arterial stiffening‐induced hemodynamic stress because the retina is a high‐flow, low‐impedance microvascular system.3, 10
Indeed, there is some evidence that arterial stiffening is associated with narrowing of retinal arterioles11, 12, 13, 14, 15 and, possibly, widening of retinal venules,13 although these studies did not adjust for potential confounders such as blood pressure,14 diet,11, 12, 13, 14, 15, 16 and physical activity.11, 12, 13, 14, 15, 16 Current evidence is also limited because the associations between arterial stiffness and flicker light‐induced retinal arteriolar and venular dilation have not yet been investigated.
In view of the above, we studied the associations between arterial stiffness and retinal arteriolar and venular diameters and flicker light‐induced dilation in the population‐based Maastricht Study. We hypothesized that greater arterial stiffness is associated with narrower retinal arterioles, possibly wider retinal venules, lower arteriolar‐to‐venular ratio, and lower retinal arteriolar and venular flicker light‐induced dilation, and that associations may be stronger in individuals with type 2 diabetes.
2. METHODS
2.1. Study population and design
We used data from The Maastricht Study, an observational prospective population‐based cohort study. The rationale and methodology have been described previously.17 In brief, the study focuses on the etiology, pathophysiology, complications, and comorbidities of type 2 diabetes and is characterized by an extensive phenotyping approach. Eligible for participation were all individuals aged between 40 and 75 years and living in the southern part of the Netherlands. Participants were recruited through mass media campaigns and from the municipal registries and the regional Diabetes Patient Registry via mailings. Recruitment was stratified according to known type 2 diabetes status, with an oversampling of individuals with type 2 diabetes, for reasons of efficiency. The present report includes cross‐sectional data from the first 3451 participants, who completed the baseline survey between November 2010 and September 2013. The examinations of each participant were performed within a time window of 3 months. The study has been approved by the institutional medical ethical committee (NL31329.068.10) and the Minister of Health, Welfare and Sports of the Netherlands (Permit 131088‐105234‐PG). All participants gave written informed consent.
2.2. Arterial stiffness
All measurements were done by trained vascular technicians unaware of the participants' clinical or diabetes mellitus status, in a dark, quiet, temperature‐controlled room (21–23°C), as described previously.18, 19 Participants were asked to refrain from smoking and drinking coffee, tea, or alcoholic beverages 3 h before the study. Participants were allowed to have a light meal (breakfast or lunch). All measurements were performed in the supine position after 10 min of rest. Talking or sleeping was not allowed during the examination. During the vascular measurements (≈45 min), brachial systolic, diastolic, and mean arterial pressure (MAP) were determined every 5 min with an oscillometric device (Accutorr Plus, Datascope Inc, Montvale, NJ, USA). The MAP and heart rate during these measurements were used in the statistical analysis. A 3‐lead ECG was recorded continuously during the measurements to facilitate automatic signal processing.
2.2.1. Local carotid arterial elastic properties
Measurements of local carotid arterial properties were done at the left common carotid artery (10‐mm proximal to the carotid bulb), with the use of an ultrasound scanner equipped with a 7.5‐MHz linear probe (MyLab 70, Esaote Europe B.V., Maastricht, the Netherlands). This setup enables the measurement of diameter, distension, and intima‐media thickness (IMT) as described previously.18, 19 Briefly, during the ultrasound measurements, a B‐mode image on the basis of 19 M‐lines was depicted on screen, and an online echo‐tracking algorithm showed real‐time anterior and posterior arterial wall displacements. The M‐mode recordings were composed of 19 simultaneous recordings at a frame rate of 498 Hz. The distance between the M‐line recording positions was 0.96 mm; thus, a total segment of 18.24 mm of each artery was covered by the scan plane. For offline processing, the radiofrequency signal was fed into a dedicated PC‐based acquisition system (ART.LAB, Esaote Europe B.V.) with a sampling frequency of 50 MHz. Data processing was performed in MatLab (version 7.5, Mathworks, Natick, MA, USA). The distension waveforms were obtained from the radio frequency data with the use of a wall‐track algorithm.18 Carotid IMT was defined as the distance of the posterior wall from the leading edge interface between lumen and intima to the leading edge interface between media and adventitia.19 The median diameter, distension, and IMT of three measurements were used in the analyses.
Local arterial elastic properties were quantified by calculating the following indices20:
Distensibility coefficient (DC)
| (1) |
Young's elastic modulus (YEM)
| (2) |
where ΔD is, distension; IMT, intima‐media thickness; and PP, brachial pulse pressure (calculated as systolic blood pressure [BP] minus diastolic BP).
2.2.2. Carotid‐femoral pulse wave velocity
Carotid‐femoral pulse wave velocity (cfPWV; in m/s) was determined according to international guidelines with the use of applanation tonometry (SphygmoCor, Atcor Medical, Sydney, NSW, Australia).21 Pressure waveforms were determined at the right common carotid arteries and right common femoral arteries. Difference in the time of pulse arrival from the R‐wave of the ECG between the two sites (transit time) was determined with the intersecting tangents algorithm. The pulse wave travel distance was calculated as 80% of the direct straight distance (measured with an infantometer) between the two arterial sites. The median of three consecutive cfPWV (defined as traveled distance/transit time) recordings was used in the analyses. Details are provided in the Appendix S1.
2.3. Retinal microvascular function measures
All participants were asked to refrain from smoking and drinking caffeine‐containing beverages 3 h before the measurement.22, 23 A light meal (breakfast and (or) lunch), low in fat content, was allowed if taken at least 90 min prior to the start of the measurements.
For retinal measurements, pupils were dilated with 0.5% tropicamide and 2.5% phenylephrine at least 15 min prior to the start of the examination.
2.3.1. Retinal microvascular diameters
All fundus photographs were taken with an auto‐focus, auto‐shot, and auto‐tracking fundus camera (Model AFC‐230; Nidek, Gamagori, Japan) in an optic disc‐centered field of view of 45° in a darkened room. Static retinal vessel analysis (one image of the left or right eye was randomly chosen per participant) was performed using the retinal health information and notification system (RHINO) software developed by the RetinaCheck group of the Technical University of Eindhoven (Eindhoven, the Netherlands).24, 25 Optic disc detection and arteriole/venule classification were corrected manually. Retinal vessel diameters were measured at 0.5–1.0 disc diameter away from the optic disc margin and were presented as central retinal arteriolar equivalent and central retinal venular equivalent (CRAE and CRVE, respectively) in measurement units (MU) and as arteriole‐to‐venule ratio (AVR). The scale factor is based on the optic disc diameter, which is assumed to be 1800 μm,26 that is, 1 MU = 1 pixel size × 1800 μm/pixel size of optic disc diameter. CRAE and CRVE represent the equivalent single‐vessel parent diameter for the six largest arterioles and largest venules in the region of interest, respectively. The calculations were based on the improved Knudtson‐Parr‐Hubbard formula.27
Fundus photographs of insufficient quality, for example, obstructed by lashes or defocused, were evaluated and discussed with a second observer and excluded on mutual agreement. We calculated the intraclass correlation coefficients for CRAE and CRVE to assess the agreement between analyses of the RHINO software with versus without manual identification of arterioles and venules using 2556 images. The intraclass correlation coefficient of CRAE was 0.910 and that of CRVE was 0.897.
2.3.2. Retinal microvascular flicker light‐induced dilation
The retinal arteriolar and venular dilation response to flicker light, which is thought to be related to nutritive demands of activated retinal neurons,28 was measured in a dimly lit room by use of the Dynamic Vessel Analyzer (DVA) (IMEDOS, Jena, Germany). Per participant, we randomly measured the left or right eye.
During the measurement, the participant was instructed and encouraged to focus on the tip of a fixated needle inside the retinal camera (FF450; Carl Zeiss GmbH, Jena, Germany), while the fundus of the eye was examined under green measuring light (530–600 nm, illumination of fundus approximately 6500 lux). A straight arteriolar or venular segment of approximately 1.5 mm in length located 0.5 to 2.0 disc diameter from the margin of the optic disc in the temporal section was examined. When the specific vessel profile was recognized, vessel diameter was automatically and continuously measured for 150 s. A baseline recording of 50 s was followed by a 40‐s flicker‐light exposure period (flicker frequency 12.5 Hz, bright‐to‐dark contrast ratio 25:1) followed by a 60‐s recovery period. The DVA automatically corrected for alterations in luminance caused by, for example, slight eye movements. During blinks and small eye movements, the registration stopped and restarted once the vessel segments were automatically re‐identified.28
The integrated DVA software (version 4.51, Imedos) automatically calculated baseline diameter and percentage dilation. Baseline diameter was calculated as the average diameter size of the 20–50 s recording and was expressed in measurement units (MU), where 1 MU is equal to 1 µm of the Gullstrand's eye.29 Percentage dilation over baseline was based on the average dilation achieved at time‐points 10 and 40 s during the flicker stimulation period. Two regression lines were drawn (at intervals 0–10 s and 10–40 s during flicker stimulation) and averaged to assess average percentage dilation. The software successfully assessed two regression lines in 95.4% of the curves; only 102 dilation curves (4.6%) were based on one regression line. The purpose of taking the average dilation was to account for interindividual variation in the curve shape during dilation. More details are provided in the Appendix S1.
2.4. Covariates
We determined glucose metabolism status (normal glucose metabolism, prediabetes, type 1 diabetes, or type 2 diabetes) based on a 75‐gram oral glucose tolerance test and use of glucose‐lowering medication according to the World Health Organization 2006 criteria.17 Education level (‘educational status’) was classified into three groups: low (none, primary or lower vocational education only), medium (intermediate general secondary, intermediate vocational or higher general secondary education), and high (higher vocational education or university level of education). Alcohol consumption was classified as non‐consumer, low‐consumer (≤7 alcoholic drinks/week for women; ≤14 alcoholic drinks/week for men), or high‐consumer (>7 alcoholic drinks/week for women; >14 alcohol drinks/week for men). We determined age, sex, smoking status (never, former, current), medication use, waist circumference, body mass index (BMI), total/high‐density lipoprotein (HDL) cholesterol ratio, triglycerides, fasting plasma glucose, 2‐h post‐load glucose, and glycated hemoglobin (HbA1c), office and ambulatory blood pressure, accelerometer‐assessed physical activity, estimated glomerular filtration rate (eGFR), albuminuria, prior cardiovascular disease, plasma biomarkers of low‐grade inflammation (i.e., high‐sensitive C‐reactive protein, serum amyloid A, interleukin‐6, interleukin‐8, and tumor necrosis factor‐alpha) and retinopathy as described previously.5, 17, 30, 31, 32 An automated refractor (Tonoref II; Nidek) was used for automated refraction and noncontact tonometry assessment in both eyes. We determined glaucoma as the use of intraocular pressure‐lowering medication or intraocular pressure greater than 21 mm Hg. We used a validated food frequency questionnaire to assess the Dutch Health Diet score (‘diet score’).33, 34 As described previously, we used questionnaires to assess income level and occupational status.35
2.5. Statistical analyses
All analyses were performed with Statistical Package for Social Sciences version 23.0 (IBM SPSS, IBM Corp, Armonk, NY, USA). We used multivariable linear regression analysis to investigate the associations between (standardized) carotid DC, YEM, and cfPWV (determinants) with (standardized) retinal microvascular diameters (i.e., CRAE, CRVE, AVR) and retinal arteriolar and venular flicker light‐induced dilation (outcomes). We checked whether measures of arterial stiffness were associated with baseline diameter in analyses of retinal microvascular flicker light‐induced percentage dilation because such associations may result in spurious estimates of associations, but this was not the case [data are not shown].
Model 1 shows crude results. In model 2 we adjusted for age, sex, glucose metabolism status (entered as dummies [i.e., type 2 diabetes, or prediabetes, or other types of diabetes versus normal glucose metabolism status]). In model 3, we additionally adjusted for mean arterial pressure and heart rate. We chose these variables as they are key potential confounders (all) or were oversampled (type 2 diabetes). In model 4, we additionally adjusted for variables whose status as potential confounders has been less firmly established (use of antihypertensive medication [yes/no], waist circumference, total cholesterol/HDL cholesterol ratio, lipid‐modifying medication, smoking status [current, ever, never], alcohol consumption status [none, low, high] and educational status [low, medium, high]).
Data were expressed as standardized regression coefficient and corresponding 95% confidence interval (95% CI). Collinearity diagnostics (i.e., tolerance <0.10 and/or variance inflation factor >10) were used to detect multicollinearity between covariates. For analyses, p‐value < .05 was considered statistically significant.
To assess whether associations differed by type 2 diabetes status (i.e., between individuals with type 2 diabetes and individuals without diabetes) or sex we tested for interaction. We excluded participants with other types of diabetes from the interaction analyses because the number of participants with other types of diabetes was small. We hypothesized a priori that associations may be stronger individuals with type 2 diabetes as under hyperglycemic circumstances the retinal microvasculature is likely more vulnerable. As most previous studies did not find interaction by sex, we, a priori, did not expect interaction by sex to be likely.11, 14 p interaction < .10 was considered statistically significant.
2.5.1. Additional analyses
First, we repeated the analysis additionally adjusting for the diet score, physical activity, and refractive error. Adjustment for these potential confounders was not included in the main analysis because data were missing in a relatively large number of participants (n = 120–488 had missing data on one or more of these variables). Second, we additionally adjusted for kidney variables (eGFR and albuminuria), prior cardiovascular disease, plasma biomarkers of low‐grade inflammation, retinopathy, and glaucoma. We adjusted for these covariates in a separate model because these variables may be confounders, but may also (partly) mediate the associations under study. Third, we substituted glucose metabolism status by fasting plasma glucose, 2‐h post‐load glucose, or HbA1c; waist circumference by BMI; mean arterial pressure measured during the vascular ultrasound measurement by 24‐h mean arterial pressure; and educational status by occupational status and income level. Fourth, when type 2 diabetes status modified associations, we repeated tests for interaction analyses with continuous measures of hyperglycemia (i.e., fasting plasma glucose, 2‐h post‐load glucose, and HbA1c).
3. RESULTS
3.1. Selection and characteristics of the study population
Figure 1 shows an overview of the study population selection.
FIGURE 1.
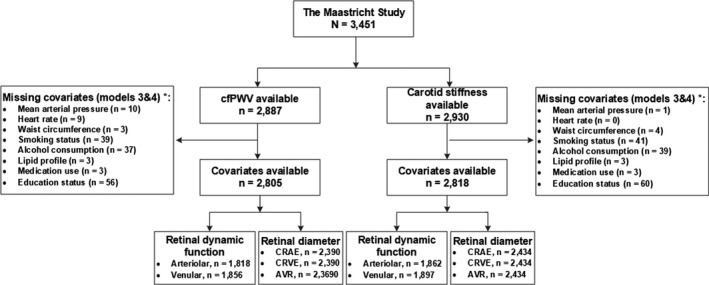
Retinal microvascular diameter and retinal microvascular flicker light‐induced dilation study population selection
Table 1 shows general characteristics according to retinal arteriolar diameter (CRAE) and Table S1 shows general characteristics according to retinal venular flicker light‐induced dilation. Overall, participants with a narrower arteriolar caliber and lower venular flicker light‐induced dilation were more often men, had a higher age, and had a more adverse cardiovascular risk profile. General characteristics of participants included in the study were highly comparable to those of participants with missing data (Table S2).
TABLE 1.
General study population characteristics according to tertiles of arteriolar diameter
| Characteristic | Total study group (N = 2434) | Tertiles of arteriolar diameter | ||
|---|---|---|---|---|
| Tertile 1 (low) (n = 811) | Tertile 2 (middle) (n = 809) | Tertile 3 (high) (n = 814) | ||
| Demographics | ||||
| Age, years | 59.8 ± 8.2 | 61.3 ± 7.9 | 59.6 ± 8.1 | 58.6 ± 8.3 |
| Men | 1252 (51.4%) | 477 (58.8%) | 414 (51.2%) | 453 (55.7%) |
| Educational status | ||||
| Low | 801 (32.9%) | 269 (33.2%) | 266 (32.9%) | 266 (32.7%) |
| Medium | 707 (29.0%) | 230 (28.4%) | 242 (29.9%) | 235 (28.9%) |
| High | 926 (38.0%) | 312 (38.5%) | 301 (37.2%) | 313 (38.5%) |
| Occupational statusa | ||||
| Low | 654 (32.2%) | 201 (29.6%) | 212 (32.1%) | 241 (35.0%) |
| Middle | 701 (34.6%) | 233 (34.3%) | 242 (36.7%) | 226 (32.8%) |
| High | 673 (33.2%) | 245 (36.1%) | 216 (31.2%) | 222 (32.2%) |
| Income per month, eurosa | 2016.8 ± 814.0 | 2084 ± 831 | 2007 ± 789 | 1959 ± 818 |
| Lifestyle factors | ||||
| Smoking status | ||||
| Never | 817 (33.6%) | 255 (31.4%) | 276 (34.1%) | 286 (35.1%) |
| Former | 1302 (53.5%) | 467 (57.6%) | 432 (53.4%) | 403 (49.5%) |
| Current | 315 (12.9%) | 89 (11.0%) | 101 (12.5%) | 125 (15.4%) |
| Alcohol consumption | ||||
| None | 440 (18.1%) | 131 (16.2%) | 135 (16.7%) | 174 (21.4%) |
| Low (women ≤7, men ≤14) | 1368 (56.2%) | 441 (54.4%) | 480 (59.3%) | 447 (54.9%) |
| High (women >7, men >14) | 626 (25.7%) | 239 (29.5%) | 194 (24.0%) | 193 (23.7%) |
| Physical activity, h/daya | 2.0 ± 0.7 | 1.9 [1.5–2.5] | 2.0 [1.5–2.4] | 1.9 [1.5–2.4] |
| Dutch Healthy diet score, points | 83.6 ± 14.6 | 82.8 ± 14.4 | 84.4 ± 14.4 | 83.7 ± 14.8 |
| Cardiovascular risk factors | ||||
| Glucose metabolism status | ||||
| Normal glucose metabolism | 1364 (56.0%) | 429 (52.9%) | 470 (58.1%) | 465 (57.1%) |
| Prediabetes | 357 (14.7%) | 130 (16.0%) | 119 (14.7%) | 108 (13.3%) |
| Type 2 diabetes | 684 (28.1%) | 238 (29.3%) | 212 (26.2%) | 234 (28.7%) |
| Other types of diabetes | 29 (1.2%) | 14 (1.7%) | 8 (1.0%) | 7 (0.9%) |
| Fasting plasma glucose, mmol/La | 5.6 [5.1–6.5] | 5.6 [5.1–6.7] | 5.5 [5.1–6.4] | 5.5 [5.0–6.5] |
| 2‐h post‐load plasma glucose, mmol/La | 6.2 [5.1–9.4] | 6.5 [5.2–9.7] | 6.2 [5.1–9.1] | 6.1 [4.9–9.5] |
| HbA1c, %a | 5.7 [5.4–6.2] | 5.7 [5.4–6.3] | 5.7 [5.4–6.2] | 5.6 [5.4–6.2] |
| HbA1c, mmol/mola | 39 [36–44] | 39 [36–45] | 39 [36–44] | 38 [36–44] |
| Glucose lowering medication | 568 (23.3%) | 201 (24.8%) | 179 (22.1%) | 188 (23.1%) |
| Waist circumference, cm | 27.0 ± 4.5 | 27.0 ± 4.3 | 27.0 ± 4.3 | 27.1 ± 4.8 |
| Body mass index, kg/m2 a | 27.0 ± 4.5 | 27.0 ± 4.3 | 27.0 ± 4.3 | 27.1 ± 4.8 |
| History of cardiovascular diseasea | 405 (16.7%) | 139 (17.3%) | 124 (15.4%) | 142 (17.6%) |
| eGFR, ml/min/1.73 m2 a | 88.3 ± 14.8 | 87.9 ± 14.9 | 88.7 ± 14.3 | 88.3 ± 15.2 |
| Albuminuria, mg/24 ha | 6.6 [4.0–11.8] | 7.2 [4.4–13.4] | 6.3 [3.8–11.3] | 6.2 [3.8–10.7] |
| Total‐to‐HDL cholesterol ratio | 3.6 ± 1.2 | 3.6 ± 1.2 | 3.6 ± 1.2 | 3.6 ± 1.1 |
| Triglycerides, mmol/L | 1.2 [0.9–1.7] | 1.2 [0.9–1.7] | 1.2 [0.9–1.7] | 1.2 [0.9–1.7] |
| Use of lipid‐modifying medication | 910 (37.4%) | 341 (42.0%) | 279 (34.5%) | 290 (35.6%) |
| Office systolic BP, mmHga | 134.1 ± 18.2 | 138.7 ± 18.3 | 134.2 ± 17.5 | 131.6 ± 18.0 |
| Office diastolic BP, mmHga | 76.2 ± 10.0 | 78.1 ± 10.1 | 76.1 ± 9.7 | 74.2 ± 9.7 |
| Mean arterial pressure, mmHg | 96.5 ± 10.2 | 98.9 ±10.4 | 96.4 ± 9.9 | 94.3 ± 9.7 |
| 24‐h mean arterial pressure, mmHga | 88.6 ± 8.0 | 90.5 ± 8.2 | 88.5 ± 7.8 | 86.7 ± 7.4 |
| Heart rate, bpm | 62.6 ± 9.4 | 62.6 ± 9.5 | 62.5 ± 9.1 | 62.7 ± 9.6 |
| Use of antihypertensive medication | 974 (40.0%) | 354 (43.6%) | 322 (39.8%) | 298 (36.6%) |
| Inflammation markersa | ||||
| C‐reactive protein, µg/ml | 1.3 [0.6–2.7] | 1.3 [0.6–2.6] | 1.2 [0.6–2.7] | 1.3 [0.6–2.9] |
| Serum amyloid A, µg/ml | 3.3 [2.1–5.4] | 3.2 [2.2–5.5] | 3.3 [2.0–5.6] | 3.4 [2.0–5.5] |
| Tumor necrosis factor α, pg/ml | 2.2 [1.9–2.6] | 2.2 [1.9–2.6] | 2.2 [1.9–2.5] | 2.2 [1.9–2.6] |
| Interleukin‐6, pg/ml | 0.6 [0.4–0.9] | 0.6 [0.4–0.9] | 0.6 [0.4–0.9] | 0.6 [0.4–0.9] |
| Interleukin‐8, pg/ml | 4.1 [3.3–5.3] | 4.2 [3.4–5.4] | 4.1 [3.2–5.2] | 4.0 [3.3–5.2] |
| Eye variables | ||||
| Retinopathya | 38 (1.7%) | 13 (1.7%) | 13 (1.7%) | 12 (1.6%) |
| Glaucomaa | 114 (5.2%) | 46 (5.7%) | 40 (4.9%) | 28 (3.4%) |
| Refractive errora | ||||
| Right eye | 0.1 [−1.5 to 1.1] | 0.0 [−2.0 to 1.0] | 0.1 [−1.50 to 1.1] | 0.1 [−1.0 to 1.3] |
| Left eye | 0.1 [−1.4 to 1.1] | −0.1 [−2.3 to 0.9] | 0.13 [−1.4 to 1.13] | 0.3 [−1.0 to 1.3] |
| Arterial stiffness | ||||
| cfPWV, m/sb | 9.0 ± 2.1 | 9.3 ± 2.2 | 8.9 ± 2.0 | 8.8 ± 2.1 |
| Carotid DC, 10−3/kPa | 14.5 ± 5.12 | 13.9 ± 5.1 | 14.3 ± 4.9 | 15.2 ± 5.4 |
| Carotid YEM, 103/kPaa | 0.73 ± 0.34 | 0.77 ± 0.35 | 0.75 ± 0.33 | 0.70 ± 0.33 |
| Retinal microvascular function | ||||
| Arteriolar diameter (CRAE), µm | 142.2 ± 20.4 | 120.2 ± 10.9 | 141.9 ± 5.2 | 164.6 ± 10.7 |
| Venular diameter (CRVE), µm | 214.4 ± 31.1 | 190.9 ± 24.5 | 213.1 ± 21.6 | 239.2 ± 25.7 |
| Arteriole‐to‐venule ratio (AVR), no unit | 0.67 ± 0.08 | 0.64 ± 0.08 | 0.67 ± 0.07 | 0.69 ± 0.07 |
| Arteriolar flicker light‐induced dilation, %c | 3.0 ± 2.8 | 3.2 ± 2.9 | 3.1 ± 2.8 | 2.8 ± 2.8 |
| Venular flicker light‐induced dilation, %c | 3.9 ± 2.2 | 3.9 ± 2.3 | 3.8 ± 2.1 | 4.0 ± 2.4 |
Data presented as mean ± standard deviation, median [interquartile range], or number (%).
Abbreviations: AVR, arterio‐to‐venule ratio; BP, blood pressure; carotid DC, carotid distensibility coefficient; carotid YEM, carotid Young elastic modulus; cfPWV, carotid ‐femoral pulse wave velocity; CRAE, central retinal arteriolar equivalent; CRVE, central retinal venular equivalent; eGFR; estimated glomerular filtration rate; HbA1c, glycated hemoglobin; HDL, high‐density lipoprotein.
Data available for occupational status, n = 2028; income level, n = 1841; body mass index, n = 2433; physical activity, n = 1946; Dutch healthy diet score, n = 2314; fasting plasma glucose, n = 2433; 2‐h post‐load plasma glucose, n = 2249; HbA1c, n = 2429; history of cardiovascular disease, n = 2418; eGFR, n = 2416; albuminuria, n = 2415; office systolic and diastolic blood pressure, n = 2432; 24‐h mean arterial pressure, n = 2169; inflammation markers, n = 2411; retinopathy, n = 2254; glaucoma, n = 2211; refractive error right eye, n = 2237, refractive error left eye n = 2229; carotid YEM, n = 2432.
Value shown for cfPWV study population with complete retinal arteriolar diameter data.
Value shown for carotid stiffness population with complete arteriolar or venular dilation data.
3.2. Arterial stiffness and retinal microvascular diameters
After adjustment, neither carotid DC, nor carotid YEM, nor cfPWV were significantly associated with CRAE (per SD, standardized beta [95% CI], respectively, −0.00 [−0.05, 0.05]; 0.02 [−0.02; 0.07]; 0.03 [−0.02; 0.08]), CRVE (−0.01 [−0.06; 0.04]; 0.02 [−0.02; 0.07]; 0.00 [−0.05; 0.05]) or AVR (0.01 [−0.04; 0.06]; 0.00 [−0.04; 0.05]; 0.04 [−0.01; 0.09]) (Table 2).
TABLE 2.
Associations of aortic and carotid stiffness with retinal microvascular diameter and flicker light‐induced dilation
| Arterial stiffness | Model | Retinal microvascular diameter | Retinal microvascular flicker light‐induced dilation | |||
|---|---|---|---|---|---|---|
|
CRAE Stβ (95%CI) |
CRVE Stβ (95%CI) |
AVR Stβ (95%CI) |
Arteriolar dilation Stβ (95%CI) |
Venular dilation Stβ (95%CI) |
||
| Carotid DC, per SD | 1 | 0.11 (0.07; 0.15) | 0.03 (−0.01; 0.07) | 0.11 (0.07; 0.15) | 0.09 (0.04;0.13) | 0.03 (−0.01; 0.08) |
| 2 | 0.07 (0.03; 0.12) | 0.00 (−0.04; 0.05) | 0.09 (0.04; 0.13) | 0.03 (−0.02; 0.08) | 0.01 (−0.04; 0.06) | |
| 3 | −0.00 (−0.05; 0.05) | −0.00 (−0.06; 0.04) | 0.01 (−0.04; 0.06) | 0.06 (0.00; 0.12) | 0.04 (−0.01; 0.10) | |
| 4 | −0.00 (−0.05; 0.05) | −0.01 (−0.06; 0.04) | 0.01 (−0.04; 0.06) | 0.06 (0.00; 0.12) | 0.04 (−0.02; 0.10) | |
| Carotid YEM, per SD | 1 | −0.08 (−0.12; −0.04) | −0.00 (−0.04; 0.04) | −0.09 (−0.13; −0.05) | −0.05 (−0.09; 0.00) | −0.02 (−0.07; 0.02) |
| 2 | −0.04 (−0.08; 0.01) | 0.02 (−0.03; 0.06) | −0.06 (−0.11; −0.02) | 0.01 (−0.04; 0.05) | −0.00 (−0.05; 0.05) | |
| 3 | 0.02 (−0.02; 0.07) | 0.03 (−0.02; 0.07) | −0.00 (−0.05; 0.04) | −0.01 (−0.06; 0.04) | −0.03 (−0.08; 0.03) | |
| 4 | 0.02 (−0.02; 0.07) | 0.02 (−0.02; 0.07) | 0.00 (−0.04; 0.05) | −0.01 (−0.06; 0.05) | −0.02 (−0.07; 0.03) | |
| cfPWV, per SD | 1 | −0.11 (−0.15; −0.07) | −0.04 (−0.08; 0.01) | −0.09 (−0.13; −0.05) | −0.08 (−0.13; −0.04) | −0.04 (−0.09; 0.01) |
| 2 | −0.05 (−0.09; 0.00) | −0.01 (−0.05; 0.04) | −0.05 (−0.10; −0.01) | 0.00 (−0.05; 0.06) | 0.00 (−0.05; 0.05) | |
| 3 | 0.03 (−0.02; 0.08) | 0.00 (−0.05; 0.05) | 0.04 (−0.01; 0.09) | −0.02 (−0.07; 0.04) | −0.03 (−0.08; 0.03) | |
| 4 | 0.03 (−0.02; 0.08) | 0.00 (−0.05; 0.05) | 0.04 (−0.01; 0.09) | −0.01 (−0.07; 0.05) | −0.02 (−0.08; 0.04) | |
Standardized regression coefficient (stβ) represents arteriolar or venular dilation in SD per SD greater arterial stiffness. For the retinal microvascular diameter population 1 SD corresponds with 5.16 × 10−3/kPa for carotid DC, 0.34 × 103/kPa for carotid YEM, 2.08 m/s for cfPWV, 20.39 µm for CRAE, 31.07 µm for CRVE and 0.08 (no unit) for AVR. For the retinal arteriolar flicker light‐induced dilation population 1 SD corresponds with 5.14 × 10−3/kPa for carotid DC, 0.37 × 103/kPa for carotid YEM, 2.07 m/s for cfPWV, 2.82% for flicker light‐induced arteriolar dilation and 2.20% for flicker light‐induced venular dilation. Bold denotes p < .05. Model 1: crude. Model 2: age, sex, and glucose metabolism status; model 3: model 2 + mean arterial pressure and heart rate; model 4: model 2 + smoking status, alcohol consumption, waist circumference, total‐to‐high density lipoprotein cholesterol ratio, lipid‐modifying and antihypertensive medication, educational status.
Abbreviations: AVR, arteriole‐to‐venule‐ratio; cfPWV, carotid‐to‐femoral pulse wave velocity; CI, confidence interval; CRAE, central retinal arteriolar equivalent; CRVE, central retinal venular equivalent; DC, distensibility coefficient; SD, standard deviation; YEM, Young's elastic modulus.
Type 2 diabetes status and sex did not modify any of the above associations (Table S3).
3.3. Arterial stiffness and flicker light‐induced retinal arteriolar and venular dilation
After adjustment, greater carotid DC was significantly associated with greater retinal arteriolar and non‐significantly, but directionally similarly, associated with greater retinal venular flicker light‐induced dilation (respectively, 0.06 [0.00; 0.12]; 0.04 [−0.02; 0.10]; Table 2 and Figure 2). In contrast, carotid YEM and cfPWV were not significantly associated with retinal arteriolar flicker light‐induced dilation (respectively, −0.01 [−0.06; 0.05] and −0.01 [−0.07; 0.05]) or with retinal venular flicker light‐induced dilation (respectively, −0.02 [−0.07; 0.03] and −0.02 [−0.078 0.04]).
FIGURE 2.
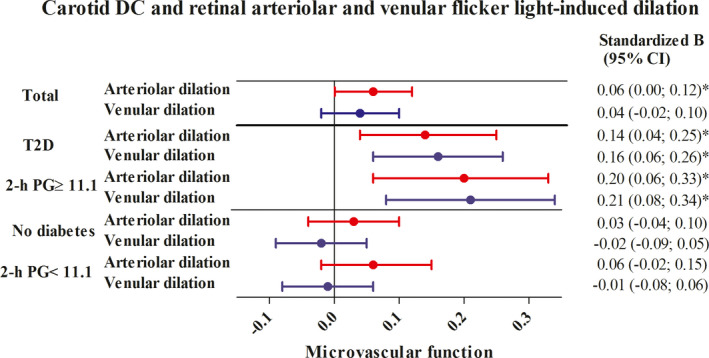
Associations between carotid DC (per SD) and retinal arteriolar and venular flicker light‐induced dilation (per SD). Standardized regression coefficient (stβ) represents the difference in arteriolar or venular dilation in SD per SD greater carotid DC. In the total population, 1 SD corresponds with 5.14 × 10−3/kPa for carotid DC, 2.82% for flicker light‐induced arteriolar dilation, and 2.20% for flicker light‐induced venular dilation. In participants with type 2 diabetes, 1 SD corresponds with 4.8 × 10−3/kPa for carotid DC, 2.7% for flicker light‐induced arteriolar dilation, and 2.3% for flicker light‐induced venular dilation in participants without diabetes. In participants without diabetes 1 SD corresponds with 5.2 × 10−3/kPa for carotid DC, 2.9% for flicker light‐induced arteriolar dilation, and 2.2% for flicker light‐induced venular dilation. For participants with higher and lower levels of 2‐h PG (≥11.1 mmol/L vs. <11.1), values per SD were quantitatively similar to values per SD for participants with type 2 diabetes and without diabetes, respectively. Variables in model in addition to carotid DC: age, sex, and glucose metabolism status, mean arterial pressure and heart rate, smoking status, alcohol consumption, waist circumference, total‐to‐high density lipoprotein cholesterol ratio, lipid‐modifying and antihypertensive medication, educational status. In stratified analyses, glucose metabolism status was not included in the model. *Indicates statistically significant (p < .05). 2‐h PG, 2‐h post‐load glucose; CI, confidence interval; DC, distensibility coefficient; SD, standard deviation; T2D, type 2 diabetes
Type 2 diabetes modified the associations between carotid DC and arteriolar and venular flicker light‐induced dilation (p interaction = .02 and p interaction = .01, respectively). Sex modified the associations between carotid DC and carotid YEM with venular flicker light‐induced dilation (p interaction = .02 and p interaction = .02, respectively; p interaction values are shown in Table S3). In participants with type 2 diabetes, but not in participants without diabetes, greater carotid DC was significantly associated with greater arteriolar and venular flicker light‐induced dilation (respectively, 0.14 [0.04; 0.25] and 0.16 [0.06; 0.26] in participants with type 2 diabetes versus 0.03 [−0.04; 0.10] and −0.02 [−0.09; 0.05] in participants without diabetes; Table S4 and Figure 2). In men, but not in women, carotid DC and YEM were significantly associated with retinal venular flicker light‐induced dilation (respectively, 0.11 [0.03; 0.19] and −0.08 [−0.16; −0.01] in men versus −0.03 [−0.11; 0.06] and 0.05 [−0.03; 0.12] in women; Table S5). There was no three‐way interaction by type 2 diabetes status and sex (p interaction > .10) (data are not shown).
3.4. Additional analyses
Quantitatively similar results were observed in a range of sensitivity analyses. First, after additional adjustment for diet score, physical activity, and refractive error, associations did not materially change (Table S6). Second, associations remained similar after additional adjustment for kidney variables, prior cardiovascular disease, plasma biomarkers of low‐grade inflammation, retinopathy, and glaucoma (Table S6). Third, associations were not materially altered when glucose metabolism status was substituted by fasting plasma glucose, 2‐h post‐load glucose or HbA1c; when waist circumference was substituted by BMI; when mean arterial pressure measured during the vascular ultrasound measurement was substituted by 24‐h mean arterial pressure; or when educational status was substituted by occupational status or income level (Table S7). Fourth, when we replaced glucose metabolism status by continuous measures of hyperglycemia, 2‐h post‐load glucose modified the association between carotid DC and retinal arteriolar and venular flicker light‐induced dilation (p interaction = .05 and p interaction = .03, respectively) and HbA1c modified the association between carotid DC and retinal venular flicker light‐induced dilation (p interaction = .07; Table S3). In stratified analyses, the only significant associations were between carotid DC and arteriolar and venular flicker light‐induced dilation in participants with WHO‐defined17 higher levels of 2‐h post‐load glucose (≥11.1 mmol/L; 0.20 [0.06; 0.33] and 0.21 [0.08; 0.34], respectively; Table S5; Figure 2).
4. DISCUSSION
The current population‐based study has three main findings. First, carotid and aortic stiffness were not significantly associated with retinal arteriolar and venular diameters. Second, carotid but not aortic stiffness was significantly associated with worse retinal arteriolar flicker light‐induced dilation in individuals with type 2 diabetes but not in individuals without diabetes. Third, carotid but not aortic stiffness was significantly associated with worse retinal venular flicker light‐induced dilation in individuals with type 2 diabetes, but not in individuals without diabetes; and in men, but not in women.
In contrast to previous studies,11, 12, 13, 14, 15 we observed no association between arterial stiffness and narrower retinal arteriolar diameters, possibly because inward remodeling (the proximate cause of arteriolar narrowing)3 occurs in a more advanced disease stage than was present in the population that we studied. Additionally, and similar to most previous studies,11, 14, 15 we observed no association of arterial stiffness with retinal venular diameters, possibly because hemodynamic stress in venules is insufficient to affect their structure.
Retinal arteriolar and venular flicker light‐induced dilation (which represent measures of microvascular function and specifically neurovascular coupling) may be more easily affected by arterial stiffness than retinal arteriolar and venular diameters (which presumably mostly reflect microvascular structure).36 Indeed, we observed that carotid stiffness was significantly associated with less retinal arteriolar and non‐significantly with less venular flicker light‐induced dilation. The observation that carotid stiffness was two‐ to threefold more strongly associated with lower flicker light‐induced dilation under hyperglycemic circumstances (i.e., in individuals with type 2 diabetes and with higher levels of 2‐h post‐load glucose) suggests that the retinal microvasculature may be more vulnerable to hemodynamic stress in the presence of hyperglycemia. Associations were likely stronger because hyperglycemia impairs both neuronal1 and endothelial cell2 function (i.e., neurovascular coupling) and intact neurovascular coupling is required for flicker light‐induced dilation.4, 6, 7
Additionally, we consider the observation that the association between greater carotid stiffness and worse retinal venular dilation was stronger in men exploratory. Further investigation of this observation is warranted.
The lack of an association of both carotid YEM and aortic stiffness with retinal microvascular flicker light‐induced dilation does not necessarily contradict the findings above. Carotid YEM reflects stiffness of the material of the vessel wall,10 but not the arterial stiffening‐induced hemodynamic stress which is thought to be detrimental to the retinal microvasculature.11, 12, 13, 14, 15 Aortic stiffening, which reflects both muscular and elastic artery remodeling (in contrast to carotid stiffening which [mostly] reflects elastic remodeling), may not have yet occurred to such an extent that greater aortic pulsatile hemodynamic stress affects the retinal microvasculature.37
Strengths of this study are the large size of this population‐based cohort study with oversampling of individuals with type 2 diabetes which enables accurate comparison of individuals with and without diabetes, the large number of potential confounders which were considered, and the standardized assessment of all variables included in this study. Carotid and aortic stiffness, as well as retinal microvascular diameters, were measured with state‐of‐the‐art methods.21, 23, 25
Limitations include the following. First, due to the cross‐sectional nature of the study, we cannot account for temporality and, therefore, causal inferences should be made with considerable caution. Although many data support the concept that arterial stiffening may lead to hemodynamic stress in the retinal microvasculature, in theory, we cannot with the current data exclude that option that arterial stiffening and retinal microvascular dysfunction reflect separate entities of macrovascular and microvascular angiopathies in patients with diabetes.3 Second, we may have underestimated the strength of the association between arterial stiffness and retinal microvascular flicker light‐induced dilation response if such an association was stronger among participants that were excluded from the study population (who generally tend to be sicker). Additionally, the use of brachial rather than carotid pulse pressure to calculate carotid DC will again tend to underestimate associations through regression dilution.10, 38 Third, even though we took an extensive set of confounders into account, we cannot fully exclude residual confounding.39 Fourth, we studied Caucasian individuals aged 40–75 years, and therefore, our results may be generalizable to such a population; whether these results also apply to other populations, for example, older populations, requires further study.40
In summary, in this population‐based study greater carotid, but not aortic, stiffness was associated with worse retinal flicker light‐induced dilation and this association was stronger in individuals with type 2 diabetes. Hence, carotid stiffness may be a determinant of retinal microvascular dysfunction.
5. PERSPECTIVES
The present population‐based study found that carotid stiffness may be a determinant of retinal microvascular dysfunction. Hence, prevention of carotid stiffness may contribute to the prevention of retinal microvascular dysfunction.
CONFLICT OF INTEREST
No potential conflicts of interest relevant to this article were reported.
AUTHOR CONTRIBUTIONS
F.C.T.H. and T.L.Z. contributed to conception and design, participated in acquisition of data, analyzed and interpreted data, drafted the manuscript (with C.D.A.S. and R.M.A.H.), revised the manuscript critically for important intellectual content, and provided final approval of the version to be published. F.C.T.H also is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. A.J.H.M.H, A.A.K., P.C.D., M.C.J.M.v.D., S.J.P.M.E., T.J.M.B., J.S.A.G.S., C.A.B.W., M.T.S., M.M.J.G., A.W., C.G.S., A.K., H.H.C.M.S., N.C.S., K.D.R. contributed to conception and design, revised the manuscript critically for important intellectual content, and provided final approval of the version to be published. T.L.Z., J.S.A.G.S., R.M.A.H. and C.D.A.S. contributed to conception and design, contributed to analyses and interpretation of data, revised the manuscript critically for important intellectual content, and provided final approval of the version to be published.
Supporting information
Table S1‐S7
ACKNOWLEDGMENTS
The authors acknowledge ZIO foundation (Vereniging Regionale HuisartsenZorg Heuvelland) for their contribution to The Maastricht Study. The researchers are indebted to the participants for their willingness to participate in the study.
Funding information
This study was supported by the European Regional Development Fund via OP‐Zuid, the Province of Limburg, the Dutch Ministry of Economic Affairs (grant 31O.041), Stichting De Weijerhorst (Maastricht, the Netherlands), the Pearl String Initiative Diabetes (Amsterdam, the Netherlands), the Cardiovascular Center (CVC, Maastricht, the Netherlands), CARIM School for Cardiovascular Diseases (Maastricht, the Netherlands), CAPHRI School for Public Health and Primary Care (Maastricht, the Netherlands), NUTRIM School for Nutrition and Translational Research in Metabolism (Maastricht, the Netherlands), Stichting Annadal (Maastricht, the Netherlands), Health Foundation Limburg (Maastricht, the Netherlands), Perimed (Järfälla, Sweden), Diabetesfonds grant 2016.22.1878 (Amersfoort, The Netherlands), Oogfonds (Utrecht, The Netherlands) and by unrestricted grants from Janssen‐Cilag B.V. (Tilburg, the Netherlands), Novo Nordisk Farma B.V. (Alphen aan den Rijn, the Netherlands), and Sanofi‐Aventis Netherlands B.V. (Gouda, the Netherlands).
DATA AVAILABILITY STATEMENT
Data are available from The Maastricht Study for any researcher who meets the criteria for access to confidential data; the corresponding author may be contacted to request data.
REFERENCES
- 1.Simo R, Stitt AW, Gardner TW. Neurodegeneration in diabetic retinopathy: does it really matter? Diabetologia. 2018;61:1902‐1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stehouwer CDA. Microvascular dysfunction and hyperglycemia: a vicious cycle with widespread consequences. Diabetes. 2018;67:1729‐1741. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell GF. Effects of central arterial aging on the structure and function of the peripheral vasculature: implications for end‐organ damage. J Appl Physiol. 1985;2008(105):1652‐1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lim M, Sasongko MB, Ikram MK, et al. Systemic associations of dynamic retinal vessel analysis: a review of current literature. Microcirculation. 2013;20:257‐268. [DOI] [PubMed] [Google Scholar]
- 5.de Clerck EEB, Schouten JSAG, Berendschot TTJM, et al. Loss of temporal peripapillary retinal nerve fibers in prediabetes or type 2 diabetes without diabetic retinopathy: the Maastricht study. Invest Ophthalmol Vis Sci. 2017;58:1017‐1027. [Google Scholar]
- 6.Metea MR, Newman EA. Glial cells dilate and constrict blood vessels: a mechanism of neurovascular coupling. J Neurosci. 2006;26:2862‐2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takano T, Tian GF, Peng W, et al. Astrocyte‐mediated control of cerebral blood flow. Nat Neurosci. 2006;9:260‐267. [DOI] [PubMed] [Google Scholar]
- 8.Sorensen BM, Houben AJ, Berendschot TT, et al. Prediabetes and type 2 diabetes are associated with generalized microvascular dysfunction: the Maastricht study. Circulation. 2016;134:1339‐1352. [DOI] [PubMed] [Google Scholar]
- 9.O'Rourke MF, Hashimoto J. Arterial stiffness: a modifiable cardiovascular risk factor? J Cardiopulm Rehabil Prev. 2008;28:225‐237. [DOI] [PubMed] [Google Scholar]
- 10.Laurent S, Cockcroft J, Van Bortel L, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588‐2605. [DOI] [PubMed] [Google Scholar]
- 11.Cheung N, Sharrett AR, Klein R, et al. Aortic distensibility and retinal arteriolar narrowing: the multi‐ethnic study of atherosclerosis. Hypertension. 2007;50:617‐622. [DOI] [PubMed] [Google Scholar]
- 12.Lin F, Zhu P, Huang F, et al. Aortic stiffness is associated with the central retinal arteriolar equivalent and retinal vascular fractal dimension in a population along the southeastern coast of China. Hypertens Res. 2015;38:342. [DOI] [PubMed] [Google Scholar]
- 13.Meyer ML, Klein BE, Klein R, et al. Central arterial stiffness and retinal vessel calibers: the atherosclerosis risk in communities study‐neurocognitive study. J Hypertens. 2020;38:266‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tapp RJ, Owen CG, Barman SA, et al. Associations of retinal microvascular diameters and tortuosity with blood pressure and arterial stiffness: United Kingdom Biobank. Hypertension. 2019;74:1383‐1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei F, Lutgarde T, Yu CC, et al. Retinal Microvasculature in relation to central hemodynamics in a Flemish population. Hypertension. 2019;74:606‐613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liao D, Wong TY, Klein R, Jones D, Hubbard L, Sharrett AR. Relationship between carotid artery stiffness and retinal arteriolar narrowing in healthy middle‐aged persons. Stroke. 2004;35:837‐842. [DOI] [PubMed] [Google Scholar]
- 17.Schram MT, Sep SJ, van der Kallen CJ, et al. The Maastricht Study: an extensive phenotyping study on determinants of type 2 diabetes, its complications and its comorbidities. Eur J Epidemiol. 2014;29:439‐451. [DOI] [PubMed] [Google Scholar]
- 18.Hermeling ERK, Kornmann LM, Reneman RS, Hoeks AP. The dicrotic notch as alternative time‐reference point to measure local pulse wave velocity in the carotid artery by means of ultrasonography. J Hypertens. 2009;27:2028‐2035. [DOI] [PubMed] [Google Scholar]
- 19.Willekes C, Hoeks AP, Bots ML, Brands PJ, Willigers JM, Reneman RS. Evaluation of off‐line automated intima‐media thickness detection of the common carotid artery based on M‐line signal processing. Ultrasound Med Biol. 1999;25:57‐64. [DOI] [PubMed] [Google Scholar]
- 20.Reneman RS, Meinders JM, Hoeks AP. Non‐invasive ultrasound in arterial wall dynamics in humans: what have we learned and what remains to be solved. Eur Heart J. 2005;26:960‐966. [DOI] [PubMed] [Google Scholar]
- 21.Van Bortel LM, Laurent S, Boutouyrie P, et al. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid‐femoral pulse wave velocity. J Hypertens. 2012;30:445‐448. [DOI] [PubMed] [Google Scholar]
- 22.Garhofer G, Resch H, Sacu S, et al. Effect of regular smoking on flicker induced retinal vasodilatation in healthy subjects. Microvasc Res. 2011;82:351‐355. [DOI] [PubMed] [Google Scholar]
- 23.Li W, Schram MT, Sorensen BM, et al. Microvascular phenotyping in the maastricht study: design, and main findings, 2010–2018. Am J Epidemiol. 2020;189(9):873‐884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bekkers EDR, Berendschot T, Romeny BT. A multi‐orientation analysis approach to retinal vessel tracking. J Math Imaging Vis. 2014;49:583‐610. [Google Scholar]
- 25.ter Haar Romeny BM, Bekkers EJ, Zhang J, et al. Braininspired algorithms for retinal image analysis. Mach Vis Appl. 2016;27:1117‐1135. [Google Scholar]
- 26.Jonas JBGG, Naumann GO. Optic disc, cup andneuroretinal rim size, configuration and correlations in normal eyes. Invest Ophthalmol Vis Sci. 1989;29:1151‐1158. [PubMed] [Google Scholar]
- 27.Knudtson MD, Lee KE, Hubbard LD, Wong TY, Klein R, Klein BE. Revised formulas for summarizing retinal vessel diameters. Curr Eye Res. 2003;27:143‐149. [DOI] [PubMed] [Google Scholar]
- 28.Nagel E, Vilser W. Flicker observation light induces diameter response in retinal arterioles: a clinical methodological study. Br J Ophthalmol. 2004;88:54‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagel E, Vilser W, Fink A, Riemer T. [Variance of retinal vessel diameter response to flicker light. A methodical clinical study]. Ophthalmologe. 2006;103:114‐119. [DOI] [PubMed] [Google Scholar]
- 30.Martens RJH, Houben A, Kooman JP, et al. Microvascular endothelial dysfunction is associated with albuminuria: the Maastricht Study. J Hypertens. 2018;36:1178‐1187. [DOI] [PubMed] [Google Scholar]
- 31.van Dooren FE, Schram MT, Schalkwijk CG, et al. Associations of low grade inflammation and endothelial dysfunction with depression ‐ the Maastricht Study. Brain Behav Immun. 2016;56:390‐396. [DOI] [PubMed] [Google Scholar]
- 32.Sörensen BM, Heide FCT, Houben AJHM, et al. Higher levels of daily physical activity are associated with better skin microvascular function in type 2 diabetes‐the Maastricht Study. Microcirculation. 2020;27:e12611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Dongen MC, Wijckmans‐Duysens NEG, den Biggelaar LJ, et al. The Maastricht FFQ: development and validation of a comprehensive food frequency questionnaire for the Maastricht study. Nutrition. 2019;62:39‐46. [DOI] [PubMed] [Google Scholar]
- 34.Looman M, Feskens EJ, de Rijk M, et al. Development and evaluation of the Dutch Healthy Diet index 2015. Public Health Nutr. 2017;20:2289‐2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qi Y, Koster A, van Boxtel M, et al. Adulthood socioeconomic position and type 2 diabetes mellitus‐a comparison of education, occupation, income, and material deprivation: the Maastricht study. Int J Environ Res Public Health. 2019;16:1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Newman A, Andrew N, Casson R. Review of the association between retinal microvascular characteristics and eye disease. Clin Exp Ophthalmol. 2018;46:531‐552. [DOI] [PubMed] [Google Scholar]
- 37.Boutouyrie P, Bussy C, Lacolley P, Girerd X, Laloux B, Laurent S. Association between local pulse pressure, mean blood pressure, and large‐artery remodeling. Circulation. 1999;100:1387‐1393. [DOI] [PubMed] [Google Scholar]
- 38.Hutcheon JA, Chiolero A, Hanley JA. Random measurement error and regression dilution bias. BMJ. 2010;340:c2289. [DOI] [PubMed] [Google Scholar]
- 39.Liang W, Zhao Y, Lee AH. An investigation of the significance of residual confounding effect. Biomed Res Int. 2014;2014:658056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li X, Wong WL, Cheung CY, et al. Racial differences in retinal vessel geometric characteristics: a multiethnic study in healthy Asians. Invest Ophthalmol Vis Sci. 2013;54:3650‐3656. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1‐S7
Data Availability Statement
Data are available from The Maastricht Study for any researcher who meets the criteria for access to confidential data; the corresponding author may be contacted to request data.


