Abstract
Background
Hepatitis C virus may cause liver inflammation and fibrosis. It is not known whether glucocorticosteroids are beneficial or harmful for patients with hepatitis C infection.
Objectives
The objectives were to evaluate the beneficial and harmful effects of glucocorticosteroids for patients with acute or chronic hepatitis C infection with or without hepatitis C related autoimmune disorders.
Search methods
Searches of The Cochrane Hepato‐Biliary Controlled Trials Register, The Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, and reference lists of relevant articles and hand searches of relevant journals were performed in July 2003. Principal authors of clinical trials were approached.
Selection criteria
Randomised clinical trials dealing with glucocorticosteroids for viral hepatitis C ‐ acute or chronic with or without autoimmune disorders.
Data collection and analysis
Data were extracted by one reviewer and validated by another. Further information was sought by correspondence with the principal investigator of the trial in case the relevant data were not published. Disagreements were solved by discussion before the meta‐analysis.
Main results
Eight trials randomised 384 patients with chronic hepatitis C to glucocorticosteroids plus interferon versus interferon plus placebo/no intervention, glucocorticosteroids versus interferon, or glucocorticosteroids versus placebo. Glucocorticosteroids treatment given as short pre‐treatment followed by interferon or as long‐term parallel treatment combined with interferon versus interferon monotherapy had no significant effect on mortality (no deaths occurred; 342 patients), virological response at six months follow‐up (RR 0.85; 95% CI 0.52 to 1.38; 38 patients), or biochemical response at six months follow‐up (RR 0.95; 95% CI 0.84 to 1.06; 307 patients). There was no significant difference in serious adverse events between combination therapy versus interferon monotherapy (RR 4.76; 95% CI 0.24 to 93.19; 342 patients). Glucocorticosteroids versus interferon had no significant effect on mortality (RR 2.33; 95% CI 0.27 to 17.80; 13 patients) or virological response at follow‐up (RR 1.17; 95% CI 0.86 to 1.58; 13 patients). We found no trials on glucocorticosteroids for acute hepatitis C.
Authors' conclusions
There is insufficient evidence neither to confirm nor exclude both beneficial and harmful effects of glucocorticosteroids for chronic hepatitis C with or without autoimmune disorders. This Review is not able to rule out potential serious adverse effects of glucocorticosteroids. Therefore, this Review cannot establish whether glucocorticosteroids treatment can be safely administrated for indications requiring glucocorticosteroids without analysing for hepatitis C virus. The effect of glucocorticosteroids for acute hepatitis C has not been examined in randomised trials.
Plain language summary
No evidence to support or refute glucocorticosteroids for viral hepatitis C
Acute infection with viral hepatitis C manifests most commonly no symptoms, but frequently results in chronic infection. Chronic hepatitis C is in most cases benign, but may progress to severe illness and liver‐related death. This review found no significant effect of glucocorticosteroids on chronic hepatitis, but the amount of data is sparse. Accordingly there is insufficient evidence to neither confirm nor exclude beneficial and harmful effects of glucocorticosteroids for hepatitis C. Further, the evidence is unclear as to whether glucocorticosteroids treatment can be safely administered for other diseases in patients with concomitant hepatitis C. The authors were unable to identify randomised clinical trials on glucocorticosteroids for acute hepatitis C.
Background
Hepatitis C is a viral infection of the liver. It was referred to as 'non‐B, parenterally transmitted' hepatitis or 'non‐A, non‐B' hepatitis until its causative agent, hepatitis C virus (HCV), was identified in 1989 (Kuo 1989). HCV infection is thought to be the most common cause of post‐transfusion hepatitis worldwide. It accounts for approximately 90 per cent of the cases in Japan, USA, and Western Europe (WHO 1997). HCV infection is widespread throughout the world, with approximately 170 million people infected (WHO 1998). Primary HCV infection is asymptomatic in most cases and only about 10 per cent of the patients report an acute illness associated with jaundice (Booth 1995; Alberti 1999). About 50 to 80 per cent of patients with HCV infection develop chronic infection (Alberti 2003). However, the potential damaging effect of hepatitis C infection develops very slowly; about 25 per cent of patients with chronic infection develop cirrhosis within 10 to 30 years, and 5 to 10 per cent of patients with cirrhosis develop hepatocellular carcinoma (HCC) (Alberti 2003). Moreover, HCV infection may induce autoimmune hepatitis and extrahepatic autoimmune disorders like mixed cryoglobulinaemia, glomerulonephritis, porphyria cutanea tarda, vitiligo, lichen planus, and malignancies like non‐Hodgkin's lymphoma (Ferri 2002; El‐Serag 2003).
An important differential diagnosis in the management of HCV infection is autoimmune hepatitis, as the two conditions may resemble each other and require different treatments. Whereas HCV infection responds to alfa‐interferon alone (Lambiase 1992) or in combination with ribavirin (Kjaergard 2003), autoimmune hepatitis generally responds well to glucocorticosteroids with or without azathioprine (Lambiase 1992; Krawitt 1996). Although reliable assays for detection of HCV RNA exist, the distinction between autoimmune hepatitis and hepatitis C infection is not always clear‐cut. The diagnosis of autoimmune hepatitis is based on a score system, which does not exclude patients with concomitant HCV infection (Alvarez 1999). Further, a small group of hepatitis C infected people develop antibodies to liver/kidney microsomal antigens (anti‐LMK), mimicking the serological antibody profile of type two autoimmune hepatitis (Alberti 1999).
Immunosuppressive therapy is the treatment of choice for autoimmune hepatitis. A rapid and sustained response to immunosuppressive therapy in patients with putative autoimmune hepatitis is considered to confirm the diagnosis (Alvarez 1999). However, in patients with viral hepatitis the effect of immunosuppressive therapy is unclear. A Cochrane review (Mellerup 2003) showed limited beneficial effects of glucocorticosteroids, as treatment before interferon for patients with chronic hepatitis B, but several studies have reported a detrimental effect of glucocorticosteroid for viral hepatitis B infection (Gregory 1976; Tygstrup 1986; Lee 1991). Glucocorticosteroids may therefore also be harmful in patients with hepatitis C infection. A retrospective study (Magrin 1994) analysed the relationship between viraemia and treatment and found that glucocorticosteroids increased viraemia in anti‐HCV positive individuals. Magy et al. (Magy 1999) showed increased HCV‐RNA replication in glucocorticosteroid treated liver transplant patients. Calleja et al. (Calleja 1996) observed that prednisone exacerbated liver necrosis in autoantibody positive patients with chronic HCV infection. Thiele et al. (Thiele 1996) observed that absence of anti‐HCV antibodies was a better predictor of a positive biochemical response to glucocorticosteroids than assessment of autoantibodies or degree of biochemical abnormalities in patients with 'non‐B' chronic hepatitis.
These findings question the use of glucocorticosteroids in patients with hepatitis C virus infection. We therefore conducted a systematic Cochrane review to examine the efficacy and the potential detrimental effects of treatment with glucocorticosteroids for patients with acute or chronic HCV infection with or without HCV related autoimmune disorders. We also wanted to assess whether the effects of glucocorticosteroids for hepatitis C could be viewed as so deleterious that screening for HCV infection be warranted before treating patients with glucocorticosteroids for non‐hepatic diseases.
Objectives
The objectives were to evaluate the beneficial and harmful effects of glucocorticosteroids therapy in acute and chronic HCV infection with or without HCV related autoimmune disorders. We also wanted to assess whether the detrimental effects of glucocorticosteroids warranted screening of patients for HCV if they had a non‐hepatic indication for glucocorticosteroid treatment.
Methods
Criteria for considering studies for this review
Types of studies
The review included all randomised clinical trials using any type of glucocorticosteroid regimen in the treatment of acute and chronic HCV infection or non‐hepatic diseases in patients with concomitant HCV infection. Both double‐, single‐, and non‐blinded trials were included, and no limitations regarding language, publication dates, and publication status were applied.
Types of participants
Male and female patients, of any age and ethnic origin who had acute or chronic hepatitis C. Acute hepatitis C was defined as: (1) serum aminotransferase above twice the upper normal value; (2) seroconversion of antibodies to HCV, or the appearance of HCV RNA in serum between two and 26 weeks after infection. Chronic hepatitis C was defined as: (1) positivity for serum antibody to HCV and/or serum HCV‐RNA and continuous elevated serum aminotransferase or chronic hepatitis documented on liver biopsy; (2) patients with non‐A, non‐B hepatitis (assessed by screening for hepatitis A virus and hepatitis B surface antigen, and antibodies to hepatitis‐B core antigen) and continuous elevated serum aminotransferase or chronic hepatitis documented on liver biopsy.
Patients were also included if they had chronic hepatitis C and autoimmune disorders or they had autoimmune hepatitis or other diseases, which are treated with glucocorticosteroids, and concomitant hepatitis C infection. Patients were excluded if they had undergone liver transplantation.
Types of interventions
The review included randomised comparisons between any type of a glucocorticosteroid (regardless of dose, duration, and mode of administration) versus no intervention, placebo, or antiviral agents (e.g., ribavirin). Co‐interventions (e.g., interferon) were allowed if received by both interventions arms.
Types of outcome measures
For all patients with hepatitis C infection, irrespective of the presence of other diseases/disorders, the following outcome were evaluated:
(1) All‐cause mortality plus liver related morbidity (histological cirrhosis assessed in a post‐treatment liver biopsy, clinical cirrhosis as defined by the authors of the individual trials, hepatocellular carcinoma, and liver transplantation). (2) Virological response: HCV‐RNA positivity using polymerase chain reaction (PCR) assay at the end of treatment and at least six months (sustained virological response) after treatment. (3) Biochemical response: Elevated serum levels of aspartate aminotransferase (AST) or alanine aminotransferase (ALT) at the end of treatment and at least six months (sustained biochemical response) after treatment. (4) Liver histology: post‐treatment liver biopsy inflammation, histological scores, or overall appearance (chronic persistent hepatitis (CPH), chronic active hepatitis (CAH), cirrhosis, or hepatocellular carcinoma (HCC)). (5) Quality of life. (6) Adverse events: adverse events as well as serious adverse events, defined as any untoward medical occurrence in a patient in either of the two described regimens, which did not necessarily have a causal relationship with the treatment, but did, however, result in a dose reduction or discontinuation of treatment. Serious adverse events were defined according to the International Committee on Harmonization Good Clinical Practice Guidelines (ICH‐GCP 1997) as any event that:
led to death;
was life‐threatening;
required inpatient hospitalisation or prolongation of existing hospitalisation;
resulted in persistent or significant disability or congenital anomaly/birth defect;
any important medical event which may have jeopardized the patient or required intervention to prevent it.
Search methods for identification of studies
We searched The Cochrane Hepato‐Biliary Group Controlled Trials Register (July 2003), The Cochrane Central Register of Controlled Trials (CENTRAL) on The Cochrane Library (Issue 2, 2003), MEDLINE (from 1966 until July 2003), and EMBASE (from 1980 until July 2003). See Appendix 1 for the search strategies. The reference list of identified studies were examined for additional trials and the list of included and excluded trials were sent to the authors of included trials asking, if they knew of more pertinent trials.
Data collection and analysis
Selection of trials for inclusion Two reviewers independently selected the trials included in the review. The selection was performed unblinded with regard to the names of the authors, investigators, institution, source, and the results.
Methodological quality The methodological quality of each trial was evaluated independently by two reviewers, using the following quality components, which have been associated with empirical evidence of bias (Schultz 1995; Moher 1998; Jüni 2001; Kjaergard 2001). Generation of the allocation sequence Adequate: table of random numbers, computer generated numbers, coin tossing or similar. Unclear: if the trial was described as randomised, but the method used for the allocation sequence generation was not described. Inadequate: if a system involving dates, names, or admittance numbers were used for the allocation of patients. Such trials were excluded. Allocation concealment Adequate: central independent unit, on‐site locked computer, sealed envelopes or similar. Unclear: if the trial was described as randomised, but the method used to conceal the allocation was not described. Inadequate: if the allocation sequence was known to the investigators who assigned participants, open table of random numbers or similar.
Double blinding Adequate: if the trial was described as double blind and the method of blinding involved identical placebo or active drugs. Unclear: if the trial was described as double blind, but the method the method of blinding was not described. Inadequate: if blinding was not performed or the method was inappropriate. Follow‐up Adequate: if the number and reasons for dropouts and withdrawals were described or if it was specified that there were no dropouts or withdrawals. Unclear: if the trial gave the impression that there had been no dropouts or withdrawals, but this was not specifically stated. Inadequate: if the number or reasons for dropouts and withdrawals were not described.
Data extraction Data were extracted by JB and validated by CG. Further information was sought by correspondence with the principal investigator of the trial in case the relevant data were not published. Disagreements were solved by discussion before the meta‐analysis. We extracted the number of patients randomised, exclusion criteria, proportion of females, mean age, number of patients with cirrhosis, HCV genotype, type, dose and duration of therapy, dropouts and withdrawals, methodological quality of the trial, and the outcome measures as described above.
Statistical methods Data were analysed by intention to treat using the last reported observed response ('carry forward') and including all patients irrespective of compliance or follow‐up. All binary outcomes were expressed as relative risks (RR) and 95% confidence intervals (CI). Rare events (mortality and serious adverse events) were also estimated by Peto odds ratio (Deeks 1999). If the results of RR and Peto odds ratio analyses gave the same overall result regarding significance, only the RR is reported. Meta‐analysis was performed using the statistical package (RevMan 4.2.3 and RevMan analyses 1.0.) provided by The Cochrane Collaboration. Data were analysed by both a random effects model (DerSimonian 1986) and a fixed effect model (DeMets 1987). If the results of both analyses gave the same overall result regarding significance, only the results of the fixed effect model analysis is reported. Funnel plot asymmetry was estimated by regression analysis (Egger 1997).
The following sensitivity analyses were intended:
The methodological quality of the included trials: adequate versus unclear or inadequate generation of allocation sequence, allocation concealment, and blinding.
Publication status: full reports versus abstracts/unpublished.
Stage of infection at inclusion (acute hepatitis C, chronic hepatitis C, or cirrhosis).
Whether the patients had autoimmune disorders related to chronic hepatitis C infection.
Whether the patients were 'naive' (not previously treated), 'relapsers' (patients with a transient virological and/or biochemical response to previous therapy), or 'non‐responders' (patients without a virological or biochemical response to previous therapy).
Results
Description of studies
We identified 735 references through the electronic searches of The Cochrane Hepato‐Biliary Group Controlled Trials Register (n = 162), The Cochrane Central Register of Controlled Trials in The Cochrane Library (n = 261), MEDLINE (n = 169), and EMBASE (n = 143). Of these references, we excluded duplicates, clearly irrelevant references, and non‐randomised studies (n = 725). No quasi‐randomised studies were identified. Through manual searches of reference lists, specialist journals, and correspondence with authors, no additional references were identified. Accordingly, 10 publications fulfilled the inclusion criteria of the present review describing a total of eight randomised clinical trials. Two of the 10 trials (Stokes 1987; Mondazzi 1993) were only published as abstracts. Types of participants In four trials (Mondazzi 1993; Chayama 1996; Guilera 2000; McHutchison 2001) all patients had chronic hepatitis C. In one trial (Mazzaro 2000) all patients were HCV‐RNA positive and in one trial (Dammacco 1994) 52/65 patients were HCV‐RNA positive. In one trial (Stokes 1987) all patients were diagnosed with chronic non‐A and non‐B hepatitis. In one trial (Liaw 1993) 42/48 patients were also diagnosed with chronic non‐A and non‐B hepatitis and 6/48 patients were diagnosed with chronic hepatitis C.
In one trial (Dammacco 1994) all patients had chronic hepatitis C and type 2 mixed cryoglobulinaemia. In one trial (Mazzaro 2000) all patients had cryoglobulinaemic glomerulonephritis. One trial (Guilera 2000) excluded patients with HCV related immunological disorders. However, five trials (Stokes 1987; Liaw 1993; Mondazzi 1993; Chayama 1996; McHutchison 2001) did not report the number of patients with HCV associated extrahepatic autoimmune disorders.
We did not identify randomised clinical trial assessing glucocorticosteroids for acute hepatitis C or for patients with chronic hepatitis C and autoimmune hepatitis or non‐hepatic diseases, which were treated with glucocorticosteroids.
Only one trial (McHutchison 2001) reported the number of included patients who were 'non‐responders' to previous interferon therapy.
Types of interventions In one trial (Stokes 1987), patients were randomised to glucocorticosteroids versus placebo. One trial (Liaw 1993) randomised patients to three different intervention arms (glucocorticosteroids plus interferon versus interferon versus no intervention). One trial (Dammacco 1994) randomised patients to four different intervention arms (glucocorticosteroids plus interferon versus interferon versus glucocorticosteroids versus no intervention). In three trials, patients were randomised to short‐term glucocorticosteroids plus interferon (Chayama 1996; Guilera 2000; McHutchison 2001) versus interferon and placebo or no intervention. In one trial (Mondazzi 1993) patients were randomised to long‐term glucocorticosteroids and interferon treatment versus interferon. In one trial (Mazzaro 2000), patients were randomised to treatment with long‐term glucocorticosteroids versus interferon.
The intervention regimes with glucocorticosteroids (prednisolone or prednisone) varied substantially among the identified trials. In summary the different regimens were:
Glucocorticosteroids versus no intervention/placebo (a) short‐term treatment (four weeks) with high dose glucocorticosteroids (45 mg/day) (Stokes 1987). (b) long‐term treatment (48 weeks) with low dose glucocorticosteroids (16 mg/day four times a week) (Dammacco 1994).
Glucocorticosteroids plus interferon versus interferon plus no intervention or placebo (a) short‐term (three to six weeks) pre‐treatment with high dose glucocorticosteroids (30 to 40 mg/day) followed by interferon (Liaw 1993; Chayama 1996; Guilera 2000; McHutchison 2001). (b) long‐term treatment with low dose glucocorticosteroids (12 mg/day for 52 weeks (Mondazzi 1993) or 16 mg/day four times a week for 48 weeks (Dammacco 1994)) in parallel with interferon.
Glucocorticosteroids versus interferon Long‐term low dose glucocorticosteroids (20 mg/day for 26 weeks (Mazzaro 2000) or 16 mg/day four times a week for 48 weeks (Dammacco 1994)) versus interferon (3 million units three times/week for 26 weeks (Mazzaro 2000) or 3 million units three times/week for 48 weeks (Dammacco 1994)).
Risk of bias in included studies
Three trials had adequate generation of the allocation sequence (Chayama 1996; Mazzaro 2000; McHutchison 2001). The allocation was adequately concealed by sealed envelopes in two trials (Chayama 1996; McHutchison 2001). Treatment with prednisolone was adequately blinded by identical placebo tablets in three trials (Stokes 1987; Guilera 2000; McHutchison 2001). Accordingly, only one trial (McHutchison 2001) used adequate methods to minimise selection and performance/outcome assessment biases. Four trials had adequate follow‐up and clearly described the numbers and reasons for dropouts and withdrawals (Dammacco 1994; Chayama 1996; Mazzaro 2000; McHutchison 2001).
Effects of interventions
Patients with chronic hepatitis C ‐ Glucocorticosteroids versus no intervention The mortality was zero in both intervention arms after maximum follow‐up and no patient from any intervention arm obtained a negative HCV‐RNA at the end of treatment (1 trial, n = 26 patients). None of the included trials provided information about biochemical response, histological response, quality of life, or adverse events.
‐ Glucocorticosteroids plus interferon versus interferon plus no intervention or placebo Mortality The mortality was zero in both intervention arms at maximum follow‐up (6 trials, n = 342 patients).
Virological response (number with positive HCV‐RNA) Glucocorticosteroids plus interferon versus interferon had no significant effect on the virological response at the end of treatment (relative risk (RR) 0.86; 95% CI 0.42 to 1.74; 1 trial, n = 26 patients) or after six months (RR 0.85; 95% CI 0.52 to 1.38; 1 trial, n = 38 patients) Biochemical response (number without normalisation of serum ALT activity) Glucocorticosteroids plus interferon versus interferon had no significant effect on the biochemical response at the end of treatment (RR 0.92; 95% CI 0.77 to 1.11; 5 trials, n = 307 patients) or after 24 to 26 weeks (RR 0.95; 95% CI 0.84 to 1.06; 5 trials, n = 307 patients).
Liver histology Glucocorticosteroids plus interferon versus interferon had no significant effect on necroinflammatory activity in the liver at the end of treatment (RR 0.38; 95% CI 0.13 to 1.11; 1 trial, n = 281 patients) or after six months (RR 0.77; 95% CI 0.57 to 1.04; 1 trial, n = 26 patients). None of the included trials provided data about quality of life.
Adverse events There was no significant difference in the occurrence of serious adverse events between glucocorticosteroids plus interferon versus interferon alone (RR 4.76; 95% CI 0.24 to 93.19; 6 trials, n = 342 patients; Fischer's exact test: chi square 0.475; P = 0.51). The two cases of serious adverse events (one patient with serious soft tissue infection requiring surgical debridement and one patient with acute exacerbation of hepatitis) occurred during pre‐treatment with prednisolone in one trial (McHutchison 2001).
We found no significant difference in the occurrence of other adverse events like fever, myalgia, headache, alopecia, depression, fatigue, nausea, irritability, and sleep disturbance between glucocorticosteroids plus interferon versus interferon alone (Dammacco 1994; Chayama 1996). However, one trial (Mondazzi 1993) reported that the flu‐like syndrome caused by interferon therapy was nearly significantly reduced by prednisolone (RR 0.14 95% CI 0.02 to 1.00; 1 trial, n = 26 patients).
Sensitivity analyses Methodological quality There was no significant difference regarding the end of treatment or sustained biochemical response in trials with adequate compared to unclear or inadequate generation of the allocation sequence (RR 0.89 versus RR 0.93 (P = 0.88) and RR 0.90 versus RR 0.96 (P = 0.62), respectively) or allocation concealment (RR 0.89 versus RR 0.93 (P = 0.88) and RR 0.90 versus RR 0.96 (P = 0.62), respectively).
There was no significant difference regarding the end of treatment or sustained biochemical response in blinded trials compared to unblinded trials (RR 0.85 versus RR 1.20 (P = 0.17), respectively and RR 1.00 versus RR 0.83 (P = 0.25), respectively).
Due to the limited number of data we were unable to perform any of the remaining planned sensitivity analyses or funnel plot analyses.
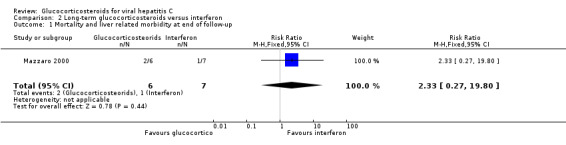
‐ Glucocorticosteroids versus interferon Mortality The mortality was zero in both intervention arms during treatment with prednisolone versus interferon (2 trials, 40 patients). After 12 months two patients in the glucocorticosteroids group and one patients in the interferon group died (RR 2.33; 95% CI 0.27 to 19.80; 1 trial, 13 patients).
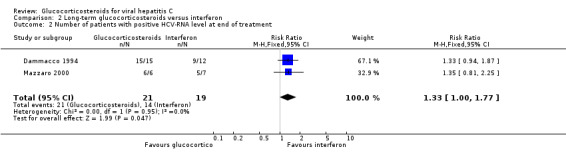
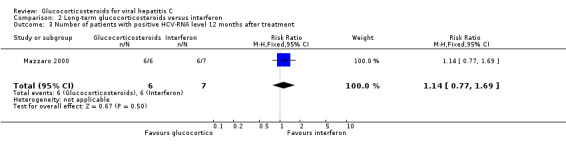
Virological response (number with positive HCV‐RNA) Compared to interferon, glucocorticosteroids significantly increased the risk of having positive HCV‐RNA at the end of treatment (RR 1.35; 95% CI 1.04 to 1.77; 2 trials, 40 patients), but not after 12 months (RR 1.17; 95% CI 0.86 to 1.58; 1 trial, 13 patients).
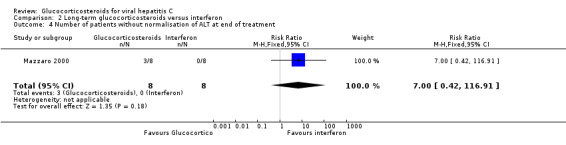
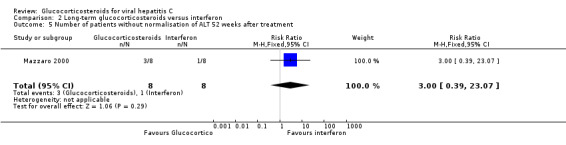
Biochemical response (number without normalisation of serum ALT activity (more than 50 IU/ml) Compared to interferon, glucocorticosteroids had no significant effect on the biochemical response at the end of treatment (RR 7.00; 95% CI 0.42 to 116.91; 1 trial, 16 patients) or after 52 weeks (RR 3.00; 95% CI 0.39 to 23.07; 1 trial, 16 patients). None of the included trials provided data about histological response, quality of life, or adverse events.
Due to the limited number of data we were unable to perform any of the remaining planned sensitivity analyses or funnel plot analyses.
Discussion
Based on this systematic review, we found no significant effects of glucocorticosteroids with or without interferon on mortality, end of treatment or end of follow‐up virological or biochemical response, or adverse events in patients with chronic hepatitis C. This review also assessed two trials on glucocorticosteroids versus interferon. No significant difference was found in mortality and end of follow‐up virological response, but interferon compared with glucocorticosteroids significantly decreased the risk of having positive HCV‐RNA at the end of treatment, but the amount of data is sparse.
The major limitations of this review are the low number of patients and the low methodological quality of the included trials. Only three trials reported adequate generation of the allocation sequence, only two trials reported adequate allocation concealment, and only three trials used adequate blinding. In fact, only one trial (McHutchison 2001) used both adequate randomisation and blinding methods, which can guard against both selection and performance/outcome assessment biases (Schultz 1995; Moher 1998; Kjaergard 2001). Further, the intervention regimes varied substantially, and in two trials all patients were diagnosed with immunological disorders (cryoglobulinaemia and glomerulonephritis), which are probably induced by HCV (Mazzaro 2000; El‐Serag 2003). Excluding these patients from our meta‐analysis did not provide any significant changes in the lack of significant effects of glucocorticosteroids, nor did glucocorticosteroids have any significant effect on these patients alone.
It may take many years for a patient with hepatitis C to develop cirrhosis, hepatocellular carcinoma, and eventually die (Alberti 1999; Brok 2003). Therefore, a further weakness of the trials included in the present review is that the follow‐up on average was six months. The limit for normalisation of ALT varied from 50 to 60 IU/L in the trials, and the limit for detectability of amplified HCV‐RNA by polymerase chain reaction (PCR) varied depending on the method. Longer follow‐up periods, identical predefined upper limit of ALT activity, and identical tests to measure virological response in the clinical trials would have increased the strength of this review.
In the included trials, glucocorticosteroids were administrated either as short pre‐treatment before interferon therapy or as long‐term treatment with interferon. We found no significant beneficial or harmful effect of either pre‐treatment or long‐term treatment with glucocorticosteroids. These data suggest that glucocorticosteroids should not be considered as additive treatment for patients with chronic hepatitis C.
We found no significant effect of glucocorticosteroids on adverse advents in our meta‐analysis. However, randomised clinical trials and meta‐analyses are weak in detecting rare adverse events. Very large observational studies are needed to detect and assess presence of rare adverse events. In general, it is difficult to get a clear picture of the adverse events that are known to be associated with glucocorticosteroids (e.g., osteoporosis, hyperglycaemia, diabetes, peptic ulcer, infection, depression, electrolyte imbalance, and weight gain) (Jarlbæk 2000; Gøtzsche 2002). The risk of adverse events during short‐term treatment with glucocorticosteroids for, e.g., asthma and rheumatoid arthritis is considered low but it is known that long‐term treatment increases the risk of adverse events (Hougardy 2000; Jarlbæk 2000). In one of the included trials (McHutchison 2001), pre‐treatment with glucocorticosteroids led to two cases of serious adverse events. No serious adverse events were observed in 39 patients giving long‐term treatment with low‐dose glucocorticosteroids (Dammacco 1994; Mondazzi 1993).
Several autoimmune disorders (e.g., mixed cryoglobulinaemia, porphyria cutanea tarda, and autoimmune hepatitis) are more frequently observed in patients with chronic hepatitis C (Ferri 2002; El‐Serag 2003). Glucocorticosteroids are often used to reduce autoimmune disorders induced by hepatitis C infection (Ferri 2002). Dammacco 1994 trial, however, found no significant effect of long‐term glucocorticosteroids on mixed cryoglobulinaemia. Further, glucocorticosteroids compared to interferon had no significant effect on cryoglobulinaemic glomerulonephritis (Mazzaro 2000), but the risk of type 2 errors in both trials is substantial. No other randomised clinical trials have assessed the efficacy of glucocorticosteroids in patients with hepatitis C infection and extrahepatic associated immune disorders or autoimmune hepatitis. Since hepatitis C infection induce autoimmune disorders, eradication of HCV‐RNA with interferon plus ribavirin seems to be first line treatment (Brok 2003; Kjaergard 2003). However, interferon may worsen or exacerbate autoimmune disorders (Dusheiko 1997; Fabbri 2003; Fabris 2003) and should be given with caution, especially to patients with positive autoantibodies (Bell 1999).
Only two trials including 40 patients assessed the effects of long‐term glucocorticosteroids versus interferon. No significant difference in mortality or biochemical response was observed. Virological response at the end of treatment showed that interferon compared to glucocorticosteroids significantly reduce the risk of having positive HCV‐RNA. However, previous evidence suggests that interferon therapy is effective in achieving viral clearance and improvement in liver biochemistry and histology in chronic hepatitis C. However, interferon also increases the risk of adverse events and reduces the quality of life (Myers 2003).
Three trials (Liaw 1993; Guilera 2000;McHutchison 2001) reported that pre‐treatment with glucocorticosteroids compared with no intervention increased viraemia, but no significant difference on viraemia were observed after subsequent interferon therapy. Whether glucocorticosteroid induced viraemia is of clinical relevance is unclear. Therefore, we were not able to determine whether patients should be screened for HCV‐RNA before starting treatment with glucocorticosteroids for non‐hepatic diseases.
We did not identify any randomised clinical trials assessing the effects of glucocorticosteroids for acute hepatitis C. However, it may be difficult to assess interventions for acute hepatitis C since 75 per cent are asymptomatic (Alter 1989).
Liver transplantation may be the ultimate treatment for patients with chronic hepatitis C who develop end‐stage liver failure. However, viraemia persists in the majority of patients who undergo liver transplantation (Filipponi 2001). Therefore, treatment aiming for eradication of HCV‐RNA may be needed even after liver transplantation. Two small randomised trials (Tisone 1999; Belli 2001) have assessed glucocorticosteroids for patients with chronic hepatitis C who underwent liver transplantation. These trials found no significant beneficial or harmful effects of glucocorticosteroids on hepatitis C recurrence or on graft rejection.
Authors' conclusions
Implications for practice.
There is insufficient evidence to confirm or exclude both beneficial and harmful effects of glucocorticosteroids for hepatitis C with or without autoimmune disorders. However, the sparse results are not promising. Therefore, we are unable to recommend glucocorticosteroids as treatment for patients with acute and chronic hepatitis C. This review cannot to rule out potential serious adverse effects of glucocorticosteroids. Therefore, this review cannot establish whether glucocorticosteroids treatment can be safely administrated for indications requiring glucocorticosteroids. Accordingly, the use of glucocorticosteroids must be based on the strength of evidence for using glucocorticosteroids for 'the other disease'.
Implications for research.
Based on the absence of significant effects in our meta‐analysis and on the knowledge from other studies that glucocorticosteroids increase the risk of adverse effects focusing on other interventions for chronic hepatitis C may be more relevant to. However, high‐quality randomised clinical trials are needed to assess the beneficial and harmful effects of glucocorticosteroids for patients with chronic hepatitis C and autoimmune hepatitis or other autoimmune disorders induced by hepatitis C infection. Such trials should be reported according to the CONSORT guidelines (www.consort‐statemant.org)
What's new
| Date | Event | Description |
|---|---|---|
| 9 November 2008 | Amended | Converted to new review format. |
Acknowledgements
We are indebted to D Nikolova, S Frederiksen, and N Salasshahri for their expert assistance in the identification of trials, and to N Frydendall and B Hansen for secretarial assistance. We also appreciate the helpful comments and suggestions from LL Gluud, B Als‐Nielsen, W Chen, and R Simmonetti. Further, we wish to give special gratitude to K Chayama, J McHutchison, and G Pozzato who provided us with additional information about the included trials they were involved in.
Appendices
Appendix 1. Search strategies
| Databases | Search Strategies | date of search |
| The Cochrane Hepato‐Biliary Group Controlled Trials Register of | #1 = RCT AND glucocorticosteroids AND hepatitis, #2 = RCT AND prednisone AND hepatitis, #3 = RCT AND prednisolone AND hepatitis, #4 = RCT AND hydrocortisone AND hepatitis, #5 = RCT AND corticosteroids AND hepatitis, #6 = RCT AND cortisone AND hepatitis, #7 = RCT AND budosonide AND hepatitis, #8 = RCT AND beclomethasone AND hepatitis, #9 = RCT AND dexamethasone AND hepatitis, #10 = RCT AND cortisol AND hepatitis, #11 = RCT AND fluocortolone AND hepatitis. | Search performed 01.07.03. 162 references were identified. |
| The Cochrane Library (Issue 2, 2003) | #1 = glucocorticosteroids AND hepatitis, #2 = prednisone AND hepatitis, #3 = prednisolone AND hepatitis, #4 = RCT AND hydrocortisone AND hepatitis, #5 = corticosteroids AND hepatitis, #6 = cortisone AND hepatitis, #7 = budosonide AND hepatitis, #8 = beclomethasone AND hepatitis, #9 = dexamethasone AND hepatitis, #10 = cortisol AND hepatitis, #11 = fluocortolone AND hepatitis. | Search performed 01.07.03. 261 references were identified. |
| MEDLINE | We used the search strategy developed by the Cochrane Collaboration (Cochrane Reviewers' Handbook 4.1.5, 11a.15 Appendix B) AND: #35 hepatitis #36 glucocorticosteroids #37 prednisone #38 prednisolone #39 hydrocortisone #40 corticosteroids #41 cortisone #42 budosonide #43 beclomethasone #44 dexamethasone #45 cortisol #46 fluocortolone #1 AND #35 AND (#36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 AND #46). | Search preformed 01.07.03. 143 references were identified. |
| EMBASE | #1 Study #2 trial #3 randomi* #4 hepatitis #5 glucocorticosteroids #6 prednisone #7 prednisolone #8 hydrocortisone #9 corticosteroids #10 cortisone #11 budosonide #12 beclomethasone #13 dexamethasone #14 cortisol #15 fluocortolone (#1 or #2) AND #3 AND #4 AND (#5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15). | Search performed 01.07.03. 169 references were identified. |
Data and analyses
Comparison 1. Glucocorticosteroids plus interferon versus interferon plus no intervention/placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of patients with positive HVC‐RNA level at end of treatment | 1 | 26 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.42, 1.74] |
| 2 Number of patients with positive HCV‐RNA level 26 weeks after treatment | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.52, 1.38] |
| 2.1 Short‐term pre‐treatment with glucocorticosteroids followed by interferon | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.52, 1.38] |
| 2.2 Long‐term treatment with glucocorticosteroids in parallel with interferon | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Number of patients without normalisation of ALT at end of treatment | 5 | 307 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.77, 1.11] |
| 3.1 Short‐term pre‐treatment with glucocorticosteroids followed by interferon | 4 | 281 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.74, 1.08] |
| 3.2 Long‐term treatment with glucocorticosteroids in parallel with interferon | 1 | 26 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.69, 2.39] |
| 4 Number of patients without normalisation of ALT 24 to 26 weeks after treatment | 5 | 307 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.84, 1.06] |
| 4.1 Short‐term pre‐treatment with glucocorticosteroids followed by interferon | 4 | 281 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.83, 1.05] |
| 4.2 Long‐term treatment with glucocorticosteroids in parallel with interferon | 1 | 26 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.64, 1.97] |
| 5 Liver histology (patients without decrease in necroinflammatory activity at end of treatment) | 1 | 26 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.13, 1.11] |
| 6 Liver histology (patients without decrease in necroinflammatory activity at end of follow‐up (26 weeks). | 1 | 26 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.57, 1.07] |
| 7 Serious adverse events | 6 | 342 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.76 [0.24, 93.19] |
| 7.1 Short‐term pre‐treatment with glucocorticosteroids followed by interferon | 4 | 281 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.76 [0.24, 93.19] |
| 7.2 Long‐term treatment with glucocorticosteroids in parallel with interferon | 2 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Adverse events | 3 | 320 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.71, 1.43] |
| 8.1 Fever | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.44, 2.39] |
| 8.2 Myalgia | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.36, 3.37] |
| 8.3 headache | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.27, 2.93] |
| 8.4 Alopecia | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.06, 12.91] |
| 8.5 Depression | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.27, 2.93] |
| 8.6 Fatigue | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.56, 2.29] |
| 8.7 Nausea | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.09 [0.75, 12.65] |
| 8.8 Irritability | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.40, 2.77] |
| 8.9 Flu‐like syndrome | 1 | 26 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.02, 1.00] |
| 8.10 Sleep disturbance | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 69.31] |
1.1. Analysis.
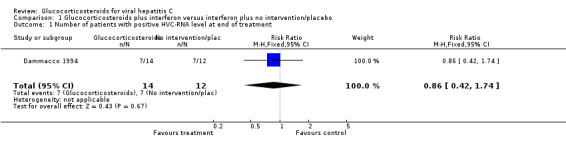
Comparison 1 Glucocorticosteroids plus interferon versus interferon plus no intervention/placebo, Outcome 1 Number of patients with positive HVC‐RNA level at end of treatment.
1.2. Analysis.
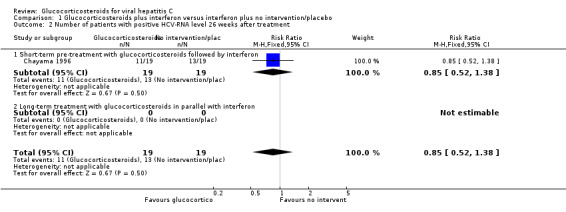
Comparison 1 Glucocorticosteroids plus interferon versus interferon plus no intervention/placebo, Outcome 2 Number of patients with positive HCV‐RNA level 26 weeks after treatment.
1.3. Analysis.
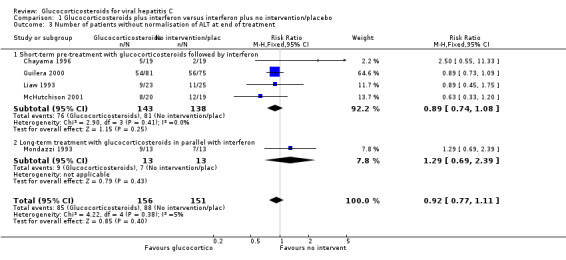
Comparison 1 Glucocorticosteroids plus interferon versus interferon plus no intervention/placebo, Outcome 3 Number of patients without normalisation of ALT at end of treatment.
1.4. Analysis.
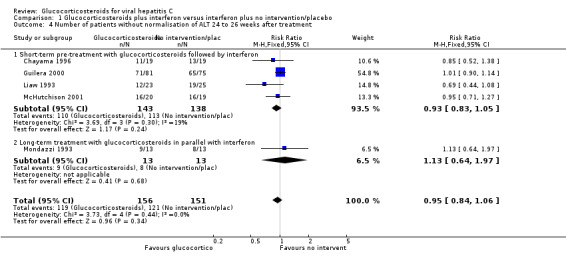
Comparison 1 Glucocorticosteroids plus interferon versus interferon plus no intervention/placebo, Outcome 4 Number of patients without normalisation of ALT 24 to 26 weeks after treatment.
1.5. Analysis.
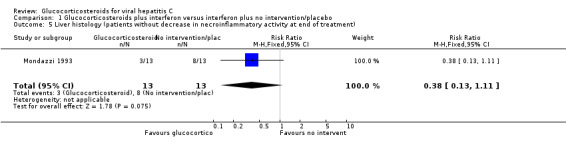
Comparison 1 Glucocorticosteroids plus interferon versus interferon plus no intervention/placebo, Outcome 5 Liver histology (patients without decrease in necroinflammatory activity at end of treatment).
1.6. Analysis.
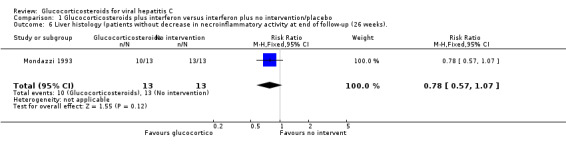
Comparison 1 Glucocorticosteroids plus interferon versus interferon plus no intervention/placebo, Outcome 6 Liver histology (patients without decrease in necroinflammatory activity at end of follow‐up (26 weeks)..
1.7. Analysis.
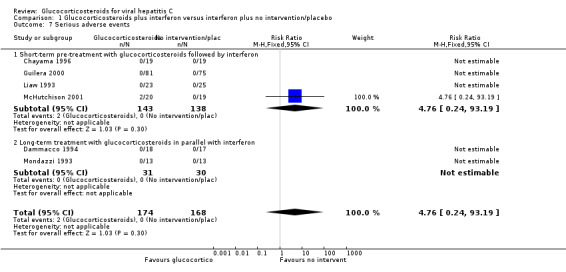
Comparison 1 Glucocorticosteroids plus interferon versus interferon plus no intervention/placebo, Outcome 7 Serious adverse events.
1.8. Analysis.
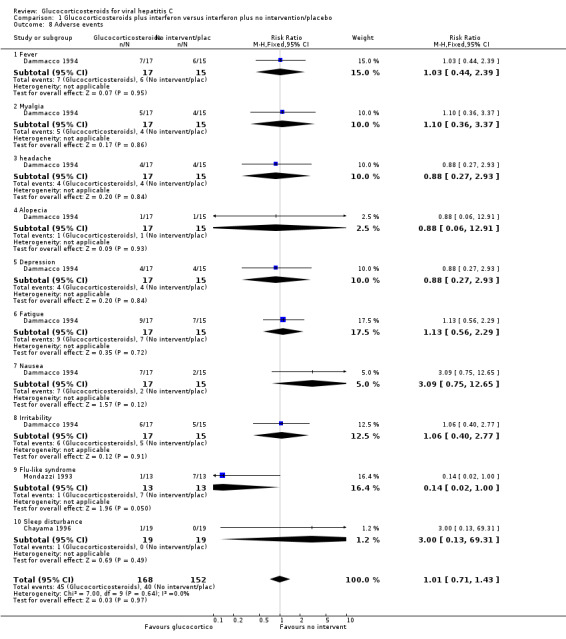
Comparison 1 Glucocorticosteroids plus interferon versus interferon plus no intervention/placebo, Outcome 8 Adverse events.
Comparison 2. Long‐term glucocorticosteroids versus interferon.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality and liver related morbidity at end of follow‐up | 1 | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.33 [0.27, 19.80] |
| 2 Number of patients with positive HCV‐RNA level at end of treatment | 2 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [1.00, 1.77] |
| 3 Number of patients with positive HCV‐RNA level 12 months after treatment | 1 | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.77, 1.69] |
| 4 Number of patients without normalisation of ALT at end of treatment | 1 | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.0 [0.42, 116.91] |
| 5 Number of patients without normalisation of ALT 52 weeks after treatment | 1 | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.39, 23.07] |
2.1. Analysis.
Comparison 2 Long‐term glucocorticosteroids versus interferon, Outcome 1 Mortality and liver related morbidity at end of follow‐up.
2.2. Analysis.
Comparison 2 Long‐term glucocorticosteroids versus interferon, Outcome 2 Number of patients with positive HCV‐RNA level at end of treatment.
2.3. Analysis.
Comparison 2 Long‐term glucocorticosteroids versus interferon, Outcome 3 Number of patients with positive HCV‐RNA level 12 months after treatment.
2.4. Analysis.
Comparison 2 Long‐term glucocorticosteroids versus interferon, Outcome 4 Number of patients without normalisation of ALT at end of treatment.
2.5. Analysis.
Comparison 2 Long‐term glucocorticosteroids versus interferon, Outcome 5 Number of patients without normalisation of ALT 52 weeks after treatment.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Chayama 1996.
| Methods | Generation of the allocation sequence: adequate (computer generated random sequence). Allocation concealment: adequate (sealed envelopes). Blinding: no blinding. Follow‐up: adequate. Follow‐up was six months after treatment. They clearly reported that there had been no dropouts or withdrawals. | |
| Participants | Thirty‐eight patients from Japan with chronic hepatitis C Inclusion criteria: histopathological evidence of chronic active hepatitis, seropositive for anti‐HCV and HCV‐RNA, and ALT levels above 50 IU/L for more than six months. Exclusion criteria: patients with apparent cirrhosis, and patients who did not receive immunosuppressive or antiviral therapy within six months before the study. Characteristics: Prednisolone‐interferon group: Male/Female: 14/5. Age (median (range)): 52 (41 to 65). Prior transfusions: 10. HCV level (median (range)): 7 (5 to 14) copies/nl. Histology with bridging fibrosis: 7. ALT (median (range)): 76 (48 to 128) IU/L. Interferon group: Male/Female: 14/5. Age (median (range)): 52 (22 to 65). Prior transfusions: 4. HCV level (median (range) ):6.5 (5 to 17) copies/nl. Histology with bridging fibrosis: 5. ALT (median (range)): 118 (60 to 250) IU/L. |
|
| Interventions | Prednisolone and interferon group:
all patients received 40 mg/day of prednisolone for three weeks and four weeks of observation without treatment. This was followed by administration of six million units/day of lymphoblastoid interferon alpha‐n1 for eight weeks. The dosage was later reduced to six million units twice weekly for 16 weeks. Interferon group: The same as the group above, but without the prednisolone priming. |
|
| Outcomes | ALT, HVC‐RNA, and adverse effects. Patients with normalization of ALT (less than 50 IU/L) and negative HCV‐RNA were considered responders. | |
| Notes | We wrote to the authors asking for more information regarding the trial, which they kindly replied to. They found no significant difference regarding quality of life in patients who received prednisolone plus interferon compared to interferon (no numerical data provided). No information on health economics was provided. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Dammacco 1994.
| Methods | Generation of the allocation sequence: unclear (not reported). Allocation concealment: unclear (not reported). Blinding: no blinding. Follow‐up: adequate. Median follow‐up was 13 months after treatment. They specified that all patients completed the study. | |
| Participants | 52 patients from Italy with type 2 mixed cryoglobulinaemia and with positive anti‐HCV and HCV‐RNA. Inclusion criteria: diagnosed cryoglobulinaemia characterised by detectable serum cryoglobulins for at least one year associated with the triad purpura‐weakness‐arthralgias and positive anti‐HCV. Exclusion criteria: Interferon or immunosuppressive therapy within the previous six months, pregnancy, concomitant illness, ascites, bleeding oesophageal varices, hepatic encephalopathy, bilirubin greater than three mg/dL, albumin less than three g/dL, prothrombin time greater than three seconds longer than the control, leukocyte count less than 3000/µL, platelet count less than 10 g/dL, and positive test for hepatitis B surface antigen. Characteristics (including patients without positive anti‐HCV): Interferon group: Male/Female: 6/9. Age (mean ± SD): 59.2 ± 7.1. ALT (IU/L): 54.1 ± 32.2. Gamma‐globulin (g/L): 2.01 ± 0.6. Anti‐HCV: 12. HCV RNA: 12. Cirrhosis: 3. Prednisolone group: Male/Female: 8/10. Age (mean ± SD): 49.2 ± 16.7. ALT (IU/L): 56.1 ± 47.2. Gamma‐globulin (g/L): 1.9 ± 0.8. Anti‐HCV: 15. HCV RNA: 15. Cirrhosis: 5. Interferon plus prednisolone group: Male/Female: 8/9. Age (mean ± SD): 56.3 ± 3.3. ALT (IU/L): 49.4 ± 41.0. Gamma‐globulin (g/L): 1.9 ± 0.5. Anti‐HCV: 11. HCV RNA: 11. Cirrhosis: 6. Control group: Male/Female: 7/8. Age (mean ± SD): 51.4 ± 5.0. ALT (IU/L): 62.0± 18.0. Gamma‐globulin (g/L): 1.8 ± 0.7. Anti‐HCV: 11. HCV RNA: 11. Cirrhosis: 4. |
|
| Interventions | Prednisolone group:
all patients received prednisolone (16 mg/day) for 48 weeks. Interferon plus prednisolone group: Same as the interferon group plus prednisolone on non‐interferon days. Interferon group: all patients received three million units of lymphoblastoid interferon alpha‐n1 three times/week for 48 weeks. Control group: received no intervention. |
|
| Outcomes | IgM, rheumatoid factor activity, ALT, HCV‐RNA and adverse events. The authors defined complete response as having a negative (non‐detectable) HCV‐RNA level. | |
| Notes | Thirteen patients were not anti‐HCV positive. They were excluded from our meta‐analysis. No information on health economics was provided. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Guilera 2000.
| Methods | Generation of the allocation sequence: unclear (not reported).
Allocation concealment:
unclear (not reported).
Blinding: yes ‐ adequately blinded with identical placebo.
Follow‐up: Inadequate. Twenty‐four weeks after treatment. They did not describe the number and reason for dropouts. However, they used an intention‐to‐treat analyses. Results were analysed on a intention‐to‐treat basis, but the authors did not no specify what is meant by this analysis and number of drop‐outs were not reported. |
|
| Participants | 156 patients from Spain with chronic hepatitis C. Inclusion criteria: ALT elevation and positive anti‐HCV for at least six months prior to the beginning of treatment, presence of HCV‐RNA in serum, and histopathological evidence of chronic hepatitis. Exclusion criteria: Patients with other causes of chronic liver diseases such as excessive ethanol consumption, current infection with hepatitis B, and metabolic or autoimmune disorders. No patients had evidence of HIV infection. Characteristics: Prednisolone ‐ interferon group: Male/Female: 56/25. Age (mean ± SD): 39 ± 11. ALT (IU/L): 123 ± 88. HCV genotype: 1: 62 Non‐1: 19. HCV RNA (copies/nanolitre): 2.0 ± 2.4. White blood cells count (nanoltire): 6.7 ± 1.9. Platelet count (nanolitre): 185 ± 48. Placebo ‐ interferon group: Male/Female: 52/23. Age (mean ± SD): 38 ± 12. ALT (IU/L): 122 ± 80. HCV genotype: 1: 61 Non‐1: 14. HCV RNA (copies/nanolitre): 1.8 ± 2.3. White blood cells count (nanolitre): 6.7 ± 1.7. Platelet count (nanolitre): 187 ± 42. |
|
| Interventions | Prednisolone plus interferon group: all patients received oral prednisolone for four weeks: 0.6 mg/kg/day for two weeks, 0.45 mg/kg/day for one week and 0.25 mg/kg/day for one week. Two weeks after withdrawal of prednisolone, recombinant interferon alpha‐2b was three million units three times/week for 48 weeks.
Treatment was stopped in patients who did not respond (ALT level) after 24 weeks on interferon therapy. Placebo plus interferon group: all patients received identical placebo followed by interferon treatment as the prednisolone group. |
|
| Outcomes | ALT, HVC‐RNA, and adverse events. Patients with normal level of ALT (not defined) and negative HCV‐RNA were considered as responders. | |
| Notes | No information on health economics was provided. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Liaw 1993.
| Methods | Generation of the allocation sequence: unclear (not reported). Allocation concealment: unclear (not reported). Blinding: no blinding. Follow‐up: Unclear. Follow‐up was 24 months after treatment. Three patients did not complete treatment in the interferon group as they were considered as drop‐outs. | |
| Participants | 48 patients from Taiwan with chronic 'non‐A, non‐B' hepatitis.
Inclusion criteria: patients aged 18 years or older and with persistent elevations of ALT for at least a year and histologic features on liver biopsy compatible with chronic 'non‐A, non‐B' hepatitis. Exclusion criteria: chronic hepatitis due to other etiologies, such as alcohol, Wilson's disease or autoimmune hepatitis. Patients treated with immunosuppressive or antiviral therapy within a year were also excluded. Characteristics: Interferon group: Male/Female: 15/10. Age (mean ± SD): 48 ± 0.4. Prior hepatitis: 5. Prior transfusions: 6. Anti‐HCV: 15. AST (IU/L) (mean ± SD): 130 ± 1.8. ALT (IU/L) (mean ± SD): 183 ± 2.1. Bilirubin (mg/dl ± SD): 0.8 ± 0.1. Gamma‐globulin (g/dl) ± SD): 1.9 ± 0.1. Cirrhosis: 8. Prednisolone group: Male/Female: 18/5. Age (mean): 47. Prior hepatitis: 6. Prior transfusions: 9. Anti‐HCV: 21. AST (IU/L) (mean ± SD): 154 ± 2.1. ALT (IU/L) (mean ± SD): 221 ± 2.3. Bilirubin (mg/dl ± SD): 1.0 ± 0.2. Gamma‐globulin (g/dl) ± SD ): 2.0 ± 0.1. Cirrhosis: 7. Control group: Male/Female: 15/7. Age (mean): 45. Prior hepatitis: 3. Prior transfusions: 4. Anti‐HCV: 21. AST (IU/L) (mean ± SD): 108 ± 1.6. ALT (IU/L) (mean ± SD): 177 ± 2.3. Bilirubin (mg/dl ± SD): 0.9 ± 0.1. Gamma‐globulin (g/dl) ± SD ): 2.0 ± 0.1. Cirrhosis: 3. |
|
| Interventions | Prednisolone group: Prednisolone 30 mg daily for three weeks, 15 mg daily for another week, followed by a 2‐week rest and starting the same regime as the fixed interferon group. Fixed dosage interferon group: all patients received recombinant interferon alpha‐2b three million units three times/week for 24 weeks. Adjustable interferon group: start as the fixed interferon group but down or up titration according to AST changes: if ALT remains normal for four weeks dosage is reduced but if relapse occur dosage is increased to the original level. Control group: no treatment for three month followed by altering treatment with three million units of recombinant interferon alpha‐2b three times/week for two weeks then two weeks with no treatment for a period of six months. |
|
| Outcomes | ALT, blood counts, bilirubin, gamma‐globulin, and adverse effects. Complete response was defined as normalisation of ALT (less than 60 IU/L). | |
| Notes | We included follow‐up 26 weeks after treatment in our meta‐analysis. Only outcome measures from three groups were reported (prednisolone, fixed interferon, and no intervention group). No information on health economics was provided. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Mazzaro 2000.
| Methods | Generation of the allocation sequence: adequate (computer generated random sequence). Allocation concealment: unclear (not reported). Blinding: no blinding. Follow‐up: Adequate. Follow‐up was 12 months after treatment. They clearly reported that there had been no dropouts or withdrawals. | |
| Participants | 13 patients form Italy with cryoglobulinaemic glomerulonephritis and positive HCV‐RNA. Inclusion criteria: patients with diagnosed cryoglobulinaemic glomerulonephritis. Diagnosis was confirmed by kidney biopsy. All patients had positive HCV‐RNA diagnosed by enzyme‐linked immunosorbent assay (ELISA). Exclusion criteria: History of intravenous drug or alcohol abuse All patients had previously been treated with corticosteroids and cyclophosphamide and were after remission enrolled in the study. Group prednisolone: Male/Female: 2/4. Age (mean ± SD): 59 ± 11. Duration of disease (years): 4 ± 2.7. ALT (IU/L): 48 ± 27. HCV‐RNA copies/nl: 1.7 ± 2.9. Group interferon: Male/Female: 3/4. Age (mean ± SD): 64 ± 11. Duration of disease (years): 5.1 ± 3.0. ALT (IU/L): 44 ± 18. HCV‐RNA copies/nl: 2.1 ± 3.1. |
|
| Interventions | All patients received methyl‐prednisolone and cyclophosphamide in the acute phase. After six to nine months patients with stationary, but active disease were randomly assigned to either interferon or prednisolone. Prednisolone group: all patients received oral prednisolone 0.2 mg/kg/day for six months. Interferon group: all patients received lymphoblastoid interferon alpha‐n1 three million units three times/week for six months. |
|
| Outcomes | Purpura score, level of cryoglobulins, rheumatoid factor, creatinine, HCV‐RNA, ALT, and side effects. A negative HCV‐RNA (less than 10 copies/ml) were defined as complete response. | |
| Notes | We wrote to the authors asking for more information regarding the trial which they kindly replied to. This trial reported that significantly more patients treated with interferon compared to prednisolone were having adverse events like fever, fatigue, and flu‐like syndrome but no numerical data were provided. They reported that cost of prednisone was 585 Euro and that the cost of interferon was 1950 Euro. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
McHutchison 2001.
| Methods | Generation of the allocation sequence: adequate (computer generated random sequence).
Allocation concealment:
adequate (sealed envelopes controlled by pharmacy).
Blinding: yes ‐ adequately blinded with identical placebo.
Follow‐up: Adequate. Twenty‐four weeks after treatment.
Six patients did not complete the treatment. Two of them were from the placebo group. Previous 'non‐responsders' to interferon therapy were stratified within the randomisation to ensure that equal numbers entered both treatment groups. |
|
| Participants | Inclusion criteria: patients aged 18 years or older, detectable hepatitis C antibodies, persistently elevated serum ALT during the 6 months before entry, prothrombin time less than three seconds prolonged, serum bilirubin less than 68 umol/L, and liver biopsy findings compatible with the diagnosis of chronic HVC infection Exclusion criteria: previous course of antiviral or immunosuppressive therapy within the preceding six months, other serious illness, pregnancy, other causes of liver disease, and history of significant alcohol intake. Characteristics: Prednisone ‐ interferon group: Male/Female: 15/5. Age (mean ± SD): 37.5 ± 6.6. Bilirubin (umol/L): 0.8 ± 0.4. Albumin (g/L): 43 ± 4. Prothrombin time (s): 12.3 ± 0.5. HCV genotype: 1: 14 Non‐1: 6. HCV RNA (MEq/ml): 7.8 ± 7.4. Cirrhosis: 3. Prior interferon treatment: 10. Placebo ‐ interferon group: Male/Female: 14/5. Age (mean ± SD): 42.5 ± 9.0. Bilirubin (umol/L): 0.8 ± 0.5. Albumin (g/L): 42 ± 4. Prothrombin time (s): 12.3 ± 0.0. HCV genotype: 1: 12 Non‐1: 7. HCV RNA (MEq/ml): 18.0 ± 26.5. Cirrhosis: 3. Prior interferon treatment: 10. |
|
| Interventions | Prednisone plus interferon group:
all patients received six weeks of a tapering dose of prednisone (60 mg, 40 mg 20 mg in two‐week intervals) followed by recombinant interferon alpha‐2b, three million units three times/week for 24 weeks. Placebo plus interferon group: all patients received identical placebo followed by interferon treatment as the prednisone group. |
|
| Outcomes | ALT, liver histology, and HCV‐RNA. Complete response was defined as normalisation of ALT (less than 55 IU/L). | |
| Notes | We wrote to the authors asking for more information regarding the trial which they kindly replied to. No information on health economics was provided. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Mondazzi 1993.
| Methods | Generation of the allocation sequence: unclear (not reported). Allocation concealment: unclear (not reported). Blinding: inadequate (no blinding). Follow‐up: Inadequate. Six months after treatment. The number or reasons for dropouts and withdrawals were not described. | |
| Participants | Patients with anti‐HCV positive chronic hepatitis. | |
| Interventions | Prednisone plus interferon group:
all patients received lymphoblastoid interferon alpha‐n1 three million units three times/week for one year plus prednisone 20 mg/day for eight weeks, 15 mg/day for two weeks, and 10 mg/day until the end of 12th month. Interferon group: was administrated as above. |
|
| Outcomes | ALT, liver histology, and flu‐syndrome. | |
| Notes | Only abstract. No information on health economics was provided. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Stokes 1987.
| Methods | Generation of the allocation sequence: unclear (not reported). Allocation concealment: unclear (not reported). Blinding: yes ‐ adequately blinded with identical placebo. Follow‐up: Inadequate. Twelve weeks after treatment. The number or reasons for dropouts and withdrawals were not described. | |
| Participants | Patients with non‐A, non‐B chronic hepatitis based on clinical criteria, negative hepatitis‐B serology, and compatible liver histology. | |
| Interventions | Prednisone group:
all patients received 60 mg/day for two weeks followed by two weeks with 30 mg/day. Placebo group: all patients received identical placebo. |
|
| Outcomes | ALT. | |
| Notes | Only abstract. No information on health economics was provided. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anonymous 1986 | Included only patients with hepatitis B. |
| Brook 1993 | Included only patients with hepatitis B. |
| Cook 1971 | Included patients with autoimmune hepatitis. |
| Giacchino 1988 | Included only patients with hepatitis B. |
| Giacchino 1995 | Included only patients with hepatitis B. |
| Gregorio 1996 | Included only patients with hepatitis B. |
| Hanson 1986 | An observational study. Not a randomised clinical trial. |
| Hoofnagle 1986 | Included only patients with hepatitis B. |
| Krogsgaard 1998 | Included only patients with chronic hepatitis B. |
| Lam 1981 | Included only patients with hepatitis B. |
| Lauta 1995 | Compared glucocorticosteroids versus glucocorticosteroids plus interferon for patients with cryoglobulinaemia and chronic hepatitis C. |
| Lee 1991 | Included only patients with hepatitis B. |
| Liaw 1994a | Included only patients with hepatitis B. |
| Magrin 1994 | Compared to different interferon treatment regimes for patients with chronic hepatitis C. |
| Manzillo 1983 | Included only patients with hepatitis B. |
| Murray 1973 | Included patients with autoimmune hepatitis. |
| Niederau 1992 | Included only patients with hepatitis B. |
| Perez 1993 | Included only patients with hepatitis B. |
| Schvarcz 1991 | A review. Not a randomised clinical trial. |
| Sjögren 1987 | Included only patients with hepatitis B. |
| Ware 1974 | Included patients with hepatitis B. |
| Ware 1981 | Included patients with hepatitis B. |
| Weller 1982 | Included only patients with hepatitis B. |
| Wu 1982 | Included only patients with hepatitis B. |
| Zarski 1994 | Included only patients with hepatitis B. |
Contributions of authors
Jesper Brok performed the searches, selected trials and studies, extracted data, and drafted and revised the review. Martin Mellerup drafted and revised the protocol and selected trials. Kim Krogsgaard revised the protocol and the review. Christian Gluud participated in the selection of trials and data extraction. Further, he revised the protocol and the review.
Sources of support
Internal sources
Copenhagen Trial Unit, Denmark.
External sources
Copenhagen Hospital Corporation Research Foundation, Denmark.
The 1991 Pharmacy Foundation, Denmark.
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Chayama 1996 {published data only}
- Chayama K, Tsubota A, Kobayashi M, Hashimoto M, Miyano Y, Koike H, et al. A pilot study of corticosteroid priming for lymphoblastoid interferon alfa in patients with chronic hepatitis C. Hepatology 1996;23(5):953‐7. [DOI] [PubMed] [Google Scholar]
Dammacco 1994 {published data only}
- Dammacco F, Sansonno D, Han JH, Shyamala V, Cornacchiulo V, Iacobelli AR, et al. Natural interferon‐alpha versus its combination with 6‐methyl‐prednisolone in the therapy of type 2 mixed cryoblobulinemia. Blood 1994;84(10):3336‐43. [PubMed] [Google Scholar]
Guilera 2000 {published data only}
- Guilera M, Forns X, Torras X, Enriques J, Coll S, Sola R, et al. Pre‐treatment with prednisolone does not improve the efficacy of subsequent alpha interferon therapy in chronic hepatitis C. Journal of Hepatology 2000;33(1):135‐41. [DOI] [PubMed] [Google Scholar]
Liaw 1993 {published data only}
- Liaw YF, Sheen IS, Lin SM, Chen TJ. Prednisolone withdrawel followed by recombinant alfa‐interferon in chronic non‐A, non‐B hepatitis. Gastroenterologia Japonica 1991;26 Suppl:3247‐50. [DOI] [PubMed] [Google Scholar]
- Liaw YF, Sheen IS, Lin SM, Chen TJ, Chu CM. Effects of prednisolone pretreatment in interferon alfa therapy for patients with chronic non‐A, non‐B hepatitis. Liver 1993;13(1):46‐50. [DOI] [PubMed] [Google Scholar]
Mazzaro 2000 {published data only}
- Mazzaro C, Panarello G, Carniello S, Faelli A, Mazzi G, Crovatto M, et al. Interferon versus steroids in patients with hepatitis C virus associated cryoglobulinaemic glomerulonephritis. Digestive and Liver Disease 2000;32(8):708‐15. [DOI] [PubMed] [Google Scholar]
McHutchison 2001 {published data only}
- McHutchison JG, Ponnudurai R, Bylund DL, Anguiano A, Pockros PJ, Modala T, et al. Prednisone withdrawel followed by interferon alpha for treatment of chronic hepatitis C infection. Journal of Clinical Gastroenterology 2001;32(2):133‐7. [DOI] [PubMed] [Google Scholar]
- McHutchison JG, Wilkes LB, Pockros PJ, Chan CS, Neuwald P, Urdea M, et al. Pulse corticosteroids therapy increases viremia (HCV RNA) in patients with chronic HCV infection [abstract]. Journal of Hepatology 1993;18 (4 pt 2):87A. [Google Scholar]
Mondazzi 1993 {published data only}
- Mondazzi L, Bellobuono A, Rezakovic I, Minola E, Idéo G. Lymphoid alpha‐IFN plus prednisone treatment of anti‐HCV positive chronic hepatitis [abstract]. Journal of Hepatology 1993;18 Suppl (1):150A. [Google Scholar]
Stokes 1987 {published data only}
- Stokes P, Lopez WC, Balart LA. Effects of short‐term corticosteroid therapy in patients with chronic non‐A, non‐B hepatitis [abstract]. Gastroenterology 1987;92(5):1783A. [Google Scholar]
References to studies excluded from this review
Anonymous 1986 {published data only}
- Anonymous. Steroids in chronic B‐hepatitis. A randomised, double‐blind, multinational trial on the effect of low‐dose, long‐term treatment on survival. Liver 1986;6(4):227‐32. [PubMed] [Google Scholar]
Brook 1993 {published data only}
- Brook MG, Main J, Yap I, Chan G, Karayiannis P, Crosey M, et al. Prednisolone withdrawal followed by lymphoblastoid interferon alpa in the therapy of adult patients with presumed childhood acquired chronic hepatitis B virus infection. Alimentary Pharmacology & Therapeutics 1993;7(3):331‐6. [DOI] [PubMed] [Google Scholar]
Cook 1971 {published data only}
- Cook GC, Mulligan R, Sherlock S. Controlled prospective trial of corticosteroid therapy in active chronic hepatitis. The Quarterly Journal of Medicine 1971;40(158):159‐85. [DOI] [PubMed] [Google Scholar]
Giacchino 1988 {published data only}
- Giacchino R, Facco F, Giambartolomei G, Navone C, Timitilli A, Cirillo C, et al. Treatment of children with chronic hepatitis B with a combinations of steroids and human lymphobastoid interferon. Chemioterapia 1988;7 Suppl:320‐5. [PubMed] [Google Scholar]
Giacchino 1995 {published data only}
- Giacchino R, Main J, Timitilli A, Giambartolomei G, Facco F, Cirillo C, et al. Dual‐centre, double‐blind, randomsed trial of lymphoblasoid interferon alpha with or without steroid pretreatment in children with chronic hepatitis B. Liver 1995;15(3):143‐8. [DOI] [PubMed] [Google Scholar]
Gregorio 1996 {published data only}
- Gregorio GV, Jara P, Hierro L, Diaz C, Vega A, Vegnente A, et al. Lymphoid interferon alfa with or without steroid pretreatment in children with chronic hepatitis B. Hepatology 1996;23(4):700‐7. [DOI] [PubMed] [Google Scholar]
Hanson 1986 {published data only}
- Hanson RG, Peters MG, Hoofnagle JH. Effects of immunosuppressive therapy with prednisolone on B and T lymphocyte function in patients with chronic type B hepatitis. Hepatology 1986;6(2):173‐9. [DOI] [PubMed] [Google Scholar]
Hoofnagle 1986 {published data only}
- Hoofnagle JH, Davis GL, Pappas SC, Hanson RG, Peters M, Avigan MI, et al. A short course of prednisolone in chronic type B hepatitis. Report of a randomised double‐blind, placebo‐controlled trial. Annals of Internal Medicine 1986;104(1):12‐7. [DOI] [PubMed] [Google Scholar]
Krogsgaard 1998 {published data only}
- Krogsgaard K, Marcellin P, Trepo C, Berthelot P, Sanchez‐Tapias JM, Bassendine M, et al. Prednisolone withdrawal therapy enhances the effect of human lymphoblastoid interferon in chronic hepatitis B. Journal of Hepatology 1996;25(6):803‐13. [DOI] [PubMed] [Google Scholar]
- Krogsgaard K, Marcellin P, Trepo C, Berthelot P, Sanchez‐Tapias JM, Bassendine M, et al. Pretreatment with prednisolone enhance the effect of human lymphoblastoid interferon in chronic hepatitis B. Ugeskrift for Laeger 1998;160(39):5657‐71. [PubMed] [Google Scholar]
Lam 1981 {published data only}
- Lam KC, Lai CL, Trepo C, Wu PC. Deleterious effect of prednisolone in HBsAg‐positive chronic active hepatitis. The New England Journal of Medicine 1981;304(7):380‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lauta 1995 {published data only}
- Lauta VM, Sangro MA. Long‐term results regarding the use of recombinant interferon alpha‐2b in the treatment of type 2 mixed essential cryoglobulinemia. Medical Oncology 1995;12(4):223‐30. [DOI] [PubMed] [Google Scholar]
Lee 1991 {published data only}
- Lee SD, Tong MJ, Wu JC, Lin HC, Tsai YT, Lo KJ. A randomised double‐blind placebo‐controlled trial of prednisolone therapy in HBeAg and HBV DNA positive Chinese patients with chronic active hepatitis B. Journal of Hepatology 1991;12(2):246‐50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Liaw 1994a {published data only}
- Liaw YF, Lin SM, Chen TJ, Chien RN, Sheen IS, Chu CM. Beneficial effect of prednisolone withdrawal followed by human lymphoblastoid pretreatment on the treatment of chronic type B hepatitis in Asians. Journal of Hepatology 1994;20(2):175‐80. [DOI] [PubMed] [Google Scholar]
- Liaw YF, Lin SM, Sheen IS, Chen TJ, Chu CM. Treatment of chronic type B hepatitis in Southeast Asia. The American Journal of Medicine 1988;29(85 (2A)):147‐9. [PubMed] [Google Scholar]
Magrin 1994 {published data only}
- Magrin S, Craxi A, Fabiano C, Simonetti RG, Fiorentino G, Marino L, et al. Hepatitis C viremia in chronic liver disease: relationship to interferon‐alpha or corticosteroid treatment. Hepatology 1994;19(2):273‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Manzillo 1983 {published data only}
- Manzillo G, Piccinino F, Sagnelli E, Izzo CM, Pasquale G, Maio G, et al. Treatment of HBsAg‐positive chronic hepatitis with corticosteroids and/or azathioprine. A prospective study. Ricerca in Clinica e in Labarotorio 1983;13(2):261‐8. [DOI] [PubMed] [Google Scholar]
Murray 1973 {published data only}
- Murray LI, Stern RB, Williams R. Controlled trial of prednisone and azathioprine in active chronic hepatitis. Lancet 1973;1(7806):735‐7. [DOI] [PubMed] [Google Scholar]
Niederau 1992 {published data only}
- Niederau C, Heintges T, Niederau M, Stremmel W, Strohmeyer G. Preospective randomised controlled trial of sequential treatment with corticoids and alpha‐interferon versus treatment with interferon‐alpha alone in patinets with chronic active hepatitis B. The European Journal of Medicine 1992;1(7):396‐402. [PubMed] [Google Scholar]
Perez 1993 {published data only}
- Perez V, Findor J, Tanno H, Sorda J. A controlled trial of high dose of interferon, alone and after prednisone withdrawal, in the treatment of chronic hepatitis B. Gut 1993;34 Suppl (2):91‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schvarcz 1991 {published data only}
- Schvarcz R. Chronic posttranfusion non‐A, non‐B hepatitis and autoimmune chronic active hepatitis‐aspects on treatment, prognosis and relation to hepatitis C virus. Scandinavian Journal of Infectious Diseases 1991;79:791‐848. [DOI] [PubMed] [Google Scholar]
Sjögren 1987 {published data only}
- Sjögren MH, Hoornagle JH, Waggoner JG. Effects of corticosteroid therapy on levels of antibody to hepatitis B core antigen in patients with chronic type B hepatitis. Hepatology 1987;7(3):582‐5. [DOI] [PubMed] [Google Scholar]
Ware 1974 {published data only}
- Ware AJ, Jones RE, Shorey JW, Combes B. A controlled trial of steroid therapy in massive hepatic necrosis. The American Journal of Gastroenterology 1974;62(2):130‐3. [PubMed] [Google Scholar]
Ware 1981 {published data only}
- Ware AJ, Cuthbert JA, Shorey JW, Gurian LE, Eigenbrodt EH, Combes B. A prospective trial of steroid therapy in severe viral hepatitis. The prognostic significance of bridging necrosis. Gastroenterology 1981;80(2):219‐24. [PubMed] [Google Scholar]
Weller 1982 {published data only}
- Weller IV, Bassendine MF, Murray AK, Craxi A, Thomas HC, Sherlock S. Effects of prednisolone/azathioprine in chronic hepatitis B viral infection. Gut 1982;23:650‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wu 1982 {published data only}
- Wu PC, Lai CL, Lam KC, HO J. Prednisole in HBsAg‐positive chronic active hepatitis: histologic evaluation in a controlled prospective study. Hepatology 1982;2(6):777‐83. [DOI] [PubMed] [Google Scholar]
Zarski 1994 {published data only}
- Zarski JP, Causse X, Cohard M, Cougnard J, Trepo C. A randomised, controlled clinical trial of interferon alpa 2b alone and with simultaneous prednisone for the treatment of chronic hepatitis B. Journal of Hepatology 1994;20(6):735‐41. [DOI] [PubMed] [Google Scholar]
Additional references
Alberti 1999
- Alberti A, Bortolotti F. Hepatitis C. In: Bircher J, Benhamou JP, McIntyre N, Rizzetto M, Rodés J editor(s). Oxfort Textbook of Clinical Hepatology. Second Edition. Vol. 1, Oxford: Oxford University Press, 1999:903‐22. [Google Scholar]
Alberti 2003
- Alberti A, Benvegùe L. Management of hepatitis C. Journal of Hepatology 2003;38 Suppl (1):104‐18. [DOI] [PubMed] [Google Scholar]
Alter 1989
- Alter HJ, Purcell RH, Shih JW. Detection of antibody to hepatitis C virus in prospectively followed transfusion recipients with acute and chronic non‐A, non‐B hepatitis. The New England Journal of Medicine 1989;321:1494‐500. [DOI] [PubMed] [Google Scholar]
Alvarez 1999
- Alvarez F, Berg PA, Bianchi FB, Bianchi L, Burroughs AK, Cancado EL, et al. International Autoimmune Hepatitis Group Report: review of criteria for diagnosis of autoimmune hepatitis. Journal of Hepatology 1999;31(5):929‐38. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bell 1999
- Bell TM, Bansal AS, Shorthouse C, Sandford N, Powell EE. Low‐titre auto‐antibodies predict autoimmune disease during interferon‐alfa treatment of chronic hepatitis C. Journal of Gastroenterology and Hepatology 1999;14(5):419‐22. [DOI] [PubMed] [Google Scholar]
Belli 2001
- Belli LS, Alberti AB, Rondinara GF, Carlis L, Corti A, Mazza E, et al. Early ribavirin treatment and avoidance of corticosteroids in hepatitis C virus (HCV)‐positive liver transplant recipients: interim report of a prospective randomized trial. Transplantation Proceedings 2001;33(1‐2):1353. [DOI] [PubMed] [Google Scholar]
Booth 1995
- Booth JC, Brown JL, Thomas HC. The management of chronic hepatitis C virus infection. Gut 1995;37(4):449‐54. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brok 2003
- Brok J, Kjaergard LL, Gluud C. Interferon alpha plus ribavirin versus interferon alpha for chronic hepatitis C ‐ an updated systematic Cochrane review. Journal of Hepatology 2003;38 Suppl (2):129A. [Google Scholar]
Calleja 1996
- Calleja JL, Albillos A, Cacho G, Iborra J, Abreu L, Escartin P. Interferon and prednisone therapy in chronic hepatitis C with non‐organ‐specific antibodies. Journal of Hepatology 1996;24(3):308‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Deeks 1999
- Deeks JJ, Bradburn MJ, Localio R, Berlin J. Much ado about nothing: meta‐analysis for rare events [abstract]. Proceedings of 2nd symposium on systematic reviews: beyond the basics. www.ihs.ox.ac.uk/csm/talks.html#p23 (accessed 16/2 2004) 1999:23.
DeMets 1987
- DeMets DL. Methods for combining randomized clinical trials: strengths and limitations. Statistics in Medicine 1987;6(3):341‐50. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
Dusheiko 1997
- Dusheiko G. Side effects of alpha interferon in chronic hepatitis C. Hepatology 1997;26 Suppl (1)(3):112‐21. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smth G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple grapical test. British Medical Journal 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
El‐Serag 2003
- El‐Serag HB, Hampel H, Yeh C, Rabeneck L. Extrahepatic manifestations of hepatitis C among United States male veterans. Hepatology 2003;36(6):1439‐45. [DOI] [PubMed] [Google Scholar]
Fabbri 2003
- Fabbri C, Jaboli MF, Giovanelli S, Azzaroli F, Pezzoli A, Accogli E, et al. Gastric autoimmune disorders in patients with chronic hepatitis C before, during and after interferon‐alpha therapy. World Journal of Gastroenterology 2003;9(7):1487‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fabris 2003
- Fabris P, Floreani A, Tositti G, Vergani D, Lalla F, Betterle C. Fabris P, Floreani A, Tositti G, Vergani D, Lalla F, Betterle C. Type 1 diabetes mellitus in patients with chronic hepatitis C before and after interferon therapy. Alimentary Pharmacology & Therapeutics 2003;18(6):549‐58. [DOI] [PubMed] [Google Scholar]
Ferri 2002
- Ferri C, Zignego AL, Pileri SA. Cryoglobulins. Journal of Clinical Pathology 2002;55:4‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Filipponi 2001
- Filipponi F, Salizzoni M, Grazi G, Pisati R, Ferrara R. Study of simulect‐based, steroid‐free immunosuppressive regimen in HCV de novo liver transplant patients: preliminary results. Transplantation Proceedings 2001;33(7‐8):3211‐12. [DOI] [PubMed] [Google Scholar]
Gregory 1976
- Gregory PB, Knauer CM, Kempson RL, Miller R. Steroid therapy in severe viral hepatitis. A double‐blind, randomized trial of methyl‐prednisolone versus placebo. The New England Journal of Medicine 1976;294(13):681‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gøtzsche 2002
- Gøtzsche PC, Johansen HK. Short‐term low‐dose corticosteroids vs placebo and nonsteroidal antiinflammatory drugs in rheumatoid arthritis. The Cochrane Library 2002, Issue 3. [DOI] [PubMed] [Google Scholar]
Hougardy 2000
- Hougardy DMC, Peterson GM, Bleasel MD, Randall CTC. Is enough attention being given to the adverse effects of corticosteroids therapy. Journal of Clinical Pharmacy and Therapeutics 2000;25:227‐34. [DOI] [PubMed] [Google Scholar]
ICH‐GCP 1997
- International Conference on Harmonisation Expert Working Group. Code of Federal Regulations & International Conference on Harmonization Guidelines. Parexel/Barnett, 1997. [Google Scholar]
Jarlbæk 2000
- Jarlbæk L, Gram LF, Arnberg A. Adverse events in short‐term steroid therapy. Which adverse events can be expected during daily administration of 20 to 40 mg prednisolone for a period of 1‐3 weeks [Bivirkninger ved kortvarig steroidbehandling]. Ugeskraeft for Laeger 2000;162(4):528‐9. [PubMed] [Google Scholar]
Jüni 2001
- Jüni P, Altman DG, Egger M. Systematic reviews in health care: assessing the quality of controlled clinical trials. British Medical Journal 2001;323(7303):42‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. [DOI] [PubMed] [Google Scholar]
Kjaergard 2003
- Kjaergard LL, Krogsgaard K, Gluud C. Ribavirin with or without alpha interferon for chronic hepatitis C. The Cochrane Library 2003, Issue 4. [DOI] [PubMed] [Google Scholar]
Krawitt 1996
- Krawitt EL. Autoimmune hepatitis [see comments]. The New England Journal of Medicine 1996;334(14):897‐903. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kuo 1989
- Kuo G, Choo QL, Alter HJ, Gitnick GL, Redeker AG, Purcell RH, et al. An assay for circulating antibodies to a major etiologic virus of human non‐A, non‐B hepatitis. Science 1989;244(4902):362‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lambiase 1992
- Lambiase L, Davis GL. Treatment of chronic hepatitis. Gastroenterology Clinics of North America 1992;21(3):659‐77. [MEDLINE: ] [PubMed] [Google Scholar]
Magy 1999
- Magy N, Cribier B, Schmitt C, Ellero B, Jaeck D, Boudjema K, et al. Effects of corticosteroids on HCV infection. International Journal of Immunopharmacology 1999;21(4):253‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mellerup 2003
- Mellerup MT, Krogsgaard K, Mathurin P, Gluud C, Poynard T. Sequential combination of glucocorticosteroids and alfa interferon versus alfa interferon alone for HBeAg‐positive chronic hepatitis B. The Cochrane Library 2003, Issue 4. [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352:609‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Myers 2003
- Myers RP, Regimbeau C, Thevenot T, Leroy V, Mathurin P, Opolon P, et al. Interferon for acute hepatitis C. The Cochrane Library 2003, Issue 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schultz 1995
- Schultz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. The Journal of the American Medical Association 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
Thiele 1996
- Thiele DL, DuCharme L, Cunningham MR, Mimms LT, Cuthbert JA, Lee WM, et al. Steroid therapy of chronic hepatitis: characteristics associated with response in anti‐hepatitis C virus‐positive and ‐negative patients. The American Journal of Gastroenterology 1996;91(2):300‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Tisone 1999
- Tisone G, Angelico M, Palmieri G, Pisani F, Anselmo A, Baiocchi L, et al. A pilot study on the safety of immunosuppression without prednisone after liver transplantation. Transplantation 1999;67(10):1308‐13. [DOI] [PubMed] [Google Scholar]
Tygstrup 1986
- Tygstrup N, Andersen PK, Juhl E. Steroids in chronic B hepatitis. A randomized, double‐blind, multinational trial on the effect of low‐dose, long‐term treatment on survival. A trial group of the European Association for the Study of the Liver. Liver 1986;6(4):227‐32. [MEDLINE: ] [PubMed] [Google Scholar]
WHO 1997
- WHO. Fact sheet N 164. http://www.who.int/inf‐fs/en/fact164.html June 1997 (accessed 16/2 2004).
WHO 1998
- WHO Press Release. Hepatitis C: 170 million affected worldwide and still no vaccine. http://www.who.int/emc/diseases/hepatiti/index.html 1 May 1998.


