Optimal responses to neuromuscular electrical stimulation interventions may be achieved by using alternative stimulation techniques and parameters, and combined strategies.
Supplemental digital content is available in the text.
Key Words: NMES, rehabilitation, nerve, pulse width, frequency, strength
Abstract
Neuromuscular electrical stimulation (NMES) applied to skeletal muscles is an effective rehabilitation and exercise training modality. However, the relatively low muscle force and rapid muscle fatigue induced by NMES limit the stimulus provided to the neuromuscular system and subsequent adaptations. We hypothesize that adaptations to NMES will be enhanced by the use of specific stimulation protocols and adjuvant interventions.
KEY POINTS
Both the magnitude and duration of mechanical tension developed during neuromuscular electrical stimulation (NMES) exercise influence neuromuscular adaptations, and these adaptations may be optimized by the use of specific stimulation protocols and adjuvant interventions.
Commonly used NMES protocols evoke relatively low muscle forces with partial muscle recruitment, and fatigue occurs rapidly, limiting the adaptive response to repeated use.
Distributed NMES techniques, using multiple electrode pairs to stimulate the muscle or nerve, show promise in producing higher forces with less fatigue and discomfort than traditional (single-channel) NMES.
Despite positive results in acute studies, the chronic effects of using alternative NMES parameters or configurations, or combining NMES with local vibration, blood flow restriction, voluntary contractions, mental imagery, or photobiomodulation therapies, remain understudied, and their clinical effectiveness remains to be determined.
INTRODUCTION
Electrical stimulation of motor axons through electrodes placed over a muscle or nerve, that is, neuromuscular electrical stimulation (NMES), can be used to generate muscular contractions. NMES can assist movement for people with incomplete muscle activation, as with functional electrical stimulation, or produce contractions with the sole purpose of evoking chronic physiological adaptations. The present review focuses on the latter application by exploring NMES as a physical training (including rehabilitation) modality. As with voluntary exercise, contractions produced by NMES repeatedly over weeks can reduce disuse atrophy and promote muscle hypertrophy and contractile function, neural adaptations, and positive health and well-being in clinical and nonclinical populations (1). NMES is, thus, a beneficial clinical tool, particularly in individuals with impaired voluntary contraction capacity.
NMES-evoked forces are often low (usually <50% of maximum muscle capacity at the highest tolerable intensity), relative to the maximal force-generating capacity of the muscle, and the evoked force declines rapidly and substantially (i.e., “fatigue” manifests). Both outcomes minimize the potential for chronic adaptive responses, being of practical benefit only to the most deconditioned of individuals. Accordingly, solutions are required that (a) increase muscle recruitment and reduce discomfort to produce more force and thus increase the physiological stimulus, (b) reduce contraction fatigue to increase work (i.e., mechanical stress) during a training session, and (c) increase the magnitude (and breadth) of adaptation to a given level of work. We hypothesize that adaptations to NMES will be enhanced by the use of specific stimulation protocols and adjuvant interventions. After a short overview of conventional NMES, we therefore report on the state of the art with regard to several important strategies that may achieve these aims. Strategies are organized as intrinsic to NMES (alternative parameters and electrode configurations) or concurrent to NMES (vibration, volition and mental imagery, blood flow restriction [BFR], and photobiomodulation therapies [PBMT]).
COMMONLY USED NMES PROTOCOLS: OUTCOMES
During NMES, current pulses are delivered through electrodes on the skin at frequencies high enough that the twitches generated by each stimulus pulse sum to produce smooth (“tetanic”) contractions (Fig. 1). Common NMES parameters include relatively short biphasic pulses (0.2–0.6 ms) delivered at 30–50 Hz, although it may be delivered at higher frequencies (e.g., 80 Hz) under some conditions. When used to improve muscle size, architecture, or function, these pulse widths and frequencies are delivered at the highest tolerable amplitude (current intensity) typically in 5- to 10-s trains (“on” period) separated by longer recovery phases (“off” period) to produce intermittent contractions, often under static (isometric) conditions. Typically, two stimulating electrodes are positioned over a muscle belly to target motor point(s) and facilitate current flow to motor axons (2). In both clinical and laboratory applications, the quadriceps femoris is the most commonly stimulated muscle due to its accessibility and functional relevance; however, NMES can be delivered over a muscle belly to activate any superficial muscle or muscle group.
Figure 1.
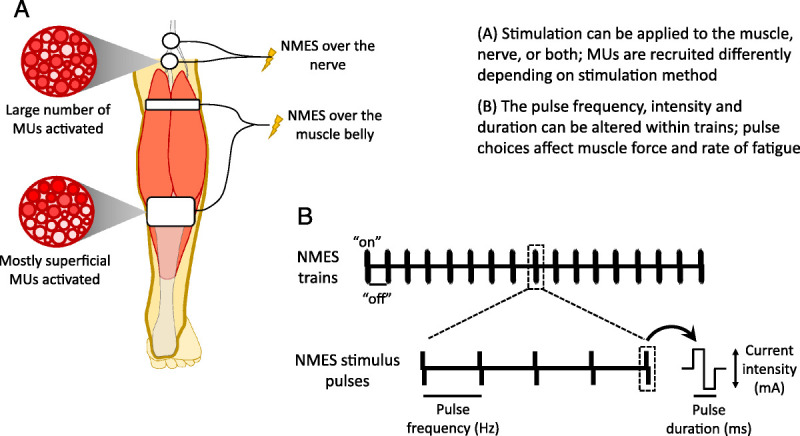
A. neuromuscular electrical stimulation (NMES) may be applied via electrodes over the muscle belly or a large nerve trunk; nerve stimulation may activate all MUs within the nerve, whereas muscle belly stimulation recruits motor units (MUs) that lie closer to the stimulating electrodes; few nerves are readily accessible, so muscle belly NMES is more commonly used. B. Pulse frequency and duration as well as current intensity may be altered to achieve different outcomes. Higher frequencies and stimulation intensities both recruit more MUs and increase MU-specific force and thus evoke higher forces, but fatigue may be more rapid. Wider pulses (e.g., 1 ms) may activate sensory fibers to enhance MU recruitment through central (reflex) pathways.
Repeated NMES use in healthy subjects can improve maximal voluntary strength, albeit less than with volitional strength training, and induce neural adaptations and muscle hypertrophy (3). Similar outcomes — often accompanied by improvements in physical function — are observed when NMES is used for rehabilitation after a period of disuse, such as after surgery (4). For individuals who are partially or completely immobilized, including those with advanced disease, even small doses of NMES effectively counteract muscle atrophy and weakness and can substantially improve general health, well-being, and quality of life (1). NMES reduces muscle atrophy and weakness after immobilization by increasing or maintaining muscle protein synthesis (5), although suppression of protein breakdown may also contribute.
ALTERNATIVE STIMULATION PARAMETERS AND ELECTRODE CONFIGURATIONS
Current Parameters: Pulse Duration and Frequency
When relatively short pulse durations (narrow pulse widths; 0.05–0.25 ms) and low frequencies (30–50 Hz) are used for NMES, the activation of motor axons is favored over sensory axons (6). Increasing both pulse duration (up to 0.5–1.0 ms) and frequency (up to 80–100 Hz), however, can generate contractions that arise at least in part through central pathways by increasing the activation of sensory axons and generating contractions via reflex pathways through the spinal cord (7). This effect of pulse duration stems from differences in the types of ion channels in motor and sensory axons, with channels on sensory axons requiring longer depolarization times before they open, and has been shown to reduce fatigue (8). Combining wide pulses with high frequencies to minimize fatigue has been trialed with mixed success. A disadvantage of delivering NMES at high frequencies is that it decreases motor axon excitability (9) and may (3) (although not always (10)) lead to an increase in metabolic cost and peripheral fatigue (11), which are counterproductive for producing fatigue-resistant contractions. Thus, there is a trade-off between using lower NMES frequencies to avoid fatigue and higher frequencies to maximize the afferent signal sent to the central nervous system, and thus contraction force. Wide-pulse high-frequency NMES may also have neuromodulation applications, as the large sensory input may increase activity in circuits that control movement and over time strengthen these circuits and improve movement outcomes of rehabilitation programs. This possibility is worthy of further study.
Stimulation Site: Muscle Belly Stimulation, Nerve Trunk, and “Interleaved” NMES
NMES commonly is applied through two electrodes, an “active” cathode and a “return” anode, placed on the skin over a muscle belly (or muscle group) to target the motor points. Delivered in this way, NMES generates contractions predominantly by depolarizing motor axons, and motor units (MUs) are recruited synchronously with each stimulus pulse (7). This contrasts with the asynchronous MU discharge that occurs during voluntary contractions, necessitating the use of high NMES frequencies to generate sufficiently strong tetanic contractions and forcing MUs to discharge at unphysiologically high rates. High MU discharge rates during NMES increase fatigue, due to increased metabolic demand on muscle fibers (3) and because MUs “drop out” as the excitability of motor axons under the stimulating electrodes decreases (9).
When applied over a muscle belly, NMES preferentially recruits superficial MUs (Fig. 1), with deeper MUs progressively recruited as stimulation intensity, and, thus, contraction strength, increases (12,13). Consequently, MU recruitment order during NMES depends on spatial distance from the stimulating electrodes (rather than Henneman's size principle, as during voluntary contractions), and MUs are recruited relatively randomly with respect to their size (and, thus, their type), contributing to fatigue. It is, therefore, challenging to recruit a sufficient number of MUs to generate strong contractions and thus to maximize the physiological stimulus before reaching either the maximal stimulator output or the limit of discomfort induced by the stimulation. This is particularly relevant for large muscles, for example, the quadriceps, and in individuals with substantial subcutaneous adipose thickness, which increases current resistance (14). Thus, although NMES delivery through two electrodes over a muscle belly is common, it is rarely optimum due to its effects on fatigue and difficulty in recruiting a sufficient number of MUs. Strategies described in this review are being developed to address these limitations.
Delivering NMES over a nerve trunk can provide a reliable alternative to stimulating over a muscle belly, although it is less common as there are fewer sites at which a nerve runs sufficiently close to the skin to be accessible to stimulation. Accessible nerves in the lower limbs include the common peroneal and tibial, at the side and back of the knee, respectively. Indeed, NMES was first applied over the common peroneal nerve in the 1960s to dorsiflex the ankle during the swing phase of walking for individuals experiencing “foot drop” after a stroke (15), and this application is still in use today. Although the femoral nerve is accessible in the femoral triangle to activate the quadriceps, there are challenges (both physical and social) in accessing the site, and it is difficult to generate stable knee extensor contractions while minimizing discomfort; it is thus not feasible in many individuals. Although NMES is rarely applied over nerves of the upper limb, the radial, median and ulnar nerves are accessible to stimulation.
One potential reason to stimulate over a nerve trunk is that MU recruitment is not superficial-to-deep, as occurs with NMES over the muscle. Instead, both superficial and deep MUs are recruited, independent of stimulation or contraction level (13). In addition, all motor axons to the muscle lie directly beneath the stimulating electrodes and are, thus, readily accessible to the applied current. Both factors markedly increase contraction strength. Delivering NMES over a nerve trunk can increase the extent to which reflex pathways through the spinal cord contribute to the evoked contraction (see point 1, “current parameters,” previously). The stimulation of sensory axons can generate contractions via reflex pathways through the spinal cord, and this recruits fatigue-resistant MUs first, according to Henneman's size principle (16). Accordingly, when stimulating the tibial nerve in individuals with complete spinal cord injury, contraction strength was reduced by ~70% when generated solely by the stimulation of motor axons, but by only ~40% when H-reflexes also contributed to the contractions (8).
An alternative approach, called “interleaved” NMES, combines NMES over the muscle and nerve to minimize fatigue by reducing MU discharge rates (17). During interleaved NMES, pulses are alternated between the two sites, thus recruiting different MUs with each alternate pulse. Accordingly, to deliver a “net” frequency of 40 Hz, pulses are delivered at 20 Hz to each site alternately, effectively reducing MU discharge rates by half. Interleaved NMES exploits the previously described spatial differences in the way NMES over the muscle and nerve recruit MUs. Interleaved NMES reduces fatigue by ~30%–40% compared with over-the-muscle or nerve alone during light tibialis anterior contractions (15% maximum voluntary contraction [MVC]) (17), with a similar effect observed in contractions up to at least 30% MVC (18). As contraction strength increases further, however, the “overlap” of MUs recruited by the muscle and nerve sites increases (10) and will theoretically eventually negate the advantage of the interleaved approach. Although interleaved NMES has proven effective for reducing fatigue for tibialis anterior, it is not known whether it will be as effective for other muscles.
Current Distribution: Spatiotemporally Distributed NMES With Multichannel Systems
The physiological shortcomings of conventional NMES, delivered through two electrodes over a muscle belly, are partially circumvented by the so-called distributed NMES (also referred to as multielectrode/multichannel/sequential/asynchronous NMES), in which multiple electrodes are spatially distributed over the same muscle belly (19) or distributed to synergistic muscles (20,21) and current pulses are rotated between electrodes. Current is then delivered at lower frequencies, sequentially to each channel (i.e., electrode pair). For example, delivering pulse trains at a frequency of 10 Hz per channel in a sequential order to four distinct channels results in a “net” NMES frequency of 40 Hz (22). As such, distributed NMES represents a valid alternative to conventional NMES for generating stronger contractions by recruiting more MUs as a result of the spatially distributed electrodes, with less fatigue due to the lower discharge rates (23). Indeed, distributed NMES over the quadriceps resulted in contractions that fatigued ~25% less than when using conventional NMES (19,22).
Distributed NMES has been applied to different lower and upper limb muscles in healthy subjects and neurological patients and studied in a variety of conditions from static single-joint actions (21) to functional movements and activities of daily living (15,20). For example, “multipath” NMES — where pulsed current is delivered to the quadriceps muscle through a wearable solution integrating large electrodes and multiple current pathways — was more effective than conventional NMES for restoring muscle strength and physical function after knee surgery (4). This may be explained by the higher contraction force, presumably through a greater and more distributed MU recruitment, achieved with multipath NMES than conventional NMES (24), but possibly also to lower discomfort and hence greater training compliance (4,24). Although the usability of a new foot drop NMES device based on surface multifield electrodes has recently been demonstrated in a clinical rehabilitation context (15), its therapeutic effects within a neurorehabilitation program remain to be confirmed. Regardless, with a careful selection of channel numbers, NMES frequency, and intensity, both neuromodulation effects and fatigue-resistant contractions could be attained. Despite good evidence in favor of spatiotemporally distributed NMES for increasing force (recruitment) and reducing fatigue, further research is needed to determine its clinical feasibility and effectiveness for different applications and patient groups.
NMES-PLUS: STRATEGIES FOR CONCURRENT USE WITH NMES
Local Vibration During NMES
The application of tendon (or muscle) vibration during NMES has received recent scrutiny because of the possibility that activation of reflexive pathways might promote recruitment of additional lower-threshold MUs for a given current intensity, that is, more MUs may be activated for a given level of discomfort. As described in detail in Supplemental Digital Content 1, http://links.lww.com/ESSR/A54, vibration (typically 0.2–2.0 mm amplitude; 20–100 Hz frequency) activates primary muscle spindle endings and thus may provide a facilitatory stimulus onto the vibrated muscle. This facilitation tends to reduce MU recruitment thresholds and increase the number of active MUs, and thus increase force of low-to-moderate intensity contractions but not of high-to-maximal intensity contractions (25). In addition, part of the facilitatory effect may arise from the activation of persistent inward currents at the motoneuron dendrites (26,27), leading to an increase in NMES-evoked force (27). Nonetheless, even relatively brief bouts of vibration applied during voluntary contractions may reduce maximal voluntary force production (28) and time to failure during submaximal exercise tasks (29). Thus, vibration of the duration required to complete a series of muscle contractions (e.g., 30–180 s) may not provide meaningful benefits or may speculatively reduce voluntary force production.
However, Magalhães and Kohn (30) observed significant increases in NMES-evoked plantar flexor torque and impulse and ongoing torque at the cessation of NMES and vibration when 2-s bursts of 100-Hz tendon vibration were superimposed onto 20-Hz NMES trains. In addition, Trajano et al. (27) imposed five 2-s, 20-Hz NMES trains at 20% MVC over 33 s of continuous 70-Hz Achilles tendon vibration and observed variable but substantial increases in torque in successive stimulations (up to ~140% after five stimulations) and ongoing torque between stimulations (during vibration) and at cessation of both NMES and vibration (e.g., Fig. 2A; note, the ongoing torque can approximate the initial NMES torque). However, although the interindividual variability of the response was not reported, Kirk et al. (31), using the same technique (but with a higher vibration frequency of 115 Hz), found an augmented response to vibration in 17 of 25 participants (varying from ~5% to >100% of initial NMES torque), indicating a substantial interindividual variability in the response. Subsequently, Bochkezanian et al. (32,33) examined the response to 30-Hz NMES trains (2 s on/2 s off) with and without 55-Hz patellar tendon vibration. In a nonclinical population (32), 8 of 16 subjects showed greater torque-time integral (i.e., total impulse) before reaching “fatigue” at 50% of initial knee extension torque when vibration was applied. Individuals with the lowest torque-time integral typically showed the best response to vibration, and the loss of MVC torque immediately after the NMES condition was overcome in the vibration condition in all participants. In people with chronic spinal cord injury (33), 4 of 9 participants showed a greater torque-time integral in the vibration condition, although there was no clear indicator as to who would or would not respond positively. Of concern in both studies was that an earlier fatigue onset and substantially reduced torque-time integral were observed in most of the remaining participants (i.e., nonresponders).
Figure 2.
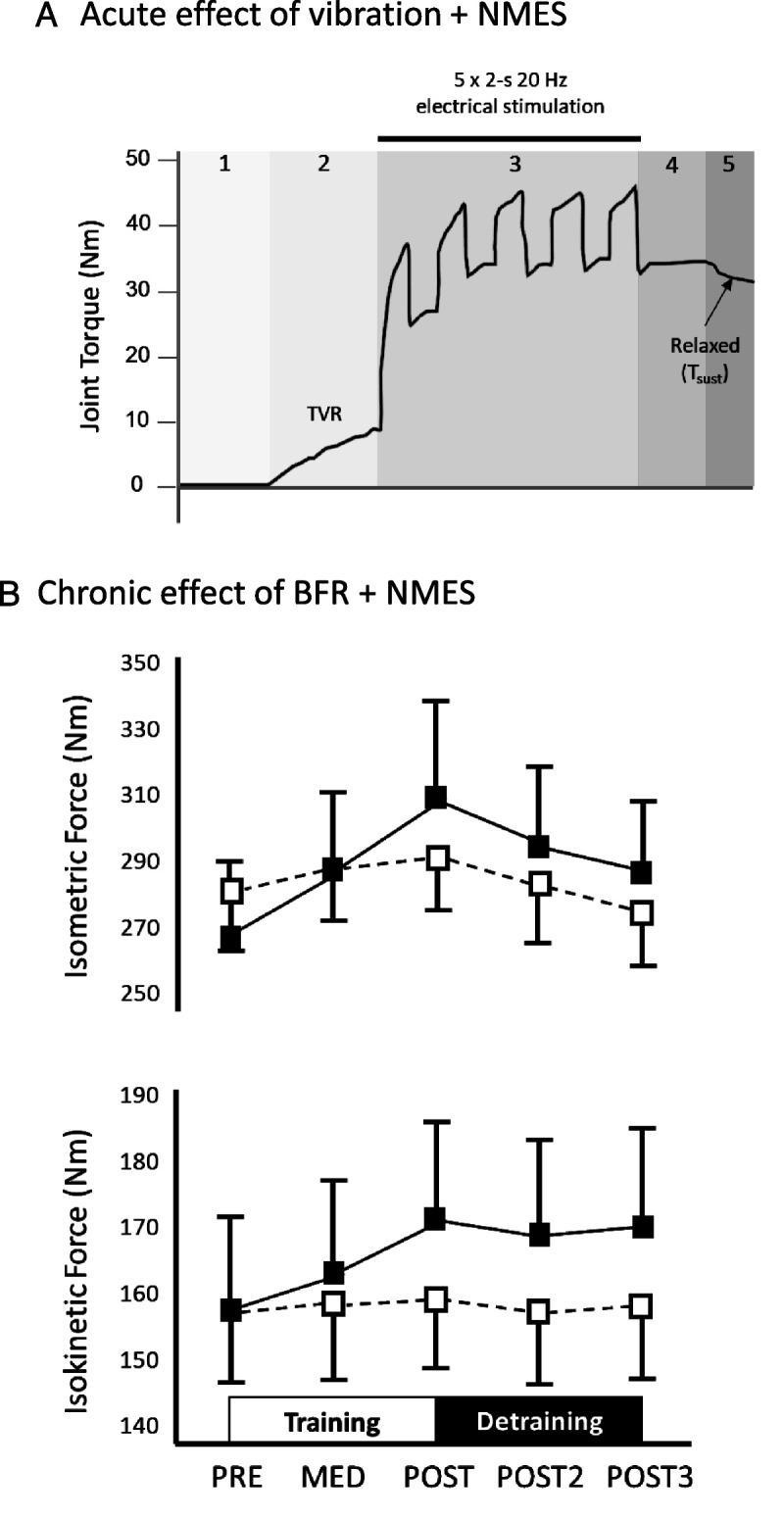
A. Effect of neuromuscular electrical stimulation (NMES) superimposed over Achilles tendon vibration. Ankle plantar flexion torque (dark solid line) is zero at rest (section 1). Application of continuous tendon vibration (section 2) induces a tendon vibration reflex (TVR) and an increase in torque. Superposition of 2-s bursts of NMES over the muscle belly generates larger torques until a maximum response is reached (section 3). Torque remains high as vibration is continued even after NMES is ceased (section 4) and tends to remain above baseline in some individuals even after vibration is ceased (section 5). This ongoing torque, or sustained torque (Tsust), indicates a motoneuron facilitation effect, possibly resulting from activation of persistent inward currents in motoneurons. B. Changes in maximal isometric (upper panel) and isokinetic (180°·s−1, lower panel) knee extension torque under NMES (open squares) and NMES + blood flow restriction (BFR, filled squares) before the training period (PRE), during the training period (MED), and immediately (POST), 1 wk (POST2), and 2 wk after the training period (POST3). Data are means ± SE. (Reprinted from 56. Copyright © 2015 American College of Sports Medicine. Used with permission.)
Based on the above data, it seems that the imposition of tendon vibration during NMES well above motor threshold (e.g., contractions ≥20% MVC) may provide significant peak torque or muscle work benefits; however, these effects may only work in approximately half of all individuals. It is not clear why such response variability exists, but a significant scope exists to vary the NMES and vibration parameters to optimize the response and, thus, to find a protocol that strongly improves torque or impulse in most individuals. As no trials exist comparing NMES with vibration to NMES alone, it is not known yet whether vibration might improve clinical outcomes.
Voluntary Contractions and Mental Imagery During NMES
As both voluntary contractions and NMES acutely enhance corticospinal excitability (16,34), and their combination can promote additional excitability, the hypothesis has been tested that continued use of NMES above motor threshold paired with voluntary muscle contraction would enhance motor output and thus muscle force production and motor recovery from neurologic injury. In stroke survivors, electromyogram (EMG)–triggered NMES (i.e., NMES delivered when voluntary activation is detected) produced better strength and functional outcomes than NMES alone (e.g., see (35)). However, subsequent randomized controlled trials were less convincing, and recent systematic reviews have not detected larger effects after EMG-triggered NMES versus NMES alone (e.g. (36)). Thus, ideal protocols in which the production of voluntary forces with NMES augments the response to NMES have not yet been defined.
A subsequent question arises as to whether the effects of NMES are augmented when performed with mental imagery. As strong efforts to imagine a contraction are commonly associated with small electrical signals, that is, an EMG signal, being detected at the target muscle without conscious effort to contract by the subject, the EMG signal can be used to trigger the timed delivery of NMES. Using this technique, Hong et al. (37) found greater improvements in muscle function (upper limb scores of Fugl-Meyer motor assessment and Barthel index) and activation of premotor, motor, and somatosensory cortices (assessed by positron emission tomography) after 4 wk (five sessions per week) of forearm extensor NMES when triggered by the EMG signal produced during mental imagery (subjects imagined vigorously waving their arm) than with NMES alone. Subsequently, these findings have been supported by some (38) but not others (39) using similar study designs and populations. Therefore, although the simultaneous use of mental imagery with NMES above motor threshold shows promise, there is currently a lack of controlled trials on which to draw firm conclusions. It also is unclear whether factors such as the imagined task or instructions, or other factors associated with the imagery process, might affect training outcomes.
Photobiomodulation Therapy Before NMES
Although increasing the stimulus applied to the muscle is an important focus of current research, it may also, at least theoretically, be of benefit to increase the duration of muscle contraction (to increase the work done) before fatigue critically limits force production within a session. With this aim, the application of light sources to the muscle before NMES has recently been trialed. Photobiomodulation therapy (PBMT) is thought to reduce muscle fatigue and resolve muscle damage after intense exercise, thereby improving physical performance (40). However, other effects, including reductions in muscle fatigue, increases in the clearance of metabolic by-products (e.g., improving blood lactate clearance), reductions in exercise-induced muscle damage (e.g., creatine kinase, C-reactive protein), and thus improvements in physical performance during exercise (i.e., increasing the number of repetitions or exercise time), might indicate a benefit to increasing training load during NMES sessions (40). Our current understanding of the mechanisms by which PBMT exerts its effects is briefly summarized in Supplemental Digital Content 2, http://links.lww.com/ESSR/A55.
Outcomes of research examining the effect of PBMT on the work completed before fatigue in an NMES session are mixed. In rat tibialis anterior, Lopes-Martins et al. (41) found significant increases in the time for the NMES-evoked torque to decrease by 50% in PBMT groups receiving 0.5 and 1.0 J·cm−2 of muscle surface, but not 2.5 J·cm−2, compared with a no-PBMT control group. Lower creatine kinase levels were observed in 1.0 and 2.5 J·cm−2 PBMT groups compared with the 0.5 J·cm−2 PBMT and control groups. In the same muscle, Santos et al. (42) found that only specific power-wavelength combinations reduced fatigue and attenuated muscle damage when applied before and in the days after fatiguing NMES contractions, indicating the effectiveness of the method only under specific conditions. Nonetheless, in humans, both small (N = 5; PBMT: 500 mW, 808 nm; NMES: 3 min continuous) (43) and large (N = 24; PBMT: 200 mW, 830 nm; NMES: 5 s on/15 s off, 45 contractions) (44) randomized, crossover studies found no effect of PBMT applied shortly before NMES on knee extensor fatigue or soreness compared with control, although PBMT was reported to reduce impairments in the enzymatic antioxidant system and reduce inflammation duration induced by a single NMES session (45). Thus, either PBMT has less effect in human than animal skeletal muscle or further tests are required using different PBMT parameters and muscle groups to determine the parameters that might have a clinically meaningful effect. Importantly, a higher charge density may be delivered to the animal muscles, partly due to lesser subcutaneous adipose tissue, smaller muscle thickness, differences in PBMT probes, and a smaller laser beam distance between application sites in animals (possibly due to use of single beam probes in animals, but a fixed distance between multiple laser beams in humans). Recommended PBMT parameters have been proposed for voluntary exercise (46) but are not yet available for NMES exercise.
BFR During NMES
In addition to increasing the total stimulus applied during NMES, improved clinical outcomes may be obtained by enhancing the postexercise adaptive response to the training. BFR during exercise may be a key intervention in this regard, particularly because of its purported benefits during low-load resistance exercise (47). BFR increases the anaerobic contribution of energy expenditure and, consequently, metabolic by-product accumulation and neuromuscular fatigue. For instance, Husmann et al. (48) showed that the decrease in maximal voluntary torque was exacerbated with BFR, the cause being both of peripheral (decrease in high-frequency doublet and occurrence of low-frequency fatigue during exercise) and central (at exercise termination) origin. As a result, type II muscle fibers, usually activated at high exercise intensities, are recruited earlier during the task, allowing BFR to up-regulate the muscle hypertrophy-signaling cascade, satellite cell cycle initiation, and growth hormone production and to promote a vascular endothelial growth factor–mediated angiogenic response (49). Relating to NMES, the level of peripheral fatigue during a 5-min continuous train of 2-Hz NMES gradually increased with increasing perfusion restriction in the triceps surae (50). Thus, BFR may induce similar physiological adaptations as more demanding exercise (such as high-load/high-intensity resistance training) and, consequently, be particularly suited to individuals who are unable to tolerate high musculoskeletal forces/stresses such as clinical populations, elderly persons, or athletes recovering from injury. Overall, although high-load strength training induces greater neural adaptations than low-load BFR resistance exercise, low-load BFR resistance exercise can induce both strength gains and muscle hypertrophy (47). When comparing low-load exercise training with BFR versus high-load strength training, recent meta-analyses have shown some inconsistency (e.g., see (47)).
Regarding chronic interventions, NMES combined with BFR improved rat soleus muscle mass more than NMES alone (51), and this was associated with activation of ERK1/2 and mTOR pathways, a finding confirmed by Natsume et al. (52) in rat gastrocnemius. In recreationally active humans, 6 wk of BFR + NMES training of the quadriceps (with four weekly sessions of 32 min) was more effective for increasing maximal knee extensor strength than BFR or NMES alone, although no significant changes in muscle mass were detected (53). In patients with spinal cord injury (incomplete tetraplegia), 6 wk of bilateral NMES of wrist extensor muscles induced a 17% greater increase in muscle cross-sectional area when combined with BFR than NMES alone (30% above resting systolic blood pressure) (54). However, when NMES was performed at a low contraction intensity (5% to 20% MVC) for relatively short periods (2 to 6 wk, two sessions per day), conflicting results were observed. Whereas Andrade et al. (55) found no changes in maximal strength, Natsume et al. (56) reported significant increases in both maximal isometric and isokinetic force production (Fig. 2B). Surprisingly, muscle hypertrophy was induced in only 2 wk in the latter study, yet this result was confirmed by a recent experiment (57) showing that NMES + BFR (2 wk, two sessions per day, five sessions per week) may prevent muscle mass loss resulting from limb disuse (using knee brace and crutches), although NMES + BFR did not preserve maximal strength. Thus, the current evidence suggests that BFR + NMES may evoke greater strength and muscle mass adaptations in human muscles than NMES alone, but these data need to be reinforced by additional human studies with larger samples and longer interventions. Of note, although severe muscle damage has been reported only rarely after BFR during voluntary contractions, further studies need to explicitly determine whether the combination of NMES and BFR may increase the risk of severe muscle damage or, potentially, kidney injury.
Nutritional Supplementation Before or After NMES
Although the reduction in physical activity resulting from illness, injury, disease, or aging decreases muscle mass and strength through a net muscle protein breakdown in skeletal muscles, food ingestion can both stimulate protein synthesis and inhibit breakdown (58). A key nutrient driving muscle acquisition is protein. Ensuring that multiple meals throughout the day contain a substantial protein bolus is considered key to muscle mass gain or preservation with disuse; ~20 g is required within each of four daily meals in younger individuals, but more is required in older individuals or those unable to maintain physical activity levels (see (59) for review). Given that (especially high-force) physical activity also is a key driver of muscle mass gain, it is of interest to determine whether the ingestion of a high-protein meal (or supplement) might enhance the effects of NMES exercise. Despite some studies testing the effects of NMES with protein supplementation against a no-intervention control, relatively few studies have compared the effects of protein supplementation additional to NMES, or vice versa. Dirks et al. (60) observed no additional effect of 60-min quadriceps NMES (after warm-up, “palpable” contraction intensity, 5 s on/10 s off) over 20 g of casein supplementation alone on quadriceps protein synthesis in older (69 yr) men. However, Dirks et al. (61) subsequently observed greater quadriceps protein synthesis (assessed as greater incorporation of phenylalanine to muscle tissue) the morning after (8 h) NMES + protein ingestion versus protein ingestion alone in older men (69 yr) who had rested in bed for 1 d. Thus, some additional effects of NMES over feeding alone have been observed under some conditions. However, no studies have yet (i) studied the effects of supplementation after higher-intensity, shorter-duration NMES exercise, (ii) compared NMES + protein to NMES alone, (iii) examined responses in younger adult or clinical populations, or (iv) conducted longitudinal investigations of the effects of supplementation on NMES-induced outcomes. Thus, despite a strong theoretical rationale, there is no current evidence that protein supplementation can enhance the effects of NMES exercise on muscle mass, strength, or other variables.
Other dietary supplements also might be considered to be of potential benefit. However, Stevenson and Dudley (62) detected no effect of creatine supplementation (5 g·d−1) when three to five sets of eccentric-concentric stimulated contractions were performed by the quadriceps twice weekly for 8 wk, despite the training itself evoking ~7% and ~10% improvements in quadriceps cross-sectional area (above nontrained control limb) in creatine and control groups, as well as improvements in maximum torque and fatigue resistance. No evidence exists for beneficial effects of creatine, or other dietary supplements, when used during NMES training, so it is not yet clear whether supplementation might augment the response to NMES training in humans. This may be an important area for future research.
CONCLUSIONS
Repeated application of NMES using traditional parameters and configurations can enhance muscle size and function and induce neural adaptations. However, traditional NMES recruits a limited portion of the muscle and induces rapid muscle fatigue (and damage), limiting the capacity to evoke moderate-high forces for prolonged periods. We propose that alternative NMES protocols combined with concurrent interventions will significantly enhance the effectiveness of NMES interventions, as summarized in Figure 3. For example, stimulation pulse duration and frequency can be increased (e.g., to 0.5–1.0 ms and 80–100 Hz) in comparison with traditional NMES protocols to better activate sensory axons, which can assist contractions by recruiting fatigue-resistant MUs through central (reflexive) pathways. Importantly, this practice has been trialed with mixed success, central recruitment occurs only in a subset of participants, and the high NMES frequencies decrease motor axon excitability and increase metabolic cost, although further developments that address these issues may be impactful. In addition, NMES methods that distribute stimulus pulses either between electrodes over a muscle and nerve, or dispersed across a muscle belly show significant promise. Rotating pulses between the multiple sites reduce MU discharge rates, and different MUs are recruited from each site, increasing the number of accessible MUs and, thus, reducing fatigue and increasing force, respectively. Acute studies demonstrate the effectiveness of these methods, although longitudinal studies are required to test them more rigorously against traditional NMES protocols in the clinical context (see Table for summary). In addition, muscle or tendon vibration imposed during NMES increases muscle force or reduces the rate of fatigue in some (~50%), but not all, individuals, and identification of optimum protocols may be of clinical value. NMES in conjunction with attempts to voluntarily contract the target muscle or mental imagery may prove beneficial over the long term; nonetheless, results are inconsistent, and the precise conditions under which they might be effective remain to be determined. Recently, PBMT imposed before NMES have shown promise in animal models for reducing fatigue during NMES sessions. However, trials in humans have yet to show an effect, suggesting that the method is either less effective in humans or that optimum protocols are yet to be found. Importantly, NMES in conjunction with BFR protocols show significant clinical promise for evoking greater strength and muscle mass adaptations than NMES alone, although additional human studies are needed with larger samples and longer interventions. With the caveat that the risk of severe muscle damage requires further exploration, BFR shows promise for use with NMES in the clinical context. Nonetheless, no current evidence exists for a benefit of nutritional supplementation strategies (e.g., protein, creatine); however, few studies exist, and study designs rarely have tested the specific effect of supplementation added to NMES training; this may be an important area of future research. Collectively, the current evidence suggests that traditional NMES interventions provide suboptimal clinical outcomes and that the use of alternative NMES protocols (e.g., distributed NMES) and concurrent interventions (vibration, voluntary contraction or mental imagery, PBMT, BFR, or nutritional supplementation) might, in some cases, enhance its effectiveness. A concerted research effort, therefore, is required to determine which practices are best suited to the widely varying contexts within which NMES is currently used.
Figure 3.
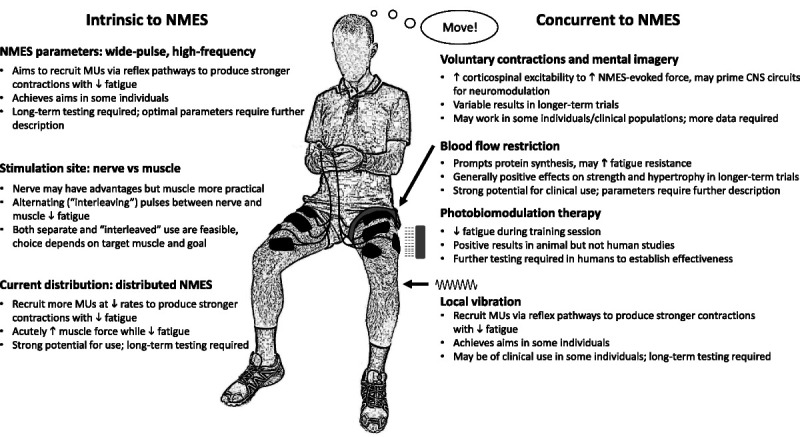
Summary of methods for increasing muscle recruitment and minimizing fatigue during, and enhancing adaptations to, neuromuscular electrical stimulation (NMES) training. Rationale for use, summary of current evidence, and indication of future potential are summarized for methods intrinsic to NMES delivery and for methods to be used concurrently with NMES. CNS, central nervous system.
Table.
Summary of the current understanding of the effects of different strategies for enhancing the effectiveness of NMES interventions
| Strategy/Solution | ↑Recruitment/Force | ↓Fatigue | ↑Magnitude of Adaptation | Observations |
|---|---|---|---|---|
| Alternative NMES parameters | ||||
| Interleaved NMES (acute effect) | + | +++ | + | Only shown for dorsiflexors; no longitudinal RCTs conducted |
| Wide-pulse NMES (acute effect) | ++ | + | + | Neuromodulatory effects observed; no longitudinal RCTs conducted |
| Distributed NMES (acute effect) | ++ | +++ | + | Neuromodulatory effects observed; no longitudinal RCTs conducted |
| Concurrent strategies | ||||
| Vibration + NMES (acute effect) | + | + | − | High individual variability in response; no longitudinal RCTs conducted |
| Voluntary contraction + NMES training (chronic effect) | ++ | − | + | Additional RCTs required |
| Imagery + NMES training (chronic effect) | − | − | + | Additional RCTs required |
| PBMT before/after NMES (acute effect) | − | − | − | No longitudinal RCTs conducted |
| BFR + NMES training (chronic effect) | ++ | − | + | Generally positive long-term adaptations |
| Nutritional supplementation + NMES (chronic effect) | − | − | − | No current favorable evidence; specific testing required |
−, no/unclear evidence; +, weak evidence; ++, moderate evidence; +++, strong evidence; BFR, blood flow restriction; NMES, neuromuscular electrical stimulation; PBMT, photobiomodulation therapy; RCT, randomized controlled trial.
Supplementary Material
Acknowledgment
The authors would like to thank Dr. Thomas Lapole for his critical feedback on parts of this article.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.acsm-essr.org).
Accepted for publication: April 23, 2021.
Editor: Marni D. Boppart, Sc.D., FACSM
Contributor Information
David F. Collins, Email: dcollins@ualberta.ca.
Guillaume Y. Millet, Email: guillaume.millet@univ-st-etienne.fr.
Marco A. Vaz, Email: marco.vaz@ufrgs.br.
Nicola A. Maffiuletti, Email: Nicola.Maffiuletti@kws.ch.
References
- 1.Jones S, Man WD, Gao W, Higginson IJ, Wilcock A, Maddocks M. Neuromuscular electrical stimulation for muscle weakness in adults with advanced disease. Cochrane Database Syst. Rev. 2016; 10:CD009419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gobbo M, Maffiuletti NA, Orizio C, Minetto MA. Muscle motor point identification is essential for optimizing neuromuscular electrical stimulation use. J. Neuroeng. Rehabil. 2014; 11(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vanderthommen M, Duchateau J. Electrical stimulation as a modality to improve performance of the neuromuscular system. Exerc. Sport Sci. Rev. 2007; 35(4):180–5. [DOI] [PubMed] [Google Scholar]
- 4.Feil S, Newell J, Minogue C, Paessler HH. The effectiveness of supplementing a standard rehabilitation program with superimposed neuromuscular electrical stimulation after anterior cruciate ligament reconstruction: a prospective, randomized, single-blind study. Am. J. Sports Med. 2011; 39(6):1238–47. [DOI] [PubMed] [Google Scholar]
- 5.Gibson J, Smith K, Rennie M. Prevention of disuse muscle atrophy by means of electrical stimulation: maintenance of protein synthesis. Lancet. 1988; 332(8614):767–70. [DOI] [PubMed] [Google Scholar]
- 6.Collins DF. Central contributions to contractions evoked by tetanic neuromuscular electrical stimulation. Exerc. Sport Sci. Rev. 2007; 35(3):102–9. [DOI] [PubMed] [Google Scholar]
- 7.Bergquist AJ, Clair JM, Collins DF. Motor unit recruitment when neuromuscular electrical stimulation is applied over a nerve trunk compared with a muscle belly: triceps surae. J. Appl. Physiol. 2011; 110(3):627–37. [DOI] [PubMed] [Google Scholar]
- 8.Bergquist AJ, Wiest MJ, Okuma Y, Collins DF. H-reflexes reduce fatigue of evoked contractions after spinal cord injury. Muscle Nerve. 2014; 50(2):224–34. [DOI] [PubMed] [Google Scholar]
- 9.Luu MJ, Jones KE, Collins DF. Decreased excitability of motor axons contributes substantially to contraction fatigability during neuromuscular electrical stimulation. Appl. Physiol. Nutr. Metabol. 2021; 46(4):346–55. [DOI] [PubMed] [Google Scholar]
- 10.Gondin J Giannesini B Vilmen C, et al. Effects of stimulation frequency and pulse duration on fatigue and metabolic cost during a single bout of neuromuscular electrical stimulation. Muscle Nerve. 2010; 41(5):667–78. [DOI] [PubMed] [Google Scholar]
- 11.Neyroud D, Dodd D, Gondin J, Maffiuletti NA, Kayser B, Place N. Wide-pulse–high-frequency neuromuscular stimulation of triceps surae induces greater muscle fatigue compared with conventional stimulation. J. Appl. Physiol. 2014; 116(10):1281–9. [DOI] [PubMed] [Google Scholar]
- 12.Mesin L, Merlo E, Merletti R, Orizio C. Investigation of motor unit recruitment during stimulated contractions of tibialis anterior muscle. J. Electromyogr. Kinesiol. 2010; 20(4):580–9. [DOI] [PubMed] [Google Scholar]
- 13.Okuma Y, Bergquist AJ, Hong M, Chan KM, Collins DF. Electrical stimulation site influences the spatial distribution of motor units recruited in tibialis anterior. Clin. Neurophysiol. 2013; 124(11):2257–63. [DOI] [PubMed] [Google Scholar]
- 14.Petrofsky J. The effect of the subcutaneous fat on the transfer of current through skin and into muscle. Med. Eng. Phys. 2008; 30(9):1168–76. [DOI] [PubMed] [Google Scholar]
- 15.Imatz-Ojanguren E Sánchez-Márquez G Asiain-Aristu JR, et al. A foot drop compensation device based on surface multi-field functional electrical stimulation—usability study in a clinical environment. J. Rehabil. Assist. Technol. Engin. 2019; 6:2055668319862141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dean JC, Clair-Auger JM, Lagerquist O, Collins DF. Asynchronous recruitment of low-threshold motor units during repetitive, low-current stimulation of the human tibial nerve. Front. Hum. Neurosci. 2014; 8:1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lou JW, Bergquist AJ, Aldayel A, Czitron J, Collins DF. Interleaved neuromuscular electrical stimulation reduces muscle fatigue. Muscle Nerve. 2017; 55(2):179–89. [DOI] [PubMed] [Google Scholar]
- 18.Ainsley EN, Barss TS, Collins DF. Contraction fatigability during interleaved neuromuscular electrical stimulation of the ankle dorsiflexors does not depend on contraction amplitude. Appl. Physiol. Nutr. Metabol. 2020; 45(9):948–56. [DOI] [PubMed] [Google Scholar]
- 19.Sayenko DG, Nguyen R, Popovic MR, Masani K. Reducing muscle fatigue during transcutaneous neuromuscular electrical stimulation by spatially and sequentially distributing electrical stimulation sources. Eur. J. Appl. Physiol. 2014; 114(4):793–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Decker M, Griffin L, Abraham L, Brandt L. Alternating stimulation of synergistic muscles during functional electrical stimulation cycling improves endurance in persons with spinal cord injury. J. Electromyogr. Kinesiol. 2010; 20(6):1163–9. [DOI] [PubMed] [Google Scholar]
- 21.Pournezam M, Andrews B, Baxendale R, Phillips G, Paul J. Reduction of muscle fatigue in man by cyclical stimulation. J. Biomed. Eng. 1988; 10(2):196–200. [DOI] [PubMed] [Google Scholar]
- 22.Bergquist AJ, Babbar V, Ali S, Popovic MR, Masani K. Fatigue reduction during aggregated and distributed sequential stimulation. Muscle Nerve. 2017; 56(2):271–81. [DOI] [PubMed] [Google Scholar]
- 23.Barss TS Ainsley EN Claveria-Gonzalez FC, et al. Utilizing physiological principles of motor unit recruitment to reduce fatigability of electrically-evoked contractions: a narrative review. Arch. Phys. Med. Rehabil. 2018; 99(4):779–91. [DOI] [PubMed] [Google Scholar]
- 24.Maffiuletti NA, Vivodtzev I, Minetto MA, Place N. A new paradigm of neuromuscular electrical stimulation for the quadriceps femoris muscle. Eur. J. Appl. Physiol. 2014; 114(6):1197–205. [DOI] [PubMed] [Google Scholar]
- 25.Souron R, Besson T, Millet GY, Lapole T. Acute and chronic neuromuscular adaptations to local vibration training. Eur. J. Appl. Physiol. 2017; 117(10):1939–64. [DOI] [PubMed] [Google Scholar]
- 26.Gorassini M, Yang JF, Siu M, Bennett DJ. Intrinsic activation of human motoneurons: possible contribution to motor unit excitation. J. Neurophysiol. 2002; 87(4):1850–8. [DOI] [PubMed] [Google Scholar]
- 27.Trajano GS, Seitz LB, Nosaka K, Blazevich AJ. Can passive stretch inhibit motoneuron facilitation in the human plantar flexors? J. Appl. Physiol. 2014; 117(12):1486–92. [DOI] [PubMed] [Google Scholar]
- 28.Barrera-Curiel A, Colquhoun RJ, Hernandez-Sarabia JA, DeFreitas JM. The effects of vibration-induced altered stretch reflex sensitivity on maximal motor unit firing properties. J. Neurophysiol. 2019; 121(6):2215–21. [DOI] [PubMed] [Google Scholar]
- 29.Mottram CJ, Maluf KS, Stephenson JL, Anderson MK, Enoka RM. Prolonged vibration of the biceps brachii tendon reduces time to failure when maintaining arm position with a submaximal load. J. Neurophysiol. 2006; 95(2):1185–93. [DOI] [PubMed] [Google Scholar]
- 30.Magalhães FH, Kohn AF. Vibration-induced extra torque during electrically-evoked contractions of the human calf muscles. J. Neuroeng. Rehabil. 2010; 7(1):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kirk BJ, Trajano GS, Pulverenti TS, Rowe G, Blazevich AJ. Neuromuscular factors contributing to reductions in muscle force after repeated, high-intensity muscular efforts. Front. Physiol. 2019; 10:783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bochkezanian V, Newton RU, Trajano GS, Vieira A, Pulverenti TS, Blazevich AJ. Effect of tendon vibration during wide-pulse neuromuscular electrical stimulation (NMES) on the decline and recovery of muscle force. BMC Neurol. 2017; 17(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bochkezanian V, Newton RU, Trajano GS, Vieira A, Pulverenti TS, Blazevich AJ. Effect of tendon vibration during wide-pulse neuromuscular electrical stimulation (NMES) on muscle force production in people with spinal cord injury (SCI). BMC Neurol. 2018; 18(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vastano R, Perez MA. Changes in motoneuron excitability during voluntary muscle activity in humans with spinal cord injury. J. Neurophysiol. 2020; 123(2):454–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Kroon J, IJzerman M, Chae J, Lankhorst G, Zilvold G. Relation between stimulation characteristics and clinical outcome in studies using electrical stimulation to improve motor control of the upper extremity in stroke. J. Rehabil. Med. 2005; 37(2):65–74. [DOI] [PubMed] [Google Scholar]
- 36.Monte-Silva K, Piscitelli D, Norouzi-Gheidari N, Batalla MAP, Archambault P, Levin MF. Electromyogram-related neuromuscular electrical stimulation for restoring wrist and hand movement in poststroke hemiplegia: a systematic review and meta-analysis. Neurorehabil. Neural Repair. 2019; 33(2):96–111. [DOI] [PubMed] [Google Scholar]
- 37.Hong IK, Choi JB, Lee JH. Cortical changes after mental imagery training combined with electromyography-triggered electrical stimulation in patients with chronic stroke. Stroke. 2012; 43(9):2506–9. [DOI] [PubMed] [Google Scholar]
- 38.You S-j, Lee JH. Effects of mental activity training linked with electromyogram-triggered electrical stimulation on paretic upper extremity motor function in chronic stroke patients: a pilot trial. Turkish J Physical Med Rehabil. 2013; 59(2):133–9. [Google Scholar]
- 39.Park J-H. Effects of mental imagery training combined electromyogram-triggered neuromuscular electrical stimulation on upper limb function and activities of daily living in patients with chronic stroke: a randomized controlled trial. Disabil. Rehabil. 2020; 42(20):2876–81. [DOI] [PubMed] [Google Scholar]
- 40.Leal-Junior ECP, Vanin AA, Miranda EF, de Carvalho PdTC, dal Corso S, Bjordal JM. Effect of phototherapy (low-level laser therapy and light-emitting diode therapy) on exercise performance and markers of exercise recovery: a systematic review with meta-analysis. Lasers Med. Sci. 2015; 30(2):925–39. [DOI] [PubMed] [Google Scholar]
- 41.Lopes-Martins RÁB Marcos RL Leonardo PS, et al. Effect of low-level laser (Ga-Al-As 655 nm) on skeletal muscle fatigue induced by electrical stimulation in rats. J. Appl. Physiol. 2006; 101(1):283–8. [DOI] [PubMed] [Google Scholar]
- 42.Santos LA Marcos RL Tomazoni SS, et al. Effects of pre-irradiation of low-level laser therapy with different doses and wavelengths in skeletal muscle performance, fatigue, and skeletal muscle damage induced by tetanic contractions in rats. Lasers Med. Sci. 2014; 29(5):1617–26. [DOI] [PubMed] [Google Scholar]
- 43.Gorgey AS, Wadee AN, Sobhi NN. The effect of low-level laser therapy on electrically induced muscle fatigue: a pilot study. Photomed. Laser Surg. 2008; 26(5):501–6. [DOI] [PubMed] [Google Scholar]
- 44.Cieśliński M, Jówko E, Sacewicz T, Cieśliński I, Płaszewski M. Low-level laser therapy and the recovery of muscle function after a single session of neuromuscular electrical stimulation: a crossover trial. Polish J Sport Tourism. 2018; 25(1):3–9. [Google Scholar]
- 45.Jówko E, Płaszewski M, Cieśliński M, Sacewicz T, Cieśliński I, Jarocka M. The effect of low level laser irradiation on oxidative stress, muscle damage and function following neuromuscular electrical stimulation. A double blind, randomised, crossover trial. BMC Sports Sci. Med. Rehabil. 2019; 11(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leal-Junior ECP, Lopes-Martins RÁB, Bjordal JM. Clinical and scientific recommendations for the use of photobiomodulation therapy in exercise performance enhancement and post-exercise recovery: current evidence and future directions. Braz. J. Phys. Ther. 2019; 23(1):71–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Duchateau J, Stragier S, Baudry S, Carpentier A. Strength training: in search of optimal strategies to maximize neuromuscular performance. Exerc. Sport Sci. Rev. 2021; 49(1):2–14. [DOI] [PubMed] [Google Scholar]
- 48.Husmann F, Mittlmeier T, Bruhn S, Zschorlich V, Behrens M. Impact of blood flow restriction exercise on muscle fatigue development and recovery. Med. Sci. Sports Exerc. 2018; 50(3):436–46. [DOI] [PubMed] [Google Scholar]
- 49.Anderson AB, Owens JG, Patterson SD, Dickens JF, LeClere LE. Blood flow restriction therapy: from development to applications. Sports Med. Arthrosc. 2019; 27(3):119–23. [DOI] [PubMed] [Google Scholar]
- 50.Cole M, Brown M. Response of the human triceps surae muscle to electrical stimulation during varying levels of blood flow restriction. Eur. J. Appl. Physiol. 2000; 82(1):39–44. [DOI] [PubMed] [Google Scholar]
- 51.Yoshikawa M, Morifuji T, Matsumoto T, Maeshige N, Tanaka M, Fujino H. Effects of combined treatment with blood flow restriction and low-current electrical stimulation on muscle hypertrophy in rats. J. Appl. Physiol. 2019; 127(5):1288–96. [DOI] [PubMed] [Google Scholar]
- 52.Natsume T, Yoshihara T, Naito H. Electromyostimulation with blood flow restriction enhances activation of mTOR and MAPK signaling pathways in rat gastrocnemius muscles. Appl. Physiol. Nutr. Metabol. 2019; 44(6):637–44. [DOI] [PubMed] [Google Scholar]
- 53.Slysz JT, Burr JF. The effects of blood flow restricted electrostimulation on strength and hypertrophy. J. Sport Rehabil. 2018; 27(3):257–62. [DOI] [PubMed] [Google Scholar]
- 54.Gorgey AS Timmons MK Dolbow DR, et al. Electrical stimulation and blood flow restriction increase wrist extensor cross-sectional area and flow meditated dilatation following spinal cord injury. Eur. J. Appl. Physiol. 2016; 116(6):1231–44. [DOI] [PubMed] [Google Scholar]
- 55.Andrade SF, Skiba GH, Krueger E, Rodacki AF. Effects of electrostimulation with blood flow restriction on muscle thickness and strength of the soleus. J. Exerc. Physiol. Online. 2016; 19(3). [Google Scholar]
- 56.Natsume T, Ozaki H, Saito AI, Abe T, Naito H. Effects of electrostimulation with blood flow restriction on muscle size and strength. Med. Sci. Sports Exerc. 2015; 47(12):2621–7. [DOI] [PubMed] [Google Scholar]
- 57.Slysz JT, Boston M, King R, Pignanelli C, Power GA, Burr JF. Blood flow restriction combined with electrical stimulation attenuates thigh muscle disuse atrophy. Med. Sci. Sports Exerc. 2021; 53(5):1033–44. [DOI] [PubMed] [Google Scholar]
- 58.Rennie MJ, Edwards RH, Halliday D, Matthews DE, Wolman SL, Millward DJ. Muscle protein synthesis measured by stable isotope techniques in man: the effects of feeding and fasting. Clin. Sci. 1982; 63(6):519–23. [DOI] [PubMed] [Google Scholar]
- 59.Dirks ML, Wall BT, van Loon LJC. Interventional strategies to combat muscle disuse atrophy in humans: focus on neuromuscular electrical stimulation and dietary protein. J. Appl. Physiol. 2018; 125(3):850–61. [DOI] [PubMed] [Google Scholar]
- 60.Dirks ML Wall BT Kramer IF, et al. A single session of neuromuscular electrical stimulation does not augment postprandial muscle protein accretion. Am. J. Physiol. Endocrinol. Metab. 2016; 311(1):E278–85. [DOI] [PubMed] [Google Scholar]
- 61.Dirks ML, Groen BB, Franssen R, van Kranenburg J, van Loon LJ. Neuromuscular electrical stimulation prior to presleep protein feeding stimulates the use of protein-derived amino acids for overnight muscle protein synthesis. J. Appl. Physiol. 2017; 122(1):20–7. [DOI] [PubMed] [Google Scholar]
- 62.Stevenson SW, Dudley GA. Dietary creatine supplementation and muscular adaptation to resistive overload. Med. Sci. Sports Exerc. 2001; 33(8):1304–10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


