Abstract
Background:
Multidrug-resistant infections complicating combat-related trauma necessitate use of broad-spectrum antimicrobials. Recent literature posits an association between vancomycin (VANC) and piperacillin-tazobactam (VPT) combination therapy and acute kidney injury (AKI). We examined whether therapy with VPT was associated with an increased risk of AKI compared to VANC and other broad-spectrum beta-lactam antibiotics (VBL) following combat-related injuries.
Methods:
Patients within the Trauma Infectious Disease Outcomes Study (TIDOS) who received ≥48 hours concomitant VPT or VBL started within 24 hours of each other were assessed. Exclusion criteria were receipt of renal replacement therapy and baseline creatinine >1.5 mg/dL. AKI was defined by meeting any of the RIFLE, AKIN or VANC Consensus Guidelines criteria 3-7 days after therapy initiation. Variables significantly associated with AKI were used in inverse probability treatment weighting to perform univariate and subsequent logistic regression multivariate modeling to determine significant risk factors for AKI.
Results:
Sixty-one patients who received VPT and 207 who received VBL were included. Both groups had a median age of 24 years and initial median creatinine of 0.7 mg/dL. The VBL patients were more likely to have sustained blast injuries (p=0.001) and received nephrotoxic agents (amphotericin [p=0.002] and aminoglycosides [p<0.001]). In the VBL group, AKI incidence was 9.7% compared to 13.1% in the VPT group (p=0.438). Multivariate analysis identified a relative risk of 1.727 (95% CI: 1.027-2.765) for AKI associated with VPT exposure. AKI severity generally met RIFLE Risk criteria and was one day in duration. Only one patient had persistent renal dysfunction 30 days after therapy completion.
Conclusion:
In this young and previously healthy, severely ill combat-injured population, VPT was associated with nearly twice the risk of AKI compared to VBL. Nevertheless, AKI was of low severity, short duration, and had high rates of renal recovery.
Keywords: Acute Kidney Injury, Combat trauma, Antibiotics
BACKGROUND
After the first 24 hours following traumatic injury, infection has historically been, and remains, a major cause of morbidity and mortality. In combat-related trauma, infectious complications are compounded by the threat of multidrug resistant (MDR) bacteria.1,2 Studies investigating the epidemiology of MDR Gram-negative infections in military trauma have shown that patients may have their first or only infection after injury be due to an MDR Gram-negative pathogen despite being previously healthy with limited healthcare exposure.3 These patients are frequently more severely injured compared to patients with no infections or infections attributed to pathogens other than MDR Gram-negative bacteria.3 This is in contrast to MDR infections in civilian counterparts where states of immune compromise, advanced age, previous hospitalizations, and long-term care facility residence are risk factors.4
Although combat trauma-related infections from the recent wars were largely attributed to Gram-negative pathogens, extremity wound infections were frequently polymicrobial with Gram-positive organisms (primarily Enterococcus spp.) also being recovered.5,6 As such, empiric antimicrobial therapy for the critically injured often includes coverage for MDR Gram-positive organisms to prevent unchecked infection in these patients with compromised physiologic reserves. Due to concern for MDR organisms, clinicians frequently employ broad-spectrum antimicrobials; however, antibiotic usage is not without risk. A growing body of literature posits an association between vancomycin and piperacillin-tazobactam (VPT) combination therapy and acute kidney injury (AKI).7–22 A similar association between concomitant vancomycin and other broad-spectrum beta-lactams (VBL) has not been as readily identified. 11–20,23,24 The significance of these findings is magnified when taking into account the association between AKI and short-term morbidity, along with both short and long-term mortality.
Multiple investigations into this phenomenon have been undertaken, but the exact relationship and pathophysiology between administration of VPT combination therapy and AKI remains undefined. Further complicating the issue, not all investigations have had similar conclusions. 7,12,16,17,23 Part of this may be attributable to limitations of previous studies, which included patients with multiple confounding risk factors for AKI development, such as comorbid medical conditions, underlying renal pathology, and chronic nephrotoxic medication use. In particular, the majority of studies had a predominant focus in non-critically ill hospitalized patients.
In comparison, the active-duty military population by virtue of baseline fitness for service requirements have relatively few medical comorbidities, decreasing confounding risk factors for AKI. No study to date has evaluated incidence of AKI related to VPT exposure in a critically-injured trauma population, which is otherwise young, healthy, and may be at a lower risk overall for adverse renal outcomes. In this study, we evaluate the rates and severity of AKI related to VPT in comparison to VBL in combat-related trauma patients.
METHODS
Study Design and Population
This was a retrospective study that utilized data collected through the Trauma Infectious Disease Outcomes Study (TIDOS)1 and analyzed incidence of AKI for all subjects treated with concomitant VPT in comparison to patients who received concomitant VBL (meropenem, imipenem-cilastatin, or cefepime). Subjects were eligible if they were active-duty personnel or DoD beneficiaries, age 18 years and older, injured during deployment requiring return via Landstuhl Regional Medical Center (LRMC, Germany), to a participating military hospital in the United States between June 1, 2009 – December 31, 2014. Participating U.S. military hospitals included National Naval Medical Center and Walter Reed Army Medical Center in the National Capital Region (merged to become Walter Reed National Military Medicine Center in September 2011) and Brooke Army Medical Center (BAMC) in San Antonio, TX. Full data regarding the TIDOS project design have been previously outlined.1 This study was approved (IRB number 351767) by the Institutional Review Board of the Uniformed Services University of the Health Sciences (Bethesda, MD). As data were collected as part of the Department of Defense Trauma Registry, informed consent was waived.
For the retrospective study herein, additional criteria for inclusion required that patients receive a minimum of 48 hours of concomitant antibiotic administration of vancomycin with the beta-lactam (piperacillin-tazobactam, cefepime, meropenem, or imipenem-cilastatin) and have creatinine data available. Initiation of both agents had to occur within 24 hours during initial U.S. hospitalization (Figure 1). The comparator VBL group size was restricted secondary to variability in reporting of AKI rates in the literature with sample size calculations utilizing conservative estimates of AKI rates. 7–10,12–17,21,23 Varying AKI rates of 10-48% in those exposed to VPT and 1-28% in the VBL group were used with an alpha of 0.05 and a fixed power of 80% to estimate the sample size for the analysis using a two-sided Z test with pooled variance. When the baseline creatinine levels of the available patient population were evaluated, the size of the VPT group was preliminarily estimated to be 43. Using that value, it was calculated that a sample size in the range of 197 to 214 was needed for the VBL comparator group. Further evaluation of the dataset identified additional subjects meeting the exposure criteria (N=61) and a VBL comparator group of 207 individuals was identified.
Figure 1:
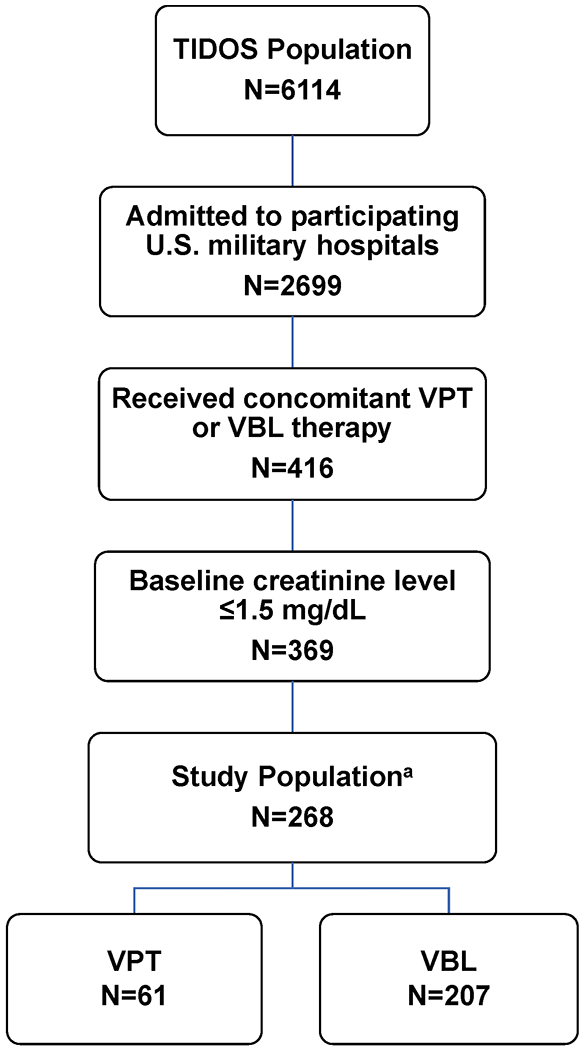
Flow diagram of study population and treatment groups. a Data available for analysis. TIDOS- Trauma Infectious Disease Outcomes Study, VPT- Vancomycin/Piperacillin-tazobactam therapy. VBL- Vancomycin/broad-spectrum beta-lactam therapy
In the instance that a participant received both antibiotics on multiple occasions, only the first episode was considered. Participants were excluded if baseline (Day 1) creatinine was greater than 1.5 mg/dL, estimated glomerular filtration rate was less than 60 mL/min, or they had no measure available. Previous or current receipt of renal replacement therapy at the time of antibiotic initiation were also measures for exclusion.
Data Collection
Data were acquired from the Department of Defense Trauma Registry and supplemented by the TIDOS infectious disease module. Included in these databases were general patient demographics, trauma information (injury patterns and severity), antimicrobial administration and duration, hospital courses, and data pertaining to infectious diagnoses.1
Patient-level data were abstracted from the electronic medical record to assess for the presence of medical comorbidities. The concurrent use of nephrotoxic agents during administration of the antimicrobial agents of interest was also assessed. Nephrotoxic agents considered were amphotericin, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, aminoglycosides, diuretics, intravenous contrast dye, and nonsteroidal anti-inflammatory drugs (NSAIDs). Electronic medical records were also reviewed for the loading and maintenance dosing for antibiotics of interest. When available, the highest vancomycin trough prior to onset of AKI or highest trough during the combination antibiotic administration, in the absence of AKI for up to seven days, were collected. The blood urea nitrogen (BUN)/creatinine values were obtained for all patients who received the dual antimicrobial therapy being investigated and levels were collected beginning the day of antimicrobial administration and continued for 96 hours following completion of vancomycin therapy. For patients with laboratory tests that had multiple measures for a given day, only the first daily measure was considered. When available, a follow-up creatinine was obtained 30 days (+/− 10 days) after antibiotic initiation.
Study Definitions
The primary outcome of interest was the development of AKI defined by any of the following: the RIFLE (Risk, Injury, Failure, Loss, End Stage Renal Disease) criteria, the Acute Kidney Injury Network (AKIN) criteria, or previously delineated definitions from the vancomycin therapeutic guidelines. 25–27 For the vancomycin therapeutic guidelines, AKI was defined as a rise in serum creatinine by >50% or >0.5 mg/dl sustained over at least two consecutive measurements. The RIFLE criteria was divided into three categories. An increase in serum creatinine 1.5 times the baseline or decrease in glomerular filtration rate (GFR) greater than 25% correlated to the RIFLE Risk category. Injury was defined as an increase in serum creatinine two times the baseline or decrease in GFR greater than 50%, and Failure as an increase in serum creatinine three times the baseline or decrease in GFR greater than 75% or serum creatinine greater than or equal to 4 mg/dl. The AKIN is similarly composed of three categories designated as stages. Stage 1 refers to an increase in serum creatinine of more than or equal to 0.3 mg/dl or increase to more than or equal to 150% to 200% (>1.5 to 2-fold) from baseline, Stage 2 is an increase in serum creatinine to more than 200% to 300% (>2 to 3-fold) from baseline, and Stage 3 is an increase in serum creatinine to more than 300% (>3 fold) from baseline or serum creatinine of more than or equal to 4 mg/dl with an acute increase of at least 0.5 mg/dl. A participant was classified as having AKI if any of the above criteria were met on combination antibiotic days 3-7 with day 1 defined as the first day of combination therapy. The time period for AKI onset was selected in keeping with previously published median onset between 3-5 days. The vancomycin loading dose was defined as the initial, higher dose of vancomycin compared to subsequent dosing. Maintenance dose referred to the first maintenance vancomycin dose, as subsequent doses were based on serum concentrations. Intravenous contrast exposure was defined as any contrasted radiographic study administered seven days prior to and 72 hrs after combination antimicrobial initiation. The GFR was calculated using the Modification of Diet in Renal Disease (MDRD) equation: GFR = 186 × Serum Creatinine−1.154 × Age−0.203 × 1.212 (if patient was black) × 0.742 (if female). Similar to Kim et. al.,8 we used the MDRD equation because of concerns about the role of weight and interpretations in the setting of potential amputations during the study period.
As amphotericin, a potential nephrotoxic agent, was frequently prescribed to wounded military personnel as part of the management of invasive fungal infections (IFIs), the occurrence of IFIs in the population was assessed. To be classified as an IFI, a wound required persistent necrosis and laboratory evidence of a filamentous fungus after ≥2 surgical debridements. Wounds that had laboratory evidence of a fungus, but did not meet IFI criteria were classified as High Suspicion of IFI (i.e., had signs/symptoms of a deep soft-tissue infection attributed to a fungus) or Low Suspicion of IFI (i.e., did not meet either IFI or High Suspicion criteria).28
Statistical Analysis
Fisher’s exact and Chi squared tests were utilized to gauge the association of categorical variables when comparing the characteristics of the VPT and VBL groups, as well as comparing characteristics of patients with and without AKI. Mann-Whitney U was used when assessing the comparison between continuous variables with the respective VPT/VBL exposure and AKI/non-AKI groups. In order to reduce confounding, variables significantly associated with outcome (i.e., AKI) were selected to calculate propensity scores. Propensity score weights were calculated using propensity score inverse for VPT and inverse of 1-propensity score for VBL. All observations were weighed by this value using inverse probability treatment weighting, while running logistic regression for univariate and multivariate models. Relative risk (RR) and 95% confidence intervals (CI) were estimated using these models. Terms significant in univariate models were utilized to create a final multivariate model determined using backward selection technique. Statistical analysis was performed with SAS version 9.3 (SAS, Cary, North Carolina).
RESULTS
Study Population
Of the initial 6,114 patients available in the TIDOS database, 369 patients had a baseline creatinine level of ≤1.5 mg/dl with 268 patients having data available for analysis (Figure 1). There was a total of 61 and 207 patients in the VPT group and VBL group, respectively. The median age of the study population was 24 years (interquartile range [IQR]: 21-28 years) with no significant difference between the VPT and VBL groups (Table 1). Initial median creatinine between the groups was likewise similar at 0.7 mg/dL (VBL IQR: 0.6-0.9 mg/dL; VPT IQR: 0.6-0.8 mg/dL). Medical comorbidities were more commonly encountered in the VBL group, but were not significant. Likewise, the injury severity score was similar between groups. Patients treated with VBL were more commonly admitted to the National Capital Region (p=0.001), sustained improvised explosive device injuries (p=0.001), and were significantly more likely to require intensive care unit (ICU) admission both at LRMC and at U.S. hospitals (p<0.001). Amphotericin and aminoglycosides were the only additional nephrotoxic agents significantly more utilized in the VBL group (p=0.002 and p<0.001, respectively). When comparing the patient population between those who developed AKI and those who did not, age at time of injury (median of 26.5 [IQR: 22-29.5] years for AKI vs 24 [IQR: 21-27] years for non-AKI; p=0.043), receipt of blood products within 24 hours post-injury (median of 21.5 [IQR: 13.5-30] units vs 15 [IQR: 7-22] units; p=0.020), U.S. military hospital admission (42.9% at BAMC vs 22.9% in the National Capital Region; p=0.021) and amphotericin exposure (28.6% vs 13.3%; p=0.032) were significant.
Table 1:
Patient Demographics by Antibiotic Groups, Vancomycin and Piperacillin-Tazobactam versus Vancomycin and Carbapenem or Cefepime combination therapy
| Characteristic, No. (%) | Total (N=268) | Vancomycin & Piperacillin-Tazobactam (N=61) | Vancomycin & Carbapenem/Cefepime (N=207) | P-value |
|---|---|---|---|---|
| Age at injury, median (IQR) | 24 (21, 28) | 24 (21, 29) | 24 (21, 28) | 0.912 |
| Male | 265 (98.9) | 60 (98.4) | 205 (99.0) | 0.541 |
| Enlisted | 238 (88.8) | 55 (90.2) | 183 (88.4) | 0.702 |
| Branch of Service | 0.017 | |||
| Army | 151 (56.3) | 38 (62.3) | 113 (54.6) | |
| Marine | 99 (36.9) | 15 (24.6) | 84 (40.6) | |
| Navy/Air Force | 14 (5.2) | 7 (11.5) | 7 (3.4) | |
| Other | 4 (1.5) | 1 (1.6) | 3 (1.5) | |
| Comorbid Conditions | ||||
| Diabetes Mellitus | 2 (0.8) | 0 | 2 (1.0) | ~1.00 |
| Hypertension | 1 (0.4) | 0 | 1 (0.5) | ~1.00 |
| Chronic Lung Disease (asthma) | 3 (1.1) | 0 | 3 (1.5) | ~1.00 |
| Chronic Renal Disease | 0 | 0 | 0 | / |
| Chronic Liver Disease | 1 (0.4) | 0 | 1 (0.5) | ~1.00 |
| Injury Cause | 0.001 | |||
| Non-Blast | 52 (19.4) | 21 (34.4) | 31 (15.0) | |
| Non-IED Blast | 24 (9.0) | 7 (11.5) | 17 (8.2) | |
| IED | 192 (71.6) | 33 (54.1) | 159 (76.8) | |
| Foot patrol Statusa | 157 (58.6) | 34 (55.7) | 123 (59.4) | 0.903 |
| Injury Severity Score, median (IQR) | 33 (22, 45) | 30 (19, 42) | 33 (22, 50) | 0.230 |
| 0 – 9 | 11 (4.1) | 4 (6.6) | 7 (3.4) | 0.226 |
| 10 – 15 | 9 (3.4) | 4 (6.6) | 5 (2.4) | |
| 16 – 25 | 67 (25.0) | 13 (21.3) | 54 (26.1) | |
| ≥26 | 181 (67.5) | 40 (65.6) | 141 (68.1) | |
| Number of Limbs Lost (trauma-related) | 0.330 | |||
| None or 1 limb | 182 (67.9) | 46 (75.4) | 136 (65.7) | |
| More than 1 limb | 86 (32.1) | 15 (24.6) | 71 (34.3) | |
| Number of Limbs Injured | 0.173 | |||
| None or 1 limb | 78 (29.1) | 22 (36.1) | 56 (27.1) | |
| More than 1 limb | 190 (70.9) | 39 (63.9) | 151 (72.9) | |
| Shock Index (SI)b | 1.0 (0.7, 1.3) | 0.9 (0.7, 1.3) | 1.0 (0.7, 1.3) | 0.481 |
| SI < 0.65 | 40 (14.9) | 8 (13.1) | 32 (15.5) | 0.722 |
| 0.65 ≤ SI < 0.8 | 42 (15.7) | 11 (18.0) | 31 (15.0) | |
| 0.8 ≤ SI | 159 (59.3) | 33 (54.1) | 126 (60.9) | |
| Mechanical Ventilation | 0.077 | |||
| LRMC Only | 63 (23.5) | 17 (27.9) | 46 (22.2) | |
| LRMC & US Hospital ≤ 1 Week | 140 (52.2) | 24 (39.3) | 116 (56.0) | |
| LRMC & US Hospital > 1 Week | 2 (0.8) | 1 (1.6) | 1 (0.5) | |
| Blood Units 24 hrs Post Injury | 15 (7, 23) | 15 (7.5, 18.5) | 15 (7, 24) | 0.580 |
| 0/Missing | 57 (21.3) | 18 (29.5) | 39 (18.8) | 0.164 |
| 1 – 9 | 62 (23.1) | 13 (21.3) | 49 (23.7) | |
| 10 – 20 | 82 (30.6) | 20 (32.8) | 62 (30.0) | |
| > 20 | 67 (25.0) | 10 (16.4) | 57 (27.5) | |
| LRMC Admission SOFA, median (IQR) | 8 (4, 11) | 6 (3, 9) | 8 (5, 11) | 0.014 |
| US Hospital Admission SOFA, median (IQR) | 5 (1, 8) | 2 (1, 6) | 5 (1, 8) | 0.004 |
| IFI Statusc | 0.164 | |||
| Non-IFI | 197 (73.5) | 52 (85.3) | 145 (70.1) | |
| IFI Low Suspicion | 26 (9.7) | 3 (4.9) | 23 (11.1) | |
| IFI High Suspicion | 23 (8.6) | 3 (4.9) | 20 (9.7) | |
| IFI | 22 (8.2) | 3 (4.9) | 19 (9.2) | |
| Intensive Care Unit (ICU) Admission | <0.001 | |||
| No ICU | 20 (7.5) | 7 (11.5) | 13 (6.3) | |
| LRMC ICU Only | 34 (12.7) | 19 (31.2) | 15 (7.3) | |
| US Hospital ICU ± LRMC ICU | 214 (79.9) | 35 (57.4) | 179 (86.5) | |
| Nephrotoxic Agents | ||||
| Intravenous Contrast | 127 (47.4) | 33 (54.1) | 94 (45.4) | 0.232 |
| ACE-I/ARBs | 5 (1.9) | 0 | 5 (2.4) | 0.592 |
| Diuretics | 26 (9.7) | 4 (6.6) | 22 (10.6) | 0.463 |
| NSAIDs | 73 (27.2) | 11 (18.0) | 62 (30.0) | 0.066 |
| Amphotericin Exposure | 40 (14.9) | 2 (3.3) | 38 (18.4) | 0.002 |
| Aminoglycosides | 88 (32.8) | 8 (13.1) | 80 (38.7) | <0.001 |
| Dosing Information | ||||
| Loading Dose, Y/N | 9 (3.4) | 3 (4.9) | 6 (2.9) | 0.430 |
| Loading Dose,d mg/day, median (IQR) | 1625 (1500, 2000) | 1500 (1500, 2000) | 1750 (1500, 2000) | 0.759 |
| Vancomycin Maintenance Dosing,e mg/day, median (IQR) | 2650 (2000, 3000) | 2000 (2000, 3000) | 3000 (2000, 3000) | 0.003 |
| Vancomycin Troughs,f μg/mL, median (IQR) | 10.8 (6.8, 16.1) | 9.4 (4.2, 14.0) | 10.9 (7.3, 16.8) | 0.080 |
| Cefepime Dosing,g mg/day, median (IQR) | 4000 (4000, 6000) | / | 4000 (4000, 6000) | / |
| Carbapenem Dosing,h mg/day, median (IQR) | 3000 (3000, 3000) | 3000 (1500, 3000) | 3000 (3000, 3000) | 0.266 |
| Mortality | 6 (2.2) | 0 | 6 (2.9) | 0.342 |
| Acute kidney injury | 28 (10.4) | 8 (13.1) | 20 (9.7) | 0.438 |
| US Military hospital | 0.001 | |||
| BAMC | 67 (25.0) | 25 (41.0) | 42 (20.3) | |
| NCR | 201 (75.0) | 36 (59.0) | 165 (79.7) |
ACE-I-angiotensin converting enzyme inhibitors, ARBs-angiotensin II receptor blockers, BAMC-Brooke Army Medical Center, IED-improvised explosive device, IFI-invasive fungal infection, IQR-interquartile range, LRMC- Landstuhl Regional Medical Center, NCR-national capital region, NSAIDs-non-steroidal anti-inflammatory drugs, SOFA-sequential organ failure assessment, VBL- vancomycin/broad-spectrum beta-lactam therapy, VPT- vancomycin/piperacillin-tazobactam therapy
Foot patrol status is missing for 17 VPT patients and 46 VBL patients.
Shock index is missing for 9 VPT patients and 18 VBL patients.
See Methods for IFI definition
Loading dose is missing for 58 VPT patients and 202 VBL patients.
The vancomycin maintenance dose refers to the first maintenance dose; subsequent doses are based on serum concentrations. Vancomycin maintenance dose is missing for 6 VBL patients.
Vancomycin troughs is missing for 16 VPT patients and 53 VBL patients.
Cefepime dosing is missing for 61 VPT patients and 197 VBL patients.
Carbapenem dosing is missing for 58 VPT patients and 18 VBL patients.
Acute Kidney Injury
Any stage AKI occurred in 8 of 61 patients (13.1%) in the VPT group and 20 of 207 in the VBL group (9.7%; p=0.438; Table 1). As military hospital admission, amphotericin exposure, and blood transfusion had significant differences between patients who did and did not develop AKI (see above), these variables were selected for propensity score weighting. Factors associated with development of AKI in univariate analysis included VPT therapy, cause of injury, number of limbs lost/injured, utilization of mechanical ventilation, and the nephrotoxic agents: aminoglycosides, NSAIDs, and intravenous contrast (Table 2). Following multivariate analysis, a RR of 1.73 (95% CI: 1.03-2.77) was associated with VPT utilization (Table 3). Additional factors from multivariate analysis associated with approximately double the risk of AKI were the loss of more than 1 limb (RR: 1.87, 95% CI: 1.16-2.88) and exposure to aminoglycosides (RR: 2.59, 95% CI: 1.61-3.92). Mechanical ventilation and NSAID administration were associated with a decreased risk of AKI. Although VPT was associated with an increased rate of AKI, there was no significant difference in overall length of hospitalization with the median duration being 45 days for patients treated with VPT and 38 days for those with VBL (p=0.573). Urine eosinophil testing was conducted only on 46% of those with AKI; all with negative results.
Table 2:
Univariate Model: Relative risk of Acute Kidney Injurya
| Characteristic | AKI (N=28), No. (%) | Non-AKI (N=240), No. (%) | Relative Risk (95% CL) | P-value |
|---|---|---|---|---|
| Antibiotic Group | 0.035 | |||
| VPT | 8 (28.6) | 53 (22.1) | 1.637 (1.036, 2.494) | |
| VBL (reference) | 20 (71.4) | 187 (77.9) | 1.00 | |
| Injury Cause | 0.040 | |||
| Non-Blast (reference) | 5 (17.9) | 47 (19.6) | 1.00 | |
| Non-IED Blast | 1 (3.6) | 23 (9.6) | 0.808 (0.236, 2.439) | |
| IED | 22 (78.6) | 170 (70.8) | 1.927 (1.029, 3.329) | |
| Injury Severity Score | 0.700 | |||
| 0 – 15 (reference) | 2 (7.1) | 18 (7.5) | 1.00 | |
| 16 – 25 | 6 (21.4) | 61 (25.4) | 1.420 (0.496, 3.463) | |
| ≥26 | 20 (71.4) | 161 (67.1) | 1.492 (0.573, 3.378) | |
| Number of Limbs Lost (trauma-related) | 0.001 | |||
| None or 1 limb (reference) | 17 (60.7) | 165 (68.8) | 1.00 | |
| More than 1 limb | 11 (39.3) | 75 (31.3) | 2.087 (1.354, 3.082) | |
| Number of Limbs Injured | 0.032 | |||
| None or 1 limb (reference) | 7 (25.0) | 71 (29.6) | 1.00 | |
| More than 1 limb | 21 (75.0) | 169 (70.4) | 1.852 (1.058, 3.046) | |
| Shock Index (SI) | 0.284 | |||
| SI < 0.65 (reference) | 4 (14.3) | 36 (15.0) | 1.00 | |
| 0.65 ≤ SI < 0.8 | 4 (14.3) | 38 (15.8) | 0.473 (0.164, 1.291) | |
| 0.8 ≤ SI | 15 (53.6) | 144 (60.0) | 0.917 (0.455, 1.765) | |
| Mechanical Ventilation | <0.001 | |||
| None (reference) | 9 (32.1) | 54 (22.5) | 1.00 | |
| LRMC Only | 5 (17.9) | 58 (24.2) | 0.273 (0.129, 0.567) | |
| LRMC & US hospital | 14 (50.0) | 128 (53.3) | 0.282 (0.160, 0.492) | |
| LRMC Admission SOFA, median (IQR) | 8 (4, 9) | 8 (4, 11) | 0.965 (0.908, 1.025) | 0.252 |
| US Hospital Admission SOFA, median (IQR) | 5.5 (1.5, 10) | 5 (1, 8) | 0.987 (0.932, 1.047) | 0.675 |
| IFI Status | 0.134 | |||
| Non-IFI (reference) | 18 (64.3) | 179 (74.6) | 1.00 | |
| IFI Low Suspicion | 2 (7.1) | 24 (10.0) | 1.057 (0.439, 2.341) | |
| IFI High Suspicion | 3 (10.7) | 20 (8.3) | 0.524 (0.168, 1.524) | |
| IFI | 5 (17.9) | 17 (7.1) | 2.020 (0.993, 3.691) | |
| Intensive Care Unit Admission | 0.104 | |||
| No ICU (reference) | 4 (14.3) | 16 (6.7) | 1.00 | |
| LRMC ICU Only | 2 (7.1) | 32 (13.3) | 0.362 (0.129, 0.976) | |
| US Hospital ICU ± LRMC ICU | 22 (78.6) | 192 (80.0) | 0.522 (0.249, 1.062) | |
| Nephrotoxic Agents | ||||
| Intravenous Contrast | 9 (32.1) | 118 (49.2) | 0.380 (0.218, 0.657) | <0.001 |
| Diuretics | 2 (7.1) | 24 (10.0) | 0.424 (0.115, 1.458) | 0.182 |
| NSAIDs | 4 (14.3) | 69 (28.8) | 0.445 (0.210, 0.920) | 0.028 |
| Aminoglycosides | 10 (35.7) | 78 (32.5) | 2.513 (1.652, 3.636) | <0.001 |
CL-confidence limit, IED-improvised explosive device, IFI-invasive fungal infection, IQR-interquartile range, LRMC- Landstuhl Regional Medical Center, NSAIDs-non-steroidal anti-inflammatory drugs, SOFA-sequential organ failure assessment, VBL- vancomycin/broad-spectrum beta-lactam therapy, VPT- vancomycin/piperacillin-tazobactam therapy
Military hospital, amphotericin exposure, and blood transfusion were used for propensity score weighting
Table 3:
Multivariate Model: Relative Risk of Acute Kidney Injurya
| Characteristic | Relative Risk (95% CL) | P-value |
|---|---|---|
| Antibiotic Group | 0.040 | |
| VPT | 1.727 (1.027, 2.765) | |
| VBL (reference) | 1.00 | |
| Number of Limbs Lost | 0.012 | |
| None or 1 limb (reference) | 1.00 | |
| More than 1 limb | 1.866 (1.155, 2.880) | |
| Mechanical Ventilation | <0.001 | |
| None (reference) | 1.00 | |
| LRMC Only | 0.282 (0.130, 0.603) | |
| LRMC & US Hospital | 0.323 (0.177, 0.583) | |
| Nephrotoxic Agents | ||
| NSAIDs | 0.445 (0.203, 0.949) | 0.036 |
| Aminoglycosides | 2.592 (1.605, 3.915) | <0.001 |
CL-confidence limit, LRMC- Landstuhl Regional Medical Center, NSAIDs-non-steroidal anti-inflammatory drugs, VBL- vancomycin/broad-spectrum beta-lactam therapy, VPT- vancomycin/piperacillin-tazobactam therapy
Military hospital, amphotericin exposure, and blood transfusion were used for propensity score weighting
The median days of therapy before onset of AKI in the VPT group was 5 days (IQR: 4-5.25 days). In contrast, the VBL group reached AKI faster with median duration of therapy before onset of 3.5 days (IQR: 3-6 days). Once AKI occurred, the duration was comparable between groups with a median duration of 1 day in the VPT group (IQR: 1-5 days) and 2 days in the VBL group (IQR: 1-3 days). Of the 28 patients who met any criterion for AKI, only 8 of 22 (36.4%) patients with available follow-up laboratory values had persistent deficits in renal function at 96 hours following completion of therapy (5 patients from the VBL group and 3 from the VPT group). Of the 3 patients with persistent renal dysfunction at 96 hours in the VPT group, one patient met RIFLE Risk and AKIN Stage 1, another RIFLE Injury and AKIN Stage 2, and the final met RIFLE Failure and AKIN Stage 3. In the VBL group, 3 patients met criteria for RIFLE Risk, but only one unique patient met criteria for RIFLE Injury, Failure, as well as AKIN Stages 1-3. Similarly, of the 21 patients who met AKI criteria with available data at 30 days after completion of combination therapy, only 1 VBL patient displayed persistent renal dysfunction. At 30 days following completion of therapy, the patient met RIFLE Injury and AKIN Stage 2 criteria.
Vancomycin maintenance dosing was significantly higher in patients who received VBL (p=0.003). Only three patients with AKI received maintenance vancomycin doses in excess of four grams in a 24-hour period (2 VPT, 1 VBL). No difference in median vancomycin troughs was seen between the groups, though there was a trend towards higher vancomycin troughs in the VBL group (p=0.080). There was also no significant difference in the vancomycin trough levels between the military hospitals: median of 12.3 μg/mL (IQR: 6.6-19.4 μg/mL) at BAMC and a median of 10.2 μg/mL (6.9-15.5 μg/mL) at hospitals in the National Capital Region (p=0.241). Of those who developed AKI and received VPT, the median vancomycin trough was 12.95 μg/mL (IQR: 10.13-17.75 μg/mL). The median vancomycin trough was 11 μg/mL (IQR: 7.3-15.2 μg/mL) for those with AKI and treated with VBL. Thirty percent of patients with AKI had vancomycin troughs >15 μg/mL prior to AKI and 7% (2 patients) had troughs >20 μg/mL.
DISCUSSION
Since 2011, when the potential association between AKI and VPT utilization was first reported, multiple studies have investigated this issue with conflicting results. 29,30 Only a fraction of studies have included the critically ill, even fewer exclusively evaluated them, and none involved combat trauma patients. 10–13,17–19,23,24,29 In this study comprised of critically ill, combat-related trauma patients, we found treatment with VPT was independently associated with close to twice the risk of AKI compared to VBL therapy.
In our analysis, the overall AKI rates for both groups were appreciably lower than the rates of AKI observed in most preceding studies, which ranged from 7.4% to 40.5% with VPT. 7,10–14,17–19,23,24,29 In studies including the critically ill and a comparator beta-lactam group (i.e., meropenem or cefepime), rates of AKI ranged from 5.3% to 28.8%.11–14,17–19,23,24 It should be noted that four of these studies were not predominantly composed of critically ill patients and the investigators did not perform separate subgroup analyses of the critically ill patients. 10,13,17,23 One possible explanation for the lower rate of AKI in our study is that our patient population was overwhelming male in gender and possibly younger. Three groups investigating the question of VPT toxicity found that females were significantly more likely to develop AKI compared to their male counterparts. 8,11,22 Regardless, the variability in AKI rates in the critically ill patient population has been noted previously and may be partly due to differences in study design.31 Attempts at making direct comparisons between studies are problematic given the incongruences in factors, such as baseline patient comorbidities, inclusion/exclusion criteria, record of concomitant nephrotoxins, time period analyzed, and definitions of AKI.32
A strength of our study is that in addition to the incidence of AKI, we also evaluated AKI severity. The overwhelming body of literature investigating the nephrotoxicity of VPT focuses only on the incidence of AKI. Consistent with studies reporting this data, 13–15,18,22 the majority of patients in our study with AKIs met RIFLE Risk criteria, while Stage 1 was the most common classification for the AKIN criteria. Only one study, which ultimately found no difference in AKI rates between VPT and VBL, was discordant and reported over 50% of AKIs meeting criteria for RIFLE Failure.11 The same study also found a statistically significant increase in patients treated with VPT requiring renal replacement therapy, albeit with very small numbers.
One of the more clinically relevant factors included is the timeframe for AKI onset. We excluded AKIs within the first 48 hours of combination antibiotic therapy due to prior data indicating median time to AKI of 3 days, and concerns that with minimal drug exposure this would be more consistent with alternative etiologies. Of the few instances in previous studies when AKI onset was reported, the range has been 3 to 8 days with VPT and as short as 2.2 to 6.3 days with VBL. 7,11,13,14,16,18,22 Only a single study has found more rapid AKI onset with VPT.14 Of note, one study reported date of AKI onset as a dichotomous variable with <3 days or >5 days, further limiting generalizations in this infrequently reported factor.11
In our population, when AKI did occur, it was short-lived and most commonly restricted to one day. Furthermore, less than one-fifth of all patients with AKI suffered persistent renal dysfunction 96 hours after therapy discontinuation. On our review, only three other studies evaluated persistence of renal dysfunction.11–13 Duration of AKI in these studies was significantly longer with 7-12 days for VPT and 9.9-10.6 days for VBL. Two studies assessed renal dysfunction at time of discharge with rates of persistent renal dysfunction at 59-92% for VPT and 78-82% for VBL. It is important to note that one of these studies included patients with baseline GFRs less than 60 mL/min and the other failed to capture additional information relevant to AKI development (e.g., sepsis, vasopressor use, and severity of illness scores).11,13 The failure of renal recovery in these patient cohorts may be due to their enrollment of a patient population at higher risk for AKI at baseline.
The relationship between VPT and AKI in a population with few medical comorbidities was also examined. Prior studies have relied on patient populations with median ages double the age of our patients and significant medical comorbidities that at baseline increase risk for AKI.33,34 With these medical comorbidities comes chronic medications that may act alone or in synergy with antibiotic therapies to increase likelihood of AKI.13 Nearly all study participants included here were young, active-duty military males with extremely low rates of medical comorbidities. This allows a unique opportunity to assess the potential nephrotoxicity of VPT versus VBL in an otherwise healthy population and further emphasizes the clinical importance of our primary outcome findings and the validity of VPT toxicity.
Interestingly, mechanical ventilation was associated with a decreased risk for AKI on multivariate analysis. Previous meta-analyses investigating the relationship between invasive mechanical ventilation and renal outcomes in the critically ill found a pooled odds ratio of 3.58 (95% CI: 1.85 to 6.92; p<0.001) for mechanical ventilation as an independent risk factor for AKI.35 One explanation for the difference in conclusions may stem from VBL recipients being significantly more likely to require admission to the ICU both at LRMC and U.S. hospitals (p<0.001). Approximately 80% of patients who received the VBL regimen were at military hospitals in the National Capital Region where 88% of the patients were admitted to the ICU. Specifically, within the trauma population, a clinical factor that often drives medical ICU admission is the need for mechanical ventilation. The finding of mechanical ventilation may not in itself be renal protective, but may instead reflect that more patients admitted to the ICU requiring mechanical ventilation were treated with the less nephrotoxic VBL regimen. The NSAID usage was similarly associated with decreased rates of AKI. Rutter et al. investigated the nephrotoxic effects of VPT versus vancomycin and piperacillin-tazobactam monotherapies with analogous results for intravenous contrast dye administration.22 It is possible the treating clinicians were less likely to dispense NSAIDs for patients perceived to be at higher risk of adverse renal outcomes, resulting in statistically protective values for what is commonly regarded as nephrotoxic. Additionally, our study was not designed or powered to evaluate these risks.
The pathophysiology underlying the potential development of AKI in relation to VPT is unknown, but its occurrence has been commonly discussed. 8,10,13–15,18,20,22,29,30 One potential hypothesis is the development of acute interstitial nephritis potentiated by vancomycin.32 Nearly half our patients with AKI underwent urine eosinophil testing, all with negative results. Unfortunately, poor sensitivity and specificity of urine eosinophils limits the negative predictive value of this testing for acute interstitial nephritis.36 Regardless, prior studies examining AKIs related to VPT have commented on the lack of urine eosinophil testing and the high percentage of negative testing is worth noting.
While the retrospective design of our study is a limitation to note, it is consistent with previous studies in the literature examining this question. Only one prospective study has been performed investigating this question and was underpowered.20 A random sample of the comparator VBL patients was utilized as a result of the need to abstract data from the medical records. Our attempt at accounting for concomitant nephrotoxins is another limitation as they were given equal weights in terms of their nephrotoxic risk. They were also accounted for in a binary fashion without consideration for duration, frequency of administration, or dosing. Vancomycin troughs were not assessed for validity in terms of appropriateness of collection time from last dose administered. The inability to further clarify the pathophysiological process driving the incidence of AKIs is a limitation of this study. While there were no positive urine eosinophil results, this is again not a sensitive test for AKI and no renal biopsies were performed. The unknown mechanism behind VPT-induced AKIs, as well as lack of renal biopsy is consistent with the current literature and further research is needed. Differences in antimicrobial stewardship practices between military hospitals also may have impacted results. In particular, patients admitted to BAMC were more likely to have received VPT therapy than were patients admitted to the National Capital Region. This may reflect admitting patterns or be associated with local site-specific antimicrobial stewardship program policies (e.g., restrictions on use of carbapenems at BAMC compared to the National Capital Region) or different injury profiles.
CONCLUSIONS
In this young and previously healthy, severely ill, combat-injured population, VPT was associated with almost twice the risk of AKI compared to VBL. While the current body of literature remains heterogeneous in its conclusions, our study makes several relevant clinical points. First, the lack of concurrent medical comorbidities, medication usage, and young age helps to support the argument that VPT is truly the driving factor behind incidence of AKI. Second, the combat-trauma patient population, despite their young age, lack of medical comorbidities and resilience, is not immune to medication-associated AKI that abounds in the civilian medical ICU literature. Reassuringly, while AKI incidence in the VPT group was 13%, the overall severity was low, the duration was short, and nearly all patients had a return to their baseline renal function. Additional research is needed not only to clarify the underlying pathophysiology of AKI associated with VPT, but also to further characterize complications and clinical relevance of this AKI.
Acknowledgements:
We are indebted to the Infectious Disease Clinical Research Program Trauma Infectious Disease Outcomes Study team of clinical coordinators, microbiology technicians, data managers, clinical site managers, and administrative support personnel for their tireless hours to ensure the success of this project. We specifically would like to thank Dan Lu, Teresa Merritt, Katrin Mende, M. Leigh Carson, Marcia G. Goodrich, and Robert Leimbach for their efforts on this project.
Funding:
Support for this work (IDCRP-024) was provided by the Infectious Disease Clinical Research Program (IDCRP), a Department of Defense (DoD) program executed by the Uniformed Services University of the Health Sciences (USU) through a cooperative agreement with The Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc. (HJF). This project has been supported with federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH), under Inter-Agency Agreement Y1-AI-5072, the Defense Health Program, U.S. DoD, under award HU0001190002, and the Department of the Navy under the Wounded, Ill, and Injured Program under award HU0001-10-1-0014.
Footnotes
Declaration of Conflicting Interest: The authors have no conflict of interest to declare.
Publisher's Disclaimer: Disclaimer: The views expressed are those of the authors and do not reflect the official views of the Uniformed Services University of the Health Sciences, Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., the National Institute of Health or the Department of Health and Human Services, Brooke Army Medical Center, Walter Reed National Military Medical Center, Landstuhl Regional Medical Center, the U.S. Army Medical Department, the U.S. Army Office of the Surgeon General, the Department of Defense, or the Departments of the Army, Navy or Air Force. Mention of trade names, commercial products, or organizations does not imply endorsement by the U.S. Government.
REFERENCES
- 1.Tribble DR, Conger NG, Fraser S, Gleeson TD, Wilkins K, Antonille T, Weintrob A, Ganesan A, Gaskins LJ, Li P, Grandits G, Landrum ML, Hospenthal DR, Millar EV, Blackbourne LH, Dunne JR, Craft D, Mende K, Wortmann GW, Herlihy R, McDonald J, Murray CK. Infection-associated clinical outcomes in hospitalized medical evacuees after traumatic injury: trauma infectious disease outcome study. J Trauma. 2011;71(1 Suppl):S33–S42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murray CK, Wilkins K, Molter NC, Yun HC, Dubick MA, Spott MA, Jenkins D, Eastridge B, Holcomb JB, Blackbourne LH, Hospenthal DR. Infections in combat casualties during Operations Iraqi and Enduring Freedom. J Trauma. 2009;66(4 Suppl):S138–S144. [DOI] [PubMed] [Google Scholar]
- 3.Campbell WR, Li P, Whitman TJ, Blyth DM, Schnaubelt ER, Mende K, Tribble DR, The Infectious Disease Clinical Research Program Trauma Infectious Disease Outcomes Study Group. Multi-drug–resistant gram-negative infections in deployment-related trauma patients. Surg Infect (Larchmt). 2017;18(3):357–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaye KS, Pogue JM. Infections caused by resistant gram-negative bacteria: epidemiology and nanagement. Pharmacotherapy. 2015;35(10):949–962. [DOI] [PubMed] [Google Scholar]
- 5.Mende K, Stewart L, Shaikh F, Bradley W, Lu D, Krauss MR, Greenberg L, Yu Q, Blyth DM, Whitman TJ, Petfield JL, Tribble DR. Microbiology of combat-related extremity wounds: Trauma Infectious Disease Outcomes Study. Diagn Microbiol Infect Dis. 2019;94(2):173–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heitkamp RA, Li P, Mende K, Demons ST, Tribble DR, Tyner SD. Association of Enterococcus spp. with severe combat extremity injury, intensive care, and polymicrobial wound infection. Surg Infect (Larchmt). 2018;19(1):95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karino S, Kaye KS, Navalkele B, Nishan B, Salim M, Solanki S, Pervaiz A, Tashtoush N, Shaikh H, Koppula S, Martin ET, Mynatt RP, Murray KP, Rybak MJ, Pogue JM. Epidemiology of acute kidney injury among patients receiving concomitant vancomycin and piperacillin-tazobactam: opportunities for antimicrobial stewardship. Antimicrob Agents Chemother. 2016;60(6):3743–3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim T, Kandiah S, Patel M, Rab S, Wong J, Xue W, Easley K, Anderson AM. Risk factors for kidney injury during vancomycin and piperacillin/tazobactam administration, including increased odds of injury with combination therapy. BMC Res Notes. 2015;8:579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hammond DA, Smith MN, Li C, Hayes SM, Lusardi K, Bookstaver PB. Systematic review and meta-analysis of acute kidney injury associated with concomitant vancomycin and piperacillin/tazobactam. Clin Infect Dis. 2017;64(5):666–674. [DOI] [PubMed] [Google Scholar]
- 10.Burgess LD, Drew RH. Comparison of the incidence of vancomycin-induced nephrotoxicity in hospitalized patients with and without concomitant piperacillin-tazobactam. Pharmacotherapy. 2014;34(7):670–676. [DOI] [PubMed] [Google Scholar]
- 11.Buckley MS, Hartsock NC, Berry AJ, Bikin DS, Richards EC, Yerondopoulos MJ, Kobic E, Wicks LM, Hammond DA. Comparison of acute kidney injury risk associated with vancomycin and concomitant piperacillin/tazobactam or cefepime in the intensive care unit. J Crit Care. 2018;48:32–38. [DOI] [PubMed] [Google Scholar]
- 12.Hammond DA, Smith MN, Painter JT, Meena NK, Lusardi K. Comparative incidence of acute kidney injury in critically ill patients receiving vancomycin with concomitant piperacillin-tazobactam or cefepime: a retrospective cohort study. Pharmacotherapy. 2016;36(5):463–471. [DOI] [PubMed] [Google Scholar]
- 13.Gomes DM, Smotherman C, Birch A, Dupree L, Della Vecchia BJ, Kraemer DF, Jankowski CA. Comparison of acute kidney injury during treatment with vancomycin in combination with piperacillin-tazobactam or cefepime. Pharmacotherapy. 2014;34(7):662–669. [DOI] [PubMed] [Google Scholar]
- 14.Navalkele B, Pogue JM, Karino S, Nishan B, Salim M, Solanki S, Pervaiz A, Tashtoush N, Shaikh H, Koppula S, Koons J, Hussain T, Perry W, Evans R, Martin ET, Mynatt RP, Murray KP, Rybak MJ, Kaye KS. Risk of acute kidney injury in patients on concomitant vancomycin and piperacillin-tazobactam compared to those on vancomycin and cefepime. Clin Infect Dis. 2017;64(2):116–123. [DOI] [PubMed] [Google Scholar]
- 15.Jeon N, Staley B, Klinker KP, Hincapie Castillo J, Winterstein AG. Acute kidney injury risk associated with piperacillin/tazobactam compared with cefepime during vancomycin therapy in hospitalised patients: a cohort study stratified by baseline kidney function. Int J Antimicrob Agents. 2017;50(1):63–67. [DOI] [PubMed] [Google Scholar]
- 16.Moenster RP, Linneman TW, Finnegan PM, Hand S, Thomas Z, McDonald JR. Acute renal failure associated with vancomycin and beta-lactams for the treatment of osteomyelitis in diabetics: piperacillin-tazobactam as compared with cefepime. Clin Microbiol Infect. 2014;20(6):O384–O389. [DOI] [PubMed] [Google Scholar]
- 17.Al Yami MS. Comparison of the incidence of acute kidney injury during treatment with vancomycin in combination with piperacillin-tazobactam or with meropenem. J Infect Public Health. 2017;10(6):770–773. [DOI] [PubMed] [Google Scholar]
- 18.Blevins AM, Lashinsky JN, McCammon C, Kollef M, Micek S, Juang P. Incidence of acute kidney injury in critically ill patients receiving vancomycin with concomitant piperacillin/tazobactam, cefepime, or meropenem. Antimicrob Agents Chemother. 2019;63(5):e02658–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schreier DJ, Kashani KB, Sakhuja A, Mara KC, Tootooni MS, Personett HA, Nelson S, Rule AD, Steckelberg JM, Tande AJ, Barreto EF. Incidence of acute kidney injury among critically ill patients with brief empiric use of antipseudomonal beta-lactams with vancomycin. Clin Infect Dis. 2019;68(9):1456–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peyko V, Smalley S, Cohen H. Prospective comparison of acute kidney injury during treatment with the combination of piperacillin-tazobactam and vancomycin versus the combination of cefepime or meropenem and vancomycin. J Pharm Pract. 2017;30(2):209–213. [DOI] [PubMed] [Google Scholar]
- 21.Giuliano CA, Patel CR, Kale-Pradhan PB. Is the combination of piperacillin-tazobactam and vancomycin associated with development of acute kidney injury? A meta-analysis. Pharmacotherapy. 2016;36(12):1217–1228. [DOI] [PubMed] [Google Scholar]
- 22.Rutter WC, Burgess DR, Talbert JC, Burgess DS. Acute kidney injury in patients treated with vancomycin and piperacillin-tazobactam: A retrospective cohort analysis. J Hosp Med. 2017;12(2):77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davies SW, Efird JT, Guidry CA, Dietch ZC, Willis RN, Shah PM, Sawyer RG. Top guns: the “Maverick” and “Goose” of empiric therapy. Surg Infect (Larchmt). 2016;17(1):38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Molina KC, Barletta JF, Hall ST, Yazdani C, Huang V. The risk of acute kidney injury in critically ill patients receiving concomitant vancomycin with piperacillin-tazobactam or cefepime. J Intensive Care Med. 2019. doi: 10.1177/0885066619828290. [DOI] [PubMed] [Google Scholar]
- 25.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P, Acute Dialysis Quality Initiative Workgroup. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8(4):R204–R212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A, Acute Kidney Injury Network. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rybak MJ, Lomaestro BM, Rotschafer JC, Moellering RC, Craig WA, Billeter M, Dalovisio JR, Levine DP. Vancomycin therapeutic guidelines: a summary of consensus recommendations from the Infectious Diseases Society of America, the American Society of Health-System Pharmacists, and the Society of Infectious Diseases Pharmacists. Clin Infect Dis. 2009;49(3):325–327. [DOI] [PubMed] [Google Scholar]
- 28.Ganesan A, Shaikh F, Bradley W, Blyth DM, Bennett D, Petfield JL, Carson ML, Wells JM, Tribble DR. Classification of trauma-associated invasive fungal infections to support wound treatment decisions. Emerg Infect Dis. 2019;25(9):1639–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Min E, Box K, Lane J, Sanchez J, Coimbra R, Doucet J, Potenza B, Wargel L. 714: Acute kidney injury in patients recieving concomitant vancomycin and piperacillin/tazobactam. Crit Care Med. 2011;39(12):200.21178536 [Google Scholar]
- 30.Hellwig T, Hammerquist R, Loecker B, Shields J. 301: Retrospective evaluation of the incidence of vancomycin and/or piperacillin-tazobactam induced acute renal failure. Crit Care Med. 2011;39(12):79. [Google Scholar]
- 31.Luther MK, Timbrook TT, Caffrey AR, Dosa D, Lodise TP, LaPlante KL. Vancomycin plus piperacillin-tazobactam and acute kidney injury in adults: a systematic review and meta-analysis. Crit Care Med. 2018;46(1):12–20. [DOI] [PubMed] [Google Scholar]
- 32.Watkins RR, Deresinski S. Increasing evidence of the nephrotoxicity of piperacillin/tazobactam and vancomycin combination therapy-what is the clinician to do? Clin Infect Dis. 2017;65(12):2137–2143. [DOI] [PubMed] [Google Scholar]
- 33.Leblanc M, Kellum JA, Gibney RT, Lieberthal W, Tumlin J, Mehta R. Risk factors for acute renal failure: inherent and modifiable risks. Curr Opin Crit Care. 2005;11(6):533–536. [DOI] [PubMed] [Google Scholar]
- 34.Pereira M, Rodrigues N, Godinho I, Gameiro J, Neves M, Gouveia J, Costa ESZ, Lopes JA. Acute kidney injury in patients with severe sepsis or septic shock: a comparison between the ‘Risk, Injury, Failure, Loss of kidney function, End-stage kidney disease’ (RIFLE), Acute Kidney Injury Network (AKIN) and Kidney Disease: Improving Global Outcomes (KDIGO) classifications. Clin Kidney J 2017;10(3):332–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van den Akker JP, Egal M, Groeneveld AB. Invasive mechanical ventilation as a risk factor for acute kidney injury in the critically ill: a systematic review and meta-analysis. Crit Care. 2013;17(3):R98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muriithi AK, Nasr SH, Leung N. Utility of urine eosinophils in the diagnosis of acute interstitial nephritis. Clin J Am Soc Nephrol. 2013;8(11):1857–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]


