Abstract
The adult mammal lacks the ability to regenerate neurons lost to retinal damage or disease in a meaningful capacity. However, previous studies from this laboratory have demonstrated that PNU-282987, an α7 nicotinic acetylcholine receptor agonist, elicits a robust neurogenic response in the adult murine retina. With eye drop application of PNU-282987, Müller glia cells re-enter the cell cycle and produce progenitor-like cells that can differentiate into various types of retinal neurons. In this study, we analyzed the regenerative capability of PNU-282987 in two retinal disease models and identified the source of newly regenerated neurons. Wild-type mice and mice with a transgenic Müller-glia lineage tracer were manipulated to mimic loss of retinal cells associated with glaucoma or photoreceptor degeneration. Following treatment with PNU-282987, the regenerative response of retinal neurons was quantified and characterized. After onset of photoreceptor degeneration, PNU-282987 was able to successfully regenerate both rod and cone photoreceptors. Quantification of this response demonstrated significant regeneration, restoring photoreceptors to near wild-type density. In mice that had glaucoma-like conditions induced, PNU-282987 treatment led to a significant increase in retinal ganglion cells. Retrograde labeling of optic nerve axon fibers demonstrated that newly regenerated axons projected into the optic nerve. Lineage tracing analysis demonstrated that these new neurons were derived from Müller glia. These results demonstrate that PNU-282987 can induce retinal regeneration in adult mice following onset of retinal damage. The ability of PNU-282987 to regenerate retinal neurons in a robust manner offers a new direction for developing novel and potentially transformative treatments to combat neurodegenerative disease.
Keywords: Mammalian regeneration, Muller glia, PNU-282987, alpha7 nicotinic acetylcholine receptor, photoreceptor, retinal ganglion cell, optic nerve
1. Introduction
Irreversible vision loss due to neurodegenerative diseases cause significant problems across social spectrums, which negatively impacts the economy and leads to undesirable biomedical consequences (Tham et al., 2014). This results in a decreased quality of life, large burdens on health care systems, and increased morbidity and mortality (Kirtland et al., 2015). Two of the leading causes of retinal degenerative diseases are glaucoma and retinitis pigmentosa (RP) (Bourne et al., 2017; Openshaw et al., 2008; Tham et al., 2014).
Glaucoma is characterized by a progressive loss of retinal ganglion cells (RGCs) and optic disc cupping that can lead to loss of vision and visual defects. The axons of the RGCs converge at the optic nerve head (ONH) to form the optic nerve, carrying visual information to the brain for further vision processing and perception. Even though glaucoma is a leading cause of blindness globally, the biological basis of glaucoma is not well understood, which significantly limits treatment options and disease outcomes (Weinreb et al., 2014). Likewise, retinitis pigmentosa (RP), a retinal disease that leads to blindness due to loss of photoreceptors (Van Soest et al., 1999; Hartong et al., 2006; Liu et al., 2015;), is very difficult to treat as it is largely a hereditary condition, with well over 100 genes implicated in the disease (Shintani et al., 2009). In mammals, photoreceptors and RGCs cannot regenerate, rendering diseases such as glaucoma and RP currently uncurable. Therefore, strategies that can regenerate physiologically functional retinal neurons in adult mammals are of significant interest (Gamm and Wong, 2015; Vetter and Hitchcock, 2017).
Teleost fish, which include zebrafish, have been studied extensively for their intrinsic retinal regenerative capabilities (Gemberling et al., 2013; Hitchcock et al., 2004; Hitchcock and Raymond, 1992; Reh and Levine, 1998; Thummel et al., 2008). In zebrafish, Müller glia (MG) are the source of retinal stem cells which generate new neurons, replacing those that have been damaged, and can lead to vision restoration (Goldman, 2014; Hitchcock and Raymond, 1992; Ramachandran et al., 2015). MG in mammals do not typically have this capability (Elsaeidi et al., 2018; Osakada et al., 2007). Instead, upon activation due to damage, mammalian MG briefly re-enter the cell cycle but do not generate retinal stem cells (Pran et al., 2017). However, previous work from this lab has demonstrated that the α7 nicotinic acetylcholine receptor agonist (α7 nAChR), PNU-282987, has the ability to stimulate MG to generate Müller-derived progenitor cells (MDPCs) and generate multiple types of new differentiated neurons in adult mammalian retinas (Webster et al., 2017, 2019).
PNU-282987 is a quinuclidine benzamide developed as a part of a screen for selective α7 nAChR agonists (Hajos et al., 2005; Walker et al., 2006) and was characterized as the most selective α7 nAChR agonist developed. Previous work demonstrated that activation of nAChRs in cultured porcine RGCs led to neuroprotection against glutamate excitotoxicity (Wehrwein et al., 2004) and intraocular injections of PNU-282987 led to robust neuroprotection against glaucoma-induced conditions in rats (Iwamoto et al., 2014a; Mata et al., 2015). However, if applied as eyedrops, PNU-282987 induces BrdU+ retinal neurons (Webster et al., 2017) from a MG lineage, and migrates in a pattern similar to interkinetic nuclear migration (Lahne and Hyde, 2016; Lenkowski and Raymond, 2014). The α7 nAChR has been localized to bipolar, amacrine, and ganglion cells in the adult mammalian neural retina (Dmitrieva et al., 2007). In the non-neuronal retina, the receptor is found on retinal pigment epithelium (RPE) (Maneu et al., 2010). Since PNU-282987 is an efficacious agonist and inhibition of the receptor with methyllycaconitine prevents BrdU incorporation following exposure to the agonist (Webster et al., 2017), it was hypothesized that RPE respond to the agonist causing MG to undergo neurogenesis (Webster et al., 2019). It was proposed that stimulation of RPE with PNU-282987 is sufficient to generate not only BrdU+ neurons, but progenitor-like cells derived from MG (Webster et al., 2019). These progenitor-like cells were shown to be genetically similar to those seen in Zebrafish regeneration (Stanchfield et al., 2020). However, it remains to be demonstrated if PNU-282987 can regenerate lost or damaged retinal neurons in specific models of retinal degeneration.
The objective of this research was to determine if PNU-282987 could stimulate regeneration of neurons in models of glaucoma and in a model of photoreceptor loss in adult mice. Damaged retinas were treated with PNU-282987 and the regenerative response was characterized. The source of new neurons was determined to be MG. These data support the hypothesis that the adult mammalian retina is capable of robust regeneration using selectively targeted compounds.
2. Methods
2.1. Animals, Transgenic Constructs, Breeding, and Genotyping
All procedures using animal subjects were conducted in accordance with the standards of the Institute of Animal Care and Use Committee (IACUC) at Western Michigan University. Wild-type (Jackson Laboratories, Bar Harbor, ME) and transgenic 129/SvJ mice (3–6 months old, both sexes) weighing between 22 and 25 g were used for these studies. Animals were housed in a normal experimental room and exposed to 12-hour light/dark cycle with free access to food and water. 129/SvJ mice carrying the RlbpCre-ER (Cre+) and Rosa-tdTomatofloxstopflox (tdTomato+) transgenes (generated and used with permission by E. Levine, Vanderbilt Univ.) were used to label Müller glia (MG) (Vazquez-Chona et al., 2009; Webster et al., 2019). Cre+/WT;tdTomato+/− mice were injected with tamoxifen, leading to tdTomato reporter expression in MG cells of the retina, as previously reported (Webster et al., 2019). Specifically, 6-week old male and female mice were injected intraperitoneally with 10 mg/mL tamoxifen in corn oil for three subsequent days for a total injection volume of 300μl tamoxifen. To generate experimental mice, Cre+/WT;tdTomato+/− males were crossed with CreWT/WT;tdTomato+/+ females. Pups were tagged, tail-clipped, and genotyped by PCR.
2.2. Hypertonic Saline Injections
Glaucomatous damage was induced by elevating the intraocular pressure following a hypertonic saline injection into the episcleral vein as previously described in rats and mice (Morrison et al., 1997; Kipfer-Kauer et al., 2010; Gossman et al., 2016). The hypertonic saline causes scarring to reduce aqueous humor outflow and increase IOP (Morrison et al., 1997). This protocol has become the standard technique used to induce glaucoma-like conditions in this lab and was chosen over other techniques due to the consistent and robust loss of RGCs that corresponds with an increase in IOP. Briefly, mice were anesthetized with a ketamine (100mg/mL)/acepromazine (10mg/mL)/xylazine (20mg/mL) injection (0.1mL per 100g body weight) and kept warm. One drop of topical anesthetic (Proparacaine Hydrochloride Ophthalmic Solution, USP, 0.5%) was applied to the cornea. Sterile 1.2M sodium chloride (25μL) was injected into the episcleral vein of one eye using a beveled microneedle until the entire episcleral vein blanched white. Typically, two injections on contralateral sides of the eye were required to blanch the entire vessel. A cotton applicator was used to apply triple antibiotic ointment (Bacitracin zinc, neomycin sulfate, polymysin B sulfate) to the injection site following injection. During recovery, animals were kept warm until normal behavior was regained. This procedure was performed once or was repeated one-week post the first injection if the intraocular pressure did not increase. Animals then remained in the animal facility for 28 days post-surgery before being treated or were euthanized.
2.3. Intraocular Pressure Measurements
Intraocular pressure (IOP) was measured using the TonoLab tonometer for rodents (Colonial Medical Supply, Windham, NH) according to the manufacturer’s protocol. Briefly, awake animals were gently restrained by hand and IOP measurements were then performed using the tonometer. For each reading, 3 separate IOP measurements were obtained, each consisting of the mean of six consecutive error-free IOP readings and averaged to represent an N of 1. All measurements were taken between 6 and 8 PM to control for diurnal variation. For experiments, IOP measurements were taken prior to saline injection, and then every two days for 28-days in control and glaucoma-induced mice, with or without PNU-282987.
2.4. N-methyl-N-nitrosourea Injections
To generate photoreceptor cell loss, N-methyl-N-nitrosourea (MNU) was used to induce retinal degeneration. MNU induces apoptosis in photoreceptors through oxidative stress, which leads to loss of mitochondria and displaced remnant synaptic ribbons. This effect is observed relatively quickly, while loss of other neurons in the INL and GCL take several months to observe (Chen et al., 2014; Lin et al., 2017; Rösch et al., 2014). Wild-type 129/SvJ mice were intraperitoneally injected with N-methyl-N-nitrosourea (60 mg/kg; ChemService, West Chester, PA), solubilized in a carrier of 0.05% acetic acid in sterile saline (Lin et al., 2017). Control animals were injected with the carrier alone. Animals were euthanized at varying time points based on treatment scheme and retinal counts were quantified and characterized.
2.5. Eye Drop Treatments, Retina Preparation, and Optic Nerve Preparation
Both eyes of all experimental animals were treated once daily with eye drops containing PNU-282987 (1 mM; Sigma, St. Louis, MO) and 5-bromo-2’-deoxyuridine (BrdU) (1 mg/mL; Sigma, St. Louis, MO) in PBS for varying amounts of time as previously described (Linn et al., 2018) and then euthanized. Verified detection of the agonist was found in the retina using LC/MS/MS after eyedrop application (Mata et al., 2015). Control animals were treated once daily with only 1 mg/mL BrdU in PBS in both eyes. Following euthanasia, the retinas and 5–7 mm of optic nerve were removed using the blunt dissection for enucleation of the mouse eye in unfixed specimens (Mahajan et al., 2011). For retrograde labeling, the optic nerves were kept attached to the retina, pinned, and fixed in 4% PFA. For other applications, the excised retinas were flat-mounted and fixed in 4% PFA. The contralateral eye was not used as a control as repetitive facial self-grooming behaviors in mice could lead to the transfer of eye drop solution between eyes.
2.6. Immunohistochemistry and Retrograde Labeling
Antibody labeling was performed as previously described (Webster et al., 2019). Antibody staining was carried out using anti-Thy1.2 antibody (1:200, abcam, Cambridge, MA), anti-BrdU antibody (1:125; abcam, Cambridge, MA), anti-Cone arrestin antibody (1:10,000; Millipore, Temecula, CA), anti-recoverin antibody (1:1000; Millipore, Temecula, CA), anti-Iba1 antibody (1:500; abcam, Cambridge, MA), or anti-RFP antibody (1:200; Rockland Antibodies and Assays, Limerick, PA). Primary antibodies were detected using Alexa Fluor 405, 488, or 594 fluorescent secondary antibodies (1:500; abcam, Cambridge, MA or Invitrogen, Waltham, MA). Nuclei were counterstained with DAPI (0.1 μg/mL; Sigma, St. Louis, MO), Sytox Green (5 mM; ThermoFisher, Waltham, MA), or Sytox Red (5 mM; ThermoFisher, Waltham, MA).
For retrograde labeling, a small triangular piece of filter paper containing the lipophilic green emitting dye (ex max = 748nm; em max=508nm), NeuroVue Jade (MTTI, West Chester, PA, USA) was used. The filter paper was inserted centrally into a longitudinal cut made to the optic nerve 2–3 mm from the ONH. The filter paper was placed in control and PNU-282987 treated transgenic mice; similar to the retrograde labeling procedure using NeuroVue dyes in other applications (Duncan et al., 2011; Saito et al., 2016). Lipophilic dyes diffuse in all processes belonging to the given neuron it touches, both retrogradely and anterogradely. After inserting the dye, the optic nerve and attached retina were kept in the dark in a 37°C incubator for 4 days in 4% PFA. After 4 days, the dye filter paper was removed, the retina and attached optic nerve were bisected with a single cut allowing the retina to be flat-mounted. The tissue was then mounted in glycerol and imaged immediately using a confocal microscope to examine lipophilic and transgenic fluorescence.
2.7. Microscopy, Cell Counting, Normalization
Flat mounts:
Flat mounted retinal tissue was visualized using a Nikon C2+ scanning laser confocal microscope (Nikon, Tokyo, Japan). Using the Z stack function of the confocal microscope, images were obtained 4 mm from the optic nerve head (ONH). This peripheral distance from the ONH was chosen as a result of previous studies that demonstrated the procedure to induce glaucoma-like conditions produced the greatest amount of damage to RGCs 4 mm from the ONH (Iwamoto et al., 2014b; Mata et al., 2015). Images were obtained throughout the entire nerve fiber layer and RGC layer in 1 μm increments to measure nerve fiber thickness and RGC density. As the distribution of RGCs vary across the mouse retina (Drager and Olsen, 1981), images were obtained from four 200 μm2 quadrants in each retina, 4 mm away from the center of the ONH and averaged. This represented an N of 1. Previous studies have averaged the number of cells in two, four, eight, ten and twenty 200 μm2 sections across each retina (Iwamoto et al., 2014b; Mata et al., 2015) to determine the most reliable averaging method. Averaging cell counts from 4 retinal sections in 4 different quadrants proved the be most accurate representative of the true cell count in the retina (Iwamoto et al., 2014a; Mata et al., 2015). The total number of Thy1.2-postive RGCs or BrdU+ cells were counted, averaged, and compared to experimental and control retinas as in previous studies (Birkholz et al., 2016; Iwamoto et al., 2014a; Mata et al., 2015; Webster et al., 2019, 2017). Quantitative analysis was performed using ImageJ software.
Retinal Sections:
Retinas were sectioned in 40 μm increments and imaged as above. DAPI-stained nuclei that colocalized with anti-BrdU or other markers were counted and normalized as a mean percentage of total cells. To measure the width of the outer nuclear layer (ONL), three different areas of a section were measured and averaged from 10 sections per retina in 4 different animals. A total of 10 flat mounted retinas were analyzed to quantify axon fasciculation. To obtain images of the axon bundles, the confocal microscope was focused on the GCL and stacked images (1 μm) were obtained throughout the entire nerve fiber layer. A line was drawn perpendicular to the axis of the axon fascicule and the length of this line was measured using ImageJ software. For lineage tracing in the optic nerve, the length of the nerve was trimmed to 6 mm and serial sections were taken every 40 μm up to the optic nerve head. To ensure that the animals expressed the transgene in all experiments and that the lack of tdTomato in the ON was not due to lack of transgenic expression, the retinas were sectioned and imaged for tdTomato. Investigators were blind to the experimental condition of the sample.
2.8. RNAseq Analysis and Bioinformatics
A previously published RNAseq dataset from out lab (Stanchfield et al., 2020) was used. This dataset described the transciptiomics of MG post PNU-282987 treatment (GEO accession ID: GSE151477). Using DESdeq2, a comparison of gene expression between untreated and multiple timepoints of treated MG was performed. The Wald test was used to generate p-values and log2 fold changes. Genes with a p-value greater than 0.05 and absolute log2 fold change greater than one were called as differentially expressed genes for each comparison. Differentially expressed genes were mapped using Morpheus (Morpheus; https://software.broadinstitute.org/morpheus) using their log2 fold changes based on genes grouped in classes according to their ontology of differentiated MG, primary retinal progenitor cells (RPCs), or neurogenic RPCs (Lahne et al., 2020).
2.9. Statistical Analysis
Statistical analysis used the Kruskal-Wallis nonparametric ANOVA, with post hoc multiple comparisons (Dunn’s test), with p< 0.01 was considered statistically different using GraphPad software (La Jolla, CA, USA).
3. Results
3.1. Incorporation of BrdU by Neurons in the Adult Mouse Retina
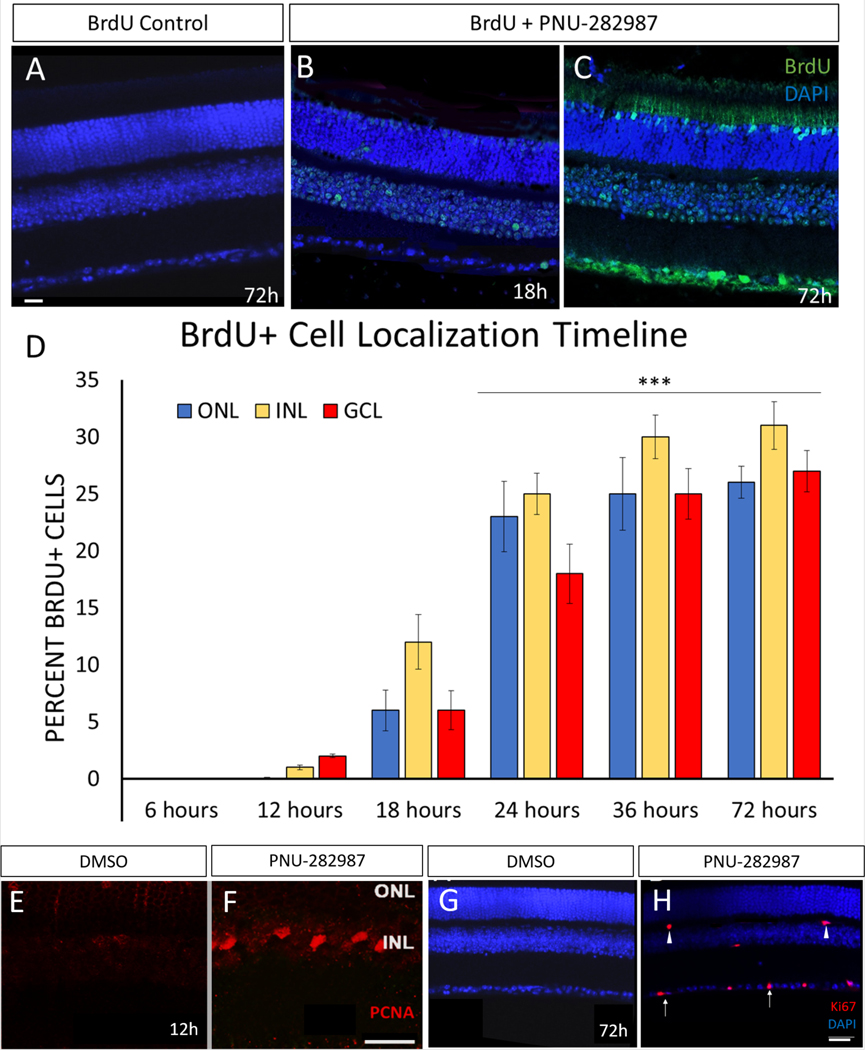
Previous studies demonstrated that PNU-282987 application results in cells entering the cell cycle as indicated by BrdU incorporation in adult rat retina (Webster et al., 2017). Daily eye drop application in rats for two weeks first led to BrdU incorporation in the inner nuclear layer (INL), then in the outer nuclear layer (ONL) and finally in the ganglion cell layer (GCL). This resembled the typical pattern of dividing MG in zebrafish regeneration, better known as interkinetic nuclear migration. For these studies using adult mice, the timeline of BrdU incorporation in the mouse was examined. Eye drops were given once daily for a period of 72 hours, and retinas were examined at multiple timepoints for BrdU incorporation. Application of BrdU alone yielded no BrdU+ cells (Figure 1A). In eyes treated with PNU-282987 and BrdU, the first BrdU-positive cells were found in the INL 12 hours post PNU −282987 treatment (Figure 1B). Interestingly, in contrast to results obtained using the rat model, there were also BrdU+ cells in the GCL at this time point. After 18 hours, BrdU+ cells were found in all three cell layers, with the greatest number of labeled cells being found in the INL. At 72 hours there was robust BrdU+ staining of cells (Figure 1C). BrdU-labeled cells were dispersed throughout all retinal cell layers, with BrdU+ labeling in 25±2.1% of the total cells in each confocal image (Figure 1D). Furthermore, other markers of cell-cycle activation were seen in PNU-282987 treated retinas. Specifically, expression of proliferating cell nuclear antigen (PCNA; Figure 1E, F) and Ki67 (Figure 1G,H) were seen during this timeline. Both Ki67 and PCNA (Figure 1E, F) appeared in the INL 12 hours post PNU-282987 application. Expression of Ki67 persisted for 72 hours and Ki67+ cells could be seen in the IPL, OPL, and GCL (Figure 1G,H).
Figure 1: Eye drop application of PNU-282987 in mice causes cell proliferation in the mouse retina.
All retinas were processed for detection of BrdU. Application of BrdU alone (A) in the adult mouse retina yields no BrdU-positive cells. Mice treated with PNU-282987 for 6 hours show no BrdU+ cells, but BrdU+ cells are detected in the INL and GCL after 12 hours of treatment (B). By 72 hours, there are BrdU+ cells in all three nuclear layers (C). At 18, 24, 36, and 72-hours post PNU-282987 application, the number of BrdU+ cells increase with consistently highest amounts of BrdU+ cells in the INL (D). To validate that the BrdU incorporation is due to cells entering the cell cycle two different makers were used. PCNA (E,F) which marks S, G2, and M was found as early as 12h after PNU-2829887 application in the INL (F) while no PCNA+ cells could be found in DMSO treated animals (E). Additionally, Ki67 which marks G1 through M was used and was found starting at 12h with few cells in the INL and by 72h had cells in the INL and GCL (H). Mice treated with DMSO did not show any expression of Ki67 (G). n=5; scale bar = 50 μm; p≤0.001.
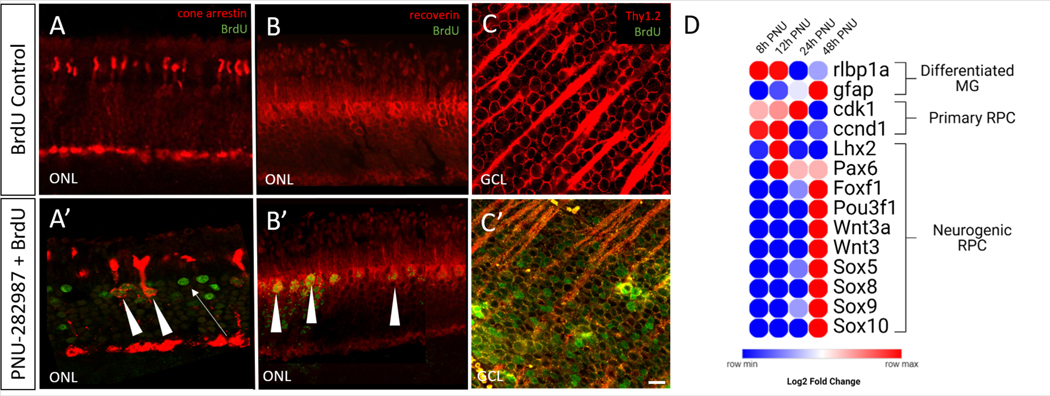
To test the hypothesis that BrdU labeling in the retina represents a neurogenic response to PNU-282987 in adult mice, experiments were designed to determine if new RGCs and new photoreceptors were induced after PNU-282987 treatment. For these studies, IHC studies were performed to co-label cells for BrdU and cell-type specific markers including; Thy1.2, which is expressed in RGCs (Barnstable and Dräger, 1984; Barres et al., 1988; Strom and Williams, 1998), cone arrestin, which is expressed in cone photoreceptors (Brown et al., 2014; Craft et al., 1994) and recoverin, which is expressed in rod and cone photoreceptors in the mouse (Dizhoor et al., 1991; Mcginnis et al., 1997). Mice were treated with PNU-282987 and BrdU for a period of two weeks while control mice received BrdU eyedrops alone. Figure 2 shows the presence of co-labeled BrdU+ cone photoreceptors (Figure 2A) and rod photoreceptors (Figure 2B) in the ONL, as well as RGCs in the GCL (Figure 2C) after PNU-282987 treatment. Not all BrdU+ cells were co-labeled in the ONL (Figure 2A’, arrowheads).
Figure 2: PNU-282987 in adult mice generates both Müller-derived progenitor cells and new differentiated neurons.
Cone arrestin (cone photoreceptors) (A), recoverin (rod and cone photoreceptors) (B) Thy1.2 (RGC) (C) labels across the retina. Application of PNU-282987 for 14 days leads to new BrdU+ cones (A’) and rods (B’) marked by arrowheads. However, not all BrdU+ cells in the ONL are differentiated neurons (arrow; A’). New RGCs are indicated by BrdU+ in the GCL (C’). RNAseq data shows that PNU-282987 activated RPE induce MG to dedifferentiate into progenitor-like cells (D). Initially, MG show high expression of rlbp1 and gfap, gene markers for differentiated MG. However, between 8 and 48 hours post PNU-282987 treatment, the MG cells switch to producing genes that are found in a neurogenic RPC, including Pax6, Pou3f1, and Sox9. n=4; scale bar = 50 μm.
We hypothesized that adult MG can dedifferentiate into progenitor-like cells to generate these new neurons. To validate this, we examined the gene expression changes in cultured MG due to PNU-282987 activation of RPE through RNAseq analysis (Stanchfield et al., 2020). Using this dataset, we examined the significant differentially expressed genes (sDEGs) that fell into a category of differentiated MG, primaryretinal progenitor cells (RPCs), or neurogenic RPCs. Using the log2 fold change values between control treated and PNU-treated samples, a heatmap was generated showing the gene expression changes in the MG over time (Figure 2G). Initially, the MG have a strong differentiated MG identity, with high expression of rlbp1a and gfap. After approximately 24h of exposure to RPE cells that had been treated with PNU-282987, the expression of these genes significantly decreased. As differentiated MG gene expression decreased, primary RPC markers, cdk1 and ccnd1, increased. Between 12h and 48h post exposure, the MG slowly start to express neurogenic RPC markers beginning with Pax6 first, followed by Lhx2. By 48h, express several important genes for neurogenic RPCs including Foxf1, several Wnt family genes, Sox family genes, and Pou3f1 were expressed. The expression of these genes was not seen in the untreated controls. The generation of progenitor-like cells and new differentiated neurons through PNU-282987 application led to the hypothesis that PNU-282987 may act as an activator of regeneration.
3.2. Inducible Model of Glaucoma in Mice
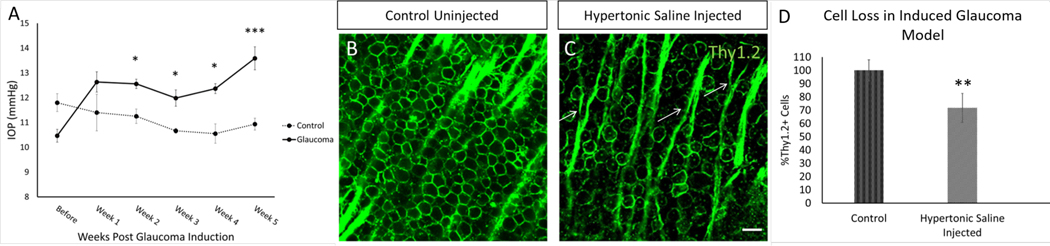
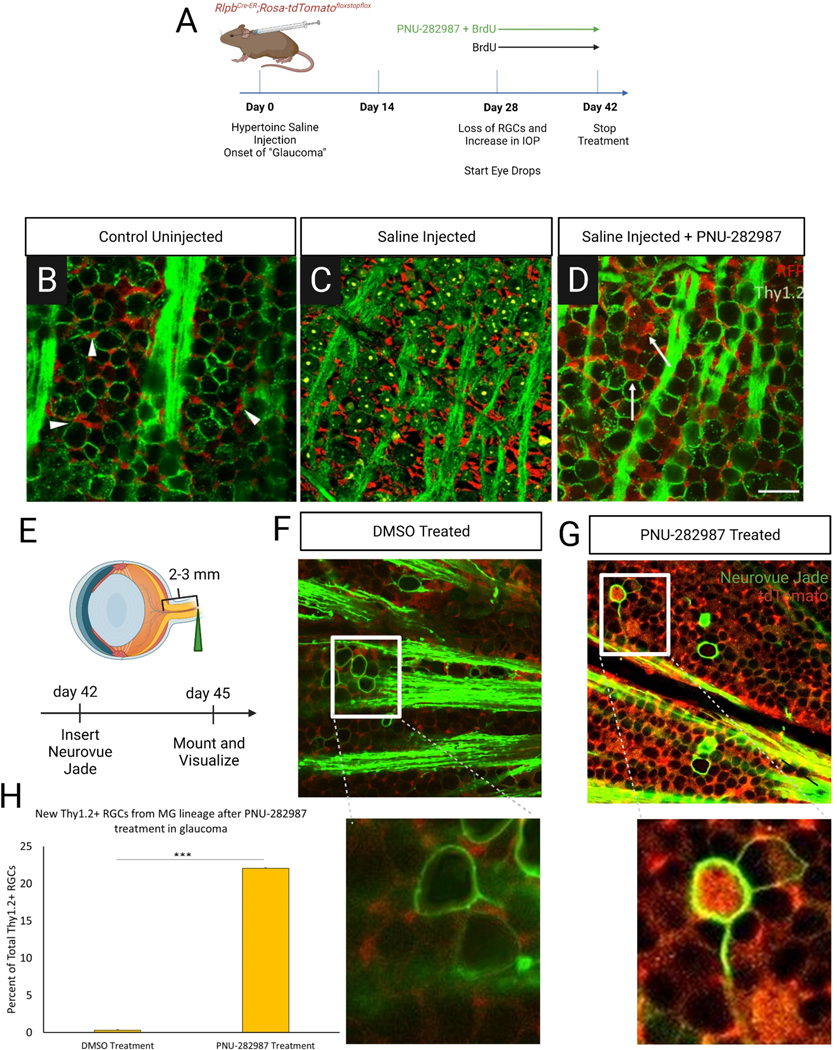
Previous studies have induced glaucomatous damage in rodents (Gossman et al., 2016) using hypertonic saline injections to induce vascular scarring and increase intraocular pressure. In Fig. 3, mice were injected with 1.2M hypertonic saline in the episcleral vein. After 28 days, the animals were euthanized, and retinal cells were viewed and analyzed. The glaucoma inducing procedure elicited a significant increase in intraocular pressure (IOP) two-weeks post injection and maintained a constant increase in IOP in the injected group across the five-week timeframe (Figure 3A). Peak IOP in the ocular hypertension group was 15.8 mmHg vs 11.2 mmHg in the control group. In conjunction with the increase in IOP, there was a significant loss of RGC density in saline-injected animals compared to un-injected animals (Figure 3B and 3C). The cell density of Thy1.2+ RGCs in the GCL was reduced by 28.31±1.5% (Figure 3D). The loss of RGCs and increased IOP is consistent with other models of glaucoma-like damage (Fedorchak et al., 2014; Jakobs et al., 2005; Morrison et al., 1997; Park et al., 2019; Schlamp et al., 2006). To determine if subsequent injections increase the IOP further and cause further loss of RGC cell density (Kipfer-Kauer et al., 2010), a second round of injections were done one-week after the first injection. These injections showed no further significant elevation in IOP nor further loss of RGC density via IHC analysis (Supplemental Figure 1) and therefore, only one day of hypertonic saline injection was performed for these studies.
Figure 3: An inducible model of glaucoma-like damage.
Chronic IOP elevation and loss of RGCs was induced by injection of 1.2M hypertonic saline into the episcleral vein of mice eyes. (A) The hypertonic saline injection group hit a peak IOP of 15.8 mmHg as compared to a peak of 11.2 mmHg in control uninjected mice after a significant increase initially two-weeks post injection which continued to increase up to five-weeks post injection. (B & C) IHC of RGCs in the retina 28 days post-injection was completed using Thy1.2. As compared to control uninjected, saline-injected animals showed a loss of RGC cell density and a defasciculation of axon bundles (arrows), both characteristics of glaucomatous damage. (D) Quantification of loss of Thy1.2-positive cells in the RGC 28 days post saline injection showed a consistent loss of about 30% of RCS cell density. N=3 for control condition and N=8 for glaucoma condition; scale bar = 50 μm; p≤0.05 (*), p≤0.001 (***).
3.3. PNU-282987 Regenerates New Ganglion Cells after Glaucomatous Damage
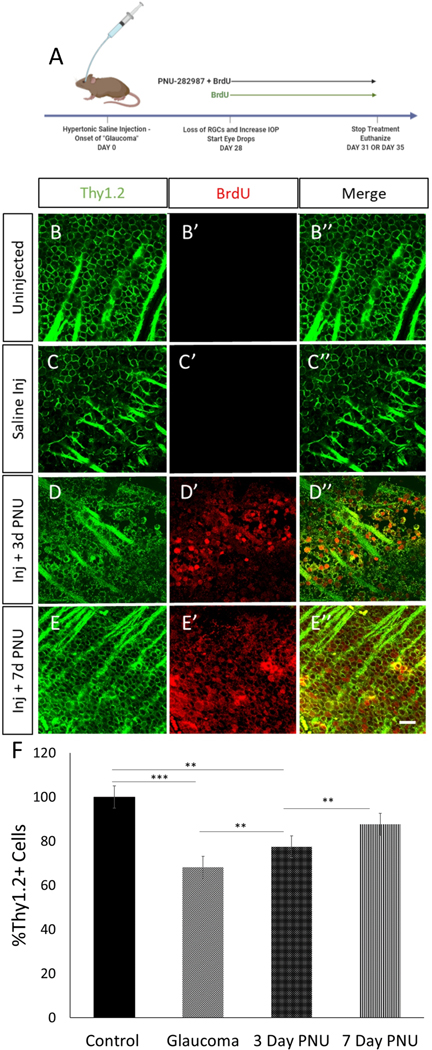
In order to assess the regenerative potential of PNU-282987, the agonist was applied to mice with glaucomatous damage. 28 days following the injection, mice were treated with once daily eyedrops for 72 hours, the time after which the first significant amounts of new BrdU+ cells appear, or for 7-days (Figure 4A). In control animals treated with BrdU alone (Figure 4B), episcleral vein injection did not elicit BrdU labeling of retinal cells, despite a loss of 30.88±1.4% of Thy1.2+ cells and despite thinning of the axon fascicles due to loss of RGCs (Figure 4C). However, mice that had induced glaucomatous damage and were treated with PNU-282987 for 3 days (Figure 4D) had Thy1.2/BrdU+ neurons present in the GCL (D”). Similarly, mice with glaucomatous damage treated with PNU-282987 for 7 days had significantly more Thy1.2/BrdU+ neurons and axons resembled control conditions (Figure 4E). Normalized counts of Thy1.2+ cells showed that treatment with PNU-282987 reduced the loss of Thy1.2+ cells to 22.59±1.05% 3 days post treatment and to 12.4±0.8% after 7 days of treatment (Fig 4F). Because the PNU-282987 treatments begin 28 days following the episcleral vein injections when GCL loss has already occurred, the presence of progressively increasing numbers of Thy1.2+, in conjunction with BrdU labeling of Thy1.2 positive cells, provides evidence that these are newly generated RGCs.
Figure 4: Glaucomatous mouse retinas treated with PNU-282987 show a regeneration of RGCs.
. Damage induction and treatment scheme shown in (A). Both uninjected and saline injected controls were treated with BrdU eye drops only. (B) Uninjected mice treated with BrdU show a normal RGC density and no BrdU expression (B’) where saline injected (C) treated with BrdU alone show a loss of RGC density. Saline injected mice treated with PNU-282987 and BrdU for 3 days (D) show new RGCs that are BrdU positive (D’) and after 7 days of treatment (E) RGC density looks like that of the uninjected controls (E’ ’). Control uninjected Thy1.2-positive RGCs were quantified and normalized to 100% (F). After glaucoma induction, an average loss of 30.8% RGCs was seen. Following 3 days of PNU-282987 treatment the average loss was reduced by 9.29% and after 7 days of treatment, loss was reduced to just 12.4% with there being no significant difference between control density and 7-day treatment density. p≤0.001 (**), p≤0.0001 (***); N=3 for each condition; scale bar = 50 μm
To determine if the source of these new RGCs could be Müller glia-derived progenitor cells (MDPC), a transgenic reporter was used for lineage tracing. The reporter makes use of a Cre-lox system in which injection of tamoxifen allows for expression of a tdTomato fluorescent reporter in mature MG cells. The induced reporter is specific to MG in the retina (Figure 5A,B) and serves as a lineage tracer, as any cells resulting from proliferating MG maintain the tdTomato expression (Webster et al., 2019). Transgenic mice were injected with hypertonic saline to induce glaucoma-like conditions. After 28 days, they were treated once daily for 14 days with PNU-2829897/BrdU or BrdU only (Figure 5C). Normal transgenic expression from a retina flat mount preparation demonstrated the tdTomato-labeled MG endfeet between RGCs (Figure 5D, arrowheads). After hypertonic saline injection, the loss of RGCs was noted, but there was no obvious loss of MG, as endfeet were still seen (Figure 5E). Interestingly, after PNU-282987 treatment, tdTomato positive Thy1.2+ RGCs were present (Figure 5F, arrows). 22.06±2.28% of examined RGCs co-labeled with tdTomato/Thy1.2 cells (Figure 5G).
Figure 5: New RGCs after PNU-282987 treatment are derived from MG.
(A) Experimental strategy for lineage tracing of regenerated RGCs. Transgenic mice expressing tdTomato in MG underwent glaucoma induction and were then treated for two weeks with either PNU-282987 and/or BrdU before undergoing IHC for Thy1.2 (RGCs) and RFP (tdTomato expression). (B) Expression of TdTom with uninjected control shows MG endfeet (arrowhead) are in the spaces between the Thy1.2+ RGCs. (C) After glaucoma induction, TdTom as MG endfeet are still evident and RGC population shows glaucomatous damage 42-days post glaucoma induction. (D) After PNU-282987 treatment in the glaucomatous retina Thy1.2/TdTom double-labeled RGCs are evident in the GCL (arrows) while MG endfeet still persist. (E) Experimental strategy for validation of lineage tracing results. Neurovue jade was inserted in optic nerves of mice that were treated with either DMSO or PNU-282987 per the strategy in (A). (F) RGC axons were retrogradely labeled with NeuroVue jade (green) and imaged for expression of the tracing dye and tdTomato (red). In retinas treated with vehicle control (F), no axons could be traced with a tdTomato-filled cell body. Increased magnification shows endfeet with normal morphology surrounded jade+ RGCs. (G) In contrast, retinas treated with PNU-282987 (G), axons could be traced that show tdTomato-filled RGC cell bodies. Upon closer magnification, full labeled jade+ RGC membrane can be seen filled with tdTom+ cell body with MG endfeet with normal morphology surrounding. Double labeling is seen (yellow) in many areas including the axon fascicles. (H) RGCs that were Thy1.2+ and RFP+ were normalized to cell counts for Thy1.2 after PNU-282987 treatment in the glaucomatous retina, indicating that around 22.06%±0.2 of RGCs post treatment are new and derived from MG. N=4 for each condition; scale bar = 50 μm.
To further establish that MDPCs were the source of new cells, RGC axons were retrogradely labeled with the green fluorescent-emitting lipophilic dye, NeuroVue. In transgenic mouse treated with a vehicle control, no labeled axons were found in the retina (Figure 5H). However, after application of PNU-282987, retrograde-labeled axons were identified that had tdTomato-positive cell bodies (Figure 5I, arrowhead).
3.4. New MG-derived Ganglion Cells Make Axons that Extend into the Optic Nerve
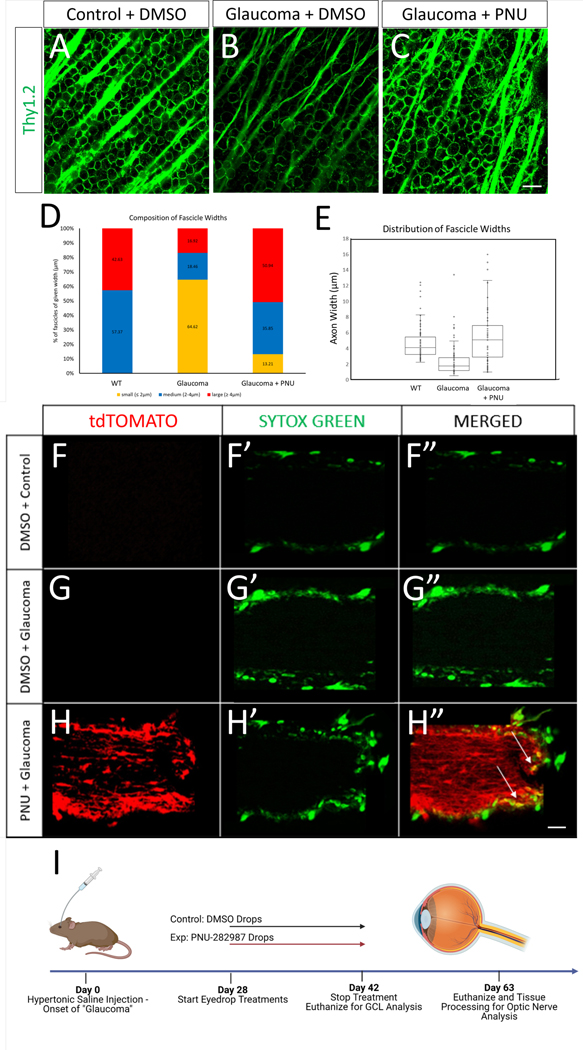
Induction of glaucoma caused changes in fasciculation of the RGC axons located near the GCL plane. Immunohistochemistry images display axon fascicles in wild type animals that are well organized into fascicles (Figure 6A), while induction of glaucoma caused defasciculation (Figure 6B). After 14-day application of PNU-282987 to glaucomatous eyes, axon fascicles appeared more like those found in control eyes (Figure 6C). Quantification of the width of axon fascicle showed three different groups: large (≥ 4 μm), medium (2–4 μm), or small (≤ 2 μm). Axon fascicle widths in the retinas from uninjured eyes consisted of the medium (57.37%) and large (42.63%) width groups (Figure 6D). Upon glaucomatous damage, 64.62% of fascicles were classified as small, with a reduction in the percentage of fascicles classified as large (16.92%) or medium (18.46%) widths (Figure 6D). After PNU-282987 application, the percentage of axons in glaucomatous eyes that were classified as having a small width decreased to 13.21% and the organization of fascicles looking indistinguishable from normal retinas. These findings suggest that the newly generated RGCs in the damaged retinas by PNU-282987 treatment extend their axons and improve fascicle organization (Figure 6D,E).
Figure 6: Glaucomatous mouse retinas treated with PNU-282987 show thicker axon fascicles and new axon projections into the optic nerve.
(A) Wild-type retinas treated with DMSO show organized axon fascicles as marked with Thy1.2. (B) 28 days after glaucoma onset, fascicles appear sparser and thinner than in wild type. (C) 7 days post PNU-282987 treatment in the glaucomatous retina, the bulk of the axon fascicles seem to be more organized and the fascicles appear more WT-like in appearance. (D) Graph representing composition of axons of given width from WT and glaucoma mice treated with DMSO or PNU-282987. (E) Distribution of axon widths in from WT and glaucoma mice treated with DMSO or PNU-282987. (F-H) New axons are projected into the optic nerve and are derived from MG. All mice were treated with tamoxifen at 2mo before experimentation. Mice were either uninjected or saline-injected and were treated with vehicle control (DMSO) or PNU-282987. No tdTomato-positive axons are seen in the transgenic model with DMSO treatment with (F) and without (G) glaucomatous-like damage. Transgenic mice (H) treated with PNU-282987 showed new axon projections derived from MG at a distance 6 mm into the optic nerve (H). In addition, the PNU-282987 treated optic nerve (H’) appears more wildtype (F’) than glaucomatous (G’). Interestingly, few Sytox green nuclei co-localized with TdTomato (H”; arrows) indicating the presence of either microglia, astrocytes, or oligodendrocytes. (I) A summary of experimental timelines for the axon fascicle and optic nerve projections. N=4 per condition; scale bar = 50 μm
To determine if the newly generated RGCs extended their axons beyond the retina into the optic nerve, the transgenic reporter in MG was used. If new RGCs were tdTomato+ and extended their axons into the optic nerve, some nerve fibers should also be positive for tdTomato. In uninjected (non-glaucomatous) tdTomato transgenic control mice, no tdTomato reporter expression was detected in the ON (Figure 6F). Likewise, saline injected transgenic mice (glaucomatous) treated with control eyedrops, illustrated no tdTomato expression in the ON (Figure 6G). However, when glaucomatous tdTomato transgenic mice were treated with PNU-282987 and left for 5 weeks, tdTomato expression was seen along the entire 6 mm length of ON (Figure 6H). Examination of the fluorescent pattern after 6-weeks revealed that glaucomatous mice treated with PNU-282987 expressed tdTomato in a pattern consistent with RGC membranes and cell bodies, while untreated controls showed tdTomato limited to MG endfeet (supplemental figure 2).
3.5. Inducible Model of Photoreceptor Degeneration in Mice
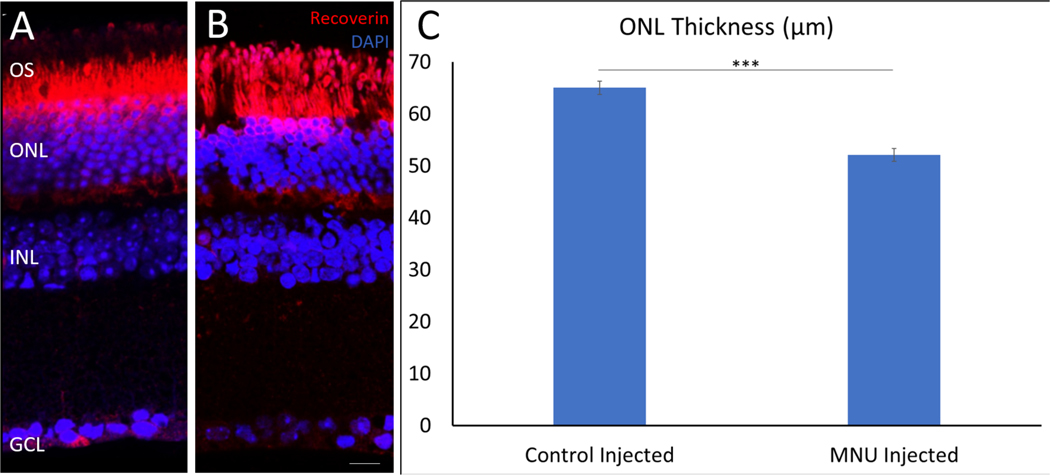
Intraperitoneal injection of MNU into wildtype and transgenic mice led to loss of photoreceptor cells as quantified with a reduction in ONL thickness 10-days post injection (Figure 7A, B). On average, the control vehicle-injected mice had a ONL thickness of 65.041 ± 3.69 μm, whereas MNU injected mice had approximately a 20% reduction in ONL thickness to 52.089 ± 1.27 μm (Figure 7C). This was consistent in all animals injected with MNU and is similar to ONL damage seen with MNU injection reported in other studies (Chen et al., 2014; Lin et al., 2017; Rösch et al., 2014; Tao et al., 2017).
Figure 7: MNU-injections cause loss of photoreceptors.
. Mice were IP injected with MNU and euthanized 10-days post injection. Compared to vehicle control injection (A) there is a significant loss of ONL thickness with MNU (B). Quantification shows that MNU-injected retinas have an ONL thickness of 52.089±1.27 μm compared to controls at 65.041±3.69 μm, a loss of around 20% (C). n=4; scale bar = 30 μm, p<0.01.
3.6. PNU-282987 Application Reverses the loss of Photoreceptors in MNU Treated Mice
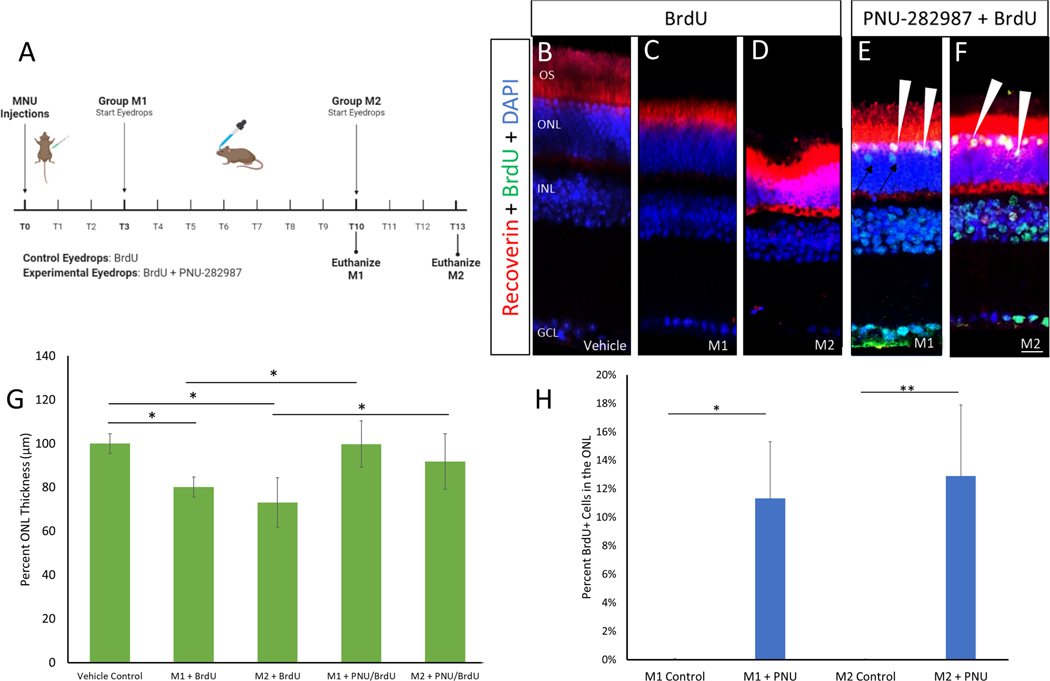
Mice were injected with MNU or a vehicle and treated with PNU-282987/BrdU, or BrdU only in one of two treatment schemes: 1) PNU-282987 drops began day 3 after MNU injections and mice were maintained for 10 days before sacrifice, (group M1) or 2) PNU-282987 drops began day 10 post MNU injections and treated for 72 hours before sacrifice, (group M2). In group M1, PNU-282987 was administered during the onset of mild MNU damage, while in group M2, PNU-282987 was administered after 10 days, when more significant photoreceptor damage typically occurred (Figure 8). Compared to the vehicle-injected control animals (Figure 8B), loss of photoreceptors was evident at 10-days (Figure 8C) as well as at 13-days (Figure 8D) in the retinas of MNU injected animals treated with BrdU drops alone. When quantified, group M1 had 80.07±4.64% of cells in the ONL compared to control retinas, while group M2 had 72.99±11.31% of cell density in the ONL compared to control conditions, representing a statistically significant loss from vehicle controls (Figure 8G). However, in group M1 eyes treated with PNU-282987, the ONL cell density was similar to controls tissue, containing 99.69±10.61% of cells that were typically found in the ONL. Similarly, in group M2 eyes treated with PNU-282987, the ONL cell density was 91.77±12.76% of expected cells in the ONL. The increase in ONL cell density observed in PNU-treated eyes compared to the BrdU-treated controls was significantly different for both groups (p<0.01, Fig. 8G).
Figure 8: PNU-282987 regenerates lost photoreceptors after photoreceptor damage.
(A) Treatment scheme for MNU-injections. On day 0, mice were injected with vehicle control or MNU. Group M1 mice were left for 3 days before starting treatment with BrdU (control) or PNU-282987/BrdU. Group M1 mice were then euthanized on day 10. Group M2 were left for 10 days before starting eyedrop treatments and were euthanized on day 13. (B-F) IHC for photoreceptors (recoverin) and DAPI showed a significant loss of ONL density in both groups M1 (C) and M2 (D) as compared to vehicle control (B). Application of PNU-282987 led to incorporation of BrdU-positive, recoverin positive cells (E,F arrowhead) as well as BrdU-positive cells that may be migrating as they are localized in the ONL but do not stain for recoverin (E, arrows). (G) ONL thickness was measured and data was normalized to vehicle control. MNU injections showed a significant loss of around 20% of ONL thickness in both groups M1 and M2. When compared to vehicle control, treatments in both M1 and M2 were no longer significantly different, indicating that they look more like wildtype conditions rather than damaged conditions. (H) Quantification of BrdU+ cells in the ONL showed integration of 11.32±4.09% in group M1 while group M2 showed 12.89±0.55% BrdU+ cells in the ONL. N=4; scale bar = 50 μm; *p<0.01, **p<0.001.
To determine if the increase in ONL cell density following PNU-282987 treated eyes was the result of regeneration, IHC was used to label and quantify the percentage of BrdU+ cells found in the ONL after each treatment type (Figure 8F). The damage caused by MNU failed to elicit a regeneration response (MI and M2), as only a few BrdU+ cells were seen (Figure 8C, 8D, 8G). However, when mice in groups M1 and M2 were treated with PNU-282987/BrdU, 11.32±4.09% (M1) and 12.89±0.55% (M2) of ONL cells were BrdU+. In each group, the majority of BrdU+ cells in the ONL were double labeled with recoverin, a photoreceptor-specific marker consistent with regeneration of photoreceptors. However, BrdU+ neurons migrate through the retina similar to interkinetic nuclear migration seen in zebrafish, which has been previously described (Webster et al., 2019, 2017). Therefore, BrdU+ cells in the ONL which were recoverin-negative (Figure 8E, arrows) may be non-photoreceptors. These regenerated photoreceptors (BrdU+/recoverin+) composed 85.31±4.5% of total BrdU+ cells in the ONL in group M1 and 88.64±6.06% of total BrdU+ cells in the ONL in group M2.
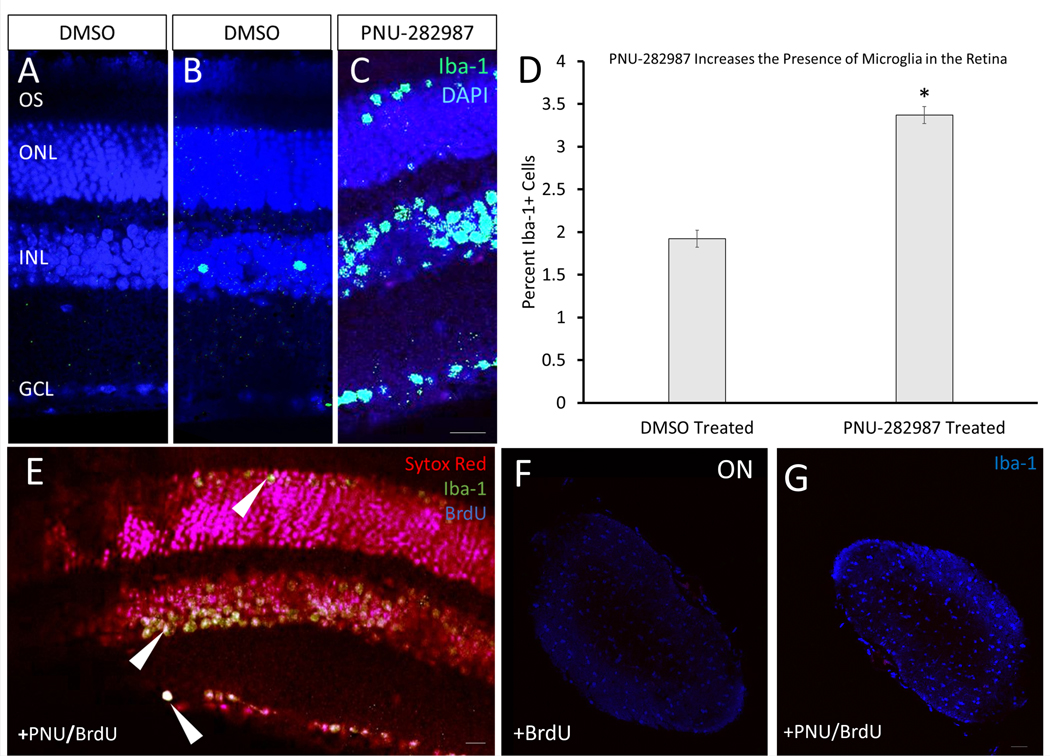
3.7. Application of PNU-282987 Leads to Increased Presence of Microglia in the Retina and Optic Nerve
Microglia are non-retinal immune cells that have been implicated in injury and regenerative responses in the retina (Fisher et al., 2014; Vecino et al., 2016). Interestingly, a small percentage (~10%) of the tdTomato positive labeling in the optic nerve may not be attributed to RGC axons, as some Sytox labeled cells that label nuclei were double-labeled cells for tdTomato (Figure 6H”; arrows). This labeling was consistent with the presence of resident microglia, astrocytes, or oligodendrocytes that occur in the optic nerve (Butt et al., 2004). IHC analysis using antibodies against Iba-1, a marker for microglia, showed that application of PNU-282987 increased the numbers of microglia in the retina (Figure 9). Appearance of microglia in vehicle control treated mice (DMSO) showed microglia localized to the INL almost exclusively (Figure 9B), while after PNU-282987 treatment, microglia were localized in the ONL, INL, and GCL (Figure 9C) at a significantly higher amount (Figure 9D). To determine if some BrdU+ cells seen after application of PNU-282987 could be explained by an increased presence of microglia, cells were triple-labeled with antibodies against BrdU, Iba-1, and the nuclear stain (Sytox Red) after addition of PNU-282987/BrdU+ eye drops for 7 days (Figure 9E). Microglia were responsible for 9.8±2.54% of total BrdU+ cells after PNU-282987 treatment. Additionally, the number of microglia in the optic nerve increased following PNU-282987 treatment (Figure 9F, 9G). Although it is clear that microglia are not responsible for the majority of PNU-282987’s regenerative effect, they likely play a role that should be investigated further.
Figure 9: Increased microglial presence in the PNU-282987-based regenerative response.
(A) Control mice were treated with DMSO vehicle and a no primary antibody control while (B) was treated with DMSO vehicle but was stained for the microglia marker Iba-1, and microglia were seen primarily in the INL. (C) Application of PNU-282987 for 7 days lead to an increased expression of Iba-1+ cells, expanding into the ONL, INL, and GCL. (D) When quantified, Iba-1+ cells increased to 3.37±0.89%, a statistically significant increase from the percentage of Iba-1+ cells found in the DMSO treated retina. (E) Using IHC, BrdU+, Iba-1+, and BrdU+/Iba-1+ cells were quantified. It was determined that new microglia, Iba+/BrdU+/DAPI (triple-labeled cells; arrowheads) compose 9.8±2.54% of BrdU+ cells from application of PNU-282987. (F&G) Mice were treated with BrdU or PNU/BrdU for 7 days and then maintained for 14 days before optic nerves were prepped for IHC. An increase of Iba-1+ microglia is seen with PNU treatment (G) when compared to controls (F) in the optic nerve 10mm back from the optic nerve head. N=6; scale bar = 50 μm; p<0.01.
4. Discussion
Regeneration in the adult mammalian retina does not typically occur. Identification of mechanisms that can trigger regeneration to produce functional new neurons capable of improving or restoring vision is of great interest in regenerative medicine. PNU-282987 has been shown to induce production of MDCPs in adult mice without injury, but it was unknown if the induction of the α7 nAChR with this compound would be sufficient to regenerate neurons lost to neurodegeneration and damage (Webster et al., 2019). Results indicate the mechanism of neurogenesis, as a result of PNU-282987 treatment in adult rats, indicate that MG proliferate in a manner like that of MG in zebrafish and chick retinal regeneration. Specifically, this study demonstrated that MG proliferation begins with BrdU+ MG in the INL. Notably, these studies provide evidence that BrdU+ cells are detected in the GCL much sooner in mice than previously seen in rat. This is consistent with other reports of regeneration in mouse (Elsaeidi et al., 2018; Pirmardan et al., 2018) where various interventions to stimulate regenerative responses in the mouse retina show a relatively rapid timeframe, similar to the results presented here (Hoang et al., 2020). Furthermore, the expression of other cell-cycle markers, including PCNA and Ki67, demonstrate cells entering the cell cycle after PNU-282987 application. Ki67 has previously been shown to be expressed in the retina following high levels of retinal damage (Kaur et al., 2011) but PNU-282987 does not elicit a caspase-mediated damage response (Webster et al., 2017). The ability of PNU-282987 to trigger generation of multiple different types of retinal neurons, as demonstrated in this and prior publications (Webster et al., 2019, 2017) makes it an interesting and exciting candidate for stimulating neuronal regeneration in adult mammalian retina.
Studies of retinal regeneration in mice over the age of 3 months in the past have not been as robust as the results obtained using PNU-282987 (Elsaeidi et al., 2018; Ramachandran et al., 2015; Yao et al., 2018, 2016). The induction of glaucomatous-type damage by elevation of IOP following injection of hypertonic saline to the episcleral vein was found to be a reliable model for ocular hypertension and RGC loss. Application of PNU-282987 after the induction of glaucomatous damage yielded significant numbers of Thy1.2-positive RGCs following 72 hours of treatment. This returned RGC counts to near normal levels, compared to control un-injected animals. The robust regeneration of RGCs induced by PNU-282987 was unexpected based on other reports of RGC regeneration in mouse. In these studies, generation of RGCs from MG required deletion of Ptbp1 (Zhou et al., 2020) or overexpression of Brn3b and Math5 (Xiao et al., 2019) using AAV-mediated vectors which transduced both neurons and glia. These previous studies did not report levels of RGCs as seen with PNU-282987 treatment. Further, these studies did not demonstrate that the reprogrammed cells were generated after treatment with a marker such as BrdU.
However, a key question is whether these new RGCs are functional? Previous literature has reported that a complex treatment scheme is required to regenerate significant axon density 5 mm from the optic nerve in a time span from between 8–10 weeks with limited restoration of function (De Lima et al., 2012). In this study, our lineage tracing analysis demonstrated that new RGC axons were capable of regenerating and extended into the optic nerve at least 6 mm beyond the ONH after only 5 weeks. HPLC MS/MS studies measuring PNU-282987 in the eye after eyedrop application verified detectable amounts of PNU-282987 in the retina as well as in deeper vasculature (Mata et al., 2015), possibly accounting for the more rapid timeline.
Literature suggests that the microenvironment of the optic nerve is inhibitory to regeneration (Yang et al., 2020). However, based on the results obtained in this study, it may be possible that PNU-282987 application triggers interactions in this environment to remove normal existing inhibition. The regeneration of RGC axons is a critical prerequisite for vision restoration as appropriate synapses in the brain must be made before significant change in visual function can occur. Further electrophysiological studies are underway to evaluate PNU-282987-based restoration of functional vision.
In these current studies, the damage caused by MNU injection was consistent with other studies (Chen et al., 2014; Lin et al., 2017; Rösch et al., 2014). Interestingly, PNU-282987 application following MNU-induced loss of photoreceptors led to restoration of photoreceptor density, although not back to pre-damage levels. This is likely due to the fact that RPE cells have been found to direct PNU-282987 based regeneration (Stanchfield et al., 2020; Webster et al., 2019) and the MNU treatment leads to notable perturbations in the RPE cells as well as to the cells in the ONL (Chen et al., 2014). In previous regeneration studies of photoreceptors, new photoreceptor cells were not produced to a level which would support vision restoration (Berry et al., 2019; Ooto et al., 2004). It remains to be seen if the regeneration of photoreceptors due to PNU-282987 supports vision restoration.
Previous studies have demonstrated that late-stage neural progenitors and MG in mice share a common gene expression profile (Blackshaw et al., 2004). A recent study performed in our lab has demonstrated a similar gene expression profile of MDPCs and MG in the PNU-282987 treated system (Stanchfield et al., 2020). Previous studies indicate the ability of MG to re-enter the cell cycle in mammals is variable and dependant on the type and extent of damage, but often does not yield a number of new cells sufficient to replace the damaged neurons (Joly et al., 2011; Ooto et al., 2004). However, studies from our lab have consistently demonstrated that eye drop application of PNU-282987 elicits denovo generation of multiple types of new neurons and yields a significant number of cells to replace damaged cells. In fact, PNU-282987 treatment was able to stimulate regeneration of both RGCs and photoreceptors in response to a significant amount of cell death due to glaucomatous damage and photoreceptor degeneration. Lineage tracing studies using a MG-reporter showed that these new neurons were derived from MG cells, a result that has until now, has not been seen in the mouse retina. This supports the hypothesis that PNU-282987 enables MG to reprogram into MDPCs, which ultimately differentiate into mature neurons. The detailed mechanism of how PNU-282987 elicits this response is currently under investigation.
Recently, literature has demonstrated varying results with manipulation of gene expression to reprogram adult rodent MG into functional neurons using similar methods. These varied results have called into question the validity of using Cre-lox systems as reliable transgenic reporters (Blackshaw and Sanes, 2021). The transgene used in this study has been previously published as being specific to MG within the neuronal retina (Vazquez-Chona et al., 2009) and more importantly, has recently been published as a rigorous transgenic line that remains limited to MG or MG-derived cells within the neuronal retina, even with excessive manipulation (Jorstad et al., 2017). However, does PNU-282987 application allow for “trans-differentiation” of MG, or does PNU-282987 simply allow the transgenic line to become “leaky”? If the transgenic line were “leaky,” PNU-282987 application would cause expression of the TdTomato reporter molecule in other non-glial cells. However, this explanation is not possible in this study based on the experimental schemes used, based on how the CreER/TdTomato system works with the induction of the system several months before experimentation and based on the chemistry behind activation of the reporter system. Though trans-differentiation is a possibility, the RNAseq results from PNU-activated MG demonstrated that the MG express several key progenitor genes including Lhx2, Pax6, and Sox9 after exposure to PNU-282987-treated RPE. Expression of Lhx2 is required to specify the eye field and works with other genes to specify retinal neurogenesis (de Melo et al., 2016; Tétreault et al., 2009). Pax6 is required for the multipotent state of RPCs and is considered the master regulator of RPCs (Marquardt et al., 2001; Raymond et al., 2006; Wan et al., 2012; Wang et al., 2012). Sox9 is known to both induce and maintain neural stem cells (Scott et al., 2010). The significant upregulation of these key genes, in conjunction with the decreased expression of differentiated MG markers, along with the previously published involvement of hbEGF/Ascl1/Lin28a genes would indicate that the MG are differentiating into MDPCs. However, single cell RNAseq is currently underway to assess the identity of the BrdU-positive and TdTomato-positive cells more directly to better answer this issue.
The increased presence and activation of microglia with PNU-282987 application is an interesting phenomenon that has been previously documented in other tissues (Hijioka et al., 2012; Ke et al., 2017; Navarro et al., 2017). Microglia, generally recognized as the resident immune cells of the CNS, play various roles in injury, inflammation, and regeneration in the retina (Butt et al., 2004; Gallina et al., 2014). During retinal regeneration in zebrafish, microglia are activated following injury and migrate to the ONL where they play a role in initiating MG reprogramming (White et al., 2017). Likewise, in chick, activation of microglia is necessary for the formation of MDPCs (Fisher et al., 2014; Gao et al., 2021). Microglia play a key role in regeneration of lower vertebrates that can regenerate on their own. Here we showed that PNU-282987 causes an increase in microglia in the mouse retina, suggesting a potential role for microglia in the PNU-282987 regenerative response. The study of the role of inflammation and microglia in this response is currently underway.
Before PNU-282987 was tested as a neurogenic compound (Webster et al., 2019, 2017), it was shown to be neuroprotective against the loss of RGCs associated with induced glaucoma when the agonist was applied before the induction procedure. (Iwamoto et al., 2014a; Mata et al., 2016, 2015; Wehrwein et al., 2004). However, these current studies focused on regeneration of retinal neurons, as PNU-282987 was applied after specific cell types in the retina were already lost or damaged. This study demonstrated regeneration of new neurons in two injury paradigms using multiple methods. While it is known that the α7 nAChRs play a role in mammalian CNS regeneration in the brain (Shabani et al., 2020), our understanding of the mechanisms by which PNU-282987 stimulates retinal regeneration remains incomplete. In addition to activation of microglia, it is known that PNU-282987 acts on retinal pigment epithelium (RPE) and in response the RPE releases signals that drive MG back into the cell cycle (Webster et al., 2019) and promote MG dedifferentiation to MDPCs (Stanchfield et al., 2020). The molecular response of the RPE with stimulation by PNU-282987 is currently under investigation.
5. Conclusions
The results from this study provides evidence of the regenerative ability of PNU-282987 to elicit new RGCs and new photoreceptors in two different retinal disease models. Specifically, PNU-282987 induced new RGCs with axons that extended into the optic nerve when it was applied after loss of RGCs occurred. In addition, PNU-282987 acted to generated new photoreceptors after loss of photoreceptors occurred following MNU injections. Using tdTomato Müller glia reporter mice, PNU-282987 driven neurogenesis was shown to be initiated from Müller glia. As the adult mammalian retina typically lacks the capability to regenerate neurons lost to many retinal diseases, these results suggest a future clinical potential for using PNU-282987 in the treatment of degenerative retinal diseases.
Supplementary Material
Hypertonic saline injections were performed either once (day 1) or twice (day 1 and day 14) and were left for 28 days. Injected retinas were compared against control uninjected retinas. IHC of Thy1.2 showed that there was a consistent loss of about 30% of RGC cell density after 28 days with either one or two injections. N=8; p≤0.001 (**).
Glaucomatous mice were treated with PNU-282987/BrdU or BrdU only for 6-weeks. Mice were euthanized and retinas were excised and fixed. After fixation, tdTomato fluorescence was examined in the GCL. A) a glaucoma-induced retina with large gaping holes where few surviving RGC bodies remain and a heavy prevalence of MG end feet. B) after PNU-282987 treatment, the tdTomato expression can be seen in some RGC membranes as well as in axons of the RGCs. N=3; scale bar = 50 μm.
Highlights:
PNU-282987 induces regeneration of adult mammalian neurons in all retinal layers
Regeneration of new RGCs after glaucoma induction
Alpha7 nAChR agonist induces BrdU positive photoreceptors after damage
Acknowledgements
A special thanks to Dr. Edward Levine for access to the Cre-ER tdTomato mice line he generated. Figures were prepared with BioRender.com. This research was funded by an NIH NEI grant awarded to C.L. Linn (#EY027970).
Footnotes
Commercial Relationships Disclosure
S.E. Webster, None; N.C. Sklar, None; J.B. Spitsbergen, None; M.L. Stanchfield, None; M.K. Webster, None; D.M. Linn, None; D. Otteson, None; C.L. Linn, None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barnstable CJ, Dräger UC, 1984. Thy-1 antigen: A ganglion cell specific marker in rodent retina. Neuroscience 11, 847–855. 10.1016/0306-4522(84)90195-7 [DOI] [PubMed] [Google Scholar]
- Barres BA, Silverstein BE, Corey DR, Chun LLY, 1988. Immunological, morphological, and electrophysiological variation among retinal ganglion cells purified by panning. Neuron 1, 791–803. 10.1016/0896-6273(88)90127-4 [DOI] [PubMed] [Google Scholar]
- Berry MH, Holt A, Salari A, Veit J, Visel M, Levitz J, Aghi K, Gaub BM, Sivyer B, Flannery JG, Isacoff EY, 2019. Restoration of high-sensitivity and adapting vision with a cone opsin. Nat. Commun. 10, 1–12. 10.1038/s41467-019-09124-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkholz PJ, Goassman CA, Webster MK, Linn DM, Linn CL, 2016. Prevention of Glaucoma-Induced Retinal Ganglion Cell Loss Using Alpha7 nAChR Agonists. J. Ophthalmic Vis. Res. 1, 1–8. [Google Scholar]
- Blackshaw S, Harpavat S, Trimarchi J, Cai L, Huang H, Kuo WP, Weber G, Lee K, Fraioli RE, Cho SH, Yung R, Asch E, Ohno-Machado L, Wong WH, Cepko CL, 2004. Genomic analysis of mouse retinal development. PLoS Biol. 2. 10.1371/journal.pbio.0020247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackshaw S, Sanes JR, 2021. Turning lead into gold: Reprogramming retinal cells to cure blindness. J. Clin. Invest. 131, 1–4. 10.1172/JCI146134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne RRA, Flaxman SR, Braithwaite T, Cicinelli MV, Das A, Jonas JB, Keeffe J, Kempen JH, Leasher J, Limburg H, Naidoo K, Pesudovs K, Resnikoff S, Silvester A, Stevens GA, Tahhan N, Wong TY, Taylor HR, 2017. Magnitude, temporal trends, and projections of the global prevalence of blindness and distance and near vision impairment : a systematic review and meta-analysis. Lancet Glob. Heal. 5. 10.1016/S2214-109X(17)30293-0 [DOI] [PubMed] [Google Scholar]
- Brown B, Weiss ER, Craft CM, 2014. Mouse cone arrestin expression pattern: Light induced translocation in cone photoreceptors Retinal degeneration View project Rhodopsin interaction with its binding partners View project. [PubMed] [Google Scholar]
- Butt AM, Pugh M, Hubbard P, James G, 2004. Functions of optic nerve glia: Axoglial signalling in physiology and pathology. Eye 18, 1110–1121. 10.1038/sj.eye.6701595 [DOI] [PubMed] [Google Scholar]
- Chen Y, Liu S, Hu D, Xing Y, Shen Y, 2014. N -methyl- N -nitrosourea-induced retinal degeneration in mice. Exp. Eye Res. 121, 102–113. 10.1016/j.exer.2013.12.019 [DOI] [PubMed] [Google Scholar]
- Craft CM, Whitmore DH, Wiechmann AF, 1994. Cone arrestin identified by targeting expression of a functional family. J. Biol. Chem. 269, 4613–4619. 10.1016/s0021-9258(17)41820-5 [DOI] [PubMed] [Google Scholar]
- De Lima S, Koriyama Y, Kurimoto T, Oliveira JT, Yin Y, Li Y, Gilbert HY, Fagiolini M, Martinez AMB, Benowitz L, 2012. Erratum: Full-length axon regeneration in the adult mouse optic nerve and partial recovery of simple visual behaviors (Proceedings of the National Academy of Sciences of the United States of America (2012) 109, 23, (9149–9154) doi: 10.1073/pnas.1119449109). Proc. Natl. Acad. Sci. U. S. A. 109, 13465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Melo J, Zibetti C, Clark BS, Hwang W, Miranda-Angulo AL, Qian J, Blackshaw S, 2016. Lhx2 is an essential factor for retinal gliogenesis and notch signaling. J. Neurosci. 36, 2391–2405. 10.1523/JNEUROSCI.3145-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dizhoor AM, Ray S, Kumar S, Niemi G, Spencer M, Brolley D, Walsh KA, Philipov PP, Hurley JB, Stryer L, 1991. Recoverin: A calcium sensitive activator of retinal rod guanylate cyclase. Science (80-. ). 4996, 915–918. 10.1126/science.1672047 [DOI] [PubMed] [Google Scholar]
- Dmitrieva NA, Strang CE, Keyser KT, 2007. Expression of alpha 7 nicotinic acetylcholine receptors by bipolar, amacrine, and ganglion cells of the rabbit retina. J. Histochem. Cytochem. 55, 461–476. 10.1369/jhc.6A7116.2006 [DOI] [PubMed] [Google Scholar]
- Drager UC, Olsen JF, 1981. Ganglion cell distribution in the retina of the mouse. IOVS 20, 285–293. [PubMed] [Google Scholar]
- Duncan J, Kersigo J, Gray B, Fritzsch B, 2011. Combining Lipophilic dye, in situ Hybridization, Immunohistochemistry, and Histology. JoVE e2451. 10.3791/2451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsaeidi XF, Macpherson XP, Mills EA, Jui XJ, Flannery XJG, Goldman D, 2018. Notch Suppression Collaborates with Ascl1 and Lin28 to Unleash a Regenerative Response in Fish Retina, But Not in Mice. J. Neurosci. 38, 2246–2261. 10.1523/JNEUROSCI.2126-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorchak MV, Conner IP, Medina CA, Wingard JB, Schuman JS, Little SR, 2014. 28-Day Intraocular Pressure Reduction With a Single Dose of Brimonidine Tartrate-Loaded Microspheres. Exp. Eye Res. 125, 210–216. 10.1016/j.exer.2014.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher AJ, Zelinka C, Gallina D, Scott M, Todd L, 2014. Reactive microglia and macrophage facilitate the formation of Muller glia-derived retinal progenitors. Glia 62, 1608–1628. 10.1002/glia.22703.Reactive [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallina D, Todd L, Fischer AJ, 2014. A comparative analysis of Muller glia-mediated regeneration in the vertebrate retina. Exp. Eye Res. 123, 121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamm D, Wong R, 2015. Report on the National Eye Institute audacious goals initiative: photoreceptor regeneration workshop. Trans Vis Sci Tech 4, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Luodan A, Huang X, Chen X, Xu H, 2021. Müller Glia-Mediated Retinal Regeneration. Mol. Neurobiol. 10.1007/s12035-020-02274-w [DOI] [PubMed] [Google Scholar]
- Gemberling M, Bailey TJ, Hyde DR, Poss KD, 2013. The zebrafish as a model for complex tissue regeneration. Trends Genet. 29, 611–620. 10.1016/j.tig.2013.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D, 2014. retina regeneration 15. 10.1038/nrn3723 [DOI] [Google Scholar]
- Gossman CA, Linn DM, Linn C, 2016. Glaucoma-inducing Procedure in an In Vivo Rat Model and Whole-mount Retina Preparation. J. Vis. Exp. 109. 10.3791/53831 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajos M, Hurst RS, Hoffmann WE, Krause M, Wall TM, Higdon NR, Groppi VE, 2005. The Selective a7 Nicotinic Acetylcholine Receptor Agonist chlorobenzamide Hydrochloride Enhances GABAergic Synaptic Activity in Brain Slices and Restores Auditory Gating Deficits in Anesthetized Rats 312, 1213–1222. 10.1124/jpet.104.076968.CHRNA7 [DOI] [PubMed] [Google Scholar]
- Hartong DT, Berson EL, Dryja TP, 2006. Retinitis pigmentosa. Lancet 368, 1795–1809. 10.1016/S0140-6736(06)69740-7 [DOI] [PubMed] [Google Scholar]
- Hijioka M, Matsushita H, Ishibashi H, Hisatsune A, Isohama Y, Katsuki H, 2012. α7 Nicotinic acetylcholine receptor agonist attenuates neuropathological changes associated with intracerebral hemorrhage in mice. Neuroscience 222. 10.1016/j.neuroscience.2012.07.024. [DOI] [PubMed] [Google Scholar]
- Hitchcock P, Ochocinska M, Sieh A, Otteson D, 2004. Persistent and injury-induced neurogenesis in the vertebrate retina 23, 183–194. 10.1016/j.preteyeres.2004.01.001 [DOI] [PubMed] [Google Scholar]
- Hitchcock P, Raymond P, 1992. Retinal Regeneration. Trends Neurosci. 15, 103–108. 10.1016/0166-2236(92)90020-9 [DOI] [PubMed] [Google Scholar]
- Hoang T, Wang J, Boyd P, Wang F, Santiago C, Jiang L, Yoo S, Lahne M, Todd LJ, Jia M, Saez C, Keuthan C, Palazzo I, Squires N, Campbell WA, Rajaii F, Parayil T, Trinh V, Kim DW, Wang G, Campbell LJ, Ash J, Fischer AJ, Hyde DR, Qian J, Blackshaw S, 2020. Gene regulatory networks controlling vertebrate retinal regeneration. Science (80-. ). 8598, eabb8598. 10.1126/science.abb8598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto K, Birkholz P, Schipper A, Mata D, Linn DM, Linn CL, 2014a. A nicotinic acetylcholine receptor agonist prevents loss of retinal ganglion cells in a glaucoma model. Investig. Ophthalmol. Vis. Sci. 55, 1078–1087. 10.1167/iovs.13-12688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto K, Birkholz P, Schipper A, Mata D, Linn DM, Linn CL, 2014b. A Nicotinic Acetylcholine Receptor Agonist Prevents Loss of Retinal Ganglion Cells in a Glaucoma Model. IVOS. 10.1167/iovs.13-12688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobs TC, Libby RT, Ben Y, John SWM, Masland RH, 2005. Retinal ganglion cell degeneration is topological but not cell type specific in DBA/2J mice. J. Cell Biol. 171, 313–325. 10.1083/jcb.200506099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly S, Pernet V, Samardzija M, Grimm C, 2011. PAX6-positive müller glia cells express cell cycle markers but do not proliferate after photoreceptor injury in the mouse retina. Glia 59, 1033–1046. 10.1002/glia.21174 [DOI] [PubMed] [Google Scholar]
- Jorstad NL, Wilken MS, Grimes WN, Wohl SG, Vandenbosch LS, Yoshimatsu T, Wong RO, Rieke F, Reh TA, 2017. Stimulation of functional neuronal regeneration from Müller glia in adult mice. Nature 548, 103–107. 10.1038/nature23283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur J, Mencl S, Sahaboglu A, Farinelli P, van Veen T, Zrenner E, Ekström P, Paquet-Durand F, Arango-Gonzalez B, 2011. Calpain and PARP activation during photoreceptor cell death in P23H and S334ter rhodopsin mutant rats. PLoS One 6, 1–11. 10.1371/journal.pone.0022181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke P, Shao B, Xu Z, Chen X, Wei W, Liu C, 2017. Activating α7 nicotinic acetylcholine receptor inhibits NLRP3 inflammasome through regulation of β-arrestin-1. CNS Neurosci Ther 23, 875–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipfer-Kauer A, McKinnon SJ, Frueh BE, Goldblum D, 2010. Distribution of amyloid precursor protein and amyloid-β in ocular hypertensive C57BL/6 mouse eyes. Curr. Eye Res. 35, 828–834. 10.3109/02713683.2010.494240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirtland K, Saaddine J, Geiss L, Thompson T, Cotch M, Lee P, 2015. Geographic disparity of severe vision loss - United States, 2009–2013. MMWR Morb Mortal Wkly Rep 64, 513–517. [PMC free article] [PubMed] [Google Scholar]
- Lahne M, Hyde DR, 2016. Interkinetic Nuclear Migration in the Regenerating Retina. Adv Exp Med Biol 854, 587–593. [DOI] [PubMed] [Google Scholar]
- Lahne M, Nagashima M, Hyde DR, Hitchcock PF, 2020. Reprogramming Müller Glia to Regenerate Retinal Neurons. Annu. Rev. Vis. Sci. 6, 1–23. 10.1146/annurev-vision-121219-081808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenkowski JR, Raymond PA, 2014. Müller glia: Stem cells for generation and regeneration of retinal neurons in teleost fish. Prog. Retin. Eye Res. 40, 94–123. 10.1016/j.preteyeres.2013.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin FL, Lin CH, Ho J. Der, Yen JL, Chang HM, Chiou GCY, Cheng YW, Hsiao G, 2017. The natural retinoprotectant chrysophanol attenuated photoreceptor cell apoptosis in an N-methyl-N-nitrosourea-induced mouse model of retinal degenaration. Sci. Rep. 7, 1–13. 10.1038/srep41086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn CL, Webster SE, Webster MK, 2018. Eye Drops for Delivery of Bioactive Compounds and BrdU to Stimulate Proliferation and Label Mitotically Active Cells in the Adult Rodent Retina. Bio-protocol 8, e3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zhang Y, He Y, Zhao J, Su G, 2015. Progress in histopathologic and pathogenesis reasarch in a retinitis pigmentosa model. Histol. Histopathol. 30, 771–779. 10.14670/HH-11-596 [DOI] [PubMed] [Google Scholar]
- Mahajan VB, Skeie JM, Assefnia AH, Mahajan M, Tsang SH, 2011. Mouse eye enucleation for remote high-throughput phenotyping. J. Vis. Exp. 1–5. 10.3791/3184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maneu V, Gerona G, Fernández L, Cuenca N, Lax P, 2010. Evidence of alpha 7 nicotinic acetylcholine receptor expression in retinal pigment epithelial cells. Vis. Neurosci. 27, 139–147. 10.1017/S0952523810000246 [DOI] [PubMed] [Google Scholar]
- Marquardt T, Ashery-padan R, Andrejewski N, Scardigli R, Guillemot F, Gruss P, Chemistry MB, Inserm C, Pasteur L, Ce I, 2001. Pax6 Is Required for the Multipotent State of Retinal Progenitor Cells. Cell 105, 43–55. 10.1016/s0968-0004(05)00043-5 [DOI] [PubMed] [Google Scholar]
- Mata D, Linn DM, Linn CL, 2015. Retinal ganglion cell neuroprotection induced by activation of alpha7 nicotinic acetylcholine receptors. Neuropharmacology 99, 337–346. 10.1016/j.neuropharm.2015.07.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata D, Linn DM, Linn CL, Universit M, 2016. Retinal ganglion cell neuroprotection induced by activation of alpha7 nicotinic acetylcholine receptor. Neuropharmacology 337–346. 10.1016/j.neuropharm.2015.07.036.Retinal [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcginnis JF, Stepanik PL, Jariangprasert S, Lerious V, 1997. Rapid Communication Functional Significance of Recoverin Localization in Multiple Retina Cell Types, J. Neurosci. Res. Wiley-Liss, Inc. 10.1002/(SICI)1097-4547(19971101)50:3 [DOI] [PubMed] [Google Scholar]
- Morrison JC, Moore CG, Deppmeier LM, Gold BG, Meshul CK, Johnson EC, 1997. A rat model of chronic pressure-induced optic nerve damage. Exp. Eye Res. 64, 85–96. 10.1006/exer.1996.0184 [DOI] [PubMed] [Google Scholar]
- Navarro E, Gonzalez-Lafuente L, Perez-Liebana I, Buendia I, Lopez-Bernado E, Sanchez-Ramos C, Prieto I, Cuadrado A, Satrustegui J, Cadenas S, Monsalve M, Lopez MG, 2017. Heme-Oxygenase I and PCG-1α Regulate Mitochondrial Biogenesis via Microglial Activation of Alpha7 Nicotinic Acetylcholine Receptors Using PNU282987. Antioxid Redox Signal 27, 93–105. [DOI] [PubMed] [Google Scholar]
- Ooto S, Akagi T, Kageyama R, Akita J, Mandai M, Honda Y, Takahashi M, 2004. Potential for neural regeneration after neurotoxic injury in the adult mammalian retina. Proc. Natl. Acad. Sci. U. S. A. 101, 13654–13659. 10.1073/pnas.0402129101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Openshaw A, Branham K, Heckenlively J, 2008. Understanding Retinitis Pigmentosa. Univ. Michigan Kellogg Eye Cent. 1–28. [Google Scholar]
- Osakada F, Ooto S, Akagi T, Mandai M, Akaike A, Takahashi M, 2007. Wnt Signaling Promotes Regeneration in the Retina of Adult Mammals 27, 4210–4219. 10.1523/JNEUROSCI.4193-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park YH, Snook JD, Ostrin EJ, Kim S, Chen R, Frankfort BJ, 2019. Transcriptomic profiles of retinal ganglion cells are defined by the magnitude of intraocular pressure elevation in adult mice. Sci. Rep. 9, 1–12. 10.1038/s41598-019-39141-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirmardan ER, Soheili Z, Samiei S, Ahmadieh H, 2018. In Vivo Evaluation of PAX6 Overexpression and NMDA Cytotoxicity to Stimulate Proliferation in the Mouse Retina. Sci. Rep. 1–10. 10.1038/s41598-018-35884-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pran S, Sardar B, Münch R, Schäfer P, Oertel P, Sykes AM, Zhu Y, Karl MO, 2017. Retinal cell death dependent reactive proliferative gliosis in the mouse retina 1–16. 10.1038/s41598-017-09743-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran R, Fausett BV, Goldman D, 2015. Ascl1a regulates Müller glia dedifferentiation and retinal regeneration through a Lin - 28-dependent, let - 7 microRNA signalling pathway 12. 10.1038/ncb2115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond PA, Barthel LK, Bernardos RL, Perkowski JJ, 2006. Molecular characterization of retinal stem cells and their niches in adult zebrafish. BMC Dev. Biol. 6, 1–17. 10.1186/1471-213X-6-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reh TA, Levine EM, 1998. Multipotential Stem Cells and Progenitors in the Vertebrate Retina. [PubMed] [Google Scholar]
- Rösch S, Johnen S, Mataruga A, Müller F, Pfarrer C, Walter P, 2014. Selective photoreceptor degeneration by intravitreal injection of N-methyl-N-nitrosourea. Investig. Ophthalmol. Vis. Sci. 55, 1711–1723. 10.1167/iovs.13-13242 [DOI] [PubMed] [Google Scholar]
- Saito Y, Miranda-Rottmann S, Ruggiu M, Park CY, Fak JJ, Zhong R, Duncan JS, Fabella BA, Junge HJ, Chen Z, Araya R, Fritzsch B, Hudspeth AJ, Darnell RB, 2016. NOVA2-mediated RNA regulation is required for axonal pathfinding during development. Elife 5, 1–29. 10.7554/eLife.14371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlamp CL, Li Y, Dietz JA, Janssen KT, Nickells RW, 2006. Progressive ganglion cell loss and optic nerve degeneration in DBA/2J mice is variable and asymmetric. BMC Neurosci. 7, 1–14. 10.1186/1471-2202-7-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott CE, Wynn SL, Sesay A, Cruz C, Cheung M, Gaviro MVG, Booth S, Gao B, Cheah KSE, Lovell-Badge R, Briscoe J, 2010. SOX9 induces and maintains neural stem cells. Nat. Neurosci. 13, 1181–1189. 10.1038/nn.2646 [DOI] [PubMed] [Google Scholar]
- Shabani Z, Mahmoudi J, Farajdokht F, Sadigh-Eteghad S, 2020. An Overview of Nicotinic Cholinergic System Signaling in Neurogenesis. Arch. Med. Res. 10.1016/j.arcmed.2020.03.014 [DOI] [PubMed] [Google Scholar]
- Shintani K, Schechtman D, Gurwood A, 2009. Review and Update: Current Treatment Trends for Patients with Retinitis Pigmentosa. Optometry 80, 384–402. 10.1016/j.optm.2008.01.026 [DOI] [PubMed] [Google Scholar]
- Stanchfield ML, Webster SE, Webster MK, Linn CL, 2020. Involvement of HB-EGF / Ascl1 / Lin28a Genes in Dedifferentiation of Adult Mammalian Müller Glia. Front. Mol. Biosci. 7, 1–17. 10.3389/fmolb.2020.00200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strom RC, Williams RW, 1998. Cell production and cell death in the generation of variation in neuron number. J. Neurosci. 18, 9948–9953. 10.1523/jneurosci.18-23-09948.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y, Chen T, Fang W, Yan Z, Yang Q, Huang Y, Yu L, Fan L, 2017. The comparative efficiency of intraperitoneal and intravitreous injection of hydrogen rich saline against N-Methyl-N-nitrosourea induced retinal degeneration: A topographic study. Front. Pharmacol. 8, 587. 10.3389/fphar.2017.00587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tétreault N, Champagne MP, Bernier G, 2009. The LIM homeobox transcription factor Lhx2 is required to specify the retina field and synergistically cooperates with Pax6 for Six6 trans-activation. Dev. Biol. 327, 541–550. 10.1016/j.ydbio.2008.12.022 [DOI] [PubMed] [Google Scholar]
- Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY, 2014. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 121, 2081–2090. 10.1016/j.ophtha.2014.05.013 [DOI] [PubMed] [Google Scholar]
- Thummel R, Kassen SC, Enright JM, Nelson CM, Montgomery JE, Hyde DR, 2008. Characterization of Muller glia and neuronal progenitors during adult zebrafish retinal regeneration 87, 433–444. 10.1016/j.exer.2008.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Soest S, Westerveld A, De Jong P, Bleeker-Wagemakers E, Bergen A, 1999. Retinitis Pigmentosa: Defined from a Molecular Point of View. Surv. Opthalmology 43, 321–334. 10.1016/S0039-6257(98)00046-0 [DOI] [PubMed] [Google Scholar]
- Vazquez-Chona FR, Clark AM, Levine EM, 2009. Rlbp1 promoter drives robust Muller glia GFP expression in transgenic mice. Invest Ophthalmol Vis Sci 50, 3996–4003. [DOI] [PubMed] [Google Scholar]
- Vecino E, Rodriguez FD, Ruzafa N, Pereiro X, Sharma SC, 2016. Glia-neuron interactions in the mammalian retina. Prog. Retin. Eye Res. 51, 1–40. 10.1016/j.preteyeres.2015.06.003 [DOI] [PubMed] [Google Scholar]
- Vetter M, Hitchcock PF, 2017. Report on the National Eye Institute audacious goals inititative: replacement of retinal ganglion cells from endogenous cell sources. Trans Vis Sci Tech 6, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DP, Wishka DG, Piotrowski DW, Jia S, Reitz SC, Yates KM, Myers JK, Vetman TN, Margolis BJ, Jacobsen EJ, Acker BA, Groppi VE, Wolfe ML, Thornburgh BA, Tinholt PM, Cortes-burgos LA, Walters RR, Hester MR, Seest EP, Dolak LA, Han F, Olson BA, Fitzgerald L, Staton BA, Raub TJ, Hajos M, Hoffmann WE, Li KS, Higdon NR, Wall TM, Hurst RS, Wong EHF, Rogers BN, 2006. Design, synthesis, structure – activity relationship, and in vivo activity of azabicyclic aryl amides as a 7 nicotinic acetylcholine receptor agonists. Bioorganic 14, 8219–8248. 10.1016/j.bmc.2006.09.019 [DOI] [PubMed] [Google Scholar]
- Wan J, Ramachandran R, Goldman D, 2012. HB-EGF Is Necessary and Sufficient for Müller Glia Dedifferentiation and Retina Regeneration. Dev. Cell 22, 334–347. 10.1016/j.devcel.2011.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Sugano E, Isago H, Murayama N, Tamai M, Tomita H, 2012. Notch signaling pathway regulates proliferation and differentiation of immortalized Müller cells under hypoxic conditions in vitro. Neuroscience 214, 171–180. 10.1016/j.neuroscience.2012.04.025 [DOI] [PubMed] [Google Scholar]
- Webster MK, Barnett BJ, Stanchfield ML, Paris JR, Webster SE, Cooley-themm CA, Levine EM, Otteson DC, Linn CL, 2019. Stimulation of Retinal Pigment Epithelium With an a 7 nAChR Agonist Leads to Muller Glia Dependent Neurogenesis in the Adult Mammalian Retina. Investig. Ophthalmol. Vis. Sci. 60, 570–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster MK, Cooley-themm CA, Barnett JD, Bach HB, Vainner M, Webster SE, Linn CL, 2017. Evidence of BrdU Positive Retinal Neurons after Application of an Alpha7 Nicotinic Acetylchoine Receptor Agonist 437–446. 10.1016/j.neuroscience.2017.01.029.Evidence [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrwein E, Thompson SA, Coulibaly SF, Linn DM, Linn CL, 2004. Acetylcholine Protection of Adult Pig Retinal Ganglion Cells from Glutamate-Induced Excitotoxicity. Invest. Ophthalmol. Vis. Sci. 45, 1531–1543. 10.1167/iovs.03-0406 [DOI] [PubMed] [Google Scholar]
- Weinreb RN, Aung T, Medeiros FA, 2014. The Pathophysiology and Treatment of Glaucoma. J. Med. Am. Assoc. 311, 1901. 10.1001/jama.2014.3192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White DT, Sengupta S, Saxena MT, Xu Q, Hanes J, Ding D, Ji H, Mumm JS, 2017. Immunomodulation-accelerated neuronal regeneration following selective rod photoreceptor cell ablation in the zebrafish retina. Proc. Natl. Acad. Sci. U. S. A. 114, E3719–E3728. 10.1073/pnas.1617721114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao D, Qiu S, Huang X, Zhang R, Lei Q, Huang W, Chen H, Gou B, Tie X, Liu S, Liu Y, Jin K, Xiang M, 2019. Directed robust generation of functional retinal ganglion cells from Müller glia. bioRxiv 735357. 10.1101/735357 [DOI] [Google Scholar]
- Yang SG, Li CP, Peng XQ, Teng ZQ, Liu CM, Zhou FQ, 2020. Strategies to Promote Long-Distance Optic Nerve Regeneration. Front. Cell. Neurosci. 14, 1–10. 10.3389/fncel.2020.00119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao K, Qiu S, Tian L, Snider WD, Flannery JG, Schaffer DV, Chen B, 2016. Wnt Regulates Proliferation and Neurogenic Potential of Muller Glial Cells via a Lin28/let-7 miRNA-Dependent Pathway in Adult Mammalian Retinas. Cell Rep. 17, 165–178. 10.1016/j.celrep.2016.08.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao K, Qiu S, Yanbin V, Park SJH, Mohns EJ, Mehta B, Liu X, Chang B, Zenisek D, Crair MC, Demb JB, Chen B, 2018. Restoration of vision after de novo genesis of rod photoreceptors in mammalian retinas. Nature. 10.1038/s41586-018-0425-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Su J, Hu X, Zhou C, Li H, Chen Z, Xiao Q, Wang B, Wu W, Sun Y, Zhou Y, Tang C, Liu F, Wang L, Feng C, Liu M, Li S, Zhang Y, Xu H, Yao H, Shi L, Yang H, 2020. Glia-to-Neuron Conversion by CRISPR-CasRx Alleviates Symptoms of Neurological Disease in Mice. Cell 181, 590–603.e16. 10.1016/j.cell.2020.03.024 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Hypertonic saline injections were performed either once (day 1) or twice (day 1 and day 14) and were left for 28 days. Injected retinas were compared against control uninjected retinas. IHC of Thy1.2 showed that there was a consistent loss of about 30% of RGC cell density after 28 days with either one or two injections. N=8; p≤0.001 (**).
Glaucomatous mice were treated with PNU-282987/BrdU or BrdU only for 6-weeks. Mice were euthanized and retinas were excised and fixed. After fixation, tdTomato fluorescence was examined in the GCL. A) a glaucoma-induced retina with large gaping holes where few surviving RGC bodies remain and a heavy prevalence of MG end feet. B) after PNU-282987 treatment, the tdTomato expression can be seen in some RGC membranes as well as in axons of the RGCs. N=3; scale bar = 50 μm.