Abstract
Background
Epilepsy is one of the most common brain disorders worldwide. It is usually hard to be identified properly, and a third of patients are drug-resistant. Genes related to the progression and prognosis of epilepsy are particularly needed to be identified.
Methods
In our study, we downloaded the Gene Expression Omnibus (GEO) microarray expression profiling dataset GSE143272. Differentially expressed genes (DEGs) with a fold change (FC) >1.2 and a P-value <0.05 were identified by GEO2R and grouped in male, female and overlapping DEGs. Functional enrichment analysis and Protein-Protein Interaction (PPI) network analysis were performed.
Results
In total, 183 DEGs overlapped (77 ups and 106 downs), 302 DEGs (185 ups and 117 downs) in the male dataset, and 750 DEGs (464 ups and 286 downs) in the female dataset were obtained from the GSE143272 dataset. These DEGs were markedly enriched under various Gene Ontology (GO) terms and Kyoto Encyclopedia of Genes and Genomes (KEGG) terms. 16 following hub genes were identified based on PPI network analysis: ADCY7, C3AR1, DEGS1, CXCL1 in male-specific DEGs, TOLLIP, ORM1, ELANE, QPCT in female-specific DEGs and FCAR, CD3G, CLEC12A, MOSPD2, CD3D, ALDH3B1, GPR97, PLAUR in overlapping DEGs.
Conclusion
This discovery-driven study may be useful to provide a novel insight into the diagnosis and treatment of epilepsy. However, more experiments are needed in the future to study the functional roles of these genes in epilepsy.
1 Introduction
Epilepsy, one of the most common brain conditions including both genetic and acquired disorders, affects at least 46 million people worldwide [1]. As a complex diagnosis consisting of multiple subtypes, it is usually hard to be identified properly. People with epilepsy have varied symptoms such as strange sensations, emotions, and behavior or convulsions, muscle spasms, and loss of consciousness when the brain sends out the wrong signals. Antiepileptic drugs are the main treatment and increasing nowadays. However, there are still up to a third of people who have drug-resistant epilepsy [2, 3]. Gender differences in epilepsy are observed in clinical and experimental researches [4]. It is suggested that the incidence of epilepsy is slightly lower in females than in males, and males have greater mortality [5]. Several studies show that unprovoked seizures and status epilepticus are more common in males as compared with females [6–9], whereas some idiopathic generalized epilepsies are more frequent in females [10–12].
Despite a number of genes and signaling pathways in the development and progression of epilepsy have been widely studied, the mechanisms underlying epilepsy are still being unraveled. Currently, the high-throughput sequencing analysis of gene expression, coupled with bioinformatics tools, becomes promising for investigating the novel genes in the initiation and evolution of diseases. In the study, we researched the human peripheral blood sample microarray dataset GSE143272 from the GEO to identify the DEGs between epilepsy patients and normal individuals by applying the bioinformatic method. Our results may provide potential biomarker candidates for clinical diagnosis and therapy of epilepsy.
2 Materials and methods
2.1 Microarray data
We downloaded the microarray expression profiling dataset GSE143272 as peripheral blood expression profiles of patients with epilepsy, deposited by Rawat C et al., from the GEO (https://www.ncbi.nlm.nih.gov/geo/). The dataset was performed on GPL10558 Illumina HumanHT-12 V4.0 expression beadchip platform. The array data for GSE143272 contained 142 samples, including 34 newly diagnosed, drug-free patients with epilepsy, 57 followed-up patients receiving antiepileptic drug monotherapy, and 50 healthy subjects. We selected the drug-free epilepsy patients and healthy control subjects, consisting of 21 male epilepsy patients, 13 female epilepsy patients, 26 male controls, and 25 female controls. All data were downloaded from the GEO freely. No ethics approval and patients’ informed consent were needed for this present study.
2.2 Identification of DEGs
The online analysis tool GEO2R was using to identify the DEGs. Genes with the specific cut-off criteria of FC >1.2 and a P-value <0.05 were considered DEGs. The epilepsy patients and healthy controls were assigned to two groups depending on gender, male and female, according to the annotation of the GSE143272. We conducted the analysis by comparing the male epilepsy patients with male controls, and female epilepsy patients with female controls, respectively. The intersecting and sex-specific genes were obtained by drawing a Venn diagram with Bio-Conductor R and package. The visual hierarchical cluster analysis was also performed, exhibiting a volcano plot of DEGs.
2.3 Functional enrichment analysis of DEGs
We used the online database webgestalt (http://www.webgestalt.org/) to reveal the functions of DEGs, by conducting the analysis including GO annotation and KEGG pathway enrichment analyses. The GO analysis consisted of three divisions: biological process (BP), cellular component (CC), and molecular function (MF). P <0.05 and an enriched gene count ≥5 were selected as the criteria for statistical significance.
2.4 Protein-Protein Interaction (PPI) network analysis
We applied the online database STRING (https://string-db.org/) to perform the PPI network analysis of evaluating the protein-protein interactions between the screened DEGs. The STRING, a PPI database, collects and assesses evidence from many sources, such as scientific literature, to integrate all known and predicted associations between proteins, including physical interactions and functional associations [13]. All PPI pairs with an interaction score of >0.9 as the threshold value were extracted. Moreover, we used Cytoscape v3.7.2 software plugin cytoHubba to calculate the degree of all nodes and construct PPI networks through different topological analyses. Cytoscape, an open source software project that integrates biomolecular interaction networks with high-throughput expression data and other molecular states into a unified conceptual framework, is one of the most powerful network biology analyses and visualization tools when applied in conjunction with large databases of protein-protein, protein-DNA, and genetic interactions [14, 15]. CytoHubba was composed by Chia-Hao Chin et al. for ranking and exploring important nodes in biological networks by their network features, which provides 11 topological analysis methods in one-stop shopping way [16]. The proteins with higher degrees are more likely to be essential proteins, and the importance of nodes within a biological network will be evaluated. These node ranking methods can be divided into two categories: local and global methods. The local-based methods are considered to be the better methods in discovering essential proteins, which only focus on the relationship between the node and its direct neighbors. Due to the heterogeneity of biological networks, it is reasonable to implement multiple methods for capturing essential proteins [16]. Thus, the genes with the top 10 highest degree values were screened by the 3 topological analyses, including maximal clique centrality (MCC), maximum neighborhood component (MNC), and Degree, all of which are local-based methods. The overlapping hub genes in the top 10 by these 3 topological methods were identified.
3 Results
3.1 Identification of DEGs
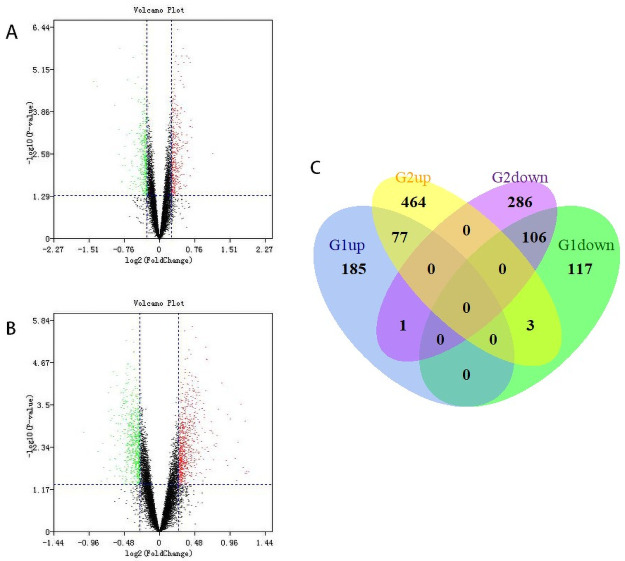
We downloaded the dataset GSE143272 from the GEO database, using GEO2R to analyze the DEGs between drug-free epilepsy patients and normal individuals. Epilepsy patients consisted of idiopathic, cryptogenic, and symptomatic epilepsy. In total, 263 upregulated and 226 downregulated DEGs were identified between male epilepsy patients and male controls, while 544 upregulated and 393 downregulated DEGs were identified between female epilepsy patients and female controls. By conducting Venn analysis, we examined the overlap among the two datasets and obtained a total of 183 (77 upregulated and 106 downregulated) DEGs. Besides, Sex-specific DEGs were screened, within which 302 (185 upregulated and 117 downregulated) DEGs were obtained in the male dataset, while 750 (464 upregulated and 286 downregulated) DEGs were obtained in the female dataset. The volcano plot and Venn diagram for the DEGs are presented in Fig 1.
Fig 1. Identification of DEGs from GSE143272.
(A) Volcano plot of DEGs in the male dataset. (B) Volcano plot of DEGs in the female dataset. Red, upregulation; green, downregulation. (C) Venn diagram of upregulated and downregulated DEGs based on the male (G1) and female (G2) datasets.
3.2 Functional enrichment analysis of DEGs
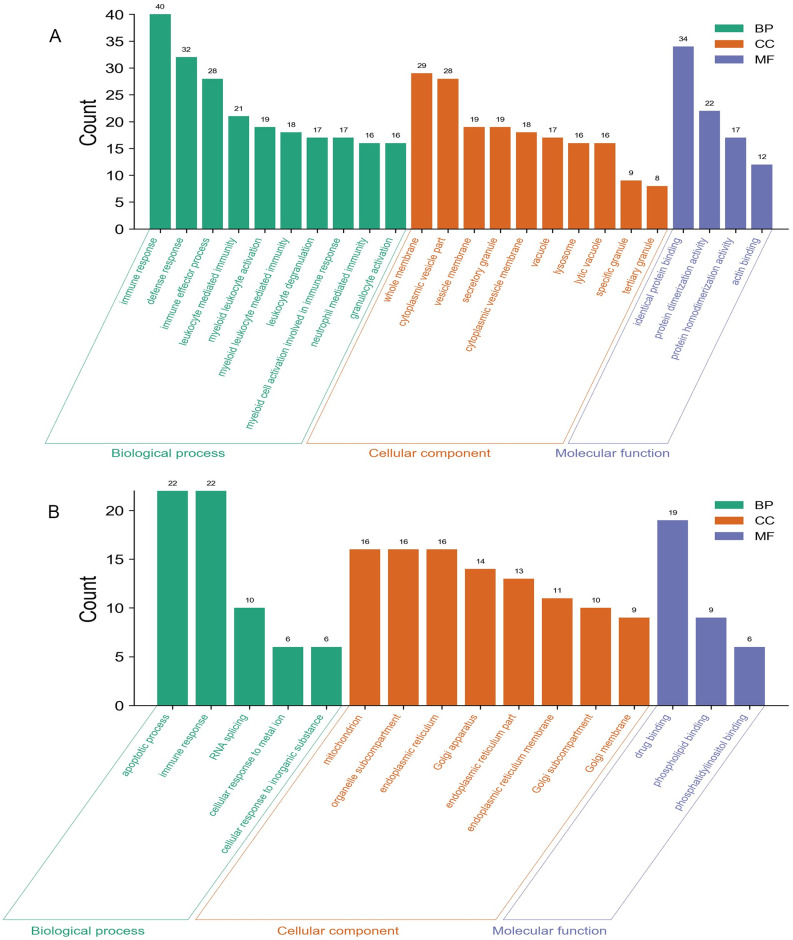
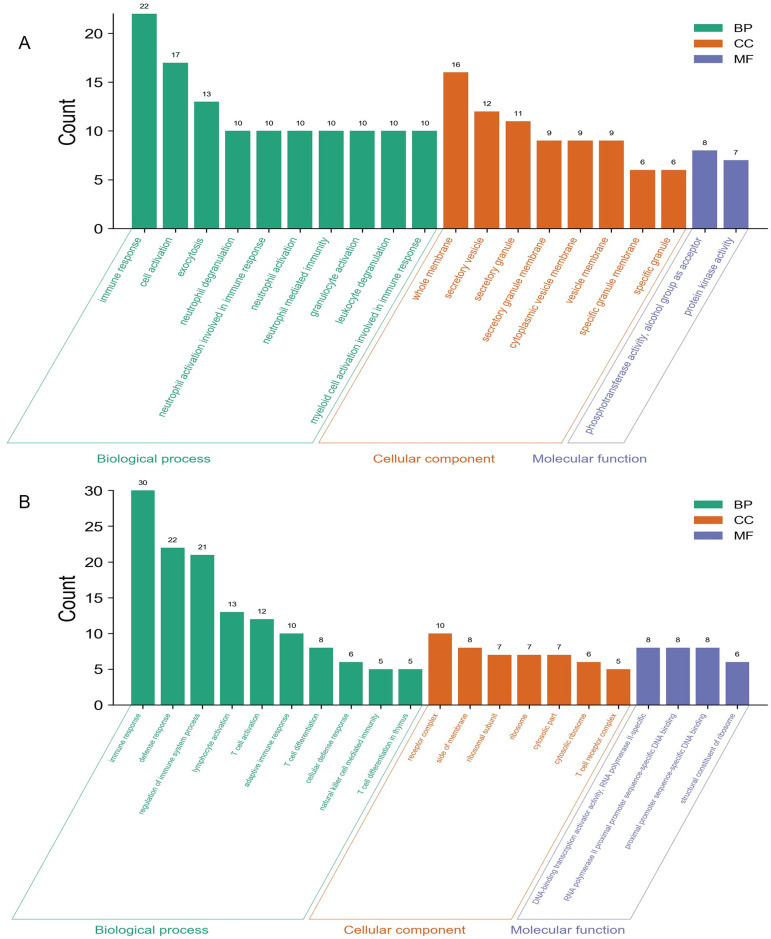
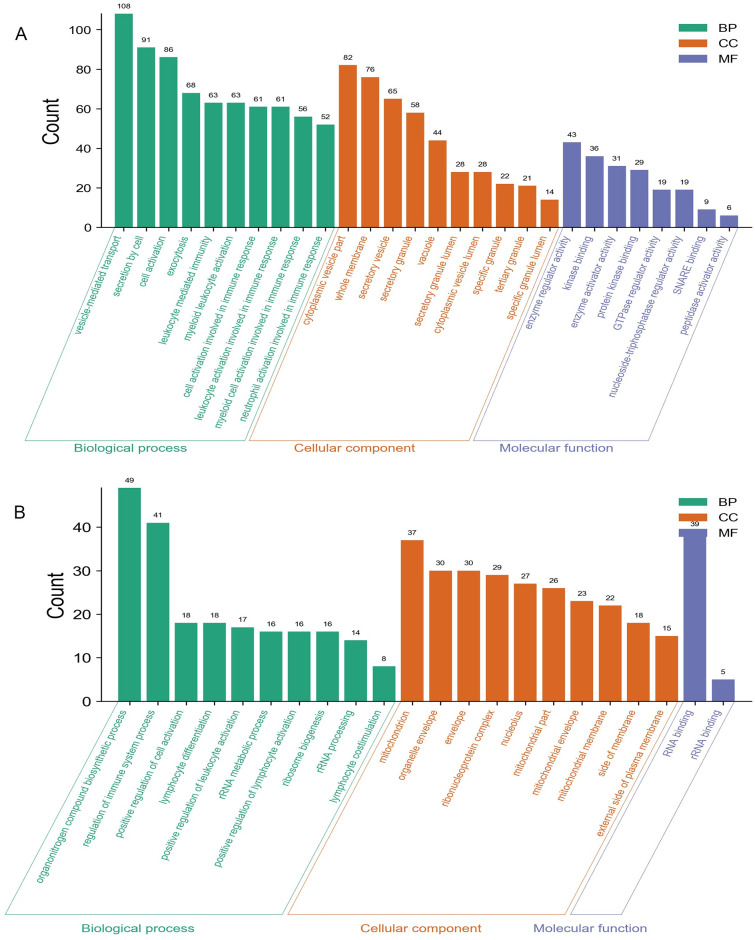
We carried out the functional enrichment analysis for the male-specific DEGs, female-specific DEGs, and overlapping DEGs. The upregulated and downregulated DEGs were analyzed respectively. Top enriched GO terms were shown in Figs 2–4. Results indicated that the male-specific upregulated DEGs were mostly enriched related to immune response in BP, cytoplasmic vesicle part in CC, and identical protein binding in MF term, while male-specific downregulated DEGs were mostly enriched related to RNA splicing in BP, mitochondrion in CC, and phospholipid binding in MF term; the female-specific upregulated DEGs were mostly enriched related to vesicle-mediated transport in BP, cytoplasmic vesicle part in CC, and enzyme activator activity in MF term, while female-specific downregulated DEGs were mostly enriched related to rRNA metabolic process in BP, ribonucleoprotein complex in CC, and RNA binding in MF term; the overlapping upregulated DEGs were mostly enriched in immune response for BP, secretory granule membrane for CC, and phosphotransferase activity, alcohol group as acceptor for MF term, while overlapping downregulated DEGs were mostly enriched in immune response for BP, T cell receptor complex for CC, and structural constituent of ribosome for MF term, respectively.
Fig 2. GO analyses of the male-specific upregulated (A) and downregulated (B).
The y-axis depicts the number of genes. The x-axis lists the enriched functional terms. The color of bars corresponds to different categories of GO analysis (green represents BP, orange represents CC, and purple represents MF).
Fig 4. GO analyses of the overlapping upregulated (A) and downregulated (B) DEGs.
The y-axis depicts the number of genes. The x-axis lists the enriched functional terms. The color of bars corresponds to different categories of GO analysis (green represents BP, orange represents CC, and purple represents MF).
Fig 3. GO analyses of the female-specific upregulated (A) and downregulated (B).
The y-axis depicts the number of genes. The x-axis lists the enriched functional terms. The color of bars corresponds to different categories of GO analysis (green represents BP, orange represents CC, and purple represents MF).
In addition, the top KEGG pathways were presented in Table 1. The male-specific upregulated and downregulated, female-specific upregulated and downregulated, overlapping upregulated and downregulated DEGs were mostly significantly enriched in sphingolipid signaling pathway, rheumatoid arthritis, leishmaniasis, natural killer cell mediated cytotoxicity, platelet activation, and structural constituent of ribosome, respectively.
Table 1. KEGG pathway enrichment analyses for DEGs.
| Gender | Pathway description | P-Value | Count | |
|---|---|---|---|---|
| Male | Upregulated | Sphingolipid signaling pathway | 0.000462 | 7 |
| Biosynthesis of amino acids | 0.001856 | 5 | ||
| Hepatocellular carcinoma | 0.003485 | 7 | ||
| Autophagy | 0.003942 | 6 | ||
| NOD-like receptor signaling pathway | 0.014277 | 6 | ||
| Downregulated | Rheumatoid arthritis | 0.000121 | 5 | |
| Chemokine signaling pathway | 0.003538 | 5 | ||
| Human cytomegalovirus infection | 0.007385 | 5 | ||
| Cytokine-cytokine receptor interaction | 0.021566 | 5 | ||
| Female | Upregulated | Leishmaniasis | 0.000008 | 11 |
| Osteoclast differentiation | 0.000076 | 13 | ||
| Chemokine signaling pathway | 0.000105 | 16 | ||
| Renal cell carcinoma | 0.000148 | 9 | ||
| Hepatitis B | 0.000252 | 13 | ||
| Systemic lupus erythematosus | 0.000437 | 12 | ||
| Kaposi sarcoma-associated herpesvirus infection | 0.000937 | 14 | ||
| Autophagy | 0.001133 | 11 | ||
| Human immunodeficiency virus 1 infection | 0.001169 | 15 | ||
| Phagosome | 0.001433 | 12 | ||
| Downregulated | Natural killer cell mediated cytotoxicity | 3.85E-07 | 13 | |
| Antigen processing and presentation | 0.000366 | 7 | ||
| Graft-versus-host disease | 0.000680 | 5 | ||
| Th1 and Th2 cell differentiation | 0.001076 | 7 | ||
| NF-kappa B signaling pathway | 0.001300 | 7 | ||
| T cell receptor signaling pathway | 0.001856 | 7 | ||
| Sphingolipid signaling pathway | 0.004458 | 7 | ||
| Hematopoietic cell lineage | 0.006826 | 6 | ||
| HIF-1 signaling pathway | 0.007894 | 6 | ||
| Overlap | Upregulated | Platelet activation | 0.000410 | 5 |
| Downregulated | Structural constituent of ribosome | 0.000097 | 6 | |
| DNA-binding transcription activator activity, RNA polymerase II-specific | 0.001188 | 8 | ||
| RNA polymerase II proximal promoter sequence-specific DNA binding | 0.002694 | 8 | ||
| proximal promoter sequence-specific DNA binding | 0.003296 | 8 | ||
3.3 PPI network analysis of DEGs
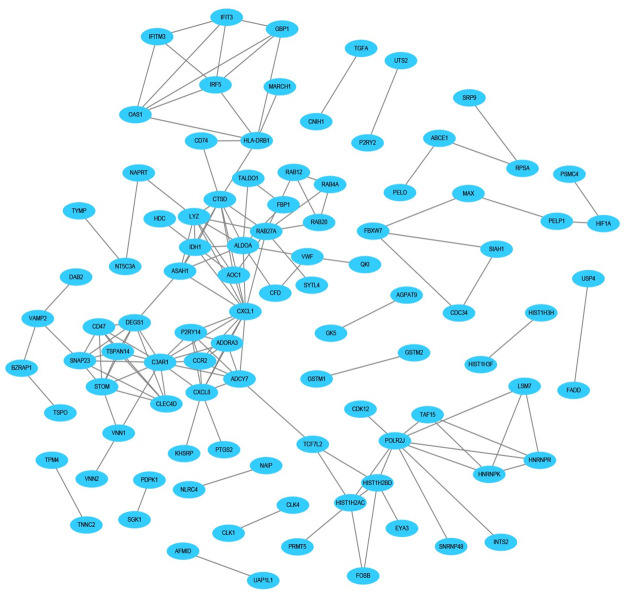
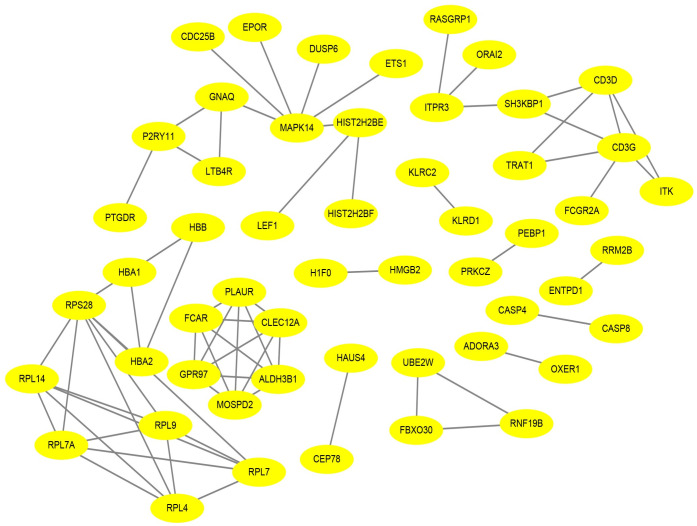
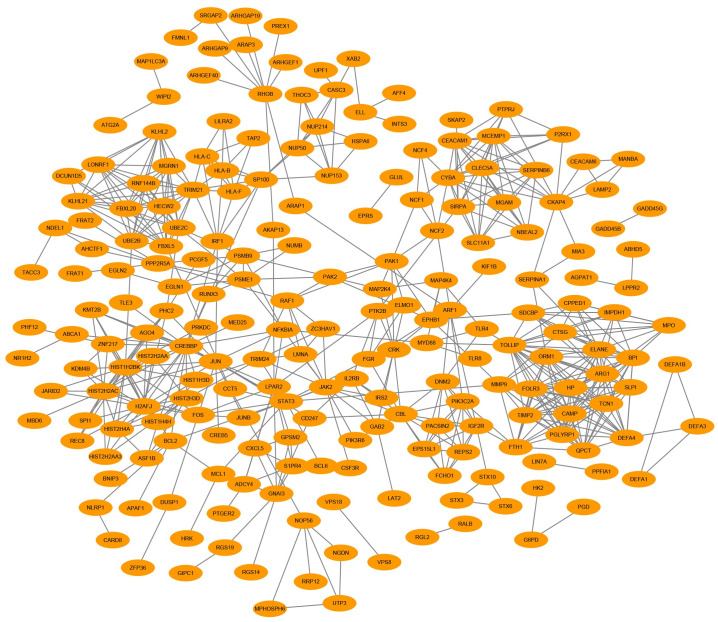
The STRING database was applied to determine the PPI networks with interaction score > 0.9 among the male-specific, female-specific, and overlapping DEGs, which were constructed via Cytoscape software, respectively (Figs 5–7).
Fig 5. Male-specific PPI network in epilepsy.
The PPI network included 96 nodes and 151 edges.
Fig 7. Male and female overlapping PPI network in epilepsy.
The PPI network included 53 nodes and 68 edges.
Fig 6. Female-specific PPI network in epilepsy.
The PPI network included 213 nodes and 604 edges.
Cytohubba plugin of Cytoscape was used to rank the top 10 nodes in the above PPI networks according to 3 topological analysis methods, including MCC, MNC, and Degree. The overlapping hub genes according to the three methods were ADCY7, C3AR1, DEGS1, CXCL1 in male-specific DEGs, TOLLIP, ORM1, ELANE, QPCT in female-specific DEGs, and FCAR, CD3G, CLEC12A, MOSPD2, CD3D, ALDH3B1, GPR97, PLAUR in overlapping DEGs (Table 2).
Table 2. Hub genes for highly differentiated expressed genes ranked in Cytohubba plugin of Cytoscape.
| Gender | Rank methods in CytoHubba | ||
|---|---|---|---|
| Male | MCC | MNC | Degree |
| ADCY7 | ADCY7 | ADCY7 | |
| ADORA3 | ADORA3 | C3AR1 | |
| P2RY14 | P2RY14 | LYZ | |
| C3AR1 | C3AR1 | POLR2J | |
| STOM | LYZ | DEGS1 | |
| CLEC4D | STOM | RAB27A | |
| SNAP23 | CLEC4D | ALDOA | |
| DEGS1 | DEGS1 | CXCL1 | |
| CXCL1 | CXCL1 | CTSD | |
| CXCL8 | CTSD | CXCL8 | |
| Female | TOLLIP | TOLLIP | TOLLIP |
| PGLYRP1 | ARG1 | ARG1 | |
| ORM1 | CREBBP | CREBBP | |
| TCN1 | ORM1 | ORM1 | |
| CAMP | BPI | BPI | |
| TIMP2 | H2AFJ | H2AFJ | |
| FOLR3 | HIST1H2BK | HIST1H2BK | |
| HP | HIST2H2AC | HIST2H2AC | |
| ELANE | ELANE | ELANE | |
| QPCT | QPCT | QPCT | |
| Overlap | FCAR | FCAR | FCAR |
| CD3G | CD3G | CD3G | |
| CLEC12A | CLEC12A | CLEC12A | |
| MOSPD2 | MOSPD2 | GNAQ | |
| MAPK14 | CD3D | MOSPD2 | |
| CD3D | ALDH3B1 | MAPK14 | |
| ALDH3B1 | GPR97 | CD3D | |
| GPR97 | HBA1 | ALDH3B1 | |
| HBA1 | PLAUR | GPR97 | |
| PLAUR | HBA2 | PLAUR | |
4 Discussion
In this study, we performed bioinformatics analysis to search for the potential key genes associated with epilepsy. Male and female epilepsy were compared to healthy controls respectively on the hypothesis that epilepsy in different genders had different mechanisms. The results showed that 302 male-specific DEGs, 750 female-specific DEGs, and 183 overlapping DEGs were successfully identified. Those DEGs were put into multi-step bioinformatic functional annotations, including GO, KEGG, and PPI analysis.
We observed that male, female and overlapping DEGs took part in similar and overlapping biological processes significantly. Most of the biological processes were involved in inflammation and immune system defense response, for example, immune response, leukocyte mediated immunity, T cell activation, etc. In KEGG analysis, sphingolipid signaling pathway was showed upregulated in male patients but downregulated in female patients. It is well known that sphingolipids, such as ceramide, sphingosine, sphingosine-1-phosphate, sphingomyelin, and gangliosides, play an important role in the regulation of steroidogenesis [17]. The sex hormones, such as androgens, estrogens, and progestogens, are thought to influence sex differences in epilepsy [18, 19]. For example, it is suggested that progesterone has anticonvulsant effects, while estradiol has neuroprotective effects along with mild anticonvulsant effects [4]; and androgen can amplify sex differences in the expression of some epileptic disorders [18]. The observation in our study indicated that sphingolipid signaling pathway may have a complex relationship with epilepsy in sex differences.
Based on the PPI networks, 16 hub genes were identified, which were ADCY7, C3AR1, DEGS1, CXCL1 in male-specific DEGs, TOLLIP, ORM1, ELANE, QPCT in female-specific DEGs and FCAR, CD3G, CLEC12A, MOSPD2, CD3D, ALDH3B1, GPR97, PLAUR in overlapping DEGs, respectively.
ADCY7 encodes a membrane-bound adenylate cyclase (AC), which is one of the most ubiquitous signal transduction molecules that catalyzes the formation of cyclic adenosine monophosphate (AMP) from adenosine triphosphate (ATP) [20, 21]. It is indicated that in the central amygdala (CeA) AC7 plays an important role in the modulation of presynaptic gamma-aminobutyric acid (GABA) release. When responding to ethanol and corticotropin-releasing factor (CRF), AC7 increases cAMP signaling and activates protein kinase A cascade, resulting in the release of presynaptic vesicular GABA [22]. GABA, formed within GABAergic axon terminals and released into the synapse, acts on receptors GABAA and GABAB. As the main inhibitory neurotransmitter in the cerebral cortex and hippocampus, GABA maintains inhibitory tension to balance nerve excitation. When the balance is disrupted, seizures may occur. Therefore, GABA agonists suppress seizures, while GABA antagonists cause seizures [23]. As the localization of AC7 was observed in the hippocampus, cerebral cortex, cerebellum, caudate-putamen, and nucleus accumbens [24], ADCY7 involving in regulation of GABA may be correlated with epilepsy.
C3AR1 is a Protein Coding gene. The protein C3a Receptor 1 encoded by this gene is an orphan G protein-coupled receptor for C3a, which is a proinflammatory mediator released during activation of the complement system [25]. The complement pathway is critical in innate immunity [26]. In Alzheimer’s patients, the overexpression of C3 and C3aR1 increases with cognitive decline and Braak staging. It has been shown that loss of C3aR1 in mice causes the rescue of tau pathology and attenuation of neuroinflammation, synaptic deficits, and neurodegeneration. C3aR1 is a critical regulator in neuronal tau pathogenesis and mediating central nervous system (CNS) immune network, as its direct target is STAT3 (signal transducers and activators of transcription) [27]. More and more evidence reveals there are common underlying mechanism mechanisms associated with network hyperexcitability and cognitive decline [28, 29]. Studies have shown that in mouse models the increased abnormal tau and amyloid-β proteins may have a synergistic effect on the occurrence of epileptic seizures [30–32]. Hyperphosphorylation of Tau has been reported in epileptic patients with different forms [33–37] and in a range of animal models of epilepsy [38–40]. Focus on C3AR1 mediating tau pathology might represent a novel opportunity to research therapy for epilepsy.
DEGS1 encodes Delta 4-Desaturase, Sphingolipid 1, a member of the membrane fatty acid desaturase family which is responsible for inserting double bonds into specific positions in fatty acids. The related pathways of DEGS1 are sphingolipid signaling pathway and sphingolipid metabolism [41, 42]. In the nervous system, sphingolipids are pivotal constituents of myelin formation in glial cells, which can improve the efficiency and speed of action potentials. Perturbations of the sphingolipid metabolism can result in rearrangements in the plasma membrane, which has been associated with the development of various neurological disorders [43–46]. It has been reported that a variant in DEGS1 leads to a novel early-onset autosomal recessive complex neurological disease with Intelligent disability, progressive spastic paraplegia, scoliosis, and epilepsy. The DEGS1 variant encodes C4-dihydroceramide desaturase, which plays an important role in a pathway of ceramide/phospholipids synthesis [41, 47]. In this study, the present findings also revealed that DEGS1 was correlated with epilepsy.
CXCL1 gene encodes a member of the CXC subfamily of chemokines. The CXCL1 protein plays a pivotal role by recruiting and activating neutrophils in inflammation when signaling through the CXCR2 receptor [48]. It is being increasingly recognized that immunity and inflammatory processes in the brain contribute to the pathogenesis of epilepsy [49–51]. The study reported that CXCL1 concentrations increase significantly after seizure onset, which results in a strong induction of chemotactic response from brain cells that recruits circulating neutrophils to the injured brain tissues [52]. The activated neutrophils can exacerbate the initial injury by the damage to the surrounding healthy area [53]. Thus, CXCL1 may be a novel therapeutic target for epilepsy.
TOLLIP encodes the Toll-interacting protein (Tollip), which is present in a complex with the interleukin-1 receptor associated kinase (IRAK). With the activation of IL-1β, the Tollip–IRAK complex is recruited and disrupted [54]. The overexpression of Tollip can impair IL-1β-induced activation of NF-κB, indicating that Tollip is an inhibitory modulator in inflammatory signaling [54, 55]. The cerebral cortex may display the highest density of Tollip protein [56]. Therefore, Tollip may be a potential target to provide neuroprotective effects by reducing neuroinflammation in epilepsy [57].
ORM1, accounting for 75% of plasma ORM, is an inflammatory factor with multiple activities [58]. Plasma concentration of ORM increases under the control of various regulatory mediators, including inflammatory stimuli, such as glucocorticoids, tumor necrosis factor (TNF)-α, interleukin (IL)-1, IL-8, IL-11, IL-6, and IL-6 related cytokines [59–61]. ORM has been revealed to have effects on immunoregulation, such as decreasing the rolling, adhesion, and migration of neutrophils [62–64]. In the ORM family, ORM1 is the unique member that could be considered as an acute-phase protein. ORM1 regulates the inflammation by contributing both anti- and pro-inflammatory signals to cytokine-mediated feedback mechanisms activated by the acute-phase response [65]. What’s more, ORM can also enhance the functional integrity of the blood-brain barrier (BBB) [66]. The study showed that decreasing the ORM1 expression may be a possible mechanism for the aggravation of BBB damage [67], while the disruption of BBB may underlie the occurrence of seizures and epilepsy [68, 69]. This study indicated that ORM1 may play important role in epilepsy.
ELANE encodes neutrophil elastase (NE), which belongs to the family of serine proteases. It has been reported that the mutations in the gene usually cause Cyclic neutropenia (CyN) and severe congenital neutropenia (SCN) [70, 71]. As NE is involved in immune responses and widely regarded as a regulatory factor in degenerative and inflammatory diseases through proteolysis of collagen-IV and elastin [72, 73], ELANE may be also associated with epilepsy.
The QPCT gene encodes glutaminyl cyclase (QC). QC is an enzyme responsible for catalyzing the posttranslational modification of N-terminal glutamate to pyroglutamate in many neuroendocrine peptides, which renders the protein more susceptible to neurotoxic [74]. Amyloid-β (Aβ) deposits have been found to be a characteristic neuropathological feature of Alzheimer’s disease (AD) [75]. N-terminally modified Aβ, pyroglutamate-amyloid-β (pE3-Aβ), is a major component of Aβ deposits specific to human AD [76, 77]. pE3-Aβ is processed by QC and/or its isoenzymes (isoQC), formed by cyclization of truncated Aβ species, rapidly aggregates and initiates other Aβ aggregates [78–80]. The formation of large amounts of pE3-Aβ has been shown to be QC-dependent. Reducing QC-dependent post-translational pE3-Aβ formation rate can in turn reduce the number of neurotoxic Aβ species [81]. As it is reported that in the pathophysiology of epilepsy Aβ may play an important role [82–84], QPCT may also serve as a novel strategy for the treatment of epilepsy.
FCAR encodes FcαRI or CD89, which is expressed on cells of the myeloid lineage [85] and is a bifunctional inhibitory/activating receptor for the Fc region of Immunoglobulin A (IgA) [86]. On one hand, FcαRI plays an anti-inflammatory role when binding to monomeric IgA and inducing inhibitory ITAMi signaling. On the other hand, when cross-linking IgA immune complexes, FcαRI mediates pro-inflammatory function, activating immune cells and leading to the elimination of pathogens [87, 88]. Neutrophilic activation is beneficial for infection; however, overabundant IgA complexes can trigger severe tissue damage causing various autoimmune diseases. Achiron A et al. found that FCAR participates in the pathogenic pathways in MS [89], which indicated FCAR may be involved in the various inflammatory responses in CNS. Targeting FcαRI might serve as a novel therapeutic strategy for epilepsy.
CD3G and CD3D encode CD3γ and CD3δ, respectively. Both CD3γ and CD3δ are part of the T-cell receptor/CD3 complex (TCR/CD3 complex) [90], which is crucial for the development, activation, and differentiation of T cells [91]. The CD3D gene defect, which occurs early in life, leads to severe immune deficiency, making a person susceptible to infection [92]. However, the CD3G mutation results in a milder clinical phenotype that is primarily autoimmune [93–95]. The severe immune deficiency may cause intracranial infection, while autoimmunity in brain may lead to autoimmune encephalitis, both of which are correlated to epilepsy [96–99]. However, the role of CD3G and CD3D in CNS is still not clear.
CLEC12A is a C-type lectin receptor (CLR) and a Src homology region 2 domain-containing phosphatase 1 and 2 (SHP-1 and -2)-associated receptor, highly expressed on human dendritic cells (DCs). CLEC12A contains a single immunoreceptor tyrosine-based inhibitory motif (ITIM) in its cytoplasmic tail. In response to chemokine CCL2, ITIM can associate with SHP-1 and SHP-2, involved in inhibitory signaling as a key molecule to deliver immature DCs to the CNS across the BBB [100]. It has been reported that DCs can initiate autoimmune demyelination and inflammation in CNS by presenting antigen to autoreactive myelin-specific T cells [101], while in the CLEC12A-KO mice the reduction of DC infiltration and demyelination was observed [102]. Thus, CLEC12A may be a promising target to inhibit seizures in brain.
MOSPD2 (motile sperm domain-containing protein 2) is the surface protein predominantly expressed on cytoplasmic membrane of human monocytes. MOSPD2 is also found in neutrophils, but not in lymphocytes. It is revealed that MOSPD2 is critical in regulating the inflammatory monocyte and neutrophil migration without activating ligands [103]. The monocyte from blood can rise macrophage in CNS, which is dominant in demyelination [104]. More and more researches suggest that chronic demyelination in multiple sclerosis can induce seizure [105–107]. As it has been demonstrated silencing or neutralizing MOSPD2 not only reduced the proportion of inflammatory monocytes in the blood significantly but also inhibited monocyte migrating into CNS [108], MOSPD2 may be a potential target for the treatment of epilepsy.
ALDH3B1 encodes the protein that belongs to the ALDH3 protein family (Aldehyde Dehydrogenase Family 3) [109–111]. It has been shown that ALDH3B1 plays a critical role in the cellular defense against oxidative stress processes and aldehyde toxicity [112, 113]. Oxidative stress toxicity and lowered antioxidant defense are considered as contributing factors in the genesis and progression of epilepsy [114–116], while epileptic seizures, especially recurrent seizures may also increase oxidative stress, which will result in treatment resistance [117–120]. Oxidative stress can lead to the occurrence of lipid peroxidation (LPO) and resulting in plenty of aldehydes, such as 4-hydroxy-2-nonenal (4HNE) [121]. In the CNS, dopamine is metabolized to 3,4-dihydroxyphenylacetaldehyde (DOPAL), while both norepinephrine and epinephrine are metabolized to 3,4-dihydroxyphenylglycol aldehyde (DOPEGAL) [122]. Aldehydes, including DOPAL, DOPEGAL, and 4HNE, are neurotoxic and involving in Parkinson’s disease (PD) and AD [123, 124]. ALDH7A1, the other member of the ALDH protein family, has been demonstrated to be related to pyridoxine-dependent epilepsy [125–127]. Thus, it is indicated that ALDH3B1 may have a protective role in various brain diseases including epilepsy.
GPR97 belongs to the G protein-coupled receptors (GPCRs), the largest receptor superfamily broadly involved in the regulation of biological processes and various diseases, including CNS disorders, such as anxiety, depression, schizophrenia, epilepsy, Alzheimer’s disease, and Parkinson’s disease [128, 129]. GPR97, expressed in immune cells and lymphatic endothelial cells [130, 131], contributes to macrophage-associated inflammation [132]. GPR97 also regulates the development of B-cell and NF-κB activity [133], which plays a critical role in encephalitogenic T cell activation [134]. The study reveals that the loss of GPR97 results in the increase of constitutive expression and activation of NF-κB pathway components, in turn causing severe inflammation and demyelination in CNS [135]. Thus, modulation of GPR97 functions or its pathway may be a potential treatment of epilepsy.
PLAUR encodes urokinase-type plasminogen activator receptor (uPAR) [136], which is a glycoprotein linked to the cell membrane by a glycosylphosphatidylinositol anchor [137]. The uPAR is a key regulator in many processes involving in not only cell signaling, proliferation, differentiation, and migration, but also tissue remodeling [137–139]. uPAR plays an important role in the early and injured brain. The uPA-uPAR complex induces axonal growth and regeneration by stimulating neuronal migration and neuritogenesis via both proteolytic and nonproteolytic events [140–142]. The dysregulation of uPA/uPAR axis is involved in various CNS disorders [139]. In rats undergoing seizures, expression of uPAR is increased in interneurons [143], while deficiency of both uPA and uPAR in mice increases seizure susceptibility [143–145]. In this study, the present findings also revealed that PLAUR was correlated with epilepsy.
Summarily, using the profile dataset and bioinformatics analysis, 16 epilepsy-associated hub genes were identified (Table 3). However, there are some limitations of this study. The lack of experimental evidence is probably the biggest limitation. In addition, the mechanism of these 16 hub genes in epilepsy is still unclear. Hence, more research should be carried out to investigate the functional roles of these hub genes in epilepsy.
Table 3. Summary of functions of hub genes.
| Hub genes | Functions |
|---|---|
| ADCY7 | Modulate the release of presynaptic GABA |
| C3AR1 | Critical regulator in neuronal tau pathogenesis and mediating CNS immune network |
| DEGS1 | Play an important role in sphingolipid signaling pathway and sphingolipid metabolism |
| CXCL1 | Recruit and activate neutrophils in inflammation |
| TOLLIP | Inhibitory modulator in inflammatory signaling |
| ORM1 | Regulate the inflammation and enhance the functional integrity of BBB |
| ELANE | Regulatory factor in degenerative and inflammatory diseases |
| QPCT | Catalyze the posttranslational modification of N-terminal glutamate of proteins to pyroglutamate in many neuroendocrine peptides |
| FCAR | Bifunctional inhibitory/activating receptor for the Fc region of IgA |
| CD3G and CD3D | Crucial for the development, activation, and differentiation of T cells |
| CLEC12A | Deliver immature DCs to the CNS across the BBB |
| MOSPD2 | Critical in regulating the inflammatory monocyte and neutrophil migration without activating ligands |
| ALDH3B1 | Play a critical role in the cellular defense against oxidative stress processes and aldehyde toxicity |
| GPR97 | Contribute to macrophage-associated inflammation; regulate the development of B-cell and NF-κB activity involving in encephalitogenic T cell activation |
| PLAUR | Key regulator involving in not only cell signaling, proliferation, differentiation, and migration, but also tissue remodeling |
5 Conclusion
In conclusion, by using the integrated bioinformatics analysis for gene expression profiles in epilepsy, we identified 16 hub genes, including sex-specific genes. These hub genes were correlated with the pathogenesis and prognosis of epilepsy. This study may contribute to further insight into epilepsy, by digging out the potential diagnostic and prognostic biomarkers, as well as therapeutic targets. Nevertheless, in the future, more research (in vivo and in vitro experiments) should be carried out to validate the functional roles of these genes in epilepsy.
Supporting information
(XLSX)
Data Availability
All relevant data are within the manuscript.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Beghi E. The Epidemiology of Epilepsy. Neuroepidemiology. 2020;54(2):185–91. Epub 2019/12/19. doi: 10.1159/000503831 . [DOI] [PubMed] [Google Scholar]
- 2.Thijs RD, Surges R, O’Brien TJ, Sander JW. Epilepsy in adults. Lancet. 2019;393(10172):689–701. Epub 2019/01/29. doi: 10.1016/S0140-6736(18)32596-0 . [DOI] [PubMed] [Google Scholar]
- 3.Fisher RS, Cross JH, French JA, Higurashi N, Hirsch E, Jansen FE, et al. Operational classification of seizure types by the International League Against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58(4):522–30. Epub 2017/03/10. doi: 10.1111/epi.13670 . [DOI] [PubMed] [Google Scholar]
- 4.Veliskova J, Desantis KA. Sex and hormonal influences on seizures and epilepsy. Horm Behav. 2013;63(2):267–77. Epub 2012/04/17. doi: 10.1016/j.yhbeh.2012.03.018 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McHugh JC, Delanty N. Epidemiology and classification of epilepsy: gender comparisons. Int Rev Neurobiol. 2008;83:11–26. Epub 2008/10/22. doi: 10.1016/S0074-7742(08)00002-0 . [DOI] [PubMed] [Google Scholar]
- 6.Hauser WA, Annegers JF, Kurland LT. Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota: 1935–1984. Epilepsia. 1993;34(3):453–68. Epub 1993/05/01. doi: 10.1111/j.1528-1157.1993.tb02586.x . [DOI] [PubMed] [Google Scholar]
- 7.Kotsopoulos IA, van Merode T, Kessels FG, de Krom MC, Knottnerus JA. Systematic review and meta-analysis of incidence studies of epilepsy and unprovoked seizures. Epilepsia. 2002;43(11):1402–9. Epub 2002/11/09. doi: 10.1046/j.1528-1157.2002.t01-1-26901.x . [DOI] [PubMed] [Google Scholar]
- 8.Coeytaux A, Jallon P, Galobardes B, Morabia A. Incidence of status epilepticus in French-speaking Switzerland: (EPISTAR). Neurology. 2000;55(5):693–7. Epub 2000/09/12. doi: 10.1212/wnl.55.5.693 . [DOI] [PubMed] [Google Scholar]
- 9.Hesdorffer DC, Logroscino G, Cascino G, Annegers JF, Hauser WA. Incidence of status epilepticus in Rochester, Minnesota, 1965–1984. Neurology. 1998;50(3):735–41. Epub 1998/04/01. doi: 10.1212/wnl.50.3.735 . [DOI] [PubMed] [Google Scholar]
- 10.Christensen J, Kjeldsen MJ, Andersen H, Friis ML, Sidenius P. Gender differences in epilepsy. Epilepsia. 2005;46(6):956–60. Epub 2005/06/11. doi: 10.1111/j.1528-1167.2005.51204.x . [DOI] [PubMed] [Google Scholar]
- 11.Kleveland G, Engelsen BA. Juvenile myoclonic epilepsy: clinical characteristics, treatment and prognosis in a Norwegian population of patients. Seizure. 1998;7(1):31–8. Epub 1998/04/21. doi: 10.1016/s1059-1311(98)90005-x . [DOI] [PubMed] [Google Scholar]
- 12.Waaler PE, Blom BH, Skeidsvoll H, Mykletun A. Prevalence, classification, and severity of epilepsy in children in western Norway. Epilepsia. 2000;41(7):802–10. Epub 2000/07/18. doi: 10.1111/j.1528-1157.2000.tb00246.x . [DOI] [PubMed] [Google Scholar]
- 13.Szklarczyk D, Gable AL, Nastou KC, Lyon D, Kirsch R, Pyysalo S, et al. The STRING database in 2021: customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021;49(D1):D605–D12. Epub 2020/11/26. doi: 10.1093/nar/gkaa1074 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13(11):2498–504. Epub 2003/11/05. doi: 10.1101/gr.1239303 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doncheva NT, Morris JH, Gorodkin J, Jensen LJ. Cytoscape StringApp: Network Analysis and Visualization of Proteomics Data. J Proteome Res. 2019;18(2):623–32. Epub 2018/11/20. doi: 10.1021/acs.jproteome.8b00702 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chin CH, Chen SH, Wu HH, Ho CW, Ko MT, Lin CY. cytoHubba: identifying hub objects and sub-networks from complex interactome. BMC Syst Biol. 2014;8Suppl 4:S11. Epub 2014/12/19. doi: 10.1186/1752-0509-8-S4-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lucki NC, Sewer MB. Multiple roles for sphingolipids in steroid hormone biosynthesis. Subcell Biochem. 2008;49:387–412. Epub 2008/08/30. doi: 10.1007/978-1-4020-8831-5_15 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frye CA. Hormonal influences on seizures: basic neurobiology. Int Rev Neurobiol. 2008;83:27–77. Epub 2008/10/22. doi: 10.1016/S0074-7742(08)00003-2 . [DOI] [PubMed] [Google Scholar]
- 19.Qureshi IA, Mehler MF. Sex, epilepsy, and epigenetics. Neurobiol Dis. 2014;72Pt B:210–6. Epub 2014/07/08. doi: 10.1016/j.nbd.2014.06.019 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dessauer CW, Watts VJ, Ostrom RS, Conti M, Dove S, Seifert R. International Union of Basic and Clinical Pharmacology. CI. Structures and Small Molecule Modulators of Mammalian Adenylyl Cyclases. Pharmacol Rev. 2017;69(2):93–139. Epub 2017/03/04. doi: 10.1124/pr.116.013078 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cooper DM, Crossthwaite AJ. Higher-order organization and regulation of adenylyl cyclases. Trends Pharmacol Sci. 2006;27(8):426–31. Epub 2006/07/06. doi: 10.1016/j.tips.2006.06.002 . [DOI] [PubMed] [Google Scholar]
- 22.Cruz MT, Bajo M, Maragnoli ME, Tabakoff B, Siggins GR, Roberto M. Type 7 Adenylyl Cyclase is Involved in the Ethanol and CRF Sensitivity of GABAergic Synapses in Mouse Central Amygdala. Front Neurosci. 2011;4:207. Epub 2011/01/25. doi: 10.3389/fnins.2010.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Treiman DM. GABAergic mechanisms in epilepsy. Epilepsia. 2001;42Suppl 3:8–12. Epub 2001/08/25. doi: 10.1046/j.1528-1157.2001.042suppl.3008.x . [DOI] [PubMed] [Google Scholar]
- 24.Mons N, Yoshimura M, Ikeda H, Hoffman PL, Tabakoff B. Immunological assessment of the distribution of type VII adenylyl cyclase in brain. Brain Res. 1998;788(1–2):251–61. Epub 1998/05/21. doi: 10.1016/s0006-8993(98)00005-5 . [DOI] [PubMed] [Google Scholar]
- 25.Coulthard LG, Woodruff TM. Is the complement activation product C3a a proinflammatory molecule? Re-evaluating the evidence and the myth. J Immunol. 2015;194(8):3542–8. Epub 2015/04/08. doi: 10.4049/jimmunol.1403068 . [DOI] [PubMed] [Google Scholar]
- 26.Veerhuis R, Nielsen HM, Tenner AJ. Complement in the brain. Mol Immunol. 2011;48(14):1592–603. Epub 2011/05/07. doi: 10.1016/j.molimm.2011.04.003 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Litvinchuk A, Wan YW, Swartzlander DB, Chen F, Cole A, Propson NE, et al. Complement C3aR Inactivation Attenuates Tau Pathology and Reverses an Immune Network Deregulated in Tauopathy Models and Alzheimer’s Disease. Neuron. 2018;100(6):1337–53 e5. Epub 2018/11/13. doi: 10.1016/j.neuron.2018.10.031 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paudel YN, Angelopoulou E, Jones NC, O’Brien TJ, Kwan P, Piperi C, et al. Tau Related Pathways as a Connecting Link between Epilepsy and Alzheimer’s Disease. ACS Chem Neurosci. 2019;10(10):4199–212. Epub 2019/09/19. doi: 10.1021/acschemneuro.9b00460 . [DOI] [PubMed] [Google Scholar]
- 29.Friedman D, Honig LS, Scarmeas N. Seizures and epilepsy in Alzheimer’s disease. CNS Neurosci Ther. 2012;18(4):285–94. Epub 2011/11/11. doi: 10.1111/j.1755-5949.2011.00251.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roberson ED, Halabisky B, Yoo JW, Yao J, Chin J, Yan F, et al. Amyloid-beta/Fyn-induced synaptic, network, and cognitive impairments depend on tau levels in multiple mouse models of Alzheimer’s disease. J Neurosci. 2011;31(2):700–11. Epub 2011/01/14. doi: 10.1523/JNEUROSCI.4152-10.2011 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palop JJ, Chin J, Roberson ED, Wang J, Thwin MT, Bien-Ly N, et al. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer’s disease. Neuron. 2007;55(5):697–711. Epub 2007/09/06. doi: 10.1016/j.neuron.2007.07.025 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scharfman HE. Alzheimer’s disease and epilepsy: insight from animal models. Future Neurol. 2012;7(2):177–92. Epub 2012/06/23. doi: 10.2217/fnl.12.8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sen A, Thom M, Martinian L, Harding B, Cross JH, Nikolic M, et al. Pathological tau tangles localize to focal cortical dysplasia in older patients. Epilepsia. 2007;48(8):1447–54. Epub 2007/04/20. doi: 10.1111/j.1528-1167.2007.01107.x . [DOI] [PubMed] [Google Scholar]
- 34.Dyment DA, Smith AC, Humphreys P, Schwartzentruber J, Beaulieu CL, Consortium FC, et al. Homozygous nonsense mutation in SYNJ1 associated with intractable epilepsy and tau pathology. Neurobiol Aging. 2015;36(2):1222 e1–5. Epub 2014/10/16. doi: 10.1016/j.neurobiolaging.2014.09.005 . [DOI] [PubMed] [Google Scholar]
- 35.Tai XY, Koepp M, Duncan JS, Fox N, Thompson P, Baxendale S, et al. Hyperphosphorylated tau in patients with refractory epilepsy correlates with cognitive decline: a study of temporal lobe resections. Brain. 2016;139(Pt 9):2441–55. Epub 2016/08/09. doi: 10.1093/brain/aww187 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu C, Russin J, Heck C, Kawata K, Adiga R, Yen W, et al. Dysregulation of PINCH signaling in mesial temporal epilepsy. J Clin Neurosci. 2017;36:43–52. Epub 2016/11/14. doi: 10.1016/j.jocn.2016.10.012 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thom M, Liu JY, Thompson P, Phadke R, Narkiewicz M, Martinian L, et al. Neurofibrillary tangle pathology and Braak staging in chronic epilepsy in relation to traumatic brain injury and hippocampal sclerosis: a post-mortem study. Brain. 2011;134(Pt 10):2969–81. Epub 2011/09/10. doi: 10.1093/brain/awr209 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crespo-Biel N, Canudas AM, Camins A, Pallas M. Kainate induces AKT, ERK and cdk5/GSK3beta pathway deregulation, phosphorylates tau protein in mouse hippocampus. Neurochem Int. 2007;50(2):435–42. Epub 2006/11/23. doi: 10.1016/j.neuint.2006.10.002 . [DOI] [PubMed] [Google Scholar]
- 39.Liang Z, Liu F, Iqbal K, Grundke-Iqbal I, Gong CX. Dysregulation of tau phosphorylation in mouse brain during excitotoxic damage. J Alzheimers Dis. 2009;17(3):531–9. Epub 2009/04/14. doi: 10.3233/JAD-2009-1069 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tian FF, Zeng C, Ma YF, Guo TH, Chen JM, Chen Y, et al. Potential roles of Cdk5/p35 and tau protein in hippocampal mossy fiber sprouting in the PTZ kindling model. Clin Lab. 2010;56(3–4):127–36. Epub 2010/05/19. . [PubMed] [Google Scholar]
- 41.Karsai G, Kraft F, Haag N, Korenke GC, Hanisch B, Othman A, et al. DEGS1-associated aberrant sphingolipid metabolism impairs nervous system function in humans. J Clin Invest. 2019;129(3):1229–39. Epub 2019/01/09. doi: 10.1172/JCI124159 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pant DC, Dorboz I, Schluter A, Fourcade S, Launay N, Joya J, et al. Loss of the sphingolipid desaturase DEGS1 causes hypomyelinating leukodystrophy. J Clin Invest. 2019;129(3):1240–56. Epub 2019/01/09. doi: 10.1172/JCI123959 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Olsen ASB, Faergeman NJ. Sphingolipids: membrane microdomains in brain development, function and neurological diseases. Open Biol. 2017;7(5). Epub 2017/06/02. doi: 10.1098/rsob.170069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grassi S, Chiricozzi E, Mauri L, Sonnino S, Prinetti A. Sphingolipids and neuronal degeneration in lysosomal storage disorders. J Neurochem. 2019;148(5):600–11. Epub 2018/07/01. doi: 10.1111/jnc.14540 . [DOI] [PubMed] [Google Scholar]
- 45.Sandhoff K. Neuronal sphingolipidoses: Membrane lipids and sphingolipid activator proteins regulate lysosomal sphingolipid catabolism. Biochimie. 2016;130:146–51. Epub 2016/10/30. doi: 10.1016/j.biochi.2016.05.004 . [DOI] [PubMed] [Google Scholar]
- 46.Hannun YA, Obeid LM. Sphingolipids and their metabolism in physiology and disease. Nat Rev Mol Cell Biol. 2018;19(3):175–91. Epub 2017/11/23. doi: 10.1038/nrm.2017.107 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dolgin V, Straussberg R, Xu R, Mileva I, Yogev Y, Khoury R, et al. DEGS1 variant causes neurological disorder. Eur J Hum Genet. 2019;27(11):1668–76. Epub 2019/06/13. doi: 10.1038/s41431-019-0444-z . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sawant KV, Poluri KM, Dutta AK, Sepuru KM, Troshkina A, Garofalo RP, et al. Chemokine CXCL1 mediated neutrophil recruitment: Role of glycosaminoglycan interactions. Sci Rep. 2016;6:33123. Epub 2016/09/15. doi: 10.1038/srep33123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vezzani A, Granata T. Brain inflammation in epilepsy: experimental and clinical evidence. Epilepsia. 2005;46(11):1724–43. Epub 2005/11/24. doi: 10.1111/j.1528-1167.2005.00298.x . [DOI] [PubMed] [Google Scholar]
- 50.Rana A, Musto AE. The role of inflammation in the development of epilepsy. J Neuroinflammation. 2018;15(1):144. Epub 2018/05/17. doi: 10.1186/s12974-018-1192-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rakhade SN, Jensen FE. Epileptogenesis in the immature brain: emerging mechanisms. Nat Rev Neurol. 2009;5(7):380–91. Epub 2009/07/07. doi: 10.1038/nrneurol.2009.80 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johnson EA, Dao TL, Guignet MA, Geddes CE, Koemeter-Cox AI, Kan RK. Increased expression of the chemokines CXCL1 and MIP-1alpha by resident brain cells precedes neutrophil infiltration in the brain following prolonged soman-induced status epilepticus in rats. J Neuroinflammation. 2011;8:41. Epub 2011/05/04. doi: 10.1186/1742-2094-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Clark RS, Carlos TM, Schiding JK, Bree M, Fireman LA, DeKosky ST, et al. Antibodies against Mac-1 attenuate neutrophil accumulation after traumatic brain injury in rats. J Neurotrauma. 1996;13(6):333–41. Epub 1996/06/01. doi: 10.1089/neu.1996.13.333 . [DOI] [PubMed] [Google Scholar]
- 54.Burns K, Clatworthy J, Martin L, Martinon F, Plumpton C, Maschera B, et al. Tollip, a new component of the IL-1RI pathway, links IRAK to the IL-1 receptor. Nat Cell Biol. 2000;2(6):346–51. Epub 2000/06/15. doi: 10.1038/35014038 . [DOI] [PubMed] [Google Scholar]
- 55.Humbert-Claude M, Duc D, Dwir D, Thieren L, Sandstrom von Tobel J, Begka C, et al. Tollip, an early regulator of the acute inflammatory response in the substantia nigra. J Neuroinflammation. 2016;13(1):303. Epub 2016/12/09. doi: 10.1186/s12974-016-0766-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Burgess DJ. Gene expression: spatial characterization of proteomes. Nat Rev Genet. 2015;16(3):129. Epub 2015/02/11. doi: 10.1038/nrg3910. [DOI] [PubMed] [Google Scholar]
- 57.Amini E, Golpich M, Farjam AS, Kamalidehghan B, Mohamed Z, Ibrahim NM, et al. Brain Lipopolysaccharide Preconditioning-Induced Gene Reprogramming Mediates a Tolerance State in Electroconvulsive Shock Model of Epilepsy. Front Pharmacol. 2018;9:416. Epub 2018/05/17. doi: 10.3389/fphar.2018.00416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou Z, Li Z, Sun Z, Zhang X, Lu L, Wang Y, et al. S100A9 and ORM1 serve as predictors of therapeutic response and prognostic factors in advanced extranodal NK/T cell lymphoma patients treated with pegaspargase/gemcitabine. Sci Rep. 2016;6:23695. Epub 2016/03/30. doi: 10.1038/srep23695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alam T, Papaconstantinou J. Interaction of acute-phase-inducible and liver-enriched nuclear factors with the promoter region of the mouse alpha 1-acid glycoprotein gene-1. Biochemistry. 1992;31(7):1928–36. Epub 1992/02/25. doi: 10.1021/bi00122a005 . [DOI] [PubMed] [Google Scholar]
- 60.Baumann H, Gauldie J. Regulation of hepatic acute phase plasma protein genes by hepatocyte stimulating factors and other mediators of inflammation. Mol Biol Med. 1990;7(2):147–59. Epub 1990/04/01. . [PubMed] [Google Scholar]
- 61.Wigmore SJ, Fearon KC, Maingay JP, Lai PB, Ross JA. Interleukin-8 can mediate acute-phase protein production by isolated human hepatocytes. Am J Physiol. 1997;273(4):E720–6. Epub 1997/11/14. doi: 10.1152/ajpendo.1997.273.4.E720 . [DOI] [PubMed] [Google Scholar]
- 62.Mestriner FL, Spiller F, Laure HJ, Souto FO, Tavares-Murta BM, Rosa JC, et al. Acute-phase protein alpha-1-acid glycoprotein mediates neutrophil migration failure in sepsis by a nitric oxide-dependent mechanism. Proc Natl Acad Sci U S A. 2007;104(49):19595–600. Epub 2007/12/01. doi: 10.1073/pnas.0709681104 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Laine E, Couderc R, Roch-Arveiller M, Vasson MP, Giroud JP, Raichvarg D. Modulation of human polymorphonuclear neutrophil functions by alpha 1-acid glycoprotein. Inflammation. 1990;14(1):1–9. Epub 1990/02/01. doi: 10.1007/BF00914025 . [DOI] [PubMed] [Google Scholar]
- 64.Spiller F, Carlos D, Souto FO, de Freitas A, Soares FS, Vieira SM, et al. alpha1-Acid glycoprotein decreases neutrophil migration and increases susceptibility to sepsis in diabetic mice. Diabetes. 2012;61(6):1584–91. Epub 2012/03/15. doi: 10.2337/db11-0825 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Luo Z, Lei H, Sun Y, Liu X, Su DF. Orosomucoid, an acute response protein with multiple modulating activities. J Physiol Biochem. 2015;71(2):329–40. Epub 2015/02/26. doi: 10.1007/s13105-015-0389-9 . [DOI] [PubMed] [Google Scholar]
- 66.Yuan W, Li G, Zeng M, Fu BM. Modulation of the blood-brain barrier permeability by plasma glycoprotein orosomucoid. Microvasc Res. 2010;80(1):148–57. Epub 2010/04/07. doi: 10.1016/j.mvr.2010.03.011 . [DOI] [PubMed] [Google Scholar]
- 67.Wu L, Jiang Y, Zhu J, Wen Z, Xu X, Xu X, et al. Orosomucoid1: Involved in vascular endothelial growth factor-induced blood-brain barrier leakage after ischemic stroke in mouse. Brain Res Bull. 2014;109:88–98. Epub 2014/09/30. doi: 10.1016/j.brainresbull.2014.09.007 . [DOI] [PubMed] [Google Scholar]
- 68.Han H, Mann A, Ekstein D, Eyal S. Breaking Bad: the Structure and Function of the Blood-Brain Barrier in Epilepsy. AAPS J. 2017;19(4):973–88. Epub 2017/05/28. doi: 10.1208/s12248-017-0096-2 . [DOI] [PubMed] [Google Scholar]
- 69.Loscher W, Friedman A. Structural, Molecular, and Functional Alterations of the Blood-Brain Barrier during Epileptogenesis and Epilepsy: A Cause, Consequence, or Both? Int J Mol Sci. 2020;21(2). Epub 2020/01/23. doi: 10.3390/ijms21020591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Horwitz M, Benson KF, Person RE, Aprikyan AG, Dale DC. Mutations in ELA2, encoding neutrophil elastase, define a 21-day biological clock in cyclic haematopoiesis. Nat Genet. 1999;23(4):433–6. Epub 1999/12/02. doi: 10.1038/70544 . [DOI] [PubMed] [Google Scholar]
- 71.Dale DC, Person RE, Bolyard AA, Aprikyan AG, Bos C, Bonilla MA, et al. Mutations in the gene encoding neutrophil elastase in congenital and cyclic neutropenia. Blood. 2000;96(7):2317–22. Epub 2000/09/26. . [PubMed] [Google Scholar]
- 72.Jenne DE. Structure of the azurocidin, proteinase 3, and neutrophil elastase genes. Implications for inflammation and vasculitis. Am J Respir Crit Care Med. 1994;150(6 Pt 2):S147–54. Epub 1994/12/01. doi: 10.1164/ajrccm/150.6_Pt_2.S147 . [DOI] [PubMed] [Google Scholar]
- 73.Korkmaz B, Horwitz MS, Jenne DE, Gauthier F. Neutrophil elastase, proteinase 3, and cathepsin G as therapeutic targets in human diseases. Pharmacol Rev. 2010;62(4):726–59. Epub 2010/11/17. doi: 10.1124/pr.110.002733 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang Z, Sun B, Zhu F. Molecular characterization of glutaminyl-peptide cyclotransferase(QPCT)in Scylla paramamosain and its role in Vibrio alginolyticus and white spot syndrome virus (WSSV) infection. Fish Shellfish Immunol. 2018;78:299–309. Epub 2018/05/02. doi: 10.1016/j.fsi.2018.04.059 . [DOI] [PubMed] [Google Scholar]
- 75.Iwatsubo T, Odaka A, Suzuki N, Mizusawa H, Nukina N, Ihara Y. Visualization of A beta 42(43) and A beta 40 in senile plaques with end-specific A beta monoclonals: evidence that an initially deposited species is A beta 42(43). Neuron. 1994;13(1):45–53. Epub 1994/07/01. doi: 10.1016/0896-6273(94)90458-8 . [DOI] [PubMed] [Google Scholar]
- 76.Saido TC, Iwatsubo T, Mann DM, Shimada H, Ihara Y, Kawashima S. Dominant and differential deposition of distinct beta-amyloid peptide species, A beta N3(pE), in senile plaques. Neuron. 1995;14(2):457–66. Epub 1995/02/01. doi: 10.1016/0896-6273(95)90301-1 . [DOI] [PubMed] [Google Scholar]
- 77.Piccini A, Russo C, Gliozzi A, Relini A, Vitali A, Borghi R, et al. beta-amyloid is different in normal aging and in Alzheimer disease. J Biol Chem. 2005;280(40):34186–92. Epub 2005/08/17. doi: 10.1074/jbc.M501694200 . [DOI] [PubMed] [Google Scholar]
- 78.Schilling S, Hoffmann T, Manhart S, Hoffmann M, Demuth HU. Glutaminyl cyclases unfold glutamyl cyclase activity under mild acid conditions. FEBS Lett. 2004;563(1–3):191–6. Epub 2004/04/06. doi: 10.1016/S0014-5793(04)00300-X . [DOI] [PubMed] [Google Scholar]
- 79.Cynis H, Rahfeld JU, Stephan A, Kehlen A, Koch B, Wermann M, et al. Isolation of an isoenzyme of human glutaminyl cyclase: retention in the Golgi complex suggests involvement in the protein maturation machinery. J Mol Biol. 2008;379(5):966–80. Epub 2008/05/20. doi: 10.1016/j.jmb.2008.03.078 . [DOI] [PubMed] [Google Scholar]
- 80.Schlenzig D, Manhart S, Cinar Y, Kleinschmidt M, Hause G, Willbold D, et al. Pyroglutamate formation influences solubility and amyloidogenicity of amyloid peptides. Biochemistry. 2009;48(29):7072–8. Epub 2009/06/13. doi: 10.1021/bi900818a . [DOI] [PubMed] [Google Scholar]
- 81.Alexandru A, Jagla W, Graubner S, Becker A, Bauscher C, Kohlmann S, et al. Selective hippocampal neurodegeneration in transgenic mice expressing small amounts of truncated Abeta is induced by pyroglutamate-Abeta formation. J Neurosci. 2011;31(36):12790–801. Epub 2011/09/09. doi: 10.1523/JNEUROSCI.1794-11.2011 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Costa C, Parnetti L, D’Amelio M, Tozzi A, Tantucci M, Romigi A, et al. Epilepsy, amyloid-beta, and D1 dopamine receptors: a possible pathogenetic link? Neurobiol Aging. 2016;48:161–71. Epub 2016/10/05. doi: 10.1016/j.neurobiolaging.2016.08.025 . [DOI] [PubMed] [Google Scholar]
- 83.Costa C, Romoli M, Liguori C, Farotti L, Eusebi P, Bedetti C, et al. Alzheimer’s disease and late-onset epilepsy of unknown origin: two faces of beta amyloid pathology. Neurobiol Aging. 2019;73:61–7. Epub 2018/10/15. doi: 10.1016/j.neurobiolaging.2018.09.006 . [DOI] [PubMed] [Google Scholar]
- 84.Chin J, Scharfman HE. Shared cognitive and behavioral impairments in epilepsy and Alzheimer’s disease and potential underlying mechanisms. Epilepsy Behav. 2013;26(3):343–51. Epub 2013/01/17. doi: 10.1016/j.yebeh.2012.11.040 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Monteiro RC, Kubagawa H, Cooper MD. Cellular distribution, regulation, and biochemical nature of an Fc alpha receptor in humans. J Exp Med. 1990;171(3):597–613. Epub 1990/03/01. doi: 10.1084/jem.171.3.597 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pasquier B, Launay P, Kanamaru Y, Moura IC, Pfirsch S, Ruffie C, et al. Identification of FcalphaRI as an inhibitory receptor that controls inflammation: dual role of FcRgamma ITAM. Immunity. 2005;22(1):31–42. Epub 2005/01/25. doi: 10.1016/j.immuni.2004.11.017 . [DOI] [PubMed] [Google Scholar]
- 87.Aleyd E, Heineke MH, van Egmond M. The era of the immunoglobulin A Fc receptor FcalphaRI; its function and potential as target in disease. Immunol Rev. 2015;268(1):123–38. Epub 2015/10/27. doi: 10.1111/imr.12337 . [DOI] [PubMed] [Google Scholar]
- 88.Breedveld A, van Egmond M. IgA and FcalphaRI: Pathological Roles and Therapeutic Opportunities. Front Immunol. 2019;10:553. Epub 2019/04/16. doi: 10.3389/fimmu.2019.00553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Achiron A, Gurevich M, Magalashvili D, Kishner I, Dolev M, Mandel M. Understanding autoimmune mechanisms in multiple sclerosis using gene expression microarrays: treatment effect and cytokine-related pathways. Clin Dev Immunol. 2004;11(3–4):299–305. Epub 2004/11/24. doi: 10.1080/17402520400001603 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mariuzza RA, Agnihotri P, Orban J. The structural basis of T-cell receptor (TCR) activation: An enduring enigma. J Biol Chem. 2020;295(4):914–25. Epub 2019/12/19. doi: 10.1074/jbc.REV119.009411 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Call ME, Wucherpfennig KW. The T cell receptor: critical role of the membrane environment in receptor assembly and function. Annu Rev Immunol. 2005;23:101–25. Epub 2005/03/18. doi: 10.1146/annurev.immunol.23.021704.115625 . [DOI] [PubMed] [Google Scholar]
- 92.de Saint Basile G, Geissmann F, Flori E, Uring-Lambert B, Soudais C, Cavazzana-Calvo M, et al. Severe combined immunodeficiency caused by deficiency in either the delta or the epsilon subunit of CD3. J Clin Invest. 2004;114(10):1512–7. Epub 2004/11/17. doi: 10.1172/JCI22588 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Arnaiz-Villena A, Timon M, Corell A, Perez-Aciego P, Martin-Villa JM, Regueiro JR. Brief report: primary immunodeficiency caused by mutations in the gene encoding the CD3-gamma subunit of the T-lymphocyte receptor. N Engl J Med. 1992;327(8):529–33. Epub 1992/08/20. doi: 10.1056/NEJM199208203270805 . [DOI] [PubMed] [Google Scholar]
- 94.Tokgoz H, Caliskan U, Keles S, Reisli I, Guiu IS, Morgan NV. Variable presentation of primary immune deficiency: two cases with CD3 gamma deficiency presenting with only autoimmunity. Pediatr Allergy Immunol. 2013;24(3):257–62. Epub 2013/04/18. doi: 10.1111/pai.12063 . [DOI] [PubMed] [Google Scholar]
- 95.Gokturk B, Keles S, Kirac M, Artac H, Tokgoz H, Guner SN, et al. CD3G gene defects in familial autoimmune thyroiditis. Scand J Immunol. 2014;80(5):354–61. Epub 2014/06/10. doi: 10.1111/sji.12200 . [DOI] [PubMed] [Google Scholar]
- 96.Klein P, Dingledine R, Aronica E, Bernard C, Blumcke I, Boison D, et al. Commonalities in epileptogenic processes from different acute brain insults: Do they translate? Epilepsia. 2018;59(1):37–66. Epub 2017/12/17. doi: 10.1111/epi.13965 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vezzani A, Fujinami RS, White HS, Preux PM, Blumcke I, Sander JW, et al. Infections, inflammation and epilepsy. Acta Neuropathol. 2016;131(2):211–34. Epub 2015/10/02. doi: 10.1007/s00401-015-1481-5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Geis C, Planaguma J, Carreno M, Graus F, Dalmau J. Autoimmune seizures and epilepsy. J Clin Invest. 2019;129(3):926–40. Epub 2019/02/05. doi: 10.1172/JCI125178 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Spatola M, Dalmau J. Seizures and risk of epilepsy in autoimmune and other inflammatory encephalitis. Curr Opin Neurol. 2017;30(3):345–53. Epub 2017/02/25. doi: 10.1097/WCO.0000000000000449 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kanazawa N. Dendritic cell immunoreceptors: C-type lectin receptors for pattern-recognition and signaling on antigen-presenting cells. J Dermatol Sci. 2007;45(2):77–86. Epub 2006/10/19. doi: 10.1016/j.jdermsci.2006.09.001 . [DOI] [PubMed] [Google Scholar]
- 101.Greter M, Heppner FL, Lemos MP, Odermatt BM, Goebels N, Laufer T, et al. Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nat Med. 2005;11(3):328–34. Epub 2005/03/01. doi: 10.1038/nm1197 . [DOI] [PubMed] [Google Scholar]
- 102.Sagar D, Singh NP, Ginwala R, Huang X, Philip R, Nagarkatti M, et al. Antibody blockade of CLEC12A delays EAE onset and attenuates disease severity by impairing myeloid cell CNS infiltration and restoring positive immunity. Sci Rep. 2017;7(1):2707. Epub 2017/06/05. doi: 10.1038/s41598-017-03027-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mendel I, Yacov N, Salem Y, Propheta-Meiran O, Ishai E, Breitbart E. Identification of Motile Sperm Domain-Containing Protein 2 as Regulator of Human Monocyte Migration. J Immunol. 2017;198(5):2125–32. Epub 2017/02/01. doi: 10.4049/jimmunol.1601662 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yamasaki R, Lu H, Butovsky O, Ohno N, Rietsch AM, Cialic R, et al. Differential roles of microglia and monocytes in the inflamed central nervous system. J Exp Med. 2014;211(8):1533–49. Epub 2014/07/09. doi: 10.1084/jem.20132477 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lapato AS, Szu JI, Hasselmann JPC, Khalaj AJ, Binder DK, Tiwari-Woodruff SK. Chronic demyelination-induced seizures. Neuroscience. 2017;346:409–22. Epub 2017/02/06. doi: 10.1016/j.neuroscience.2017.01.035 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Poser CM, Brinar VV. Epilepsy and multiple sclerosis. Epilepsy Behav. 2003;4(1):6–12. Epub 2003/03/01. doi: 10.1016/s1525-5050(02)00646-7 . [DOI] [PubMed] [Google Scholar]
- 107.Calabrese M. Multiple sclerosis and epilepsy: much more than a coincidence. Eur J Neurol. 2018;25(9):1103–4. Epub 2018/06/23. doi: 10.1111/ene.13727 . [DOI] [PubMed] [Google Scholar]
- 108.Yacov N, Kafri P, Salem Y, Propheta-Meiran O, Feldman B, Breitbart E, et al. MOSPD2 is a therapeutic target for the treatment of CNS inflammation. Clin Exp Immunol. 2020;201(2):105–20. Epub 2020/05/01. doi: 10.1111/cei.13448 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vasiliou V, Bairoch A, Tipton KF, Nebert DW. Eukaryotic aldehyde dehydrogenase (ALDH) genes: human polymorphisms, and recommended nomenclature based on divergent evolution and chromosomal mapping. Pharmacogenetics. 1999;9(4):421–34. Epub 2000/04/26. . [PubMed] [Google Scholar]
- 110.Hsu LC, Chang WC, Yoshida A. Cloning of a cDNA encoding human ALDH7, a new member of the aldehyde dehydrogenase family. Gene. 1994;151(1–2):285–9. Epub 1994/12/30. doi: 10.1016/0378-1119(94)90672-6 . [DOI] [PubMed] [Google Scholar]
- 111.Hsu LC, Chang WC, Yoshida A. Human aldehyde dehydrogenase genes, ALDH7 and ALDH8: genomic organization and gene structure comparison. Gene. 1997;189(1):89–94. Epub 1997/04/11. doi: 10.1016/s0378-1119(96)00839-6 . [DOI] [PubMed] [Google Scholar]
- 112.Marchitti SA, Brocker C, Orlicky DJ, Vasiliou V. Molecular characterization, expression analysis, and role of ALDH3B1 in the cellular protection against oxidative stress. Free Radic Biol Med. 2010;49(9):1432–43. Epub 2010/08/12. doi: 10.1016/j.freeradbiomed.2010.08.004 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Marchitti SA, Orlicky DJ, Vasiliou V. Expression and initial characterization of human ALDH3B1. Biochem Biophys Res Commun. 2007;356(3):792–8. Epub 2007/03/27. doi: 10.1016/j.bbrc.2007.03.046 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fedin AI, Starykh EV, Torshin DV. [Oxidative stress in epilepsy]. Zh Nevrol Psikhiatr Im S S Korsakova. 2019;119(1):97–101. Epub 2019/02/20. doi: 10.17116/jnevro201911901197 . [DOI] [PubMed] [Google Scholar]
- 115.Aguiar CC, Almeida AB, Araujo PV, de Abreu RN, Chaves EM, do Vale OC, et al. Oxidative stress and epilepsy: literature review. Oxid Med Cell Longev. 2012;2012:795259. Epub 2012/08/01. doi: 10.1155/2012/795259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pearson-Smith JN, Patel M. Metabolic Dysfunction and Oxidative Stress in Epilepsy. Int J Mol Sci. 2017;18(11). Epub 2017/11/09. doi: 10.3390/ijms18112365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Maes M, Supasitthumrong T, Limotai C, Michelin AP, Matsumoto AK, de Oliveira Semao L, et al. Increased Oxidative Stress Toxicity and Lowered Antioxidant Defenses in Temporal Lobe Epilepsy and Mesial Temporal Sclerosis: Associations with Psychiatric Comorbidities. Mol Neurobiol. 2020;57(8):3334–48. Epub 2020/06/10. doi: 10.1007/s12035-020-01949-8 . [DOI] [PubMed] [Google Scholar]
- 118.Sudha K, Rao AV, Rao A. Oxidative stress and antioxidants in epilepsy. Clin Chim Acta. 2001;303(1–2):19–24. Epub 2001/02/13. doi: 10.1016/s0009-8981(00)00337-5 . [DOI] [PubMed] [Google Scholar]
- 119.Puttachary S, Sharma S, Stark S, Thippeswamy T. Seizure-induced oxidative stress in temporal lobe epilepsy. Biomed Res Int. 2015;2015:745613. Epub 2015/02/05. doi: 10.1155/2015/745613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Waldbaum S, Patel M. Mitochondria, oxidative stress, and temporal lobe epilepsy. Epilepsy Res. 2010;88(1):23–45. Epub 2009/10/24. doi: 10.1016/j.eplepsyres.2009.09.020 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11(1):81–128. Epub 1991/01/01. doi: 10.1016/0891-5849(91)90192-6 . [DOI] [PubMed] [Google Scholar]
- 122.Marchitti SA, Deitrich RA, Vasiliou V. Neurotoxicity and metabolism of the catecholamine-derived 3,4-dihydroxyphenylacetaldehyde and 3,4-dihydroxyphenylglycolaldehyde: the role of aldehyde dehydrogenase. Pharmacol Rev. 2007;59(2):125–50. Epub 2007/03/24. doi: 10.1124/pr.59.2.1 . [DOI] [PubMed] [Google Scholar]
- 123.Mattammal MB, Haring JH, Chung HD, Raghu G, Strong R. An endogenous dopaminergic neurotoxin: implication for Parkinson’s disease. Neurodegeneration. 1995;4(3):271–81. Epub 1995/09/01. doi: 10.1016/1055-8330(95)90016-0 . [DOI] [PubMed] [Google Scholar]
- 124.Fukuda M, Kanou F, Shimada N, Sawabe M, Saito Y, Murayama S, et al. Elevated levels of 4-hydroxynonenal-histidine Michael adduct in the hippocampi of patients with Alzheimer’s disease. Biomed Res. 2009;30(4):227–33. Epub 2009/09/05. doi: 10.2220/biomedres.30.227 . [DOI] [PubMed] [Google Scholar]
- 125.Mills PB, Footitt EJ, Mills KA, Tuschl K, Aylett S, Varadkar S, et al. Genotypic and phenotypic spectrum of pyridoxine-dependent epilepsy (ALDH7A1 deficiency). Brain. 2010;133(Pt 7):2148–59. Epub 2010/06/18. doi: 10.1093/brain/awq143 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mills PB, Struys E, Jakobs C, Plecko B, Baxter P, Baumgartner M, et al. Mutations in antiquitin in individuals with pyridoxine-dependent seizures. Nat Med. 2006;12(3):307–9. Epub 2006/02/24. doi: 10.1038/nm1366 . [DOI] [PubMed] [Google Scholar]
- 127.Laciak AR, Korasick DA, Wyatt JW, Gates KS, Tanner JJ. Structural and biochemical consequences of pyridoxine-dependent epilepsy mutations that target the aldehyde binding site of aldehyde dehydrogenase ALDH7A1. FEBS J. 2020;287(1):173–89. Epub 2019/07/16. doi: 10.1111/febs.14997 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jalink K, Moolenaar WH. G protein-coupled receptors: the inside story. Bioessays. 2010;32(1):13–6. Epub 2009/12/19. doi: 10.1002/bies.200900153 . [DOI] [PubMed] [Google Scholar]
- 129.Alavi MS, Shamsizadeh A, Azhdari-Zarmehri H, Roohbakhsh A. Orphan G protein-coupled receptors: The role in CNS disorders. Biomed Pharmacother. 2018;98:222–32. Epub 2017/12/22. doi: 10.1016/j.biopha.2017.12.056 . [DOI] [PubMed] [Google Scholar]
- 130.Shi JP, Li XN, Zhang XY, Du B, Jiang WZ, Liu MY, et al. Gpr97 Is Dispensable for Inflammation in OVA-Induced Asthmatic Mice. PLoS One. 2015;10(7):e0131461. Epub 2015/07/02. doi: 10.1371/journal.pone.0131461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Peng YM, van de Garde MD, Cheng KF, Baars PA, Remmerswaal EB, van Lier RA, et al. Specific expression of GPR56 by human cytotoxic lymphocytes. J Leukoc Biol. 2011;90(4):735–40. Epub 2011/07/05. doi: 10.1189/jlb.0211092 . [DOI] [PubMed] [Google Scholar]
- 132.Shi J, Zhang X, Wang S, Wang J, Du B, Wang Z, et al. Gpr97 is dispensable for metabolic syndrome but is involved in macrophage inflammation in high-fat diet-induced obesity in mice. Sci Rep. 2016;6:24649. Epub 2016/04/20. doi: 10.1038/srep24649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang JJ, Zhang LL, Zhang HX, Shen CL, Lu SY, Kuang Y, et al. Gpr97 is essential for the follicular versus marginal zone B-lymphocyte fate decision. Cell Death Dis. 2013;4:e853. Epub 2013/10/12. doi: 10.1038/cddis.2013.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tak PP, Firestein GS. NF-kappaB: a key role in inflammatory diseases. J Clin Invest. 2001;107(1):7–11. Epub 2001/01/03. doi: 10.1172/JCI11830 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang J, Wang X, Chen X, Lu S, Kuang Y, Fei J, et al. Gpr97/Adgrg3 ameliorates experimental autoimmune encephalomyelitis by regulating cytokine expression. Acta Biochim Biophys Sin (Shanghai). 2018;50(7):666–75. Epub 2018/06/04. doi: 10.1093/abbs/gmy060 . [DOI] [PubMed] [Google Scholar]
- 136.Campbell DB, Li C, Sutcliffe JS, Persico AM, Levitt P. Genetic evidence implicating multiple genes in the MET receptor tyrosine kinase pathway in autism spectrum disorder. Autism Res. 2008;1(3):159–68. Epub 2009/04/11. doi: 10.1002/aur.27 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Smith HW, Marshall CJ. Regulation of cell signalling by uPAR. Nat Rev Mol Cell Biol. 2010;11(1):23–36. Epub 2009/12/23. doi: 10.1038/nrm2821 . [DOI] [PubMed] [Google Scholar]
- 138.Gorrasi A, Petrone AM, Li Santi A, Alfieri M, Montuori N, Ragno P. New Pieces in the Puzzle of uPAR Role in Cell Migration Mechanisms. Cells. 2020;9(12). Epub 2020/12/02. doi: 10.3390/cells9122531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Baart VM, Houvast RD, de Geus-Oei LF, Quax PHA, Kuppen PJK, Vahrmeijer AL, et al. Molecular imaging of the urokinase plasminogen activator receptor: opportunities beyond cancer. EJNMMI Res. 2020;10(1):87. Epub 2020/07/30. doi: 10.1186/s13550-020-00673-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Merino P, Diaz A, Jeanneret V, Wu F, Torre E, Cheng L, et al. Urokinase-type Plasminogen Activator (uPA) Binding to the uPA Receptor (uPAR) Promotes Axonal Regeneration in the Central Nervous System. J Biol Chem. 2017;292(7):2741–53. Epub 2016/12/18. doi: 10.1074/jbc.M116.761650 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lino N, Fiore L, Rapacioli M, Teruel L, Flores V, Scicolone G, et al. uPA-uPAR molecular complex is involved in cell signaling during neuronal migration and neuritogenesis. Dev Dyn. 2014;243(5):676–89. Epub 2014/02/01. doi: 10.1002/dvdy.24114 . [DOI] [PubMed] [Google Scholar]
- 142.Semina E, Rubina K, Sysoeva V, Rysenkova K, Klimovich P, Plekhanova O, et al. Urokinase and urokinase receptor participate in regulation of neuronal migration, axon growth and branching. Eur J Cell Biol. 2016;95(9):295–310. Epub 2016/06/22. doi: 10.1016/j.ejcb.2016.05.003 . [DOI] [PubMed] [Google Scholar]
- 143.Lahtinen L, Huusko N, Myohanen H, Lehtivarjo AK, Pellinen R, Turunen MP, et al. Expression of urokinase-type plasminogen activator receptor is increased during epileptogenesis in the rat hippocampus. Neuroscience. 2009;163(1):316–28. Epub 2009/06/17. doi: 10.1016/j.neuroscience.2009.06.019 . [DOI] [PubMed] [Google Scholar]
- 144.Ndode-Ekane XE, Pitkanen A. Urokinase-type plasminogen activator receptor modulates epileptogenesis in mouse model of temporal lobe epilepsy. Mol Neurobiol. 2013;47(3):914–37. Epub 2012/12/25. doi: 10.1007/s12035-012-8386-2 . [DOI] [PubMed] [Google Scholar]
- 145.Powell EM, Campbell DB, Stanwood GD, Davis C, Noebels JL, Levitt P. Genetic disruption of cortical interneuron development causes region- and GABA cell type-specific deficits, epilepsy, and behavioral dysfunction. J Neurosci. 2003;23(2):622–31. Epub 2003/01/21. doi: 10.1523/JNEUROSCI.23-02-00622.2003 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the manuscript.