Abstract
Anticancer drugs play an important role in reducing mortality rates and increasing life expectancy in cancer patients. Treatments include monotherapy and/or a combination of radiation therapy, chemotherapy, hormone therapy, or immunotherapy. Despite great advances in drug development, some of these treatments have been shown to induce cardiotoxicity directly affecting heart function and structure, as well as accelerating the development of cardiovascular disease. Such side effects restrict treatment options and can negatively affect disease management. Consequently, when managing cancer patients, it is vital to understand the mechanisms causing cardiotoxicity to better monitor heart function, develop preventative measures against cardiotoxicity, and treat heart failure when it occurs in this patient population. This review discusses the role and mechanism of major chemotherapy agents with principal cardiovascular complications in cancer patients.
Keywords: cancer, chemotherapy, trastuzumab, doxorubicin, cardiotoxicity
Introduction and background
According to the American Cancer Society, cancer is one of the most prevalent healthcare challenges worldwide with 1.7 million cancer diagnoses in the United States in 2017 [1]. Although advancements in cancer therapy have significantly decreased patient mortality [2,3], unfortunately, some cancer treatments can damage the heart, a condition known as cardiotoxicity. High blood pressure, arrhythmias, and heart failure can be caused or exacerbated by chemotherapy and radiation therapy, as well as by newer forms of cancer treatment such as targeted therapies and immunotherapies [4]. As a result, to treat patients holistically and facilitate a better prognosis, cardiologists and oncologists must use an interdisciplinary approach: patients exposed to anticancer treatments require cardiovascular evaluation, risk analysis, prevention and mitigation of cardiac injury and cardiotoxicity, and their cardiac function must be monitored during and long after the therapy. Thus, cardio-oncology is an emerging discipline [5] and an essential part of a comprehensive approach to cancer treatment.
Review
Definition of cardiotoxicity
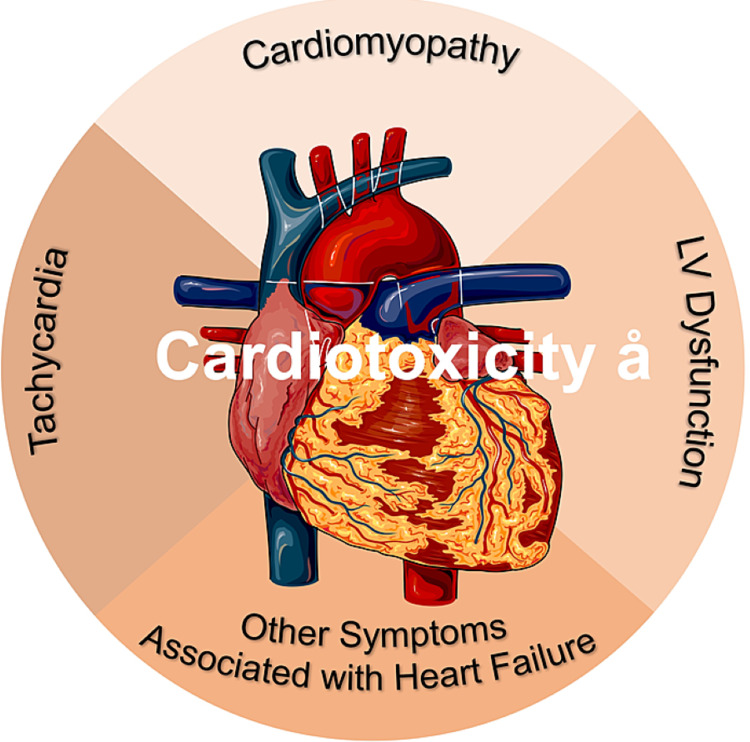
Cardiotoxicity is a general term used to describe toxicity that can directly or indirectly affect the heart: directly, by damaging the heart structure; and indirectly, through thrombogenic states and hemodynamic alterations of blood flow [6]. The Cardiac Review and Evaluation Committee defines cardiotoxicity as the presence of one or more of the following conditions in patients who have received anticancer treatments [7]: (1) cardiomyopathy characterized by decreased left ventricular ejection fraction (LVEF) or the more severe abnormal ventricular septal motion; (2) heart failure symptoms; (3) tachycardia; (4) decrease in the minimum LVEF to less than 55% accompanied by signs and symptoms of heart failure (Figure 1) [7].
Figure 1. Cardiotoxicity.
According to the Cardiac Review and Evaluation Committee, cardiotoxicity leads to cardiomyopathy, tachycardia, heart failure, and LV dysfunction.
LV: left ventricular
Additionally, according to the American Society of Echocardiography and the European Association of Cardiovascular Imaging [8], LVEF decrease can be categorized either as symptomatic or asymptomatic depending on its reversibility. Improvement to within 5% points of the baseline is considered reversible; improvement to ≥10% points from the nadir but remaining >5% points below the baseline is considered partially reversible; and improvement to <10% points from the nadir and remaining >5% points below the baseline is considered irreversible.
Furthermore, global systolic longitudinal myocardial strain (GLS) has been reported to accurately predict a subsequent decrease in LVEF [9]. A relative percentage GLS reduction of >15% from the baseline is considered abnormal and a marker of early left ventricular (LV) subclinical dysfunction. [10] However, neither GLS use nor its cutoff point to predict cardiotoxicity has been standardized [11].
Cardiotoxicity induced by chemotherapy
New therapies for managing neoplasms/tumors greatly extend the survival of cancer patients, in many cases making cancer a chronic pathology such as diabetes or systemic hypertension. Yet, these therapies have severe side effects [12].
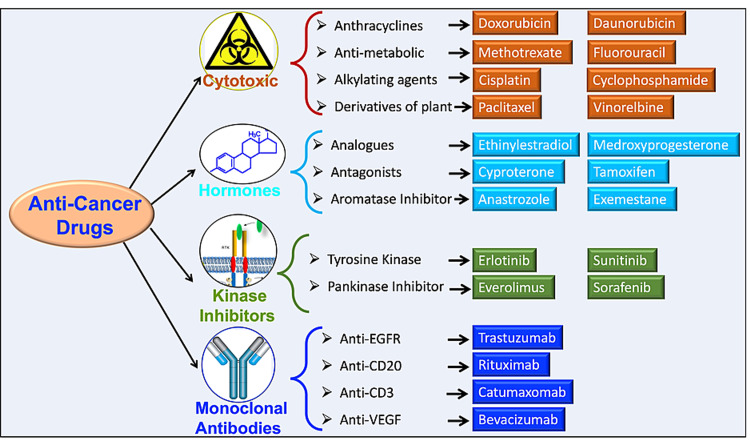
Chemotherapy is one of the most effective therapies for cancer treatment [2,3]. Figure 2 summarizes the classification of antineoplastic agents used as chemotherapy with representative examples of each class. Chemotherapy inhibits cell division through the action of many different types of cytotoxic drugs, hormonal agents, protein kinase inhibitors, and monoclonal antibodies. Yet, these agents are not only toxic to cancer cells they are also toxic to noncancerous tissues [13]. For the cardiovascular system, chemotherapy-induced complications were first reported in 1967 when pediatric leukemia patients, on high doses of anthracycline, developed heart failure [14].
Recently, it has been reported that doxorubicin, an anthracycline (Figure 2), can cause dose-dependent cardiotoxicity [15]. Along the same lines, a meta-analysis (based on several scientific journals, including the European Society of Cardiology, Association of Medical Scientific Societies of Germany, and the European Society of Medical Oncology) evaluated the cardiotoxicity of antineoplastic agents and how this limits their usefulness. This study reported that some cancer treatments are cardiotoxic and can trigger lethal complications as late as four years after treatment. The authors described that doxorubicin, given at a high dose (500 mg/m2), can cause cardiac complications in up to 36% of patients [16]. Similarly, the monoclonal antibody drug trastuzumab also causes cardiovascular toxic effects in up to 5% of patients. Therefore, clinicians need to be thoroughly aware of the cardiovascular toxic effects of anticancer drugs to be able to diagnose them early and not jeopardize the overall success of the treatment [7].
Figure 2. Classes of antineoplastic agents used as chemotherapy and representatives of each class.
Anticancer drugs are divided into four groups: (1) cytotoxic (e.g., anthracyclines, antimetabolic, alkylating agents, and derivatives of plants); (2) kinase inhibitors (e.g., analogs, antagonists, and aromatase inhibitors; (3) hormones, (e.g., tyrosine kinase and pan-kinase inhibitors); and (4) monoclonal antibodies (e.g., trastuzumab, anti-EGF, anti-CD20, anti-CD3, and anti-VEGF).
EGF: epidermal growth factor; CD: cluster of differentiation; VEGF: vascular endothelial growth factor
The individual management of patients requiring anthracyclines remains a challenge due to the uncertainty in cardiotoxicity predictors. A systematic review and meta-analysis of 18 studies regarding the incidence and chemotherapy predictors with anthracyclines in patients with cancer included 49,017 cancer patients, of whom 22,815 patients were treated with anthracyclines [17]. After an average follow-up of nine years, clinically evident cardiotoxicity occurred in 12% of patients, while subclinical cardiotoxicity developed in 24% of patients. Evaluation of the independent risk factors of cardiotoxicity showed that the cumulative doses of anthracycline were consistently an accurate and robust indicator of cardiotoxicity. Thus, anthracyclines present a significant risk of cardiotoxicity, especially when given at high cumulative doses [17].
To identify the effect of acute treatment with anthracycline on cardiotoxicity in children under 16 years of age with malignant childhood diseases, a cohort study was carried out among 110 children (between the ages of one month and 16 years) using anthracycline (doxorubicin). The incidence of anthracycline-induced cardiotoxicity was alarming. Within one month of doxorubicin treatment, the incidence of cardiac dysfunction was up to 14%, and after one year of treatment, the incidence increased to 25%. Thus, long-term follow-up is essential to diagnose late manifestations [18].
Another cohort study reviewed 105 breast cancer cases with anthracycline chemotherapy or a combination of anthracycline and the monoclonal antibody trastuzumab. One and four years after the start of chemotherapy, patients were clinically evaluated and tested with a baseline echocardiogram, as well as for systolic and diastolic function. Although subclinical, the incidence of myocardiopathy due to anthracycline was higher after four years following the first treatment (6%). Even more so, the combination of anthracyclines and trastuzumab further exacerbated the myocardial damage (more incidences of cardiomyopathy, diastolic dysfunction, and a greater drop in the LVEF) when compared to anthracyclines alone [19]. Diastolic dysfunction preceded or was associated with all cases of cardiomyopathy; therefore, more studies are required to determine whether diastolic dysfunction might be an early marker that identifies patients with a higher risk of developing cardiomyopathy.
These studies suggest that cardiotoxicity can occur within a wide window (as early as during treatment and as late as four years after the end of chemotherapy) [20], manifesting as many conditions: (1) acute or subacute, developing between the onset of treatment and two weeks after completion; and (2) chronic, developing at least one year after completion of therapy. Chronic cardiotoxicity can then be divided into two states: early chronic cardiotoxicity if it occurs during the first year after therapy and late cardiotoxicity if it occurs in the subsequent years after the end of therapy [21,22].
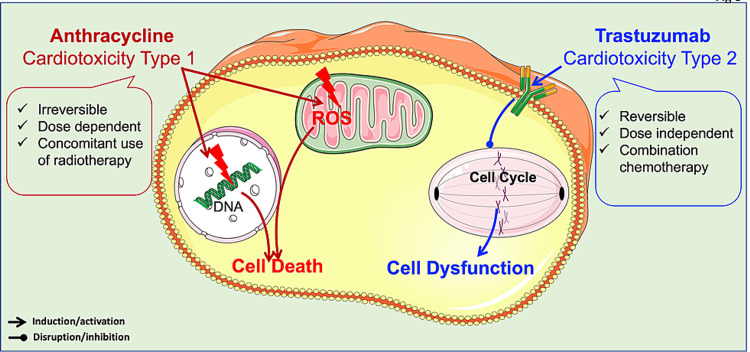
Among the antineoplastic agents, the drugs most prone to cardiotoxicity are classified into two types (Figure 3): (1) Type 1: cardiotoxicity via anthracycline-like mechanisms. Its cardiac toxicity is dose-dependent and produces irreversible cardiac damage. (2) Type II: cardiotoxicity via trastuzumab-like mechanisms. These cause reversible cardiac damage, allowing chemotherapy to be halted until the patient recovers, and then restarted if indicated. This is achieved because there are no ultrastructural changes in myocytes [23,24].
Figure 3. Types of cardiotoxicity.
Cardiotoxicity is classified into type 1 and type 2. Type I is irreversible and dose-dependent, whereas type 2 is reversible and dose-independent.
In this review, we focus on the cardiotoxicity mechanisms produced by the treatment with anthracyclines or trastuzumab.
Anthracyclines and cardiotoxicity
Anthracyclines are a group of cytotoxic antibiotics that were initially extracted from the Streptomyces bacterium [25]. They are very effective drugs against a wide spectrum of solid and hematological malignancies and are a part of many treatment regimens [26]. Nevertheless, cardiotoxicity remains one of the main elements that limits their use. The main risk factors for developing cardiac failure from exposure to these cytotoxic agents are accumulated doses, age greater than 70, early or simultaneous irradiation, use of other drugs that damage the myocyte, and a history of heart diseases [27].
The mechanisms by which anthracyclines damage the heart are probably multifactorial [28]. The release of free radicals, alteration in iron homeostasis, changes in intracellular calcium, and mitochondrial dysfunction are some of the effects produced by anthracyclines [29]. The best-known mechanism is the pathway of damage mediated by free radicals. The reduction of the quinone group of the anthracycline generates a semiquinone radical that oxidizes rapidly, generating superoxide radicals that produce hydrogen peroxide. In turn, hydrogen peroxide interacts with the myocardium. Because the myocardium expresses a relatively lower amount of superoxide dismutase and catalase, its only defense is glutathione peroxidase, which itself is reduced by anthracyclines [30,31].
When ferric iron forms a complex with doxorubicin, it generates more free radicals, which, in turn, convert ferrous iron to ferric iron, a vicious circle that can damages mitochondrial and nuclear membranes, the cell membrane, and the endoplasmic reticulum, leading to an intracellular calcium decrease and reducing heart contractility [30,31].
Proinflammatory cytokines are also related to the cardiovascular side effects of anthracyclines because they induce the release of histamine, tumor necrosis factor-alpha, and interleukin-2, proteins that induce dilated cardiomyopathy in addition to beta-adrenergic dysfunction [32].
Although the previously mentioned mechanisms are widely studied, the interaction of reactive oxygen species (ROS) with cellular elements and the formation of free radicals (induced by anthracyclines) are not the mechanisms directly responsible for the cellular injury. This was demonstrated for the first time by Lyu et al. [33] who used mouse embryos exposed to anthracyclines and observed DNA breaks and cell death dependent on the presence of DNA topoisomerase II beta (TOP2B). Based on this finding, Zhang et al. conducted studies on cardiac tissue from wild-type (WT) versus TOP2B-knockout (KO) mice treated with doxorubicin. They demonstrated that the first step toward cardiomyocyte damage is independent of ROS and depends on a complex formed by the TOP2B-ROS (generated by anthracycline)-DNA. This complex leads to the suppression of transcription factors (via the activation of p53), DNA degradation, and inevitable apoptosis due to mitochondrial dysfunction [15]. In contrast to controls, the mutant animals (lacking TOP2B) exposed to doxorubicin did not display acute, chronic cardiac injury or the reduction of the LVEF [34]. Thus, the depletion of cardiac TOP2B should prevent doxorubicin-induced cardiotoxicity while preserving its tumor-killing effect.
Recently, several mechanisms have been described by which anthracyclines cause cardiotoxicity; these mechanisms are still under investigation. Anthracyclines show an affinity for cardiolipin, a cofactor of the respiratory chain enzymes (i.e., cytochrome c oxidase, NADH, and oxidoreductase). Cardiolipin possesses a high density of phospholipids and, thus, a higher affinity to anthracyclines, especially doxorubicin. A cardiolipin-doxorubicin complex can damage the inner mitochondrial membrane and inhibit oxidative phosphorylation, thereby losing its function as a cofactor [35]. Another cell component with which anthracyclines interact to cause cardiotoxicity is the protein titin. This protein comprises part of the sarcomere in striated muscle, serving as a scaffold for the assembly of myofilament proteins in the sarcomere, as well as mediating the passive contractile forces [36]. Anthracyclines degrade titin and alter sarcomeric cardiac structure (sarcopenia) through the loss and disorganization of sarcomere myofibrils, sarcoplasmic reticulum dilatation, mitochondrial edema, and cytoplasmic vacuolization. Therefore, titin degradation can lead to progressive diastolic and systolic dysfunction, with the suppression of transcription of sarcomere proteins [37,38]. In addition, anthracyclines deplete reserves of GATA binding protein 4 (GATA4), a transcriptional factor that regulates the apoptotic pathway and preserves mitochondrial function, and is a potent regulator of cardiac gene activity [39].
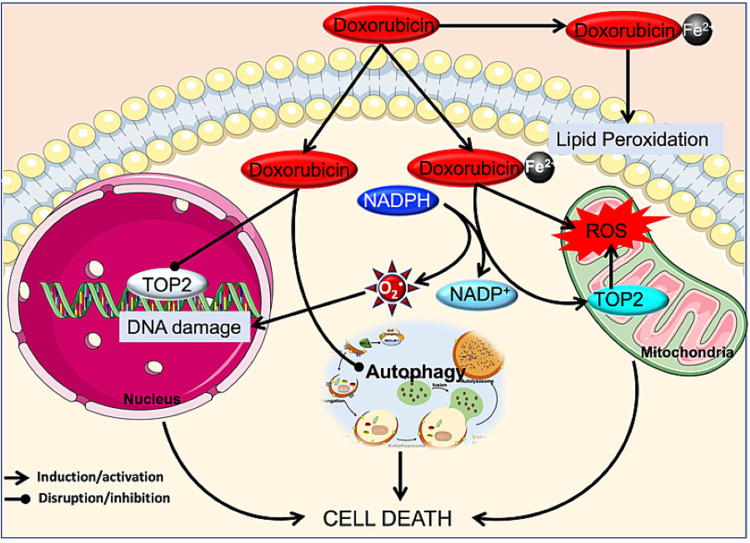
Furthermore, doxorubicin can contribute to cardiotoxicity by disrupting autophagy, a programmed cell death pathway, independent of apoptosis and necrosis. In a doxorubicin-induced heart failure model in rats, it has been concluded that doxorubicin damages the mitochondria of cardiomyocytes, which then leads to heart failure through the induction of pathological autophagy. The inhibition of autophagy has reduced doxorubicin-induced mitochondrial injury and rescued heart function [40]. Similarly, in vitro treatment of cardiomyocytes with doxorubicin induces autophagy and leads to cardiomyocyte death [41]. Overexpressing GATA4 in the cardiomyocytes inhibited doxorubicin-induced autophagy, reducing cardiomyocyte apoptosis [42]. In contrast, it has been shown that activation of autophagy through starvation prior to doxorubicin administration mitigates the acute cardiotoxicity of this drug. This mitigation may occur partly because fasting restores and strengthens myocardial autophagic flux, which reduces the negative impact of doxorubicin on the cardiomyocytes. Based on these findings, patients on doxorubicin might be able to prevent or reduce the risk of cardiotoxicity through fasting or caloric restriction [43,44]. Additionally, induction of autophagy using rapamycin has shown a prospective cardioprotective role against doxorubicin-induced cardiotoxicity. This highlights rapamycin as a plausible adjuvant therapy possessing a high therapeutic index to counteract and improve the life-threatening impediment of doxorubicin actions in clinical practice [45]. The discrepancy between these studies might be due to the use of doxorubicin either in vivo or in vitro. Furthermore, the majority of the studies in the literature use short-term and high-dose doxorubicin exposure, which does not reflect the chronic clinical use of doxorubicin. Li et al. overcame this issue by giving multiple injections of doxorubicin at doses used clinically [46] to demonstrate that treatment with clinical doses of doxorubicin blocks autophagy flux in cardiomyocytes by impairing lysosomal acidity and function. Thus, the reduction of autophagy induction protects against doxorubicin-induced cardiotoxicity. Even though numerous studies have investigated molecular mechanisms of doxorubicin cardiomyopathy, a single, unifying model of pathogenesis remains elusive. Thus, there is a need to establish a specific model that mimics the clinical dose of doxorubicin in vivo using different animal models. The mechanism of action of doxorubicin is summarized in Figure 4.
Figure 4. Cardiotoxicity by doxorubicin.
Doxorubicin acts on TOP2B causing breaks in genomic and mitochondrial DNA, and, as a consequence, elevating ROS. Additionally, ferric iron forms a complex with doxorubicin that generates even more free radicals via NADPH oxidase and lipid peroxidation. Furthermore, doxorubicin disrupts autophagy flux in cardiomyocytes by impairing lysosome acidity and function. All these mechanisms impair cardiomyocytes and are cardiotoxic.
TOP2B: DNA topoisomerase II beta; ROS: reactive oxygen species. O2.: superoxide anion. Fe2+: ferric anion. NADPH: nicotinamide adenine dinucleotide phosphate
Trastuzumab and cardiotoxicity
Although the pathophysiology of cardiac dysfunction associated with trastuzumab is not entirely clear, various mechanisms have been proposed to explain it. On one hand, trastuzumab, as a monoclonal antibody, can initiate antibody-dependent cellular cytotoxicity that can affect cardiomyocytes, increasing cardiac toxicity [47,48]. On the other hand, various arguments support the idea that human epidermal growth factor 2 (HER2) contributes to cardiotoxicity [49]. Trastuzumab inhibits the proliferation of human tumor cells overexpressing the HER2 receptor that is expressed on cardiac cells and plays an essential role in the proliferation, growth, and survival of cardiomyocytes; thus, the HER2 signaling pathway controls cardiac development and function [50] and is essential to prevent the development of cardiomyopathy. The deletion of HER2 has led to multiple features of dilated cardiomyopathy, including cavities dilation, thinning of the cardiac wall, and decreased contractility [50]. In the absence of HER2 function, cardiomyocytes are not able to activate survival pathways and thus accumulate ROS, leading to cardiac dysfunction [51]. Additionally, reduction of HER2 activity dampens the extracellular signal-regulated kinase (MEK/ERK) signaling pathway, inducing apoptosis [52]. MEK/ERK inhibition through the trastuzumab-induced reduction in HER2 leads to an increase in the number of mitochondrial permeability transition pores (mPTP) with subsequently increased sensitivity to Ca2+ overload, excessive production of cytotoxic ROS, and suppressed gap junction permeability. These factors culminate in myocyte injury [53]. Additionally, patients with a mutation that regulates the MEK/ERK activity display hypertrophic cardiomyopathy [54]. Overall, these findings indicate that trastuzumab regulates MEK/ERK pathway, a pathway important to stimulate proliferation and survival and to protect the function of myocytes [55].
The ratio between antiapoptotic and proapoptotic stimuli is a key regulator of mitochondrial function in cardiomyocytes. Trastuzumab induces cardiotoxicity by downregulating the antiapoptotic protein, B-cell lymphoma-extra large (BCL-XL), and by upregulating the proapoptotic protein B-cell lymphoma-extra small (BCL-XS). [56]
Phosphoinositide 3-kinase (PI3K) and mammalian target of rapamycin (mTOR) are key elements in HER2 downstream signaling. The PI3Ks phosphorylate phosphatidylinositol 4,5 bisphosphate (PIP2) to phosphatidylinositol 3,4,4-triphosphate (PIP3), which, in turn, leads to the phosphorylation of Akt, a serine/threonine kinase which has an impact on cancer cell cycling, survivalc, and growth [57-59]. mTOR is a serine/threonine protein kinase, which is found downstream of PI3K. The role of the PI3K/mTOR in cardiac structure and function is very well established [60]; therefore, the deregulation of this pathway by trastuzumab leads to cardiotoxicity.
During cancer, when HER2 is overexpressed, mitogen-activated protein kinase (MAPK) becomes hyperactive [61]. Abnormalities in the MAPK signaling pathway play a critical role in the development of cancer [62]. However, MAPK signaling also drives the pathogenesis of cardiac diseases such as cardiac hypertrophy and cardiac remodeling after myocardial infarction [63]. Because of this important role, the cardiotoxicity of trastuzumab may be related to the drug’s inhibition of MAPK.
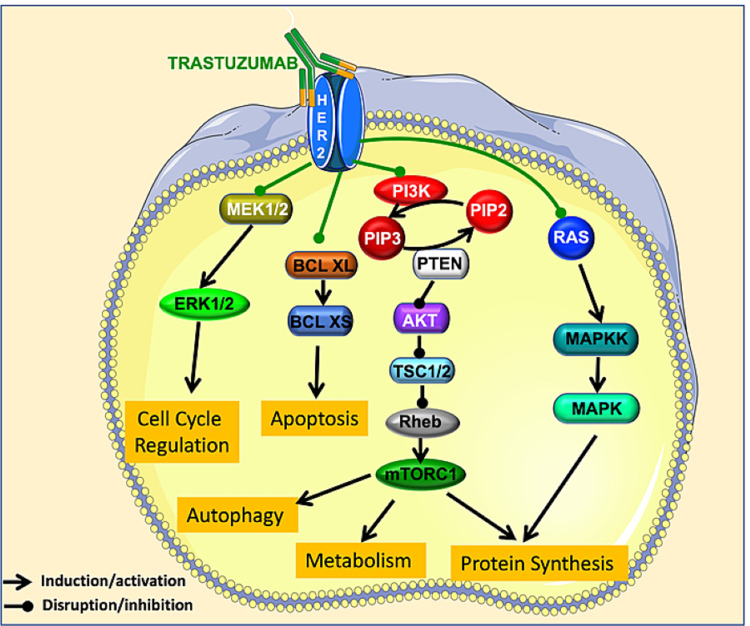
Clinically, the cardiotoxic effects of trastuzumab can manifest as asymptomatic (decreases in LVEF) or symptomatic (congestive heart failure) which can lead to death [64]. The mechanism of action of trastuzumab is summarized in Figure 5.
Figure 5. Cardiotoxicity by trastuzumab.
Trastuzumab acts on HER2, decreasing signaling via the MEK/ERK pathway and causing apoptosis. It also downregulates the antiapoptotic protein BCL-XL and upregulates the proapoptotic protein BCL-XS; impairs the PI3K/mTOR pathway, causing decrease in autophagy and protein synthesis; and decreases the MAPK activity, dampening protein synthesis. Together, these impairments are cardiotoxic
HER2: human epidermal growth factor 2; BCL-XL: B-cell lymphoma-extra large; BCL-XS: B-cell lymphoma-extra small; PIP2: phosphorylate phosphatidylinositol 4,5 bisphosphate; PIP3: phosphatidylinositol 3,4,4-triphosphate; MAPK: mitogen-activated protein kinase; mTOR: mammalian target of rapamycin
Prevention and cardiotoxicity treatment
To date, as there are no specific treatments for cardiac insufficiency induced by chemotherapy, the treatment of choice for congestive heart failure remains angiotensin-converting enzyme inhibitors ACEI (enalapril), beta-blockers (metoprolol), and diuretics (carvedilol). For instance, enalapril and metoprolol have been shown to reduce cardiotoxicity in patients with elevated concentrations of troponin I due to chemotherapy. These agents prevented the reduction in the LVEF [65]. Numerous basic science studies and clinical trials have shown that administration of ACEI had a cardioprotective effect with reduced morbidity and mortality in animal models and patients of acute and chronic chemotherapy-induced cardiotoxicity [66,67]. However, the sample size used in all studies that aimed to evaluate the preventive effect of ACEI on the heart during chemotherapy is small and needs to be increased to confirm the preventive outcome. Additionally, it has been suggested that beta-blockers can prevent trastuzumab-related cardiotoxicity by promoting ERK signaling [68]. However, more studies are needed to further evaluate the cardioprotective effects of beta-blockers in chemotherapy-induced cardiotoxicity.
Although some of these drugs attenuate the decrease in LVEF, they have no effect on GLS or cardiac biomarkers. Even though GLS may be a more sensitive tool to detect early cardiotoxicity because there is no specific standardized guidance for GLS [11], it is recommended that cancer treatment should not be stopped, interrupted, or reduced in dose based on GLS reduction alone [10]. Recently, other treatments have been introduced such as antioxidant drugs (probucol), bioactive compounds (inorganic nitrates), and aerobic exercise training.
Anthracycline-induced cytotoxicity is mainly due to the generation of ROS in the cardiomyocytes which results in LV dysfunction. In animal models of cardiotoxicity associated with doxorubicin, the antioxidant probucol was effective in preventing LV dysfunction and could be a potential therapeutic tool to combat cardiotoxicity [69]. Additionally, carvedilol is prescribed as cardioprotective due to its antioxidant properties via increasing levels of GATA4 [70]. Along the same lines, ranolazine, a drug used to treat chronic angina, showed cardioprotective effects in both animals and patients undergoing chemotherapy by suppressing ROS production [71-73]. Recently, cardio-oncology research has been focusing on the redox compound hydrogen sulfide (H2S). Exogenous H2S has been shown to protect against cardiotoxicity; however, less is known about its adverse effect so further basic and clinical research will be needed to assess the safety of H2S [74,75]. Nevertheless, not all antioxidants are equally effective. Vitamin E, also known for its antioxidant effects, appears not to prevent ventricular dysfunction in long-term experimental and clinical trials [71].
Dexrazoxane is an iron chelator known to prevent the formation of excess hydroxyl radicals, thereby decreasing the incidence of cardiac insufficiency and boosting LV function. Dexrazoxane is the only Food and Drug Administration-approved drug for chemotherapy-induced cardiotoxicity. Its protective effect was observed in patients receiving high doses of doxorubicin (300 mg/m2). One of its protective mechanisms is associated with suppression of anthracycline-induced troponin elevation, a key player in myocytes death. Another mechanism by which dexrazoxane likely produces its beneficial effect is through preventing doxorubicin binding to TOP2B, thereby preventing DNA breaks and cell death [39,76]. It has been reported that dexrazoxane may attenuate the chemotherapeutic efficacy of doxorubicin but recent studies have shown that dexrazoxane does not interfere with the antitumor activity nor does it reduce the progression or overall survival, which are the key endpoints of cancer studies [75].
Other reports showed that there is an association between dexrazoxane and induction of a second tumor [76]. These results were questionable, especially because the statistical method applied in these studies was not appropriate and importantly because at a clinical level the examination and the follow-up of more than 1,000 patients showed that this association was not confirmed [77,78]. Based on the clinical data outcome, dexrazoxane could present as the most appropriate cardioprotective drug to use in combination with anticancer drugs.
Inorganic nitrate is a bioactive compound that can be reduced into nitrite and nitric oxide in vivo. Once reduced, it could have therapeutic properties for diseases related to nitric oxide bioavailability disruption. Recently, it was shown that administration of inorganic nitrates to mice during doxorubicin therapy (at a rate of 400% of what is recommended by the World Health Organization) decreased ventricular dysfunction, cell death, oxidative stress, and mitochondrial damage, without reducing the antineoplastic effect of doxorubicin [79].
Another way to reduce anthracycline and trastuzumab cardiotoxicity is with aerobic exercise. In addition to its many benefits in cancer management [80-82], aerobic exercise training has been shown to increase systolic and diastolic function, diminishing pathological restructuring of cardiac tissue, thereby preventing dilatation of the left ventricle. At the same time, aerobic exercise increases resistance to fatigue in patients with cardiac insufficiency [83-85]. It also protects the heart against oxygen free radicals by activating endogenous antioxidant processes and increasing the expression of antioxidant enzymes that decrease their production [69]. Dolinsky et al. have demonstrated that intense aerobic exercise training for only eight weeks decreased doxorubicin-induced cardiac damage [86]. Aerobic exercise training has not only attenuated the adverse LV remodeling but also reduced the level of atrial natriuretic peptide and lipid peroxidation byproducts [85]. Additionally, aerobic exercise can regulate proapoptotic signals by decreasing the expression of p53 (an apoptotic mediator) and increasing GATA4 [70]. Although several studies have obtained positive results after testing the role of aerobic exercise in preventing chemotherapy-induced cardiotoxicity [86,87], we need more information to define in greater detail the effects of aerobic exercise as a means of preventing cancer therapy-induced cardiotoxicity [88].
Another way to prevent and monitor the early signs of cardiotoxicity is echocardiographic measurements. These advanced echocardiographic measurements are preferred, when available, to serve as the basis for clinical decisions when performed with adequate expertise performing cardiac safety studies [89]. In the clinical setting, cardiac imaging surveillance is used for the early detection of cardiotoxicity. Recent advances in molecular imaging of apoptosis and tissue characterization by cardiac magnetic resonance imaging (MRI) allow early detection of patients at high risk for developing cardiotoxicity prior to a drop in LVEF. Therefore, cardiac MRI is the gold standard for determining cardiac volumes and function because of the superior image quality [90]. Current guidelines recommend this imaging modality for the confirmation of cancer therapy-related cardiac dysfunction, mainly when echocardiographic- or radionuclide-derived LVEF is uncertain [8].
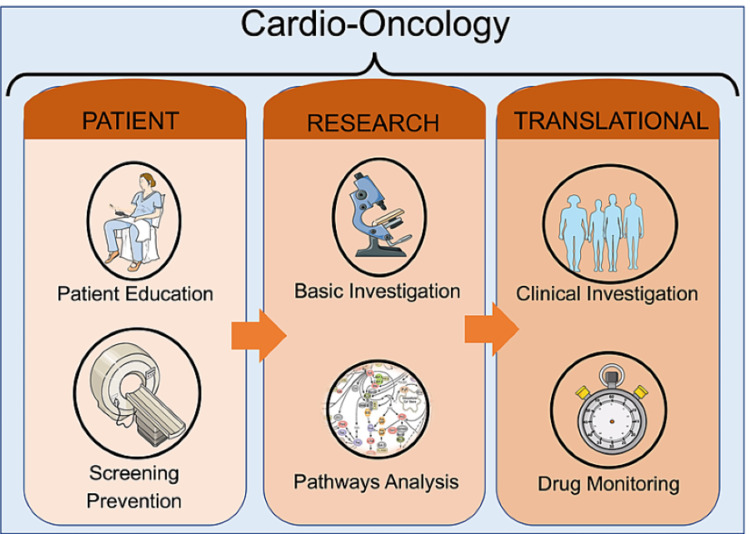
With the increasing number of cancer survivors, often burdened with pre-existing or new cardiovascular disease or risk factors, the need has arisen for a new specialty in the field of cardiovascular care that can assess and treat these patients. This specialty must combine cardiologists and oncologists. In the same way, all healthcare providers involved in the care of patients with cancer and heart disease should be fully aware of the adverse impact of cardiovascular disease on the survival of these patients. Collaboration is necessary to mitigate the effect of cardiovascular toxicity associated with these anticancer therapies that otherwise save lives. Cardio-oncologists can play a fundamental role in combining the two specialties by creating a comprehensive plan to address comorbidities and providing guidance for choosing the optimal treatment. The cardio-oncologist unit should focus on three aspects: (a) patient education and drug screening, which is a way to detect the cardiotoxicity in its earliest stages; (b) basic investigation and pathway analysis that addresses cardiotoxicity as a consequence of cancer therapy and discusses the prevention, diagnosis, and management of cardiovascular disease in patients with cancer; and (c) translational clinical investigation and drug monitoring. These aspects and their importance to the cardio-oncology field are summarized in Figure 6.
Figure 6. A comprehensive plan for the cardio-oncologist to address comorbidities when personalizing treatment for a cancer patient.
Patient education and drug screening are key because they allow the cardio-oncologist to detect cardiotoxicity in its earliest stages. Additionally, pathway analysis should point to new techniques for managing cardiovascular disease in cancer patients. Finally, translational investigation and drug monitoring must be incorporated into any cardio-oncology program.
Conclusions
Cardiotoxicity represents a side effect for patients receiving chemotherapy and increases with other concomitant risk factors; however, in some cases, it can be prevented or limited. The methods to combat cardiotoxicity range from pharmacological interventions that limit cardiac restructuring to nonpharmacological ones such as aerobic exercise, which gives us a simple method of preserving the cardiac function in exposed patients. New methods are needed to provide novel and promising instruments for the detection of cardioprotective gene modulators that are affected by antineoplastic drugs. This could lead to a change in the current definition of cardiotoxicity from a clinical definition to a subclinical one based on earlier, sensitive, and specific biomarkers.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
Funding Statement
D. Castañeda is supported by the TWD Rise award R25 GM061331, S.K. Choi by the Basic Science Research Program of the National Research Foundation of Korea (NRF) (NRF-2018R1D1A1B07041820), and M. Kassan by the AHA-18CDA34030155 and 7R01HL150360-02.
Footnotes
The authors have declared that no competing interests exist.
References
- 1.Cancer Statistics, 2017. Siegel RL, Miller KD, Jemal A. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Anticipating the "silver tsunami": prevalence trajectories and comorbidity burden among older cancer survivors in the United States. Bluethmann SM, Mariotto AB, Rowland JH. Cancer Epidemiol Biomarkers Prev. 2016;25:1029–1036. doi: 10.1158/1055-9965.EPI-16-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clinical Cancer Advances 2018: annual report on progress against cancer from the American Society of Clinical Oncology. Heymach J, Krilov L, Alberg A, et al. J Clin Oncol. 2018;36:1020–1044. doi: 10.1200/JCO.2017.77.0446. [DOI] [PubMed] [Google Scholar]
- 4.[2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines] Zamorano JL, Lancellotti P, Muñoz DR, et al. Kardiol Pol. 2016;74:1193–1233. doi: 10.5603/KP.2016.0156. [DOI] [PubMed] [Google Scholar]
- 5.Cardio-oncology: a new discipline in medicine to lead us into truly integrative care. Clarke E, Lenihan D. Future Cardiol. 2015;11:359–361. doi: 10.2217/fca.15.55. [DOI] [PubMed] [Google Scholar]
- 6.Kang JY. Casarett and Doull's Toxicology: The Basic Science of Poisons. New York, NY: McGraw-Hill; 1996. Toxic responses of the heart and vascular systems; pp. 487–527. [Google Scholar]
- 7.Cardiac dysfunction in the trastuzumab clinical trials experience. Seidman A, Hudis C, Pierri MK, et al. J Clin Oncol. 2002;20:1215–1221. doi: 10.1200/JCO.2002.20.5.1215. [DOI] [PubMed] [Google Scholar]
- 8.Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Plana JC, Galderisi M, Barac A, et al. Eur Heart J Cardiovasc Imaging. 2014;15:1063–1093. doi: 10.1093/ehjci/jeu192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Assessment of echocardiography and biomarkers for the extended prediction of cardiotoxicity in patients treated with anthracyclines, taxanes, and trastuzumab. Sawaya H, Sebag IA, Plana JC, et al. Circ Cardiovasc Imaging. 2012;5:596–603. doi: 10.1161/CIRCIMAGING.112.973321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: the task force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC) Zamorano JL, Lancellotti P, Rodriguez Muñoz D, et al. Eur Heart J. 2016;37:2768–2801. doi: 10.1093/eurheartj/ehw211. [DOI] [PubMed] [Google Scholar]
- 11.Global longitudinal strain accuracy for cardiotoxicity prediction in a cohort of breast cancer patients during anthracycline and/or trastuzumab treatment. Gripp EA, Oliveira GE, Feijó LA, Garcia MI, Xavier SS, Sousa AS. Arq Bras Cardiol. 2018;110:140–150. doi: 10.5935/abc.20180021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Health profiles in 5836 long-term cancer survivors. Schultz PN, Beck ML, Stava C, Vassilopoulou-Sellin R. Int J Cancer. 2003;104:488–495. doi: 10.1002/ijc.10981. [DOI] [PubMed] [Google Scholar]
- 13.Ritter JM, Rang HP, Flower R, Henderson G. Oxford, UK: Elsevier Health Sciences; 2014. Rang & Dale's pharmacology. [Google Scholar]
- 14.Childhood leukemic heart disease. A study of 116 hearts of children dying of leukemia. Sumners JE, Johnson WW, Ainger LE. Circulation. 1969;40:575–581. doi: 10.1161/01.cir.40.4.575. [DOI] [PubMed] [Google Scholar]
- 15.Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Zhang S, Liu X, Bawa-Khalfe T, Lu LS, Lyu YL, Liu LF, Yeh ET. Nat Med. 2012;18:1639–1642. doi: 10.1038/nm.2919. [DOI] [PubMed] [Google Scholar]
- 16.Cardiotoxicity and oncological treatments. Schlitt A, Jordan K, Vordermark D, Schwamborn J, Langer T, Thomssen C. Dtsch Arztebl Int. 2014;111:161–168. doi: 10.3238/arztebl.2014.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Review and meta-analysis of incidence and clinical predictors of anthracycline cardiotoxicity. Lotrionte M, Biondi-Zoccai G, Abbate A, et al. Am J Cardiol. 2013;112:1980–1984. doi: 10.1016/j.amjcard.2013.08.026. [DOI] [PubMed] [Google Scholar]
- 18.Anthracycline-induced cardiotoxicity: prospective cohort study from Pakistan. Shaikh AS, Saleem AF, Mohsin SS, Alam MM, Ahmed MA. BMJ Open. 2013;3:0. doi: 10.1136/bmjopen-2013-003663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anthracycline- and trastuzumab-induced cardiotoxicity: a retrospective study. Hamirani Y, Fanous I, Kramer CM, Wong A, Salerno M, Dillon P. Med Oncol. 2016;33:82. doi: 10.1007/s12032-016-0797-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chemotherapy-induced cardiotoxicity. Florescu M, Cinteza M, Vinereanu D. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3749765/ Maedica (Bucur) 2013;8:59–67. [PMC free article] [PubMed] [Google Scholar]
- 21.Chemotherapy induced cardiomyopathy: pathogenesis, monitoring and management. Shakir DK, Rasul KI. J Clin Med Res. 2009;1:8–12. doi: 10.4021/jocmr2009.02.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cardiotoxicity of chemotherapeutic agents: incidence, treatment and prevention. Pai VB, Nahata MC. Drug Saf. 2000;22:263–302. doi: 10.2165/00002018-200022040-00002. [DOI] [PubMed] [Google Scholar]
- 23.Reversibility of trastuzumab cardiotoxicity: is the concept alive and well? Ewer MS, Tan-Chiu E. J Clin Oncol. 2007;25:5532–5533. doi: 10.1200/JCO.2007.14.0657. [DOI] [PubMed] [Google Scholar]
- 24.Braña IE, Zamora E, Oristrell G, Tabernero J. Side Effects of Medical Cancer Therapy. Cham, Switzerland: Springer; 2018. Cardiotoxicity; pp. 367–406. [Google Scholar]
- 25.The discovery of daunorubicin. Di Marco A, Cassinelli G, Arcamone F. https://pubmed.ncbi.nlm.nih.gov/7049379/ Cancer Treat Rep. 1981;65 Suppl 4:3–8. [PubMed] [Google Scholar]
- 26.Epidermal growth factor receptor targeting in cancer. Mendelsohn J, Baselga J. Semin Oncol. 2006;33:369–385. doi: 10.1053/j.seminoncol.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 27.Angiogenesis inhibitors in clinical development; where are we now and where are we going? Eskens FA. Br J Cancer. 2004;90:1–7. doi: 10.1038/sj.bjc.6601401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tyrosine kinases as targets for cancer therapy. Krause DS, Van Etten RA. N Engl J Med. 2005;353:172–187. doi: 10.1056/NEJMra044389. [DOI] [PubMed] [Google Scholar]
- 29.Anthracycline-containing chemotherapy causes long-term impairment of mitochondrial respiration and increased reactive oxygen species release in skeletal muscle. Gouspillou G, Scheede-Bergdahl C, Spendiff S, et al. Sci Rep. 2015;5:8717. doi: 10.1038/srep08717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.In reply to 'Cardiotoxicity of the cancer therapeutic agent imatinib mesylate'. Atallah E, Kantarjian H, Cortes J. Nat Med. 2007;13:14–16. doi: 10.1038/nm0107-14. [DOI] [PubMed] [Google Scholar]
- 31.Cardiotoxicity of the cancer therapeutic agent imatinib mesylate. Kerkelä R, Grazette L, Yacobi R, et al. Nat Med. 2006;12:908–916. doi: 10.1038/nm1446. [DOI] [PubMed] [Google Scholar]
- 32.Phase II study of weekly docetaxel and trastuzumab for patients with HER-2-overexpressing metastatic breast cancer. Esteva FJ, Valero V, Booser D, et al. J Clin Oncol. 2002;20:1800–1808. doi: 10.1200/JCO.2002.07.058. [DOI] [PubMed] [Google Scholar]
- 33.Role of topoisomerase II beta in the expression of developmentally regulated genes. Lyu YL, Lin CP, Azarova AM, Cai L, Wang JC, Liu LF. Mol Cell Biol. 2006;26:7929–7941. doi: 10.1128/MCB.00617-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reduction of doxorubicin cardiotoxicity by prolonged continuous intravenous infusion. Legha SS, Benjamin RS, Mackay B, et al. Ann Intern Med. 1982;96:133–139. doi: 10.7326/0003-4819-96-2-133. [DOI] [PubMed] [Google Scholar]
- 35.Doxorubicin-induced cardiotoxicity: from bioenergetic failure and cell death to cardiomyopathy. Carvalho FS, Burgeiro A, Garcia R, Moreno AJ, Carvalho RA, Oliveira PJ. Med Res Rev. 2014;34:106–135. doi: 10.1002/med.21280. [DOI] [PubMed] [Google Scholar]
- 36.Review focus series: sarcomeric proteins as key elements in integrated control of cardiac function. Solaro RJ, de Tombe PP. Cardiovasc Res. 2008;77:616–618. doi: 10.1093/cvr/cvn004. [DOI] [PubMed] [Google Scholar]
- 37.Changes in phospholipid content and myocardial calcium-independent phospholipase A2 activity during chronic anthracycline administration. McHowat J, Swift LM, Crown KN, Sarvazyan NA. J Pharmacol Exp Ther. 2004;311:736–741. doi: 10.1124/jpet.104.069419. [DOI] [PubMed] [Google Scholar]
- 38.Anthracyclines induce calpain-dependent titin proteolysis and necrosis in cardiomyocytes. Lim CC, Zuppinger C, Guo X, et al. J Biol Chem. 2004;279:8290–8299. doi: 10.1074/jbc.M308033200. [DOI] [PubMed] [Google Scholar]
- 39.Mechanisms of anthracycline cardiac injury: can we identify strategies for cardioprotection? Sawyer DB, Peng X, Chen B, Pentassuglia L, Lim CC. Prog Cardiovasc Dis. 2010;53:105–113. doi: 10.1016/j.pcad.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adriamycin-induced autophagic cardiomyocyte death plays a pathogenic role in a rat model of heart failure. Lu L, Wu W, Yan J, Li X, Yu H, Yu X. Int J Cardiol. 2009;134:82–90. doi: 10.1016/j.ijcard.2008.01.043. [DOI] [PubMed] [Google Scholar]
- 41.Resveratrol attenuates doxorubicin-induced cardiomyocyte death via inhibition of p70 S6 kinase 1-mediated autophagy. Xu X, Chen K, Kobayashi S, Timm D, Liang Q. J Pharmacol Exp Ther. 2012;341:183–195. doi: 10.1124/jpet.111.189589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Transcription factor GATA4 inhibits doxorubicin-induced autophagy and cardiomyocyte death. Kobayashi S, Volden P, Timm D, Mao K, Xu X, Liang Q. J Biol Chem. 2010;285:793–804. doi: 10.1074/jbc.M109.070037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prior starvation mitigates acute doxorubicin cardiotoxicity through restoration of autophagy in affected cardiomyocytes. Kawaguchi T, Takemura G, Kanamori H, et al. Cardiovasc Res. 2012;96:456–465. doi: 10.1093/cvr/cvs282. [DOI] [PubMed] [Google Scholar]
- 44.Can short-term fasting protect against doxorubicin-induced cardiotoxicity? Dirks-Naylor AJ, Kouzi SA, Yang S, et al. World J Biol Chem. 2014;5:269–274. doi: 10.4331/wjbc.v5.i3.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Autophagy upregulation promotes survival and attenuates doxorubicin-induced cardiotoxicity. Sishi BJ, Loos B, van Rooyen J, Engelbrecht AM. Biochem Pharmacol. 2013;85:124–134. doi: 10.1016/j.bcp.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 46.Doxorubicin blocks cardiomyocyte autophagic flux by inhibiting lysosome acidification. Li DL, Wang ZV, Ding G, et al. Circulation. 2016;133:1668–1687. doi: 10.1161/CIRCULATIONAHA.115.017443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pilot study of the mechanism of action of preoperative trastuzumab in patients with primary operable breast tumors overexpressing HER2. Gennari R, Menard S, Fagnoni F, et al. Clin Cancer Res. 2004;10:5650–5655. doi: 10.1158/1078-0432.CCR-04-0225. [DOI] [PubMed] [Google Scholar]
- 48.Predicting and preventing cardiotoxicity in the era of breast cancer targeted therapies. Novel molecular tools for clinical issues. Zambelli A, Della Porta MG, Eleuteri E, De Giuli L, Catalano O, Tondini C, Riccardi A. Breast. 2011;20:176–183. doi: 10.1016/j.breast.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 49.Neuregulin receptor ErbB2 localization at T-tubule in cardiac and skeletal muscle. Ueda H, Oikawa A, Nakamura A, Terasawa F, Kawagishi K, Moriizumi T. J Histochem Cytochem. 2005;53:87–91. doi: 10.1177/002215540505300110. [DOI] [PubMed] [Google Scholar]
- 50.Toxoplasma gondii infection promotes epithelial barrier dysfunction of Caco-2 cells. Briceño MP, Nascimento LA, Nogueira NP, et al. J Histochem Cytochem. 2016;64:459–469. doi: 10.1369/0022155416656349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Trastuzumab-induced cardiac dysfunction: a 'dual-hit'. Zeglinski M, Ludke A, Jassal DS, Singal PK. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3209542/ Exp Clin Cardiol. 2011;16:70–74. [PMC free article] [PubMed] [Google Scholar]
- 52.Anti-tumor activity of GW572016: a dual tyrosine kinase inhibitor blocks EGF activation of EGFR/erbB2 and downstream Erk1/2 and AKT pathways. Xia W, Mullin RJ, Keith BR, et al. Oncogene. 2002;21:6255–6263. doi: 10.1038/sj.onc.1205794. [DOI] [PubMed] [Google Scholar]
- 53.Cardiotoxicity mechanisms of the combination of BRAF-inhibitors and MEK-inhibitors. Bronte E, Bronte G, Novo G, Rinaldi G, Bronte F, Passiglia F, Russo A. Pharmacol Ther. 2018;192:65–73. doi: 10.1016/j.pharmthera.2018.06.017. [DOI] [PubMed] [Google Scholar]
- 54.Mitogen-activated protein kinase signaling in the heart: angels versus demons in a heart-breaking tale. Rose BA, Force T, Wang Y. Physiol Rev. 2010;90:1507–1546. doi: 10.1152/physrev.00054.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.The extracellular signal-regulated kinase: multiple substrates regulate diverse cellular functions. Yoon S, Seger R. Growth Factors. 2006;24:21–44. doi: 10.1080/02699050500284218. [DOI] [PubMed] [Google Scholar]
- 56.Inhibition of ErbB2 causes mitochondrial dysfunction in cardiomyocytes: implications for herceptin-induced cardiomyopathy. Grazette LP, Boecker W, Matsui T, Semigran M, Force TL, Hajjar RJ, Rosenzweig A. J Am Coll Cardiol. 2004;44:2231–2238. doi: 10.1016/j.jacc.2004.08.066. [DOI] [PubMed] [Google Scholar]
- 57.Class I PI3K in oncogenic cellular transformation. Zhao L, Vogt PK. Oncogene. 2008;27:5486–5496. doi: 10.1038/onc.2008.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Sarbassov DD, Ali SM, Sengupta S, et al. Mol Cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 59.Next-generation mTOR inhibitors in clinical oncology: how pathway complexity informs therapeutic strategy. Wander SA, Hennessy BT, Slingerland JM. J Clin Invest. 2011;121:1231–1241. doi: 10.1172/JCI44145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cardiac regulation by phosphoinositide 3-kinases and PTEN. Oudit GY, Penninger JM. Cardiovasc Res. 2009;82:250–260. doi: 10.1093/cvr/cvp014. [DOI] [PubMed] [Google Scholar]
- 61.Inhibition of HER2/neu (erbB-2) and mitogen-activated protein kinases enhances tamoxifen action against HER2-overexpressing, tamoxifen-resistant breast cancer cells. Kurokawa H, Lenferink AE, Simpson JF, Pisacane PI, Sliwkowski MX, Forbes JT, Arteaga CL. https://cancerres.aacrjournals.org/content/60/20/5887.long. Cancer Res. 2000;60:5887–5894. [PubMed] [Google Scholar]
- 62.MAP kinase signalling pathways in cancer. Dhillon AS, Hagan S, Rath O, Kolch W. Oncogene. 2007;26:3279–3290. doi: 10.1038/sj.onc.1210421. [DOI] [PubMed] [Google Scholar]
- 63.MAPK signalling in cardiovascular health and disease: molecular mechanisms and therapeutic targets. Muslin AJ. Clin Sci (Lond) 2008;115:203–218. doi: 10.1042/CS20070430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.The acute effect of trastuzumab infusion on ECG parameters in metastatic breast cancer patients. Yavas O, Yazici M, Eren O, Oyan B. https://pubmed.ncbi.nlm.nih.gov/17990147/ Swiss Med Wkly. 2007;137:556–558. doi: 10.4414/smw.2007.11899. [DOI] [PubMed] [Google Scholar]
- 65.Cardioprotective effect of metoprolol and enalapril in doxorubicin-treated lymphoma patients: a prospective, parallel-group, randomized, controlled study with 36-month follow-up. Georgakopoulos P, Roussou P, Matsakas E, et al. Am J Hematol. 2010;85:894–896. doi: 10.1002/ajh.21840. [DOI] [PubMed] [Google Scholar]
- 66.Effects of angiotensin-converting enzyme inhibitor on delayed-onset doxorubicin-induced cardiotoxicity. Boucek RJ Jr, Steele A, Miracle A, Atkinson J. Cardiovasc Toxicol. 2003;3:319–329. doi: 10.1385/ct:3:4:319. [DOI] [PubMed] [Google Scholar]
- 67.ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure) Hunt SA. J Am Coll Cardiol. 2005;46:0–82. doi: 10.1016/j.jacc.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 68.The β-blocker Nebivolol Is a GRK/β-arrestin biased agonist. Erickson CE, Gul R, Blessing CP, et al. PLoS One. 2013;8:0. doi: 10.1371/journal.pone.0071980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Anthracycline cardiotoxicity: prevalence, pathogenesis and treatment. Volkova M, Russell R 3rd. Curr Cardiol Rev. 2011;7:214–220. doi: 10.2174/157340311799960645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Anthracycline cardiotoxicity: from bench to bedside. Gianni L, Herman EH, Lipshultz SE, Minotti G, Sarvazyan N, Sawyer DB. J Clin Oncol. 2008;26:3777–3784. doi: 10.1200/JCO.2007.14.9401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Effects of ranolazine in a model of doxorubicin-induced left ventricle diastolic dysfunction. Cappetta D, Esposito G, Coppini R, et al. Br J Pharmacol. 2017;174:3696–3712. doi: 10.1111/bph.13791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pharmacology at work for cardio-oncology: ranolazine to treat early cardiotoxicity induced by antitumor drugs. Minotti G. J Pharmacol Exp Ther. 2013;346:343–349. doi: 10.1124/jpet.113.204057. [DOI] [PubMed] [Google Scholar]
- 73.Elevated cytosolic Na+ increases mitochondrial formation of reactive oxygen species in failing cardiac myocytes. Kohlhaas M, Liu T, Knopp A, et al. Circulation. 2010;121:1606–1613. doi: 10.1161/CIRCULATIONAHA.109.914911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pathophysiology of anthracycline cardiotoxicity. Mele D, Tocchetti CG, Pagliaro P, et al. J Cardiovasc Med (Hagerstown) 2016;17 Suppl 1:0. doi: 10.2459/JCM.0000000000000378. [DOI] [PubMed] [Google Scholar]
- 75.Preventing antiblastic drug-related cardiomyopathy: old and new therapeutic strategies. Cadeddu C, Mercurio V, Spallarossa P, et al. J Cardiovasc Med (Hagerstown) 2016;17 Suppl 1 Special issue on Cardiotoxicity from Antiblastic Drugs and Cardioprotection:0–75. doi: 10.2459/JCM.0000000000000382. [DOI] [PubMed] [Google Scholar]
- 76.Anthracyclines and heart failure. Sawyer DB. N Engl J Med. 2013;368:1154–1156. doi: 10.1056/NEJMcibr1214975. [DOI] [PubMed] [Google Scholar]
- 77.Cardioprotection with dexrazoxane for doxorubicin-containing therapy in advanced breast cancer. Swain SM, Whaley FS, Gerber MC, et al. J Clin Oncol. 1997;15:1318–1332. doi: 10.1200/JCO.1997.15.4.1318. [DOI] [PubMed] [Google Scholar]
- 78.Absence of secondary malignant neoplasms in children with high-risk acute lymphoblastic leukemia treated with dexrazoxane. Barry EV, Vrooman LM, Dahlberg SE, et al. J Clin Oncol. 2008;26:1106–1111. doi: 10.1200/JCO.2007.12.2481. [DOI] [PubMed] [Google Scholar]
- 79.Dexrazoxane exposure and risk of secondary acute myeloid leukemia in pediatric oncology patients. Seif AE, Walker DM, Li Y, et al. Pediatr Blood Cancer. 2015;62:704–709. doi: 10.1002/pbc.25043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dietary inorganic nitrate alleviates doxorubicin cardiotoxicity: mechanisms and implications. Xi L, Zhu SG, Das A, et al. Nitric Oxide. 2012;26:274–284. doi: 10.1016/j.niox.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Modulation of anthracycline-induced cardiotoxicity by aerobic exercise in breast cancer: current evidence and underlying mechanisms. Scott JM, Khakoo A, Mackey JR, Haykowsky MJ, Douglas PS, Jones LW. Circulation. 2011;124:642–650. doi: 10.1161/CIRCULATIONAHA.111.021774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. Schmitz KH, Holtzman J, Courneya KS, Mâsse LC, Duval S, Kane R. Cancer Epidemiol Biomarkers Prev. 2005;14:1588–1595. doi: 10.1158/1055-9965.EPI-04-0703. [DOI] [PubMed] [Google Scholar]
- 83.Physical exercise and quality of life following cancer diagnosis: a literature review. Courneya KS, Friedenreich CM. Ann Behav Med. 1999;21:171–179. doi: 10.1007/BF02908298. [DOI] [PubMed] [Google Scholar]
- 84.Effects of exercise on breast cancer patients and survivors: a systematic review and meta-analysis. McNeely ML, Campbell KL, Rowe BH, Klassen TP, Mackey JR, Courneya KS. CMAJ. 2006;175:34–41. doi: 10.1503/cmaj.051073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Physical exercise improves the functional capacity and quality of life in patients with heart failure. Bocalini DS, dos Santos L, Serra AJ. Clinics (Sao Paulo) 2008;63:437–442. doi: 10.1590/S1807-59322008000400005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Both aerobic exercise and resveratrol supplementation attenuate doxorubicin-induced cardiac injury in mice. Dolinsky VW, Rogan KJ, Sung MM, et al. Am J Physiol Endocrinol Metab. 2013;305:0–53. doi: 10.1152/ajpendo.00044.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Adjuvant trastuzumab induces ventricular remodeling despite aerobic exercise training. Haykowsky MJ, Mackey JR, Thompson RB, Jones LW, Paterson DI. Clin Cancer Res. 2009;15:4963–4967. doi: 10.1158/1078-0432.CCR-09-0628. [DOI] [PubMed] [Google Scholar]
- 88.Exercise therapy as treatment for cardiovascular and oncologic disease after a diagnosis of early-stage cancer. Scott JM, Koelwyn GJ, Hornsby WE, Khouri M, Peppercorn J, Douglas PS, Jones LW. Semin Oncol. 2013;40:218–228. doi: 10.1053/j.seminoncol.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 89.Definitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Voigt JU, Pedrizzetti G, Lysyansky P, et al. Eur Heart J Cardiovasc Imaging. 2015;16:1–11. doi: 10.1093/ehjci/jeu184. [DOI] [PubMed] [Google Scholar]
- 90.Reduction in sample size for studies of remodeling in heart failure by the use of cardiovascular magnetic resonance. Bellenger NG, Davies LC, Francis JM, Coats AJ, Pennell DJ. J Cardiovasc Magn Reson. 2000;2:271–278. doi: 10.3109/10976640009148691. [DOI] [PubMed] [Google Scholar]