Abstract
Objectives:
Older adults with serious mental illness (SMI) are more likely to have high body mass index (BMI) and chronic conditions such as cardiovascular disease and diabetes. A sedentary lifestyle, which may be attributed to pharmacologic side effects and the symptoms of mental illness, has been difficult to treat. Patients experiencing negative symptoms (e.g., apathy, anhedonia) may be more likely to exercise in a group setting with activities that are designed to stimulate the mind and encourage engagement. “Exergames,” or exercise-based videogames, are an interactive and stimulating method to provide aerobic activities. Exercise has also been shown to reduce the symptoms of depression. The purpose of this study is to evaluate the impact of a 10-week exergame program on depressive and negative symptoms in older adults with SMI.
Materials and Methods:
A single-group pretest posttest study was conducted with 52 older adults diagnosed with SMI. Participants engaged in group exergame activities for 50-minute sessions three times a week for 10 weeks. The Patient Reported Outcome Measurement Information System (PROMIS) and the Scale for the Assessment of Negative Symptoms (SANS) were conducted at enrollment, 5 weeks, and 10 weeks.
Results:
Participants achieved statistically significant reductions in self-reported depressive symptoms (−0.83, LL −1.46, UL −0.12) and observed negative symptoms (−5.29, LL −7.67, UL −3.14) over a 10-week period.
Conclusions:
Our results suggest utilization of exergames as an adjunct treatment can be an effective, engaging, and cost-efficient method to reducing depressive and negative symptoms in older adults with SMI.
Keywords: exergame, serious mental illness, negative symptoms, depression, exercise video game, outpatient and transitional mental health
Introduction:
Worldwide, the number of people age 60 or greater is projected to more than double by 2050 (United Nations, 2017). Nearly 11% of the world’s population in 2017 was estimated to be living with a mental health disorder (Ritchie & Roser, 2018). Serious mental illnesses (SMIs) are characterized as distinct conditions that require routine management, produce functional impairment, and interfere with a person’s quality of life (Substance Abuse Mental Health Services Administration, 2016). Individuals that typically meet the criteria of SMI have illnesses that include schizophrenia, schizoaffective disorder, psychotic disorders, major depressive disorders, bipolar disorders, and borderline personality disorder (Ruggeri, Leese, Thornicroft, Bisoffi, & Tansella, 2000). Patients with serious mental illness are burdened with more than double the prevalence of obesity and four times the prevalence of diabetes compared to the general population in the U.S. (Cook, et al., 2016). Patients with SMI die on average 20 years before their non-afflicted peers (Liu, 2017). Their premature deaths are frequently a result of preventable illnesses, including cardiovascular disease, pulmonary disease and unnatural causes including suicide (Liu, 2017). Comorbidities in adults with serious mental illness may be due to metabolic effects of psychiatric medications, sociodemographic issues and lack of exercise (Allison et al., 2009) (Bartels, 2011).
In 2011, the American Heart Association (AHA) endorsed the potential of exergames as a new and innovative technology that could engage populations who are otherwise inactive (Lieberman et al., 2011). Monedero, Murphy and O’Gorman found that active video games were significantly more enjoyable than traditional exercise and provided the moderate to vigorous physical activity needed to meet the recommendations of the AHA and American College of Sports Medicine (Monedero, Murphy & O’Gorman, 2017). Exergames were found to minimized boredom and provided multiple high-intensity short training segments, which are more effective at promoting cardiovascular health than low intensity prolonged aerobic activities (Stanton & Happell, 2014). Exergames have been shown to enhance social well-being, reduce loneliness, increase positive attitudes towards others and improve social connections in older adults (Li et al., 2018a). A systematic review utilizing various populations found exergames improved overall positive psychological and behavioral outcomes while enhancing user motivation (Matallaoui et al., 2017). Exercise has been found to reduce depression in all age groups (Ashdown-Franks et al., 2020) and exergames improved game participation attendance and in-session engagement, enhancing aerobic fitness in patients with schizophrenia (Kimhy et al., 2016). Exergames provide positive social contact and improve motivation in patients with SMI, including schizophrenia, major depression, post-traumatic stress disorder, bipolar disorder and other psychosis (Dobbins et al., 2018).
While there is evidence to show exercise can reduce depressive symptoms and rates of metabolic disease in the general population, and that group exergames can improve social interactions and motivation in patients with SMI, there is a lack of evidence in the literature exploring exergames and its effects on depressive symptoms in patients with SMI. In this study, we explore the impact of exergames on depressive symptoms in older adults with SMI, defined for this study as patients diagnosed with schizophrenia, schizoaffective disorder, bipolar disorder, anxiety disorder, post-traumatic stress disorder (PTSD), and/or major depression. The shortened lifespan and higher rates of chronic comorbidities starting at 45 years of age (Bahorik, Satre, Kline-Simon, Weisner, & Campbell 2017) lead us to include individuals 45 years or older in our analysis. We hypothesized that statistically significant reductions in negative and depressive symptoms would be detected after participants with SMI engaged in a 10 week exergame program.
Materials and Methods:
Potential participants were recruited by referrals from the community, direct contact of potential subjects (who previously gave consent to be contacted for future research), and posting of flyers at the outpatient mental health clinic and the transitional residential facility. Initial eligibility screening was conducted by phone or in person with the PI or Research Assistant (RA). All subjects who self-referred, or were referred to or recruited by the PI or RA were assessed by brief interview (In person or on the telephone) for the following eligibility criteria: English speaking, 45 years old or older, have a diagnosis of serious mental illness (e.g. schizophrenia, schizoaffective disorder, bipolar disorder, psychotic disorder, anxiety disorder, PTSD, or major depression) or have symptoms which indicate risk for psychosis. Symptoms which indicate risk for psychosis include attenuated psychotic symptoms, such as changes in mood or thought, odd perceptions or beliefs, or a decrement in behavioral or social functioning. Participants self-disclosed their diagnosis. All participants passed a capacity to consent test based on comprehension of the consent form and gave consent for use of their information in our study with all data fully anonymized. A convenience sample was recruited from either a transitional residential facility for older adults with SMI or an outpatient assertive community treatment center. For health and safety reasons, subjects were excluded if they had known medical conditions or other physical problems that required special attention to engage in an exercise program (e.g. prior myocardial infarction, uncontrolled hypertension, history of angioplasty, history of angina, or use of nitroglycerin to treat angina).
Participants played an active video game using the Kinect for Xbox 360 gaming system (Microsoft, Redmond, WA) in 50-minute sessions, three times a week for 10 weeks. The Kinect for XBox 360 system does not require participants to use a controller. Instead, participants used their body to directly control the game via full-body tracking sensors that mirror movements. Approximately six feet of free space between the participant and the X-Box Kinect sensor was required. The sessions took place in a multi-purpose room at a transitional residential facility or an outpatient clinic utilizing flexible use space and a television, both of which are common in these environments. The gaming console itself is small and easily stored under or behind the television.
The games offered a variety of intensities and each group started at the beginner level. Participants were educated on warning signs of overexertion (e.g., shortness of breath, dizziness) and told to stop exercising if these occurred. The PI or her staff were present during all sessions to assist with set up and monitor participants. Each 10-week group was started with Bowling from the Kinect Sports DVD. Participants were allowed to choose from 10 games at the following session, with all participants eventually playing the same 10 games. These included: Kinect Sports (bowling, baseball, golf, tennis, and skiing), Kinect Dance Central 2, Kinect Adventures (River Rush and 20,000 Leaks), and Kinect Your Shape Fitness Evolved (Tai-Chi and Body Focus Workouts). One session a week focused on games that promoted coordination and balance, a second session focused on games that promoted aerobic activity, and the third session focused on games that promoted strength and flexibility. Participants initiated the 10-week sessions with single player turns so they could learn how to play the games. Some multiplayer games, such as skiing, allowed for groups of up to six players to be engaged at the same time. As the participants became more adept, multiplayer games were encouraged so participants could play for longer periods of time. On the rare occasion that only one participant was in attendance, the study team participated in the group. Participants were paid 5 dollars at the end of each session for the time needed to complete the assessment.
Measures:
In addition to completing questionnaires and assessments about clinical and sociodemographic information, participants were assessed with the Patient Reported Outcome Measurement Information System (PROMIS)- 29 Profile V1.0 and the Scale for the Assessment of Negative Symptoms (SANS).
The PROMIS-29 Profile V1.0, referred to as PROMIS throughout this study, was developed to provide a validated measurement tool for patient-reported outcomes to be utilized across studies and conditions, allowing for the comparison of findings (Bevans et al., 2014). The PROMIS includes 28 questions scored using a Likert scale of 1–5 points, with higher scores associated with more depressive symptoms. The tool includes multiple questions in each category of Physical Function, Anxiety, Depression, Fatigue, Social Roles/Activities, and Pain Interference experienced over the past seven days. The final 29th question asks for a 0–10 pain scale rating average for the past 7 days. These scores are then summed for an overall score used to indicate levels of depressive symptoms in the study sample. While only one section with 4 questions directly addressed depression, (“feeling worthless,” “helpless,” “depressed,” and “hopeless”), other questions addressed common manifestations or contributory factors of depression (“worries overwhelmed,” “feeling fatigued,” “trouble starting things because I am tired,” “how run-down did you feel,” “ability to do regular personal and household responsibilities,” “sleep quality and difficulty falling asleep”) (Bevans et al., 2014). The PROMIS has been shown to be a reliable, precise and valid measure of multiple health related outcomes including depression (Cella et al., 2010).
The SANS was performed by a trained interviewer. The SANS includes questions that allow the interviewer to assess patient’s orientation, insight and judgment, mental status, affective flattening or blunting, alogia, avolition-apathy, anhedonia-asociality, and attention. The patient’s negative symptoms are then scored by the interviewer on a 0–5 point Likert scale with 0 indicating no and 5 indicating severe negative symptoms. These 25 symptom scores are then totaled for an overall score.
The SANS (Andreasen, 1989) is the most widely used rating scale for negative symptoms in patients with schizophrenia (Kirkpatrick et al., 2006). The SANS shows excellent interrater reliability (Andreasen, 1982), has been demonstrated to be a reliable (Mueser et al., 1994) and valid (Rabany et al., 2010) scale when used to assess negative symptoms in schizophrenia, and can record negative symptoms over time in response to treatments (Andreasen, 1989). Furthermore, the SANS was developed to define and describe a wide range of symptoms that can be used with a variety of diagnostic categories (Andreasen, 1989).
Assessments were completed prior to starting the exergame program and after 5 and 10 weeks of the program. Participants self-reported information on the PROMIS measure and a trained interviewer conducted the SANS.
Analysis:
Multilevel linear regression models were used to test change across time for all outcome measures (Hox, 2010)(Singer & Willett, 2003). An advantage of the multilevel regression approach over more traditional repeated measures analysis of variance (RMANOVA) that is often used for this analysis, is that multilevel regression models can be estimated when predictors are continuous as well as categorical (as expected for RMANOVA). The effects of predictors at baseline (the first assessment), and the effects of predictors of the change trajectory can be estimated with multilevel regression models. In addition, multilevel regression can provide unbiased estimates of change or of the effect of predictors on change even if some assessments of the outcome are missing or participants drop out from the study. This is possible through the use of Full Information Maximum Likelihood (FIML) (Schafer & Graham, 2002) with the Expectation-Maximization (EM) algorithm (Muthen & Shedden, 1999).
This method provides unbiased parameter estimates as long as the missingness is ignorable. Even if participants only provide data at the initial assessment, their data contributes to the estimation of the intercept (e.g., mean at baseline) and intercept variance. Participants contribute information to the analysis for as many times as they provide data. The missing data that did occur is assumed to be missing at random (one type of “ignorable”) (Enders, 2006; Enders, 2010; Schafer, 1997). This assumption is a reasonable assumption for the outcomes of interests, because it is unlikely that participants did not provide assessments of the measures because of the values that would have been reported had they been assessed.
Distributions of the PROMIS depression and SANS outcome measures were non-normal (i.e., depression is ordinal with a 1–5 scale, SANS is right-skewed), and so the general linear model assumption of normal errors was not justified (Hox, 2010; Singer & Willett, 2003). Therefore, and in order to allow interpretation of effects on the original scales for the PROMIS depression and SANS scores, estimation was carried out with a bootstrap in order to draw inferences about statistical significance. The bootstrap (Wehrens et al., 2000; Wood, 2005; Zhu, 1997) may be carried out in several ways: with inference based on a z-test using bootstrapped standard errors assuming normality, percentile-based confidence intervals, or nonparametric bias-corrected confidence intervals (BC CI). The present analyses employed the nonparametric bootstrap in order to obtain non-parametric bias-corrected bootstrapped confidence intervals using 5,000 repetitions. In using bootstrapped confidence intervals to draw conclusions about statistical significance for our quantitative outcome, a regression coefficient or other effect was considered to be significant if zero was not in the interval. For all analyses, estimation was carried out with Stata/SE Release 16, (StataCorp, 2019) using a two-sided alpha of 0.05.
Results:
A total of 52 participants were included in the analyses. As indicated in table 1, the majority of participants were male and the average age for the overall sample was 59.2 (49 – 71; 5.3). Twenty-seven participants were current smokers, 16 were past smokers, and 9 never smoked. The mean number of sessions attended was 17 out of 30 total (SD=9). Twenty-nine percent of participants completed at least 75% of the sessions and 62% of participants completed at least 50% of the sessions. Three participants completed all 30 sessions. Eight 10-week waves were offered and the average number of participants per wave was 5 (SD=.74).
Table 1:
Characteristics of Participants, n=52
| Characteristic | % (n) or Mean (SD) |
|---|---|
| Age(years) | 59.2 (49 – 71; 5.3) |
| Male | 61.5 (32) |
| Female | 38.5 (20) |
| White | 50 (26) |
| Black/African American | 25 (13) |
| Latinx | 1.9 (1) |
| Native American | 1.9 (1) |
| Asian | 11.5 (6) |
| Other | 9.6 (5) |
| First Language English | 88.7 (47) |
| Past Substance Use Disorder | 16 (30) |
| Body Mass Index | 28.8 (5.9)* |
| Schizophrenia | 44.2 (23) |
| Schizoaffective | 5.8 (3) |
| Major Depression | 21.2 (11) |
| Anxiety | 1.9 (1) |
| Bipolar Disorder | 9.6 (5) |
| Psychosis (Not Otherwise Specified) | 7.7 (4) |
| Post-Traumatic Stress Disorder | 9.6 (5) |
BMI is listed for 51 participants. Missing data for one participant.
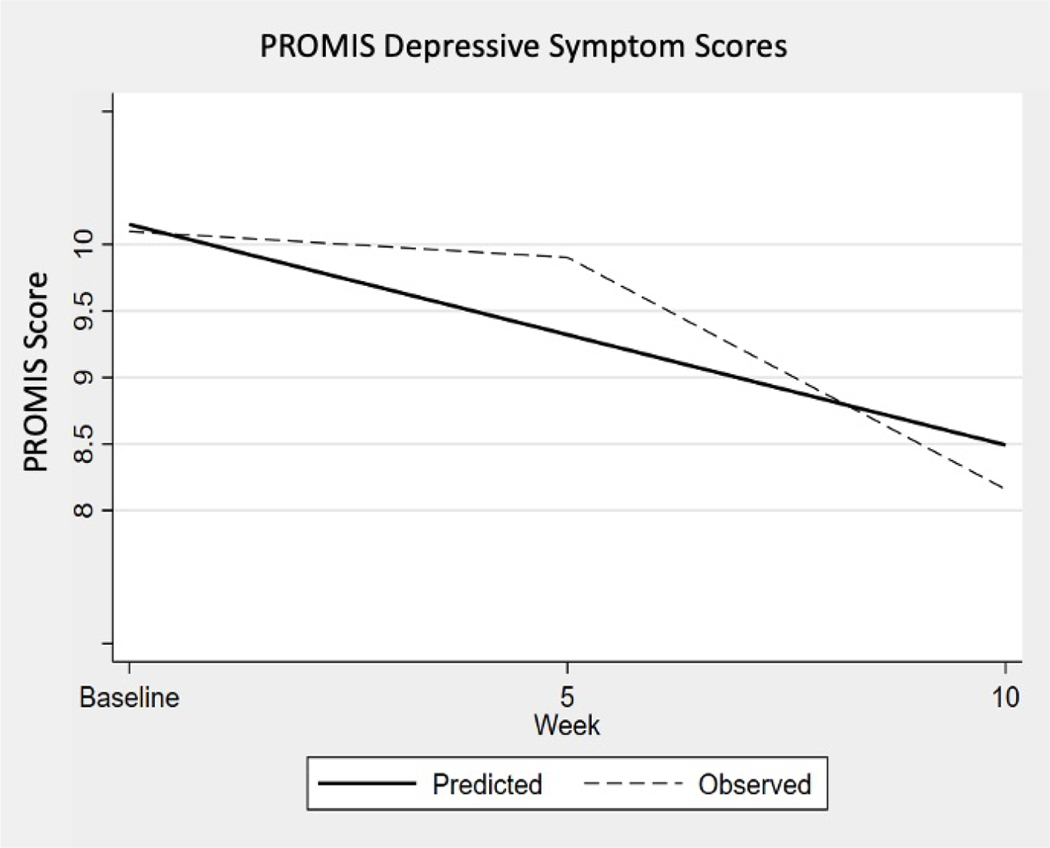
Results of the multilevel linear regression are in table 2 and illustrated in figures 1 and 2. These changes in psychiatric symptoms were found to be significant with a 95% confidence interval. Neither confidence interval contained zero, indicating a significant change occurred. Analyzing the PROMIS data, the predicted mean score from the linear analysis for the sample at enrollment was 10.15 points. For each additional 5 weeks of engaging in the physical activity program, self-reported depression scores decreased on average 0.83 points, or 1.66 points over the entire 10 week study period as illustrated in Figure 1, PROMIS Depressive Symptom Scores. The mean raw score for depressive symptoms (10.15 points) at enrollment corresponds to a T-score of 58.9 (SE 2.3) and the decrease of 1.66 points (8.49) for the 10 week period corresponds to a T-score of 55.7 (SE 2.3) (HealthMeasures, 2020). The score indicates the sample is one standard deviation above the average for the United states general population.
Table 2:
Change in psychiatric symptoms over a 10 week physical activity program:
| Statistical Model Data Source: | Estimate | 95% CI | |
|---|---|---|---|
| Lower Bound | Upper Bound | ||
| PROMIS | |||
| Initial Score | 10.15 | 9.37 | 10.83 |
| Coefficient | −0.83 | −1.46* | −0.12* |
| SANS | |||
| Initial Score | 26.89 | 23.32 | 30.46 |
| Coefficient | −5.29 | −7.67* | −3.14* |
Lower and upper limits for the nonparametric bootstrapped bias-corrected confidence interval (5,000 repetitions): If zero is in the interval, the coefficient is not significant (two-sided alpha = .05)
Figure 1:
Patient-Reported Outcome Measures Information System Scores: observed decline in mean scores at baseline, 5-week, and 10-week intervals, with predicted decline based on the nonparametric bootstrap with 5,000 repetitions. A statistically significant reduction of depressive symptoms as measured with the PROMIS was observed for this study sample at both 5 and 10 week intervals.
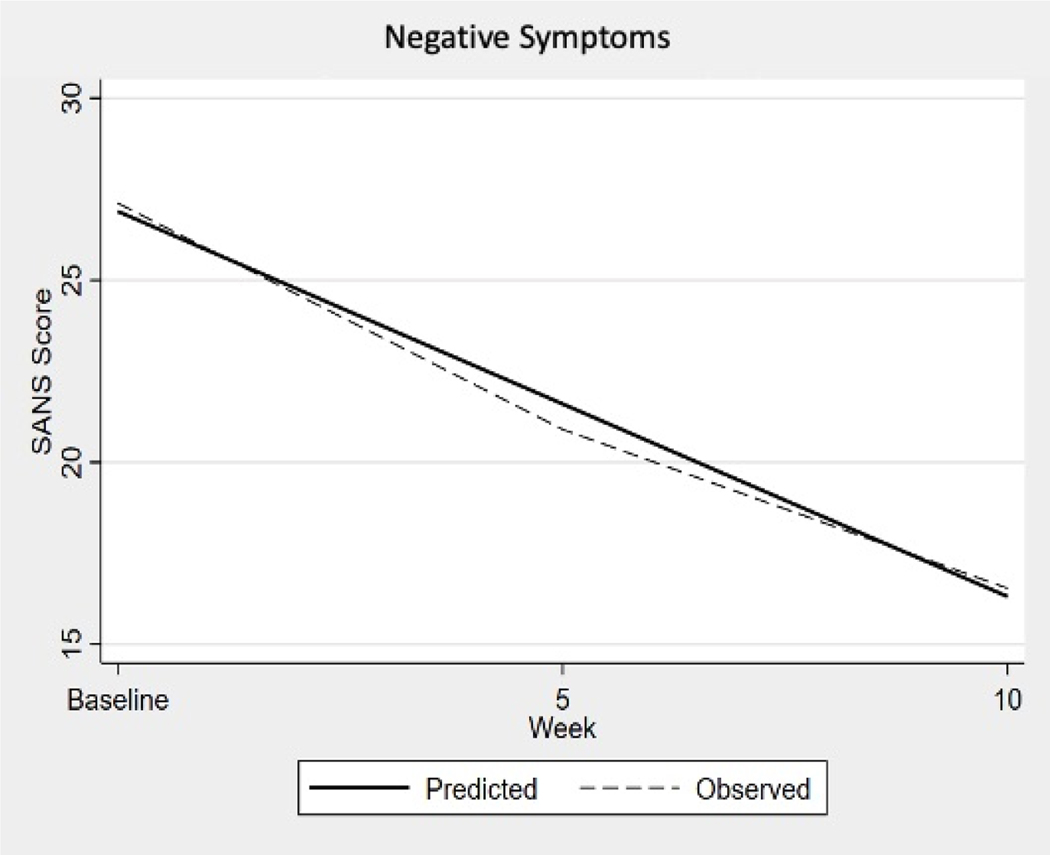
Figure 2:
Scale for the Assessment of Negative Symptoms: observed decline in mean scores at baseline, 5-week, and 10-week intervals, with predicted decline based on the nonparametric bootstrap with 5,000 repetitions. A statistically significant reduction of negative symptoms as measured with the SANS was observed for this study sample at both 5 and 10 week intervals.
For the SANS data, the predicted mean score from the linear analysis for the sample at enrollment was 26.89. For each additional 5 weeks of engaging in the physical activity program, investigator-assessed psychiatric symptom scores decreased on average 5.29 points, or 10.58 points over the entire 10 week study period as illustrated in Figure 2, Negative Symptoms. The average SANS score at enrollment was 26.89 points and that score correlates to a Clinical Global Impression (CGI) score of “borderline” level of illness (SANS score between 15–63) (Levine & Leucht, 2013). The decrease of 10.58 points for the 10 week period would leave the average SANS score still in the “borderline” level of illness but on the upper limit.
Discussion:
This study is the first to specifically evaluate the utilization of exergames and their effects on depressive and negative symptoms in older adults with SMI while in a transitional care or outpatient setting. We theorized that the process of play, social interactions, and physical engagement provided by exergames over a 10-week period would have a positive impact on older adult patients with SMI and their mental health. We utilized two reliable, validated and commonly implemented mental health assessment tools to show the impact of exergames on depressive and negative symptoms. Our results showed a statistically significant effect in reducing self-reported depressive symptoms and observed negative symptoms in older adults with serious mental illness. These findings suggest group exergame activity programs offered on-site at a mental health transitional care facility or outpatient clinic is an effective adjunct modality in reducing negative symptoms of depression in this older patient population with SMI.
Similar to our findings, a recent RCT with over one hundred older adult participants experiencing subsyndromal depression compared exergames to the comparable traditional physical activity for a six-week one hour per week frequency and found a statistically significant greater reduction in depressive symptoms in the exergame group (Li et al., 2018b). The authors concluded exergames are more beneficial for reducing subsyndromal depression in older adults when compared to traditional exercise. This study assessed a similar older adult patient population, with less acute depression, utilizing an analogous intervention and found results comparable to ours.
Implications for practice:
Utilization of an exergame regimen stands out from traditional modalities because it is relatively easy to incorporate into the daily routine of a treatment program with little cost burden on staff to facilitate (Groessl et al., 2009; Leutwyler et al., 2018). Our exergame program is cost reducing when compared to a traditional physical activity program. The initial cost of an appropriate gaming console can be purchased new for approximately $250 (Amazon at time of publishing) (Amazon.com, 2020). A comparable ten-week traditional exercise program performed by a “Healthcare Support Worker” (US Department of Labor Bureau of Labor Statistics, 2020) would cost $577.20 to implement and would not be reusable for future patients. Our research team’s experience suggests sessions could easily be run as peer lead groups.
In 2015, Waldman-Levi, Bar-Haim, & Katz suggested play be incorporated into the concept of wellbeing for older adults and that play may help increase feelings of positive self-concept and empowerment (Waldman-Levi et al., 2015). Improving these playful feelings may be why exercise reduced negative and depressive symptoms in our older adult population with SMI.
Limitations:
Our study was limited by the loss to follow up of patients who did not complete all three rounds of surveys. These missing data were accommodated using the multilevel regression method of analysis. Our study design lacked a control group which weakens the argument for a causative change from exergames use. However, our results are comparable to other researchers’ findings using similar modalities with similar patient populations. Our study used only one highly trained researcher to perform the SANS interviews, assuring a high level of intrarater and interrater reliability.
Conclusion:
We concluded the use of exergames for older adults with SMI was associated with a reduction of depressive symptoms and therefore utilization of exergames as an adjunct modality in this patient population should be implemented clinically, cost effectively and safely to improve patient outcomes. The results of this study can be used to support further research and funding utilizing an RCT design of exergames vs traditional exercise in patients with SMI. Our findings contribute to the growing body of literature indicating play and social connections, facilitated by exergames, can lead to an improvement in patients’ depressive symptoms among older adults with SMI.
Acknowledgments
Declaration of interest statements:
This work was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI under Grant (number KL2 TR0000143), the UCSF Claude D. Pepper Center (4P30AG044281-04), the UCSF Academic Senate Individual Investigator Award (no number), the UCSF School of Nursing (no number), the Symptom Management Faculty Scholars Program under Grant (number 1-P30-NR011934-0), and the National Institute on Aging under Grant (number K23AG044438).
Contributor Information
Michael Heinbach, UCSF Medical Center, 505 Parnassus Ave, San Francisco, CA 94143.
Astrid J. Block, UCSF School of Nursing, Department of Physiological Nursing, 2 Koret Way, Room N611C, 6th Floor, Campus Box 0610, San Francisco, CA 94143-0610.
Erin M. Hubbard, Department of Physiological Nursing, University of California, San Francisco, San Francisco, CA 94143-0610.
Janine K Cataldo, UCSF Department of Physiological Nursing, 2 Koret Way, N611Q, San Francisco, CA 94143-0610.
Bruce A. Cooper, UCSF Dept. of Physiological Nursing, Room N631, 2 Koret Way | San Francisco, CA 94143-0610.
Heather Leutwyler, Department of Physiological Nursing, University of California, San Francisco, 2 Koret Way, N631A, Box 0610, San Francisco, California, 94143-0610.
References
- Allison DB, Newcomer JW, Dunn AL, Blumenthal JA, Fabricatore AN, Daumit GL, Cope MB, Riley WT, Vreeland B, Hibbeln JR, & Alpert JE (2009). Obesity among those with mental disorders: A national institute of mental health meeting report. American Journal of Preventive Medicine, 36(4), 341–350. 10.1016/j.amepre.2008.11.020 [DOI] [PubMed] [Google Scholar]
- Amazon.com. (2020). Xbox one S 1TB all-digital edition console. Amazon.com. https://www.amazon.com/Xbox-All-Digital-Console-Disc-Free-Gaming/dp/B07XQXZXJC/ref=sr_1_1?dchild=1&keywords=xbox&qid=1588284422&sr=8-1 [Google Scholar]
- American Psychological Association. (2009). Proficiency in psychology: Assessment and treatment of serious mental illness. Washington, DC: https://www.apa.org/practice/resources/smi-proficiency.pdf [Google Scholar]
- Andreasen NC (1982). Negative symptoms in schizophrenia. definition and reliability. Arch Gen Psychiatry, 39(7), 784–8. https://www.ncbi.nlm.nih.gov/pubmed/7165477 [DOI] [PubMed] [Google Scholar]
- Andreasen NC (1989). The scale for the assessment of negative symptoms (SANS): Conceptual and theoretical foundations. British Journal of Psychiatry, 155(S7), 49–52. 10.1192/S0007125000291496 [DOI] [PubMed] [Google Scholar]
- Ashdown-Franks G, Firth J, Carney R, Carvalho AF, Hallgren M, Koyanagi A, Rosenbaum S, Schuch FB, Smith L, Solmi M, Vancampfort D, & Stubbs B. (2020). Exercise as medicine for mental and substance use disorders: A meta-review of the benefits for neuropsychiatric and cognitive outcomes. Sports Medicine (Auckland, N.Z.), 50(1), 151–170. 10.1007/s40279-019-01187-6 [DOI] [PubMed] [Google Scholar]
- Bartels SJ (2011). Commentary: The forgotten older adult with serious mental illness: The final challenge in achieving the promise of olmstead? Journal of Aging & Social Policy, 23(3), 244–257. doi: 10.1080/08959420.2011.579497 [DOI] [PubMed] [Google Scholar]
- Bevans M, Ross A, & Cella D. (2014). Patient-reported outcomes measurement information system (PROMIS): Efficient, standardized tools to measure self-reported health and quality of life. Nursing Outlook, 62(5), 339–345. 10.1016/j.outlook.2014.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, Amtmann D, Bode R, Buysse D, Choi S, Cook K, DeVellis R, DeWalt D, Fries JF, Gershon R, Hahn EA, Lai J, Pilkonis P, Revicki D, . . . Hays R. (2010). The patient-reported outcomes measurement information system (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. Journal of Clinical Epidemiology, 63(11), 1179–1194. 10.1016/j.jclinepi.2010.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JA, Razzano L, Jonikas JA, Swarbrick MA, Steigman PJ, Hamilton MM, Carter TM, & Santos AB (2016). Correlates of co-occurring diabetes and obesity among community mental health program members with serious mental illnesses. Psychiatric Services, 67(11), 1269–1271. [DOI] [PubMed] [Google Scholar]
- Dickerson FB, Brown CH, Kreyenbuhl JA, Fang L, Goldberg RW, Wohlheiter K, & Dixon LB (2006). Obesity among individuals with serious mental illness. Acta Psychiatrica Scandinavica, 113(4), 306–313. 10.1111/j.1600-0447.2005.00637.x [DOI] [PubMed] [Google Scholar]
- Dobbins S, Hubbard E, Flentje A, Dawson-Rose C, & Leutwyler H. (2018). Play provides social connection for older adults with serious mental illness: A grounded theory analysis of a 10-week exergame intervention. Aging & Mental Health,, 1–8. 10.1080/13607863.2018.1544218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders CK (2006). A primer on the use of modern missing-data methods in psychosomatic medicine research. Psychosomatic Medicine, 68(3), 427–436. 10.1097/01.psy.0000221275.75056.d8 [DOI] [PubMed] [Google Scholar]
- Enders CK (2010). Applied missing data analysis. Guilford Publications Inc. M.U.A. [Google Scholar]
- Groessl EJ, Kaplan RM, Blair SN, Rejeski WJ, Katula JA, King AC, Fielding RA, Glynn NW, & Pahor M. (2009). A cost analysis of a physical activity intervention for older adults. Journal of Physical Activity & Health, 6(6), 767–774. https://www.ncbi.nlm.nih.gov/pubmed/20101920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- HealthMeasures (2020). PROMIS Adult Profile Scoring Manual: Patient-Reported Outcomes Measurement Information System.[PDF file]. Northwestern University. Retrieved from http://www.healthmeasures.net/images/PROMIS/manuals/PROMIS_Adult_Profile_Scoring_Manual.pdf [Google Scholar]
- Hox JJ (2010). Multilevel analysis: Techniques and applications (7th ed.). Routledge Academic: Taylor & Francis Group. [Google Scholar]
- Jeste DV, Alexopoulos GS, Bartels SJ, Cummings JL, Gallo JJ, Gottlieb GL, Halpain MC, Palmer BW, Patterson TL, Reynolds CF, & Lebowitz BD (1999). Consensus statement on the upcoming crisis in geriatric mental health: Research agenda for the next 2 decades. Archives of General Psychiatry, 56(9), 848–853. 10.1001/archpsyc.56.9.848 [DOI] [PubMed] [Google Scholar]
- Kimhy D, Khan S, Ayanrouh L, Chang RW, Hansen MC, Lister A, Ballon JS, Vakhrusheva J, Armstrong HF, Bartels MN, & Sloan RP (2016). Use of active-play video games to enhance aerobic fitness in schizophrenia: Feasibility, safety, and adherence. Psychiatric Services, 67(2), 240–243. 10.1176/appi.ps.201400523 [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Fenton WS, Carpenter J, William T, & Marder SR (2006). The NIMH-MATRICS consensus statement on negative symptoms. Schizophrenia Bulletin, 32(2), 214–219. 10.1093/schbul/sbj053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutwyler H, Hubbard E, Cooper B, & Dowling G. (2018). Impact of a pilot videogame-based physical activity program on walking speed in adults with schizophrenia. Community Mental Health Journal, 54(6), 735–739. 10.1007/s10597-017-0208-6 [DOI] [PubMed] [Google Scholar]
- Levine SZ, & Leucht S. (2013). Identifying clinically meaningful symptom response cut-off values on the SANS in predominant negative symptoms. Schizophr Res. 2013;145(1–3):125–127. doi: 10.1016/j.schres.2012.12.032 [DOI] [PubMed] [Google Scholar]
- Li J, Erdt M, Chen LX, Cao YY, Lee SQ, & Theng YL (2018a). The social effects of exergames on older adults: Systematic review and metric analysis. Journal of Medical Internet Research, 20(6), e10486. 10.2196/10486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Theng Y, Foo S, & Xu X. (2018b). Exergames vs. traditional exercise: Investigating the influencing mechanism of platform effect on subthreshold depression among older adults. Aging & Mental Health, 22(12), 1634–1641. 10.1080/13607863.2017.1385722 [DOI] [PubMed] [Google Scholar]
- Lieberman DA, Chamberlin B, Medina E Jr, Franklin BA, Sanner BM, & Vafiadis DK (2011). The power of play: Innovations in getting active summit 2011: A science panel proceedings report from the american heart association. Circulation, 123(21), 2507–2516. 10.1161/CIR.0b013e318219661d [DOI] [PubMed] [Google Scholar]
- Liu NH, Daumit GL, Dua T, Aquila R, Charlson F, Cuijpers P, Druss B, Dudek K, Freeman M, Fujii C, Gaebel W, Hegerl U, Levav I, Laursen T, Ma H, Maj M, Medina-Mora M, Nordentoft M, Prabhakaran D, Pratt K, Prince M, Thara R, Shiers D, Susser E, Thornicroft G, Wahlbeck K, Wassie A, Whiteford H, Saxena S. (2017) Excess mortality in persons with severe mental disorders: a multilevel intervention framework and priorities for clinical practice, policy and research agendas. World Psychiatry. 2017;16(1):30–40. doi: 10.1002/wps.20384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matallaoui A, Koivisto J, Hamari J, & Zarnekow R. (2017). How effective is “Exergamification”? A systematic review on the effectiveness of gamification features in exergames. University of Hawai’i at Manoa. 10.24251/HICSS.2017.402 [DOI] [Google Scholar]
- Monedero J, Murphy EE, & O’Gorman DJ (2017). Energy expenditure and affect responses to different types of active video game and exercise. Plos One, 12(5), 1–13. 10.1371/journal.pone.0176213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueser KT, Sayers SL, Schooler NR, Mance RM, & Haas GL (1994). A multisite investigation of the reliability of the scale for the assessment of negative symptoms. American Journal of Psychiatry, 151(10), 1453–1462. 10.1176/ajp.151.10.1453 [DOI] [PubMed] [Google Scholar]
- Muthen B, & Shedden K. (1999). Finite mixture modeling with mixture outcomes using the EM algorithm. Biometrics, 55(2), 463–469. 10.1111/j.0006-341X.1999.00463.x [DOI] [PubMed] [Google Scholar]
- Rabany L, Weiser M, Werbeloff N, & Levkovitz Y. (2010). Assessment of negative symptoms and depression in schizophrenia: Revision of the SANS and how it relates to the PANSS and CDSS. Schizophrenia Research, 126(1), 226–230. 10.1016/j.schres.2010.09.023 [DOI] [PubMed] [Google Scholar]
- Ritchie H, & Roser M. (2018). Mental health. OurWorldInData.org. Retrieved from: https://ourworldindata.org/mental-health#citation [Google Scholar]
- Ruggeri M, Leese M, Thornicroft G, Bisoffi G, Tansella M. Definition and prevalence of severe and persistent mental illness. The British Journal of Psychiatry. 2000;177(2):149–155. doi: 10.1192/bjp.177.2.149. [DOI] [PubMed] [Google Scholar]
- Schafer JL (1997). Analysis of incomplete multivariate data. Chapman & Hall. [Google Scholar]
- Schafer JL, & Graham JW (2002). Missing data: Our view of the state of the art. Psychological Methods, 7(2), 147–177. 10.1037/1082-989X.7.2.147 [DOI] [PubMed] [Google Scholar]
- Singer JD, & Willett JB (2003). Applied longitudinal data analysis: Modeling change and event occurrence. Oxford University Press. [Google Scholar]
- Stanton R, & Happell B. (2014). A systematic review of the aerobic exercise program variables for people with schizophrenia. Current Sports Medicine Reports, 13(4), 260–266. 10.1249/JSR.0000000000000069 [DOI] [PubMed] [Google Scholar]
- Substance Abuse Mental Health Services Administration (SAMHSA). (2016). Behind the term: Serious mental illness (Report 283– 12–3702). Washington, DC: Development Services Group, Inc. Retrieved from: https://nrepp.samhsa.gov/Docs/Literatures/Behind_the_Term_Serious%20%20Mental%20Illness.pdf [Google Scholar]
- StataCorp. (2019). Stata statistical software [computer software]. College Station, TX: [Google Scholar]
- Tiihonen J, Lönnqvist J, Wahlbeck K, Klaukka T, Niskanen L, Tanskanen A, & Haukka J. (2009). 11-year follow-up of mortality in patients with schizophrenia: A population-based cohort study. Lancet, The, 374(9690), 620–627. 10.1016/S0140-6736(09)60742-X [DOI] [PubMed] [Google Scholar]
- United Nations. World population prospects: The 2017 revision. United Nations. 2017. [Google Scholar]
- US Department of Labor. Bureau of Labor Statistics. (2020). May 2019 national occupational employment and wage estimates united states. https://www.bls.gov/oes/current/oes_nat.htm#29-0000
- Waldman-Levi A, Erez AB, & Katz N. (2015). Healthy aging is reflected in well-being, participation, playfulness, and cognitive-emotional functioning. Healthy Aging Research, 4(1), 1. 10.12715/har.2015.4.8 [DOI] [Google Scholar]
- Wehrens R, Putter H, & Buydens LMC (2000). The bootstrap: A tutorial. Chemometrics and Intelligent Laboratory Systems, 54(1), 35–52. 10.1016/S0169-7439(00)00102-7 [DOI] [Google Scholar]
- Wood M. (2005). Bootstrapped confidence intervals as an approach to statistical inference. Organizational Research Methods, 8(4), 454–470. 10.1177/1094428105280059 [DOI] [Google Scholar]
- Zhu W. (1997). Making bootstrap statistical inferences: A tutorial. Research Quarterly for Exercise and Sport, 68(1), 44–55. 10.1080/02701367.1997.10608865 [DOI] [PubMed] [Google Scholar]