This cross-sectional study characterizes the frequency and changes over time (2009-2020) of opioid prescriptions following Mohs micrographic surgery.
Key Points
Question
Have patients obtained opioids following Mohs micrographic surgery at different rates between 2009 and 2020?
Findings
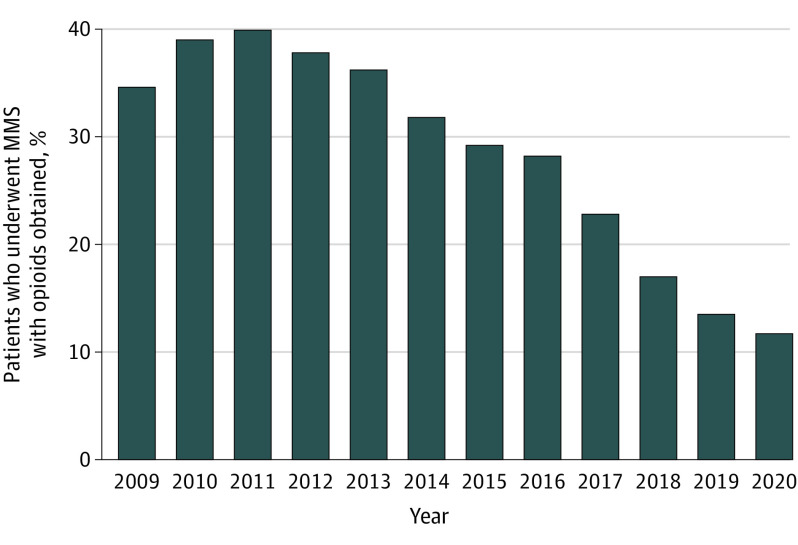
In this cross-sectional study of database claims, the proportion of patients obtaining an opioid prescription after Mohs micrographic surgery increased from 2009 (34.6%) to 2011 (39.6%) and then declined each year, reaching a low in 2020 (11.7%). The most common opioids during this time frame were hydrocodone, codeine, oxycodone, and tramadol.
Meaning
The downward trend reflects patients’ and dermatologic surgeons’ increased recognition of opioid prescription risks and public health warnings regarding the opioid epidemic.
Abstract
Importance
To curtail the opioid epidemic, physicians have been advised to limit opioid prescriptions.
Objective
To characterize the frequency and changes over time (2009-2020) of opioid prescriptions following Mohs micrographic surgery.
Design, Setting, and Participants
This cross-sectional study using Optum Clinformatics DataMart (Optum CDM), a nationally representative insurance claims database, included patients aged 18 years and older who had Mohs micrographic surgery insurance claims in the Optum CDM database from 2009 to 2020. Data were analyzed from November 11, 2020, to March 30, 2021.
Exposures
Opioid prescription following Mohs surgery.
Main Outcomes and Measures
The primary outcome was the proportion of patients who underwent Mohs surgery and obtained an opioid prescription within 2 days of surgery. Secondary outcomes included type and opioid quantity prescribed.
Results
Among 358 012 patients with Mohs micrographic surgery claims (mean [SD] age, 69 [13] years; 205 609 [57.4%] were men), the proportion of patients obtaining an opioid prescription after Mohs micrographic surgery increased from 2009 (34.6%) to 2011 (39.6%). This proportion then declined each year, reaching a low of 11.7% in 2020 (27.9% absolute decrease from 2011 to 2020). Hydrocodone, codeine, oxycodone, and tramadol were the 4 most commonly prescribed opioids. By 2020, hydrocodone was obtained less (2009: 47.5%; 2011: 67.1%; 2020: 45.4%; 21.7% absolute decrease from 2011 to 2020) and tramadol was obtained more (2009: 1.6%; 2020: 27.9%; 26.3% absolute increase from 2009 to 2020).
Conclusions and Relevance
In this cross-sectional study of Mohs micrographic surgery claims, patients obtained fewer postsurgery opioid prescriptions over the study period, suggesting responsiveness of patients and dermatologic surgeons to public health concerns regarding the opioid epidemic. During this decline, prescriptions for hydrocodone decreased and tramadol increased.
Introduction
Among dermatologists, dermatologic surgeons prescribe the majority of opioids.1,2 Most dermatologic surgeons limit opioid supplies to 4 to 5 days.1,2 However, it has been demonstrated that even short courses of opioids may significantly increase addiction risk.3 Despite evidence that nonopioid analgesics control pain after Mohs micrographic surgery (MMS)4,5 and consensus recommendations to reserve opioids as a second-line treatment for postsurgery pain,6,7 15.5% to 58% of patients still receive an opioid prescription after MMS or conventional excisions.4,8,9
Adequate patient pain management must be delicately balanced with the potential negative consequences of prescription opioids. From 1999 to 2011, opioid pharmaceutical sales in the US increased 400%, and prescription opioid–related deaths surpassed deaths due to heroin and cocaine combined.10 In 2016, the US Department of Health and Human Services declared the opioid epidemic a public health emergency,11 and the Centers for Disease Control and Prevention released guidelines to curtail unnecessary opioid prescriptions.12 Unfortunately, overdose deaths involving prescription opioids continued to increase even after these measures.13 Given the ongoing public health emergency, multiple federal government agencies stress the importance of safe opioid prescribing practices across all medical specialties.14
To our knowledge, nationwide patterns of opioid prescriptions among dermatologic surgeons have not been studied over time. We sought to assess the rates at which patients obtain opioid medications after MMS over the past decade to establish national trends. We additionally characterized opioid type and quantity supplied. Ascertaining national trends over time further informs the ongoing discussion on optimal opioid prescribing patterns in dermatologic surgery.
Methods
Data Set
This study was deemed exempt from approval and patient informed consent by the Institutional Review Board at the University of Pennsylvania owing to the use of deidentified data. This cross-sectional study used Optum Clinformatics Data Mart (Optum CDM) to evaluate paid insurance database claims for skin cancers treated in the US from January 1, 2009, to June 1, 2020. Optum CDM is a Health Insurance Portability and Accountability Act–compliant, deidentified insurance claims database that contains both Medicare Advantage members and members of a single national insurance company. Optum CDM data and US census data have similar representation of sex, age, race and ethnicity, and geography.15 This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Study Cohort
Patients were included if they were 18 years or older and had a claim for MMS, as defined by Current Procedural Terminology codes 17311 and 17313. If patients had more than 1 MMS claim, only the first was included in the analysis. Patients who underwent MMS were counted as obtaining a surgery-related opioid prescription if they had an associated pharmaceutical claim for an opioid within 2 days of the MMS claim.16 The primary outcome was whether or not an opioid analgesic was obtained after MMS. Secondary outcomes included the quantity and type of opioids prescribed.
For patients undergoing MMS for more than 1 surgical site in a single billing encounter, surgical details such as skin cancer type and location could not be specified with coding. Therefore, 2 cohorts were created, the first containing all patients, regardless of multisite surgery, and the second containing only single-site MMS procedures (see Table 1).
Table 1. Demographic Data and Surgical Characteristics.
| Characteristic | Patients (n = 358 012), No. (%) | ||
|---|---|---|---|
| Received opioids | Did not receive opioids | Overall cohort | |
| Demographic | |||
| No. | 93 046 | 264 966 | 358 012 |
| Age, mean (SD), y | 65 (13) | 70 (12) | 69 (3) |
| Sex | |||
| Male | 51 369 (58.2) | 154 240 (55.2) | 205 609 (57.4) |
| Female | 41 668 (44.8) | 110 715 (41.8) | 142 383 (42.6) |
| Race and ethnicity | |||
| Asian | 718 (0.9) | 2059 (0.9) | 2777 (0.9) |
| Black | 4781 (5.8) | 7863 (3.5) | 12 644 (4.1) |
| Hispanic | 3220 (3.9) | 8815 (4.0) | 12 035 (3.9) |
| White | 74 343 (89.5) | 204 096 (91.6) | 278 439 (91.0) |
| Unknown | 9984 (10.7) | 42 133 (15.9) | 52 117 (14.6) |
| Information for patients with single-site MMS only | |||
| No. | 79 982 | 229 150 | 309 132 |
| Skin cancer type | |||
| BCC | 41 132 (51.4) | 124 765 (54.5) | 165 897 (53.7) |
| SCC | 15 091 (18.9) | 54 748 (23.9) | 69 839 (22.6) |
| SCCIS | 2316 (2.9) | 13 650 (6.0) | 15 966 (5.2) |
| MM | 500 (0.6) | 639 (0.3) | 1139 (0.4) |
| MIS | 306 (0.4) | 819 (0.4) | 1125 (0.4) |
| Unspecified | 20 621 (25.8) | 34 507 (15.1) | 55 128 (17.8) |
| MCC | 16 (0.02) | 22 (0.01) | 38 (0.01) |
| Tumor location | |||
| Face | 45 217 (56.5) | 132 544 (57.8) | 177 761 (57.5) |
| Scalp/neck | 7377 (9.2) | 25 173 (11.0) | 32 550 (10.5) |
| Ear | 7072 (8.8) | 19 202 (8.4) | 26 274 (8.5) |
| Eye | 4308 (5.4) | 6999 (3.0) | 11 307 (3.7) |
| Lip | 4233 (5.3) | 8163 (3.6) | 12 396 (4.0) |
| Trunk | 2182 (2.7) | 7183 (3.1) | 9365 (3.0) |
| Upper limb | 3474 (4.3) | 12 910 (5.6) | 16 384 (5.3) |
| Lower limb | 2676 (3.4) | 10 990 (4.8) | 13 666 (4.4) |
| Other | 3443 (4.3) | 5986 (2.6) | 9429 (3.1) |
Abbreviations: BCC, basal cell carcinoma; MCC, Merkel cell carcinoma; MIS, melanoma in situ; MM, malignant melanoma; MMS, Mohs micrographic surgery; SCC, squamous cell carcinoma; SCCIS, squamous cell carcinoma in situ.
Similar to previous studies using Optum CDM, opioid National Drug Codes were used to extract opioid data.17 Patients were excluded if they had claims for long-term (greater than 30 continuous days) opioid treatment such as cancer-related pain, opioid substance use disorder (naloxone, buprenorphine, methadone), and migraine relief (opioid plus caffeine). Opioid medications were grouped under their active ingredient (hydrocodone, tramadol, codeine, oxycodone, propoxyphene, and “other,” which included hydromorphone, morphine, fentanyl, meperidine, tapentadol, oxymorphone, and pentazocine).18
Statistical Analysis
Statistical analysis was conducted on the entire cohort. Descriptive statistics were used to describe the study population and surgery characteristics. The frequency of patients obtaining an opioid after MMS, including the type and quantity, was calculated by year. To understand the difference in frequency between years, the absolute and percentage changes were calculated. Absolute percentage change is denoted as “absolute increase/decrease,” and percentage change, defined as the difference in frequency divided by the original frequency, is denoted as “percentage increase/decrease.”
Multivariable logistic regression was done with the entire cohort to evaluate associations between the dependent outcome (whether a patient obtained an opioid prescription) and several factors: year; race and ethnicity (Asian, Black, Hispanic, White, unspecified), sex (male, female), age in years at surgery, and geographic location (US Census geographic division). Statistical significance was determined by 2-sided P values less than .05 for the multivariable logistic regression. Statistical analyses were performed using Stata, version 16 (StataCorp LLC). Data were analyzed from November 11, 2020, to March 30, 2021.
Results
Study Cohort
From 2009 to 2020, Optum CDM included 358 012 MMS claims. Of patients with MMS claims, the mean (SD) age was 69 (13) years, and 205 609 (57.4%) were men. Demographic data and surgical characteristics are shown in Table 1.
Frequency by Year, 2009 to 2020
A total of 93 046 of 358 012 patients (26.0%) who underwent MMS from 2009 to 2020 had an associated opioid claim. The proportion of patients who underwent MMS with opioid claims rose from 34.6% in 2009 to a peak of 39.6% in 2011, then decreased annually to a rate of 11.7% in 2020 (Figure 1).
Figure 1. Percentage of Patients Who Underwent Mohs Micrographic Surgery (MMS) With an Opioid Prescription Obtained by Year, 2009 to 2020.
Odds of an opioid claim in patients who underwent MMS decreased 61% per calendar year (odds ratio [OR], 0.39 per calendar year; 95% CI, 0.37-0.41; P < .001). Highest and lowest ORs for geographic division were West South Central (OR, 2.71; 95% CI, 2.61-2.80; P < .001) and New England (OR, 0.42; 95% CI, 0.40-0.45; P < .001). Logistic regression results are illustrated in Table 2.
Table 2. Logistic Regression Results of Entire Cohort.
| Variable | Odds ratio of obtaining an opioid after MMS (95% CI) | P value |
|---|---|---|
| MMS site | ||
| 19-39 | 1 [Reference] | NA |
| 40-64 | 0.98 (0.94-1.03) | .40 |
| ≥65 | 0.47 (0.45-0.49) | <.001a |
| Sex | ||
| Females | 1 [Reference] | NA |
| Males | 0.93 (0.92-0.95) | <.001a |
| Race and ethnicity | ||
| Racial and ethnic minority groupsb | 1 [Reference] | NA |
| White | 0.91 (0.90-0.93) | <.001a |
| Geographic location | ||
| East North Central | 1 [Reference] | NA |
| East South Central | 2.55 (2.44-2.66) | <.001a |
| West North Central | 0.57 (0.55-0.60) | <.001a |
| West South Central | 2.71 (2.61-2.80) | <.001a |
| Middle Atlantic | 0.48 (0.46-0.50) | <.001a |
| Mountain | 2.00 (1.93-2.07) | <.001a |
| Pacific | 1.33 (1.28-1.38) | <.001a |
| Unknown | 1.03 (0.78-1.37) | .81 |
| South Atlantic | 1.85 (1.79-1.90) | <.001a |
| New England | 0.42 (0.40-0.45) | <.001a |
Abbreviations: MMS, Mohs micrographic surgery; NA, not applicable.
P value indicates statistical significance.
Racial and ethnic minority groups includes Asian, Black, Hispanic, and unspecified.
Opioid Days Supplied by Year, 2009 to 2020
From 2009 to 2020, the mean (SD) and median (range) number of opioid days supplied per MMS encounter were 4.0 (5.1) days and 3.0 (1-90) days, respectively (Table 3). The mean number of days supplied remained relatively unchanged from 2009 to 2020. Of note, by 2020 a plurality (45.4%) of opioid prescriptions provided 2 days’ worth of opioids or less, a substantial change from 36.4% of prescriptions in 2009.
Table 3. Opioid Days Supplied by Year.
| Year | No. of days supplied, mean (SD) | Opioid supply (days supplied), % | |||
|---|---|---|---|---|---|
| ≤2 d | 3-5 d | 5-7 d | >1 wk | ||
| 2009 | 3.9 (4.5) | 36.4 | 40.6 | 17.0 | 4.5 |
| 2010 | 3.8 (4.5) | 38.7 | 39.5 | 15.8 | 4.4 |
| 2011 | 3.8 (4.7) | 38.6 | 39.1 | 15.9 | 4.8 |
| 2012 | 3.9 (4.9) | 40.6 | 35.7 | 16.4 | 5.4 |
| 2013 | 4.0 (5.2) | 40.3 | 36.1 | 15.6 | 5.8 |
| 2014 | 4.0 (5.0) | 38.9 | 37.3 | 15.0 | 6.0 |
| 2015 | 4.1 (5.2) | 39.4 | 35.7 | 16.4 | 6.0 |
| 2016 | 4.1 (5.1) | 37.9 | 38.1 | 15.3 | 6.1 |
| 2017 | 4.0 (5.3) | 40.4 | 37.6 | 14.2 | 5.7 |
| 2018 | 4.1 (5.6) | 41.1 | 37.1 | 13.6 | 5.3 |
| 2019 | 4.2 (5.6) | 41.6 | 36.1 | 13.9 | 5.3 |
| 2020 | 4.1 (5.6) | 45.4 | 34.9 | 11.2 | 5.3 |
Opioid Group by Year, 2009 to 2020
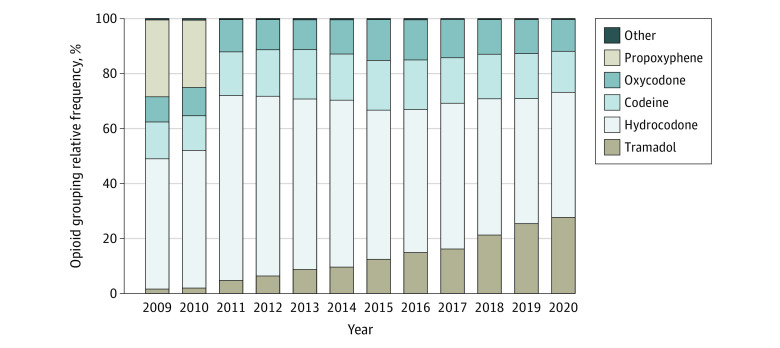
Figure 2 depicts the relative frequencies of opioid active ingredients from 2009 to 2020. Overall, the 4 most commonly obtained opioids were hydrocodone (51 135 of 93 046 [55.0%]), codeine (15 148 of 93 046 [16.3%]), oxycodone (11 163 of 93 046 [12.0%]), and tramadol (10 828 of 93 046 [11.6%]).
Figure 2. Opioid Active Ingredient Relative Frequency by Year, 2009 to 2020.
Other refers to opioids containing hydromorphone, morphine, fentanyl, meperidine, tapentadol, oxymorphone, and pentazocine.
Hydrocodone prescriptions comprised 47.5% of all opioids prescribed in 2009 and 67.1% of all opioids in 2011, an increase of 19.6% (percentage increase of 41.2% from 2009 to 2011). They then decreased to 45.4% of all opioids in 2020, an absolute decline of 21.7% (percentage decrease of 30.6% from 2011 to 2020) (Figure 2). Tramadol prescriptions increased from 1.6% in 2009 to 27.9% in 2020, an absolute increase of 26.3% (percentage increase of 1643% from 2009 to 2020). Propoxyphene constituted 28.0% and 24.5% of opioids prescribed in 2009 and 2010, respectively, but dropped to 0% from 2011 onward. The US Food and Drug Administration recalled propoxyphene in 2011 due to cardiotoxicity.19,20
Discussion
This study demonstrates substantial changes in the frequency and types of opioids that Mohs surgeons prescribed between 2009 and 2020. After reaching a peak of 39.6% in 2011, the proportion of patients who underwent MMS with opioid prescriptions decreased annually, reaching a low of 11.7% in 2020. While Mohs surgeons remained consistent in prescribing short-term supplies of opioids during the study period, the types of medications prescribed shifted over time from hydrocodone to tramadol.
Post-MMS opioid trends demonstrate a 5.0% absolute increase from 2009 to 2011 (percentage increase of 14.6%), consistent with overall national trends during this time frame. In 2009, it was estimated that there were 79.5 opioid prescriptions written for every 100 people in the US.21 From 1999 to 2010, opioid prescription sales dramatically increased 4-fold,22 and medical use of opioids increased.23 While countless factors influenced the opioid epidemic, experts suggest that drug companies’ unethical claims22 and incorrect medical research24,25 caused this spike in prescription opioid sales and usage. More specific to dermatology, 1 retrospective single-center study8 reported that in 2010, 35% of an institution’s dermatologic surgery patients received an opioid prescription after their surgical encounter. This parallels our study, which reports that 34.6% to 39.6% of patients who underwent MMS obtained an opioid from 2009 to 2010.
Our trends illustrate a 27.9% absolute decrease in frequency of opioids obtained after MMS from 2011 to 2020 (percentage decrease of 70.4%). Outside of dermatology, national pharmaceutical claims analysis demonstrated a decline in opioid prescriptions as early as 2012.26,27 In 2016, the US Department of Health and Human Services declared the opioid epidemic a national emergency.11 This study illustrates that decreasing trends specific to MMS predated general trends by approximately 1 year. From 2010 to 2015, there was growing evidence in the literature questioning the safety of opioids.28,29 Outside of academia, news outlets and the media increasingly publicized the opioid epidemic.30 These influences may have discouraged clinicians and patients from prescribing and obtaining opioids, respectively.
Within the field of dermatologic surgery, single-center studies indicated similar trends, questioning the necessity for opioids after cutaneous surgery. One center’s study comparing opioid prescribing rates from 2010 to 2017 demonstrated a 23% decrease in opioid prescriptions during this time frame.31 Quality improvement studies at another institution exhibited a 53% decrease in post–dermatologic surgery opioid prescriptions with a notable subsequent decrease in postoperative pain complaints within a 6-month time frame in 2018.4 Another single-center study reported that only 15.5% of patients who underwent MMS received an opioid prescription in 2020.9 Furthermore, studies emerged in 2011 demonstrating that over-the-counter pain medications may outperform opioids in post–dermatologic surgery pain management.5,32 The national decline in opioid prescriptions witnessed in this study may be explained by a multitude of factors, as no formal opioid reduction initiatives occurred within dermatology until March 2020.7
Geographic division was significantly associated with the likelihood of filling an opioid after MMS (Table 2; eFigure in the Supplement). MMS encounters in Southern US regions (East and West South Central, South Atlantic, Mountain geographic divisions) were associated with a significantly increased chance of filling an opioid. Decreased odds in Northern US regions (New England and Middle Atlantic geographic divisions) are consistent with geographic trends listed in Medicare-based dermatologic opioid studies.1,2 While southern states have higher frequencies of MMS encounters with associated opioid claims, they also have higher dermatologic surgical volume overall.2 Therefore, southern regions contribute significantly to opioid prescriptions after MMS. Attitudes toward opioid prescriptions may be different overall in the south, as southern states consistently have more opioid claims than other US regions across all medical specialties.33 Regardless of geographic location, the frequency of MMS encounters with opioids filled has decreased across every geographic location from 2012 to 2020 (eFigure in the Supplement).
The mean number of opioid days supplied with each prescription remained relatively low (3.9-4.2 mean opioid days supplied). Other dermatologic opioid studies based on Medicare data reveal similar mean opioid days supplied (3.9-4.4 days supplied).1,2 Single-center studies indicate that roughly half of patients who undergo MMS do not take any of their prescribed opioid.9,34 Medicare-based data specific to dermatology estimates that at least 493 204 opioid pills are wasted, and our data approximates that roughly 707 520 pills may remain unused in dermatologic surgery.1,34 Because postoperative pain in MMS only lasts a mean of 3 days,12,16,35,36,37 dermatologic surgeons should continue to limit the number of pills prescribed when an opioid is required.
Hydrocodone was the most commonly obtained opioid after MMS from 2009 to 2020 (55.0% of all opioid prescriptions). Hydrocodone prescriptions peaked in 2011, comprising 67.1% of all opioids after MMS. Hydrocodone’s steady climb from 2009 to 2011 is consistent with trends outside dermatology.38 From 2007 to 2014, hydrocodone was the most prescribed drug in the US,39 a noteworthy statistic given that it is one of the most addictive opioid analgesics.40 In 2014, the US Food and Drug Administration changed hydrocodone’s categorization from a schedule class III drug (moderate/low physical dependence and/or high psychological dependence) to a schedule class II drug (high abuse potential; may lead to severe psychological and/or physical dependence, considered “dangerous”).39 In our data set, hydrocodone prescriptions after MMS declined by 21.7% from 2012 to 2020.
Tramadol use after MMS increased dramatically from 2009 to 2020 (absolute increase of 26.3%, percentage increase of 1643%). Early studies suggested tramadol was a safer opioid, with low to no risk of respiratory depression and addiction.41,42 Compared with hydrocodone’s status as a schedule class II narcotic, tramadol is a schedule class IV (low potential for abuse/dependence).43 Clinicians most likely opt to prescribe the “safer” pain control option, as tramadol sales have grown from 2005 to 2015.44 However, recent studies suggest that tramadol may not be as safe as previously asserted. Respiratory depression and addiction transpire more frequently with tramadol use than originally postulated.45 Furthermore, tramadol may not be more effective than ibuprofen for short-term pain relief,45 so the pain control benefits may not outweigh the risks of addiction.
Limitations
This study must be interpreted within the context of its limitations. This study analyzed opioid claims and therefore cannot account for unfilled opioid prescriptions, opioids not taken, or those obtained without a prescription. Although Optum CDM is representative of the US population,15 information may not be generalizable to uninsured individuals and those using Medicaid. Additionally, this data set cannot accurately determine the number of prescribing Mohs surgeons because of coding limitations.
Conclusions
This cross-sectional study demonstrates a considerable decrease in frequency of opioids obtained after MMS. The type of opioids obtained changed dramatically as well. The highly addictive opioid hydrocodone was obtained less frequently over time, while tramadol opioid prescriptions increased. Dermatologists should continue to conservatively prescribe opioids when necessary, using clinician judgment in each individualized situation and using over-the-counter and alternative medications as first-line analgesics whenever possible.6,7
eTable. Frequency of MMS encounters with opioids filled by geographic location (US Census Division) and year
References
- 1.Cao S, Karmouta R, Li DG, Din RS, Mostaghimi A. Opioid prescribing patterns and complications in the dermatology Medicare population. JAMA Dermatol. 2018;154(3):317-322. doi: 10.1001/jamadermatol.2017.5835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feng H, Kakpovbia E, Petriceks AP, Feng PW, Geronemus RG. Characteristics of opioid prescriptions by Mohs surgeons in the Medicare population. Dermatol Surg. 2020;46(3):335-340. doi: 10.1097/DSS.0000000000002038 [DOI] [PubMed] [Google Scholar]
- 3.Shah A, Hayes CJ, Martin BC. Characteristics of initial prescription episodes and likelihood of long-term opioid use—United States, 2006-2015. MMWR Morb Mortal Wkly Rep. 2017;66(10):265-269. doi: 10.15585/mmwr.mm6610a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raji K, Yeung H, Bein AA, Lequeux-Nalovic KG. Dermatologic surgeons can positively impact the opioid epidemic: a quality improvement study of pain management in dermatology surgery. Dermatol Surg. 2020;46(5):635-638. doi: 10.1097/DSS.0000000000002198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sniezek PJ, Brodland DG, Zitelli JA. A randomized controlled trial comparing acetaminophen, acetaminophen and ibuprofen, and acetaminophen and codeine for postoperative pain relief after Mohs surgery and cutaneous reconstruction. Dermatol Surg. 2011;37(7):1007-1013. doi: 10.1111/j.1524-4725.2011.02022.x [DOI] [PubMed] [Google Scholar]
- 6.Donigan JM, Srivastava D, Maher I, et al. Opioid prescribing recommendations after Mohs micrographic surgery and reconstruction: a Delphi consensus. Dermatol Surg. 2021;47(2):167-169. doi: 10.1097/DSS.0000000000002551 [DOI] [PubMed] [Google Scholar]
- 7.McLawhorn JM, Stephany MP, Bruhn WE, et al. ; Opioid-Prescribing in Dermatology Workgroup . An expert panel consensus on opioid-prescribing guidelines for dermatologic procedures. J Am Acad Dermatol. 2020;82(3):700-708. doi: 10.1016/j.jaad.2019.09.080 [DOI] [PubMed] [Google Scholar]
- 8.Harris K, Calder S, Larsen B, et al. Opioid prescribing patterns after Mohs micrographic surgery and standard excision: a survey of American Society for Dermatologic Surgery members and a chart review at a single institution. Dermatol Surg. 2014;40(8):906-911. doi: 10.1097/DSS.0000000000000073 [DOI] [PubMed] [Google Scholar]
- 9.Eikenberg JD, Taylor S, Lockhart ER, Prickett K, Phillips MA. Postoperative pain after Mohs surgery: physician perceptions and how those perceptions influence opioid prescribing practices. Dermatol Surg. 2021;47(2):170-173. doi: 10.1097/DSS.0000000000002465 [DOI] [PubMed] [Google Scholar]
- 10.Guy GP Jr, Zhang K, Bohm MK, et al. Vital signs: changes in opioid prescribing in the United States, 2006-2015. MMWR Morb Mortal Wkly Rep. 2017;66(26):697-704. doi: 10.15585/mmwr.mm6626a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.The Lancet . The opioid crisis in the USA: a public health emergency. Lancet. 2017;390(10107):2016. doi: 10.1016/S0140-6736(17)32808-8 [DOI] [PubMed] [Google Scholar]
- 12.Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA. 2016;315(15):1624-1645. doi: 10.1001/jama.2016.1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention . CDC Wonder. Accessed August 17, 2021. https://wonder.cdc.gov/
- 14.Soelberg CD, Brown RE Jr, Du Vivier D, Meyer JE, Ramachandran BK. The US opioid crisis: current federal and state legal issues. Anesth Analg. 2017;125(5):1675-1681. doi: 10.1213/ANE.0000000000002403 [DOI] [PubMed] [Google Scholar]
- 15.Lukowiak TM, Aizman L, Perz A, et al. Association of age, sex, race, and geographic region with variation of the ratio of basal cell to cutaneous squamous cell carcinomas in the United States. JAMA Dermatol. 2020;156(11):1192-1198. doi: 10.1001/jamadermatol.2020.2571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Firoz BF, Goldberg LH, Arnon O, Mamelak AJ. An analysis of pain and analgesia after Mohs micrographic surgery. J Am Acad Dermatol. 2010;63(1):79-86. doi: 10.1016/j.jaad.2009.10.049 [DOI] [PubMed] [Google Scholar]
- 17.Noe MH, Shin DB, Wehner MR, Margolis DJ, Gelfand JM. Opioid prescribing in adults with and without psoriasis. J Am Acad Dermatol. 2020;83(6):1777-1779. doi: 10.1016/j.jaad.2020.03.080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dart RC, Surratt HL, Cicero TJ, et al. Trends in opioid analgesic abuse and mortality in the United States. N Engl J Med. 2015;372(3):241-248. doi: 10.1056/NEJMsa1406143 [DOI] [PubMed] [Google Scholar]
- 19.Barkin RL, Barkin SJ, Barkin DS. Propoxyphene (dextropropoxyphene): a critical review of a weak opioid analgesic that should remain in antiquity. Am J Ther. 2006;13(6):534-542. doi: 10.1097/01.mjt.0000253850.86480.fb [DOI] [PubMed] [Google Scholar]
- 20.Raffa RB, Burmeister JJ, Yuvasheva E, Pergolizzi JV Jr. QTc interval prolongation by d-propoxyphene: what about other analgesics? Expert Opin Pharmacother. 2012;13(10):1397-1409. doi: 10.1517/14656566.2012.682150 [DOI] [PubMed] [Google Scholar]
- 21.CDC National Center for Injury Prevention and Control . Annual surveillance report of drug-related risks and outcomes. United States, 2019. Accessed August 17, 2021. https://www.cdc.gov/drugoverdose/pdf/pubs/2019-cdc-drug-surveillance-report.pdf
- 22.Centers for Disease Control and Prevention (CDC) . Vital signs: overdoses of prescription opioid pain relievers—United States, 1999-2008. MMWR Morb Mortal Wkly Rep. 2011;60(43):1487-1492. [PubMed] [Google Scholar]
- 23.Atluri S, Sudarshan G, Manchikanti L. Assessment of the trends in medical use and misuse of opioid analgesics from 2004 to 2011. Pain Physician. 2014;17(2):E119-E128. doi: 10.36076/ppj.2014/17/E119 [DOI] [PubMed] [Google Scholar]
- 24.Porter J, Jick H. Addiction rare in patients treated with narcotics. N Engl J Med. 1980;302(2):123. doi: 10.1056/NEJM198001103020221 [DOI] [PubMed] [Google Scholar]
- 25.Portenoy RK, Foley KM. Chronic use of opioid analgesics in non-malignant pain: report of 38 cases. Pain. 1986;25(2):171-186. doi: 10.1016/0304-3959(86)90091-6 [DOI] [PubMed] [Google Scholar]
- 26.Bohnert ASB, Guy GP Jr, Losby JL. Opioid prescribing in the United States before and after the Centers for Disease Control and Prevention’s 2016 opioid guideline. Ann Intern Med. 2018;169(6):367-375. doi: 10.7326/M18-1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guy GP Jr, Zhang K, Schieber LZ, Young R, Dowell D. County-level opioid prescribing in the United States, 2015 and 2017. JAMA Intern Med. 2019;179(4):574-576. doi: 10.1001/jamainternmed.2018.6989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152(2):85-92. doi: 10.7326/0003-4819-152-2-201001190-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller M, Barber CW, Leatherman S, et al. Prescription opioid duration of action and the risk of unintentional overdose among patients receiving opioid therapy. JAMA Intern Med. 2015;175(4):608-615. doi: 10.1001/jamainternmed.2014.8071 [DOI] [PubMed] [Google Scholar]
- 30.McGinty EE, Stone EM, Kennedy-Hendricks A, Barry CL. Stigmatizing language in news media coverage of the opioid epidemic: implications for public health. Prev Med. 2019;124:110-114. doi: 10.1016/j.ypmed.2019.03.018 [DOI] [PubMed] [Google Scholar]
- 31.Donigan JM, Franco AI, Stoddard GJ, et al. Opioid prescribing patterns after micrographic surgery: a follow-up retrospective chart review. Dermatol Surg. 2019;45(4):508-513. doi: 10.1097/DSS.0000000000001725 [DOI] [PubMed] [Google Scholar]
- 32.Glass JS, Hardy CL, Meeks NM, Carroll BT. Acute pain management in dermatology: risk assessment and treatment. J Am Acad Dermatol. 2015;73(4):543-560. doi: 10.1016/j.jaad.2015.04.050 [DOI] [PubMed] [Google Scholar]
- 33.McDonald DC, Carlson K, Izrael D. Geographic variation in opioid prescribing in the US. J Pain. 2012;13(10):988-996. doi: 10.1016/j.jpain.2012.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harris K, Curtis J, Larsen B, et al. Opioid pain medication use after dermatologic surgery: a prospective observational study of 212 dermatologic surgery patients. JAMA Dermatol. 2013;149(3):317-321. doi: 10.1001/jamadermatol.2013.1871 [DOI] [PubMed] [Google Scholar]
- 35.Lopez JJ, Warner NS, Arpey CJ, et al. Opioid prescribing for acute postoperative pain after cutaneous surgery. J Am Acad Dermatol. 2019;80(3):743-748. doi: 10.1016/j.jaad.2018.09.032 [DOI] [PubMed] [Google Scholar]
- 36.Chen AF, Landy DC, Kumetz E, Smith G, Weiss E, Saleeby ER. Prediction of postoperative pain after Mohs micrographic surgery with 2 validated pain anxiety scales. Dermatol Surg. 2015;41(1):40-47. doi: 10.1097/DSS.0000000000000224 [DOI] [PubMed] [Google Scholar]
- 37.Limthongkul B, Samie F, Humphreys TR. Assessment of postoperative pain after Mohs micrographic surgery. Dermatol Surg. 2013;39(6):857-863. doi: 10.1111/dsu.12166 [DOI] [PubMed] [Google Scholar]
- 38.Manchikanti L, Singh A. Therapeutic opioids: a ten-year perspective on the complexities and complications of the escalating use, abuse, and nonmedical use of opioids. Pain Physician. 2008;11(2)(suppl):S63-S88. doi: 10.36076/ppj.2008/11/S63 [DOI] [PubMed] [Google Scholar]
- 39.Kroll D. New rules for hydrocodone: what you should know. Forbes. August 22, 2014. Accessed August 17, 2021. https://www.forbes.com/sites/davidkroll/2014/08/22/what-you-need-to-know-about-new-restrictions-on-hydrocodone-combinations
- 40.Miller NS, Greenfeld A. Patient characteristics and risks factors for development of dependence on hydrocodone and oxycodone. Am J Ther. 2004;11(1):26-32. doi: 10.1097/00045391-200401000-00008 [DOI] [PubMed] [Google Scholar]
- 41.Preston KL, Jasinski DR, Testa M. Abuse potential and pharmacological comparison of tramadol and morphine. Drug Alcohol Depend. 1991;27(1):7-17. doi: 10.1016/0376-8716(91)90081-9 [DOI] [PubMed] [Google Scholar]
- 42.Centers for Disease Control and Prevention . Calculating total daily dose of opioids for safer dosage. Accessed August 17, 2021. https://www.cdc.gov/drugoverdose/pdf/calculating_total_daily_dose-a.pdf
- 43.Harrigan TM. Schedules of controlled substances: placement of tramadol into schedule IV. Published November 4, 2013. Accessed August 17, 2021. https://www.deadiversion.usdoj.gov/fed_regs/rules/2013/fr1104.htm
- 44.Bigal LM, Bibeau K, Dunbar S. Tramadol prescription over a 4-year period in the USA. Curr Pain Headache Rep. 2019;23(10):76. doi: 10.1007/s11916-019-0777-x [DOI] [PubMed] [Google Scholar]
- 45.Shiratsuchi T, Ogawa S. Weak opioids. Article in Japanese. Nihon Rinsho. 2001;59(9):1795-1799. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Frequency of MMS encounters with opioids filled by geographic location (US Census Division) and year