Abstract
Background
Virus-associated respiratory infections are in the spotlight with the emergence of SARS-CoV-2 and the expanding use of multiplex PCR (mPCR). The impact of molecular testing as a point-of-care test (POCT) in the emergency department (ED) is still unclear.
Objectives
To compare the impact of a syndromic test performed in the ED as a POCT and in the central laboratory on length of stay (LOS), antibiotic use and single-room assignment.
Methods
From 19 November 2019 to 9 March 2020, adults with acute respiratory illness seeking care in the ED of a large hospital were enrolled, with mPCR performed with a weekly alternation in the ED as a POCT (week A) or in the central laboratory (week B).
Results
474 patients were analysed: 275 during A weeks and 199 during B weeks. Patient characteristics were similar. The hospital LOS (median 7 days during week A versus 7 days during week B, P = 0.29), the proportion of patients with ED-LOS <1 day (63% versus 60%, P = 0.57) and ED antibiotic prescription (59% versus 58%, P = 0.92) were not significantly different. Patients in the POCT arm were more frequently assigned a single room when having a positive PCR for influenza, respiratory syncytial virus and metapneumovirus [52/70 (74%) versus 19/38 (50%) in the central testing arm, P = 0.012].
Conclusions
Syndromic testing performed in the ED compared with the central laboratory failed to reduce the LOS or antibiotic consumption in patients with acute respiratory illness, but was associated with an increased single-room assignment among patients in whom a significant respiratory pathogen was detected.
Introduction
Worldwide, respiratory tract infections are one of the leading causes of emergency department (ED) visits1 and also one of the leading causes of infectious-disease-related deaths.2 Among the causative pathogens of pneumonia and respiratory infections, the role of viruses has recently been highlighted by the emergence of syndromic testing.3 Specifically, the frequency and role of non-influenza respiratory viruses has been increasingly recognized in recent years.4–11 Because antibiotic overuse drives antimicrobial resistance, it is essential to quickly document the pathogen responsible for lower respiratory tract infections and decide whether antibiotic treatment is required. The number of rapid syndromic testing assays (<2 h) has been expanding in the past few years,12 but they continue to be used primarily in central laboratories despite their utility as a point-of-care test (POCT). The guidelines of the Infectious Diseases Society of America recently included multiplex PCR (mPCR) as one of the diagnostic tools to manage patients with lower respiratory tract infections who require hospitalization, whether or not they are immunosuppressed.13
Several recent studies have evaluated the benefits of using rapid syndromic testing assays as POCTs, including decreased antibiotic use, decreased need for complementary examinations, reduced duration of hospitalization and increased antiviral use for influenza-related infections. Some of the results of these studies conflict, but two studies have shown substantial reductions in lengths of hospital stay and either decreases in the use of antibiotics or increases in the numbers of patients receiving a single dose of antibiotics.14–16 However, a limitation of these two studies was that they often compared the use of rapid syndromic testing as a POCT targeting multiple viruses and some atypical bacteria with the use of a classic central laboratory approach without the use of rapid syndromic testing (i.e. systematically testing for influenza but testing for other respiratory viruses only on physician request). Thus, the observed benefits could be explained both by the delay of results and by the systematic detection of all respiratory viruses.
Isolation management is becoming an topic of great interest in the context of the COVID-19 pandemic and may be complexified by the potential co-circulation of SARS-CoV-2 with other respiratory viruses.17,18 POCTs have the potential to contribute substantially in this regard by reducing the duration of probabilistic isolation precautions in the ED and providing the result in a timeframe compatible with room assignment. This last point is of importance as single room availability is limited and can lead to double room misattribution for influenza-positive patients, as observed in our previous study for up to 75% of influenza-positive patients.19
We therefore designed a monocentric, prospective, controlled clinical trial to measure the impact of using the same rapid syndromic test as a POCT versus using it in the central laboratory. The outcomes measured were antibiotic consumption, hospital and ED lengths of stay and assignment of patients to single rooms.
Patients and methods
Ethics
The research was conducted in accordance with the Declaration of Helsinki and it was approved by the local ethics committee (CEERB N2019-050). In accordance with French law and ethics regulations for observational studies, written consent was not required but patients were informed of the study to ensure their non-opposition to the research.
Study design and participants
We performed a monocentric, prospective, controlled, clinical trial at the 850 bed Bichat-Claude Bernard university hospital ED in Paris, France. The hospital has 455 single rooms and 276 double rooms. The study design comprised two periods: (i) the POCT weeks (A weeks), with mPCR testing performed in the ED, 24 h per day and 7 days per week; and the (ii) the central laboratory weeks (B weeks), with mPCR testing performed in the hospital’s central virology laboratory each weekday between 8 am and 5 pm and each Saturday between 8 am and 1 pm, but not overnight or between 1 pm on Saturday and Monday 8 am. These two periods were alternated weekly (i.e. week A, week B, A, B, etc.), the first week was randomly chosen as a POCT week.
Due to the purpose of monitoring the benefits of the shortened delay in the POCT arm, the study was designed as an open study and the physicians were not blinded from which was the ongoing arm.
Patients were included during the 2019–20 winter period, from 19 November 2019 to 9 March 2020. The latter date corresponds to the emergence of the COVID-19 pandemic and the French national recommendation to perform SARS-CoV-2 systematic testing among patients seeking care in the ED. Before this date, no patients suspected of having COVID-19 were admitted to the hospital ED; instead, they were sent to a dedicated unit in the infectious diseases department.
The included patients were adults (aged >18 years) presenting symptoms compatible with a respiratory infection according to the definition of influenza-like illness (ILI) provided by the European Centre for Disease Prevention and Control20,21 with an Emergency Severity Index22 (ESI) of level 1, 2 or 3. Patients were hospitalized for the following reasons: oxygen therapy or a CRB65 score ≥1.
Demographic and clinical data were collected at recruitment; outcome data were collected retrospectively from electronic medical records. We collected all the data on a standard case report form on a protected server.
Procedures
For respiratory viruses testing, we used the QIAstat-Dx Respiratory Panel V2 (Qiagen, Hilden, Germany). This rapid multiplex PCR assay allows the detection of 22 viral and bacterial respiratory targets, including: influenza (A and B); parainfluenza viruses 1 to 4 (PIV); rhinovirus/enteroviruses (HRV); respiratory syncytial viruses (RSV) A and B; human metapneumovirus (hMPV); adenoviruses (ADV); human coronaviruses HKU1, OC43, NL63 and 229E; bocavirus; Mycoplasma pneumoniae; Legionella pneumophila; and Bordetella pertussis. Results are provided within about 1 h. The QIAstat-Dx Respiratory Panel V2 is a closed mPCR system allowing respiratory virus detection from nasopharyngeal swabs with single-use cartridges that can be filled directly with the dry swab into the swab port for POCT use, or with 300 μL of viral transport medium in the liquid port, for central laboratory use, as previously described.23 In case of internal control errors, the test was performed a second time under the same conditions. Internal quality controls were used by the laboratory technician in both the central laboratory and the ED to ensure the results were reproducible between arms. During the central testing arm (B weeks), the analyser was operated by a laboratory technician in the hospital’s central virology laboratory. During the POCT arm (A weeks), it was operated by trained ED physicians (n = 30) using the swab port and analyser located in the ED.
Outcomes
The primary outcome measure was the hospital length of stay (LOS). The LOS has been calculated as the number of the number of days from entry to the ED (day 0) to the day of patient departure or death. The follow-up period was 30 days and no patients were still hospitalized at this point. Secondary outcomes were the proportion of ED lengths of stay (ED-LOS) <1 day, antibiotic prescriptions and assignment of patients to a single room or a double room with a blocked bed (meaning that the second bed could not be used) in individuals with a recommendation for isolation. The overall disease severity was also assessed for each patient using the CRB65 score.24 The type of admission (hospital ward or ICU) was also retrieved. Of note, local recommendation for isolation requires that all patients with a positive test for an influenza virus, RSV or hMPV be assigned to a single room. Patients remained in their ED examination single rooms or ED hospitalization ward in single rooms until the virological results were made available.
Statistical analysis
Sample size was calculated based on the primary outcome, with an estimated reduction in LOS of 2 days, an SD of 7 days, with 90% power and a risk α at 5%. The average LOS of patients was 7 days, in accordance with the previous studies.14,15,25 The difference of 2 days was chosen by taking into account the two previous studies showing an overall reduction ranging from 0.8 to 1.1 days when comparing mPCR POC testing with classical virology testing in the central laboratory14,15 and a post hoc analysis showing a reduction of 2.8 days when providing the result in <1.6 h.26 Thus, the number of required subjects was 518, with 259 patients per arm. Baseline characteristics within each group were summarized using appropriate descriptive statistics. We used linear regression for the primary outcome LOS and reported mean difference with 95% CI from univariable analysis and for a multivariable model including sex, age and CRB 65 score as covariables. Logistic regression was performed for analysis of the impact of POCT on ED-LOS <1 day and single-room assignment in individuals with a recommendation for isolation. OR and 95% CI are reported using a normal approximation. Missing data were not replaced in the final dataset. The P values corresponded to the Wald statistic and a threshold of 0.05 was used for statistical significance. Statistical analysis and data management were performed in R version 4.0.3.
Results
Baseline characteristics
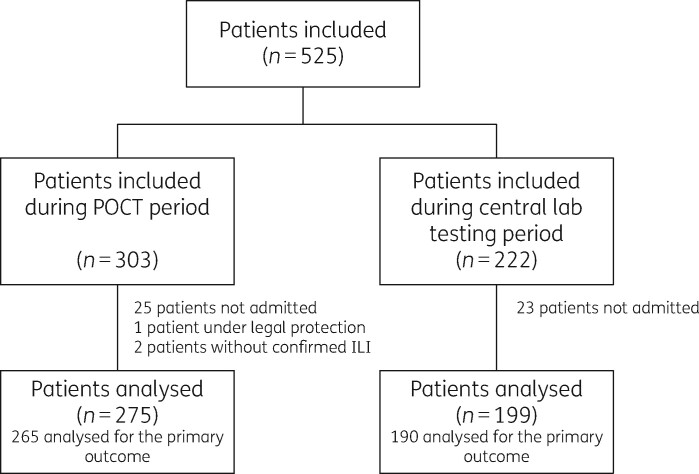
A total of 525 patients were included during the study period. Among them, 48 were excluded because they were discharged without hospitalization and 3 were excluded because of legal protection (N = 1) or absence of ILI (N = 2). Of the 474 patients that were analysed, 275 were analysed for the POCT periods (9 weeks) and 199 for central laboratory testing periods (8 weeks) (Figure 1). Patient characteristics in both arms were similar (Table 1). The median age was 73 years (IQR: 62–85 years), with a majority of men (56%, n = 266). Between the two arms, patients had no statistically significant differences in initial clinical presentation (Table 1).
Figure 1.
Study flow chart. ED, emergency department; ILI, influenza-like illness.
Table 1.
Comparison of patients’ baseline characteristics and prognosis
| Variables | All |
Point-of-care testing |
Central testing |
P valuea |
|---|---|---|---|---|
| (n = 474) | (n = 275) | (n = 199) | ||
| Patient characteristics | ||||
| Male, n (%) | 266 (56) | 154 (56) | 112 (56) | 1 |
| Age (years), median (IQR) | 73 (62–85) | 73 (62–84) | 72 (60–85) | 0.41 |
| Temperature (°C), median (IQR) | 37 (37–38) | 37 (37–38) | 37 (36–38) | 0.48 |
| Systolic blood pressure (mmHg), median (IQR) | 131 (113–149) | 131 (114–151) | 129 (112–145) | 0.11 |
| SpO2 (%), median (IQR) | 95 (92–97) | 95 (91–97) | 95 (93–98) | 0.51 |
| Heart rate (bpm), median (IQR) | 94 (81–110) | 95 (81–110) | 92 (82–107) | 0.18 |
| Respiratory rate (min), median (IQR) | 19 (16–24) | 20 (17–25) | 18 (16–22) | 0.09 |
| Duration of symptoms before ED visit (days), median (IQR) | 2 (1–4) | 2 (1–4) | 2 (1–4) | 0.65 |
| CRB-65 score, median (IQR) | 1 (1–1) | 1 (1–2) | 1 (1–1) | 0.14 |
| Charlson Score, median (IQR) | 4 (3–6) | 5 (3–6) | 4 (3–6) | 0.12 |
| PCR turnaround times (h), median (IQR) | 1.1 (1.1–1.1) | 19.6 (12.7–39.2) | ||
| Virological results | ||||
| Negative PCR, n (%) | 288 (61) | 155 (56) | 133 (67) | 0.02 |
| Positive PCR, n (%) | 186 (39) | 120 (44) | 66 (33) | 0.02 |
| Coinfections, n (%) | 13 (3) | 7 (3) | 6 (3) | 0.78 |
| Identified pathogens | 0.25 | |||
| Influenza A | 49 (25) | 35 (28) | 14 (20) | |
| Influenza B | 6 (3) | 4 (3) | 2 (3) | |
| Entero/rhinovirus | 54 (27) | 32 (25) | 22 (31) | |
| Metapneumovirus | 23 (12) | 16 (13) | 7 (10) | |
| RSV | 30 (15) | 15 (12) | 15 (21) | |
| Parainfluenza | 11 (6) | 8 (6) | 3 (4) | |
| Coronavirus | 19 (10) | 15 (12) | 4 (6) | |
| M. pneumoniae | 3 (2) | 1 (1) | 2 (3) | |
| L. pneumophila | 1 (1) | 0 (0) | 1 (1) | |
| Bocavirus | 1 (1) | 1 (1) | 0 (0) | |
| Patient management | ||||
| Isolation | ||||
| Single room or double room with blocked bed, n (%) | 207 (44) | 122(44) | 85 (43) | 0.61 |
| Double room, n (%) | 267 (56) | 153 (56) | 114 (57) | |
| Orientation | ||||
| Medical ward | 206 (43) | 117 (43) | 89 (45) | 0.39 |
| ICU | 29 (6) | 18 (7) | 11 (6) | |
| Transfer to another hospital | 171 (36) | 103 (38) | 68 (35) | |
| ED ward | 118 (25) | 63 (23) | 55 (28) | |
| Antibiotics before ED visit (n = 471) | 62 (13) | 38 (14) | 24 (12) | 0.81 |
| Antibiotics at the ED (n = 472) | 275 (58) | 160 (59) | 115 (58) | 0.92 |
| Oseltamivir | 44 (9) | 30 (11) | 14 (7) | 0.15 |
| Patient outcomes (n = 455) | ||||
| LOSb (days) | 7 (3–14) | 7 (3–14) | 7 (2–13) | 0.29 |
| ED-LOS <1 day | 291 (61) | 172 (63) | 119 (60) | 0.57 |
| Patients still hospitalized at day 30 | 41 (9) | 26 (9) | 15 (8) | 0.51 |
| Patient death during hospitalization | 37 (8) | 25 (9) | 12 (6) | 0.3 |
Values shown are n (%) or median (IQR). ED, emergency department; LOS, length of stay; RSV, respiratory syncytial virus.
P value of the Fisher test for categorical variables, or Wilcoxon test for quantitative variables.
Nineteen patients are missing due to an unknown length of stay; the length of stay for patients still hospitalized on D30 was set at 30 days.
The median turnaround time for mPCR results was 1.1 h (IQR: 1.1–1.1) and 19.6 h (IQR: 12.7–39.2) in the POCT and central testing arms, respectively (P < 0.001). A total of 186 (39.2%) patients had a positive PCR for at least one respiratory pathogen, including 13 (3%) cases of pathogen codetection. The identified pathogens included 55 influenza virus (30%, including 49 influenza A and 6 influenza B), 54 HRV (30%), 30 RSV (15%), 23 hMPV (12%), 19 human coronaviruses (10%), 11 parainfluenza (6%), 3 M. pneumoniae (2%), 1 L. pneumophila (1%) and 1 bocavirus (1%). No statistically significant differences were observed between the arms in the’ distribution of pathogens (Table 1).
Primary outcomes
For the primary outcome, hospital LOS, we did not observe any difference between the POCT and the central testing arms. The mean difference adjusted for sex and age was 0.3 days (95% CI, −1.2 to 2.8; P = 0.66). The median LOS stay was 7 days (IQR: 3–14) in the POCT arm and 7 days (IQR: 2–13) in the central testing arm. The multivariable analysis showed that the severity, as assessed by CRB65 score, was associated with a longer LOS (OR 1.2 for one each point; 95% CI, 1.1–2.4; P = 0.04) (Table 2).
Table 2.
Factors associated with length of stay (univariable and multivariable linear regression)
| Univariable analysis (n = 455) |
Multivariable analysis (n = 440) |
||||
|---|---|---|---|---|---|
| Variables | Median (IQR) | Estimation (95% CI) | P valuea | Estimation (95% CI) | P valueb |
| Study arm | |||||
| Central testing | 7 (2–13) | 0 (ref) | 0.34 | 0 (ref) | |
| POCT | 7 (3–14) | 0.8 (−0.9 to 2.6) | 0.3 (−1.2 to 1.8) | 0.66 | |
| Sex | |||||
| M | 7 (2–13) | 0 (ref) | 0.59 | 0 (ref) | |
| F | 8 (3–15) | 0.5 (−1.2 to 2.2) | 0.9 (−0.6 to 2.4) | 0.24 | |
| Age (/10 years) | 0.7 (0.2 to 1.2) | 0.003 | 0.3 (−0.2 to 0.9) | 0.23 | |
| Symptom duration before ED visit (days) | 0.1 (−0.1 to 0.3) | 0.22 | |||
| CRB65 score | 2.6 (1.5 to 3.7) | <0.001 | 1.2 (0.1 to 2.4) | 0.041 | |
| Charlson’s score | 0.5 (0.2 to 0.9) | 0.005 | |||
| Antibiotics before ED | 8 (2–18) | 1.6 (−0.9 to 4.1) | 0.21 | ||
| Administered antibiotics in ED | 7 (3–14) | 0.9 (−0.8 to 2.6) | 0.31 | ||
| Oseltamivir | 6 (1–13) | −0.2 (−3.1 to 2.7) | 0.88 | ||
| Medical ward | 9 (5–15) | 0 (ref) | <0.001 | 0 (ref) | |
| ICU | 25 (11–30) | 9 (6 to 12.1) | 8.9 (5.8 to −12.1) | <0.001 | |
| Transfer | 9 (6–15) | 0.9 (−1.0 to 2.8) | 0.4 (−1.6 to 2.3) | 0.72 | |
| ED ward | 1 (1–2) | −8.3 (−10.1 to −6.5) | −8 (−9.8 to −6.1) | <0.001 | |
| Isolation | |||||
| Single room | 8 (4–15) | 0 (ref) | 0.39 | ||
| Double room with blocked bed | 6 (2–13) | −1.2 (−2.9 to 0.5) | |||
| Double room | 10 (8–10) | 0.3 (−7.2 to 7.7) | |||
ED, emergency department; POCT, point-of-care testing.
P value of the Likelihood Ratio Test.
P value of the Wald test.
In the univariable analysis, the proportions of patients with ED-LOS <1 day were not significantly different between the two arms (OR 1.1; 95% CI, 0.8–1.6) (Table 3). No statistically significant differences were observed between the POCT testing arm and the central testing arm regarding the duration of antibiotic treatment. We observed no statistically significant difference regarding antibiotic prescription or ward of admission between the two arms (Table 1). The median durations of treatment were similar in both arms: 2 days for third-generation cephalosporin (P = 0.14), 2 days for macrolides (P = 0.43) and 5 days for amoxicillin (P = 0.77).
Table 3.
Factors associated with ED-LOS <1 day (univariable logistic regression)
| Univariable analysis |
||
|---|---|---|
| Variables | OR (95% CI) | P valuea |
| Study arm | ||
| Central testing | 1 (ref) | 0.54 |
| POCT | 1.1 (0.8–1.6) | |
| Sex | ||
| M | 1 (ref) | 0.89 |
| F | 1.0 (0.7–1.4) | |
| Age (years) | 1.0 (1.0–1.0) | 0.92 |
| Symptom duration before ED visit (days) | 1.1 (0.9–1.4) | 0.44 |
| CRB65 score | 1.0 (0.9–1.1) | 0.76 |
| Charlson’s score | 1.3 (0.7–2.3) | 0.4 |
| Antibiotics before ED | 1.0 (0.7–1.4) | 0.92 |
| Oseltamivir | 1.4 (0.7–2.8) | 0.3 |
| Isolation | ||
| Single room | 1 (ref) | 0.3 |
| Double room with a blocked bed | 0.8 (0.5–1.1) | |
| Double room | 1.3 (0.3–9.6) | |
ED, emergency department; POCT, point-of-care testing.
P value of the Wald test.
Overall, 207 and 7 patients were admitted to a single room or a double room with a blocked bed, respectively. For clarity purposes, these two populations are referred to as single-room admission in the rest of the manuscript. Patients in the POCT arm were more frequently assigned to a single room after admission when having a positive PCR for influenza, RSV and metapneumovirus pathogens [52/70 (74%) in the POCT arm versus 19/38 (50%) in the central testing arm, P = 0.019, OR, 2.9; 95% CI, 1.3–6.7; P = 0.012]. Multivariable analysis confirmed these associations between POCT testing and single-room assignment in patients with a recommendation for single room assignment (OR, 2.9; 95% CI, 1.3–6.8; P = 0.014) (Table 4). No statistically significant differences were observed for patients admitted to single rooms with either a negative PCR or a positive PCR for any other virus between the POCT and central testing arm [66/161 patients (34%) in the POCT arm versus 70/205 patients (40%) in the central testing arm, P = 0.36] (Table 5).
Table 4.
Factors associated with the prescription of suitable single-room assignment in patients with a recommendation for single-room assignment (univariable and multivariable logistic regression)
| Univariable analysis (n = 108) |
Multivariable analysis |
|||
|---|---|---|---|---|
| Variables | OR (95% CI) | P valuea | OR (95% CI) | P valueb |
| Study arm | ||||
| Central testing | 1 (ref) | 0.012 | 1 (ref) | |
| POCT | 2.9 (1.3–6.7) | 2.9 (1.3–6.8) | 0.014 | |
| Sex | ||||
| M | 1 (ref) | 0.62 | 1 (ref) | |
| F | 0.8 (0.4–1.8) | 0.9 (0.4–2.2) | 0.86 | |
| Age (years) | 1.0 (1.0–1.0) | 0.95 | 1.0 (1.0–1.0) | 0.79 |
| Symptoms duration before ED visit (days) | 1.0 (0.9–1.2) | 0.89 | ||
| CRB65 score | 1.0 (0.6–1.8) | 1 | ||
| Charlson’s score | 1.0 (0.8–1.2) | 0.89 | ||
| Antibiotics before ED | 0.9 (0.3–2.5) | 0.84 | ||
| ED antibiotics administration | 0.6 (0.2–1.3) | 0.18 | ||
| Oseltamivir | 1.0 (0.4–2.3) | 0.93 | ||
ED, emergency department; POCT, point-of-care-testing.
P value of the Likelihood Ratio Test.
P value of the Wald test.
Table 5.
Multiplex PCR result and single room assignment of patients isolated by pathogen
| Characteristic | Single room assignment, POCT period, n/N (%) | Single room assignment, central testing period, n/N (%) | P valuea |
|---|---|---|---|
| Overall single room assignment | 122/275 (44) | 85/199 (43) | 0.72 |
| Pathogen with local recommendation for single room assignmentb | 52/70 (74) | 19/38 (50) | 0.019 |
| Influenza A | 27/35 (77) | 8/14 (57) | 0.18 |
| Influenza B | 4/4 (100) | 1/2 (50) | 0.33 |
| Metapneumovirus | 11/16 (69) | 1/7 (14) | 0.027 |
| RSV | 10/15 (67) | 9/15 (60) | 1 |
| Unrequired single room assignment | 70/205 (34) | 66/161 (40) | 0.36 |
| Rhinovirus | 7/28 (25) | 8/19 (42) | 0.34 |
| Parainfluenza | 2/8 (25) | 2/3 (67) | 0.49 |
| Coronavirus | 8/13 (62) | 2/3 (67) | 1 |
| Negative PCR | 53/155 (34) | 53/133 (40) | 0.33 |
POCT, point-of-care testing; RSV, respiratory syncytial virus.
P value of the Fisher test.
There was a footnote marker but no corresponding footnote.
Discussion
This large, pragmatic, quasi-randomized prospective controlled trial compared the impact of a uniform mPCR respiratory pathogen testing approach performed as a POCT in the ED with testing performed in a central virology laboratory. The median delay to result decreased from 19.6 h in the central laboratory arm to 1.1 h in the POCT arm. POCT mPCR did not shorten hospital LOS or reduce prescription of antibiotic treatment. However, a higher rate of compliance with local isolation recommendations in terms of assigning patients to single rooms was observed. Thus, POCT increased the proportion of patients isolated due to a positive PCR for influenza, RSV, or metapneumovirus, from 50% in the central laboratory arm to 74% in the POCT arm (P = 0.019).
Our results regarding LOS and antibiotic use are inconsistent with the findings of two previous studies14,15 that assessed the benefits of both quicker results and detection of a wider range of pathogens. We expected a decrease in antibiotic use in the POCT arm owing to the rapid diagnosis of viral respiratory infections. However, a viral infection does not exclude a bacterial coinfection7,27 and viro-bacterial coinfections may be associated with more severe prognoses.8,10
To our knowledge, very few studies have assessed the impact of rapid molecular testing on patient isolation for respiratory viruses.14,28 However our study showed a positive impact on compliance with preventive measures by increasing single-room assignment in the downstream unit among patients with a viral infection that requires isolation. These measures are critical for reducing nosocomial transmission and outbreaks. This is particularly relevant because nosocomial acquisition of influenza is associated with more severe cases and substantially increases hospital LOS.29,30 The same impact should also be expected for other viruses, but very few data are available aside from data related to influenza.10,11
The emergence of SARS-CoV-2 has highlighted the need for more efficient respiratory infection control measures. In this context, our results are in line with a study18 that demonstrated that rapid POCT for SARS-CoV-2 decreased the time to availability of results compared with laboratory PCR (1.7 versus 21.3 h); this reduction was associated with improved infection control measures and reduced patient time in the ED before hospitalization. It is likely that SARS-CoV-2 will continue to circulate and burden health systems for years to come. Thus, forthcoming testing strategies will be impacted by the potential co-circulation of SARS-CoV-2 with the other major respiratory pathogens, such as influenza, coupled with uncertainty about the duration of protection after infection or potential vaccination. In those strategies, it will be critical to be able to accurately detect pathogens of concern and isolate patients appropriately.
Our study has several limitations. Firstly, it is a monocentric study conducted in a centre with prior experience in systematic mPCR testing. During the previous winter, POCT had already been introduced for a more restricted population,25 so the ED physicians were already trained in clinical use of those results. Our findings should be replicated in further studies in other centres because some results may be dependent on the specific health care system and local setting. Secondly, due to the study’s objective, it was not possible to blind physicians from the POCT or central laboratory randomization group. Thirdly, the use of randomized weekly periods may not be ideal, as the epidemiological situation can change from one week to another. This resulted in an imbalanced number of patients between the POCT and central laboratory arms, which is explained by the odd number of weeks between the two arms as well as the study stopping early due to the COVID-19 pandemic. Moreover, the winter epidemic started during a POCT week, increasing the number of inclusions in the POCT arm at the beginning of the study. We did not observe significantly different distributions of patients or pathogens between the two time periods, but there was a higher proportion of positive cases in the POCT arm (44% versus 33% in the central laboratory arm). Additionally, individual randomization would have blurred the potential effects of ED length duration and room assignments. Only half of the patients each day would have benefited from the shorter time to results. The other half would have been placed blindly, thus potentially depriving some patients of a more appropriate room assignment.
In conclusion, the use of an mPCR POCT for adults with acute respiratory illness seeking care in the ED failed to reduce the LOS or antibiotic consumption. On the other hand, the mPCR POCT approach was associated with an increased single-room assignment among patients positive for a significant respiratory pathogen. Routine mPCR POCT for respiratory viruses should be more widely introduced into diagnostic pathways for adults presenting to hospitals with acute respiratory infections to improve compliance with preventive measures and reduce nosocomial outbreaks.
Acknowledgements
ED influenza management study group
Luisa COLOSI, Romain HELLMANN, Thomas PAVLOVLSKY, Daniel Aiham GHAZALI, Maéva RENOUX, Laure FALQUE-PIERROTIN, Élise DUPEYRAT, Camille RAVAUT, Parfait KOUADIO, Laurent PEREIRA, Romain NGUYEN, Stéphanie ANTONIOL POSTIC, Anne Sophie CHAULET, Vittiaroat ING, Philippe KENWAY, Swanie GIGOT, Karine GAUFFRIAUD, Kadir KALKA, Philippe MOUJAOUI, Lorène RADOU, Richard CLERY, Michèle MACAUX.
Virology laboratory team
Houria ICHOU, Florence DAMOND, Vincent MACKIEWICZ, Charlotte CHARPENTIER, Valentine Marie FERRE, Quentin LE HINGRAT, Manuella Mireille ONAMBELE GUINDI, Lucile LARROUY.
Clinical research unit
Naima BELDJOUDI.
Funding
This study was supported by QIAGEN. The funder had no role in the study conception, design, conduct, data analysis, or manuscript preparation. The corresponding author had full access to all data and the final responsibility to submit for publication.
Transparency declarations
D.B. and B.V. have received funds for speaking at symposia organized on behalf of QIAGEN and have also received funds for research from QIAGEN. BV also received funds for symposia from BioMérieux, Gilead and Hologic. The remaining authors have none to declare. Editorial support was provided by Doxastic LLC and funded by QIAGEN. This article forms part of a Supplement sponsored by QIAGEN.
References
- 1.Afilalo M, Stern E, Oughton M.. Evaluation and management of seasonal influenza in the emergency department. Emerg Med Clin North Am 2012; 30: 271–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iuliano AD, Roguski KM, Chang HH. et al. Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet 2018; 391: 1285–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Legand A, Briand S, Shindo N. et al. Addressing the public health burden of respiratory viruses: the Battle against Respiratory Viruses (BRaVe) initiative. Future Virol 2013; 8: 953–68. [Google Scholar]
- 4.van Asten L, van den Wijngaard C, van Pelt W. et al. Mortality attributable to 9 common infections: significant effect of influenza A, respiratory syncytial virus, influenza B, norovirus, and parainfluenza in elderly persons. J Infect Dis 2012; 206: 628–39. [DOI] [PubMed] [Google Scholar]
- 5.Yu X, Lu R, Wang Z. et al. Etiology and clinical characterization of respiratory virus infections in adult patients attending an emergency department in Beijing. PLoS One 2012; 7: e32174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Das D, Le Floch H, Houhou N. et al. Viruses detected by systematic multiplex polymerase chain reaction in adults with suspected community-acquired pneumonia attending emergency departments in France. Clin Microbiol Infect 2015; 21: 608.e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi S-H, Hong S-B, Ko G-B. et al. Viral infection in patients with severe pneumonia requiring intensive care unit admission. Am J Respir Crit Care Med 2012; 186: 325–32. [DOI] [PubMed] [Google Scholar]
- 8.Voiriot G, Visseaux B, Cohen J. et al. Viral-bacterial coinfection affects the presentation and alters the prognosis of severe community-acquired pneumonia. Crit Care 2016; 20: 375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Visseaux B, Burdet C, Voiriot G. et al. Prevalence of respiratory viruses among adults, by season, age, respiratory tract region and type of medical unit in Paris, France, from 2011 to 2016. PLoS One 2017; 12: e0180888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loubet P, Voiriot G, Houhou-Fidouh N. et al. Impact of respiratory viruses in hospital-acquired pneumonia in the intensive care unit: single-center retrospective study. J Clin Virol 2017; 91: 52–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hong H-L, Hong S-B, Ko G-B. et al. Viral infection is not uncommon in adult patients with severe hospital-acquired pneumonia. PLoS One 2014; 9: e95865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bouzid D, Zanella M-C, Kerneis S. et al. Rapid diagnostic tests for infectious diseases in the emergency department. Clin Microbiol Infect 2021; 27: 182–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uyeki TM, Bernstein HH, Bradley JS. et al. Clinical practice guidelines by the Infectious Diseases Society of America: 2018 update on diagnosis, treatment, chemoprophylaxis, and institutional outbreak management of seasonal influenzaa. Clin Infect Dis 2019; 68: e1–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brendish NJ, Malachira AK, Armstrong L. et al. Routine molecular point-of-care testing for respiratory viruses in adults presenting to hospital with acute respiratory illness (ResPOC): a pragmatic, open-label, randomised controlled trial. Lancet Respir Med 2017; 5: 401–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shengchen D, Gu X, Fan G. et al. Evaluation of a molecular point-of-care testing for viral and atypical pathogens on intravenous antibiotic duration in hospitalized adults with lower respiratory tract infection: a randomized clinical trial. Clin Microbiol Infect 2019; 25: 1415–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andrews D, Chetty Y, Cooper BS. et al. Multiplex PCR point of care testing versus routine, laboratory-based testing in the treatment of adults with respiratory tract infections: a quasi-randomised study assessing impact on length of stay and antimicrobial use. BMC Infect Dis 2017; 17: 671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burrel S, Hausfater P, Dres M. et al. Co-infection of SARS-CoV-2 with other respiratory viruses and performance of lower respiratory tract samples for the diagnosis of COVID-19. Int J Infect Dis 2021; 102: 10–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hingrat QL, Bouzid D, Choquet C. et al. Viral epidemiology and SARS-CoV-2 co-infections with other respiratory viruses during the first COVID-19 wave in Paris, France. Influenza Other Respir Viruses 2021; 15: 425–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bouzid D, Visseaux B, Perozziello A. et al. Factors associated with single-room assignment among patients admitted through the emergency department during influenza epidemics. PLoS One 2020; 15: e0237214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32018D0945.
- 21.Casalegno J-S, Eibach D, Valette M. et al. Performance of influenza case definitions for influenza community surveillance: based on the French influenza surveillance network GROG, 2009–14. Euro Surveill 2017; 22: 30504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eitel DR, Travers DA, Rosenau AM. et al. The emergency severity index triage algorithm version 2 is reliable and valid. Acad Emerg Med 2003; 10: 1070–80. [DOI] [PubMed] [Google Scholar]
- 23.Boers SA, Melchers WJG, Peters CJA. et al. Multicenter evaluation of QIAstat-Dx respiratory panel V2 for detection of viral and bacterial respiratory pathogens. J Clin Microbiol 2020; 58: e01793-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lim W, van der Eerden MM, Laing R. et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax 2003; 58: 377–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bouzid D, Lucet J-C, Duval X. et al. Multiplex PCR implementation as point-of-care testing in a French emergency department. J Hosp Infect 2020; 105: 337–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brendish NJ, Malachira AK, Beard KR. et al. Impact of turnaround time on outcome with point-of-care testing for respiratory viruses: a post hoc analysis from a randomised controlled trial. Eur Respir J 2018; 52: 1800555. [DOI] [PubMed] [Google Scholar]
- 27.Kalil AC, Thomas PG.. Influenza virus-related critical illness: pathophysiology and epidemiology. Crit Care 2019; 23: 258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berry L, Lansbury L, Gale L. et al. Point of care testing of Influenza A/B and RSV in an adult respiratory assessment unit is associated with improvement in isolation practices and reduction in hospital length of stay. J Med Microbiol 2020; 69: 697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salgado CD, Farr BM, Hall KK. et al. Influenza in the acute hospital setting. Lancet Infect Dis 2002; 2: 145–55. [DOI] [PubMed] [Google Scholar]
- 30.Mendoza-García JL, Quirós-González V, Jiménez-Rodríguez M. et al. [Impact of nosocomial transmission of influenza virus in an acute hospital]. Rev Esp Salud Publica 2018; 92: e201808014. [PubMed] [Google Scholar]