Abstract
INTRODUCTION:
Brain tissue was adversely affected by renal ischemia-reperfusion injury (renal IRI) in several studies. Moreover, we are awareness that kidney diseases are gender dependent, but there is not enough evidence of the impact of gender on renal IRI-induced brain injury. Hence, this study was designed to investigate gender differences in renal IRI-induced brain tissue injury in adult rats.
MATERIALS AND METHODS:
Forty Wistar rats (four groups) include two main groups (20 male and 20 female). Each of them was divided into two subgroups including 1 and 2: male and female sham-operated groups and 3and 4: male and female ischemia (ISC) groups were exposed to renal ischemia for 45 min and then 24 h reperfusion (male and female ISC 24 h). Sham groups were exposed to surgery without ischemia process. After reperfusion time, blood samples were obtained for the renal function measurements. The kidney and brain were removed and were fixed in a 10% formalin solution for pathological assessment. The left kidney was used to measure malondialdehyde (MDA) and nitrite.
RESULTS:
Renal IRI increased significantly levels of creatinine, blood urea nitrogen, kidney weight, and damage score in both genders (P < 0.05). Furthermore, brain injuries were significantly higher following 24 h of reperfusion in male and female groups. Serum nitrite level and MDA concentration of female rats decreased significantly in ISC 24 h group (P < 0.05) but not in male rats.
CONCLUSION:
The brain tissue of both genders, male and female, is affected by renal IRI as a remote organ. Female sex hormones may indicate a protective role against IR by the nitric oxide pathway and antioxidant signaling.
Keywords: Brain tissue, ischemia-reperfusion injury, remote organ
Introduction
The high mortality and morbidity rate during acute kidney injury (AKI) mainly refers to extrarenal complications. Renal ischemia-reperfusion injury (renal IRI) occurs following of renal surgeries, transplantation, sepsis, and partial nephrectomy.[1,2,3] During the IRI injury, reactive oxygen species (ROS) and release of vasoactive factors promote the inflammatory process locally and systemically. The release of chemokines, cytokines, ROS, peroxynitrite, and prostaglandins in the circulatory system results in remote organ injury such as liver, pancreas, lung, and brain tissues, referred to as “multiple organ dysfunction syndrome.”[3,4,5] Some studies documented the damage of the central nervous system during kidney diseases. Brain insult in the different levels of structure, biochemistry, inflammation, and behavior reported renal ischemia consequence.[6,7,8,9,10,11] Inflammatory and functional changes of male mice’s brain following ischemic AKI have been shown by Liu et al.[10] Most of these researches have been done on male rats, but it is certain that kidney diseases are gender related.[12,13,14,15,16] In this regard, Park et al. showed that kidney dysfunction occurs just 30 min after bilateral renal ischemia in males but in females after 60 min of ischemia. Hence, renal ischemia progression is faster in males. Moreover, the males had a higher mortality rate than females after kidney IR.[17] In another study, females showed lower levels of blood urea nitrogen (BUN) and serum creatinine (Cr) and less severe tubular necrosis following kidney IRI compared to males.[18] Gender difference in renal IRI is related to important factors including sex hormones testosterone and estrogen or ratio of testosterone/estrogen.[16] Moreover, studies suggest that gender influences the severity of brain damages and recovery of them. The male gender was more susceptible to ischemic stroke, cerebral hemorrhage, and vascular injury. It is known that the female sex hormone, estrogen, provides a protective effect in brain ischemia and stroke in male and female.[19] Estrogen starts a cascade of cellular and subcellular reactions after the ischemia causes stability of blood–brain barrier (BBB) and decrease of brain edema. In fact, the estrogen is an antioxidant and vasodilatory hormone that prevents inflammation and lipid peroxidation and increases the cerebral blood flow. Supplementation of estrogen in animals likely improves the memory function after ischemia.[19] Based on these information, renal IRI and brain damages are distinctly affected by gender. On the other side, most studies on renal IRI-induced remote organ injury were done in the male gender. Hence, the present study was designed to investigate gender differences in brain injuries following bilateral renal IRI (45 min) and reperfusion (24 h) in adult male and female rats. We consider this issue to know whether gender will affect histology of the brain following renal IRI or not. The answer to this question can determine the possibility of gender-specific treatments for this issue.
Materials and Methods
Animals
Forty adult Wistar rats including 20 male and 20 female animals (weighting 200 ± 20 g) (Animal Center, Hormozgan University of Medical Sciences, Bandar Abbas, Iran) were used and maintained under standard conditions with 12 h light/12 h dark cycle. All animals had free access to standard rat chow and water. The protocol of the study was approved by the Ethics Committee of Hormozgan University of Medical Sciences (Ethics code: (IR.HUMS.REC.1398.423)).
Surgery and experimental protocol
The rats were assigned to four groups (n = 10 in each groups) including: group 1: sham-operated male, Group 2: sham-operated female, and 3 and 4 Groups: male and female rats were exposed to renal ischemia for 45 min and then 24 h reperfusion (male and female ISC 24 h).
To induce bilateral renal IRI model, the animals were weighed and injected ketamine (75 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.) to anesthetize them. Two small incisions were made in the dorsal area of the animals. The fascia was carefully removed and kidneys were taken out healthy. Both renal artery and vein were clamped at the renal hilum for 45 min. Then, the clamps were removed and blood flowed to kidney again. The kidneys were observed for about 10 min to ensure reperfusion. Then, all incisions were closed and rats were kept in the animal room during the reperfusion phase, 24 h. At the end of reperfusion time, the animals were anesthetized again and blood samples were drawn via heart puncture. In sham-operated rats, all procedures were done except clamping of renal vessels. Thereafter, rats were sacrificed and removed kidney and brain tissue samples. To obtain serum sample, blood sample was centrifuged at 2,500 g for 10 min and stored at −80°C for further processing. The serum levels of BUN, Cr, nitrite, and malondialdehyde (MDA) were measured.
The kidneys and brain tissues were weighted immediately after removal of the body. The kidney weight (KW) was normalized to the body weight (BW) and reported as tissue kW/100 g of BW. The right kidney and brain tissues were fixed in 10% formalin for histopathological damages investigation. The left kidney was transferred into liquid nitrogen very quickly and then was stored at −80°C refrigerator until MDA and nitrite measurement.
Biochemical assay
The serum levels of Cr and BUN were determined, using quantitative diagnostic kits (Pars Azmoon, Iran). Serum and kidney nitrite levels (stable metabolite of NO) were measured using an assay kit (Promega Corporation, USA). The nitrite concentration of samples was determined by comparison with the nitrite standard reference curve. The serum and kidney levels of MDA were quantified according to the thiobarbituric acid method.[20]
Histopathological procedures
After fixing of the kidney and brain tissue in 10% formalin solution, they embedded in paraffin for histopathological staining, hematoxylin and eosin stain. The kidney damage evaluated such as the presence of tubular atrophy, ischemic necrosis, inflammation, vessel congestion, hyaline casts, vacuolization, and debris. Damage intensity and percentage were scored as 1–4, while score zero was assigned to normal tissue. Presence of congestion, necrosis, and gliosis evaluated to examine the brain tissue damage.
Statistical analysis
The data were presented as mean ± standard error of mean differences between groups in serum levels of BUN, Cr, NO, and MDA, and kidney levels of MDA and NO, KW, were compared with each other by one-way analysis of variance, followed by Turkey’s post hoc test. Due to the qualitative nature of scoring, Kruskal–Wallis and Mann–Whitney tests were used to compare the pathological damage score of the groups. Values of P < 0.05 were considered statistically significant.
Results
The effect of renal ischemia-reperfusion injury on biochemical and pathological markers in kidneys of male and female rats
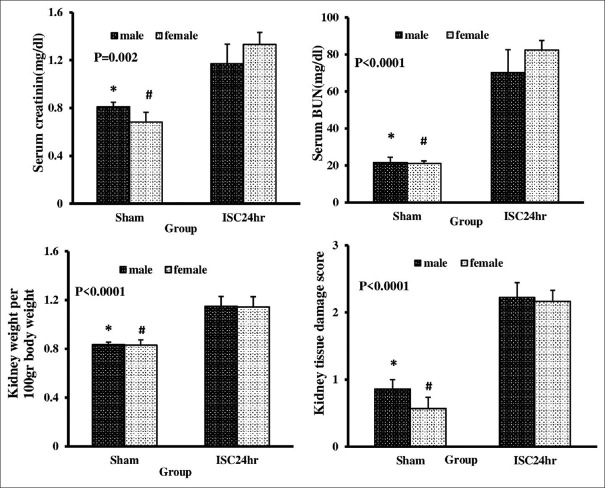
Renal IRI increased significantly levels of Cr, BUN, KW, and damage score in both of gender (P < 0.05). Hence, it is proved that the kidneys of both genders are structurally and functionally affected by renal IRI [Figures 1 and 2].
Figure 1.
Serum and kidney levels of blood urea nitrogen and creatinine, kidney weight, and kidney tissue damage score in sham and ISC 24 h groups in both genders. *Significant difference between male groups of sham and ISC 24 h, #Significant difference between sham and ISC 24 h groups in female gender (P < 0.05)
Figure 2.
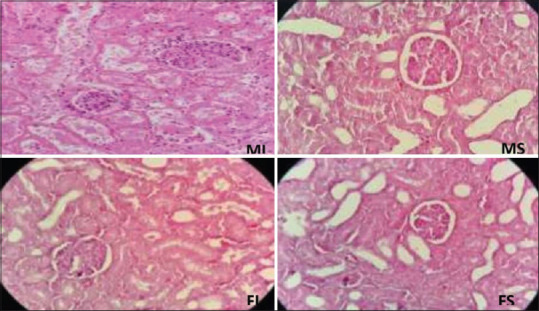
The effect of ischemia-reperfusion on renal histologic injury. Male sham, female sham, male ischemia, and female ischemia
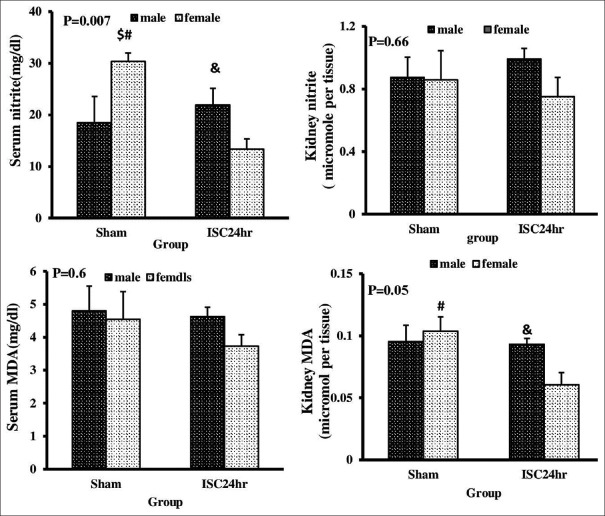
The effect of renal ischemia-reperfusion injury on nitrite and malondialdehyde levels of serum and kidney in male and female rats
The renal MDA concentration and serum nitrite level decreased significantly in the female group after renal IR, but this observation was not in the male group. These factors showed a statistically significant difference between genders. Renal MDA concentration and serum nitrite concentration were remarkably reduced in ischemic female rats compared to ischemic male rats. However, there was no statistically significant difference in serum MDA and renal nitrite concentration after renal IR and we did not also observe gender difference in these parameters [Figure 3].
Figure 3.
Serum and kidney levels of nitrite and malondialdehyde in sham and ISC 24 h groups in both genders. #Significant difference between sham and ISC 24 h groups in female gender. &Significant difference between male and female groups of ISC24 h. $Significant difference between male and female groups of sham (P < 0.05)
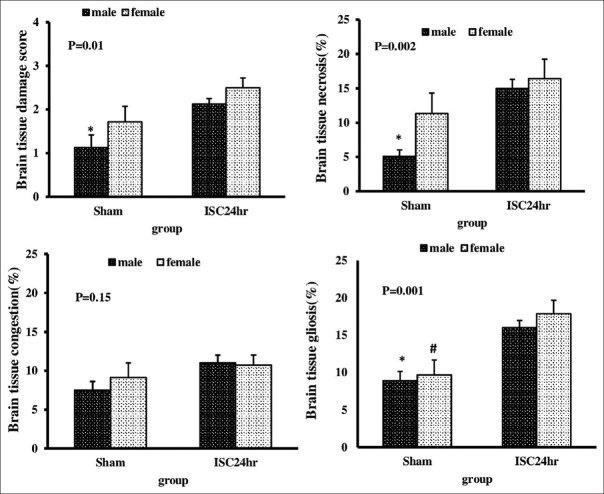
The effect of renal ischemia-reperfusion injury on brain damage score in male and female rats
Brain damage score had a significant increase in male rats during 24 h reperfusion time, but this observation was not in female groups (P < 0.05) [Figures 4 and 5]. In males, necrosis and gliosis took place significantly after 24 h of reperfusion, whereas in females, just gliosis injuries were shown.
Figure 4.
Degree of brain tissue damage in sham and ISC24 h groups in both genders. *Significant difference between sham and ISC24 h groups in male gender. #Significant difference between sham and ISC24 h groups in female gender (P < 0.05)
Figure 5.
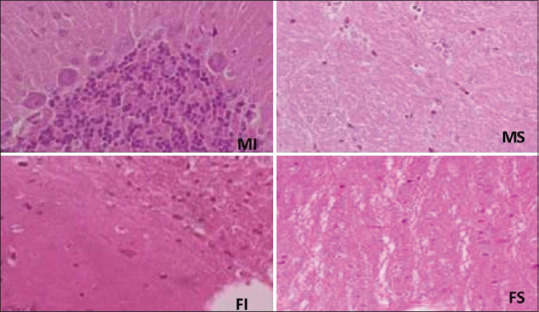
Brain tissue histologic damages following renal ischemia-reperfusion injury. Male sham, female sham, male ischemia, and female ischemia
Discussion
Based on high mortality in AKI patients and brain injury following the renal IRI, we examined the influence of renal IRI-induced brain injury in male and female rats. The main result of this study was the brain histology damage in male and female rats during renal IRI. This renal IRI model results in raise of Cr, BUN, KW, and kidney damage score as indexes of renal functional and structural insult in both genders that showed our result is in line with the other researches.[9,17,18,21] Also in both genders, histology of kidney proved tubular damages such as necrosis, atrophy, presence of hyaline cast, vacuolization, inflammation, and congestion of vessels. In line with this result, Fekete et al. and Park et al. showed that levels of serum BUN and Cr increase continuously during the different times of 2, 16, and 24 h after renal reperfusion. Furthermore, they indicated these parameters and kidney tissue damage scores were higher in males than females.[18,21] In the study of Muller, gender difference was not observed in kidney damage score, which was consistent with our study.[22] Hence, it can be considered that the course length of ischemia and reperfusion is important in renal IRI-induced gender difference.
It is believed that nitric oxide (NO), as a product of nitric oxide synthases (NOSs), regulates blood flow gender differently.[23,24] NO is produced by three isoforms of NOSs with names endothelial NO synthase (eNOS), inducible NO synthase (iNOS), and neuronal NO synthase. Some characteristics are known for NO such as antiapoptotic, anti-inflammatory, and vasodilator factors.[16] In this study, we examined nitrite as a stable metabolite of NO for the evaluation of endothelial dysfunction. There are gender differences in serum nitrite concentration between the groups. Interestingly, the reduction of level of serum nitrite following the renal IRI was done only in female gender. NO is abundant in the circulatory system and the kidney tissue and the physiological and pathological functions of NO are mediated through three isoforms of NOS (eNOS, iNOS, and nNOS). It is certain that there are multiple pathways of NO generation in tissues and blood due to the different sources of NO in biological tissues and plasma. Therefore, in the ischemic model of this study, renal nitrite changes have started in female gender, but the plasma level has not changed yet. The observations of changes in serum nitrite may depend on gender, the severity of the tissue damage, and reperfusion time. In addition, the inhibition of NO release can significantly increase renal vascular resistance and reduce renal blood flow.[25] Increased iNOS activity promotes pro-inflammatory cytokines and peroxynitrite formation, leading to lipid peroxidation. On the other side, eNOS production can protect the kidney damage through suppresses inflammatory responses. One of the largest meta-analyses to evaluate gender differences in the progression of kidney disease on 11,000 patients from 60 different studies revealed that females show slower progress in various kidney diseases, such as chronic kidney disease, polycystic kidney disease, and membranous glomerulonephritis compared to males.[14] Based on the studies, female sex hormones can indicate a protective role against RIR. The administration of estrogen attenuates renal dysfunctions in ischemia-reperfusion injury, characterized by NO pathway through the reduction in iNOS activity and promoting eNOS in female animals.[26,27] In line with these results, our previous study indicated that treatment with estradiol valerate can influence renal hemodynamic and improve renal blood flow through the NO pathway.[25] In this study, estrogen might be reduced nitrite during ischemia by inhibition of iNOS, in a gender-dependent manner. Therefore, the effects of NO on kidney structure and function can be beneficial or harmful and depend on its concentration, NOS activity as well as the role of sex hormones.
We evaluated the MDA levels of serum and kidneys as the end products of cellular lipid peroxidation. Some works documented increments of MDA level during IRI due to stress oxidative conditions.[28,29] In our research, we observed a decrease in kidney level of MDA in the female group and there was a sex difference between both genders but did not significantly change the serum level of MDA in any groups. In line with our research, MDA level had not significantly changed after 24 h of renal reperfusion in the studies of Rasoulian et al. and Askaripour et al.[30,31] Besides, in our previous study in male rats, after 72 h of reperfusion, we did not observe a significant change in the serum and kidney levels of MDA.[32,33] It is thought that increasing of MDA concentration occurs in initial times of renal reperfusion, but it returns to near-normal levels 24 h later.[34] It is known that sex difference in kidney levels of MDA may be related to antioxidant properties of the female sex hormones, estrogen, that can reduce free radicals.[35] Hence, increased activity of superoxide dismutase or other antioxidant enzymes leads to a reduction in the MDA level.[36] Moreover, as previously discussed about the reduction of female nitrite level following IRI, we think that the decrement of kidney MDA level in female gender may be due to the increased antioxidant enzymes and reduction of iNOS activity by estrogen in the present study.
The key point is patients with acute and chronic renal failure often exhibit neurological complications such as cerebrovascular disease, neuropathy, and cognitive injuries. Cytokine-induced damage, infiltration of leukocytes, oxidative stress, and imbalance of water and electrolytes may promote brain injuries during renal IRI.[11] In our study, there are significant damages in brain tissue after 24 h of renal reperfusion in male rats. Pathological examination distinguished the presence of brain vascular congestion, gliosis, and tissue necrosis. Our data are in line with Chou et al. and Liu et al.’s studies that had created renal IRI (60 min) followed with 2 and 24 h reperfusion in mice and the result of Firouzjaei et al. in male Sprague–Dawley rats. Their result showed brain injuries by increasing activated microglial cells (gliosis), BBB permeability, neuronal pyknosis, and the elevation of levels of pro-inflammatory chemokine in the cerebral cortex and hippocampus and severe memory loss.[7,8,10] Moreover, ischemia injury with 24-h reperfusion induced systemic and neuronal inflammation and neuronal apoptosis in the hippocampus.[9] It is known that the hippocampus is responsible for memory and learning.[7] Hence, these changes progress losing of memory. The mechanism of these deleterious effects refers to the release systemic inflammatory factors such as interleukin (IL)-1B, IL-8, and tumor necrosis factor-alpha during AKI and caused the brain dysfunction. Moreover, in the cerebral cortex and corpus callosum of the brain, raised glial fibrillary acidic protein is a special marker of cellular inflammation in AKI complication. Finally, these inflammatory responses can damage the endothelium, BBB, and blood–cerebrospinal fluid barrier that are responsible for cerebral homeostasis. AKI induced-inflammatory cascade responses trigger disruption of BBB integrity and then by increasing of the BBB permeability and changes in neuronal homeostasis can enter disorderly proteins, amino acids, more inflammatory cells, more cytokines, and complement into the brain tissue. In our previous study, male brain tissue was more susceptible compare to female brain tissue following 3 h renal reperfusion.[37] Furthermore, a model of unilateral carotid occlusion for 3 h on male and female gerbils and induction of brain ischemia showed less severe brain injuries in female compared to male, 24 h later.[38]
Conclusion
It seems that the gender difference of renal IRI-induced brain pathophysiology is related to several factors such as reperfusion times and surgery model and may be affected by the influence of ischemia situation on NO system and oxidant and antioxidant balance in male and female animals. Moreover, the key point is estrogen, as an antioxidant agent, can improve renal AKI-induced brain damage by reducing oxidative stress. The protective effects of estradiol against IR appear to be mediated by the NO pathway and antioxidant signaling. Gender differences in kidney dysfunction and remote organ after renal IRI may relate to different procedures of inducing of renal IRI, animals species, and also may be affected by reperfusion time.
Financial support and sponsorship
The project was supported by the Deputy of Research of Hormozgan University of Medical Sciences.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Nongnuch A, Panorchan K, Davenport A. Brain-kidney crosstalk. Crit Care. 2014;18:225. doi: 10.1186/cc13907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paladino JD, Hotchkiss JR, Rabb H. Acute kidney injury and lung dysfunction: A paradigm for remote organ effects of kidney disease? Microvasc Res. 2009;77:8–12. doi: 10.1016/j.mvr.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kao CC, Yang WS, Fang JT, Liu KD, Wu VC. Remote organ failure in acute kidney injury. J Formos Med Assoc. 2019;118:859–66. doi: 10.1016/j.jfma.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 4.Malek M, Nematbakhsh M. Renal ischemia/reperfusion injury; from pathophysiology to treatment. J Renal Inj Prev. 2015;4:20–7. doi: 10.12861/jrip.2015.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dorweiler B, Pruefer D, Andrasi TB, Maksan SM, Schmiedt W, Neufang A, et al. Ischemia- reperfusion injury. Eur J Trauma Emerg Surg. 2007;33:600–12. doi: 10.1007/s00068-007-7152-z. [DOI] [PubMed] [Google Scholar]
- 6.Kovalčíková A, Gyurászová M, Vavrincová-Yaghi D, Vavrinec P, Tóthová Ĺ, Boor P, et al. Oxidative stress in the brain caused by acute kidney injury. Metab Brain Dis. 2018;33:961–7. doi: 10.1007/s11011-018-0204-8. [DOI] [PubMed] [Google Scholar]
- 7.Firouzjaei MA, Haghani M, Moosavi SM. Renal ischemia/reperfusion induced learning and memory deficit in the rat: Insights into underlying molecular and cellular mechanisms. Brain Res. 2019;1719:263–73. doi: 10.1016/j.brainres.2019.05.018. [DOI] [PubMed] [Google Scholar]
- 8.Chou AH, Lee CM, Chen CY, Liou JT, Liu FC, Chen YL, et al. Hippocampal transcriptional dysregulation after renal ischemia and reperfusion. Brain Res. 2014;1582:197–210. doi: 10.1016/j.brainres.2014.07.030. [DOI] [PubMed] [Google Scholar]
- 9.Karimi N, Haghani M, Noorafshan A, Moosavi SM. Structural and functional disorders of hippocampus following ischemia/reperfusion in lower limbs and kidneys. Neuroscience. 2017;358:238–48. doi: 10.1016/j.neuroscience.2017.06.058. [DOI] [PubMed] [Google Scholar]
- 10.Liu M, Liang Y, Chigurupati S, Lathia JD, Pletnikov M, Sun Z, et al. Acute kidney injury leads to inflammation and functional changes in the brain. J Am Soc Nephrol. 2008;19:1360–70. doi: 10.1681/ASN.2007080901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu R, Kiernan MC, Murray A, Rosner MH, Ronco C. Kidney-brain crosstalk in the acute and chronic setting. Nat Rev Nephron. 2015;11:707. doi: 10.1038/nrneph.2015.131. [DOI] [PubMed] [Google Scholar]
- 12.Saberi S, Dehghani A, Nematbakhsh M. Role of Mas receptor in renal blood flow response to angiotensin-(1-7) in ovariectomized estradiol treated rats. Res Pharm Sci. 2016;11:65–72. [PMC free article] [PubMed] [Google Scholar]
- 13.Takayama J, Takaoka M, Sugino Y, Yamamoto Y, Ohkita M, Matsumura Y. Sex difference in ischemic acute renal failure in rats: Approach by proteomic analysis. Biol Pharm Bull. 2007;30:1905–12. doi: 10.1248/bpb.30.1905. [DOI] [PubMed] [Google Scholar]
- 14.Neugarten J, Acharya A, Silbiger SR. Effect of gender on the progression of nondiabetic renal disease: A meta-analysis. J Am Soc Nephrol. 2000;11:319–29. doi: 10.1681/ASN.V112319. [DOI] [PubMed] [Google Scholar]
- 15.Dehghani A, Saberi S, Nematbakhsh M. Role of mas receptor antagonist a799 in renal blood flow response to ang 1-7 after bradykinin administration in ovariectomized estradiol-treated rats. Adv Pharmacol Sci 2015. 2015 doi: 10.1155/2015/801053. 801053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kher A, Meldrum KK, Wang M, Tsai BM, Pitcher JM, Meldrum DR. Cellular and molecular mechanisms of sex differences in renal ischemia-reperfusion injury. Cardiovasc Res. 2005;67:594–603. doi: 10.1016/j.cardiores.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Park KM, Kim JI, Ahn Y, Bonventre AJ, Bonventre JV. Testosterone is responsible for enhanced susceptibility of males to ischemic renal injury. J Biol Chem. 2004;279:52282–92. doi: 10.1074/jbc.M407629200. [DOI] [PubMed] [Google Scholar]
- 18.Fekete A, Vannay Á, Vér Á, Rusai K, Muller V, Reusz G, et al. Sex differences in heat shock protein 72 expression and localization in rats following renal ischemia-reperfusion injury. Am J Physiol Renal Physiol. 2006;291:F806–11. doi: 10.1152/ajprenal.00080.2006. [DOI] [PubMed] [Google Scholar]
- 19.Vagnerova K, Koerner IP, Hurn PD. Gender and the injured brain. Anesth Analg. 2008;107:201–14. doi: 10.1213/ane.0b013e31817326a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Satoh K. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin Chim Acta. 1978;90:37–43. doi: 10.1016/0009-8981(78)90081-5. [DOI] [PubMed] [Google Scholar]
- 21.Fekete A, Vannay Á, Vér Á, Vásárhelyi B, Müller V, Ouyang N, et al. Sex differences in the alterations of Na+, K+-ATPase following ischaemia–reperfusion injury in the rat kidney. J Physiol. 2004;555:471–80. doi: 10.1113/jphysiol.2003.054825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Müller V, Losonczy G, Heemann U, Vannay Á, Fekete A, Reusz G, et al. Sexual dimorphism in renal ischemia-reperfusion injury in rats: possible role of endothelin. Kidney Int. 2002;62:1364–71. doi: 10.1111/j.1523-1755.2002.kid590.x. [DOI] [PubMed] [Google Scholar]
- 23.Weiner CP, Lizasoain I, Baylis SA, Knowles RG, Charles IG, Moncada S. Induction of calcium-dependent nitric oxide synthases by sex hormones. Proc Natl Acad Sci U S A. 1994;91:5212–6. doi: 10.1073/pnas.91.11.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayashi T, Ishikawa T, Yamada K, Kuzuya M, Naito M, Hidaka H, et al. Biphasic effect of estrogen on neuronal constitutive nitric oxide synthase via Ca2+-calmodulin dependent mechanism. Biochem Biophys Res Commun. 1994;203:1013–9. doi: 10.1006/bbrc.1994.2283. [DOI] [PubMed] [Google Scholar]
- 25.Dehghani A, Saberi S, Nematbakhsh M. Nitric oxide synthases blockade; L-NAME and estradiol alter renal blood flow response to angiotensin 1-7 in ovariectomized rats. Indian J Physiol Pharmacol. 2015;59:266–74. [Google Scholar]
- 26.Shibata Y, Takaoka M, Maekawa D, Kuwahara C, Matsumura Y. Involvement of nitric oxide in the suppressive effect of 17beta-estradiol on endothelin-1 overproduction in ischemic acute renal failure. J Cardiovasc Pharmaco. 2004;44:S459–61. doi: 10.1097/01.fjc.0000166315.38258.e1. [DOI] [PubMed] [Google Scholar]
- 27.Lekontseva O, Chakrabarti S, Jiang Y, Cheung CC, Davidge ST. Role of neuronal nitric-oxide synthase in estrogen-induced relaxation in rat resistance arteries. J Pharmacol Exp Ther. 2011;339:367–75. doi: 10.1124/jpet.111.183798. [DOI] [PubMed] [Google Scholar]
- 28.Demirbilek S, Karaman A, Baykarabulut A, Akin M, Gürünlüoğlu K, TÜRkmen E, et al. Polyenylphosphatidylcholine pretreatment ameliorates ischemic acute renal injury in rats. Int J Urol. 2006;13:747–53. doi: 10.1111/j.1442-2042.2006.01397.x. [DOI] [PubMed] [Google Scholar]
- 29.Bernardi RM, Constantino L, Machado RA, Vuolo F, Budni P, Ritter C, et al. N-acetylcysteine and deferrioxamine protects against acute renal failure induced by ischemia/reperfusion in rats. Rev Bras Ter Intensiva. 2012;24:219–23. [PubMed] [Google Scholar]
- 30.Rasoulian B, Jafari M, Noroozzadeh A, Mehrani H, Wahhab-Aghai H, Hashemi-Madani S, et al. Effects of ischemia-reperfusion on rat renal tissue antioxidant systems and lipid peroxidation. Acta Med Iran. 2008;46:353–60. [Google Scholar]
- 31.Askaripour M, Tabatabaei S, Hosseini F, Varzi H. Effect of purslane (Portulaca oleracea) on renal ischemia/reperfusion injury in rat. Int J Pharm Pharm Sci. 2015;7:467–71. [Google Scholar]
- 32.Azarkish F, Nematbakhsh M, Fazilati M, Talebi A, Pilehvarian AA, Pezeshki Z, et al. N-acetylcysteine prevents kidney and lung disturbances in renal ischemia/reperfusion injury in rat. Int J Prev Med. 2013;4:1139–46. [PMC free article] [PubMed] [Google Scholar]
- 33.Moeini M, Nematbakhsh M, Fazilati M, Talebi A, Pilehvarian AA, Azarkish F, et al. Protective role of recombinant human erythropoietin in kidney and lung injury following renal bilateral ischemia-reperfusion in rat model. Int J Prev Med. 2013;4:648–55. [PMC free article] [PubMed] [Google Scholar]
- 34.Tucci Junior S, Carvalho RM, Celini FM, Cologna AJ, Suaid HJ, Tirapelli LF, et al. Renal ischemia and reperfusion injury: Influence of chorpromazine on renal function and lipid peroxidation. Acta Cir Bras. 2008;23(Suppl 1):42–6. doi: 10.1590/s0102-86502008000700008. [DOI] [PubMed] [Google Scholar]
- 35.Strehlow K, Rotter S, Wassmann S, Adam O, Grohé C, Laufs K, et al. Modulation of antioxidant enzyme expression and function by estrogen. Circ Res. 2003;93:170–7. doi: 10.1161/01.RES.0000082334.17947.11. [DOI] [PubMed] [Google Scholar]
- 36.Kim J, Kil IS, Seok YM, Yang ES, Kim DK, Lim DG, et al. Orchiectomy attenuates post-ischemic oxidative stress and ischemia/reperfusion injury in mice. A role for manganese superoxide dismutase. J Biol Chem. 2006;281:20349–56. doi: 10.1074/jbc.M512740200. [DOI] [PubMed] [Google Scholar]
- 37.Armin F, Azarkish F, Atash Ab Parvar A, Dehghani A. The effect of gender on brain tissue changes induced by renal ischemia-reperfusion injury in adult rats. Int Electron J Med. 2019;8:113–8. [Google Scholar]
- 38.Hall ED, Pazara KE, Linseman KL. Sex differences in postischemic neuronal necrosis in gerbils. J Cereb Blood Flow Metab. 1991;11:292–8. doi: 10.1038/jcbfm.1991.61. [DOI] [PubMed] [Google Scholar]