Abstract
Liposomes are essentially a subtype of nanoparticles comprising a hydrophobic tail and a hydrophilic head constituting a phospholipid membrane. The spherical or multilayered spherical structures of liposomes are highly rich in lipid contents with numerous criteria for their classification, including structural features, structural parameters, and size, synthesis methods, preparation, and drug loading. Despite various liposomal applications, such as drug, vaccine/gene delivery, biosensors fabrication, diagnosis, and food products applications, their use encounters many limitations due to physico-chemical instability as their stability is vigorously affected by the constituting ingredients wherein cholesterol performs a vital role in the stability of the liposomal membrane. It has well established that cholesterol exerts its impact by controlling fluidity, permeability, membrane strength, elasticity and stiffness, transition temperature (Tm), drug retention, phospholipid packing, and plasma stability. Although the undetermined optimum amount of cholesterol for preparing a stable and controlled release vehicle has been the downside, but researchers are still focused on cholesterol as a promising material for the stability of liposomes necessitating explanation for the stability promotion of liposomes. Herein, the prior art pertaining to the liposomal appliances, especially for drug delivery in cancer therapy, and their stability emphasizing the roles of cholesterol.
Keywords: liposome, lipids, compounds, cholesterol, stability
Introduction
Increasing advances in nanotechnology and nanoscience have raised great hopes in the field of biomedicine. Due to their unique, multifaceted and flexible properties, nanomaterials circumvents many challenges in diverse fields of medicine, including health, diagnosis, and treatment (Liu et al., 2020; Naskar and Kim, 2021), nanoliposomes being one of the most widely used nanoparticles in biomedicine. Liposomes are lipid bilayer spherical membranes that provide both hydrophilic and hydrophobic environments. Adjustability, flexibility, variety of ingredients, ease of functionalization, tunability of the number of layers/sizes, biocompatibility, and biodegradability have turned liposomes into incredible structures in medicine, especially drug delivery (Aguilar-Pérez et al., 2020; Trucillo et al., 2020; Kashapov et al., 2021), most notable use of these structures being in cosmetics and drug delivery. Consequently, various liposome-based products have been commercialized to date, with the approval from United States Food and Drug Administration (FDA) (Yuba, 2020; Barenholz, 2021). Liposomes, a type of lipid-based nanoparticle, have a great assortment each of which in turn offers unique properties. However, there are still barriers and challenges related to lipid nanoparticles, the most critical being their stability (Yu et al., 2021). In the present review, an attempt has been made to comprehensively deliberate liposomes in terms of structure, function, and stability starting initially with an introduction to lipid structures.
Lipid Nanoparticles
Lipid molecules are one of the crucial elements of life and lipid-based nanoparticles comprise a broad range according to their application, components, shape, and fabrication methods; they possess numerous advantages over polymeric-based nanoparticles. Besides topical usage, agent delivery is ascribed to as a significant application of lipid-based nanoparticles. Being a physiological analog to the cellular membrane, liposomes have superior biocompatibility compared to polymeric-based nanoparticles, leading to a more acceptable biomedical application choices (Müller et al., 2000).
As highly adaptable nanoparticles, lipidic nanoparticles can be deployed for a wide varieties of delivery with a bit of condescension. Liposomes and niosomes, as the phospholipid and amphipathic lipids constituents, respectively, are the most known lipid-based formulation. According to previous studies, liposomes could be engineered purposefully with favorable parameters. However, kinetic stability is the primary limitation of vesicular lipid-based nanoparticles, including liposomes (Battaglia and Ugazio, 2019). The most applicable lipid-based nanoparticles are presented in Table 1.
TABLE 1.
Lipid-based structures.
| Types | Structural features | Structural compositions |
|---|---|---|
| Liposomes (Pan et al., 2002; Thompson et al., 2006; Yavlovich et al., 2009; Chen et al., 2012) | 1- Similarity to the cell membrane | DSPC- HSPC- DPPC-DOPEPC- EPCSPC-DMPC-DOPCCholesterol |
| 2- One or more concentric lipid bilayers enclosing an equal number of aqueous portions | ||
| Emulsions (Pan et al., 2002; Cardenia et al., 2011; Lu et al., 2012) | 1- Composed of two different phases, i.e., diffuse and continuous | PC- EPC – DOPC- DMPC DPPC- POPC- DSPC |
| 2- A liquid in liquid colloid structure | ||
| Composed of two immiscible liquids (usually oil and water). Liquid-liquid emulsions are mainly divided into two categories: water in oil (W/O) and oil in water (O/W) in which the oil phase and the aqueous phase form the continuous phase, respectively | ||
| Micelle (Ashok et al., 2004; Faustino et al., 2011; Deng et al., 2012; Saadat et al., 2014) | 1- The formation structure is based on the accumulation of diffused surfactant molecules in a colloidal liquid | PC- DSPE- DOPE- EPCGlycolic acid-lecithin |
| 2- Conventional and reverse micelles can be fabricated | ||
| Conventional micelles: hydrophobic tails assembled in the center of the micelles | ||
| Reverse micelle: the hydrophobic end is oriented towards the solvent, and the hydrophilic heads are gathered next to the center of the micelle | ||
| Cochleates (Pawar et al., 2015; Mannino and Lu, 2018; Asprea et al., 2019) | 1- Multilayered structures | PS and PC |
| 2- Composed of extensive and continuous two fat sheet layers | ||
| 3- Stable structures with the two- valence phospholipid sediments of natural materials | ||
| 4- It can be made of negatively charged phospholipids and a divalent cation | ||
| 5- It can be used for delivering the hydrophobic and hydrophilic drug molecules; positively and negatively charged | ||
| SLN (solid lipid nanoparticles) (Shah et al., 2007; Fang et al., 2008; Mehnert and Mäder, 2012) | 1- Nanostructures with the solid core of the particle, which are mainly made of lipids, for delivering nucleic acids, proteins, and drugs | Tween 80- soybean phospholipids- SPC- squalene- precirol- PF68- glyceryl palmito-stearate |
| Nanostructured lipid carriers (NLC) (Müller et al., 2002; Müller et al., 2007) | 1- SLN modified structures | 1- Tween 80-phospholipids- glyceryl palmito stearate- glycyrrhizin- propylene glycol monostearate- lecithin, poloxamer 800, polyglyceryl-3methyl -glucose di-stearate, SDS, SDC, oleic acid, alpha-tocopherol/vitamin E, corn oil- squalene |
| 2- NLC, or oil-loaded SLN, contains lipid droplets that are partially crystallized and have a less regular or amorphous solid crystal structure to overcome the limitations of SLN |
Note: DMPC, Dipalmitoyl phosphatidylcholine; DOPC, Dioleoyl-sn-glycero-3-phosphocholine; DOPE, Dioleoyl phosphatidylethanolamine; DOPEPC, Dioleoyl phosphatidylethanolamine phosphatidylcholine; DPPC, Dipalmitoyl phosphatidylcholine; DSPC, Distearoyl-sn-glycero-3-phosphocholin; DSPE, Distearoyl-sn-glycero-3-phosphorylethanolamine; EPC, Ethanolamine phosphatidylcholine; HSPC, Hydro Soy phosphatidylcholine; PC, phosphatidylcholine; POPC, Palmitoyl-oleoyl-sn-glycero-phosphocholine; PS, Phosphatidylserine; SDC, Sodium deoxycholate; SDS, Sodium dodecyl sulfate; SPC, Sphingosyl phosphorylcholine.
Liposome Structure
Liposome structure has been initially described by the British hematologist, Alec D Bangham in 1961. From the terminology point of view, liposome consists of “Lipos” and “Soma,” attributed to fat and body, respectively. The cell membrane’s bilayer lipid structure has been determined using electron microscope images, proving their unmistakable resemblance to plasmalemma (Dua et al., 2012; Hashemzadeh et al., 2020a). Liposomes were recruited as a drug carrier for the first time in the early 1990s. It has since been concluded that the low percentages inclusion of lipid-bonded polymers (called polymer-lipids) in liposomes’ structure could increase blood circulation in vivo. Structurally, liposomes are concentric bleeder vesicles in which a membranous lipid bilayer surrounds an aqueous volume. Typically, the bilayer lipid membrane comprise phospholipids containing a hydrophobic tail and a hydrophilic head (Rovira-Bru et al., 2002).
According to the phospholipid’s properties, the final structure represents an amphiphilic feature (Dua et al., 2012). Due to the unique structure, both natural and synthetic phospholipids-based liposomes are considered vesicles or drug-carrying systems. The spherical or multilayered spherical design of the fabricated liposomes is highly reliant on the amount and the kind of lipid components. According to a concentric form, the bilayer lipid formation arrangement constitutes an equal number of water chambers (Chetoni et al., 2004; Choi and Maibach, 2005; Pavelić et al., 2005).
In view of the utmost similarity to the cell membrane, the liposomes have been described as an appropriate membrane model to reveal the fundamental nature of cell membranes with assorted appliances (Wong et al., 2001; Laouini et al., 2012). The self-assembly of diacyl-chain phospholipids in aqueous solutions can form spherical bilayer structures referred to as liposomes. Because of encapsulating an extensive aqueous environment, liposomal structures can load almost any type of hydrophilic molecules (Lebègue et al., 2015; Vakili-Ghartavol et al., 2020; Wu et al., 2021). Liposome’s internal hydrophilic part can protect loaded drugs from the host body’s destructive factors that ultimately minimize the unwanted side effects. Besides the internal aqueous environment, hydrophobic substances can also be embedded between the lipid membranes or adsorb on the liposome surface (Xu et al., 2007; Silverman et al., 2013; Chen et al., 2014; Eloy et al., 2014; Jain et al., 2014). The essential liposome-like structures are presented in Table 2.
TABLE 2.
Liposome-like structures.
| Liposomes -like structure | Description |
|---|---|
| Niosome | Niosomes are attributed to carriers consisting of nonionic surfactants through cholesterol hydration (Sankhyan and Pawar, 2012; Puras et al., 2014; Arora, 2016) |
| Phytosome | Phytosomes are made from plant compounds. Phytosomes are lipid nanocarriers produced by phospholipids’ binding to polyphenols in organic solvents (Kidd, 2009; Jain et al., 2010; Pawar and Bhangale, 2015; Abd El-Fattah et al., 2017) |
| Virosomes | Virosomes are spherical shape structures with a mono/bilayer phospholipid-based membrane. The embedded central cavity of these structures is used to loading the therapeutic molecules such as nucleic acids, proteins, and drugs (Felnerova et al., 2004; Daemen et al., 2005) |
| BODIPYsome | The aza-BODIPY lipid is the building block that is self-assembled into a BODIPYsome vesicle structure capable of stable N.I.R. J-aggregation (Cheng et al., 2019) |
| DQAsomes | DQAsomes are vesicular structures composed of amphiphiles, decolinium (Zupančič et al., 2014; Weissig, 2015; Bae et al., 2018) |
| Archaeosomes | Archaeosomes is a new family of liposomes. They have been made of one or more ether lipids that are unique to the Archaea constitute domain. These types of structures are found in Archaeobacteria. Achaean-type lipids consist of archaeol (diether) and/or caldarchaeol (tetraether) core structures (Réthoré et al., 2007; Benvegnu et al., 2009; Kaur et al., 2016) |
| Ethosomes | Ethosomes are phospholipid nanovesicles. These structures are composed of flexible bilayers phospholipid, with a relatively high ethanol concentration (20–45%), glycols, and water. Transdermal delivery is considered the main application of the Ethosomes (Dayan and Touitou, 2000; Ainbinder et al., 2010) |
The Packing Parameter
As described, liposomes are lipid-based structures that constitute one or more bilayer phospholipids which can encapsulate aqueous media. Liposome formation begins by dispersing phospholipids in water, leading to interactions between phospholipids and water (Anwekar et al., 2011), PP being a critical criterion determining the formation of liposome.
PP is described as the ratio between the cross-section of the amphiphile hydrophobic part (hydrocarbon chains of phospholipids or the hydrocarbon rings of sterols) and the cross-section of the hydrophilic part (the amphiphile head group). Liposome-forming lipids are considered amphiphilic structures with a PP of 0.74–1.0. In this regard, HSPC (PP: 0.8) and DSPE–PEG (PP: 0.487) have been addressed as liposome and non-liposome forming lipids, respectively. For DSPE-PEG, the low PP implied the presence of an extensive polar head group due to the large (45 mer) polyethylene glycol (PEG) moiety that prevents liposome-structure formation. The head group in this molecule is highly flexible (Nagarajan, 2002; Garbuzenko et al., 2005; Barenholz, 2016; Maritim et al., 2021). The most common phospholipids and cholesterol deployed to prepare liposomes with respect to their transition temperature (Tm) and molecular weights are presented in Table 3.
TABLE 3.
The common compounds deployed to prepare liposomes.
| Phospholipids | Molecular Formula | Electrical Charge | Tc (°C) | Mol. Wt. |
|---|---|---|---|---|
| Dilauryl phosphotidyl choline (DLPC) (Jung et al., 2005) | C32H64NO8P | −1 | −1 | 633 |
| Dimyristoyl phosphotidyl choline (DMPC) (Akabori and Nagle, 2015) | C36H72NO8P | 0 | 23 | 678 |
| Dipalmitoyl phosphotidyl choline (DPPC) (Jimbo et al., 2016) | C40H80NO8P | 0 | 41 | 734 |
| Dioleolyl phosphotidy l choline (DOPC) (Chibowski and Szcześ, 2016) | C44H84NO8P | - | −20 | 786 |
| Dilauryl phosphotidyl ethanolamine (DLPE) (Benz et al., 2004) | C29H58NO8P | - | 30.5 | 579.75 |
| Dipalmitoyl phosphotidyl choline (DPPC) (Gullapalli et al., 2008) | C40H80NO8P | −1 | 41 | 734.053 |
| Distearoyl phosphotidyl choline (DSPC) (Hashemzadeh et al., 2020b) | C44H88NO8P | 0 | 58 | 790 |
| Dioleolyl phosphotidy l choline (DOPC) (Attwood et al., 2013) | C44H84NO8P | 0 | −16.5 | 786 |
| Dimyristoyl phosphotidyl ethanolamine (DMPE) (Li et al., 2015) | C33H66NO8P | 0 | 50 | 635.85 |
| Distearoyl phosphotidyl ethanolamine (DSPE) (Seo et al., 2011) | C41H82NO8P | 0 | - | 748 |
| Dilauryl phosphotidyl glycerol (DLPG) (Jacoby et al., 2015) | C30H58O10 PN a | −1 | 4 | 633 |
| Dicetyl phosphate (DCP) (Chupin et al., 2002) | C32H67O4P | −1 | - | 546.85 |
| Dioleoyl phosphatidyl ethanolamine (DOPE) (Evjen et al., 2011) | C41H78NO8P | - | −16 | 744 |
| 1,2-dioleoyl-3 trimethylammoniumpropane (DOTAP) (Caracciolo et al., 2005) | C42H80ClNO4 | - | - | 698.5 |
| Dioleoyl phosphatidylserine (DOPS) (Okamoto et al., 2008) | C42H78NO10P | - | −10 | 788 |
| Hydrogenated soybean phosphatidylcholine (HSPC) (Kitayama et al., 2014) | C42H84NO8P | - | 52 | 762.1 |
| Cholesterol (Bennett et al., 2009) | C27H46O | 0 | - | 386.65354 |
Types of Liposomes
Liposomes are highly versatile compounds and they can be fabricated with various combinations, their diversity and properties vary in structure, size, shape, and surface properties. One of the classification types of liposomes is founded on their size and amount of layers, for example, unilamellar and multilamellar liposomes. They can be subdivided into four main categories based on structural parameters: multilamellar liposomes/vesicles (MLV), oligolamellar vesicles (OLV), multilamellar liposomes/vesicles (MVV), and unilamellar vesicles (ULV). Furthermore, the ULV can be divided into giant unilamellar liposomes/vesicles (GUV) groups, large unilamellar liposomes/vesicles (LUV), medium unilamellar vesicles (MUL), and small unilamellar liposomes/vesicles (SUV), and based on size (Walde and Ichikawa, 2001; Gabriëls and Plaizier-Vercammen, 2003; Wagner et al., 2006; Drulis-Kawa and Dorotkiewicz-Jach, 2010; Baykal-Caglar et al., 2012; Garg and K. Goyal, 2014).
Despite the aforementioned classification of various liposomes, many liposome characteristics cannot be deduced, including synthesis techniques or their applications. Since the introduction of liposomes as lipid-carrying structures, many methods have been proposed for their fabrication. Each has some advantages and disadvantages that can be exploited depending on the practical function. The process of liposome making strongly affects the final properties, so special attention should be paid to the construction method. Liposomes are referred to as a very miscellaneous structure in terms of their charge and size, the ultimate size and electrical charge of the fabricated liposome being highly reliant on the manufacturing method and the types of phospholipid deployed. In this regard, liposomes synthesis methods can be categorized as dehydration and rehydration (DRV), reverse phase evaporation (REV), particularly for SUL, OLV, and MLV liposomes, vesicles prepared by extrusion technique (VET), and frozen and thawed (FAT) for MLV preparation (Zhang and Pawelchak, 2000; Scott and Jones, 2001; Xia and Xu, 2005; Zaru et al., 2007; Akbarzadeh et al., 2013; Pradhan et al., 2015). Assorted liposome types, according to the fabrication methods, are presented in Table 4.
TABLE 4.
Liposome classification based on the synthesis method.
| Techniques | Features | Advantages | Disadvantages |
|---|---|---|---|
| Extrusion technique (Al-Remawi et al., 2017; Wang et al., 2017) | 1- Different pore size filters depending on the need | - | - |
| 2- Producing LUV or nanoliposomes is based on the pore-size of the filters | |||
| Sonication (Nasrabadi et al., 2016; Veneti et al., 2016; Yu and Tang, 2016) | 1- The most widely used technique for the preparation and production of liposomes and nanoliposomes | - | 1- Low internal volume/encapsulation efficiency2- Low ability to remove large molecules and metal pollution from the probe tip |
| 2- One of the most straightforward techniques to size reduction and producing nano-liposomes | |||
| 3- Probe sonication and bath sonication are the main techniques | |||
| Microfluidization[(Alizadeh et al., 2015; Yu et al., 2015; Davidson et al., 2016; Devrim et al., 2016; Saliba et al., 2016; Tabatabaei Mirakabad et al., 2016)] | 1- This method is used in the pharmaceutical industry to produce liposomes and pharmaceutical emulsions | 1- Possibility of producing a large volume of liposomes2- Ability to adjust the average size of the liposomes3- High efficiency (up to 70%) | - |
| 2- A chamber of microfluidizer contains a divided pressure stream | |||
| 3- Recruiting microfluidizer | |||
| 4- without potentially toxic solvents | |||
| Heating method (Mozafari, 2005; Mozafari et al., 2007; Panahi et al., 2017; Hadavi et al., 2020; Khosravi-Darani and Sheida Aarabi) | 1- It can be used for nanoliposomal production | 1- It does not have the disadvantage of other methods, such as: Not using toxic solvents like ethyl ether, containing methanol and chloroform2- Not using high pressures | - |
| 2- Decreases the production time and cost on an industrial scale which has received much attention | |||
| 3- Produce hollow micro-liposomes (HM-liposomes) that can be used as vectors in drug and gene transfer | |||
| Freeze-drying (lyophilization) (Chen et al., 2010a; Franzé et al., 2018) | 1- Based on removing water from products in the frozen state | - | - |
| 2- This step takes place at very low pressures | |||
| 3- This method can solve the long-term stability problem of the solvent | |||
| 4- The uses of trehalose can help liposomes retain as much as 100% of their original contents, so trehalose (a carbohydrate) in this method can be used as a freeze protectant | |||
| REV (Otake et al., 2001; Kafle et al., 2020) | 1- A one-step method for producing liposomes that do not make any toxic organic solvent | - | - |
| 2- This method can lead to the production of L.U.V.s with the diameters of 0.1–1.2 μm | |||
| 3- Have a high ability to trap both water-soluble and oil-soluble substances | |||
| Solvent dispersion method (Mozafari, 2005; Dua et al., 2012; Akbarzadeh et al., 2013) | Includes ether injection and 1- ethanol injection methodsAt the ether injection method, a solution of lipids dissolved in diethyl ether or ether/methanol mixture was injected gently into the aqueous solution. The temperature should be set at approximately 55–65°C, or the experiment should perform under reduced pressure. In later stages, under vacuum condition, ether is removed from the environment, and eventually, liposome will be fabricated | Ether injection method1- Heterogeneous nature of the synthesized liposomes (70–190 nm)2- Exposure of compounds to relatively high temperatures3- Exposure of encapsulated compounds to organic solventsEthanol injection method1- Heterogeneous products (30–110 nm)2- The fabricated liposomes are very dilute3- It is difficult to remove all ethanol from the environment due to the formation of azeotrope with water. Failure to completely remove ethanol from the reaction medium and formation of an azeotrope with water may lead to inactivation of biologically different activities | |
| 2- ethanol injection method: In this method, ethanol’s lipid solution is rapidly injected into a vast excess of the buffer. At this point, The M.L.V.s are formed immediately |
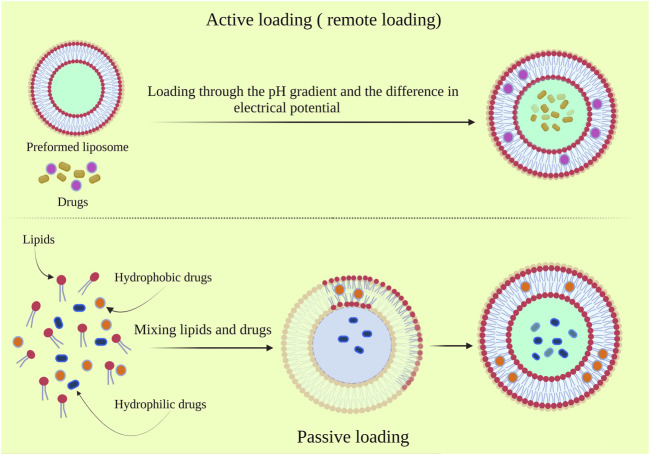
Regarding the production of liposome, in addition to the fabrication method, drug loading should also be considered. In general, drug loading is performed via two standard procedures, passive and active loading, which affects the amount and quality of the loaded drug and some extent on the liposome properties. In the active loading method, known as the remote loading method, the drug molecules are loaded into the fabricated liposome. The pH gradient and electrical potential difference across the liposomal membrane are the most known underlying mechanisms dictating the active drug loading (Figure 1). The active loading method has provided various advantages over passive loading methods, including high efficiency and loading capacity, decreasing the loaded drug’s leakage, and reducing the drug’s shrinking during storage. Another outstanding feature of this method is the possibility of drug loading after carrier formation due to the use of the flexibility of constitutive lipid. It is also possible to prevent the degradation of the biologically active compounds during the preparative process (Barratt, 2003; Anwekar et al., 2011; Agrawal et al., 2012; Burton et al., 2015).
FIGURE 1.
The active and passive drug loading on the liposomes.
It has been well known that liposome characteristics are highly dependent on lipid composition. Surface charge, particle size, and the preparation method are among the most outstanding influenced features by lipid combination. Besides, the effective properties of bilayer structure, including rigidity, fluidity, and electrical charge, can be determined through bilayer component selection. In this regard, natural-based liposomes fabricated from unsaturated phosphatidylcholine species, including egg or soybean phosphatidylcholine, have provided bilayer structures with highly permeable and low stable properties. However, saturated-phospholipids-based liposomes such as dipalmitoyl phosphatidylcholine lead to rigid and almost impermeable bilayer structures (AllenLiposomes, 1997; Sahoo and Labhasetwar, 2003). The most common liposomal system, in terms of its composition constituent, are presented in Table 5.
TABLE 5.
liposome classification according to composition constituents.
| Types | Feature |
|---|---|
| Conventional liposome | Spontaneously self-assembled phospholipids (Neutral/negative charge) in an aqueous medium. The fabricated liposomes have surrounded an aqueous medium (Zhao et al., 2005; Zaru et al., 2007; Meure et al., 2008) |
| Fusogenic liposome (FL) | The liposomal system is based on reconstitute Sendai virus (Nakanishi et al., 2000; Kunisawa et al., 2005) |
| Cationic liposome | Liposomes containing the cationic lipid (Dokka et al., 2000; Radwan Almofti et al., 2003; Sioud and Sørensen, 2003) |
| Long circulatory liposome | The obtained products can improve tissues localization. These types of liposomes are liposomes with neutral high transition temperature (Moghimi et al., 2001; Awasthi et al., 2003; Metselaar et al., 2003; Moghimi and Szebeni, 2003) |
| P.H. sensitive liposome | These liposomes usually contain phosphatidylethanolamine (P.E.) and titratable stabilizing amphiphiles that become unstable under acidic conditions (Simões et al., 2004) |
| Immunoliposome | Long-circulating liposomes that may contain monoclonal antibodies or their fragments (Fab ') (Park et al., 2004; Hua, 2013) |
Application of Liposomes
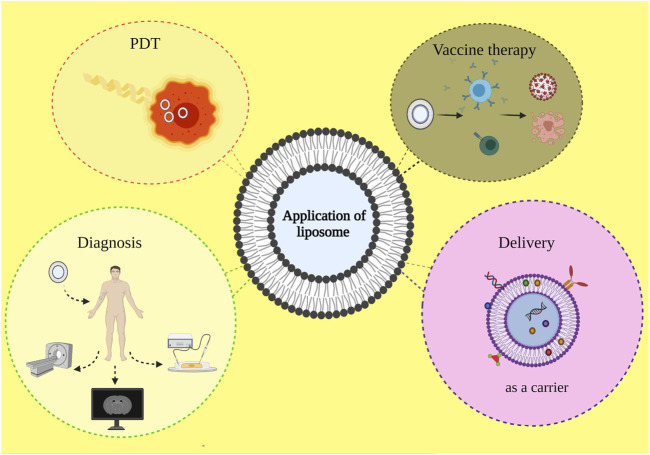
As highly versatile nanoparticles, liposomes have been considered for many biomedical applications (Figure 2). Liposome as cholesterol and natural-non-toxic phospholipids-based spherical shape vesicles has provided many biomedical opportunities, especially for drug delivery ascribed to their biocompatibility, appropriate size, and suitable hydrophobic and hydrophilic character. Besides, the cosmetics industry has been vigorously affected by liposome formulations as they proposed many unique properties for drug carriers (Figueroa-Robles et al., 2020; Matole et al., 2020). On the other hand, the great potential application of liposomes should not be underestimated in the food and farming industries. Numerous studies have been conducting regarding liposome encapsulation to develop appropriate delivery systems to entrap unstable compounds. The ensnared invertebrate ingredients such as antimicrobials, antioxidants, hydrophobic, and hydrophilic compounds in liposome particles could be used to targeted delivery and prevent composition and functionality disruption (Benech et al., 2002; Shehata et al., 2008; Atrooz, 2011).
FIGURE 2.
Diverse biomedical applications of liposome-based structure.
The nanoliposome is a favorable particle to develop cancer drug delivery systems due to its unique properties, including biocompatibility, biodegradability, and hydrophilic and lipophilic drug loading capability. Liposome-based structures have owned the most commercial delivery systems worldwide. There is various ongoing research regarding improvement drug toxicity and specific targeting with liposomes (Akbarzadeh et al., 2013).
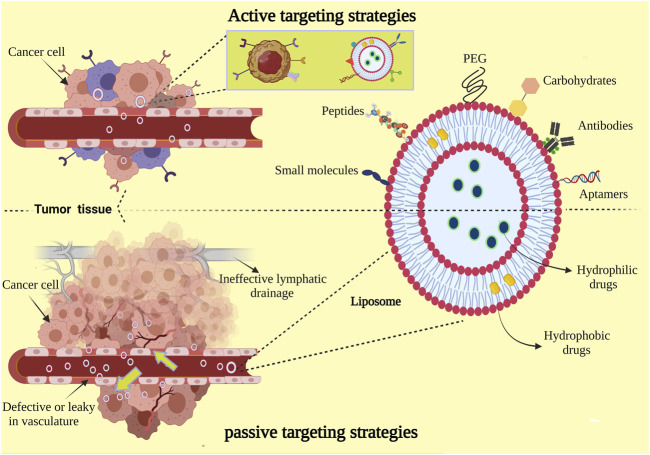
In view of the admirable properties mentioned for liposomes, it has been extensively studied in drug delivery to cancerous and tumor tissues via two main approaches in terms of design to target tumor tissues: passive targeting and active targeting (Figure 3). Passive targeting is reliant on the physiological characteristics of the tumor and the size of the nanoparticles. Cancer cells overexpress vascular endothelial growth factor (VEGF) due to their very high metabolism, which leads to excessive angiogenesis in tumor tissue. The vascular pores in the tumor tissue are larger than normal tissue, and the appropriate size of the liposomes enable them to circulate for a longer time in the circulatory system, so the anti-cancer drug nanosystem could target the tumor tissue (Zhu et al., 2017; Jeon et al., 2020; Liu et al., 2021). In addition, after the drug delivery system enters the cancerous tissue due to defects in the lymphatic system, the retention time of nanoparticles increases, which is not possible for the small drug molecules (Attia et al., 2019). In this method, the nanosystem is also coated with a biocompatible PEG polymer, which causes the escape of the reticuloendothelial (RES) system and increases the circulation time in the circulatory system; effect of PEG is through shielding liposomes from opsonization (Suk et al., 2016; Nunes et al., 2019).
FIGURE 3.
Passive targeting and active targeting. Liposomes can be surface functionalized to dedicate stealth through PEGylation and to meliorate receptor-mediated endocytosis by utilizing targeting ligands such as antibodies, peptides, proteins, carbohydrates, Aptamer, and various other small molecules. PEGylation prolongs liposomal circulation half-life in vivo. Types of drugs based on whether they are hydrophobic or hydrophilic can be encapsulated into the aqueous lumen, incorporated into the lipid bilayer, or conjugated to the liposome surface.
Despite the relatively good performance of passive targeting in increasing drug delivery to cancerous tissues, the optimum amount may still not reach the target tissue, or the drug may leak into normal tissues. Hence, researchers are using the active targeting method to enhance drug delivery to the target tissue. The basis of this method can be attributed to the functionalization of the liposome surface. Cancer cells need more nutrients because of their distinct metabolism. Therefore, some surface receptors are overexpressed on the surface of these cells. Targeted drug delivery to the cancerous tissue can be performed using this feature through specifically functionalizing liposome surface (Dana et al., 2020; Montaseri et al., 2020; Raj et al., 2021).
A comprehensive overview of liposomes’ applications in biomedicine is summarized in Table 6 according to the type of liposomes used.
TABLE 6.
The use of liposome in biomedical applications.
| Liposome type | Applications | Studies |
|---|---|---|
| Conventional liposome | Drug delivery | Improving the therapeutic index of encapsulated doxorubicin (Hilmer et al., 2004; Papagiannaros et al., 2006) |
| Improving the therapeutic index of encapsulated amphotericin (Bern et al., 2006; Ellis et al., 2009) | ||
| Liposomes have been used for sustained release cytarabine (Domínguez et al., 2005; Benesch and Urban, 2008) | ||
| Cosmetics | Liposome has been recruited for‐ 4 ‐ n ‐ butyl resorcinol 0.1% cream encapsulation for the treatment of melasma (Huh et al., 2010) | |
| Liposomes have been used for skin wound healing application as antifibrogenic effects through IFN-alpha 2b encapsulation as cream formulation (Fuchsluger et al., 2006) | ||
| Using liposomal hydrogel with polyvinylpyrrolidone iodine in the topical treatment of partial-thickness burn wounds (Homann et al., 2007) | ||
| Vaccine adjuvant | NE and HBsAg in protein and DNA + protein have been entrapped into liposomes to induce immune response for hepatitis E and hepatitis B (Shrivastava et al., 2009) | |
| Lipid A-containing liposomes: A non-toxic adjuvant for the malaria sporozoite vaccine in humans (Alving et al., 2012) | ||
| CAF01 liposomes as a mucosal vaccine adjuvant (Christensen et al., 2010) | ||
| Sensors and Biosensors | Polydiacetylene liposome arrays for selective potassium detection (Lee et al., 2008) | |
| Immobilized-liposome sensor system for detection of proteins under stress conditions (Jung et al., 2007) | ||
| Liposome to detect acute leukemia based on electrochemical cell sensor (Ghazizadeh and Neshastehriz, 2020) | ||
| Vaccine therapy | BLP25 liposome vaccine to treat non-small cell lung and prostate cancers (North and Butts, 2005) | |
| P5 HER2/neu-derived peptide conjugated to liposomes containing M.P.L. adjuvant as an effective prophylactic vaccine formulation for breast cancer (Farzad et al., 2019) | ||
| Liposomes containing interferon-gamma as an adjuvant in tumor cell vaccines (Van Slooten et al., 2000) | ||
| Fusogenic liposome (FL) | Labeling | Novel fusogenic liposomes for fluorescent cell labeling and membrane modification (Csiszár et al., 2010) |
| Fusogenic liposomes for cell membrane labeling and visualization (Kleusch et al., 2012) | ||
| Intracellular delivery of carboxyl coated CdTe quantum dots mediated by fusogenic liposomes (Lira et al., 2013) | ||
| Gene transfer | Gene transfer with fusogenic liposomes containing vesicular stomatitis Virus G Glycoprotein (Shoji et al., 2004) | |
| Subcellular trafficking of antisense oligonucleotides and down‐regulation of BCL‐2 gene expression in human melanoma cells using a fusogenic liposome delivery system (Hu et al., 2002) | ||
| Transferring maternally administered fusogenic liposome‐DNA complexes into monkey fetuses in a pregnancy model (Hirano et al., 2002) | ||
| Drug delivery | Fusogenic liposomes to deliver mucosal insulin (Goto et al., 2006) | |
| Fusogenic liposomes containing diphtheria toxin fragment A to suppress tumor growth (Fang et al., 2005) | ||
| Transferring antibodies and doxorubicin to the cytoplasm based on fusogenic liposomes for the metastatic treatment of breast cancer (Deng et al., 2017) | ||
| Vaccine | Fusogenic liposome (F.L.) containing non-methylated CpG motif to increase antigen-specific immunity in mice (Yoshikawa et al., 2006a) | |
| Fusogenic liposomes (F.L.) can efficiently deliver exogenous antigen through the cytoplasm into the MHC class I processing pathway (Nakanishi et al., 2000) | ||
| Fusogenic liposomes have the potential to be used as an effective vaccine carrier for peptide vaccination to induce cytotoxic T lymphocyte (CTL) response (Sugita et al., 2005) | ||
| Vaccine therapy | Membrane fusogenic liposomes vaccine against melanoma that can produce both systemic immune and CTL responses (Qiang et al., 2004) | |
| Versatile cancer immunotherapy using vaccine of fusogenic liposomes containing tumor cell-lysate against murine B16BL6 melanoma (Yoshikawa et al., 2006b) | ||
| Cationic liposome | Gene delivery | Cationic liposomes to transmit human cystic fibrosis transmembrane conductance regulator (CFTR) gene to mouse models of cystic fibrosis (CF) (Lee et al., 2012) |
| Cationic liposomes for co-delivery of small interfering R.N.A. and a M.E.K. inhibitor for enhanced anticancer efficacy (Kang et al., 2011) | ||
| Trilysinoyl polyamide-based cationic liposomes for systemic co-delivery of siRNA and an anticancer drug [ (Shim et al., 2011) | ||
| Drug delivery | Cationic liposomal doxorubicin (LPs-DOX) and paclitaxel (LPs-PTX) via electrostatic force to tumor cells (TRAMP-C1, B16) and HUVEC cells in vitro (Chen et al., 2010b) | |
| targeted delivery of cationic liposome-encapsulated paclitaxel (EndoTAG-1) for treating CNV (Gross et al., 2013) | ||
| Cationic liposomes containing doxilagainst human SKOV-3 ovarian adenocarcinoma xenograft rat model (Jung et al., 2009) | ||
| Vaccine adjuvant | Cationic liposomes containing mycobacterial lipids as Th1 Adjuvant (Rosenkrands et al., 2005) | |
| Cationic liposomes based on dimethyldioctadecylammonium and synthetic cord factor from M. tuberculosis (trehalose 6,6′dibehenate)—A adjuvant inducing both strong CMI and antibody responses (Davidsen et al., 2005) | ||
| Cationic liposomes as a potent adjuvant for DNA vaccine of human immunodeficiency virus type 1 (Qiao et al., 2016) | ||
| Vaccine therapy | Cationic liposomes as an antitumor autologous lewis lung cancer cell vaccine engineered to secrete mouse Interleukin 27 (Zhang et al., 2013) | |
| Cationic liposome-based synthetic long-peptide vaccines lead to strongly activate functional, antigen-specific CD8 + CD4 + T cells and induce in vivo cytotoxicity against melanoma and HPV-induced tumors in mice (Varypataki et al., 2017) | ||
| Dendritic cells pulsed with tumor extract–cationic liposome complex increase the induction of cytotoxic T lymphocytes in mouse malignant glioma (Aoki et al., 2001) | ||
| Long circulatory liposome | Photodynamic therapy (PDT) | Glucuronatemodified (also known as long-circulating liposomes) liposomalized BPD-MA into Balb/c mice bearing Meth A sarcoma by PDT (Ichikawa et al., 2005) |
| Anti-angiogenic PDT using long-circulating liposomes modified with peptide-specific to angiogenic vessels as a carrier for delivering photosensitizer to angiogenic endothelial cells (Ichikawa et al., 2004) | ||
| Cancer therapy | Liposomes containing lipid derivatives of polyethylene glycol (sterically stabilized liposomes), known as long-circulating liposomes, were used to transfer doxorubicin to squamous cell lung carcinoma through specific antibodies attached to the liposome surface (Bakker-Woudenberg, 2002) | |
| Marqibo®:long circulating liposomes containing vincristine sulfate (Zhang et al., 2016) | ||
| Long circulatory liposomes containing adriamycin were used in Colon 26 NL-17 carcinoma bearing mice and especially angiogenic site (Maeda et al., 2004) | ||
| Gene delivery | pH-sensitive liposomes to transfer plasmid D.N.A. into mammalian cell lines (Chen et al., 2013) | |
| Encapsulation of a plasmid containing the E. coli chloramphenicol acetyltransferase gene in a pH-sensitive liposome and improvement of its transfection conditions (Torchilin, 2006) | ||
| DNA entrapped pH-sensitive liposomes as a system for the transformation of tobacco mesophyll protoplasts (Hahn and Friedt, 2012) | ||
| pH-sensitive liposome | Gene delivery | PH-sensitive liposomes have been used as an intelligent system for the delivery of antisense oligonucleotides (Fattal et al., 2004) |
| Anionic pH-sensitive liposomes were used as a delivery system for the smart delivery of antisense oligonucleotides (Fattal et al., 2004) | ||
| pH-sensitive liposomes containing antisense oligodeoxynucleotide, ribozyme, and acyclic nucleoside phosphonate analogs for enhanced inhibition of HIV1 replication in macrophages (Düzgünes et al., 2001) | ||
| Antibiotic Delivery | pH-sensitive liposomes to encapsulate gentamicin as an intracellular delivery system and improving its activity against intracellular pathogens (Cordeiro et al., 2000) | |
| pH-sensitive nystatin liposomes increased the antifungal activity against cryptococcus neoformans (Nasti et al., 2006) | ||
| Endolysin LysRODI anti-staphylococcal encapsulation in pH-sensitive liposomes provides targeted delivery under mildly acidic conditions (Portilla et al., 2020) | ||
| Proteins and peptides delivery | The pH-sensitive stealth liposome was used to deliver a therapeutic peptide to its nuclear site of action (Ducat et al., 2011) | |
| A pH-sensitive polymer-liposome-based antigen system was used as an interferon-γ gene delivery system lipoplex for efficient cancer immunotherapy (Yuba et al., 2015) | ||
| Immunoliposome | Drug delivery | Immunoliposomes can deliver high amounts of the 10B atoms into tumor cells and exert a cytotoxic effect by thermal neutrons (B.T.S. Thirumamagal et al., 2006) |
| The dual peptides-modified liposomes loading VEGF siRNA and D.T.X. can inhibit glioma cell growth in a synergistic manner (Yang et al., 2014) | ||
| Liposomes containing doxorubicin and NGR peptide targeting aminopeptidase N, a marker of angiogenic endothelial cells, were used to treat neuroblastoma (NB) in SCID mice (Pastorino et al., 2003) | ||
| Diagnosis | Liposomes coated with polyethylene glycol and modified with monoclonal antibody 2C5 were used as contrast agents for the diagnosis and molecular imaging of tumor by SPECT/CT (Silindir et al., 2013) | |
| Reporter DNA encapsulated liposomes and surface modified with biotin-labeled polyethylene glycol (PEG) phospholipid can be used as a surrogate to quantify the target protein using real-time PCR (He et al., 2012) | ||
| A liposomal immunosensor was used to monitor insulin and manage diabetes (Ho et al., 2006) | ||
| An immunoliposome complex containing an anti-transferrin receptor single-chain antibody fragment (TfRscFv) decorating the surface and containing the contrast agent gadopentetate dimeglumine (“gad-d") was used to increase the sensitivity of detection of lung metastases (Freedman et al., 2009) | ||
| IL-13-liposome-Gd-DTPA can cross the BBB and detect glioma at an early stage (Liu et al., 2016) |
Advantages and Disadvantages of Liposomes
Like any other carrier, liposomes have provided some advantages and disadvantages, too. The benefits of liposomes have been mentioned briefly in this text. As amphiphilic and non-ionic structures particle, liposomes offer an exceptional opportunity to deliver both water and lipid-soluble drugs. This feature has a significant priority in the pharmaceutical industry to develop new formulations (Abdelkader et al., 2014; Joshi et al., 2016). On the other hand, their inimitable structure frame enables researchers to fabricate both sustained and targeted drug delivery systems through controlling permeability, rigidity, size, and surface functionalization (Daraee et al., 2016; Jain and Jain, 2016). Although liposomes can be administered through different routes, they are composed of biocompatible ingredients (Mansoori et al., 2012). One of the main limitations of drug distribution systems is the importance of biodegradable drug transport, which can be overcome through liposomal delivery that prevents drug oxidation (Manconi et al., 2016). It has been reported that liposomes can promote drug pharmacokinetics by eliminating and circulation life (Bhatt et al., 2018).
Despite all advantages, liposome-based structures have some limitation that prevents their widespread clinical use. The most significant obstacle is attributed to their physical and chemical stabilities (Bakker-Woudenberg, 2002). Among other essential impediments, low solubility in aqueous solutions (Li et al., 2019), short half-life time in body environment (Kshirsagar et al., 2005), high production cost (Noble et al., 2014), challenges in various tissue targeting due to the relatively large size of liposomes may cause (Santos et al., 2005), leakage and fusion of the loaded drugs (Joly et al., 2011), phospholipids oxidation and hydrolysis (Jain and Jain, 2016), rapidly detection by RES system (Daraee et al., 2016), and allergic reactions to some liposomal compounds (Mansoori et al., 2012), can be mentioned.
Liposome Stability
One of the most critical challenges in liposome application is attributed to the relative instability in aqueous dispersions. The physical and chemical instability of liposomes can lead to unwanted side effects and efficacy reduction (Scrimgeour et al., 2005; Toh and Chiu, 2013); oxidation and hydrolysis are two primary mechanisms in the degradation of liposomes that cause chemical instability (Carlson et al., 2006; Jung et al., 2006; Frenzel and Steffen-Heins, 2015). The oxidation process is very probable due to free radicals in fatty acids as an intrinsic compound where unsaturated fatty acids are more susceptible than the saturated fatty acids to this mechanism (Anderson and Omri, 2004; Tan et al., 2016). In the presence of acid- or baseـ catalyst, this process can occur either at the 1-acyl or at the 2-acyl position, which subsequently leads to lysophospholipids through free fatty acids generation, eventually undergoing hydrolysis to free fatty acids and creating phosphoglycerols (Patel and Panda, 2012; Frenzel and Steffen-Heins, 2015). The combination of membrane bilayers, aggregation, reduced retaining of encapsulated materials, and alteration can be mentioned as the causes for physical instability in liposomes (Karmali and Chaudhuri, 2007; Shim et al., 2013; Rahdar et al., 2019).
The administrated liposome fate and stability can be strongly affected by liposomes’ physicochemical characteristics, including bilayer membrane composition, size, rigidity, and charge (Portet and Dimova, 2010; Ghanbarzadeh et al., 2013; Ib and Corredig, 2013). The surface charge of liposomes can be positive, negative, or without electrical charge based on functional groups on liposomes’ surface at the environmental pH. As such, net-charge liposomes have represented the highest tendency to accumulate in the target tissue following systematic administration due to the reticuloendothelial system’s low clearance. On the other hand, cationic liposomes are often used to transport negatively charged nucleic acids (Xia and Xu, 2005; El-Samaligy et al., 2006a; Demetzos, 2008; Fujisawa et al., 2012). The methods used to prepare liposomes significantly affect physical properties, such as the coating of active compounds’ size and efficiency. Various phenomena such as aggregation and mixing can affect material properties, such as particle size and particle size distribution, attributed to the accumulation of liposomal particles. It has been reported that particle size reduction can be used for achieving bioactive compounds with optimal bioavailability due to increasing specific surface area (Campbell et al., 2001; Miao et al., 2002; Ulrich, 2002; Silva et al., 2011). The smaller the liposomal size, easier it is to cross the membrane, but it can affect the liposomal properties, including decreasing the amount and efficiency of drug loading and reducing liposome stability by increasing the surface energy. The smaller the particle size distribution, the more uniform is the liposomes’ size and the more similar would be the products’ overall characteristics (Marsh, 2001; Yamauchi et al., 2007; Drin et al., 2008).
Another critical factor affecting the stability of liposomes is referred to lipid composition. Studies have shown that controlling solute retention by liposomes and circulation half-life can be strongly affected by liposomal membrane fluidity and composition manipulation. One of the most critical barriers to prevent the transfer of liposomal drugs from the bench scale to the pharmaceutical market is their physical and chemical instability during production and storing. The mechanically robust and well-filled bilayers diminish the oxidizing and hydrolyzing agents that eventually improve the structure stability through size distribution (Kunzelmann-Marche et al., 2002; Liu and Krieger, 2002; López-Revuelta et al., 2006; Giulimondi et al., 2019); composition of the bilayer membrane is one of the influential and essential factors in liposome stability. The lipid selection during liposome fabrication should be commensurate with the carrier’s composition (Jiménez-Escrig and Sánchez-Muniz, 2000; Scheffer et al., 2005; Zhao et al., 2015; Ricci et al., 2016).
The Effect of Cholesterol on Liposome Membrane Stability
Cholesterol is an organic sterol molecule with amphiphilic nature. Structurally, this molecule has a hydroxyl group that can form hydrogen bonds with phospholipids and bulky steroid ring with a flexible carbohydrate. Cholesterol is a 27-carbon molecule found in the eukaryotic cell membrane and its concentration in the cell membrane is about 30–50 mol% of the entire lipid compounds. Various vital roles have been attributed to cholesterol, including membrane permeability regulation, elasticity and stiffness, and membrane strength. Cholesterol is the most used sterol in the formulation of liposomes which can prevent liposome aggregation and improve the liposomal membrane’s stability (Jiménez-Escrig and Sánchez-Muniz, 2000; Scheffer et al., 2005; Sun et al., 2007; Ricci et al., 2016; Trucillo et al., 2017).
In view of the spherical 3-D structure, liposomes have shown more realistic fluidity and cell membrane mobility than lipid monolayers. Sterols, such as ergosterol, stigmasterol, lanosterol, β-sitosterol and cholesterol, have been added to liposomes to modulate membrane fluidity and improve the stability of the phospholipids bilayer and decrease permeation of the encapsulated active compounds. The sterol molecule is located inside the phospholipid’s bilayer. The sterol carbohydrate tail at C17 intermingles with the hydrophobic fatty acyl chains, while the sterol hydroxyl group attaches to the hydrophilic head group of phospholipids (Socaciu et al., 2000; Sodt et al., 2015; Zhao et al., 2015; Giulimondi et al., 2019). Cholesterol plays a vital role in the liposome’s composition. It is one of the most critical structural components in the mammalian cell plasma membrane. It has been proven that the fluidity and permeability of the artificial vesicle are strongly affected by cholesterol through hydrogen binding to fatty acids that eventually lead to increasing the cohesiveness and mechanical strength. Cholesterol is a critical regulator of lipid bilayer dynamics, and it is essential for the normal functioning of the cells. The reduction of passive permeability to small molecules results from cholesterol interaction with membrane phospholipids, increasing the membrane cohesion. According to experimental studies, cholesterol adding to liposome bilayers can prevent lipid exchange that can be counted as an additional stabilizing effect (Sun et al., 2007; Ricci et al., 2016; Trucillo et al., 2017).
The Relationship Between Cholesterol and Transition Temperature
Another significant factor in the arrangement of bilayers in the liposome is the degree of saturation in phospholipids and the chain length. The presence of sterols such as cholesterol, β-sitosterol, and related plant sterols can lead to the pre-transition temperature peak’s disappearance. The gel-to-liquid crystalline phase Tm has been reported to decrease by addition of the sterols. It can be stated that the orientation of cholesterol in the lipid bilayer has a determinative effect on the reduction of head group accumulation. In the low Tm, cholesterol leads to the crystallization of the hydrocarbon chains into the inflexible crystalline gel phase. While in the high Tm, the rigid cholesterol molecule can limit the movement of the hydrocarbon chains. It can be concluded that, by increasing the sterol concentration, decreases the enthalpy of the main phase transition (Silva et al., 2011; Wu et al., 2012; Ricci et al., 2016).
For example, in the case of the PC molecule, it has been stated that cholesterol at concentrations of more than 25% leads to an order-liquid phase, which is essential for the mobility of membrane compounds; inferred that cholesterol at high concentrations causes the decouples of positional and conformational degrees of freedom of phospholipid molecules. On the other hand, at low cholesterol concentrations below 10%, its effect on the bilayer phospholipid is very different; only a slight decrease in the Tm, the main phase transfer occurs. This means that cholesterol at low concentrations cannot disrupt the crystalline order, nor can it ultimately induce the acyl chains in the liquid phase. Cholesterol, therefore, functions as a surfactant molecule, which causes the formation of lipid domains thus increasing the dynamic membrane heterogeneity (Trandum et al., 2000; Pinilla et al., 2020).
The Role of Cholesterol in Retention of Drugs and Modulation of Phospholipid Packing
The cholesterol content has a critical role in drug retention as it implies its effect by raising the phospholipid packing, leading to bilayer permeability reduction to non-electrolytic and dielectrolysis solvents (Dos Santos et al., 2002; Johnston et al., 2007). It is believed that the underlying mechanism in a biological milieu may be related to the phospholipid and high-density lipoproteins exchange of the low-cholesterol or no-cholesterol content liposomes while in cholesterol-encompassing liposomes, the phospholipid motility has been limited that prevents lipoproteins loss (Kunzelmann-Marche et al., 2002; Liu and Krieger, 2002; López-Revuelta et al., 2006).
The phospholipid packing, the membrane fluidity, and the surface charge of liposomes can be modulated and adapted by the cholesterol content, affecting the particle size, encapsulation efficiency, and final morphology (Zhao et al., 2015; Ohvo-Rekilà et al., 2002). Studies have shown that cholesterol as a nonionic molecule has revealed interesting effects on the zeta potential, especially increasing the highest zeta potential of cationic liposomes. The proposed mechanism is reliant on the membrane structure transition and the molecular filling state for the cholesterol-induced charge boosting of cationic liposomes. Cholesterol can induce the phase transition from the crystalline state to the liquid-ordered (LO) phase (Lv et al., 2006; Aramaki et al., 2016). Increasing the amount of cholesterol content in the membrane of liposomes can increase the mean size of liposomes. Due to the hydrophobic nature, cholesterol structure can easily interact with the phospholipid hydrophobic acyl chain through hydrogen bonds and hydrophobic interactions. The ring structure of cholesterol is comparatively inflexible, and its presence stabilizes the protracted straight-chain arrangement of saturated fatty acids via van der Waals interactions (Lee et al., 2005).
The Role of Cholesterol-Containing Liposomes in Plasma Stability
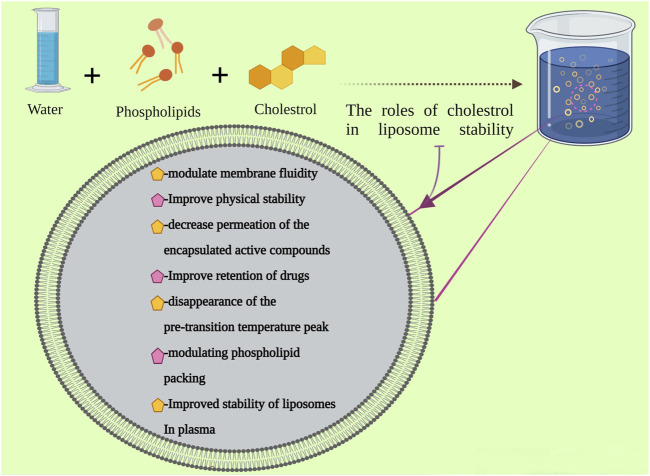
Cholesterol has a very significant effect on the structural stability of liposomes in plasma (Figure 4). It can reduce the interaction of liposomes with several proteins, leading to less susceptibility to phospholipase, reduce the phospholipid’s loss of high-density lipoproteins, change the membrane enzyme activities, inhibit digestion by macrophages, and inhibit fusion with specific cell types. By changing some liposome features through composition, mostly cholesterol modification, blood clearance and tissue distribution of intravenously injected liposomes can be expected. The cholesterol modification of liposomes has an inhibitory effect on the reticuloendothelial uptake. This modification reduces their interaction with several proteins and probably with serum components, which may affect liposomes’ tissue uptake (D’Avanzo et al., 2011; Johnstone et al., 2001; Moghimi and Patel, 2002). The cholesterol-based liposomal products are presented in Table 7.
FIGURE 4.
The liposome preparation and the role of cholesterol in its stability.
TABLE 7.
Cholesterol-based liposomal products.
| Drug compounds | Composition | The purpose of using cholesterol |
|---|---|---|
| Vitamin E (Samuni et al., 2000) | EPC + PUFA + cholesterol | 1- Increasing the storage time by reducing physical and chemical changes: a- Decreasing the lipid-bilayer hydration |
| b- Reduction in oxidative levels | ||
| 5(6)carboxyfluorescein 5(6) (CF) (Liang et al., 2007) | DPPC + cholesterol | 1- Increasing physical stability and reducing liposome deformity |
| Epirubicin (Wang et al., 2010) | CHCS + phosphatidylcholine + cholesterol | 1- Physical stability |
| 2- Drug release | ||
| Doxorubicin (Zhao et al., 2007) | mPEG-DSPE + HSPC + cholesterol | 1- Inhibiting aggregation through the steric barrier |
| 2- Prolonged blood circulation in vivo | ||
| CF (Abu-Dahab et al., 2001) | DPPC + LC-Biotin-DPPE + cholesterol | 1- Enhancing the stability |
| Paclitaxel (Yang et al., 2007) | SPC + EPC + PE + DSPC + DPPC + HPC + cholesterol | 1- Improving physicochemical stability |
| Vinorelbine (Semple et al., 2005) | SM + cholesterol | 1- Improving drug retention |
| 2- Prolongation of plasma | ||
| 3- Circulation time | ||
| 4- Improving therapeutic activity | ||
| Curcumin (Chen et al., 2009) | DMPC + DMPG + cholesterol | 1- Improving the bioavailability and efficacy of drug-containing liposomes |
| Vincristine (Liang et al., 2008) | PC + OQCMC + cholesterol | 1- Good physical form |
| 2- Thermal stability | ||
| 3- Excellent solubility in water | ||
| 4- High effectiveness in drug encapsulation | ||
| Tenofovir (Xu et al., 2011) | DMPC + DPPC + DSPC+ DPTAP + cholesterol |
1- Reducing the membrane permeability |
| Amphotericin B (Matsuoka and Murata, 2002) | POPC + EPC + FCCP + cholesterol | 1- Preventing the formation of ion channels in the crystalline phase of the membrane of the liquid |
| 1- Inhibiting AmB-induced membrane permeability | ||
| Minoxidil (Mx) (López-Pinto et al., 2005) | α-DPPC + cholesterol | 1- Increasing the drug-entrapment percentage, due to the stabilizing effect of cholesterol into the lipid bilayers |
| 2- An increase in the mean particle size | ||
| 3- Eliminating the phase-transition temperature (Tc) peak of DPPC and thus the range of the gel state of vesicles increased | ||
| 4- Preventing the partial dilution of the bilayers | ||
| 5- Decreasing permeability and making more rigid | ||
| CLX (celecoxib) (Deniz et al., 2010) | DSPC + cholesterol | 1- Reduction of phase transition temperature (Tm) |
| 2- Encapsulation efficiency, loading, and releasing CLX decreased with the increasing cholesterol content | ||
| 3- Increasing drug retention | ||
| Acetazolamide (Hathout et al., 2007) | PC + holesterol + SA + DP | 1- Increasing drug loading by 2- Increasing cholesterol |
| 3- Increasing Physical stability | ||
| 4- Increasing retained drug | ||
| Silymarin (El-Samaligy et al., 2006b) | Lecithin Soya + SA + DP + cholesterol | 2- Adding cholesterol beyond a specific limit produced a decrease in encapsulation efficiency |
| Dithranol (Agarwal et al., 2001) | phosphatidyl choline + DCP + cholesterol | 3- 1- Increasing entrapment efficiency of dithranol |
| Ciprofloxacin (Hosny, 2010) | PC + cholesterol | 1- Optimal encapsulation efficiency by increasing a certain amount of C.H. content |
| 2- Improving prolonged drug | ||
| 3- Agent helping to control drug release |
Note: CF, carboxyfluorescein; CLX, celecoxib; EPC, Ethanolamine phosphatidylcholine; PUFA, Polyunsaturated fatty acids; DPPC, Dipalmitoyl phosphatidylcholine; PEG-DSPE, Distearoyl-sn-glycero-3-phosphoethanolamine-Poly(ethylene glycol glycol, HSPC: Hydrogenated soybean phosphatidylcholine; LC-Biotin-DPPE, N((biotinyl)amino)hexanoyl)dipalmitoyl-l-α-phosphatidylethanolamine; SPC, Sphingosyl phosphorylcholine; PE, phosphatidyl-ethanolamine; DSPC, Distearoyl L-3-phosphatidylcholine; HPC, hydrogenated soybean phosphatidylcholine; SM, sphingomyelin; DMPC, dimyristoylphosphatidylcholine; DMPG, dimyristoylphosphatidylglycerol; PC, phosphatidylcholine; OQCMC, octadecyl quaternized carboxymethyl chitosan; DPTAP, 1,2-dipalmitoyl-3-trimethylammonium-propane; POPC, 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine; EPC, Egg yolk phosphatidylcholine; FCCP, carbonyl cyanid-p-trifluoro-methoxyphenyl hydrazone; α-DPPC, α-dipalmitoylphosphatidylcholine; SA, stearylamine; DP, dicetyl phosphate; DCP, Dicetyl phosphate.
The Optimum Cholesterol Concentration for Liposome’s Stability
The importance of cholesterol in liposome stability has been described earlier and schematically shows in Figure 3. However, the optimum concentration of cholesterol to attain a suitable formulation has not yet been elucidated. To make stable and regulated drug discharge means, lipids and cholesterol ratio screening arrangements in different studies can help. For the preparation of liposomes, some phospholipids are blended with varying molar ratios of cholesterol. The results of numerous studies have shown that the maximum amount of cholesterol that can be integrated into reconstructed bilayers is assumed to be ∼50 mol%. The most frequently used proportion is the 2: 1 ratio (e.g., two parts of lipids and one part of cholesterol) or 1: 1 ratio; although the underlying reasons for using these ratios is still unknown (Marsh, 2001; Liang et al., 2007; Briuglia et al., 2015).
The essential mechanisms of the liposomal interaction with cells according to both, in vitro and in vivo studies have been summarized as:
1- Particular interfaces with cell-surface components such as electrostatic bonds and imprecise interactions, including feeble hydrophobic bonds
2- Endocytosis caused by cells of the reticuloendothelial system (RES), comprising neutrophils and macrophages
3- Combination with the plasma cell membrane by inclusion of the lipid bilayer of the liposome into the plasma membrane (Akbarzadeh et al., 2013)
Adjusting cholesterol levels can be a very influential factor in controlling the liposomal stability. This factor can be crucial in designing liposomes for practical use in biological systems in vivo and in vitro (Epstein et al., 2008). The most important commercial liposomes are depicted in Table 8.
TABLE 8.
Commercial cholesterol-based liposomes.
| Commercial name | Composition | Type of drug | Application |
|---|---|---|---|
| LADR (small-sized liposomal Adriamycin) (Chen et al., 2005) | Cholesterol + egg phosphatidylcholine | doxorubicin HCl | Anti-Cancer |
| AmBisome (Dynarowicz-Łątka et al., 2003) | HSPC + DSPG + cholesterol | Amphotericin B | Anti-fungal |
| Doxil®/Caelyx®a (Wibroe et al., 2016) | HSPC + cholesterol + DSPE-PEG2,000 | Doxorubicin | Anti-Cancer |
| Myocet™ (Collier et al., 2017) | EPC + cholesterol | Citrate conjugated doxorubicin | Anti-Cancer |
| Marqibo® (Silverman and Deitcher, 2013) | Sphingomyelin + cholesterol | vincristine sulfate | Anti-Cancer |
| Abelcet® (Husain et al., 2010) | DMPC + DMPG | Amphotericin B | Anti-fungal |
| DaunoXome® (Lowis et al., 2006) | DSPC + cholesterol | Daunorubicin | Anti-Cancer |
| Depocyt® (Phuphanich et al., 2007; Crommelin et al., 2020) | Cholesterol + triolein + DOPC + DPPG | Cytarabine | Anti-Cancer |
| Lipo-dox (Huang et al., 2018; Weng et al., 2019) | DSPC + cholesterol + PEG 2000-DSPE | Doxorubicin | Anti-Cancer |
| Visudyne (Barnes et al., 2010; Jain et al., 2016; Rizvi et al., 2019) | EPG + DMPC | Verteporfin | PDT |
| DepoDur (Peravali et al., 2014) | Cholesterol + Triolein + DOPC + DPPG | Morphine sulfate | Pain control and management |
DSPG, Distearoyl-sn-glycero-3-phosphoglycerol.
Clinical Trials
Unlike most nanoparticles, which encounter serious challenges in entering the clinic for various reasons, including safety issues, liposomes are well accepted in the clinic, first liposome-based drug being approved was Doxil®[(Anselmo and Mitragotri, 2015; Bulbake et al., 2017; Singh et al., 2020)], a liposome-based Doxorubicin formulation that has been FDA approved in 1995 for the United States market to treat ovarian cancer and AIDS-related Kaposi’s sarcoma. Subsequently, various other liposomal-based drugs have been commercialized, such as DaunoXome®, for the delivery of daunorubicin, approved in 1996 to manage advanced HIV-associated Kaposi’s sarcoma and several different formulations (Khadke et al., 2020).
Currently, many efforts are underway to develop lipid formulations for entry in to the clinics in various fields. To examine the clinical phase studies of liposome-based structures, the term “liposome” was searched from the PubMed database pertaining to various studies related to the clinical phases in the last year. In one study, the effect of liposomal amphotericin B formulation as an antifungal agent was investigated in patients with hematological malignancies with neutropenia and persistent fever (Yoshida et al., 2020). Because fungal infections subsequent to the chemotherapy in patients with neutropenia are considered a severe complication, and as the use of common antifungal drugs is highly toxic, so eliminating this life-threatening complication is very important. The usage of 3 mg/kg/day liposomal amphotericin B concentration for patients was compared with itraconazole; results showed no difference between the liposomal amphotericin B and itraconazole regarding the efficacy and safety in antifungal therapy in hematological malignancy patients (Yoshida et al., 2020).
Liposomal compounds are known to be very effective in treating cancer. Recently, another liposomal system has been proposed for the treatment of acute myeloid leukemia wherein the Vyxeos liposome in phase III clinical was examined. This liposomal system comprise two different topoisomerase II inhibitors known as daunorubicin and cytarabine, which included 1 and 0.44 mg in every 1 unit of liposome formulation. The study was performed on elderly patients with untreated acute myeloid leukemia where a higher survival rate for patients treated with the liposomal formulation was discerned than for standard chemotherapy, although some side effects have been reported for the use of this formulation (Tzogani et al., 2020).
Conclusion
Health and medical issues and problems have always been one of the main areas of interest for scientists. Applying new methods for solving medical problems requires introducing new and efficient materials and tools that address related questions and issues. Liposomes have been extensively studied since their introduction, and their potential biomedical use has been well demonstrated. The unique properties of liposomes, including biocompatibility, biodegradability, amphiphilic nature, low toxicity, non-ionicity, sustained release, and active targeting, have made them one of the most widely used nanoparticles. Currently, the most commercialized nanoparticles in the field of drug delivery and cosmetics are related to liposomes. However, they still have some shortcomings that need to be addressed for clinical and pharmaceutical use. As mentioned earlier, since Doxil® introduced in 1995, many efforts have been made to bring these versatile nanomaterials to the clinic; however, their structure still needs to be optimized to reduce some complications. One approach to increase liposomes’ efficiency, especially concerning cancers therapy, is the use of RGD which can be anchored to liposome surfaces or via self-assembly means (Cheng and Ji, 2019). This strategy has been proposed to reduce the side effects of liposomal drugs, as the clinical trial sometimes suffers from, including acute infusion reaction and hand-foot syndrome (HFS). Hence future studies must include new procedures to reduce safety problems (He and Tang, 2018; Cheng and Ji, 2019). Since varying combinations of liposomes can provide unique and distinctive properties, exciting suggestions have been propositioned in the field of novel drug delivery systems, such as nose-to-brain direct drug transport using liposome formulation based on DTE and DTP ingredients (Hong et al., 2019).
However, one of the most critical challenges that liposomes encounter is their physical and chemical instability. Various factors such as environmental conditions, manufacturing method, components characteristics, lipid type, and the absence or presence of cholesterol affect liposomes’ stability. The vital need for the development of liposomes with high stability significantly impacts their clinical application. Cholesterol has been shown to increase liposome stability through various mechanisms, including increased retention time, modulating phospholipid packing, Tm, and plasma stability. However, the optimal amount of cholesterol has not yet been identified. Future field research should be based on the principle of finding the optimal amount of cholesterol in liposome production. Notably, cholesterol still tend to maintain membrane’s fluidity as its concentration is increased and decreased. Therefore, the amount of cholesterol and the type of liposome constituents need further investigation. In this context, simulation and computational studies may prove to be very helpful. Hashemzadeh et al. (2020a) have investigated DSPC and DPSM effect on liposome stability through a simulation study which revealed that DSPC preserves its structure shape due to the cylindrical geometric structure and small-size head group, while the DPSM was causing liposome turnover into micelle structure because of its conical geometric design with the larger head group. Such studies regarding cholesterol’s effect on liposome stability via simulation investigations would be highly desirable.
Author Contributions
All authors contributed to the conception and the main idea of the work. MJ, NB, FB, HK, AH, and FM drafted the main text, figures, and tables. MJ supervised the work and provided the comments and additional scientific information. MS and AC-A also reviewed and revised the text. All authors read and approved the final version of the work to be published.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Abd El-Fattah A. I., Fathy M. M., Ali Z. Y., El-Garawany A. E.-R. A., Mohamed E. K. (2017). Enhanced Therapeutic Benefit of Quercetin-Loaded Phytosome Nanoparticles in Ovariectomized Rats. Chemico-Biological Interactions 271, 30–38. 10.1016/j.cbi.2017.04.026 [DOI] [PubMed] [Google Scholar]
- Abdelkader H., Alani A. W. G., Alany R. G. (2014). Recent Advances in Non-ionic Surfactant Vesicles (Niosomes): Self-Assembly, Fabrication, Characterization, Drug Delivery Applications and Limitations. Drug Deliv. 21 (2), 87–100. 10.3109/10717544.2013.838077 [DOI] [PubMed] [Google Scholar]
- Abu-Dahab R., Schäfer U. F., Lehr C.-M. (2001). Lectin-functionalized Liposomes for Pulmonary Drug Delivery: Effect of Nebulization on Stability and Bioadhesion. Eur. J. Pharm. Sci. 14 (1), 37–46. 10.1016/s0928-0987(01)00147-6 [DOI] [PubMed] [Google Scholar]
- Agarwal R., Katare O. P., Vyas S. P. (2001). Preparation and In Vitro Evaluation of Liposomal/niosomal Delivery Systems for Antipsoriatic Drug Dithranol. Int. J. Pharm. 228 (1-2), 43–52. 10.1016/s0378-5173(01)00810-9 [DOI] [PubMed] [Google Scholar]
- Agrawal M. M., Jawade S., Khan S. (2012). A Review on Liposome. Int. J. Adv. Res. Pharm. Bio Sci. 2 (1), 453–465. [Google Scholar]
- Aguilar-Pérez K., Avilés-Castrillo J., Medina D. I., Parra-Saldivar R., Iqbal H. (2020). Insight into Nanoliposomes as Smart Nanocarriers for Greening the Twenty-First century Biomedical Settings. Front. Bioeng. Biotechnol. 8, 1441. 10.3389/fbioe.2020.579536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainbinder D., Paolino D., Fresta M., Touitou E. (2010). Drug Delivery Applications with Ethosomes. J Biomed. Nanotechnol 6 (5), 558–568. 10.1166/jbn.2010.1152 [DOI] [PubMed] [Google Scholar]
- Akabori K., Nagle J. F. (2015). Structure of the DMPC Lipid Bilayer Ripple Phase. Soft matter 11 (5), 918–926. 10.1039/c4sm02335h [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbarzadeh A., Rezaei-Sadabady R., Davaran S., Joo S. W., Zarghami N., Hanifehpour Y., et al. (2013). Liposome: Classification, Preparation, and Applications. Nanoscale Res. Lett. 8 (1), 102. 10.1186/1556-276X-8-102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Remawi M., Elsayed A., Maghrabi I., Hamaidi M., Jaber N. (2017). Chitosan/lecithin Liposomal Nanovesicles as an Oral Insulin Delivery System. Pharm. Dev. Technol. 22 (3), 390–398. 10.1080/10837450.2016.1213745 [DOI] [PubMed] [Google Scholar]
- Alizadeh E., Akbarzadeh A., Eslaminejad M. B., Barzegar A., Hashemzadeh S., Nejati-Koshki K., et al. (2015). Up Regulation of Liver-Enriched Transcription Factors HNF4a and HNF6 and Liver-specific MicroRNA (miR-122) by Inhibition of Let-7b in Mesenchymal Stem Cells. Chem. Biol. Drug Des. 85 (3), 268–279. 10.1111/cbdd.12398 [DOI] [PubMed] [Google Scholar]
- AllenLiposomes T. M. (1997). Liposomen. DrugsSuppl 54, 8–14. 10.2165/00003495-199700544-00004 [DOI] [Google Scholar]
- Alving C. R., Rao M., Steers N. J., Matyas G. R., Mayorov A. V. (2012). Liposomes Containing Lipid A: an Effective, Safe, Generic Adjuvant System for Synthetic Vaccines. Expert Rev. Vaccin. 11 (6), 733–744. 10.1586/erv.12.35 [DOI] [PubMed] [Google Scholar]
- Anderson M., Omri A. (2004). The Effect of Different Lipid Components on the In Vitro Stability and Release Kinetics of Liposome Formulations. Drug Deliv. 11 (1), 33–39. 10.1080/10717540490265243 [DOI] [PubMed] [Google Scholar]
- Anselmo A. C., Mitragotri S. (2015). A Review of Clinical Translation of Inorganic Nanoparticles. Aaps J. 17 (5), 1041–1054. 10.1208/s12248-015-9780-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwekar H., Patel S., Singhai A. (2011). Liposome-as Drug Carriers. Int. J. Pharm. Life Sci., 2.(7) [Google Scholar]
- Aoki H., Mizuno M., Natsume A., Tsugawa T., Tsujimura K., Takahashi T., et al. (2001). Dendritic Cells Pulsed with Tumor Extract-Cationic Liposome Complex Increase the Induction of Cytotoxic T Lymphocytes in Mouse Brain Tumor. Cancer Immunol. Immunother. 50 (9), 463–468. 10.1007/s002620100220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aramaki K., Watanabe Y., Takahashi J., Tsuji Y., Ogata A., Konno Y. (2016). Charge Boosting Effect of Cholesterol on Cationic Liposomes. Colloids Surf. A: Physicochemical Eng. Aspects 506, 732–738. 10.1016/j.colsurfa.2016.07.040 [DOI] [Google Scholar]
- Arora R. (2016). Advances in Niosome as a Drug Carrier: a Review. Asian J. Pharmaceutics (Ajp) Free full text articles Asian J Pharm, 1.(1) [Google Scholar]
- Ashok B., Arleth L., Hjelm R. P., Rubinstein I., Önyüksel H. (2004). In Vitro characterization of PEGylated Phospholipid Micelles for Improved Drug Solubilization: Effects of PEG Chain Length and PC Incorporation. J. Pharm. Sci. 93 (10), 2476–2487. 10.1002/jps.20150 [DOI] [PubMed] [Google Scholar]
- Asprea M., Tatini F., Piazzini V., Rossi F., Bergonzi M., Bilia A. (2019). Stable, Monodisperse, and Highly Cell-Permeating Nanocochleates from Natural Soy Lecithin Liposomes. Pharmaceutics 11 (1), 34. 10.3390/pharmaceutics11010034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atrooz O. M. (2011). Efects of Alkylresorcinolic Lipids Obtained from Acetonic Extract of Jordanian Wheat Grains on Liposome Properties. Int. J. Biol. Chem. 5 (5), 314–321. 10.3923/ijbc.2011.314.321 [DOI] [Google Scholar]
- Attia M. F., Anton N., Wallyn J., Omran Z., Vandamme T. F. (2019). An Overview of Active and Passive Targeting Strategies to Improve the Nanocarriers Efficiency to Tumour Sites. J. Pharm. Pharmacol. 71 (8), 1185–1198. 10.1111/jphp.13098 [DOI] [PubMed] [Google Scholar]
- Attwood S., Choi Y., Leonenko Z. (2013). Preparation of DOPC and DPPC Supported Planar Lipid Bilayers for Atomic Force Microscopy and Atomic Force Spectroscopy. Ijms 14 (2), 3514–3539. 10.3390/ijms14023514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awasthi V. D., Garcia D., Goins B. A., Phillips W. T. (2003). Circulation and Biodistribution Profiles of Long-Circulating PEG-Liposomes of Various Sizes in Rabbits. Int. J. Pharm. 253 (1-2), 121–132. 10.1016/s0378-5173(02)00703-2 [DOI] [PubMed] [Google Scholar]
- Bae Y., Jung M. K., Mun J. Y., Mallick S., Song S. J., Kim D. M., et al. (2018). DQAsomes Nanoparticles Promote Osteogenic Differentiation of Human Adipose-Derived Mesenchymal Stem Cells. Bull. Korean Chem. Soc. 39 (1), 97–104. 10.1002/bkcs.11355 [DOI] [Google Scholar]
- Bakker-Woudenberg I. A. J. M. (2002). Long-circulating Sterically Stabilized Liposomes as Carriers of Agents for Treatment of Infection or for Imaging Infectious Foci. Int. J. Antimicrob. Agents 19 (4), 299–311. 10.1016/s0924-8579(02)00021-3 [DOI] [PubMed] [Google Scholar]
- Barenholz Y. (2016). Chapter 13. Doxil - the First FDA-Approved Nano-Drug: from Basics via CMC, Cell Culture and Animal Studies to Clinical Use. Nanomedicines: Des. Deliv. Detect. 51, 315–345. 10.1039/9781782622536-00315 [DOI] [Google Scholar]
- Barenholz Y. (2021). Doxil - the First FDA-Approved Nano-Drug: From an Idea to a Product. Handbook harnessing Biomater. nanomedicine, 463–528. Jenny Stanford Publishing. 10.1201/9781003125259-16 [DOI] [Google Scholar]
- Barnes L. D., Giuliano E. A., Ota J. (2010). Cellular Localization of Visudyne as a Function of Time after Local Injection in an In Vivo Model of Squamous Cell Carcinoma: an Investigation into Tumor Cell Death. Vet. Ophthalmol. 13 (3), 158–165. 10.1111/j.1463-5224.2010.00775.x [DOI] [PubMed] [Google Scholar]
- Barratt G. (2003). Colloidal Drug Carriers: Achievements and Perspectives. Cell Mol. Life Sci. CMLS 60 (1), 21–37. 10.1007/s000180300002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia L., Ugazio E. (2019). Lipid nano- and microparticles overview of patent-related research. J. Nanomater. 2019, 2834941. 10.1155/2019/2834941 [DOI] [Google Scholar]
- Baykal-Caglar E., Hassan-Zadeh E., Saremi B., Huang J. (2012). Preparation of Giant Unilamellar Vesicles from Damp Lipid Film for Better Lipid Compositional Uniformity. Biochim. Biophys. Acta (Bba) - Biomembranes 1818 (11), 2598–2604. 10.1016/j.bbamem.2012.05.023 [DOI] [PubMed] [Google Scholar]
- Benech R.-O., Kheadr E. E., Laridi R., Lacroix C., Fliss I. (2002). Inhibition of Listeria Innocua in Cheddar Cheese by Addition of Nisin Z in Liposomes or by In Situ Production in Mixed Culture. Appl. Environ. Microbiol. 68 (8), 3683–3690. 10.1128/aem.68.8.3683-3690.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benesch M., Urban C. (2008). Liposomal Cytarabine for Leukemic and Lymphomatous Meningitis: Recent Developments. Expert Opin. Pharmacother. 9 (2), 301–309. 10.1517/14656566.9.2.301 [DOI] [PubMed] [Google Scholar]
- Bennett W. F. D., MacCallum J. L., Hinner M. J., Marrink S. J., Tieleman D. P. (2009). Molecular View of Cholesterol Flip-Flop and Chemical Potential in Different Membrane Environments. J. Am. Chem. Soc. 131 (35), 12714–12720. 10.1021/ja903529f [DOI] [PubMed] [Google Scholar]
- Benvegnu T., Lemiègre L., Cammas-Marion S. (2009). New Generation of Liposomes Called Archaeosomes Based on Natural or Synthetic Archaeal Lipids as Innovative Formulations for Drug Delivery. Ddf 3 (3), 206–220. 10.2174/187221109789105630 [DOI] [PubMed] [Google Scholar]
- Benz M., Gutsmann T., Chen N., Tadmor R., Israelachvili J. (2004). Correlation of AFM and SFA Measurements Concerning the Stability of Supported Lipid Bilayers. Biophysical J. 86 (2), 870–879. 10.1016/s0006-3495(04)74162-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bern C., Adler-Moore J., Berenguer J., Boelaert M., den Boer M., Davidson R. N., et al. (2006). Liposomal Amphotericin B for the Treatment of Visceral Leishmaniasis. Clin. Infect. Dis. 43 (7), 917–924. 10.1086/507530 [DOI] [PubMed] [Google Scholar]
- Bhatt P., Lalani R., Vhora I., Patil S., Amrutiya J., Misra A., et al. (2018). Liposomes Encapsulating Native and Cyclodextrin Enclosed Paclitaxel: Enhanced Loading Efficiency and its Pharmacokinetic Evaluation. Int. J. pharmaceutics 536 (1), 95–107. 10.1016/j.ijpharm.2017.11.048 [DOI] [PubMed] [Google Scholar]
- Briuglia M.-L., Rotella C., McFarlane A., Lamprou D. A. (2015). Influence of Cholesterol on Liposome Stability and on In Vitro Drug Release. Drug Deliv. Transl. Res. 5 (3), 231–242. 10.1007/s13346-015-0220-8 [DOI] [PubMed] [Google Scholar]
- B.T.S. Thirumamagal B., Zhao X. B., Bandyopadhyaya A. K., Sureshbabu Narayanasamy S., Johnsamuel J., Tiwari R., et al. (2006). Receptor-targeted Liposomal Delivery of boron-containing Cholesterol Mimics for boron Neutron Capture Therapy (BNCT). Bioconjug. Chem. 17 (5), 1141–1150. 10.1021/bc060075d [DOI] [PubMed] [Google Scholar]
- Bulbake U., Doppalapudi S., Kommineni N., Khan W. (2017). Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics 9 (2), 12. 10.3390/pharmaceutics9020012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton A. J., Giguère S., Berghaus L. J., Hondalus M. K., Arnold R. D. (2015). Efficacy of Liposomal Gentamicin against Rhodococcus Equi in a Mouse Infection Model and Colocalization with R. Equi in Equine Alveolar Macrophages. Vet. Microbiol. 176 (3-4), 292–300. 10.1016/j.vetmic.2015.01.015 [DOI] [PubMed] [Google Scholar]
- Campbell R. B., Balasubramanian S. V., Straubinger R. M. (2001). Influence of Cationic Lipids on the Stability and Membrane Properties of Paclitaxel‐containing Liposomes. J. Pharm. Sci. 90 (8), 1091–1105. 10.1002/jps.1063 [DOI] [PubMed] [Google Scholar]
- Caracciolo G., Pozzi D., Amenitsch H., Caminiti R. (2005). Multicomponent Cationic Lipid−DNA Complex Formation: Role of Lipid Mixing. Langmuir 21 (25), 11582–11587. 10.1021/la052077c [DOI] [PubMed] [Google Scholar]
- Cardenia V., Waraho T., Rodriguez‐Estrada M. T., Julian McClements D., Decker E. A. (2011). Antioxidant and Prooxidant Activity Behavior of Phospholipids in Stripped Soybean Oil‐in‐Water Emulsions. J. Am. Oil Chem. Soc. 88 (9), 1409–1416. 10.1007/s11746-011-1807-y [DOI] [Google Scholar]
- Carlson D. L., Than K. D., Roberts A. L. (2006). Acid- and Base-Catalyzed Hydrolysis of Chloroacetamide Herbicides. J. Agric. Food Chem. 54 (13), 4740–4750. 10.1021/jf0530704 [DOI] [PubMed] [Google Scholar]
- Chen C., Johnston T. D., Jeon H., Gedaly R., McHugh P. P., Burke T. G., et al. (2009). An In Vitro Study of Liposomal Curcumin: Stability, Toxicity and Biological Activity in Human Lymphocytes and Epstein-Barr Virus-Transformed Human B-Cells. Int. J. Pharm. 366 (1-2), 133–139. 10.1016/j.ijpharm.2008.09.009 [DOI] [PubMed] [Google Scholar]
- Chen C., Han D., Cai C., Tang X. (2010). An Overview of Liposome Lyophilization and its Future Potential. J. controlled release 142 (3), 299–311. 10.1016/j.jconrel.2009.10.024 [DOI] [PubMed] [Google Scholar]
- Chen J.-H., Ling R., Yao Q., Li Y., Chen T., Wang Z., et al. (2005). Effect of Small-Sized Liposomal Adriamycin Administered by Various Routes on a Metastatic Breast Cancer Model. Endocr. Relat. Cancer 12 (1), 93–100. 10.1677/erc.1.00871 [DOI] [PubMed] [Google Scholar]
- Chen J., Lu W.-L., Gu W., Lu S.-S., Chen Z.-P., Cai B.-C., et al. (2014). Drug-in-cyclodextrin-in-liposomes: A Promising Delivery System for Hydrophobic Drugs. Expert Opin. Drug Deliv. 11 (4), 565–577. 10.1517/17425247.2014.884557 [DOI] [PubMed] [Google Scholar]
- Chen X., Wang X., Wang Y., Yang L., Hu J., Xiao W., et al. (2010). Improved Tumor-Targeting Drug Delivery and Therapeutic Efficacy by Cationic Liposome Modified with Truncated bFGF Peptide. J. controlled release 145 (1), 17–25. 10.1016/j.jconrel.2010.03.007 [DOI] [PubMed] [Google Scholar]
- Chen Y., Sun J., Lu Y., Tao C., Huang J., Zhang H., et al. (2013). Complexes Containing Cationic and Anionic pH-Sensitive Liposomes: Comparative Study of Factors Influencing Plasmid DNA Gene Delivery to Tumors. Int. J. Nanomedicine 8, 1573–1593. 10.2147/IJN.S42800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Wu Q., Zhang Z., Yuan L., Liu X., Zhou L. (2012). Preparation of Curcumin-Loaded Liposomes and Evaluation of Their Skin Permeation and Pharmacodynamics. Molecules 17 (5), 5972–5987. 10.3390/molecules17055972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng M. H. Y., Harmatys K. M., Charron D. M., Chen J., Zheng G. (2019). Stable J‐Aggregation of an aza‐BODIPY‐Lipid in a Liposome for Optical Cancer Imaging. Angew. Chem. 131 (38), 13528–13533. 10.1002/ange.201907754 [DOI] [PubMed] [Google Scholar]
- Cheng Y., Ji Y. (2019). RGD-modified Polymer and Liposome Nanovehicles: Recent Research Progress for Drug Delivery in Cancer Therapeutics. Eur. J. Pharm. Sci. 128, 8–17. 10.1016/j.ejps.2018.11.023 [DOI] [PubMed] [Google Scholar]
- Chetoni P., Rossi S., Burgalassi S., Monti D., Mariotti S., Saettone M. F. (2004). Comparison of Liposome-Encapsulated Acyclovir with Acyclovir Ointment: Ocular Pharmacokinetics in Rabbits. J. Ocul. Pharmacol. Ther. 20 (2), 169–177. 10.1089/108076804773710849 [DOI] [PubMed] [Google Scholar]
- Chibowski E., Szcześ A. (2016). Zeta Potential and Surface Charge of DPPC and DOPC Liposomes in the Presence of PLC Enzyme. Adsorption 22 (4-6), 755–765. 10.1007/s10450-016-9767-z [DOI] [Google Scholar]
- Choi M. J., Maibach H. I. (2005). Liposomes and Niosomes as Topical Drug Delivery Systems. Skin Pharmacol. Physiol. 18 (5), 209–219. 10.1159/000086666 [DOI] [PubMed] [Google Scholar]
- Christensen D., Foged C., Rosenkrands I., Lundberg C. V., Andersen P., Agger E. M., et al. (2010). CAF01 Liposomes as a Mucosal Vaccine Adjuvant: In Vitro and In Vivo Investigations. Int. J. pharmaceutics 390 (1), 19–24. 10.1016/j.ijpharm.2009.10.043 [DOI] [PubMed] [Google Scholar]
- Chupin V., Boots J. W. P., Killian J. A., Demel R. A., de Kruijff B. (2002). Thermotropic Phase Behavior of Monoglyceride-Dicetylphosphate Dispersions and Interactions with Proteins: a 2H and 31P NMR Study. Biophysical J. 82 (2), 843–851. 10.1016/s0006-3495(02)75446-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier M. A., Bachelder E. M., Ainslie K. M. (2017). Electrosprayed Myocet-like Liposomes: an Alternative to Traditional Liposome Production. Pharm. Res. 34 (2), 419–426. 10.1007/s11095-016-2072-4 [DOI] [PubMed] [Google Scholar]
- Cordeiro C., Wiseman D. J., Lutwyche P., Uh M., Evans J. C., Finlay B. B., et al. (2000). Antibacterial Efficacy of Gentamicin Encapsulated in pH-Sensitive Liposomes against an In Vivo Salmonella enterica Serovar Typhimurium Intracellular Infection Model. Antimicrob. Agents Chemother. 44 (3), 533–539. 10.1128/aac.44.3.533-539.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crommelin D. J. A., van Hoogevest P., Storm G. (2020). The Role of Liposomes in Clinical Nanomedicine Development. What Now? Now what?. J. Controlled Release 318, 256–263. 10.1016/j.jconrel.2019.12.023 [DOI] [PubMed] [Google Scholar]
- Csiszár A., Hersch N., Dieluweit S., Biehl R., Merkel R., Hoffmann B. (2010). Novel Fusogenic Liposomes for Fluorescent Cell Labeling and Membrane Modification. Bioconjug. Chem. 21 (3), 537–543. 10.1021/bc900470y [DOI] [PubMed] [Google Scholar]
- D'Avanzo N., Hyrc K., Enkvetchakul D., Covey D. F., Nichols C. G. (2011). Enantioselective Protein-Sterol Interactions Mediate Regulation of Both Prokaryotic and Eukaryotic Inward Rectifier K+ Channels by Cholesterol. PLoS One 6 (4), e19393. 10.1371/journal.pone.0019393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daemen T., Demare A., Bungener L., Dejonge J., Huckriede A., Wilschut J. (2005). Virosomes for Antigen and DNA Delivery. Adv. Drug Deliv. Rev. 57 (3), 451–463. 10.1016/j.addr.2004.09.005 [DOI] [PubMed] [Google Scholar]
- Dana P., Bunthot S., Suktham K., Surassmo S., Yata T., Namdee K., et al. (2020). Active Targeting Liposome-PLGA Composite for Cisplatin Delivery against Cervical Cancer. Colloids Surf. B: Biointerfaces 196, 111270. 10.1016/j.colsurfb.2020.111270 [DOI] [PubMed] [Google Scholar]
- Daraee H., Etemadi A., Kouhi M., Alimirzalu S., Akbarzadeh A. (2016). Application of Liposomes in Medicine and Drug Delivery. Artif. Cell Nanomedicine, Biotechnol. 44 (1), 381–391. 10.3109/21691401.2014.953633 [DOI] [PubMed] [Google Scholar]
- Davidsen J., Rosenkrands I., Christensen D., Vangala A., Kirby D., Perrie Y., et al. (2005). Characterization of Cationic Liposomes Based on Dimethyldioctadecylammonium and Synthetic Cord Factor from M. tuberculosis (Trehalose 6,6'-Dibehenate)-A Novel Adjuvant Inducing Both strong CMI and Antibody Responses. Biochim. Biophys. Acta 1718 (1-2), 22–31. 10.1016/j.bbamem.2005.10.011 [DOI] [PubMed] [Google Scholar]
- Davidson E. M., Haroutounian S., Kagan L., Naveh M., Aharon A., Ginosar Y. (2016). A Novel Proliposomal Ropivacaine Oil. Anesth. Analgesia 122 (5), 1663–1672. 10.1213/ane.0000000000001200 [DOI] [PubMed] [Google Scholar]
- Dayan N., Touitou E. (2000). Carriers for Skin Delivery of Trihexyphenidyl HCl: Ethosomes vs. Liposomes. Biomaterials 21 (18), 1879–1885. 10.1016/s0142-9612(00)00063-6 [DOI] [PubMed] [Google Scholar]
- Demetzos C. (2008). Differential Scanning Calorimetry (DSC): a Tool to Study the thermal Behavior of Lipid Bilayers and Liposomal Stability. J. liposome Res. 18 (3), 159–173. 10.1080/08982100802310261 [DOI] [PubMed] [Google Scholar]
- Deng H., Song K., Zhao X., Li Y., Wang F., Zhang J., et al. (2017). Tumor Microenvironment Activated Membrane Fusogenic Liposome with Speedy Antibody and Doxorubicin Delivery for Synergistic Treatment of Metastatic Tumors. ACS Appl. Mater. Inter. 9 (11), 9315–9326. 10.1021/acsami.6b14683 [DOI] [PubMed] [Google Scholar]
- Deng M., Tu N., Bai F., Wang L. (2012). Surface Functionalization of Hydrophobic Nanocrystals with One Particle Per Micelle for Bioapplications. Chem. Mater. 24 (13), 2592–2597. 10.1021/cm301285g [DOI] [Google Scholar]
- Deniz A., Sade A., Severcan F., Keskin D., Tezcaner A., Banerjee S. (2010). Celecoxib-loaded Liposomes: Effect of Cholesterol on Encapsulation and In Vitro Release Characteristics. Biosci. Rep. 30 (5), 365–373. 10.1042/bsr20090104 [DOI] [PubMed] [Google Scholar]
- Devrim B., Kara A., Vural İ., Bozkır A. (2016). Lysozyme-loaded Lipid-Polymer Hybrid Nanoparticles: Preparation, Characterization and Colloidal Stability Evaluation. Drug Dev. Ind. Pharm. 42 (11), 1865–1876. 10.1080/03639045.2016.1180392 [DOI] [PubMed] [Google Scholar]
- Dokka S., Toledo D., Shi X., Castranova V., Rojanasakul Y. (2000). Oxygen Radical-Mediated Pulmonary Toxicity Induced by Some Cationic Liposomes. Pharm. Res. 17 (5), 521–525. 10.1023/a:1007504613351 [DOI] [PubMed] [Google Scholar]
- Domínguez A. R., Hidalgo D. O., Garrido R. V., Sánchez E. T. (2005). Liposomal Cytarabine (DepoCyte) for the Treatment of Neoplastic Meningitis. Clin. Transl Oncol. 7 (6), 232–238. 10.1007/bf02710168 [DOI] [PubMed] [Google Scholar]
- Dos Santos N., Mayer L. D., Abraham S. A., Gallagher R. C., Cox K. A. K., Tardi P. G., et al. (2002). Improved Retention of Idarubicin after Intravenous Injection Obtained for Cholesterol-free Liposomes. Biochim. Biophys. Acta (Bba) - Biomembranes 1561 (2), 188–201. 10.1016/s0005-2736(02)00345-0 [DOI] [PubMed] [Google Scholar]
- Drin G., Morello V., Casella J.-F., Gounon P., Antonny B. (2008). Asymmetric Tethering of Flat and Curved Lipid Membranes by a Golgin. Science 320 (5876), 670–673. 10.1126/science.1155821 [DOI] [PubMed] [Google Scholar]
- Drulis-Kawa Z., Dorotkiewicz-Jach A. (2010). Liposomes as Delivery Systems for Antibiotics. Int. J. Pharm. 387 (1-2), 187–198. 10.1016/j.ijpharm.2009.11.033 [DOI] [PubMed] [Google Scholar]
- Dua J., Rana A., Bhandari A. (2012). Liposome: Methods of Preparation and Applications. Int. J. Pharm. Stud. Res. 3 (2), 14–20. [Google Scholar]
- Ducat E., Deprez J., Gillet A., Noël A., Evrard B., Peulen O., et al. (2011). Nuclear Delivery of a Therapeutic Peptide by Long Circulating pH-Sensitive Liposomes: Benefits over Classical Vesicles. Int. J. pharmaceutics 420 (2), 319–332. 10.1016/j.ijpharm.2011.08.034 [DOI] [PubMed] [Google Scholar]
- Düzgünes N., Simões S., Slepushkin V., Pretzer E., Rossi J. J., De Clercq E., et al. (2001). Enhanced Inhibition of HIV-1 Replication in Macrophages by Antisense Oligonucleotides, Ribozymes and Acyclic Nucleoside Phosphonate Analogs Delivered in pH-Sensitive Liposomes. Nucleosides Nucleotides Nucleic Acids 20 (4-7), 515–523. 10.1081/NCN-100002327 [DOI] [PubMed] [Google Scholar]
- Dynarowicz-Łątka P., Seoane R., Minones J., Jr, Velo M., Minones J. (2003). Study of Penetration of Amphotericin B into Cholesterol or Ergosterol Containing Dipalmitoyl Phosphatidylcholine Langmuir Monolayers. Colloids Surf. B: Biointerfaces 27 (2-3), 249–263. [Google Scholar]
- El-Samaligy M. S., Afifi N. N., Mahmoud E. A. (2006). Evaluation of Hybrid Liposomes-Encapsulated Silymarin Regarding Physical Stability and In Vivo Performance. Int. J. Pharm. 319 (1-2), 121–129. 10.1016/j.ijpharm.2006.04.023 [DOI] [PubMed] [Google Scholar]
- El-Samaligy M. S., Afifi N. N., Mahmoud E. A. (2006). Increasing Bioavailability of Silymarin Using a Buccal Liposomal Delivery System: Preparation and Experimental Design Investigation. Int. J. Pharm. 308 (1-2), 140–148. 10.1016/j.ijpharm.2005.11.006 [DOI] [PubMed] [Google Scholar]
- Ellis M., Bernsen R., Ali-Zadeh H., Kristensen J., Hedström U., Poughias L., et al. (2009). A Safety and Feasibility Study Comparing an Intermittent High Dose with a Daily Standard Dose of Liposomal Amphotericin B for Persistent Neutropenic Fever. J. Med. Microbiol. 58 (11), 1474–1485. 10.1099/jmm.0.012401-0 [DOI] [PubMed] [Google Scholar]
- Eloy J. O., Claro de Souza M., Petrilli R., Barcellos J. P. A., Lee R. J., Marchetti J. M. (2014). Liposomes as Carriers of Hydrophilic Small Molecule Drugs: Strategies to Enhance Encapsulation and Delivery. Colloids Surf. B: Biointerfaces 123, 345–363. 10.1016/j.colsurfb.2014.09.029 [DOI] [PubMed] [Google Scholar]
- Epstein H., Gutman D., Cohen-Sela E., Haber E., Elmalak O., Koroukhov N., et al. (2008). Preparation of Alendronate Liposomes for Enhanced Stability and Bioactivity: In Vitro and In Vivo Characterization. Aaps J. 10 (4), 505–515. 10.1208/s12248-008-9060-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evjen T. J., Nilssen E. A., Barnert S., Schubert R., Brandl M., Fossheim S. L. (2011). Ultrasound-mediated Destabilization and Drug Release from Liposomes Comprising Dioleoylphosphatidylethanolamine. Eur. J. Pharm. Sci. 42 (4), 380–386. 10.1016/j.ejps.2011.01.002 [DOI] [PubMed] [Google Scholar]
- Fang J.-Y., Fang C.-L., Liu C.-H., Su Y.-H. (2008). Lipid Nanoparticles as Vehicles for Topical Psoralen Delivery: Solid Lipid Nanoparticles (SLN) versus Nanostructured Lipid Carriers (NLC). Eur. J. Pharmaceutics Biopharmaceutics 70 (2), 633–640. 10.1016/j.ejpb.2008.05.008 [DOI] [PubMed] [Google Scholar]
- Fang J.-Y., Hung C.-F., Hwang T.-L., Huang Y.-L. (2005). Physicochemical Characteristics Andin Vivodeposition of Liposome-Encapsulated tea Catechins by Topical and Intratumor Administrations. J. Drug Target. 13 (1), 19–27. 10.1080/10611860400015977 [DOI] [PubMed] [Google Scholar]
- Farzad N., Barati N., Momtazi-Borojeni A. A., Yazdani M., Arab A., Razazan A., et al. (2019). P435 HER2/neu-Derived Peptide Conjugated to Liposomes Containing DOPE as an Effective Prophylactic Vaccine Formulation for Breast Cancer. Artif. Cell Nanomedicine, Biotechnol. 47 (1), 664–672. 10.1080/21691401.2019.1576702 [DOI] [PubMed] [Google Scholar]
- Fattal E., Couvreur P., Dubernet C. (2004). "Smart" Delivery of Antisense Oligonucleotides by Anionic pH-Sensitive Liposomes. Adv. Drug Deliv. Rev. 56 (7), 931–946. 10.1016/j.addr.2003.10.037 [DOI] [PubMed] [Google Scholar]
- Faustino C. M. C., Calado A. R. T., Garcia-Rio L. (2011). Mixed Micelle Formation between Amino Acid-Based Surfactants and Phospholipids. J. Colloid Interf. Sci. 359 (2), 493–498. 10.1016/j.jcis.2011.04.016 [DOI] [PubMed] [Google Scholar]
- Felnerova D., Viret J.-F., Glück R., Moser C. (2004). Liposomes and Virosomes as Delivery Systems for Antigens, Nucleic Acids and Drugs. Curr. Opin. Biotechnol. 15 (6), 518–529. 10.1016/j.copbio.2004.10.005 [DOI] [PubMed] [Google Scholar]
- Figueroa-Robles A., Antunes-Ricardo M., Guajardo-Flores D. (2020). Encapsulation of Phenolic Compounds with Liposomal Improvement in the Cosmetic Industry. Int. J. Pharm. 593, 120125. 10.1016/j.ijpharm.2020.120125 [DOI] [PubMed] [Google Scholar]
- Franzé S., Selmin F., Samaritani E., Minghetti P., Cilurzo F. (2018). Lyophilization of Liposomal Formulations: Still Necessary, Still Challenging. Pharmaceutics 10 (3), 139. 10.3390/pharmaceutics10030139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman M., Chang E. H., Zhou Q., Pirollo K. F. (2009). Nanodelivery of MRI Contrast Agent Enhances Sensitivity of Detection of Lung Cancer Metastases. Acad. Radiol. 16 (5), 627–637. 10.1016/j.acra.2008.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenzel M., Steffen-Heins A. (2015). Whey Protein Coating Increases Bilayer Rigidity and Stability of Liposomes in Food-like Matrices. Food Chem. 173, 1090–1099. 10.1016/j.foodchem.2014.10.076 [DOI] [PubMed] [Google Scholar]
- Fuchsluger T. A., Hintschich C., Steuhl K.-P., Meller D. (2006). Adjuvante topische Interferon-α-2b-Therapie bei epithelialen Tumoren der Augenoberfläche. Ophthalmologe 103 (2), 124–128. 10.1007/s00347-005-1249-8 [DOI] [PubMed] [Google Scholar]
- Fujisawa T., Miyai H., Hironaka K., Tsukamoto T., Tahara K., Tozuka Y., et al. (2012). Liposomal Diclofenac Eye Drop Formulations Targeting the Retina: Formulation Stability Improvement Using Surface Modification of Liposomes. Int. J. Pharm. 436 (1-2), 564–567. 10.1016/j.ijpharm.2012.07.024 [DOI] [PubMed] [Google Scholar]
- Gabriëls M., Plaizier-Vercammen J. (2003). Physical and Chemical Evaluation of Liposomes, Containing Artesunate. J. Pharm. Biomed. Anal. 31 (4), 655–667. 10.1016/s0731-7085(02)00678-7 [DOI] [PubMed] [Google Scholar]
- Garbuzenko O., Barenholz Y., Priev A. (2005). Effect of Grafted PEG on Liposome Size and on Compressibility and Packing of Lipid Bilayer. Chem. Phys. Lipids 135 (2), 117–129. 10.1016/j.chemphyslip.2005.02.003 [DOI] [PubMed] [Google Scholar]
- Garg T., K. Goyal A. (2014). Liposomes: Targeted and Controlled Delivery System. Ddl 4 (1), 62–71. 10.2174/22103031113036660015 [DOI] [Google Scholar]
- Ghanbarzadeh S., Valizadeh H., Zakeri-Milani P. (2013). The Effects of Lyophilization on the Physico-Chemical Stability of Sirolimus Liposomes. Adv. Pharm. Bull. 3 (1), 25–29. 10.5681/apb.2013.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazizadeh E., Neshastehriz A. (2020). Different Liposome Patterns to Detection of Acute Leukemia Based on Electrochemical Cell Sensor. Analytica Chim. Acta. [DOI] [PubMed] [Google Scholar]
- Giulimondi F., Digiacomo L., Pozzi D., Palchetti S., Vulpis E., Capriotti A. L., et al. (2019). Interplay of Protein corona and Immune Cells Controls Blood Residency of Liposomes. Nat. Commun. 10 (1), 3686–3711. 10.1038/s41467-019-11642-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto T., Morishita M., Nishimura K., Nakanishi M., Kato A., Ehara J., et al. (2006). Novel Mucosal Insulin Delivery Systems Based on Fusogenic Liposomes. Pharm. Res. 23 (2), 384–391. 10.1007/s11095-005-9175-7 [DOI] [PubMed] [Google Scholar]
- Gross N., Ranjbar M., Evers C., Hua J., Martin G., Schulze B., et al. (2013). Choroidal Neovascularization Reduced by Targeted Drug Delivery with Cationic Liposome-Encapsulated Paclitaxel or Targeted Photodynamic Therapy with Verteporfin Encapsulated in Cationic Liposomes. Mol. Vis. 19, 54–61. [PMC free article] [PubMed] [Google Scholar]
- Gullapalli R. R., Demirel M. C., Butler P. J. (2008). Molecular Dynamics Simulations of DiI-C18(3) in a DPPC Lipid Bilayer. Phys. Chem. Chem. Phys. 10 (24), 3548–3560. 10.1039/b716979e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadavi R., Jafari S. M., Katouzian I. (2020). Nanoliposomal Encapsulation of Saffron Bioactive Compounds; Characterization and Optimization. Int. J. Biol. Macromolecules 164, 4046–4053. 10.1016/j.ijbiomac.2020.09.028 [DOI] [PubMed] [Google Scholar]
- Hahn V., Friedt W. (2012). Important Characters by Recombinant DNA Technology. Prog. Bot. Stuctural Bot. Physiol. Genet. Taxonomy Geobotany/Fortschritte der Botanik Struktur Physiologie Genetik Systematik Geobotanik 53, 181. [Google Scholar]
- Hashemzadeh H., Javadi H., Darvishi M. H. (2020). Study of Structural Stability and Formation Mechanisms in DSPC and DPSM Liposomes: A Coarse-Grained Molecular Dynamics Simulation. Sci. Rep. 10 (1), 1837–1910. 10.1038/s41598-020-58730-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashemzadeh H., Javadi H., Darvishi M. H. (2020). Study of Structural Stability and Formation Mechanisms in DSPC and DPSM Liposomes: A Coarse-Grained Molecular Dynamics Simulation. Sci. Rep. 10 (1), 1837. 10.1038/s41598-020-58730-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hathout R. M., Mansour S., Mortada N. D., Guinedi A. S. (2007). Liposomes as an Ocular Delivery System for Acetazolamide: In Vitro and In Vivo Studies. Aaps Pharmscitech 8 (1), E1–E12. 10.1208/pt0801001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J., Evers D. L., O’Leary T. J., Mason J. T. (2012). Immunoliposome-PCR: a Generic Ultrasensitive Quantitative Antigen Detection System. J. nanobiotechnology 10 (1), 26. 10.1186/1477-3155-10-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He K., Tang M. (2018). Safety of Novel Liposomal Drugs for Cancer Treatment: Advances and Prospects. Chemico-Biological Interactions 295, 13–19. 10.1016/j.cbi.2017.09.006 [DOI] [PubMed] [Google Scholar]
- Hilmer S. N., Cogger V. C., Muller M., Le Couteur D. G. (2004). The Hepatic Pharmacokinetics of Doxorubicin and Liposomal Doxorubicin. Drug Metab. Dispos 32 (8), 794–799. 10.1124/dmd.32.8.794 [DOI] [PubMed] [Google Scholar]
- Hirano M., Nakamura S., Mitsunaga F., Okada M., Shimuzu K., Imamura T. (2002). Transfer of Maternally Administered Fusogenic Liposome-DNA Complexes into Monkey Fetuses in a Pregnancy Model. J. Gene Med. 4 (5), 560–566. 10.1002/jgm.289 [DOI] [PubMed] [Google Scholar]
- Ho J. A., Zeng S. C., Huang M. R., Kuo H. Y. (2006). Development of Liposomal Immunosensor for the Measurement of Insulin with Femtomole Detection. Anal. Chim. Acta 556 (1), 127–132. 10.1016/j.aca.2005.08.074 [DOI] [PubMed] [Google Scholar]
- Homann H.-H., Rosbach O., Moll W., Vogt P. M., Germann G., Hopp M., et al. (2007). A Liposome Hydrogel with Polyvinyl-Pyrrolidone Iodine in the Local Treatment of Partial-Thickness Burn Wounds. Ann. Plast. Surg. 59 (4), 423–427. 10.1097/sap.0b013e3180326fcf [DOI] [PubMed] [Google Scholar]
- Hong S.-S., Oh K. T., Choi H.-G., Lim S.-J. (2019). Liposomal Formulations for Nose-To-Brain Delivery: Recent Advances and Future Perspectives. Pharmaceutics 11 (10), 540. 10.3390/pharmaceutics11100540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosny K. M. (2010). Ciprofloxacin as Ocular Liposomal Hydrogel. Aaps Pharmscitech 11 (1), 241–246. 10.1208/s12249-009-9373-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q., Bally M. B., Madden T. D. (2002). Subcellular Trafficking of Antisense Oligonucleotides and Down-Regulation of Bcl-2 Gene Expression in Human Melanoma Cells Using a Fusogenic Liposome Delivery System. Nucleic Acids Res. 30 (16), 3632–3641. 10.1093/nar/gkf448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua S. (2013). Targeting Sites of Inflammation: Intercellular Adhesion Molecule-1 as a Target for Novel Inflammatory Therapies. Front. Pharmacol. 4, 127. 10.3389/fphar.2013.00127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F.-Y. J., Hung C.-C., Chang C.-W., Chao J.-H., Hsieh B.-T. (2018). Evaluation of Injectable Chitosan-Based Co-cross-linking Hydrogel for Local Delivery of 188Re-LIPO-DOX to Breast-Tumor-Bearing Mouse Model. Anticancer Res. 38 (8), 4651–4659. 10.21873/anticanres.12770 [DOI] [PubMed] [Google Scholar]
- Huh S. Y., Shin J. W., Na J. I., Huh C. H., Youn S. W., Park K. C. (2010). Efficacy and Safety of Liposome-Encapsulated 4-N-Butylresorcinol 0.1% Cream for the Treatment of Melasma: A Randomized Controlled Split-Face Trial. J. Dermatol. 37 (4), 311–315. 10.1111/j.1346-8138.2010.00787.x [DOI] [PubMed] [Google Scholar]
- Husain S., Capitano B., Corcoran T., Studer S. M., Crespo M., Johnson B., et al. (2010). Intrapulmonary Disposition of Amphotericin B after Aerosolized Delivery of Amphotericin B Lipid Complex (Abelcet; ABLC) in Lung Transplant Recipients. Transplantation 90 (11), 1215–1219. 10.1097/tp.0b013e3181f995ea [DOI] [PubMed] [Google Scholar]
- Ib G., Corredig M. (2013). Storage Stability and Physical Characteristics of tea-polyphenol-bearing Nanoliposomes Prepared with Milk Fat Globule Membrane Phospholipids. J. Agric. Food Chem. 61 (13), 3242–3251. [DOI] [PubMed] [Google Scholar]
- Ichikawa K., Hikita T., Maeda N., Takeuchi Y., Namba Y., Oku N. (2004). PEGylation of Liposome Decreases the Susceptibility of Liposomal Drug in Cancer Photodynamic Therapy. Biol. Pharm. Bull. 27 (3), 443–444. 10.1248/bpb.27.443 [DOI] [PubMed] [Google Scholar]
- Ichikawa K., Hikita T., Maeda N., Yonezawa S., Takeuchi Y., Asai T., et al. (2005). Antiangiogenic Photodynamic Therapy (PDT) by Using Long-Circulating Liposomes Modified with Peptide Specific to Angiogenic Vessels. Biochim. Biophys. Acta (Bba) - Biomembranes 1669 (1), 69–74. 10.1016/j.bbamem.2005.02.003 [DOI] [PubMed] [Google Scholar]
- Jacoby G., Cohen K., Barkan K., Talmon Y., Peer D., Beck R. (2015). Metastability in Lipid Based Particles Exhibits Temporally Deterministic and Controllable Behavior. Sci. Rep. 5, 9481. 10.1038/srep09481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain A., Jain S. K. (2016). In Vitro release Kinetics Model Fitting of Liposomes: An Insight. Chem. Phys. Lipids 201, 28–40. 10.1016/j.chemphyslip.2016.10.005 [DOI] [PubMed] [Google Scholar]
- Jain A. K., Thanki K., Jain S. (2014). Solidified Self-Nanoemulsifying Formulation for Oral Delivery of Combinatorial Therapeutic Regimen: Part I. Formulation Development, Statistical Optimization, and In Vitro Characterization. Pharm. Res. 31 (4), 923–945. 10.1007/s11095-013-1213-2 [DOI] [PubMed] [Google Scholar]
- Jain M., Zellweger M., Frobert A., Valentin J., Bergh H. v. d., Wagnières G., et al. (2016). Intra-Arterial Drug and Light Delivery for Photodynamic Therapy Using Visudyne: Implication for Atherosclerotic Plaque Treatment. Front. Physiol. 7, 400. 10.3389/fphys.2016.00400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain N., Gupta B. P., Thakur N., Jain R., Banweer J., Jain D. K., et al. (2010). Phytosome: a Novel Drug Delivery System for Herbal Medicine. Int. J. Pharm. Sci. Drug Res. 2 (4), 224–228. [Google Scholar]
- Jeon M., Kim G., Lee W., Im H-J. (2020). Development of Theranostic PEGylated Liposomal Au-Liposome for Effective Tumor Passive Targeting and Photothermal Therapy. Soc. Nucl. Med 61, 1076. [Google Scholar]
- Jimbo T., Sakuma Y., Urakami N., Ziherl P., Imai M. (2016). Role of Inverse-Cone-Shape Lipids in Temperature-Controlled Self-Reproduction of Binary Vesicles. Biophysical J. 110 (7), 1551–1562. 10.1016/j.bpj.2016.02.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez-Escrig A., Sánchez-Muniz F. J. (2000). Dietary Fibre from Edible Seaweeds: Chemical Structure, Physicochemical Properties and Effects on Cholesterol Metabolism. Nutr. Res. 20 (4), 585–598. 10.1016/s0271-5317(00)00149-4 [DOI] [Google Scholar]
- Johnston M. J. W., Semple S. C., Klimuk S. K., Ansell S., Maurer N., Cullis P. R. (2007). Characterization of the Drug Retention and Pharmacokinetic Properties of Liposomal Nanoparticles Containing Dihydrosphingomyelin. Biochim. Biophys. Acta (Bba) - Biomembranes 1768 (5), 1121–1127. 10.1016/j.bbamem.2007.01.019 [DOI] [PubMed] [Google Scholar]
- Johnstone S. A., Masin D., Mayer L., Bally M. B. (2001). Surface-associated Serum Proteins Inhibit the Uptake of Phosphatidylserine and Poly(ethylene Glycol) Liposomes by Mouse Macrophages. Biochim. Biophys. Acta (Bba) - Biomembranes 1513 (1), 25–37. 10.1016/s0005-2736(01)00292-9 [DOI] [PubMed] [Google Scholar]
- Joly F., Ray-Coquard I., Fabbro M., Donoghoe M., Boman K., Sugimoto A., et al. (2011). Decreased Hypersensitivity Reactions with Carboplatin-Pegylated Liposomal Doxorubicin Compared to Carboplatin-Paclitaxel Combination: Analysis from the GCIG CALYPSO Relapsing Ovarian Cancer Trial. Gynecol. Oncol. 122 (2), 226–232. 10.1016/j.ygyno.2011.04.019 [DOI] [PubMed] [Google Scholar]
- Joshi S., Hussain M. T., Roces C. B., Anderluzzi G., Kastner E., Salmaso S., et al. (2016). Microfluidics Based Manufacture of Liposomes Simultaneously Entrapping Hydrophilic and Lipophilic Drugs. Int. J. pharmaceutics 514 (1), 160–168. 10.1016/j.ijpharm.2016.09.027 [DOI] [PubMed] [Google Scholar]
- Jung H.-S., Ishii H., Shimanouchi T., Umakoshi H., Kuboi R. (2007). Immobilized-liposome Sensor System for Detection of Proteins under Stress Conditions. Membrane 32 (5), 294–301. 10.5360/membrane.32 [DOI] [Google Scholar]
- Jung J. H., Ree M., Kim H. (2006). Acid-and Base-Catalyzed Hydrolyses of Aliphatic Polycarbonates and Polyesters. Catal. Today 115 (1-4), 283–287. 10.1016/j.cattod.2006.02.060 [DOI] [Google Scholar]
- Jung S.-Y., Holden M. A., Cremer P. S., Collier C. P. (2005). Two-Component Membrane Lithography via Lipid Backfilling. ChemPhysChem. 6 (3), 423–426. 10.1002/cphc.200400540 [DOI] [PubMed] [Google Scholar]
- Jung S. H., Jung S. H., Seong H., Cho S. H., Jeong K. S., Shin B. C. (2009). Polyethylene Glycol-Complexed Cationic Liposome for Enhanced Cellular Uptake and Anticancer Activity. Int. J. Pharm. 382 (1-2), 254–261. 10.1016/j.ijpharm.2009.08.002 [DOI] [PubMed] [Google Scholar]
- Park J. W., Benz C. C., Martin F. J. (2004). in Future Directions of Liposome-And Immunoliposome-Based Cancer Therapeutics. Seminars in Oncology (Elsevier; ). [DOI] [PubMed] [Google Scholar]
- Kafle A., Akamatsu M., Bhadani A., Sakai K., Kaise C., Kaneko T., et al. (2020). Phase Behavior of the Bilayers Containing Hydrogenated Soy Lecithin and β-sitosteryl Sulfate. Langmuir. 10.1021/acs.langmuir.0c00472 [DOI] [PubMed] [Google Scholar]
- Kang S. H., Cho H.-J., Shim G., Lee S., Kim S.-H., Choi H.-G., et al. (2011). Cationic Liposomal Co-delivery of Small Interfering RNA and a MEK Inhibitor for Enhanced Anticancer Efficacy. Pharm. Res. 28 (12), 3069–3078. 10.1007/s11095-011-0569-4 [DOI] [PubMed] [Google Scholar]
- Karmali P. P., Chaudhuri A. (2007). Cationic Liposomes as Non-viral Carriers of Gene Medicines: Resolved Issues, Open Questions, and Future Promises. Med. Res. Rev. 27 (5), 696–722. 10.1002/med.20090 [DOI] [PubMed] [Google Scholar]
- Kashapov R., Ibragimova A., Pavlov R., Gabdrakhmanov D., Kashapova N., Burilova E., et al. (2021). Nanocarriers for Biomedicine: From Lipid Formulations to Inorganic and Hybrid Nanoparticles. Ijms 22 (13), 7055. 10.3390/ijms22137055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur G., Garg T., Rath G., Goyal A. K. (2016). Archaeosomes: an Excellent Carrier for Drug and Cell Delivery. Drug Deliv. 23 (7), 2497–2512. 10.3109/10717544.2015.1019653 [DOI] [PubMed] [Google Scholar]
- Khadke S., Roces C. B., Donaghey R., Giacobbo V., Su Y., Perrie Y. (2020). Scalable Solvent-free Production of Liposomes. J. Pharm. Pharmacol. 72 (10), 1328–1340. 10.1111/jphp.13329 [DOI] [PubMed] [Google Scholar]
- Khosravi-Darani K., Zahed O., Aarabi S. (2018). Antioxidant activity of Zataria multiflora boiss. Essential oil encapsulated in nanoliposome in broth media and minced beef. Pak. J. Biotechnol. 15 (2), 365–375. [Google Scholar]
- Kidd P. M. (2009). Bioavailability and Activity of Phytosome Complexes from Botanical Polyphenols: the Silymarin, Curcumin, green tea, and Grape Seed Extracts. Altern. Med. Rev. 14 (3), 226–246. [PubMed] [Google Scholar]
- Kitayama H., Takechi Y., Tamai N., Matsuki H., Yomota C., Saito H. (2014). Thermotropic Phase Behavior of Hydrogenated Soybean Phosphatidylcholine-Cholesterol Binary Liposome Membrane. Chem. Pharm. Bull. 62 (1), 58–63. 10.1248/cpb.c13-00587 [DOI] [PubMed] [Google Scholar]
- Kleusch C., Hersch N., Hoffmann B., Merkel R., Csiszár A. (2012). Fluorescent Lipids: Functional Parts of Fusogenic Liposomes and Tools for Cell Membrane Labeling and Visualization. Molecules 17 (1), 1055–1073. 10.3390/molecules17011055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kshirsagar N. A., Pandya S. K., Kirodian G. B., Sanath S. (2005). Liposomal Drug Delivery System from Laboratory to Clinic. J. Postgrad. Med. 51 Suppl 1 (5), S5–S15. [PubMed] [Google Scholar]
- Kunisawa J., Masuda T., Katayama K., Yoshikawa T., Tsutsumi Y., Akashi M., et al. (2005). Fusogenic Liposome Delivers Encapsulated Nanoparticles for Cytosolic Controlled Gene Release. J. controlled release 105 (3), 344–353. 10.1016/j.jconrel.2005.03.020 [DOI] [PubMed] [Google Scholar]
- Kunzelmann-Marche C., Freyssinet J.-M., Martínez M. C. (2002). Loss of Plasma Membrane Phospholipid Asymmetry Requires Raft Integrity. J. Biol. Chem. 277 (22), 19876–19881. 10.1074/jbc.m200324200 [DOI] [PubMed] [Google Scholar]
- Laouini A., Jaafar-Maalej C., Limayem-Blouza I., Sfar S., Charcosset C., Fessi H. (2012). Preparation, Characterization and Applications of Liposomes: State of the Art. J Coll. Sci. Biotechnol. 1 (2), 147–168. 10.1166/jcsb.2012.1020 [DOI] [Google Scholar]
- Lebègue E., Anderson C. M., Dick J. E., Webb L. J., Bard A. J. (2015). Electrochemical Detection of Single Phospholipid Vesicle Collisions at a Pt Ultramicroelectrode. Langmuir 31 (42), 11734–11739. 10.1021/acs.langmuir.5b03123 [DOI] [PubMed] [Google Scholar]
- Lee C. M., Flynn R., Hollywood J. A., Scallan M. F., Harrison P. T. (2012). Correction of the ΔF508 Mutation in the Cystic Fibrosis Transmembrane Conductance Regulator Gene by Zinc-Finger Nuclease Homology-Directed Repair. BioResearch open access 1 (3), 99–108. 10.1089/biores.2012.0218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Kim H.-J., Kim J. (2008). Polydiacetylene Liposome Arrays for Selective Potassium Detection. J. Am. Chem. Soc. 130 (15), 5010–5011. 10.1021/ja709996c [DOI] [PubMed] [Google Scholar]
- Lee S. C., Lee K. E., Kim J. J., Lim S. H. (2005). The Effect of Cholesterol in the Liposome Bilayer on the Stabilization of Incorporated Retinol. J. Liposome Res. 15 (3-4), 157–166. 10.1080/08982100500364131 [DOI] [PubMed] [Google Scholar]
- Li J., Wang X., Zhang T., Wang C., Huang Z., Luo X., et al. (2015). A Review on Phospholipids and Their Main Applications in Drug Delivery Systems. Asian J. Pharm. Sci. 10 (2), 81–98. 10.1016/j.ajps.2014.09.004 [DOI] [Google Scholar]
- Li M., Du C., Guo N., Teng Y., Meng X., Sun H., et al. (2019). Composition Design and Medical Application of Liposomes. Eur. J. Med. Chem. 164, 640–653. 10.1016/j.ejmech.2019.01.007 [DOI] [PubMed] [Google Scholar]
- Liang L. P. T. H. J., Chung T. W., Liu Y. Y. H. D. Z. (2007). Liposomes Incorporated with Cholesterol for Drug Release Triggered by Magnetic Field. J. Med. Biol. Eng. 27 (1), 29–34. [Google Scholar]
- Liang X. F., Wang H. J., Luo H., Tian H., Zhang B. B., Hao L. J., et al. (2008). Characterization of Novel Multifunctional Cationic Polymeric Liposomes Formed from Octadecyl Quaternized Carboxymethyl Chitosan/cholesterol and Drug Encapsulation. Langmuir 24 (14), 7147–7153. 10.1021/la703775a [DOI] [PubMed] [Google Scholar]
- Lira R. B., Seabra M. A. B. L., Matos A. L. L., Vasconcelos J. V., Bezerra D. P., de Paula E., et al. (2013). Studies on Intracellular Delivery of Carboxyl-Coated CdTe Quantum Dots Mediated by Fusogenic Liposomes. J. Mater. Chem. B 1 (34), 4297–4305. 10.1039/c3tb20245c [DOI] [PubMed] [Google Scholar]
- Liu B., Krieger M. (2002). Highly Purified Scavenger Receptor Class B, Type I Reconstituted into Phosphatidylcholine/cholesterol Liposomes Mediates High Affinity High Density Lipoprotein Binding and Selective Lipid Uptake. J. Biol. Chem. 277 (37), 34125–34135. 10.1074/jbc.m204265200 [DOI] [PubMed] [Google Scholar]
- Liu W. L., Zou M. Z., Qin S. Y., Cheng Y. J., Ma Y. H., Sun Y. X., et al. (2020). Recent Advances of Cell Membrane‐Coated Nanomaterials for Biomedical Applications. Adv. Funct. Mater. 30 (39), 2003559. 10.1002/adfm.202003559 [DOI] [Google Scholar]
- Liu X., Madhankumar A. B., Miller P. A., Duck K. A., Hafenstein S., Rizk E., et al. (2016). MRI Contrast Agent for Targeting Glioma: Interleukin-13 Labeled Liposome Encapsulating Gadolinium-DTPA. Neuro Oncol. 18 (5), 691–699. 10.1093/neuonc/nov263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Castro Bravo K. M., Liu J. (2021). Targeted Liposomal Drug Delivery: a Nanoscience and Biophysical Perspective. Nanoscale Horiz. 6 (2), 78–94. 10.1039/d0nh00605j [DOI] [PubMed] [Google Scholar]
- López-Pinto J. M., González-Rodríguez M. L., Rabasco A. M. (2005). Effect of Cholesterol and Ethanol on Dermal Delivery from DPPC Liposomes. Int. J. pharmaceutics 298 (1), 1–12. 10.1016/j.ijpharm.2005.02.021 [DOI] [PubMed] [Google Scholar]
- López-Revuelta A., Sánchez-Gallego J. I., Hernández-Hernández A., Sánchez-Yagüe J., Llanillo M. (2006). Membrane Cholesterol Contents Influence the Protective Effects of Quercetin and Rutin in Erythrocytes Damaged by Oxidative Stress. Chemico-biological interactions 161 (1), 79–91. 10.1016/j.cbi.2006.03.004 [DOI] [PubMed] [Google Scholar]
- Lowis S., Lewis I., Lewis I., Elsworth A., Weston C., Doz F., et al. (2006). A Phase I Study of Intravenous Liposomal Daunorubicin (DaunoXome) in Paediatric Patients with Relapsed or Resistant Solid Tumours. Br. J. Cancer 95 (5), 571–580. 10.1038/sj.bjc.6603288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu F. S. H., Nielsen N. S., Baron C. P., Jensen L. H. S., Jacobsen C. (2012). Physico-chemical Properties of marine Phospholipid Emulsions. J. Am. Oil Chem. Soc. 89 (11), 2011–2024. 10.1007/s11746-012-2105-z [DOI] [Google Scholar]
- Lv H., Zhang S., Wang B., Cui S., Yan J. (2006). Toxicity of Cationic Lipids and Cationic Polymers in Gene Delivery. J. Controlled Release 114 (1), 100–109. 10.1016/j.jconrel.2006.04.014 [DOI] [PubMed] [Google Scholar]
- Maeda N., Takeuchi Y., Takada M., Sadzuka Y., Namba Y., Oku N. (2004). Anti-neovascular Therapy by Use of Tumor Neovasculature-Targeted Long-Circulating Liposome. J. controlled release 100 (1), 41–52. 10.1016/j.jconrel.2004.07.033 [DOI] [PubMed] [Google Scholar]
- Manconi M., Marongiu F., Castangia I., Manca M. L., Caddeo C., Tuberoso C. I. G., et al. (2016). Polymer-associated Liposomes for the Oral Delivery of Grape Pomace Extract. Colloids Surf. B: biointerfaces 146, 910–917. 10.1016/j.colsurfb.2016.07.043 [DOI] [PubMed] [Google Scholar]
- Mannino R., Lu R. (2018). Cochleates Made with Soy Phosphatidylserine. Google Patents. [Google Scholar]
- Mansoori M., Agrawal S., Jawade S., Khan M. (2012). A Review on Liposome. Int. J. Adv. Res. Pharm. Bio-sciences 2 (4), 453–464. [Google Scholar]
- Maritim S., Boulas P., Lin Y. (2021). Comprehensive Analysis of Liposome Formulation Parameters and Their Influence on Encapsulation, Stability and Drug Release in Glibenclamide Liposomes. Int. J. Pharmaceutics 592, 120051. 10.1016/j.ijpharm.2020.120051 [DOI] [PubMed] [Google Scholar]
- Marsh D. (2001). Elastic Constants of Polymer-Grafted Lipid Membranes. Biophysical J. 81 (4), 2154–2162. 10.1016/s0006-3495(01)75863-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matole V., Shegaonkar A., Kumbhar S., Thorat Y., Hosmani A. (2020). Need of Liposomes as a Novel Drug Delivery System. Res. J. Pharm. Dosage Forms Technology 12 (4), 285–294. 10.5958/0975-4377.2020.00047.6 [DOI] [Google Scholar]
- Matsuoka S., Murata M. (2002). Cholesterol Markedly Reduces Ion Permeability Induced by Membrane-Bound Amphotericin B.. Biochim. Biophys. Acta (Bba) - Biomembranes 1564 (2), 429–434. 10.1016/s0005-2736(02)00491-1 [DOI] [PubMed] [Google Scholar]
- Mehnert W., Mäder K. (2012). Solid Lipid Nanoparticles. Adv. Drug Deliv. Rev. 64, 83–101. 10.1016/j.addr.2012.09.021 [DOI] [PubMed] [Google Scholar]
- Metselaar J. M., Wauben M. H. M., Wagenaar-Hilbers J. P. A., Boerman O. C., Storm G. (2003). Complete Remission of Experimental Arthritis by Joint Targeting of Glucocorticoids with Long-Circulating Liposomes. Arthritis Rheum. 48 (7), 2059–2066. 10.1002/art.11140 [DOI] [PubMed] [Google Scholar]
- Meure L. A., Foster N. R., Dehghani F. (2008). Conventional and Dense Gas Techniques for the Production of Liposomes: a Review. Aaps Pharmscitech 9 (3), 798. 10.1208/s12249-008-9097-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao L., Nielsen M., Thewalt J., Ipsen J. H., Bloom M., Zuckermann M. J., et al. (2002). From Lanosterol to Cholesterol: Structural Evolution and Differential Effects on Lipid Bilayers. Biophysical J. 82 (3), 1429–1444. 10.1016/s0006-3495(02)75497-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghimi S. M., Hunter A. C., Murray J. C. (2001). Long-circulating and Target-specific Nanoparticles: Theory to Practice. Pharmacol. Rev. 53 (2), 283–318. [PubMed] [Google Scholar]
- Moghimi S. M., Patel H. M. (2002). Modulation of Murine Liver Macrophage Clearance of Liposomes by Diethylstilbestrol. The Effect of Vesicle Surface Charge and a Role for the Complement Receptor Mac-1 (CD11b/CD18) of Newly Recruited Macrophages in Liposome Recognition. J. Control. Release 78 (1-3), 55–65. 10.1016/s0168-3659(01)00481-3 [DOI] [PubMed] [Google Scholar]
- Moghimi S. M., Szebeni J. (2003). Stealth Liposomes and Long Circulating Nanoparticles: Critical Issues in Pharmacokinetics, Opsonization and Protein-Binding Properties. Prog. lipid Res. 42 (6), 463–478. 10.1016/s0163-7827(03)00033-x [DOI] [PubMed] [Google Scholar]
- Montaseri H., Kruger C. A., Abrahamse H. (2020). Review: Organic Nanoparticle Based Active Targeting for Photodynamic Therapy Treatment of Breast Cancer Cells. Oncotarget 11 (22), 2120–2136. 10.18632/oncotarget.27596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozafari M. R. (2005). Liposomes: an Overview of Manufacturing Techniques. Cell Mol Biol Lett 10 (4), 711–719. [PubMed] [Google Scholar]
- Mozafari M. R., Reed C. J., Rostron C. (2007). Cytotoxicity Evaluation of Anionic Nanoliposomes and Nanolipoplexes Prepared by the Heating Method without Employing Volatile Solvents and Detergents. Pharmazie 62 (3), 205–209. [PubMed] [Google Scholar]
- Müller R. H., Mäder K., Gohla S. (2000). Solid Lipid Nanoparticles (SLN) for Controlled Drug Delivery - a Review of the State of the Art. Eur. J. Pharm. Biopharm. 50 (1), 161–177. 10.1016/s0939-6411(00)00087-4 [DOI] [PubMed] [Google Scholar]
- Müller R. H., Radtke M., Wissing S. A. (2002). Solid Lipid Nanoparticles (SLN) and Nanostructured Lipid Carriers (NLC) in Cosmetic and Dermatological Preparations. Adv. Drug Deliv. Rev. 54, S131–S155. 10.1016/s0169-409x(02)00118-7 [DOI] [PubMed] [Google Scholar]
- Müller R., Petersen R., Hommoss A., Pardeike J. (2007). Nanostructured Lipid Carriers (NLC) in Cosmetic Dermal Products☆. Adv. Drug Deliv. Rev. 59 (6), 522–530. 10.1016/j.addr.2007.04.012 [DOI] [PubMed] [Google Scholar]
- Nagarajan R. (2002). Molecular Packing Parameter and Surfactant Self-Assembly: The Neglected Role of the Surfactant Tail†. Langmuir 18 (1), 31–38. 10.1021/la010831y [DOI] [Google Scholar]
- Nakanishi T., Hayashi A., Kunisawa J., Tsutsumi Y., Tanaka K., Yashiro-Ohtani Y., et al. (2000). Fusogenic Liposomes Efficiently Deliver Exogenous Antigen through the Cytoplasm into the MHC Class I Processing Pathway. Eur. J. Immunol. 30 (6), 1740–1747. [DOI] [PubMed] [Google Scholar]
- Naskar A., Kim K.-s. (2021). Potential novel food-related and biomedical applications of nanomaterials combined with bacteriocins. Pharmaceutics 13, 86. 10.3390/pharmaceutics13010086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasrabadi H. T., Abbasi E., Davaran S., Kouhi M., Akbarzadeh A. (2016). Bimetallic Nanoparticles: Preparation, Properties, and Biomedical Applications. Artif. Cell Nanomedicine, Biotechnol. 44 (1), 376–380. 10.3109/21691401.2014.953632 [DOI] [PubMed] [Google Scholar]
- Nasti T. H., Khan M. A., Owais M. (2006). Enhanced Efficacy of pH-Sensitive Nystatin Liposomes against Cryptococcus Neoformans in Murine Model. J. Antimicrob. Chemother. 57 (2), 349–352. 10.1093/jac/dki454 [DOI] [PubMed] [Google Scholar]
- Noble G. T., Stefanick J. F., Ashley J. D., Kiziltepe T., Bilgicer B. (2014). Ligand-targeted Liposome Design: Challenges and Fundamental Considerations. Trends Biotechnology 32 (1), 32–45. 10.1016/j.tibtech.2013.09.007 [DOI] [PubMed] [Google Scholar]
- North S., Butts C. (2005). Vaccination with BLP25 Liposome Vaccine to Treat Non-small Cell Lung and Prostate Cancers. Expert Rev. Vaccin. 4 (3), 249–257. 10.1586/14760584.4.3.249 [DOI] [PubMed] [Google Scholar]
- Nunes S. S., Fernandes R. S., Cavalcante C. H., da Costa César I., Leite E. A., Lopes S. C. A., et al. (2019). Influence of PEG Coating on the Biodistribution and Tumor Accumulation of pH-Sensitive Liposomes. Drug Deliv. Transl. Res. 9 (1), 123–130. 10.1007/s13346-018-0583-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohvo-Rekilà H., Ramstedt B., Leppimäki P., Slotte J. P. (2002). Cholesterol Interactions with Phospholipids in Membranes. Prog. lipid Res. 41 (1), 66–97. 10.1016/s0163-7827(01)00020-0 [DOI] [PubMed] [Google Scholar]
- Okamoto Y., Masum S. M., Miyazawa H., Yamazaki M. (2008). Low-pH-induced Transformation of Bilayer Membrane into Bicontinuous Cubic Phase in Dioleoylphosphatidylserine/monoolein Membranes. Langmuir 24 (7), 3400–3406. 10.1021/la7036795 [DOI] [PubMed] [Google Scholar]
- Otake K., Imura T., Sakai H., Abe M. (2001). Development of a New Preparation Method of Liposomes Using Supercritical Carbon Dioxide. langmuir 17 (13), 3898–3901. 10.1021/la010122k [DOI] [Google Scholar]
- Pan L. G., Tomás M. C., Añón M. C. (2002). Effect of sunflower Lecithins on the Stability of Water-In-Oil and Oil-In-Water Emulsions. J. Surfact Deterg 5 (2), 135–143. 10.1007/s11743-002-0213-1 [DOI] [Google Scholar]
- Panahi Y., Farshbaf M., Mohammadhosseini M., Mirahadi M., Khalilov R., Saghfi S., et al. (2017). Recent Advances on Liposomal Nanoparticles: Synthesis, Characterization and Biomedical Applications. Artif. Cell Nanomedicine, Biotechnol. 45 (4), 788–799. 10.1080/21691401.2017.1282496 [DOI] [PubMed] [Google Scholar]
- Papagiannaros A., Hatziantoniou S., Dimas K., Papaioannou G. T., Demetzos C. (2006). A Liposomal Formulation of Doxorubicin, Composed of Hexadecylphosphocholine (HePC): Physicochemical Characterization and Cytotoxic Activity against Human Cancer Cell Lines. Biomed. Pharmacother. 60 (1), 36–42. 10.1016/j.biopha.2005.09.001 [DOI] [PubMed] [Google Scholar]
- Pastorino F., Brignole C., Marimpietri D., Cilli M., Gambini C., Ribatti D., et al. (2003). Vascular Damage and Anti-angiogenic Effects of Tumor Vessel-Targeted Liposomal Chemotherapy. Cancer Res. 63 (21), 7400–7409. [PubMed] [Google Scholar]
- Patel N., Panda S. (2012). Liposome Drug Delivery System: a Critic Review. JPSBR 2 (4), 169–175. [Google Scholar]
- Pavelić Z., Skalko-Basnet N., Jalsenjak I. (2005). Characterisation and In Vitro Evaluation of Bioadhesive Liposome Gels for Local Therapy of Vaginitis. Int. J. Pharm. 301 (1-2), 140–148. 10.1016/j.ijpharm.2005.05.022 [DOI] [PubMed] [Google Scholar]
- Pawar A., Bothiraja C., Shaikh K., Mali A. (2015). An Insight into Cochleates, a Potential Drug Delivery System. RSC Adv. 5 (99), 81188–81202. 10.1039/c5ra08550k [DOI] [Google Scholar]
- Pawar H. A., Bhangale B. D. (2015). Phytosome as a Novel Biomedicine: a Microencapsulated Drug Delivery System. J. Bioanal. Biomed. 7 (1), 6–12. [Google Scholar]
- Peravali R., Brock R., Bright E., Mills P., Petty D., Alberts J. (2014). Enhancing the Enhanced Recovery Program in Colorectal Surgery - Use of Extended-Release Epidural Morphine (DepoDur). Ann. Coloproctol. 30 (4), 186. 10.3393/ac.2014.30.4.186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phuphanich S., Maria B., Braeckman R., Chamberlain M. (2007). A Pharmacokinetic Study of Intra-CSF Administered Encapsulated Cytarabine (DepoCyt) for the Treatment of Neoplastic Meningitis in Patients with Leukemia, Lymphoma, or Solid Tumors as Part of a Phase III Study. J. Neurooncol. 81 (2), 201–208. 10.1007/s11060-006-9218-x [DOI] [PubMed] [Google Scholar]
- Pinilla C. M. B., Reque P. M., Brandelli A. (2020). Effect of Oleic Acid, Cholesterol, and Octadecylamine on Membrane Stability of Freeze-Dried Liposomes Encapsulating Natural Antimicrobials. Food Bioproc. Technol 13 (4), 599–610. 10.1007/s11947-020-02419-8 [DOI] [Google Scholar]
- Portet T., Dimova R. (2010). A New Method for Measuring Edge Tensions and Stability of Lipid Bilayers: Effect of Membrane Composition. Biophysical J. 99 (10), 3264–3273. 10.1016/j.bpj.2010.09.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portilla S., Fernández L., Gutiérrez D., Rodríguez A., García P. (2020). Encapsulation of the Antistaphylococcal Endolysin LysRODI in pH-Sensitive Liposomes. Antibiotics 9 (5), 242. 10.3390/antibiotics9050242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan B., Kumar N., Saha S., Roy A. (2015). Liposome: Method of Preparation, Advantages, Evaluation and its Application. J. Appl. Pharm. Res. 3 (3), 01–08. [Google Scholar]
- Puras G., Mashal M., Zárate J., Agirre M., Ojeda E., Grijalvo S., et al. (2014). A Novel Cationic Niosome Formulation for Gene Delivery to the Retina. J. Controlled Release 174, 27–36. 10.1016/j.jconrel.2013.11.004 [DOI] [PubMed] [Google Scholar]
- Qiang L., Yi J., Fu-De C. (2004). Melanoma Vaccine Based on the Vector of Membrane Fusogenic Liposomes. Pharmazie 59 (4), 263–267. [PubMed] [Google Scholar]
- Qiao C., Liu J., Yang J., Li Y., Weng J., Shao Y., et al. (2016). Enhanced Non-inflammasome Mediated Immune Responses by Mannosylated Zwitterionic-Based Cationic Liposomes for HIV DNA Vaccines. Biomaterials 85, 1–17. 10.1016/j.biomaterials.2016.01.054 [DOI] [PubMed] [Google Scholar]
- Radwan Almofti M., Harashima H., Shinohara Y., Almofti A., Baba Y., Kiwada H. (2003). Cationic Liposome-Mediated Gene Delivery: Biophysical Study and Mechanism of Internalization. Arch. Biochem. Biophys. 410 (2), 246–253. 10.1016/s0003-9861(02)00725-7 [DOI] [PubMed] [Google Scholar]
- Rahdar A., Sayyadi K., Sayyadi J., Yaghobi Z. (2019). Nano-gels: A Versatile Nano-Carrier Platform for Drug Delivery Systems: A Mini Review. Nanomedicine Res. J. 4 (1), 1–9. [Google Scholar]
- Réthoré G., Montier T., Le Gall T., Delepine P., Cammas-Marion S., Lemiègre L., et al. (2007). Archaeosomes Based on Synthetic Tetraether-like Lipids as Novel Versatile Gene Delivery Systems. Chem. Commun. 20, 2054–2056. 10.1039/b618568a() [DOI] [PubMed] [Google Scholar]
- Ricci M., Oliva R., Del Vecchio P., Paolantoni M., Morresi A., Sassi P. (2016). DMSO-induced Perturbation of Thermotropic Properties of Cholesterol-Containing DPPC Liposomes. Biochim. Biophys. Acta (Bba) - Biomembranes 1858 (12), 3024–3031. 10.1016/j.bbamem.2016.09.012 [DOI] [PubMed] [Google Scholar]
- Rizvi I., Nath S., Obaid G., Ruhi M. K., Moore K., Bano S., et al. (2019). A Combination of Visudyne and a Lipid-Anchored Liposomal Formulation of Benzoporphyrin Derivative Enhances Photodynamic Therapy Efficacy in a 3D Model for Ovarian Cancer. Photochem. Photobiol. 95 (1), 419–429. 10.1111/php.13066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkrands I., Agger E. M., Olsen A. W., Korsholm K. S., Andersen C. S., Jensen K. T., et al. (2005). Cationic Liposomes Containing Mycobacterial Lipids: a New Powerful Th1 Adjuvant System. Infect. Immun. 73 (9), 5817–5826. 10.1128/iai.73.9.5817-5826.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovira-Bru M., Thompson D. H., Szleifer I. (2002). Size and Structure of Spontaneously Forming Liposomes in lipid/PEG-Lipid Mixtures. Biophysical J. 83 (5), 2419–2439. 10.1016/s0006-3495(02)75255-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj S., Khurana S., Choudhari R., Kesari K. K., Kamal M. A., Garg N., et al. (Editors) (2021). Specific Targeting Cancer Cells with Nanoparticles and Drug Delivery in Cancer Therapy. Seminars in Cancer Biology (Elsevier; ). [DOI] [PubMed] [Google Scholar]
- Saadat E., Amini M., Khoshayand M. R., Dinarvand R., Dorkoosh F. A. (2014). Synthesis and Optimization of a Novel Polymeric Micelle Based on Hyaluronic Acid and Phospholipids for Delivery of Paclitaxel, In Vitro and In-Vivo Evaluation. Int. J. Pharm. 475 (1-2), 163–173. 10.1016/j.ijpharm.2014.08.030 [DOI] [PubMed] [Google Scholar]
- Sahoo S. K., Labhasetwar V. (2003). Nanotech Approaches to Drug Delivery and Imaging. Drug Discov. Today 8 (24), 1112–1120. 10.1016/s1359-6446(03)02903-9 [DOI] [PubMed] [Google Scholar]
- Saliba A.-E., Vonkova I., Deghou S., Ceschia S., Tischer C., Kugler K. G., et al. (2016). A Protocol for the Systematic and Quantitative Measurement of Protein-Lipid Interactions Using the Liposome-Microarray-Based Assay. Nat. Protoc. 11 (6), 1021–1038. 10.1038/nprot.2016.059 [DOI] [PubMed] [Google Scholar]
- Samuni A. M., Lipman A., Barenholz Y. (2000). Damage to Liposomal Lipids: protection by Antioxidants and Cholesterol-Mediated Dehydration. Chem. Phys. Lipids 105 (2), 121–134. 10.1016/s0009-3084(99)00136-x [DOI] [PubMed] [Google Scholar]
- Sankhyan A., Pawar P. (2012). Recent Trends in Niosome as Vesicular Drug Delivery System. J. Appl. Pharm. Sci. 2 (6), 20–32. [Google Scholar]
- Santos H. d. L., Lopes M. L., Maggio B., Ciancaglini P. (2005). Na,K-ATPase Reconstituted in Liposomes: Effects of Lipid Composition on Hydrolytic Activity and Enzyme Orientation. Colloids Surf. B: Biointerfaces 41 (4), 239–248. 10.1016/j.colsurfb.2004.12.013 [DOI] [PubMed] [Google Scholar]
- Scheffer L., Solomonov I., Jan Weygand M., Kjaer K., Leiserowitz L., Addadi L. (2005). Structure of Cholesterol/ceramide Monolayer Mixtures: Implications to the Molecular Organization of Lipid Rafts. Biophysical J. 88 (5), 3381–3391. 10.1529/biophysj.104.051870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott M. J., Jones M. N. (2001). The Interaction of Phospholipid Liposomes with Zinc Citrate Particles: a Microcalorimetric Investigation. Colloids and Surfaces A:. Physicochemical Eng. Aspects 182 (1-3), 247–256. 10.1016/s0927-7757(00)00827-x [DOI] [Google Scholar]
- Scrimgeour C., Gao Y., Oh W. Y., Shahidi F. (2005). Chemistry of Fatty Acids. Bailey's Ind. oil fat Prod., 1–40. 10.1002/047167849x.bio005 [DOI] [Google Scholar]
- Semple S. C., Leone R., Wang J., Leng E. C., Klimuk S. K., Eisenhardt M. L., et al. (2005). Optimization and Characterization of a Sphingomyelin/cholesterol Liposome Formulation of Vinorelbine with Promising Antitumor Activity. J. Pharm. Sci. 94 (5), 1024–1038. 10.1002/jps.20332 [DOI] [PubMed] [Google Scholar]
- Seo J. W., Qin S., Mahakian L. M., Watson K. D., Kheirolomoom A., Ferrara K. W. (2011). Positron Emission Tomography Imaging of the Stability of Cu-64 Labeled Dipalmitoyl and Distearoyl Lipids in Liposomes. J. controlled release 151 (1), 28–34. 10.1016/j.jconrel.2011.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah K. A., Date A. A., Joshi M. D., Patravale V. B. (2007). Solid Lipid Nanoparticles (SLN) of Tretinoin: Potential in Topical Delivery. Int. J. Pharm. 345 (1-2), 163–171. 10.1016/j.ijpharm.2007.05.061 [DOI] [PubMed] [Google Scholar]
- Shehata T., Ogawara K., Higaki K., Kimura T. (2008). Prolongation of Residence Time of Liposome by Surface-Modification with Mixture of Hydrophilic Polymers. Int. J. Pharm. 359 (1-2), 272–279. 10.1016/j.ijpharm.2008.04.004 [DOI] [PubMed] [Google Scholar]
- Shim G., Han S.-E., Yu Y.-H., Lee S., Lee H. Y., Kim K., et al. (2011). Trilysinoyl Oleylamide-Based Cationic Liposomes for Systemic Co-delivery of siRNA and an Anticancer Drug. J. controlled release 155 (1), 60–66. 10.1016/j.jconrel.2010.10.017 [DOI] [PubMed] [Google Scholar]
- Shim G., Kim M.-G., Park J. Y., Oh Y.-K. (2013). Application of Cationic Liposomes for Delivery of Nucleic Acids. Asian J. Pharm. Sci. 8 (2), 72–80. 10.1016/j.ajps.2013.07.009 [DOI] [Google Scholar]
- Shoji J. i., Tanihara Y., Uchiyama T., Kawai A. (2004). Preparation of Virosomes Coated with the Vesicular Stomatitis Virus Glycoprotein as Efficient Gene Transfer Vehicles for Animal Cells. Microbiol. Immunol. 48 (3), 163–174. 10.1111/j.1348-0421.2004.tb03502.x [DOI] [PubMed] [Google Scholar]
- Shrivastava S., Lole K. S., Tripathy A. S., Shaligram U. S., Arankalle V. A. (2009). Development of Candidate Combination Vaccine for Hepatitis E and Hepatitis B: a Liposome Encapsulation Approach. Vaccine 27 (47), 6582–6588. 10.1016/j.vaccine.2009.08.033 [DOI] [PubMed] [Google Scholar]
- Silindir M., Erdoğan S., Özer A. Y., Doğan A. L., Tuncel M., Uğur Ö., et al. (2013). Nanosized Multifunctional Liposomes for Tumor Diagnosis and Molecular Imaging by SPECT/CT. J. Liposome Res. 23 (1), 20–27. 10.3109/08982104.2012.722107 [DOI] [PubMed] [Google Scholar]
- Silva C., Aranda F. J., Ortiz A., Martínez V., Carvajal M., Teruel J. A. (2011). Molecular Aspects of the Interaction between Plants Sterols and DPPC Bilayers. J. Colloid Interf. Sci. 358 (1), 192–201. 10.1016/j.jcis.2011.02.048 [DOI] [PubMed] [Google Scholar]
- Silverman J. A., Reynolds L., Deitcher S. R. (2013). Pharmacokinetics and Pharmacodynamics of Vincristine Sulfate Liposome Injection (VSLI) in Adults with Acute Lymphoblastic Leukemia. J. Clin. Pharmacol. 53 (11), 1139–1145. 10.1002/jcph.155 [DOI] [PubMed] [Google Scholar]
- Silverman J. A., Deitcher S. R. (2013). Marqibo (Vincristine Sulfate Liposome Injection) Improves the Pharmacokinetics and Pharmacodynamics of Vincristine. Cancer Chemother. Pharmacol. 71 (3), 555–564. 10.1007/s00280-012-2042-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simões S., Moreira J. N., Fonseca C., Düzgüneş N., de Lima M. C. P. (2004). On the Formulation of pH-Sensitive Liposomes with Long Circulation Times. Adv. Drug Deliv. Rev. 56 (7), 947–965. 10.1016/j.addr.2003.10.038 [DOI] [PubMed] [Google Scholar]
- Singh D., Dilnawaz F., Sahoo S. K. (2020). Challenges of Moving Theranostic Nanomedicine into the Clinic. Future Med. 10.2217/nnm-2019-0401 [DOI] [PubMed] [Google Scholar]
- Sioud M., Sørensen D. R. (2003). Cationic Liposome-Mediated Delivery of siRNAs in Adult Mice. Biochem. biophysical Res. Commun. 312 (4), 1220–1225. 10.1016/j.bbrc.2003.11.057 [DOI] [PubMed] [Google Scholar]
- Socaciu C., Jessel R., Diehl H. A. (2000). Competitive Carotenoid and Cholesterol Incorporation into Liposomes: Effects on Membrane Phase Transition, Fluidity, Polarity and Anisotropy. Chem. Phys. Lipids 106 (1), 79–88. 10.1016/s0009-3084(00)00135-3 [DOI] [PubMed] [Google Scholar]
- Sodt A. J., Pastor R. W., Lyman E. (2015). Hexagonal Substructure and Hydrogen Bonding in Liquid-Ordered Phases Containing Palmitoyl Sphingomyelin. Biophysical J. 109 (5), 948–955. 10.1016/j.bpj.2015.07.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita T., Yoshikawa T., Gao J.-Q., Shimokawa M., Oda A., Niwa T., et al. (2005). Fusogenic Liposome Can Be Used as an Effective Vaccine Carrier for Peptide Vaccination to Induce Cytotoxic T Lymphocyte (CTL) Response. Biol. Pharm. Bull. 28 (1), 192–193. 10.1248/bpb.28.192 [DOI] [PubMed] [Google Scholar]
- Suk J. S., Xu Q., Kim N., Hanes J., Ensign L. M. (2016). PEGylation as a Strategy for Improving Nanoparticle-Based Drug and Gene Delivery. Adv. Drug Deliv. Rev. 99 (Pt A), 28–51. 10.1016/j.addr.2015.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M., Northup N., Marga F., Huber T., Byfield F. J., Levitan I., et al. (2007). The Effect of Cellular Cholesterol on Membrane-Cytoskeleton Adhesion. J. Cel. Sci. 120 (13), 2223–2231. 10.1242/jcs.001370 [DOI] [PubMed] [Google Scholar]
- Tabatabaei Mirakabad F. S., Akbarzadeh A., Milani M., Zarghami N., Taheri-Anganeh M., Zeighamian V., et al. (2016). A Comparison between the Cytotoxic Effects of Pure Curcumin and Curcumin-Loaded PLGA-PEG Nanoparticles on the MCF-7 Human Breast Cancer Cell Line. Artif. Cell Nanomedicine, Biotechnol. 44 (1), 423–430. 10.3109/21691401.2014.955108 [DOI] [PubMed] [Google Scholar]
- Tan C., Feng B., Zhang X., Xia W., Xia S. (2016). Biopolymer-coated Liposomes by Electrostatic Adsorption of Chitosan (Chitosomes) as Novel Delivery Systems for Carotenoids. Food hydrocolloids 52, 774–784. 10.1016/j.foodhyd.2015.08.016 [DOI] [Google Scholar]
- Thompson A. K., Haisman D., Singh H. (2006). Physical Stability of Liposomes Prepared from Milk Fat Globule Membrane and Soya Phospholipids. J. Agric. Food Chem. 54 (17), 6390–6397. 10.1021/jf0605695 [DOI] [PubMed] [Google Scholar]
- Toh M.-R., Chiu G. N. C. (2013). Liposomes as Sterile Preparations and Limitations of Sterilisation Techniques in Liposomal Manufacturing. Asian J. Pharm. Sci. 8 (2), 88–95. 10.1016/j.ajps.2013.07.011 [DOI] [Google Scholar]
- Torchilin V. P. (2006). Recent Approaches to Intracellular Delivery of Drugs and DNA and Organelle Targeting. Annu. Rev. Biomed. Eng. 8, 343–375. 10.1146/annurev.bioeng.8.061505.095735 [DOI] [PubMed] [Google Scholar]
- Trandum C., Westh P., Jørgensen K., Mouritsen O. G. (2000). A Thermodynamic Study of the Effects of Cholesterol on the Interaction between Liposomes and Ethanol. Biophysical J. 78 (5), 2486–2492. 10.1016/s0006-3495(00)76793-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trucillo P., Campardelli R., Reverchon E. (2020). Liposomes: From Bangham to Supercritical Fluids. Processes 8 (9), 1022. 10.3390/pr8091022 [DOI] [Google Scholar]
- Trucillo P., Campardelli R., Reverchon E. (2017). Supercritical CO 2 Assisted Liposomes Formation: Optimization of the Lipidic Layer for an Efficient Hydrophilic Drug Loading. J. Co2 utilization 18, 181–188. 10.1016/j.jcou.2017.02.001 [DOI] [Google Scholar]
- Tzogani K., Penttilä K., Lapveteläinen T., Hemmings R., Koenig J., Freire J., et al. (2020). EMA Review of Daunorubicin and Cytarabine Encapsulated in Liposomes (Vyxeos, CPX-351) for the Treatment of Adults with Newly Diagnosed, Therapy-Related Acute Myeloid Leukemia or Acute Myeloid Leukemia with Myelodysplasia-Related Changes. Oncologist 25 (9), e1414–e20. 10.1634/theoncologist.2019-0785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich A. S. (2002). Biophysical Aspects of Using Liposomes as Delivery Vehicles. Biosci. Rep. 22 (2), 129–150. 10.1023/a:1020178304031 [DOI] [PubMed] [Google Scholar]
- Vakili-Ghartavol R., Rezayat S. M., Faridi-Majidi R., Sadri K., Jaafari M. R. (2020). Optimization of Docetaxel Loading Conditions in Liposomes: Proposing Potential Products for Metastatic Breast Carcinoma Chemotherapy. Sci. Rep. 10 (1), 5569. 10.1038/s41598-020-62501-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Slooten M. L., Storm G., Zoephel A., Küpcü Z., Boerman O., Crommelin D. J. A., et al. (2000). Liposomes Containing Interferon-Gamma as Adjuvant in Tumor Cell Vaccines. Pharm. Res. 17 (1), 42–48. 10.1023/a:1007514424253 [DOI] [PubMed] [Google Scholar]
- Varypataki E. M., Benne N., Bouwstra J., Jiskoot W., Ossendorp F. (2017). Efficient Eradication of Established Tumors in Mice with Cationic Liposome-Based Synthetic Long-Peptide Vaccines. Cancer Immunol. Res. 5 (3), 222–233. 10.1158/2326-6066.cir-16-0283 [DOI] [PubMed] [Google Scholar]
- Veneti E., Tu R. S., Auguste D. T. (2016). RGD-targeted Liposome Binding and Uptake on Breast Cancer Cells Is Dependent on Elastin Linker Secondary Structure. Bioconjug. Chem. 27 (8), 1813–1821. 10.1021/acs.bioconjchem.6b00205 [DOI] [PubMed] [Google Scholar]
- Wagner A., Platzgummer M., Kreismayr G., Quendler H., Stiegler G., Ferko B., et al. (2006). GMP Production of Liposomes-A New Industrial Approach. J. liposome Res. 16 (3), 311–319. 10.1080/08982100600851086 [DOI] [PubMed] [Google Scholar]
- Walde P., Ichikawa S. (2001). Enzymes inside Lipid Vesicles: Preparation, Reactivity and Applications. Biomol. Eng. 18 (4), 143–177. 10.1016/s1389-0344(01)00088-0 [DOI] [PubMed] [Google Scholar]
- Wang Y., Tu S., Li R., Yang X., Liu L., Zhang Q. (2010). Cholesterol Succinyl Chitosan Anchored Liposomes: Preparation, Characterization, Physical Stability, and Drug Release Behavior. Nanomedicine: Nanotechnology, Biol. Med. 6 (3), 471–477. 10.1016/j.nano.2009.09.005 [DOI] [PubMed] [Google Scholar]
- Wang Y., Wang S., Firempong C. K., Zhang H., Wang M., Zhang Y., et al. (2017). Enhanced Solubility and Bioavailability of Naringenin via Liposomal Nanoformulation: Preparation and In Vitro and In Vivo Evaluations. Aaps Pharmscitech 18 (3), 586–594. 10.1208/s12249-016-0537-8 [DOI] [PubMed] [Google Scholar]
- Weissig V. (2015). DQAsomes as the Prototype of Mitochondria-Targeted Pharmaceutical Nanocarriers: Preparation, Characterization, and Use. Mitochondrial Med. Springer, 1–11. 10.1007/978-1-4939-2288-8_1 [DOI] [PubMed] [Google Scholar]
- Weng C.-S., Wu C.-C., Chen T.-C., Chen J.-R., Huang C.-Y., Chang C.-L. (2019). Retrospective Analysis of Comparative Outcomes in Recurrent Platinum-Sensitive Ovarian Cancer Treated with Pegylated Liposomal Doxorubicin (Lipo-Dox) and Carboplatin versus Paclitaxel and Carboplatin. Cmar 11, 9899–9905. 10.2147/cmar.s217329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wibroe P. P., Ahmadvand D., Oghabian M. A., Yaghmur A., Moghimi S. M. (2016). An Integrated Assessment of Morphology, Size, and Complement Activation of the PEGylated Liposomal Doxorubicin Products Doxil, Caelyx, DOXOrubicin, and SinaDoxosome. J. Controlled Release 221, 1–8. 10.1016/j.jconrel.2015.11.021 [DOI] [PubMed] [Google Scholar]
- Wong J. P., Nagata L. P., Dale R. M. (2001). Liposome-encapsulated Nucleic Acid-Based Drugs and Vaccines as Novel Antiviral Agents. Recent Dev. Antivir. Res., 175–191. [Google Scholar]
- Wu R. G., Dai J. D., Wu F. G., Zhang X. H., Li W. H., Wang Y. R. (2012). Competitive Molecular Interaction Among Paeonol-Loaded Liposomes: Differential Scanning Calorimetry and Synchrotron X-ray Diffraction Studies. Int. J. Pharm. 438 (1-2), 91–97. 10.1016/j.ijpharm.2012.08.052 [DOI] [PubMed] [Google Scholar]
- Wu X., Dai X., Liao Y., Sheng M., Shi X. (2021). Investigation on Drug Entrapment Location in Liposomes and Transfersomes Based on Molecular Dynamics Simulation. J. Mol. Model. 27 (4), 1–10. 10.1007/s00894-021-04722-3 [DOI] [PubMed] [Google Scholar]
- Xia S., Xu S. (2005). Ferrous Sulfate Liposomes: Preparation, Stability and Application in Fluid Milk. Food Res. Int. 38 (3), 289–296. 10.1016/j.foodres.2004.04.010 [DOI] [Google Scholar]
- Xu Q., Tanaka Y., Czernuszka J. T. (2007). Encapsulation and Release of a Hydrophobic Drug from Hydroxyapatite Coated Liposomes. Biomaterials 28 (16), 2687–2694. 10.1016/j.biomaterials.2007.02.007 [DOI] [PubMed] [Google Scholar]
- Xu X., Khan M. A., Burgess D. J. (2011). A Quality by Design (QbD) Case Study on Liposomes Containing Hydrophilic API: I. Formulation, Processing Design and Risk Assessment. Int. J. Pharm. 419 (1-2), 52–59. 10.1016/j.ijpharm.2011.07.012 [DOI] [PubMed] [Google Scholar]
- Yamauchi M., Tsutsumi K., Abe M., Uosaki Y., Nakakura M., Aoki N. (2007). Release of Drugs from Liposomes Varies with Particle Size. Biol. Pharm. Bull. 30 (5), 963–966. 10.1248/bpb.30.963 [DOI] [PubMed] [Google Scholar]
- Yang T., Cui F.-D., Choi M.-K., Lin H., Chung S.-J., Shim C.-K., et al. (2007). Liposome Formulation of Paclitaxel with Enhanced Solubility and Stability. Drug Deliv. 14 (5), 301–308. 10.1080/10717540601098799 [DOI] [PubMed] [Google Scholar]
- Yang Z.-Z., Li J.-Q., Wang Z.-Z., Dong D.-W., Qi X.-R. (2014). Tumor-targeting Dual Peptides-Modified Cationic Liposomes for Delivery of siRNA and Docetaxel to Gliomas. Biomaterials 35 (19), 5226–5239. 10.1016/j.biomaterials.2014.03.017 [DOI] [PubMed] [Google Scholar]
- Yavlovich A., Singh A., Tarasov S., Capala J., Blumenthal R., Puri A. (2009). Design of Liposomes Containing Photopolymerizable Phospholipids for Triggered Release of Contents. J. Therm. Anal. Calorim. 98 (1), 97–104. 10.1007/s10973-009-0228-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida I., Saito A. M., Tanaka S., Choi I., Hidaka M., Miyata Y., et al. (2020). Intravenous Itraconazole Compared with Liposomal Amphotericin B as Empirical Antifungal Therapy in Patients with Neutropaenia and Persistent Fever. Mycoses 63 (8), 794–801. 10.1111/myc.13100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa T., Imazu S., Gao J.-Q., Hayashi K., Tsuda Y., Okada N., et al. (2006). Non-methylated CpG Motif Packaged into Fusogenic Liposomes Enhance Antigen-specific Immunity in Mice. Biol. Pharm. Bull. 29 (1), 105–109. 10.1248/bpb.29.105 [DOI] [PubMed] [Google Scholar]
- Yoshikawa T., Okada N., Tsujino M., Gao J.-Q., Hayashi A., Tsutsumi Y., et al. (2006). Vaccine Efficacy of Fusogenic Liposomes Containing Tumor Cell-Lysate against Murine B16BL6 Melanoma. Biol. Pharm. Bull. 29 (1), 100–104. 10.1248/bpb.29.100 [DOI] [PubMed] [Google Scholar]
- Yu F., Tang X. (2016). Novel Long-Circulating Liposomes Consisting of PEG Modified β-Sitosterol for Gambogic Acid Delivery. j nanosci nanotechnol 16 (3), 3115–3121. 10.1166/jnn.2016.12405 [DOI] [PubMed] [Google Scholar]
- Yu J. Y., Chuesiang P., Shin G. H., Park H. J. (2021). Post-processing Techniques for the Improvement of Liposome Stability. Pharmaceutics 13 (7), 1023. 10.3390/pharmaceutics13071023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T., Ji P., Zhao W. M. (2015). Preparation of Baicalein Liposome-Lyophilized Powder and its Pharmacokinetics Study. Zhong Yao Cai 38 (11), 2404–2407. [PubMed] [Google Scholar]
- Yuba E. (2020). Development of Functional Liposomes by Modification of Stimuli-Responsive Materials and Their Biomedical Applications. J. Mater. Chem. B 8 (6), 1093–1107. 10.1039/c9tb02470k [DOI] [PubMed] [Google Scholar]
- Yuba E., Kanda Y., Yoshizaki Y., Teranishi R., Harada A., Sugiura K., et al. (2015). pH-sensitive Polymer-Liposome-Based Antigen Delivery Systems Potentiated with Interferon-γ Gene Lipoplex for Efficient Cancer Immunotherapy. Biomaterials 67, 214–224. 10.1016/j.biomaterials.2015.07.031 [DOI] [PubMed] [Google Scholar]
- Zaru M., Mourtas S., Klepetsanis P., Fadda A. M., Antimisiaris S. G. (2007). Liposomes for Drug Delivery to the Lungs by Nebulization. Eur. J. Pharmaceutics Biopharmaceutics 67 (3), 655–666. 10.1016/j.ejpb.2007.04.005 [DOI] [PubMed] [Google Scholar]
- Zhang J., Chen Y., Li X., Liang X., Luo X. (2016). The Influence of Different Long-Circulating Materials on the Pharmacokinetics of Liposomal Vincristine Sulfate. Int. J. Nanomedicine 11, 4187–4197. 10.2147/IJN.S109547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Tian H., Li C., Cheng L., Zhang S., Zhang X., et al. (2013). Antitumor Effects Obtained by Autologous Lewis Lung Cancer Cell Vaccine Engineered to Secrete Mouse Interleukin 27 by Means of Cationic Liposome. Mol. Immunol. 55 (3-4), 264–274. 10.1016/j.molimm.2013.02.006 [DOI] [PubMed] [Google Scholar]
- Zhang J. A., Pawelchak J. (2000). Effect of pH, Ionic Strength and Oxygen burden on the Chemical Stability of EPC/cholesterol Liposomes under Accelerated Conditions. Part 1: Lipid Hydrolysis. Eur. J. Pharm. Biopharm. 50 (3), 357–364. 10.1016/s0939-6411(00)00127-2 [DOI] [PubMed] [Google Scholar]
- Zhao L., Temelli F., Curtis J. M., Chen L. (2015). Preparation of Liposomes Using Supercritical Carbon Dioxide Technology: Effects of Phospholipids and Sterols. Food Res. Int. 77, 63–72. 10.1016/j.foodres.2015.07.006 [DOI] [Google Scholar]
- Zhao X. B., Muthusamy N., Byrd J. C., Lee R. J. (2007). Cholesterol as a Bilayer Anchor for PEGylation and Targeting Ligand in Folate‐receptor‐targeted Liposomes. J. Pharm. Sci. 96 (9), 2424–2435. 10.1002/jps.20885 [DOI] [PubMed] [Google Scholar]
- Zhao Y.-Z., Liang H.-D., Mei X.-G., Halliwell M. (2005). Preparation, Characterization and In Vivo Observation of Phospholipid-Based Gas-Filled Microbubbles Containing Hirudin. Ultrasound Med. Biol. 31 (9), 1237–1243. 10.1016/j.ultrasmedbio.2005.05.007 [DOI] [PubMed] [Google Scholar]
- Zhu R., Wang Z., Liang P., He X., Zhuang X., Huang R., et al. (2017). Efficient VEGF Targeting Delivery of DOX Using Bevacizumab Conjugated SiO2@LDH for Anti-neuroblastoma Therapy. Acta Biomater. 63, 163–180. 10.1016/j.actbio.2017.09.009 [DOI] [PubMed] [Google Scholar]
- Zupančič Š., Kocbek P., Zariwala M. G., Renshaw D., Gul M. O., Elsaid Z., et al. (2014). Design and Development of Novel Mitochondrial Targeted Nanocarriers, DQAsomes for Curcumin Inhalation. Mol. Pharm. 11 (7), 2334–2345. 10.1021/mp500003q [DOI] [PubMed] [Google Scholar]