Key Points
Question
What were the maturation, functional patency, and intervention rates for autogenous arteriovenous fistulas in the prospective, multicenter Hemodialysis Fistula Maturation cohort study?
Findings
In this case series including 602 participants, the autogenous arteriovenous fistula maturation rates were 67% at 6 months and 76% at 12 months after creation for the participants with kidney failure, although almost one-third required an intervention to facilitate maturation or treat a complication. The functional patency rate for the autogenous arteriovenous fistulas that matured was 75% at 2 years, although almost one-half of the fistulas required an intervention to maintain use or treat a complication.
Meaning
The findings of this study suggest the maturation and functional patency rates for autogenous hemodialysis arteriovenous fistulas are suboptimal, but reasonable considering the alternatives, and require a substantial number of interventions to both facilitate maturation and to maintain use after successful cannulation.
Abstract
Importance
National initiatives have emphasized the use of autogenous arteriovenous fistulas (AVFs) for hemodialysis, but their purported benefits have been questioned.
Objective
To examine AVF usability, longer-term functional patency, and remedial procedures to facilitate maturation, manage complications, or maintain patency in the Hemodialysis Fistula Maturation (HFM) Study.
Design, Setting, and Participants
The HFM Study was a multicenter (n = 7) prospective National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases cohort study performed to identify factors associated with AVF maturation. A total of 602 participants were enrolled (dialysis, kidney failure: 380; predialysis, chronic kidney disease [CKD]: 222) with AVF maturation ascertained for 535 (kidney failure, 353; CKD, 182) participants.
Interventions
All clinical decisions regarding AVF management were deferred to the individual centers, but remedial interventions were discouraged within 6 weeks of creation.
Main Outcomes and Measures
In this case series analysis, the primary outcome was unassisted maturation. Functional patency, freedom from intervention, and participant survival were summarized using Kaplan-Meier analysis.
Results
Most participants evaluated (n = 535) were men (372 [69.5%]) and had diabetes (311 [58.1%]); mean (SD) age was 54.6 (13.6) years. Almost two-thirds of the AVFs created (342 of 535 [64%]) were in the upper arm. The AVF maturation rates for the kidney failure vs CKD participants were 29% vs 10% at 3 months, 67% vs 38% at 6 months, and 76% vs 58% at 12 months. Several participants with kidney failure (133 [37.7%]) and CKD (63 [34.6%]) underwent interventions to facilitate maturation or manage complications before maturation. The median time from access creation to maturation was 115 days (interquartile range [IQR], 86-171 days) but differed by initial indication (CKD, 170 days; IQR, 113-269 days; kidney failure, 105 days; IQR, 81-137 days). The functional patency for the AVFs that matured at 1 year was 87% (95% CI, 83.2%-90.2%) and at 2 years, 75% (95% CI, 69.7%-79.7%), and there was no significant difference for those receiving interventions before maturation. Almost half (188 [47.5%]) of the AVFs that matured had further intervention to maintain patency or treat complications.
Conclusions and Relevance
The findings of this study suggest that AVF remains an accepted hemodialysis access option, although both its maturation and continued use require a moderate number of interventions to maintain patency and treat the associated complications.
This case series examines the usability of autogenous arteriovenous fistulas for hemodialysis in patients with chronic kidney disease and kidney failure.
Introduction
The creation and maintenance of a functional arteriovenous hemodialysis access is a problem for the expanding population of patients with kidney failure. The original National Kidney Foundation’s Dialysis Outcome Quality Initiative recommended the autogenous arteriovenous fistula (AVF) over the prosthetic alternative (arteriovenous graft) and a tunneled dialysis catheter (TDC) based on its apparent benefits, which included improved patency, decreased morbidity, decreased mortality, and cost.1 Furthermore, these guidelines defined a target incidence (≥50%) and prevalence (>40%) for AVFs across the US. The Centers for Medicare & Medicaid Services implemented the National Access Vascular Initiative (2003-2006), known as the Fistula First Breakthrough Initiative, to achieve the Dialysis Outcome Quality Initiative targets for AVFs with an updated prevalence target of 66% by 2009.2
This increased emphasis on AVFs resulted in the unintended consequences of a higher rate of nonmaturation and TDC use across the US.3,4,5,6,7 Dember et al8 reported a 61% rate of AVF nonmaturation from the Dialysis Access Consortium Fistula Trial (DAC), a multicenter National Institutes of Health National Institute of Diabetes and Diabetes and Kidney Diseases (NIH NIDDK) randomized trial that evaluated clopidogrel as an intervention to prevent early thrombosis of newly created AVFs. The high maturation failure rate observed in the DAC partially led the NIH NIDDK to create the Hemodialysis Fistula Maturation (HFM) Consortium, a group of 7 clinical centers charged with evaluating the influences of vascular anatomy, biology, clinical attributes, and health care processes on AVF maturation. This study, initiated in 2010 and enrolling 602 participants, has dissected the various elements in each of these domains and elucidated their association with AVF maturation.9,10,11,12,13,14,15 However, a detailed analysis of the time course of AVF maturation and secondary failure, coupled with an examination of the surgical and endovascular interventions used to promote and maintain AVF patency, has not been reported.
The HFM data set, initially encompassing patients with dialysis-dependent kidney failure and those with predialysis chronic kidney disease (CKD), offers the opportunity to examine the temporal progression of access creation and maintenance that characterizes these distinct populations. Therefore, the present case-series analysis was undertaken to examine AVF maturation, longer-term patency, and remedial procedures to facilitate maturation, manage complications, or maintain patency in the HFM cohort. With the increasing emphasis in the recent National Kidney Foundation’s Dialysis Outcome Quality Initiative guideline on selecting the “right access, in the right patient, at the right time, for the right reasons,”16 these findings may provide important insights to assist clinical decision-making for this challenging population.
Methods
HFM Design and Participants
The HFM collaboration was a multicenter, prospective cohort study funded by the NIH NIDDK that was designed to elucidate the clinical and biological factors associated with AVF maturation.17 The study focused on the domains of clinical care, histologic characteristics, physiological testing, and ultrasonography-based imaging. The primary outcome measure was unassisted AVF maturation using predefined criteria. All clinical decisions were deferred to the individual centers and clinicians with the exception that interventions were discouraged within the first 6 weeks after access creation. Only single-stage AVFs were included (eg, 2-stage brachial-basilic AVFs were excluded) owing to the presumed biological differences associated with their maturation. The HFM Study was approved by each center’s institutional review board, and all participants provided written informed consent. Participants were not recruited with an advertised stipend, but financial compensation was inconsistent across the centers: some were provided a small stipend at the time of their clinic appointment. This study followed the reporting guideline for case series.
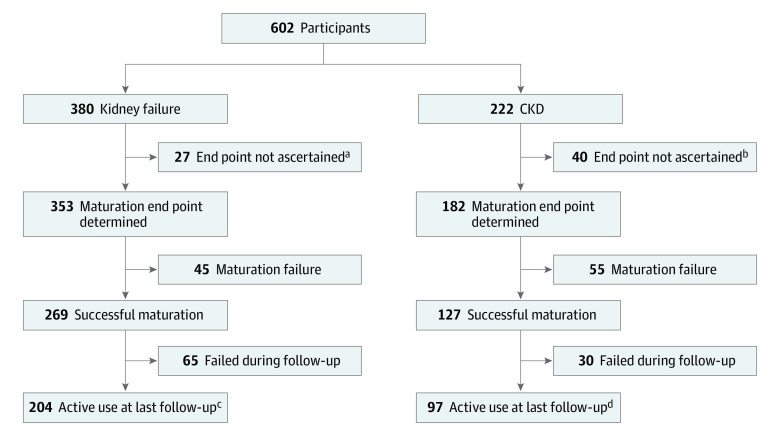
A total of 602 participants were enrolled, and a graphic representation of all the participants is highlighted in Figure 1; eFigure 1 in the Supplement, categorized by initial dialysis status (kidney failure vs CKD). Participants were stratified by their enrollment dialysis status because of the potential different access strategies related to the immediate vs imminent need for dialysis. Autogenous arteriovenous fistula maturation (N = 535) was ascertained for 353 kidney failure and 182 CKD participants. Autogenous arteriovenous fistula maturation was not determined for 67 participants (kidney failure, 27; CKD = 40) who had either died, changed kidney replacement modality (eg, peritoneal dialysis or transplant), failed to progress to hemodialysis, or were lost to follow-up. Functional AVF patency was determined until access abandonment among the participants (n = 396) whose AVFs were ascertained to be mature (kidney failure, 269; CKD, 127).
Figure 1. Flow Diagram of the Participants.
A flow diagram of the participants enrolled in the study, as categorized by their initial dialysis status (kidney failure vs chronic kidney disease [CKD]), is shown for the autogenous arteriovenous fistula (AVFs) that were ascertained, the AVFs that matured, and the AVFs that were used for dialysis until abandonment.
aDeath (n = 19), alternative dialysis modality (n = 3), lost to follow-up (n = 5).
bDeath (n = 8), alternative dialysis modality (n = 6), lost to follow-up (n = 2), never started dialysis (n = 24).
cMedian follow-up, 26 months (interquartile range, 17-37 months).
dMedian follow-up, 28 months (interquartile range, 18-39 months).
Baseline demographic characteristics and comorbidities were established using predefined criteria.17 Participant race and ethnicity was self-reported and included because of potential differences in maturation and functional patency. The access configuration was classified based on preoperative ultrasonographic assessment of the donor artery and outflow vein along with the location of the planned cannulation zone (eg, forearm vs upper arm). The forearm accesses (artery-vein) included the radial/ulnar-cephalic and radial/ulnar-basilic configurations, and upper arm AVFs included proximal radial/brachial-cephalic and proximal radial/brachial-basilic/brachial configurations.
Outcome Measures
All participants were monitored monthly throughout the study or until 3 months after AVF abandonment, with median follow-up of 26 months (interquartile range [IQR], 17-37 months) for patients with kidney failure and 28 months (IQR, 18-39 months) for those with CKD. The status of the study access and the participant’s dialysis prescription were determined along with any complications, interventions, or hospitalizations.
Autogenous arteriovenous fistulas maturation was defined as access use for effective dialysis using 2 needles for 75% or more of the dialysis sessions over 4 weeks.17 The maturation criteria had to be satisfied within 9 months of access creation or within 8 weeks of the initial cannulation attempt for participants who did not initiate dialysis by 9 months after AVF creation. Unassisted maturation was defined as maturation before any endovascular or surgical intervention on the access to facilitate maturation and/or manage complications. Diagnostic imaging studies alone (eg, fistulagram, duplex ultrasonography) were not considered interventions. Any uncertain maturation outcomes were adjudicated by a dedicated committee using the participant’s entire study record. Functional access patency was defined as freedom from abandonment after AVF maturation. Autogenous arteriovenous fistulas were classified as abandoned if the treating nephrologist or surgeon determined that the AVF was unsuitable for future use and an alternative vascular access was required. Functional access patency was not a formal end point in the original study design, but an operational definition, and access use was tracked for all AVFs until after abandonment or the end of the study.
Statistical Analysis
Participant and procedure characteristics are presented as number and percentage for categorical variables and mean (SD) and median (IQR) for continuous variables. Cumulative incidence of maturation was derived from dates of surgery and committee ascertainment of maturation status. Kaplan-Meier methods were used to summarize functional patency, freedom from intervention, and participant survival. The log-rank test was used to assess whether postmaturation outcomes differed between the kidney failure and CKD groups and the association of prematuration intervention with time to abandonment. All analyses were performed on the original study data using R, version 4.0.2 (R Foundation for Statistical Computing). All tests were 2-sided and performed at a significance level of .05.
Results
The participant baseline demographics, comorbidities, access history, and access configurations are provided in Table 1 for participants with kidney failure and CKD who reached a maturation end point (overall, 535; kidney failure, 353 [66.0%]; CKD, 182 [34.0%]). Participants included 372 men (69.5%) and 163 women (30.5%); mean (SD) age was 54.6 (13.6) years. Most participants had diabetes (311 [58.1%]). The racial distribution was relatively equal overall (Black, 243 [45.4%]; White, 244 [45.6%]; other, 48 [9.0%] [no further breakdown available]), but varied by dialysis status (kidney failure: Black, 178 [50.4%]; White, 141 [39.9%]; CKD: Black, 65 [35.7%]; White, 103 [56.6%]). The overall mean (SD) body mass index was 30.3 (7.6) (calculated as weight in kilograms divided by height in meters squared), and a substantial proportion of participants had coronary artery disease (135 [25.2%]) and peripheral artery disease (77 [14.4%]). Most participants with kidney failure were undergoing dialysis through a TDC at the time of enrollment, and almost one-third (115 [32.6%]) had a prior permanent access. The target cannulation site for almost two-thirds (342 [64%]) of study access procedures involved the upper arm with the brachial/ulnar/radial-cephalic configuration being the most common (kidney failure, 36.8%; CKD, 47.8%). The frequency of the forearm radial/ulnar-cephalic accesses was comparable for the groups, but the incidence of brachial/radial/ulnar-basilic/brachial appeared to be higher among participants with kidney failure (90 [25.5%]) compared with the CKD group of participants (35 [19.2%]).
Table 1. Participant Baseline Characteristics and Configuration of Study AVFs.
| Characteristic | No. (%) | ||
|---|---|---|---|
| Overall | Kidney failure | CKD | |
| No. | 535 | 353 | 182 |
| Demographic characteristics | |||
| Age, y | |||
| Mean (SD) | 54.6 (13.6) | 53.2 (14.2) | 57.4 (11.7) |
| Median (IQR) | 56 (47-64) | 54 (43-63) | 58 (51-65) |
| Sex | |||
| Female | 163 (30.5) | 103 (29.2) | 60 (17.) |
| Male | 372 (69.5) | 250 (70.8) | 122 (67.0) |
| Race | |||
| Black | 243 (45.4) | 178 (50.4) | 65 (35.7) |
| White | 244 (45.6) | 141 (39.9) | 103 (56.6) |
| Othera | 48 (9.0) | 34 (9.6) | 14 (7.7) |
| Comorbidities | |||
| Diabetes | 311 (58.1) | 189 (53.5) | 122 (67.0) |
| Coronary artery disease | 135 (25.2) | 79 (22.4) | 56 (30.8) |
| Peripheral artery disease | 77 (14.4) | 47 (13.3) | 30 (16.5) |
| BMI | |||
| Mean (SD) | 30.3 (7.6) | 29.4 (7.5) | 31.9 (7.6) |
| Median (IQR) | 29 (24-35) | 28 (24-34) | 32 (27-37) |
| Access history | |||
| Current dialysis catheter, No/total No. (%)b | 338/533 (63.4) | 338/351 (96.3) | 0 |
| Prior permanent access | 119 (22.2) | 115 (32.6) | 4 (2.2) |
| AVF configuration (artery-vein) | |||
| Radial/ulnar, forearm cephalic | 153 (28.6) | 101 (28.6) | 52 (28.6) |
| Radial/ulnar, forearm basilic | 40 (7.5) | 32 (9.1) | 8 (4.4) |
| Brachial/ulnar/radial, upper arm cephalic | 217 (40.6) | 130 (36.8) | 87 (47.8) |
| Brachial/ulnar/radial, upper arm basilic/brachial | 125 (23.4) | 90 (25.5) | 35 (19.2) |
Abbreviations: AVF, arteriovenous fistula; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CKD, chronic kidney disease; IQR, interquartile range.
Participant race and ethnicity was self-reported and included because of potential differences in maturation and functional patency. No further breakdown of the category of “other” is available.
Data were not available on all participants.
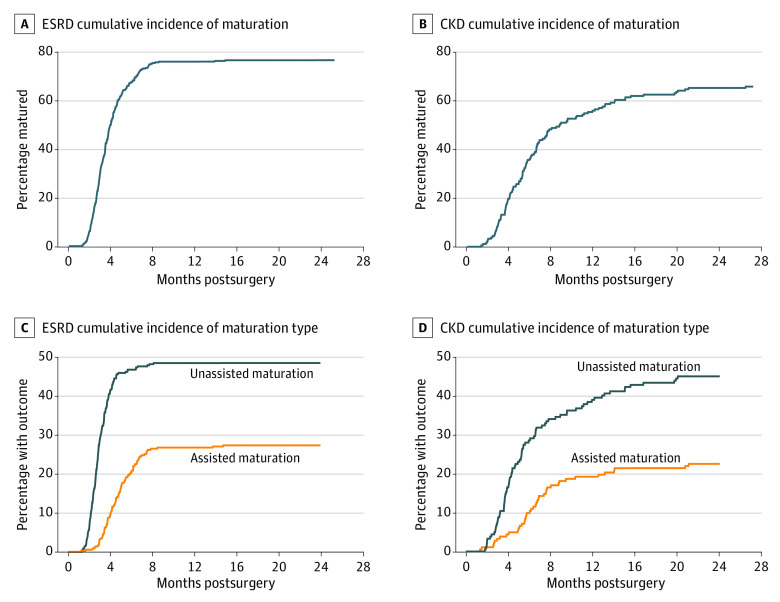
The cumulative incidence of AVF maturation is shown for both the kidney failure and CKD participants in Figure 2A. The corresponding maturation rates for the kidney failure vs CKD participants were 29% vs 10% at 3 months, 67% vs 38% at 6 months, and 76% vs 58% at 12 months. The timeline for maturation in the CKD group was delayed as expected due to the fact that maturation could not be determined until the patients with CKD had progressed to kidney failure and dialysis was attempted. Approximately one-third of both the kidney failure (133 [37.7%]) and CKD (63 [34.6%]) participants underwent intervention to facilitate maturation or manage access complications before ascertainment (Table 2). Intervention for an AVF stenosis was most common for both groups (kidney failure, 26.3%; CKD, 22.5%); while the other interventions to facilitate maturation or manage complications were substantially less common (access vein branch > central vein stenosis > thrombosis) and did not differ meaningfully between groups.
Figure 2. Cumulative Incidence of Maturation in Participants Shown by Their Initial Dialysis Status.
A, The cumulative incidence of autogenous arteriovenous fistula (AVF) maturation is shown for participants with kidney failure (A) and chronic kidney disease (B) who were evaluated. B, The cumulative incidence of unassisted and assisted AVF maturation is shown for participants with kidney failure (C) and chronic kidney disease (D) who were evaluated.
Table 2. Interventions to Facilitate Maturation or Manage Complications.
| Indication | Kidney failure (n = 353) | CKD (n = 182) | All participants with matured study AVF (n = 396) | |||
|---|---|---|---|---|---|---|
| Before maturation | Before maturation | After maturation | ||||
| No. of events | Participants, No. (%) | No. of events | Participants, No. (%) | No. of events | Participants, No. (%) | |
| Thrombosis | 18 | 18 (5.1) | 6 | 6 (3.3) | 73 | 53 (13.4) |
| Aneurysm | 2 | 2 (0.6) | 0 | 0 | 37 | 33 (8.3) |
| Bleeding/hematoma | 1 | 1 (0.3) | 2 | 2 (1.1) | 27 | 24 (6.1) |
| Infection | 2 | 1 (0.3) | 1 | 1 (0.5) | 3 | 3 (0.8) |
| Arterial inflow stenosis | 11 | 8 (2.3) | 5 | 5 (2.7) | 32 | 23 (5.8) |
| AVF vein stenosis | 119 | 93 (26.3) | 51 | 41 (22.5) | 359 | 153 (38.6) |
| Central vein stenosis | 25 | 22 (6.2) | 9 | 9 (4.9) | 123 | 58 (14.6) |
| Accessory vein branch | 25 | 23 (6.5) | 12 | 12 (6.6) | 14 | 13 (3.3) |
| Inability to cannulate | 17 | 17 (4.8) | 9 | 8 (4.4) | 4 | 4 (1.0) |
| Hand ischemia | 13 | 11 (3.1) | 7 | (6 (3.3) | 5 | 5 (1.3) |
| Noninfectious fluid collection | 3 | 3 (0.8) | 0 | 0 (0) | 4 | (3 (0.8) |
| Total interventions | 186a | 133 (37.7) | 88b | 63 (34.6) | 477c | 188 (47.5) |
Abbreviations: AVF, arteriovenous fistula; CKD, chronic kidney disease.
Events per intervention, mean (SD), 1.27 (0.54).
Events per intervention, mean (SD), 1.16 (0.37).
Events per intervention, mean (SD), 43 (0.66).
The associated unassisted and assisted maturation rates are shown in Figure 2B. Forty-nine percent of the participants with kidney failure successfully used their AVFs without intervention at 12 months, and 27% of the participants with kidney failure underwent an intervention before successful maturation. Maturation in the CKD group was expectedly delayed, with 39% achieving unassisted maturation and 19% achieving assisted maturation at 12 months. The median time from access creation to maturation was 115 days (IQR, 86-171 days) for all participants with maturation but differed by initial indication (CKD, 170 days [IQR, 113-269 days]; kidney failure, 105 days [IQR, 81-137 days]). Participants with kidney failure required a TDC for a mean of 2.9 months (median, 2 [IQR, 2-4] months) before access ascertainment. A total of 133 participants (37.7%) with kidney failure and 61 (33.5%) of those with CKD required at least 1 inpatient hospitalization for any cause before AVF ascertainment.
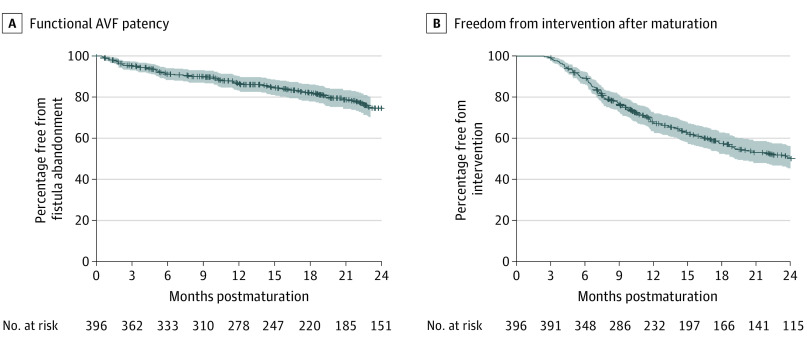
The functional patency rate for the AVFs that matured is depicted in Figure 3A with corresponding rates of 87% (95% CI, 83.2%-90.2%; SEM, 1.8%) at 1 year and 75% (95% CI, 69.7%-79.7%; SEM, 2.6%) at 2 years. The patency rates for the kidney failure and CKD groups (eFigure 2 in the Supplement) were similar, so the cohorts were combined for postmaturation analysis.
Figure 3. Functional Patency and Freedom From Intervention After Autogenous Arteriovenous Fistula (AVF) Maturation.
A, The functional patency for participants whose AVF was successfully used for dialysis. The kidney failure and chronic kidney disease groups were combined, given the lack of significant difference between these groups (eFigure 2 in the Supplement). B, The freedom from intervention for the participants after successful AVF maturation. The shaded areas indicate 95% CIs.
Functional patency was not significantly different between the AVFs that received interventions before maturation (eFigure 3 in the Supplement). Almost half (188 [47.5%]) underwent some type of intervention to maintain patency and/or treat access-related complications (Table 2). Interventions performed for AVF stenoses (153 [38.6%]) accounted for the largest percentage followed by procedures for central vein stenosis (58 [14.6%]) and thrombosis (53 [13.4%]). The corresponding freedom-from-intervention rates were 67% (95% CI, 62.8%-72.3%; SEM, 2.4%) at 1 year and 50% (95% CI, 44.8%-55.8%; SEM, 2.8%) at 2 years. More than half of participants with kidney failure (167 [62.1%]) and CKD (72 [56.7%]) with a functional study AVF (mean follow-up, 26 months for kidney failure and 28 months for CKD groups), required inpatient hospitalization for any cause after their access was ascertained to be mature. The corresponding survival rates for the kidney failure vs CKD participants were 92% (95% CI, 89.0%-95.1%) vs 84% (95% CI, 79.8%-88.6%) at 12 months, 74% (95% CI, 68.1%-80.7%) vs 97% (95% CI, 93.6%-99.6%) at 24 months, and 85% (95% CI, 79.3%-92.0%) vs 76% (95% CI, 67.7%-85.2%) at 36 months (eFigure 4 in the Supplement).
Discussion
The results of this study help define the natural history of AVFs, detailing the timeline of their clinical maturation, functional patency, and the associated intervention rates. The AVF maturation rate at 6 months was 67% for participants with kidney failure, although almost one-third of these participants required some type of intervention to facilitate maturation and/or manage complications. The ultimate maturation and intervention rates for CKD participants were comparable; however, the timeline was more protracted given the need to initiate dialysis before measuring outcomes. The AVF functional patency following maturation was reasonable, with a 2-year rate of 75%, but almost half of the study participants underwent some type of intervention to maintain use. The associated participant morbidity in terms of TDC use, inpatient hospitalizations, and mortality was substantial. The importance of these findings is notable in light of the multicenter, prospective cohort design of this large, comprehensive study and the substantial resources and intensive data collection algorithms that were used.
It may be helpful to put the current data in the context of similar studies. The clinical maturation rate for AVFs in our study was greater than those in the DAC.8 Notably, “failure to obtain suitability for dialysis” was the major secondary outcome measure in the DAC and was defined by the inability to meet specific dialysis criteria between 150 and 180 days after access placement. The AVF was not suitable for dialysis in 61% of the participants (clopidogrel, 61.8%; placebo, 59.5%) in the DAC. This finding corresponds to a 39% maturation rate at 6 months, which is markedly lower than the 67% rate reported in our study for the participants with kidney failure at the same time point. However, the incidence of forearm AVFs was higher in the DAC (54% vs 36%) and the frequency of remedial interventions to facilitate maturation in the DAC was reported to be low, although the exact number was not provided.
The DAC study was completed before the current widespread use of interventions to facilitate AVF maturation8; thus, their maturation rate was comparable to the unassisted maturation rate reported in our study. Pisoni et al18 reported from the Dialysis Outcomes and Practice Patterns Study that the prevalence of forearm AVFs has decreased in the US over the time frame of the DAC and HFM studies, while maturation incidence was greater for upper arm AVFs. Our AVF maturation was consistent with those reported by Al-Jaishi et al19 from their systematic review and meta-analysis examining AVF patency rates. They reported that the primary AVF failure rate (eg, immediate AVF failure within 72 hours, early dialysis suitability failure, and late dialysis suitability failure) was 23% (95% CI, 18%-28%; 37 cohorts; 7393 AVFs), although they stated that the estimate should be treated with caution owing to the high degree of heterogeneity within the studies. Similarly, our results are consistent with those of Woodside et al20 from the US Renal Data System that reported 36.2% of the AVFs were never used among the 45 087 AVFs identified in the database.
The successful maturation rates observed in the present study were associated with a significant cost. The incidence of remedial interventions to facilitate maturation and/or manage complications was consistent with the 44% rate reported by Lee at al21 among more than 7000 patients, aged 67 years or older, who initiated dialysis in the US Renal Data System between 2010 and 2012. Patients in their study who received intervention had a higher risk of both primary access loss and need for postmaturation interventions. The obligatory maturation time in our study (kidney failure, 105 days) was consistent with findings from the US Renal Data System that documented a median time of 111 days between AVF creation and usability.20 This maturation period typically mandates dialysis through a TDC for patients with kidney failure and places them at risk for the spectrum of catheter-related complications, including sepsis, hospitalization, and death. Not unexpectedly, delayed AVF maturation or the need for remedial interventions is associated with additional catheter-dependent dialysis time.22
The Dialysis Outcomes and Practice Patterns Study reported that AVFs not used within the first 6 months after placement were associated with a 53% higher mortality rate within the ensuing 6 months.23 The appropriate duration for AVF maturation and number of remedial interventions remains undefined; however, the maturation rate increased from 67% at 6 months to 76% at 12 months among participants with kidney failure in the present study. From a practical standpoint, it seems worthwhile to begin investigating the next access option at 6 months if the AVF has not matured. There is clearly a substantial financial cost associated with AVF maturation that is increased with remedial interventions and/or complications, although these data were not collected in the present study. In addition, there is a substantial psychological cost to the patient associated with delayed maturation that should not be underestimated.
The functional patency rate for the AVFs that matured in this study was consistent with those reported from administrative databases. For example, the Dialysis Outcomes and Practice Patterns Study reported a cumulative functional patency rate of 88% at 1 year, which was similar across all sites (eg, US, Japan, Europe, Australia, and New Zealand).23 The consistency across regions was particularly striking given the marked differences in access configuration (eg, forearm vs arm) and unassisted patency. In comparison, the meta-analysis by Al-Jaishi et al19 described a functional patency rate of 71% (95% CI, 64%-78%; 18 cohorts; 3558 AVFs) at 1 year, which was somewhat lower but included primary failures (23%) in their calculation. In our analysis, the crude estimate of a participant with kidney failure possessing a functional access 2 years after creation would be approximately 66% (maturation at 1 year × cumulative patency at 1 year). Maintaining a functional AVF in our study was associated with a continued need for remedial interventions similar to the initial requirement to achieve maturation. Brooke et al24 examined the cost-effectiveness of repeated interventions among 720 AVFs over an 8-year period. They reported that 56% of the patients required at least 1 intervention to maintain patency and that the clinical effectiveness decreased with each intervention, although it was not cost-effective to create a new access until after the second intervention. The need for remedial interventions before maturation was not associated with the subsequent functional patency rate in our study. Lee et al21 identified similar results from the US Renal Data System, although 2 small studies had reported contrasting findings.22,25
The findings of our study may help optimize clinical care by providing a benchmark for AVFs. It is generally accepted that a mature AVF is the optimal dialysis access. Approximately 65% of the patients in the US dialyze through an AVF.26 However, access care has evolved over the past 3 decades since the publication of the original National Kidney Foundation’s Dialysis Outcome Quality Initiative guidelines,1 shifting from an emphasis on AVFs to an emphasis on access functionality and the appreciation that the access choice should be tailored to the individual patient’s need. The present study was singularly focused on the process of AVF maturation and did not include a comparison arteriovenous graft or TDC group, so it is impossible to comment on their relative risks or benefits. The remaining challenge is to select the ideal access type and configuration, optimizing the likelihood of success while reducing the number of remedial interventions and the duration of catheter dependence.
Limitations
This study has limitations. The study was performed by clinicians with a dedicated interest in hemodialysis access, primarily at academic medical centers. The primary objective of the study was to identify predictors of AVF maturation, and accordingly, the participants were all deemed to be reasonable candidates for an AVF creation. It is conceivable that the criteria for enrollment were liberalized and participants with a low likelihood of successful maturation were enrolled. However, the early thrombosis rate (5.3% within 18 days) and the maturation rate were both good and consistent with other reports in the literature.27 The maturation end point was indeterminate in a small proportion of participants who were excluded from analysis; thus, it is conceivable that their outcomes could have adversely affected the results. In addition, only single-stage AVF procedures were included in the HFM Study despite the increasing application of a 2-stage brachial basilic approach.28,29
Conclusions
The findings of this study suggest that the AVF remains a reasonable option for patients who require access for hemodialysis access, although both their maturation and continued use require a moderate number of interventions to maintain patency and treat associated complications.
eFigure 1. Monthly Renal Replacement Status
eFigure 2. Functional Patency by Initial Dialysis Status
eFigure 3. Functional Patency by Assisted Maturation
eFigure 4. Participant Survival
References
- 1.National Kidney Foundation–Dialysis Outcomes Quality Initiative . NKF-DOQI clinical practice guidelines for vascular access. Am J Kidney Dis. 1997;30(4)(suppl 3):S150-S191. [PubMed] [Google Scholar]
- 2.Gold JA, Hoffman K. Fistula First: the National Vascular Access Improvement Initiative. WMJ. 2006;105(3):71-73. [PubMed] [Google Scholar]
- 3.Huijbregts HJ, Bots ML, Moll FL, Blankestijn PJ; CIMINO members . Hospital specific aspects predominantly determine primary failure of hemodialysis arteriovenous fistulas. J Vasc Surg. 2007;45(5):962-967. doi: 10.1016/j.jvs.2007.01.014 [DOI] [PubMed] [Google Scholar]
- 4.Lok CE, Allon M, Moist L, Oliver MJ, Shah H, Zimmerman D. Risk equation determining unsuccessful cannulation events and failure to maturation in arteriovenous fistulas (REDUCE FTM I). J Am Soc Nephrol. 2006;17(11):3204-3212. doi: 10.1681/ASN.2006030190 [DOI] [PubMed] [Google Scholar]
- 5.McLafferty RB, Pryor RW III, Johnson CM, Ramsey DE, Hodgson KJ. Outcome of a comprehensive follow-up program to enhance maturation of autogenous arteriovenous hemodialysis access. J Vasc Surg. 2007;45(5):981-985. doi: 10.1016/j.jvs.2007.01.013 [DOI] [PubMed] [Google Scholar]
- 6.Danese MD, Liu Z, Griffiths RI, et al. Catheter use is high even among hemodialysis patients with a fistula or graft. Kidney Int. 2006;70(8):1482-1485. doi: 10.1038/sj.ki.5001786 [DOI] [PubMed] [Google Scholar]
- 7.Lacson E Jr, Lazarus JM, Himmelfarb J, Ikizler TA, Hakim RM. Balancing fistula first with catheters last. Am J Kidney Dis. 2007;50(3):379-395. doi: 10.1053/j.ajkd.2007.06.006 [DOI] [PubMed] [Google Scholar]
- 8.Dember LM, Beck GJ, Allon M, et al. ; Dialysis Access Consortium Study Group . Effect of clopidogrel on early failure of arteriovenous fistulas for hemodialysis: a randomized controlled trial. JAMA. 2008;299(18):2164-2171. doi: 10.1001/jama.299.18.2164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allon M, Imrey PB, Cheung AK, et al. ; Hemodialysis Fistula Maturation (HFM) Study Group . Relationships between clinical processes and arteriovenous fistula cannulation and maturation: a multicenter prospective cohort study. Am J Kidney Dis. 2018;71(5):677-689. doi: 10.1053/j.ajkd.2017.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robbin ML, Greene T, Allon M, et al. ; Hemodialysis Fistula Maturation Study Group . Prediction of arteriovenous fistula clinical maturation from postoperative ultrasound measurements: findings from the Hemodialysis Fistula Maturation Study. J Am Soc Nephrol. 2018;29(11):2735-2744. doi: 10.1681/ASN.2017111225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alpers CE, Imrey PB, Hudkins KL, et al. ; Hemodialysis Fistula Maturation Study Group . histopathology of veins obtained at hemodialysis arteriovenous fistula creation surgery. J Am Soc Nephrol. 2017;28(10):3076-3088. doi: 10.1681/ASN.2016050598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allon M, Greene T, Dember LM, et al. ; Hemodialysis Fistula Maturation Study Group . Association between preoperative vascular function and postoperative arteriovenous fistula development. J Am Soc Nephrol. 2016;27(12):3788-3795. doi: 10.1681/ASN.2015020141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheung AK, Imrey PB, Alpers CE, et al. ; Hemodialysis Fistula Maturation Study Group . Intimal hyperplasia, stenosis, and arteriovenous fistula maturation failure in the Hemodialysis Fistula Maturation Study. J Am Soc Nephrol. 2017;28(10):3005-3013. doi: 10.1681/ASN.2016121355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farber A, Cheng TW, Nimmich A, et al. Femoral vein transposition is a durable hemodialysis access for patients who have exhausted upper extremity options. J Vasc Surg. 2020;71(3):929-936. doi: 10.1016/j.jvs.2019.07.062 [DOI] [PubMed] [Google Scholar]
- 15.Huber TS, Larive B, Imrey PB, et al. ; HFM Study Group . Access-related hand ischemia and the Hemodialysis Fistula Maturation Study. J Vasc Surg. 2016;64(4):1050-1058.e1. doi: 10.1016/j.jvs.2016.03.449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lok CE, Huber TS, Lee T, et al. ; National Kidney Foundation . 2019 Update. Am J Kidney Dis. 2020;75(4)(suppl 2):S1-S164. doi: 10.1053/j.ajkd.2019.12.001 [DOI] [PubMed] [Google Scholar]
- 17.Dember LM, Imrey PB, Beck GJ, et al. ; Hemodialysis Fistula Maturation Study Group . Objectives and design of the hemodialysis fistula maturation study. Am J Kidney Dis. 2014;63(1):104-112. doi: 10.1053/j.ajkd.2013.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pisoni RL, Zepel L, Fluck R, et al. International differences in the location and use of arteriovenous accesses created for hemodialysis: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis. 2018;71(4):469-478. doi: 10.1053/j.ajkd.2017.09.012 [DOI] [PubMed] [Google Scholar]
- 19.Al-Jaishi AA, Oliver MJ, Thomas SM, et al. Patency rates of the arteriovenous fistula for hemodialysis: a systematic review and meta-analysis. Am J Kidney Dis. 2014;63(3):464-478. doi: 10.1053/j.ajkd.2013.08.023 [DOI] [PubMed] [Google Scholar]
- 20.Woodside KJ, Bell S, Mukhopadhyay P, et al. Arteriovenous fistula maturation in prevalent hemodialysis patients in the United States: a national study. Am J Kidney Dis. 2018;71(6):793-801. doi: 10.1053/j.ajkd.2017.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee T, Qian JZ, Zhang Y, Thamer M, Allon M. Long-term outcomes of arteriovenous fistulas with unassisted versus assisted maturation: a retrospective national hemodialysis cohort study. J Am Soc Nephrol. 2019;30(11):2209-2218. doi: 10.1681/ASN.2019030318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harms JC, Rangarajan S, Young CJ, Barker-Finkel J, Allon M. Outcomes of arteriovenous fistulas and grafts with or without intervention before successful use. J Vasc Surg. 2016;64(1):155-162. doi: 10.1016/j.jvs.2016.02.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pisoni RL, Zepel L, Zhao J, et al. International comparisons of native arteriovenous fistula patency and time to becoming catheter-free: findings from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis. 2021;77(2):245-254. doi: 10.1053/j.ajkd.2020.06.020 [DOI] [PubMed] [Google Scholar]
- 24.Brooke BS, Griffin CL, Kraiss LW, Kim J, Nelson R. Cost-effectiveness of repeated interventions on failing arteriovenous fistulas. J Vasc Surg. 2019;70(5):1620-1628. doi: 10.1016/j.jvs.2019.01.085 [DOI] [PubMed] [Google Scholar]
- 25.Lee T, Ullah A, Allon M, et al. Decreased cumulative access survival in arteriovenous fistulas requiring interventions to promote maturation. Clin J Am Soc Nephrol. 2011;6(3):575-581. doi: 10.2215/CJN.06630810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saran R, Li Y, Robinson B, et al. US Renal Data System 2015 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2016;67(3 suppl 1):S1-S305. doi: 10.1053/j.ajkd.2015.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farber A, Imrey PB, Huber TS, et al. ; HFM Study Group . Multiple preoperative and intraoperative factors predict early fistula thrombosis in the Hemodialysis Fistula Maturation Study. J Vasc Surg. 2016;63(1):163-70.e6. doi: 10.1016/j.jvs.2015.07.086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jun Yan Wee I, Mohamed IH, Patel A, Choong AMTL. A systematic review and meta-analysis of one-stage versus two-stage brachiobasilic arteriovenous fistula creation. J Vasc Surg. 2018;68(1):285-297. doi: 10.1016/j.jvs.2018.03.428 [DOI] [PubMed] [Google Scholar]
- 29.Sheta M, Hakmei J, London M, et al. One- versus two-stage transposed brachiobasilic arteriovenous fistulae: A review of the current state of the art. J Vasc Access. 2020;21(3):281-286. doi: 10.1177/1129729819862694 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Monthly Renal Replacement Status
eFigure 2. Functional Patency by Initial Dialysis Status
eFigure 3. Functional Patency by Assisted Maturation
eFigure 4. Participant Survival