Clinical Implications.
Compared with non–eosinophilic gastrotintestinal disease (EGID) coronavirus disease 2019 (COVID-19) positive patients, EGID COVID-19 positive individuals stayed longer in the hospital, yet had a lower hazard of in-patient mortality. This analysis suggests that EGID may provide a protective effect against severe COVID-19 outcomes.
In the United States, the coronavirus disease 2019 (COVID-19) pandemic was the third leading cause of death in 2020.1 Peripheral eosinophilia is hypothesized to play a protective role in COVID-19.2 Yet, little is known about eosinophilic gastrotintestinal disease (EGID) and COVID-19 outcomes. Th2 mucosal responses of patients with EGID may protect against severe effects of COVID-19 by reducing viral entry into cells.3 , 4 We hypothesized that EGID would be protective against severe outcomes in COVID-19 infections. We reviewed administrative data from an extensive central medical system in the United States to identify all COVID-19 cases and compared hospitalization rates, ventilator dependence, and death between patients with and without EGID.
Data were used from the Cerner COVID-19 De-Identified Data Cohort. This cohort contains patient encounter-level information and is a subset of the larger Cerner Real-World Data cohort. Our primary cohort of COVID-19 positive patients identified those having either a diagnosis code of COVID-19 infection or a recent positive severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) lab result by nucleic acid amplication with probe detection. For comparison with a larger sample of patients, a secondary cohort used records of patients identified as having a diagnosis code of possible exposure or infection of COVID-19 or a recent positive lab result for possible COVID-19–related testing. Patients with COVID-19 indications spanning from December 2019 to September 2020 were included. Their demographic and clinical characteristics are provided in Tables E1 and E2 (available in this article’s Online Repository at www.jaci-inpractice.org).
The outcomes of interest included the categorical hospitalization, the nonparametric continuous maximum length of stay (LOS), and survival times to invasive ventilator dependence (IVD) and in-hospital mortality. The primary predictor of interest was a history of EGID. Demographic predictors of interest were age, sex, race and ethnicity, insurance, and US geographical region. Clinical predictors included EGID-related symptoms and procedures, associated atopic diseases, conditions related to adverse COVID-19 outcomes, and EGID medications.
A nearest-neighbor “greedy” matching method was used to obtain matched controls. Demographically/clinically similar patients were matched by EGID diagnosis status in a 4:1 ratio on age, gender, race and ethnicity, insurance, geographical region, atopic conditions, and comorbidities known to be associated with worse COVID-19 outcomes. Unadjusted outcomes, between EGID and matched non-EGID patients, were compared with χ2 tests and Wilcoxon rank-sum tests for hospitalization and maximum LOS, respectively. Survival curves of time to IVD and in-hospital mortality, by EGID status, were compared with log-rank χ2 tests. For adjusted associations of EGID status with the outcomes of interest, regression models were employed. Hospitalization was fit with a Poisson model, maximum LOS with an exponential model, and time to IVD and in-hospital mortality with a Cox-proportional hazards model. All models were adjusted for EGID symptoms, procedures, and medications. From the Cox models, adjusted survival curves were plotted for the time to event outcomes, with different lines for EGID and non-EGID patients. For comparison, all previous analyses were repeated on the larger COVID-19 exposed and positive cohort.
Analyses were conducted on the primary cohort of COVID-19 positive patients (Table I ). Patients with EGID, compared with matched non-EGID patients, had higher percentages of hospitalization (53.6% vs 44.6%, P = .09) and stayed longer in the hospital (median maximum LOS: 1.5 vs 0.3, P = .062). Non-EGID were intubated faster and died relatively faster, although these findings were not statistically significant in the unadjusted analysis. When adjusting for confounding variables, patients with EGID did exhibit again higher hospitalization and maximum LOS, yet a lower hazard of IVD (adjusted hazard ratio [aHR]: 0.95, 95% confidence interval [CI] = 0.50-1.79) and in-hospital mortality (aHR: 0.38, 95% CI = 0.11-1.39). Again, these results were not significant.
Table I.
Associations of EGID (and matched∗ non-EGID) with COVID-19 outcomes among COVID-19 positive patients (EGID: 125; matched controls: 500) and COVID-19 exposed and positive patients (EGID: 432; matched controls: 1728)
| Variables | Hospitalization | Maximum LOS (d) | Time to invasive ventilator dependence (d) | Time to in-hospital mortality (d) |
|---|---|---|---|---|
| COVID-19 positive | ||||
| Unadjusted† | n (%‡) | Median [Q1-Q3] | Mean§ (95% CI) | Mean§ (95% CI) |
| Non-EGID | 223 (44.6) | 0.3 (0.1, 4.8) | 111 (102, 120) | 162 (111, 213) |
| EGID | 67 (53.6) | 1.5 (0.1, 6.1) | 142 (122, 162) | 167 (156, 178) |
| Adjusted‖ | aIRR¶ (95% CI) | # (95% CI) | aHR∗∗ (95% CI) | aHR∗∗ (95% CI) |
| Non-EGID | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| EGID | 1.21 (0.98, 1.49) | 1.31 (0.83, 2.06) | 0.95 (0.50, 1.79) | 0.38 (0.11, 1.39) |
| COVID-19 exposed and positive | ||||
| Unadjusted†† | n (%) | Median [Q1-Q3] | Mean (95% CI) | Mean (95% CI) |
| Non-EGID | 846 (49.0) | 0.9 (0.1, 4.0) | 191 (171, 211) | 225 (207, 243) |
| EGID | 229 (53.0) | 1.3 (0.2, 4.2) | 222 (199, 245) | 260 (250, 270) |
| Adjusted | aIRR (95% CI) | (95% CI) | aHR (95% CI) | aHR (95% CI) |
| Non-EGID | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| EGID | 1.02 (0.91, 1.14) | 1.08 (0.85, 1.36) | 0.61 (0.38, 0.99) | 0.28 (0.09, 0.85) |
aHR, Adjusted hazard ratio; aIRR, adjusted incidence risk ratio; CI, confidence interval; EGID, eosinophilic gastrotintestinal disease; EoE, eosinophilic esophagitis; LOS, length of stay.
Bold values indicate statistical significance.
Matched on age, gender, race and ethnicity, insurance, region, atopic conditions, comorbidities known to be associated with worse COVID-19 outcomes.
Hospitalization compared with the χ2 test, maximum LOS compared with the Wilcoxon rank-sum test, time to event survival curves compared with the log-rank test (hospitalization P: .09, maximum LOS P: .062, time to invasive ventilator dependence (IVD) P: .62, time to in-hospital mortality P: .067).
Column percentages.
Mean survival times (Wald 95% CIs) restricted to the highest survival time per EGID group (median not used because survival probability did not drop below 50%).
Adjusted for symptoms, procedures, and EGID medications.
Adjusted incidence risk ratio.
Adjusted exponentiated coefficient.
Adjusted hazard ratio.
Hospitalization compared with the χ2 test, maximum LOS compared with the Wilcoxon rank-sum test, time to event survival curves compared with the log-rank test (hospitalization P: .15, maximum LOS P: .02, time to IVD P: .11, time to in-hospital mortality P: .01).
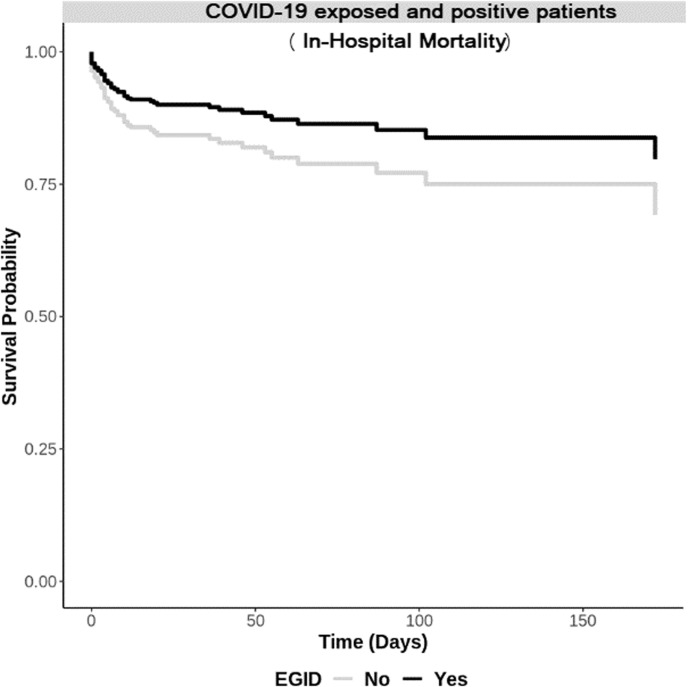
Analyses were repeated on the larger COVID-19 exposed and positive cohort (Table I). Patients with EGID, compared with non-EGID patients, had higher hospitalization, significantly higher maximum LOS (median maximum LOS: 1.3 vs 0.9, P = .02), intubated slower, and died relatively slower (mean survival time: 260 vs 225, P = .01). Patients with EGID had a signficantly lower hazard of IVD (aHR: 0.61, 95% CI = 0.38-0.99) and in-hospital mortality (aHR: 0.28, 95% CI = 0.09-0.85) than non-EGID patients. Adjusted curves are provided in Figure 1 .
Figure 1.
Adjusted curves of survival from in-hospital mortality among COVID-19 exposed and positive patients by eosinophilic gastrotintestinal disease (EGID) status.
This study examined the largest cohort of EGID patients with COVID-19 infection to date. Even after controlling for COVID-19–related comorbidities, patients with EGID were still found to have similar infection course and lower IVD and in-hospital mortality than non-EGID patients. These results imply that there is some mechanistic feature of EGID that leads to a less severe COVID-19 course.
An observational Italian study on patients with EGID found no reports of COVID-19 diagnosis among the 130 of 145 patients contacted.5 In a similar analysis of 1526 cases of COVID-19, asthma and inhaled corticosteroids were not associated with increased risk for severe outcomes.6 Thus, atopy and EGID may offer a protective immune response.
In support of our findings, a worldwide registry of patients with EGID found COVID-19 infections to appear mild to moderate.7 One possible explanation for milder infection is that because of upregulated IL-13 in EGID, there might be a decrease in expression of ACE2 and TMPRSS2 on epithelial cells in patient with EGID, which are critical for SARS-CoV-2 infection.8 Low tissue ACE2/TMPRSS2 levels have been demonstrated in esophageal tissue of eosinophilic esophagitis.4
Eosinophils also play a fundamental role in antiviral responses. Eosinophil-derived neurotoxin is a ribonuclease with antiviral activity, and peripheral eosinophilia is associated with more favorable outcomes in COVID-19.9
Limitations of this study include the lack of availability of serum absolute eosinophil count before and at the time of COVID-19 infection, duration of EGID, and not knowing if the eosinophilia in the gastrotintestinal tract is primary or secondary. Also, a quarter of patients in this study were nonadults, and we know that COVID-19 outcomes are better in children than adults. All of these confounders could have influenced the results of this study. Nonetheless, a major strength of this study is that the size of the Cerner COVID-19 De-Identified Data Cohort made it possible to study COVID-19 outcomes in patients with EGID on a large scale, despite the fact that it is a rare disease.
In conclusion, our analysis supports previous findings that EGID may provide a protective effect against severe COVID-19 outcomes. Although no specific conclusions can be made about mechanisms driving these observations, it is plausible that the reduced expression of ACE2/TMPRSS2 and the eosinophilic disease itself may play a protective role in COVID-19 mortality, and this should be assessed in future studies.
Acknowledgment
The authors acknowledge Cerner and Amazon Web Services for awarding FQ free data access and computation capabilities.
Footnotes
No funding was received for this work.
Conflicts of Interest: M. Chehade has received research funding from the National Institutes of Health (NIH) (R01-AI140133, U54-AI117804), American Partnership for Eosinophilic Disorders/American Academy of Allergy, Asthma & Immunology, and Danone; clinical trial funding from Regeneron, Allakos, Shire, and AstraZeneca; and consulting fees from Regeneron, Allakos, Adare, Shire/Takeda, AstraZeneca, Sanofi, and Bristol Myers Squibb. E. S. Dellon has received research funding from Adare/Ellodi, Allakos, AstraZeneca, GSK, Meritage, Miraca, Nutricia, Celgene/Receptos/BMS, Regeneron, and Shire/Takeda; consultant fees from Abbott, Abbvie, Adare/ Ellodi, Aimmune, Allakos, Amgen, Arena, AstraZeneca, Avir, Biorasi, Calypso, Celgene/Receptos/BMS, Celldex, Eli Lilly, EsoCap, GSK, Gossamer Bio, Holoclara, Landos, Morphic, Parexel/Calyx, Regeneron, Revolo, Robarts/Alimentiv, Salix, Sanofi, and Shire/Takeda; and educational grants from Allakos, Banner, and Holoclara. K. A. Peterson had received research funding from NIH, Allakos, Chobani, Regeneron-Sanofi, AstraZeneca, Bristol Meyers Squibb, and Celegene; and speaker or advisory fees from Allakos, Eli Lilly, Medscape, Alladapt, Ellodi, Takeda, Regeneron-Sanofi, AstraZeneca, and Bristol Meyers Squibb. She has stock options in Nexeos. The rest of the authors declare that they have no relevant conflicts of interest.
Online Repository
Table E1.
Demographic and clinical characteristics of COVID-19 positive patients by EGID status
| Characteristic | EGID |
Matched† controls (non-EGID) |
Overall non-EGID population |
P value‡ |
|---|---|---|---|---|
| n (%∗) | n (%∗) | n (%∗) | ||
| Total | 125 | 500 | 173,594 | |
| Age median [Q1-Q3] | 44 [19-60] | 42 [22-60] | 49 [31-64] | .83§ |
| Age categorized | .09 | |||
| <18 | 28 (22.4) | 80 (16.0) | 14,299 (8.2) | |
| 18-29 | 16 (12.8) | 91 (18.2) | 25,275 (14.6) | |
| 30-44 | 19 (15.2) | 110 (22.0) | 36,124 (20.8) | |
| 45-59 | 30 (24.0) | 93 (18.6) | 41,508 (23.9) | |
| 60-74 | 21 (16.8) | 97 (19.4) | 34,414 (19.8) | |
| ≥75 | 11 (8.8) | 29 (5.8) | 21,974 (12.7) | |
| Female | 64 (51.2) | 265 (53.0) | 90,527 (52.1) | .80 |
| Race and Ethnicity | .87 | |||
| White | 71 (56.8) | 268 (53.6) | 50,921 (29.3) | |
| Black | 15 (12.0) | 57 (11.4) | 28,538 (16.4) | |
| Hispanic or Latino | 31 (24.8) | 136 (27.2) | 76,068 (43.8) | |
| Other | 8 (6.4) | 39 (7.8) | 18,067 (10.4) | |
| Insurance | .96 | |||
| Private | 38 (30.4) | 163 (32.6) | 58,800 (33.9) | |
| Medicaid | 24 (19.2) | 96 (19.2) | 24,742 (14.3) | |
| Medicare | 24 (19.2) | 96 (19.2) | 32,073 (18.5) | |
| Other | 39 (31.2) | 145 (29.0) | 57,979 (33.4) | |
| Region | .78 | |||
| Midwest | 43 (34.4) | 169 (33.8) | 45,513 (26.2) | |
| Northeast | 21 (16.8) | 100 (20.0) | 16,385 (9.4) | |
| Southeast | 29 (23.2) | 120 (24.0) | 58,299 (33.6) | |
| West | 32 (25.6) | 111 (22.2) | 53,397 (30.8) | |
| Comorbidities known to be associated with worse COVID-19 outcomes | ||||
| Type 2 diabetes | 26 (20.8) | 113 (22.6) | 44,316 (25.5) | .76 |
| Hypertension | 68 (54.4) | 259 (51.8) | 71,943 (41.4) | .67 |
| Obesity | 36 (28.8) | 137 (27.4) | 27,939 (16.1) | .84 |
| Coronary artery disease | 15 (12.0) | 57 (11.4) | 12,754 (7.3) | .98 |
| Heart failure | 17 (13.6) | 72 (14.4) | 14,707 (8.5) | .93 |
| Chronic obstructive pulmonary disease | 23 (18.4) | 100 (20.0) | 19,748 (11.4) | .78 |
| Chronic kidney disease | 21 (16.8) | 81 (16.2) | 19,139 (11.0) | .98 |
| Eosinophilia | 0 (0.0) | 0 (0.0) | 0 (0.0) | NA |
| Atopic conditions | ||||
| Asthma | 53 (42.4) | 222 (44.4) | 28,948 (16.7) | .76 |
| Allergic rhinitis | 16 (12.8) | 67 (13.4) | 3233 (1.9) | .98 |
| Atopic dermatitis | 16 (12.8) | 70 (14.0) | 8800 (5.1) | .84 |
| IgE-mediated food allergy | 2 (1.6) | 10 (2.0) | 217 (0.1) | >.99 |
| Urticaria | 1 (0.8) | 7 (1.4) | 1916 (1.1) | >.99‖ |
| Mast cell activation syndrome | 0 (0.0) | 0 (0.0) | 0 (0.0) | NA |
| Symptom codes | ||||
| Dysphagia | 34 (27.2) | 24 (4.8) | 4743 (2.7) | <.001 |
| Food impaction | 8 (6.4) | 0 (0.0) | 139 (0.1) | <.001‖ |
| Reflux/heartburn | 81 (64.8) | 126 (25.2) | 26,025 (15.0) | <.001 |
| Chest pain | 43 (34.4) | 115 (23.0) | 29,599 (17.1) | .01 |
| Abdominal pain | 64 (51.2) | 121 (24.2) | 31,130 (17.9) | <.001 |
| Nausea/vomiting | 58 (46.4) | 113 (22.6) | 26,619 (15.3) | <.001 |
| Weight loss | 9 (7.2) | 6 (1.2) | 1917 (1.1) | <.001 |
| Failure to thrive | 30 (24.0) | 33 (6.6) | 6416 (3.7) | <.001 |
| Maladaptive eating behavior | 0 (0.0) | 0 (0.0) | 82 (0.0) | NA |
| Feeding difficulties | 13 (10.4) | 7 (1.4) | 516 (0.3) | <.001 |
| Procedures and complications | ||||
| Esophageal stricture | 16 (12.8) | 2 (0.4) | 819 (0.5) | <.001 |
| Esophageal dilation | 7 (5.6) | 3 (0.6) | 444 (0.3) | <.001 |
| Esophageal perforation | 1 (0.8) | 0 (0.0) | 24 (0.0) | .20‖ |
| Type of EGID | ||||
| EoE | 89 (71.2) | NA | NA | NA |
| Eosinophilic gastritis | 21 (16.8) | NA | NA | NA |
| Eosinophilic gastroenteritis | 11 (8.8) | NA | NA | NA |
| Eosinophilic colitis | 9 (7.2) | NA | NA | NA |
| EGID medications | ||||
| Proton pump inhibitor | 17 (13.6) | 23 (4.6) | 2393 (1.4) | .001 |
| Topical steroids | 2 (1.6) | 0 (0.0) | 42 (0.0) | .04‖ |
| Enteral release budesonide | 0 (0.0) | 0 (0.0) | 2 (0.0) | NA |
| Systemic steroids | 0 (0.0) | 7 (1.4) | 557 (0.3) | .35‖ |
| Montelukast | 1 (0.8) | 10 (2.0) | 527 (0.3) | .70‖ |
| Cromolyn | 0 (0.0) | 0 (0.0) | 0 (0.0) | NA |
| 6MP (6-mercaptopurine) | 0 (0.0) | 0 (0.0) | 27 (0.0) | NA |
| Infliximab | 0 (0.0) | 0 (0.0) | 3 (0.0) | NA |
| Vedolizumab | 0 (0.0) | 0 (0.0) | 0 (0.0) | NA |
| Omalizumab | 0 (0.0) | 0 (0.0) | 11 (0.0) | NA |
| Mepolizumab | 0 (0.0) | 0 (0.0) | 5 (0.0) | NA |
| Reslizumab | 0 (0.0) | 0 (0.0) | 22 (0.0) | NA |
| Benralizumab | 0 (0.0) | 0 (0.0) | 5 (0.0) | NA |
| Dupilumab | 0 (0.0) | 0 (0.0) | 4 (0.0) | NA |
| Methotrexate | 0 (0.0) | 1 (0.2) | 80 (0.0) | >.99‖ |
| Tacrolimus | 1 (0.8) | 1 (0.2) | 86 (0.0) | .36‖ |
| Mycophenylate | 0 (0.0) | 0 (0.0) | 32 (0.0) | NA |
EGID, Eosinophilic gastrotintestinal disease; EoE, eosinophilic esophagitis; NA, not available.
Bold values indicate statistical significance.
Column percentages (except when otherwise noted).
Matching on age, gender, race and ethnicity, insurance, region, atopic conditions, comorbidities known to be associated with worse COVID-19 outcomes.
χ2 test (except where otherwise noted) comparing EGID with matched controls.
Wilcoxon rank-sum test.
Fisher’s exact test.
Table E2.
Demographic and clinical characteristics of COVID-19 exposed and positive patients by EGID status
| Characteristic | EGID |
Matched† controls (non-EGID) |
Overall non-EGID population |
P value‡ |
|---|---|---|---|---|
| n (%∗) | n (%∗) | n (%∗) | ||
| Total | 432 | 1728 | 484,417 | |
| Age median [Q1-Q3] | 40 [17-59] | 40 [19-60] | 48 [28-65] | .50§ |
| Age categorized | .39 | |||
| <18 | 113 (26.2) | 401 (23.2) | 55,199 (11.4) | |
| 18-29 | 60 (13.9) | 256 (14.8) | 73,624 (15.2) | |
| 30-44 | 65 (15.0) | 314 (18.2) | 95,000 (19.6) | |
| 45-59 | 88 (20.4) | 306 (17.7) | 99,871 (20.6) | |
| 60-74 | 69 (16.0) | 302 (17.5) | 93,721 (19.3) | |
| ≥75 | 37 (8.6) | 149 (8.6) | 67,002 (13.8) | |
| Female | 206 (47.7) | 808 (46.8) | 260,075 (53.7) | .77 |
| Race and Ethnicity | .93 | |||
| White | 274 (63.4) | 1124 (65.0) | 197,102 (40.7) | |
| Black | 41 (9.5) | 156 (9.0) | 70,241 (14.5) | |
| Hispanic or Latino | 90 (20.8) | 348 (20.1) | 170,212 (35.1) | |
| Other | 27 (6.2) | 100 (5.8) | 46,862 (9.7) | |
| Insurance | .90 | |||
| Private | 148 (34.3) | 601 (34.8) | 160,614 (33.2) | |
| Medicaid | 64 (14.8) | 238 (13.8) | 71,924 (14.8) | |
| Medicare | 82 (19.0) | 349 (20.2) | 91,071 (18.8) | |
| Other | 138 (31.9) | 540 (31.2) | 160,808 (33.2) | |
| Region | .92 | |||
| Midwest | 137 (31.7) | 568 (32.9) | 114,530 (23.6) | |
| Northeast | 100 (23.1) | 406 (23.5) | 50,273 (10.4) | |
| Southeast | 67 (15.5) | 270 (15.6) | 153,269 (31.6) | |
| West | 128 (29.6) | 484 (28.0) | 166,345 (34.3) | |
| Comorbidities known to be associated with worse COVID-19 outcomes | ||||
| Type 2 diabetes | 82 (19.0) | 349 (20.2) | 109,508 (22.6) | .62 |
| Hypertension | 190 (44.0) | 761 (44.0) | 203,719 (42.1) | >.99 |
| Obesity | 104 (24.1) | 412 (23.8) | 73,339 (15.1) | .97 |
| Coronary artery disease | 56 (13.0) | 244 (14.1) | 41,405 (8.5) | .59 |
| Heart failure | 47 (10.9) | 189 (10.9) | 53,425 (11.0) | >.99 |
| Chronic obstructive pulmonary disease | 84 (19.4) | 309 (17.9) | 69,238 (14.3) | .49 |
| Chronic kidney disease | 56 (13.0) | 224 (13.0) | 57,805 (11.9) | >.99 |
| Eosinophilia | 0 (0.0) | 0 (0.0) | 0 (0.0) | NA |
| Atopic conditions | ||||
| Asthma | 183 (42.4) | 725 (42.0) | 96,323 (19.9) | .92 |
| Allergic rhinitis | 44 (10.2) | 164 (9.5) | 9406 (1.9) | .73 |
| Atopic dermatitis | 68 (15.7) | 266 (15.4) | 27,786 (5.7) | .92 |
| IgE-mediated food allergy | 6 (1.4) | 23 (1.3) | 565 (0.1) | >.99 |
| Urticaria | 10 (2.3) | 33 (1.9) | 5730 (1.2) | .73 |
| Mast cell activation syndrome | 0 (0.0) | 0 (0.0) | 0 (0.0) | NA |
| Symptom codes | ||||
| Dysphagia | 108 (25.0) | 75 (4.3) | 14,600 (3.0) | <.001 |
| Food impaction | 50 (11.6) | 2 (0.1) | 638 (0.1) | <.001‖ |
| Reflux/heartburn | 248 (57.4) | 394 (22.8) | 82,728 (17.1) | <.001 |
| Chest pain | 132 (30.6) | 363 (21.0) | 91,156 (18.8) | <.001 |
| Abdominal pain | 203 (47.0) | 411 (23.8) | 96,862 (20.0) | <.001 |
| Nausea/vomiting | 189 (43.8) | 391 (22.6) | 83,133 (17.2) | <.001 |
| Weight loss | 30 (6.9) | 29 (1.7) | 7089 (1.5) | <.001 |
| Failure to thrive | 88 (20.4) | 123 (7.1) | 19,944 (4.1) | <.001 |
| Maladaptive eating behavior | 0 (0.0) | 4 (0.2) | 210 (0.0) | .60‖ |
| Feeding difficulties | 25 (5.8) | 27 (1.6) | 1647 (0.3) | <.001 |
| Procedures and complications | ||||
| Esophageal stricture | 70 (16.2) | 18 (1.0) | 3475 (0.7) | <.001 |
| Esophageal dilation | 31 (7.2) | 14 (0.8) | 1348 (0.3) | <.001 |
| Esophageal perforation | 1 (0.2) | 0 (0.0) | 112 (0.0) | .2‖ |
| Type of EGID | ||||
| EoE | 318 (73.6) | NA | NA | NA |
| Eosinophilic gastritis | 75 (17.4) | NA | NA | NA |
| Eosinophilic gastroenteritis | 32 (7.4) | NA | NA | NA |
| Eosinophilic colitis | 24 (5.6) | NA | NA | NA |
| EGID medications | ||||
| Proton pump inhibitor | 39 (9.0) | 42 (2.4) | 7451 (1.5) | <.001 |
| Topical steroids | 2 (0.5) | 0 (0.0) | 137 (0.0) | .04‖ |
| Enteral release budesonide | 0 (0.0) | 0 (0.0) | 4 (0.0) | NA |
| Systemic steroids | 3 (0.7) | 12 (0.7) | 1795 (0.4) | >.99 |
| Montelukast | 4 (0.9) | 17 (1.0) | 1561 (0.3) | >.99 |
| Cromolyn | 0 (0.0) | 0 (0.0) | 0 (0.0) | NA |
| 6MP (6-mercaptopurine) | 0 (0.0) | 1 (0.1) | 98 (0.0) | >.99‖ |
| Infliximab | 0 (0.0) | 0 (0.0) | 19 (0.0) | NA |
| Vedolizumab | 0 (0.0) | 0 (0.0) | 7 (0.0) | NA |
| Omalizumab | 0 (0.0) | 1 (0.1) | 23 (0.0) | >.99‖ |
| Mepolizumab | 0 (0.0) | 1 (0.1) | 15 (0.0) | >.99‖ |
| Reslizumab | 0 (0.0) | 0 (0.0) | 0 (0.0) | NA |
| Benralizumab | 0 (0.0) | 0 (0.0) | 5 (0.0) | NA |
| Dupilumab | 0 (0.0) | 0 (0.0) | 15 (0.0) | NA |
| Methotrexate | 2 (0.5) | 2 (0.1) | 221 (0.0) | .18‖ |
| Tacrolimus | 2 (0.5) | 3 (0.2) | 243 (0.1) | .26‖ |
| Mycophenylate | 0 (0.0) | 0 (0.0) | 76 (0.0) | NA |
EGID, Eosinophilic gastrotintestinal disease; EoE, eosinophilic esophagitis; NA, not available.
Bold values indicate statistical significance.
Column percentages (except when otherwise noted).
Matching on age, gender, race and ethnicity, insurance, region, atopic conditions, comorbidities known to be associated with worse COVID-19 outcomes.
χ2 test (except where otherwise noted) comparing EGID with matched controls.
Wilcoxon rank-sum test.
Fisher’s exact test.
References
- 1.Ahmad F.B., Anderson R.N. The leading causes of death in the US for 2020. JAMA. 2021;325:1829. doi: 10.1001/jama.2021.5469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferastraoaru D., Hudes G., Jerschow E., Jariwala S., Karagic M., de Vos G., et al. Eosinophilia in asthma patients is protective against severe COVID-19 illness. J Allergy Clin Immunol Pract. 2021;9:1152–1162.e3. doi: 10.1016/j.jaip.2020.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Franceschini L., Macchiarelli R., Rentini S., Biviano I., Farsi A. Eosinophilic esophagitis: is the Th2 inflammation protective against the severe form of COVID-19? Eur J Gastroenterol Hepatol. 2020;32:1583. doi: 10.1097/MEG.0000000000001909. [DOI] [PubMed] [Google Scholar]
- 4.Chiang A.W., Duong L.D., Shoda T., Nhu Q.M., Ruffner M., Hara T., et al. Type 2 immunity and age modify gene expression of coronavirus-induced disease 2019 receptors in eosinophilic gastrointestinal disorders. J Pediatr Gastroenterol Nutr. 2021;72:718. doi: 10.1097/MPG.0000000000003032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Savarino E., Lorenzon G., Ghisa M., Laserra G., Barberio B., Maniero D., et al. Lack of complications in patients with eosinophilic gastrointestinal diseases during SARS-CoV-2 outbreak. J Allergy Clin Immunol Pract. 2020;8:2790–2792. doi: 10.1016/j.jaip.2020.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chhiba K.D., Patel G.B., Vu T.H., Chen M.M., Guo A., Kudlaty E., et al. Prevalence and characterization of asthma in hospitalized and nonhospitalized patients with COVID-19. J Allergy Clin Immunol. 2020;146:307–314. doi: 10.1016/j.jaci.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zevit N., Chehade M., Leung J., Dellon E.S. COVID-19 infections in the eosinophilic GI disease population are not severe: initial report from a global online registry. Gastroenterology. 2021;160(Suppl):S251. [Google Scholar]
- 8.Kimura H., Francisco D., Conway M., Martinez F.D., Vercelli D., Polverino F., et al. Type 2 inflammation modulates ACE2 and TMPRSS2 in airway epithelial cells. J Allergy Clin Immunol. 2020;146:80–88. doi: 10.1016/j.jaci.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perlini S., Ciprandi G., Castagnoli R., Licari A., Marseglia G.L. Eosinopenia could be a relevant prognostic biomarker in patients with coronavirus disease 2019. Allergy Asthma Proc. 2020;41:e80–e82. doi: 10.2500/aap.2020.41.200079. [DOI] [PubMed] [Google Scholar]