Introduction
On May 15, 2018, the Federal Drug Administration (FDA) approved once daily tenofovir/emtricitabine (TDF/FTC) for use as preexposure prophylaxis (PrEP) for adolescents (weighing greater than 35kgs) engaging in high-risk sexual behavior (Gilead, 2018). This decision was largely based on the Adolescent Medicine Trials Network for HIV/AIDS Interventions (ATN) 113 which demonstrated that PrEP was well tolerated among the adolescents, age 15–17, enrolled in the study (Hosek, 2017). The use of TDF/FTC for PrEP in adults (18 and older) was approved by the FDA in 2012 after its safety and efficacy had been demonstrated in multiple international clinical trials (FDA, 2016). Iniciativa Profilaxis Pre-Exposición (iPrEx), Partners PrEP, and Pre-exposure Option for Reducing HIV in the UK: Immediate or Deferred (PROUD) demonstrated that if taken daily, PrEP is at least 92% effective in preventing HIV transmission among men and transgender women who have sex with men and sero-discordant couples (Baeten, 2016; Grant, 2010; McCormack, 2016).
Since 2012, there has been mounting pressure from the adolescent health community to make PrEP accessible to individuals under 18 years of age (Burda, 2015). The Centers for Disease Control and Prevention (CDC) reported that in 2016, youth aged 13 to 24 accounted for 21% of all new HIV diagnoses in the United States (Hess, 2016). In New York City in 2016, there were 882 new HIV diagnoses among individuals 13 to 29 years of age, out of a total of 2279 new infections (39%). Seventy-nine of those new diagnoses were among individuals aged 13–19 years of age. Since the majority of HIV infections occur among individuals between the ages of 20 and 29 years, health care providers need to consider educating patients about HIV prevention and provide biomedical interventions prior to the patient’s 20th birthday (HIV Epidemiology and Field Services Program, 2017) (Ocfemia, 2018). Additionally, adolescents, especially minors, take many sexual risks in pursuit of autonomy and self-exploration with concurrent poorly developed impulse control (Steinberg, 2009). It is clear that adolescents are, at least, as much in need of PrEP as individuals of other age groups.
Adolescent Provider PrEP Knowledge and Attitudes
In a recent sample of 162 surveyed adolescent providers, 93.2% of the providers had heard of PrEP, however, only 35.2% of these providers indicated that they had previously ever prescribed PrEP. Sixty-five percent of the providers surveyed stated that they were “willing to prescribe PrEP to adolescents ages 13–17” and this indicator was associated with the provider having enough knowledge to safely provide PrEP to adolescents (Hart-Cooper, 2018). Mullins and colleagues conducted a mixed method study that included 56 clinicians within the ATN who provided care to HIV positive adolescents, and their attitudes towards providing PrEP to at-risk HIV uninfected adolescents (Mullins, 2017). The primary outcomes of the study included 1) intention to provide PrEP to at-risk adults over 18 years of age and at-risk adolescents under 18 years of age and 2) actual prescriptions to adults and adolescents in at-risk groups. Significantly more clinicians had prescribed PrEP to adult men who have sex with men (MSM) than to adolescent MSM. The authors found that clinicians identified two significant barriers to prescribing PrEP to adolescents: the perceived need for a multidisciplinary team and inclusion of behavioral interventions in the visit. These results suggested that even providers who were knowledgeable about PrEP needed a “brief, streamlined and effective behavioral intervention that can be delivered with PrEP” (Mullins, 2017).
Similar findings were presented in a study of examining perceived barriers and facilitators to prescribing PrEP among Boston HIV care providers caring for adults. While the majority of providers understood PrEP to be efficacious, they expressed desire for readily accessible, simple guidance regarding the provision of PrEP that would not require significant time and effort (Krakower, 2014).
In 2017, a web-based survey among pediatric (n=35) and internal medicine providers at a large urban medical center in Upper Manhattan revealed that although pediatric providers reported taking a sexual history on majority (68.5%) of their patients above the age of 13, only 14% of these providers felt comfortable assessing these patients’ eligibility for PrEP. A consistent barrier to providing PrEP across all groups of providers was lack of formal training (Zucker, 2018).
The FDA approval of an adolescent indication for PrEP, removed an important barrier to providing PrEP to adolescents nationwide. With this development, the priority now shifts to considering implementation challenges, opportunities, and determinants of success. This calls for setting educational standards for providers, establishing PrEP guidelines specific to adolescents, offering support for providers prescribing PrEP to adolescents or information regarding referrals should adolescent providers need to outsource the provision of PrEP for their patients.
To respond to these challenges, we undertook the development of an evidence-based curriculum for the purpose of training healthcare practitioners how to provide HIV prevention interventions, including PrEP, to an adolescent population.
Methods
A comprehensive provider curriculum was developed focused on adolescent-specific issues related to PrEP screening and monitoring. A literature review was conducted to identify learning needs of adolescent providers, identify key domains and to design the curriculum. The articles were retrieved from medical, public health, nursing and adolescent health journals via PubMed and SCOPUS. The search terms used were HIV Prevention, Pre-Exposure Prophylaxis, Healthcare Provider, Implementation, Adolescent Health and Primary Care. Medical/Nursing education and adult learning guides were used in the development of the curriculum (Brenner, 2010; Diamond, 1998; Joyce and Showers, 2002).
The Adolescent HIV Prevention Curriculum was piloted on September 21st 2017 during one day training session. The purpose of this pilot was to construct the flow of the curriculum and obtain feedback about the content.
An expert panel made up of both nursing/medical professionals and public health officials was contacted to review the curriculum to determine content validity (Figure 1).
Figure 1.
The institution and titles of expert panel members
The seven experts are leaders in the field of HIV Prevention, Adolescent Health and Medical/Nursing Education. They were asked to review the content of the curriculum and evaluate for importance, clarity and relevance. To determine scale-level content validity, a three-point scale was constructed (ie: 1=not relevant, 2=somewhat relevant, 3=very relevant). A ratings guide was developed through Qualtrics and the experts were asked to log their responses electronically. An expert selecting “somewhat relevant” or “somewhat clear” for a reviewed section was categorized as an affirmative. All sections that received a 78% affirmative response were deemed valid (Polit, Beck, and Owen 2007).
Results
Curriculum Content
Eight domains were identified as essential for inclusion in the curriculum: 1) What is PrEP/PEP?, 2) Overview of PrEP Efficacy- Clinical Trials, 3) PrEP Eligibility, 4) PEP Eligibility, 5) Sexual History Taking in the PrEP Era, 6) Can Adolescents Access PrEP?, 7) How to conduct an Adolescent PrEP Medical Visit and 8) Benefits and Insurance Navigation.
The curriculum starts with a comprehensive overview of the PrEP efficacy clinical trials and reviews of some of the real-world demonstration projects indicating PrEP’s effectiveness. It is recommended that clinicians receive an overview of the published PrEP clinical trials and demonstration projects which will provide context to questions that may arise with patients or their caretakers. Having ready access to comprehensive yet concise data supporting the safety, efficacy and tolerability of PrEP both in research and real-world settings is important for any clinician prescribing PrEP.
Additionally, this curriculum includes an in-depth look at how to assess if a patient is a candidate for HIV prevention biomedical interventions. In the United States, HIV risk varies based on location as well as age; the curriculum encourages providers to develop a thoughtful and individualized approach in their practice that incorporates the CDC and local DOH guidelines but, importantly, also includes careful behavioral screening of patients they serve as well as examination of local HIV and STD incidence rates. Instruction on how to assess for prospective risk, especially when working with adolescents, is included. The adolescent in front of a clinician may answer no to every question about historic risk behaviors but over the next year, may begin to explore their sexuality through engaging in risky sex.
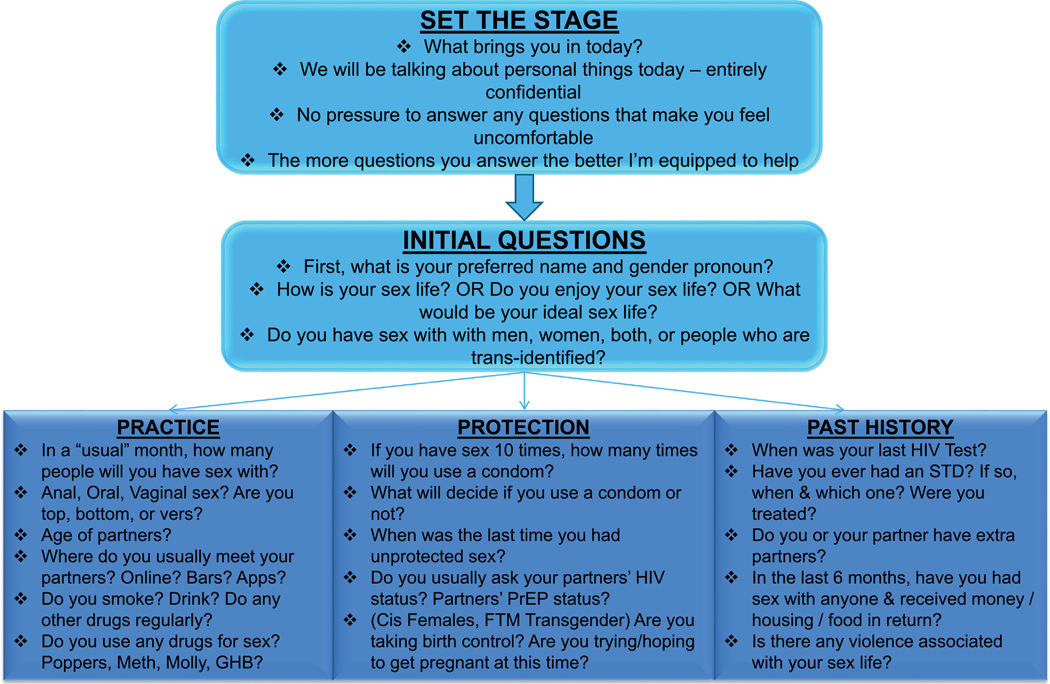
The final curriculum includes tips on how to conduct an adolescent PrEP focused sexual history by expanding on the CDC’s 5Ps (CDC, 2005) (Figure 2). The algorithm guides providers through questions that could be important in establishing a patient’s risk profile. When initiating a PrEP focused sexual history, we recommend starting with “what is your ideal sex life”. This helps to establish with the adolescent patient that they can feel free to discuss with the provider what they imagine their sex life to be like once they start PrEP. It not only gives the provider insight into what patient’s desires are, but also sets a positive and optimistic tone for the visit. Conducting the sexual history in a sex-positive manner increases patient comfort and will ultimately lead to better retention.
Figure 2.
Sexual history algorithm FTM female to male PrEP pre-exposure prophylaxis STD sexually transmitted disease
We also recommend including a brief self-administered sexual history as part of the adolescent clinic visit. This is not designed to replace a comprehensive sexual history but as an opportunity for the adolescent to reveal behaviors that they may not feel comfortable revealing to a provider or coordinator.
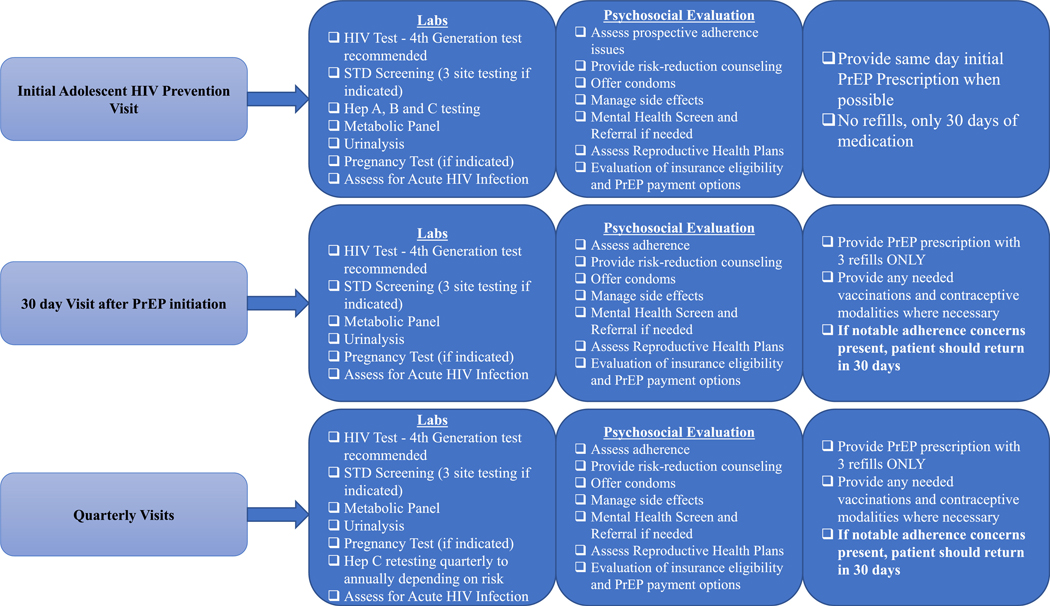
A key component of the curriculum was a development of a detailed checklist of evaluations conducted at each visit as well as visit schedule (Figure 3). The aim is to provide guidance on how to conduct a PrEP visit within the time constraints of the clinic setting. Our graphic, adapted from New York City Department of Health guidelines (New York State AIDS Institute, 2018) provides a quick overview of the visit components separated by areas or tasks (labs, psychosocial evaluation, next steps) to encourage task sharing between providers and PrEP navigator/coordinator or social worker in order to create efficiencies and save time. Due to the increasing incidence of sexually transmitted infections (STIs), especially in New York City, we recommend testing for STIs at every PrEP visit, including the one month follow up (CDC, 2017; NYC, 2017). Not only does this offer an opportunity for test of cure should there have been an STI diagnosis at the first visit but it can have the beneficial effect of normalizing 3 site STI testing for patients. We also recommend self-collection GC/CT testing when possible (Taylor, 2013).
Figure 3.
Adolescent pre-exposure prophylaxis visit schedule Hep hepatitis abs laboratory tests PrEP pre-exposure prophylaxis STD sexually transmitted disease
Curriculum Pilot
The curriculum was tested during a 2-day training of 54 staff from eight high-school based health clinics. Staff members consisted of 14 healthcare providers, social workers, registered nurses, medical assistants, health educators and the clinics’ front desk staff. In a post-training assessment, the majority of providers found the content helpful but wished the curriculum included more training on how to conduct a PrEP-focused sexual history. The providers stated that, although they had been working in the field of adolescent health for some time, their sexual histories typically focused more on family planning concerns and they stated that very rarely did they have young MSM seeking services in their school-based clinics. Based on this feedback, a PrEP-focused sexual history was added to the final curriculum prior to expert panel review.
Expert Panel Review
There was 100% agreement among all seven experts in the relevance and importance of each of these sections in the curriculum. One expert indicated that the section entitled “Overview of PrEP Efficacy” was unclear but the overall affirmative response rate for this section was greater than 78% indicating its validity (Table 1)
TABLE 1:
Adolescent PrEP Curriculum Expert Panel Evaluation with Item Level Content Validity
| Curriculum Sections | Inclusion | Clarity | Relevance | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Not Clear at all | Somewhat Clear | Very Clear | Item CVI | Not Relevant at All | Somewhat Relevant | Very Relevant | Item CVI | ||
| What is PrEP/PEP? | 100% | 16.67% | 83.33% | 1.00 | 100.00% | 1.00 | |||
| Overview of PrEP Efficacy - Clinial Trials | 100% | 16.67% | 50.00% | 33.33% | 0.86 | 100.00% | 1.00 | ||
| Who should be on PrEP? | 100% | 100.00% | 1.00 | 100.00% | 1.00 | ||||
| Who should take PEP? | 100% | 100.00% | 1.00 | 16.67% | 83.33% | 1.00 | |||
| Sexual History and PrEP | 100% | 16.67% | 83.33% | 1.00 | 100.00% | 1.00 | |||
| Can Adolescents Access PrEP? | 100% | 33.33% | 66.67% | 1.00 | 100.00% | 1.00 | |||
| How to conduct an Adolescent PrEP Medical Visit | 100% | 33.33% | 66.67% | 1.00 | 100.00% | 1.00 | |||
| Benefits/Insurance Navigation | 100% | 33.33% | 66.67% | 1.00 | 100.00% | 1.00 | |||
| Mean (Item-level) Content Validity Index | 1.000 | 0.983 | 1.000 | ||||||
The experts unanimously agreed that the content was appropriate for a professionally diverse audience.
Discussion
A comprehensive curriculum on the provision of HIV prevention modalities to adolescents has been successfully developed, piloted, and validated by a panel of experts. While the provision of PrEP can be as simple as writing a prescription, more is needed to engage and assess an adolescent in order to administer a multicomponent HIV prevention intervention. This curriculum contains content validated components that should be included when training clinicians how to administer PrEP to adolescents. It has been shown that certain misconceptions regarding PrEP efficacy, for example, have led providers away from prescribing PrEP to their patients (Silapaswan, 2017). While recognizing this, our curriculum seeks to shape providers attitudes regarding integrating PrEP into their adolescent health practices by initially describing the efficacy trials. We then give providers the concrete skills needed to implement a PrEP assessment, PrEP initiation and the PrEP follow up.
Adherence to PrEP is critical to its efficacy and it undoubtedly will be what adolescents struggle with the most (Grant, 2014) (Hoesk, 2016). Healthcare providers planning to provide PrEP to adolescents must incorporate adherence assessments into their routine care. These assessments do not necessarily have to be completed by a healthcare provider but must be included in routine visits to ensure quality care. We recommend that if adherence concerns arise, the adolescent patient should return to clinic monthly for evaluation and only receive 30 days of medication at a time to ensure there has been no HIV seroconversion. The development and implementation of methods to track and support adherence using technology-based tools that appeal to adolescents, are under way in a variety of settings (Koss, 2017; Fuch, 2018; Hightow-Weidman, 2017).
Toxicities related to PrEP such as renal function decline and bone mineral density (BMD) loss are rare although particularly worrisome in an adolescent population. Providers need to understand the nature of these toxicities when prescribing PrEP therefore inclusion of these topics in the training is crucial (Hosek, 2017). In our curriculum, we educate providers on the clinical trials examining bone mineral density loss and renal function decline with the understanding that more research is needed in a younger population.
Finally, as previously mentioned, one of clinicians’ concerns with providing PrEP to adolescents is navigating how the adolescent can ultimately receive the medication (Mullins, 2017). Insurance coverage and the concern of parental involvement is a prohibitory barrier for adolescents receiving PrEP. It becomes very important for clinicians to understand how their adolescent patients can receive PrEP beyond simply writing the prescription. Education and training about the resources available to cover provision of PrEP to adolescents is critically important to retention. Our curriculum provides healthcare providers with strategies in navigating payment for PrEP in an adolescent community although this may vary at state and local levels.
Consensus from the expert panel supports the dissemination of this Adolescent PrEP Curriculum. It is recommended that when training clinicians on the provision of PrEP to adolescents that all eight domains presented in this curriculum be included.
A limitation of this paper is a smaller sized expert panel of seven experts. Further quantitative evaluation of the curriculum’s effectiveness is needed through implementation and pre/post testing. These efforts are underway. Additionally, content needs to be developed on how best to engage the parents of adolescents in the administration of PrEP. Another limitation of our curriculum is the lack of supporting data associated with same-day PrEP starts, which we recommend in the visit schedule. New York City Department of Health and other health departments have recommended starting PrEP at the initial visit as a way of avoiding potential barriers that may arise in the follow up to a prescription (NYC, 2014)(Denver Public Health, 2017). There have been arguments that starting PrEP on the same day as the first visit without having access to HIV negative confirmatory test result can overlook a potential acute HIV infection and therefore lead to the chance of a resistant HIV virus. Experts have estimated that resistance to TDF/FTC while on PrEP can develop within 15–540 days and in the presence of an acute HIV infection would only occur in approximately 25% of the infections (Dimitrov, 2016)(Lehman, 2015). We contend that same-day PrEP starts are effective in ensuring patients initiate PrEP and we also conduct a repeat HIV test at the one month visit as a failsafe to catch any possible acute HIV infections. More research regarding retention outcomes is necessary on this topic.
Conclusion
We have learned a great deal in the first 6 years of PrEP implementation in the United States, and this can be applied to the roll out of PrEP for adolescents. However, adolescents have a unique set of needs when it comes to a biomedical intervention for HIV prevention. The healthcare community, for the first time, has an intervention that can prevent the transmission of HIV among at-risk individuals if taken correctly. Given the high rates of HIV in the younger age groups, we have a considerable responsibility to determine how to efficiently and safely provide this intervention to youth. PrEP Implementation in a younger population requires a new set of youth focused guidelines, teaching points, and educational methodologies. The validated curriculum developed through the stakeholder driven process may be an important contribution. Preventing HIV transmission in adolescents is a national and international priority and is undoubtedly an essential step necessary to end the HIV epidemic.
To access our validated curriculum, please use the following link prevention.nyc/learn/adolescent-prep/
Acknowledgments
Sources of Support:
Dr. Jason Zucker is supported by the training grant “Training in Pediatric Infectious Diseases” National Institute of Allergy and Infectious Diseases at the National Institutes of Health [T32AI007531]
Contributor Information
Caroline Carnevale, Yale University HIV Prevention Program, New York Presbyterian Hospital’s Comprehensive Health Program.
Jason Zucker, Division of Infectious Diseases, Departments of Internal Medicine and Pediatrics Columbia University Medical Center, New York, NY.
Julie A Womack, University.
Jane Dixon, Nursing, Yale University.
Alwyn Cohall, Clinical Public Health & Clinical Pediatrics, Mailman School of Public Health & Columbia University Medical Center.
Magdalena E. Sobieszczyk, Division of Infectious Diseases, Department of Internal Medicine Columbia University Medical Center, New York, NY.
Peter Gordon, Division of Infectious Diseases, Department of Internal Medicine Columbia University Medical Center, New York, NY.
References
- Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, . . . Celum C, (2012). Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. New England Journal of Medicine, 367(5), 399–410. doi: 10.1056/NEJMoa1108524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benner P, Sutphen M, Leonard V, & Day L. (2010). Educating nurses: A call for radical transformation. [DOI] [PubMed] [Google Scholar]
- Burda JP (2015). PrEP and our youth: Implications in law and policy. Colum.J.Gender & L, 30, 295. [Google Scholar]
- Centers for Disease Control and Prevention. (2005). A guide to taking a sexual history US Department of Health and Human Services, Centers for Disease Control and Prevention. [Google Scholar]
- Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2016. Atlanta: U.S. Department of Health and Human Services; 2017. [Google Scholar]
- Diamond RM (1998). Designing and assessing courses and curricula: A practical guide. the jossey-bass higher and adult education series. ERIC. [Google Scholar]
- Dimitrov DT, Boily M, Hallett TB, Albert J, Boucher C, Mellors JW, . . . van de Vijver David AMC. (2016). How much do we know about drug resistance due to PrEP use? Analysis of experts’ opinion and its influence on the projected public health impact. PloS One, 11, e0158620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federal Drug Administration. (2012). FDA approves first drug for reducing the risk of sexually acquired HIV infection. Retrieved from http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm312210.htm [Google Scholar]
- Fuchs JD, Stojanovski K, Vittinghoff E, McMahan VM, Hosek SG, Amico KR, . . . Lester RT (2018). A mobile health strategy to support adherence to antiretroviral preexposure prophylaxis. AIDS Patient Care and STDs, 32(3), 104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilead Science I. (2018, May 15). U.S. food and drug administration approves expanded indication for truvada® (emtricitabine and tenofovir disoproxil fumarate) for reducing the risk of acquiring HIV-1 in adolescents. (Press Release). doi:http://www.gilead.com/news/press-releases/2018/5/us-food-and-drug-administration-approves-expanded-indication-for-truvada-emtricitabine-and-tenofovir-disoproxil-fumarate-for-reducing-the-risk-of-acquiring-hiv1-in-adolescents
- Grant RM, Anderson PL, McMahan V, Liu A, Amico KR, Mehrotra M, . . . Glidden DV (2014). Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: A cohort study. The Lancet Infectious Diseases, 14(9), 820–829. doi: 10.1016/S1473-3099(14)70847-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, . . . Glidden DV (2010). Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. New England Journal of Medicine, 363(27), 2587–2599. doi: 10.1056/NEJMoa1011205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart-Cooper GD, Allen I, Irwin CE, & Scott H. (2018). Adolescent health providers’ willingness to prescribe pre-exposure prophylaxis (PrEP) to youth at risk of HIV infection in the United States. Journal of Adolescent Health. [DOI] [PubMed] [Google Scholar]
- Hess KL, Johnson AS, Hu X, Li J, Wu B, Yu C, . . . Gerstle J. (2017). Diagnoses of HIV infection in the United States and dependent areas, 2016. [Google Scholar]
- Hightow-Weidman LB, Muessig KE, Bauermeister JA, LeGrand S, & Fiellin LE (2017). The future of digital games for HIV prevention and care. Current Opinion in HIV and AIDS, 12(5), 501–507. doi: 10.1097/COH.0000000000000399 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosek S, Celum C, Wilson C, Kapogiannis B, Delany-Moretlwe S, Bekker L. (2016). Preventing HIV among adolescents with oral PrEP: Observations and challenges in the united states and south africa. Journal of the International AIDS Society, 19(7) Retrieved from http://www.jiasociety.org/index.php/jias/article/view/21107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosek SG, Landovitz RJ, Kapogiannis B, Siberry GK, Rudy B, Rutledge B, . . . Zimet G. (2017). Safety and feasibility of antiretroviral preexposure prophylaxis for adolescent men who have sex with men aged 15 to 17 years in the United States. JAMA Pediatr, 171(11), 1063–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce BR, & Showers B. (2002). Student achievement through staff development. [Google Scholar]
- Koss CA, Hosek SG, Bacchetti P, Anderson PL, Liu AY, Horng H, . . . Saberi P. (2017). Comparison of measures of adherence to human immunodeficiency virus preexposure prophylaxis among adolescent and young men who have sex with men in the United States. Clinical Infectious Diseases, 66, 213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakower D, Ware N, Mitty JA, Maloney K, & Mayer KH (2014). HIV providers’ perceived barriers and facilitators to implementing pre-exposure prophylaxis in care settings: A qualitative study. AIDS and Behavior, 18(9), 1712–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman DA, Baeten JM, McCoy CO, Weis JF, Peterson D, Mbara G, . . . Marzinke MA (2015). Risk of drug resistance among persons acquiring HIV within a randomized clinical trial of single-or dual-agent preexposure prophylaxis. The Journal of Infectious Diseases, 211(8), 1211–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack S, Dunn DT, Desai M, Dolling DI, Gafos M, Gilson R, . . . Gill ON (2016). Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): Effectiveness results from the pilot phase of a pragmatic open-label randomised trial. The Lancet, 387(10013), 53–60. doi: 10.1016/S0140-6736(15)00056-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins TLK, Zimet G, Lally M, Xu J, Thornton S, Kahn JA, & Adolescent Medicine Trials Network for HIV/AIDS Interventions. (2017). HIV care providers’ intentions to prescribe and actual prescription of pre-exposure prophylaxis to at-risk adolescents and adults. AIDS Patient Care and STDs, 31(12), 504–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIV Epidemiology and Field Service Program. HIV Surveillance Annual Report, 2016. New York City Department of Health and Mental Hygiene: New York, NY. December 2017 [Google Scholar]
- The New York City Department of Health and Mental Hygiene Bureau of Sexually Transmitted Disease Control Quarterly Report Vol. 15, No. 1 March 2017. [Google Scholar]
- New York State Department of Health AIDS Institute, & New York State Department of Health AIDS Institute. (2014). Guidance for the use of pre-exposure prophylaxis (PrEP) to prevent HIV transmission. [Google Scholar]
- Ocfemia MCB, Dunville R, Zhang T, Barrios LC, & Oster AM (2018). HIV diagnoses among persons aged 13–29 years - united states, 2010–2014. MMWR.Morbidity and Mortality Weekly Report, 67(7), 212–215. doi: 10.15585/mmwr.mm6707a2 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polit DF, Beck CT, & Owen SV (2007). Is the CVI an acceptable indicator of content validity? appraisal and recommendations. Research in Nursing & Health, 30(4), 459–467. [DOI] [PubMed] [Google Scholar]
- Silapaswan A, Krakower D, & Mayer KH (2017). Pre-exposure prophylaxis: A narrative review of provider behavior and interventions to increase PrEP implementation in primary care. Journal of General Internal Medicine, 32(2), 192–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. (2009). Should the science of adolescent brain development inform public policy? American Psychologist, 64(8), 739. [DOI] [PubMed] [Google Scholar]
- Taylor D, Lunny C, Wong T, Gilbert M, Li N, Lester R, . . . Ogilvie G. (2013). Self-collected versus clinician-collected sampling for sexually transmitted infections: A systematic review and meta-analysis protocol. Systematic Reviews, 2, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Food and Drug Administration FDA approves first drug for reducing the risk of sexually acquired HIV infection. Author, Silver Spring, MD: 2012. (Retrieved from) https://www.fda.gov/downloads/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/UCM312290.pdf [Google Scholar]
- Zucker J, Carnevale C, Gold J, Borsa A, Scherer M, Cohall A,…, Olender S. [Oral Presentation] “An Online Survey of HIV Testing and Pre-Exposure Prophylaxis Attitudes and Practice Habits among Physicians at an Academic Medical Center”. 13th International Conference on HIV Treatment and Prevention Adherence June 6, 2018. Miami Beach, Florida. [Google Scholar]