Recurrence of bacterial vaginosis is common with few therapeutic solutions. A prolonged antimicrobial regimen incorporating antibiofilm activity produced encouraging results in 105 women with recurrent disease.
Background
Recurrence of bacterial vaginosis (RBV) is a major challenge to effective therapy. Women experiencing intractable and frequent recurrences are ill-served by available treatment options, such as both antimicrobial and use of probiotics.
Methods
One hundred five women with RBV failing all recommended regimens seen in the clinic were prescribed combination oral nitroimidazole 500 mg twice a day for 7 days and simultaneous boric acid 600 mg daily per vagina therapy for 30 days; thereafter, they were prescribed twice-weekly vaginal metronidazole gel for 5 months in an attempt to prevent recurrence and followed by a 6-month observation period. Results reflect standard of clinic care in this uncontrolled retrospective cohort analysis.
Results
An initial regimen of nitroimidazole and simultaneous but prolonged vaginal boric acid achieved a satisfactory response (BV cure ≤2 Amsel criteria) in 92 of 93 available patients. Thereafter, a maintenance metronidazole gel prevented symptomatic BV recurrence in 69.6% of compliant patients at 6-month follow-up. Long-term cure at a 12-month follow-up was demonstrated in almost 69% of women reaching the 6-month observation phase. Vaginal candidiasis frequently complicated prolonged antibiotic prophylaxis requiring frequent antifungal rescue or prophylaxis. Frequent loss to follow-up in this long-term study influenced efficacy evaluation.
Conclusions
In the absence of new antimicrobials or proven probiotic regimens, women with RBV may benefit from a prolonged drug-intensive antimicrobial regimen incorporating antibiofilm activity until newer measures are available. Additional randomized, control studies are needed.
Bacterial vaginosis (BV) with an extensive worldwide distribution is the commonest cause of vaginitis in women of reproductive age.1,2 Despite its global prevalence and significance by virtue of multiple complications, definitive understanding of BV pathophysiology is still not available.3 Considerable progress in understanding BV transmission and microbiology has occurred, resulting in new diagnostic methodologies; however, therapeutic advances have not been forthcoming.4–6 Standard-of-care drug treatment recommendations and guidelines have not substantially changed in 3 decades.7 Adding to the frustration of treating refractory intractable symptomatic disease, women frequently present with recurrent symptomatic episodes, often >4 attacks annually, and even fewer guidance or therapeutic options are available.7–10 Needless to say, the emotional and economic consequences of recurrent BV (RBV) are enormous and a source of considerable frustration to both practitioners and patients.2
The Wayne State University Vaginitis Clinic situated in the heart of Detroit has been the dominant local destination of women with RBV and over the years afforded an opportunity to study epidemiology, diagnosis, and treatment of BV. We recently encountered an increase of women with RBV, many of whom had participated in multiple treatment trials aimed at curing or controlling these recurrent episodes.11,12 The frustration expressed by these unfortunate patients remains enormous.13 In the absence of new therapeutic options, we developed a long-term strategy to at least control symptomatic RBV, and encouraging results are presented. Data presented reflect a management strategy implemented rather than a prospective randomized controlled trial.
METHODS
Rationale for Protocol Selection
One hundred five patients were premenopausal women with RBV who were not invited or solicited to participate in a study, but predominantly women already in the clinic roster having participated in multiple previous BV treatment studies including a partner treatment study (NCT No. 02209519). Moreover, this was not an institutional review board–sanctioned prospective study, which would have mandated an untreated or conventional therapy control group. The latter were definitely not options in this antibiotic-experienced population; rather, the regimen represented a clinical attempt to offer some hope and an alternative to previous failing treatment regimens. This was not a planned microbiologic or molecular-based study, and informed consent was not obtained. Women were enthusiastic to try something different. This report represents a summary of our experience of 105 women with RBV who were offered a new strategy for managing their recurrent episodes of BV after enjoying no long-term and often no short-term benefit from all previous treatment regimens and options.
We based the regimen upon 2 previous studies in our clinic. The first study evaluated long-term twice-weekly metronidazole vaginal gel use in which continued use of this maintenance regimen was reasonably effective in controlling RBV, that is, preventing symptomatic BV recurrence but only while prophylaxis continued and long-term efficacy or cure was uncommon.14 The use of maintenance vaginal metronidazole twice weekly is now widely used to control or prevent RBV and is safe but serves as only half a step forward.7
The second study to influence clinic strategy was based on the original observation that vaginal boric acid 600 mg daily given after conventional oral metronidazole and followed by maintenance suppressive metronidazole gel offered even additional benefits.15 Although the 2 studies are not directly comparable, there was some evidence to suggest that the 2 drug actions were additive. The benefit of boric acid was postulated to be the result of BV-related biofilm removal, allowing metronidazole access to pathogens previously inaccessible and hidden in the biofilm sanctuary.16–18 Accordingly, the optimal time to administer boric acid is concomitantly with the nitroimidazole agent.
The regimen is shown in Figure 1, consisted of oral nitroimidazole (tinidazole or metronidazole) 500 mg twice a day for 7 days starting on the same day as vaginal boric acid 600 mg daily, which is given for 30 days. Follow-up visit occurred on days 30 to 35, and if asymptomatic and with ≤2 Amsel criteria for BV, twice daily metronidazole gel 0.75% twice weekly was then prescribed for 5 months, with follow-up visits after 2 and 5 additional months or in the presence of genital symptoms. A history of recurrent vulvovaginal candidiasis (VVC) or routinely obtained positive Candida cultures prompted the use of prophylactic fluconazole 150 mg once weekly. After 5 months of this regimen, a further visit was required, and if asymptomatic and in remission (≤2 Amsel criteria), patients were followed up every 3 months for a further 6 months (observation phase).
Figure 1.
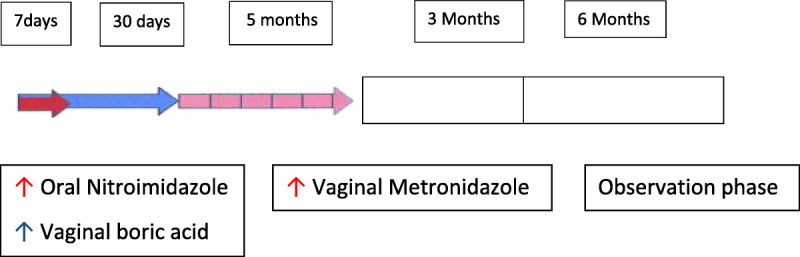
Clinical RBV strategy.
No intervention regarding sexual activity, frequency, or use of condoms was instituted. At each visit, a full pelvic examination was performed, and vaginal secretion pH measurement, whiff test, saline and 10% KOH microscopy, and Sabouraud agar cultures were obtained. Because the aforementioned protocol was considered standard clinic care, no additional samples were obtained for research purposes. A retrospective chart review of women with RBV who received the long-term suppressive regimen was undertaken with approval of the Wayne State University Medical Institutional Review Board (No. 074418MIE).
Study Population
Data are presented of the first 105 women seen for RBV between August 2014 and May 2019 who agreed to receive combination therapy. Of the 105 patients included in the analysis, 84 (80%) were African American and 21 (20%) were White. The mean age at the time of enrollment was 36.5 years (range, 18 to 60 years), with peak age being 30 to 40 years. Of note, nearly half the patients had been enrolled in previous clinical research studies (48.6%) conducted in our clinic, and all had a history of at least 3 episodes of BV in the previous 12 months. In a recent unrelated study of RBV conducted in our clinic, we reported that 26% of women with RBV receiving metronidazole 500 mg twice a day for 7 days had refractory, symptomatic BV (≥3 Amsel criteria) when seen 1 week after therapy reflecting frequent clinical resistance to conventional therapy in this clinic population.19
The mean body mass index of the 105 patients was 27.6 kg/m2, with a range of 17.4 to 43.6 kg/m2. Thus, 36% were in normal body mass index range, and 56.5% were in the preobesity and obesity range.
RESULTS
Outcome of Initial Phase: Simultaneous Nitroimidazole and Boric Acid Therapy
Of the 105 patients whose data were reviewed, 12 patients were shortly lost to follow-up; thus, data are presented for 93 women. At the first follow-up visit (mean, 32 days), only 1 in 93 patient was refractory and remained symptomatic and with Amsel 4/4 criteria. The other 92 patients were entirely asymptomatic and with ≤2 Amsel criteria (Fig. 2).
Figure 2.
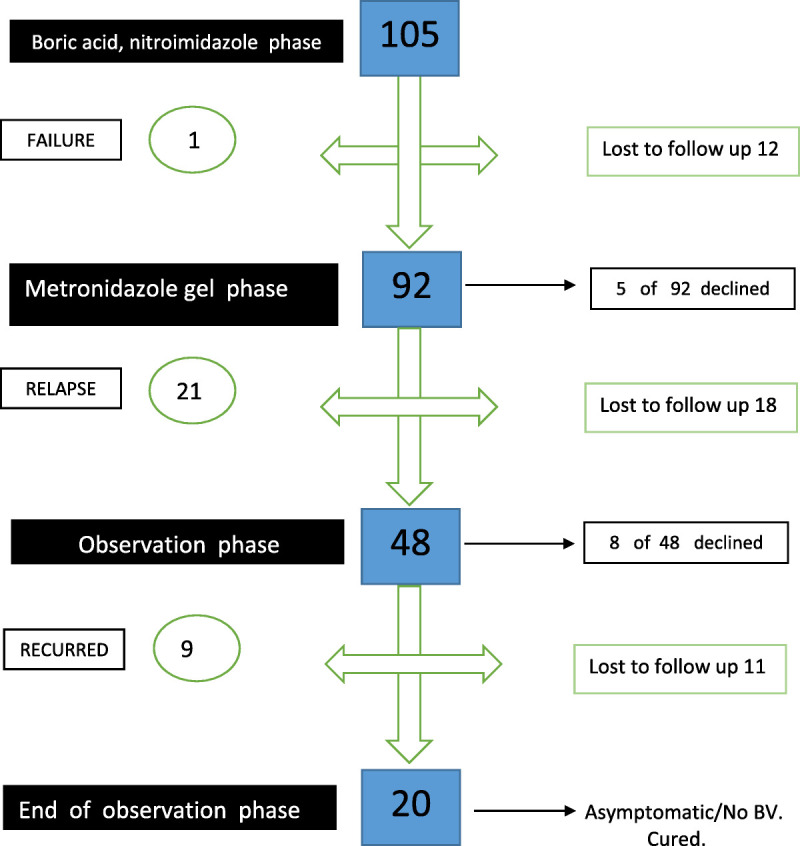
Longitudinal outcome of enrolled subjects with RBV.
Maintenance Phase (5 Months) With Twice-Weekly Metronidazole Gel
Five patients declined to participate in the maintenance phase. In addition, 18 additional patients were subsequently lost to follow-up, leaving 69 patients followed up and evaluated. Twenty-one (30.4%) of 169 patients relapsed with symptomatic BV, and 48 (69.6%) patients remained in full remission 6 months after enrollment.
Observation Phase
Eight women declined further participation because of logistic difficulties, and 40 women entered the observation phase for 6 months of follow-up. Unfortunately, 11 additional women were lost to follow-up, leaving 29 patients who were evaluable. Nine (31.0%) of 29 women developed a symptomatic relapse of BV, and 20 (69%) of 29 remained in full remission 6 months after discontinuing all therapies.
Overall Results
Of the 105 women enrolled, 12 were lost to follow-up during the initial dual drug treatment phase, 18 were lost to follow-up during the maintenance treatment phase, and 11 were lost to follow-up during the observation phase, for a total of 41 patients who were lost to follow-up and 13 women who withdrew. One patient only failed the oral antimicrobial and vaginal boric acid phase. The largest number recurred during maintenance suppressive period (n = 21), and finally, 9 patients recurred during the observation phase. A total of 31 failed with florid BV for 12 months. Overall, 20 women who were compliant and returned for follow-up remained BV-free for 1 year and were externally grateful.
Complications and Tolerance
Throughout the 6-month treatment and the 6-month observation phase, 52 of 105 patients had at least one positive yeast culture. Eighty-seven percent (87%) of isolates were identified as Candida albicans, including 3 women with fluconazole resistance. Four patients had Candida glabrata, most positive cultures emerged during metronidazole gel treatment. Patients with symptoms of VVC and accompanying positive cultures were placed on maintenance weekly fluconazole therapy.
Vaginal boric acid was extremely well tolerated despite nightly use for 30 days. No complaints of intolerance were received, and no discontinuation or interruption of therapy occurred.
DISCUSSION
The most striking finding of the study was the dramatic effect obtained with use of combination oral nitroimidazole and topical boric acid in achieving symptomatic relief, microscopy, and pH reversal after 30 days of therapy. Only a single patient in this difficult population was refractory to this therapy.
We compared the 32-day follow-up data with results obtained in a historic but recent control group of women, with identical demographic results regarding age and race, who were studied at our clinic and followed up for 30 days after a similar 7-day nitroimidazole therapy with and without boric acid and results were published.19 Among 85 referred women with RBV, only 36 achieved short remission (30 days) after nitroimidazole-only therapy. The odds ratio indicating benefits of combining boric acid with nitroimidazole was 124 (95% confidence interval, 17–930; Fisher exact test performed using GraphPad Prism version 6.07 for Windows; GraphPad Software, La Jolla, CA; www.graphpad.com).
Unfortunately, definitive microbiome research studies were not performed and are needed in the future. We are unable to define definitive prognostic details at the completion of the boric acid therapy. Even pH measurement was unreliable because recent boric acid within the previous 24 hours could elevate the pH, given that boric acid is only a weak acid and also a bacteriostatic agent. it is most important to note that we are unable to determine which patients might achieve long-term remission without requiring the metronidazole gel maintenance. We are similarly unable to conclude the optimal dose or duration of boric acid daily therapy. The prolonged therapy was based on past experience in our clinic using a similar duration of therapy.15 Boric acid was extremely well tolerated. Previous unpublished data obtained in our clinic indicated that boric acid therapy alone in the absence of antibiotic therapy, although reducing odor, was not efficient in controlling or curing BV. However, fewer than 30 doses of boric acid could easily be as effective. Another advantage of boric acid was its fungistatic effect, preventing yeast superinfection during the initial early treatment phase. Uniformly, patient satisfaction at the end of boric acid therapy was remarkably positive, as was saline microscopy evaluation. Boric acid is rarely available in standard pharmacies but easily and inexpensively obtained from compounding pharmacies.
The twice-weekly metronidazole therapy was prescribed with the intent of using minimal antibiotic measures capable of suppressing any residual or persisting anaerobic pathogens without suppressing Lactobacillus species recovery in restoring the normal vagina microbiota.14 We chose metronidazole rather than clindamycin for this prolonged period because of the more favorable profile of metronidazole in allowing lactobacilli to proliferate. The duration of the metronidazole prophylaxis chosen was entirely arbitrary. Clearly, the most vulnerable period of this treatment strategy emerged between 2 and 6 months after entering the protocol. Recurrence rates were highest, reaching 31% during this period and accompanied by a high risk of Candida species superinfection. Anecdotally, asymptomatic women with elevated vaginal pH and microscopy that identified dysbiosis were most likely to recur.19 On the other hand, several women with entirely normal clinical parameters still recurred. The post–boric acid phase may well be the optimal time to initiate effective probiotic therapy.
The majority of women completing 6 months of suppressive or antibiotic prophylactic therapy remained asymptomatic and clinically cured for at least 6 months after cessation of antibacterial therapy (69%). Late recurrence could be the result of relapse or reinfection. Unfortunately, details of sexual behavior were not collected.
Recurrence rates of BV are highly variable depending on the population studied.8–10 Accordingly, identical treatment regimens may result in conflicting outcomes. Of the numerous prognostic factors that affect and bias long-term recurrence, remission, and cure rates, a history of confirmed RBV episodes is of major significance and was present in all patients in our study. In addition to differences in therapy prescribed, a major confounding factor in long-term recurrence studies lasting longer than 6 months is the role of sexual transmission in BV causation; hence, BV recurrence could be the result of either relapse or reinfection. The possibility of sexual reinfection profoundly limits the efficacy evaluation of therapeutic regimens correcting vaginal dysbiosis. In the present study, we were dealing with a biased, highly selected population of women with therapy-resistant RBV. Only women with drug regimen failures were referred to the Vaginitis Clinic and enrolled into multiple studies. Inevitably, women who likely responded well over time dropped out. As mentioned previously, almost half the patients in the present studied had participated and failed previous studies, representing a major accrual bias.
The study is also biased by the dominance of African American women, a function of the clinic being situated in a city with a considerable majority of Black citizens. In previous studies of recurrent VVC conducted in our clinic, slightly more than half the enrolled patients were Whites. Of note, almost two-thirds of patients enrolled in this RBV study provided a history of post–antibiotic-treated BV-induced VVC. Not surprisingly, Candida superinfection was common during the metronidazole gel twice-weekly maintenance phase requiring frequent fluconazole intervention and weekly prophylactic prescription, which was effective in preventing breakthrough VVC symptomatic episodes.
The limitations of our clinical experience include the absence of a control group or placebo in each phase of therapy, measures not feasible in this frustrated population. In addition, high follow-up loss was evident, but this was a 12-month period of follow-up, most of whom work and who were not paid to participate or receive any compensation.
In summary, clinicians worldwide daily are faced by women plagued by recurring symptoms, with manifestation playing havoc with their sexual lives.2 The medical community has no current answer other than repeat prescriptions using 2 drug classes with frequent adverse effects. While the search for probiotics continues, we still have nothing to offer, although recently encouraging results were published using Lactin-V (phase 2b trial), which unfortunately is not available commercially or currently Food and Drug Administration approved.20 Understanding the failure of probiotic therapy is also lacking. Recently, a vaginal fluid transfer study offers a new option in the future for women with RBV, but more information is necessary.21 The clinic experience presented herein represents only a modest improvement in therapy but an acceptable chance of control and cure. The optimal use of vaginal boric acid, dose, duration, and administration in combination with antibiotics is worthy of further study in prospective randomized studies.
Footnotes
Conflict of Interest and Sources of Funding: J.D.S. has served as a consultant for Scynexis Pharmaceuticals and Mycovia Pharmaceutical. All other authors have no relevant conflict of interest to disclose. The authors report no sources of support.
Contributor Information
Sarvani Surapaneni, Email: gr8809@wayne.edu.
Robert Akins, Email: Rakins@med.wayne.edu.
REFERENCES
- 1.Kenyon C, Colebunders R, Grucitti T. The global epidemiology of bacterial vaginosis: A systematic review. Am J Obstet Gynecol 2013; 209:505–523. [DOI] [PubMed] [Google Scholar]
- 2.Peebles K Velloza J Balkud JE, et al. High global burden and costs of bacterial vaginosis: A systematic review and meta-analysis. Sex Transm Dis 2019; 46:304–311. [DOI] [PubMed] [Google Scholar]
- 3.Muzny CA Taylor CM Swords WE, et al. An updated conceptual model on the pathogenesis of bacterial vaginosis. J Infect Dis 2019; 220:1399–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thompson A Timm K Borders N, et al. Diagnostic performance of two molecular assays for the detection of vaginitis in symptomatic women. Eur J Clin Microbiol Infect Dis 2020; 39:39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwebke JR Gaydos CA Nyirjesy P, et al. Diagnostic performance of a molecular test versus clinician assessment of vaginitis. J Clin Microbiol 2018; 56:e00252–e00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neal CM Kus LH Eckert LO, et al. Noncandidal vaginitis: A comprehensive approach to diagnosis and management. Am J Obstet Gynecol 2020; 222:114–122. [DOI] [PubMed] [Google Scholar]
- 7.Workowski KA Bolan GA, Centers for Disease Control and Prevention . Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015; 64(RR-03):1–137. Erratum in: MMWR Recomm Rep 2015; 64(33):924. [PMC free article] [PubMed] [Google Scholar]
- 8.Sobel JD, Schmitt C, Meriwether C. Long-term follow-up of patients with bacterial vaginosis treated with oral metronidazole and topical clindamycin. J Infect Dis 1993; 167:783–784. [DOI] [PubMed] [Google Scholar]
- 9.Bradshaw CS Morton AN Hocking J, et al. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J Infect Dis 2006; 193:1478–01486. [DOI] [PubMed] [Google Scholar]
- 10.Bradshaw CS, Sobel JD. Current treatment of bacterial vaginosis—Limitations and need for innovation. J Infect Dis 2016; 214(Suppl 1):S14–S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiao B Wu C Song W, et al. Association analysis on recurrence of bacterial vaginosis revealed microbes and clinical variables important for treatment outcome. Front Cell Infect Microbial 2019; 9:1–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Powell AM, Nyirjesy P. Recurrent vulvovaginitis. Best Pract Res Clin Obstet Gynaecol 2014; 28:967–976. [DOI] [PubMed] [Google Scholar]
- 13.Faught BM, Reyes S. Characterization and treatment of recurrent bacterial vaginosis. J Womens Health (Larchmt) 2019; 9:1218–1226. [DOI] [PubMed] [Google Scholar]
- 14.Sobel JD Ferris D Schwebke T, et al. Suppressive antibacterial therapy with 0.75% metronidazole vaginal gel to prevent recurrent bacterial vaginosis. Am J Obstet Gynecol 2006; 194:1283–1289. [DOI] [PubMed] [Google Scholar]
- 15.Reichman O, Akins R, Sobel JD. Boric acid addition to suppression antimicrobial therapy for recurrent bacterial vaginosis. Sex Transm Dis 2009; 36:732–734. [DOI] [PubMed] [Google Scholar]
- 16.Swidsinski A Mendling W Loening-Baucke V, et al. Adherent biofilms in bacterial vaginosis. Obstet Gynecol 2005; 106(5Pt 1):1013–1023. [DOI] [PubMed] [Google Scholar]
- 17.Swidsinski A Mendling W Loening-Baucke V, et al. An adherent Gardnerella vaginalis biofilm persists on the vaginal epithelium after standard therapy with oral metronidazole. Am J Obstet Gynecol 2008; 198:97e.1–97e.6. [DOI] [PubMed] [Google Scholar]
- 18.Muzny CA, Schwebke JR. Biofilms: An underappreciated mechanism of treatment failure and recurrence in vaginal infections. Clin Infect Dis 2015; 61:601–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sobel JD Kaur N Woznicki NA, et al. Prognostic indicators of recurrence of bacterial vaginosis. J Clin Microbiol 2019; 57:e00227–e00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen CR Wierzbicki MR French AL, et al. Randomized trial of Lactin-V to prevent recurrence of bacterial vaginosis. N Engl J Med 2020; 382:1906–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lev-Sagie A Goldman-Wohl D Cohen Y, et al. Vaginal microbiome transplantation in women with intractable bacterial vaginosis. Nat Med 2019; 25:1500–1504. [DOI] [PubMed] [Google Scholar]


