Abstract
After the introduction of shunt treatment for the management of childhood hydrocephalus, a wide variety of complications related to this treatment modality have been recognized. The entity of slit ventricle syndrome (alternatively, symptomatic ventricular coaptation) is one of them, is frequently encountered in the pediatric population and its symptom complex resembles that of shunt failure. We conducted research on PubMed®, MEDLINE®, and Web of Science®, using the keywords: “slit ventricles,” “slit ventricle syndrome,” “SVS” and “ventricular coaptation.” The aim of our review was to trace the advances made through the past decades, concerning our knowledge about the clinical characteristics, pathophysiology, and treatment options of this entity. The discrepancy among researchers about the offending etiology and the optimum treatment algorithm of this entity, as well as the necessity of an updated concept regarding shunt over drainage is analyzed. The multiple treatment modalities proposed and pathophysiologic mechanisms implicated for the treatment of slit ventricle syndrome illustrate the complexity of this entity. Consequently, the issue requires more detailed evaluation. In this review, we comment on all the main facets related to shunt over drainage and the resultant slit ventricle syndrome.
Keywords: Anti-siphon device, programmable valve, slit ventricle, upgrade of opening pressure
Introduction
Before the 1950s, the outcome of patients with hydrocephalus who remained untreated was extremely dismal. More precisely, approximately 49% of patients had died by the end of the 20-year observation period and among the survivors, only 38% had an intelligence quotient > 85.[1,2] The modern era of management for hydrocephalic patients began in 1949 with Nulsen and Spitz inserting a unidirectional, pressure-responsive valve into a ventriculo-venous shunt system.[3,4,5]
The first case report of over-drainage of cerebrospinal fluid was probably that reported by Dandy in 1932, who described the sudden and rapid drainage of cerebrospinal fluid (CSF) leading after surgery to intracranial hypotension, along with ventricular collapse. However, Becker, in 1968, was the first to introduce the term “over-drainage” in the literature.[6,7] A few years later, Portnoy emphasized on the role of postural change (siphoning) in the genesis of over-drainage and developed a control device for its management.[6,7]
The introduction of shunting devices substantially improved the natural history of the entity named hydrocephalus but was accompanied by a constellation of complications. Indeed, although the development of valve-regulated shunts has decreased the morbidity of children suffering from hydrocephalus and improved their neuro-cognitive outcome,[8,9,10] a wide variety of long-term complications arose, not being able to be predicted from the beginning. The most common and most chronic of these newly recognized conditions is the association of chronic headaches with the existence of a shunt. Severe headache disorder in patients with shunts and small ventricular dimensions has been termed the “slit-ventricle syndrome,”[11] but, undoubtedly, is not a single pathological entity. Instead, several different pathophysiological mechanisms are considered to be implicated, which could potentially underlie this constellation of findings.[12] Slit ventricle syndrome is currently universally accepted to be one of those complications[13] and its severity and complexity are indicated by the extended number of literature referrals and papers that are dedicated to it, even since 1980 and so on.[14,15,16,17,18]
Materials and Methods
Our review included articles, including case reports, previous reviews, technical notes, and clinical studies, centered on the entity of slit ventricle syndrome (SVS). We conducted research on PubMed®, MEDLINE®, and Web of Science®, using the keywords: “slit ventricles,” “slit ventricle syndrome,” “SVS” and “ventricular coaptation.” At first, we used Web of Science to conduct a bibliometric analysis of the results and thus identify the most prominent articles on this topic. Besides, we utilized PubMed® and MEDLINE® to enrich our results with the latest published articles.
Discussion
SVS is a chronic complication of ventriculoperitoneal shunt placement for the treatment of hydrocephalus. It is observed in 4%–37% of patients who undergo shunt procedures.[12,14,18,19,20]
A common and not completely resolved point of controversy and confusion among researchers, which reflects in the formulation of a treatment algorithm, is the demarcation of a consensus about the definition of slit ventricle syndrome. A commonly encountered pitfall in the vast majority of papers dedicated to slit ventricle syndrome emerges from the fact that it is rarely specified which clinical pattern case reports are referring to.[1] Radiologically, up to 50% of shunted children may fulfill the criteria of the entity of slit ventricle and the patients suffering from headaches may be categorized under at least 5 clinical groups.[21,22,23] This ambiguity could be considered a possible source of the confusion related to slit ventricle syndrome. As a consequence, it could be regarded as a potential obstacle, which is related to the inability to formulate widely accepted treatment algorithms.
The most widely accepted clinical patterns which may be associated radiologically with slit ventricles are: True over-drainage with negative pressure, an on-off symptom complex, recurring proximal ventricular dysfunction, chronic subdural collections due to shunt over-drainage and headaches unrelated to shunt function. Reviewing literature reports regarding SVS, we encountered an early report of researcher’s experience with this condition, who defined SVS as a triad involving the following: Intermittent headaches lasting 10–90 min, small ventricles on imaging studies, and slow refilling of the pumping mechanism of the valve.[11] Lage considered that the most appropriate term to describe this entity was “symptomatic ventricular collapse.” The relevant clinical picture was consisting of headache and vomiting, along with different degrees of altered consciousness, combined with imaging findings showing very small ventricles and slow filling of the valve reservoir.[6] Nowadays, according to the literature, the underlying pathophysiologic mechanism that is presumed to be responsible for over-drainage is related to what has become known as “shunt-related headaches.” Most researchers agree with the recommendation that the true slit ventricle syndrome is mainly a manifestation of the on-off symptom complex. Based on that, their recommendation is that this should be known as the non-compliant ventricle syndrome. The presentation is a constellation of intermittent clinical features, consisting of shunt obstruction, slit-like appearance of ventricles on computed tomography scanning and slow refill of shunt pumping devices.
A great controversy exists regarding the exact pathophysiologic substrate of this entity, which definitively interacts with the adoption of the most efficient treatment strategy.
Another important issue is the clinical status of the majority of these patients suffering from SVS. Regardless of the predominant mode of their clinical presentation, most of them report from their medical history an operation for shunt placement during their first year of life.[24] The onset of symptomatology varies, with the vast majority of them being referred between 3–5 years after first shunt introduction. The concepts of shunt-related headaches and slit ventricle syndrome, with which the former is associated, have been accepted almost universally, though with subtle differences. Cheok has published a historical timeline of siphoning, over-drainage, and slit ventricle syndrome.[7]
An interesting report regarding the pathophysiology of the syndrome and the possible role of a lumbo-peritoneal shunt in its management is presented by Khorasani et al.,[3] and is supported by another report.[25] More precisely, they supported the theory that by introducing a shunt into a closed system, like the ventricular cavities, the system is supposed to adjust until a new equilibrium is reached. After the insertion of an intraventricular catheter, an equilibrium must be reached between forces tending to dilate and contract the ventricle. Forces that are destined to contract the ventricles include increased elastance over time due to maturation-associated changes such as myelination and glial proliferation. This is supported by the fact that the peak prevalence of SVS exists between 5 and 10 years of age in children who have been shunted for the first time at infancy.[26,27] Another force resisting ventricular dilation in patients with ventriculo-peritoneal shunt (VPS) is the increase in elastance attributed to subependymal gliosis promoted by the long-term presence of ventricular drainage.[28] Along with the increased elastance of the brain parenchyma over time causing the ventricles to collapse, patients with a VPS often have depressed intraventricular pulse pressure. This is considered to be an important force in dilating the ventricles. Hydraulic and mechanical mismatching of CSF shunts to individual patients may lead to over-drainage of CSF, resulting in the development of the entity of SVS.
Shunt over-drainage represents a pathophysiologic entity that is related with considerable controversy when its pathogenetic substrate is being investigated. According to our current scientific data, we could support the hypothesis that drainage of CSF should not be regarded as the sole mechanism that underlies this condition. A fact that is against the theory that the only implicated factor is CSF over-drainage relates to the recording that intracranial pressure (ICP) levels are often disproportionately elevated.[29] A very well presented and documented analysis of the most prevalent underlying etiologies is written by Ros et al.,[6] which recognizes the following theories as the pathophysiologic substrate of SVS:
Theory of acquired craniocerebral disproportion (supra and/or infratentorial)
This theory proposed that slit ventricle syndrome should be considered as a consequence of early suture ossification, which was a secondary effect associated with drainage of CSF via a working shunt. Insertion of a VPS in newborns and infants has as a consequence the remodeling of the skull, which leads to a craniocerebral disproportion. In the majority of the aforementioned patients, a shunt has been inserted during the first year of life and they were associated with remarkable microcephaly. Based on the doctrine of Monro-Kellie, we could explain the resultant intracranial hypertension, along with the existence of tonsillar herniation below the foramen magnum, which is described as acquired or secondary Chiari I. Imaging finding consistent with tonsillar herniation were, initially, more frequently encountered in patients harboring lumboperitoneal, rather than ventriculoperitoneal shunts. However, Rekate[8] was unable to verify this proposed relationship between lumboperitoneal shunts and Chiari I, a finding that is in contradistinction to that theory. However, the management protocol that is considered to be the most suitable for the patients that are categorized under this entity (acquired craniocerebral disproportion) is the expansion of the supratentorial cranial compartment, while the existing shunt is maintained.
Theory of periventricular gliosis or “stiff ventricle”
The development of gliotic scar tissue around the ventricles has been considered as been associated with the existence of a chronic CSF drainage system. When insertion of a new ventricular catheter is performed, this process could result in an increased resistance when canulation of the ventricular system is attempted. This is called “stiff ventricle” and it may be an obstacle to the development of ventricular enlargement when failure of the shunt system is verified. The pathological finding of periventricular gliosis could be the equivalent, in anatomical terms, to a system that is characterized by the presence of increased elastance. Contrary to that theory are studies centered on patients that are characterized by overdrainage, which did not reveal significant differences related to the existence of reactive astroglia in the periventricular white matter when normal subjects were compared with children who had died due to shunt failure and associated ventricular dilatation.[8] Additionally, If the inability of the ventricular system to increase its dimensions was merely associated with periventricular gliosis, this would not permit the transmission of pressure. Because of that, a pressure gradient would be established. This concept was not verified by experimental work which compared intraventricular pressure with intraparenchymal pressure using bolus infusion.[8]
Theory of venous congestion and increased cerebral elastance
Patients who are subject to episodes of hypotension which is alternated with hypertension of shunt overdrainage are amenable to the development of a state characterized by reduced compliance, or, in different words, increased elastance or stiffness. This pathological situation renders patients particularly susceptible to minor alterations of ICP measurements. Elastance constitutes an intrinsic property of the brain and it refers to the resistance to distortion (viscoelastic properties). It is largely determined by the volume of the venous compartment, along with the functionality of the mechanisms that determine the venous outflow of the cerebrum. Indeed, when overdrainage of CSF is evident, this is related to ICP values below zero this fact is associated, based on Monro-Kellie doctrine, with venous congestion. This pathophysiologic cascade leads to increased brain elastance. When shunt failure occurs, ICP increases and this may provoke the development of increased transmural pressure, with the resultant sudden collapse of bridging veins to the major venous sinuses. This sequence of events could be the generative cause for the development of a model of positive feedback between venous and intracranial hypertension. This mechanism could only support the concept of treatment of a group of patients suffering from shunt overdrainage via the aid of a lumboperitoneal shunt.
Theory of ventricular isolation
This theory has been suggested based on the changes on the morphometric characteristics of the ventricles that are encountered in cases of chronically inserted drainage systems. When overdrainage is evident, asymmetry in the dimensions of the ventricular system may develop, which consists of collapse of the ventricular system harboring the central catheter, resulting in its occlusion. Additionally, enlargement of the contralateral ventricle may ensue. This sequence of events may be transitory, a concept that is compatible with the intermittent nature of the clinical presentation which characterizes the slit ventricle syndrome. When the distal tip of the central catheter is occluded, the CSF compartment that refers to the ventricular system becomes entrapped, and this could be related to the contralateral ventricular enlargement, along with the resultant intracranial hypertension. Based on that concept, slit ventricle syndrome could be regarded as synonymous to ventricular isolation. This model provides support to the management plan which consists of treating a specific subgroup of patients suffering from shunt overdrainage by the establishment of communication of the isolated compartments with the rest of the ventricular system via the aid of neuroendoscopy.
Theory of capillary absorption laziness
Some researchers consider that in patients who manifest chronic overdrainage (in the setting of a ventriculoperitoneal shunting), the pressure within the ventricles, as well as within the subarachnoid space of the cerebrum ranges in low, or even in negative, settings.[6] When acute shunt malfunction becomes evident, there is no effective compensatory mechanism for the acute management of the increased intraventricular and extracellular fluid pressures. This condition is presumed to lead to cerebral edema due to the accumulation of extracellular fluid.
Theory of the pulsatile vector for shunt over-drainage
A newly proposed theory about the development of the slit ventricle syndrome is centered on an alteration in the transmission of the arterial cerebral pulsatility to the CSF. It mainly refers to patients with shunt systems draining CSF from the extrathecal compartment. According to this hypothesis, the treatment of shunt overdrainage could be based on the reduction of the pulse wave transmission to outside the extracranial compartment. To achieve that, they proposed the use of devices capable to regulate the antigravitational drainage, to prevent the uncontrolled drainage of the CSF during activities of daily living.
Theory of the siphon effect
The theory of the siphon effect justifies the management of over-drainage by systems to offset the siphon effect, the so-called antisiphon devices, or other systems that increase the resistance to drainage across the valve.[30,31,32] These antisiphon systems could be used preventively in the first shunt implantation or at the time of revision due to malfunction, attempting to reduce the rates of ventricular catheter obstruction and the probability of developing symptomatic shunt over-drainage.[33] Conclusively, it appears that multifactorial pathophysiology should be responsible for the development of SVS.[1,3] These newer theories about the genesis of SVS share in common with previous studies the demographic characteristics and risk factors of the patients included in this group. More precisely, they mention that symptoms more often appear before the age of 10 years, generally between the ages of 2 and 5 years,[34] though others have reported a peak at 6.5 years.[35] A clinical observation related to slit ventricle syndrome is that it is less common in children with hydrocephalus associated with cerebral palsy and relevant atrophy.[29] Buxton reported a mean age at diagnosis of 7–9 years,[21] although the transition from normal ventricles to ventricular collapse can occur later and is therefore difficult to be predicted with accuracy.[36] One of the most widely accepted risk factors is shunt implantation during the first months of life.[29,34,37] Other risk factors are related to hemorrhage[38] (posthemorrhagic hydrocephalus of the newborn or premature infant), infection (neonatal meningitis), valve malfunction (more common in valves that have been functioning well for several years),[20] and the hydrodynamic properties of the valve.[20] The latter issue is more evident with the use of low differential pressure opening valves that is considered by some researchers some the most undisputed predictor for the development of slit ventricle syndrome.[39] The fact that the time of first intervention for treatment of hydrocephalus is an important part of the history is supported by a recent review article.[40]
Another important issue in the management of the entity of SVS is the surgical treatment of this entity and the orientation of our treatment goals. Based on our current knowledge about the pathophysiology of SVS, the standard treatment for shunt over-drainage is the reduction of CSF flow across the valve system. This can be accomplished by placement of a device that counteracts the siphon effect, ideally additionally increasing the valve opening pressure,[20] or by using self-adjusting valves or flow control systems.[39] A large cohort studies centered on the relevant efficacy of flow control versus anti-siphon valves[37] concluded that early development of SVS could be addressed by raising the opening pressure of the inserted valve early in the clinical course of the patient, an option that is easily to be accomplished when a programmable valve is inserted.[37,41]
Despite these modern treatment options, the rate of recurrence continues to be relatively high and other treatment options should be incorporated. One of them involves repositioning catheters or shunt transferring, placement of a complete second shunt system, “Y” connectors between the two ventricular catheters, or a single multi-perforated catheter in both lateral ventricles (trans-septal catheter). These procedures can be made easier with neuronavigation or neuroendoscopy.[42,43,44]
Another alternative option, in the case previously proposed therapeutic measures have failed, is considered to be the insertion of lumboperitoneal shunts and the performance of cranial expansion surgeries, which can increase the buffering capacity of the brain.[28,45,46,47] An additional role for these systems relates to their capacity to circumvent VPS mechanics and to directly drain the SAS.[48] Drainage of CSF through the subarachnoid space permits a certain degree of ventricular dilatation by creating a favorable pressure gradient through the cortical mantle.[3] Lumboperitoneal shunts can be used alone or in combination with the third ventriculostomy, or other treatment modalities, depending on the underlying pathology.[14] An interesting case report, referring that only bilateral shunting of the ventricular system was able to eliminate the symptoms permanently,[49] is recently reported.
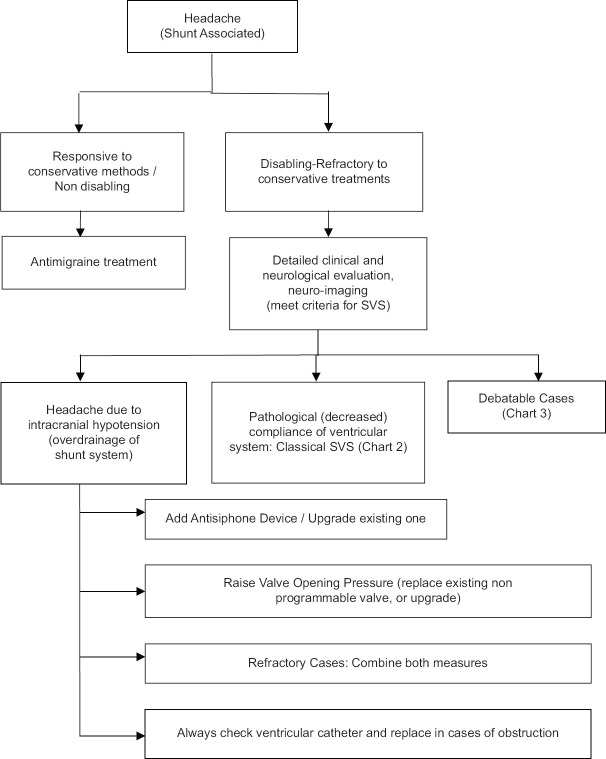
In case that the treatment armamentarium that is available for SVS (e.g., shunt revision, cranial expansion, lumboperitoneal shunt) does not work,[47,48,49] there are studies which have described endoscopic procedures for the treatment of SVS. These include shunt replacement, shunt removal, and endoscopic third ventriculostomy (ETV), which are safe and effective treatment options.[20,50,51] There are previous reports who support the concept of performing ETV as an effective strategy for the management of SVS that occurs following shunt placement.[46] Another recent report[52] supports the effectiveness of aqueductoplasty in the treatment of isolated fourth ventricle when is considered to be combined with slit-ventricle morphology on imaging studies. An algorithm, which indicates most currently accepted treatment options and alternatives associated with the different clinical manifestations of this syndrome, as well as its associated pathophysiologic substrate, follows [Figures 1-3].
Figure 1.
Depicts a widely accepted algorithm for the management (conservative and surgical) of the clinical entity of headaches in children harboring a ventriculoperitoneal shunt
Figure 3.
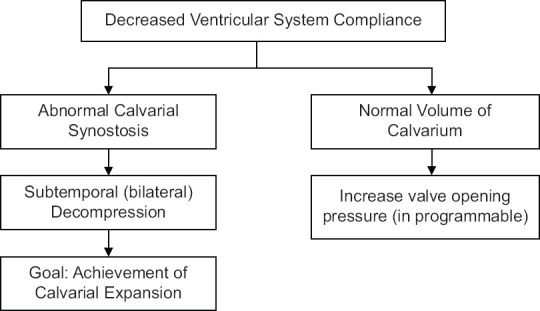
Presents a management protocol in cases of patients harboring a ventriculoperitoneal shunt and in whom decreased ventricular compliance is anticipated
Regarding the definition of the syndrome, the triad of headache, small ventricles, and slow valve refilling conform to the typical diagnostic criteria. Nonetheless, we have encountered different or atypical forms of presentation, as well as apparently silent forms but with intracranial hypertension.[53,54]
As far as the issue of the definition of the term SVS is considered, it is a common concept that there are different forms of presentation, as well as types of shunt over-drainage. Besides that, we could support the concept that the classifications proposed by Rekate, Khorasani, and Olson, albeit only coincide in some of the types, even though they are obviously using different terminology.
The pathophysiology of this condition is considered to be the key element in our effort to improve the efficacy of our treatment strategies. Currently, there is no theory that can unify the variously proposed models. A possible explanation should be that in any particular case, there should be more than one pathophysiological mechanism, perhaps with one predominating, and it is evident that this one should suggest the main treatment strategy. Despite progress in valve technology, CSF over-drainage continues to be one of the most usual complications in shunted hydrocephalus.[55] A multi-centered comparison of different treatment strategies for groups of patients who fulfill the same diagnostic criteria would be beneficial, which, in turn, would enhance our ultimate goal, which is improvement in clinical practice and patient care.[56]
In cases of refractory shunt over drainage, a final treatment option that is adopted is the execution of cranial expansion procedures. A variety of techniques have been employed the oldest and most basic technique utilized in patients suffering from secondary craniosynostosis related to a CSF is suturectomy.[57] Later on, a more complex surgical concept was introduced, which involved subtemporal decompression of 4 cm × 6 cm, which was eventually combined with a dural opening.[58,59] Nowadays, other surgical alternatives are introduced, including cranial vault expansion,[45,60] which are considered as more suitable for patients who are shunted early in their life. These patients commonly develop secondary craniosynostosis and microcephaly. Furthermore, this treatment option is suggested in cases of shunt over drainage without ventricular dilatation,[1] as well as in syndromic craniosynostosis. The current trend is not to consider these techniques as first-line management of slit ventricle syndrome. As such, they are usually selected for cases in which we are unable to adequately control CSF drainage via the selection of any type of valvular mechanism.[6]
Based on our research, we mention that propose ETV is proposed as a suitable alternative for this entity. It is remarkable that the success of this technique is irregular depending on the pathology that caused the hydrocephalus in the first place. Also, ETV is difficult to perform in these patients because of the collapse of the ventricular system. Researchers state that the cranial expansion technique in SVS cases allows ventricular expansion and favors the ETV procedure. Some cases have been treated this way with good results, but this approach is highly controversial and cannot be considered an alternative for all the cases.
In order to assume a thorough perspective of the entity of the slit ventricle syndrome, any figures or percentages would be helpful to depict the distribution of patients in diagnostic and therapeutic perspectives. It would be a helpful adjunct for our research to know how many patients with chronic headache and existing shunts got symptom-free after simple pressure adjusting of shunt valves and how many patients are refractory to conservative measures and go to cranial expansion surgery. To the best of our knowledge, we haven’t heard the answer yet according to our opinion, this is due to the lack of concordant data.
According to our opinion, several definitions are rendered mandatory to clarify the relevant entities which are discussed in this manuscript. As already discussed, the term “Classical” slit ventricle syndrome refers to the original definition of the condition and required three things (as previously depicted) and included the failure of refilling of the pumping mechanism. Many and maybe most contemporary valves do not have a pumping chamber and that definition is no longer applicable. According to our opinion, to define the condition of slit ventricle syndrome we should use the following definition: “SVS is a syndrome of intractable headaches in shunted patients with small ventricle.”
According to Olson,[1] slit ventricle syndrome should probably be referred to as the non-compliant ventricle syndrome, to avoid confusion of radiological “slit ventricles” with the syndrome. He also supports the concept that as the true slit ventricle syndrome is represented by the on-off symptom complex, this should be known as the noncompliant ventricle syndrome to avoid confusion.[21] It is estimated that 6%–22% of children with imaging characteristics compatible with slit ventricles and associated headaches may suffer from the non-compliant ventricle syndrome.[45,58]
Another pathologic entity whose definition remains an unresolved problem is the shunt over-drainage syndrome. Nowadays, the most accepted concept of shunt over-drainage is related to what has become known as “shunt-related headaches.” Rekate[8] considered it to be an entity characterized by the appearance of severe headache, i.e. that which interferes with activities of daily living, in patients harboring a CSF shunt valve and normal or smaller than normal ventricles.
The charts that are included in our manuscript do recognize that there are several sub diagnoses for this syndrome. Figure 1 essentially does this by calling the SVS “Headache (Shunt Associated). On Figure 1 there is a block for headaches that respond to conservative treatments such as antimigraine medication. These patients do not, therefore, meet the criteria for SVS being “Disabling-refractory” headaches. Basically, then SVS patients may require management of the shunt or the hydrocephalus. At this point, we recognize three subtypes of SVS.
Low ICP (similar to post spinal tap)
Pathological with non-responsive ventricles called “classical” SVS. This condition seems to be “Normal Volume Hydrocephalus” as discussed in the text relative to reference 28 by Engle and Carmel. This problem is called “Ambiguous” cases in Figure 2. Rather than any of the other names that are given they then use the title “Classical” SVS. It would be less confusing to use either Normal Volume Hydrocephalus or their own name pathological compliance
Shunt over drainage syndrome
Figure 2.
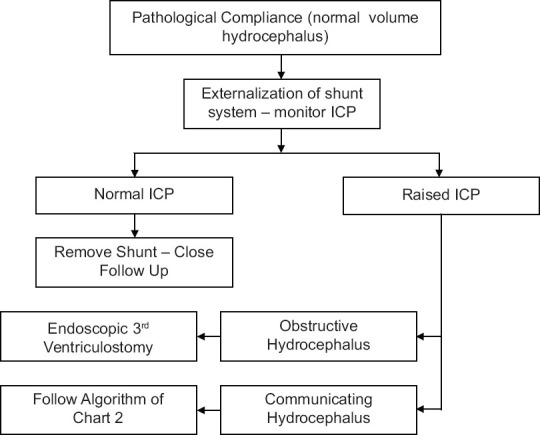
Refers to a proposed management algorithm in cases which harbor a ventriculoperitoneal shunt and the presence of increased intracranial pressure is equivocal
Figure 2 is an algorithm for the management of “ambiguous cases.” As in the Dandy classification, we recognize two subtypes, “obstructive” and “communicating.” Unfortunately, this too may become confusing. To Dandy and most papers now “communicating” relates to flow from the ventricles to the spinal subarachnoid space. It does not mean that the CSF can go from the ventricles to the cortical subarachnoid space. It would be better to ventricles expand or ventricles do not expand to clarify.[49]
In Figure 3, we limit the management of “classical” SVS to the manipulation of the valve. This is the usual treatment and often works. There are times however when it is necessary to access the cortical subarachnoid space as well as the ventricle in this treatment. This is dealt with in the discussion related to the lumbar shunts and cisterna magna shunts.[3,40]
Shunt infusion study is still used in some institutes, although it constitutes an old diagnostic method. Actually, some researcher says it is the only method to determine the true functional state of the shunt in situ. Clinical practice has shown that accurate diagnosis of shunt malfunction is a clinical problem presenting many challenges.[61,62] More precisely, shunt malfunction can come to clinical attention with nontypical symptomatology and/or ventricular dimensions that do not differ from normal. Moreover, there is a subgroup of children which may not be clinically affected, but, on the other hand, they do not demonstrate the anticipated decrease of ventricular dimensions, along with the widening of external CSF spaces following a seemingly successful shunt placement. When the shunt-opening pressure is low or is lowered (in cases of programmable valves) and the previously recorded situation is our case, this constitutes a more difficult to resolve the clinical challenge.[63,64,65]
When we are confronted with equivocal cases, an option is to perform open shunt revision with intra-operative shunt testing however, this is very invasive method. Serial follow-up examination could be another option. However, this has the drawback of adding an element of delay in treatment, or long-term neglect, especially in patients with complex postural over-drainage or intermittent obstruction.[66,67,68,69] Shunt testing in vivo using shunt infusion studies has been reported as a diagnostic adjunct for over 25 years and is utilized, in selected cases, in clinical practice.[70,71,72,73] It is an accurate and relatively low-invasive method that helps us to assess shunt function and detect over-drainage, underdrainage as well as proximal or distal blockage.
It seems that the shunt infusion study is an accurate and radiation-free diagnostic tool which provides a quantitative shunt assessment in the pediatric population, aiming to rule out or prove not clinically evident shunt malfunction. We consider that silent shunt dysfunction simply represents another form of compensated, even though not sufficiently treated, chronic hydrocephalus. This situation is accompanied by all devastating consequences to the development of the child, so shunt infusion studies constitute an important tool for the pediatric neurosurgeon. The recent bibliographic review considers that it provides a significant degree of certainty that children with shunted hydrocephalus either are harboring working shunts, or revision is necessary. A clinical benefit accompanied by radiologic improvement is clearly demonstrated.[61]
Results
Currently, based on a review of the literature, it seems that the most common practice for treatment of shunt over drainage, and consequently for the management of the slit ventricle syndrome, remains the decrease of the amount of CSF that is drained through the valvular mechanism. The most efficacious means to achieve that goal, apart from using an anti-siphon device, consists of increasing the valve opening pressure,[1,11,20] either by upgrading the opening pressure of an existing programmable valve or by replacing the existing one with one that is adjustable.[41]
Despite advancement in our knowledge about the pathophysiology of the slit ventricle syndrome, there is no widespread consensus regarding the underlying substrate of the definition and etiology of headaches that are recorded in shunted patients.[8] Although upgrade of the valve opening pressure and incorporation of an antisiphon device constitutes a strategy commonly used, there are reports that mention that about 20% of these patients exhibit no improvement, or only a temporary response is recorded. A detailed investigation to clarify the pathophysiologic basis of the patient’s headaches is undertaken, before the adoption of more invasive treatment options. This includes the investigation of the relationship of headaches to the adequacy of shunt function and ICP. Therefore, definement of the relationship of ICP measurements and shunt function to the headaches is of paramount importance. Studies have been performed, centered on chronic monitoring of ICP in these patients suffering from shunt-related headaches, and five syndromes of shunt-related headaches have been delimited. Most importantly, it seems that each syndrome leads to specific treatment strategies.[12] Briefly, these syndromes are named intracranial hypotension, intermittent proximal obstruction, increased ICP with a working shunt: Cephalocranial disproportion, shunt failure without ventricular enlargement, and Shunt-related migraine. All these data undermine the role of shunt function test in the algorithm.
Recent publications have suggested that the evidence about over-drainage and its consequences should not be overestimated, although their role is relatively robust, necessitating that more focused trials and more examination of the topic is essential.[73]
Conclusions
The vast majority of patients treated for hydrocephalus with the implantation of a valve mechanism are chronically shunted patients suffering from frequent headaches. Nevertheless, it is a common concept that these patients have an acceptable quality of life without requiring surgical intervention. Unfortunately, a small but undefined percentage of them complain of severe, frequent, or even daily headaches, which influence their daily living and require some form of interference to ameliorate the clinical consequences of this entity. Shunt over-drainage and its associated morbidity have existed ever since hydrocephalus started to be treated with different shunt systems. Indeed, technological advancement, leading to the development of self-regulated valve systems, programmable valves, and anti-siphon devices are, in great part, the result of our efforts to find controlled and well-adjusted CSF drainage methods, resembling the homeostasis of the normal brain. Despite a large number of relevant publications and the great advances in understanding the pathogenesis and treatment of this entity by many researchers over the last 60 years, important issues still exist concerning the definition of the entity, its classification, and the pathophysiological proposals behind the various treatment algorithms.
A comprehensive literature review reveals that the topic of shunt over-drainage remains a complex and unresolved issue. In order to overcome this frustrating problem, our main target should be the acquisition of improved knowledge and definition of the syndrome, including the precise definition of the constellation of signs and symptoms, and its different patterns of presentation (clinical and its radiological correlates). This could enhance our effort in order to make it possible to identify (that is, to diagnose) and treat, and even better, prevent the evolution of this entity, as early as possible. Besides that, it would be helpful if we could be able to evaluate in a standardized manner the results of the different treatment strategies and protocols that are included in our current therapeutic armamentarium, as well as the overall state and clinical and neurological condition of the patients after treatment, focusing on symptom control. Scientific advancement, leading to the acquisition of self-regulated valve systems, programmable valves, and anti-siphon devices, mainly affects our efforts to invent controlled and well-adjusted CSF drainage methods resembling the homeostasis of the normal brain.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Olson S. The problematic slit ventricle syndrome. A review of the literature and proposed algorithm for treatment. Pediatr Neurosurg. 2004;40:264–9. doi: 10.1159/000083738. [DOI] [PubMed] [Google Scholar]
- 2.Laurence KM, Coates S. The natural history of hydrocephalus. Detailed analysis of 182 unoperated cases. Arch Dis Child. 1962;37:345–62. doi: 10.1136/adc.37.194.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khorasani L, Sikorski CW, Frim DM. Lumbar CSF shunting preferentially drains the cerebral subarachnoid over the ventricular spaces: Implications for the treatment of slit ventricle syndrome. Pediatr Neurosurg. 2004;40:270–6. doi: 10.1159/000083739. [DOI] [PubMed] [Google Scholar]
- 4.Nulsen F, Spitz E. Treatment of hydrocephalus by direct shunt from ventricle to jugular vein. Surg Forum. 1952;2:399–403. [PubMed] [Google Scholar]
- 5.Aschoff A, Kremer P, Hashemi B, Kunze S. The scientific history of hydrocephalus and its treatment. Neurosurg Rev. 1999;22:67–93. doi: 10.1007/s101430050035. [DOI] [PubMed] [Google Scholar]
- 6.Ros B, Iglesias S, Martín Á, Carrasco A, Ibáñez G, Arráez MA. Shunt overdrainage syndrome: Review of the literature. Neurosurg Rev. 2018;41:969–81. doi: 10.1007/s10143-017-0849-5. [DOI] [PubMed] [Google Scholar]
- 7.Cheok S, Chen J, Lazareff J. The truth and coherence behind the concept of overdrainage of cerebrospinal fluid in hydrocephalic patients. Childs Nerv Syst. 2014;30:599–606. doi: 10.1007/s00381-013-2327-x. [DOI] [PubMed] [Google Scholar]
- 8.Rekate HL. Shunt-related headaches: The slit ventricle syndromes. Childs Nerv Syst. 2008;24:423–30. doi: 10.1007/s00381-008-0579-7. [DOI] [PubMed] [Google Scholar]
- 9.Nulsen FE, Becker DP. Control of hydrocephalus by valve-regulated shunt. J Neurosurg. 1967;26:362–74. doi: 10.3171/jns.1967.26.3.0362. [DOI] [PubMed] [Google Scholar]
- 10.Nulsen FE, Spitz EB. Treatment of hydrocephalus by direct shunt from ventricle to jugular vain. Surg Forum. 1951:399–403. PMID: 14931257. [PubMed] [Google Scholar]
- 11.Hyde-Rowan MD, Rekate HL, Nulsen FE. Reexpansion of previously collapsed ventricles: The slit ventricle syndrome. J Neurosurg. 1982;56:536–9. doi: 10.3171/jns.1982.56.4.0536. [DOI] [PubMed] [Google Scholar]
- 12.Rekate HL. Classification of slit-ventricle syndromes using intracranial pressure monitoring. Pediatr Neurosurg. 1993;19:15–20. doi: 10.1159/000120694. [DOI] [PubMed] [Google Scholar]
- 13.Goldstein I, LaMarca V, Abbott R, Jallo GI. Slit ventricle syndrome: Overview of pathophysiology and treatment? Internet J Neurosurg. 2001;1 doi: 10.1136/bcr-2017-223861. [Google Scholar]
- 14.Benzel EC, Reeves JD, Kesterson L, Hadden TA. Slit ventricle syndrome in children: Clinical presentation and treatment. Acta Neurochir (Wien) 1992;117:7–14. doi: 10.1007/BF01400628. [DOI] [PubMed] [Google Scholar]
- 15.Di Rocco C. Is the slit ventricle syndrome always a slit ventricle syndrome? Childs Nerv Syst. 1994;10:49–58. doi: 10.1007/BF00313585. [DOI] [PubMed] [Google Scholar]
- 16.Walker ML, Fried A, Petronio J. Diagnosis and treatment of the slit ventricle syndrome. Neurosurg Clin N Am. 1993;4:707–14. [PubMed] [Google Scholar]
- 17.Serlo W, Heikkinen E, Saukkonen AL, von Wendt L. Classification and management of the slit ventricle syndrome. Childs Nerv Syst. 1985;1:194–9. doi: 10.1007/BF00270761. [DOI] [PubMed] [Google Scholar]
- 18.Serlo W, Saukkonen AL, Heikkinen E, von Wendt L. The incidence and management of the slit ventricle syndrome. Acta Neurochir (Wien) 1989;99:113–6. doi: 10.1007/BF01402318. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto T, Takeuchi K, Nagata Y, Mizuno A, Wakabayashi T. Novel endoscopic technique for inserting a sheath into a slit ventricle: The water-slide technique. World Neurosurg. 2021;145:1–4. doi: 10.1016/j.wneu.2020.08.206. [DOI] [PubMed] [Google Scholar]
- 20.Chernov MF, Kamikawa S, Yamane F, Ishihara S, Hori T. Neurofiberscope-guided management of slit-ventricle syndrome due to shunt placement. J Neurosurg. 2005;102:260–7. doi: 10.3171/ped.2005.102.3.0260. [DOI] [PubMed] [Google Scholar]
- 21.Buxton N, Punt J. Subtemporal decompression: The treatment of noncompliant ventricle syndrome. Neurosurgery. 1999;44:513–8. doi: 10.1097/00006123-199903000-00045. [DOI] [PubMed] [Google Scholar]
- 22.Obana WG, Raskin NH, Cogen PH, Szymanski JA, Edwards MS. Antimigraine treatment for slit ventricle syndrome. Neurosurgery. 1990;27:760–3. doi: 10.1097/00006123-199011000-00014. [DOI] [PubMed] [Google Scholar]
- 23.Linder M, Diehl J, Sklar FH. Subtemporal decompressions for shunt-dependent ventricles: Mechanism of action. Surg Neurol. 1983;19:520–3. doi: 10.1016/0090-3019(83)90375-0. [DOI] [PubMed] [Google Scholar]
- 24.Bruce DA, Weprin B. The slit ventricle syndrome. Neurosurg Clin N Am. 2001;12:709–17, viii. [PubMed] [Google Scholar]
- 25.Sood S, Barrett RJ, Powell T, Ham SD. The role of lumbar shunts in the management of slit ventricles: Does the slit-ventricle syndrome exist? J Neurosurg. 2005;103:119–23. doi: 10.3171/ped.2005.103.2.0119. [DOI] [PubMed] [Google Scholar]
- 26.Oi S, Matsumoto S. Infantile hydrocephalus and the slit ventricle syndrome in early infancy. Childs Nerv Syst. 1987;3:145–50. doi: 10.1007/BF00717890. [DOI] [PubMed] [Google Scholar]
- 27.Cardoso ER, Del Bigio MR, Schroeder G. Age-dependent changes of cerebral ventricular size. Part I: Review of intracranial fluid collections. Acta Neurochir (Wien) 1989;97:40–6. doi: 10.1007/BF01577738. [DOI] [PubMed] [Google Scholar]
- 28.Engel M, Carmel PW, Chutorian AM. Increased intraventricular pressure without ventriculomegaly in children with shunts: ’Normal volume’ hydrocephalus. Neurosurgery. 1979;5:549–52. doi: 10.1227/00006123-197911000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Di Rocco C, Massimi L, Tamburrini G. Shunts vs endoscopic third ventriculostomy in infants: Are there different types and/or rates of complications? A review. Childs Nerv Syst. 2006;22:1573–89. doi: 10.1007/s00381-006-0194-4. [DOI] [PubMed] [Google Scholar]
- 30.Kajimoto Y, Ohta T, Miyake H, Matsukawa M, Ogawa D, Nagao K, et al. Posture-related changes in the pressure environment of the ventriculoperitoneal shunt system. J Neurosurg. 2000;93:614–7. doi: 10.3171/jns.2000.93.4.0614. [DOI] [PubMed] [Google Scholar]
- 31.Pinto FC, Pereira RM, Saad F, Teixeira MJ. Performance of fixed-pressure valve with antisiphon device SPHERA(®) in hydrocephalus treatment and overdrainage prevention. Arq Neuropsiquiat. 2012;70:704–9. doi: 10.1590/s0004-282x2012000900011. [DOI] [PubMed] [Google Scholar]
- 32.Sotelo J. The hydrokinetic parameters of shunts for hydrocephalus might be inadequate. Surg Neurol Int. 2012;3:40. doi: 10.4103/2152-7806.94292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gruber RW, Roehrig B. Prevention of ventricular catheter obstruction and slit ventricle syndrome by the prophylactic use of the Integra antisiphon device in shunt therapy for pediatric hypertensive hydrocephalus: A 25-year follow-up study. J Neurosurg Pediatr. 2010;5:4–16. doi: 10.3171/2008.7.17690. [DOI] [PubMed] [Google Scholar]
- 34.Fattal-Valevski A, Beni-Adani L, Constantini S. Short-term dexamethasone treatment for symptomatic slit ventricle syndrome. Childs Nerv Syst. 2005;21:981–4. doi: 10.1007/s00381-004-1132-y. [DOI] [PubMed] [Google Scholar]
- 35.Major O, Fedorcsák I, Sipos L, Hantos P, Kónya E, Dobronyi I, et al. Slit-ventricle syndrome in shunt operated children. Acta Neurochir (Wien) 1994;127:69–72. doi: 10.1007/BF01808550. [DOI] [PubMed] [Google Scholar]
- 36.Liniger P, Marchand S, Kaiser GL. Flow control versus antisiphon valves: Late results concerning slit ventricles and slit-ventricle syndrome. Eur J Pediatr Surg. 2003;13(Suppl 1):S3–6. doi: 10.1055/s-2003-44749. [DOI] [PubMed] [Google Scholar]
- 37.Albright AL, Tyler-Kabara E. Slit-ventricle syndrome secondary to shunt-induced suture ossification. Neurosurgery. 2001;48:764–9. doi: 10.1097/00006123-200104000-00013. [DOI] [PubMed] [Google Scholar]
- 38.Breimer GE, Sival DA, Hoving EW. Low-pressure valves in hydrocephalic children: A retrospective analysis. Childs Nerv Syst. 2012;28:469–73. doi: 10.1007/s00381-011-1664-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kan P, Walker ML, Drake JM, Kestle JR. Predicting slit-like ventricles in children on the basis of baseline characteristics at the time of shunt insertion. J Neurosurg. 2007;106(5 Suppl):347–9. doi: 10.3171/ped.2007.106.5.347. [DOI] [PubMed] [Google Scholar]
- 40.Rekate HL. Hydrocephalus in infants: The unique biomechanics and why they matter. Childs Nerv Syst. 2020;36:1713–28. doi: 10.1007/s00381-020-04683-7. [DOI] [PubMed] [Google Scholar]
- 41.Rohde V, Mayfrank L, Ramakers VT, Gilsbach JM. Four-year experience with the routine use of the programmable Hakim valve in the management of children with hydrocephalus. Acta Neurochir (Wien) 1998;140:1127–34. doi: 10.1007/s007010050226. [DOI] [PubMed] [Google Scholar]
- 42.Atalay B, Yilmaz C, Cekinmez M, Altinors N, Caner H. Treatment of hydrocephalus with functionally isolated ventricles. Acta Neurochir (Wien) 2006;148:1293–6. doi: 10.1007/s00701-006-0906-2. [DOI] [PubMed] [Google Scholar]
- 43.Gil Z, Siomin V, Beni-Adani L, Sira B, Constantini S. Ventricular catheter placement in children with hydrocephalus and small ventricles: The use of a frameless neuronavigation system. Childs Nerv Syst. 2002;18:26–9. doi: 10.1007/s00381-001-0550-3. [DOI] [PubMed] [Google Scholar]
- 44.Steinbok P, Poskitt KJ, Cochrane DD, Kestle JR. Prevention of postshunting ventricular asymmetry by transseptal placement of ventricular catheters. A randomized study. Pediatr Neurosurg. 1994;21:59–64. doi: 10.1159/000120816. [DOI] [PubMed] [Google Scholar]
- 45.Epstein F, Lapras C, Wisoff JH. ‘Slit-ventricle syndrome’: Etiology and treatment. Pediatr Neurosci. 1988;14:5–10. [PubMed] [Google Scholar]
- 46.Butler WE, Khan SA. The application of controlled intracranial hypertension in slit ventricle syndrome patients with obstructive hydrocephalus and shunt malfunction. Pediatr Neurosurg. 2001;35:305–10. doi: 10.1159/000050442. [DOI] [PubMed] [Google Scholar]
- 47.Eide PK, Helseth E, Due-Tønnessen B, Lundar T. Changes in intracranial pressure after calvarial expansion surgery in children with slit ventricle syndrome. Pediatr Neurosurg. 2001;35:195–204. doi: 10.1159/000050421. [DOI] [PubMed] [Google Scholar]
- 48.Le H, Yamini B, Frim DM. Lumboperitoneal shunting as a treatment for slit ventricle syndrome. Pediatr Neurosurg. 2002;36:178–82. doi: 10.1159/000056054. [DOI] [PubMed] [Google Scholar]
- 49.Mencser Z, Kopniczky Z, Kis D, Barzo P. Slit ventricle as a neurosurgical emergency: Case report and review of literature. World Neurosurg. 2019;130:493–8. doi: 10.1016/j.wneu.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 50.Baskin JJ, Manwaring KH, Rekate HL. Ventricular shunt removal: The ultimate treatment of the slit ventricle syndrome. J Neurosurg. 1998;88:478–84. doi: 10.3171/jns.1998.88.3.0478. [DOI] [PubMed] [Google Scholar]
- 51.Zheng J, Chen G, Xiao Q, Huang Y, Guo Y. Endoscopy in the treatment of slit ventricle syndrome. Exp Ther Med. 2017;14:3381–6. doi: 10.3892/etm.2017.4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Waqar M, Ellenbogen JR, Mallucci C. Endoscopic third ventriculostomy for shunt malfunction in children: A review. J Clin Neurosci. 2018;51:6–11. doi: 10.1016/j.jocn.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 53.Tirado-Caballero J, Rivero-Garvia M, Moreno-Madueño G, Gómez-González E, Márquez-Rivas J. Cranial expansion and aqueductoplasty for combined isolated fourth ventricle and slit-ventricle syndrome: A surgical alternative. Childs Nerv Syst. 2021;37:885–94. doi: 10.1007/s00381-020-04939-2. [DOI] [PubMed] [Google Scholar]
- 54.Katz DM, Trobe JD, Muraszko KM, Dauser RC. Shunt failure without ventriculomegaly proclaimed by ophthalmic findings. J Neurosurg. 1994;81:721–5. doi: 10.3171/jns.1994.81.5.0721. [DOI] [PubMed] [Google Scholar]
- 55.Nguyen TN, Polomeno RC, Farmer JP, Montes JL. Ophthalmic complications of slit-ventricle syndrome in children. Ophthalmology. 2002;109:520–4. doi: 10.1016/s0161-6420(01)00985-x. [DOI] [PubMed] [Google Scholar]
- 56.Tschan CA, Antes S, Huthmann A, Vulcu S, Oertel J, Wagner W. Overcoming CSF overdrainage with the adjustable gravitational valve proSA. Acta Neurochir (Wien) 2014;156:767–76. doi: 10.1007/s00701-013-1934-3. [DOI] [PubMed] [Google Scholar]
- 57.Meling TR, Tiller C, Due-Tonnessen BJ, Egge A, Eide PK, Frosile KF, et al. Audits can improve neurosurgical practise – illustrated by third ventriculostomy. Pediatr Neurosurg. 2007;43:482–7. doi: 10.1159/000108791. [DOI] [PubMed] [Google Scholar]
- 58.Andersson H, Carlsson CA. The surgical management of myelomeningocele with a preliminary report of 31 cases. Acta Paediatr Scand. 1966;55:626–35. doi: 10.1111/j.1651-2227.1966.tb15263.x. [DOI] [PubMed] [Google Scholar]
- 59.Holness RO, Hoffman HJ, Hendrick EB. Subtemporal decompression for the slit-ventricle syndrome after shunting in hydrocephalic children. Childs Brain. 1979;5:137–44. doi: 10.1159/000119812. [DOI] [PubMed] [Google Scholar]
- 60.Walsh JW, James HE. Subtemporal craniectomy and elevation of shunt valve opening pressure in the management of small ventricle-induced cerebrospinal fluid shunt dysfunction. Neurosurgery. 1982;10:698–703. doi: 10.1227/00006123-198206010-00004. [DOI] [PubMed] [Google Scholar]
- 61.Epstein FJ, Fleischer AS, Hochwald GM, Ransohoff J. Subtemporal craniectomy for recurrent shunt obstruction secondary to small ventricles. J Neurosurg. 1974;41:29–31. doi: 10.3171/jns.1974.41.1.0029. [DOI] [PubMed] [Google Scholar]
- 62.Dias SF, Lalou AD, Spang R, Haas-Lude K, Garnett M, Fernandez H, et al. Value of computerized shunt infusion study in assessment of pediatric hydrocephalus shunt function-a two center cross-sectional study. Childs Nerv Syst. 2020;36:59–71. doi: 10.1007/s00381-019-04264-3. [DOI] [PubMed] [Google Scholar]
- 63.Lalou AD, Czosnyka M, Garnett MR, Nabbanja E, Petrella G, Hutchinson PJ, et al. Shunt infusion studies: Impact on patient outcome, including health economics. Acta Neurochirurgica. 2020;162:1019–31. doi: 10.1007/s00701-020-04212-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boyle TP, Nigrovic LE. Radiographic evaluation of pediatric cerebrospinal fluid shunt malfunction in the emergency setting. Pediatr Emerg Care. 2015;31:435–40. doi: 10.1097/PEC.0000000000000462. [DOI] [PubMed] [Google Scholar]
- 65.Goeser CD, McLeary MS, Young LW. Diagnostic imaging of ventriculoperitoneal shunt malfunctions and complications. Radiographics. 1998;18:635–51. doi: 10.1148/radiographics.18.3.9599388. [DOI] [PubMed] [Google Scholar]
- 66.Nabbanja E, Pickard JD, Lalou AD, Czosnyka ZH. Use of CSF infusion studies to unblock occluded hydrocephalus ventricular shunt catheters: A preliminary report of two patients. BMJ Case Rep 2018. 2018:1–4. doi: 10.1136/bcr-2017-223861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dupepe EB, Hopson B, Johnston JM, Rozzelle CJ, Jerry Oakes W, Blount JP, et al. Rate of shunt revision as a function of age in patients with shunted hydrocephalus due to myelomeningocele. Neurosurg Focus. 2016;41:E6. doi: 10.3171/2016.8.FOCUS16257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Limbrick DD, Jr, Baird LC, Klimo P, Jr, Riva-Cambrin J, Flannery AM. Pediatric Hydrocephalus Systematic R, Evidence-Based Guidelines Task F. Pediatric hydrocephalus: Systematic literature review and evidence-based guidelines. Part 4: Cerebrospinal fluid shunt or endoscopic third ventriculostomy for the treatment of hydrocephalus in children. J Neurosurg Pediatr. 2014;14(Suppl 1):30–4. doi: 10.3171/2014.7.PEDS14324. [DOI] [PubMed] [Google Scholar]
- 69.Paulsen AH, Lundar T, Lindegaard KF. Pediatric hydrocephalus: 40-year outcomes in 128 hydrocephalic patients treated with shunts during childhood. Assessment of surgical outcome, work participation, and health-related quality of life. J Neurosurg Pediatr. 2015;16:633–41. doi: 10.3171/2015.5.PEDS14532. [DOI] [PubMed] [Google Scholar]
- 70.Richards HK, Seeley HM, Pickard JD. Who should perform shunt surgery? Data from the United Kingdom Shunt Registry. Cerebrospinal Fluid Res. 2009;6:181–6. [Google Scholar]
- 71.Kasprowicz M, Lalou DA, Czosnyka M, Garnett M, Czosnyka Z. Intracranial pressure, its components and cerebrospinal fluid pressure-volume compensation. Acta Neurol Scand. 2016;134:168–80. doi: 10.1111/ane.12541. [DOI] [PubMed] [Google Scholar]
- 72.Petrella G, Czosnyka M, Keong N, Pickard JD, Czosnyka Z. How does CSF dynamics change after shunting? Acta Neurol Scand. 2008;118:182–8. doi: 10.1111/j.1600-0404.2008.01041.x. [DOI] [PubMed] [Google Scholar]
- 73.Petrella G, Czosnyka M, Smielewski P, Allin D, Guazzo EP, Pickard JD, et al. In vivo assessment of hydrocephalus shunt. Acta Neurol Scand. 2009;120:317–23. doi: 10.1111/j.1600-0404.2009.01176.x. [DOI] [PubMed] [Google Scholar]