Abstract
The objective of this study was to determine the expression and functional role of a long noncoding RNA (lncRNA) MIAT (myocardial infarction–associated transcript) in leiomyoma pathogenesis. Leiomyoma compared with myometrium (n = 66) expressed significantly more MIAT that was independent of race/ethnicity and menstrual cycle phase but dependent on MED12 (mediator complex subunit 12) mutation status. Leiomyomas bearing the MED12 mutation expressed higher levels of MIAT and lower levels of microRNA 29 family (miR-29a, -b, and -c) compared with MED12 wild-type leiomyomas. Using luciferase reporter activity and RNA immunoprecipitation analysis, MIAT was shown to sponge the miR-29 family. In a 3-dimensional spheroid culture system, transient transfection of MIAT siRNA in leiomyoma smooth muscle cell (LSMC) spheroids resulted in upregulation of miR-29 family and downregulation of miR-29 targets, collagen type I (COL1A1), collagen type III (COL3A1), and TGF-β3 (transforming growth factor β-3). Treatment of LSMC spheroids with TGF-β3 induced COL1A1, COL3A1, and MIAT levels, but repressed miR-29 family expression. Knockdown of MIAT in LSMC spheroids blocked the effects of TGF-β3 on the induction of COL1A1 and COL3A1 expression. Collectively, these results underscore the physiological significance of MIAT in extracellular matrix accumulation in leiomyoma.
Keywords: leiomyoma, MIAT, miR-29, TGF-β3, MED12 mutation
Leiomyomas are benign fibrotic uterine tumors developing in ~70% women during reproductive years. These tumors account for more than 30% of all hysterectomies performed in the United States annually due to limited availability of medical therapies (1-4). Although their etiology remains unknown, the growth of leiomyomas is dependent on ovarian steroids, and they are characterized by excess accumulation of extracellular matrix (ECM) with a higher prevalence and symptom severity in African Americans (5-7).
Microarray, next-generation RNA sequencing and proteomic approaches have identified overexpression of a number of protein-coding genes in leiomyomas including COL1A1 (Collagen Type I Alpha 1 Chain), COL3A1 (Collagen Type III Alpha 1 Chain), and TGF-β3 (transforming growth factor-β3) (2). The products of these genes play essential roles in ECM deposition and leiomyoma progression (2). TGF-β3 is a profibrotic cytokine which through activation of signaling pathways such as Smad2/3 (SMAD family member 2/3), MAPK/ERK (mitogen activated protein kinase/extracellular signal–regulated kinase), PI3K/Akt (phosphatidylinositol 3-kinases/protein kinase B), and the inhibition of expression of matrix metalloproteinases (MMPs), induces the accumulation of ECM components including COL1A1 and COL3A1 (8-11). Moreover, accumulated genomic studies have implicated many chromosomal rearrangements and mutation associated genes which are related to leiomyomas development and progression. The driver mutations for fibroid include MED12 (mediator complex subunit 12), HMGA2 (high-mobility group AT-hook 2), FH (fumarate hydratase) and COL4A5/6 (collagen, type IV, alpha 5, and alpha 6) (12-14). MED12 mutations in exon 2 occur at a frequency of up to 80% in leiomyomas causing abnormal activation of Wnt/β-catenin signaling, sex steroid receptor signaling, cell proliferation, and fibrosis-associated gene expression in leiomyoma (12, 15, 16).
In addition to abnormal expression of protein-coding genes and activation of cell signaling pathways, several studies have revealed misexpression of nonprotein coding RNAs in leiomyomas (17-19). To date, high-throughput sequencing has identified a large number of noncoding RNAs including long (lncRNAs; >200 nucleotides long) and small (sncRNAs; 17-120 nucleotides long) noncoding RNAs which are involved in various disease processes, including tumorigenesis and tissue fibrosis (20-24). Using new-generation RNA-seq our group reported differential expression of several sncRNAs and lncRNAs in leiomyomas, which may play key roles in leiomyoma development and progression (18, 19). We also reported that leiomyomas express significantly lower levels of miR-29c, resulting in an increase in expression of miR-29c targets such as COL1A1, COL3A1, CDK2, and TGF-β3 (25-27). Other groups also reported lower expression of miR-29a and miR-29b with an associated increase in expression of collagen (28, 29).
LncRNAs are >200 nucleotides in length and are transcribed from different genomic loci, representing sense or antisense regions, overlapping sequences with part or entire genomic sequence, as well as intergenic and intronic regions of protein-coding genes (20, 30). Multiple lines of evidence suggest that sncRNAs, more specifically miRNAs as well as lncRNAs, play key roles in regulating the expression of protein-coding genes through transcriptional, post-transcriptional, and epigenetic mechanisms (20, 30). In addition, lncRNAs serve as miRNA sponges or competing endogenous RNAs (ceRNAs) in both sequence-dependent and sequence-independent manners thus preventing the availability of miRNAs to target mRNAs (31-33). Through these mechanisms miRNAs and lncRNAs in a cell- and tissue-specific manner regulate various cellular activities under physiological conditions, and their altered expression has been associated with a wide range of disorders, including tumorigenesis and tissue fibrosis (31-33).
The functional role of lncRNAs in leiomyoma pathogenesis has recently started to be explored. Cao et al. reported that the lncRNA H19 is aberrantly increased in leiomyomas and is linked to increased HMGA2 expression, and several genes related to proliferation, inflammation, and ECM deposition via TET3 (Tet methylcytosine dioxygenase 3)-mediated epigenetic modification (34). We recently reported that the lncRNA XIST (X-inactive specific transcript) was overexpressed in leiomyoma and acted as a sponge of miR-29c and miR-200c, resulting in increased expression of their targets including COL1A1, COL3A1, and FN1 (fibronectin) (35). Because the miR-29 family is one of the pivotal miRNAs regulating the composition of ECM and is uniformly downregulated in leiomyoma, we sought to determine other lncRNAs which could sponge the miR-29 family.
Previous work showed a lncRNA MIAT (myocardial infarction–associated transcript) functions as a competing endogenous RNA for several miRNAs including the miR-29 family and caused fibrosis in various fibrotic diseases (36-38). Moreover, we previously reported that miR-29c is uniformly downregulated in all fibroid specimens tested (25). However, in our previous study, the mutation status of the fibroids and the expression of other members of the miR-29 family was not determined. Based on this and our previous RNA-seq results (GSE100338), which also revealed increased expression of MIAT in leiomyomas (18), we hypothesized that MIAT might also play a role in leiomyoma pathogenesis by sequestering the miR-29 family, which would result in its downregulation and upregulation of miR-29 target genes which are critical for ECM deposition. We tested this hypothesis through analysis of large numbers of paired leiomyoma specimens, and functional testing using a 3-dimensional culture system which is considered to better represent the in vivo state by maintaining physiological cell to cell interaction and chemical gradients of oxygen, nutrients, and catabolites (39, 40), and extended our previous study by measuring the expression of other members of the miR-29 family (miR-29a/miR-29-b) in wild-type and MED12-mutated fibroids.
We previously reported that TGF-β3 inhibited the expression of miR-29c through an epigenetic mechanism, resulting in fibrosis which is the hallmark of fibroids (26). TGF-β3 is an established profibrotic growth factor previously shown to be upregulated by several groups in fibroids (41). Therefore, in the present study we also determined if the effects of TGF-β3 on miR-29 regulation was mediated by MIAT.
Material and Methods
Tissue Collection
Portions of uterine leiomyomas and paired myometrium were obtained from patients (n = 66) who had not been taking any hormonal medications for at least 3 months prior to surgery at Harbor-UCLA Medical Center. Prior approval from the Institutional Review Board (#036247) at the Lundquist Institute at Harbor-UCLA Medical Center was obtained. Informed consent was obtained from all the patients participating in the study prior to surgery. The paired tissues were obtained from Caucasians (n = 12), African Americans (n = 24), Hispanics (n = 23), and Asians (n = 7). The mean age of patients was 45 ± 3.1 years with a range of 30-54 years. The menstrual cycle phase was determined by histologic analysis of hematoxylin and eosin–stained endometrial sections (42), with 31 specimens being identified as in the proliferative phase and 16 specimens in the secretory phase. The MED12 mutation status was determined by polymerase chain reaction (PCR) amplification and Sanger sequencing. Of the specimens sequenced, 43 fibroids had the MED12 mutations (65.2%) with no mutations in the myometrium. The leiomyomas used in this study ranged in size from 3 to 5 cm in diameter and were intramural. The tissues were either snap frozen and stored in liquid nitrogen for further analysis or used for isolation of leiomyoma smooth muscle cell (LSMCs) as previously described (43, 44).
MED12 Mutation Analysis
Genomic DNA from leiomyomas and paired myometrial specimens was extracted from 100 mg of freshly frozen tissue using MagaZorb DNA Mini-Prep Kit (Promega, Madison, WI) according to the manufacturer’s protocol. PCR amplification and Sanger sequencing (Laragen Inc. Culver City, CA) was performed to investigate the MED12 exon 2 mutations using the primer sequences in the 5′-3′ direction: sense, GCCCTTTCACCTTGTTCCTT and antisense, TGTCCCTATAAGTCTTCCCAACC. PCR products were sequenced using Big Dye Terminator v.3.1 sequencing chemistry and the sequences were analyzed with the Software ChromasPro 2.1.8 and compared with the MED12 reference sequence (NG_012808 and NM_005120).
Reagents and Spheroid Cell Culture
LSMCs were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum until reaching confluence with a change of medium every 2 to 3 days. Cells at passages p1 to p3 were used for all experiments. Isolated LSMCs were plated in 6-well plates (1.5 × 105 cells/well) which were coated with 0.5% agarose gel and incubated 48 hours for spheroid formation with size ranging from 50 µm to 250 µm in diameter (35). For blocking TGF-β type I receptor, LSMC spheroids were treated with 5 μM SB431542 (Cayman Chemical, Ann Arbor, MI) for 48 hours. Cell culture experiments were performed at least 3 times using LSMCs obtained from different patients. Overall, 16 LSMCs were used for the in vitro experiments. All supplies for the isolation and cell culture were purchased from Sigma-Aldrich (St. Louis, MO), Invitrogen (Carlsbad, CA), and Fisher Scientific (Atlanta, GA).
siRNA Transfection
Prior to the formation of spheroids, primary LSMCs were transfected with 50 nM siRNA negative control (siNC) or siRNA against MIAT (siMIAT; 5′-CUUGCUAUGAGCAGGAUCU-3′) using PureFection transfection reagent (System Biosciences, Inc. Mountain View, CA) according to the manufacturer’s protocol.
RNA Isolation and qPCR Analysis
Total RNA was isolated from leiomyoma and matched myometrium using Trizol (Thermo Fisher Scientific, Waltham, MA) and RNA concentration and integrity was determined using a Nanodrop 2000c spectrophotometer (Thermo Scientific) and Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA) according to manufacturer’s protocols as previously described (19). Subsequently, a 1-μg RNA sample was reverse transcribed using random primers for MIAT, COL1A1, and COL3A1. Primer design for the miR-29 family and PCR conditions have been described previously (45). Quantitative PCR was carried out using SYBR gene expression master mixes (Applied Biosystems, Carlsbad, CA). Reactions were incubated for 10 minutes at 95°C followed by 40 cycles for 15 seconds at 95°C and 1 minute at 60°C. Levels of mRNA and miRNA were quantified using the Invitrogen StepOne System and normalized to FBXW2 (46) and RNU6B, respectively. All reactions were run in triplicate and relative expression was determined using the comparative cycle threshold method (2–ΔΔCq), as recommended by the supplier (Applied Biosystems). Abundance values was expressed as fold change compared with the corresponding control group. The primer sequences in the 5′-3′ direction used for MIAT detection are sense, TCAGGATGGTGCACTCTCAG and antisense, TGTCTCCATTTGCTCAGTGC. For COL1A1 are sense, CCAATGGTGCTCCTGGTATT and antisense, GTTCACCGCTGTTACCCTT. For COL3A1 are sense, ATTATTTTGGCACAACAGGAAGCT and antisense, TCCGCATAGGACTGACCAAGAT. For TGF-β3 are sense, CGGGCTTTGGACACCAATTA and antisense, GGGCGCACACAGCAGTTC. For FBXW2 are sense, CCTCGTCTCTAAACAGTGGAATAA and antisense, GCGTCCTGAACAGAATCATCTA. For miR-29a are sense, TCCAGTTTTTTTTTTTTTTTAACCGA and antisense, CGCAGTAGCACCATCTGA. For miR-29b are sense, GGTCCAGTTTTTTTTTTTTTTTAACAC and antisense, CAGTAGCACCATTTGAAATCAG. For miR-29c are sense, GCAGTAGCACCATTTGAAATC and antisense, GGTCCAGTTTTTTTTTTTTTTTAACC. For RNU6B are sense, ATTGGAACGATACAGAGAAGATTAG and antisense, AATATGGAACGCTTCACGAAT.
Immunoblotting
Total protein isolated from LSMC spheroids was subjected to immunoblotting as previously described (26, 47). Briefly, samples were suspended in RIPA buffer containing 1 mM EDTA and EGTA (Boston BioProducts, Ashland, MA) supplemented with 1 mM phenylmethylsulfonyl fluoride and a complete protease inhibitor mixture (Roche Diagnostics, Indianapolis, IN), sonicated, and centrifuged at 4°C for 10 minutes at 14 000 rpm. The concentration of protein was determined using the BCA™ Protein Assay Kit (Thermo Scientific Pierce, Rockford, IL). Equal aliquots (30 µg) of total protein for each sample was denatured with sodium dodecyl sulfate-polyacrylamide gel electrophoresis sample buffer and separated by electrophoresis on a sodium dodecyl sulfate polyacrylamide gel. After transferring the samples to a nitrocellulose membrane, the membrane was blocked with Tris-buffered saline-Tween + 5% milk and probed with the following primary antibodies: COL1A1 (1:3000; RRID:AB_2882107) (48), COL3A1 (1:3000; RRID:AB_2879158) (49), and TGF-β3 (1:300; RRID:AB_10611929) (50). The membranes were washed with Tris-buffered saline containing 0.1% Tween-20 wash buffer after each antibody incubation cycle. SuperSignal West Pico Chemiluminescent Substrate™ (Thermo Scientific Pierce) was used for detection, and photographic emulsion was used to identify the protein bands, which were subsequently quantified by densitometry. The membranes were also stripped and probed with glyceraldehyde 3-phosphate dehydrogenase antibody (1:1000; RRID:AB_10847862) (51) serving as the loading control. The densities of the specific protein bands were determined using image J program (http://imagej.nih.gov/ij/), normalized to glyceraldehyde 3-phosphate dehydrogenase or a band obtained from staining the membrane with Ponceau S. Results were obtained from 5 experiments using cells isolated from different patients in each set and expressed as mean ± standard error of the mean (SEM) as a ratio relative to the control group designated as 1.
Reporter Plasmid Construction
Recombinant luciferase reporter plasmid pEZX-MT01(MIAT) was constructed by insertion of a EcoRI/Xho1-digested PCR amplified fragment of MIAT (NR_033319; +8038/+8512) covering 2 miR-29 family binding sites into the downstream of the luciferase reporter pEZX-MT01 (GeneCopoeia, Rockville, MD). The fragment of MIAT was amplified using primers with the following sequences: forward primer 5′-GGTGGTGAATTCCAGTTTAGGGACAGGTCAAAGG-3′, reverse primer 5′-GGTGGTCTCGAGCCAACTCTCCCACTAGGCTATAA-3′.
Luciferase Reporter Assays
LSMCs were seeded in 6-well plates and at 70% to 80% subconfluence were transfected with 50 nM 2′-O-methoxyethyl modified pre-miR-29a, -b, -c oligonucleotides, and the corresponding pre-miR negative control (NC) (Applied Biosystems, Carlsbad, CA) using the PureFection transfection reagent as previously described (27). At the same time, the cells were co-transfected with a luciferase reporter plasmid (1 μg/well) pEZX-MT01 (Control) or pEZX-MT01(MIAT). After 48 hours of transfection Firefly and Renilla luciferase activities were measured using the Dual-Luciferase Reporter Assay System (Promega, Madison, WI). Firefly luciferase activity was normalized to Renilla luciferase activity. The level of induction was reported as mean ± SEM of 3 experiments performed in triplicate using cells isolated from different patients in each set and compared with a ratio in cells transfected with negative control independently set as 1.
RNA Immunoprecipitation
RNA immunoprecipitation (RIP) assay was performed using an EZ-Magna RIP RNA Binding Protein Immunoprecipitation Kit according to the protocol of manufacturer (Millipore, Burlington, MA). Briefly, fresh specimens of leiomyoma and matched myometrium were lysed in RIP lysis buffer, followed by incubation with RIP buffer containing magnetic bead–bound human anti-argonaute 2 (Ago2) antibody (RRID:AB_10807962) (52) or negative control normal mouse immunoglobulin G (RRID:AB_490557) (53). Next, the samples were incubated with proteinase K to digest protein and the immunoprecipitated RNA was isolated. The precipitated RNA was subjected to qPCR analysis to detect the level of target sequences. The assay was performed 3 times using specimens collected from 3 different patients.
Statistical Analysis
Throughout the text, all data are presented as mean ± SEM and analyzed by PRISM software (Graph-Pad, San Diego, CA). Dataset normality was determined by the Kolmogorov–Smirnoff test, Shapiro–Wilk test, D’Agostino and Pearson test, and Anderson–Darling test. Data presented in Fig. 1 are not normally distributed and therefore nonparametric tests are used for data analysis. Comparisons involving 2 groups were analyzed using the Wilcoxon matched-pairs signed rank test (Fig. 1A) or Mann–Whitney test (Fig. 1B-F) as appropriate. For normally distributed data (Figs 2-4) comparisons involving 2 groups were analyzed using unpaired Student’s t-tests, and 1-way analysis of variance was used for comparisons involving multiple groups. Statistical significance was established at P < .05.
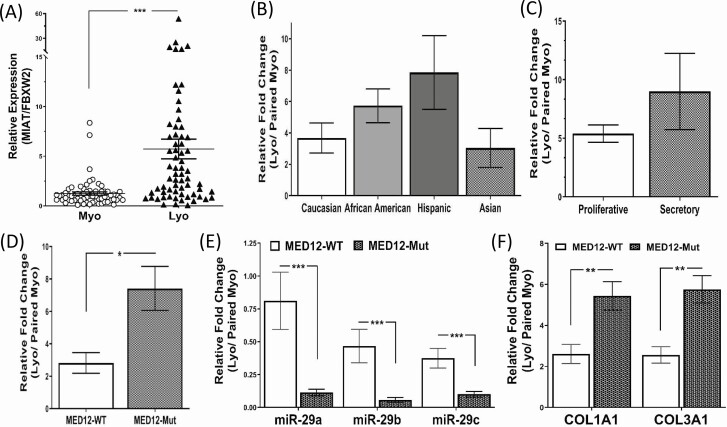
Figure 1.
(A) The relative expression of MIAT in 66 paired myometrium (Myo) and leiomyoma (Lyo). The results in this and subsequent figures are presented as mean ± SEM. *P < .05, **P < .01, and ***P < .001. (B) Relative MIAT levels expressed as fold change (Lyo/paired Myo) based on race/ethnicity in Caucasian (n = 12), African American (n = 24), Hispanics (n = 23), and Asians (n = 7); (C) menstrual cycle phase in proliferative phase (n = 31) and secretory phase (n = 16); (D) MED12 mutation status in MED12 wild type (n = 23) and MED12 mutated (n = 43). (E,F) Relative levels of miR-29 family (E), COL1A1 and COL3A1 (F) expressed as fold change (Lyo/ paired Myo) in MED12 wild type (n = 23) and MED12 mutations (n = 43).
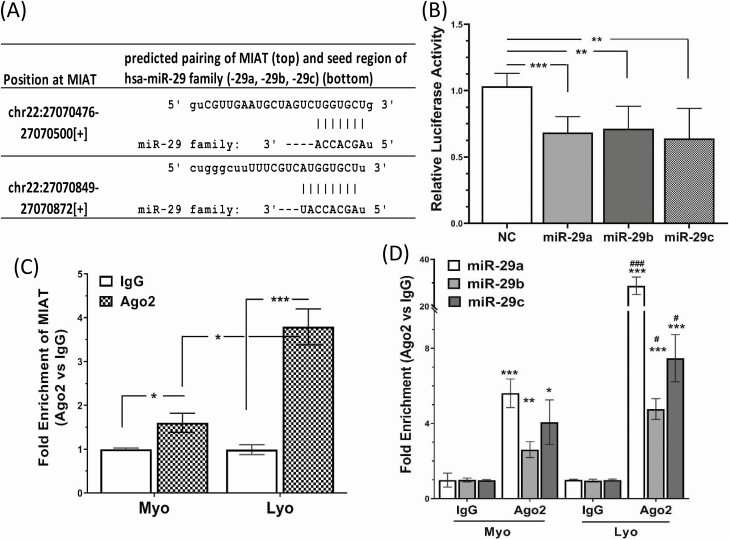
Figure 2.
(A) Sequence alignment with the coordinated positions of MIAT with miR-29 family. (B) Shows the relative luciferase activity in isolated LSMCs transfected with Renilla and firefly luciferase reporter pEZX-MT01 (Control) or pEZX-MT01(MIAT). Cells were also cotransfected with pre-miR-29 family (miR-29a, -b, -c; 50 nM) or scramble oligonucleotides (NC). Ratio of firefly to Renilla was determined after 48 hours and reported as relative luciferase activity compared with NC, which was independently set as 1. The results are presented as mean ± SEM and analyzed using 1-way analysis of variance. **P < .01 and ***P < .001. (C,D) Show the results of RNA immunoprecipitation assay with Ago2 antibody which was conducted to confirm the association between MIAT and miR-29 family using lysates from fresh specimens of leiomyoma (Lyo) and matched myometrium (Myo). The expression of MIAT (C) and miR-29 family (D) from purified RNA was determined by qRT-PCR. The results are presented as mean ± SEM and analyzed using unpaired t test. *P < .05, **P < .01, and ***P < .001 vs IgG, #P < .05, ###P < .001 vs Ago2 of the Myo group.
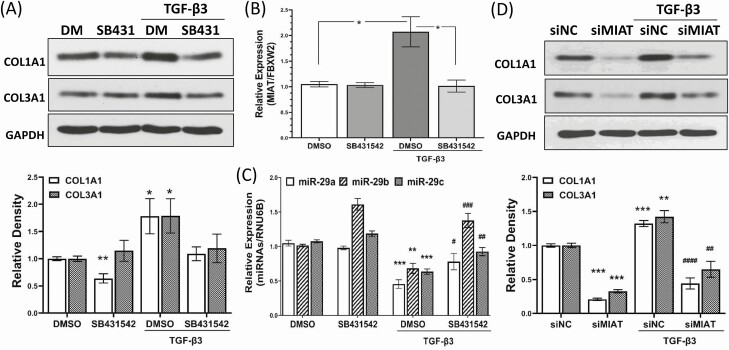
Figure 4.
(A-C) Representative of Western blot analysis of COL1A1 and COL3A1 proteins with relative band densities shown below (A); qRT-PCR analysis of MIAT (B) and miR-29 family (C) in LSMC spheroids following treatment with DMSO or SB431542 (5 μM; TGF-β type I receptor inhibitor) with TGF-β3 (10 ng/mL) for 48 hours. (D) LSMC spheroids were transfected with siRNA scrambled oligonucleotides (siNC) or MIAT siRNA oligonucleotides (siMIAT) for 96 hours with TGF-β3 (10 ng/mL) treatment for the last 48 hours and the levels of COL1A1 and COL3A1 proteins were determined with the relative density histograms shown below. The results were obtained from 4 experiments using cells isolated from different patients in each set and analyzed using unpaired t test with presentation as mean ± SEM. *P < .05, **P < .01, and ***P < .001 vs DMSO group (A and C) or siNC group (D); #P < .05, ##P < .01, and ###P < .001 vs TGF-β3 group (C) or siNC group of TGF-β3 treatment (D).
Results
Using paired leiomyoma and matched myometrium (n = 66) the expression of the lncRNA MIAT was determined by qRT-PCR. As shown in Fig. 1A, leiomyomas expressed significantly higher levels of MIAT (55/66 pairs) than myometrium. We next analyzed the MIAT expression in terms of fold change (leiomyoma vs matched myometrium) based on race/ethnicity, menstrual cycle phase, and MED12 mutation status. The analysis indicated that there was no significant correlation between MIAT expression with either race/ethnicity or menstrual cycle phase (Fig. 1B and 1C). However, the analysis in terms of MED12 mutation status indicated that MIAT was expressed in greater abundance in leiomyomas bearing the MED12 mutation (n = 43) compared with MED12 wild-type leiomyomas (n = 23) (Fig. 1D). Among the MED12-mutated specimens, missense mutations in MED12 exon 2 were the most frequent alteration (36/43 pairs), followed by in-frame insertion–deletion type mutations (7/43 pairs). The missense mutations in exon 2 included c.130G>C (p.Gly44Arg) (4/36 pairs), c.130G>A (p.Gly44Ser) (7/36 pairs), c.130G>T (p.Gly44Cys) (3/36 pairs), c.131G>C (p.Gly44Ala) (1/36 pairs), c.131G>A (p.Gly44Asp) (15/36 pairs), and c.131G>T (p.Gly44Val) (6/36 pairs). There were no correlations between MIAT levels with different types of MED12 missense mutations in the specimens analyzed. We further detected and analyzed the levels of miR-29 family based on MED12 mutation status. This analysis indicated that the expression of entire miR-29 family was lower in fibroids than in myometrium and this downregulation was greater in fibroids bearing the MED12 mutation than the MED12 wild-type specimens. As would be expected, the mRNA levels of COL1A1 and COL3A1, which we previously reported as target genes of miR-29 (25, 54), were more upregulated in fibroids bearing the MED12 mutation (Fig. 1E and 1F).
A computational algorithm for prediction of an interaction between lncRNAs and miRNAs (55, 56) indicated that there is complementary base pairing between MIAT and seed regions of miR-29 family (Fig. 2A). Using luciferase reporter assay we confirmed that MIAT could target miR-29 family in LSMC spheroids (Fig. 2B). In addition, using RNA immunoprecipitation with AGO2 antibody we demonstrated the physical association between MIAT and the miR-29 family in paired leiomyoma and myometrium (Fig. 2C and 2D). Among the 8 Argonaute family members, only AGO2 (Argonaute 2) has endonuclease activity in humans and therefore plays a central role in RNA silencing processes (57). Our results indicated a greater interaction between AGO2, MIAT, and miR-29 family in leiomyomas compared with matched myometrium (Fig. 2C and 2D), suggesting a sponge effect of MIAT on the miR-29 family.
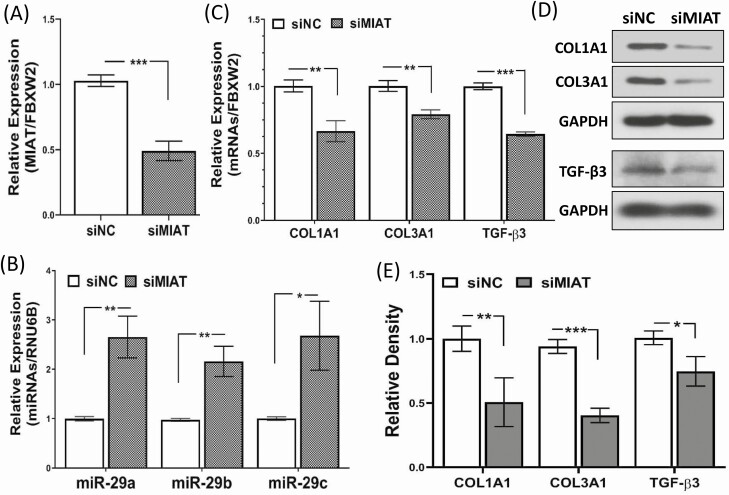
To further demonstrate the role of MIAT in regulating miR-29 we transfected LSMC spheroids with MIAT siRNA. The MIAT levels were reduced by 51% in response to MIAT siRNA transfection and there was a significant increase in the expression of miR-29 family (Fig. 3A and 3B), a decrease in COL1A1, COL3A1, and TGF-β3 mRNA, and suppression of their protein levels in primary LSMC spheroids (Fig. 3C-3E).
Figure 3.
The effect of MIAT knockdown through transfection of LSMC spheroids with siRNA scrambled oligonucleotides (siNC) or siRNA against MIAT (siMIAT) for 96 hours on MIAT expression (A), miR-29 family expression (B), RNA levels of COL1A1, COL3A1 and TGF-β3 (C) and their protein levels determined by western blot (D) along with the relative band density analysis (E). The results were obtained from 4 experiments using cells isolated from different patients in each set and presented as mean ± SEM. P values are analyzed using unpaired t test and indicated as *P < .05, **P < .01, and ***P < .001.
We further determined the relationship between MIAT and the miR-29 family following TGF-β3 treatment in cultured LSMC spheroids. As shown in Fig. 4A-4C the protein abundance of COL1A1 and COL3A1 was increased following TGF-β3 treatment, and this effect was blocked by a TGFβ type I receptor inhibitor (SB431542) (Fig. 4A). Treatment of LSMC spheroids with TGF-β3 resulted in induction of MIAT (Fig. 4B), but repression of miR-29 family expression (Fig. 4C). The effect of TGF-β3 on miR-29 and MIAT expression was blocked by SB431542 (Fig. 4C).
The functional relevance of MIAT in mediating the effects of TGF-β3 on the induction of COL1A1 and COL3A1 was determined in LSMC spheroids. As shown in Fig. 4D, knockdown of MIAT by siRNA blocked the induction of COL1A1 and COL3A1 protein by TGF-β3, suggesting that MIAT plays an essential role in TGF-β3-mediated collagen accumulation.
Discussion
In this study we provide evidence for the first time that (1) leiomyomas overexpress MIAT compared with matched myometrium; (2) MED12-mutated leiomyomas express higher levels of MIAT and lower levels of miR-29 family than MED12 wild-type leiomyomas; (3) MIAT acts as a sponge for the miR-29 family in leiomyomas; (4) knockdown of MIAT results in upregulation of the miR-29 family, and downregulation of COL1A1, COL3A1, and TGF-β3 expression in LSMC spheroids; (5) TGF-β3 induces MIAT, but represses the miR-29 family via TGF-β type I receptor dependent mechanism in LSMC spheroids; (6) the accumulation of COL1A1 and COL3A1 induced by TGF-β3 in LSMC spheroids is blocked by MIAT knockdown. Collectively, these findings support the hypothesis that downregulation of the miR-29 family in fibroids is in part due to overexpression of MIAT which sequesters it with resultant upregulation of miR-29 target genes which are critical for ECM deposition.
Our group’s focus has been on the regulatory mechanism underlying aberrant gene expression in fibroids. We reported differential expression of lncRNA (18) and short noncoding RNAs in fibroids (19). Among the differentially expressed noncoding RNAs, the miR-29 family is of particular physiological significance as it primarily targets ECM genes. Our group (25) and others (28, 29) reported that the expression of miR-29 is downregulated in fibroids. We have identified a number of mechanisms underlying the downregulation of miR-29c in fibroids including epigenetic mechanisms involving increased DNMT1 and DNMT3A expression inducing methylation of miR-29c promoter (25, 26, 58), NF-κB signaling, and SP1 (25). Here we provide a novel mechanism involving the lncRNA MIAT, which acts a ceRNA of the miR-29 family in fibroids. Our data indicate that there is an activation of an MIAT/miR-29/TGF-β3/collagen network in fibroids (Fig. 4E) wherein overexpression of MIAT sequesters miR-29 leading to its downregulation. Downregulation of miR-29 not only leads to upregulation of its target genes, which include various collagen subtypes, but also upregulation of TGF-β3, which we previously reported is a target of miR-29 in fibroids (26). Thus, through a miRNA and a growth factor–dependent mechanism the expression of collagen is stimulated in fibroids. Similar to our findings, other groups reported that the induction of MIAT in diabetic retinopathy leads to sponging of miR-29b levels with resultant promotion of Sp1 expression, which is a target gene of miR-29 family (59). In another report, higher MIAT levels in gastric cancer by acting as an endogenous miR-29a sponge regulated histone deacetylase 4 (HDAC4) expression (60). More recently, we reported that prenatal exposure to nicotine induced offspring cardiac expression of MIAT, resulting in sequestration of the miR-29 family and upregulation of collagens (37).
LncRNAs can exert their effect on gene expression through mechanism other than their sponging effects such as serving as precursors of miRNAs (61), as competitors of miRNAs by binding to shared targets (61), as antisense of protein coding genes (30), as mediator of promoter methylation of target genes (62), as enhancers to upregulate gene transcription via the mediator complex (63), as scaffolds for histone-modifying complexes (64), and as signaling molecules via exosomes in the circulation which can act at different sites (65). To date none of these mechanisms of lncRNA action have been explored in fibroids and warrant further investigation.
Here we show that MED12 mutation can influence the expression of ncRNAs through yet to be determined mechanisms and alter the expression of protein coding genes which are critical in fibroid pathogenesis. Recent studies have demonstrated that oncogenic mutations in exon 2 of the MED12 gene occurs in up to 80% of leiomyomas (66, 67), and transgenic mice with a conditional mutation of MED12 develop leiomyoma-like tumors in the uterus (68). MED12 encodes a subunit of the Mediator complex, which can trigger either repression (via polymerase II repression) or promotion of gene transcription (69). Somatic MED12 mutations have been linked to the induction of Wnt/β-catenin signaling pathway due to the feedback transactivation between β-catenin and MED12 (70-72). Moreover, MED12 is related to focal adhesion, ECM receptor interaction, and the modulation of hedgehog signaling (66, 73). In addition, MED12 is required for the estrogen receptor-α expression and regulation of the TGF-β receptor signaling (74, 75). However, the role of MED12 in leiomyoma pathogenesis and progression remains unclear. MED12-mutated leiomyomas tend to be multiple and smaller in size than MED12 wild-type leiomyomas (76). Our data indicate that fibroids bearing the MED12 mutation express higher levels of MIAT, lower levels of miR-29, and consequently greater expression of COL1A1 and COL3A1 mRNA levels. We recently showed that TDO2 was expressed in greater abundance in MED12-mutated fibroids (77), and others reported greater expression of RAD51b and WNT4 in MED12-mutated fibroids (70, 78). The mechanism by which MED12 mutation influence the expression of these genes remains to be determined.
Our present study for the first time indicates that TGF-β3 exerts its fibrotic effects in part through MIAT. In this scheme, TGF-β3 stimulates the expression of MIAT, which then sequesters miR-29 with final induction of COL1A1 and COL3A1 expression, which are key components of ECM in leiomyomas (79, 80). In our previous publication we showed the presence of a positive feedback loop between miR-29c and TGF-β3 (26). As such lower expression of miR-29c in fibroids would result in upregulation of TGF-β3, which is a target gene of miR-29c (26), thus setting up a continuous cycle of fibrosis leading to fibroid growth. Our findings are in agreement with prior studies in cardiac fibroblast cells where TGF-β1 a major regulator of cardiac fibrosis, repressed the expression of miR-29 family (81), and in kidney tubular epithelial cell where miR-29b was negatively regulated by TGF-β1/Smad3 signaling (82). In a mouse model of myocardial infarction, MIAT was significantly elevated, reducing the expression of miR-24, and thereby upregulating the expression levels of miR-24 target genes, Furin, and TGF-β1 (36).
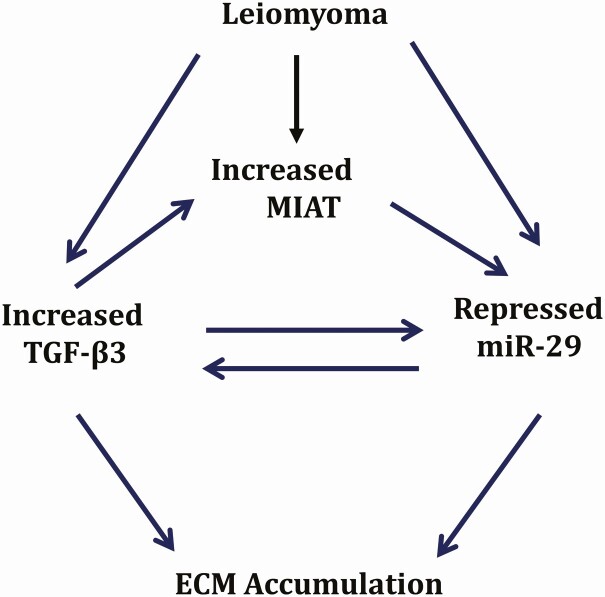
In summary, our data provide evidence for the presence of a profibrotic network involving MIAT/miR-29/TGF-β3/collagens in leiomyomas. In this scheme (Fig. 5) the network has an inherently built-in positive feedback loop which allows for continuous fibrosis and growth of fibroid tumors. In this pathogenesis model (Fig. 5), downregulation of miR-29 in fibroid can be attributed in part to overexpression of MIAT, which directly binds to miR-29 lowering its levels and upregulating the expression levels of miR-29 target genes including TGF-β3 and collagens, and TGF-β3 in turn stimulates the expression of MIAT. These data suggest targeting lncRNAs which regulate the expression of critical miRNAs in fibroid pathogenesis such as miR-29 family could provide another approach for treatment of leiomyomas.
Figure 5.
Schematic diagram representing our working model in which leiomyoma are characterized by reduced miR-29 family and elevated expression of TGF-β3 and the lncRNA MIAT, which causes an increase in extracellular matrix accumulation.
Acknowledgments
Financial Support: We acknowledge funding support from National Institutes of Health (HD088868, HD100529 and HD101852).
Author Contributions: T.-D.C. contributed to study design, conducted experiments, data analysis, interpretation, and wrote the manuscript. D.Q. and D.B. conducted experiments and reviewed the manuscript. O.K. designed, interpreted the studies, and revised the manuscript. All authors read and approved the final version of the manuscript.
Glossary
Abbreviations
- COL1A1
collagen type I
- COL3A1
collagen type III
- ECM
extracellular matrix
- lncRNA
long noncoding RNA
- LSMC
leiomyoma smooth muscle cell
- MED12
mediator complex subunit 12
- MIAT
myocardial infarction associated transcript
- MMP
matrix metalloproteinase
- NC
negative control
- PCR
polymerase chain reaction
- RIP
RNA immunoprecipitation
- SEM
standard error of the mean
- TGF
transforming growth factor
Additional Information
Disclosures: All authors have nothing to disclose, and no conflict of interests existed.
Data Availability
The datasets generated or analyzed during the current study are included in this published article or in the data repositories listed in References.
References
- 1. Doherty L, Mutlu L, Sinclair D, Taylor H. Uterine fibroids: clinical manifestations and contemporary management. Reprod Sci. 2014;21(9):1067-1092. [DOI] [PubMed] [Google Scholar]
- 2. Segars JH, Parrott EC, Nagel JD, et al. Proceedings from the Third National Institutes of Health International Congress on Advances in Uterine Leiomyoma Research: comprehensive review, conference summary and future recommendations. Hum Reprod Update. 2014;20(3):309-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Catherino WH, Eltoukhi HM, Al-Hendy A. Racial and ethnic differences in the pathogenesis and clinical manifestations of uterine leiomyoma. Semin Reprod Med. 2013;31(5):370-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bulun SE. Uterine fibroids. N Engl J Med. 2013;369(14):1344-1355. [DOI] [PubMed] [Google Scholar]
- 5. Islam MS, Ciavattini A, Petraglia F, Castellucci M, Ciarmela P. Extracellular matrix in uterine leiomyoma pathogenesis: a potential target for future therapeutics. Hum Reprod Update. 2018;24(1):59-85. [DOI] [PubMed] [Google Scholar]
- 6. Chegini N. Proinflammatory and profibrotic mediators: principal effectors of leiomyoma development as a fibrotic disorder. Semin Reprod Med. 2010;28(3):180-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stewart EA, Cookson CL, Gandolfo RA, Schulze-Rath R. Epidemiology of uterine fibroids: a systematic review. BJOG. 2017;124(10):1501-1512. [DOI] [PubMed] [Google Scholar]
- 8. Halder SK, Goodwin JS, Al-Hendy A. 1,25-Dihydroxyvitamin D3 reduces TGF-beta3-induced fibrosis-related gene expression in human uterine leiomyoma cells. J Clin Endocrinol Metab. 2011;96(4):E754-E762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Norian JM, Malik M, Parker CY, et al. Transforming growth factor beta3 regulates the versican variants in the extracellular matrix-rich uterine leiomyomas. Reprod Sci. 2009;16(12):1153-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Salama SA, Diaz-Arrastia CR, Kilic GS, Kamel MW. 2-Methoxyestradiol causes functional repression of transforming growth factor β3 signaling by ameliorating Smad and non-Smad signaling pathways in immortalized uterine fibroid cells. Fertil Steril. 2012;98(1):178-184. [DOI] [PubMed] [Google Scholar]
- 11. Joseph DS, Malik M, Nurudeen S, Catherino WH. Myometrial cells undergo fibrotic transformation under the influence of transforming growth factor beta-3. Fertil Steril. 2010;93(5):1500-1508. [DOI] [PubMed] [Google Scholar]
- 12. Yatsenko SA, Mittal P, Wood-Trageser MA, et al. Highly heterogeneous genomic landscape of uterine leiomyomas by whole exome sequencing and genome-wide arrays. Fertil Steril. 2017;107(2):457-466.e9. [DOI] [PubMed] [Google Scholar]
- 13. Gallagher CS, Morton CC. Genetic association studies in uterine fibroids: risk alleles presage the path to personalized therapies. Semin Reprod Med. 2016;34(4):235-241. [DOI] [PubMed] [Google Scholar]
- 14. Liegl-Atzwanger B, Heitzer E, Flicker K, et al. Exploring chromosomal abnormalities and genetic changes in uterine smooth muscle tumors. Mod Pathol. 2016;29(10):1262-1277. [DOI] [PubMed] [Google Scholar]
- 15. Al-Hendy A, Laknaur A, Diamond MP, Ismail N, Boyer TG, Halder SK. Silencing Med12 gene reduces proliferation of human leiomyoma cells mediated via Wnt/β-catenin signaling pathway. Endocrinology. 2017;158(3):592-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. El Andaloussi A, Al-Hendy A, Ismail N, Boyer TG, Halder SK. Introduction of somatic mutation in MED12 induces Wnt4/β-catenin and disrupts autophagy in human uterine myometrial cell. Reprod Sci. 2020;27(3):823-832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guo H, Zhang X, Dong R, et al. Integrated analysis of long noncoding RNAs and mRNAs reveals their potential roles in the pathogenesis of uterine leiomyomas. Oncotarget. 2014;5(18):8625-8636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chuang TD, Khorram O. Expression profiling of lncRNAs, miRNAs, and mRNAs and their differential expression in leiomyoma using next-generation RNA sequencing. Reprod Sci. 2018;25(2):246-255. [DOI] [PubMed] [Google Scholar]
- 19. Chuang TD, Xie Y, Yan W, Khorram O. Next-generation sequencing reveals differentially expressed small noncoding RNAs in uterine leiomyoma. Fertil Steril. 2018;109(5):919-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Beermann J, Piccoli MT, Viereck J, Thum T. Non-coding RNAs in development and disease: background, mechanisms, and therapeutic approaches. Physiol Rev. 2016;96(4):1297-1325. [DOI] [PubMed] [Google Scholar]
- 21. Jiang C, Li X, Zhao H, Liu H. Long non-coding RNAs: potential new biomarkers for predicting tumor invasion and metastasis. Mol Cancer. 2016;15(1):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li J, Tian H, Yang J, Gong Z. Long noncoding RNAs regulate cell growth, proliferation, and apoptosis. DNA Cell Biol. 2016;35(9):459-470. [DOI] [PubMed] [Google Scholar]
- 23. Ulitsky I, Bartel DP. lincRNAs: genomics, evolution, and mechanisms. Cell. 2013;154(1):26-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cao MX, Jiang YP, Tang YL, Liang XH. The crosstalk between lncRNA and microRNA in cancer metastasis: orchestrating the epithelial-mesenchymal plasticity. Oncotarget. 2017;8(7):12472-12483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chuang TD, Khorram O. Mechanisms underlying aberrant expression of miR-29c in uterine leiomyoma. Fertil Steril. 2016;105(1):236-45.e1. [DOI] [PubMed] [Google Scholar]
- 26. Chuang TD, Khorram O. Cross-talk between miR-29c and transforming growth factor-β3 is mediated by an epigenetic mechanism in leiomyoma. Fertil Steril. 2019;112(6):1180-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chuang TD, Khorram O. Regulation of cell cycle regulatory proteins by microRNAs in uterine leiomyoma. Reprod Sci. 2019;26(2):250-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Qiang W, Liu Z, Serna VA, et al. Down-regulation of miR-29b is essential for pathogenesis of uterine leiomyoma. Endocrinology. 2014;155(3):663-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Marsh EE, Steinberg ML, Parker JB, Wu J, Chakravarti D, Bulun SE. Decreased expression of microRNA-29 family in leiomyoma contributes to increased major fibrillar collagen production. Fertil Steril. 2016;106(3):766-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dey BK, Mueller AC, Dutta A. Long non-coding RNAs as emerging regulators of differentiation, development, and disease. Transcription. 2014;5(4):e944014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ergun S, Oztuzcu S. Oncocers: ceRNA-mediated cross-talk by sponging miRNAs in oncogenic pathways. Tumour Biol. 2015;36(5):3129-3136. [DOI] [PubMed] [Google Scholar]
- 32. Paci P, Colombo T, Farina L. Computational analysis identifies a sponge interaction network between long non-coding RNAs and messenger RNAs in human breast cancer. BMC Syst Biol. 2014;8:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Thomson DW, Dinger ME. Endogenous microRNA sponges: evidence and controversy. Nat Rev Genet. 2016;17(5):272-283. [DOI] [PubMed] [Google Scholar]
- 34. Cao T, Jiang Y, Wang Z, et al. H19 lncRNA identified as a master regulator of genes that drive uterine leiomyomas. Oncogene. 2019;38(27):5356-5366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chuang TD, Rehan A, Khorram O. Functional role of the long noncoding RNA X-inactive specific transcript in leiomyoma pathogenesis. Fertil Steril. 2021;115(1):238-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Qu X, Du Y, Shu Y, et al. MIAT is a pro-fibrotic long non-coding RNA governing cardiac fibrosis in post-infarct myocardium. Sci Rep. 2017;7:42657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chuang TD, Ansari A, Yu C, et al. Mechanism underlying increased cardiac extracellular matrix deposition in perinatal nicotine-exposed offspring. Am J Physiol Heart Circ Physiol. 2020;319(3):H651-H660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bijkerk R, Au YW, Stam W, et al. Long non-coding RNAs rian and miat mediate myofibroblast formation in kidney fibrosis. Front Pharmacol. 2019;10:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hirschhaeuser F, Menne H, Dittfeld C, West J, Mueller-Klieser W, Kunz-Schughart LA. Multicellular tumor spheroids: an underestimated tool is catching up again. J Biotechnol. 2010;148(1):3-15. [DOI] [PubMed] [Google Scholar]
- 40. Breslin S, O’Driscoll L. The relevance of using 3D cell cultures, in addition to 2D monolayer cultures, when evaluating breast cancer drug sensitivity and resistance. Oncotarget. 2016;7(29):45745-45756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ciebiera M, Wlodarczyk M, Wrzosek M, et al. Role of transforming growth factor beta in uterine fibroid biology. Int J Mol Sci. 2017;18(11):2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. Am J Obstet Gynecol. 1975;122(2):262-263. [DOI] [PubMed] [Google Scholar]
- 43. Chuang TD, Luo X, Panda H, Chegini N. miR-93/106b and their host gene, MCM7, are differentially expressed in leiomyomas and functionally target F3 and IL-8. Mol Endocrinol. 2012;26(6):1028-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chuang TD, Panda H, Luo X, Chegini N. miR-200c is aberrantly expressed in leiomyomas in an ethnic-dependent manner and targets ZEBs, VEGFA, TIMP2, and FBLN5. Endocr Relat Cancer. 2012;19(4):541-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Busk PK. A tool for design of primers for microRNA-specific quantitative RT-qPCR. BMC Bioinformatics 2014;15:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Almeida TA, Quispe-Ricalde A, Montes de Oca F, Foronda P, Hernández MM. A high-throughput open-array qPCR gene panel to identify housekeeping genes suitable for myometrium and leiomyoma expression analysis. Gynecol Oncol. 2014;134(1):138-143. [DOI] [PubMed] [Google Scholar]
- 47. Chuang TD, Rehan A, Khorram O. Tranilast induces MiR-200c expression through blockade of RelA/p65 activity in leiomyoma smooth muscle cells. Fertil Steril. 2020;113(6):1308-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. RRID:AB_2882107. https://scicrunch.org/resolver/AB_2882107 [Google Scholar]
- 49. RRID:AB_2879158. https://scicrunch.org/resolver/AB_2879158 [Google Scholar]
- 50. RRID:AB_10611929. https://scicrunch.org/resolver/AB_10611929 [Google Scholar]
- 51. RRID:AB_10847862. https://scicrunch.org/resolver/AB_10847862 [Google Scholar]
- 52. RRID:AB_10807962. https://scicrunch.org/resolver/AB_10807962 [Google Scholar]
- 53. RRID:AB_490557. https://scicrunch.org/resolver/AB_490557 [Google Scholar]
- 54. Chuang TD, Sakurai R, Gong M, Khorram O, Rehan VK. Role of miR-29 in mediating offspring lung phenotype in a rodent model of intrauterine growth restriction. Am J Physiol Regul Integr Comp Physiol. 2018;315(5):R1017-R1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Li JH, Liu S, Zhou H, Qu LH, Yang JH. starBase v2.0: decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res. 2014;42(Database issue):D92-D97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yang JH, Li JH, Shao P, Zhou H, Chen YQ, Qu LH. starBase: a database for exploring microRNA-mRNA interaction maps from Argonaute CLIP-Seq and Degradome-Seq data. Nucleic Acids Res. 2011;39(Database issue):D202-D209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kupferschmidt K. A lethal dose of RNA. Science (New York, NY). 2013;341(6147):732-733. [DOI] [PubMed] [Google Scholar]
- 58. Chuang TD, Khorram O. Tranilast inhibits genes functionally involved in cell proliferation, fibrosis, and epigenetic regulation and epigenetically induces miR-29c expression in leiomyoma cells. Reprod Sci. 2017;24(9):1253-1263. [DOI] [PubMed] [Google Scholar]
- 59. Zhang J, Chen M, Chen J, et al. Long non-coding RNA MIAT acts as a biomarker in diabetic retinopathy by absorbing miR-29b and regulating cell apoptosis. Bioscience Reports. 2017;37(2):BSR20170036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Li Y, Wang K, Wei Y, et al. lncRNA-MIAT regulates cell biological behaviors in gastric cancer through a mechanism involving the miR-29a-3p/HDAC4 axis. Oncol Rep. 2017;38(6):3465-3472. [DOI] [PubMed] [Google Scholar]
- 61. Yoon JH, Abdelmohsen K, Gorospe M. Functional interactions among microRNAs and long noncoding RNAs. Semin Cell Dev Biol. 2014;34:9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tufarelli C, Stanley JA, Garrick D, et al. Transcription of antisense RNA leading to gene silencing and methylation as a novel cause of human genetic disease. Nat Genet. 2003;34(2):157-165. [DOI] [PubMed] [Google Scholar]
- 63. Ørom UA, Derrien T, Guigo R, Shiekhattar R. Long noncoding RNAs as enhancers of gene expression. Cold Spring Harb Symp Quant Biol. 2010;75:325-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Moore A. Trusts target private patients. Health Serv J. 2013;123(6354):10-11. [PubMed] [Google Scholar]
- 65. Huang X, Yuan T, Tschannen M, et al. Characterization of human plasma-derived exosomal RNAs by deep sequencing. BMC Genomics. 2013;14:319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mäkinen N, Mehine M, Tolvanen J, et al. MED12, the mediator complex subunit 12 gene, is mutated at high frequency in uterine leiomyomas. Science. 2011;334(6053):252-255. [DOI] [PubMed] [Google Scholar]
- 67. McGuire MM, Yatsenko A, Hoffner L, Jones M, Surti U, Rajkovic A. Whole exome sequencing in a random sample of North American women with leiomyomas identifies MED12 mutations in majority of uterine leiomyomas. PLoS One. 2012;7(3):e33251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Mittal P, Shin YH, Yatsenko SA, Castro CA, Surti U, Rajkovic A. Med12 gain-of-function mutation causes leiomyomas and genomic instability. J Clin Invest. 2015;125(8):3280-3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Croce S, Chibon F. MED12 and uterine smooth muscle oncogenesis: State of the art and perspectives. Eur J Cancer. 2015;51(12):1603-1610. [DOI] [PubMed] [Google Scholar]
- 70. Markowski DN, Bartnitzke S, Löning T, Drieschner N, Helmke BM, Bullerdiek J. MED12 mutations in uterine fibroids – their relationship to cytogenetic subgroups. Int J Cancer. 2012;131(7):1528-1536. [DOI] [PubMed] [Google Scholar]
- 71. Kim S, Xu X, Hecht A, Boyer TG. Mediator is a transducer of Wnt/beta-catenin signaling. J Biol Chem. 2006;281(20):14066-14075. [DOI] [PubMed] [Google Scholar]
- 72. Rocha PP, Scholze M, Bleiss W, Schrewe H. Med12 is essential for early mouse development and for canonical Wnt and Wnt/PCP signaling. Development. 2010;137(16):2723-2731. [DOI] [PubMed] [Google Scholar]
- 73. Zhou H, Kim S, Ishii S, Boyer TG. Mediator modulates Gli3-dependent Sonic hedgehog signaling. Mol Cell Biol. 2006;26(23):8667-8682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Prenzel T, Kramer F, Bedi U, Nagarajan S, Beissbarth T, Johnsen SA. Cohesin is required for expression of the estrogen receptor-alpha (ESR1) gene. Epigenetics Chromatin. 2012;5(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Huang S, Hölzel M, Knijnenburg T, et al. MED12 controls the response to multiple cancer drugs through regulation of TGF-β receptor signaling. Cell. 2012;151(5):937-950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Heinonen HR, Sarvilinna NS, Sjöberg J, et al. MED12 mutation frequency in unselected sporadic uterine leiomyomas. Fertil Steril. 2014;102(4):1137-1142. [DOI] [PubMed] [Google Scholar]
- 77. Chuang TD, Quintanilla D, Boos D, Khorram O. Tryptophan catabolism is dysregulated in leiomyomas. Fertil Steril. 2021;S0015-0282(21)00434-9. doi: 10.1016/j.fertnstert.2021.05.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Mehine M, Kaasinen E, Mäkinen N, et al. Characterization of uterine leiomyomas by whole-genome sequencing. N Engl J Med. 2013;369(1):43-53. [DOI] [PubMed] [Google Scholar]
- 79. Behera MA, Feng L, Yonish B, Catherino W, Jung SH, Leppert P. Thrombospondin-1 and thrombospondin-2 mRNA and TSP-1 and TSP-2 protein expression in uterine fibroids and correlation to the genes COL1A1 and COL3A1 and to the collagen cross-link hydroxyproline. Reprod Sci. 2007;14(8 Suppl):63-76. [DOI] [PubMed] [Google Scholar]
- 80. Cirilo PD, Marchi FA, Barros Filho Mde C, et al. An integrative genomic and transcriptomic analysis reveals potential targets associated with cell proliferation in uterine leiomyomas. PLoS One. 2013;8(3):e57901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. van Rooij E, Sutherland LB, Thatcher JE, et al. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci U S A. 2008;105(35):13027-13032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Qin W, Chung AC, Huang XR, et al. TGF-beta/Smad3 signaling promotes renal fibrosis by inhibiting miR-29. J Am Soc Nephrol 2011;22(8):1462-1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated or analyzed during the current study are included in this published article or in the data repositories listed in References.