Eosinophilic esophagitis (EoE) is a chronic, inflammatory disease of the esophagus that is responsive to medical treatments or dietary restrictions.1 With respect to dietary treatments, the choice of which protein to eliminate is complicated by the lack of effective testing. This has led to treatments of empiric elimination of one to six food antigens. Because symptoms often do not correlate with mucosal inflammation, usually serial esophageal mucosal assessments at intervals of greater than 6 to 8 weeks using sedated endoscopy have become the standard of care.1
The use of this approach creates two clinical concerns related to the number of endoscopies and the time required for food reintroductions to occur. First, performance of a large number of sedated procedures incurs a significant potential financial cost, risk, and lost time from school and/or work. For instance, clinical practice suggests that after the initial diagnostic endoscopy, many assessments may be needed to establish the correct diet. This has been substantiated in other studies in which subjects incurred six to eight endoscopies during development of the treatment plan. Second, the reintroduction of foods into the diet typically occurs after a food is trialed for a minimum of 6 to 8 weeks.1 For example, if six foods were eliminated, reintroduction could take greater than 12 months. Because the kinetics of EoE relapse after an allergen introduction are not well-known, it is difficult to determine optimal timing.
To address these concerns, we hypothesized that serial unsedated transnasal esophagoscopy (TN-Eso) would be well-tolerated and could allow the determination of how quickly mucosal eosinophilia develops after a single-food reintroduction. Satisfactory use of TN-Eso at more frequent intervals may improve a patient and family’s quality of life and potentially increase interest in dietary therapies.
To address this, we used TN-Eso to biopsy esophageal tissue in children who were undergoing standard of care changes in dietary treatments. Recently unsedated emerging technologies including TN-Eso with or without virtual reality distraction has been shown to be an effective alternative to sedated endoscopy.2–5 Transnasal esophagoscopy provides biopsy samples similar to those obtained during traditional endoscopy, as well as high patient satisfaction, decreased cost, no anesthesia, and lower risk.6
In this single-site pilot study, pediatric and young adult EoE subjects undergoing standard of care single-food introductions were enrolled to assess the esophagus every 2 weeks after this single-food introduction. Subjects with EoE were eligible for enrollment if they met inclusion criteria of (1) age 5 to 22 years, 2) histologic remission on esophageal biopsies demonstrating less than 15 eosinophils/high power field (eos/HPF) within the past year; and (3) treatment with a four-food or less elimination diet. Exclusion criteria included an inability to undergo serial TN-Eso, current secondary treatment with corticosteroids, or significant symptoms. At 2 and 4 weeks after the single-food introduction, subjects underwent TN-Eso with virtual reality distraction.3 Six weeks after that introduction, subjects chose either sedated esophagoscopy with biopsy or TN-Eso. Eosinophil enumeration was performed on four proximal and four distal mucosal biopsies by a board-certified pathologist. Subjects completed baseline and post-TN-Eso satisfaction, diet adherence, and EoE symptom questionnaires. Statistical analysis was performed by evaluating 95% confidence intervals for binomial proportion to evaluate evidence of differences between a standard of care time interval at 6 weeks compared with 2 and 4 weeks. The study was conducted as a pilot study of the Consortium of Eosinophilic Disease Researchers under institutional review board approval (COMIRB 16–2315/NCT03342391). Enrollment was halted because of research restrictions evoked by the COVID-19 pandemic.
Between June 2017 and March 2020, six subjects met criteria for recruitment. Five subjects aged 11 to 18 years enrolled in the study (four males and one female). Four of the five had prior TN-Eso (range, 0–3) and all had undergone prior sedated endoscopy (range, 1–7). All subjects reported daily administration of the single-food introduction. All subjects completed the study and chose the standard of care 6-week endoscopy as unsedated TN-Eso. Two subjects reported no adverse events, three reported expected symptoms such as brief gagging, choking, or abdominal pain, and none experienced an event graded as a significant adverse event.3 Average duration of the TN-Eso was 277 seconds (SD, 50 seconds; range 192–360 seconds).
Figure 1 shows that two subjects had increased mucosal eosinophilia at 2 weeks (after introduction of dairy or egg) and another two at week 4 (after introduction of egg or soy). These four subjects all had ongoing EoE (>15 eos/HPF) at week 6. For one subject at week 2 with a soy re-introduction, peak eosinophil count decreased to less than 15 eos/HPF at week 4 but rose to greater than 15 eos/HPF again at week 6 (Figure 1). Of the 30 TN-Eso–ederived samples, 16 were found to have lamina propria present. Statistical analysis found no difference between EoE recurrence detection with esophageal biopsy at 4 weeks compared with 6 weeks (Figure 2).
FIGURE 1.
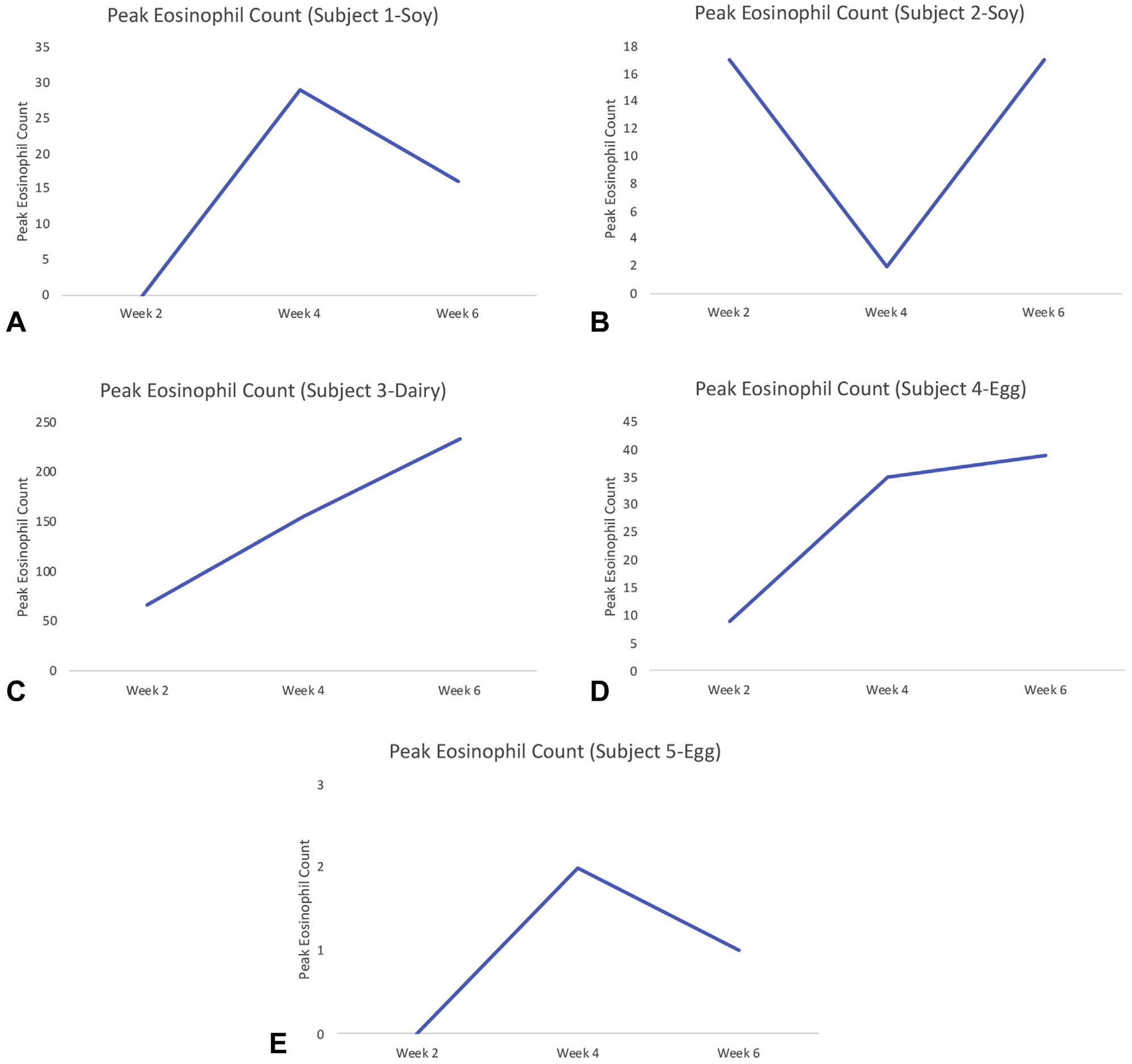
Peak esophageal eosinophil count versus time interval from food introduction for each subject during unsedated transnasal esophagoscopy.
FIGURE 2.
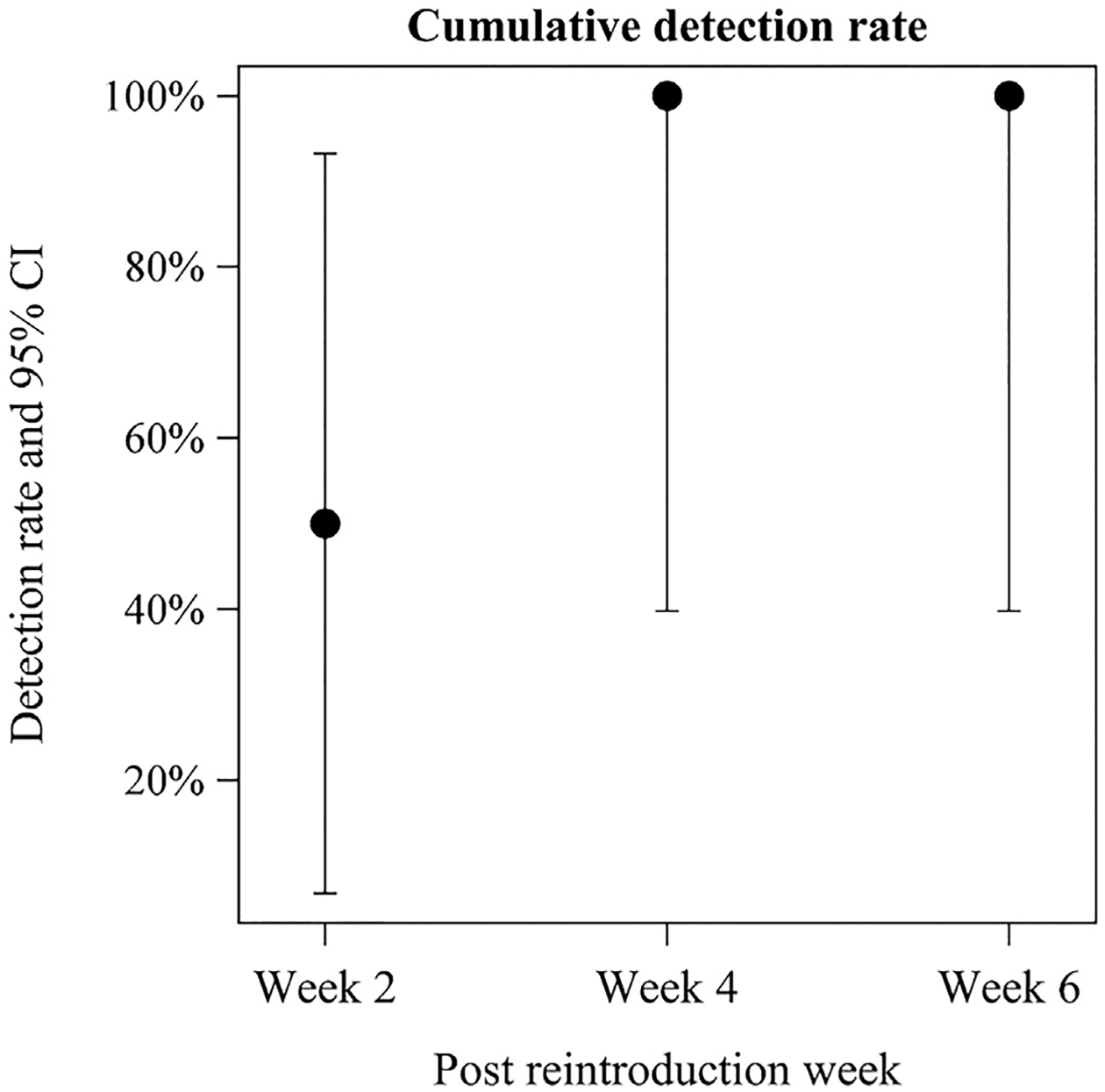
Cumulative percent detection rate of eosinophilic esophagitis recurrence in subjects using transnasal esophagoscopy after food introduction at 2, 4, and 6 weeks. CI, confidence interval.
We report three clinically relevant findings. First, TN-Eso was well-tolerated and preferred by patients and families during a single-food reintroduction. During the study, two TN-Esos were performed on pediatric patients during a single month. For the final assessment at week 6, all subjects chose a third TN-Eso over sedated endoscopy. Second, analysis of TN-Eso–ederived tissues revealed a rapid return of eosinophilia as early as 2 weeks after single-food exposure. No previous pediatric study examined dietary reintroduction therapeutic kinetics using non-elemental dietary therapies of mucosal eosinophilia in EoE.7,8 Two adult studies included allergen reintroduction during elemental dietary therapy and another evaluated the effectiveness of atopy patch testing.8,9 Our findings suggest the possibility that the timing of food reintroductions could be shortened by 50% to 75%, compared with the traditional waiting period, before repeating endoscopies. Third, in sensitized EoE patients, these findings demonstrate a rapid mucosal eosinophil recurrence during a food challenge. Although our study was limited by the number of enrolled subjects and the subject’s preference bias based on previous TN-Eso exposure, our findings support future studies examining a shortened challenge period and the use of TN-Eso to aid in this process.
Clinical Implications.
Mucosal eosinophilia after a single allergen exposure occurs more quickly than previously thought. Use of unsedated transnasal esophagoscopy with virtual reality distraction may permit quicker establishment of a suitable eosinophilic esophagitis treatment.
Acknowledgments
The Consortium of Eosinophilic Disease Researchers (CEGIR) (U54 AI117804) is part of the Rare Disease Clinical Research Network (RDCRN), an initiative of the Office of Rare Diseases Research (ORDR), National Center for Advancing Translational Sciences (NCATS), and is funded through collaboration between National Institute of Allergy and Infectious Diseases (NIAID), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), and National Center for Advancing Translational Sciences (NCATS). CEGIR is also supported by patient advocacy groups including American Partnership for Eosinophilic Disorders (APFED), Campaign Urging Research for Eosinophilic Diseases (CURED), and Eosinophilic Family Coalition (EFC). As a member of the RDCRN, CEGIR is also supported by its Data Management and Coordinating Center (DMCC) (U2CTR002818).
Footnotes
Conflicts of interest: J.A. Friedlander is president and chief medical officer of EvoEndo (formerly known as Triple Endoscopy, Inc). He is coinventor of University of Colorado patents pending AU 2016283112, CA 2990182, US 15/887438, EP 16815420.1, JP 2017–566710, PCT/US2018/067152, US 15/853521, PCT/US2018/067152, EP 18890819.9, JP 2020–534933, HK 62021030047, US 15/850939, PCT/US2019/034954, US 16/428408, US 16/573567, US 17/218,614, PCT/US2019/051523, JP 2021–514596, AU 2019345256, CA 3,113,229, EP 19861561,9, and IL 281522, related to endoscopic methods, distraction techniques, and technologies. These are related to unsedated transnasal endoscopy and distraction technologies. M.E. Rothenberg is a consultant for Pulm One, Spoon Guru, ClostraBio, Serpin Pharm, Allakos, Celgene, Astra Zeneca, Arena Pharmaceuticals, Ellodi Pharma, GlaxoSmithKline, Regeneron/Sanofi, Guidepoint, and Suvretta Capital Management, and has an equity interest in the first five listed, and royalties from reslizumab (Teva Pharmaceuticals), PEESSv2 (Mapi Research Trust), and UpToDate. He is an inventor of patents owned by Cincinnati Children’s Hospital. G.T. Furuta is cofounder of EnteroTrack and is a consultant for Abbvie. The rest of the authors declare that they have no relevant conflicts of interest.
REFERENCES
- 1.Hirano I, Chan ES, Rank MA, Sharaf RN, Stollman NH, Stukus DR, et al. AGA Institute and the Joint Task Force on Allergy-Immunology Practice Parameters Clinical Guidelines for the Management of Eosinophilic Esophagitis. Gastroenterology 2020;158:1776–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friedlander JA, DeBoer EM, Soden JS, Furuta GT, Menard-Katcher CD, Atkins D, et al. Unsedated transnasal esophagoscopy for monitoring therapy in pediatric eosinophilic esophagitis. Gastrointest Endosc 2016;83: 299–306.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nguyen N, Lavery WJ, Capocelli KE, Smith C, DeBoer EM, Deterding R, et al. Transnasal endoscopy in unsedated children with eosinophilic esophagitis using virtual reality video goggles. Clin Gastroenterol Hepatol 2019;17:2455–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Venkatesh RD, Dellon ES. This string’s attached: the esophageal string test for detecting disease activity in eosinophilic esophagitis. Gastroenterology 2020;159: 2244–5. [DOI] [PubMed] [Google Scholar]
- 5.Philpott H, Nandurkar S, Royce SG, Gibson PR. Ultrathin unsedated transnasal gastroscopy in monitoring eosinophilic esophagitis. J Gastroenterol Hepatol 2016;31:590–4. [DOI] [PubMed] [Google Scholar]
- 6.Scherer C, Sosensky P, Schulman-Green D, Levy M, Smith C, Friedlander J, et al. Pediatric patients’ and parents’ perspectives of unsedated transnasal endoscopy in eosinophilic esophagitis: a qualitative descriptive study. J Pediatr Gastroenterol Nutr 2021;72:558–62. [DOI] [PubMed] [Google Scholar]
- 7.Lucendo AJ, Miehlke S, Schlag C, Vieth M, von Arnim U, Molina-Infante J, et al. Efficacy of budesonide orodispersible tablets as induction therapy for eosinophilic esophagitis in a randomized placebo-controlled trial. Gastroenterology 2019;157:74–86.e15. [DOI] [PubMed] [Google Scholar]
- 8.Peterson KA, Byrne KR, Vinson LA, Ying J, Boynton KK, Fang JC, et al. Elemental diet induces histologic response in adult eosinophilic esophagitis. Am J Gastroenterol 2013;108:759–66. [DOI] [PubMed] [Google Scholar]
- 9.Eckmann JD, Ravi K, Katzka DA, Davis DR, See JA, Geno DR, et al. Efficacy of atopy patch testing in directed dietary therapy of eosinophilic esophagitis: a pilot study. Dig Dis Sci 2018;63:694–702. [DOI] [PubMed] [Google Scholar]


