Abstract
Background
This is an updated version of the original Cochrane Review published in 2014.
Epilepsy is a common neurological condition characterised by recurrent seizures. Pharmacological treatment remains the first choice to control epilepsy. Sulthiame (STM) is widely used as an antiepileptic drug in Europe and Israel. In this review, we have presented a summary of evidence for the use of STM as monotherapy in epilepsy.
Objectives
To assess the efficacy and side effect profile of STM as monotherapy when compared with placebo or another antiepileptic drug for people with epilepsy.
Search methods
We searched the following databases on 13 April 2020: the Cochrane Register of Studies (CRS Web), MEDLINE (Ovid, 1946 to 10 April 2020). CRS Web includes randomised or quasi‐randomised controlled trials from PubMed, Embase, ClinicalTrials.gov, the World Health Organization International Clinical Trials Registry Platform, the Cochrane Central Register of Controlled Trials (CENTRAL), and the specialised registers of Cochrane Review Groups including Cochrane Epilepsy. We imposed no language restrictions. We contacted the manufacturers of STM and researchers in the field to ask about ongoing and unpublished studies.
Selection criteria
Randomised controlled monotherapy trials of STM in people of any age with epilepsy of any aetiology.
Data collection and analysis
We followed standard Cochrane methodology. Two review authors independently selected trials for inclusion and extracted the relevant data. We assessed the following outcomes: treatment withdrawal; seizure‐free at six months; adverse effects; and quality of life scoring. We conducted the primary analyses by intention‐to‐treat where possible, and presented a narrative analysis of the data.
Main results
We included four studies involving a total of 355 participants: three studies (209 participants) with a diagnosis of benign epilepsy of childhood with centrotemporal spikes (BECTS), and one study (146 participants) with a diagnosis of generalised tonic‐clonic seizures (GTCS). STM was given as monotherapy compared with placebo and with levetiracetam in the BECTS studies, and compared with phenytoin in the GTCS study. An English translation of the full text of one of the BECTS studies could not be found, and analysis of this study was based solely on the English translation of the abstract.
For the primary outcome, the total number of dropouts caused either by seizure recurrence or adverse reaction was significantly higher in the levetiracetam treatment arm compared to the STM treatment arm (RR 0.32, 95% Cl 0.10 to 1.03; 1 study, 43 participants; low‐certainty evidence). For the secondary outcomes for this comparison, results for seizure freedom were inconclusive (RR 1.12, 95% Cl 0.88 to 1.44; 1 study, 43 participants; low‐certainty evidence).
Reporting of adverse effects was incomplete. Participants receiving STM were significantly less likely to develop gingival hyperplasia than participants receiving phenytoin in the GTCS study (RR 0.03, 95% CI 0.00 to 0.58; 1 study, 146 participants; low‐certainty evidence). No further statistically significant adverse events were noted when STM was compared with phenytoin or placebo. The most common adverse events were related to behavioural disturbances when STM was compared with levetiracetam (RR 0.95, 95% Cl 0.59 to 1.55; 1 study, 43 participants; low‐certainty evidence), with the same incidence in both groups. No data were reported for quality of life.
Overall, we assessed one study at high risk of bias and one study at unclear bias across the seven domains, mainly due to lack of information regarding study design. Only one trial reported effective methods for blinding. The risk of bias assessments for the other two studies ranged from low to high. We rated the overall certainty of the evidence for the outcomes as low using the GRADE approach.
Authors' conclusions
This review provides insufficient information to inform clinical practice. Small sample sizes, poor methodological quality, and lack of data on important outcome measures precluded any meaningful conclusions regarding the efficacy and tolerability of sulthiame as monotherapy in epilepsy. More trials, recruiting larger populations, over longer periods, are needed to determine whether sulthiame has a clinical use.
Keywords: Humans, Anticonvulsants, Anticonvulsants/therapeutic use, Epilepsy, Epilepsy/drug therapy, Quality of Life, Randomized Controlled Trials as Topic, Thiazines, Thiazines/therapeutic use
Plain language summary
Sulthiame monotherapy for epilepsy
Background
Epilepsy is a common neurological condition characterised by recurrent seizures. Sulthiame (STM) is widely used as an antiepileptic drug in Europe and Israel.
Review aims
This review aimed to determine the efficacy and side effect profile of STM as monotherapy, when compared with placebo or another antiepileptic drug for people with epilepsy.
Results
We found four randomised controlled trials (studies in which participants are randomly assigned to one of two or more treatment groups) involving a total of 355 participants that looked at the effectiveness and tolerability of sulthiame used as a single treatment in epilepsy. Three studies were conducted on a common form of childhood epilepsy known as benign epilepsy of childhood with centrotemporal spikes, and one study was conducted on generalised tonic‐clonic seizures, a type of seizure that starts on both sides of the brain and causes stiffness or twitching throughout the body. Based on the available evidence, we could draw no meaningful conclusions on the effectiveness or tolerability of sulthiame as a single treatment in epilepsy.
Quality of the evidence
The quality of the evidence is limited by small sizes of the groups being studied, significant risk of bias, and incomplete data on important outcome measures, as well as by the lack of an English translation of the full‐text manuscript of one study.
Conclusions
Further high‐quality research is needed to fully evaluate the effectiveness and tolerability of sulthiame as a single treatment in epilepsy.
The evidence is current to April 2020.
Summary of findings
Summary of findings 1. Sulthiame compared to placebo for epilepsy.
| Sulthiame compared to placebo for epilepsy | ||||||
|
Patient or population: participants with epilepsy Settings: hospital Intervention: sulthiame Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Sulthiame | |||||
| Treatment withdrawal | 0 per 1000 (0 to 0) | 0 per 1000 (0 to 0) | N/A | 100 (2 studies) | N/A | Data for treatment withdrawal not reported. |
| Seizure freedom | 0 per 1000 (0 to 0) | 0 per 1000 (0 to 0) | N/A | 100 (2 studies) | N/A | Data for seizure freedom not reported. |
| All adverse effects | 0 per 1000 (0 to 0) | 0 per 1000 (0 to 0) | N/A | 100 (2 studies) | N/A | Data for adverse effects not reported. |
| *The basis for the assumed risk1(e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; N/A: not applicable | ||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Assumed risk is calculated as the event rate in the control group per 1000 people (number of events divided by the number of participants receiving control treatment).
Summary of findings 2. Sulthiame compared with levetiracetam for epilepsy.
| Sulthiame compared with levetiracetam for epilepsy | ||||||
|
Patient or population: participants with epilepsy (BECTS) Settings: hospital Intervention: sulthiame Comparison: levetiracetam | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Sulthiame | |||||
| Treatment withdrawal | 429 per 1000 |
137 per 1000 (43 to 442) |
RR 0.32 (0.10 to 1.03) | 43 (1 study) | ⊕⊕⊝⊝ Low1,2 | Single, small study. Data for number of treatment withdrawal provided. RR < 1 indicates outcome is more likely in control group. |
| Seizure freedom | 809 per 1000 |
906 per 1000 (712 to 1165) |
RR 1.12 (0.88 to 1.44) |
43 (1 study) | ⊕⊕⊝⊝ Low1,2 |
Single, small study. Data for seizure freedom provided. RR > 1 indicates outcome is more likely in sulthiame group. |
| Adverse effects (general symptoms) | 714 per 1000 |
771 per 1000 (543 to 1100) |
RR 1.08 (0.76 to 1.54) | 43 (1 study) | ⊕⊕⊝⊝ Low1,2 | Single, small study. Data for number of adverse events provided. RR > 1 indicates outcome is more likely in sulthiame group. |
| Adverse effects (central nervous system) | 619 per 1000 |
724 per 1000 (477 to 1108) |
RR 1.17 (0.77 to 1.79) | 43 (1 study) | ⊕⊕⊝⊝ Low1,2 | Single, small study. Data for number of adverse events provided. RR > 1 indicates outcome is more likely in sulthiame group. |
| Adverse effects (behaviour) | 619 per 1000 |
588 per 1000 (365 to 959) |
RR 0.95 (0.59 to 1.55) | 43 (1 study) | ⊕⊕⊝⊝ Low1,2 | Single, small study. Data for number of adverse events provided. RR < 1 indicates outcome is more likely in control group. |
| Adverse effects (airways) | 238 per 1000 |
636 per 1000 (279 to 1457) |
RR 2.67 (1.17 to 6.12) | 43 (1 study) | ⊕⊕⊝⊝ Low1,2 | Single, small study. Data for number of adverse events provided. RR > 1 indicates outcome is more likely in sulthiame group. |
| Adverse effects (cardiac) | 0 per 1000 |
0 per 1000 (0 to 0) |
RR 2.87 (0.12 to 66.75) | 43 (1 study) | ⊕⊕⊝⊝ Low1,2 | Single, small study. Data for number of adverse events provided. RR > 1 indicates outcome is more likely in sulthiame group. |
| Adverse effects (gastrointestinal) | 576 per 1000 |
438 per 1000 (213 to 899) |
RR 0.76 (0.37 to 1.56) | 43 (1 study) | ⊕⊕⊝⊝ Low1,2 | Single, small study. Data for number of adverse events provided. RR < 1 indicates outcome is more likely in control group. |
| Adverse effects (bones and muscles) | 191 per 1000 |
182 per 1000 (52 to 636) |
RR 0.95 (0.27 to 3.33) | 43 (1 study) | ⊕⊕⊝⊝ Low1,2 | Single, small study. Data for number of adverse events provided. RR < 1 indicates outcome is more likely in control group. |
| Adverse effects (others) | 191 per 1000 |
227 per 1000 (71 to 735) |
RR 1.19 (0.37 to 3.85) | 43 (1 study) | ⊕⊕⊝⊝ Low1,2 | Single, small study. Data for number of adverse events provided. RR > 1 indicates outcome is more likely in sulthiame group. |
| *The basis for the assumed risk3 (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BECTS: benign epilepsy of childhood with centrotemporal spikes; CI: confidence interval; RR: risk ratio | ||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded once for risk of bias: some included studies had incomplete methodological information. 2Downgraded once for imprecision: number of events does not suffice for optimal information size. 3Assumed risk is calculated as the event rate in the control group per 1000 people (number of events divided by the number of participants receiving control treatment).
Summary of findings 3. Sulthiame compared with phenytoin for epilepsy.
| Sulthiame compared with phenytoin for epilepsy | ||||||
|
Patient or population: participants with epilepsy (GTCS) Settings: hospital Intervention: sulthiame Comparison: phenytoin | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Sulthiame | |||||
| Treatment withdrawal |
0 per 1000 (0 to 0) |
0 per 1000 (0 to 0) |
N/A | 146 (1 study) | N/A | Data for treatment withdrawal not reported. |
| Seizure freedom | ‐ | ‐ | N/A | 146 (1 study) | N/A | Data for seizure freedom not reported. |
| All adverse effects | 250 per 1000 | 263 per 1000 (135 to 517) | RR 1.05 (0.54 to 2.07) | 146 (1 study) | ⊕⊕⊝⊝ Low1,2 | Single study. Data for number of adverse events provided. Unclear if data on adverse events reflect number of events or number of participants experiencing an event. RR > 1 indicates outcome is more likely in sulthiame group. |
| Paraesthesia | 0 per 1000 | 123 per 1000 (0 to 0) | RR 8.32 (0.51 to 135.82) | 146 (1 study) | ⊕⊕⊝⊝ Low1,2 | Single study. Data for number of adverse events provided. Unclear if data on adverse events reflect number of events or number of participants experiencing an event. RR > 1 indicates outcome is more likely in sulthiame group. |
| Dizziness | 0 per 1000 | 44 per 1000 (0 to 0) | RR 3.16 (0.18 to 55.62) | 146 (1 study) | ⊕⊕⊝⊝ Low1,2 | Single study. Data for number of adverse events provided. Unclear if data on adverse events reflect number of events or number of participants experiencing an event. RR > 1 indicates outcome is more likely in sulthiame group. |
| Headache | 0 per 1000 | 18 per 1000 (0 to 0) | RR 1.43 (0.07 to 29.15) | 146 (1 study) | ⊕⊕⊝⊝ Low1,2 | Single study. Data for number of adverse events provided. Unclear if data on adverse events reflect number of events or number of participants experiencing an event. RR > 1 indicates outcome is more likely in sulthiame group. |
| Anorexia | 63 per 1000 | 26 per 1000 (4 to 152) | RR 0.42 (0.07 to 2.41) | 146 (1 study) | ⊕⊕⊝⊝ Low1,2 | Single study. Data for number of adverse events provided. Unclear if data on adverse events reflect number of events or number of participants experiencing an event. RR < 1 indicates outcome is more likely in control group. |
| Rash | 31 per 1000 | 9 per 1000 (1 to 135) | RR 0.28 (0.02 to 4.36) | 146 (1 study) | ⊕⊕⊝⊝ Low1,2 | Single study. Data for number of adverse events provided. Unclear if data on adverse events reflect number of events or number of participants experiencing an event. RR < 1 indicates outcome is more likely in control group. |
| Gingival hyperplasia | 125 per 1000 | 4 per 1000 (0 to 72) | RR 0.03 (0.00 to 0.58) | 146 (1 study) | ⊕⊕⊝⊝ Low1,2 | Single study. Data for number of adverse events provided. Unclear if data on adverse events reflect number of events or number of participants experiencing an event. RR < 1 indicates outcome is more likely in control group. |
| Other | 31 per 1000 | 43 per 1000 (5 to 359) | RR 1.40 (0.17 to 11.59) | 146 (1 study) | ⊕⊕⊝⊝ Low1,2 | Single study. Data for number of adverse events provided. Unclear if data on adverse events reflect number of events or number of participants experiencing an event. RR > 1 indicates outcome is more likely in sulthiame group. |
| *The basis for the assumed risk3(e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). GTCS: generalised tonic‐clonic seizures; CI: confidence interval; RR: risk ratio | ||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded once for risk of bias: one included study had incomplete methodological information. 2Downgraded once for imprecision: number of events does not suffice for optimal information size. 3Assumed risk is calculated as the event rate in the control group per 1000 people (number of events divided by the number of participants receiving control treatment).
Background
This review is an update of a Cochrane Review previously published in 2014 (Milburn‐McNulty 2014).
Description of the condition
Epilepsy is a common neurological condition characterised by recurrent seizures. It has an estimated worldwide prevalence of between eight and 10 per 1000 of the general population (WHO 2001). Most people with epilepsy will respond well to conventional antiepileptic drugs (AEDs) (ILAE 1997), although around 30% will not achieve remission despite trying numerous AEDs, often in combination (Sander 1993; Schmidt 1995; Brodie 1996).
Description of the intervention
Sulthiame (STM) is a sulphonamide that is usually taken two to three times per day in tablet form. STM was initially investigated for use in epilepsy in clinical trials in the 1960s (Griffiths 1964), but was never licenced widely as a treatment for epilepsy. However, it is currently widely used as an AED in some European countries and in Israel (Gross‐Selbeck 2001; Koepp 2002; Engler 2003; Ben‐Zeev 2004; Chahem 2007). Pharmacological treatment remains the first choice for controlling epilepsy, although recent decades have seen advances in vagal stimulation, Panebianco 2015; Panebianco 2016, and surgery (West 2019).
When used as monotherapy, STM has been reported to reduce the occurrence of seizures and electroencephalographic (EEG) discharges in study participants with benign epilepsy of childhood with centrotemporal spikes (BECTS) (Rating 2000; Bast 2003; Ben‐Zeev 2004; Wirrell 2008), benign partial epilepsy of childhood (Engler 2003; Ben‐Zeev 2004), symptomatic, localisation‐related epilepsy and juvenile myoclonic epilepsy (Ben‐Zeev 2004), as well as adults with drug‐resistant epilepsy and learning disabilities (Koepp 2002). In addition, STM as an add‐on therapy has been reported to reduce seizure activity in participants with drug‐resistant epilepsy (Livingston 1967; Chahem 2007; Miyajima 2009). Reported adverse effects of STM include deterioration of reading ability, memory, attention skills, and mathematical ability (Wirrell 2008), mixed respiratory and metabolic acidosis (Weissbach 2010), and crystalluria (Go 2005).
How the intervention might work
At the time of the writing of this review, no studies have systematically reviewed the literature on the mechanism of action of STM. Early studies suggest that the main antiepileptic properties of STM are indirect and due to a pharmacokinetic interaction with phenytoin (PHT): by inhibiting the parahydroxylation of phenytoin by hepatic enzymes, STM increases the serum levels and half‐life of PHT when taken in combination (Houghton 1974). More recent studies have found that STM produces a modest intracellular acidosis in central neurons via its action as a carbonic anhydrase inhibitor, thereby reducing the frequency of action potentials and epileptiform bursts (Leniger 2002).
Why it is important to do this review
This review, which is an update of a previous Cochrane Review (Milburn‐McNulty 2014), aims to summarise evidence from randomised controlled trials where the efficacy and tolerability of STM in monotherapy for participants with epilepsy has been investigated, in order to inform the use of this drug and decisions about its further assessment. A separate Cochrane Review has addressed the use of STM as an add‐on treatment (Bresnahan 2019).
Objectives
To assess the efficacy and side effect profile of STM as monotherapy when compared with placebo or another antiepileptic drug for people with epilepsy.
Methods
Criteria for considering studies for this review
Types of studies
We included trials that met all of the following criteria.
Randomised controlled trials, in which an adequate method of concealment of randomisation was used (e.g. allocation of sequentially sealed packages of medication; sealed, opaque envelopes; telephone randomisation).
Double‐blinded, single‐blinded, or unblinded trials.
Placebo‐controlled or actively controlled trials.
Parallel‐group and cross‐over studies. For cross‐over studies, we treated the first treatment period as a parallel trial, for the purposes of analysis of efficacy and safety data (i.e. only data from the first treatment period were used).
Types of participants
Individuals with epilepsy of any aetiology.
Persons of any age.
Types of interventions
For the active intervention group, STM taken as monotherapy.
For the control group, placebo or another AED taken as monotherapy.
Types of outcome measures
Primary outcomes
Treatment withdrawal. We chose the proportion of participants who had treatment withdrawn during the course of the treatment period as a measure of global effectiveness. This outcome reflects both efficacy and tolerability, as treatment may be withdrawn because of continued seizures, adverse effects, or a combination of both. This outcome is recommended by the Commission on Antiepileptic Drugs of the International League Against Epilepsy as the primary outcome measure in monotherapy trials (Commission on Antiepileptic Drugs 1998).
Secondary outcomes
Seizure freedom. The proportion of people with complete cessation of seizures during the treatment period.
Adverse effects. Any reported adverse effects such as, but not limited to, deterioration in cognitive ability, crystalluria, or respiratory and metabolic acidosis. We will assess both the proportion of any adverse effect and the proportion of each individual adverse effect.
Quality of life (QoL). Overall improvement or deterioration in QoL as assessed by validated and reliable rating scales.
Search methods for identification of studies
Electronic searches
Searches were run for the original review on 28 August 2012. Subsequent searches were run on 24 October 2014, 7 March 2018, and 12 September 2017. For the latest update, we searched the following databases on 13 April 2020.
Cochrane Register of Studies (CRS Web) using the search strategy shown in Appendix 1.
MEDLINE (Ovid, 1946 to 10 April 2020) using the search strategy shown in Appendix 2.
We imposed no language restrictions.
CRS Web includes randomised or quasi‐randomised controlled trials from PubMed, Embase, US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov, the World Health Organization International Clinical Trials Registry Platform, the Cochrane Central Register of Controlled Trials (CENTRAL), and the specialised registers of Cochrane Review Groups including Cochrane Epilepsy. We previously searched Scopus as a substitute for Embase, using the search strategy shown in Appendix 3, but because of CRS Web, this was no longer necessary.
Searching other resources
We checked the reference lists of retrieved reports for additional reports of relevant studies, including conference proceedings. We contacted the manufacturers of STM and colleagues in the field to ask for information about ongoing or unpublished studies.
Data collection and analysis
Selection of studies
Two review authors (PM‐M and MP) independently assessed studies for inclusion. Any disagreements were resolved by discussion or through arbitration with the third review author (AGM) if necessary. Two review authors (PM‐M and MP) independently extracted data from and assessed risk of bias of the included trials, with any disagreements resolved by discussion.
Data extraction and management
Two review authors (PM‐M and MP) independently extracted data from the included trials and assessed study design and demographic makeup of the participants, in addition to the outcomes listed in the Types of outcome measures section. All outcome measure data were separated into intervention group and control group data. Any disagreements were resolved by discussion or through arbitration with the third review author (AGM) if necessary.
Methods and trial design
Method of randomisation.
Method of allocation concealment.
Method of blinding.
Cross‐over or parallel trial.
Duration of study.
Duration of baseline period.
Duration of treatment period.
Duration of 'washout' period for cross‐over studies.
Dose of STM.
Description of how adverse effects were reported.
Source of funding.
Participants and demographic information
Number of participants in the intervention group.
Number of participants in the control group.
Study setting.
Country in which study was performed.
Age.
Sex.
Ethnicity.
Whether treatment‐naive (i.e. has the participant taken any AEDs previously, and if so, which ones?).
Diagnostic criteria.
Types of seizures and epilepsy.
Number of seizures before the start of treatment.
Outcomes
Time to treatment failure: number of events, time to treatment failure, and reason for treatment failure.
Time to 12‐month remission: number of events and time to 12‐month remission.
Proportion seizure‐free at 12 months: number of events.
Adverse effects: number of events, and categorisation into specific adverse effects.
Overall improvement or deterioration in quality of life: type of scale used, score before and after intervention, and time postintervention quality of life scoring repeated.
Assessment of risk of bias in included studies
Two review authors (PM‐M and MP) independently assessed the risk of bias for each trial using the Cochrane risk of bias tool as described in the Cochrane Handbook for Systematic Review of Interventions (Higgins 2011). Any disagreements were resolved by discussion or through arbitration with the third review author (AGM) if necessary. We completed a risk of bias table for each included study in Review Manager 5 (Review Manager 2020). We rated each included study as having a low, high, or unclear risk of bias on several domains applicable to randomised controlled trials, as follows.
Random sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants and personnel (performance bias).
Blinding of outcome assessment (detection bias).
Incomplete outcome data (attrition bias).
Selective outcome reporting (reporting bias).
Other sources of bias.
Measures of treatment effect
We expressed dichotomous data as risk ratios (RRs) with 95% confidence intervals (CIs), and planned to express time‐to‐event outcomes as hazard ratios (HRs) with 95% CIs. We planned that if HRs were not reported directly, we would use previously reported methods to approximate these values (Parmar 1998; Williamson 2002). For quality of life data, we planned to use mean differences (MDs) with 95% CIs.
Unit of analysis issues
We analysed parallel‐arm and cross‐over design studies in separate subgroups.
Dealing with missing data
We planned to implement an intention‐to‐treat analysis for all primary and secondary outcomes. We planned to calculate any missing statistics from the raw data when possible.
Assessment of heterogeneity
We planned to assess methodological heterogeneity by comparing trials based on the items outlined in the Methods and trial design section in Data extraction and management, and to assess clinical heterogeneity by comparing trials based on the items outlined in the Participants and demographic information section in Data extraction and management. If we found a forest plot to be appropriate, we would perform a visual inspection to identify any inconsistencies amongst studies and quantify this using the I2 statistic, using the following parameters as a guide.
0% to 40%: might not be important.
30% to 60%: may represent moderate heterogeneity.
50% to 90%: may represent substantial heterogeneity.
75% to 100%: considerable heterogeneity.
Assessment of reporting biases
We requested protocols for all of the included studies to enable a comparison of our outcomes of interest. In the case of sufficient randomised controlled trials, we would prepare a funnel plot to help identify publication bias, further investigating any visual asymmetry by exploratory analysis. We attempted to obtain source data for all studies included in the analysis to assess any non‐reported outcomes. We investigated outcome reporting bias using the Outcome Reporting Bias In Trials (ORBIT) matrix system for benefit outcomes (Kirkham 2010).
Data synthesis
We planned to analyse data in a meta‐analysis using a fixed‐effect model within Review Manager 5 (Review Manager 2020), provided this was clinically appropriate and there was no evidence of substantial heterogeneity. If we found evidence of substantial heterogeneity, we would explore the factors for heterogeneity; if substantial heterogeneity could not be readily explained, we would use a random‐effects model to perform meta‐analysis. Our primary analysis was intention‐to‐treat, in which all participants are included in the intervention groups to which they have been allocated, irrespective of whether they had received the treatment. We analysed data as set out in Measures of treatment effect. We analysed different control groups separately. A P value of less than 0.05 qualified statistical significance.
We did not undertake meta‐analysis in this review update as no more than one study was combined together. A narrative analysis of the data is presented.
Subgroup analysis and investigation of heterogeneity
We planned to separately assess the effects of STM in participants with focal epilepsy and in those with generalised epilepsy. We planned to separately assess the effects of different doses of STM. Given the small number of included studies, we were unable to conduct any meaningful subgroup analysis.
Sensitivity analysis
We planned to conduct the following sensitivity analyses to test the robustness of the meta‐analysis, where possible.
Repeating the analysis with exclusion of unpublished studies.
Repeating the analysis with exclusion of studies published only as abstracts.
These sensitivity analyses were not required to be conducted in the current review, as all included studies were published journal articles.
Summary of findings and assessment of the certainty of the evidence
We created summary of findings tables using GRADEpro GDT software, and employed the GRADE approach to assess the certainty of evidence for the following outcomes: treatment withdrawal, seizure freedom, and adverse effects (Schünemann 2013).
Results
Description of studies
Results of the search
The electronic searches identified a total of 170 references (Figure 1). After the removal of duplicates and irrelevant records, we screened the remaining 18 records, identifying 8 potentially eligible studies. After screening the full‐text publications for these 8 studies, we included one study (Characteristics of included studies), and excluded 7 studies (Characteristics of excluded studies).
1.

Study flow diagram
Included studies
We included one additional study in this update (Borggraefe 2013; 43 participants). A total of four studies met our inclusion criteria (Rating 1999; Li 2000; Basnec 2005; Borggraefe 2013), comprising 355 participants. One study, Rating 1999, accounted for two of the search results: Rating 1999 and Rating 2000; each publication reported data for the same study. Four additional references (Borggraefe 2015; Tracke 2016; Tracke 2017; Tracke 2018) relate to a new study (Borggraefe 2013).
Rating 1999 was a multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group study. Children between the ages of three and 10 years, weighing between 10 and 50 kg and with a diagnosis of BECTS with at least two seizures in the past six months, were recruited into the study. Children with severe organic disease, acute porphyria, a history of mental illness, relevant hypersensitivity reactions, relevant renal, thyroid, or hepatic dysfunction, and somatic signs of puberty or AED treatment after the age of six months (unless treatment was provided for less than six months) were excluded. A total of 66 participants were randomly assigned: 31 participants received STM, and 35 received placebo. Participants in the intervention group had a median (range) age of 8.2 years (3.9 to 10.7 years); participants in the placebo group had a median (range) age of 8.4 years (3.1 to 10.3 years). Interquartile age was not reported. There were 16 (52%) males and 15 (48%) females in the intervention group and 24 (69%) males and 11 (31%) females in the control group. After a six‐month historic baseline period, during which participants kept a seizure diary but received no intervention or placebo, participants were randomly assigned to receive STM (5 mg/kg/d) or placebo during a six‐month treatment phase. No titration period was provided. Seizure activity was recorded by participants in a diary, and assessments occurred at screening, on day 14, on day 28, after three months, and at the end of the six‐month treatment phase. On day 14, assessment consisted of physical and neurological examinations, review of seizure diaries, and evaluation of adverse effects, intercurrent illnesses, and medications. During subsequent reviews, assessment included laboratory tests such as STM plasma levels and awake and asleep EEG changes.
Li 2000 was a multicentre, randomised, double‐blind, active‐controlled (phenytoin (PHT)), parallel‐group study in which an additional third group received STM openly. Individuals with generalised tonic‐clonic seizures (GTCS) who had experienced a seizure within three to six months of the study start date were recruited into the study. Participants in the intervention group had a mean (standard deviation (SD)) age of 29.53 (15.09) years; participants in the placebo group had a mean (SD) age of 34.91 (15.12) years. Participants in the open group had a mean (SD) age of 27.69 (16.76) years. Thirty‐two participants in the intervention group received STM, and 32 participants in the control group received PHT; a further 82 participants in the open group received STM. The intervention group included 18 (56%) males and 14 (44%) females. The treatment phase lasted for six months; however, no information was provided on loading or titration periods, or whether a historic baseline period was included. The control group included 17 (53%) males and 15 (47%) females. The open group included 57 (70%) males and 25 (30%) females. At the start of the trial, participants were randomly assigned to receive STM (100 to 200 mg/d) or PHT (300 mg/d) as a double‐blind treatment, or STM (100 to 200 mg/d) as an open treatment. Treatment continued for six months, during which seizure frequency and adverse effects were measured on a monthly basis. Laboratory tests consisting of blood count, liver function, kidney function, electrocardiogram (ECG), and EEG were carried out before treatment commenced and after the six‐month treatment period had been completed.
Providing a robust analysis of Basnec 2005, which was published in Croatian, is difficult because of the lack of a reliable English translation of the full text. We will aim to obtain an English translation of the full text in future updates of this review and will discuss below information that could be obtained from the English translation of the abstract. Basnec 2005 was a multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group study. Details on dosing and study phases were not provided. 34 participants were randomly assigned. Children between the ages of three and 11 years who had experienced a single seizure only and had received no AED treatment were recruited into the study. Participants received STM or placebo, and data were collected on the proportion of participants who withdrew from treatment; proportion of participants who experienced a second seizure within six months; proportion of participants who experienced a second seizure after six months; and proportion of participants who experienced status epilepticus.
Borggraefe 2013 was a multicentre (from 47 German centres), prospective, double‐blinded, randomised controlled trial, conducted over a period of 26 months. Children between six and 12 years of age with a body weight of between 19 and 45 kg, with a diagnosis of BECTS, who had experienced two or more seizures in the past six months were recruited into the study. A total of 44 participants were randomly assigned to STM (6 mg/kg bodyweight) or levetiracetam (LEV) (30 mg/kg bodyweight) treatment group. Forty‐three participants were analysed (one participant was excluded due to a protocol violation). Children with Landau‐Kleffner syndrome and epileptic encephalopathy with continuous spike and wave during sleep, and relevant medical conditions (i.e. hepatopathy, nephropathy, disorders of the heart, endocrine system, and metabolic diseases) were excluded. Participants in the intervention group had a mean (SD) age of 9 years (1.5); participants in the control group had a mean (SD) age of 8.7 years (1.7). A total of 22 participants received STM, and 21 received LEV. The intervention group included 12 (54.5%) males and 10 (45.5%) females; the control group included 15 (71.4%) males and 6 (28.6%) females. Medication was started at a dosage of 2 mg/kg bodyweight (STM) or 10 mg/kg bodyweight (LEV), and was increased weekly by increments of 2 and 10 mg/kg bodyweight weekly to a final dosage of 6 and 30 mg/kg bodyweight, respectively. After a total observation period of 24 weeks whilst children took the recommended study dosage, the study was unblinded, and the local principal investigator and participants could choose to continue the treatment or not. Explorative data analysis was performed to investigate the number of total dropouts, participants who experienced seizure freedom, and occurrence of adverse events.
Excluded studies
Three studies administered STM to participants with epilepsy but did not include a placebo group (not randomised controlled trials) (Ingram 1963; Griffiths 1964; Livingston 1967). One study compared STM versus placebo as an add‐on therapy in epilepsy (Debus 2004). One study compared STM versus placebo as monotherapy in healthy participants with no history of epilepsy, measuring axonal excitability of cortical neurons as a primary outcome (Groppa 2006). One study assessed the effects of STM in both epileptic and non‐epileptic participants (Moffatt 1970). One study that compared STM versus placebo as monotherapy in participants with BECTS was abandoned (ISRCTN66730162 2011).
Risk of bias in included studies
Summaries of our judgements for each risk of bias domain, across the included studies, are presented in Figure 2 and Figure 3. Reasons supporting our judgements, including quotations taken directly from the text publications and specific review author comments, can be found in the risk of bias tables in Characteristics of included studies.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Rating 1999 stated that participants were divided into blocks of four according to a pre‐prepared list. The study authors did not explain how this list was formulated or how each block of four was assigned to treatment or placebo. The control group included a high proportion of males compared with females (69% versus 31%). Li 2000 provided no information on how allocation was determined. The open group included a high proportion of males compared with females (70% versus 30%). Basnec 2005 did not provide information on how allocation was determined. Borggraefe 2013 stated that the randomisations list was generated at the central randomisations centre of the study medication manufacturer using permuted blocks. Participants were allocated to one to the two treatment groups in a 1:1 ratio.
Blinding
In Rating 1999, each participant had his or her designation held in a sealed, coded envelope that was kept by an investigator for emergency use only. Li 2000 and Basnec 2005 provided no information on how blinding was performed. Borggraefe 2013 provided no details regarding blinding of participants, personnel, and outcome assessors.
Incomplete outcome data
Rating 1999 was terminated early after an interim analysis found superiority in the intervention group. Two participants from each group were removed from the study at this point. Rating 1999 reported the following withdrawals from the study after randomisation: six participants in the intervention group (four because of seizure and two due to early termination of the study) and 25 participants in the placebo group (21 because of seizure, two due to withdrawal of parental consent, and two as a result of early termination of the study). The paper defined the following events as treatment failure events: participants experiencing a first seizure after a seven‐day run‐in period; having intolerable adverse effects; developing another epileptic syndrome; or being terminated from the trial by their parents or by themselves. It is unclear based on the published paper whether an intention‐to‐treat analysis was performed. Li 2000 provided no information on treatment withdrawal. All participants who were randomly assigned were included in the final analysis. It is plausible that this indicates that no participants withdrew from treatment; however, this is not explicitly stated in the publication. Basnec 2005 stated that four participants withdrew from treatment during the study, but to which groups these participants had been assigned is not reported. Analysis was performed without these participants, rather than by intention‐to‐treat approach. Borggraefe 2013 stated that one participant in the control group was excluded from the final analysis due to a protocol violation. Attrition was fully reported, and intention‐to‐treat analysis was used.
Selective reporting
Rating 1999 reported that a total of 31 participants withdrew from treatment, but it is not stated whether intention‐to‐treat analysis was performed. The total number of adverse effects experienced by each group was reported; however, data on the individual frequency of each adverse effect were not reported. Data were not reported on time to treatment withdrawal; proportion of participants with a reduction in seizure frequency of 50% or greater; proportion of participants seizure‐free at 12 months; or quality of life scale scores. Li 2000 provided no data on time to treatment withdrawal; proportion of participants who withdrew from treatment; proportion of participants with a reduction in seizure frequency of 50% or greater; proportion of participants seizure‐free at 12 months; or quality of life scale scores. Adverse effects, including individual frequencies of each adverse effect, were reported; however, data for the intervention group were combined with data from a much larger, unblinded, open group that had an unusually large proportion of male participants compared with the other groups. It is unclear whether data provided on adverse effects relate to number of events or number of participants experiencing an adverse effect. It was not possible to assess this domain for Basnec 2005 because a reliable English language full‐text version of the study was not available. In Borggraefe 2013, all expected and prespecified outcomes were reported in the results section. We have also presented outcome reporting bias for all studies in an ORBIT table (Figure 4).
4.
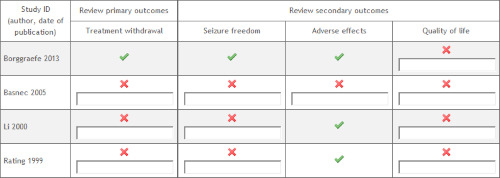
ORBIT table
Other potential sources of bias
Rating 1999 utilised clearly stated inclusion and exclusion criteria. Li 2000 provided clear inclusion criteria based on a recent history of GTCS; however, no information was provided on inclusion or exclusion criteria based on participant age, weight, comorbidities, or previous AED use. The study authors combined treatment and open groups when performing an analysis of adverse effects, providing no separate data for the intervention group alone. Basnec 2005 provided clear inclusion criteria, based on age and a diagnosis of BECTS with a single seizure and no AED treatment, in the abstract; however, as an English version of the full text was not available, it was not possible to fully appraise the inclusion and exclusion criteria for this study. Borggraefe 2013 utilised clearly stated inclusion and exclusion criteria. This study was funded in part by UCB Pharma SA.
Effects of interventions
See: Table 1; Table 2; Table 3
See Table 1; Table 2; Table 3.
Sulthiame versus placebo
Two included studies involving a total of 100 randomised participants compared sulthiame versus placebo (Rating 1999; Basnec 2005).
Primary outcome
Treatment withdrawal
No data were reported for this outcome.
Secondary outcomes
Seizure freedom
No data were reported for this outcome.
Adverse effects
Basnec 2005 provided no data on adverse effects in the abstract.
Rating 1999 did not report the number of participants who experienced adverse effects. They did report a total of 60 events (1.9 events per participant) in the intervention group and 33 events (0.9 events per participant) in the placebo group. Adverse effects that occurred more than once included leukopenia, loss of strength, and fatigue.
Quality of life
No data were reported for this outcome.
Sulthiame versus levetiracetam
One included study involving a total of 43 randomised participants compared sulthiame versus levetiracetam (Borggraefe 2013).
Primary outcome
Treatment withdrawal
Borggraefe 2013 presented data for this outcome (Analysis 1.1), reporting that 3 of 22 participants in the STM group and 9 of 21 participants in the LEV group withdrew from treatment (risk ratio (RR) 0.32, 95% confidence interval (Cl) 0.10 to 1.02). The total number of dropouts caused either by seizure recurrence or adverse reaction was therefore significantly higher in the LEV treatment arm compared to the STM treatment arm.
1.1. Analysis.

Comparison 1: Sulthiame versus levetiracetam, Outcome 1: Treatment withdrawal
Secondary outcomes
Seizure freedom
Borggraefe 2013 presented data for this outcome (Analysis 1.2), reporting that 20 participants in the STM group and 17 in the LEV group attained seizure freedom (RR 1.12, 95% Cl 0.88 to 1.44). However, the analysis indicated that the results were inconclusive, and we were unable to determine whether there was an effect of sulthiame on seizure freedom.
1.2. Analysis.

Comparison 1: Sulthiame versus levetiracetam, Outcome 2: Seizure freedom
Adverse effects
Borggraefe 2013 reported that total adverse reactions were higher in participants receiving LEV compared to those receiving STM, though not reaching statistical significance (23.8% and 4.5%, respectively). General symptoms (weight gain or loss, sleep disorder, fatigue, skin lesions, hiccup) were as follows: 17 in the STM group versus 15 in the LEV group (RR 1.08, 95% Cl 0.76 to 1.54). The following individual adverse effects were reported.
Central nervous system: 16 in STM group versus 13 in LEV group (RR 1.17, 95% CI 0.77 to 1.79).
Behaviour: 13 in STM group versus 13 in LEV group (RR 0.95, 95% CI 0.59 to 1.55).
Airways: 14 in STM group versus 5 in LEV group (RR 2.67, 95% CI 1.17 to 6.12).
Cardiac: 1 in STM group versus 0 in LEV group (RR 2.87, 95% CI 0.12 to 66.75).
Gastrointestinal: 8 in STM group versus 10 in LEV group (RR 0.76, 95% CI 0.37 to 1.56).
Bones and muscles: 4 in STM group versus 4 in LEV group (RR 0.95, 95% CI 0.27 to 3.33).
Others: 5 in STM group versus 4 in LEV group (RR 1.19, 95% CI 0.37 to 3.85).
Quality of life
No data were reported for this outcome.
Sulthiame versus phenytoin
One included study involving a total of 146 randomised participants compared sulthiame versus phenytoin (Li 2000).
Primary outcome
Treatment withdrawal
No data were reported for this outcome.
Secondary outcomes
Seizure freedom
No data were reported for this outcome.
Adverse effects
Li 2000 reported a total of 30 adverse effects in the combined intervention and open group, and 8 in the PHT group. The overall RR for STM compared with PHT was 1.05 (95% CI 0.54 to 2.07).
The following individual adverse effects were reported.
Paraesthesia: 14 in STM groups versus 0 in PHT group (RR 8.32, 95% CI 0.51 to 135.82).
Dizziness: 5 in STM groups versus 0 in PHT group (RR 3.16, 95% CI 0.18 to 55.62).
Headache: 2 in STM groups versus 0 in PHT group (RR 1.43, 95% CI 0.07 to 29.15).
Anorexia: 3 in STM groups versus 2 in PHT group (RR 0.42, 95% CI 0.07 to 2.41).
Rash: 1 in STM groups versus 1 in PHT group (RR 0.28, 95% CI 0.02 to 4.36).
Gingival hyperplasia: 0 in STM groups versus 4 in PHT group (RR 0.03, 95% CI 0.00 to 0.58).
Others: 5 in STM groups versus 1 in PHT group (RR 1.40, 95% CI 0.17 to 11.59).
No significant difference in the total number of adverse effects was noted between the two groups (P = 0.88), and gingival hyperplasia was the only individual adverse effect showing a significant difference between the STM and PHT groups (P = 0.02).
Quality of life
No data were reported for this outcome.
Discussion
Summary of main results
We included four published studies in the review. Both Rating 1999 and Basnec 2005 compared STM as monotherapy versus placebo for treatment of BECTS. Li 2000 compared STM versus PHT for treatment of GTCS. Borggraefe 2013 compared STM versus LEV for treatment of BECTS. Li 2000 was published in Chinese, and an English translation of the full manuscript was obtained for the purposes of this review. Basnec 2005 was published in Croatian. At the time of publication of this review, the English translation of the abstract but not the full manuscript was available. None of the included studies reported data on quality of life scale scores.
Borggraefe 2013 reported data on treatment withdrawal: the total number of dropouts caused either by seizure recurrence or adverse reaction was significantly higher in the LEV treatment arm compared to the STM treatment arm. Borggraefe 2013 presented data on the proportion of participants seizure‐free at six months. The results for whether there was an effect of sulthiame for seizure freedom were inconclusive.
The adverse effects of STM compared with placebo were reported incompletely by Rating 1999, precluding the drawing of any meaningful conclusions. Li 2000 reported data on adverse effects of STM compared with PHT, in which the intervention group was combined with a large group of participants who received STM openly and comprised a disproportionately large number of males. Li 2000 reported data suggesting that there was no significant difference in the overall occurrence of adverse effects between STM and PHT groups; however, the occurrence of gingival hyperplasia was significantly greater in the PHT group. The study reported no significant difference in the occurrence of paraesthesia, dizziness, headache, anorexia, rash, or other adverse effects between the STM and PHT groups. Basnec 2005 reported none of our prespecified outcomes. Borggraefe 2013 reported data on adverse effects of STM compared with LEV. Total adverse reactions was higher in the LEV group compared to the STM group, though not reaching statistical significance. The most common adverse events were related to behavioural disturbances (depression, restlessness, irritability, anxiety, change of personality, aggressive behaviour), with the same incidence in both groups. In addition, there was a higher incidence of adverse events affecting the airways (shortness of breathing, tachypnoea, coughing, irritability of upper airway tract) in the STM group. The study reported no significant difference between groups for the other adverse reactions: general symptoms (weight gain or loss, sleep disorder, fatigue, skin lesions, hiccup), central nervous system (headache, vertigo, tremor, paraesthesia, ataxia, diplopia, amnesia, impairment of alertness) (Analysis 1.4), cardiac (pain, tachycardia), gastrointestinal (abdominal pain, loss of appetite, indigestion, nausea, vomiting, diarrhoea), bones and muscles (bone and joint pain, muscle pain), or other adverse effects (gingivitis, alopecia, gingival bleeding, hyperacusis, dry skin).
1.4. Analysis.

Comparison 1: Sulthiame versus levetiracetam, Outcome 4: Central nervous system (headache, vertigo, tremor, paraesthesia, ataxia, diplopia, amnesia, impairment of alertness)
Overall completeness and applicability of evidence
This review included two studies (Rating 1999; Basnec 2005) that compared STM as monotherapy versus placebo in participants with a diagnosis of BECTS; one study that compared STM as monotherapy versus PHT in participants with a history of GTCS (Li 2000); and one study that compared STM as monotherapy versus LEV in children with BECTS (Borggraefe 2013). We were unable to adequately assess the methodological quality and the full range of data of Basnec 2005 because a reliable English translation of the published paper could not be obtained. Rating 1999 clearly stated their inclusion and exclusion criteria and provided a satisfactory explanation of a methodologically sound randomised controlled trial.
We downgraded the certainty of evidence to low for the primary outcome treatment withdrawal. The certainty of evidence for the secondary outcomes, seizure freedom and adverse effects, was low. We downgraded the certainty of the evidence to low because of small sample size, lack of clarity on whether intention‐to‐treat analysis was performed, and the absence of important outcome measures.
Given that data on important outcome measures were lacking in some studies, sample sizes were small, and methodology was unclear in the included studies, little clinical relevance can be attributed to this review at this time.
Quality of the evidence
Overall, we assessed one study at high risk of bias and one study at unclear bias across the seven domains, mainly due to lack of information regarding study design. Only one trial reported effective methods for blinding. The risk of bias assessments for the other two studies ranged from low to high.
We used the GRADE approach to rate the certainty of evidence for each outcome. The assessments are presented in a summary of findings tables (see Table 1; Table 2; Table 3). We judged the certainty of the evidence for adverse effects as low given that the numbers of events reported were insufficient to draw any conclusions.
Potential biases in the review process
Although we requested protocols for all of the included studies, the time frame in which the majority of the studies were conducted made retrieval of these difficult. This could lead to potential bias through omitted information to which we did not have access.
Agreements and disagreements with other studies or reviews
We found no reviews or published information on the use of sulthiame as monotherapy for epilepsy.
Authors' conclusions
Implications for practice.
We could draw no meaningful conclusions regarding the efficacy or safety of sulthiame as monotherapy in epilepsy based on the available evidence.
Implications for research.
Studies should report data on time to treatment withdrawal; proportion of participants achieving a reduction in seizure frequency of 50% or greater; proportion of participants seizure‐free at 12 months; and quality of life scale scores, in addition to adverse effects, to facilitate meaningful meta‐analysis.
What's new
| Date | Event | Description |
|---|---|---|
| 3 September 2021 | New citation required but conclusions have not changed | Conclusions remain unchanged. |
| 3 September 2021 | New search has been performed | Searches updated 13 April 2020; one new study included (Borggraefe 2013). |
History
Protocol first published: Issue 9, 2012 Review first published: Issue 3, 2014
| Date | Event | Description |
|---|---|---|
| 13 April 2020 | New citation required but conclusions have not changed | Conclusions remain unchanged. |
Acknowledgements
We thank Graham Powell and Graham Sills for their contributions to the original protocol and to the previous version of this review.
Appendices
Appendix 1. CRS Web search strategy
1. (sulthiame OR sultiame OR Ospolot) AND CENTRAL:TARGET
2. MESH DESCRIPTOR Epilepsy EXPLODE ALL AND CENTRAL:TARGET
3. MESH DESCRIPTOR Seizures EXPLODE ALL AND CENTRAL:TARGET
4. (epilep* OR seizure* OR convuls*):AB,KW,MC,MH,TI AND CENTRAL:TARGET
5. #2 OR #3 OR #4 AND CENTRAL:TARGET
6. #1 AND #5
Appendix 2. MEDLINE (Ovid) search strategy
This strategy includes a modification of the Cochrane highly sensitive search strategy for identifying randomised trials (Lefebvre 2021).
1. (sulthiame or sultiame or Ospolot).tw.
2. exp Epilepsy/
3. exp Seizures/
4. (epilep$ or seizure$ or convuls$).tw.
5. 2 or 3 or 4
6. exp *Pre‐Eclampsia/ or exp *Eclampsia/
7. 5 not 6
8. (randomized controlled trial or controlled clinical trial or pragmatic clinical trial).pt. or (randomi?ed or placebo or randomly).ab.
9. clinical trials as topic.sh.
10. trial.ti.
11. 8 or 9 or 10
12. exp animals/ not humans.sh.
13. 11 not 12
14. 1 and 7 and 13
15. remove duplicates from 14
Appendix 3. Scopus search strategy
((TITLE‐ABS‐KEY(sulthiame OR sultiame OR Ospolot)) AND ((TITLE‐ABS‐KEY(epilep* OR "infantile spasm" OR seizure OR convuls* OR (syndrome W/2 (aicardi OR angelman OR doose OR dravet OR janz OR jeavons OR "landau kleffner" OR "lennox gastaut" OR ohtahara OR panayiotopoulos OR rasmussen OR rett OR "sturge weber" OR tassinari OR "unverricht lundborg" OR west)) OR "ring chromosome 20" OR "R20" OR "myoclonic encephalopathy" OR "pyridoxine dependency") AND NOT (TITLE(*eclampsia) OR INDEXTERMS(*eclampsia))) OR (TITLE‐ABS‐KEY(lafora* W/4 (disease OR epilep*)) AND NOT (TITLE(dog OR canine) OR INDEXTERMS(dog OR canine)))) and (TITLE((randomiz* OR randomis* OR controlled OR placebo OR blind* OR unblind* OR "parallel group" OR crossover OR "cross over" OR cluster OR "head to head") PRE/2 (trial OR method OR procedure OR study)) OR ABS((randomiz* OR randomis* OR controlled OR placebo OR blind* OR unblind* OR "parallel group" OR crossover OR "cross over" OR cluster OR "head to head") PRE/2 (trial OR method OR procedure OR study)))) AND NOT (TITLE((adjunct* OR "add‐on" OR "add on" OR adjuvant* OR combination* OR polytherap*) AND NOT (monotherap* OR alone OR singl*)))
Data and analyses
Comparison 1. Sulthiame versus levetiracetam.
1.3. Analysis.

Comparison 1: Sulthiame versus levetiracetam, Outcome 3: General symptoms (weight gain or loss, sleep disorder, fatigue, skin lesions, hiccups)
1.5. Analysis.

Comparison 1: Sulthiame versus levetiracetam, Outcome 5: Behaviour (depression, restlessness, irritability, anxiety, change of personality, aggressive behaviour)
1.6. Analysis.

Comparison 1: Sulthiame versus levetiracetam, Outcome 6: Airways (shortness of breathing, tachypnoea, coughing, irritability of upper airway tract)
1.7. Analysis.

Comparison 1: Sulthiame versus levetiracetam, Outcome 7: Cardiac (pain, tachycardia)
1.8. Analysis.

Comparison 1: Sulthiame versus levetiracetam, Outcome 8: Gastrointestinal (abdominal pain, loss of appetite, indigestion, nausea, vomiting, diarrhoea)
1.9. Analysis.

Comparison 1: Sulthiame versus levetiracetam, Outcome 9: Bones and muscles (bone and joint pain, muscle pain)
1.10. Analysis.

Comparison 1: Sulthiame versus levetiracetam, Outcome 10: Others (gingivitis, alopecia, gingival bleeding, hyperacusis, dry skin)
Comparison 2. Sulthiame versus phenytoin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 All adverse effects | 1 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.54, 2.07] |
| 2.2 Paraesthesia | 1 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.32 [0.51, 135.82] |
| 2.3 Dizziness | 1 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.16 [0.18, 55.62] |
| 2.4 Headache | 1 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.07, 29.15] |
| 2.5 Anorexia | 1 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.07, 2.41] |
| 2.6 Rash | 1 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.02, 4.36] |
| 2.7 Gingival hyperplasia | 1 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.03 [0.00, 0.58] |
| 2.8 Other | 1 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.17, 11.59] |
2.1. Analysis.

Comparison 2: Sulthiame versus phenytoin, Outcome 1: All adverse effects
2.2. Analysis.

Comparison 2: Sulthiame versus phenytoin, Outcome 2: Paraesthesia
2.3. Analysis.

Comparison 2: Sulthiame versus phenytoin, Outcome 3: Dizziness
2.4. Analysis.

Comparison 2: Sulthiame versus phenytoin, Outcome 4: Headache
2.5. Analysis.

Comparison 2: Sulthiame versus phenytoin, Outcome 5: Anorexia
2.6. Analysis.

Comparison 2: Sulthiame versus phenytoin, Outcome 6: Rash
2.7. Analysis.

Comparison 2: Sulthiame versus phenytoin, Outcome 7: Gingival hyperplasia
2.8. Analysis.

Comparison 2: Sulthiame versus phenytoin, Outcome 8: Other
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Basnec 2005.
| Study characteristics | ||
| Methods | Placebo‐controlled, parallel‐group, double‐blind randomised controlled trial | |
| Participants | Participants between 3 and 11 years of age with a diagnosis of BECTS who had experienced only 1 seizure and had received no AED 34 participants were randomly assigned. Unclear how many participants were allocated to each group. Males versus females ‐ not stated |
|
| Interventions | Sulthiame versus placebo | |
| Outcomes |
|
|
| Notes | At the time of publication of this review, a full‐text English translation was unavailable; the above information was taken from the English translation of the abstract only. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unable to fully assess risk of bias because of lack of English language full‐text manuscript |
| Allocation concealment (selection bias) | Unclear risk | Unable to fully assess risk of bias because of lack of English language full‐text manuscript |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unable to fully assess risk of bias because of lack of English language full‐text manuscript |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unable to fully assess risk of bias because of lack of English language full‐text manuscript |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unable to fully assess risk of bias because of lack of English language full‐text manuscript |
| Selective reporting (reporting bias) | Unclear risk | Unable to fully assess risk of bias because of lack of English language full‐text manuscript There was no protocol available to check a priori outcomes |
| Other bias | Unclear risk | Unable to fully assess risk of bias because of lack of English language full‐text manuscript |
Borggraefe 2013.
| Study characteristics | ||
| Methods | Multicentre, prospective, double‐blinded, randomised controlled trial conducted over a period of 26 months between July 2006 and September 2008 | |
| Participants | Children with benign partial epilepsy with centro‐temporal spikes who had experienced 2 or more seizures within 6 months prior to study entry were eligible. Number of participants randomly assigned: 44, with 43 remaining for the final analysis from 47 German centres Number of participants in each group:
Mean (SD) age, years: intervention group: 9.0 (1.5); control group: 8.7 (1.7) |
|
| Interventions | Sulthiame (6 mg/kg body weight) versus levetiracetam (30 mg/kg body weight) | |
| Outcomes | Adverse effects Treatment response defined as:
|
|
| Notes | Borggraefe 2015, Tracke 2016, Tracke 2017, and Tracke 2018 are additional references to this study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation list was generated at the central randomisation centre of the study medication manufacturer using permuted blocks (block size 10). |
| Allocation concealment (selection bias) | Low risk | Participants allocated to 1 to the 2 treatment groups in a 1:1 ratio. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No details were provided regarding the blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No details were provided regarding the blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition was fully reported, and intention‐to‐treat analysis was used. 1 participant of the control group was excluded from the final analysis due to a protocol violation. |
| Selective reporting (reporting bias) | Low risk | Protocol unavailable, but it appeared that all expected and prespecified outcomes had been reported in the results section. |
| Other bias | Unclear risk | This study was funded in part by UCB Phama SA. |
Li 2000.
| Study characteristics | ||
| Methods | Active‐controlled, parallel‐group, double‐blind randomised controlled trial with a third group receiving sulthiame openly No historical baseline period, 6‐month treatment phase |
|
| Participants | Individuals with GTCS who had experienced a seizure within 3 to 6 months of the study start date Number of participants randomly assigned: 146 Number of participants in each group:
Mean (SD) age, years: intervention group: 29.53 (15.09); control group: 34.91 (15.12): open group: 27.69 (16.76) |
|
| Interventions | Sulthiame (100 to 200 mg/d) versus phenytoin (100 mg 3 times a day) | |
| Outcomes | Adverse effects Treatment response defined as:
Laboratory tests pre‐study and poststudy:
|
|
| Notes | No information provided regarding participants withdrawing from the trial after randomisation. All participants were subsequently included in the analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Method of randomisation not stated. High proportion of males versus females in open group. |
| Allocation concealment (selection bias) | High risk | Method of allocation concealment not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Method of blinding not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Method of blinding not stated. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No data provided on participants withdrawing from treatment. |
| Selective reporting (reporting bias) | High risk | No data provided on proportion of participants with a reduction in seizure frequency of 50% or greater; proportion of participants seizure‐free at 12 months; or quality of life scale scores. Data provided on adverse effects include blinded and unblinded participants. It is unclear whether data provided on adverse effects relate to number of events or number of participants experiencing an adverse effect. No protocol was available to check a priori outcomes. |
| Other bias | High risk | Incomplete information on inclusion and exclusion criteria, and treatment and open groups were combined when analysis of adverse effects was performed; separate data for the intervention group alone not provided. |
Rating 1999.
| Study characteristics | ||
| Methods | Placebo‐controlled, parallel‐group, double‐blind randomised controlled trial 6‐month historical baseline followed by 6‐month treatment phase |
|
| Participants | Children between 3 and 10 years of age with a diagnosis of BECTS and at least 2 seizures in the past 6 months Number of participants randomly assigned (n = 66):
Median (range) age, years: intervention group: 8.2 (3.9 to 10.7); control group: 8.4 (3.1 to 10.3) |
|
| Interventions | Sulthiame (5 mg/kg/d) versus placebo | |
| Outcomes |
|
|
| Notes | 31 participants (6 from the intervention group and 25 from the control group) withdrew from the study, as follows:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Partial explanation of how participants were allocated to groups provided. |
| Allocation concealment (selection bias) | Low risk | Clear explanation of how allocation was concealed provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Clear explanation of blinding process provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Clear explanation of blinding process provided. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Number of participants withdrawing from treatment provided, but does not state whether intention‐to‐treat analysis was performed. |
| Selective reporting (reporting bias) | High risk | Inclusion and exclusion criteria clearly stated. No data on proportion of participants with a reduction in seizure frequency of 50% or greater; proportion of participants seizure‐free at 12 months; or quality of life scale scores. Incomplete data on adverse effects No protocol was available to check a priori outcomes. |
| Other bias | Low risk | None detected. |
AED: antiepileptic drug BECTS: benign epilepsy of childhood with centrotemporal spikes ECG: electrocardiogram EEG: electroencephalographic GTCS: generalised tonic‐clonic seizures
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Debus 2004 | STM used as add‐on therapy in the intervention group. |
| Griffiths 1964 | Not a randomised controlled trial |
| Groppa 2006 | Study on the effect of STM as monotherapy on axonal excitability of cortical neurons in participants with no history of epilepsy |
| Ingram 1963 | Not a randomised controlled trial |
| ISRCTN66730162 2011 | Trial abandoned. |
| Livingston 1967 | Not a randomised controlled trial |
| Moffatt 1970 | Study assessing the effects of STM on aggressive behaviour in both epileptic and non‐epileptic participants |
STM: sulthiame
Differences between protocol and review
Addition of search strategy for Scopus database.
We renamed the outcomes to ensure that our terminology was consistent with other reviews produced by Cochrane Epilepsy.
We had planned to conduct sensitivity analyses. Specifically, we had intended to repeat the meta‐analyses whilst excluding unpublished studies and then whilst excluding studies that had been published only as abstracts. All of the included studies were published as full‐length journal articles; therefore, neither sensitivity analysis was necessary.
Contributions of authors
For the latest update, PM‐M and MP assessed studies for inclusion, evaluated the methodological quality of studies, extracted data, performed analysis of the data, and composed the final document.
AGM supervised the update.
Sources of support
Internal sources
No sources of support provided
External sources
National Institute for Health Research, UK
Declarations of interest
PM‐M: none known.
MP: none known.
AGM is partly funded by the National Institute for Health Research Applied Research Collaboration North West Coast (NIHR ARC NWC). A consortium of pharmaceutical companies (GSK, EISAI, UCB Pharma) funded the National Audit of Seizure Management in Hospitals (NASH) through grants paid to the University of Liverpool.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Basnec 2005 {published data only (unpublished sought but not used)}
- Basnec A, Skarpa D, Barisic N, Jurin M, Mucic-Pucic B. The risk of second seizure in children with benign childhood epilepsy with centrotemporal spikes without treatment - a prospective study [Rizik javljanja drugog napadaja u neliječene djece s benignom parcijalnom epilepsijom s centrotemporalnim šiljaka - prospektivno istraživanje]. Acta Medica Croatica 2005;59(1):59-62. [PMID: ] [PubMed] [Google Scholar]
Borggraefe 2013 {published data only}97864911
- Borggraefe I, Bonfert M, Bast T, Neubauer BA, Schotten KJ, Massmann K, et al. Levetiracetam vs. sulthiame in benign epilepsy with centrotemporal spikes in childhood: a double-blinded, randomized, controlled trial (German HEAD Study). European Journal of Paediatric Neurology 2013;17(5):507-14. [DOI: 10.1016/j.ejpn.2013.03.014] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Borggraefe I, Bonfert M, Gerstl L, Heinen F, Neubauer B. A double-blinded, randomized evaluation of neuropsychological and behavioral changes in children with benign epilepsy with centrotemporal spikes treated either with levetiracetam or sulthiame. Epilepsy Currents 2015;15(Suppl 1):278, Abstract no: 2.04. [Google Scholar]
- Tacke M, Borggraefe I, Gerstl L, Heinen F, Vill K, Bonfert M, et al. Effects of levetiracetam and sulthiame on EEG in benign epilepsy with centrotemporal spikes: a randomized controlled trial. Seizure 2018;56:115-20. [DOI: 10.1016/j.seizure.2018.01.015] [PMID: ] [DOI] [PubMed]
- Tacke M, Gerstl L, Heinen F, Heukaeufer I, Bonfert M, Bast T, et al. Effect of anticonvulsive treatment on neuropsychological performance in children with BECTS. European Journal of Paediatric Neurology 2016;20(6):874-9. [DOI: 10.1016/j.ejpn.2016.07.015] [PMID: ] [DOI] [PubMed]
- Tacke M. EEG changes in rolandic epilepsy under treatment with levetiracetam and sulthiame. European Journal of Paediatric Neurology 2017;21(Suppl 1):e97, Abstract no: P2-5. [DOI: 10.1016/j.ejpn.2017.04.733] [DOI] [PubMed] [Google Scholar]
Li 2000 {published data only (unpublished sought but not used)}
- Li Jimin, Li Zuohan, Jin Yongqing, Pan Daoming, Xu Peixi, Hu Dameng, Xu Min. A randomized controlled multicenter clinical study on sulthiame in the treatment of GTCS. Journal of Clinical Neurology 2000;13(6):345-7. [Google Scholar]
Rating 1999 {published data only (unpublished sought but not used)}
- Bast T, Völp A, Wolf C, Rating D. The influence of sulthiame on EEG in children with benign epilepsy with centrotemporal spikes (BECTS). Epilepsia 2003;44(2):215-20. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Rating D, Wolf C, Bast T. Sulthiame as monotherapy in children with benign childhood epilepsy with centrotemporal spikes: a 6-month randomized, double-blind, placebo-controlled study. Epilepsia 2000;41(10):1284-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Rating D, Wolf C. Sulthiame vs placebo in the treatment of benign epilepsy with centrotemporal spikes ("rolandic" epilepsy). Epilepsia 1999;40(Suppl 2):163. [Google Scholar]
References to studies excluded from this review
Debus 2004 {published data only}
- Debus OM, Kurlemann G. Sulthiame in the primary therapy of West syndrome: a randomized, double-blind placebo-controlled add-on trial on baseline pyridoxine medication. Epilepsia 2004;45(2):103-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Griffiths 1964 {published data only}
- Griffiths AW, Sylvester PE. Ospolot - a clinical trial of a new anticonvulsant. British Journal of Psychiatry 1964;110:261-6. [DOI: 10.1192/bjp.110.465.261] [PMID: ] [DOI] [PubMed] [Google Scholar]
Groppa 2006 {published data only}
- Groppa S, Siniatchkin M, Siebner H, Stephani U. Reduction of motor cortex excitability by the anticonvulsant drug sulthiame: a TMS study. Epilepsia 2006;47(Suppl 3):123-4, Abstract no: p474. [Google Scholar]
- Siniatchkin M, Groppa S, Siebner H, Stephani U. A single dose of sulthiame induces a selective increase in resting motor threshold in human motor cortex: a transcranial magnetic stimulation study. Epilepsy Research 2006;72(1):18-24. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ingram 1963 {published data only}
- Ingram TT, Ratcliffe SG. Clinical trial of Ospolot in epilepsy. Developmental Medicine and Child Neurology 1963;5:313-5. [PMID: ] [PubMed] [Google Scholar]
ISRCTN66730162 2011 {published data only}
- ISRCTN66730162. Investigating the relationship between sleep disturbance and learning in children with benign epilepsy of childhood with centrotemporal spikes (BECCTS): a randomised double blind placebo controlled crossover trial. isrctn.com/ISRCTN66730162. [www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2011-001571-39]
Livingston 1967 {published data only}
- Livingston S, Villamater C, Sakata Y. Ospolot (sulthiame) in epilepsy. Diseases of the Nervous System 1967;28(4):259-63. [PMID: ] [PubMed] [Google Scholar]
Moffatt 1970 {published data only}
- Moffatt WR, Siddiqui AR, MacKay DN. The use of sulthiame with disturbed mentally subnormal patients. British Journal of Psychiatry 1970;117(541):673-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Additional references
Bast 2003
- Bast T, Volp A, Wolf C, Rating D, Sulthiame Study Group. The influence of sulthiame on EEG in children with benign childhood epilepsy with centrotemporal spikes (BECTS). Epilepsia 2003;44(2):215-20. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ben‐Zeev 2004
- Ben-Zeev B, Watemberg N, Lerman P, Barash I, Brand N, Lerman-Sagie T. Sulthiame in childhood epilepsy. Paediatrics International 2004;46(5):521-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bresnahan 2019
- Bresnahan R, Martin‐McGill KJ, Milburn‐McNulty P, Powell G, Sills GJ, Marson AG. Sulthiame add-on therapy for epilepsy. Cochrane Database of Systematic Reviews 2019, Issue 8. Art. No: CD009472. [DOI: 10.1002/14651858.CD009472.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brodie 1996
- Brodie MJ, Dichter MA. Antiepileptic drugs. New England Journal of Medicine 1996;334(3):168-75. [PMID: ] [DOI] [PubMed] [Google Scholar]
Chahem 2007
- Chahem J, Bauer J. Treatment of epilepsy with third-line antiepileptic drugs: felbamate, tiagabine and sulthiame. Der Nervenarzt 2007;78(12):1407-12. [PMID: ] [DOI] [PubMed] [Google Scholar]
Commission on Antiepileptic Drugs 1998
- Commission on Antiepileptic Drugs. Considerations on designing clinical trials to evaluate the place of new antiepileptic drugs in the treatment of newly diagnosed and chronic patients with epilepsy. Epilepsia 1998;39(7):799-803. [PMID: ] [DOI] [PubMed] [Google Scholar]
Engler 2003
- Engler F, Maeder-Ingvar M, Roulet E, Deonna T. Treatment with sulthiame (ospolot) in benign partial epilepsy of childhood and related syndromes: an open clinical and EEG study. Neuropediatrics 2003;34(2):105-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Go 2005
- Go T. Effect of antiepileptic drug polytherapy on crystalluria. Pediatric Neurology 2005;32(2):113-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT [GRADEpro Guideline Development Tool [Software]]. McMaster University, 2020 (developed by Evidence Prime, Inc.), 2020. Available from gradepro.org.
Gross‐Selbeck 2001
- Gross-Selbeck G. Current treatment strategies for childhood seizures and epilepsy [Derzeitige behandlungsstrategien bei anfällen und epilepsien im kindesalter]. Monatsschrift fur Kinderheilkunde 2001;149(11):1174-9. [DOI: 10.1007/s001120170041] [DOI] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Houghton 1974
- Houghton GW, Richens A. Inhibition of phenytoin metabolism by sulthiame in epileptic patients. British Journal of Clinical Pharmacology 1974;1(1):59–66. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
ILAE 1997
- International League Against Epilepsy. ILAE Commission Report. The epidemiology of the epilepsies: future directions. Epilepsia 1997;38(5):614-8. [PMID: ] [PubMed] [Google Scholar]
Kirkham 2010
- Kirkham JJ, Dwan KM, Altman DG, Gamble C, Dodd S, Smyth R, et al. The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. BMJ 2010;340:c365. [DOI: 10.1136/bmj.c365] [PMID: ] [DOI] [PubMed] [Google Scholar]
Koepp 2002
- Koepp MJ, Patsalos PN, Sander JWAS. Sulthiame in adults with refractory epilepsy and learning disability: an open trial. Epilepsy Research 2002;50(3):277-82. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lefebvre 2021
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al. Technical Supplement to Chapter 4: Searching for and selecting studies. Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Leniger 2002
- Leniger T, Wiemann M, Bingmann D, Widman G, Hufnagel A, Bonnet U. Carbonic anhydrase inhibitor sulthiame reduces intracellular pH and epileptiform activity of hippocampal CA3 neurons. Epilepsia 2002;43(5):469-74. [PMID: ] [DOI] [PubMed] [Google Scholar]
Miyajima 2009
- Miyajima T, Kumada T, Kimura N, Mikuni T, Fujii T. Sulthiame treatment for patients with intractable epilepsy. No To Hattatsu 2009;41(1):17-20. [DOI: 10.11251/ojjscn.41.17] [DOI] [PubMed] [Google Scholar]
Panebianco 2015
- Panebianco M, Rigby A, Weston J, Marson AG. Vagus nerve stimulation for partial seizures. Cochrane Database of Systematic Reviews 2015, Issue 4. Art. No: CD002896. [DOI: 10.1002/14651858.CD002896.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Panebianco 2016
- Panebianco M, Zavanone C, Dupont S, Restivo DA, Pavone A. Vagus nerve stimulation therapy in partial epilepsy: a review. Acta Neurologica Belgica 2016;116(3):241-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815-34. [PMID: ] [DOI] [PubMed] [Google Scholar]
Rating 2000
- Rating D, Wolf C, Bast T. Sulthiame as monotherapy in children with benign childhood epilepsy with centrotemporal spikes: a 6-month randomized, double-blind, placebo-controlled study. Sulthiame Study Group. Epilepsia 2000;41(10):1284-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Review Manager (RevMan). Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Sander 1993
- Sander JW. Some aspects of prognosis in the epilepsies: a review. Epilepsia 1993;34(6):1007-16. [PMID: ] [DOI] [PubMed] [Google Scholar]
Schmidt 1995
- Schmidt D, Gram L. Monotherapy versus polytherapy in epilepsy: a reappraisal. CNS Drugs 1995;3:194-208. [DOI: 10.2165/00023210-199503030-00005] [DOI] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Weissbach 2010
- Weissbach A, Tirosh I, Scheuerman O, Hoffer V, Garty BZ. Respiratory alkalosis and metabolic acidosis in a child treated with sulthiame. Pediatric Emergency Care 2010;26(10):752-3. [PMID: ] [DOI] [PubMed] [Google Scholar]
West 2019
- West S, Nevitt SJ, Cotton J, Gandhi S, Weston J, Sudan A, et al. Surgery for epilepsy. Cochrane Database of Systematic Reviews 2019, Issue 6. Art. No: CD010541. [DOI: 10.1002/14651858.CD010541.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2001
- World Health Organization. Epilepsy: Aetiology, Epidemiology and Prognosis. Geneva: World Health Organization, 2001. [Google Scholar]
Williamson 2002
- Williamson PR, Smith CT, Hutton JL, Marson AG. Aggregate data meta-analysis with time to event outcomes. Statistics in Medicine 2002;21(22):3337-51. [PMID: ] [DOI] [PubMed] [Google Scholar]
Wirrell 2008
- Wirrell E, Sherman EMS, Vanmastrigt R, Hamiwka L. Deterioration in cognitive function in children with benign epilepsy of childhood with central temporal spikes treated with sulthiame. Journal of Child Neurology 2008;23(1):14-21. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Milburn‐McNulty 2012
- Milburn‐McNulty P, Powell G, Sills GJ, Marson AG. Sulthiame monotherapy for epilepsy. Cochrane Database of Systematic Reviews 2012, Issue 9. Art. No: CD010062. [DOI: 10.1002/14651858.CD010062] [DOI] [PMC free article] [PubMed] [Google Scholar]
Milburn‐McNulty 2014
- Milburn-McNulty P, Powell G, Sills GJ, Marson AG. Sulthiame monotherapy for epilepsy. Cochrane Database of Systematic Reviews 2014, Issue 3. Art. No: CD010062. [DOI: 10.1002/14651858.CD010062.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


