Abstract
Exogenous neurotrophins reduce neuronal atrophy and promote regeneration following spinal cord injury but little is known about the endogenous expression of neurotrophins and their Trk receptors in the injured spinal cord. For this purpose, we used the larval lamprey because it recovers from complete spinal transection and axons regenerate selectively in their correct paths. We cloned lamprey neurotrophin (NT) and its two Trk receptors and assessed their mRNA expression by in situ hybridization and QRT-PCR in control animals and after spinal cord transection. Control lampreys showed a longitudinal array of NT-expressing neurons along length of the spinal cord. At 2 weeks post-transection, NT expression was downregulated in neurons close to the transection, but was little affected remote from the lesion. By 4 weeks, NT expression returned to control levels in spinal cord neurons rostral and caudal to the lesion, although it was upregulated in reactive microglia at 14 and 30 days post-transection. Double-label in situ hybridization for Trk1 and Trk 2 showed that Trk transcripts were expressed in several giant reticulospinal neurons, including the Mauthner neurons. After spinal cord transection, Trk 1 mRNA expression was downregulated, but Trk 2 mRNA expression was not changed or was increased. Thus, our data suggest that spinal cord injury in larval lampreys modulate expression of endogenous neurotrophin and induces proliferation of macrophage/microglial cells that express neurotrophin.
Keywords: spinal cord injury, identifiable neurons, neurotrophins, molecular cloning, in situ hybridization, microglia, lamprey
Complete spinal cord injury (SCI) in humans and other mammals leads to irreversible paralysis below the level of injury. This is due to failure of axonal regeneration in the CNS. By contrast, in the lamprey, after spinal cord transection initial paralysis is followed in several weeks by impressive functional recovery (Rovainen, 1976, Selzer, 1978, Wood and Cohen, 1979, Cohen et al., 1988, Davis and McClellan, 1993). The recovery involves regeneration of spinal axons (Yin and Selzer, 1983, Davis and McClellan, 1994) and the formation of synaptic connections between the regenerated axons and neurons distal to the lesion (Wood and Cohen, 1979, Mackler and Selzer, 1987). Larval lampreys that may be 3 – 5 years old or more and in stable neurological state because the reticulospinal axons have been long developed the entire length of the spinal cord, neuron proliferation stopped after embryonic phase and no a new neurons added to brain or spinal cord at this stage(Rovainen, 1979, Vidal Pizarro et al., 2004, Villar-Cheda et al., 2006, Barreiro-Iglesias et al., 2008). All this mean that there is no confusion over whether an observed phenomenon represents development or regeneration. This is in contrast to developing mammalian CNS where addition of a new neurons and axonal growth continued during regeneration, therefore there always the risk of confusing regeneration mechanisms with those of development.
The difference in regenerative abilities between mammals and lampreys might be related in part to differences in expression of neurotrophic factors in the spinal cords of mammals and lampreys, just as differences in regenerative abilities between PNS and CNS axons in mammals (Chen et al., 2007) have been attributed in part to the relatively limited upregulation of neurotrophic factors in the spinal cord compared with the marked increases in NGF and GDNF mRNA levels observed in Schwann cells after peripheral nerve injuries (Boyd and Gordon, 2003, Gordon et al., 2003).
Neurotrophins (NTs) comprise a family of growth factors that play critical roles in the development, maintenance, survival, and death of the nervous system. They include nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3) and neurotrophin-4/5 (NT-4/5) (Ip and Yancopoulos, 1994, Lewin and Barde, 1996, Bibel and Barde, 2000). NTs activate two different receptor classes, the tropomyosin-related kinase (Trk) family of receptor tyrosine kinases and the p75 neurotrophin receptor, a member of the tumor necrosis factor (TNF) receptor superfamily. Unlike the non-selective p75, which has a similar affinity for all neurotrophins, each Trk receptor selectively binds a different neurotrophin, with NGF activating TrkA, BDNF and NT-4/5 activating TrkB, and NT-3 activating TrkC. NT-3 can also activate TrkA and TrkB in some contexts. For reviews of NT signaling, see (Kaplan and Miller, 2000, Patapoutian and Reichardt, 2001, Huang and Reichardt, 2003, Benito-Gutierrez et al., 2006). The mammalian NTs were originally identified as neuronal survival factors during development, but they also play important roles in the survival and the growth of injured nerves. After SCI, glial cells, neurons, meningeal cells and Schwann cells all contribute to increased intraspinal NGF and BDNF levels (Widenfalk et al., 2001, Brown et al., 2004). Following injury, BDNF-immunopositivity increased over time in astrocytes and microglia/macrophages at the injury site (Dougherty et al., 2000, Ikeda et al., 2001) and among nearby motor and sensory neurons (Ikeda et al., 2001).
Endogenous expression of neurotrophins and their Trk receptors in the injured spinal cord is well documented in mammalian CNS but not in lamprey. Therefore, we cloned the sole lamprey neurotrophin and its two known Trk receptors from lamprey CNS, and studied the effects of SCI on their expression in individual identified neurons by wholemount in situ hybridization and quantitative real-time RT-PCR.
Experimental procedures
Animals
Wild-type larval lampreys (Petromyzon marinus), 12–14 cm in length (4–5 years old) were obtained from streams feeding lake Michigan and maintained in fresh water tanks at 16°C until the day of surgery.
Spinal cord transection
Animals were anesthetized by immersion in 0.1% tricaine methanesulfonate, and the spinal cord exposed from the dorsal midline at the level of the fifth gill. Complete transection of the spinal cord was performed with Castroviejo scissors under microscopic vision, after which the wound was allowed to air dry over ice for one hour. Animals recovered in fresh water tanks at room temperature. At specified recovery times, animals were re-anesthetized, and the brains were removed for in situ hybridization. Experiments were carried out on 40 large larval lampreys that were either untransected (N=10) or permitted to recover 14 days (N=10), 30 days (N=10) or four months (N=10) post-transection. Experiments were approved by the Institutional Animal Care and Use Committee at University of Pennsylvania.
Cloning and sequencing of lamprey neurotrophin and Trks cDNAs from lamprey CNS
Total RNA from lamprey brain and spinal cord was isolated using Trizol reagent (Invitrogen). The first strand cDNA synthesis reaction from total RNA was catalyzed by Superscript II Reverse Transcriptase and oligo-dT18 primers. For PCR cloning, oligonucleotide primers were designed based on the published river lamprey neurotrophin (NT) and Trk sequences (Hallbook et al., 1998). Cloning procedures were the same as reported previously for netrin, UNC-5 and semaphorins (Shifman and Selzer, 2000a, Shifman and Selzer, 2006). Sequencing was carried out on an Applied Biosystems 3730XL sequencer with BigDye Taq FS Terminator V 3.1. The final sequence was confirmed from both strands. Sequence analysis and comparison was done through BLAST on NCBI.
Riboprobe synthesis
Lamprey NT and Trk cDNAs were cloned in pGEM-T Easy vector (Promega), linearized with restriction enzymes, and gel-purified. The linearized plasmids were used as templates for antisense and sense digoxigenin- (DIG-) and fluorescein- (FITC-) labeled riboprobe synthesis. The cloned cDNA for lamprey neurotrophin was used as a template to generate digoxigenin-(DIG-) labeled sense and antisense riboprobes for in situ hybridization on spinal cord. Trk 1 and Trk 2 probes were DIG- and FITC- labeled for in situ hybridization of brain wholemount preparations in control and spinal-transected animals. The neurotrophin (NT) (GenBank accession number EU 449949) cRNA probe was transcribed from a full-length sequence, and Trk1 and Trk2 probes were transcribed from cloned partial sequences (GenBank accession numbers EU449950 and EU449951, respectively). The transcription reaction was carried out with the Epicentre AmpliScribe High Yield kit. DIG and FITC incorporation into probes was controlled by dot blots. The length and integrity of the probes was examined by gel electrophoresis. DIG- and FITC- labeled sense RNA probes were used as internal controls. The NT and Trk 2 probes were labeled with DIG. The Trk 1 probe was labeled with FITC.
Fluorescent in situ hybridization to wholemounted lamprey brain
For in situ hybridization, animals were anesthetized and their brains removed and stripped of choroid plexus, uncovering the 3rd and fourth ventricles. The posterior and cerebrotectal commisures were cut along the dorsal midline and the alar plates were deflected laterally and pinned flat to a small strip of Sylgard. Brains were fixed in 4% paraformaldehyde 3 hr R/T, washed in PTw (0.1% Tween 20 in PBS) at 4°C and then in 50% and 100% methanol, and stored at − 20° C. From 100% methanol, the tissue was transferred serially to 70%, 50% and 25% methanols and washed four times, 30 minutes each wash with PTw, and prehybridized for 1 hr at 50°C in hybridization solution (50% deionized formamide; 5× SSC; 100 µg/ml Torula yeast RNA; 100 µg/ml wheat germ tRNA; 50 µg/ml heparin; 0.1% Tween 20). This was followed by hybridization overnight at 50°C in the same solution plus 1 µg/ml each of digoxigenin- and fluorescein-labeled probes. Hybridized specimens were washed in fresh hybridization solution at the hybridization temperature, in 50% hybridization solution/50% PTw, in PTw at room temperature and in maleate buffer at room temperature. Brains were incubated for 1 h in Maleate buffer (+ 0.2% Tween 20) with 2% Blocking reagent (Roche). Mouse anti-fluorescein (Roche, 1:200) and sheep anti-DIG (Roche, 1:400) antibodies in 2% blocking reagent were applied to the tissue for 2 days at 4°C. Brains were then washed in Maleate buffer + 0.2% Tween 20 at room temperature and blocked again for 1 hour in Maleate buffer + 0.2% Tween 20 with 2% Blocking reagent followed by incubation with secondary antibody (Alexa 488 donkey anti-mouse and goat anti-sheep Alexa 594; Invitrogen) overnight at 4° C. After incubation, brains were washed in PTw and mounted using Prolong (Invitrogen). Images were captured digitally using an AxioCam CCD Video Camera (Zeiss) attached to a Zeiss Axioskop microscope with AxioVision software and scale bars were added. Images were imported into Adobe Photoshop CS 2 (Adobe Systems, Inc., San Jose, CA). The images were cropped and adjusted for brightness and contrast, and labels were added.
Wholemount spinal cord in situ hybridization
Hybridization of DIG-labeled NT riboprobes to wholemounted lamprey spinal cord was performed by methods described previously (Shifman and Selzer, 2000b). Images were captured digitally and processed as above. In order to avoid artifactual differences in labeling strength among spinal cords from different times after transection, the times that animals were lesioned were staggered and positive controls included for all time points, so that all the spinal cords were processed at the same time and under the same conditions.
Quantitative real-time RT-PCR assessment of NT mRNA expression in the spinal cord after transection
RNA extraction
Total RNA was extracted at 2 weeks and 1 month post-transection from segments of spinal cord rostral to (including injury epicenter) and 10 mm caudal to the lesion, from the remaining caudal spinal cord, and from sham-operated cords, using Trizol reagent (Invitrogen) according to the manufacturer’s instructions. RNA was suspended in diethylpyrocarbonate (DEPC)-treated water, followed by treatment with DNAse 1, using the DNA-Free Kit (Ambion, TX, USA) to remove any traces of contaminating genomic DNA. The yield and purity of RNA were checked spectrophotometrically at 260 and 280 nm. Integrity of the RNA was determined by the presence of 28S and 18S ribosomal RNA in electrophoretic samples through 1.0% agarose gels. The first strand cDNA synthesis reaction from total RNA was catalyzed by Superscript II Reverse Transcriptase (Invitrogen) and random primers according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA).
Primer design
The DNA sequence of lamprey hypoxanthine-guanine phosphoribosyltransferase (HPRT 1; Genbank accession number FJ155927) was found using the MegaBLAST program to search a lamprey Trace Archive database that has been developed to store the raw genomic data underlying all of the sequences generated by genome projects. The cloning and sequencing of lamprey neurotrophin and neurotrophin receptors Trk1 and Trk2 is reported above. Cloning and sequencing of lamprey glyceraldehyde 3-phosphate dehydrogenase (GAPDH, Genbank accession number AAT70328) and 18S rRNA sequence (Genbank accession number M97575) was reported previously (Stock and Whitt, 1992, Pancer et al., 2004). Sequences sharing homology with NT, Trk 1, Trk 2, GAPDH, 18S rRNA and HPRT were amplified by RT-PCR. Only the open reading frames, or CDS coding for proteins, was chosen from these sequences. The primers were designed using Primer Express 3 software (Applied Biosystems CA, USA) according to the user’s manual. The specificity of primers was confirmed by homology search against the GenBank database. The sequences of primers are shown in Table 1. All the primers were synthesized by IDT, Inc. (Coralville, IA, USA).
Table.
| Gene (GenBank Accession #) |
Sequence 5’-3’ | Fragment size(bp) |
|---|---|---|
| NT (EU 449949) Forward primer Reverse primer |
GAGGCTGCGGACAGACATTAC AATGGCCCCAGCACTTTCT |
91 |
| HPRT 1 (U03700) Forward primer Reverse primer |
GCGCTCAACCGCAACAC CAGTAGCTCTTGAGTCGGATGAAG |
68 |
| 18S rRNA (M97575) Forward primer Reverse primer |
GTAGTTGGTGGAGCGATTTGTCT GGCCGCGTAGCTAGTTAGCA |
75 |
| GAPDH(AAT70328) Forward primer Reverse primer |
TGCAAAGCACGTCATCATCTC TTCTCGTGGTTTACTCCCATCA |
72 |
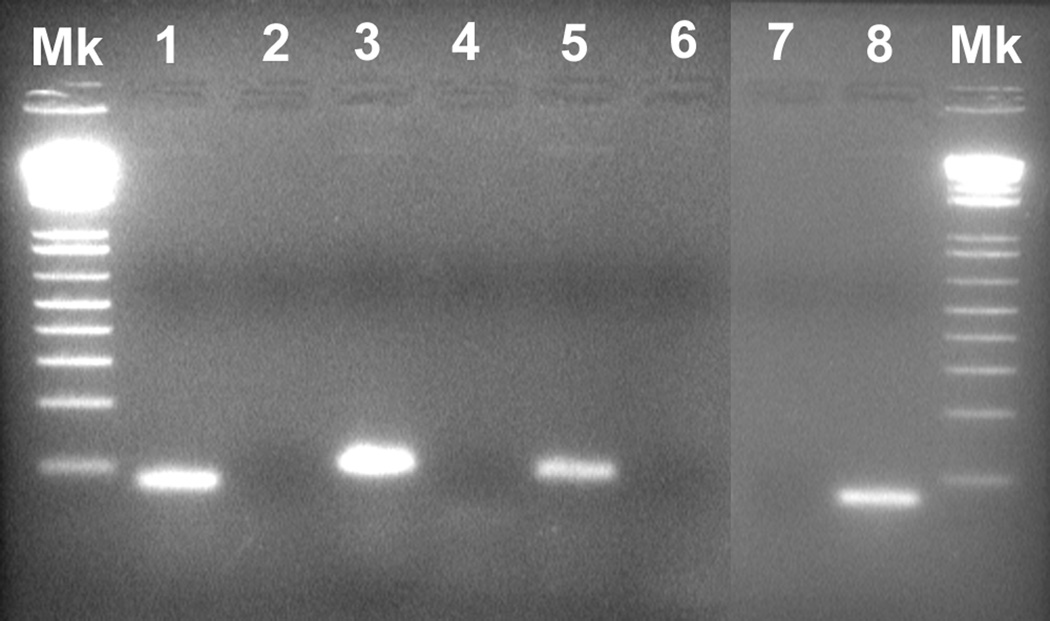
To test the specificity of primers for lamprey-specific genes, nonquantitative PCRs were performed using 2 µl of first-strand synthesis cDNA from lamprey spinal cord RNA. The 50-µl PCR reaction contained the following additional components: 0.4 µM of each primer, 10 µl 5X Green GoTaq® Flexi Buffer (Promega, WI) and 1.25 U GoTaq® DNA polymerase (Promega, WI). The PCR reactions were run in a themocycler GeneAmp® PCR System 9700 (Applied Biosystems) using an initial denature temperature of 95°C for 4 min, 35 cycles of 95°C for 45 s, 52°C for 45 s, 72°C for 1 min, and a final extension at 72°C for 10 min. A total of 20 µl of final PCR product was separated in a 1.5% agarose gel, stained with ethidium bromide, and photographed (Fig. 1).
Figure 1. PCR amplification of lamprey NT cDNA.
Gel electrophoresis of PCR products from lamprey spinal cord cDNA yielded single bands at the expected sizes in base pairs (bp) for each target gene. No band was observed in the PCR products of the no template control (NTC). The neuritrophin (NT) product was 91 bp, HPRT 1 was 68 bp, 18S rRNA was 75 bp and GAPDH was 72 bp. Molecular weight marker (Mk) was 1 Kb Plus (Invitrogen). Lanes are labeled as follows: #1 - 18S rRNA, #2 – NTC, #3 – NT, #4 – NTC, #5 – GAPDH, #6 – NTC, #7 - NTC, #8 – HPRT 1.
Differences between samples due to material losses, differences in RT yields and PCR inhibition were compensate for. Normalization included an endogenous control gene. Although there is no universal control gene, expressed at a constant level under all conditions and in all tissues, we tested the well known endogenous control genes GAPDH, HPRT 1 and 18S rRNA, in our preliminary experiments and found that GAPDH had constant expression in all the samples (data not shown) and was therefore selected as the best endogenous control gene.
Quantitative RT-PCR
Quantitative real-time PCR was performed at the Quantitative PCR Facility of Virus and Molecular Core Services of the University of Pennsylvania using an ABI PRISM 7500 Fast Real-Time PCR System (Applied Biosystems) under 9600 Emulation Mode using the following conditions: 50°C for 2 min, 95°C for 10 min, 40 cycles of 95°C for 15 s, 60°C for 1 min and 1 cycle 95°C for 15 s, 60°C for 1 min, 95°C for 15 s, 60°C for 15 s. The PCR reagents, except primers, were from the Power SYBR® Green PCR Master Mix (Applied Biosystems). The PCR for the target (NT) and endogenous control (GAPDH) were performed in separate tubes in triplicate on cDNA samples in a MicroAmp Optical 96-well reaction plate (Applied Biosystems) according to the manufacturer’s instructions. A Power SYBR® Green PCR Master Mix was mixed with 200 nM forward and reverse primer for NT (300nM for GAPDH) and 10 ng cDNA (100 ng cDNA for NT). From this mixture, an aliquot of 20 µl was placed in each of three wells. Each plate contained a no-template control (NTC), in which DEPC water replaced the cDNA, as well as 5 serial concentrations of cDNA standard to allow calculation of a standard curve.
Standard curve construction
Standard curves were prepared for both the target and the endogenous reference. Standard curves were based on real-time PCR of known quantifies of cDNA synthesized from spinal cord RNA containing the sequences of interest. Each standard curve was generated based on five 10-fold serial dilutions of a cDNA (100, 10, 1, 0.1, 0.01 ng), quantified by a spectrophotometer. The reaction kinetic was represented by an amplification curve, in which a region where the fluorescent increases exponentially was observed. For each gene, the PCR cycle number at which the fluorescence crosses a threshold (CT value) was correlated to the amount of starting template by constructing a standard curve of CT values plotted against the logarithm of input DNA copy quantity (log amoles). The standard curves for all the genes analyzed were linear over a range of at least 0.00096–29.29.4 amoles with a linear correlation coefficient > 0.987.
Data analysis
A relative standard curve method was used to quantify the different time-point NT mRNAs. NTC and DNA standards were run in the same plate with cDNA from injured spinal cord of each of the different sample regions (rostral, 10 mm caudal and caudal) at different times after injury. For each sample, the amount of target and endogenous reference was determined from the appropriate standard curve. The data were analyzed as described in “Relative Quantification Getting Started Guide (Applied Biosystems). Results were analyzed by two-way ANOVA with Bonferroni post-hoc tests using Prism, version 5 (GraphPad, USA). Experiments were performed four times, with each assay run in triplicate, and the results were expressed as the fold-difference in the level of normalized NT mRNA expression in control spinal cord and after transection.
Lectin histochemistry
To identify microglial cells, wholemounts were labeled with GSA isolectin I-B4 after neurotrophin - labeled cells were revealed colorimetrically by in situ hybridization. The spinal cord wholemounts were washed in PBS and incubated with Fluorescein-labeled GSL I – isolectin B4 (5µg/ml Griffonia (Bandeiraea) simplicifolia lectin I GSL I-B4, Vector), which is a specific marker for microglia and labels D-galactose residues that are expressed by both resting and activated microglial cells (Streit and Kreutzberg, 1987, Streit, 1990, Boya et al., 1991). Each step was followed by washing three times for 10 min with PBS. Wholemounts and sections were coverslipped in VECTASHIELD® Mounting Medium (Vector, USA), and observed under a Carl Zeiss fluorescence microscope. Control for lectin staining consisted of (a) treating tissue prior to staining with α-galactosidase from coffee beans (Sigma) at 1 u/ml in phosphate-citrate buffer (pH 5.1) for 2 hours at 37°C; and (b) incubating with GSA I-B4 in the presence of 0.1 M melibiose (6-0-α-D-galactopyranosyl-D-glucose) to saturate lectin binding sites and prevent interaction with sugars of tissue components.
Cell counting and determination of microglia numbers
The numbers of I-B4 and NT-positive I-B4-labeled cells were counted in the transected lamprey spinal cord and in control lamprey at different time points (14 and 30 days and 4 months post-transection). This was achieved by capturing five (approximately 210 µm × 180 µm) adjacent fields of view in the area of the highest microglia density at a magnification of × 400 approximately 0.5 mm caudal (or rostral) to the center of the site of transection of the spinal cord of each animal. Our preliminary observation indicated that observed numbers of I-B4-labeled microglial cells and NT–labeled microglial cells were similar in caudal and rostral parts of transected spinal cord (up to 20 mm from the transection site). Therefore, we averaged the cell counts from rostral and caudal parts and presented them as one total cell count.
Statistics
The statistics were performed using Prism, version 5 (GraphPad, USA) applying a two way ANOVA followed by Bonferroni post-test to analyze changes in the number of NT-positive GSA I-B4-labeled cells after 14 and 30 days and 4 months post-lesion and to compare numbers of NT-positive GSA I-B4-labeled cells at each time point to the number of GSA I-B4-labeled cells. Results are presented in bar graphs as means ± standard deviation.
Results
Cloning of lamprey neurotrophin and its high-affinity Trk receptors
The ability of exogenous and endogenous neurotrophins to enhance axonal regeneration and/or prevent neuronal death after spinal cord injury has been documented in mammalian CNS but not yet in lamprey. Therefore, we cloned the lamprey neurotrophin receptor homologues and lamprey neurotrophin by RT-PCR. Homology search by BLAST software on NCBI distinguished sea lamprey genes for two putative high affinity neurotrophin receptors, Trk 1 and Trk 2. These encode proteins that are highly homologous (100% amino acid identity) to the previously cloned Trk 1 and Trk 2 of the river lamprey Lampetra fluviatilis (Hallbook et al., 1998) but only 47% amino acid identity to the chicken and Xenopus type 3 neurotrophin tyrosin kinase receptors and 50% amino acid identity to the human TrkC. Homology search by BLAST also revealed a putative lamprey neurotrophin that was highly homologous to the previously cloned neurotrophin of the river lamprey (Hallbook et al., 1998) (85% amino acid identity) but had only 40% amino acid identity to chicken, Xenopus and human neurotrophin 3 (NT3).
Localization of neurotrophin mRNA expression in control and transected spinal cord
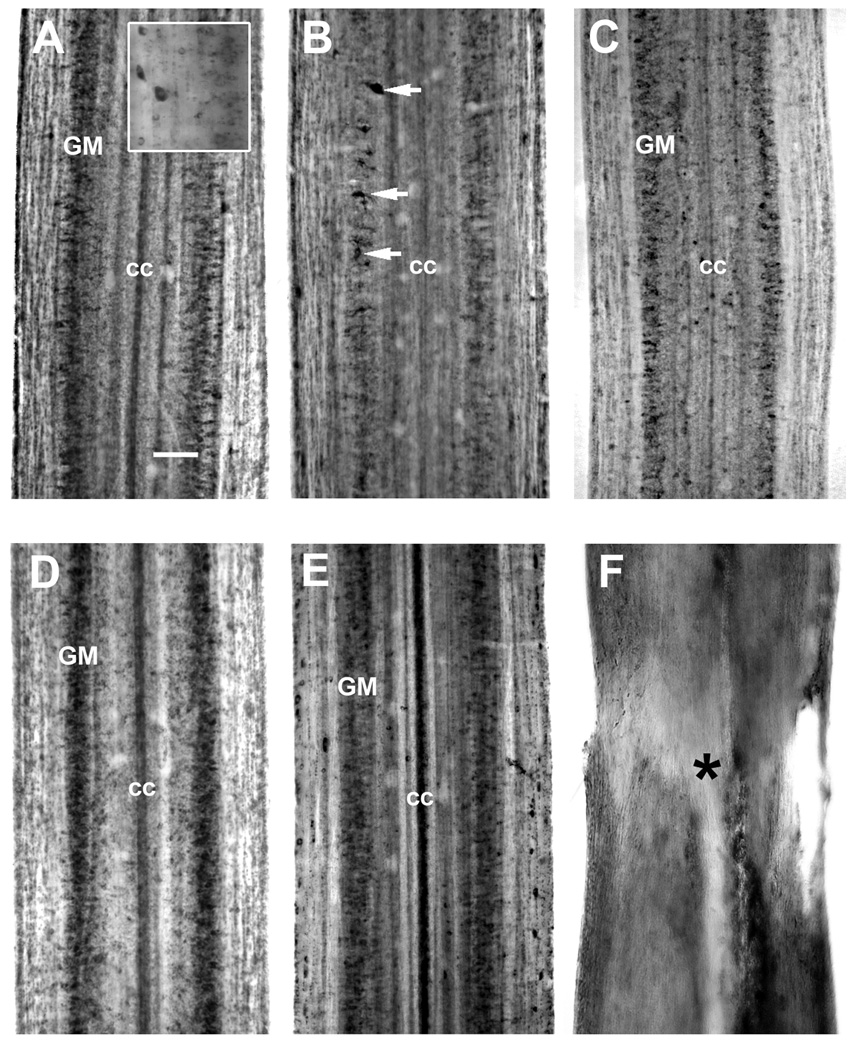
NT mRNA was moderately expressed throughout the spinal cord of control animals in medium sized neurons along the lateral grey matter (Fig. 2A). The spatiotemporal expression of NT mRNA was analyzed by wholemount in situ hybridization at 14 and 30 days after spinal cord transection, consistent with the times when axons are dying back and then regenerating through the proximal stump, respectively. At 14 days post-transection, NT mRNA expression was downregulated in the rostral spinal cord close to the transection site and only a few neurons continued to express NT mRNA (Fig. 2B). The intensity of NT-specific in situ hybridization signal and the number of NT-producing neurons were noticeably reduced caudal to the transection (Fig. 2C). However, expression of NT mRNA more than 20 mm caudal to the transection was not changed significantly (data not shown). By 30 days post-transection, NT mRNA expression was present in medium sized neurons of the spinal gray matter in a pattern resembling that in the uninjured control. NT mRNA expression level appeared slightly upregulated both rostral (Fig. 2D) and caudal to the transection (Fig. 2E). The transection site was easily identified in wholemount spinal cord preparations by the narrowing of the spinal cord and widening of the central canal (Fig. 2F). Neurons were absent in the transection zone(Lurie and Selzer, 1991a, b, Lurie et al., 1994) and NT mRNA signal was generally not detected at the lesion site (Fig. 2F).
Figure 2. Expression of neurotrophin mRNA in the spinal cord after transection.
In situ hybridization was performed in spinal cord wholemounts. Rostral is top in all micrographs. The entire width of the cord is seen. A, in situ hybridization in wholemounted control spinal cord shows moderate intensity labeling for lamprey neurotrophin (NT) in the medium size neurons of the spinal gray matter (GM). The ependymal cells lining the central canal (cc) may also express NT. Insert – high magnification of NT-expressing neurons. B and C. NT mRNA expression 14 days after spinal cord transection. NT mRNA expression was downregulated up to 10 mm rostral (B) and caudal (C) to transection in the neurons of the spinal gray matter (GM). While some cells (white arrows) continued expressing of NT mRNA, many other cells stopped expressing it. Downregulation of NT mRNA expression was more prominent in spinal cord rostral to transection. D and E. By four weeks post-transection, NT mRNA expressing neurons of the spinal gray matter (GM) were found both rostral (D) and caudal (E) to the transection site and expression was upregulated in rostral part near the lesion site compared to control animals (A). The intensity of labeling was also above control levels up to 10 mm caudal to the transection. cc – central canal. F. The absence of NT mRNA hybridization signal in the transection zone after SCI. The transection site (asterisk) was easily identified in wholemount spinal cord preparations by the narrowing of the spinal cord and widening of the central canal. High background seen on these frames (B – F) is due to nonspecific binding of label to cellular debris and blood cells entering the transection site. Scale bar, 100 µm (A).
Quantitation of temporal and spatial changes in NT expression after spinal cord transection
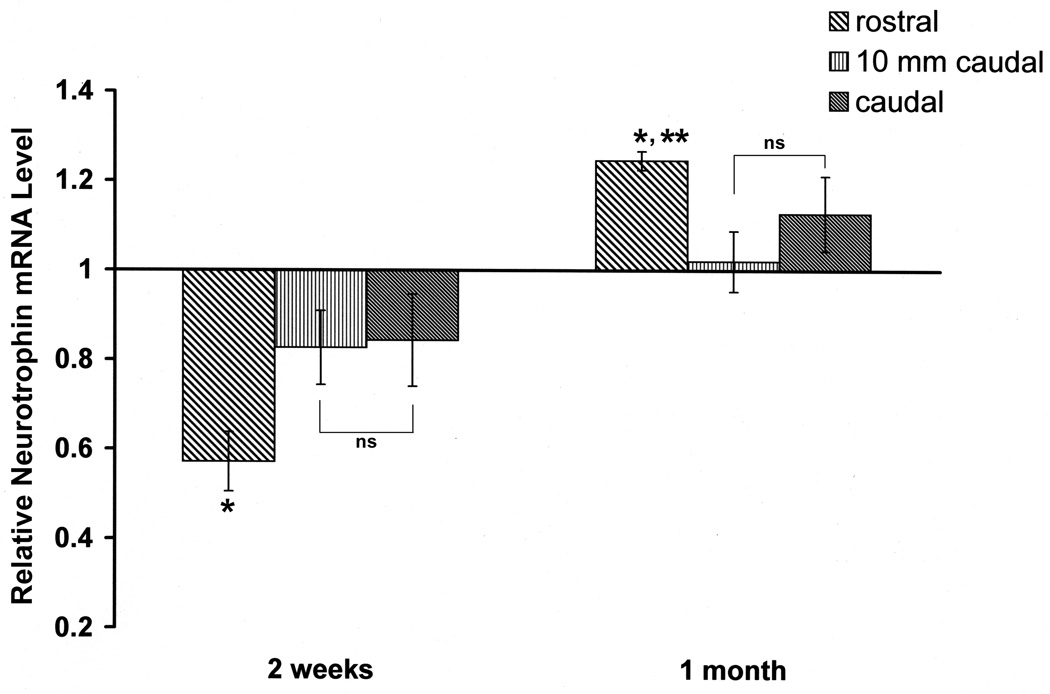
The in situ expression data were confirmed by quantitative PCR, which showed downregulation of NT expression at 14 days and slight upregulation at 30 days after spinal transection. NT mRNA was quantified in the sections of spinal cord representing: 1, the injury epicenter and cord just rostral to the transection; 2, at 10 mm caudal to the transection; 3, the rest of the cord caudally. The changes in NT mRNA expression in these regions are shown in Figure 3. The data were expressed as the fold difference in the level of NT mRNA expression compared to control spinal cord. At 14 days post-transection, NT mRNA was decreased 43% rostral to the injury, 17% 10 mm caudal to the transection and 16% beyond 10 mm caudal to the transection. These changes were significant at P<0.05 by two-way ANOVA. However, by 30 days after SCI, NT mRNA expression was increased 25% over of control levels rostral but not caudal to the injury.
Figure 3. Relative changes of mRNA expression of NT post-transection in spinal cord rostral to the lesion (including the transection site), the first 10 mm caudal to the transection, and further caudal tissue.
The time course of relative changes of mRNA expression in spinal cord after injury was determined by quantitative real-time RT–PCR. The level of NT mRNA expression was calculated after normalizing against the GADPH mRNA level in each sample and is presented as the fold-difference in the level of NT mRNA expression after transection compared with the untransected control sample, which was assigned a value of 1. Significantly different: * rostral vs 10 mm caudal 2 weeks - P < 0.001; * rostral vs caudal 2 weeks - P < 0.001; * rostral vs 10 mm caudal 1 month - P < 0.001; ** rostral vs caudal 1 month - P <0.05; non-significantly (ns) different: 10 mm caudal vs caudal - P > 0.05.
Identification of the non-neuronal NT-expressing cell types in lamprey spinal cord after injury
During our examination of neurotrophin expression in the naïve and transected spinal cord using in situ hybridization, we detected populations of small cells that expressed NT (Figs. 4 and 5). They were located on the surface of the spinal cord, although some of them were located just below the cord surface. We hypothesize, based on these cells sizes and shapes that they may be microglial cells (Streit and Kreutzberg, 1987, Streit, 1990, Boya et al., 1991).
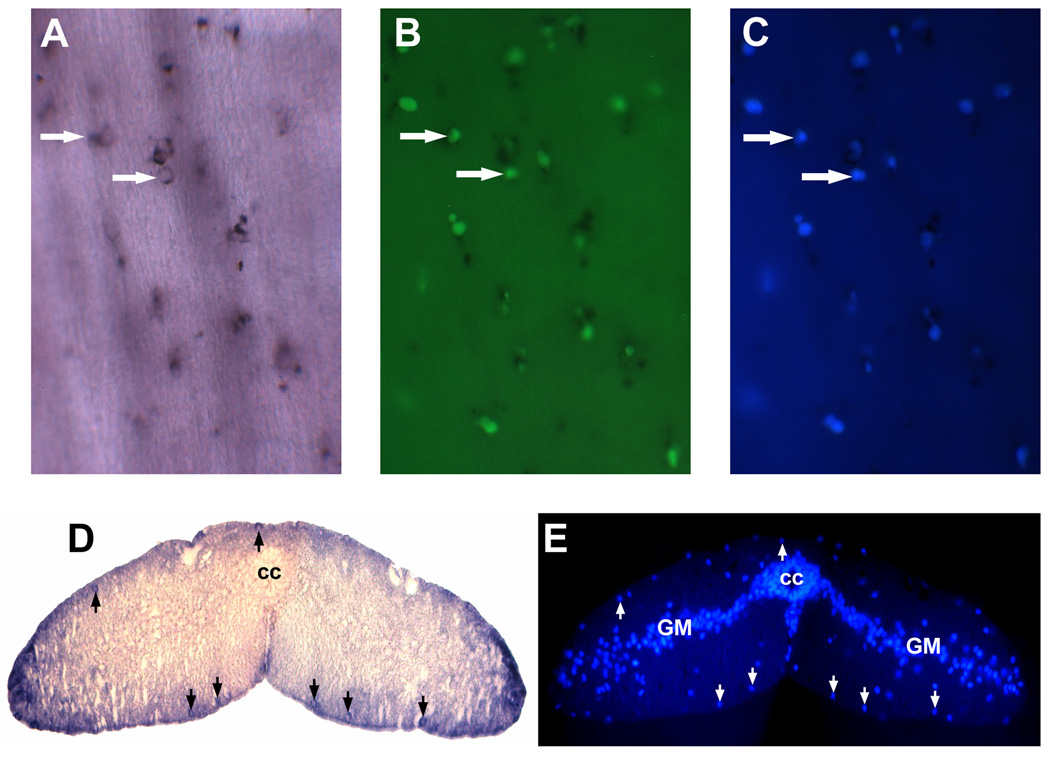
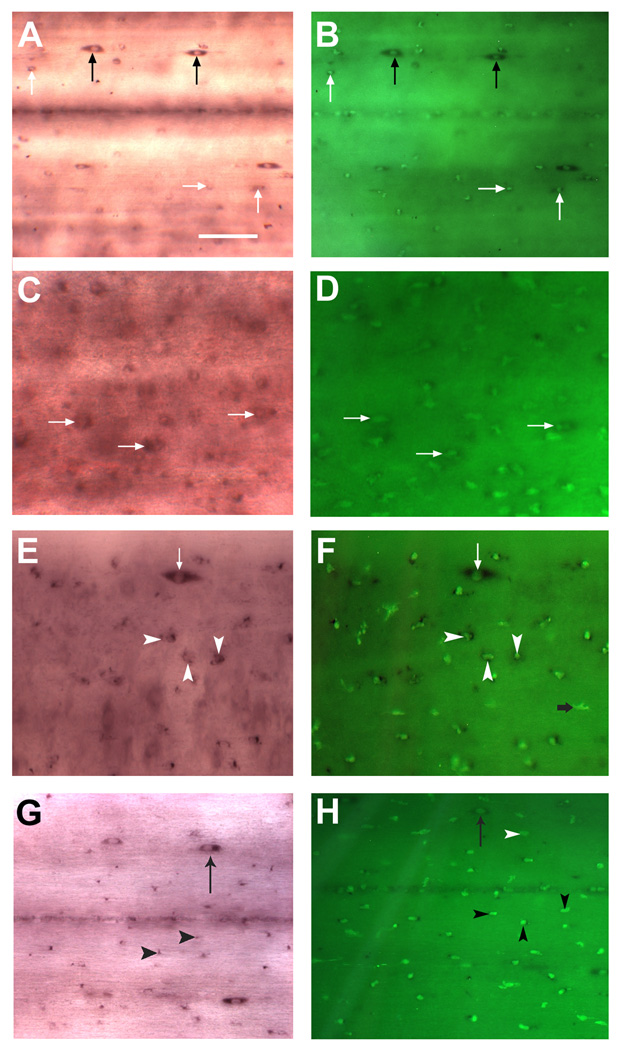
Figure 4. Identification of non-neuronal NT-expressing cells after spinal cord transection.
A–C, The same NT-expressing cells (revealed by wholemount in situ hybridization; white arrows, panel A) were also labeled with IB4 lectin (white arrows in B) and DAPI(white arrows in C); D and E showed that NT-expressing cells were located close to the spinal cord surface (black arrows in D) and were stained simultaneously with DAPI (white arrows in E) that excluded possibility of non-specific NT staining of non-cellular structures. Spinal gray matter (GM). Central canal (cc).
Figure 5. Microglial expression of NT after spinal cord transection.
A, In situ hybridization in control spinal cord revealed the presence of two distinct populations of NT-expressing cells - the small elliptic NT-expressing cells (black arrows) and small cells with rounded bodies (no larger than 5 – 10 um) and without obvious neuronal morphology (white arrows). Scale bar, 20 µm. B, isolectin B4 histochemistry on the same preparation revealed several NT–expressing, lectin-labeled cells in control spinal cord (white arrows). Note that elongated NT - expressing cells (black arrow) do not labeled with IB4 and therefore not a microglial cells. C, Two weeks after spinal cord transection, increased numbers of small, rounded cells (white arrows), reminiscent of activated microglia/macrophages, were labeled with the NT probe. High background seen on this frame (C) is due to nonspecific binding of label to cellular debris and blood cells entering the transection site. (D). A dense accumulation of reactive macrophages/microglial cells, as evidenced by increasing IB4 lectin reactivity, was detected in spinal cord two weeks after SCI. Most of the lectin-labeled cells also co-expressed NT mRNA (white arrows). E and F, 4 weeks after transection, numerous small, round macrophages/microglial cells (white arrowheads) could be detected by labeling with IB4 lectin, and they also were labeled with the NT probe. On the other hand, larger, elongated NT-expressing cells were never labeled with IB4 lectin (white arrows). G and H, macrophages/microglial expression of NT four months after spinal cord transection. Localization and shape of NT-expressing microglia/macrophages resembled that at one month. H, numerous macrophages/microglial cells (black arrowheads) as judged by intense IB4 lectin reactivity were seen in the spinal cord 4 months after injury. White arrowhead depicted microglia/macrophages cell do not expressing NT.
Identification of microglial cell in lamprey spinal cord
Fluorescein-labeled GSL I–isolectin B4, which is commonly used to identify macrophages/microglial cells in spinal cord and brain of mammals (Streit and Kreutzberg, 1987, Streit, 1990, Boya et al., 1991) was used here to determine whether the neurotrophin-labeled cells in the lamprey spinal cord are microglial cells. Lectin histochemistry was performed after NT in situ hybridization and cell nucleus was stained with DAPI to distinguish cells from autofluorescence artifacts (Fig. 4A–C). We showed that the same NT-expressing cells were also labeled with IB4 lectin and DAPI (Fig. 4A–C), therefore, identified these NT-expressing cells as microglia. NT-expressing cells were located close to the spinal cord surface and stained simultaneously with DAPI that excluded possibility of non-specific NT staining of non-cellular structures(Figs. 4D and E). The specificity of the lectin histochemical staining was confirmed by the complete elimination of staining if the isolectin B4 was pre-incubated in the presence of 0.1 M melibiose, or if the tissue was treated with α-galactosidase from coffee beans (Sigma) prior to staining (data not shown).
Microglial cell activation and upregulation of NT mRNA expression in microglia after spinal cord transection
Fluorescein-labeled IB4 was used to analyze the microglial response in the control spinal cord and caudal and rostral to the lesion site at 14 days, 30 days and 4 months after transection.
In control cord, two distinct populations of NT-expressing cells were found on the surface of control spinal cord or just below the cord surface. First was a population of a small cells with rounded bodies (no larger than 5 – 10 µm) and without obvious neuronal morphology (Fig. 5A). Because these NT-expressing cells were also labeled with IB4 isolectin (Fig. 5B), we identified these NT-expressing cells as microglia. In addition, several lectin-positive but NT-negative cells were seen in control spinal cord, indicating that microglial cells might be present in control, untransected spinal cord that do not express NT (Fig. 5B). We also detected few of the elliptic NT-expressing cells (Fig. 5A,B and E – H). These cells were bigger size and were not labeled by GSL I–isolectin B4 which indicated that they probably were small neurons or other non-neuronal cells but not microglia.
A detailed analysis of temporal changes of NT mRNA expression in microglial cells following injury is provided in Figure 5 and its legend. In the first two weeks post-transection, activated microglia was observed in the region rostral and caudal to the lesion center. These cells had very small rounded cell bodies and no cells larger than 5 µm or possessing obvious neuronal morphology were labeled with NT (Fig. 5C and D). During this time, the number of NT-expressing cells increased significantly 0.5–5 mm rostral and caudal to the injury site (Fig. 5C and Fig. 6B), accompanied by rapid accumulation of reactive microglial cells, as evidenced by increasing IB4 isolectin reactivity (Fig. 5D and Fig. 6A). Microglial NT-expression was further examined 30 days after spinal cord transection. The localization, shapes and numbers of IB4 isolectin-labeled microglial cells and also NT-positive microglia resembled those at 14 days (Figs. 5E and F). Four months after SCI, distribution of NT-expressing IB4 isolectin-labeled microglial cells was not changed compared to 14 and 30 days time points (Figs. 5G and H). However, the small spindle-shaped cells that we observed in control spinal cord (Fig. 5A) were detected again at 30 days and four months after SCI (Fig. 5E and G). They were NT-positive but because they were not labeled by IB4 lectin (Fig. 5F – H), they are likely to be small neurons and not microglia cells.
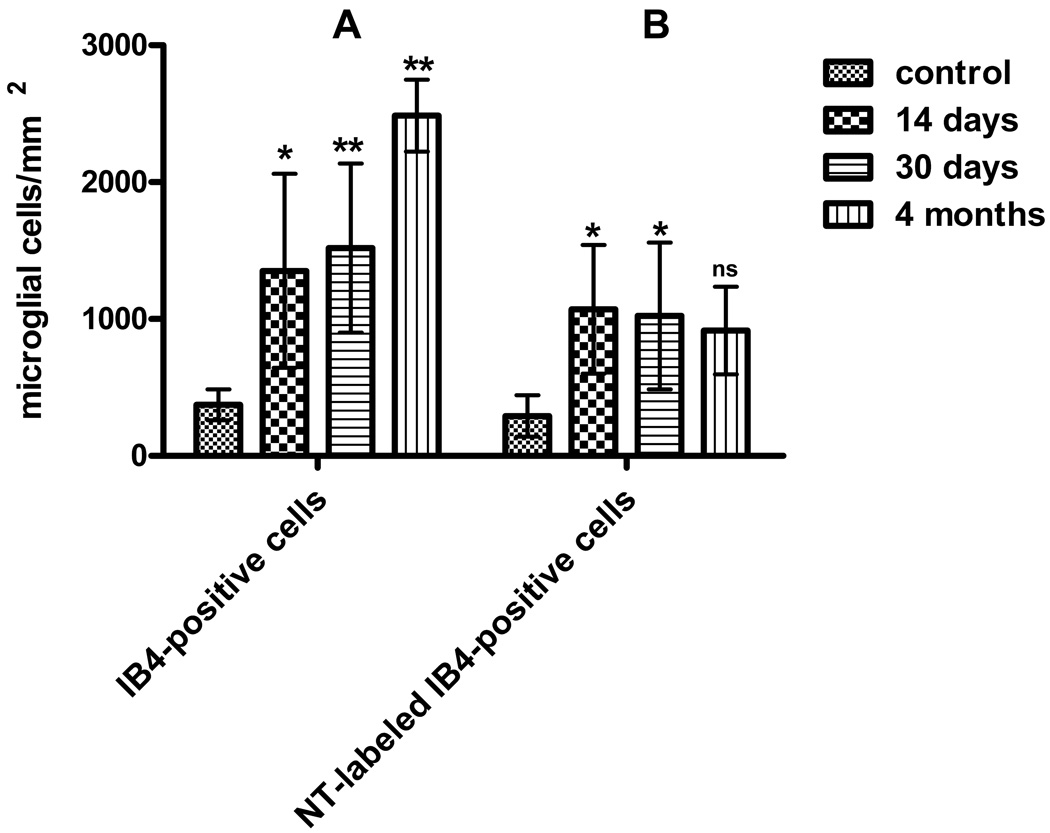
Figure 6. Microglial proliferation and NT expression in response to spinal cord transection.
A, Increase in the number of isolectin B4 -labeled microglial cells in the spinal cord between 0.5 and 5 mm rostral and caudal to the lesion site at 14, 30 days and 4 months post-lesion. P-values are indicated as follows: * P < 0.05 and ** P < 0.001 compare to control. Differences between 14 days and 30 days numbers are not significant. Differences between 14 days and 4 months and between 30 days and 4 months numbers are significant(P<0.01). B. NT expression in microglial cells post-transection. The numbers of NT-expressing microglial cells at 2, 4 weeks and 4 months post-transection are compared between control and lesioned spinal cord between 0.5 and 5 mm rostral and caudal to the lesion site. Control vs. 2 weeks: * P<0.05; control vs. 4 weeks: P<0.05; control vs. 4 months: P>0.05, ns, not significant; 2 weeks vs. 4 weeks: P>0.05, ns, not significant. Differences between 14 days and 4 months and between 30 days and 4 months numbers are not significant. Data are presented as means and standard deviations and analyzed using a two way ANOVA followed by a Bonferroni post-test.
The time course and dynamic of NT–expressing microglial cells in the spinal cord after injury
A significant 4-fold increase in total numbers of IB4 -labeled activated microglia cells was observed in transected spinal cord at 14 days (p<0.05, mean=1352/mm2) and 30 days after injury (p<0.05, mean=1519/mm2) as compared to control tissue (mean=374.4/mm2; Fig. 6A). However, the total numbers of activated microglia did not change significantly between 14 and 30 days after spinal cord transection (Fig. 6A). This microglia proliferation continued even 4 months after SCI (p<0.001, mean=2486/mm2), that constitute almost 7-fold increase compared to control cords (see Fig. 6A). In parallel with morphological signs of activation, reactive microglia appeared to upregulate NT expression after SCI (Fig. 6B). A significant accumulation of NT-expressing cells was seen two weeks after transection (p<0.05, mean=1071/mm2) and one month after SCI (p<0.05, mean=1023/mm2) as compared to control tissue (mean=292.3/mm2; Fig. 6B). However, after 4 months of survival, the numbers of NT-expressing cells in lamprey spinal cord decreased (mean=917/mm2, Fig. 6B).
Neuronal localization of Trk neurotrophin receptors mRNA in control and transected animals
Neurotrophins function through high-affinity tyrosine kinase (Trk) receptors to promote growth and survival of cells in the nervous system. To investigate the role of Trk receptors in the lamprey nervous system, we cloned two receptors, Trk 1 and Trk 2, which represent ancestral Trk genes in the early vertebrate lineage (Hallbook et al., 1998). In control animals Trk 1 and Trk 2 mRNAs were present in some reticulospinal neurons, including the Mauthner neurons (Mth), the B1, B3 and B4 neurons of the bulbar region, in the I1 and I2 neurons of the isthmic region (Fig. 7A and B), and in the M1 and M2 neurons of the mesencephalon (data not shown). Moreover, expressions of Trk 1 and Trk 2 were co-localized in almost all of these large reticulospinal neurons (Fig. 7C).
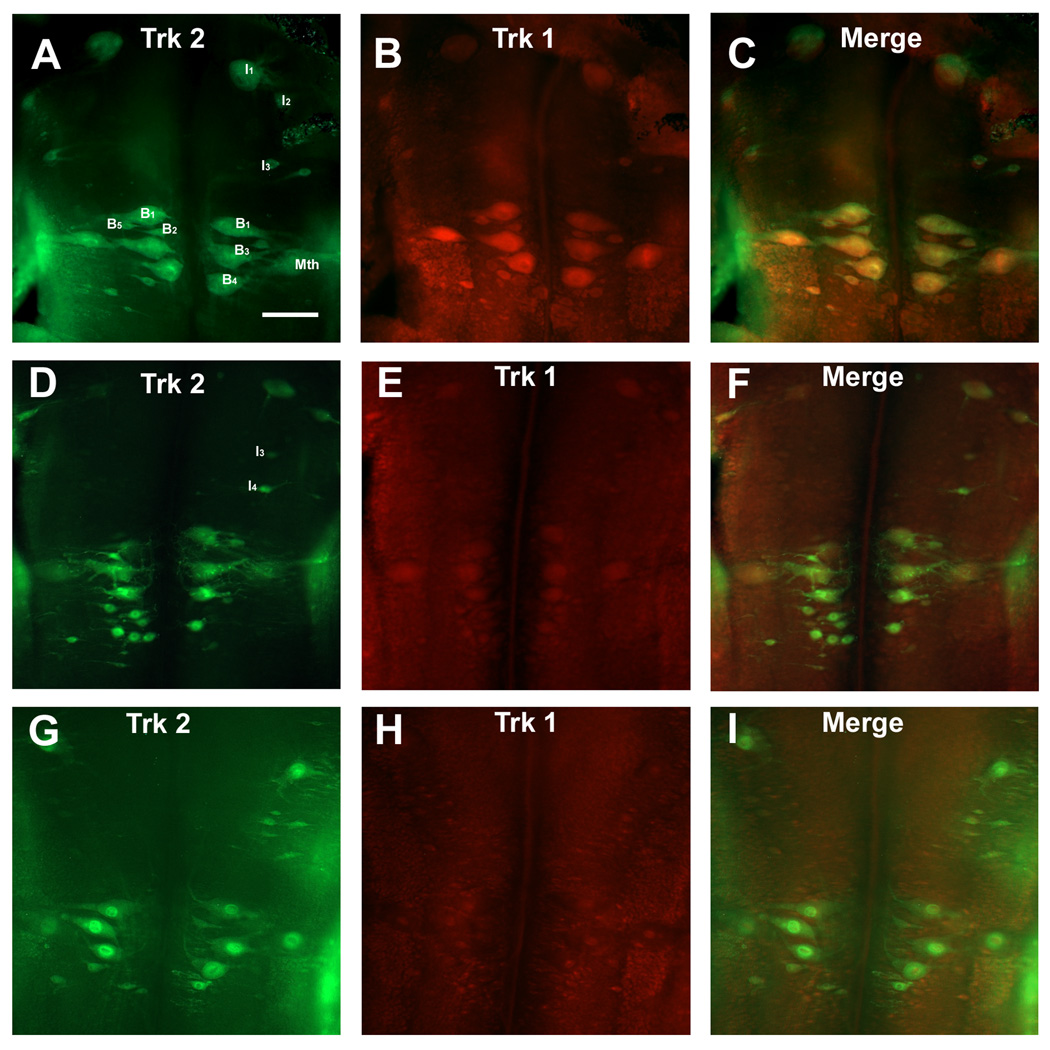
Figure 7. Expression of the Trk receptors in reticulospinal neurons after spinal cord transection.
A – C.In situ hybridization for Trk 1 and Trk 2 mRNA in control animals. The rhombencephalon shows strong Trk 1(B) and Trk 2(A) expression in the Mauthner neurons (Mth), in the I1 and I2 neurons and in B neurons of the bulbar region(B1, B3, B4, B5) and their expression co-localize in almost all reticulospinal neurons (C). Scale bar – 100 µm.
D – F. At two weeks post-transection, reticulospinal neurons downregulate Trk 1 expression while Trk 2 expression did not change. G – I. At four weeks after transection, many giant identified reticulospinal neurons express Trk 2 receptor whereas expression of Trk 1 was greatly diminished and almost undetectable.
Changes in Trk 1 and Trk 2 expression after spinal cord transection
In order to examine whether neurotrophin receptors might be involved in the regenerative response of spinal-projecting neurons, in situ hybridization for Trk 1 and Trk 2 mRNA was performed at 14 and 30 days post-transection.
The identified large reticulospinal neurons in the lamprey brainstem differ from one another in their regenerative abilities and could be divided in two broad categories – “good regenerators” (probability of regeneration greater than 50%) and “bad regenerators” (probability of regeneration less than 20%) (Jacobs et al., 1997). In control animals both categories of reticulospinal neurons expressed Trk 1 and Trk 2 receptors and their expressions co-localized in almost all reticulospinal neurons - both “good-regenerating” (e.g., B2 and B5 Müller cells) and “bad-regenerating” (Mth, I1, B1, B3 and B4 neurons; Fig. 7A – C). After spinal cord transection reticulospinal neurons tended to downregulate Trk 1 expression, while Trk 2 expression did not change (Fig. 7D – I). All giant identified reticulospinal neurons expressed Trk 2 at 14 and 30 days after axotomy (Fig. 7D and G), whereas expression of Trk 1 was greatly diminished and by 30 days, Trk 1 expression was almost undetectable (Fig. 7H). There were no consistent differences in expression patterns of Trk 2 receptor among individual identified reticulospinal neurons. Mauthner neurons, known to be a “bad regenerators,” continued to express Trk 2 mRNA at significant levels two weeks and four weeks after spinal cord transection (Fig. 7D and G). Identified neurons of the isthmic region (I1– I4) and bulbar region (B1–B5) co-expressed Trk 1 and Trk 2 mRNA only in control animals. This was because Trk 1-labeled I neurons were never seen in transected animals. However, at two weeks post-transection, Trk 2 mRNA fluorescent signal was increased in B neurons (B1, B3, B4; Fig. 7D). Four weeks after SCI increased levels of Trk 2 mRNA fluorescent signal was detected in axotomized I neurons (I1, I3 and I4; Fig. 7G). Thus there was a tendency for axotomy to be followed by decreased expression of Trk 1 and increased expression of Trk 2.
Discussion
Sea lampreys may have one neurotrophin, but at least two Trks
We cloned sea lamprey (Petromyzon marinus) NT and its receptors Trk 1 and Trk 2 and showed that they have very high levels of sequence homology with corresponding molecules in river lamprey (Lampetra fluviatilis) (Hallbook et al., 1998). Moreover our probe of a partial sequence genomic library suggested that there were no other NT sequences. The distribution and expression levels of Trks were similar to those reported in river lamprey (Hallbook et al., 1998). As in river lamprey, NT in situ hybridization showed low to moderate levels of expression in spinal cord (Hallbook et al., 1998), although the expression levels in our larval sea lampreys were higher than those in adult river lampreys (Hallbook et al., 1998). Similarly, in mammals, levels of NGF, BDNF and NT-3 were higher in embryonic than in adult spinal cords (Nakamura and Bregman, 2001).
Responses of NT and Trk expression to spinal cord transection
Identified giant reticulospinal neurons in the lamprey brainstem differ from one another in their regenerative abilities and could be divided in two broad categories - “good regenerators,” which have probabilities of regeneration greater than 50% and “bad regenerators,” whose regeneration rates are under 20% (Jacobs et al., 1997). We hypothesize that preferential expression of neurotrophin receptors in “good regenerators neurons” would enhanced their regenerating abilities similarly to mammals, where better ability of the PNS axons to regenerate compared to CNS correlates with expression of neurotrophins and their receptors post-axotomy (Ip et al., 1991, Ebadi, 1997, Lee et al., 2001, Chen et al., 2007, Priestley, 2007). While mRNAs for both Trk 1 and Trk 2 receptors were prominently expressed in control animals, only Trk 2 expression was observed after SCI, remaining unchanged at 2 and 4 weeks post-transection. The functional significance of Trk 1 downregulation after SCI is not known, but the constancy of Trk 2 expression in lamprey reticulospinal neurons is similar to the lack of change observed in TrkB and TrkC expression in the axotomized corticospinal and rubrospinal neurons of rat (Giehl et al., 2001, Liebl et al., 2001). Both “good regenerators” and “bad regenerators” neurons in lamprey brain expressed Trk 2 after spinal cord transection. Our data therefore, could be interpreted that neurotrophin might not be directly involved in modulating the regenerative abilities of reticulospinal neurons in lampreys.
Another line of evidences pointed against direct neurotrophin involvement in the lamprey spinal cord regeneration, was our experiments conducted to study the injury-induced neurotrophin response in the lamprey spinal cord after transection. Quantitative RT-RCR and in situ hybridization data demonstrated that at 2 weeks post-transection, NT expression was downregulated in neurons of the spinal cord but had returned to pre-transection levels by 4 weeks post-transection. Unlike NT expression patterns of lamprey, expression of NTs in the adult rat increased transiently after spinal cord injury, with the numbers of NGF-, BDNF- and NT-3-expressing cells peaking between 6 hours and 7 days post-injury (Ikeda et al., 2001, Nakamura and Bregman, 2001, Widenfalk et al., 2001). A closer parallel to the lamprey NT expression pattern was seen in the neonatal rat spinal cord, where NGF, BDNF and NT-3 mRNA expression did not change early (2 hour – 24 hours) after injury and either decreased at 1 and 2 weeks after injury (Nakamura and Bregman, 2001) or did not change (Widenfalk et al., 2001). The present study involved large larval lampreys, and it is not known whether the pattern of NT expression would be different in the adult.
Microglia cells activation after spinal cord injury
Two populations of non-neuronal cells have been previously described in the lamprey spinal cord: ependymal cells surrounding the central canal, and glial cells located mostly in gray matter (Schultz et al., 1956, Bertolini, 1964). In lampreys, glial cells express keratin intermediate filaments (Merrick et al., 1995) rather than glial fibrillary acidic protein (GFAP), the intermediate filament found in the astrocytes of most vertebrates. Lamprey nervous system lack myelin (Bullock et al., 1984) and therefore, oligodendrocytes are absent in lamprey. We reported previously (Shifman and Selzer, 2007, Shifman et al., 2009) detection of a new population of non-neuronal cells, microglia, in the lamprey spinal cord. In this report we confirmed this finding by using lectin to identified macrophages/microglial cells. GSL I – isolectin B4 (Griffonia (Bandeiraea) simplicifolia lectin I) is a specific marker for microglia and labels D-galactose residues that are expressed by both resting and activated microglial cells (Streit and Kreutzberg, 1987, Streit, 1990, Boya et al., 1991).
Microglia in the mammalian central nervous system exist in three distinct forms known as amoeboid, ramified and reactive/activated microglia, which serve different functional roles(Napoli and Neumann, 2009, Graeber and Streit, 2010, Prinz and Mildner, 2011). In response to injury, quiescent ramified microglia transform into active ‘brain macrophages’ otherwise known as reactive microglia (Streit, 1996, 2002a, Graeber, 2010, Loane and Byrnes, 2010). A highly characteristic feature of microglial activation and reactive microgliosis induced by acute neural injury is the massive, but usually transient expansion of the microglial cell population(Streit, 2002b, a, Graeber and Streit, 2010). Activated microglia is involved in acute CNS injury and microglial activation has been documented in the mammalian spinal cord after injury(Dusart and Schwab, 1994, Koshinaga and Whittemore, 1995, Popovich et al., 1997, Leme and Chadi, 2001, Jones and Tuszynski, 2002, Morino et al., 2003, Sroga et al., 2003, Gomes-Leal et al., 2004, Wu et al., 2005, Hains and Waxman, 2006, Mueller et al., 2006). Similar microglial reaction was described in the present study, a marked elevation and expansion of isolectin-B4 microglial labeling after SCI in lampreys. The microglial reaction appeared to continue for at least another four months (Fig. 6). Microglial activation is characterized by a number of features, including a morphological transformation of individual microglia, induction of a wide range of myeloid markers, trophic factors, cytokines, free radicals and nitric oxide, and the acquisition of a phagocytic phenotype (Streit, 1994, 1996, Xu et al., 1998, Streit, 2002b, a, Hashimoto et al., 2005, Ladeby et al., 2005, Mueller et al., 2006). Macrophages/microglial cells may have important functions for axonal regrowth by secreting axonal growth inhibiting and/or promoting molecules (Streit, 1994, 1996, Rabchevsky and Streit, 1997, Dougherty et al., 2000, Batchelor et al., 2002, Streit, 2002b, a, Sandvig et al., 2004). Similar data showing expression of Sema3 and RGM in microglial cells were reported by us previously (Shifman and Selzer, 2007, Shifman et al., 2009). In this report we described a population of the lamprey microglial cells that expressed neurotrophin mRNA after SCI and steady upregulation of NT mRNA expression continued for at least one month after injury. Our findings are in agreement with previous reports describing increased numbers of macrophages/microglia cells expressing neurotrophins after spinal cord injury (Dougherty et al., 2000, Ikeda et al., 2001, Widenfalk et al., 2001, Brown et al., 2004).
In the normal adult mammalian CNS, microglia is distributed throughout the neural parenchyma and constitutes a cell population that shows a slow turnover from either proliferation of the resident microglia or recruitment of bone marrow-derived cells through an intact blood– brain barrier (Graeber and Streit, 2010, Prinz and Mildner, 2011). In the injured CNS, microglia/macrophages were activated and increased in numbers from either proliferation of the resident microglia (distributed throughout the neural parenchyma) or recruitment of bone marrow-derived cells through a blood–brain barrier (Streit, 2002a, Graeber and Streit, 2010, Loane and Byrnes, 2010). If lamprey microglia/macrophages cells originated from proliferation of the resident microglia located in neural parenchyma, we would find them throughout the whole spinal cord. However, NT-expressing cells were almost exclusively located on the surface of lamprey spinal cord that might point to their origin as blood-born cells. Lamprey spinal cord does not have blood vessels in the parenchyma (Schultz et al., 1956) and therefore, microglia/macrophages cells originated from blood would be restricted to spinal cord external layer. Origin and nature of the lamprey microglia/macrophage cells warrant additional investigation that will be goal of our next article.
We can only speculate about the functions of NT expressed by microglia after spinal cord transection. Because NT is a diffusible protein, it is possible that it can affect regenerating axons that are located far away from spinal cord surface. Moreover, because expression of NT in spinal cord parenchyma was down-regulated after SCI (see above), enhanced microglial NT expression may somehow counterbalance NT concentration decreases in spinal cord.
Acknowledgements
This work was supported by NIH grant R01 NS38537 to MES
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Barreiro-Iglesias A, Villar-Cheda B, Abalo XM, Anadon R, Rodicio MC. The early scaffold of axon tracts in the brain of a primitive vertebrate, the sea lamprey. Brain Res Bull. 2008;75:42–52. doi: 10.1016/j.brainresbull.2007.07.020. [DOI] [PubMed] [Google Scholar]
- Batchelor PE, Porritt MJ, Martinello P, Parish CL, Liberatore GT, Donnan GA, Howells DW. Macrophages and Microglia Produce Local Trophic Gradients That Stimulate Axonal Sprouting Toward but Not beyond the Wound Edge. Mol Cell Neurosci. 2002;21:436–453. doi: 10.1006/mcne.2002.1185. [DOI] [PubMed] [Google Scholar]
- Benito-Gutierrez E, Garcia-Fernandez J, Comella JX. Origin and evolution of the Trk family of neurotrophic receptors. Molecular and Cellular Neuroscience. 2006;31:179–192. doi: 10.1016/j.mcn.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Bertolini B. Ultrastructure of the Spinal Cord of the Lamprey. J Ultrastruct Res. 1964;11:1–24. doi: 10.1016/s0022-5320(64)80089-7. [DOI] [PubMed] [Google Scholar]
- Bibel M, Barde Y-A. Neurotrophins: key regulators of cell fate and cell shape in the vertebrate nervous system. Genes Dev. 2000;14:2919–2937. doi: 10.1101/gad.841400. [DOI] [PubMed] [Google Scholar]
- Boya J, Carbonell AL, Calvo JL, Borregon A. Microglial cells in the central nervous system of the rabbit and rat: cytochemical identification using two different lectins. Acta Anat (Basel) 1991;140:250–253. doi: 10.1159/000147064. [DOI] [PubMed] [Google Scholar]
- Boyd JG, Gordon T. Neurotrophic factors and their receptors in axonal regeneration and functional recovery after peripheral nerve injury. Mol Neurobiol. 2003;27:277–324. doi: 10.1385/MN:27:3:277. [DOI] [PubMed] [Google Scholar]
- Brown A, Ricci MJ, Weaver LC. NGF message and protein distribution in the injured rat spinal cord. Experimental Neurology. 2004;188:115–127. doi: 10.1016/j.expneurol.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Bullock TH, Moore JK, Fields RD. Evolution of myelin sheaths: both lamprey and hagfish lack myelin. Neurosci Lett. 1984;48:145–148. doi: 10.1016/0304-3940(84)90010-7. [DOI] [PubMed] [Google Scholar]
- Chen Z-L, Yu W-M, Strickland S. Peripheral Regeneration. Annual Review of Neuroscience. 2007;30:209–233. doi: 10.1146/annurev.neuro.30.051606.094337. [DOI] [PubMed] [Google Scholar]
- Cohen AH, Mackler SA, Selzer ME. Behavioral recovery following spinal transection: functional regeneration in the lamprey CNS. Trends in Neuroscience. 1988;11:227–231. doi: 10.1016/0166-2236(88)90131-2. [DOI] [PubMed] [Google Scholar]
- Davis GR, McClellan AD. Extent and time course of restoration of descending brainstem projections in spinal cord-transected lamprey. J Comp Neurol. 1994;344:65–82. doi: 10.1002/cne.903440106. [DOI] [PubMed] [Google Scholar]
- Davis GRJ, McClellan AD. Time course of anatomical regeneration of descending brainstem neurons and behavioral recovery in spinal-transected lamprey. Brain Res. 1993;602:131–137. doi: 10.1016/0006-8993(93)90252-i. [DOI] [PubMed] [Google Scholar]
- Dougherty KD, Dreyfus CF, Black IB. Brain-Derived Neurotrophic Factor in Astrocytes, Oligodendrocytes, and Microglia/Macrophages after Spinal Cord Injury. Neurobiology of Disease. 2000;7:574–585. doi: 10.1006/nbdi.2000.0318. [DOI] [PubMed] [Google Scholar]
- Dusart I, Schwab ME. Secondary cell death and the inflammatory reaction after dorsal hemisection of the rat spinal cord. Eur J Neurosci. 1994;6:712–724. doi: 10.1111/j.1460-9568.1994.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Ebadi M, Bashir RM, Heidrick ML, Hamada FM, Refaey HE, Hamed A, Helal G, Baxi MD, Cerutis DR, Lassi NK. Neurotrophins and their receptors in nerve injury and repair. Neurochem Int. 1997;30:347–374. doi: 10.1016/s0197-0186(96)00071-x. [DOI] [PubMed] [Google Scholar]
- Giehl KM, Rohrig S, Bonatz H, Gutjahr M, Leiner B, Bartke I, Yan Q, Reichardt LF, Backus C, Welcher AA, Dethleffsen K, Mestres P, Meyer M. Endogenous Brain-Derived Neurotrophic Factor and Neurotrophin-3 Antagonistically Regulate Survival of Axotomized Corticospinal Neurons In Vivo. J Neurosci. 2001;21:3492–3502. doi: 10.1523/JNEUROSCI.21-10-03492.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes-Leal W, Corkill DJ, Freire MA, Picanco-Diniz CW, Perry VH. Astrocytosis, microglia activation, oligodendrocyte degeneration, and pyknosis following acute spinal cord injury. Experimental Neurology. 2004;190:456–467. doi: 10.1016/j.expneurol.2004.06.028. [DOI] [PubMed] [Google Scholar]
- Gordon T, Sulaiman O, Boyd JG. Experimental strategies to promote functional recovery after peripheral nerve injuries. J Peripher Nerv Syst. 2003;8:236–250. doi: 10.1111/j.1085-9489.2003.03029.x. [DOI] [PubMed] [Google Scholar]
- Graeber MB. Changing face of microglia. Science. 2010;330:783–788. doi: 10.1126/science.1190929. [DOI] [PubMed] [Google Scholar]
- Graeber MB, Streit WJ. Microglia: biology and pathology. Acta Neuropathol. 2010;119:89–105. doi: 10.1007/s00401-009-0622-0. [DOI] [PubMed] [Google Scholar]
- Hains BC, Waxman SG. Activated microglia contribute to the maintenance of chronic pain after spinal cord injury. J Neurosci. 2006;26:4308–4317. doi: 10.1523/JNEUROSCI.0003-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallbook F, Lundin LG, Kullander K. Lampetra fluviatilis neurotrophin homolog, descendant of a neurotrophin ancestor, discloses the early molecular evolution of neurotrophins in the vertebrate subphylum. J Neurosci. 1998;18:8700–8711. doi: 10.1523/JNEUROSCI.18-21-08700.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto M, Nitta A, Fukumitsu H, Nomoto H, Shen L, Furukawa S. Inflammation-induced GDNF improves locomotor function after spinal cord injury. Neuroreport. 2005;16:99–102. doi: 10.1097/00001756-200502080-00004. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. TRK RECEPTORS: ROLES IN NEURONAL SIGNAL TRANSDUCTION. Annual Review of Biochemistry. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- Ikeda O, Murakami M, Ino H, Yamazaki M, Nemoto T, Koda M, Nakayama C, Moriya H. Acute up-regulation of brain-derived neurotrophic factor expression resulting from experimentally induced injury in the rat spinal cord. Acta Neuropathol (Berl) 2001;102:239–245. doi: 10.1007/s004010000357. [DOI] [PubMed] [Google Scholar]
- Ip NY, Maisonpierre P, Alderson R, Friedman B, Furth ME, Panayotatos N, Squinto S, Yancopoulos GD, Lindsay RM. The neurotrophins and CNTF: specificity of action towards PNS and CNS neurons. J Physiol. 1991;85:123–130. [PubMed] [Google Scholar]
- Ip NY, Yancopoulos GD. Neurotrophic factors and their receptors. Ann Neurol. 1994;35:S13–S16. doi: 10.1002/ana.410350706. [DOI] [PubMed] [Google Scholar]
- Jacobs AJ, Swain GP, Snedeker JA, Pijak DS, Gladstone LJ, Selzer ME. Recovery of neurofilament expression selectively in regenerating reticulospinal neurons. Journal of Neuroscience. 1997;17:5206–5220. doi: 10.1523/JNEUROSCI.17-13-05206.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LL, Tuszynski MH. Spinal cord injury elicits expression of keratan sulfate proteoglycans by macrophages, reactive microglia, and oligodendrocyte progenitors. J Neurosci. 2002;22:4611–4624. doi: 10.1523/JNEUROSCI.22-11-04611.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan DR, Miller FD. Neurotrophin signal transduction in the nervous system. Current Opinion in Neurobiology. 2000;10:381–391. doi: 10.1016/s0959-4388(00)00092-1. [DOI] [PubMed] [Google Scholar]
- Koshinaga M, Whittemore SR. The temporal and spatial activation of microglia in fiber tracts undergoing anterograde and retrograde degeneration following spinal cord lesion. J Neurotrauma. 1995;12:209–222. doi: 10.1089/neu.1995.12.209. [DOI] [PubMed] [Google Scholar]
- Ladeby R, Wirenfeldt M, Garcia-Ovejero D, Fenger C, Dissing-Olesen L, Dalmau I, Finsen B. Microglial cell population dynamics in the injured adult central nervous system. Brain Research Reviews. 2005;48:196–206. doi: 10.1016/j.brainresrev.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Lee P-G, Zhuo H, Helke CJ. Axotomy alters neurotrophin and neurotrophin receptor mRNAs in the vagus nerve and nodose ganglion of the rat. Molecular Brain Research. 2001;87:31–41. doi: 10.1016/s0169-328x(00)00277-1. [DOI] [PubMed] [Google Scholar]
- Leme RJ, Chadi G. Distant microglial and astroglial activation secondary to experimental spinal cord lesion. Arq Neuropsiquiatr. 2001;59:483–492. doi: 10.1590/s0004-282x2001000400002. [DOI] [PubMed] [Google Scholar]
- Lewin GR, Barde YA. Physiology of the Neurotrophins. Annual Review of Neuroscience. 1996;19:289–317. doi: 10.1146/annurev.ne.19.030196.001445. [DOI] [PubMed] [Google Scholar]
- Liebl DJ, Huang W, Young W, Parada LF. Regulation of Trk Receptors Following Contusion of the Rat Spinal Cord. Experimental Neurology. 2001;167:15–26. doi: 10.1006/exnr.2000.7548. [DOI] [PubMed] [Google Scholar]
- Loane DJ, Byrnes KR. Role of Microglia in Neurotrauma. Neurotherapeutics. 2010;7:366–377. doi: 10.1016/j.nurt.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lurie DI, Pijak DS, Selzer ME. The structure of reticulospinal axon growth cones and their cellular environment during regeneration in the lamprey spinal cord. J Comp Neurol. 1994;344:559–580. doi: 10.1002/cne.903440406. [DOI] [PubMed] [Google Scholar]
- Lurie DI, Selzer ME. The need for cellular elements during axonal regeneration in the sea lamprey spinal cord. Exp Neurol. 1991a;112:64–71. doi: 10.1016/0014-4886(91)90114-r. [DOI] [PubMed] [Google Scholar]
- Lurie DI, Selzer ME. Preferential regeneration of spinal axons through the scar in hemisected lamprey spinal cord. J Comp Neurol. 1991b;313:669–679. doi: 10.1002/cne.903130410. [DOI] [PubMed] [Google Scholar]
- Mackler SA, Selzer ME. Specificity of synaptic regeneration in the spinal cord of the larval sea lamprey. J Physiol (Lond) 1987;388:183–198. doi: 10.1113/jphysiol.1987.sp016609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrick SE, Pleasure SJ, Lurie DI, Pijak DS, Selzer ME, Lee VM. Glial cells of the lamprey nervous system contain keratin-like proteins. Journal of Comparative Neurology. 1995;355:199–210. doi: 10.1002/cne.903550204. [DOI] [PubMed] [Google Scholar]
- Morino T, Ogata T, Horiuchi H, Takeba J, Okumura H, Miyazaki T, Yamamoto H. Delayed neuronal damage related to microglia proliferation after mild spinal cord compression injury. Neurosci Res. 2003;46:309–318. doi: 10.1016/s0168-0102(03)00095-6. [DOI] [PubMed] [Google Scholar]
- Mueller CA, Schluesener HJ, Conrad S, Pietsch T, Schwab JM. Spinal cord injury-induced expression of the immune-regulatory chemokine interleukin-16 caused by activated microglia/macrophages and CD8+ cells. J Neurosurg Spine. 2006;4:233–240. doi: 10.3171/spi.2006.4.3.233. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Bregman BS. Differences in neurotrophic factor gene expression profiles between neonate and adult rat spinal cord after injury. Exp Neurol. 2001;169:407–415. doi: 10.1006/exnr.2001.7670. [DOI] [PubMed] [Google Scholar]
- Napoli I, Neumann H. Microglial clearance function in health and disease. Neuroscience. 2009;158:1030–1038. doi: 10.1016/j.neuroscience.2008.06.046. [DOI] [PubMed] [Google Scholar]
- Pancer Z, Amemiya CT, Ehrhardt GR, Ceitlin J, Gartland GL, Cooper MD. Somatic diversification of variable lymphocyte receptors in the agnathan sea lamprey. Nature. 2004;430:174–180. doi: 10.1038/nature02740. [DOI] [PubMed] [Google Scholar]
- Patapoutian A, Reichardt LF. Trk receptors: mediators of neurotrophin action. Current Opinion in Neurobiology. 2001;11:272–280. doi: 10.1016/s0959-4388(00)00208-7. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Wei P, Stokes BT. Cellular inflammatory response after spinal cord injury in Sprague-Dawley and Lewis rats. Journal of Comparative Neurology. 1997;377:443–464. doi: 10.1002/(sici)1096-9861(19970120)377:3<443::aid-cne10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Priestley JV. Promoting anatomical plasticity and recovery of function after traumatic injury to the central or peripheral nervous system. Brain. 2007;130:895–897. doi: 10.1093/brain/awm041. [DOI] [PubMed] [Google Scholar]
- Prinz M, Mildner A. Microglia in the CNS: Immigrants from another world. Glia. 2011;59:177–187. doi: 10.1002/glia.21104. [DOI] [PubMed] [Google Scholar]
- Rabchevsky AG, Streit WJ. Grafting of cultured microglial cells into the lesioned spinal cord of adult rats enhances neurite outgrowth. J Neurosci Res. 1997;47:34–48. [PubMed] [Google Scholar]
- Rovainen CM. Regeneration of Müller and Mauthner axons after spinal transection in larval lampreys. Journal of Comparative Neurology. 1976;168:545–554. doi: 10.1002/cne.901680407. [DOI] [PubMed] [Google Scholar]
- Rovainen CM. Neurobiology of lampreys. Physiol Rev. 1979;59:1007–1077. doi: 10.1152/physrev.1979.59.4.1007. [DOI] [PubMed] [Google Scholar]
- Sandvig A, Berry M, Barrett LB, Butt A, Logan A. Myelin-, reactive glia-, and scar-derived CNS axon growth inhibitors: Expression, receptor signaling, and correlation with axon regeneration. Glia. 2004;46:225–251. doi: 10.1002/glia.10315. [DOI] [PubMed] [Google Scholar]
- Schultz R, Berkowitz EC, Pease DC. The electron microscopy of the lamprey spinal cord. J Morphol. 1956;98:251–273. [Google Scholar]
- Selzer ME. Mechanisms of functional recovery and regeneration after spinal cord transection in larval sea lamprey. J Physiol (Lond) 1978;277:395–408. doi: 10.1113/jphysiol.1978.sp012280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shifman MI, Selzer ME. Expression of Netrin Receptor UNC-5 in Lamprey Brain; Modulation by Spinal Cord Transection. Neurorehabilitation and Neural Repair. 2000a;14:49–58. doi: 10.1177/154596830001400106. [DOI] [PubMed] [Google Scholar]
- Shifman MI, Selzer ME. In situ hybridization in wholemounted lamprey spinal cord:localization of netrin mRNA expression. Journal of Neuroscience Methods. 2000b;104 doi: 10.1016/s0165-0270(00)00316-2. 19-15. [DOI] [PubMed] [Google Scholar]
- Shifman MI, Selzer ME. Semaphorins and their Receptors in Lamprey CNS: cloning, phylogenetic analysis and developmental changes during metamorphosis. JComp Neurol. 2006;497:115–132. doi: 10.1002/cne.20990. [DOI] [PubMed] [Google Scholar]
- Shifman MI, Selzer ME. Differential expression of Class 3 and 4 semaphorins and netrin in the lamprey spinal cord during regeneration. The Journal of Comparative Neurology. 2007;501:631–646. doi: 10.1002/cne.21283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shifman MI, Yumul RE, Laramore C, Selzer ME. Expression of the repulsive guidance molecule RGM and its receptor neogenin after spinal cord injury in sea lamprey. Experimental Neurology. 2009;217:242–251. doi: 10.1016/j.expneurol.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sroga JM, Jones TB, Kigerl KA, McGaughy VM, Popovich PG. Rats and mice exhibit distinct inflammatory reactions after spinal cord injury. J Comp Neurol. 2003;462:223–240. doi: 10.1002/cne.10736. [DOI] [PubMed] [Google Scholar]
- Stock DW, Whitt GS. Evidence from 18S ribosomal RNA sequences that lampreys and hagfishes form a natural group. Science. 1992;257:787–789. doi: 10.1126/science.1496398. [DOI] [PubMed] [Google Scholar]
- Streit W. An improved staining method for rat microglial cells using the lectin from Griffonia simplicifolia (GSA I-B4) J Histochem Cytochem. 1990;38:1683–1686. doi: 10.1177/38.11.2212623. [DOI] [PubMed] [Google Scholar]
- Streit WJ. The role of microglia in regeneration. Eur Arch Otorhinolaryngol. 1994:S69–S70. doi: 10.1007/978-3-642-85090-5_19. [DOI] [PubMed] [Google Scholar]
- Streit WJ. The role of microglia in brain injury. Neurotoxicology. 1996;17:671–678. [PubMed] [Google Scholar]
- Streit WJ. Microglia and the response to brain injury. Ernst Schering Res Found Workshop. 2002a:11–24. doi: 10.1007/978-3-662-05073-6_2. [DOI] [PubMed] [Google Scholar]
- Streit WJ. Microglia as neuroprotective, immunocompetent cells of the CNS. Glia. 2002b;40:133–139. doi: 10.1002/glia.10154. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Kreutzberg GW. Lectin binding by resting and reactive microglia. J Neurocytol. 1987;16:249–260. doi: 10.1007/BF01795308. [DOI] [PubMed] [Google Scholar]
- Vidal Pizarro I, Swain GP, Selzer ME. Cell proliferation in the lamprey central nervous system. The Journal of Comparative Neurology. 2004;469:298–310. doi: 10.1002/cne.11013. [DOI] [PubMed] [Google Scholar]
- Villar-Cheda B, Perez-Costas E, Melendez-Ferro M, Abalo XM, Rodriguez-Munoz R, Anadon R, Rodicio MC. Cell proliferation in the forebrain and midbrain of the sea lamprey. J Comp Neurol. 2006;494:986–1006. doi: 10.1002/cne.20851. [DOI] [PubMed] [Google Scholar]
- Widenfalk J, Lundstromer K, Jubran M, Brene S, Olson L. Neurotrophic Factors and Receptors in the Immature and Adult Spinal Cord after Mechanical Injury or Kainic Acid. J Neurosci. 2001;21:3457–3475. doi: 10.1523/JNEUROSCI.21-10-03457.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood MR, Cohen MJ. Synaptic regeneration in identified neurons of the lamprey spinal cords. Science. 1979;206:344–347. doi: 10.1126/science.482943. [DOI] [PubMed] [Google Scholar]
- Wu D, Miyamoto O, Shibuya S, Okada M, Igawa H, Janjua NA, Norimatsu H, Itano T. Different expression of macrophages and microglia in rat spinal cord contusion injury model at morphological and regional levels. Acta Med Okayama. 2005;59:121–127. doi: 10.18926/AMO/31950. [DOI] [PubMed] [Google Scholar]
- Xu M, Ng YK, Leong SK. Induction of microglial reaction and expression of nitric oxide synthase I in the nucleus dorsalis and red nucleus following lower thoracic spinal cord hemisection. Brain Res. 1998;808:23–30. doi: 10.1016/s0006-8993(98)00787-2. [DOI] [PubMed] [Google Scholar]
- Yin HS, Selzer ME. Axonal regeneration in lamprey spinal cord. J Neurosci. 1983;3:1135–1144. doi: 10.1523/JNEUROSCI.03-06-01135.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]