Abstract
Elevated fetal hemoglobin (HbF) is associated with reduced severity of sickle cell disease. Therefore, γ-globin protein levels and F-cell (HbF-positive red blood cell) percentages are used for estimation of clinical benefit. Here, we monitored transplantation-related changes in HbF and F-cell percentages for rhesus macaques (Macaca mulatta) following total body irradiation or busulfan conditioning prior to CD34+ cell transplantation. HbF protein expression peaked in the first 4–9 weeks posttransplant (0.99%–2.53%), and F-cells increased in the first 6–17 weeks posttransplant (8.7%–45.3%). HbF and F-cell ratios gradually decreased and stabilized to levels similar to those of control animals (1.96 ± 1.97% for F cells and 0.49 ± 0.19% γ-globin expression) 4–7 months post-transplant. These findings confirm and expand on previous reports of transient induction in HbF and F-cell percentages in rhesus macaques following CD34+ cell transplantation, an observation that must be taken into consideration when evaluating therapeutic strategies that aim to specifically elevate HbF expression, which are currently in clinical development. Published by Elsevier Inc. on behalf of ISEH – Society for Hematology and Stem Cells.
Sickle cell disease (SCD) is a monogenic disorder caused by a missense mutation in the β-globin gene, which results in the production of sickle hemoglobin (HbS, α2βS2) that polymerizes under hypoxic conditions. Sickle erythrocytes occlude small blood vessels leading to ischemic tissue damage, resulting in severe pain and/or organ failure [1]. High fetal hemoglobin (HbF, α2γ2) levels are clinically beneficial for SCD patients as HbF inhibits deoxy HbS polymerization. Patients with hereditary persistence of fetal hemoglobin (HPFH) mutations and a typical evenly distributed 30% HbF expression are clinically asymptomatic despite having high HbS levels [2]. Additionally, prolonged survival [3] and lower pain frequency [4] have been reported in patients with elevated HbF levels.
In the human fetus, HbF is the predominant form of hemoglobin after the first trimester of gestation, and it gradually decreases and is replaced with adult hemoglobin (HbA, α2β2) after birth. The contribution of HbF to total globin production in the adult is normally less than 1% and is restricted to a small subset of red blood cells (RBCs), referred to as F-cells [5]. Various approaches to induce γ-globin expression have been evaluated in preclinical models, including the use of chemical agents such as hydroxyurea, which has been successfully employed in patients, or ex vivo γ-globin repressor silencing/disruption, or forced chromatin looping (reviewed in [6]). In addition, recent improvements in genome editing tools, particularly CRISPR/Cas9, have paved the way for precise and cost-effective editing of hematopoietic stem cells (HSCs) to induce HbF for transplantation purposes. F-cell (HbF-positive RBC) and total γ-globin expression percentages are generally used along with the HbF/F cell ratio to evaluate potential clinical outcome, but this method falsely assumes that every F-cell has the same amount of HbF [7]. F-cell percentage cannot be a direct estimation of HbF levels, as the same percentage of F-cells can account for different HbF expression levels. Understanding the relationship between F-cell percentages and total HbF protein levels will be critical in establishing appropriate preclinical benchmarks for both parameters in the development of gene therapy strategies to increase HbF levels.
Methods
Here we describe the transplant-related changes in F-cells and HbF ratios in a rhesus macaque model (Macaca mulatta) transplanted with granulocyte colony-stimulating factor- and plerixafor-mobilized CD34+ cells marked with chimeric HIV-1-based lentiviral vectors [8,9]. To evaluate gene marking in a separate study conducted in the same animals, rhesus CD34+ cells were transduced with lentiviral vectors encoding GFP/YFP reporter gene (DEFM, DFRM, ZJ32, and ZJ50) or human globin (ZJ20, ZJ30, ZJ32, and ZJ50), which can be separately detected in reversed-phase high-performance liquid chromatography (RP-HPLC). Neither vector is expected to have an impact on rhesus HbF levels. Animals were conditioned with a myeloablative dose (10 Gy) of total-body irradiation (TBI) (DEFM, DFRM, ZJ20, and ZJ30) or busulfan treatment (4 days of 5.5 mg/kg) (ZJ32 and ZJ50) prior to autologous transplantation of mobilized CD34+ HSCs. Peripheral blood was collected at different intervals, and RBCs were subjected to flow cytometry F-cell analysis [10] using primary HbF-specific antibody (No. 551796, BD Biosciences, La Jolla, CA) and allophycocyanin (APC)-conjugated secondary antibody (No. 550874, BD Biosciences), and RP-HPLC using an Aeris 3.6-μm Widepore C4 200 (250 × 4.6 μm, Phenomenex, Torrance, CA) column for γ-globin protein level determination [11].
Results
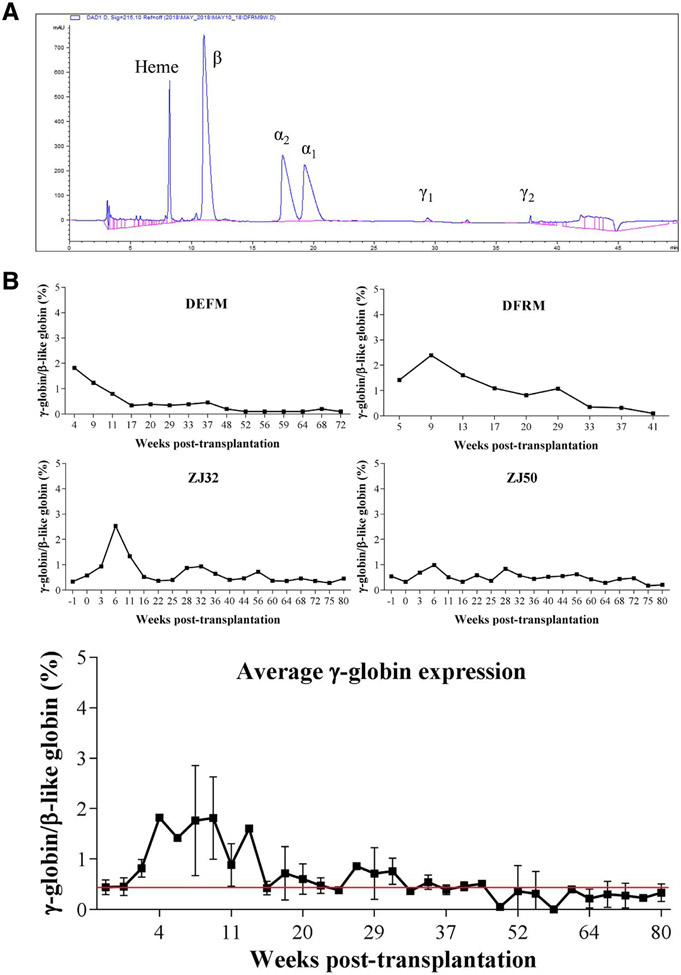
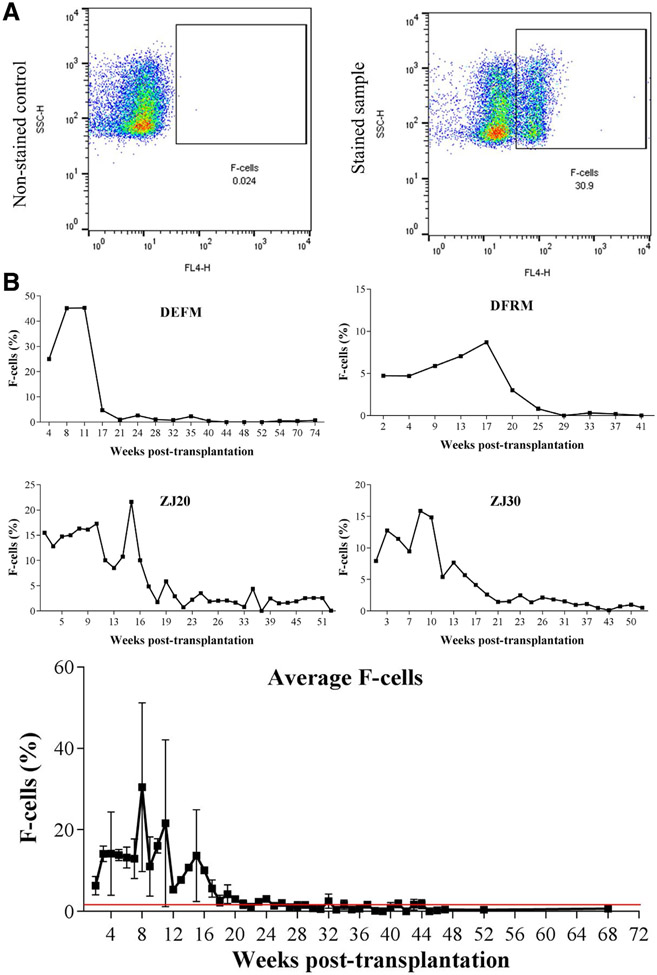
Regardless of the conditioning regimen used, there was a rapid drop in total hemoglobin levels and subsequent increase in reticulocyte percentages in transplanted rhesus monkeys during the first 2 months post-transplantation (Supplementary Figure E1, online only, available at www.exphem.org). However, both total hemoglobin and reticulocyte count reached pre-transplantation levels around 50 days post-transplantation. γ-globin expression at the protein level peaked 4–9 weeks post-transplantation (Figure 1, 0.99%–2.53%) in both TBI and busulfan conditioned animals, and g-globin levels gradually decreased and stabilized around the average expression levels for control non-transplanted animals (0.49 ± 0.19%, n = 10) 15–20 weeks post-transplant. In contrast, F-cell ratios (γ-globin-positive cells/total RBCs determined by flow cytometry) exhibited greater magnitude and variability, although they followed the same pattern as γ-globin expression profiles (Figure 2). F-cell ratios increased 6–17 weeks post-transplantation (8.7%–45.3%) and stabilized to an average F-cell ratio of control un-transplanted animals (1.96 ± 1.97%, n = 31) around 20–30 weeks post-transplantation. In line with the current data, Humbert and colleagues have reported similar transient induction in F-cell ratio (11%–42%) and γ-globin RNA expression (6.5%–39.9%) in pigtailed macaques (Macaca nemestrina) after HSC transplantation [12].
Figure 1.
(A) Representative chromatogram of RP-HPLC indicating globin chains analyzed. (B) Percentage of γ-globin/β-like globin protein expression in total body irradiated (DEFM and DFRM) and busulfan conditioned (ZJ32 and ZJ50) rhesus monkeys (Macaca mulatta) transplanted with CD34+ cells. The results were analyzed by RP-HPLC. The average γ-globin/β-like globin protein expression for 10 non-transplanted control animals (0.49 ± 0.19%) is represented by the red line.
Figure 2.
(A) Representative flow plots of non-stained and stained samples revealing the gating strategy for F-cell analysis by flow cytometry. (B) F-Cell ratios in total body irradiated (DEFM, DFRM, ZJ20 and ZJ30) rhesus monkeys (Macaca mulatta) transplanted with CD34+ cells. The results were analyzed with FlowJo V10 software. The average F-cell ratio determined from 31 non-transplanted control animals (1.96 ± 1.97%) is represented by the red line.
Discussion
Because rhesus macaques are a limited resource, the monkeys used in the present study were used to evaluate both conditioning-related HbF induction and lentiviral gene marking. We observed efficient transduction in rhesus CD34+ cells in vitro (average vector copy number per cell [VCN] of 0.5–9.8) and detected engraftment of gene-modified cells in all transplanted animals (VCN of 0.01–0.29) at 3 months post-transplantation. Lentiviral transduction does not affect HbF levels in ex vivo erythroid differentiated human [13] and rhesus CD34+ [11] cells, except in cases where the vector is explicitly designed to do so, for example, by conveying the γ-globin gene itself, inhibitory sequences for regulatory genes such as BCL11A, or a looping construct targeting the locus control region and γ-globin promoter. We have found that rhesus monkeys display acquired hematopoietic stress following conditioning and subsequent HSC transplantation. This condition promotes rapid stem cell proliferation and hematopoiesis to reconstitute a depleted blood network and bone marrow environment, which mimics fetal-like hematopoiesis. Whether cells proliferate because of high HbF levels or high proliferation rates induce HbF expression is not fully understood. Understanding the stress erythropoietic response of HbF induction, which is specific to humans and Old World monkeys, might provide greater insight into globin switching mechanisms. These results align with previous reports presenting HbF induction in conditions leading to rapid erythroid regeneration, such as transient erythroblastogenia of childhood [13], erythropoietin treatment of baboons [14], and bone marrow transplantation [15]. Because elevated HbF levels reduce the complications and severity of SCD and β-thalassemia, γ-globin gene addition [16] and targeted induction strategies [5] have been evaluated in preclinical models and are currently being investigated in clinical trials (ClinicalTrials.gov Identifiers: NCT02186418 and NCT03282656). On the basis of the data presented here and in previously published works, the results of these approaches should be evaluated after hematopoietic development has stabilized to have long-term clinical benefit. In addition, new strategies aiming to increase HbF levels must be evaluated at the protein levels as F-cell analysis might overestimate the effects because cells with even a small amount of HbF are scored positive.
Our data suggest that autologous transplantation alone can transiently induce HbF levels only up to 2.53% in rhesus macaques. This percentage is not expected to provide any therapeutic benefit in the setting of β-hemoglobinopathies, and therefore, potential gene therapy approaches aimed at directly or indirectly augmenting HbF levels can only be analyzed directly 5 months following HSC transplantation in the rhesus macaque model. It is worthwhile to note that patients with sickle cell disease often have HbF levels that are elevated compared with those of healthy individuals, without any detectable HPFH mutation [17]. Hemolysis of sickle erythrocytes results in reduced average red cell half-life, placing additional demands on erythropoiesis that may induce fetal hemoglobin expression by the same mechanism(s) responsible for the HbF induction observed under the conditions discussed above. As gene therapy strategies designed to increase fetal hemoglobin are specifically in development for sickle cell disease, distinguishing stress-erythropoiesis-related fetal induction from that arising from the genetic intervention should be important for estimating added therapeutic benefit in this patient population.
Altogether, the results of this current study indicate that HSC transplantation induces HbF production at the protein level and F-cell fraction in the early post-transplantation phase. This induction should be taken into consideration in Old World monkey models and clinical studies aiming to enhance HbF levels. Although there seems to be a relative correlation between F cells and HbF production, one parameter is not necessarily a good estimation for the other [18]. Therefore, analysis of both F cells and HbF in preclinical and clinical studies should provide a better prediction of possible clinical outcomes.
Supplementary Material
Acknowledgments
This work was supported by the Intramural Research Program of the NHLBI and the NIDDK at the NIH. We thank animal staffs and veterinarians at the NIH Animal Center for their support. We thank Dr. Duck-Yeon Lee from the NHLBI Biochemistry Core for RP-HPLC analysis help.
References
- 1.Piel FB, Steinberg MH, Rees DC. Sickle cell disease. N Engl J Med. 2017;376:1561–1573. [DOI] [PubMed] [Google Scholar]
- 2.Akinsheye I, Alsultan A, Solovieff N, et al. Fetal hemoglobin in sickle cell anemia. Blood. 2011;118:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Platt OS, Brambilla DJ, Rosse WF, et al. Mortality in sickle cell disease—Life expectancy and risk factors for early death. N Engl J Med. 1994;330:1639–1644. [DOI] [PubMed] [Google Scholar]
- 4.Platt OS, Thorington BD, Brambilla DJ, et al. Pain in sickle cell disease: Rates and risk factors. N Engl J Med. 1991;325:11–16. [DOI] [PubMed] [Google Scholar]
- 5.Demirci S, Uchida N, Tisdale JF. Gene therapy for sickle cell disease: An update. Cytotherapy. 2018;20:899–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Orkin SH, Bauer DE. Emerging genetic therapy for sickle cell disease. Annu Rev Med. 2019;70:257–271. [DOI] [PubMed] [Google Scholar]
- 7.Maier-Redelsperger M, Noguchi CT, de Montalembert M, et al. Variation in fetal hemoglobin parameters and predicted hemoglobin S polymerization in sickle cell children in the first two years of life: Parisian Prospective Study on Sickle Cell Disease. Blood. 1994;84:3182–3188. [PubMed] [Google Scholar]
- 8.Uchida N, Hargrove PW, Lap CJ, et al. High-efficiency transduction of rhesus hematopoietic repopulating cells by a modified HIV1-based lentiviral vector. Mol Ther. 2012;20:1882–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uchida N, Washington KN, Hayakawa J, et al. Development of a human immunodeficiency virus type 1-based lentiviral vector that allows efficient transduction of both human and rhesus blood cells. J Virol. 2009;83:9854–9862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Renneville A, Van Galen P, Canver MC, et al. EHMT1 and EHMT2 inhibition induces fetal hemoglobin expression. Blood. 2015;126:1930–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Demirci S, Bhardwaj SK, Uchida N, et al. Robust erythroid differentiation system for rhesus hematopoietic progenitor cells allowing preclinical screening of genetic treatment strategies for the hemoglobinopathies. Cytotherapy. 2018;20:1278–1287. [DOI] [PubMed] [Google Scholar]
- 12.Humbert O, Peterson CW, Norgaard ZK, Radtke S, Kiem HP. A nonhuman primate transplantation model to evaluate hematopoietic stem cell gene editing strategies for β-hemoglobinopathies. Mol Ther Methods Clin Dev. 2018;8:75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Papayannopoulou T, Vichinsky E, Stamatoyannopoulos G. Fetal Hb Production during acute erythroid expansion: I. Observations in patients with transient erythroblastopenia and post-phlebotomy. Br J Haematol. 1980;44:535–546. [DOI] [PubMed] [Google Scholar]
- 14.Al-Khatti A, Veith RW, Papayannopoulou T, Fritsch EF, Gold-wasser E, Stamatoyannopoulos G. Stimulation of fetal hemoglobin synthesis by erythropoietin in baboons. N Engl J Med. 1987;317:415–420. [DOI] [PubMed] [Google Scholar]
- 15.Ferster A, Corazza F, Vertongen F, et al. Transplanted sickle-cell disease patients with autologous bone marrow recovery after graft failure develop increased levels of fetal haemoglobin which corrects disease severity. Br J Haematol. 1995;90:804–808. [DOI] [PubMed] [Google Scholar]
- 16.Zhao HF, Abraham A, Kim Y-S, et al. Lentiviral transfer of γ-globin with fusion gene NUP98–HOXA10HD expands hematopoietic stem cells and ameliorates murine β-thalassemia. Mol Ther. 2017;25:593–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leonard A, Bonifacino A, Dominical VM, et al. Bone marrow characterization in sickle cell disease: Inflammation and stress erythropoiesis lead to suboptimal CD34 recovery. Br J Haematol. 2019. 10.1111/bjh.15902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Franco RS, Yasin Z, Palascak MB, Ciraolo P, Joiner CH, Rucknagel DL. The effect of fetal hemoglobin on the survival characteristics of sickle cells. Blood. 2006;108:1073–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.