Abstract
Affinity of the mineralocorticoid receptor (MR) is similar for aldosterone and the glucocorticoids (GC) cortisol and corticosterone, which circulate at concentrations far exceeding those of aldosterone. 11β-hydroxysteroid dehydrogenase type 2 (11βHSD2) inactivation of GC within the immediate vicinity of the MR is credited with prereceptor specificity for aldosterone in cells coexpressing MR and 11βHSD2. 11βHSD2 efficacy is also critical to other recently described 11βHSD2 substrates. The aim of this work was to address doubts that low levels of expression of 11βHSD2 in aldosterone target tissues suffice to prevent the initiation of gene transcription by the MR activated by physiological concentrations of corticosterone. Cell models stably expressing an MR/Gaussia luciferase reporter and various levels of constitutive or induced 11βHSD2 at concentrations lower than those in rat kidney homogenates and microsomes were produced. Aldosterone and corticosterone were equipotent transactivators of the MR reporter gene in cells without 11βHSD2. Rate of conversion of tritiated corticosterone to 11-dehydrocorticosterone increased and corticosterone-induced nuclear translocation of MR decreased, as 11βHSD2 expression increased. The 50% maximal MR activation for the reporter gene stimulation by corticosterone rose with increasing 11βHSD2 expression, shifting the steroid dose-response curve for corticosterone-induced MR transactivation to the right. Several stable cell lines expressing an easily and reproducibly measured MR reporter system and consistent incremental amounts of 11βHSD2 protein were produced and used to document that 11βHSD2 within low physiological levels inactivates relevant concentrations of GC and decreases MR transactivation by GC in a dose-dependent fashion, laying to rest doubts of the efficacy of this enzyme.
Keywords: 11βHSD2, mineralocorticoid receptor, glucocorticoids, corticosterone, Gaussia luciferase
The mineralocorticoid receptor (MR) is a member of the steroid-thyroid hormone receptor superfamily of ligand-dependent transcription factors and has diverse functions. It is unique among steroid hormone receptors in that it has 3 primary physiological agonists: aldosterone, cortisol, and corticosterone [1]. Corticosterone is the main glucocorticoid (GC) in animals that do not express the 17-hydroxylase in the zona fasciculata, including laboratory rats and mice. Before the mineralocorticoid and the glucocorticoid receptors (GRs) were cloned, it was thought that the MR was expressed primarily in epithelial cells involved in electrolyte and water transport such as those of the renal collecting tubule and colon, and that there were 2 corticosteroid receptors, one with 10-fold higher affinity than the other, reviewed in [2]. On cloning of the MR and GR, it was demonstrated that the MR was the high-affinity corticosteroid receptor and that it had a similar affinity for corticosterone, cortisol, and aldosterone of about 0.5 to 1 nM [3]. At the time it was also known that total circulating GC levels are 1000-fold higher and free, nonprotein-bound GC concentrations are 100-fold higher than those of aldosterone, providing a clear stoichiometric advantage to the GC, yet clearly aldosterone, not corticosterone or cortisol, acted through the MR to ensure water and electrolyte homeostasis [3-5]. At the time the interconversion of cortisol and cortisone, and 11-dehydrocorticosterone by 11β-hydroxysteroid dehydrogenase (11βHSD), enzymatic activity was being studied in the context of patients with a hereditary form of hypertension, apparent mineralocorticoid excess [6]. In addition to the hypertension, hypokalemia, and alkalosis expected of excessive aldosterone production, these patients had low aldosterone and low urinary ratios of cortisol plus cortisol metabolites to cortisone plus cortisone metabolites, indicating that they had deficient 11βHSD activity. Within months of the demonstration that the MR had an equal affinity for aldosterone and the GC, 2 different laboratories demonstrated that 11βHSD was coexpressed with the MR in aldosterone target tissues and provided selectivity to the MR by converting the more abundant cortisol and corticosterone into inactive cortisone and 11-dehydrocorticosterone and their metabolites, respectively [7, 8]. Within a few years, 2 11βHSD enzymes were cloned and characterized by several laboratories, as reviewed in [2]. 11βHSD2 is a high-affinity, NAD+-dependent, obligate hydroxysteroid dehydrogenase (HSD) with a Michaelis constant (4-14 nM) for corticosterone and cortisol that is low enough to be relevant to their circulating levels [9]. The products, 11-dehydrocorticosterone and cortisone, are inactive as ligands for the MR and GR. 11βHSD2 is expressed with the MR in aldosterone target cells, where it confers aldosterone specificity to the MR over the much more abundant GCs.
Despite the large amount of evidence accrued from clinical and experimental studies that is generally accepted [2], others have reported studies casting doubt that the low levels of endogenous 11βHSD2 in the kidney in vivo are sufficient to confer prereceptor specificity for aldosterone to the MR given the large amounts of free circulating GC [10, 11], reviewed in [12]. To address reservations about whether expression and catalytic activity of 11βHSD2 is sufficient to prevent corticosterone binding and transcriptional activation of the MR, we produced several stable cell lines that constitutively express different levels of 11βHSD2 below those found in the kidney and others that expressed it on induction to provide consistent graded concentrations of the enzyme and characterized its catalytic efficacy for the conversion of active GC to their inactive 11-HSD metabolites. These cells were also engineered with an MR reporter gene to demonstrate the efficacy of different expression levels of the 11βHSD2 enzyme on MR transcriptional activation by physiologically relevant concentrations of corticosterone and aldosterone.
Materials and Methods
[1,2-3H]-Corticosterone was purchased from American Radiolabeled and unlabeled steroids from Steraloids. Channeled thin-layer chromatography plates (silica gel GF254, 60 Å) were obtained from Analtech and reagent-grade solvents from Fisher Scientific. A bicinchoninic acid kit from Pierce Biotechnology was used to measure protein concentrations. Solvents and other reagents were purchased from Millipore Sigma. The antibodies for 11βHSD2 (C.E.G.S., University of Mississippi Medical Center, catalog No. 2147, RRID:AB_2892988; https://antibodyregistry.org/search.php?q=AB_2892988) and MR (DSHB catalog No. rMR1-18 1D5, RRID:AB_1157909; https://antibodyregistry.org/search.php?q=AB_1157909) were developed in house against the recombinant rat 11βHSD2 protein in sheep [13] and the rat MR protein in mice, respectively [14, 15]. Peroxidase-conjugated rabbit antibodies against β-actin and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were obtained from Proteintech, catalog Nos. HRP-60008, RRID:AB_2819183; https://www.ptglab.com/products/ACTB-Antibody-HRP-60008.htm and HRP-60004, RRID:AB_2737588; https://scicrunch.org/resolver/RRID:AB_2737588, respectively. The antilamin A/C antibody (DSHB catalog No. MANLAC1[4A7], RRID:AB_2618203; https://scicrunch.org/scicrunch/resolver/RRID:AB_2618203) was from Developmental Studies and Hybridoma Bank (DSHB, University of Iowa), MANLAC1 (4A7). The horseradish peroxidase (HRP)-conjugated donkey antisheep secondary antibody was purchased from Jackson ImmunoResearch Labs (catalog No. 713-001-003, RRID:AB_2340702; https://www.jacksonimmuno.com/catalog/products/713-001-003).
Production, Culture, and Characterization of Stable Cell-Line Models
Chinese hamster ovary cells (CHO) (CLS catalog No. 603479/p746_CHO, RRID:CVCL_0213; https://scicrunch.org/scicrunch/resolver/CVCL_0213?i=5dc224499898c958a92d4333) were grown in Dulbecco’s modified Eagle’s medium supplemented with 5% newborn calf serum until 50% confluent, were transduced with a lentivirus carrying the rat MR complementary DNA (cDNA) (pWPT-rMR), cloned, then transduced with a lentivirus with a reporter gene Gaussia luciferase (G-luc) and 3 hormone response elements (HREs; pBM14-TAT3-Gluc), and selected with 0.5 mg/mL of G418 (geneticin) as previously described [16]. The resulting CHO-rMR-pBM14-TAT3-Gluc cells were then transduced by a lentivirus pCDH-CMV-.r11βHSD2 (SBI, System Biosciences). Additional cells were also transduced with an all-in-one tetracycline (tet)-inducible plasmid (pCW57.1, Addgene.org plasmid 41393, from the laboratory of Dr David Root) carrying the rat 11βHSD2 cDNA. Transduced cells were selected with 5-µg/mL puromycin. The CHO-rMR-pBM14-TAT3-Gluc cells were transduced with pCDH-puro-rβ11HSD2 for 1, 2, or 3 times to produce 3 stably transduced cells with progressively greater 11βHSD2 expression. The resulting stably transfected cell lines are designated CHO-rMR-pBM14-TAT3-Gluc r11βHSD2 (×1-3) and CHO-rMR-pBM14-pTAT3-Gluc.tet-inducible-r11βHSD2.
CV1 monkey kidney cells (CLS catalog No. 605471/p715_CV-1, RRID:CVCL_0229; https://scicrunch.org/scicrunch/resolver/CVCL_0229?i=5fbef78f0143b73be5149fff) stably transduced with a lentivirus containing a hormone-response element from the mouse mammary tumor virus (MMTV) and a G-luc reporter gene (pBM14-MMTV-Gluc) were kindly provided by Dr William E. Rainey [17]. These cells were transduced with a lentivirus carrying the rat MR (pWPT-rMR) cDNA and the resulting CV1-rMR-MMTV-Gluc cells were transduced with a tet-inducible plasmid (pCW57.1-r11βHSD2) carrying the rat 11βHSD2 cDNA and a puromycin selection gene. Antibiotic selection with puromycin and G418 produced the stably transduced CV1-rMR MMTV-Gluc.tet-inducible-r11βHSD2. Cells were maintained in Dulbecco’s modified Eagle’s medium containing 10% fetal calf serum (FCS) under a humidified atmosphere of 5% CO2, at 37 ºC. CV1-rMR MMTV-Gluc.tet-inducible-r11βHSD2 and CHO-rMR-pBM14-pTAT3-Gluc.tet-inducible-r11βHSD2 cells were incubated in media with several concentrations of doxycycline (doxy) 0.1 to 1.0 µg/mL for 48 hours to induce 11βHSD2 expression before experiments. Before each experiment the cells were transferred to medium in which the FCS had been treated with 1% charcoal to remove steroids.
Western Blot Analyses
The levels of 11βHSD2 protein expression in each stably transfected cell line was measured by Western blot. Cells were cultured in 6-well plates until subconfluent. The CV1-rMR-MMTV-Gluc.tet-inducible-r11βHSD2 and CHO-rMR-pBM14-pTAT3-Gluc.tet-inducible-r11βHSD2 cells were treated with doxy at several doses, 0 to 1.0 µg/mL, for 48 hours to provide a stable induction of 11βHSD2. Cells were solubilized in a mixture of ice-cold radioimmunoprecipitation assay buffer and 1× protease inhibitor (Thermo Fisher). The cell lysates were centrifuged, the supernatants mixed with 2× Laemmli buffer, and then heated at 65 °C for 20 minutes. The proteins were separated using 12.5% sodium dodecyl sulfate–polyacrylamide gel electrophoresis gels and transferred electrophoretically to polyvinylidene difluoride membrane (EMD Millipore). The membranes were blocked using 1% bovine serum albumin for 1 hour and then incubated in 1% bovine serum albumin containing the rat 11βHSD2 antibody (sheep antirat, 1:3000 dilution) overnight at 4 °C. The membranes were further incubated with HRP-conjugated secondary antibody (donkey antisheep, 1:5000 dilution) for 1 hour at room temperature and then washed in Tris-buffered saline. Chemiluminescence was performed for visualization using a luminal reagent prepared as described by Haan and Behrmann [18]. Protein bands were imaged with a ChemiDoc imager (Bio-Rad). The membranes were stripped and reincubated with a HRP-conjugated anti–β-actin antibody (rabbit, 1:10 000 dilution) for protein normalization. The quantification of signal densities from triplicate wells was performed by Image J software (National Institutes of Health).
Gaussia Luciferase Assay for the Reporter Gene
Cell lines were grown in 96-well plates (0.2 mL/well) using phenol-red free growth media until confluent, then changed to 1% charcoal-treated FCS media plus steroid ligand overnight. CHO-rMR-pBM14-TAT3-Gluc-r11βHSD2 (×1-3) were incubated overnight in steroid stripped media with 0.1- to 1000-nM aldosterone or corticosterone and 10-µM (100-fold excess) mifepristone to prevent GR activation and transactivation of the reporter HRE. Cells stably transduced with the tet-inducible plasmid pCW57.1-r11βHSD2were incubated for 48 hours without or with 0- to 1.0-µg/mL doxy, then incubated with 1% charcoal-treated FCS media containing the same amounts of doxy plus aldosterone or corticosterone overnight with suppression of GR transactivation with mifepristone as described earlier. All experiments were performed in quadruplicate. After overnight incubation with or without an MR agonist, 25 µL of media was used for G-luc analysis with 50 µL of the substrate coelenterazine (diluted 1:100 in 50-mM Tris and 150-mM NaCl buffer) [16]. Luminescence was measured with a BMG microplate reader according to the manufacturer’s instructions. The ligand concentration that produced 50% maximal MR activation (EC50) was calculated from the dose-response curves.
Conversion of Corticosterone to 11-Dehydrocorticosterone
To assess the enzymatic activity, CV1-rMR-MMTV-Gluc and CHO-rMR-pTAT3-Gluc cells stably transduced with tet-inducible plasmid pCW57.1-r11βHSD2 were seeded on 24-well plates (0.5 mL/well) and treated for 48 hours with different doses of doxy. Cells were serum-starved for 1 hour, then the media were replaced with fresh phenol red-free media containing 1% charcoal-stripped FCS and 500 000 cpm of [1,23H]-corticosterone per well. After a 2-hour incubation, the supernatants were collected in glass tubes, mixed well with 2 mL of methylene chloride, the aqueous phase aspirated and discarded, 20 µg of unlabeled corticosterone and 11-dehydrocorticosterone added, and the samples dried by evaporation under vacuum. Steroids were then dissolved in 50-µL isopropanol and separated on channeled silica-gel thin-layer chromatography plates using acetone-methylene chloride (18:82). Areas corresponding to the steroids were located under UV light, scraped, and eluted with 0.5 mL of isopropanol and counted using a liquid scintillation counter. All experiments were performed in quadruplicate. The 4 types of CHO-rMR-TAT3-Gluc cell lines created by titrated infection (0, 1, 2, and 3 times) with lentivirus carrying the 11βHSD2 were grown then seeded on a 12-well plate (1 mL/well) and cultured until subconfluent. The media were replaced with media containing 500 000 cpi of [3H]-corticosterone and the steroid extraction and measurements performed as described earlier.
Nuclear Translocation of the Mineralocorticoid Receptor Induced by Aldosterone and Corticosterone
The CHO-rMR-pBM14-pTAT3-Gluc.tet-inducible-r11βHSD2 cells grown in 10-cm dishes were incubated without and with doxy at the indicated doses for 48 hours, serum-starved overnight with the same concentration of doxy, and then treated with 10-nM aldosterone or corticosterone for 1 hour. After trypsinization and phosphate-buffered saline wash, a portion of the whole cells was taken and lysed to measure the11βHSD2 and MR in total protein. The remaining cells were homogenized in ice-cold sucrose 0.25 M, HEPES 20 mM, Molybdate 20-mM buffer (pH 7.4) supplemented with a protease inhibitor cocktail (Goldbio.com), and centrifuged at 800g for 10 minutes. The pellet was resuspended in an isolation buffer containing 1.8-M sucrose and 0.5% Igepal with HEPES and molybdate, centrifuged at 60 000g for 40 minutes to pellet the nuclei. The nuclear pellet was washed once with the same buffer and lysed. The supernatant of the 800g spin was further centrifuged at 100 000g for 1 hour to separate the cytosolic fraction in the supernatant. All centrifugations were performed at 4 °C. Protein concentration of all fractions was determined by bicinchoninic acid kit. To assess the purity of the nuclear and cytosolic fractions, we performed immunoblotting for Lamin A/C and GAPDH, nuclear and cytosolic markers, respectively.
Statistical Analysis
Results are expressed as mean ± SEM. Differences between a single data set and a grouped data set were analyzed by 1-way and 2-way analysis of variance, respectively, followed by Bonferroni multiple comparisons. The differences were considered significant at P less than .05. Statistical analyses were performed using GraphPad/Prism (v6 for Windows software; GraphPad Software).
Results
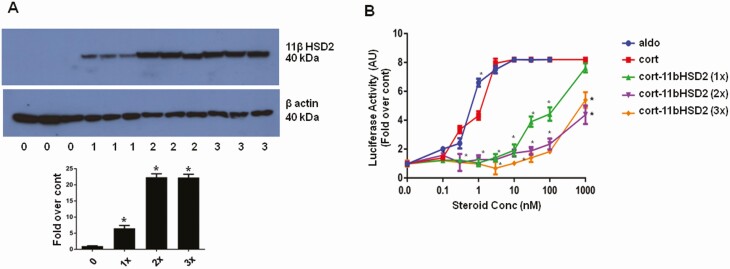
Consistent with a previous report [19], endogenous expression of 11βHSD2 mRNA and protein were not detected in the CHO and CV1 cells by reverse-transcriptase polymerase chain reaction and Western blot analysis (data not shown). Expression of the MR/G-luc was stable as reported for our original model cell [15]. 11βHSD2 protein levels analyzed by Western blot were reproducibly commensurate with the times the CHO-rMR-pBM14-TAT3-Gluc cells were stably transduced with the lentivirus carrying the rat Hsd11b2 cDNA (Fig. 1A), and concentration of doxy used to induce the expression of the gene (Fig. 2). To elucidate the effect of different levels of 11βHSD2 expression on agonist-induced MR transactivation, luciferase activity was measured in the media of the cell models after stimulation with aldosterone or corticosterone at concentrations from 0 to 1000 nM. Mifepristone was added to block binding of corticosterone to endogenous GR to determine the effect of different levels of 11βHSD2 protein on the ability of the steroids to stimulate MR transcriptional activity (Fig. 1B). MR transactivation was enhanced similarly by aldosterone or corticosterone in cells that did not express 11βHSD2 (red and blue lines). Corticosterone-induced MR transcriptional activity was significantly attenuated in an 11βHSD2 concentration-dependent manner, with the level of inactivation correlating with the concentration of enzyme (see Fig. 1A and 1B). A single transduction with the pCDH-puro-r11βHSD2 lentivirus containing the entire coding region of the rat 11βHSD2 was enough to decrease the EC50 for corticosterone by 2 orders of magnitude.
Figure 1.
A, Western blot analysis of rat 11β-hydroxysteroid dehydrogenase type 2 (11βHSD2) protein in cell lysates of control: CHO-rMR-pBM14-TAT3-Gluc 1 (0); CHO-rMR-pBM14-TAT3-Gluc r11HSD2 (1×), CHO-rMR-pBM14-TAT3-Gluc r11HSD2 (2×) cells, and CHO-rMR-pBM14-TAT3-Gluc r11HSD2 (3×) cells [3]. B, Corticosterone-induced mineralocorticoid receptor transactivation assessed by luciferase secretion into the media by stably transfected CHO-rMR-pBM14-TAT3-Gluc cells infected 0 (red and blue lines), 1, 2, or 3 times with a virus carrying 11βHSD2. For A and B, mean values are based on data from quadruplicate wells for each concentration of steroids from 3 separate experiments and expressed as fold over control (0), without doxycycline. Results are shown as mean ± SEM. *P less than .05 vs control. Results are shown as mean ± SEM. *P less than .05 vs the same concentration of corticosterone in control cells without doxycycline.
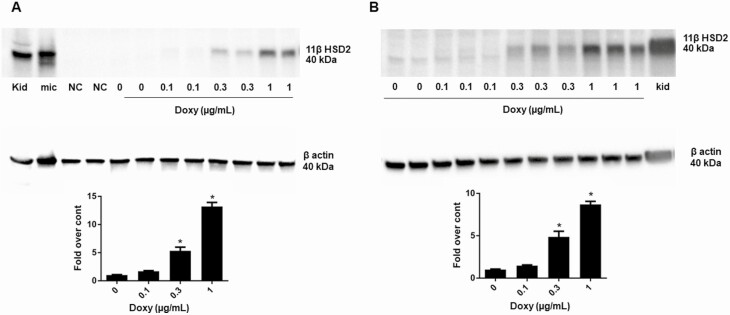
Figure 2.
11β-hydroxysteroid dehydrogenase type 2 (11βHSD2) protein measured by Western blot analysis in lysates of A, CV1-rMR MMTV-Gluc.tet-inducible-r11βHSD2, and B, CHO-rMR-pBM14-pTAT3-Gluc.tet-inducible-r11βHSD2cells incubated with graded concentrations of doxycycline for 48 hours. Kidney homogenates and kidney microsomes were used as controls. A total of 20 µg of protein was used in all lanes. Results are shown as mean ± SEM. *P less than .05 vs control (0), without doxycycline.
To demonstrate that expression of 11βHSD2 protein in the several model cells was within low physiological ranges reported in other aldosterone target tissues, it was compared to its relatively high endogenous expression in rat kidney homogenate and microsomes. Fig. 2A and 2B are representative Western blots of 11βHSD2 protein in CV1-rMR MMTV-Gluc.tet-inducible-r11βHSD2 and CHO-rMR-pBM14-pTAT3-Gluc.tet-inducible-r11βHSD2 cells treated with graded doses of doxy. Doxy at 0.3 and 1.0 µg/mL dose-dependently increased the 11βHSD2 protein in these stably transduced cells. The same amount of total protein, 20 µg, was loaded in each lane. The proportion of 11βHSD2 protein in the model cells was stable (not shown) and lower than in rat kidney homogenates and rat kidney microsomes.
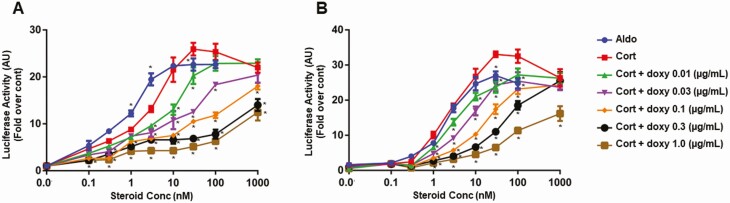
Fig. 3 shows the results of representative MR transactivation assays using a Gluc reporter construct in CV1-rMR MMTV-Gluc.tet-inducible-r11βHSD2 (Fig. 3A) and CHO-rMR-pBM14-pTAT3-Gluc.tet-inducible-r11βHSD2 (Fig. 3B) cells. The general patterns of transactivation were similar in the 2 cell lines. Corticosterone-stimulated MR transactivation of the luciferase reporter gene significantly decreased with increasing doxy concentration in the incubation media, commensurate with an increase in 11βHSD2 protein measured in Fig. 2, shifting the curves to the right in a dose-responsive manner. The EC50 for transactivation by aldosterone in the CV1-rMR MMTV-Gluc.tet-inducible-r11βHSD2 cells was about 0.9 nM, and by corticosterone was 3.0 nM without doxy (see Fig. 3A), and after 11βHSD2 induction with 0.01, 0.03, 0.1, 0.3, and 1 µg/mL of doxy, the EC50 for corticosterone was significantly increased to 29.29, 30.0, 29.87, 78.51, and 164.5 nM, respectively. The EC50 in CHO-rMR-pBM14-pTAT3-Gluc.tet-inducible-r11βHSD2 for aldosterone was 2.28 nM, similar to the EC50 for corticosterone without doxy, 2.84 nM. Induction of 11βHSD2 with 0.01, 0.03, 0.1, 0.3, and 1 µg/mL of doxy resulted in a significant increase in EC50 values for corticosterone to 2.98, 9.8, 10.2, 51.06, and 56.43 nM, respectively (see Fig. 3B).
Figure 3.
Corticosterone-induced mineralocorticoid receptor transactivation in A, CV1-rMR MMTV-Gluc.tet-inducible-r11βHSD2, and B, CHO-rMR-pBM14-pTAT3-Gluc.tet-inducible-r11βHSD2cells assessed by a Gaussia luciferase reporter gene system. Results are shown as mean ± SEM. *P less than .05 vs the same concentration of corticosterone in control cells without doxycycline (doxy). Mean values based on data from quadruplicate wells for each concentration of steroids from 3 separate experiments were plotted and expressed as fold over control.
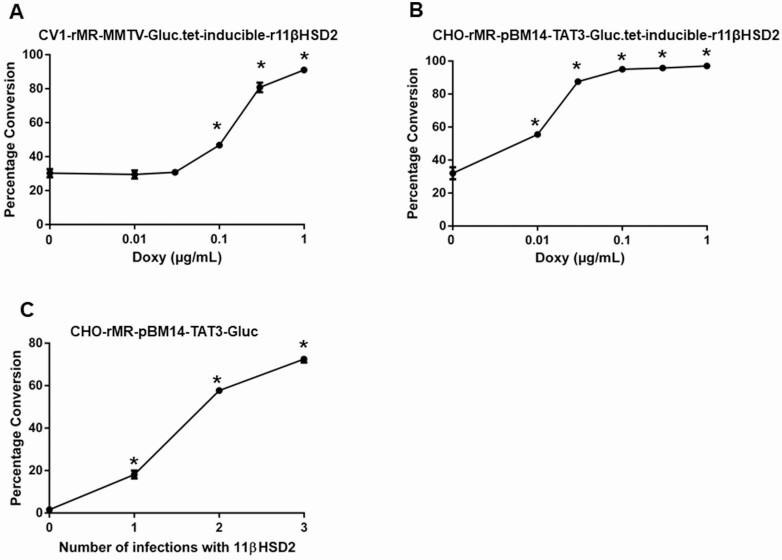
Dose-dependent activity of the 11βHSD2 in the model cells was assessed by performing a radioactive substrate conversion assay. Fig. 4A and 4B are representative results of the conversion of tritiated corticosterone to 11-dehydrocorticosterone in CV1-rMR MMTV-Gluc.tet-inducible-r11βHSD2 (see Fig. 4A), and CHO-rMR-pBM14-pTAT3-Gluc.tet-inducible-r11βHSD2 (see Fig. 4B) cells as a function of the concentration of doxy. Conversion increased commensurate with the dose of doxy in both cell lines. The efficacy of induction was greater in the CHO-rMR-pBM14-pTAT3-Gluc.tet-inducible-r11βHSD2 cells. Similarly, conversion increased significantly with each successive infection with the lentivirus carrying the r11βHSD2 cDNA in CHO-rMR-pBM14-TAT3-Gluc cells (Fig. 4C).
Figure 4.
Determination of dose-dependent enzymatic activity by measuring the conversion of corticosterone to 11-dehydrocorticosterone by cells with variable amounts of enzyme. 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2) is induced by graded doses of doxycycline (doxy) indicated by the x-axis in A, CV1-rMR MMTV-Gluc.tet-inducible-r11βHSD2, and B, CHO-rMR MMTV-Gluc.tet-inducible-r11βHSD2 cells. C, CHO-rMR-pBM14-pTAT3-Gluc.tet-inducible-r11βHSD2 cells stably infected 1 to 3 times with the virus 11βHSD2. Results are presented as the mean of percentage of conversion ± SEM. *P less than .05 vs control (0), without doxycycline induction for A and B and without infection of the complementary DNA for r11βHSD2. Mean values based on data from quadruplicate wells were plotted.
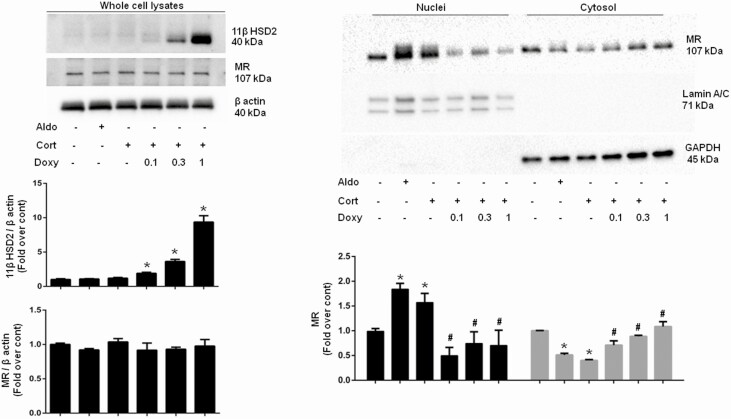
Fig. 5 represents the results of experiments demonstrating the effect of increasing levels of 11βHSD2 expression on corticosterone-induced MR nuclear translocation in CHO-rMR-pBM14-pTAT3-Gluc.tet-inducible-r11βHSD2 cells by isolating nuclei and cytosolic fractions and measuring MR by Western blot. 11βHSD2 was undetected in whole-cell lysates (upper left panel) in the absence of doxy, then increased after 48 hours of incubation with increasing concentrations of the inducing agent. As in the original model cell [15], MR/G-luc expression was stable and not significantly affected by different 11βHSD2 levels. MR expression levels in the nuclear fraction was significantly and similarly increased by corticosterone and aldosterone (upper right panel) in the absence of doxy, when 11βHSD2 was undetected in whole-cell lysates. Treatment with 0.1, 0.3, and 1 µg/mL of doxy markedly attenuated the corticosterone-induced movement of the MR from cytosol into the nucleus. The lowest dose of doxy produced minimally detectable expression of the enzyme 11βHSD2, yet significantly inhibited the nuclear transport and consequent activation of the MR by corticosterone. Lamin A/C and GAPDH are nuclear and cytosolic markers, respectively, and were measured to determine the purity of the isolated cell fractions.
Figure 5.
Nuclear translocation of the mineralocorticoid receptor (MR) in CHO-rMR-pBM14-pTAT3-Gluc.tet-inducible-r11βHSD2 cells induced by 10-nM aldosterone or corticosterone for 1 hour. Cells were lysed and the nuclear and cytosol fractions separated by centrifugation. Laminin A/C and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) are markers for nuclei and cytosol, respectively. *P less than .05 vs no treatment control, and #P less than .05 vs corticosterone, without doxycycline.
Discussion
Circulating levels of the major physiological agonists of the MR, aldosterone and the GCs cortisol and corticosterone, are regulated by distinct physiological signals including the renin-angiotensin-aldosterone system and hypothalamic-pituitary-adrenal axis, respectively. As the affinity of the MR for the GCs is 10-fold that of the GR, in cells with both receptors but no 11βHSD2, only MRs are occupied at lower concentrations of GCs [1]. 11βHSD1 and 11βHSD2 further modulate intracellular concentrations of the GCs, thus their occupation of both MR and GR. The effect of GC binding to the MR is gene, cell-type, and physiological context dependent. It may activate gene transcription as does aldosterone or maintain the MR in a quiescent state unless there are secondary factors, in particular inflammation and oxidative stress, that render the GC a full agonist for the MR [20].
The premise that 11βHSD2 protects the MR from inappropriate activation by GCs has been challenged primarily from data from in vivo studies and those using whole-organ homogenates [12]. In vitro systems have limitations; however, they allow the determination of specific enzyme and receptor functions and interactions within the milieu in which they function. For example, in the kidney, MR expression is limited to only a few cell types; expression of 11βHSD2 is even more limited and occurs in cells also expressing MR [15, 21, 22]. In vitro models such as ours for this report reflect the nature of aldosterone target cells in which MR and 11βHSD2 are coexpressed and produce their physiological effects, not the whole organ or organism.
Our cells used for these studies model the aldosterone target cell. Like the aldosterone-target MR of the renal tubular epithelium of patients who are genetically deficient in 11βHSD2 who suffer from apparent mineralocorticoid excess described earlier, the transcriptional effect of GC and aldosterone in the aldosterone target cells is the same. Therefore, studies in which model cells are incubated with both ligands were not conducted. Aldosterone and corticosterone were equally potent in producing transactivation of the MR reporter gene in our model cells that did not express the enzyme 11βHSD2. While generally accepted, the concept that 11βHSD2 in aldosterone target cells confers aldosterone specificity to the MR by inactivating GCs has been disputed on the grounds of the small amount of enzyme relative to steroid substrate. Study of the enzymatic characteristics of 11βHSD2 in vivo has been difficult because of its restricted distribution and expression, as well as coexpression with 11βHSD1 in several tissues, including the kidney. The results presented herein dispel doubts about the efficacy of low levels of 11βHSD2 in inactivating corticosterone and preventing its activation of MR transcriptional activities.
Corticosterone- and aldosterone-stimulated MR transactivation in model cells that did not express 11βHSD2 were the same. The HRE used for the transcription reporter for these studies is shared by the MR and GR, as are most in vivo. The affinity of cortisol and corticosterone for the GR is about one-tenth that for the MR, thus in cells lacking 11βHSD2 the GR is activated at higher steroid concentrations after most or all MRs are occupied. Addition of the GR antagonist mifepristone to the media limited transcription of the reporter gene by the GR activated by the highest concentrations of corticosterone in the culture media.
Total basal serum concentrations of corticosterone in the rat are about 5 to 20 µg/dL, or 0.14 to 0.58 µM; most corticosterone is protein-bound and cannot enter the cell. Free GC ranges typically from 14 to 60 nM, concentrations that still exceeded that of aldosterone by 100-fold. The lower concentrations of corticosterone used for the MR transactivation experiments are well within basal physiological concentrations; the highest concentration used is within stress concentrations reported to saturate and cause product inhibition of the 11βHSD2 [2]. Thus, both the expression levels of 11βHSD2 and the concentrations of corticosterone were within physiological limits. Sequential increments in 11βHSD2 protein in our cell models shifted the corticosterone-induced activation curves toward the right and increased the EC50 for corticosterone for MR transcriptional activity, indicating that the levels of 11βHSD2 transcription, translation, and activity are within the functional dynamic range relevant for prereceptor reduction of GC binding to the MR.
The plots for the proportion of corticosterone converted to 11-dehydrocorticosterone also indicated that the enzyme expression and concentrations of corticosterone studied were in a dynamic range. It is not necessary that a large proportion of the total GCs within the cell be inactivated. The 11βHSD2 protein spans the membrane of the endoplasmic reticulum and nucleus so that the C-terminus comprising the catalytic domain and structural features that promote a close association with the MR are in close proximity in the cytoplasm [23]. Inactivation of the GC need only occur within the microenvironment of the receptor (reviewed in [2]). It has been suggested that in tissues in which 11βHSD1 and 11βHSD2 are both expressed, for example, the aortic endothelium where 11βHSD2 expression is lower than that of 11βHSD1, the net activity within the cell is that of a reductase [24, 25]. However, in addition to the affinity of 11βHSD2 for the active steroids being an order of magnitude greater than that of 11βHSD1 for 11-dehydrocorticosterone and cortisone, the catalytic site of 11βHSD2 is in the cytosol, that of the 11βHSD1 is in the endoplasmic reticulum lumen. Thus, the net tissue or cell 11βHSD activity does not necessarily reflect that of the microenvironment of the receptor. Our present studies confirm that low physiological levels of the rat 11βHSD2 inactivate physiologically relevant concentrations of corticosterone and thereby prevent corticosterone from activating the MR and initiating the recruitment of proteins required for its translocation into the nucleus, where it can initiate transcription. Translocation of the MR from the cytosol to the nucleus is a separate event from its nuclear transcription activities. Both require ligand binding and activation of the receptor [26].
The effect of 11βHSD2 on MR-mediated effects are more complex than preventing inappropriate activation of MR by GCs. In addition to limiting GC access to the MR, 11βHSD2 limits access of GCs to the GR. GRs are expressed in many, though not all, cells that express MR, including some kidney tubular epithelial cells, and many GR-mediated transcriptional and functional activities have profound, often oppositional effects on those of the MR [27]. MR and GR share most, but not all, HRE on the DNA, as well as cell- and context-specific chaperone proteins and transcription coregulators. MR most frequently activates gene transcription; GR may activate or suppress gene transcription depending on the context and cell type [1]. Thus at higher concentrations of GCs, activation of GR represses transcription at some HREs that MR activates [28]. The same cotranscription factor may have different effects depending on the steroid receptor. ELL (eleven-nineteen lysine-rich leukemia) is a coactivator for the ligand-bound MR, but a corepressor for the ligand-bound GR [29]. 11β-HSD2 reduces the amount of GC available to bind to the GR, thus protecting the transcriptional activity of the MR at the level of HRE binding. In the brain, where 11βHSD2 is limited to very few neurons, basal levels of GCs activate the MR, which mediates essential trophic processes and neuron activation. High-stress levels of GCs activate the GR, which dampens many MR-mediated effects and modulates the stress response [30].
In conclusion, the results of these enzyme function and transactivation studies demonstrate that the low physiological levels of 11βHSD2 convert physiological amounts of corticosterone to 11-dehydrocorticosterone and prevent transactivation of the MR by corticosterone, dispelling doubts about its efficacy. The importance of this work is not limited to the MR and GR. The search for the mechanism for extrinsic ligand specificity for the MR diverted attention from other crucial functions of the 11βHSD2. The affinity of 11βHSD2 and 11βHSD1 for other endogenous and exogenous sterols is similar or greater than for the GC, as reviewed in [2]. Among these are bile and cholesterol metabolites and adrenal androgens. 11βHSD2 also regulates concentrations of 7β, 27-dihydroxycholesterol, an agonist of the retinoid-related orphan receptor-γ [31], and catalyzes the formation of the active adrenal androgens 11-ketotestosterone and 11-ketodihydrotestosterone from their inactive 11β-hydroxy forms [32]. Estrogens formed from 11-keto androgens by aromatization are potent activators of the estrogen receptor [33]. While their circulating levels may be negligible [33], like aldosterone, they may be physiologically relevant within the cells where they are formed. Our findings suggest that the low levels of 11βHSD2 will prove to be physiologically relevant in the context of these less well-studied substrates as well.
Glossary
Abbreviations
- 11βHSD2
11β-hydroxysteroid dehydrogenase type 2
- CHO
Chinese hamster ovary cells
- cDNA
complementary DNA
- doxy
doxycycline
- EC50
50% maximal mineralocorticoid receptor activation
- FCS
fetal calf serum
- GC
glucocorticoid
- G-luc
Gaussia luciferase
- GR
glucocorticoid receptor
- HRE
hormone response element
- HRP
horseradish peroxidase
- HSD
hydroxysteroid dehydrogenase
- MMTV
mouse mammary tumor virus
- MR
mineralocorticoid receptor
- tet
tetracycline
Acknowledgments
Financial Support: This work was supported by the National Heart, Lung and Blood Institute (grant No. R01 HL144847), the National Institute of General Medical Sciences (grant No. 1U54GM115428), and the Department of Veteran Affairs (grant No. BX004681).
Additional Information
Disclosures: The authors have nothing to disclose.
Data Availability
Some or all data generated or analyzed during this study are included in this published article. Data sharing is not applicable to this article because no data sets were generated or analyzed during the present study.
References
- 1. Gomez-Sanchez EP. Brain mineralocorticoid receptors in cognition and cardiovascular homeostasis. Steroids. 2014;91:20-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gomez-Sanchez EP, Gomez-Sanchez CE. 11β-hydroxysteroid dehydrogenases: a growing multi-tasking family. Mol Cell Endocrinol. 2021;526:111210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arriza JL, Weinberger C, Cerelli G, et al. Cloning of human mineralocorticoid receptor complementary DNA: structural and functional kinship with the glucocorticoid receptor. Science. 1987;237(4812):268-275. [DOI] [PubMed] [Google Scholar]
- 4. Sheppard K, Funder JW. Mineralocorticoid specificity of renal type I receptors: in vivo binding studies. Am J Physiol. 1987;252(2 Pt 1):E224-E229. [DOI] [PubMed] [Google Scholar]
- 5. Sheppard K, Funder JW. Type I receptors in parotid, colon, and pituitary are aldosterone selective in vivo. Am J Physiol. 1987;253(4 Pt 1):E467-E471. [DOI] [PubMed] [Google Scholar]
- 6. Ulick S, Levine LS, Gunczler P, et al. A syndrome of apparent mineralocorticoid excess associated with defects in the peripheral metabolism of cortisol. J Clin Endocrinol Metab. 1979;49(5):757-764. [DOI] [PubMed] [Google Scholar]
- 7. Funder JW, Pearce PT, Smith R, Smith AI. Mineralocorticoid action: target tissue specificity is enzyme, not receptor, mediated. Science. 1988;242(4878):583-585. [DOI] [PubMed] [Google Scholar]
- 8. Edwards CR, Stewart PM, Burt D, et al. Localisation of 11 beta-hydroxysteroid dehydrogenase–tissue specific protector of the mineralocorticoid receptor. Lancet. 1988;2(8618):986-989. [DOI] [PubMed] [Google Scholar]
- 9. Krozowski ZS, Rundle SE, Wallace C, et al. Immunolocalization of renal mineralocorticoid receptors with an antiserum against a peptide deduced from the complementary deoxyribonucleic acid sequence. Endocrinology. 1989;125(1):192-198. [DOI] [PubMed] [Google Scholar]
- 10. Funder J, Myles K. Exclusion of corticosterone from epithelial mineralocorticoid receptors is insufficient for selectivity of aldosterone action: in vivo binding studies. Endocrinology. 1996;137(12):5264-5268. [DOI] [PubMed] [Google Scholar]
- 11. Brem AS, Bina RB, King TC, Morris DJ. Localization of 2 11beta-OH steroid dehydrogenase isoforms in aortic endothelial cells. Hypertension. 1998;31(1 Pt 2):459-462. [DOI] [PubMed] [Google Scholar]
- 12. Morris DJ, Latif SA, Brem AS. Interactions of mineralocorticoids and glucocorticoids in epithelial target tissues revisited. Steroids. 2009;74(1):1-6. [DOI] [PubMed] [Google Scholar]
- 13. Gomez-Sanchez EP, Ganjam V, Chen YJ, Liu Y, Clark SA, Gomez-Sanchez CE. The 11beta hydroxysteroid dehydrogenase 2 exists as an inactive dimer. Steroids. 2001;66(11):845-848. [DOI] [PubMed] [Google Scholar]
- 14. Zhou MY, Gomez-Sanchez EP, Cox DL, Cosby D, Gomez-Sanchez CE. Cloning, expression, and tissue distribution of the rat nicotinamide adenine dinucleotide-dependent 11 beta-hydroxysteroid dehydrogenase. Endocrinology. 1995;136(9):3729-3734. [DOI] [PubMed] [Google Scholar]
- 15. Gomez-Sanchez EP, Ganjam V, Chen YJ, et al. Regulation of 11 beta-hydroxysteroid dehydrogenase enzymes in the rat kidney by estradiol. Am J Physiol Endocrinol Metab. 2003;285(2):E272-E279. [DOI] [PubMed] [Google Scholar]
- 16. Kuppusamy M, Gomez-Sanchez EP, Beloate LN, et al. Interaction of the mineralocorticoid receptor with RACK1 and its role in aldosterone signaling. Endocrinology. 2017;158(7):2367-2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Campana C, Rege J, Turcu AF, et al. Development of a novel cell based androgen screening model. J Steroid Biochem Mol Biol. 2016;156:17-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Haan C, Behrmann I. A cost effective non-commercial ECL-solution for Western blot detections yielding strong signals and low background. J Immunol Methods. 2007;318(1-2):11-19. [DOI] [PubMed] [Google Scholar]
- 19. Morita H, Zhou M, Foecking MF, Gomez-Sanchez EP, Cozza EN, Gomez-Sanchez CE. 11 beta-Hydroxysteroid dehydrogenase type 2 complementary deoxyribonucleic acid stably transfected into Chinese hamster ovary cells: specific inhibition by 11 alpha-hydroxyprogesterone. Endocrinology. 1996;137(6):2308-2314. [DOI] [PubMed] [Google Scholar]
- 20. Mihailidou AS, Loan Le TY, Mardini M, Funder JW. Glucocorticoids activate cardiac mineralocorticoid receptors during experimental myocardial infarction. Hypertension. 2009;54(6):1306-1312. [DOI] [PubMed] [Google Scholar]
- 21. Shimojo M, Ricketts ML, Petrelli MD, et al. Immunodetection of 11 beta-hydroxysteroid dehydrogenase type 2 in human mineralocorticoid target tissues: evidence for nuclear localization. Endocrinology. 1997;138(3):1305-1311. [DOI] [PubMed] [Google Scholar]
- 22. Hirasawa G, Sasano H, Suzuki T, et al. 11Beta-hydroxysteroid dehydrogenase type 2 and mineralocorticoid receptor in human fetal development. J Clin Endocrinol Metab. 1999;84(4):1453-1458. [DOI] [PubMed] [Google Scholar]
- 23. Odermatt A, Arnold P, Frey FJ. The intracellular localization of the mineralocorticoid receptor is regulated by 11beta-hydroxysteroid dehydrogenase type 2. J Biol Chem. 2001;276(30):28484-28492. [DOI] [PubMed] [Google Scholar]
- 24. Christy C, Hadoke PW, Paterson JM, Mullins JJ, Seckl JR, Walker BR. 11beta-hydroxysteroid dehydrogenase type 2 in mouse aorta: localization and influence on response to glucocorticoids. Hypertension. 2003;42(4):580-587. [DOI] [PubMed] [Google Scholar]
- 25. Gong R, Morris DJ, Brem AS. Variable expression of 11beta hydroxysteroid dehydrogenase (11beta-HSD) isoforms in vascular endothelial cells. Steroids. 2008;73(11):1187-1196. [DOI] [PubMed] [Google Scholar]
- 26. Galigniana MD, Erlejman AG, Monte M, Gomez-Sanchez C, Piwien-Pilipuk G. The hsp90-FKBP52 complex links the mineralocorticoid receptor to motor proteins and persists bound to the receptor in early nuclear events. Mol Cell Biol. 2010;30(5):1285-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gomez-Sanchez E, Gomez-Sanchez CE. The multifaceted mineralocorticoid receptor. Compr Physiol. 2014;4(3):965-994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pascual-Le Tallec L, Lombès M. The mineralocorticoid receptor: a journey exploring its diversity and specificity of action. Mol Endocrinol. 2005;19(9):2211-2221. [DOI] [PubMed] [Google Scholar]
- 29. Pascual-Le Tallec L, Simone F, Viengchareun S, Meduri G, Thirman MJ, Lombès M. The elongation factor ELL (eleven-nineteen lysine-rich leukemia) is a selective coregulator for steroid receptor functions. Mol Endocrinol. 2005;19(5):1158-1169. [DOI] [PubMed] [Google Scholar]
- 30. de Kloet ER, Meijer OC, de Nicola AF, de Rijk RH, Joëls M. Importance of the brain corticosteroid receptor balance in metaplasticity, cognitive performance and neuro-inflammation. Front Neuroendocrinol. 2018;49:124-145. [DOI] [PubMed] [Google Scholar]
- 31. Beck KR, Inderbinen SG, Kanagaratnam S, et al. 11β-Hydroxysteroid dehydrogenases control access of 7β,27-dihydroxycholesterol to retinoid-related orphan receptor γ. J Lipid Res. 2019;60(9):1535-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Storbeck KH, Bloem LM, Africander D, Schloms L, Swart P, Swart AC. 11β-Hydroxydihydrotestosterone and 11-ketodihydrotestosterone, novel C19 steroids with androgenic activity: a putative role in castration resistant prostate cancer? Mol Cell Endocrinol. 2013;377(1-2):135-146. [DOI] [PubMed] [Google Scholar]
- 33. Barnard L, Schiffer L, Louw du-Toit R, et al. 11-oxygenated estrogens are a novel class of human estrogens but do not contribute to the circulating estrogen pool. Endocrinology. 2021;162(3):bqaa231. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data generated or analyzed during this study are included in this published article. Data sharing is not applicable to this article because no data sets were generated or analyzed during the present study.