Abstract
Molecular designs merging circularly polarized luminescence (CPL) and thermally activated delayed fluorescence (CP-TADF) using the concept of chiral perturbation appeared recently as a cornerstone for the development of efficient CP-organic light emitting diodes (CP-OLED). Such devices could strongly increase the energy efficiency and performances of conventional OLED displays, in which 50% of the emitted light is often lost due to the use of antiglare filters. In this context, herein, ten couples of enantiomers derived from novel chiral emitter designs are reported, exhibiting CPL, TADF, and aggregation induced enhancement emission properties (AIEE). Representing the first structure properties relationship investigation for CP-TADF materials, this thorough experimental and theoretical work highlights crucial findings on the key structural and electronic parameters (isomerism, nature of the carbazole substituents) governing the synergy between CPL and TADF properties. To conclude this study, the first top emission CP-OLED is elaborated as a new approach of generating CP light in comparison with classical bottom-emission CP-OLED architecture. Indeed, the top-emission configuration represents the only relevant device architecture for future microdisplay applications. Thereby, in addition to offer molecular guidelines to combine efficiently TADF and CPL properties, this study opens new avenues toward practical applications for CP-OLEDs.
Keywords: aggregation induced emission, circularly polarized luminescence, OLED, thermally activated delayed fluorescence
1. Introduction
OLEDs appear as one of the most promising technologies for lighting devices or screen displays. Based on the use of organic compounds as emissive dopants embedded within a matrix to emit light after electrical excitation, various advantages can be put forward for display application compared to standard LCDs. Most common among them are a higher contrast ratio given by OLEDs, a faster response time, a minimal thickness, to name but a few. However, the main issue encountered with the use of organic materials as emitters in OLEDs resides in the deexcitation pathways. In OLEDs, due to quantum mechanics, 25% of the electrical energy is converted into singlet state excitons and 75% into triplet state excitons. Thus, for prompt fluorescence (PF) emitters, up to 25% of the energy input can be theoretically converted into light through fluorescent decay, while the remaining 75% excitons, in the triplet state, will be lost through non-radiative deexcitation. Thus, most fluorophores are not implemented in OLED devices because they only emit PF. In order to harvest both singlet and triplet excitons and convert those into light, phosphorescent compounds can also be used, taking advantage of intersystem crossing (ISC). However, they are often sensitive, expensive, rare metal-based compounds, or display low quantum yield. In this context, the use of thermally activated delayed fluorescence (TADF) materials has emerged as one of the best alternatives to construct efficient OLED devices.[1] TADF occurs when the energy difference between singlet and triplet states of the molecule is small enough, so that a rISC (reverse intercrossing system) allows triplet states to be converted into singlet ones. This opens up the possibility to design OLEDs with a theoretical quantum efficiency of 100%. For this reason, various molecular designs have been developed to produce molecules exhibiting energetically close lowest singlet state (S1) and lowest triplet state (T1) while maintaining as high a quantum yield as possible.[2] Because the singlet-triplet energy gap (ΔEST) is proportional to the highest occupied-lowest unoccupied molecular orbital (HOMO-LUMO) integral overlap, the most widespread strategy to minimize this ΔEST consists in using a twisted donor-acceptor (D-A) system, where HOMO and LUMO are spatially separated.[3]
In parallel to this approach, the design of efficient circularly polarized luminescent (CPL) emitters emerged as an important gateway to improve efficiency while decreasing the power consumption of portable OLED displays.[4] As opposed to lighting devices, displays need antiglare filters to avoid reflection of external light sources off their emitting surface (sunlight or public lighting for instance). The most commonly used filters are built with a quarter-wave plate and a polarizer. Unfortunately, such an architecture reduces the intensity of non-polarized light emitted by a conventional OLED display by at least 50%. A solution to this significant energy loss could lie in mastering the design of efficient CP-OLEDs able to emit polarized light. This way, with a high degree of polarization, the light emitted by the display could pass through the antiglare filter layers without any attenuation. Therefore, merging CPL and TADF into a single fluorophore recently appeared as the new frontier in the design of next-generation emissive dopants for display applications.[5] Molecular designs enabling the efficient combination of those properties in an organic molecule remain scarce (see Figure 1) because of the challenge that lies in obtaining high quantum yield (Φ), low ΔEST (for TADF emission) and good chiroptical properties (gabs for absorption and glum for emission, see Supporting Information, page 18) at the same time. Hirata and coworkers were the first to report a CP-TADF pair of enantiomers in 2015, relying on a strategy where an asymmetric center is sandwiched in a TADF active donor-acceptor (D-A) chromophore.[6] These enantiomers possessing moderate quantum yields and a long delayed fluorescence lifetime were not applied as emitters in an OLED device despite their promising chiroptical properties (notably, a dissymmetry factor glum = 1.1 × 10−3 measured in toluene solution).
Figure 1.
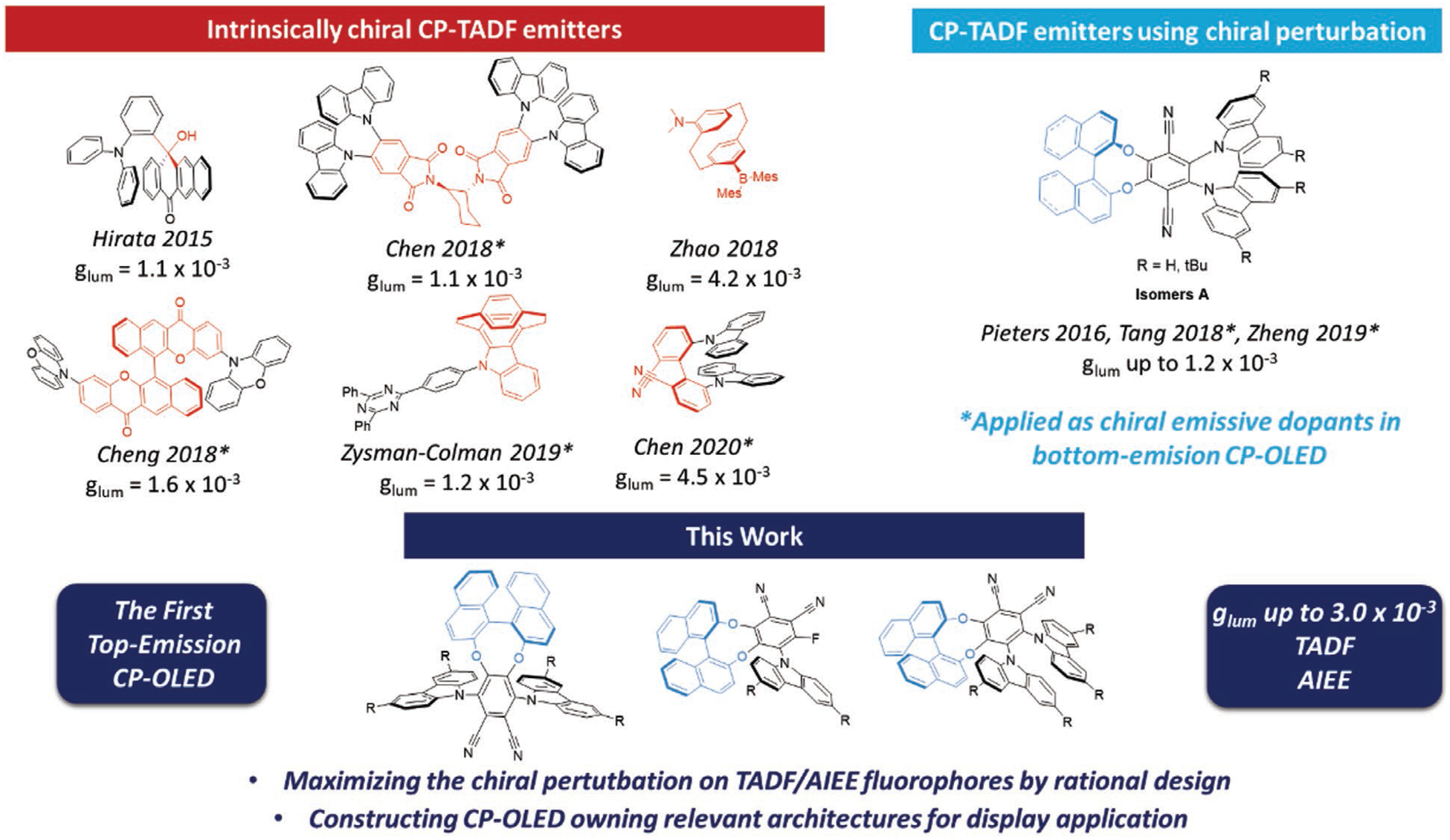
Molecular designs reported so far to construct CP-TADF molecules (glum values reported in toluene solution) and new molecular designs explored in this work.
Then, our group published a new molecular design in 2016 using the concept of chiral perturbation, by tethering an enantiopure BINOL unit to a TADF chromophore.[7] A prototype compound displaying good quantum yield in solution (53% in degassed toluene) and glum = 1.3 × 10−3 was applied as the emitter in an OLED exhibiting an external quantum efficiency (EQE) of 9.1% (see isomer A, Figure 1). The high racemization barrier of the chiral perturbing unit has allowed to maintain the optical purity of the compound during device fabrication by thermal evaporating method, demonstrating the potential of such molecules in the construction of efficient CP-OLEDs. Using this molecular design, several other research groups have synthesized chiral TADF emitters, demonstrated their aggregation induced enhancement (AIEE) properties, and used them to construct efficient bottom-emission CP-OLEDs.[8] With BINOL derivatives as the acceptor, Cheng and workers have constructed CP-TADF molecules exhibiting glum values of up to 1.6 × 10−3.[9] Another innovative molecular design involving two TADF chromophores templated by a chiral 1,2-diaminocyclohexane unit was subsequently developed by Chen and coworkers.[10] Thereafter, other intrinsically chiral TADF chromophores were reported. Zhao and coworkers reported the synthesis of chiral [2.2]-paracyclophane TADF emitters which, in spite of their attractive photophysical and chiroptical properties, were not used as emitters for the construction of CP-OLED.[11] Zysman-Colman et al. have used a chiral carbazolophane donor unit (indolo[2.2]paracyclophane) to design CP-TADF molecules showing interesting chiroptical properties (glum = 1.3 × 10−3 in toluene solution) and high electroluminescent performances (EQE up to 17%).[12] Finally, the most recent molecular design to construct CP-TADF molecules has been described by the group of Chen. Their elegant approach relies on the so-called chiral-emitting-skeleton strategy where two fluorophores with no chirality and no TADF were linked together in order to produce axially chiral blue emitting CP-TADF molecule with high glum values of 4.5 × 10−3 in solution.[13] To summarize, so far, despite their more cost and time-effective synthetic pathways, CP-TADF molecules based on chiral perturbation are still lagging behind intrinsically chiral TADF molecules, notably in terms of dissymmetry factor values (glum). Surprisingly, a systematic investigation of key electronic and structural parameters (such as isomerism and symmetry of functionalization) in CP-TADF molecules remains unexplored. With the aim to overcome this limitation, we have designed novel chiral architectures and performed the first structure-properties relationship study of this family of molecules. In this paper, we describe novel molecular designs to construct CP-TADF molecules (Figure 2) based on chiral perturbation with the idea of shortening the distance between the active chromophore and the chiral perturbing unit to enhance the chiroptical properties. Ten pairs of chiral TADF molecules exhibiting aggregation-induced enhanced emission (AIEE) were synthesized in order to evaluate the impact of isomerism and carbazole substitution on the photophysical and chiroptical properties. This first structure-properties relationship study in CP-TADF materials notably resulted in glum values up to 3.0 × 10−3, an unprecedented level for CPL molecules based on chiral perturbation strategy, providing valuable information for the development of such chiral emitters. Furthermore, we demonstrate for the first time the feasibility of top-emission CP-OLED assembly. Indeed, only bottom-emission CP-OLEDs built on glass substrates have been developed so far.[4] While these CP-OLED architectures obviously comply with the glass panel display manufacturing chain, their integration into future photonic devices may prove difficult, and even impossible for those involving microtechnologies at silicon wafer level, such as optical communication or high resolution microdisplays. To address this issue, we present an innovative top-emission CP-OLED architecture suitable for wafer level processing and enabling CP light emission. As a proof of concept, a top-emission CP-OLED integrating novel CP-thermally activated delayed fluorescence (TADF) emitters has been designed and manufactured on the basis of current high resolution microdisplay silicon wafer level technology.
Figure 2.
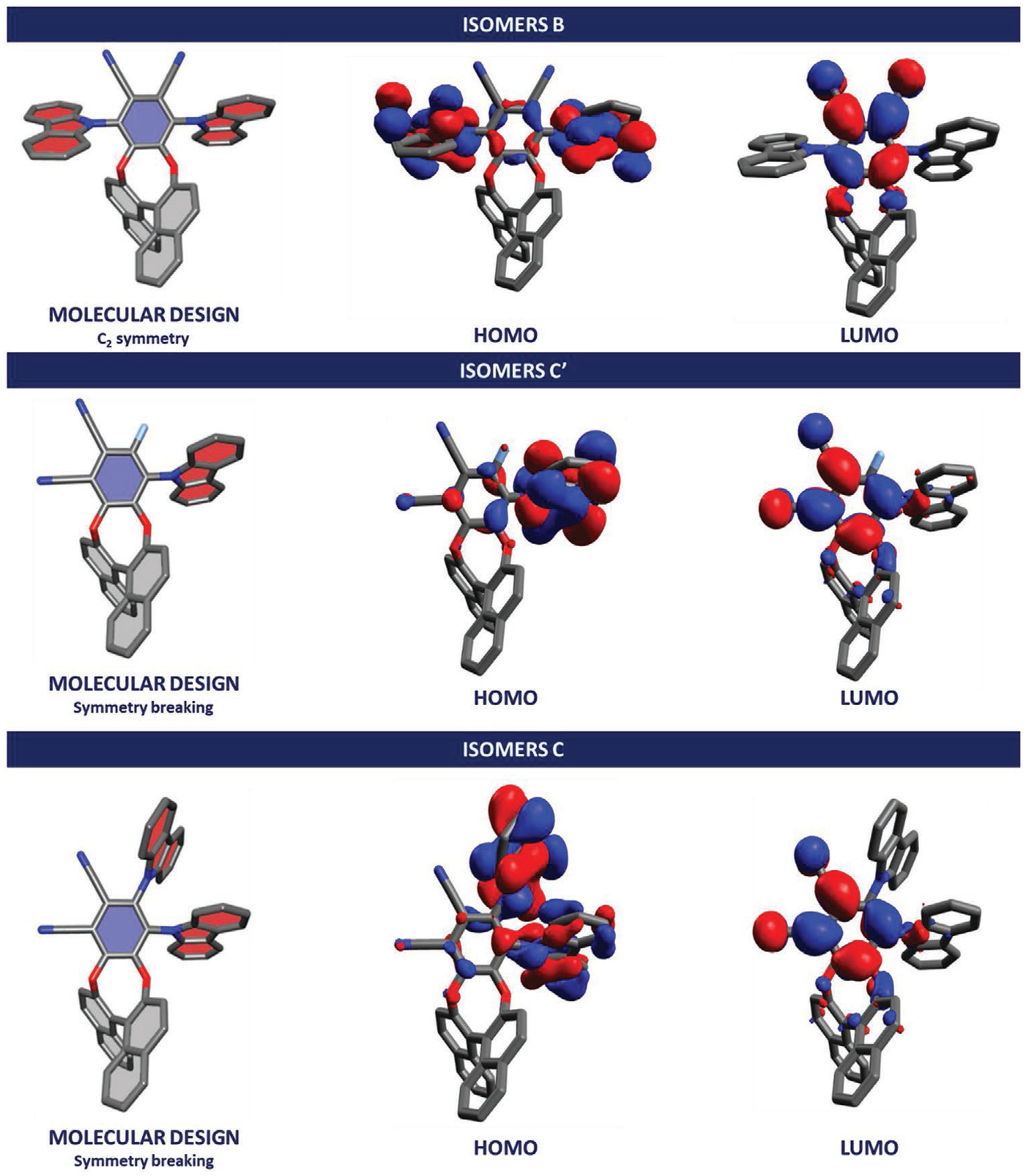
Isomers of CP-TADF molecules investigated in this study.
2. Results and Discussion
TADF molecules with CPL emission characteristics using the concept of chiral perturbation (Isomer A, Figure 1) have been developed in our group previously.[7] This powerful and flexible molecular design was then used by other groups to construct molecules exhibiting higher quantum yields or different emission colors with photoluminescence dissymmetry factors (glum) ranging from 0.5 to 1.2 × 10−3 (Figure 1).
Because the final application of these chiral emitters is related to their use as emissive dopants in CP-OLEDs, real-life benefits from these compounds can only be reached by a rational increase of their luminescence dissymmetry factors while maintaining equally high efficiency through TADF and AIEE phenomena. In this context, we have designed novel families of binaphthyl based TADF molecules which can be seen as structural isomers of the parent compounds derived from isomer A (see isomer B, C′ and C Figure 2). In the first novel molecular design, the BINOL unit is placed at position 4,5 of a 1,2-phthalonitrile unit and the electron donors at position 3,6 (isomer B, an example of this molecular design is B1, Figure 2). Compared to the initial molecular design (isomer A, see Figure 1), shortening the distance between the chiral unit to the frontier orbitals of the active chromophore was expected to enhance the effect of chiral perturbation on the TADF D-A-D system (see HOMO-LUMO representation Figure 2).
Moreover, due to the steric congestion between the D-A-D system and the chiral unit, the D-A-D system may adopt a twisted conformation (see molecular design, Figure 2) which can enhance the chiroptical properties. Geometry optimization reveals that C-H-π intramolecular noncovalent interactions between hydrogens in position 3 and 3’ of the BINOL moiety and the carbazole aromatic rings restricts free rotations in this architecture leading to a global minimum (Figure S94, Supporting Information, page 94, IGM analysis.),[14] albeit not sufficiently well as to render the twisted D-A-D system configurationally stable (see also the crystal structures of B1 Figure S102 (Supporting Information), page 102). In the second family of architectures, the chiral unit is placed at position 3,4 of the 1,2-phthalonitrile core, while acceptor and donor moieties are at position 5 for isomer C′ (an example of this design is C′1) and 5,6 for isomer C (an example of this design is C1). DFT calculations show (Figure 2) that these isomers might possess a relatively low energy difference between the triplet and singlet excited states (ΔEST) due to the important spatial separation of their HOMO and LUMO orbitals (see Figure 2).
In the case of isomers C′ (Figure 2, left) shortening the distance between the D-A active chromophore and the chiral unit compared to isomer A was expected to have a positive effect on the efficiency of the chiral perturbation from the BINOL unit onto the active fluorophore. For isomer C, although the addition of a second carbazole unit may lead to an enhancement of the photophysical properties (increasing ε value and overall rigidity of the fluorophore), this may also imply a lower efficiency of the chiral perturbation due to spatial diffusion of the HOMO orbital coefficients on the two carbazole units. Based on these novel molecular designs, we have synthesized ten pairs of enantiomers involving differently substituted carbazoles in order to test experimentally our initial hypotheses, and to study the impact of the electron donor’s nature on photophysical (QY, fluorescent decay, color emission) and chiroptical (gabs, glum) properties.
2.1. Synthesis
To synthesize derivatives of isomer B, we started from commercially available tetrafluorophthalonitrile 1 and enantiopure BINOL (R)- or (S)-2. Using already described reaction conditions, intermediates (R)- and (S)- 3 and 4 were produced through a SNAr reaction at room temperature (see Scheme 1).[7] A selectivity of 100:13 in favor of isomer 3 for this step was determined by 19F NMR (see Figure S103 in the Supporting Information).
Scheme 1.
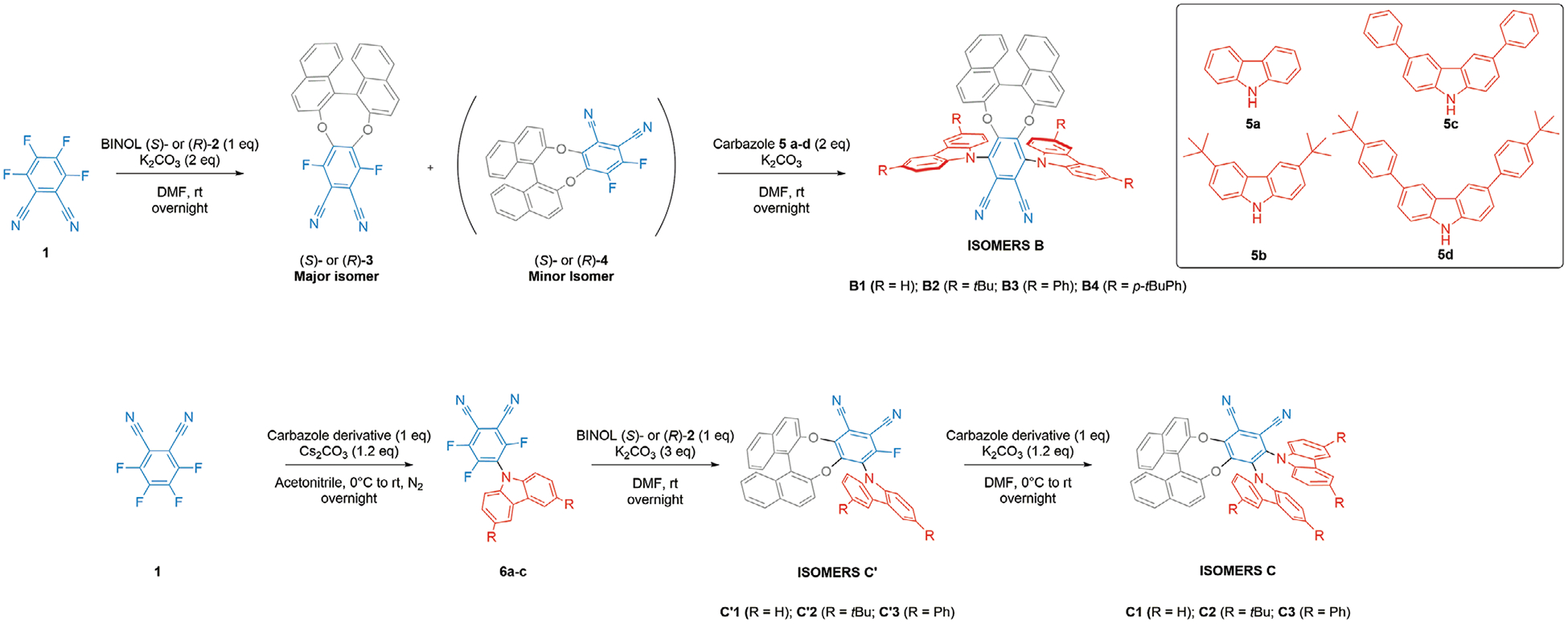
Synthesis of isomers B, C′ and C.
This regioselectivity was found to increase when lowering the reaction temperature (100:8 at 0 °C, see Figure S104 in the Supporting Information). From intermediate 3, different carbazole donors 5a-d were introduced using K2CO3 as a base and DMF as the solvent. Compounds B1–4 were thus obtained in a straightforward synthesis with isolated yields ranging from 20% to 87% depending on the nature of the carbazole moiety (see Supporting Information, page 6 for details). This difference in yields can be explained by the weak solubility of some carbazole derivatives (5d for instance), or separation issues due to coelution of impurities with the target compounds. However, all these syntheses can also be performed using a one-pot sequential approach, albeit with a slight drop in yields compared to the two-step sequences. Because the the SNAr reaction between 1 and 2 largely favors regioisomer 3, another synthetic pathway was sought out in order to obtain isomers C. The selected strategy first involved the monofunctionalisation of 1 with carbazole 5a-c (see Scheme 1,). A rapid screening of bases and solvents enabled us to obtain 6a-c as major products (see Table S1 in the Supporting Information, page 15). However, we were not able to separate these products from small amounts of unreacted tetrafluorophthalonitrile 1. Therefore, the crude product mixture was directly engaged in the second step, reacting it with enantiopure BINOL in DMF using K2CO3 as a base in order to obtain pure molecules C′1–3 in reasonable yields after flash-chromatography purification (30–55% depending on the carbazole). Finally, the synthesis of derivatives of isomers C (C1-C3), involving a final step using classical conditions for such SNAr reactions between molecules C′1-C′3 and carbazoles 5a-c, was achieved in excellent yields.
2.2. Photophysics
The different molecules were studied in toluene solution and display obvious similarity in absorption spectra, in the high-energy UV region (300–340 nm accounting for π-π transitions located on the carbazole and the binaphtyl units). Although A1, B1, C′1, and C1 feature the same components (BINOL, carbazole and phthalonitrile), significant differences due to isomerism arise on the low energy broad band spreading 340–450 nm (see Figure 3). This absorption region can be readily attributed to a charge transfer electronic transition from carbazole(s) unit(s) to phtalonitrile in comparison to isomer A1. The latter displays a well-defined absorption band centered at 395 nm, C′1 and C1 show similar behavior (respectively located at 375 and 380 nm), whereas B1 exhibits a structureless band around 345 nm. The wavelength shift of the charge transfer absorption band can be attributed to changes of the electrical transition dipolar moment between different isomers (see Table S5 in the Supporting Information, page 93).
Figure 3.
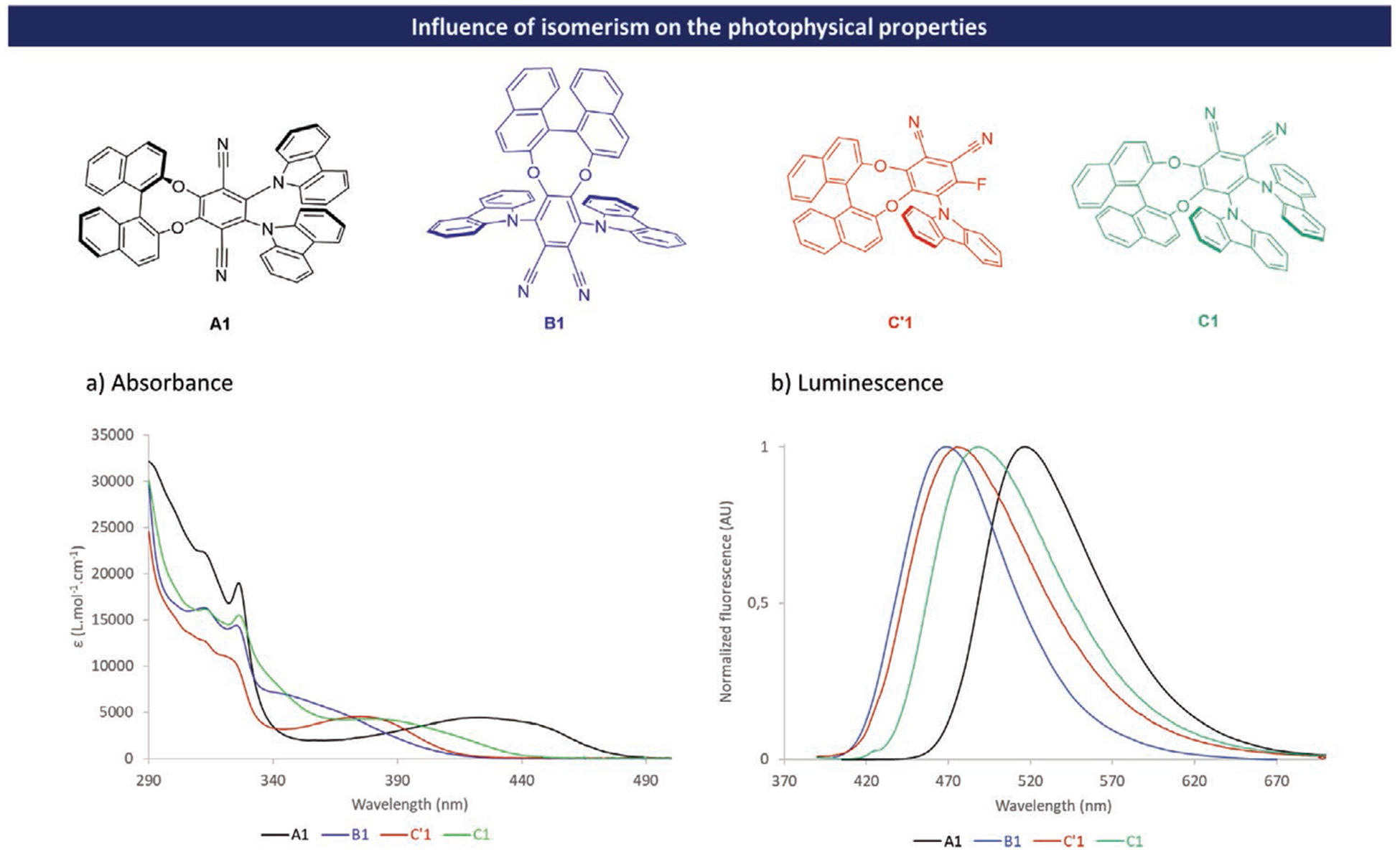
Influence of isomerism on the photophysical properties: a) absorption (C = 1 × 10−3 m in toluene); b) luminescence (C = 10 × 10−6 m in toluene); A1 in black, B1 in blue, C′1 in red, and C1 in green.
TD-DFT calculations were used to simulate the absorption spectra and to determine the MOs involved in each transition (see Figures S74–S82 in the Supporting Information). The electronic spectra and the electronic transitions were analyzed using the Multiwfn 3.7 program.[15] The broad absorption band at the longest wavelength region in compounds A1, B1, and C1 corresponds to the combination of different transitions. For A1 and C1 they originate mainly from HOMO → LUMO and HOMO-2 → LUMO excitations. For B1 it arises from HOMO → LUMO and HOMO-1 → LUMO excitations. Finally, for C′1, the absorption band originates from HOMO → LUMO excitation (see Figure S89 in the Supporting Information). These absorption bands have a π → π* nature and are caused by intramolecular charge transfer from electron donor to the phthalonitrile unit for S0 → S1 (see Figure S93 in the Supporting Information) in all compounds and for S0 → S2 in A1 and C1. In B1, upon S0 → S2 excitation a charge-transfer occurs from the electron donor to the phthalonitrile and BINOL units, while in C′1, the charge transfer occurs from the electron donor and BINOL units to the phthalonitrile unit. Compared to A1 which possesses a maximum emission wavelength at ca 520 nm, B1 possesses a significantly blue-shifted fluorescence emission with a λem max at 470 nm whereas the color emission of the C1 is situated in-between, with a maximum emission wavelength at ca. 490 nm. Because C’1 possesses a lower number of donor unit compared to C1, the HOMO-LUMO gap tends to increase, and as a result, the emission of C’1 is blue-shifted compared to the one of C1 by around 10 nm (λem max = 480 nm for C′1). In order to evaluate the impact of isomerism on the ΔEST, the energy level of S1 and T1 states were calculated using the def2-TZVP Functional with PBE0 Basis Set. Those calculations suggest that B1, C′1, and C1 exhibit quite low ΔEST values of 54 meV for B1, 13 meV for C′1, and 136 meV for C1. In contrast, the ΔEST for A1, which was already experimentally demonstrated to be TADF active,[7] reaches the value of 171 meV.
The influence of the carbazole on the photophysical properties was then studied. As expected, the addition of tBu and phenyl substituents in positions 3,6 of the carbazole units tends to increase the energy level of the HOMO resulting in a red shift of both absorption bands in the visible region and emission colors with λem max at ca 470, 490, 505, and 515 nm for B1, B2, B3, and B4 respectively (See Figure S4 in the Supporting Information). More interestingly, a dramatic impact of the substitution of the carbazole donor unit was observed on quantum yields of both prompt (ΦPF) and delayed fluorescence (ΦDF). Indeed, whereas the quantum yield of B1 was found to be relatively low in toluene solution (with a value of ΦPF around 2% measured in aerated solution), the presence of tBu in positions 3,6 of the carbazole donors in B2 gave significantly higher ΦPF with a value of 11%. Substitution by phenyl groups had an even more beneficial effect on the QY with values of ΦPF of 14% and 19% calculated for B3 and B4, respectively. Fluorescence quantum yields were also measured in the absence of triplet-quenching oxygen for a rapid evaluation of the potential TADF emission properties of molecules B1-B4. A non-significant increase of the QY was observed for B1 in degassed solution. By contrast, ΦDF (ΦDF = ΦAr–ΦO2) of respectively 3%, 7% and 11% were determined for B2, B3, and B4 respectively, when measuring their QY in the absence of oxygen. In order to investigate the emission properties of this series of compounds in solid state we prepared thin films by drop-casting toluene solutions of molecules B1-B4 as a dopants (0.1% wt) in a poly(methyl methacrylate) matrix on quartz slides. Using an integration sphere to determine the absolute QY in thin films, ΦF values of 7%, 16%, 18% and 30% were measured for B1, B2, B3, and B4, respectively. Finally, the TADF emission properties of all four compounds were confirmed by the observation of bi-exponential fluorescence decays. Generally, delayed fluorescence lifetime was found to be between 10 and 45 μs depending on carbazole substitution (see Table 1 for details and Figures S54–S63 in the Supporting Information for decays). In addition, TD-DFT calculations carried out for the optimized S1 and T1 state geometries reveal an important opposite rotation direction of the carbazole substituent group upon excitation for S1 and T1 states relative to the ground state S0 (see Figure S92 in the Supporting Information).
Table 1.
Summary of optical and chiroptical properties for the B, C′, and C isomer series.
| Compound | λabs S0→S1 [nm] | λem max [nm] | ΦF (solid) | τPF [ns]; τDF [μs] | ΔESTa) [meV] | gabs × 103 (at λabs max) | glum × 103 (at λem max) |
|---|---|---|---|---|---|---|---|
| B1 | 345 | 469 | 7 | 8; 10 | 310 (54) | 1.1 | 2.1 |
| B2 | 358 | 490 | 16 | 23; 45 | 180 (51) | 1.0 | 1.6 |
| B3 | 364 | 506 | 18 | 22; 39 | 160 (17) | 1.0 | 0.2 |
| B4 | 370 | 516 | 30 | 43; 16 | 100 (22) | 1.0 | <0.1 |
| C’1 | 375 | 481 | 29 | 10; 6 | 280 (13) | 0.9 | 1.1 |
| C’2 | 393 | 504 | 46 | 12; 19 | 130 (53) | 0.8 | 1.0 |
| C’3 | 395 | 510 | 42 | 11; 17 | 110 (9) | 0.9 | 1.1 |
| C1 | 380 | 493 | 25 | 11; 18 | 220 (136) | <0.1 | <0.1 |
| C2 | 396 | 511 | 31 | 11; 40 | 110 (70) | <0.1 | <0.1 |
| C3 | 400 | 519 | 47 | 15; 22 | 100 (75) | 0.7 | 0.7 |
Estimated experimental ΔEST measured in 2-methyltetrahydrofurane at 77 K (see Figures S17–20 and Table S2, Supporting Information) and theoretical values calculated using the def2-TZVP Functional with PBE0 Basis Set (in brackets).
Influence of the carbazole substitutions on the photophysical properties of series C’ was also evaluated (See Figure S5 in the Supporting Information). As expected, introduction of tBu and phenyl substituents led to a red shift of the visible region absorption bands (see Figure S2 in the Supporting Information) corresponding to the HOMO-LUMO transition. Such a red shift is also observed on the maximum emission wavelengths (λem max = 480 nm for C′1, 504 nm for C′2 and 510 nm for C’3, See Figure S5 in the Supporting Information). Molecule C′1 has a ΦPF of 14% and a ΦDF of 6% with a QY reaching almost 20% in degassed toluene solution. Similar values were obtained for C′2 and C′3 with a highest value recorded for C′3 with a QY of 22% in degassed toluene solution. Interestingly, the QY measured in thin films were found to significantly increase to 29%, 46% and 42% for C′1, C′2, and C′3 respectively. These striking differences between QY values in solution and in thin films might be explained by restrained molecular motion in the polymer matrix. Compared to isomers A, B and C (where differences between QY in degassed toluene solutions and in thin films are much less pronounced), the donor unit in the C′ series possesses a much higher degree of freedom due the lower steric congestion. Therefore, for isomers C′, the conformational disorder leading to nonradiative processes during relaxation of the excited state in solution might be limited in the thin film state. Finally, measurement of fluorescent decays has confirmed TADF emission of the molecules in this series, with τDF = 6, 19, and 17 μs for C′1, C′2, and C′3, respectively.
For Isomers C (See Figure S6 in the Supporting Information), as in the preceding series, a red shift of the emission bands is observed when tBu and phenyl substituents are incorporated in positions 3,6 of the carbazole moiety. Interestingly, the measured values of ΦDF were much higher in this series than for the C’ series. Indeed, while ΦPF exhibit very similar values of QY ranging from 13% to 20% when compared to isomers C′, ΦDF tend to be higher in the C series, reaching values of 6%, 7% and 20% respectively for C1, C2, and C3. In thin films, these relatively high quantum yields were confirmed, as well as the TADF emission, with τDF comprised between 18 and 40 μs.
Compounds derived from isomer A were shown to display aggregation induced emission enhancement properties.[8] In order to evaluate the potential AIEE properties of the novel B, C′, and C isomers, we have studied the fluorescence emission of each compound in solution as a function of the proportion of water (fw) in THF. For every compound, in THF/water mixtures of up to fw = 0.5, we observed a decrease of the emission compared to the initial conditions (fw = 0). We then observed an upsurge of fluorescence for fw > 0.6 (see Figures S36–S46 in the Supporting Information). In all cases, the novel isomers B, C’ and C display AIEE properties, with a particularly pronounced increase of fluorescence intensity in the case of C′1, with an I/I0 ratio of 5.6.
2.3. Chiroptical Properties
As mentioned in the introduction, one of our objectives in this study was to evaluate the influence of isomerism on the chiroptical properties of the title TADF emitters. At the ground state, the efficiency of the chiral perturbation provided by the BINOL unit can be evaluated looking at the dissymmetry factor gabs values corresponding to the S0 → S1 transition (see Figure 4). Comparison of these values for A1, B1, C′1, and C1 highlights striking differences (see Figures S74, S75, S79, and S82 in the Supporting Information). For isomer A, a gabs value of 0.53 × 10−3 was measured at the λmax of the S0 → S1 transition (situated around 430 nm). For B1, theoretical computations show that the charge-transfer band corresponds to an overlap of two transitions (S0 → S1 and S0 → S2) that can be experimentally observed thanks to the Cotton effect at 390 nm (See ECD spectra of B1 Figure S22 in the Supporting Information). The gabs value can be evaluated around 3 × 10−3 at 400 nm for S0 → S1, whereas it only reaches 0.8 × 10−3 (370 nm) for S0 → S2. The high value of gabs for S0 → S1 highlights the efficiency of the chiral perturbation at fundamental state for this molecule. In the case of isomer C, DFT calculations have shown that the HOMO possesses a higher orbital coefficient on the farthest carbazole from the BINOL.
Figure 4.
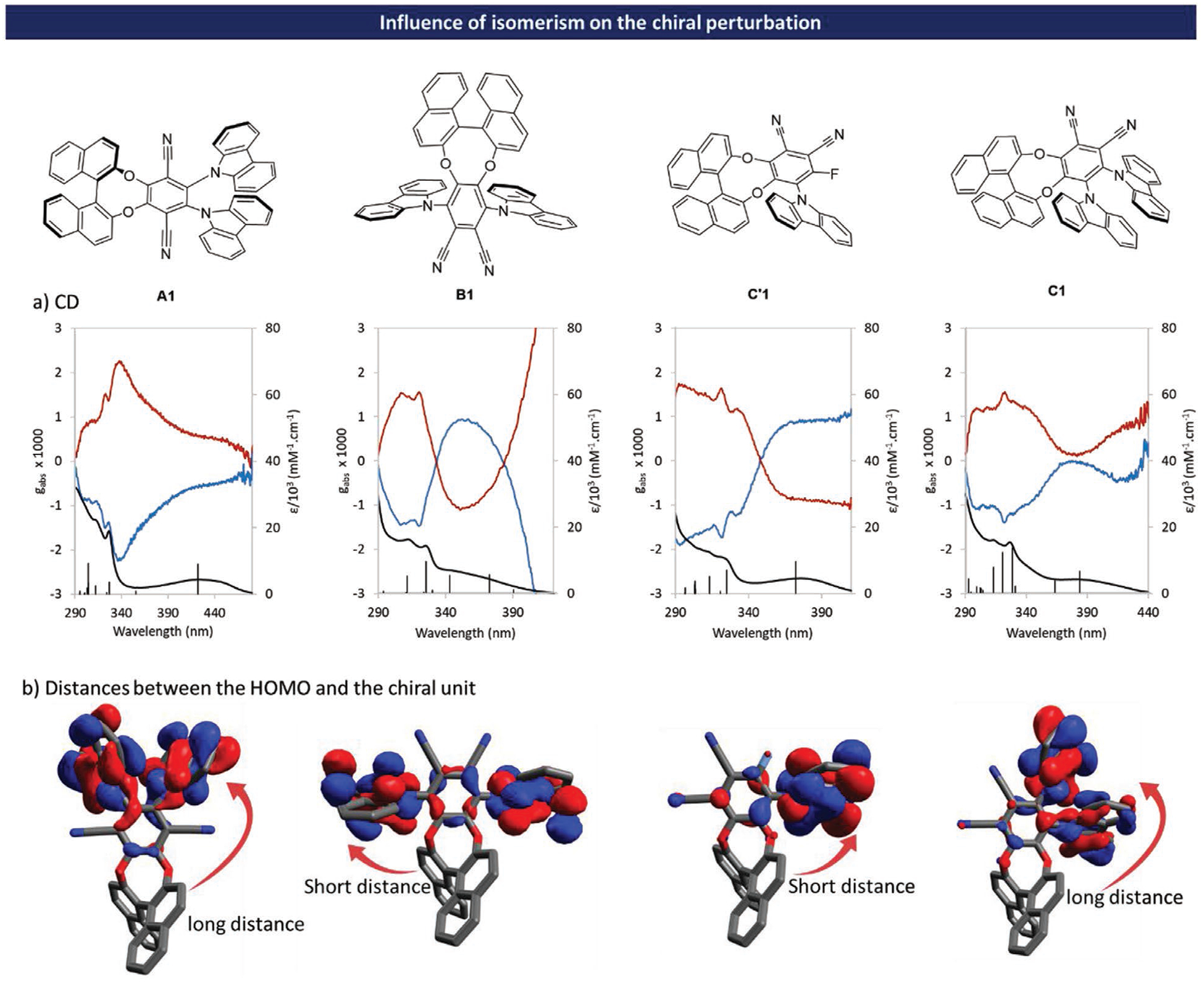
Influence of isomerism on chiroptical properties: a) UV (in black) and gabs (color) spectra measured in toluene at 1 × 10−3 m, oscillator strengths are plotted to visualize transitions but are not scaled. For gabs = f (λ) blue traces correspond to molecules with (R)- configuration and red traces correspond to molecules with (S)-configuration; b) HOMO of the four different isomers.
This means that the barycenter of the active chromophore in C1 is further away from the chiral unit than in C′1. For this reason, the dissymmetry factors for C1 are much lower in the charge transfer area, ranging between 0 and 0.8 × 10−3 (depending on the transition observed), than the ones measured for C′1, which reach 1 × 10−3.
In order to have a deeper understanding of the parameters governing the efficiency of the chiral perturbation, the magnitude of magnetic and electric transition dipole moments (respectively μm and μe) and their relative orientations for the S0 → S1 and S0 → S2 (see Tables S5 and S6 in the Supporting Information) transitions was determined computationally for all isomers. Indeed, theoretically, chiroptical properties are related to μm and μe, as well as θ, the angle between these vectors ( Tables S5 and S6, Supporting Information).[16] This calculated value enables us to understand the influence and efficiency of the transition responsible in the first transition observed. Owing to the C2 symmetry, a parallel orientation of μm and μe (z axis) is obtained for the S0 → S1 transition (Figure 5) in A1 and B1 with a cancellation of components in the other directions (i.e., the x- and y axis). For this reason, designs based on isomers A and B display an optimal orientation of transition dipole moments to maximize the chiral perturbation and therefore the chiroptical properties (with a value of θ = 0° for A1 and θ = 180° for B1).
Figure 5.
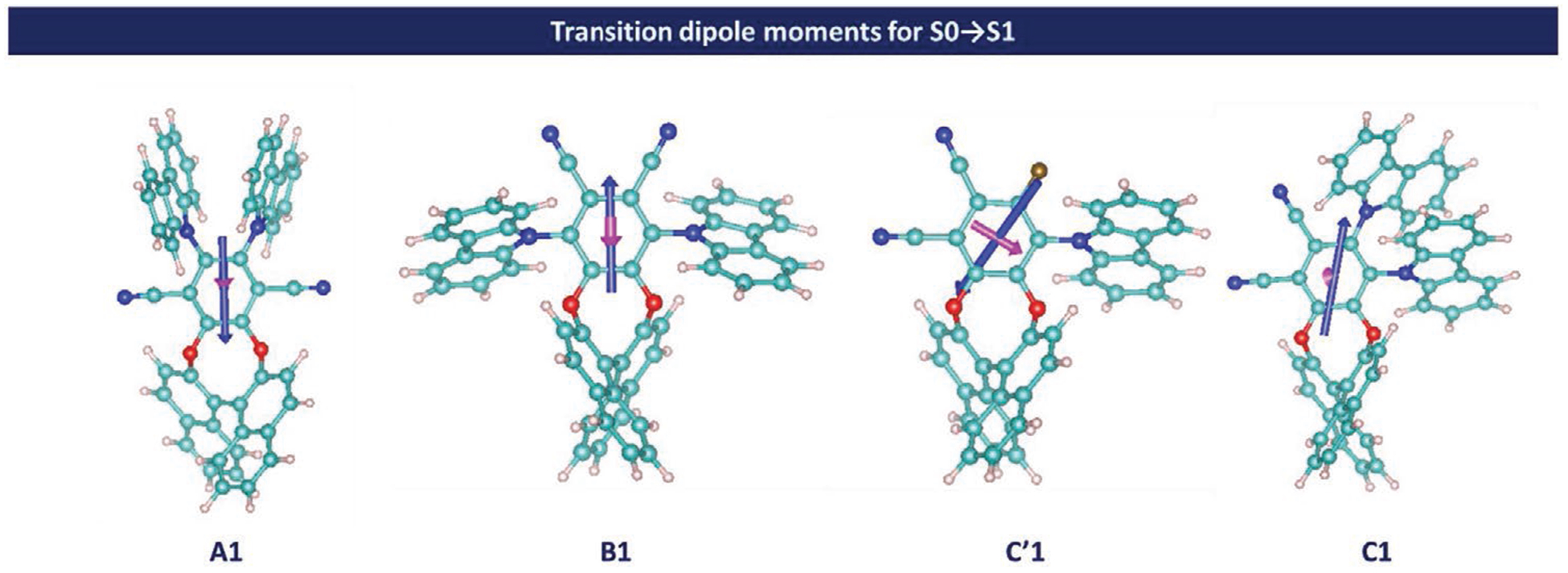
Schematic representation of electric (blue) and magnetic (magenta) transition dipole moments of the S0 → S1 transition.
The higher value of gabs for B1 compared to A1 accounts for a higher value of |μm| and a lower value of |μe| (see Table S5 in the Supporting Information). For C’1 and C1, the relative orientation of the transition dipole moments are less favorable to maximize the g values (θ = 69° for C1 and θ = 94° for C′1). However, for C′1, the magnitude of the electric and magnetic dipole transition moment vectors |μe| and |μm| are enhanced by factors of 2.9 and 3.8 respectively compared to B1. That is the reason why gabs values corresponding to S0 → S1 transition remain relatively high for C’1. Despite of the more favorable orientation of the electric and magnetic dipole transition moment vectors, the very low value of |μm| explain the experimental value of gabs for C1.
In order to study the impact of isomerism on the chiroptical properties at the excited states, the glum values have been plotted as a function of wavelength (see Figure 6).
Figure 6.
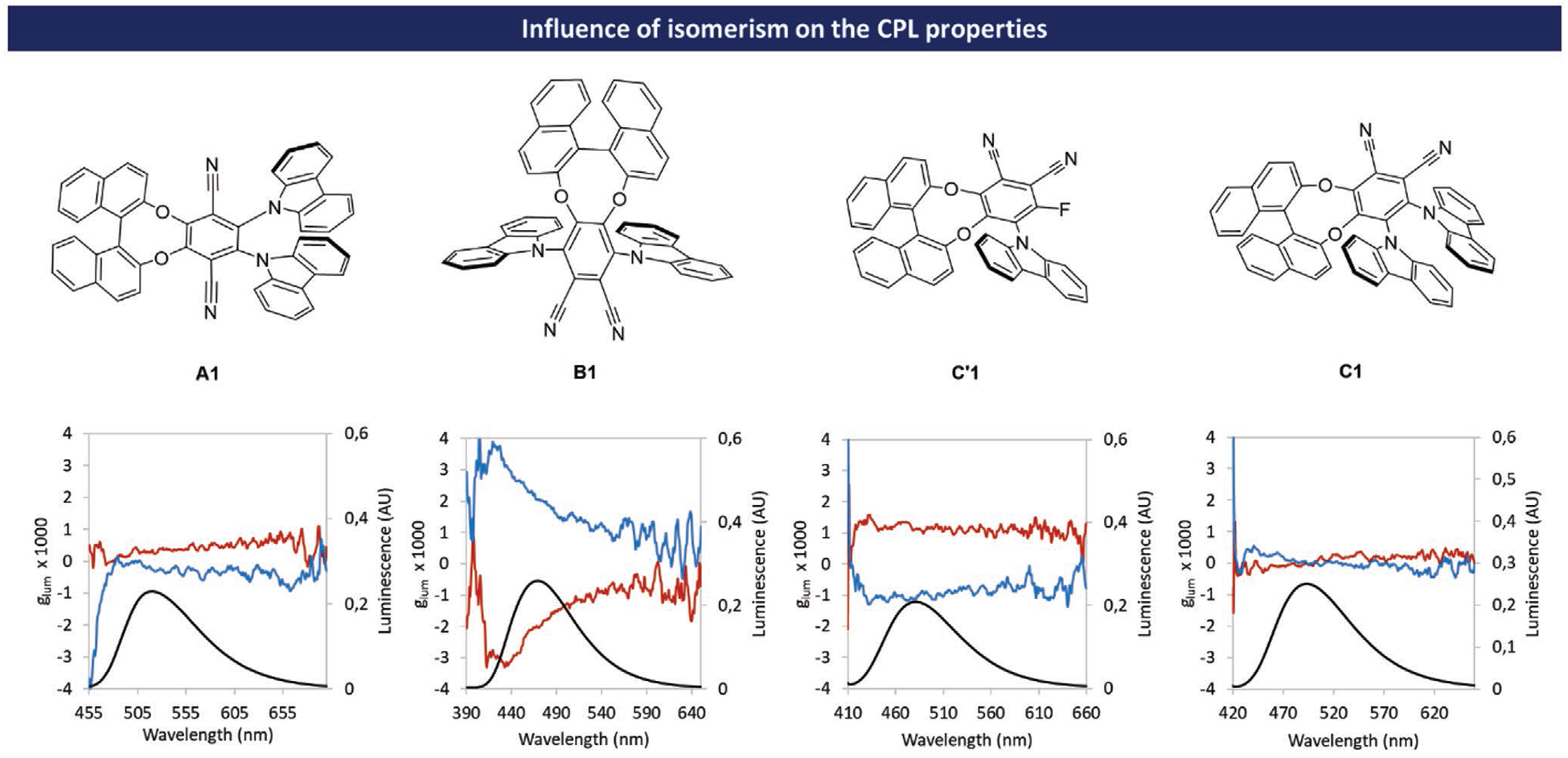
Influence of isomerism on the chiroptical properties at the excited state. glum = f (λ) in toluene solution, blue traces correspond to molecules with (R)- configuration and red traces correspond to molecules with (S)-configuration; black traces correspond to fluorescence spectra.
For A1, glum remains almost constant along the emission band centered at 520 nm with a value of 0.4 × 10−3.[17] Surprisingly, for isomer B1, the glum value tends to decrease with the emission wavelength. Relatively high values of glum were measured with a maximum of 3.0 × 10−3 at the beginning of the emission band and a value of 2.0 × 10−3 at 472 nm (λem max). For isomer C′1 the values of glum remain roughly constant along the emission band centered at 493 nm with a value of 1.0 × 10−3. Isomers C1 shows very low values of glum nearing zero at λem max with an unusual bisignate pattern. The influence of the carbazole substituents on chiroptical properties was also studied. This influence was found to be extremely important in the isomer B series (see Figure 7 and simulated ECD spectra Figures S84–S87 in the Supporting Information).
Figure 7.
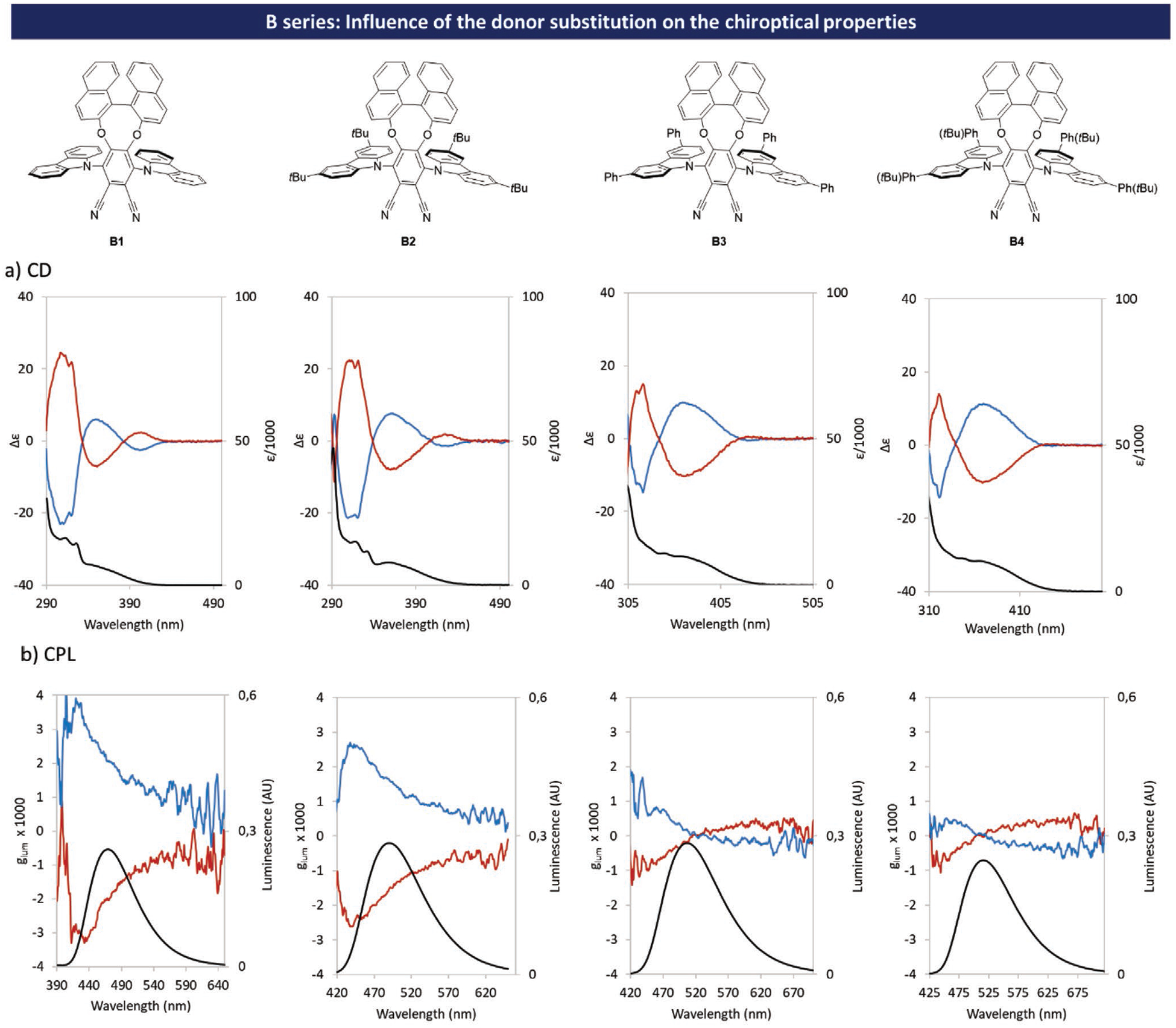
Influence of carbazole substitution on chiroptical properties in the isomer B series: a) absorbance (black trace) and CD (measured at C = 1 × 10−3 m in toluene); b) CPL and fluorescence (fluorescence spectra measured at optical density near 0.2 in toluene); blue traces correspond to molecules with (R)-configuration and red traces correspond to molecules with (S)-configuration.
While the addition of tBu substituents did not drastically change the shape of either gabs or glum as a function of the wavelengths, replacing them with phenyl groups led to a significant change in terms of chiroptical properties at the fundamental and excited states. Whereas for S0 → S2 transitions gabs values remain almost the same for all molecules in the B series, we can observe a disappearance of the Cotton effect on ECD spectra for B3 and B4. This suggests that substitution of electron donor units by phenyl groups is detrimental to the efficiency of the chiral perturbation, as was confirmed by the lower value of |μm| calculated for S0 → S1 transition of B4 compared to B1 (see Table S5 in the Supporting Information). This can also be seen in CPL measurements. In contrast to B1 and B2, glum = f (λ) curves of B3 and B4 adopt a sigmoidal shape (leading to a sign inversion of CPL along the emission band) and relatively lower intrinsic glum values between + 0.75 × 10−3 and − 0.5 × 10−3 for (R)-B3 and + 0.5 × 10−3 and − 0.5 10−3 for (R)-B4) are observed. Although glum values are relatively low, these two compounds exhibit a quite rare CPL sign modulation as a function of the emission wavelength. Because B1 exhibits the highest value of glum in this series, we have synthesized an analogue B1r with an 5,5,6,6,7,7,8,8-octahydro-1,1-bi-2-naphthol moiety as chiral unit (see Figures S95–S97 in the Supporting Information). This structural modification did not lead to significant changes in both optical and chiroptical properties.[8b–c]
For the C′ series the influence of the carbazole substituents on the chiroptical properties was found to be negligible (see Figures S26–S28 in the Supporting Information). Indeed, compounds C′2 and C′3 bearing tBu or phenyl groups respectively in positions 3,6 show quite similar chiroptical properties to those of the parent molecule C′1, with gabs values of around 0.8 × 10−3 at λabs max for S0 → S1 transition, and glum values of 1.0 × 10−3 at λem max. Indeed, theoretical investigations show that the nature of the substituent on the carbazole has little influence on the relative orientation and magnitudes of |μe| and |μm| (see Table S5 in the Supporting Information).
On the other hand, in the C series, the introduction of phenyl groups in position 3,6 of the carbazoles leads to an important enhancement of chiroptical properties as seen in molecule C3 (see Figure 8).
Figure 8.
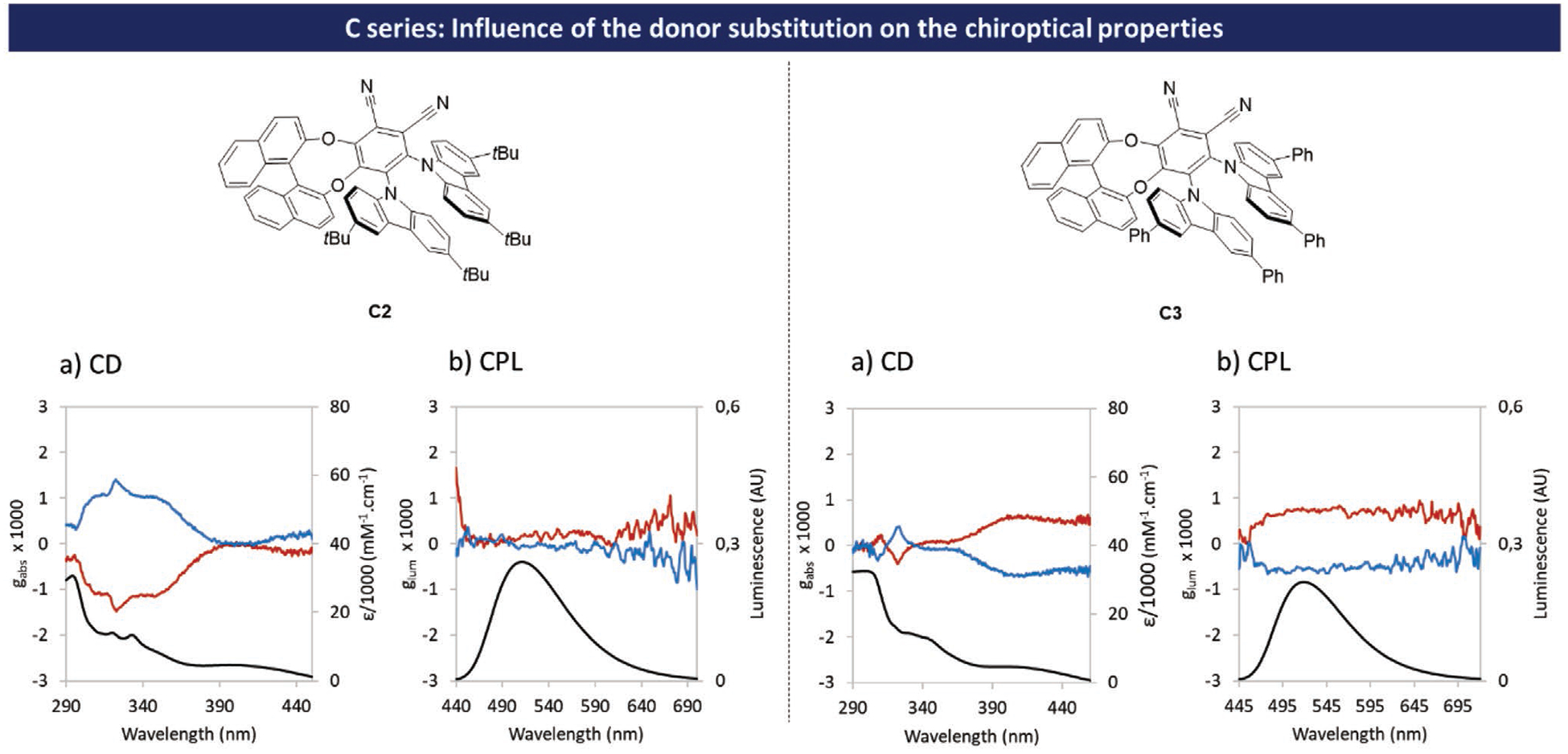
Influence of carbazole substitution in the isomer C series on chiroptical properties in toluene solutions (fluorescence and CPL spectra measured at optical density near 0.2; UV and CD spectra measured at 1 × 10−3 m; black traces correspond to absorbance or fluorescence (for CD and CPL respectively), red traces correspond to molecules with (S)-configuration and blue traces correspond to molecules with (R)-configuration.
Indeed, while the introduction of tBu groups did not drastically change the values of chiroptical properties of C2 versus C1, molecule C3 exhibits much higher gabs and glum values (gabs = 0.7 × 10−3 at λabs max S0 → S1 transition and glum = 0.7 × 10−3 at λem max). This difference can be explained by a change in the orientation of the transition dipole moments. Indeed for C3, the angle θ between |μe| and |μm| for S0 → S1 transition was calculated to be 103°, much higher than that of C1 (≈69°) and C2 (≈49°) leading to a sign inversion observable on the ECD spectra in the S0 → S1 transition region.
Optical and chiroptical properties of the ten new enantiomer pairs are reported in Table 1. Excitation of these compounds in the UV region covers the blue to green area in the visible spectrum, exhibiting TADF properties and quantum yields of up to 47% in the thin film state. Moreover, eight of them display relatively important signals in ECD on the S0 → S1 transition, as well as circularly polarized luminescence. glum values range from 0.2 × 10−3 for compound B3 to 3 × 10−3 for compound B1, the latter currently holding the highest value measured for chiral perturbation in a small organic compound.
These results demonstrate the important impact of both isomerism and carbazole substitution on the chiroptical properties of such chiral D-A chromophores. They highlight the high potential for these novel molecular designs based on isomers B and C′ to imbue TADF molecules with valuable chiroptical properties.
2.3.1. Top-Emission CP-OLED Devices
So far, CP-OLEDs demonstrated by various research groups have exclusively focused on bottom-emission devices.[4] Despite very promising chiroptical characteristics as well as emission efficiencies, these devices are unfortunately unsuitable for current microelectronics technologies based on silicon wafer processing, which limits their application to large glass-panel displays. For instance, the bottom-emission CP-OLED technology does not allow the manufacturing of high-resolution displays that require depositing arrays of very small pixel pitch (typically less than 10 μm) onto a silicon-based CMOS driven integrated circuit used to control the emission of each individual OLED pixel for efficient video stream monitoring. As a result, only technologies based on the processing of top-emission OLEDs should be considered for these applications. In a top-emission OLED, the electroluminescent light is subjected to various multi-reflections inside the OLED stack (see Figure S98 in the Supporting Information) making the control of emitted light polarization particularly challenging. That is why our purpose here is mainly assessing the feasibility of a top-emission CP-OLEDs through proofs of concept, rather than targeting maximum CP-OLED emission current efficiency and EQE. Because molecule C′3 presents the best compromise between optical (ΦF, ΔEST) and chiroptical properties (glum = 1.1 × 10−3), it was used as the emitter to build the first top-emission CP-OLEDs. Several CP-OLEDs have thus been processed for this study. Details of the OLED processing procedure are provided in the Supplementary Information. The typical OLED stack integrating C′3 chiral molecules is presented in Figure 9a. Figure 9c,d summarize the typical optoelectronic characteristics obtained with top-emission CP-OLEDs integrating either (S)-C′3 or (R)-C′3 chiral dopants. The emission spectra of these CP-OLEDs (induced by the chiral dopant) show green emission both centered at λpeak = 510 nm (Figure 9). The corresponding I–V–L characteristics denote a clear rectifier diode behavior, low leakage current (≤1 μA cm−2) and luminance performances of 6000 cd m−2 under 11V for the device involving the (R)-C′3 chiral dopant. Overall, the OLED current efficiency can reach 2.5 cd A−1 with a corresponding external quantum efficiency EQE = 0.8% in the nominal luminance operating range of a display (500–1000 cd m−2).
Figure 9.
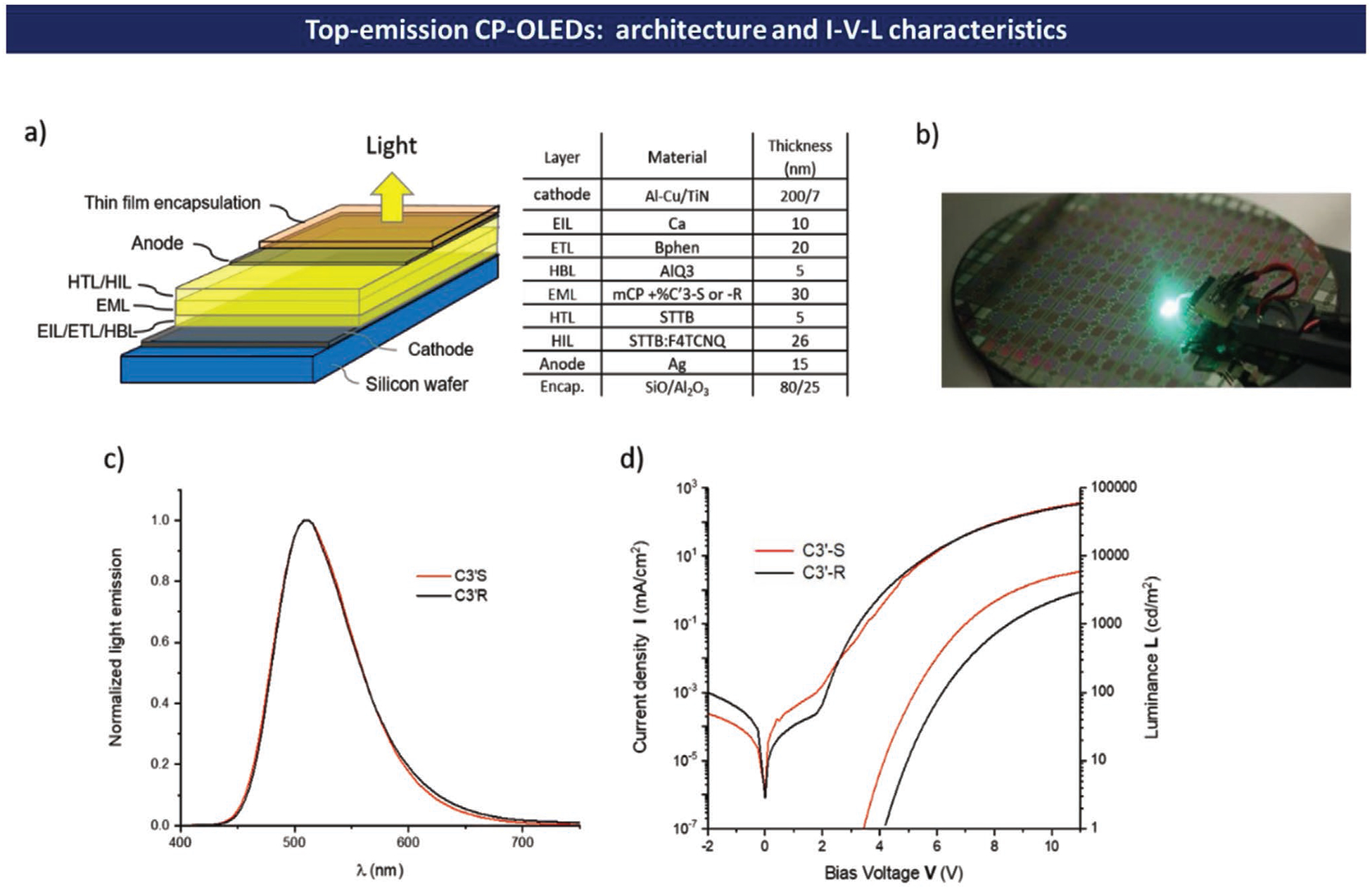
a) Typical thin film stack used to build a top-emission CP-OLEDs with the (R)-C′3 and (S)-C′3-S chiral dopants synthesized in this work; b) 8-inch wafer level-processed CP-OLEDs showing an individual CP-OLED under test; c) Light emission spectrum of CP-OLEDs integrating either (S)-C′3 or (R)-C′3 chiral dopants; d) Corresponding I–V–L characteristics.
Chiroptical properties of these devices were measured in a second step using a CPL spectrometer. SiO/Al2O3-encapsulated single layers of pure chiral dopant (30 nm-thick, glass substrate) and corresponding CP-OLEDs were systematically compared. The most important result highlighted in Figure 10 is that the maximum dissymmetry factor |g| first measured on neat dopant single layers (|glum| = 1.2 × 10−3) is globally preserved after integration of the same dopant into a top-emission OLED (|gEl| = 1.0 10−3).[18] Chiroptical properties at the excited state were measured on the same apparatus for three different states (toluene solution, pure thin film and dopant in top emission OLED), and no significant difference in the g values (glum and gEL) was observed.
Figure 10.
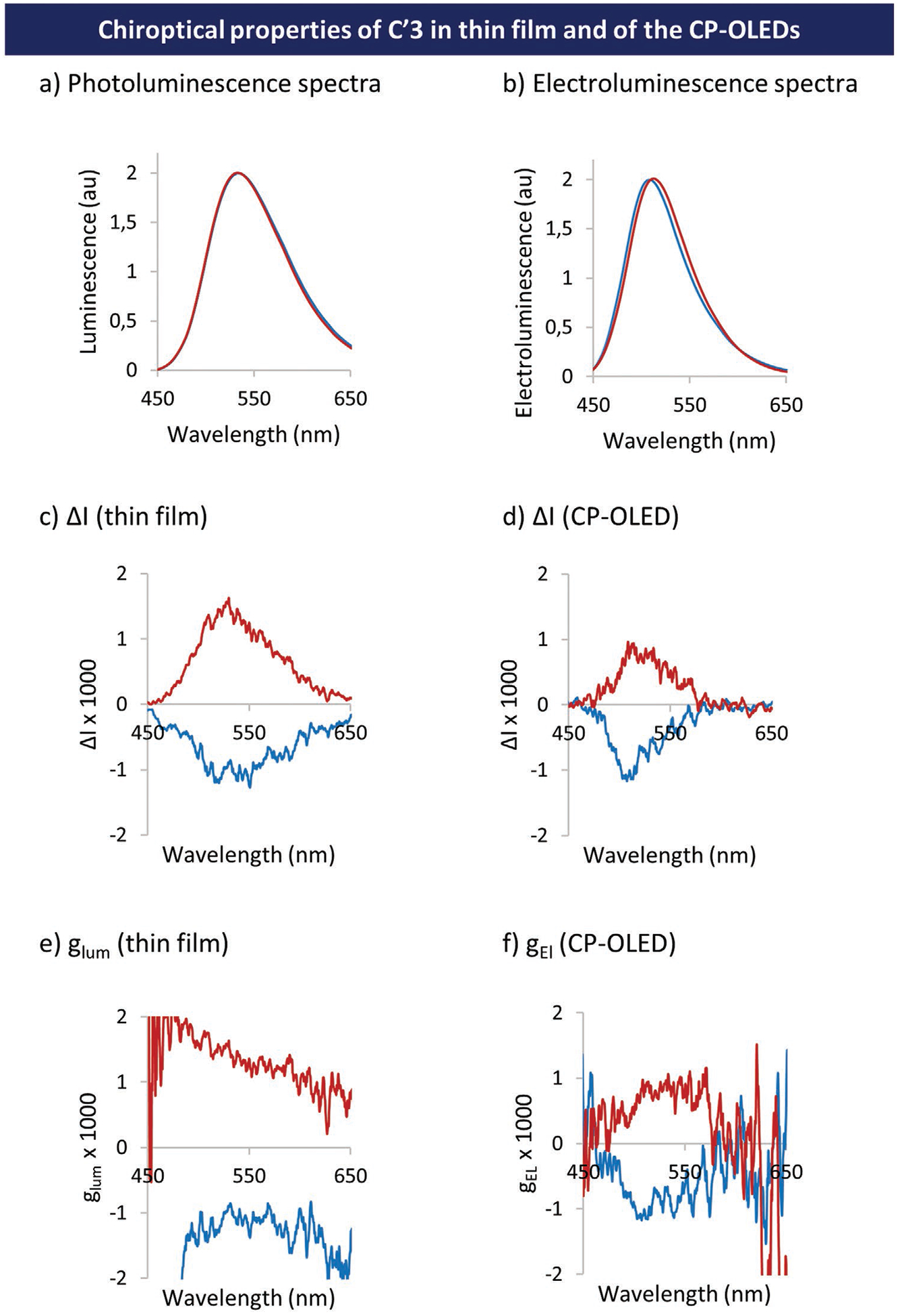
a) Photoluminescence response of single layers of pure C′3; b) electroluminescence response of the top-emission CP-OLEDs; c) ΔI = f (λ) curves related to single layers of pure C′3; d) ΔI = f (λ) curves related to the electroluminescence generated by the CP-OLEDs; e) glum = f (λ) curves obtained for single layers of pure C′3; f) gEl = f (λ) curves related to the electroluminescence generated by the top-emission CP-OLEDs; red traces correspond to molecules with (S)-configuration and blue traces correspond to molecules with (R)-configuration.
This result clearly demonstrates for the first time the possibility of manufacturing top-emission CP-OLEDs able to preserve ~80% of the circular polarization induced by the chiral emitter dopant. Because of the particular optical behavior of top-emission OLED stacks, the emission spectra I(λ) and the dissymmetry factor spectra g(λ) of single layers of pure chiral dopant (C′3) and CP-OLED are different, and CP-OLED spectra appear systematically narrower. This behavior was expected since the OLED stack, with its two reflective electrodes, behaves as a selective optical band pass filter which reduces the OLED emission spectrum bandwidth. The central wavelength of such band pass filters depends on the thickness of the organic stack and, to a lower extent, on the thickness of the semi-transparent electrode. Ideally, the organic stack thickness must be thoroughly tuned so that the central wavelength perfectly matches the emission peak of the single EML layer. This optical tuning is very sensitive to any layer thickness error since a deviation of only 1 nm can induce up to a ≈5 nm spectral shift. In these first CP-OLED experiments, the optical cavity thickness was not fully optimized, hence the slight shift between the emission peaks of the single layer (λpeak = 532 nm) and the CP-OLED (λpeak = 510 nm). These first proofs of concept show that top-emitting CP-OLEDs could be indeed developed for high resolution microdisplay applications. However, they do not match so far the OLED specifications for microdisplays where at least 10 times higher current efficiency (≥20 cd A−1) and EQE (≥10%) are generally expected in the display nominal luminance operating range. Considerable room for improvement exists including i) the use of a host matrix providing much more efficient charge injection (mCP is rather resistive) and lower emission voltage threshold matching the capacities of CMOS driven integrated circuits (typically 2.5 V), ii) higher chiral dopant concentration for enhanced luminance, and iii) to a lower extent, a better alignment of the OLED optical cavity. Indeed, unlike bottom-emission OLEDs, the top-emission OLED architecture induces light spectral filtering (optical cavity effect) which reduces the light extraction efficiency. Calculations detailed in the supporting information (see Figure S99 in the Supporting Information) show that a better cavity alignment would have increased the emission intensity of our CP-OLEDs by around 15%.[19]
3. Conclusion
To summarize, we have described several innovative molecular designs of CP-TADF emitters using the chiral perturbation strategy. This first systematic study of the structure-(chiro) optical properties relationship focuses on the impact of isomerism of the electron donor relative to the chiral fragment, with the aim of identifying the key parameters that govern the absorption and luminescence dissymmetry factors (gabs and glum) in these TADF and AIEE emitters. For each design, at least one carbazole unit is placed next to the binaphthyl unit to maximize the chiral perturbation efficiency. Among them, molecules derived from the C′ series possess interesting ΦF (solid) including a TADF component, and good glum values, up to 1.1 × 10−3 in toluene solution, due to a high magnetic dipole moment, |μm| (for the S0 → S1 transition). Increasing the electron density on the carbazole donor using tBu, phenyl or tBu-phenyl groups successfully enhances TADF efficiency without impacting the intensity of CPL. For the C series, adding one additional carbazole unit dramatically decreases the chiroptical properties due to an unfavorable orientation of the electric and magnetic dipole moments. Such an orientation can however modulate the electron density on both carbazole fragments (C3), resulting in higher values of dissymmetry factors of up to 0.62 × 10−3 for gabs and 0.66 × 10−3 for glum. Finally, having both electron donors close to the chiral unit in the B series affords the best chiroptical properties for a chiral perturbation-based molecule, despite moderate quantum yields. Indeed, a glum value up to 3.0 × 10−3 was recorded for B1, due to a favorable orientation of the transition dipole moment, together with high values of |μm|. However, modulating the electron density on carbazole fragments (B3, B4) is detrimental to CPL properties in this series, even though optical properties are still enhanced significantly.
Overall, this experimental and theoretical study shows that the interplay between TADF and CPL properties is far from trivial and proves difficult to anticipate. Our investigation notably highlights the importance of maximizing the value of |μm| and the resulting angle between the electric and magnetic dipole moments, θ, to find the best compromise between TADF and CPL efficiencies. In terms of applications, we have achieved the first proof of concept of circularly polarized top-emission OLEDs. The proposed device architecture as well as its manufacturing technology were shown to preserve 80% of the initial circular polarization of chiral emitters. Moreover, these devices meet the requirements of silicon-based high-resolution microdisplay technology, paving the way to other microelectronics-related photonics applications. Ongoing work is dedicated to performance improvements of top-emission CP-OLEDs, notably in order to optimize the stack and find new solutions to limit the reflection of light inside these devices to improve the degree of electroluminescence polarization.
Supplementary Material
Acknowledgements
G.P. thanks the SCBM, the Labex CHARMMMAT (ANR-11-LABX-0039), the “Programme Transverse de Competences du CEA” (POLEM project), and the ANR (iChiralight project, ANR-19-CE07-0040) for funding and support. David Buisson, Elodie Marcon, Céline Chollet, Amélie Goudet, and Sabrina Lebrequier are also warmly acknowledged for their contribution in this work (HPLC analysis and purification). G.M. thanks the NIH, Minority Biomedical Research Support (Grant No. 1SC3GM089589-08), and the Henry Dreyfus Teacher-Scholar Award for financial support. G.C. and G.P. thanks Arnaud Brosseau for his precious help for fluorescent decay measurements.
Footnotes
Supporting Information
Supporting Information is available from the Wiley Online Library or from the author.
Conflict of Interest
The authors declare no conflict of interest.
Contributor Information
Lucas Frédéric, Université Paris-Saclay, CEA, INRAE, Département Médicaments et Technologies pour la Santé (DMTS) SCBM, Gif-sur-Yvette F-91191, France.
Alaric Desmarchelier, Université Paris-Saclay, CEA, INRAE, Département Médicaments et Technologies pour la Santé (DMTS) SCBM, Gif-sur-Yvette F-91191, France.
Romain Plais, Université Paris-Saclay, CEA, INRAE, Département Médicaments et Technologies pour la Santé (DMTS) SCBM, Gif-sur-Yvette F-91191, France.
Leonid Lavnevich, Université Paris-Saclay, CEA, INRAE, Département Médicaments et Technologies pour la Santé (DMTS) SCBM, Gif-sur-Yvette F-91191, France.
Gilles Muller, Department of Chemistry, San Jose Staté University, One Washington Square, San José, CA 95192-0101, USA.
Cassie Villafuerte, Department of Chemistry, San Jose Staté University, One Washington Square, San José, CA 95192-0101, USA.
Gilles Clavier, Université Paris-Saclay, ENS Paris-Saclay, CNRS, PPSM, Cachan 94235, France.
Etienne Quesnel, Université Grenoble-Alpes, CEA, LETI, MINATEC Campus, 17 rue des Martyrs, Grenoble 38054, France.
Benoit Racine, Université Grenoble-Alpes, CEA, LETI, MINATEC Campus, 17 rue des Martyrs, Grenoble 38054, France.
Sylvia Meunier-Della-Gatta, Université Grenoble-Alpes, CEA, LETI, MINATEC Campus, 17 rue des Martyrs, Grenoble 38054, France.
Jean-Pierre Dognon, Université Paris-Saclay, CEA, CNRS, NIMBE, Gif-sur-Yvette 91191, France.
Pierre Thuéry, Université Paris-Saclay, CEA, CNRS, NIMBE, Gif-sur-Yvette 91191, France.
Jeanne Crassous, Université de Rennes, CNRS, ISCR – UMR 6226, Rennes 35042, France.
Ludovic Favereau, Université de Rennes, CNRS, ISCR – UMR 6226, Rennes 35042, France.
Grégory Pieters, Université Paris-Saclay, CEA, INRAE, Département Médicaments et Technologies pour la Santé (DMTS) SCBM, Gif-sur-Yvette F-91191, France.
References
- [1].Wong MY, Zysman-Colman E, Adv. Mater 2017, 29, 1605444. [DOI] [PubMed] [Google Scholar]
- [2].Im Y, Kim M, Cho YJ, Seo J-A, Yook KS, Lee JY, Chem. Mater 2017, 29, 1946. [Google Scholar]
- [3].Uoyama H, Goushi K, Shizu K, Nomura H, Adachi C, Nature 2012, 492, 234. [DOI] [PubMed] [Google Scholar]
- [4].Zhang D-W, Li M, Chen C-F, Chem. Soc. Rev 2020, 49, 1331. [DOI] [PubMed] [Google Scholar]
- [5].Pieters G, Frederic L, in Circularly Polarized Luminescence of Isolated Small Organic Molecules, (Ed: Mori T), Springer; Singapore, Singapore: 2020, Ch. 13. [Google Scholar]
- [6].Imagawa T, Hirata S, Totani K, Watanabe T, Vacha M, Chem. Commun 2015, 51, 13268. [DOI] [PubMed] [Google Scholar]
- [7].Feuillastre S, Pauton M, Gao L, Desmarchelier A, Riives AJ, Prim D, Tondelier D, Geffroy B, Muller G, Clavier G, Pieters G. g., J. Am. Chem. Soc 2016, 138, 3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].a) Song F, Xu Z, Zhang Q, Zhao Z, Zhang H, Zhao W, Qiu Z, Qi C, Zhang H, Sung HHY, Williams ID, Lam JWY, Zhao Z, Qin A, Ma D, Tang BZ, Adv. Funct. Mater 2018, 28, 1800051; [Google Scholar]; b) Wu Z-G, Han H-B, Yan Z-P, Luo X-F, Wang Y, Zheng Y-X, Zuo J-L, Pan Y, Adv. Mater 2019, 31, 1900524; [DOI] [PubMed] [Google Scholar]; c) Sun S, Wang J, Chen L, Chen R, Jin J, Chen C, Chen S, Xie G, Zheng C, Huang W, J. Mater. Chem. C 2019, 7, 14511; [Google Scholar]; d) Wu Z-G, Yan Z-P, Luo X-F, Yuan L, Liang W-Q, Wang Y, Zheng Y-X, Zuo J-L, Pan Y, J. Mater. Chem. C 2019, 7, 7045. [Google Scholar]
- [9].Wang Y, Zhang Y, Hu W, Quan Y, Li Y, Cheng Y, ACS Appl. Mater. Interfaces 2019, 11, 26165. [DOI] [PubMed] [Google Scholar]
- [10].a) Li M, Li S-H, Zhang D, Cai M, Duan L, Fung M-K, Chen C-F, Angew. Chem., Int. Ed 2018, 57, 2889; [DOI] [PubMed] [Google Scholar]; b) Wang Y-F, Lu H-Y, Chen C, Li M, Chen C-F, Org. Electron 2019, 70, 71. [Google Scholar]
- [11].Zhang M-Y, Li Z-Y, Lu B, Wang Y, Ma Y-D, Zhao C-H, Org. Lett 2018, 20, 6868. [DOI] [PubMed] [Google Scholar]
- [12].Sharma N, Spuling E, Mattern CM, Li W, Fuhr O, Tsuchiya Y, Adachi C, Bräse S, Samuel IDW, Zysman-Colman E, Chem. Sci 2019, 10, 6689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Li M, Wang Y-F, Zhang D, Duan L, Chen C-F, Angew. Chem., Int. Ed 2020, 59, 3500. [DOI] [PubMed] [Google Scholar]
- [14].Lefebvre C, Rubez G, Khartabil H, Boisson J-C, Contreras-García J, Hénon E, Phys. Chem. Chem. Phys 2017, 19, 17928. [DOI] [PubMed] [Google Scholar]
- [15].Lu T, Chen F, J. Comput. Chem 2012, 33, 580. [DOI] [PubMed] [Google Scholar]
- [16].a) Tanaka H, Ikenosako M, Kato Y, Fujiki M, Inoue Y, Mori T, Commun. Chem 2018, 1, 38; [Google Scholar]; b) Sánchez-Carnerero EM, Agarrabeitia AR, Moreno F, Maroto BL, Muller G, Ortiz MJ, de la Moya S, Chem. - Eur. J 2015, 21, 13488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17]. As reported in reference 7, glum values of +/− 1.2 × 10−3 were determined for A1 using a homemade CPL spectrophotometer. Using a commercially available CPL spectrophotometer, glum values around +/− 0.4 × 10−3 were measured (see Figure 6).
- [18]. By contrast with the results described in reference 8a, but in accordance, with results reported in references 8b and 8c, for molecule A1, B1, and C′3 no appreciable chiroptical properties amplification was found in thin film (measuring CPL responses of pure thin films fabricated by spin coating or thermal evaporating method). In our hands, glum values of compounds R/S-BN-DCB described in reference 8a in diluted toluene solution (C = 10−5) and in pure thin film (fabricated using thermal evaporating method) were found to be <10−3 (measured with two different CPL spectrometers using different samples).
- [19].Note that for this proof-of-concept top emission CP-OLED, we have chosen to use Ca as the electron injection layer in order to limit light reflection, and therefore maximize the chance to conserve the circularly polarized electroluminescence generated by the EML. This may also significantly contribute to decrease the efficiency of the device (in terms of EQE).; a) Zinna F, Giovanella U, Bari LD, Adv. Mater 2015, 27, 1791; [DOI] [PubMed] [Google Scholar]; b) Zinna F, Pasini M, Galeotti F, Botta C, Di Bari L, Giovanella U, Adv. Funct. Mater 2017, 27, 1603719. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


