Abstract
Background
Osteoarthritis (OA) is a common form of arthritis and is often associated with significant disability and impaired quality of life. This is an update of a Cochrane review first published in 2001 and previously updated in 2005.
Objectives
To review randomized controlled trials (RCTs) evaluating the effectiveness and toxicity of glucosamine in OA.
Search methods
We searched CENTRAL and the Cochrane Database of Systematic Reviews (The Cochrane Library), MEDLINE, PREMEDLINE, EMBASE, AMED, ACP Journal Club, DARE (to January 2008); contacted content experts, and handsearched reference lists and pertinent review articles.
Selection criteria
RCTs evaluating the effectiveness and safety of glucosamine in OA.
Data collection and analysis
Data abstraction was performed independently by two review authors and investigators were contacted for missing data.
Main results
This update includes 25 studies with 4963 patients. Analysis restricted to studies with adequate allocation concealment failed to show any benefit of glucosamine for pain (based on a pooled measure of different pain scales) and WOMAC pain, function and stiffness subscales; however, it was found to be better than placebo using the Lequesne index (standardized mean difference (SMD) ‐0.54; 95% confidence interval (CI) ‐0.96 to ‐0.12). Collectively, the 25 RCTs favoured glucosamine with a 22% (change from baseline) improvement in pain (SMD ‐0.47; 95% CI ‐0.72 to ‐0.23) and a 11% (change from baseline) improvement in function using the Lequesne index (SMD ‐0.47; 95% CI ‐0.82 to ‐0.12). However, the results were not uniformly positive and the reasons for this remain unexplained. WOMAC pain, function and stiffness outcomes did not reach statistical significance.
RCTs in which the Rotta preparation of glucosamine was compared to placebo found glucosamine superior for pain (SMD ‐1.11; 95% CI ‐1.66 to ‐0.57) and function (Lequesne index SMD ‐0.47; 95% CI ‐0.82 to ‐0.12). Pooled results for pain (SMD ‐0.05; 95% CI ‐0.15 to 0.05) and function using the WOMAC index (SMD ‐0.01; 95% CI ‐0.13 to 0.10) in those RCTs using a non‐Rotta preparation of glucosamine did not reach statistical significance. Two RCTs using the Rotta preparation showed that glucosamine was able to slow radiological progression of OA of the knee over a three‐year period (mean difference (MD) 0.32; 95% CI 0.05 to 0.58).
Glucosamine was as safe as placebo in terms of the number of participants reporting adverse reactions (relative risk ratio 0.99; 95% CI 0.91 to 1.07).
Authors' conclusions
Pooled results from studies using a non‐Rotta preparation or adequate allocation concealment failed to show benefit in pain and WOMAC function while those studies evaluating the Rotta preparation showed that glucosamine was superior to placebo in the treatment of pain and functional impairment resulting from symptomatic OA.
Plain language summary
Glucosamine for osteoarthritis
This summary of a Cochrane review presents what we know from research about the effect of glucosamine on osteoarthritis.
People with osteoarthritis who take glucosamine:
‐ may reduce their pain
‐ may improve their physical function
‐ will probably not have side effects
What is osteoarthritis and glucosamine?
Osteoarthritis (OA) is the most common form of arthritis that can affect the hands, hips, shoulders and knees. In OA, the cartilage that protects the ends of the bones breaks down and causes pain and swelling. Drug and non‐drug treatments are used to relieve pain and/or swelling.
Glucosamine can be found naturally in the body and is used by the body as one of the building blocks of cartilage. Glucosamine can also be taken as a pill as a supplement to the diet, or sometimes as an injection. It can come in combination with other supplements (such as chondroitin), or by itself in the form of glucosamine hydrochloride or sulphate. The usual dose recommended on packages is 1500 mg per day or 500 mg three times a day.
In Europe, glucosamine is prescribed by health care providers. But in North America, people can buy glucosamine supplements without a prescription. This means that, in North America, glucosamine is not regulated and the pills may or may not truly contain the amount described on the label.
Best estimate of what happens after about 6 months
Pain: The high quality studies showed that pain improved about the same whether people took glucosamine or fake pills. If all of the studies are examined (including low quality and old studies), then glucosamine improved pain more than fake pills.
People who took fake pills had a pain score of 7 points on a 0 to 100 scale. Pain may improve by 10 more points with glucosamine than with fake pills.
Studies testing only the Rotta brand of glucosamine (including low quality and older studies) showed that glucosamine improved pain more than fake pills. People who took fake pills had a pain score of 6 points on a 0 to 20 scale. People who took the Rotta brand of glucosamine rated their pain 3 points lower than people who did not take glucosamine.
Function: The high quality studies show that glucosamine improved function more than fake pills when measured by one type of scale, but improved the same amount as fake pills when measured by another scale.
Studies testing only the Rotta brand of glucosamine (including low quality and older studies) showed that glucosamine improved function more than fake pills. People who took fake pills had a function score of 22 points on a 0 to 68 scale. People who took the Rotta brand of glucosamine had their ability to function improve by 2 points compared to people who did not take glucosamine.
There was no difference in the number of people who had side effects. Side effects mainly included stomach upset and other joint pain.
Summary of findings
Background
Osteoarthritis (OA) is the most common form of arthritis, and it is often associated with significant disability and an impaired quality of life ( Badley 1995; Moralestorres 1996; Scott 1993; Towheed 1998). An estimated 12.1% of Americans aged 25 years and older (nearly 21 million persons in 1990) have clinical signs and symptoms of OA (Lawrence 1998). Among US adults aged 30 years or older, symptomatic disease in the knee occurs in approximately 6% and symptomatic disease in the hip occurs in approximately 3% (Felson 2000). OA of the hip and knee can be especially disabling to lower extremity functioning because the hip and knee are large weight‐bearing joints (Liang 1984). Advanced OA of the hip or knee is the most common reason for elective joint replacement (Hochberg 1996).
Although there are no curative therapies currently available for OA, individualized treatment programs are available to help relieve pain and stiffness and to maintain or improve functional status (ACR 2000; Creamer 1998; Hochberg 1995a; Hochberg 1995b). Treatment strategies for OA have included both non‐pharmacological and pharmacological modalities (Creamer 1997). Non‐pharmacological therapy is considered to be the foundation for the successful medical management of OA (Felson 1998; Puett 1994). These modalities include weight reduction (if obese), physiotherapy (for example muscle strengthening), and occupational therapy (for example, use of assistive devices for ambulation).
Non‐steroidal anti‐inflammatory drugs (NSAIDs) are considered by many physicians to be the preferred agents for the pharmacological management of OA. In support of this, randomized controlled trials (RCTs) attest to the superior efficacy of NSAIDs as compared with placebo (Towheed 1997a; Towheed 1997b; Towheed 2002). However, there are certain disadvantages of routinely using NSAIDs in OA. For example, all NSAIDs (non‐selective and COX‐2 selective) are associated with significant potential toxicity, particularly in the elderly population (Deviere 2002; Gabriel 1991; Garner 2002; Griffin 1991; Wright 2002). COX‐2 selective inhibitors have also been associated with an increased risk of cardiovascular disease. Rofecoxib, a COX‐2 selective inhibitor, was recently withdrawn from the world market due to concerns in this regard (Sibbald 2004).
There is also a concern that NSAIDs may be toxic to articular cartilage (Herman 1986) and that they may accelerate the course of OA of the hip (Rashad 1989). Given that OA is the most prevalent form of arthritis and that the number of persons affected with OA will increase significantly in the near future, finding alternative, safer pharmacological therapies for OA is of considerable importance.
Recent additions to the options for pharmacological therapy of OA have included biological compounds, such as hyaluronans, chondroitin sulfate, and glucosamine (Lozada 1997). Although not yet proven, these compounds may also potentially be chondroprotective, in that they may favourably modify the natural progression and course of OA. Glucosamine compounds, in particular, have attracted a great deal of attention, mostly in the lay press and less so in the scientific literature (Anderson 2005; Barclay 1998; Da Camara 1998; Towheed 1999; Towheed 2000; Towheed 2002; Towheed 2003, Towheed 2007). There appears to be controversy as to the relative efficacy of glucosamine, and as to whether glucosamine can indeed modify the progression of OA (Reginister 2003).
For the purposes of this review, GS refers to glucosamine sulfate and GH refers to glucosamine hydrochloride, whereas glucosamine refers to both compounds. GS is a natural substance and is the building block of the ground substance of the articular cartilage, the proteoglycans. The rationale for the use of GS in OA is based largely on in‐vitro and animal models of osteoarthritis. For example, GS has been shown to normalize cartilage metabolism, rebuild experimentally damaged cartilage, and demonstrate mild anti‐inflammatory properties (Bassleer 1992; McCarty 1994; Roden 1956; Rovati 1993; Setnikar 1991a; Vidal 1978).
Objectives
To assess the effectiveness and toxicity of glucosamine in the pharmacological management of OA. Both symptomatic effectiveness and structural effectiveness (that is, delay in radiological progression of OA) were evaluated.
Methods
Criteria for considering studies for this review
Types of studies
Two levels of criteria were used to identify all relevant studies for this review. The first criteria were used to screen all citations that involved glucosamine in the management of OA. The second criteria were used to identify those studies that met the following additional requirements: 1) RCTs evaluating the efficacy and toxicity of glucosamine in OA, 2) both placebo‐based and comparative studies were eligible, 3) both single‐blinded and double‐blinded trials were eligible, 4) studies to be included in the quantitative portion of the review (meta‐analysis) must have presented suitable quantitative data for pooling across trials, 5) studies that enrolled participants with OA at any body site were eligible with the only exception being studies that evaluated glucosamine in temporomandibular joint (TMJ) disorders, 6) only studies which evaluated glucosamine‐only preparations were included (studies which evaluated combination products containing glucosamine in association with other active compounds, for example chondroitin, were excluded), 7) glucosamine could have been administered by any route.
Types of participants
All adult (of age 18 years and older) humans with a diagnosis of either primary or secondary OA at any site, including the axial and peripheral skeleton. Studies that evaluated glucosamine in disorders of the temporomandibular joint (TMJ) were excluded from this review. Disorders of the TMJ have not been typically managed by arthritis experts and TMJ disease differs from other joints that are affected by OA in terms of its epidemiology and management.
Types of interventions
Only studies that evaluated the efficacy or toxicity, or both, of glucosamine in OA were eligible. Both placebo‐controlled and comparative RCTs were included.
Types of outcome measures
At least one outcome measure must have been used to measure response to treatment. The main outcome measures of pain, range of motion, functional assessment, and global assessment satisfied this criterion. The hierarchy of outcomes (Bellamy 1997) that were extracted consisted of: 1) pain measured by any method, and 2) functional assessment measured by a validated health status questionnaire (for example, the WOMAC), 3) patient global assessment, 4) physician global assessment, and 5) range of motion of study joint. Structural benefits, defined as the ability of glucosamine to delay the natural radiological progression of OA, were studied. Toxicity of glucosamine was also considered to represent a relevant outcome measure (measured by the number of participants reporting any adverse events and by the number of participant withdrawals due to toxicity).
Search methods for identification of studies
A MEDLINE search (1966 to November 1999) was used to identify all relevant RCTs for the first version of this Cochrane Review. For the second version of the Cochrane Review, all searches were updated (in January 2005). The same MEDLINE search strategy was extended for this updated version of the review (up to week 1, January 2008). MEDLINE In‐Process and other non‐indexed citations were also searched (January 2008); MEDLINE Daily Update was searched (January 2008). In addition, the Cochrane Central Register of Controlled Trials (CENTRAL) and the Cochrane Database of Systematic Reviews (CDSR) (The Cochrane Library), American College of Physicians (ACP) Journal Club, and Database of Abstracts of Reviews of Effectiveness (DARE) were searched (up to January 2008); Allied and Complementary Medicine (AMED) was searched (1985 to January 2008); and EMBASE was searched (1980 to week 2, January 2008). No language or age restrictions were used for any of the electronic searches. Reference lists of all identified citations were manually searched. In addition, letters were sent to authors and content experts for assistance in retrieving additional RCTs, especially those that were unpublished. A major manufacturer of glucosamine (Rotta Pharm) was also contacted for additional trials. For this version of the Cochrane Review, the search strategy was updated in January 2008.
The electronic search strategies for MEDLINE; EMBASE; CENTRAL, CDSR, ACP Journal Club, DARE; and AMED can be found in Appendix 1.
Data collection and analysis
Study identification (identifying citations that involve GS or GH in the therapy of OA)
Two review authors (TT and TP or LM) used the screening criteria to review all identified citations independently. All citations identified by either investigator were retrieved and analyzed for suitability. Authors of abstracts were contacted requesting the full manuscript, including the raw and final data incorporating the results.
Study selection (screening identified citations to see if they meet our additional criteria)
Two review authors (TT and TP or LM) reviewed each relevant citation independently to see if it met the selection criteria described previously. At this stage, an emphasis was placed on selecting RCTs and excluding non‐randomized treatment studies. If the randomization status was not clear, the article was withheld pending clarification from the principal author. In situations where authors were not available, then a consensus was reached amongst the investigators.
Data Extraction Two review authors (TT and TP or LM) independently reviewed each RCT and extracted the raw data (for example, trial characteristics, participant demographics, outcome variables, results, features of trial quality) by using a standardized form. If outcome data were not reported in a form suitable for quantitative pooling in a meta‐analysis, the primary author was contacted for access to this information. Data on adverse effects were also extracted from the RCTs. A consensus method was used in the event of disagreement.
Assessment of methodological quality The individual criteria designed for a validated scale (Jadad 1996) along with the criterion of allocation concealment were used to assess the methodological quality of the 25 glucosamine RCTs that were included in this version of the Cochrane review. The reporting of the following individual components were assessed: description and method of generation of the randomization sequence, description and method of blinding, the number of and reasons for withdrawals and dropouts in both groups, and the method of allocation concealment. Each criterion was rated as 'met' or 'not met'. For generation of the randomization sequence, appropriate methods of randomization included random number tables or computer generated sequences; date of birth or hospital numbers were considered inappropriate randomization methods. Identical placebo or an active placebo were considered appropriate methods of double‐blinding. For allocation concealment, 'met' meant that allocation concealment was adequately described in the RCT, while 'not met' meant an unclear or inadequate allocation concealment status.
Data analysis For quantitative outcome data, standardized mean differences (SMD) were used to pool across RCTs (Hedges 1985; Petitti 1994). It is important to note that we used the end of study means and standard deviations for the meta‐analyses. If either of these were not available in the trial report, the principal author was contacted for additional information. In the absence of this information, we estimated the end of study standard deviation by using the baseline standard deviation. For categorical outcome data with two categories, the relative risk ratio (RR) was calculated (Petitti 1994). Heterogeneity was tested with a Chi2 test and the I2 statistic, available in RevMan 5.0. A fixed‐effect model was used unless heterogeneity was significant (P < 0.10), in which case the random‐effects model was used.
Sensitivity and subgroup analysis An ad‐hoc sensitivity analysis was carried out to see if effect sizes varied on pain, function, and radiologic measures when only those studies with adequate allocation concealment were analyzed. An ad‐hoc subgroup analysis was performed on those studies using the Rotta preparation of glucosamine versus those that did not use the Rotta preparation to determine if the effect sizes varied on pain and function.
Results
Description of studies
Results of search strategy The MEDLINE search strategy (1966 to November 1999) resulted in a total of 61 citations. From these 61 citations, 11 RCTs were identified that evaluated the efficacy of glucosamine (Crolle 1980; D'ambrosio 1981; Drovanti 1980; Houpt 1999; Muller‐FassBender 94; Noack 1994; Pujalte 1980; Qiu 1998; Reichelt 1994; Vaz 1982; Vajaradul 1981). The updated MEDLINE search (1996 to week 3, November 2004) resulted in a total of 201 citations. An additional four RCTs were identified from this search (Hughes 2002; Pavelka 2002; Reginster 2001; Rindone 2000). The EMBASE search (1980 to week 28, 2003) resulted in a total of 207 citations, of which one additional RCT was identified (Zenk 2002). Another EMBASE search (1996 to week 2, 2005) resulted in a total of 280 references from which one additional RCT was identified (Usha 2004). The MEDLINE in‐Process and other non‐Indexed citations search (January 6, 2005) resulted in eight citations containing two additional RCTs (Cibere 2004; McAlindon 2004). MEDLINE daily update (November 17, 2004) did not identify any relevant citations. The CENTRAL,CDSR, ACP Journal Club, DARE search (October 2004) identified a total of 52 citations, but no additional RCTs were found. AMED identified 24 citations, but no additional RCTs were found. One unpublished full manuscript reporting a RCT was sent to us by Rotta Pharm; a reference to this study was available in abstract form (Rovati 1997). This study was included in the meta‐analysis since the full manuscript contained all the relevant details necessary for its critical appraisal and for inclusion in the quantitative portion of the review. Rotta Pharm also sent us three unpublished brief technical reports reporting RCTs evaluating their GS preparation. These three brief unpublished technical reports were not included in the meta‐analyses. No additional RCTs were identified by contact with content experts. Therefore, collectively the search strategies resulted in a total of 20 English language RCTs (19 published and one unpublished) that were included in the meta‐analysis.
For the January 2008 update of the electronic searches, the search strategies identified an additional five RCTs that met the inclusion criteria for this review (Clegg 2006; Herrero‐Beaumont 2007; Mehta 2007; Qiu 2005; Rozendaal 2008).
Trial demographics and features The 25 identified RCTs that were included in the meta‐analysis are listed in the 'Characteristics of included studies' table. The years of publication (or reporting date) ranged from 1980 to 2008. Six RCTs were published in the 1980s, six were published (or reported) in the 1990s, eight were published in the years 2000 to 2004, and five were published in the years 2005 to 2008. All were double‐blind, randomized parallel‐group trials and contained a total of 4963 adults with a mean age of 60.7 years (69% were female). A total of 1905 participants were randomized to treatment with glucosamine and 3058 were randomized to the comparator groups (placebo or active comparator). The mean number of participants randomized in each of the 25 RCTs was 198 (76 to glucosamine groups and 122 to comparator groups). The mean number completing each of the RCTs was 164 (83% completed the RCTs). The mean trial duration was 25.5 weeks. The country of origin (number of RCTs) included: Italy (3), Germany (3), Canada (2), France (1), Thailand (1), China (2), Portugal (2), USA (4), UK (1), Philippines (1), Belgium (1), India (2), the Czech Republic (1), Spain (1), and the Netherlands (1). One RCT included participants from both Spain and Portugal (Herrero‐Beaumont 2007). Only six North American RCTs were found (Cibere 2004; Clegg 2006; Houpt 1999; McAlindon 2004; Rindone 2000; Zenk 2002). All of the RCTs were published in the English language with the exception of the Qiu study (Qiu 2005), which was published in Chinese but had an English abstract.
Twenty of the 25 RCTs compared glucosamine to a placebo (18 used GS and two used GH), whereas, five RCTs compared glucosamine to an NSAID (ibuprofen in three, piroxicam in one, and celecoxib in one). Two studies compared glucosamine to both an NSAID and placebo (Clegg 2006; Rovati 1997). Only one RCT directly compared glucosamine to acetaminophen (Herrero‐Beaumont 2007). Only one RCT directly compared GS with GH (Qiu 2005). One RCT (Mehta 2007) compared GS with a polyherbal supplement. The method of administration of glucosamine was quite variable in the RCTs. Twenty‐one exclusively used an oral route, two used an intra‐articular (IA) route, three used an intramuscular (IM) route, one used an intravenous (IV) route, and two used multiple routes (IM or IA and IM or IV). The dosage of glucosamine used in the RCTs was also quite variable. In the 21 RCTs using an oral route, the dosage was 1500 mg/day, which was administered as either 1500 mg once daily (six RCTs), as 500 mg three times per day (14 RCTs), or as 750 mg two times per day (one RCT). In the RCTs using parenteral routes (IM, IA, IV), 400 mg of GS was administered either once daily (two RCTs), or twice weekly (one RCT).
The type and site of OA evaluated was also heterogeneous in the 25 RCTs. Twenty RCTs evaluated the knee exclusively, two evaluated OA at multiple sites (knee, hip, others), and two did not specify the location of OA that was being evaluated. Only one RCT exclusively evaluated the hip (Rozendaal 2008). Participants with only primary OA were evaluated by eight RCTs; 17 RCTs did not make clear the distinction between primary and secondary OA. The method of classification of OA was not described in 14 RCTs, whereas, five used the Lequesne's Index, eight used the American College of Rheumatology (ACR) classification criteria, and two RCTs used both the ACR and Lequesne classification criteria. Radiographs of the target joints were obtained at baseline in 19 of the RCTs. Fourteen of these RCTs used published validated x‐ray classification criteria for OA.
The outcome variables (number of RCTs) included: pain (25), range of motion (6), functional status (20), global patient derived (4), global investigator derived (11), radiographic assessment for changes in cartilage thickness (3), and health related quality of life (1). Eleven RCTs used the WOMAC instrument. Eight RCTs used the Lequesne Index.
The majority of the RCTs (14/25, or 56%) had some form of relationship (by evaluating the Rotta brand of GS or some other affiliation) with Rotta Pharm, an Italian pharmaceutical manufacturer of GS. Ten RCTs did not use the Rotta brand of glucosamine (Cibere 2004; Clegg 2006; Houpt 1999; Hughes 2002; Mehta 2007; Rindone 2000; Rozendaal 2008; Qiu 2005; Usha 2004; Zenk 2002). One RCT used both a non‐Rotta glucosamine preparation (about 90% of participants received this) and a Rotta glucosamine preparation (received by about 10% of participants) (McAlindon 2004). Fourteen of the RCTs (56%) only evaluated the Rotta brand of GS.
The four identified RCTs that were not included in the meta‐analysis are listed in the 'Characteristics of excluded studies' table (Braham 2003; Magi 1997; Pipitone 1997; Rovati 1993). Reasons for their exclusion are also outlined in this table.
Risk of bias in included studies
An assessment of each study against the individual methodological quality criteria described in the methods section is provided in the table of methodological quality assessment (Table 3).
1. Methodological quality assessment.
| Study | Method of allocation concealment described and appropriate | Study described as 'randomized' | Method of randomization described and appropriate | Study described as 'double blind' | Method of blinding described and appropriate | # and reason for withdrawals described in each group |
| Cibere 2004 | M | M | M | M | M | M |
| Clegg 2006 | M | M | M | M | M | NM |
| Crolle 1980 | NM | M | NM | M | M | NM |
| D'ambrosio 1981 | NM | M | NM | M | M | NM |
| Drovanti 1980 | NM | M | NM | M | M | NM |
| Herrero‐Beaumont 2007 | M | M | M | M | M | M |
| Houpt 1999 | M | M | NM | M | M | NM |
| Hughes 2002 | M | M | M | M | M | M |
| McAlindon 2004 | M | M | M | M | M | M |
| Mehta 2007 | NM | M | NM | M | M | M |
| Muller‐FassBender 94 | M | M | M | M | M | M |
| Noack 1994 | M | M | M | M | M | M |
| Pavelka 2002 | M | M | M | M | M | M |
| Pujalte 1980 | NM | M | NM | M | M | M |
| Qiu 1998 | NM | M | NM | M | M | M |
| Qiu 2005 | NM | M | NM | NM | NM | M |
| Reginster 2001 | M | M | M | M | NM | M |
| Reichelt 1994 | NM | M | NM | M | M | M |
| Rindone 2000 | NM | M | M | M | NM | NM |
| Rovati 1997 | M | M | M | M | M | M |
| Rozendaal 2008 | M | M | M | M | M | M |
| Usha 2004 | NM | M | NM | M | M | M |
| Vajaradul 1981 | NM | M | NM | M | M | M |
| Vaz 1982 | NM | M | NM | M | NM | M |
| Zenk 2002 | M | M | NM | M | M | M |
M = criteria met; NM = criteria not met
All studies were reported as 'randomized', but only 12 studies described the actual method of randomization. Twenty‐one of the 25 included studies reported an appropriate method of blinding.
Most studies (76%) reported the reasons for withdrawals and dropouts in both comparator groups. Only 13 RCTs (52%) were rated as having adequate allocation concealment, whereas 12 RCTs (48%) were rated as having inadequate or unclear allocation concealment. There is evidence that inadequate allocation concealment may result in an overestimation of the true treatment effect (Schulz 1995a). A post‐hoc sensitivity analysis was undertaken to assess the results in only those studies where an appropriate method of allocation concealment was described.
Effects of interventions
Summary of findings for the main comparison. Summary of findings (adequate allocation concealment).
| Glucosamine versus placebo for treating osteoarthritis | ||||||
|
Patient or population: patients with treating osteoarthritis Settings: Intervention: Glucosamine versus placebo (adequate allocation concealment) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Glucosamine versus placebo (adequate allocation concealment) | |||||
| Pain based on WOMAC pain scale Scale from: 0 (no pain) to 20 (worst pain). (follow‐up: mean 6 months) | The mean pain based on womac pain scale in the control groups was 6.6 points | The mean Pain based on WOMAC pain scale in the intervention groups was 0.7 lower (1.5 lower to 0.17 higher) | 2173 (11) | ⊕⊕⊕⊝ moderate1 | SMD ‐0.16 (95% CI ‐0.36 to 0.04, see outcome 3.1). Relative per cent change from baseline is ‐7% (95% CI ‐17%, 1.8%). Note that when all studies are pooled together, the result is statistically significant: SMD=‐0.47 (95% CI ‐0.72, ‐0.23, see outcome 1.1). This corresponds to a 10 point improvement on a 0 to 100 scale. When studies using the Rotta preparation are pooled together, the result is statistically significant: SMD=‐1.11 ( 95% CI ‐1.66, ‐0.57, see outcome 4.1 and Summary of Findings Table 2). | |
| WOMAC Function Subscale Scale from: 0 to 68. (follow‐up: median 6 months) | The mean womac function subscale in the control groups was 31.6 points | The mean WOMAC Function Subscale in the intervention groups was 1.02 lower (2.04 lower to 0 higher) | 2017 (9) | ⊕⊕⊕⊕ high | SMD ‐0.08 (95% CI ‐0.17 to 0.00, see outcome 3.6). Relative per cent change from baseline is ‐3% (95% CI ‐6%, 0%). Note that when studies using the Rotta preparation are pooled together, the SMD is ‐0.19 (95% CI ‐0.35, ‐0.03, see outcome 4.6) which is statistically significant. See Summary of Findings Table 2. | |
| Physician Global Assessment 100 mm visual analogue scale. Scale from: 0 to 100. (follow‐up: 6 months) | The mean physician global assessment in the control groups was 37.1 mm | The mean Physician Global Assessment in the intervention groups was 0.80 higher (2.78 lower to 4.38 higher) | 630 (1) | ⊕⊕⊕⊝ moderate1,2 | MD 0.80 (95% CI ‐2.78, 4.38). Relative per cent change from baseline is ‐1.5% (95% CI ‐5.4%, 8.5%)See Results section, #10. | |
| Patient Global Assessment (patients rated whether they were better at the end of trial than at the start of the trial) (follow‐up: mean 6 months) | Medium risk population | RR 1.21 (0.8 to 1.82) | 118 (1) | ⊕⊕⊕⊝ moderate3 | ||
| 40 per 100 | 48 per 100 (32 to 73) | |||||
| Minimum Joint Space Width mm (follow‐up: mean 3 years) | The mean minimum joint space width in the control groups was 3.55 mm | The mean Minimum Joint Space Width in the intervention groups was 0.32 higher (0.05 to 0.58 higher) | 414 (2) | ⊕⊕⊕⊕ high | MD 0.32 (95% CI 0.05 to 0.58, see outcome 3.8). | |
| Toxicity (Number of Patients Reporting Adverse Events) | Medium risk population | RR 0.99 (0.91 to 1.07) | 1640 (9) | ⊕⊕⊕⊕ high | ||
| 53 per 100 | 53 per 100 (48 to 57) | |||||
| Toxicity (Number of Withdrawals due to Adverse Events) | Medium risk population | RR 0.76 (0.54 to 1.07) | 2435 (12) | ⊕⊕⊕⊝ moderate4 | ||
| 4 per 100 | 3 per 100 (2 to 5) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidance High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Heterogeneity fairly high; I‐squared=79%
2 Only 1 study and wide confidence interval
3 Few events; only 1 study
4 Confidence interval crosses threshold for appreciable benefit or harm of a RRR or RRI of 25%. Also, number of events is less than 300.
Summary of findings 2. Summary of findings (Rotta preparation).
| Glucosamine versus placebo (Rotta preparation) for treating osteoarthritis | ||||||
|
Patient or population: patients with treating osteoarthritis Settings: Intervention: Glucosamine versus placebo (Rotta preparation) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Glucosamine versus placebo (Rotta preparation) | |||||
| Pain based on WOMAC scale Scale from: 0 (no pain) to 20 (worst pain). (follow‐up: mean 6 months) | The mean pain based on womac scale in the control groups was 6 points | The mean Pain based on WOMAC scale in the intervention groups was 3.33 lower (4.98 to 1.71 lower) | 940 (8) | ⊕⊕⊝⊝ low1,2 | Statistically significant. SMD ‐1.11 (‐1.66 to ‐0.57, see outcome 4.1). Relative per cent change from baseline is ‐42.2% (95% CI ‐63.0%, ‐21.6%). | |
| WOMAC Function Subscale Scale from: 0 to 68. (follow‐up: mean 6 months) | The mean womac function subscale in the control groups was 21.66 points | The mean WOMAC Function Subscale in the intervention groups was 2.07 lower (3.81 to 0.33 lower) | 624 (3) | ⊕⊕⊕⊕ high | Statistically significant. SMD ‐0.19 (‐0.35 to ‐0.03, see outcome 4.6). Relative per cent change from baseline is ‐7.6% (95% CI ‐14.0%, ‐1.2%). | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; | ||||||
| GRADE Working Group grades of evidance High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 High heterogeneity, I‐squared=92%
2 Funnel plot asymmetrical in favour of positive studies
1. Comparing GS or GH versus placebo: results from 18 RCTs were pooled for the outcome variable of reduction in pain, where pain was measured by a number of different methods (Cibere 2004; Clegg 2006; Crolle 1980; D'ambrosio 1981; Drovanti 1980; Herrero‐Beaumont 2007; Houpt 1999; Hughes 2002; McAlindon 2004; Pavelka 2002; Pujalte 1980; Reginster 2001; Rindone 2000; Rovati 1997; Rozendaal 2008; Usha 2004; Vajaradul 1981; Zenk 2002). The summary standardized mean difference (SMD) (random‐effects model) was ‐0.47 (95% CI ‐0.72 to ‐0.23). A negative SMD in this case meant that glucosamine was significantly superior to placebo in terms of its ability to reduce levels of pain. The relative per cent change from baseline was 22% (using McAlindon 2004 in the calculation as the most representative study).
2. Comparing GS to placebo for the Lequesne Index scores: results from five RCTs were pooled (Herrero‐Beaumont 2007; Noack 1994; Pavelka 2002; Reichelt 1994, Rovati 1997). The summary SMD (random‐effects model) was ‐0.47 (95% CI ‐0.82 to ‐0.12). A negative SMD in this case meant that glucosamine was significantly superior to placebo in terms of its ability to improve Lequesne Index scores. The relative per cent change from baseline was 11% (using Herrero‐Beaumont 2007 in the calculation as the most representative study).
3. Comparing GS to placebo for Lequesne Index scores in which the outcome was dichotomous (per cent responders based on change in Lequesne Index): relative risk ratios (RR) were pooled across two RCTs (Noack 1994; Reichelt 1994). The summary RR (fixed‐effect model) for likelihood of being a responder was 1.52 (95% CI 1.20 to 1.91).
4. Comparing GS or GH to placebo for WOMAC pain subscale scores: the summary SMD (fixed‐effect model) for 10 RCTs (Cibere 2004; Clegg 2006; Herrero‐Beaumont 2007; Houpt 1999; Hughes 2002; McAlindon 2004; Pavelka 2002; Reginster 2001; Rozendaal 2008; Zenk 2002) was ‐0.06 (95% CI ‐0.14 to 0.03). In this outcome there was no statistical difference between glucosamine and placebo.
5. Comparing GS or GH to placebo for WOMAC stiffness subscale scores: the summary SMD (fixed‐effect model) for seven RCTs (Cibere 2004; Clegg 2006; Houpt 1999; Hughes 2002; Pavelka 2002; Rozendaal 2008; Zenk 2002) was ‐0.02 (95% CI ‐0.13 to 0.08). In this outcome there was no statistical difference between glucosamine and placebo.
6. Comparing GS or GH to placebo for WOMAC function subscale scores: the summary SMD (fixed‐effect model) for 10 RCTs (Cibere 2004; Clegg 2006; Herrero‐Beaumont 2007; Houpt 1999; Hughes 2002; McAlindon 2004; Pavelka 2002; Reginster 2001; Rozendaal 2008; Zenk 2002) was ‐0.08 (95% CI ‐0.17 to 0.00). In this outcome there was no statistical difference between glucosamine and placebo.
7. Comparing GS or GH to placebo for WOMAC total scores: the summary SMD (fixed‐effect model) for six RCTs (Cibere 2004; Herrero‐Beaumont 2007; Houpt 1999; Pavelka 2002; Reginster 2001; Zenk 2002) was ‐0.18 (95% CI ‐0.31 to ‐ 0.05). In this outcome there was a statistical difference favoring glucosamine over placebo.
8. Comparing GS to placebo for changes in minimum joint space width for the knee or hip: the summary mean difference (MD) (fixed‐effect model) for two RCTs (Pavelka 2002; Reginster 2001) measuring minimum joint space width at the knee was 0.32 (95% CI 0.05 to 0.58). This was statistically significant in favour of glucosamine and showed that glucosamine slowed the natural radiological progression of OA. Rozendaal 2008 measured minimum joint space width changes in the hip and found inconsistent results in the four sites they measured; however, none of the 95% CI achieved a minimal clinically important change of 0.25 mm.
9. Comparing GS to placebo for patient global assessment: Clegg 2006 measured the patient assessment of disease status score on a 0 to 100 scale and found a non‐statistically significant result (mean difference 1.10, 95% CI ‐2.77 to 4.97). Houpt 1999 asked patients to respond to whether they felt better than at the start of the trial and also found a non‐statistically significant result (RR 1.21, 95% CI 0.80 to 1.82).
10. Comparing GS to placebo for physician global assessment: Clegg 2006 measured the physician assessment of disease status score on a 0 to 100 scale and found a non‐statistically significant result (mean difference 0.80, 95% CI ‐2.78 to 4.38). This corresponds to a relative per cent change from baseline of ‐1.5% (95% CI ‐5.4%, 8.5%). The outcome measured in Drovanti 1980 and Pujalte 1980 was a physician rating of a good or excellent response of their patients. The pooled RR was 2.26 (95% CI 1.49 to 3.43) in favour of glucosamine.
11. Comparing GS to an NSAID (piroxicam, ibuprofen, celecoxib) for the outcome variable of pain, where pain was measured by a number of different methods: the summary pooled SMD (random‐effects model) for four RCTs was ‐0.27 (95% CI ‐0.65 to 0.11) (Clegg 2006; Qiu 1998; Rovati 1997; Vaz 1982). In this outcome there was no statistical difference between glucosamine and NSAIDs.
12. Comparing GS to an NSAID (piroxicam, ibuprofen) for the outcome variable of change in the Lequesne Index scores: the summary SMD (random‐effects model) for two pooled studies was ‐0.36 (95% CI ‐1.07 to 0.35) (Muller‐FassBender 94; Rovati 1997). In this outcome there was no statistical difference between glucosamine and placebo.
One RCT (Mehta 2007) compared GS to a polyherbal supplement (reparagen) and, as such, this unique comparator was not included in the meta‐analyses described above. In this study, a 20% reduction in WOMAC pain was found in 84% of glucosamine participants and 94% of participants taking the polyherbal supplement at eight weeks. The difference between the two groups was not statistically significant.
Sensitivity analysis
Adequate allocation concealment
13. Comparing GS or GH versus placebo in studies with adequate allocation concealment: results from 11 RCTs were pooled for the outcome variable of reduction in pain, where pain was measured by a number of different methods (Cibere 2004; Clegg 2006; Herrero‐Beaumont 2007; Houpt 1999; Hughes 2002; McAlindon 2004; Pavelka 2002; Reginster 2001; Rovati 1997; Rozendaal 2008; Zenk 2002). The summary SMD (random‐effects model) was ‐0.16 (95% CI ‐0.36 to 0.04). In this outcome there was no statistical difference between glucosamine and placebo. This corresponds to a decrease in the treatment group of 0.70 points (95% CI 1.5 points lower to 0.17 points higher) on a 0 to 20 WOMAC scale (using McAlindon 2004 for the calculation). The relative per cent change from baseline was ‐7% (95% CI ‐17%, 1.8%).
14. Comparing GS to placebo in studies with adequate allocation concealment for the Lequesne Index scores: results from four RCTs were pooled (Herrero‐Beaumont 2007; Noack 1994; Rovati 1997; Pavelka 2002). The summary SMD (random‐effects model) was ‐0.54 (95% CI ‐0.96 to ‐0.12). A negative SMD in this case meant that glucosamine was significantly superior to placebo in terms of its ability to improve Lequesne index scores.
15. Comparing GS to placebo in studies with adequate allocation concealment for Lequesne Index scores in which the outcome was dichotomous (per cent responders based on change in Lequesne index): The summary RR (fixed‐effect model) from one RCT (Noack 1994) for likelihood of being a responder was 1.43 (95% CI 1.08 to 1.91).
16. Comparing GS or GH to placebo in studies with adequate allocation concealment for WOMAC pain subscale scores: the summary SMD (fixed‐effect model) for 10 RCTs (Cibere 2004; Clegg 2006; Herrero‐Beaumont 2007; Houpt 1999; Hughes 2002; McAlindon 2004; Pavelka 2002; Reginster 2001; Rozendaal 2008; Zenk 2002) was ‐0.06 (95% CI ‐0.14 to 0.03). In this outcome there was no statistical difference between glucosamine and placebo. This corresponds to a decrease in the treatment group of 0.25 points (95% CI 0.59 points lower to 0.13 points higher) on a 0 to 20 WOMAC scale (using McAlindon 2004 for the calculation).
17. Comparing GS or GH to placebo in studies with adequate allocation concealment for WOMAC stiffness subscale scores: The summary SMD (fixed‐effect model) for seven RCTs (Cibere 2004; Clegg 2006; Houpt 1999; Hughes 2002; Pavelka 2002; Rozendaal 2008; Zenk 2002) was ‐0.02 (95% CI ‐0.13 to 0.08). In this outcome there was no statistical difference between glucosamine and placebo.
18. Comparing GS or GH to placebo in studies with adequate allocation concealment for WOMAC function subscale scores: the summary SMD (fixed‐effect model) for 10 RCTs (Cibere 2004; Clegg 2006; Herrero‐Beaumont 2007; Houpt 1999; Hughes 2002; McAlindon 2004; Pavelka 2002; Reginster 2001; Rozendaal 2008; Zenk 2002) was ‐0.08 (95% CI ‐0.17 to 0.00). In this outcome there was no statistical difference between glucosamine and placebo. This corresponds to a decrease in the treatment group of 1.02 points (95% CI 2.04 points lower to 0 points higher) on a 0 to 68 WOMAC scale. The relative per cent change from baseline was ‐3% (95% CI ‐6%, 0%).
19. Comparing GS or GH to placebo in studies with adequate allocation concealment for WOMAC total scores: the summary SMD (fixed‐effect model) for six RCTs (Cibere 2004; Herrero‐Beaumont 2007; Houpt 1999; Pavelka 2002; Reginster 2001; Zenk 2002) was ‐0.18 (95% CI ‐0.31 to ‐ 0.05). In this outcome, there was a statistically significant difference which favored glucosamine over placebo.
20. Comparing GS to placebo for changes in minimum joint space width for the knee or hip: the summary mean difference (MD) (fixed‐effect model) for two RCTs (Pavelka 2002; Reginster 2001) measuring the minimum joint space width at the knee was 0.32 (95% CI 0.05 to 0.58). This was statistically significant in favour of glucosamine and showed that glucosamine slowed the natural radiological progression of OA. Rozendaal 2008 measured minimum joint space width changes in the hip and found inconsistent results in the four sites they measured; however, none of the 95% CIs achieved a minimal clinically important change of 0.25 mm.
The main outcomes for this comparison are summarized in the Summary of findings table 1.
Subgroup analysis
Rotta preparation 21. Comparing GS to placebo in studies using the Rotta preparation for pain: results from eight RCTs were pooled (Crolle 1980; D'ambrosio 1981; Drovanti 1980; Herrero‐Beaumont 2007; Pavelka 2002; Pujalte 1980; Reginster 2001; Rovati 1997) for the outcome variable of reduction in pain. The summary SMD (random‐effects model) was ‐1.11 (95% CI ‐1.66 to ‐0.57). A negative SMD in this case meant that glucosamine was significantly superior to placebo in terms of its ability to reduce pain. The relative per cent change from baseline was ‐42.2% (95% CI ‐63.0%, ‐21.6%) (using Herrero‐Beaumont 2007 in the calculation as the most representative study).
22. Comparing GS to placebo in studies using the Rotta preparation for the Lequesne Index scores: results from five RCTs were pooled (Herrero‐Beaumont 2007; Noack 1994; Pavelka 2002; Reichelt 1994; Rovati 1997). The summary SMD (random‐effects model) was ‐0.47 (95% CI ‐0.82 to ‐0.12). A negative SMD in this case meant that glucosamine was significantly superior to placebo in terms of its ability to improve Lequesne Index scores.
23. Comparing GS to placebo in studies using the Rotta preparation for Lequesne Index scores in which the outcome was dichotomous (per cent responders based on change in Lequesne Index): the summary RR (fixed‐effect model) from two RCTs (Noack 1994; Reichelt 1994) for likelihood of being a responder was 1.52 (95% CI 1.20 to 1.91).
24. Comparing GS to placebo in studies using the Rotta preparation for WOMAC pain subscale scores: results from three RCTs were pooled (Herrero‐Beaumont 2007; Pavelka 2002; Reginster 2001). The summary SMD (fixed‐effect model) was ‐0.17 (95% CI ‐0.32 to ‐0.01). In this outcome glucosamine was significantly superior to placebo in terms of its ability to improve WOMAC pain scores.
25. Comparing GS to placebo in studies using the Rotta preparation for WOMAC stiffness subscale scores: the result from one RCT (Pavelka 2002) was ‐0.22 (95% CI ‐0.50 to 0.06). In this outcome there was no statistical difference between glucosamine and placebo.
26. Comparing GS to placebo in studies using the Rotta preparation for WOMAC function subscale scores: results from three RCTs were pooled (Herrero‐Beaumont 2007; Pavelka 2002; Reginster 2001). The summary SMD (fixed‐effect model) was ‐0.19 (95% CI ‐0.35 to ‐0.03). In this outcome glucosamine was significantly superior to placebo in terms of its ability to improve WOMAC function scores. The relative per cent change from baseline was ‐7.6% (95% CI ‐14.0%, ‐1.2%) (using Herrero‐Beaumont 2007 in the calculation as the most representative study).
27. Comparing GS to placebo using the Rotta preparation for WOMAC total scores: the summary SMD (fixed‐effect model) for three RCTs (Herrero‐Beaumont 2007; Pavelka 2002; Reginster 2001) was ‐0.25 (95% CI ‐0.40 to ‐0.09). A negative SMD in this case meant that glucosamine was statistically significantly superior to placebo.
The main outcomes for this comparison are summarized in the Summary of findings table 2.
Non‐Rotta preparation 28. Comparing GS to placebo in studies using a non‐Rotta preparation for pain: results from 10 RCTs were pooled (Cibere 2004; Clegg 2006; Houpt 1999; Hughes 2002; McAlindon 2004; Rindone 2000; Rozendaal 2008; Usha 2004; Vajaradul 1981; Zenk 2002) for the outcome variable of reduction in pain. The summary SMD (fixed‐effect model) was ‐0.05 (95% CI ‐0.15 to 0.05). In this outcome there was no statistical difference between glucosamine and placebo.
29. Comparing GS to placebo in studies using a non‐Rotta preparation for WOMAC pain subscale scores: results from seven RCTs were pooled (Cibere 2004; Clegg 2006; Hughes 2002; Houpt 1999; McAlindon 2004; Rozendaal 2008; Zenk 2002). The summary SMD (fixed‐effect model) was ‐0.01 (95% CI ‐0.11 to 0.10). In this outcome there was no statistical difference between glucosamine and placebo.
30. Comparing GS to placebo in studies using a non‐Rotta preparation for WOMAC stiffness subscale scores: results from six RCTs were pooled (Cibere 2004; Clegg 2006; Houpt 1999; Hughes 2002; Rozendaal 2008; Zenk 2002). The summary SMD (fixed‐effect model) was 0.01 (95% CI ‐0.10 to 0.13). In this outcome there was no statistical difference between glucosamine and placebo.
31. Comparing GS to placebo in studies using a non‐Rotta preparation for WOMAC function subscale scores: results from six RCTs were pooled (Cibere 2004; Clegg 2006; Houpt 1999; Hughes 2002; Rozendaal 2008; Zenk 2002). The summary SMD (fixed‐effect model) was ‐0.01 (95% CI ‐0.13 to 0.10). In this outcome there was no statistical difference between glucosamine and placebo.
32. Comparing GS or GH to placebo using the non‐Rotta preparation for WOMAC total scores: the summary SMD (fixed‐effect model) for three RCTs (Cibere 2004; Houpt 1999; Zenk 2002) was ‐0.02 (95% CI ‐0.27 to 0.22). In this outcome there was no statistical difference between glucosamine and placebo.
Toxicity of glucosamine in OA
The safety profile of glucosamine in the 25 RCTs was excellent. For example, out of the 1883 participants randomized to glucosamine treatment in the RCTs, only 62 (3.3%) were withdrawn because of toxicity. The total number of participants reporting an adverse reaction was 436 (30%) based on 21 RCTs (n = 1451). In the 1482 participants randomized to a placebo group, 70 (4.7%) were withdrawn because of toxicity and 418 (39%) reported an adverse reaction (n = 1061). Therefore, glucosamine was as safe as placebo.
33. Comparing GS or GH to placebo for number of participants reporting adverse reactions: the summary RR (fixed‐effect model) for 13 RCTs was 0.99 (95% CI 0.91 to 1.07).
34. Comparing GS or GH to placebo for number of withdrawals due to toxicity: the summary RR (fixed‐effect model) for 12 RCTs was 0.76 (95% CI 0.55 to 1.05).
35. Comparing GS to NSAIDs for number of participants reporting adverse reactions: the summary RR (fixed‐effect model) for four RCTs was 0.29 (95% CI 0.19 to 0.44). Therefore, GS was significantly less likely than NSAIDs to produce adverse reactions.
36. Comparing GS to NSAIDs for number of withdrawals due to toxicity: the summary RR (random‐effects model) for four RCTs was 0.16 (95% CI 0.02 to 1.46). Therefore, glucosamine was significantly less likely than NSAIDs to result in withdrawal due to toxicity.
Discussion
The pooled SMD for pain reduction comparing glucosamine to placebo was 0.47, which represents a moderate clinically significant treatment benefit in favour of glucosamine and a relative difference in the change from baseline of 22%. SMDs can be interpreted as effect sizes. Cohen defined an effect size of 0.20 as small, of 0.50 as moderate, and of 0.80 or greater as large (Cohen 1977). For function, measured by the Lequesne Index, the pooled SMD compared to placebo favoured glucosamine (SMD ‐0.47, 95% CI ‐0.82 to ‐0.12). This corresponds to a difference in the change from baseline between glucosamine and placebo of 1.2 units on the Lequesne scale, or 11% of baseline. The RCTs comparing GS to an NSAID collectively suggest that GS produces similar symptomatic benefits as NSAIDs, but with a much lower probability of resultant adverse reactions. These results are very important and suggest that glucosamine therapy may represent a significant breakthrough in the pharmacological management of OA. However, not all outcomes and not all RCTs demonstrated a consistent superiority of glucosamine over placebo. For example, in all of the analyzed RCTs collectively, none of the WOMAC outcomes of pain, function and stiffness showed a superiority of glucosamine over placebo. Furthermore, analysis restricted to those studies with adequate allocation concealment did not demonstrate a superiority of glucosamine over placebo for pain (based on a pooled measure of different pain scales), WOMAC pain or WOMAC function. Therefore, the results in favor of glucosamine are mixed, and further research is still needed to clarify the true efficacy of glucosamine in OA.
Subgroup analyses of the Rotta preparation showed significant benefit over placebo in terms of pain and in the outcomes of the Lequesne index as well as in the WOMAC pain and function scores. For non‐Rotta preparations, the pooled results show that there was no statistically significant difference in terms of pain and WOMAC pain, stiffness and function scores.
Although most of the RCTs show clear superiority of glucosamine over placebo in OA, seven RCTs failed to show that glucosamine was better than placebo (Cibere 2004; Clegg 2006; Houpt 1999; Hughes 2002; McAlindon 2004; Rindone 2000; Rozendaal 2008). It is not entirely clear how to reconcile the negative results from these RCTs with the favorable results from the other RCTs (McAlindon 2003; Towheed 2003; Towheed 2007). However, the seven negative RCTs did not use the Rotta brand of GS and also had either a limited or no affiliation with a manufacturer of glucosamine. (The trial by McAlindon 2004 did use the Rotta preparation but in only about 10% of the participants treated with glucosamine.) The study by Houpt 1999 was the first RCT that used glucosamine hydrochloride rather than glucosamine sulfate. Two subsequent RCTs also used the GH preparation. In the Clegg study (Clegg 2006) GH was no better than placebo overall. However, exploratory analyses suggested that the combination of glucosamine and chondroitin sulfate may be effective in the subgroup of patients with moderate to severe knee pain. In the Qiu (Qiu 2005) study, GS and GH were directly compared and the results showed that there was no significant differences in either efficacy or safety between the two glucosamine preparations. The study by Rindone 2000 had a mostly male population of enrolled participants (only 5% were female, which contrasts with the average female inclusion rate in the remaining RCTs of 69%). Furthermore, participants in this study tended to be older and heavier, with a longer duration of OA, and also had a greater degree of radiographic severity than participants evaluated in other RCTs. One additional important aspect of the Rindone 2000 study was that participants who were taking other analgesics at baseline were instructed to continue them for the duration of the study. However, no details are provided with respect to the actual usage of these analgesics during the course of the study or at the end of the study. The use of additional therapeutic interventions may have resulted in less power to be able to detect any significant differences in efficacy. The study by Hughes (Hughes 2002) had almost 50% of the participants taking NSAIDs at baseline and these participants were allowed to continue using these drugs during the course of the trial. Once again, it is possible that the high frequency of additional therapy allowed may have affected the power to be able to detect any significant differences in efficacy.
Perhaps the major limitation with extrapolating the generally favorable results from the glucosamine RCTs lies in the fact that most of the studies (56%) in the Cochrane review exclusively evaluated the prescription medicine made by the Rotta Pharmaceutical Company (a GS preparation that is approved as a prescription drug for OA in the European Union countries). In North America, glucosamine is not considered a conventional prescription drug, rather it is considered as a dietary supplement which is widely available as an over‐the‐counter preparation. Since the content and purity of the various over‐the‐counter preparations is known to vary markedly, the relative efficacy and safety of the various preparations may also vary markedly (Adebowale 2000; Consumer Reports 2002; Deal 1999; Russell 2002). Adebowale (Adebowale 2000) analyzed the actual content of glucosamine and chondroitin within several products in the marketplace with the objective of determining if the content deviated from the label claim. The amounts of glucosamine and chondroitin found after analysis were significantly different from the label claims in some products, with deviations ranging from as low as 0% to over 115%. Russell (Russell 2002) assessed the content of active ingredients in over‐the‐counter GS preparations. The amount of free base varied from 41% to 108% of the mg content stated on the label; the amount of glucosamine varied from 59% to 138% even when expressed as sulfate. These authors concluded that if GS is to be used as a therapeutic agent, it is important that the products conform to a standard in their description.
Glucosamine therapy for OA was found to be relatively well tolerated with a safety profile similar to placebo, and significantly better than NSAIDs. An open study carried out by 252 physicians throughout Portugal evaluated the tolerability of GS in 1208 patients (Tapadinhas 1982). Patients were treated with GS for a mean duration of 50 days (range 13 to 99 days). Eighty‐eight per cent of the study population were free of any adverse effects. In the 12% experiencing adverse effects, the reactions were generally mild in severity and predominantly affected the gastrointestinal tract. All of the reported complaints were reversible after discontinuation of GS.
In a series of letters to the Editor of the Lancet, a number of possible adverse reactions with glucosamine were pointed out. Chan (Chan 2001) expressed concern about the potential adverse effects of long‐term glucosamine therapy on glucose homeostasis, citing evidence that glucosamine may increase insulin resistance and/or impair insulin secretion (Monauni 2000). They also described a case of a patient with metastatic insulinoma who achieved control of hypoglycemic episodes after starting GS for OA. They cautioned that glucose monitoring is warranted in people on long‐term therapy, and that glucosamine should be used cautiously in patients with diabetes. These concerns have not appeared to be problems in the RCTs, however they do warrant further investigation and monitoring. A recently published three‐month RCT further evaluated the possible effects of glucosamine supplementation on glycemic control in a selected population of patients with type 2 diabetes mellitus (Scroggie 2003). The main outcome measure was hemoglobin A1c levels before and after 90 days of therapy. This study demonstrated that oral glucosamine supplementation did not result in clinically significant alterations in glucose metabolism in patients with type 2 diabetes mellitus. A recently published prospective study by Tannis 2004 found that glucosamine supplementation, with normal recommended dosages, did not cause glucose intolerance in healthy adults. Pham et al (Pham 2007) reported that oral glucosamine in doses used to treat OA worsens insulin resistance in people without a known abnormality of glucose homeostasis. The methodology involved measuring fasting insulin and glucose, and vascular elasticity measurements using pulse wave analysis. Individuals with underlying poorer insulin sensitivity are at risk of worsening insulin resistance and vascular function with the use of glucosamine. Biggee et al (Biggee 2007) also reported that glucosamine ingestion may affect glucose levels and consequent glucose uptake in patients who have untreated diabetes or glucose intolerance. In contrast, Muniyappa et al (Muniyappa 2006) reported that oral glucosamine in standard doses for six weeks does not cause or significantly worsen insulin resistance or endothelial dysfunction in lean or obese subjects.
In the first report regarding the safety of glucosamine therapy in pregnancy, Sivojelezova et al (Sivojelezova 2007) were able to track the pregnancy‐related outcomes of 54 women who used glucosamine during pregnancy. These limited data found no increased risk for major malformations or other adverse fetal effects following the use of glucosamine.
There are additional reports of possible glucosamine‐related toxicity. Goldstein (Goldstein 2001) speculated that glucosamine may contribute to the development of atherosclerosis by stimulating proteoglycan production by the smooth muscle cells in the arterial wall. Although the theory seems intriguing, there is as yet no experimental or clinical evidence to support its validity. Swinburne (Swinburne 2001) reported the observation that glucosamine has a powerful stimulatory effect on the growth rate and toughness of the nails. The clinical significance of this observation as it pertains to the treatment of OA is not clear. Tallia (Tallia 2002) reported a case of a possible asthma exacerbation associated with use of an oral glucosamine‐chondroitin supplement. According to the authors, the cause and effect association in this case could not be confirmed definitively. Matheu (Matheu 1999) reported a case of an immediate hypersensitivity reaction to glucosamine sulfate, manifesting with angioedema. Glucosamine therapy may also be associated with a risk of acute interstitial nephritis (Audimoolam 2006).
The meta‐analysis by McAlindon 2000 provided a useful critical analysis of some of the pertinent methodological concerns associated with the published glucosamine trials. Their main finding was that although glucosamine is likely to be an effective therapy for OA, the actual degree of symptomatic benefit is probably less than that predicted by the available trials. This is because of methodological flaws in many of the published trials. These flaws have been associated with exaggerated estimates of treatment benefit. Methodological flaws identified in the trials included inadequate allocation concealment, absence of intention‐to‐treat analyses, and publication bias. Although it seems unlikely, it is still possible that these biases could significantly negate the supposed efficacy of glucosamine. The main differences between this systematic review and the McAlindon review are that this is a Cochrane Review and it includes the comparators, as opposed to the glucosamine versus placebo comparison alone. Also, the Cochrane review has been updated as of January 2008.
Another meta‐analysis was published by Richy 2003 comparing the structural and symptomatic efficacy of glucosamine and chondroitin in relation to placebo. Fifteen placebo‐controlled RCTs, published or performed between 1980 and March 2002, were analyzed. The results of this high quality meta‐analysis demonstrated that there was a highly significant structural and symptomatic efficacy of glucosamine on all outcomes studied, including joint space narrowing and WOMAC. The safety of glucosamine was excellent and was similar to placebo. The results of our Cochrane review corroborate the results obtained by Richy 2003.
The 12‐week study by McAlindon (McAlindon 2004) deserves special mention since it is the only trial that was conducted exclusively over the Internet. Participants were recruited and followed entirely over the Internet. This is a unique method of conduct of a RCT and the authors have nicely demonstrated the efficiency and methodological aspects of this approach. The study by Cibere (Cibere 2004) also deserves special mention since it is the only trial that was designed as a glucosamine discontinuation trial. All eligible participants must have been actively using glucosamine for OA for at least one month prior to study entry and they must also have reported having at least a moderate improvement in knee pain since starting on glucosamine. The results of this well‐conducted study were negative and failed to demonstrate efficacy of continuing glucosamine versus a placebo over a 24‐week period.
A recently published systematic review and analysis by Vlad et al (Vlad 2007) attempted to identify factors that best explain the marked heterogeneity in trials of glucosamine in OA. They concluded that the heterogeneity with glucosamine is larger than would be expected by chance alone, and that GH is not effective. Among trials with industry involvement, effect sizes were consistently higher. Potential explanations included different glucosamine preparations, inadequate allocation concealment, and industry bias. These are some of the same issues that our Cochrane Review has also identified to be of significant importance in the proper interpretation of RCTs evaluating glucosamine in OA.
The mechanisms of action of glucosamine in OA are not known. However, glucosamine is a natural substance and a 'building block' of the glycosaminoglycans (GAG) and glycoproteins in the ground substance of the articular cartilage. In vitro studies have shown that adding GS to human chondrocytes results in increased proteoglycan synthesis (Bassleer 1992; Bassleer 1998; Setnikar 1992). GS has a beneficial effect on animal models of experimental arthritis and may also have anti‐inflammatory properties (Conrozier 1998; Setnikar 1991a; Setnikar 1991b; Setnikar 2001). It has recently been speculated that the sulfate moiety of GS may actually mediate the clinical benefit of GS in OA (Hoffer 2002).
Pharmacokinetic studies on glucosamine and GS have been carried out primarily by investigators from the Rotta Research Laboratory in Italy (the sponsor of most of the RCTs reviewed here). Early work with 14C‐glucosamine administered intravenously or orally in rats indicated that, after intravenous administration, radioactivity in the plasma declined in the first 30 minutes and reached a tissue peak in the second hour and then started to disappear, with half disappearance at 28 hours. Radioactivity was detected in all tissues (primarily liver and kidney), and early in skeletal tissues including cartilage (Setnikar 1984). About 50% of the expired 14C radioactivity appeared as expired CO 2 and 35% in the urine. After oral administration, there was only a small fecal excretion but 85% of the 14C radioactivity appeared as CO 2 , indicating that there was extensive breakdown of the 14C‐glucosamine into smaller fragments. There were no measurements of free 14C‐glucosamine reported in this study.
Pharmacokinetic studies in dog and in man (Setnikar 1986) with intravenously administered 14C‐GS showed that there was a very rapid disappearance of the radioactivity due to glucosamine (t ½ = 0.28 hr) and that this was rapidly replaced by radioactivity originating from the plasma proteins, into which the 14C‐glucosamine and its metabolites are incorporated. Free 14C‐glucosamine was looked for but could not be detected, apparently at any time point, according to this report. The findings were similar with intramuscularly administered 14C‐GS (Setnikar 1993). Only a very small proportion of the 14C radioactivity was found in the femoral cartilage compared to other organs. In the earlier study by Setnikar et al (Setnikar 1986), large amounts of non‐radioactive GS were also administered intravenously and orally to six human volunteers. The ninhydrin amino‐acid analyzer method was used for detection of the serum glucosamine (Optica instrument) with a detection limit of about 10 ug/ml. After 800 mg GS was administered intravenously, plasma levels fell rapidly with a first disappearance rate or half life (t ½) of 6.5 minutes and a terminal rate (t ½) of 2.1 hours. However, after oral administration of a single dose of 6000 mg of GS to the human volunteers, plasma glucosamine was below the detection limit at all tested times. It should be noted that the usual oral dosage of GS in the reviewed RCTs was 1500 mg/day. Further studies in six healthy human male volunteers were done with 14C‐GS administered in single doses by the intravenous, intramuscular, and oral routes (Setnikar 1993). After intravenous administration, the radioactivity due to glucosamine declined rapidly with an initial t ½ of 0.28 hr. After one to two hours, the plasma radioactivity originated entirely from plasma proteins. Less than 1% of the radioactivity was found in the feces, and 28% was found in the urine. The pharmacokinetics of intramuscular administration of 14C‐GS were similar to the intravenous route. Free glucosamine was not detected in the plasma. Much lower levels of radioactivity were found after oral administration, apparently due to metabolism to carbon dioxide (CO 2 ), water and urea (Setnikar 1993).
More recent pharmacokinetic studies of glucosamine were reported by Biggee (Biggee 2006), Laverty (Laverty 2005), and Persiani (Persiani 2005a; Persiani 2005b). Biggee et al (Biggee 2006) used high performance liquid chromatography with a high sensitivity Metrohm‐Peak instrument for pulsed amperometric measurement of human serum glucosamine. The maximum concentration achieved after oral ingestion of 1500 mg of the Rotta preparation of glucosamine was only 11.5 uM (range 1.9 to 11.5 uM). Previous studies by these same authors have shown that, at this maximum concentration of 11.5 uM, one would expect that no more than 2% of the galactosamine incorporated into chondroitin sulfate would be derived from incubations of glucosamine with cultured human chondrocytes (Mroz 2004). The authors concluded that insignificant trace amounts of glucosamine enter human serum after oral ingestion and that this amount is far below any amount that might contribute directly to chondroitin synthesis. Therefore, based on this study, glucosamine probably does not act in OA simply by acting as a 'building block' for the synthesis of glycosaminoglycans in the articular cartilage. Previously published in vitro studies generally used very high concentrations of glucosamine (> 100 uM) to show positive effects in terms of the enhanced synthesis of glycosaminoglycans in the articular cartilage.
Laverty et al (Laverty 2005) reported the first published animal data confirming that free glucosamine can be detected in the synovial fluid after administration. They measured free glucosamine concentrations both in the serum and in the synovial fluid of the radiocarpal joints in horses using a sensitive fluorophore‐assisted carbohydrate electrophoresis. Two methods of administration of glucosamine were evaluated (via a nasogastric tube and intravenous administration). Following nasogastric administration, the maximum serum concentration of glucosamine was only 6.1 uM (range 4.4 to 7.6 uM). This is a result similar to that obtained by Biggee et al (Biggee 2006). Following intravenous administration, the maximum concentration of glucosamine in serum reached 300 uM. Synovial fluid concentrations reached 9 to 15 uM with intravenous dosing, and 0.3 to 0.7 uM with nasogastric dosing. The authors concluded that clinically relevant dosing of glucosamine in the horse results in serum and synovial fluid concentrations that are at least 500 fold lower than those reported to modify chondrocyte activities in tissue and cell culture experiments. Persiani and colleagues (Persiani 2005a; Persiani 2005b) used a liquid chromatography method with mass spectrometry detection for the determination of glucosamine in the plasma of humans who ingested clinically relevant dosages of glucosamine. Maximum serum concentrations were again found to be only in the 10 uM range. Preliminary data from these authors were reported in an abstract from the 2005 EULAR meeting (Persiani 2005b). For the first time, free glucosamine was detected in the knee synovial fluid in two patients with OA who were assayed 24 hours post oral ingestion in a 15‐day treatment course (1500 mg/day of GS), in concentrations of 497 and 1978 ng/ml. In these two patients, there was a good correlation between the plasma and synovial fluid levels. One cannot exclude the possibility that the observed low sustained concentrations of glucosamine in the synovial joint may have biological properties that are therapeutic in OA (for example anti‐inflammatory effects or inhibition of IL‐1 related effects, or both) (Largo 2003; Shikhman 2001).
Three RCTs have evaluated the ability of glucosamine to protect the cartilage from further loss, as defined by changes in radiographic joint cartilage width of the knee (Pavelka 2002; Reginster 2001) and hip (Rozendaal 2008). The two well‐conducted prospective three‐year RCTs by Pavelka (Pavelka 2002) and Reginster (Reginster 2001) showed favourable responses with no significant progression of cartilage loss in participants assigned to therapy with GS. These RCTs represent the first clinical evidence that glucosamine may indeed modify the natural radiological progression of OA of the knee (Reginister 2003). The most recent study by Rozendaal et al (Rozendaal 2008) was not able to confirm a beneficial effect of a non‐Rotta preparation of GS on radiological progression in participants with OA of the hip.
Authors' conclusions
Implications for practice.
The previous review from 2005, with 20 studies and 2570 participants, showed that glucosamine sulphate taken orally in amounts of 1500 mg/day produced a 28% (per cent change from baseline) benefit in pain and an increase in function of 21% (per cent change in Lequesne Index from baseline) in osteoarthritis, without side effects.
If only the best designed studies are included, the benefit in pain and WOMAC function is no longer present; as shown in this update which includes 25 studies and 4963 patients. Inclusion of five new studies reduces the overall benefit on pain to 22% and function to 11% in the Lequesne Index. Pooled results from studies using a non‐Rotta preparation or adequate allocation concealment failed to show benefit in pain and WOMAC function, while those studies evaluating the Rotta preparation showed that glucosamine was superior to placebo in the treatment of pain and functional impairment resulting from symptomatic OA. WOMAC outcomes of pain and function showed a superiority of glucosamine over placebo for only the Rotta preparation of glucosamine.
Some studies suggest the Rotta preparation of glucosamine sulfate may slow radiological progression of OA of the knee over a three‐year period. The ability of glucosamine to improve symptoms and delay radiological progression of OA affecting other joint sites also needs further research.
Glucosamine was as safe as placebo.
Implications for research.
Despite the availability of several RCTs, there are still a number of questions that remain unanswered regarding the use of glucosamine in OA. These questions represent areas for further research. First, are the various over‐the‐counter glucosamine preparations sold by different manufacturers equally effective (and equally safe) in the therapy of OA? Is there any further benefit obtained by using mixed glucosamine preparations that contain additional therapeutic products, such as chondroitin sulfate. Second, why are the trials no longer uniformly positive? Third, is glucosamine helpful for all patients with OA, involving different joints and at different stages of severity? Fourth, are the dose and route of administration of glucosamine important in maximizing efficacy and minimizing toxicity? Fifth, how does glucosamine work in the treatment of OA? Sixth, what are the patient‐specific predictors of favorable effects on radiological progression of OA?
What's new
| Date | Event | Description |
|---|---|---|
| 12 November 2008 | New search has been performed | 5 new studies included in this update. |
History
Review first published: Issue 1, 2000
| Date | Event | Description |
|---|---|---|
| 18 June 2008 | Amended | Converted to new review format. C040‐R |
| 23 February 2005 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We would like to thank Jessie McGowan for her assistance in providing the electronic search of the literature. The authors would also like to thank Lindi Jiang and Xiaohong Zhou for their assistance with data abstraction. Additionally, the authors would like to thank the Cochrane Musculoskeletal Editorial Team for their thoughtful comments and suggestions.
Appendices
Appendix 1. Electronic search strategy
1. exp osteoarthritis/ 2. (degenerative adj2 arthritis). tw. 3. osteoarthr$.tw. 4. or/1‐3 5. exp glucosamine/ 6. glucosamine.rn, tw. 7. acetylglucosamine.tw, rn. 8. n‐acetylglucosamine.tw. 9. n‐acetyl‐d‐glucosamine.tw. 10. or/5‐9 11. 4 and 10 12. limit 11 to human
Data and analyses
Comparison 1. Glucosamine versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain | 18 | 2543 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐0.72, ‐0.23] |
| 2 Lequesne Index | 5 | 951 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐0.82, ‐0.12] |
| 3 Lequesne Index | 2 | 407 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [1.20, 1.91] |
| 4 WOMAC Pain Subscale | 10 | 2017 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.14, 0.03] |
| 5 WOMAC Stiffness Subscale | 7 | 1390 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.13, 0.08] |
| 6 WOMAC Function Subscale | 10 | 2017 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.08 [‐0.17, 0.00] |
| 7 WOMAC Total | 6 | 882 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.18 [‐0.31, ‐0.05] |
| 8 Mean Joint Space Width | 1 | 212 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.20, 0.34] |
| 9 Minimum Joint Space Width | 2 | 414 | Mean Difference (IV, Fixed, 95% CI) | 0.32 [0.05, 0.58] |
| 10 Patient global assessment of disease status score (0‐100mm scale) | 1 | 630 | Mean Difference (IV, Fixed, 95% CI) | 1.10 [‐2.77, 4.97] |
| 11 Patient global assessment ‐ number responding they are better than at start of trial | 1 | 118 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.80, 1.82] |
| 12 Physician global assessment of disease status score (0‐100) | 1 | 630 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [‐2.78, 4.38] |
| 13 Physician global assessment of good or excellent response | 2 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.26 [1.49, 3.43] |
| 14 Osteoarthritis Research Society International Responder Criteria (OARSI) | 3 | 918 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.83, 1.83] |
| 15 Toxicity (Number of Patients Reporting Adverse Events) | 16 | 2117 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.91, 1.07] |
| 16 Toxicity (Number of Withdrawals due to Adverse Events) | 20 | 2970 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.55, 1.05] |
1.1. Analysis.
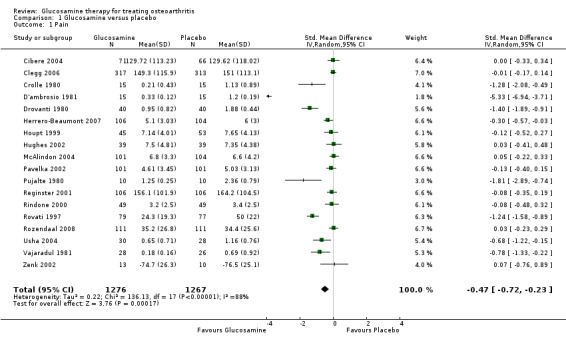
Comparison 1 Glucosamine versus placebo, Outcome 1 Pain.
1.2. Analysis.
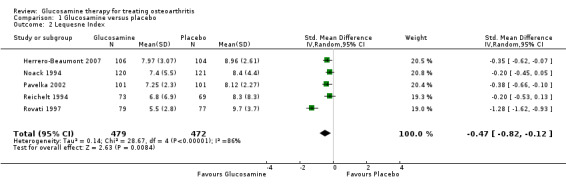
Comparison 1 Glucosamine versus placebo, Outcome 2 Lequesne Index.
1.3. Analysis.

Comparison 1 Glucosamine versus placebo, Outcome 3 Lequesne Index.
1.4. Analysis.

Comparison 1 Glucosamine versus placebo, Outcome 4 WOMAC Pain Subscale.
1.5. Analysis.
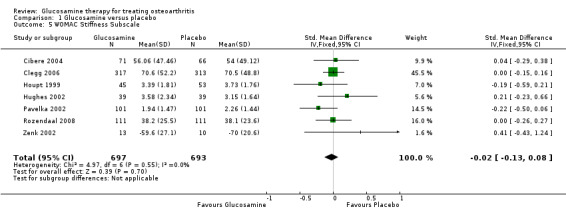
Comparison 1 Glucosamine versus placebo, Outcome 5 WOMAC Stiffness Subscale.
1.6. Analysis.

Comparison 1 Glucosamine versus placebo, Outcome 6 WOMAC Function Subscale.
1.7. Analysis.
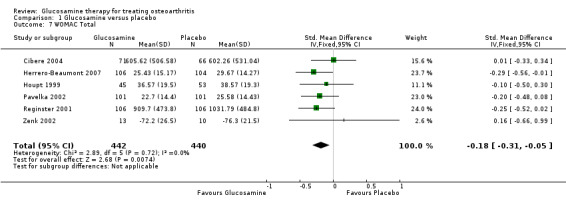
Comparison 1 Glucosamine versus placebo, Outcome 7 WOMAC Total.
1.8. Analysis.
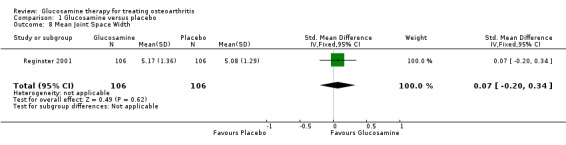
Comparison 1 Glucosamine versus placebo, Outcome 8 Mean Joint Space Width.
1.9. Analysis.
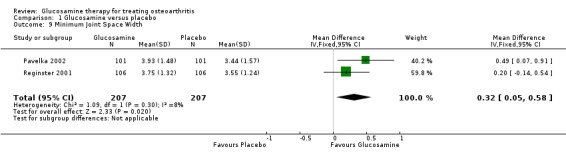
Comparison 1 Glucosamine versus placebo, Outcome 9 Minimum Joint Space Width.
1.10. Analysis.
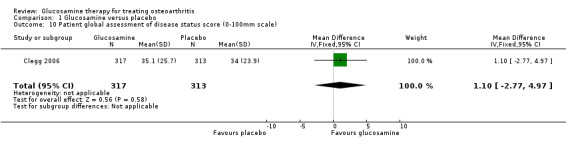
Comparison 1 Glucosamine versus placebo, Outcome 10 Patient global assessment of disease status score (0‐100mm scale).
1.11. Analysis.
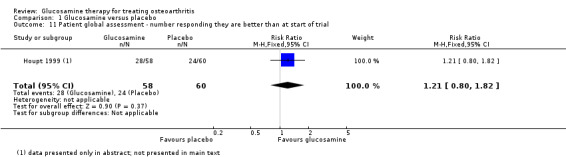
Comparison 1 Glucosamine versus placebo, Outcome 11 Patient global assessment ‐ number responding they are better than at start of trial.
1.12. Analysis.

Comparison 1 Glucosamine versus placebo, Outcome 12 Physician global assessment of disease status score (0‐100).
1.13. Analysis.
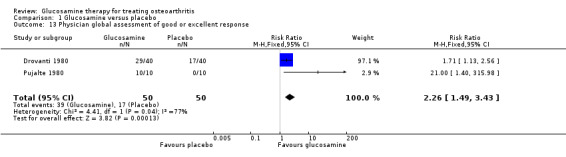
Comparison 1 Glucosamine versus placebo, Outcome 13 Physician global assessment of good or excellent response.
1.14. Analysis.

Comparison 1 Glucosamine versus placebo, Outcome 14 Osteoarthritis Research Society International Responder Criteria (OARSI).
1.15. Analysis.

Comparison 1 Glucosamine versus placebo, Outcome 15 Toxicity (Number of Patients Reporting Adverse Events).
1.16. Analysis.
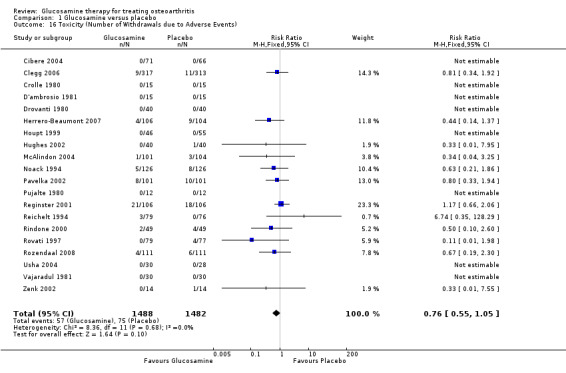
Comparison 1 Glucosamine versus placebo, Outcome 16 Toxicity (Number of Withdrawals due to Adverse Events).
Comparison 2. Glucosamine versus NSAIDs (piroxicam, ibuprofen, celecoxib).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain | 4 | 997 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.65, 0.11] |
| 2 Lequesne Index | 2 | 345 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.36 [‐1.07, 0.35] |
| 3 Toxicity (Number of Patients Reporting Adverse Events) | 4 | 580 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.19, 0.44] |
| 4 Toxicity (Number of Withdrawals due to Adverse Events) | 5 | 1215 | Risk Ratio (M‐H, Random, 95% CI) | 0.16 [0.02, 1.46] |
2.1. Analysis.

Comparison 2 Glucosamine versus NSAIDs (piroxicam, ibuprofen, celecoxib), Outcome 1 Pain.
2.2. Analysis.

Comparison 2 Glucosamine versus NSAIDs (piroxicam, ibuprofen, celecoxib), Outcome 2 Lequesne Index.
2.3. Analysis.
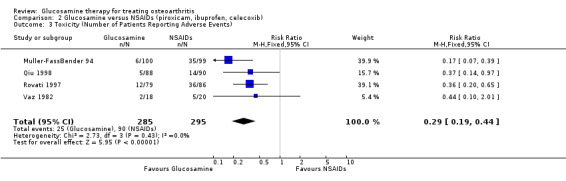
Comparison 2 Glucosamine versus NSAIDs (piroxicam, ibuprofen, celecoxib), Outcome 3 Toxicity (Number of Patients Reporting Adverse Events).
2.4. Analysis.

Comparison 2 Glucosamine versus NSAIDs (piroxicam, ibuprofen, celecoxib), Outcome 4 Toxicity (Number of Withdrawals due to Adverse Events).
Comparison 3. Glucosamine versus placebo (adequate allocation concealment).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain | 11 | 2173 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.36, 0.04] |
| 2 Lequesne Index | 4 | 809 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐0.96, ‐0.12] |
| 3 Lequesne Index | 1 | 252 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [1.08, 1.91] |
| 4 WOMAC Pain Subscale | 10 | 2017 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.14, 0.03] |
| 5 WOMAC Stiffness Subscale | 7 | 1390 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.13, 0.08] |
| 6 WOMAC Function Subscale | 10 | 2017 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.08 [‐0.17, 0.00] |
| 7 WOMAC Total | 6 | 882 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.18 [‐0.31, ‐0.05] |
| 8 Minimum Joint Space Width | 2 | 414 | Mean Difference (IV, Fixed, 95% CI) | 0.32 [0.05, 0.58] |
| 9 Toxicity (Number of Patients Reporting Adverse Events) | 9 | 1640 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.91, 1.07] |
| 10 Toxicity (Number of Withdrawals due to Adverse Events) | 12 | 2435 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.54, 1.07] |
3.1. Analysis.
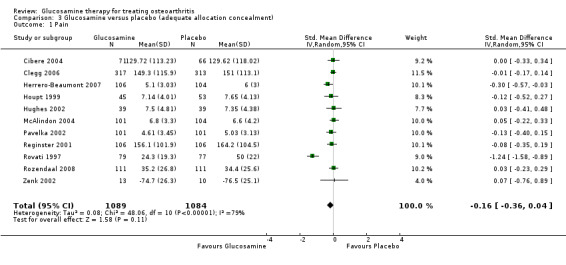
Comparison 3 Glucosamine versus placebo (adequate allocation concealment), Outcome 1 Pain.
3.2. Analysis.

Comparison 3 Glucosamine versus placebo (adequate allocation concealment), Outcome 2 Lequesne Index.
3.3. Analysis.

Comparison 3 Glucosamine versus placebo (adequate allocation concealment), Outcome 3 Lequesne Index.
3.4. Analysis.
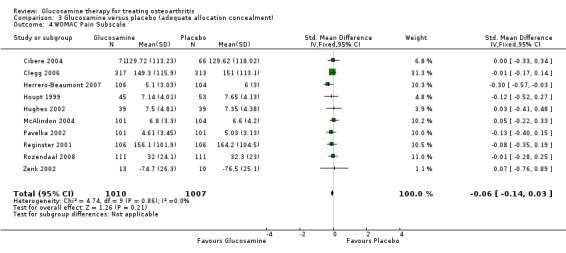
Comparison 3 Glucosamine versus placebo (adequate allocation concealment), Outcome 4 WOMAC Pain Subscale.
3.5. Analysis.

Comparison 3 Glucosamine versus placebo (adequate allocation concealment), Outcome 5 WOMAC Stiffness Subscale.
3.6. Analysis.
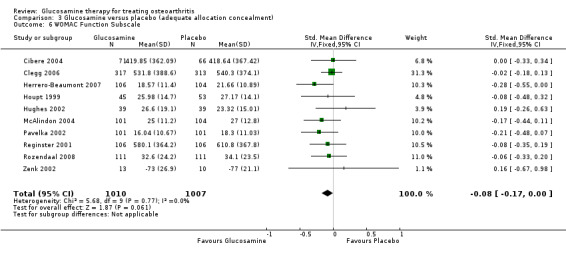
Comparison 3 Glucosamine versus placebo (adequate allocation concealment), Outcome 6 WOMAC Function Subscale.
3.7. Analysis.
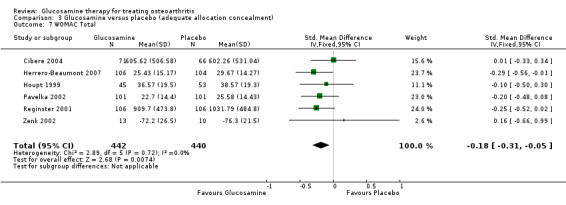
Comparison 3 Glucosamine versus placebo (adequate allocation concealment), Outcome 7 WOMAC Total.
3.8. Analysis.

Comparison 3 Glucosamine versus placebo (adequate allocation concealment), Outcome 8 Minimum Joint Space Width.
3.9. Analysis.
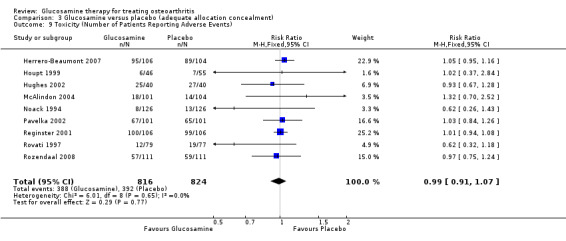
Comparison 3 Glucosamine versus placebo (adequate allocation concealment), Outcome 9 Toxicity (Number of Patients Reporting Adverse Events).
3.10. Analysis.
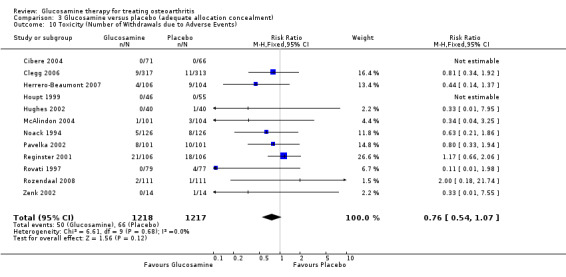
Comparison 3 Glucosamine versus placebo (adequate allocation concealment), Outcome 10 Toxicity (Number of Withdrawals due to Adverse Events).
Comparison 4. Glucosamine versus placebo (Rotta preparation).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain | 8 | 940 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.11 [‐1.66, ‐0.57] |
| 2 Lequesne Index | 5 | 951 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐0.82, ‐0.12] |
| 3 Lequesne Index | 2 | 407 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [1.20, 1.91] |
| 4 WOMAC Pain Subscale | 3 | 624 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.17 [‐0.32, ‐0.01] |
| 5 WOMAC Stiffness Subscale | 1 | 202 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.22 [‐0.50, 0.06] |
| 6 WOMAC Function Subscale | 3 | 624 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.19 [‐0.35, ‐0.03] |
| 7 WOMAC Total | 4 | 626 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.25 [‐0.40, ‐0.09] |
4.1. Analysis.

Comparison 4 Glucosamine versus placebo (Rotta preparation), Outcome 1 Pain.
4.2. Analysis.
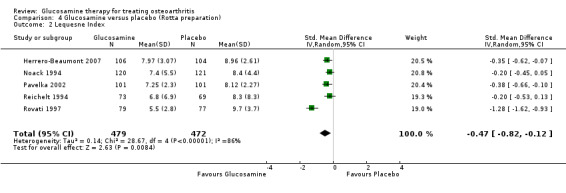
Comparison 4 Glucosamine versus placebo (Rotta preparation), Outcome 2 Lequesne Index.
4.3. Analysis.
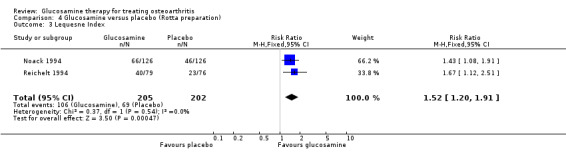
Comparison 4 Glucosamine versus placebo (Rotta preparation), Outcome 3 Lequesne Index.
4.4. Analysis.
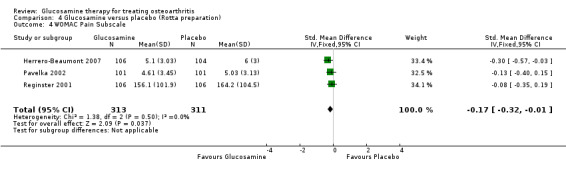
Comparison 4 Glucosamine versus placebo (Rotta preparation), Outcome 4 WOMAC Pain Subscale.
4.5. Analysis.
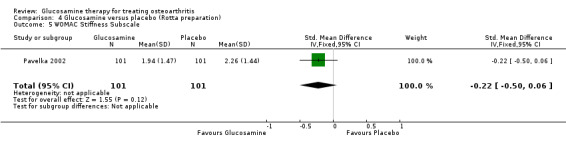
Comparison 4 Glucosamine versus placebo (Rotta preparation), Outcome 5 WOMAC Stiffness Subscale.
4.6. Analysis.
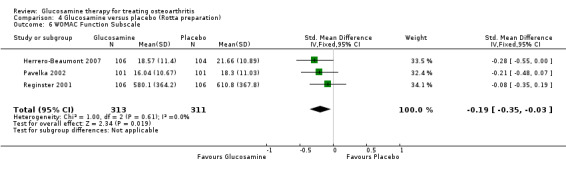
Comparison 4 Glucosamine versus placebo (Rotta preparation), Outcome 6 WOMAC Function Subscale.
4.7. Analysis.

Comparison 4 Glucosamine versus placebo (Rotta preparation), Outcome 7 WOMAC Total.
Comparison 5. Glucosamine versus placebo (non‐Rotta preparation).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain | 10 | 1603 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.15, 0.05] |
| 2 WOMAC Total | 3 | 258 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.27, 0.22] |
| 3 WOMAC Pain Subscale | 7 | 1393 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.11, 0.10] |
| 4 WOMAC Stiffness Subscale | 6 | 1188 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.10, 0.13] |
| 5 WOMAC Function Subscale | 6 | 1188 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.13, 0.10] |
5.1. Analysis.

Comparison 5 Glucosamine versus placebo (non‐Rotta preparation), Outcome 1 Pain.
5.2. Analysis.
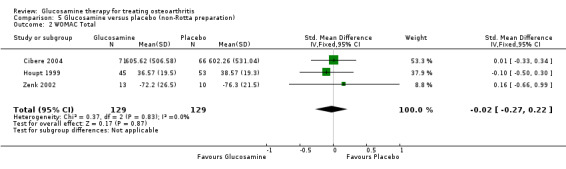
Comparison 5 Glucosamine versus placebo (non‐Rotta preparation), Outcome 2 WOMAC Total.
5.3. Analysis.
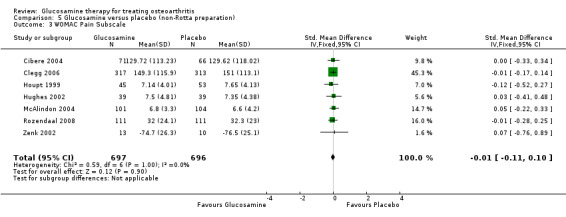
Comparison 5 Glucosamine versus placebo (non‐Rotta preparation), Outcome 3 WOMAC Pain Subscale.
5.4. Analysis.
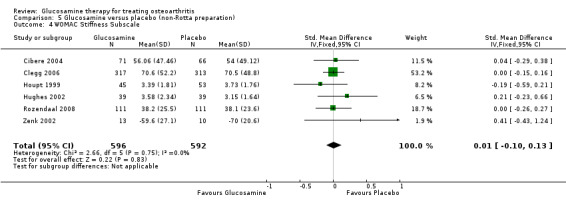
Comparison 5 Glucosamine versus placebo (non‐Rotta preparation), Outcome 4 WOMAC Stiffness Subscale.
5.5. Analysis.
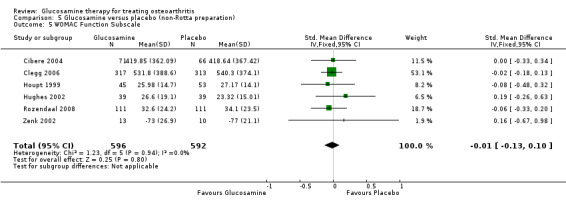
Comparison 5 Glucosamine versus placebo (non‐Rotta preparation), Outcome 5 WOMAC Function Subscale.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Cibere 2004.
| Methods | Randomized controlled trial Double blinded Discontinuation trial Multicenter trial Parallel group | |
| Participants | Outpatients with OA of the knee Country: Canada N = 137 In order to qualify for study entry, subjects must have been users of glucosamine and have experienced at least a moderate improvement in knee pain since starting on glucosamine Mean age = 64.5 years Female: 60% Male: 40% | |
| Interventions | GS = 1500 mg/day vs placebo for 24 weeks | |
| Outcomes | Proportion of disease flares Time to disease flare and severity of disease flare WOMAC pain WOMAC stiffness WOMAC function WOMAC total Analgesic medication usage | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Clegg 2006.
| Methods | Randomized controlled trial Double blinded Multicenter (NIH Funded in the USA) Parallel group | |
| Participants | Outpatients with symptomatic OA of the knee Country: USA N = 1583 Mean age = 58.6 years Female: 64% Male: 36% | |
| Interventions | GH 500 mg PO TID versus chondroitin sulfate 1200 mg/day versus GH + chondroitin sulfate versus Celebrex 200 mg/day versus placebo Duration: 24 weeks | |
| Outcomes | WOMAC pain, OMERACT‐OARSI responders, WOMAC stiffness, WOMAC function, HAQ Disability, HAQ pain, Patient global assessment, Physician's global assessment of disease status, joint swelling, effusion, usage of acetaminophen | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Crolle 1980.
| Methods | Randomized controlled trial Double blinded Single center Parallel group | |
| Participants | Inpatients with osteoarthritis Country: Italy N = 30 mean age: 72.7 years Female: 73% Male: 27% | |
| Interventions | GS = 400 mg OD IM (12) intra‐articular injection given once every 7 days followed by GS = 500 mg TID versus IM OD (piperazine plus chlorbutanol) x 7 days followed two weeks of oral placebo Duration = 3 weeks | |
| Outcomes | Pain (at rest) Pain (active range of motion) Pain (passive range of motion) scored from 0 to 3 scale Restricted function, time to walk 20 m Safety | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
D'ambrosio 1981.
| Methods | Randomized controlled trial Double blinded Single center Parallel group | |
| Participants | Inpatients with chronic degenerative osteoarthrotic disorders (site not specified) Country: Italy N = 30 Mean age = 74.7 years Female: 77% Male: 23% | |
| Interventions | GS 400 mg OD (IV or IM) for 7 days followed by two weeks of GS 500 mg PO TID versus (piperazine plus chlorbutanol) x 7 days followed two weeks of oral placebo Duration: 3 weeks | |
| Outcomes | Pain (at rest) Pain (during passive range of motion ) Pain (active range of motion) 0 to 3 scale Functional limitation 0 to 3 scale Safety | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Drovanti 1980.
| Methods | Randomized controlled trial Double blinded Single Center Parallel group | |
| Participants | Inpatients with osteoarthritis at multiple sites Country: Italy N = 80 Mean age: 60 years Female: 78% Male: 22% | |
| Interventions | GS 500 mg PO TID versus placebo PO TID Duration: 4 weeks | |
| Outcomes | Pain (0 to 4 scale) Joint tenderness Swelling Restriction of active movement and restriction of passive movement Physician global rating Safety | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Herrero‐Beaumont 2007.
| Methods | Randomized controlled trial Double blinded Multicenter Parallel group | |
| Participants | Outpatients with primary symptomatic OA of the knee Country: Spain and Portugal N = 325 Mean age: 63.9 years 87% female 13% male | |
| Interventions | GS 1500 mg/day versus acetaminophen 3 gm/day versus placebo Duration: 24 weeks | |
| Outcomes | Lequesne Index, WOMAC total WOMAC pain, WOMAC function, OARSI A and B responder criteria, Global‐patient derived | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Houpt 1999.
| Methods | Randomized controlled trial Double blinded Single center Parallel group | |
| Participants | Outpatients with primary osteoarthritis of the knee Country: Canada N = 118 Mean age: 64.5 years Female: 62% Male: 38% Age range: 40 to 85 years | |
| Interventions | GH 500 mg PO TID versus placebo PO TID Duration: 8 weeks with an additional 8 weeks follow‐up period (open label) | |
| Outcomes | WOMAC Daily pain diary Global patient derived Improvement on knee examination Use of acetaminophen Perception as to what group the subject were randomized to Safety | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Hughes 2002.
| Methods | Randomized controlled trial Double blinded Single Center Parallel group | |
| Participants | Outpatients with osteoarthritis of the knee Country: UK N = 80 Mean age = 62.3 years Female: 68% Male: 32% | |
| Interventions | GS 500 mg PO TID versus placebo PO TID Duration: 6 months | |
| Outcomes | VAS global pain VAS pain on movement VAS pain at rest WOMAC pain WOMAC stiffness WOMAC function McGill Pain Questionnaire (Affective) McGill Pain Questionnaire (Sensory) OARSI Response Criteria Knee range of motion | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
McAlindon 2004.
| Methods | Randomized controlled Internet conducted trial Double blinded Single center Parallel group | |
| Participants | Outpatients with osteoarthritis of the knee Country: USA N = 205 Mean age = NA 64% female 36% male | |
| Interventions | GS 1500 mg/day versus placebo Duration: 12 weeks | |
| Outcomes | WOMAC pain WOMAC stiffness WOMAC function WOMAC total Changes in analgesic usage | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Mehta 2007.
| Methods | Randomized controlled trial Double blinded Multicenter Parallel group | |
| Participants | Outpatients with OA of the knee Country: India N = 95 Female: 75% Male: 25% Mean age = 53.5 years | |
| Interventions | GS 750 mg PO BID versus Reparagen (a polyherbal) PO BID Duration: 8 weeks | |
| Outcomes | WOMAC pain WOMAC function WOMAC stiffness WOMAC total OMERACT/OARSI responder, VAS pain, Rescue medication usage Patient global assessment Physician global assessment | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Muller‐FassBender 94.
| Methods | Randomized controlled trial Double blinded Multicenter Parallel group | |
| Participants | Inpatients with active osteoarthritis of the knee Country :Germany N = 200 Mean age: 54 years Female: 48% Male: 52% | |
| Interventions | GS 500 mg PO TID versus Ibuprofen 400 PO TID Duration: 4 weeks | |
| Outcomes | Lequesne Index Global investigator derived Safety | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Noack 1994.
| Methods | Randomized controlled trial Double blinded Multicenter Parallel group | |
| Participants | Outpatients with osteoarthritis of the knee Country: Germany N = 252 Mean age: 55 years Female: 60% Male: 40% | |
| Interventions | GS 500 mg PO TID versus placebo PO TID Duration: 4 weeks | |
| Outcomes | Lequesne Index Global investigator derived Safety | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Pavelka 2002.
| Methods | Randomized controlled trial Double blinded Parallel group Single center | |
| Participants | Outpatients with osteoarthritis of the knee Country: Prague, Czech Republic N = 202 Mean age = 62.3 years Female: 78% Male: 22% | |
| Interventions | GS 1500 mg PO OD versus placebo PO OD Duration: 3 years | |
| Outcomes | WOMAC pain WOMAC stiffness WOMAC function WOMAC total Lequesne Index Joint space narrowing (x‐ray) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Pujalte 1980.
| Methods | Randomized controlled trial Double blinded Single center Parallel group | |
| Participants | Outpatients with established osteoarthrosis of the knee Country: Philippines N = 20 Mean age: 61.7 years Female: 85% Male: 15% | |
| Interventions | GS 500 mg PO TID versus placebo PO TID Duration: 8 weeks | |
| Outcomes | Articular pain (1 to 4 scale) Joint tenderness (1 to 4 scale) Joint swelling (1 to 4 scale) Restricted movement (1 to 4 scale) Global investigator derived Safety | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Qiu 1998.
| Methods | Randomized controlled trial Double blinded Multicenter Parallel group | |
| Participants | Outpatients with osteoarthritis of the knee Country: China N = 178 Mean age: 56.4 years Female: 79% Male: 21% | |
| Interventions | GS 500 mg PO TID versus ibuprofen 400 mg PO TID Duration: 4 weeks of treatment and 2 weeks of follow up | |
| Outcomes | Knee pain (at rest, movement, tenderness) 0 to 3 scale Knee swelling 0 to 3 scale Global investigator derived Safety | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Qiu 2005.
| Methods | Randomized controlled trial Parallel group Multicenter | |
| Participants | Outpatients with osteoarthritis of the knee Country: China N =142 Mean age = 57 ± 10 years Female: 92% Male: 8% | |
| Interventions | GS 500 mg PO TID versus GH 480 mg PO TID Duration: 4 weeks | |
| Outcomes | Knee pain at rest, on movement and pressure, swelling, morning stiffness, and walking ability according to Lequesne criteria. Adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Reginster 2001.
| Methods | Randomized controlled trial Double blinded Parallel group Single center | |
| Participants | Outpatients with osteoarthritis of the knee Country: Belgium N = 212 Mean age: 65.8 years Female: 76% Male: 24% | |
| Interventions | GS 1500 mg PO OD versus placebo PO OD Duration: 3 years | |
| Outcomes | WOMAC pain WOMAC stiffness WOMAC function Mean joint space narrowing (x‐ray) Minimum joint space narrowing (x‐ray) WOMAC total | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Reichelt 1994.
| Methods | Randomized controlled trial Double blinded Multicenter Parallel group | |
| Participants | Outpatients with osteoarthritis of the knee Country: Germany N = 155 Mean age: 56.5 years Female: 65% Male: 35% | |
| Interventions | GS 400 mg IM x two per week versus placebo IM x two per week Duration: 6 weeks of treatment and two weeks of follow up | |
| Outcomes | Lequesne Index Global investigator derived Safety | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Rindone 2000.
| Methods | Randomized controlled trial Double blinded Parallel group Single center | |
| Participants | Outpatients with osteoarthritis of the knee Country: USA N = 114 Female: 5% Male: 95% | |
| Interventions | GS 500 mg PO TID versus placebo PO TID Duration: 2 months | |
| Outcomes | VAS pain at rest VAS pain while walking | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Rovati 1997.
| Methods | Randomized controlled trial Double blinded Multicenter Parallel group This study is unpublished at this time (the authors have provided us data from this study in the form of a full manuscript ). Preliminary data from the study were also presented in abstract form by Rovati LC, 1997 | |
| Participants | Outpatients with primary osteoarthritis of the knee Country: France N = 319 Mean age: 65.5 years Female: 75% Male: 25% Age range = 45‐85 years | |
| Interventions | GS 1500 mg per day versus piroxicam 20 mg per day versus the combination of the two versus double placebo Duration 12 weeks of treatment and 8 weeks of follow up | |
| Outcomes | Lequesne Index Pain (VAS) Safety | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Rozendaal 2008.
| Methods | Randomized controlled trial Double blinded Parallel group Single center | |
| Participants | Outpatients with osteoarthritis of the hip Country: Netherlands N = 222 69% female 31% male Mean age = 63.4 years | |
| Interventions | GS 1500 mg per day versus placebo Duration: 2 years | |
| Outcomes | WOMAC pain, WOMAC stiffness, WOMAC function, VAS pain, joint space narrowing, usage of pain medication | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Usha 2004.
| Methods | Randomized controlled trial Double blinded Parallel group Single center |
|
| Participants | Outpatients with mild to moderate osteoarthritis of the knee Country: India N =118 Mean age = 51 years 60% female 40% male | |
| Interventions | GS versus MSM versus GS+MSM versus placebo Duration: 12 weeks | |
| Outcomes | Pain, Lequesne Index, walk time, joint swelling, usage of rescue analgesics, global patient, global investigator | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Vajaradul 1981.
| Methods | Controlled trial (query randomized) Double blinded Multicenter Parallel group | |
| Participants | Outpatients with osteoarthritis of the knee Country: Thailand N = 60 Mean age: 52.6 years Female: 83% Male: 17% | |
| Interventions | GS IA weekly versus placebo IA weekly Duration: 5 weeks and 4 weeks follow up | |
| Outcomes | Pain spontaneous (0 to 2 scale) Pain on standing (0 to 2 scale) Pain on walking (0 to 2 scale) Range of motion Time to climb five steps Knee joint circumference Safety | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Vaz 1982.
| Methods | Randomized controlled trial Double blinded Single center Parallel group | |
| Participants | Outpatients with osteoarthritis of the knee Country: Portugal N = 40 Mean age: 57.8 years Female: 74% Male: 26% | |
| Interventions | GS 500 mg PO TID versus ibuprofen 400 mg PO TID Duration: 8 weeks | |
| Outcomes | Articular pain (0 to 3 scale) Swelling Global investigator derived | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Zenk 2002.
| Methods | Randomized controlled trial Double blinded Parallel group Single center | |
| Participants | Outpatients with osteoarthritis of the knee and/or hip Country: USA N = 42 Mean age = 59 years Female: 81% Male: 19% | |
| Interventions | GS 500 mg PO TID versus placebo versus milk protein concentrate 2000 mg PO TID Duration: 6 weeks | |
| Outcomes | WOMAC pain WOMAC stiffness WOMAC function WOMAC total | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
GS = Glucosamine sulfate GH = Glucosamine hydrochloride OD = per day IM = Intramuscular IA = intrarticular IV = Intravenous TID = three times per day BID = two times per day PO = oral WOMAC = Western Ontario and McMaster Universities Osteoarthritis Index OARSI = Osteoarthritis Research Society International Responder Criteria MSM = methylsulfonylmethane NA = not available
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Braham 2003 | This RCT compared glucosamine to placebo in subjects who experienced regular knee pain. However, the cause of the knee pain was not clearly established, and may possibly not have been caused by OA of the knee. |
| Magi 1997 | Unpublished brief technical report prepared by Rottapharm primarily for regulatory authorities. Report describes a pilot long‐term feasibility study in subjects with OA of the knee. This was a phase 3 RCT. |
| Pipitone 1997 | Unpublished brief technical report prepared by Rottapharm primarily for regulatory authorities. Report describes a pilot long‐term feasibility study in subjects with OA of the knee. This was a phase 3 RCT. |
| Rovati 1993 | Unpublished brief technical report prepared by Rottapharm primarily for regulatory authorities. Report describes a pilot short‐term in subjects with OA of the spine. This was a phase 3 RCT. Some of the data from this study was published in abstract form by Foerster KK in the 2000 September Supplemental Issue of Arthritis and Rheumatism. |
Contributions of authors
TT is responsible for the overall content of this review
TP did data abstraction and quality assessment and reviewed the inclusion criteria for selected trials and wrote some of the discussion regarding the pharmacokinetics of glucosamine
LM did data abstraction, assisted with data analysis and helped with the preparation of the final version
JH critically reviewed the results and helped to draft the final version of this review
BS was involved in quality assessment
VW provided analytic support, helped in the preparation of the final document and reviewed the final version
MH critically reviewed the results and helped to draft the final version of this review
GW provided methodological and statistical support
Sources of support
Internal sources
Queens University Cochrane Network Site. Kingston, Ontario, Canada.
University of Ottawa, Canada.
External sources
No sources of support supplied
Declarations of interest
The current version of the 'Glucosamine for osteoarthritis' review on The Cochrane Library was last revised in 2005.
Dr Tassos Anastassiades submitted patents (Pub. No. US 2002/0045597 A1 and Pub. No. US 6,479,469 B2) for glucosamine type substances in August 2000. The patent protection is for chemically synthesized compounds with substitutions of chain length larger than the naturally occurring acetyl group in glucosamine.
Dr Tanveer Towheed was an invited speaker at the November 2002 ACR meeting. The accredited event, a post‐meeting symposium, was coordinated by the CME department at the Case Western Reserve School of Medicine. Rotta Pharmaceuticals (whose product is glucosamine sulfate) provided an unrestricted educational grant in support of the program. Dr Towheed presented a review of all available published meta‐analyses of various pharmacological therapies for OA. He received a one time honorarium for speaking at this symposium. There is no other association with Rotta Pharmaceuticals, or any other potential conflict of interest.
JB Houpt received an unrestricted grant‐in‐aid of research from Wampole Canada Inc for completion of a trial published in 1999. No industry or other support has been received since.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Cibere 2004 {published data only}
- Cibere J, Kopec JA, Thorne A, Singer J, Canvin J, Robinson DB, et al. Randomized, double‐blind, placebo‐controlled glucosamine discontinuation trial in knee osteoarthritis. Arthritis and Rheumatism (Arthritis Care and Research) 2004;51(2):738‐45. [DOI] [PubMed] [Google Scholar]
Clegg 2006 {published data only}
- Clegg DO, Reda DJ, Harris CL, Klein MA, O'Dell JR, Hooper MM, et al. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. New England Journal of Medicine 2006;354:795‐808. [DOI] [PubMed] [Google Scholar]
Crolle 1980 {published data only}
- Crolle G, D'Este E. Glucosamine sulphate for the management of arthrosis: a controlled clinical investigation. Current Medical Research & Opinion 1980;2:104‐9. [DOI] [PubMed] [Google Scholar]
D'ambrosio 1981 {published data only}
- D'Ambrosio E, Casa B, Bompani R, Scali G, Sclai M. Glucosamine sulphate: a controlled clinical investigation in arthrosis. Pharmatherapeutica 1981;2(8):504‐8. [PubMed] [Google Scholar]
Drovanti 1980 {published data only}
- Drovanti A, Bignamini AA, Rovati L. Therapeutic activity of oral glucosamine sulfate in osteoarthrosis: A placebo‐controlled double‐blind investigation. Clinical Therapeutics 1980;3(4):260‐72. [PubMed] [Google Scholar]
Herrero‐Beaumont 2007 {published data only}
- Herrero‐Beaumont G, Ivorra JAR, Trabado M, Blanco FJ, Benito P, Martin‐Mola E, et al. Glucosamine sulfate in the treatment of knee osteoarthritis symptoms. Arthritis and Rheumatism 2007;56(2):555‐67. [DOI] [PubMed] [Google Scholar]
Houpt 1999 {published data only}
- Houpt J, McMillan R, Wein C, Paget‐Dellio SD. Effect of glucosamine hydrochloride in the treatment of pain of osteoarthritis of the knee. Journal of Rheumatology 1999;26(11):2423‐2430. [PubMed] [Google Scholar]
Hughes 2002 {published data only}
- Hughes R, Carr A. A randomized, double‐blind, placebo‐controlled trial of glucosamine sulphate as an analgesic in osteoarthritis of the knee. Rheumatology 2002;41:279‐84. [DOI] [PubMed] [Google Scholar]
McAlindon 2004 {published data only}
- McAlindon T, Formica M, LaValley M, Lehmer M, Kabbara K. Effectiveness of Glucosamine for symptoms of knee osteoarthritis: Results from an Internet‐based randomized double‐blind controlled trial. American Journal of Medicine 2004;117:643‐49. [DOI] [PubMed] [Google Scholar]
Mehta 2007 {published data only}
- Mehta K, Gala J, Bhasale S, Naik S, Modak M, Thakur H, et al. Comparison of glucosamine sulfate and a polyherbal supplement for the relief of osteoarthritis of the knee: a randomized controlled trial. BMC Complementary and Alternative Medicine 2007;7(34):1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Muller‐FassBender 94 {published data only}
- Muller‐Fasbender H, Bach GL, Haase W, Rovati L, Setnikar I. Glucosamine sulfate compared to ibuprofen in osteoarthritis of the knee. Osteoarthritis and Cartilage 1994;2:61‐9. [DOI] [PubMed] [Google Scholar]
Noack 1994 {published data only}
- Noack W, Fischer M, Forster K, Rovati L, Setnikar I. Glucosamine sulfate in osteoarthritis of the knee. Osteoarthritis and Cartilage 1994;2:51‐9. [DOI] [PubMed] [Google Scholar]
Pavelka 2002 {published data only}
- Pavelka K, Gatterova J, Olejarova M, Machacek S, Giacovelli G, Rovati LC. Glucosamine sulfate use and delay of progression of knee osteoarthritis. A 3‐year, randomized, placebo‐controlled, double‐blind study. Archives of Internal Medicine 2002;162:2113‐23. [DOI] [PubMed] [Google Scholar]
Pujalte 1980 {published data only}
- Pujalte JM, Llavore EP, Ylescupidez FR. Double‐blind clinical trial evaluation of oral glucosamine sulphate in the basic treatment of osteoarthrosis. Current Medical Research and Opinion 1980;7(2):110‐14. [DOI] [PubMed] [Google Scholar]
Qiu 1998 {published data only}
- Qiu GX, Gao SN, Giacovelli G, Rovati L, Setnikar I. Efficacy and safety of glucosamine sulfate versus ibuprofen in patients with knee osteoarthritis. Arzneimittelforschung/Drug Resistance Updates 1998;48(1):469‐74. [PubMed] [Google Scholar]
Qiu 2005 {published data only}
- Qiu G, Weng X, Zhang K, Zhou Y, Wang LS, Li W, et al. A multicentral, randomized, controlled clinical trial of glucosamine hydrochloride/sulfate in the treatment of knee osteoarthritis. National Medical Journal of China 2005;85(43):3067‐70. [PubMed] [Google Scholar]
Reginster 2001 {published data only}
- Reginster JY, Deroisy R, Rovati LC, Lee RL, Lejeune E, Bruyere O, et al. Long‐term effects of glucosamine sulphate on osteoarthritis progression: a randomized, placebo‐controlled clinical trial. Lancet 2001;357:251‐6. [DOI] [PubMed] [Google Scholar]
Reichelt 1994 {published data only}
- Reichelt A, Forster KK, Fischer M, Rovati L, Setnikar I. Efficacy and safety of intramuscular glucosamine sulfate in osteoarthritis of the knee. Arzneimmittelforschung/Drug Resistance Updates 1994;44(1):75‐80. [PubMed] [Google Scholar]
Rindone 2000 {published data only}
- Rindone JP, Hiller D, Collacott E, Nordhaugen N, Arriola G. Randomized, controlled trial of glucosamine for treating osteoarthritis of the knee. Western Journal of Medicine 2000;172:91‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rovati 1997 {published and unpublished data}
- Rovati LC. The clinical profile of glucosamine sulfate as a selective symptom‐modifying drug in osteoarthritis: current data and perspectives. Osteoarthritis and Cartilage 1997;5:72. [Google Scholar]
Rozendaal 2008 {published data only}
- Rozendaal RM, Koes BW, m van Osch G, Uitterlinden EJ, Garling EH, Willemsen SP, et al. Effect of glucosamine sulfate on hip osteoarthritis. Annals of Internal Medicine 2008;148:268‐77. [DOI] [PubMed] [Google Scholar]
Usha 2004 {published data only}
- Usha PR, Naidu MUR. Randomized, double‐blind, parallel, placebo‐controlled study of oral glucosamine, methylsulfonylmethane and their combination in osteoarthritis. Clinical Drug Investigation 2004;24(6):353‐63. [DOI] [PubMed] [Google Scholar]
Vajaradul 1981 {published data only}
- Vajaradul Y. Double‐blind clinical evaluation of intra‐articular glucosamine in outpatients with gonarthrosis. Clinical Therapeutics 1981;3(5):336‐43. [PubMed] [Google Scholar]
Vaz 1982 {published data only}
- Vaz AL. Double‐blind clinical evaluation of the relative efficacy of ibuprofen and glucosamine sulphate in the management of osteoarthrosis of the knee in out‐patients. Current Medical Research and Opinion 1982;8:145‐9. [DOI] [PubMed] [Google Scholar]
Zenk 2002 {published data only}
- Zenk JL, Helmer TR, Kuskowski MA. The effects of milk protein concentrate on the symptoms of osteoarthritis in adults: an exploratory, randomized, double‐blind, placebo‐controlled trial. Current Therapeutic Research 2002;63(7):430‐42. [Google Scholar]
References to studies excluded from this review
Braham 2003 {published data only}
- Braham R, Dawson B, Goodman C. The effect of glucosamine supplementation on people experiencing regular knee pain. British Journal of Sports Medicine 2003;37:45‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Magi 1997 {unpublished data only}
- Magi D, Giacovelli G, Rovati LC. Clinical Study on the activity of glucosamine sulfate on osteoarthritis, in comparison with placebo, in patients suffering from osteoarthritis of the knee by means of imaging techniques (NMR, ecotomography, radiology) and computer processing of the such obtained images. Unpublished technical report prepared by Rottapharm 1997.
Pipitone 1997 {unpublished data only}
- Pipitone V, Giacovelli G, Rovati LC. Clinical study on the activity of glucosamine sulfate on the evolution of osteoarthritis in patients with osteoarthritis of the knee. Unpublished technical report prepared by Rottapharm 1997.
Rovati 1993 {unpublished data only}
- Rovati LC, Poma A, Biavati G, et al. A multicenter, randomized, double‐blind, parallel‐group study to investigate efficacy and safety of oral glucosamine sulfate in osteoarthritis of the spine. Unpublished technical report prepared by Rottapharm 1993.
Additional references
ACR 2000
- American College of Rheumatology (ACR) Subcommittee on osteoarthritis guidelines. Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. Arthritis and Rheumatism 2000;43:1905‐15. [DOI] [PubMed] [Google Scholar]
Adebowale 2000
- Adebowale AO, Cox DS, Liang Z, Eddington ND. Analysis of glucosamine and chondroitin sulfate content in marketed products and the caco‐2 permeability of chondroitin sulfate raw materials. Journal of the American Nutraceutical Association 2000;3(1):Spring Issue. [Google Scholar]
Anderson 2005
- Anderson JW, Nicolosi RJ, Borzelleca JF. Glucosamine effects in humans: a review of effects on glucose metabolism, side effects, safety considerations and efficacy. Food and Chemical Toxicology 2005;43:187‐201. [DOI] [PubMed] [Google Scholar]
Audimoolam 2006
- Audimoolam VK, Bhandari S. Acute interstitial nephritis induced by glucosamine. Nephrology Dialysis Transplantation 2006;21(7):2031. [DOI] [PubMed] [Google Scholar]
Badley 1995
- Badley EM. The effect of osteoarthritis on disability and health care use in Canada. Journal of Rheumatology 1995;22 Suppl 43:19‐22. [PubMed] [Google Scholar]
Barclay 1998
- Barclay TS, Tsourounis C, McCart GM. Glucosamine. Annals of Pharmacotherapy 1998;32:574‐9. [DOI] [PubMed] [Google Scholar]
Bassleer 1992
- Bassleer C, Henrotin Y, Franchimont P. In‐vitro evaluation of drugs proposed as chondroprotective agents. International Journal of Tissue Reactions 1992;14:231‐41. [PubMed] [Google Scholar]
Bassleer 1998
- Bassleer C, Rovati L, Franchimont P. Stimulation of proteoglycan production by glucosamine sulfate in chondrocytes isolated from human osteoarthritic cartilage in vitro. Osteoarthritis and Cartilage 1998;6:427‐34. [DOI] [PubMed] [Google Scholar]
Bellamy 1997
- Bellamy N, Kirwan J, Boers M, et al. Recommendations for a core set of outcome measures for future phase III clinical trials in knee, hip and hand osteoarthritis. Journal of Rheumatology 1997;24:799‐802. [PubMed] [Google Scholar]
Biggee 2006
- Biggee E, Blinn CM, McAlindon TE, Nuite M, Silbert JE. Low levels of human serum glucosamine after ingestion of glucosamine sulphate relative to capability for peripheral effectiveness. Annals of the Rheumatic Diseases 2006;65:222‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Biggee 2007
- Biggee BA, Blinn CM, Nuite M, Silbert JE, McAlindon TE. Effects of oral glucosamine sulphate on serum glucose and insulin during an oral glucose tolerance test of subjects with osteoarthritis. Annals of the Rheumatic Diseases 2007;66(2):260‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chan 2001
- Chan NN, Baldeweg S, Tan TM, Hurel S. Glucosamine sulphate and osteoarthritis (letter). Lancet 2001;357:1617. [DOI] [PubMed] [Google Scholar]
Cohen 1977
- Cohen J. Statistical power analysis for the behavioural sciences. Statistical power analysis for the behavioural sciences. New York: Academic Press, 1977. [Google Scholar]
Conrozier 1998
- Conrozier T, Mathieu P, Piperno M, et al. Glucosamine sulfate significantly reduced cartilage destruction in a rabbit model os osteoarthritis. Arthritis and Rheumatism 1998;41 Suppl:147. [Google Scholar]
Consumer Reports 2002
- No authors listed. Joint Remedies. Consumer Reports 2002;67(1):18‐21. [PubMed] [Google Scholar]
Creamer 1997
- Creamer P, Hochberg MC. Osteoarthritis. Lancet 1997;350:503‐8. [DOI] [PubMed] [Google Scholar]
Creamer 1998
- Creamer P, Flores R, Hochberg MC. Management of osteoarthritis in older adults. Clinics in Geriatric Medicine 1998;14(3):434‐54. [PubMed] [Google Scholar]
Da Camara 1998
- Camara C, Dowless GV. Glucosamine sulfate for osteoarthritis. Annals of Pharmacotherapy 1998;32:580‐7. [DOI] [PubMed] [Google Scholar]
Deal 1999
- Deal CL, Moskowitz RW. Neutraceuticals as therapeutic agents in osteoarthritis. The role of glucosamine, chrondroitin sulfate and collagen hydrolysate. Rheumatic Diseases Clinics of North America 1999;25:379‐95. [DOI] [PubMed] [Google Scholar]
Deviere 2002
- Deviere J. Do selective cyclo‐oxygenase inhibitors eliminate the adverse events associated with nonsteroidal anti‐inflammatory drug therapy?. European Journal of Gastroenterology and Hepatology 2002;14 Suppl 1:29‐33. [PubMed] [Google Scholar]
Felson 1998
- Felson D. Nonmedicinal therapies for osteoarthritis. Bulletin on the Rheumatic Diseases 1998;47(2):1‐7. [PubMed] [Google Scholar]
Felson 2000
- Felson DT, Lawrence RC, Dieppe PA, Hirsch R, Helmick CG. Osteoarthritis: new insights. Part 1: the disease and its prevalence and impact. Annals of Internal Medicine 2000;133(8):635‐46. [DOI] [PubMed] [Google Scholar]
Gabriel 1991
- Gabriel S, Jaakkimainen L, Bombardier C. Risk factors for serious gastrointestinal complications related to use of non‐steroidal anti‐inflammatory drugs. A meta‐analysis. Annals of Internal Medicine 1991;115:787‐96. [DOI] [PubMed] [Google Scholar]
Garner 2002
- Garner S, Fidan D, Frankish R, Judd M, Shea B, Towheed T, Wells G, Tugwell P. Celecoxib for rheumatoid arthritis. Cochrane Database of Systematic Reviews 2002, Issue 4. [DOI: 10.1002/14651858.CD003831] [DOI] [PubMed] [Google Scholar]
Goldstein 2001
- Goldstein MR. Glucosamine sulphate and osteoarthritis (letter). Lancet 2001;357:1617‐8. [DOI] [PubMed] [Google Scholar]
Griffin 1991
- Griffin M, Piper J, Daugherty J, et al. Non‐steroidal anti‐inflammatory drug use and increased risk for peptic ulcer disease in elderly persons. Annals of Internal Medicine 1991;114:257‐63. [DOI] [PubMed] [Google Scholar]
Hedges 1985
- Hedges L, Olkin I. Statistical methods for meta‐analysis. San Diego, CA: Academic Press, 1995. [Google Scholar]
Herman 1986
- Herman J, Appel A, Khosla R, et al. The in vitro effect of select classes of non‐steroidal anti‐inflammatory drugs on normal cartilage metabolism. Journal of Rheumatology 1986;13:1014‐8. [PubMed] [Google Scholar]
Hochberg 1995a
- Hochberg MC, Altman R, Brandt K, et al. Guidelines for the medical management of osteoarthritis. Part 1. Osteoarthritis of the hip. Arthritis and Rheumatism 1995;38(11):1535‐40. [DOI] [PubMed] [Google Scholar]
Hochberg 1995b
- Hochberg MC, Altman R, Brandt K, et al. Guidelines for the medical management of osteoarthritis. Part 2. Osteoarthritis of the knee. Arthritis and Rheumatism 1995;38(11):1541‐7. [DOI] [PubMed] [Google Scholar]
Hochberg 1996
- Hochberg MC, Perlmutter D, Hudson J, et al. Preferences in the management of osteoarthritis of the hip and knee: Results of a survey of community‐based rheumatologists in the United States. Arthritis Care and Research 1996;9(3):170‐6. [DOI] [PubMed] [Google Scholar]
Hoffer 2002
- Hoffer LJ, Kaplan LN, Hamadeh MJ, Grigoriu AC, Baron M. Sulfate could mediate the therapeutic effect of glucosamine sulfate. Metabolism 2002;50(7):767‐70. [DOI] [PubMed] [Google Scholar]
Jadad 1996
- Jadad A, Moore A, Carrol D, et al. Assessing the quality of reports of randomized trials: is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. [DOI] [PubMed] [Google Scholar]
Largo 2003
- Largo R, Alvarez‐Soria MA, Diez‐Ortego I, et al. Glucosamine inhibits IL‐1 beta induced NFKB activation in human osteoarthritic chondrocytes. Osteoarthritis and Cartilage 2003;11:290‐8. [DOI] [PubMed] [Google Scholar]
Laverty 2005
- Laverty S, Sandy JD, Celeste C, Vachon P, Marier JF, Plaas AHK. Synovial fluid levels and serum pharmacokinetics in a large animal model following treatment with oral glucosamine at clinically relevant doses. Arthritis and Rheumatism 2005;52:181‐91. [DOI] [PubMed] [Google Scholar]
Lawrence 1998
- Lawrence RC, Helmick CG, Arnett FC, Deyo RA, Felson DT, Giannini EH. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis and Rheumatism 1998;41(5):778‐99. [DOI] [PubMed] [Google Scholar]
Liang 1984
- Liang M, Larsen M, Thomson M, Eaton H, Mcnamara E, Katz R, et al. Costs and outcoes in rheumatoid arthritis and osteoarthritis. Arthritis and Rheumatism 1984;27(5):522‐9. [DOI] [PubMed] [Google Scholar]
Lozada 1997
- Lozada C, Altman R. Chrondroprotection in osteoarthritis. Bulletin on the Rheumatic Diseases 1997;46:5‐7. [PubMed] [Google Scholar]
Matheu 1999
- Matheu V, Gracia Bara MT, Pelta R, Vivas E, Rubio M. Immediate‐hypersensitivity reaction to glucosamine sulfate. Allergy 1999;54:643‐50. [DOI] [PubMed] [Google Scholar]
McAlindon 2000
- McAlindon TE, LaValley MP, Gulin JP, Felson DT. Glucosamine and Chondroitin for Treatment of Osteoarthritis. JAMA 2000;283(11):1469‐1475. [DOI] [PubMed] [Google Scholar]
McAlindon 2003
- McAlindon T. Why are clinical trials of glucosamine no longer uniformly positive?. Rheumatic Diseases Clinics of North America 2003;29(4):789‐801. [DOI] [PubMed] [Google Scholar]
McCarty 1994
- McCarty M. The neglect of gulcosamine as a treatment for osteoarthritis. A personal perspective. Medical Hypotheses 1994;42:323‐327. [DOI] [PubMed] [Google Scholar]
Monauni 2000
- Monauni T, Zenti MG, Cretti A, Daniels MC, Targher G, Caruso B, et al. Effects of glucosamine infusion on insulin secretion and insulin action on humans. Diabetes 2000;49(6):926‐35. [DOI] [PubMed] [Google Scholar]
Moralestorres 1996
- Moralestorres J, Reginster J, Hochberg MC. Rheumatic and musculoskeletal diseases and impared quality of life. A challenge for rheumatologists. Journal of Rheumatology 1996;23(1):1‐3. [PubMed] [Google Scholar]
Mroz 2004
- Mroz PJ, Silbert JE. Use of 3H‐glucosamine and 35S‐sulfate with cultured human chondrocytes to determine the effect of glucosamine concentration on formation of chondroitin sulfate. Arthritis and Rheumatism 2004;50:3574‐9. [DOI] [PubMed] [Google Scholar]
Muniyappa 2006
- Muniyappa R, Karne RJ, Hall G, Crandon SK, Bronstein JA, Ver MR, et al. Oral glucosamine for 6 weeks at standard doses does not cause or worsen insulin resistance or endothelial dysfunction in lean or obese subjects. Diabetes 2006;55(11):3142‐50. [DOI] [PubMed] [Google Scholar]
Persiani 2005a
- Persiani S, Roda E, Rovati LC, Locatelli M, Giacovelli G, Roda A. Glucosamine oral bioavailability and plasma pharmacokinetics after increasing doses of crystalline glucosamine sulfate in man. Osteoarthritis and Cartilage 2005;13:1041‐9. [DOI] [PubMed] [Google Scholar]
Persiani 2005b
- Persiani S, Rotini R, Rovati LC, Locatelli M, Roda A. Glucosamine plasma and synovial fluid concentrations before and oral administration of crystalline glucosamine sulfate in man. Annals of the Rheumatic Diseases. 2005; Vol. 64, issue Suppl III:490.
Petitti 1994
- Petitti D. Meta‐analysis, Decision analysis, and Cost‐Effectiveness analysis. Monographs in Epidemiology and Biostatistics. Vol. 24, Oxford University Press, 1994. [Google Scholar]
Pham 2007
- Pham T, Cornea A, Blick KE, Jenkins A, Scofield RH. Oral glucosamine in doses used to treat osteoarthritis worsens insulin resistance. American Journal of the Medical Sciences 2007;333(6):333‐9. [DOI] [PubMed] [Google Scholar]
Puett 1994
- Puett D, Griffin M. Published trials of nonmedicinal and noninvasive therapies for hip and knee osteoarthritis. Annals of Internal Medicine 1994;121:133‐40. [DOI] [PubMed] [Google Scholar]
Rashad 1989
- Rashad S, Revell P, Hemingway A, Low F, Rainsford K, Walker F. Effect of non‐steroidal anti‐inflammatory drugs on the course of osteoarthritis. Lancet 1989;2:519‐22. [DOI] [PubMed] [Google Scholar]
Reginister 2003
- Reginister J, Bruyere O, Lecart M, Henroitin Y. Naturocetic (glucosamine and chondroitin sulfate) compounds as structure‐modifying drugs in the treatment of osteoarthritis. Current Opinion in Rheumatology 2003;15(5):651‐5. [DOI] [PubMed] [Google Scholar]
Richy 2003
- Richy F, Bruyere O, Ethgen O, Cucherat M, Henrotin, Reginster JY. Structural and symptomatic efficacy of glucosamine and chondroitin in knee osteoarthritis. A comprehensive meta‐analysis. Archives of Internal Medicine 2003;163:1514‐22. [DOI] [PubMed] [Google Scholar]
Roden 1956
- Roden L. Effect of hexosamines on the synthesis of chrondroitin sulphruic acid in vitro. Arkhiv Foer Kemi 1956;10:345‐352. [Google Scholar]
Russell 2002
- Russell AS, Aghazadeh‐Habashi A, Jamali F. Active ingredient consistency of commercially available glucosamine sulfate products. Journal of Rheumatology 2002;29(11):2407‐9. [PubMed] [Google Scholar]
Schulz 1995a
- Schulz KF, Chalmers I, Hayes RJ, Altman D. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408‐12. [DOI] [PubMed] [Google Scholar]
Scott 1993
- Scott JC, Hochberg MC. Arthritic and other musculoskeletal diseases. In: Brownson RC, Remington PL, Davis JR editor(s). Chronic Disease Epidemiology and Control. Washington: American Public Health Association, 1993. [Google Scholar]
Scroggie 2003
- Scroggie DA, Albright A, Harris MD. The effect of glucosamine‐chondroitin supplementation on glycosylated hemaglobin levels in patients with type 2 diabetes mellitus: a placebo‐controlled, double‐blinded, randomized controlled trial. Archives of Internal Medicine 2003;163(13):1587‐90. [DOI] [PubMed] [Google Scholar]
Setnikar 1984
- Setnikar I, Giachetti C, Zanolo G. Absorption, distribution and excretion of radioactivity after a single intravenous or oral administration of 14C‐glucosamine to the rat. Pharmatherapeutica 1984;3:523‐30. [PubMed] [Google Scholar]
Setnikar 1986
- Setnikar I, Giachetti C, Zanolo G. Pharmacokinetics of glucosamine in dog and man. Arzneimittelforschung 1986;36:729‐35. [PubMed] [Google Scholar]
Setnikar 1991a
- Setnikar I, Pacini M, Revel L. Antiarthritic effects of glucosamine sulfate studied in animal mdoels. Arzneimittelforschung 1991;41:542‐5. [PubMed] [Google Scholar]
Setnikar 1991b
- Setnikar I, Cereda R, pacini MA, Revel L. Antireactive properties of glucosamine sulfate. Arneimittelforschung 1991;41:157‐61. [PubMed] [Google Scholar]
Setnikar 1992
- Setnikar I. Antireactive properties of chondroprotective drugs. International Journal of Tissue Reactions 1992;24:253‐61. [PubMed] [Google Scholar]
Setnikar 1993
- Setnikar I, Palumbo R, Canali S, Zanolo G. Pharmacokinetics of glucosamine in man. Arzneimittelforschung 1993;43:1109‐13. [PubMed] [Google Scholar]
Setnikar 2001
- Setnikar I, Rovati LC. Absorption, distribution, metabolism and excretion of glucosamine sulfate. Arzneimittelforschung/Drug Resistance Updates 2001;51(2):699‐725. [DOI] [PubMed] [Google Scholar]
Shikhman 2001
- Shikhman AR, Kuhn K, Alaaeddine N, Lotz M. N‐acetylglucosamine prevents IL‐1 beta‐mediated activation of human chondrocytes. Journal of Immunology 2001;166:5155‐60. [DOI] [PubMed] [Google Scholar]
Sibbald 2004
- Sibbald B. Rofecoxib (Vioxx) voluntarily withdrawn from market. Canadian Medical Association Journal 2004;171(9):1027‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sivojelezova 2007
- Sivojelezova A, Koren G, Einarson A. Glucosamine use in pregnancy: an evaluation of pregnancy outcome. Journal of Women's Health 2007;16(3):345‐8. [DOI] [PubMed] [Google Scholar]
Swinburne 2001
- Swinburne LM. Glucosamine sulphate and osteoarthritis (letter). Lancet 2001;357:1617. [DOI] [PubMed] [Google Scholar]
Tallia 2002
- Tallia AF, Cardone DA. Asthma exacerbation associated with glucosamine‐chondroitin supplement. The Journal of the American Board of Family Practice 2002;15:481‐4. [PubMed] [Google Scholar]
Tannis 2004
- Tannis AJ, Barban J, Conquer JA. Effect of glucosamine supplementation on fasting and non‐fasting plasma glucose and serum insulin concentration in healthy individuals. Osteoarthritis and Cartilage 2004;12(6):506‐11. [DOI] [PubMed] [Google Scholar]
Tapadinhas 1982
- Tapadinhas MJ, Rivera IC, Bignamini AA. Oral glucosamine sulphate in the management of arthrosi: report on a mult‐centre open investigation in Portugal. Pharmacotherapeutica 1982;3:157‐68. [PubMed] [Google Scholar]
Towheed 1997a
- Towheed TE, Hochberg MC. A systematic review of randomized controlled trials of pharmacological therapy in osteoarthritis of the hip. Journal of Rheumatology 1997;24:549‐57. [PubMed] [Google Scholar]
Towheed 1997b
- Towheed TE, Hochberg MC. A systematic review of randomized controlled trials of pharmacological therapy in osteoarthritis of the knee, with an emphasis on trial methodology. Seminars in Arthritis and Rheumatism 1997;26:755‐70. [DOI] [PubMed] [Google Scholar]
Towheed 1998
- Towheed TE. Impact of musculoskeletal disorders in Canada. Annals of the Royal College of Physicians and Surgeons of Canada 1998;31(5):229‐32. [Google Scholar]
Towheed 1999
- Towheed TE, Anatassiades TP. Glucosamine therapy for osteoarthritis (editorial). Journal of Rheumatology 1999;26(11):2294‐7. [PubMed] [Google Scholar]
Towheed 2000
- Towheed TE. Glucosamine and chondroitin for treating symptoms of osteoarthritis: evidence is widely touted but incomplete. JAMA 2000;283(11):1483‐4. [DOI] [PubMed] [Google Scholar]
Towheed 2002
- Towheed TE. Published meta‐analyses of pharmacological therapies for osteoarthritis. Osteoarthritis and Cartilage 2002;10(11):836‐7. [DOI] [PubMed] [Google Scholar]
Towheed 2003
- Towheed TE. Current Status of glucosamine therapy in osteoarthritis. Arthritis and Rheumatism 2003;49(4):601‐4. [DOI] [PubMed] [Google Scholar]
Towheed 2007
- Towheed TE, Anastassiades T. Glucosamine therapy for osteoarthritis: An update. Journal of Rheumatology 2007;34(9):1787‐90. [PubMed] [Google Scholar]
Vidal 1978
- Vidal Y, Plana R, Bizzarri D, Rovati AL. Articular cartilage pharmacology. In vitro studies on glucosamine and non‐steroidal anti‐inflammatory drugs. Pharmacological Research Communications 1978;10:557‐69. [DOI] [PubMed] [Google Scholar]
Vlad 2007
- Vlad SC, LaValley MP, McAlindon TE, Felson DT. Glucosamine for pain in osteoarthritis: why do trial results differ?. Arthritis and Rheumatism 2007;56(7):2105‐10. [DOI] [PubMed] [Google Scholar]
Wright 2002
- Wright JM. The double‐edged sword of COX‐2 selective NSAIDs. Canadian Medical Association Journal 2002;167(10):1131‐7. [PMC free article] [PubMed] [Google Scholar]


