Abstract
BACKGROUND:
Although the use of 17-alpha-hydroxyprogesterone caproate is one of the most commonly used strategies to reduce the risk of preterm birth since its approval by the Food and Drug Administration in 2011, there has been controversy recently that there may be no benefit associated with its use in singleton pregnancies in women with a prior history of spontaneous preterm birth. However, very few of these investigations evaluated the use of intramuscular progesterone in twin pregnancies. A few studies that used 17-alpha-hydroxyprogesterone caproate in twin pregnancies had mainly included unselected twin pregnancies. Although a twin pregnancy is independently associated with an increased likelihood of preterm birth, the primary indication for the use of supplemental progesterone in pregnancy is prior history of spontaneous preterm birth. Therefore, our investigation of weekly intramuscular progesterone in twin pregnancies with this birth history best addresses this question using a selected cohort.
OBJECTIVE:
To assess whether weekly 17-alpha-hydroxyprogesterone caproate prevents recurrent preterm birth in women with a current twin pregnancy and a prior singleton spontaneous preterm birth.
STUDY DESIGN:
This was a retrospective cohort study of women with twin pregnancy and a prior singleton spontaneous preterm birth in 2 institutions between January 2005 and December 2016. One group (intervention group) consisted of women who received weekly 17-alpha-hydroxyprogesterone caproate, whereas the other (control group) did not.
The primary outcome was twin spontaneous preterm birth <34 weeks compared with odds ratio and adjusted odds ratio, adjusting for potential confounders. Secondary outcomes included composite neonatal morbidity such as respiratory distress syndrome, intraventricular hemorrhage, necrotizing enterocolitis, admission to the neonatal intensive care nursery, and fetal or neonatal death before hospital discharge.
RESULTS:
A total of 79 patients were included; 27 women received weekly 17-alpha-hydroxyprogesterone caproate and 52 did not. There were no statistically significant differences in maternal demographics except for age. Spontaneous preterm birth <34 weeks occurred in 16 patients (59%) in the intervention group vs 24 (46%) in the control group (odds ratio, 1.69; 95% confidence interval, 0.68–4.54). Composite neonatal morbidity occurred in 20 pregnancies (74%) in the intervention group and 41 pregnancies (79%) in the control group (odds ratio, 0.76; 95% confidence interval, 0.27–2.12). There remained no differences in outcomes after adjusting for potential confounders.
CONCLUSION:
In our study, weekly 17-alpha-hydroxyprogesterone caproate did not prevent spontaneous preterm birth or neonatal morbidity in women with twins and a prior singleton spontaneous preterm birth; however, further research with larger numbers and prospective design is needed.
Keywords: 17-OHPC, preterm birth, progesterone, retrospective cohort, twin pregnancy
Twin pregnancy is a major risk factor for preterm birth (PTB). More than half of twin pregnancies are delivered preterm.1 In 2016, twin pregnancies accounted for approximately 3% of all births in the United States but made up approximately 20% of all the PTBs.2 Despite the substantial risk of PTB in twin pregnancies, limited evidence-based interventions are available to prevent this outcome.
A prior spontaneous PTB (sPTB) markedly increases the risk of recurrent PTB. The administration of weekly 17-alpha-hydroxyprogesterone caproate
Editor’s Choice: The administration of weekly 17-alpha-hydroxyprogesterone caproate (17-OHPC) starting between 16 and 20 weeks of gestation to prevent recurrent PTB has been widely used for more than a decade. It has been previously associated with a decreased risk of PTB and neonatal morbidity.3 However, the efficacy of this intervention has been largely confined to singleton pregnancies. Several studies have been performed on the use of 17-OHPC in twin pregnancies, collectively showing no benefit.4–10 A recent meta-analysis11 also found that weekly 17-OHPC in unselected twin pregnancies did not prolong pregnancies or improve neonatal outcomes. In fact, some studies have raised concern regarding the use of 17-OHPC in twin pregnancies, noting an earlier gestational age of membrane rupture9 or of delivery10 in twins who received 17-OHPC compared with those who did not.
The main shortcoming with most studies that assessed the use of 17-OHPC in twin pregnancies was that they mainly included unselected twin pregnancies. One study did not initiate 17-OHPC until the third trimester,8 whereas another assessed the use of 17-OHPC in asymptomatic twin pregnancies with short cervical length.10 A history of singleton sPTB is the primary indication for the use of 17-OHPC; hence, the best way to assess its efficacy in twins would be to select only women with this obstetrical history. To the best of our knowledge, only 2 previous studies have examined the efficacy of 17-OHPC in women carrying twins with a prior singleton sPTB. One was a secondary analysis12 of 29 patients from a randomized controlled trial, whereas the other included a subgroup of twins with prior sPTB from a meta-analysis13 that examined progesterone use in twins. AJOG MFM at a Glance
Why was this study conducted?
There is insufficient evidence regarding the use of 17-alpha-hydroxyprogesterone caproate in women carrying twins with a prior spontaneous preterm birth of a singleton pregnancy; hence, we performed a retrospective cohort study examining this important clinical issue.
Key findings
Weekly 17-alpha-hydroxyprogesterone caproate starting at 16–20 weeks’ gestation did not decrease spontaneous preterm birth or composite neonatal morbidity in twins with a prior spontaneous preterm birth.
What does this add to what is known?
Given the recent developments regarding 17-alpha-hydroxyprogesterone caproate, this study adds to the mounting evidence indicating the possible ineffectiveness of intramuscular progesterone in the prevention of spontaneous preterm birth.
The administration of 17-OHP did not decrease PTB or improve neonatal outcomes in either study. Therefore, our objective was to evaluate the efficacy of 17-OHPC compared with no 17-OHPC in twin pregnancies in women with a prior singleton PTB.
Materials and Methods
This was a retrospective cohort study of women carrying a twin pregnancy between Jan. 1, 2005, and Dec. 31, 2017. Patient charts at the following 2 hospitals were reviewed: Christiana Care Health System (Newark, DE) and Thomas Jefferson University Hospital (Philadelphia, PA). Patient charts were obtained from ultrasonography and obstetrical databases at each institution. Institutional review board (IRB) approval was obtained before the study started. The study received approval by the IRB at Christiana Hospital on May 20, 2016, (IRB #36088) by expedited review with a waiver of consent and the Health Insurance Portability and Accountability Act authorization. The study was exempt from IRB approval at Jefferson on Oct. 10, 2017, on the basis that it met the criteria as posing minimal to no risk to subjects as a retrospective review of deidentified data.
Patients were included if they delivered a twin pregnancy at either institution and had a prior singleton. pregnancy complicated by sPTB between 16 0/7 weeks and 36 6/7 weeks. Patients were excluded if they had only a prior sPTB of a multifetal pregnancy, used vaginal progesterone during the index pregnancy, underwent fetal reduction, received a diagnosis of a major congenital anomaly in either twin prenatally or postnatally, delivered at an outside hospital, or had incomplete medical records.
The intervention group consisted of women who were prescribed a dose of 250-mg intramuscular 17-OHPC weekly between 16 and 36 weeks during the twin pregnancy, whereas the control group did not receive 17-OHPC. The administration of 17-OHPC to mothers with a prior singleton sPTB and existing twin pregnancy was performed at the discretion of the managing provider. Maternal charts were reviewed for the following variables: age, race, body mass index (kg/ m2), tobacco use during pregnancy, number of prior singleton sPTB, use of cervical pessary or placement of a cervical cerclage, chorionicity, and administration of corticosteroids for fetal lung maturity. Indication for delivery was assessed as spontaneous or indicated.
The primary outcome was sPTB <34 weeks. The main secondary outcome was a composite neonatal morbidity that consisted of respiratory distress syndrome, intraventricular hemorrhage, necrotizing enterocolitis, admission to the neonatal intensive care nursery, and fetal or neonatal death before hospital discharge. Delivery outcomes included gestational age at delivery, mode of delivery, and birthweight. Other PTB outcomes included PTB <32 weeks, <28 weeks, and <24 weeks.
Categorical variables were compared between the 2 groups using chi-square analysis or Fisher exact test as appropriate. Mann-Whitney U test was used to compare continuous variables. Unadjusted and adjusted odds ratios (ORs) for the outcomes of PTB and composite neonatal morbidity were determined with multiple logistic regression. SPSS v.25 (IBM Corp, Armonk, NY) and GraphPad Prism 7 software (GraphPad Software, La Jolla, CA) were used for statistical analysis.
Results
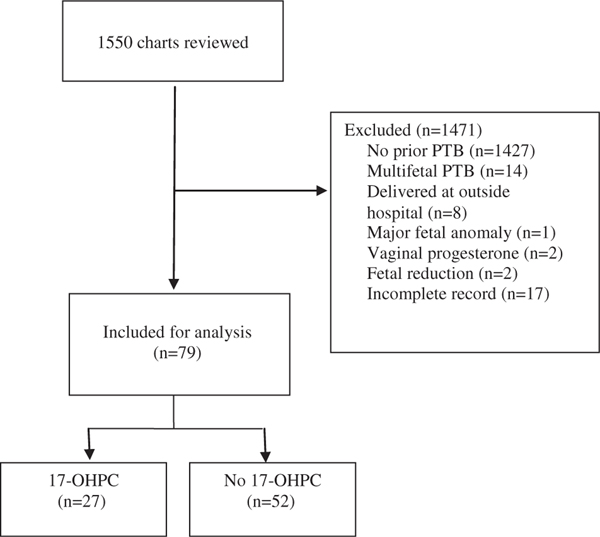
A total of 1550 patient charts were reviewed. Patients were excluded if they had no prior singleton sPTB (n1427), had prior PTB of a multifetal gestation (n14), had incomplete medical records (n17), delivered at an outside hospital (n8), received vaginal progesterone (n2), underwent fetal reduction (n2), or had a major fetal anomaly (n1; VATER sequence) (Figure).
FIGURE. Flowchart of study population.
17-OHPC, 17-alpha-hydroxyprogesterone caproate; PTB, preterm birth.
Ward et al. 17-OHPC in twins and prior preterm birth. AJOG MFM 2020.
A total of 79 women with twin pregnancy and prior singleton sPTB were included for analysis; 27 patients received weekly 17-OHPC injections and 52 did not. Maternal demographics are presented in Table 1. No significant differences were found between the 2 groups with the exception of maternal age (32.2±4.7 in the intervention group vs 29±5.5 in the control group) (P014). Patients in the intervention group were also more likely to have multiple prior PTB compared with the control group, although this difference did not reach statistical significance (37% vs 17%, respectively) (P.09).
TABLE 1.
Maternal characteristics
| Characteristic | 17-OHPC group (n=27) | No 17-OHPC group (n=52) | P value |
|---|---|---|---|
| Age | 32.2±4.7 | 29±5.5 | .014 |
| Race | .09 | ||
| White | 9 (33) | 22 (42) | |
| African American | 14 (52) | 29 (56) | |
| Hispanic | 3 (11) | 0 (0) | |
| Other | 1 (4) | 1 (2) | |
| Body mass index (kg/m2) | 32.5±7.4 | 33.3±7.2 | .55 |
| Tobacco use | 5 (19) | 13 (25) | .58 |
| Number of prior PTB | .09 | ||
| 1 | 17 (63) | 43 (83) | |
| >1 | 10 (37) | 9 (17) | |
| Cervical pessary | 0 (0) | 1 (2) | .99 |
| Cervical cerclage | 3 (11) | 3 (6) | .41 |
| Chorionicity | .29 | ||
| Monochorionic | 5 (19) | 5 (10) | |
| Dichorionic | 22 (81) | 47 (90) | |
| Corticosteroids for fetal maturity | 16 (59) | 23 (44) | .24 |
Data are expressed as n (%) and mean±standard deviation.
17-OHPC, 17-alpha-hydroxyprogesterone caproate; PTB, preterm birth.
Ward et al. 17-OHPC in twins and prior preterm birth. AJOG MFM 2020.
Weekly 17-OHPC did not decrease the frequency of the primary outcome. The rate of sPTB before 34 weeks was 59% in the intervention group and 46% in the control group (OR, 1.69; 95% confidence interval [CI], 0.68–4.54). There were no differences in sPTB <32 weeks (33% vs 31% [OR, 1.12; 95% CI, 0.42–3.06]), <28 weeks (18% vs 19% [OR, 0.95; 95% CI, 0.32–3.18]), or <24 weeks (11% vs 13% [OR, 0.8; 95% CI, 0.21–3.42]) between the intervention and control groups, respectively (Table 2). These findings were not altered with multiple logistic regression that adjusted for maternal age, race, or number of prior PTB (Table 3).
TABLE 2.
Delivery outcomes
| Characteristic | 17-OHPC group (n=27) | No 17-OHPC group (n=52) | P value |
|---|---|---|---|
| Gestational age at delivery | 32.1±5.1 | 31.9±5.4 | .9 |
| Spontaneous | 22 | 38 | |
| Indicated | 5 | 14 | |
| Delivery <34 wk | 17 | 27 | .47 |
| Spontaneous | 16(59) | 24 (46) | |
| Indicated | 1 (6) | 3(11) | |
| Delivery <32 wk | 9 | 18 | .99 |
| Spontaneous | 9(33) | 16(31) | .8 |
| Indicated | 0(0) | 2(11) | |
| Delivery <28 wk | 5 | 11 | .99 |
| Spontaneous | 5(18) | 10(19) | .99 |
| Indicated | 0(0) | 1 (9) | |
| Delivery <24 wk | 3 | 7 | .99 |
| Spontaneous | 3(11) | 7(13) | .99 |
| Indicated | 0(0) | 0(0) | |
| Mode of delivery | .99 | ||
| Vaginal | 15 (56) | 29 (56) | |
| Cesarean | 12 (44) | 23 (44) |
Data are expressed as n (%) and mean±standard deviation.
17-OHPC, 17-alpha-hydroxyprogesterone caproate.
Ward et al. 17-OHPC in twins and prior preterm birth. AJOG MFM 2020.
TABLE 3.
Unadjusted and adjusted delivery outcomes
| Delivery outcome: sPTB rate | 17-OHPC (n=21) | No 17-OHPC (n=38) | P value | OR | Adjusted ORa |
|---|---|---|---|---|---|
| sPTB <34 wk | 16(73) | 24 (63) | .45 | 1.56(0.49–4.90) | 1.75 (0.48–6.39) |
| sPTB <32 wk | 9(41) | 16(42) | .93 | 0.95 (0.33–2.77) | 0.84 (0.25–2.84) |
| sPTB <28 wk | 5(23) | 10(26) | .76 | 0.82 (0.24–2.82) | 1.59 (0.59–4.25) |
| sPTB <24 wk | 3(14) | 7(18) | .63 | 0.70(0.16–3.04) | 0.47(0.10–2.19) |
| Delivery outcome: overall PTB rate | 17-OHPC (n=27) | No 17-OHPC (n=52) | P value | OR | Adjusted ORa |
| sPTB <34 wk | 17(63) | 27 (52) | .35 | 1.57(0.61–4.08) | 2.69 (0.85–8.54) |
| sPTB <32 wk | 9(33) | 18(35) | .91 | 0.94 (0.35–2.52) | 1.02 (0.33–3.13) |
| sPTB <28 wk | 5(19) | 11 (21) | .78 | 0.85 (0.26–2.75) | 0.67(0.16–2.76) |
| sPTB <24 wk | 3(11) | 7(14) | .76 | 0.80(0.19–3.39) | 0.79(0.16–3.85) |
| Delivery outcome: neonatal | 17-OHPC (n=54) | No 17-OHPC (n=104) | P value | OR | Adjusted ORa |
| Composite neonatal morbidity and mortality (y/n) | 38 (70) | 77 (74) | .62 | 0.83 (0.40–1.73) | 1.54 (0.63–3.74) |
Delivery outcomes among patients with twin pregnancy and prior spontaneous preterm birth treated with 17-alpha-hydroxyprogesterone caproate vs those not treated with 17-alpha-hydroxyprogesterone caproate.
Data are expressed as n (%) or odds ratio (95% confidence interval). P value for 2-sided Pearson chi-square analysis.
17-OHPC, 17-alpha-hydroxyprogesterone caproate; OR, odds ratio; PTB, preterm birth; sPTB, spontaneous preterm birth.
Adjusted for maternal age, race, and number of prior spontaneous preterm births.
Ward et al. 17-OHPC in twins and prior preterm birth. AJOG MFM 2020.
Neonatal outcomes are summarized did not decrease the rate of composite 70% of the intervention group and in 74% of the control group. The administration of 17-OHPC did not prevent any of the individual components of the secondary outcome. Mean birthweight was 1786 g and 1795 g for the intervention and control groups, respectively (P.95). Cesarean delivery was performed in 44% of the pregnancies in each group (P.99).
Comment
Principal findings
In this study, weekly administration of 17-OHPC in women with twin pregnancy and prior singleton sPTB did not decrease early PTB or composite neonatal morbidity compared with women who did not receive 17-OHPC. These findings did not change when logistic regression was used.
Result implications on the use of supplemental 17-OHPC in clinical practice
Our results support those of previous studies, 4–11 which show the inefficacy of 17-OHPC to prevent recurrent PTB or improve neonatal outcomes in twin pregnancies. Only 2 previous studies assessed twin pregnancies with prior singleton PTB. A secondary analysis12 of a randomized controlled trial of 17-OHPC in twin pregnancies6 assessed 29 patients with a prior PTB and found no benefit with 17-OHPC. In addition, a meta-analysis by Schuit et al13 included a subgroup analysis on the use of 17-OHPC among certain subgroups of twin pregnancies, one of them being women with a history of prior sPTB. In this subgroup, they found that both 17-OHPC injections and vaginal progesterone showed no benefit on adverse perinatal outcome, time to delivery, or death. However, this subgroup of patients within this larger study was a retrospective aggregate of just 171 patients across 6 different studies with varied patient populations and study inclusion and/or exclusion criteria. Although the data presented in this large meta-analysis potentiate the evidence against the use of 17-OHPC in unselected twin pregnancies, there is still no consensus in the clinical setting regarding its use in select twin pregnancies with a prior sPTB history. Therefore, a primary investigation on the use of progesterone in this specific population is still needed to reproduce this preliminary evidence and better inform clinical decision making.
Strengths and limitations
Our study has some weaknesses. This was a retrospective study; thus, we could not determine causation, and 17-OHPC was administered at the discretion of the treating obstetrical provider. Our sample size was small, and we could not exclude a type II error, but trends were in the direction of possible harm, not of benefit. Patients with multiple prior PTBs were more likely to receive 17-OHPC, although this difference did not reach statistical significance (P.09). However, even after adjusting for this variable, there was still no benefit with 17-OHPC. We were unable to assess the compliance with 17-OHPC injections in the intervention group. We also did not have information about cervical length.
The major strength of this study is that to the best of our knowledge this is the first study on 17-OHPC efficacy in twin pregnancies with a prior spontaneous preterm birth that is not a secondary or subgroup analysis. Given some of the well-documented potential shortcomings in subgroup analyses,14,15 our study substantially adds to the scarce existing data on the topic and to other less specific groups of women with twin pregnancies treated with 17-OHPC.4–11 Recently, in the largest randomized trial on 17-OHPC thus far, 17-OHPC was also found to be ineffective in preventing PTB in women with singleton gestation and prior PTB.16
Conclusion
Our data shows that the use of 17-OHPC in women with a twin pregnancy and prior singleton sPTB does not decrease recurrent PTB <34 weeks or neonatal morbidity and mortality.
Given the findings of this study combined with others, 4–13 at this time there does not seem to be a cohort of twin pregnancies for which 17-OHPC is beneficial.
TABLE 4.
Neonatal outcomes
| Characteristic | 17-OHPC (n=54) | No 17-OHPC (n=104) | P value |
|---|---|---|---|
| Birthweight | 1786.1±775.3 | 1794.5±802.8 | NS |
| Composite neonatal outcome | 38 (70) | 77 (74) | NS |
| RDS | 18(33) | 22 (21) | NS |
| IVH | 7(13) | 7(13) | NS |
| NEC | 4(7) | 2(4) | NS |
| Perinatal death | 6(11) | 14(15) | NS |
| NICU | 34 (63) | 65 (63) | NS |
Data are expressed as n (%) and mean±standard deviation.
17-OHPC, 17-alpha-hydroxyprogesterone caproate; IVH, intraventricular hemorrhage; NICU, neonatal intensive care unit; NS, not significant; RDS, respiratory distress syndrome.
Ward et al. 17-OHPC in twins and prior preterm birth. AJOG MFM 2020.
Acknowledgments
R.C.B. is supported by the National Institutes of Health grant T32GM008562, this study otherwise received no financial support.
Footnotes
The authors report no conflict of interest.
References
- 1.National Collaborating Centre for Women’s and Children’s Health (UK). Multiple pregnancy: the management of twin and triplet pregnancies in the antenatal period. London: RCOG Press; 2011. [PubMed] [Google Scholar]
- 2.Martin JA, Hamilton BE, Osterman MJK, Driscoll AK, Drake P. Births: final data for 2016. Natl Vital Stat Rep 2018;67:1–55. [PubMed] [Google Scholar]
- 3.Meis PJ, Klebanoff M, Thom E, et al. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med 2003;348:2379–85. [DOI] [PubMed] [Google Scholar]
- 4.Awwad J, Usta IM, Ghazeeri G, et al. A randomised controlled double-blind clinical trial of 17-hydroxyprogesterone caproate for the prevention of preterm birth in twin gestation (PROGESTWIN): evidence for reduced neonatal morbidity. BJOG 2015;122: 71–9. [DOI] [PubMed] [Google Scholar]
- 5.Briery CM, Veillon EW, Klauser CK, et al. Progesterone does not prevent preterm births in women with twins. South Med J 2009;102: 900–4. [DOI] [PubMed] [Google Scholar]
- 6.Combs CA, Garite T, Maurel K, Das A,Porto M; Obstetrix Collaborative Research Network. 17-Hydroxyprogesterone caproate for twin pregnancy: a double-blind, randomized clinical trial. Am J Obstet Gynecol 2011;204: 221.e1–8. [DOI] [PubMed] [Google Scholar]
- 7.Rouse DJ, Caritis SN, Peaceman AM, et al. A trial of 17-alpha-hydroxyprogesterone caproate to prevent prematurity in twins. N Engl J Med 2007;357:454–61. [DOI] [PubMed] [Google Scholar]
- 8.Hartikainen-Sorri AL, Kauppila A, Tuimala R. Inefficacy of 17 alpha-hydroxyprogesterone caproate in the prevention of prematurity in twin pregnancy. Obstet Gynecol 1980;56:692–5. [PubMed] [Google Scholar]
- 9.Lim AC, Schuit E, Bloemenkamp K, et al. 17a-hydroxyprogesterone caproate for the prevention of adverse neonatal outcome in multiple pregnancies: a randomized controlled trial. Obstet Gynecol 2011;118:513–20. [DOI] [PubMed] [Google Scholar]
- 10.Senat MV, Porcher R, Winer N, et al. Prevention of preterm delivery by 17 alpha-hydroxyprogesterone caproate in asymptomatic twin pregnancies with a short cervix: a randomized controlled trial. Am J Obstet Gynecol 2013;208:194e1–8. [DOI] [PubMed] [Google Scholar]
- 11.Dodd JM, Grivell RM, O’Brien CM, Dowswell T, Deussen AR. Prenatal administration of progestogens for preventing spontaneous preterm birth in women with a multiple pregnancy. Cochrane Database Syst Rev 2017;10:CD012024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Combs CA, Garite TJ, Maurel K, Cebrik D; for the Obstetrix Collaborative Research. Network. 17-Hydroxyprogesterone caproate for women with history of preterm birth in a prior pregnancy and twins in a current pregnancy. Am J Obstet Gynecol 2012;206(1):S213.(Suppl). [Google Scholar]
- 13.Schuit E, Stock S, Rode L, et al. Effectiveness of progestogens to improve perinatal outcome in twin pregnancies: an individual participant data meta-analysis. BJOG 2015;122:27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kent DM, Rothwell PM, Ioannidis JPA, Altman DG, Hayward RA. Assessing and reporting heterogeneity in treatment effects in clinical trials: a proposal. Trials 2010;11: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klebanoff MA. 17 Alpha-hydroxyprogesterone caproate for preterm prevention: issues in subgroup analysis. Am J Obstet Gynecol 2016;214:306–7. [DOI] [PubMed] [Google Scholar]
- 16.Blackwell SC, Gyamfi-Bannerman C, Biggio JR, et al. 17-OHPC to Prevent Recurrent Preterm Birth in Singleton Gestations (PROLONG Study): a multicenter, international, randomized double-blind trial. Am J Perinatol 2020;37:127–36. [DOI] [PubMed] [Google Scholar]