Abstract
Background
To research the first-order features of apparent diffusion coefficient (ADC) values on diffusion-weighted magnetic resonance imaging (DWI) in maxillofacial malignant mesenchymal tumours.
Methods
The clinical data of 12 patients with rare malignant mesenchymal tumours of the maxillofacial region (6 cases of sarcoma and 6 cases of lymphoma) treated in the hospital from May 2018 to June 2020 and were confirmed by postoperative pathology were retrospectively analyzed. The patients were all examined by 1.5T magnetic resonance imaging. PyRadiomics were used to extract radiomics imaging first-order features. Group differences in quantitative variables were examined using independent-samples t-tests.
Results
The voxels number of ADCmean and ADCmedian of sarcoma tissues were 44.9124 and 44.2064, respectively, significantly higher than those in lymphoma tissues (ADCmean (− 68.8379) and ADCmedian (− 74.0045)), the difference considered statistically significant, so do the ADCkurt and ADCskew.
Conclusions
The statistical difference of ADCmean and ADCmedian is significant, it is consistent with the outcome of the manual measurement of the ADC mean value of the most significant cross-section of twelve cases of lymphoma. Development of tumour volume based on the ADC parameter map of DWI demonstrates that the first-order ADC radiomics features analysis can provide new imaging markers for the differentiation of maxillofacial sarcoma and lymphoma. Therefore, first-order ADC features of ADCkurt combined ADCskew may improve the diagnosis level.
Keywords: Maxillofacial, Mesenchymal tumours, MRI, DWI, Radiomics feature, First-order feature
Background
The rapid development of MRI in the maxillofacial region has improved the recognition and diagnosis of maxillofacial tumours significantly. The vast majority of malignant maxillofacial tumours originate from epithelial tissue, and mesenchymal tumours are rare. Therefore, the imaging manifestations of these diseases are not well understood, and the rate of clinical misdiagnosis is high. Different types of tumours have different clinical treatment methods. Sarcoma is generally entirely removed by surgery. Chemotherapy is the standard lymphoma treatment. Therefore, it is vital to determine the type of tumour before operating. DWI could provide essential biomarkers in several kinds of tumours [1, 2].
Radiomics is a new technology whereby algorithms automatically extract and transform a large amount of representative imaging data into exploitable feature spaces that reflect the microscopic characteristics of tumours [3, 4]. MRI could detect and locate the focus and monitor the disease progression that a biopsy cannot [5, 6]. Recently, radiomics has played an important role in the identification of imaging biomarkers and clinical management [7–11]. However, there are few reports on the application and related literature of maxillofacial mesenchymal tumours. In this paper, several rare cases of mesenchymal tumours were analysed retrospectively to provide more reliable clinical evidence for the differential diagnosis of sarcoma and lymphoma. First-order features radiomics analysis, as an emerging tool in MRI analysis [12, 13], could provide insight into tumour heterogeneity and was valuable for differentiating tumour type [14–19].
In this study, the first-order features of the volume of interests (VOI) were extracted from DWI-ADC parameters. These results, together with the mean ADC were analysed for characterisation of rare malignant mesenchymal tumours in the maxillofacial region.
Methods
Patients and MRI acquisition parameters
Patients
Twelve patients were retrospectively reviewed (seven men, five women; mean age was 54 [range 13–85] years) with a biopsy-proven malignant mesenchymal tumour. The sample patients had preoperative MRI scans between May 2018 and June 2020, and the pathological differentiation could be determined. The sample included six sarcomas and six lymphomas, including one low-grade central osteosarcoma of the left zygoma, three well-differentiated chondrosarcomas of the jaw, two leiomyosarcoma of neck and jaw, two well-differentiated diffuse large B-cell tumour of the buccal region, one B-cell lymphoma related marginal area of tongue mucosa and three non-Hodgkin's follicular lymphoma of the parotid gland. Only patients with sarcomas showed varying degrees of pain and limited facet joint movement, which had no specific clinical symptoms.
The inclusion criteria: biopsy-proven malignant mesenchymal tumour without the concomitant disease. The exclusion criteria were as follows: without definitive post-operative information on pathological characteristics, a minimum tumour diameter < 5 mm, poor MRI quality.
This study was conducted in accordance with the Helsinki Declaration of 1975, as revised in 2013 and approved by the ethics committee of China-Japan Union Hospital of Jilin University in May 2018. All patients signed an informed consent form for inclusion in the study.
MRI acquisition parameters
1.5-T Siemens Avanto with an eight-channel phased-array neck coil was used in this study. The patient's head was secured. Non-contrast axial, sagittal and coronal FS-T2WI sequences acquired in multiple breath-holds were obtained by the following parameters: a repetition/echo time of 5080/87 ms, a slice thickness/interslice gap of 4.0/0.4 mm, 20 slices and a matrix of 256 × 320. Axial T1-weighted images were also acquired in multiple breath-holds. Diffusion-weighted images were obtained in the coronal plane. Following the image acquisition, a pixel-wise ADC map was generated by the inbuilt software using b values of 800 s/mm2. All patients received a 15-ml intravenous bolus injection of gadodiamide (GE Healthcare Ireland Limited, County Cork, Republic of Ireland). The contrast imaging was performed using a fat-suppressed three-dimensional (3D) T1-weighted volumetric interpolated breath-hold examination sequence after the injection.
The shape, size, signal, bone destruction, adjacent tissue relationship on MRI were evaluated. Besides, the ADC map was generated based on DWI, and the sampling was selected to measure the ADC value at the maximum level of the lesion. The lesions were resected surgically in all eight patients. Histopathological and immunohistochemical staining (IHC) was performed postoperatively.
MRI and radiomics analysis
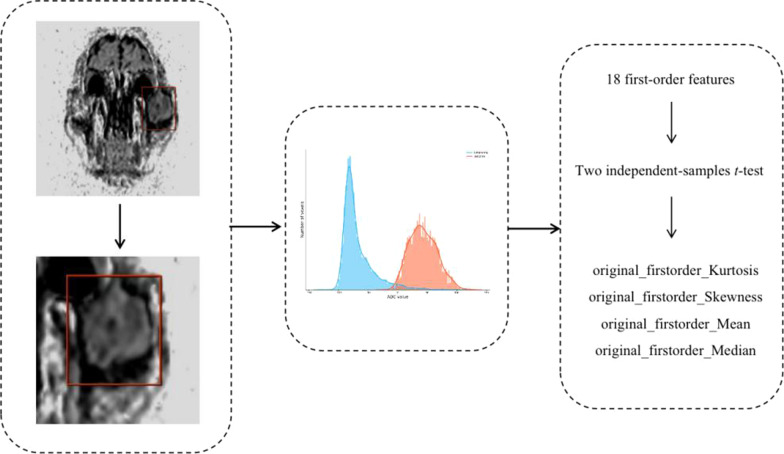
Dr Wise Multimodal Research Platform was used for radiomics analysis. An open-source python package called PyRadiomics (2.2.0) was used for extraction of features. The platform supports feature extraction used to calculate single values per feature for a region of interest (ROI) (‘segment-based’) or generate feature maps (‘voxel-based’) (Fig. 1).
Fig. 1.
First-order ADC radiomics analysis scheme used in this study
Delineation of tumour ROI
The tumour regions in the primary dataset were labeled manually by two experts. In the case of disagreement, a third opinion was requested. The DWI-ADC parameter diagram scan was selected as the labeling image, then tumour tissue was classified.
Extracting features from MRI scans
A B-spline interpolation resampling was used and the anisotropic voxels were resampled to form isotropic voxels of 2.0 mm × 2.0 mm × 2.0 mm. The MRI images were then normalised by centring it at the mean with standard deviation.
(s = 100; μχ represents mean value; σ represents standard deviation).
Eighteen first-order features were obtained from the original images based on the pixel value extracted from each ROI: Energy, Total Energy, Mean Absolute Deviation, Robust Mean Absolute Deviation, Entropy, 10Percentile, 90Percentile, Minimum, Maximum, Mean, Median, Interquartile Range, Range, Root Mean Squared, Skewness, Kurtosis, Variance and Uniformity.
Statistical analysis
The research data was normally distributed with homogeneity of variance. All data analyses were carried out using SPSS 16.0 (IBM Corp., Armonk, NY, USA). Group differences in quantitative variables were compared using student’s t-test. A P-value < 0.05 was considered statistically significant.
Results
Imaging and radiomics features
The general characteristics of the twelve study participants were listed in Table 1. The sarcomas showed slightly higher signal intensity with b = 800 s/mm2, while ADC images showed a slightly lower signal intensity on DWI images. The mean ADC for all patients with sarcoma was approximately 1.40 × 10−3mm2/s, while the mean ADC for patients with lymphoma was approximately 0.49 × 10−3mm2/s. A total of 18 first-order features were obtained. ADC first-order radiomics features were as follows: The mean voxels number of ADCkurt and ADCskew values of sarcomas were 4.1834 and 0.4956 respectively, the values of lymphomas were 9.6219 and 1.8514 respectively. The ADCkurt and ADCskew differed significantly between sarcoma and lymphoma (P < 0.05). The voxels number of ADCmean and ADCmedian of sarcomas were 44.9124 and 44.2064 respectively, which is significantly higher than that of lymphoma (ADCmean = − 68.8379 and ADCmedian = − 74.0045), the difference was statistically significant, shown in Table 2.
Table 1.
The clinical and MRI characteristics of patients (n = 12)
| MR1 scan | Sex | Age | Region | ADC valuc (l0 3 mm2/s) | Pathological and IHC |
|---|---|---|---|---|---|
| Patient 1 | F | 26 | Zygoma | 1.12 | spindie shaped tumor cells and scattered in trabecular bone tissue and bone like matrix tissue; Ki-67 (10–20%), CK (−), SMA and CD99 (+) |
| Patient 2 | F | 40 | Jaw | 1.56 | a large number of chondrocytes with obvious heteromorphism and bone septum; viaentin (+), S-100 and CK (−), Ki-67(60%) |
| Patient 3 | M | 56 | Jaw | 1.54 | A large number of chondrocytes; S-100(−), Viacntin(+), CK(−), Ki-67(50%) |
| Patient 4 | M | 85 | Neck | 1.25 | A large number of spindle cells arranged in bundles or vortices, and the cells were slightly deformed, with visible nuclei and mitotic images; viaentin (+), S-100 and CK (−), Ki-67(30%) |
| Patient 5 | F | 62 | Jaw | 1.36 | A large number of spindle cells; Ki-67(10–20%), CK(−), SMA (+) |
| Patient 6 | F | 59 | Jaw | 1.55 | a large number of chondrocytes with obvious heteromorphism and bone septum; viaentin (+), Ki-67(30%) |
| Patient 7 | F | 62 | Buccal | 0.56 | Lymphoid hyperplasia; PCK(−), EMA(−), CD20(+), CD79a(+), PAX-5(+), CD3(−), CD38(+), CyclinDI(−), MUMl(−), CD30(−), CD 10(+), Bcl-6(−), Bcl-2(−), CD23(−), CD5(+), Ki67(70%) |
| Patient 8 | M | 85 | Tongue | 0.31 | Lymphoid hyperplasia, destroyed lymphoid follicles structure; CD3T(+), BCL-6(−), BCL-2(+), CD 10(−), cyclinDl(−), CD79a(+), Pan-5(+), kappa(−), Ki-67(< 10%) |
| Patient 9 | M | 56 | Parotid gland | 0.37 | Lymphoid hyperplasia with obvious heteromorphism; CD3(+), CD20(+), BCL-2(+), BCL-6(+), CD 10(+), Muml(+), PAX-5(+), CD79a(+), Ki-67(70 -80%) |
| Patient 10 | M | 67 | Parotid gland | 0.54 | Lymphoid hyperplasia, tumor cells infiltrated glands in some areas, serous acini and adipose tissue display; CD20(+), CD 10 (+), CD3 partial cells (+), CD21 showed FDC network, BCL-6 (+), BCL-2 (+), CD38 germinal center positive (+) |
| Patient 11 | M | 13 | Parotid gland | 0.57 | Lymphoid hyperplasia, CD3T(+), CD10(−), BCL-6(+), BCL-2(+), CD79a(+), Ki-67(40–50%) |
| Patient 12 | M | 40 | Buccal | 0.61 | Lymphoid hyperplasia, CD3T(+), CD20(−), CD10(−), BCL-6(+), BCL-2(+), CD3 partial cells (+), Ki-67(20%) |
Table 2.
Results of the two-sample t-test (ADCkurt, ADCskew, ADCmean and ADCmedian)
| First-order features | ADCkurt | ADCskew | ADCmean | ADCmedian |
|---|---|---|---|---|
| P-value | 0.02 | 0.02 | 0.01 | 0.01 |
A P-value < 0.05 was considered statistically significant
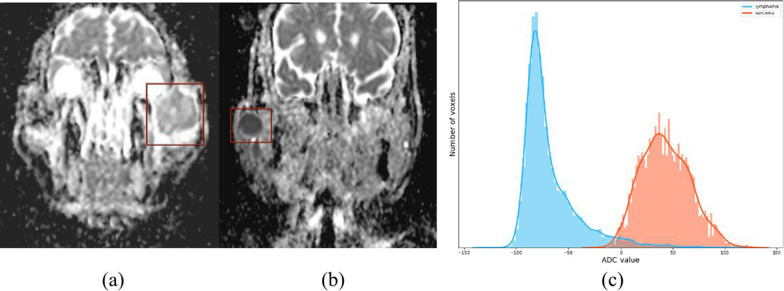
Figure 2a shows a low-grade central osteosarcoma of left zygoma with slightly higher ADC value; Fig. 2b shows a non-Hodgkin's follicular lymphoma of the right parotid gland with low value. Figure 2c shows that lymphoma correlates with an ADCmean in the lower range, positive skew (i.e. high skewness) and a steep curve (i.e. high kurtosis). Sarcoma correlates with a higher ADCmean value, a negative skew (i.e. low skewness), and a flatter shape (i.e. low kurtosis).
Fig. 2.
DWI-ADC images of a scarcoma and b lymphoma. a A 28 years old female patient with a sarcoma within the left zygoma, DWI-ADC image the lesion appears relatively inhomogeneous slightly higher signal compared to the adjacent muscle. ADC value is about 1.12 mm2/s; b a 67 years old male patient with a lymphoma within the right parotid gland, DWI-ADC image the lesion appears relatively homogeneous lower signal compared to the adjacent muscle. ADC value is about 0.54 mm2/s; c lymphoma correlating with an ADCmean in the lower range, positive skew (high skewness), and a steep curve (high kurtosis). Sarcoma correlating with a higher ADCmean value, a negative skew (low skewness), and a flatter shape (low kurtosis)
Discussion
In this study, the ADCkurt, and ADCskew were significant predictive factors. The ADCmean and ADCmedian were significantly different with statistical significance, the result is consistent with the mean of ADC value. The average ADC value of sarcomas is approximately 1.40 × 10–3 mm2/s, and the average ADC value of lymphomas is approximately 0.49 × 10–3 mm2/s. Maeda et al. [20] and Wang et al. [21] found that the average ADC value of lymphoma was lower than head and neck malignant tumours. Furthermore, Sumi et al. [22] reported that an ADC smaller than 0.560 × 10−3 mm2/s could differentiate pharyngeal lymphomas from pharyngeal carcinomas. These results are consistent with our study. Also, the mean voxels number of ADCmean and ADCmedian of sarcomas were 44.9124 and 44.2064, respectively, which is significantly higher than that of lymphoma (ADCmean = − 68.8379 and ADCmedian = − 74.0045), the difference was statistically significant. These results are consistent with the consequences of manual measurement of the maximum cross-section ADC of twelve cases. However, the previous study is limited to the review of ADC; there is no distinction between single lymphoma and sarcoma. The cases in this study are rare, which may enrich the clinical data and further improve the understanding of the radiomics features of mesenchymal tumours.
This study found that ADCkurt and ADCskew differed significantly between sarcoma and lymphoma. Lymphoma correlated with an ADCmean in the lower range. Sarcoma correlated with a higher ADCmean value. This study demonstrates that first-order ADC radiomics analysis, used with the average ADC value to improve diagnostic accuracy, could provide new imaging markers for the differentiation of maxillofacial sarcoma and lymphoma. However, more cases need to be studied. Furthermore, there is theoretical support for studying the difference between the first-order characteristics of sarcoma and lymphoma. Besides, Lisson et al. [23] identified several first-order parameters in order to differentiate enchondroma and low-grade osteosarcoma. Meyer et al. [24] reported that texture analysis parameters derived from MRIs could reflect the Ki67 index in soft tissue sarcoma. So, radiomics analysis can reflect microstructure differences between these tumour entities.
DWI has been used in head and neck tumor imaging and is considered as a sensitive marker for monitoring treatment response in head and neck cancer [25]. While many researchers focused on the interpretation of conventional CT and MRI, the MRI's imaging extraction feature presents an intriguing way to differentiate sarcoma and lymphoma [26, 27]. Suo et al. [28] reported that ADCskew, ADCkurt and ADCmean differed between benign bladder lesions and bladder carcinoma. Wang et al. [29] applied first-order ADC texture analysis in order to differentiate lymphoma from metastatic nodes in the head and neck region.
DWI could detect non-invasively the diffusion of water molecules in living tissues [30]. The characteristics of the dense distribution of lymphoma cells and small extracellular space can decrease the ADC value [22]. However, cystic degeneration and necrosis are common in sarcomas, and ADC value is slightly higher [31]. Although ADC value is helpful for clinical identification of maxillofacial lymphoma and other diseases, the rate of clinical misdiagnosis is high because of a low incidence rate and typical clinical manifestations. Therefore, more diagnostic criteria are needed, thus increasing the accuracy of clinical diagnosis. Conventional MRI examinations mainly reflect the shape, composition and water molecule diffusion of a tumour. Texture analysis can extract and quantify the grey level, roughness and homogeneity of lesions that cannot be distinguished by the naked eye [32]. That allows the practitioner to reflect on the characteristics of lesions more comprehensively and carefully [33]. Recent studies found that histogram-based parameters reflected the different histopathological features in several tumour entities [13, 34]. This relationship maybe help to better characterise tumours through radiological imaging and aid in differentiating between tumour types [35].
Based on the number of cases and features selected in this paper, 18 kinds of first-order features of lesions were extracted for analysis. First-order statistics describe the distribution of voxel intensities. Skewness measures the asymmetry of the distribution of values. A higher kurtosis reflected that distribution mass is concentrated towards the tail(s) rather than towards the mean. Lower kurtosis implies the reverse. Fine texture usually appears in healthy tissue, while rough texture highlights the heterogeneity of the tumour. In this study, the first-order feature analysis of rare cases showed that sarcoma and lymphoma were different in malignant mesenchymal tumours of the maxillofacial region. Therefore, radiomics analysis can provide quantitative parameters in the tumour ROI.
Limitations
First, the number of patients was limited. Analysing a more significant number of patients’ textural parameters may have shown vital information related to tumour characteristics. Second, the differences relating to the imaging parameters and image viewer remain unknown. Future studies of this nature should include a more significant number of cases.
Conclusions
In conclusion, the development of tumour volume based on the ADC parameter map of DWI first-order ADC radiomics analysis makes it possible to provide new imaging markers for the differentiation of maxillofacial sarcoma and lymphoma. Primarily, the feature parameters ADCkurt and ADCskew, combined with the mean ADC value, effectively improves the diagnostic level. However, due to the low incidence rate and a limited number of cases, this study explored only first-order features that were closely related to malignant mesenchymal tumours. It can be concluded from the results of the study that tumour classification can be predicted based on radiomics features, and further study is recommended.
Acknowledgements
We would like to express our gratitude to all those who helped us during the writing of this manuscript.
Abbreviations
- MRI
Magnetic resonance imaging
- DWI
Diffusion-weighted imaging
- ADC
Apparent diffusion coefficient
- VOI
Volume of interests
- ROI
Region of interest
- IHC
Immunohistochemical
Authors' contributions
YBT, HCC, LS, LT and GYY conceived of the study, and YBT, ZXW and DJ participated in its design and coordination and YBT helped to draft the manuscript. All authors read and approved the final manuscript.
Funding
Health and Health Technology Innovation Project of Jilin Province (2018J078); Discipline Layout Project of Natural Science Foundation of Technology Department of Jilin Province (20200201373JC).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Declarations
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Surov A, Meyer HJ, Wienke A. Correlation between apparent diffusion coefficient (ADC) and cellularity is different in several tumors: a meta-analysis. Oncotarget. 2017;8(35):59492–59499. doi: 10.18632/oncotarget.17752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ravanelli M, Grammatica A, Tononcelli E, Morello R, Leali M, Battocchio S, Agazzi GM, Buglione di Monale E, Bastia M, Maroldi R, Nicolai P, Farina D. Correlation between human papillomavirus status and quantitative MR imaging parameters including diffusion-weighted imaging and texture features in oropharyngeal carcinoma. AJNR Am J Neuroradiol. 2018;39(10):1878–1883. doi: 10.3174/ajnr.A5792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gillies RJ, Kinahan PE, Hricak H. Radiomics: images are more than pictures, they are data. Radiology. 2016;278(2):563–577. doi: 10.1148/radiol.2015151169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lubner MG, Smith AD, Sandrasegaran K, Sahani DV, Pickhardt PJ. CT texture analysis: definitions, applications, biologic correlates, and challenges. Radiographics. 2017;37(5):1483–1503. doi: 10.1148/rg.2017170056. [DOI] [PubMed] [Google Scholar]
- 5.Soufi M, Arimura H, Nakamoto T, Hirose TA, Ohga S, Umezu Y, Honda H, Sasaki T. Exploration of temporal stability and prognostic power of radiomic features based on electronic portal imaging device images. Phys Med. 2018;46:32–44. doi: 10.1016/j.ejmp.2017.11.037. [DOI] [PubMed] [Google Scholar]
- 6.Li Q, Kim J, Balagurunathan Y, Liu Y, Latifi K, Stringfield O, Garcia A, Moros EG, Dilling TJ, Schabath MB, Ye Z, Gillies RJ. Imaging features from pretreatment CT scans are associated with clinical outcomes in nonsmall-cell lung cancer patients treated with stereotactic body radiotherapy. Med Phys. 2017;44(8):4341–4349. doi: 10.1002/mp.12309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou W, Zhang L, Wang K, Chen S, Wang G, Liu Z, Liang C. Malignancy characterization of hepatocellular carcinomas based on texture analysis of contrast-enhanced MR images. J Magn Reson Imaging. 2017;45(5):1476–1484. doi: 10.1002/jmri.25454. [DOI] [PubMed] [Google Scholar]
- 8.Li Z, Mao Y, Huang W, Li H, Zhu J, Li W, Li B. Texture-based classification of different single liver lesion based on SPAIR T2W MRI images. BMC Med Imaging. 2017;17(1):42. doi: 10.1186/s12880-017-0212-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim AY, Kim YK, Lee MW, Park MJ, Hwang J, Lee MH, Lee JW. Detection of hepatocellular carcinoma in gadoxetic acid-enhanced MRI and diffusion-weighted MRI with respect to the severity of liver cirrhosis. Acta Radiol. 2012;53(8):830–838. doi: 10.1258/ar.2012.120099. [DOI] [PubMed] [Google Scholar]
- 10.Makanyanga J, Ganeshan B, Rodriguez-Justo M, Bhatnagar G, Groves A, Halligan S, Miles K, Taylor SA. MRI texture analysis (MRTA) of T2-weighted images in Crohn's disease may provide information on histological and MRI disease activity in patients undergoing ileal resection. Eur Radiol. 2017;27(2):589–597. doi: 10.1007/s00330-016-4324-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li M, Fu S, Zhu Y, Liu Z, Chen S, Lu L, Liang C. Computed tomography texture analysis to facilitate therapeutic decision making in hepatocellular carcinoma. Oncotarget. 2016;7(11):13248–13259. doi: 10.18632/oncotarget.7467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davnall F, Yip CS, Ljungqvist G, Selmi M, Ng F, Sanghera B, Ganeshan B, Miles KA, Cook GJ, Goh V. Assessment of tumor heterogeneity: an emerging imaging tool for clinical practice? Insights Imaging. 2012;3(6):573–589. doi: 10.1007/s13244-012-0196-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Just N. Improving tumour heterogeneity MRI assessment with histograms. Br J Cancer. 2014;111(12):2205–2213. doi: 10.1038/bjc.2014.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Downey K, Riches SF, Morgan VA, Giles SL, Attygalle AD, Ind TE, Barton DP, Shepherd JH, deSouza NM. Relationship between imaging biomarkers of stage I cervical cancer and poor-prognosis histologic features: quantitative histogram analysis of diffusion-weighted MR images. AJR Am J Roentgenol. 2013;200(2):314–320. doi: 10.2214/AJR.12.9545. [DOI] [PubMed] [Google Scholar]
- 15.Woo S, Cho JY, Kim SY, Kim SH. Histogram analysis of apparent diffusion coefficient map of diffusion-weighted MRI in endometrial cancer: a preliminary correlation study with histological grade. Acta Radiol. 2014;55(10):1270–1277. doi: 10.1177/0284185113514967. [DOI] [PubMed] [Google Scholar]
- 16.Takahashi M, Kozawa E, Tanisaka M, Hasegawa K, Yasuda M, Sakai F. Utility of histogram analysis of apparent diffusion coefficient maps obtained using 3.0T MRI for distinguishing uterine carcinosarcoma from endometrial carcinoma. J Magn Reson Imaging. 2016;43(6):1301–1307. doi: 10.1002/jmri.25103. [DOI] [PubMed] [Google Scholar]
- 17.Hao Y, Pan C, Chen W, Li T, Zhu W, Qi J. Differentiation between malignant and benign thyroid nodules and stratification of papillary thyroid cancer with aggressive histological features: Whole-lesion diffusion-weighted imaging histogram analysis. J Magn Reson Imaging. 2016;44(6):1546–1555. doi: 10.1002/jmri.25290. [DOI] [PubMed] [Google Scholar]
- 18.Liang H-Y, Huang Y-Q, Yang Z-X, Zeng M-S, Rao S-X. Potential of MR histogram analyses for prediction of response to chemotherapy in patients with colorectal hepatic metastases. Eur Radiol. 2016;26(7):2009–2018. doi: 10.1007/s00330-015-4043-2. [DOI] [PubMed] [Google Scholar]
- 19.Kyriazi S, Collins DJ, Messiou C, Pennert K, Davidson RL, Giles SL, Kaye SB, Desouza NM. Metastatic ovarian and primary peritoneal cancer: assessing chemotherapy response with diffusion-weighted MR imaging-value of histogram analysis of apparent diffusion coefficients. Radiology. 2011;261(1):182–192. doi: 10.1148/radiol.11110577. [DOI] [PubMed] [Google Scholar]
- 20.Maeda M, Kato H, Sakuma H, Maier SE, Takeda K. Usefulness of the apparent diffusion coefficient in line scan diffusion-weighted imaging for distinguishing between squamous cell carcinomas and malignant lymphomas of the head and neck. AJNR Am J Neuroradiol. 2005;26(5):1186–1192. [PMC free article] [PubMed] [Google Scholar]
- 21.Wang J, Takashima S, Takayama F, Kawakami S, Saito A, Matsushita T, Momose M, Ishiyama T. Head and neck lesions: characterization with diffusion-weighted echo-planar MR imaging. Radiology. 2001;220(3):621–630. doi: 10.1148/radiol.2202010063. [DOI] [PubMed] [Google Scholar]
- 22.Sumi M, Ichikawa Y, Nakamura T. Diagnostic ability of apparent diffusion coefficients for lymphomas and carcinomas in the pharynx. Eur Radiol. 2007;17(10):2631–2637. doi: 10.1007/s00330-007-0588-z. [DOI] [PubMed] [Google Scholar]
- 23.Lisson CS, Lisson CG, Flosdorf K, Mayer-Steinacker R, Schultheiss M, von Baer A, Barth TFE, Beer AJ, Baumhauer M, Meier R, Beer M, Schmidt SA. Diagnostic value of MRI-based 3D texture analysis for tissue characterisation and discrimination of low-grade chondrosarcoma from enchondroma: a pilot study. Eur Radiol. 2018;28(2):468–477. doi: 10.1007/s00330-017-5014-6. [DOI] [PubMed] [Google Scholar]
- 24.Meyer HJ, Renatus K, Höhn AK, Hamerla G, Schopow N, Fakler J, Josten C, Surov A. Texture analysis parameters derived from T1-and T2-weighted magnetic resonance images can reflect Ki67 index in soft tissue sarcoma. Surg Oncol. 2019;30:92–97. doi: 10.1016/j.suronc.2019.06.006. [DOI] [PubMed] [Google Scholar]
- 25.Kim S, Loevner L, Quon H, Sherman E, Weinstein G, Kilger A, Poptani H. Diffusion-weighted magnetic resonance imaging for predicting and detecting early response to chemoradiation therapy of squamous cell carcinomas of the head and neck. Clin Cancer Res. 2009;15(3):986–994. doi: 10.1158/1078-0432.CCR-08-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kierans AS, Doshi AM, Dunst D, Popiolek D, Blank SV, Rosenkrantz AB. Retrospective assessment of histogram-based diffusion metrics for differentiating benign and malignant endometrial lesions. J Comput Assist Tomogr. 2016;40(5):723–729. doi: 10.1097/RCT.0000000000000430. [DOI] [PubMed] [Google Scholar]
- 27.Suo S, Zhang K, Cao M, Suo X, Hua J, Geng X, Chen J, Zhuang Z, Ji X, Lu Q, Wang H, Xu J. Characterization of breast masses as benign or malignant at 3.0T MRI with whole-lesion histogram analysis of the apparent diffusion coefficient. J Magn Reson Imaging. 2016;43(4):894–902. doi: 10.1002/jmri.25043. [DOI] [PubMed] [Google Scholar]
- 28.Suo ST, Chen XX, Fan Y, Wu LM, Yao QY, Cao MQ, Liu Q, Xu JR. Histogram analysis of apparent diffusion coefficient at 3.0 T in urinary bladder lesions: correlation with pathologic findings. Acad Radiol. 2014;21(8):1027–1034. doi: 10.1016/j.acra.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 29.Wang YJ, Xu XQ, Hu H, Su GY, Shen J, Shi HB, Wu FY. Histogram analysis of apparent diffusion coefficient maps for the differentiation between lymphoma and metastatic lymph nodes of squamous cell carcinoma in head and neck region. Acta Radiol. 2018;59(6):672–680. doi: 10.1177/0284185117730688. [DOI] [PubMed] [Google Scholar]
- 30.Inoue T, Kozawa E, Ishikawa M, Okada H. Application of magnetic resonance imaging in the evaluation of nutritional status: a literature review with focus on dialysis patients. Nutrients. 2021;13(6):2037. doi: 10.3390/nu13062037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shen Y, Zhong Y, Wang H, et al. MR imaging features of benign retroperitoneal extra-adrenal paragangliomas. Sci Rep. 2017;7(1):4517. doi: 10.1038/s41598-017-04753-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Incoronato M, Aiello M, Infante T, Cavaliere C, Grimaldi AM, Mirabelli P, Monti S, Salvatore M. Radiogenomic analysis of oncological data: a technical survey. Int J Mol Sci. 2017;18(4):805. doi: 10.3390/ijms18040805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lambin P, Leijenaar RTH, Deist TM, Peerlings J, de Jong EEC, van Timmeren J, Sanduleanu S, Larue RTHM, Even AJG, Jochems A, van Wijk Y, Woodruff H, van Soest J, Lustberg T, Roelofs E, van Elmpt W, Dekker A, Mottaghy FM, Wildberger JE, Walsh S. Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol. 2017;14(12):749–762. doi: 10.1038/nrclinonc.2017.141. [DOI] [PubMed] [Google Scholar]
- 34.Schob S, Meyer HJ, Pazaitis N, Schramm D, Bremicker K, Exner M, Höhn AK, Garnov N, Surov A. ADC histogram analysis of cervical cancer aids detecting lymphatic metastases-a preliminary study. Mol Imaging Biol. 2017;19(6):953–962. doi: 10.1007/s11307-017-1073-y. [DOI] [PubMed] [Google Scholar]
- 35.Liu S, Zhang Y, Chen L, Guan W, Guan Y, Ge Y, He J, Zhou Z. Whole-lesion apparent diffusion coefficient histogram analysis: significance in T and N staging of gastric cancers. BMC Cancer. 2017;17(7):665. doi: 10.1186/s12885-017-3622-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.