Abstract
The asymmetric hydrogenation of heteroarenes has recently emerged as an effective strategy for the direct access to enantioenriched, saturated heterocycles. Although several homogeneous catalyst systems have been extensively developed for the hydrogenation of heteroarenes with high levels of chemo- and stereoselectivity, the development of mild conditions that allow for efficient and stereoselective hydrogenation of a broad range of substrates remains a challenge. This Perspective highlights recent advances in homogeneous catalysis of heteroarene hydrogenation as inspiration for the further development of asymmetric hydrogenation catalysts, and addresses underdeveloped areas and limitations of the current technology.
Keywords: hydrogenation, asymmetric catalysis, heterocycles, heteroarenes, homogeneous catalysts
Graphical Abstract
1. INTRODUCTION
Heterocycles are important structural motifs found frequently in natural products1 and industrial products such as pharmaceuticals,2 and agrochemicals.3 Nitrogen heterocycles constitute approximately 59% of recent FDA-approved small-molecule drugs, with an average of two to three nitrogen atoms per drug.2a In the context of pharmaceutical development, a significant positive correlation exists between key structural elements of drug candidates, such as degree of saturation and number of stereogenic centers, with observed clinical success.2c,d Thus, the ability to access stereochemically complex heterocyclic scaffolds has been of great interest in recent years. Considering the ubiquity of both aromatic and saturated heterocycles in pharmaceuticals, the direct access to enantiopure heterocycles via heteroarene hydrogenation continues to be an important research area in both academia and industry.2
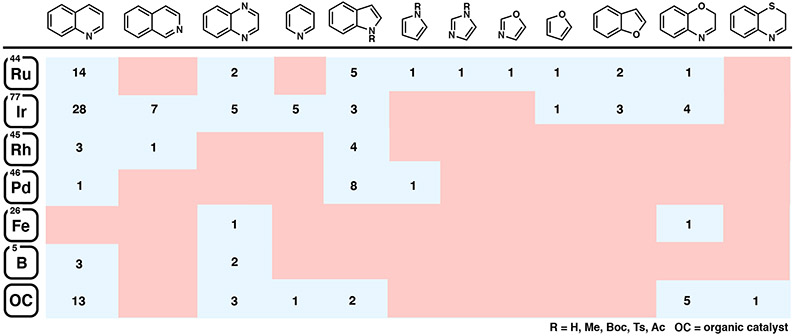
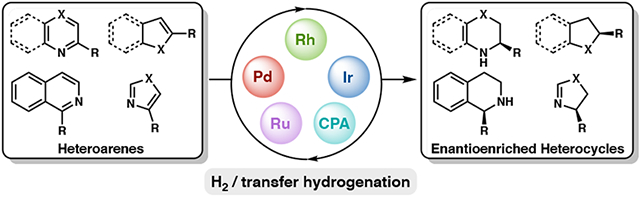
While significant progress has been made in the area of asymmetric heteroarene hydrogenation, these transformations continue to pose a challenge for catalytic processes, ostensibly due to the high energetic cost of breaking aromaticity, and the presence of heteroatoms that may poison and deactivate the catalyst.4 Nevertheless, the asymmetric hydrogenation of heteroarenes, including quinolines, isoquinolines, quinoxalines, pyridines, indoles, furans, and benzoxazines has been extensively explored, and several comprehensive reviews have been published on this subject (Figure 1).4 The hydrogenation of many common heteroarenes can be achieved using a variety of catalyst systems, including homogeneous and heterogeneous catalysts, such as metal nanoparticles.4d Among these, homogeneous catalyst systems have found widespread application for the asymmetric hydrogenation of heteroarenes, often providing access to different enantioenriched motifs with a simple adjustment of the chiral ligand.4,5 This Perspective is focused on highlighting homogeneous catalyst systems that have recently been developed for the asymmetric hydrogenation of heteroarenes, evaluating the general relationships between different catalyst complexes and their reactivity for various heterocycles. The following sections will feature reports that have been published since 2011, as previous reports have already been discussed comprehensively in prior review articles.4
Figure 1.
Overview of the total number of published reports on the asymmetric hydrogenation of common heteroarenes (≥90% ee, minimum of three substrates).
2. GENERAL MECHANISTIC CONSIDERATIONS
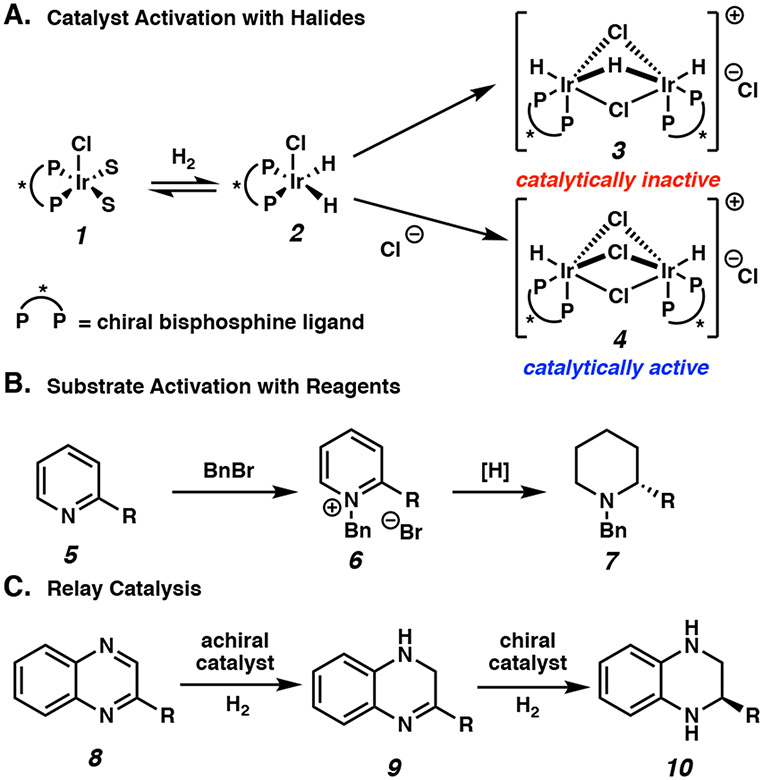
Heteroarenes pose a significant challenge for asymmetric catalysis due to their inherent thermodynamic stability and the tendency of both reactants and products to deactivate catalysts. Three general strategies have been employed to overcome these difficulties: catalyst activation, substrate activation, and relay catalysis.4a Catalyst activation involves either the preformation of the active catalyst or the addition of reagents to form a more active catalyst species in situ. For instance, the addition of halide sources to an iridium precatalyst was reported by Mashima and coworkers to prevent the irreversible formation of catalytically inactive dimeric iridium hydride species 3 (Scheme 1A).6 The substrate activation approach introduces reagents to overcome the inherent aromatic stability of heteroarenes through in situ generation or pre-formation of activated substrates, such as quinolinium or pyridinium salts (e.g. 6, Scheme 1B).7 Finally, relay catalysis involves the use of two or more catalysts for the asymmetric hydrogenation of heteroarenes. For example, an achiral transition metal catalyst is often employed to induce initial partial hydrogenation of the substrate 8, followed by an enantioselective hydrogenation of an intermediate such as imine 9 aided by a chiral Brønsted acid catalyst (Scheme 1C).8 Overall, these distinct strategies enable high stereoselectivity and functional group tolerance in the asymmetric hydrogenation of heteroarenes.
Scheme 1.
Examples for the Asymmetric Hydrogenation of Heteroarenes
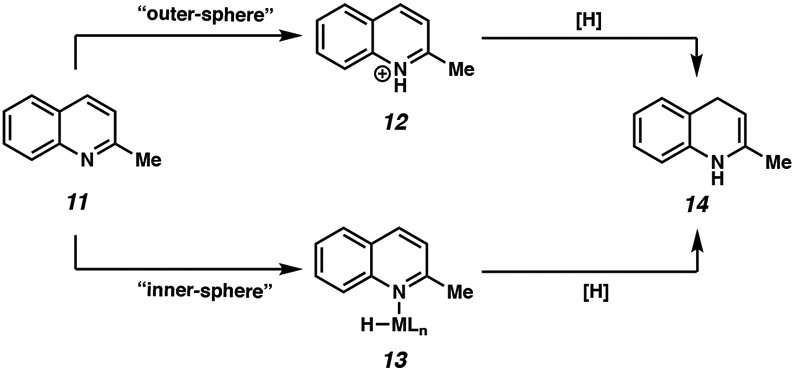
The general mechanism of the asymmetric hydrogenation of heteroarenes can be thought of to involve the following steps (depending on the degree of unsaturation): formation of the active catalyst species, hydride addition from the catalyst to the substrate, and regeneration of the catalyst from a hydrogen source.9 However, several questions regarding the mechanism of asymmetric hydrogenation that could be addressed include the order of hydride addition (1,2- vs. 1,4-addition), substrate coordination to the catalyst (inner- vs. outer-sphere), the rate-determining step, and the enantiodetermining step.4a-d,9c For instance, both inner-sphere and outer-sphere processes have been proposed to explain the mechanism for the iridium-catalyzed hydrogenation of 2-methylquinoline 11 (Scheme 2).9b In an outer-sphere pathway, protonation of the substrate occurs to form iminium intermediate 12, followed by external hydride delivery to the activated substrate from the transition metal center. In contrast, an inner-sphere process involves a substrate that is bound to the metal hydride species (13) that undergoes a hydride transfer step. While other catalyst systems have been proposed to undergo an outer-sphere mechanism for the hydrogenation of various heterocycles (vide infra), other studies also support an inner-sphere coordination of the substrate to the catalyst.10 Thus, the coordinating ability of different heteroatom-containing substrates to the catalyst complex is not explicitly defined, and therefore difficult to predict how it would behave under different hydrogenation systems.
Scheme 2.
Possible Mechanistic Pathways for the Hydrogenation of 2-Methylquinoline 11
Although the asymmetric hydrogenation of heteroarenes is often difficult to monitor due to the use of high-pressure reactors, several mechanistic studies have been conducted using empirical data and computational modeling to elucidate the mechanisms of transition metal-catalyzed and organic-catalyzed heteroarene hydrogenation reactions. The proposed catalytic cycles of these studies will be addressed in the following sections of this Perspective for each transition metal and organic catalyst system.
3. RUTHENIUM-CATALYZED ASYMMETRIC HYDROGENATION
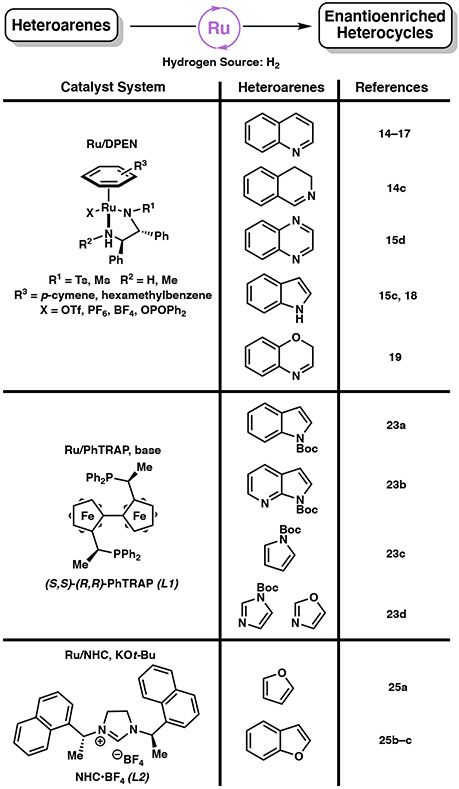
With regard to homogeneous catalysis, ruthenium was among the first transition metals explored in asymmetric hydrogenation reactions. In 1995, Noyori and coworkers introduced a Ru(II) catalyst with a chiral diamine ligand that promotes the highly stereoselective reduction of aromatic ketones through an asymmetric transfer hydrogenation process.11 Shortly after, this catalyst system was applied to the asymmetric hydrogenation of imines with a formic acid–triethylamine mixture as the hydrogen source.12 Since then, several established ruthenium catalyst systems with common ligand scaffolds were developed for the asymmetric hydrogenation of heteroarenes (Figure 2), as well as the hydrogenation of carbocyclic aromatic compounds.13
Figure 2.
Common Ru-based catalyst systems for the asymmetric hydrogenation of heteroarenes (≥90% ee, minimum three substrates). Only one enantiomer of ligand shown for simplicity.
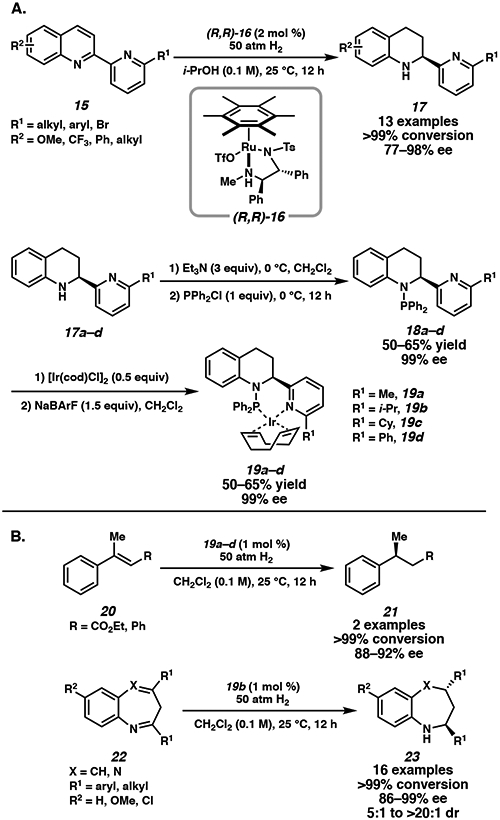
The air-stable Ru/TsDPEN catalyst (TsDPEN = N-(p-toluenesulfonyl)-1,2-diphenylethylenediamine), initially developed by Fan and Chan for the hydrogenation of quinolines, has found widespread applications in the asymmetric hydrogenation of N-heterocycles (Figure 2).14-19 In these reports, hydrogen gas is activated by the catalyst to reduce quinolines at ambient temperatures in ionic liquids and even under solvent-free conditions. Subsequent studies also utilize this system for sequential reductive amination and asymmetric hydrogenation cascade reactions of quinoline derivatives to access structurally diverse scaffolds.15 Since 2011, the Ru/TsDPEN catalyst system has evolved with important applications toward the development of novel chiral ligands. Fan and coworkers demonstrated that chiral Ru/TsDPEN catalysts can reduce 2,2’-bisquinoline and bisquinoxaline derivatives with high stereoselectivity.16 More recently, the system was applied to hydrogenate 2-(pyridine-2-yl)quinoline derivatives for the synthesis of novel N,P-ligands (Scheme 3).17 Using chiral catalyst complex 16 (Scheme 3A), selective hydrogenation of the quinoline ring was observed in a range of substrates 15 with high enantioselectivities. Although ortho-substituents on the pyridine ring are necessary to prevent catalyst deactivation, this requirement further enables the tuning of the steric effect of the chiral ligand generated.
Scheme 3.
Ru-Catalyzed Asymmetric Hydrogenation of Quinolines and Applications of Hydrogenated Products
The hydrogenated products 17a–d can then be transformed into a novel class of chiral N,P-ligands by treatment with PPh2Cl in NEt3. After recrystallization, these ligands are then treated with [Ir(COD)Cl]2, followed by anion metathesis to yield chiral iridium complexes 19a–d.17 These catalysts are then successfully used for the asymmetric hydrogenation of olefins and seven-membered cyclic imines (Scheme 3B). Notably, replacement of the phenyl substituent on the pyridine ring of 19d with an alkyl group (19a–c) improves the enantioselectivity of the hydrogenation of trisubstituted olefin 20 to 21 in 88–92% ee. Catalyst 19b is further utilized in the asymmetric hydrogenation of benzazepines and benzodiazepines, providing products 23 in up to 99% ee and 20:1 dr. The efficient synthesis of a novel class of chiral ligands through the asymmetric hydrogenation of heteroarenes is a promising application of this hydrogenation technology. The Ru/TsDPEN catalyst system is also effective for the asymmetric hydrogenation of unprotected indoles, which traditionally require protection of the indole nitrogen to prevent catalyst deactivation,18 C(3)-substituted benzoxazines,19 and a variety of polycyclic heteroarenes.20
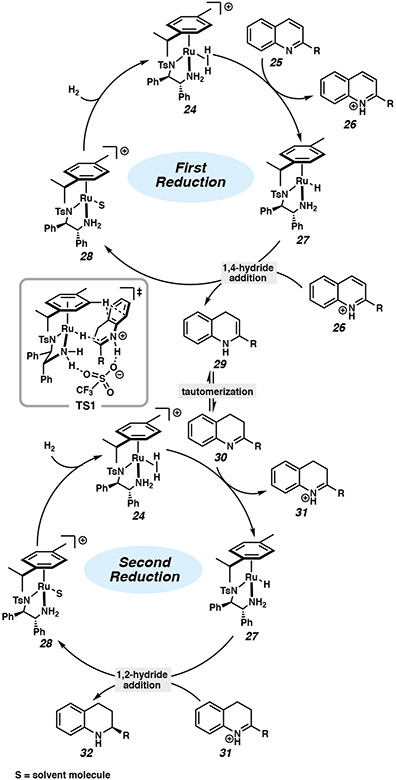
Based on experimental results and theoretical calculations, Fan and coworkers propose a catalytic cycle for the asymmetric hydrogenation of quinolines with Ru/TsDPEN catalysts (Figure 3). The Ru(II) catalyst activates molecular H2 to form dihydrogen complex 24. After heterolytic cleavage of H2 to generate complex 27 and the activated substrate 26, subsequent 1,4-hydride addition affords an enamine intermediate 29.4e,15a Tautomerization then occurs to form imine 30, which is protonated by complex 24 to produce iminium 31. Intermediate 31 undergoes an asymmetric 1,2-hydride transfer from complex 27 to deliver product 32 via TS1. The enantioselectivity is proposed to originate from the attractive CH/π interaction between the η6-arene ligand and the carbocyclic ring of the dihydroquinoline via a 10-membered transition state with participation of the triflate anion, as shown in TS1.15a,21
Figure 3.
Proposed catalytic cycle for the asymmetric hydrogenation of quinolines with a Ru/TsDPEN catalyst.
Although Ru/TsDPEN systems are efficient catalysts for the hydrogenation of bicyclic aromatic compounds, preformation of the active catalyst is required. Alternatively, other common chiral bisphosphine ligands that are explored in ruthenium-catalyzed asymmetric hydrogenations include PhTRAP (2,2”-bis[1-(diphenylphosphino)-ethyl]-1,1”-biferrocene), a C2 symmetric biferrocene framework.22 Kuwano and coworkers demonstrate that ruthenium catalysts bearing PhTRAP ligand (L1) are productive in the hydrogenation of N-Boc-protected indoles (Scheme 4A), as well as substituted N-Boc-protected pyrroles and imidazoles (Scheme 4B, C).23 Considering the high aromatic stability of single-ring aromatic compounds, the exhaustive hydrogenation of trisubstituted pyrroles to yield chiral pyrrolidines under this catalyst manifold is a significant advancement.4a,23c The Ru/PhTRAP catalyst can also be applied to the hydrogenation of other 5-membered heterocycles such as disubstituted imidazoles and oxazoles, however, only partial hydrogenation is observed in these cases.23d
Scheme 4.
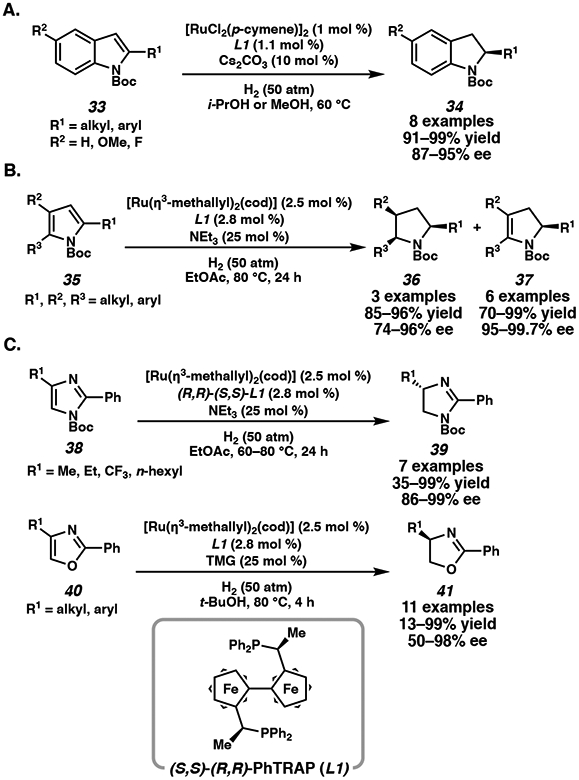
Ru-Catalyzed Asymmetric Hydrogenation of Heteroarenes with PhTRAP ligand L1
Finally, ruthenium N-heterocyclic carbene (NHC) complexes have found many applications in the transfer hydrogenation of ketones, nitriles, as well as the regioselective hydrogenation of heterocycles.24 Glorius and coworkers first demonstrated the regioselective hydrogenation of the aromatic carbocyclic ring of quinoxalines, albeit with moderate enantioselectivity. They observed that the identity of NHC ligand was critical in determining the regioselectivity of hydrogenation, and found that these catalytic systems can also be applied toward the asymmetric hydrogenation of furans and benzofurans.25 Using chiral NHC ligand SINpEt•HBF4 (L2), the asymmetric hydrogenation of disubstituted furans and 2-substituted benzofurans proceeded in high yields and enantioselectivities (Scheme 5). A wide range of substituted furans were hydrogenated with this catalyst system, demonstrating a significant correlation between strongly electron-withdrawing groups on the substrate and diminished enantioselectivity.25a Overall, the Ru/NHC complex demonstrated its capability to hydrogenate a range of heteroarenes, particularly oxygen-containing heterocycles, allowing direct access to natural metabolites such as (+)-corsifuran A (Scheme 5A). Further studies of the Ru/L2 catalyst system by Glorius and coworkers revealed that hydrogenation of the naphthyl substituents of the NHC ligand was a key step in accessing the active catalyst.26
Scheme 5.
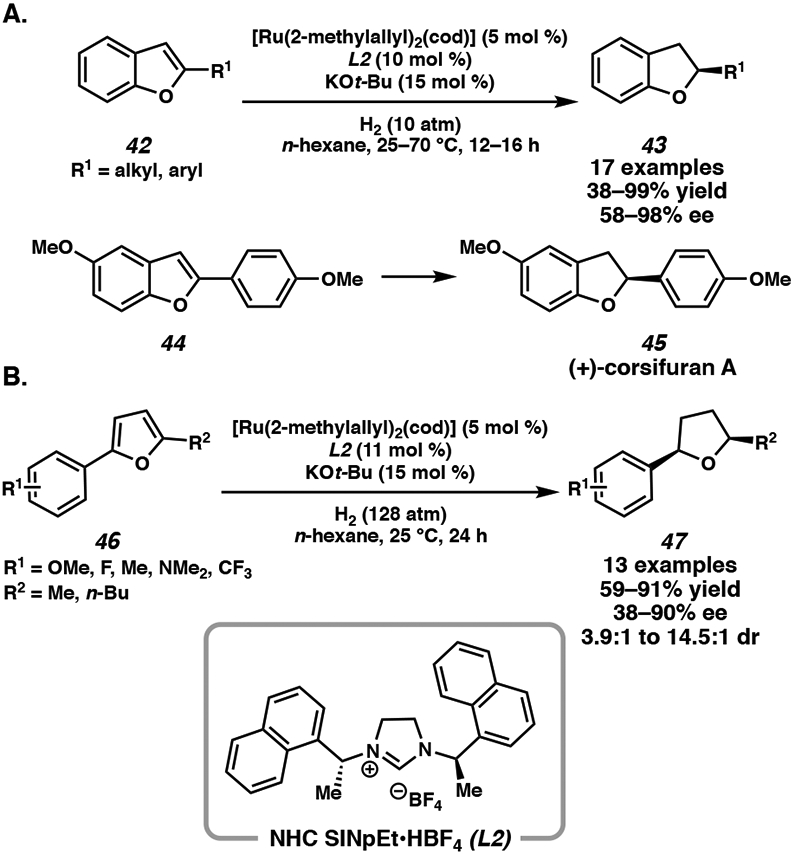
Ru-Catalyzed Asymmetric Hydrogenation of Furans and Benzofurans with NHC ligand L2
4. IRIDIUM-CATALYZED ASYMMETRIC HYDROGENATION
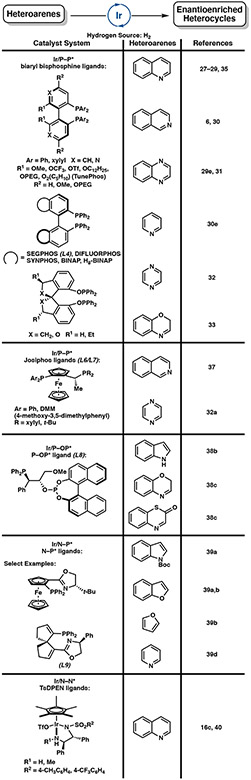
Following the initial disclosure of the enantioselective hydrogenation of quinolines using [Ir(cod)Cl]2 and bisphosphine ligand MeO-BIPHEP (L3) in 2003 (Scheme 6),27 much attention has been devoted toward the development of the iridium-catalyzed hydrogenation of heteroarenes. Most studies have focused on the exploration of different types of activating agents to reduce substrate aromaticity, as well as to prevent the irreversible formation of catalytically inactive dimeric iridium hydride species 3.6,27 Common ligand scaffolds utilized in this transformation include atropisomeric biaryl bisphosphine ligands, as well as phosphine-phosphite ligands, N,P-ligands, and chiral diamine ligands that have been explored for the asymmetric hydrogenation of a range of heterocycles (Figure 4).
Scheme 6.
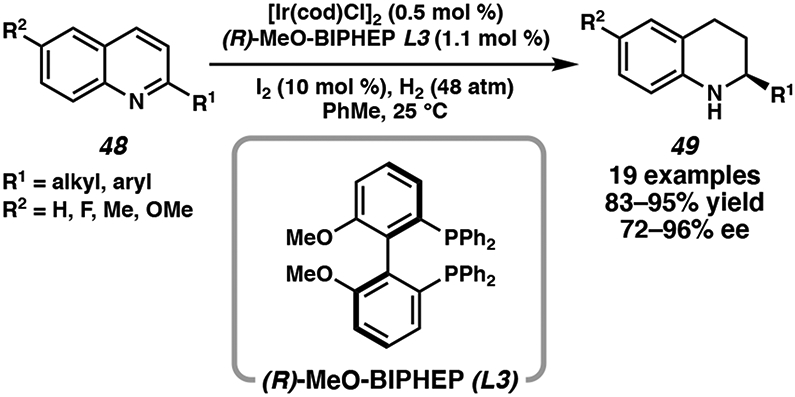
First Reported Ir-Catalyzed Asymmetric Hydrogenation of Quinolines
Figure 4.
Common Ir-based catalyst systems for the asymmetric hydrogenation of heteroarenes (≥90% ee, minimum three substrates). Only one enantiomer of ligand shown for simplicity.
The biaryl bisphosphine ligand scaffold is most common in iridium-catalyzed asymmetric hydrogenation due to its excellent steric and electronic tunability. Structural variations of the ligand include alteration of P-substitution and modifications of the phenyl backbone, including the synthesis of supramolecular chiral ligands appended with crown ethers that induce strong complexation between the ligand and alkali cations.28 Since 2011, the combination of iridium and biaryl bisphosphine ligands has been extensively studied by Zhou, Agbossou-Niedercorn, and Mashima in the asymmetric hydrogenation of heteroarenes, including quinolines,29 isoquinolines,6,30 quinoxalines,29e,31 pyridines,30e pyrazines,32 and benzoxazines.33 Iodine, chloroformates, amines, and Brønsted acids are common activating reagents for enhancing catalytic activity of these heterocyclic substrates.
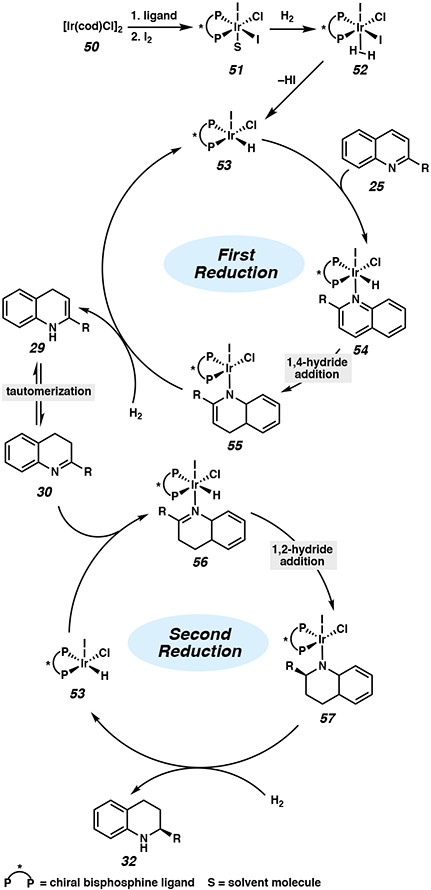
The mechanism and the role of iodine as an activator was initially proposed by Zhou and Li (Figure 5).27b Starting from [Ir(cod)Cl]2, the addition of I2 oxidizes the Ir(I) precursor to an Ir(III) species 51. Subsequent heterolytic cleavage of H2 with release of HI forms catalytically active Ir(III) complex 53. The heteroarene then coordinates to generate octahedral complex 54, followed by a 1,4-hydride transfer to form intermediate 55. An additional molecule of H2 regenerates the iridium hydride species 53 and protonates the enamine, which can isomerize to imine 30 and undergo a second reduction. The enantiodetermining step is proposed to be a 1,2-hydride addition that establishes the stereogenic center, with the addition of H2 releasing the product and regenerating Ir(III) species 53. It is unclear, however, whether substrate coordination to the metal center occurs, as other studies have proposed an outer-sphere pathway for the homogeneous iridium-catalyzed hydrogenation of quinolines and isoquinolines (vide supra).6,9b
Figure 5.
Proposed catalytic cycle for the Ir-catalyzed asymmetric hydrogenation of quinolines with I2 as additive.
Recently, Zhou and coworkers employed trichloroisocyanuric acid (TCCA) as a traceless activating reagent for the iridium-catalyzed hydrogenation of isoquinolines and pyridines (Scheme 7). This method circumvents the additional steps of installing and removing the N-acyl group to activate the substrates. Pairing [Ir(cod)Cl]2 and bisphosphine ligand (R)-SEGPHOS (L4), a range of disubstituted isoquinolines and pyridines were hydrogenated in high yield and excellent enantioselectivity.30e The use of TCCA as a halogen-bond activator gave the highest enantioselectivity compared to N-chlorosuccinimide or N-iodosuccinimide. Notably, 2,3,6-trisubstituted pyridines 60 were also converted to chiral piperidines 61 with higher enantioselectivity than previous methods, which often require more activated pyridinium salts as substrates.7
Scheme 7.
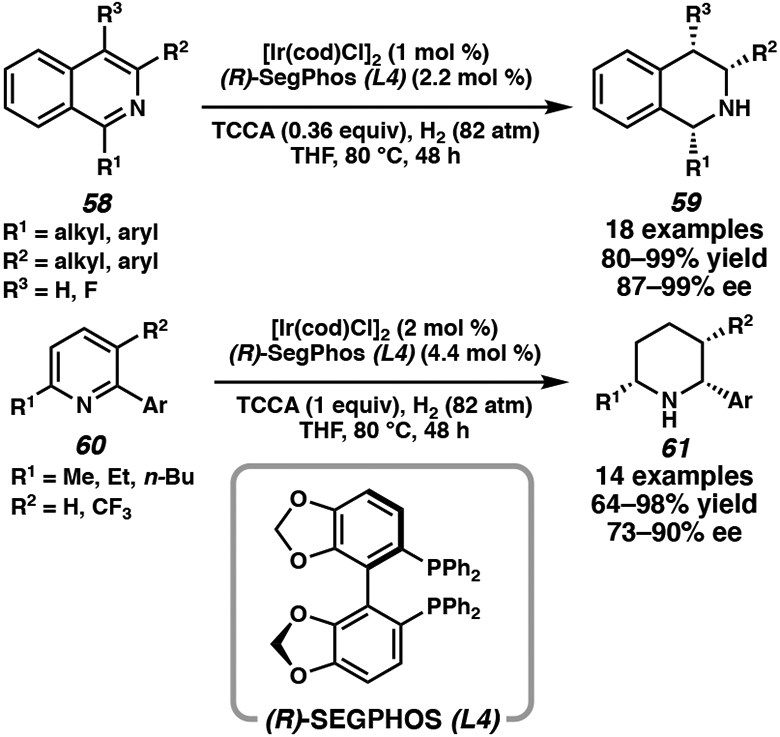
Ir-Catalyzed Asymmetric Hydrogenation of Isoquinolines and Pyridines using TCCA as the Activator
Extending BINAP-derived ligand scaffolds, biaryl spirocyclic ligands have emerged as powerful tools for asymmetric catalysis due to their higher rigidity.34 Nagorny and coworkers reported that chiral spiroketal-based bisphosphinite ligand (SPIRAPO) (L5) is effective in the asymmetric hydrogenation of quinolines, quinoxalines, and benzoxazinones (Scheme 8).35 Using 10 mol % I2 and low catalyst loadings, the synthesis of a range of enantioenriched saturated heterocycles was achieved at 24 atm H2 and room temperature.
Scheme 8.
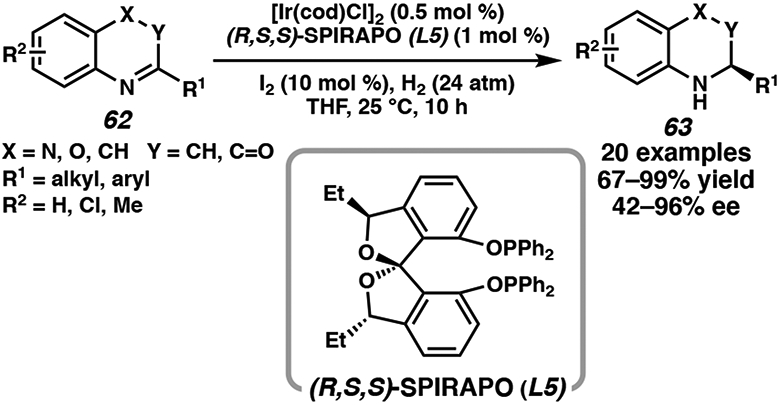
Ir-Catalyzed Asymmetric Hydrogenation of Quinolines, Quinoxalines, and Benzoxazinones
While there has been extensive development of biaryl bisphosphine (vide supra) and phosphoramidite36 ligands for iridium-catalyzed hydrogenation, ferrocenyl-based Josiphos ligands are also efficacious ligand scaffolds for N-heterocycles, imparting excellent enantioselectivity and diastereoselectivity.37 Inspired by the iridium catalyst system employed in the ether-directed asymmetric imine reduction en route to the herbicide metolachlor,10 Stoltz and coworkers explored the potential of directing groups as iridium catalyst chelating agents for the directed hydrogenation to a specific face of the substrate. Toward the total synthesis of the complex bis-tetrahydroisoquinoline alkaloid jorumycin, the hydroxymethyl functionality was utilized as a directing group for the iridium-catalyzed asymmetric hydrogenation of bis-isoquinoline 64 to generate pentacyclic intermediate 65 in one step as a single diastereomer in 88% ee (Scheme 9).37
Scheme 9.
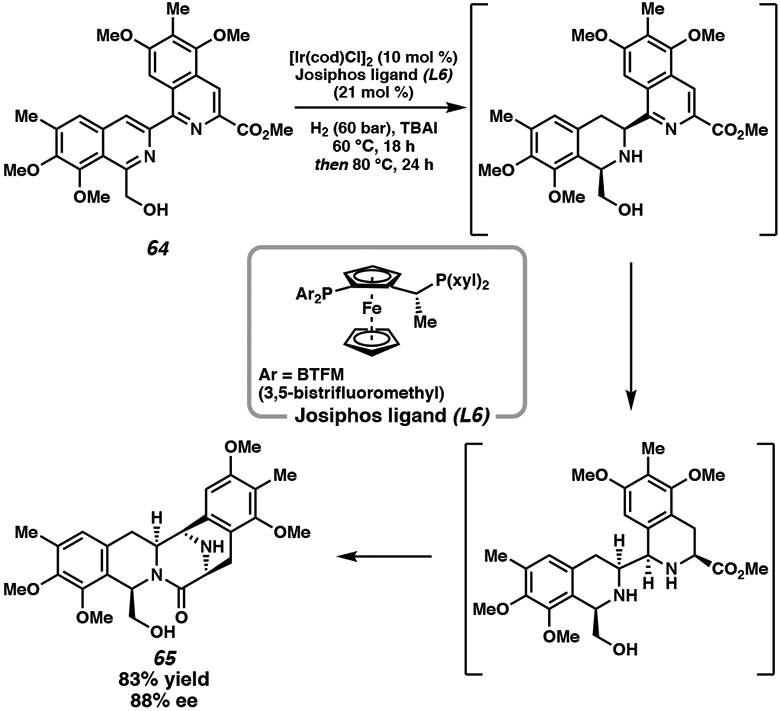
Key Ir-Catalyzed Enantioselective Hydrogenation Step in the Total Synthesis of Jorumycin
Utilizing directing groups is an underdeveloped activation strategy for the asymmetric hydrogenation of N-heterocycles, as Lewis basic functional groups are not as well tolerated with previous hydrogenation methods.30 However, Stoltz and coworkers have extended this application of using a hydroxymethyl directing group toward the asymmetric hydrogenation of a range of 1,3-disubstituted isoquinolines. Using 1.25 mol % of [Ir(cod)Cl]2 and 3 mol % of chiral Josiphos ligand (L7), a range of differentially substituted isoquinolines were hydrogenated in high yields and enantio- and diastereoselectivity (Scheme 10).37b Heterocyclic substituents, such as furan and thiophene, at the C(3) position were also tolerated in this transformation. Interestingly, altering the directing groups at the C(1) position of the isoquinoline lowered the levels of conversions but maintained similar levels of stereoselectivity.
Scheme 10.
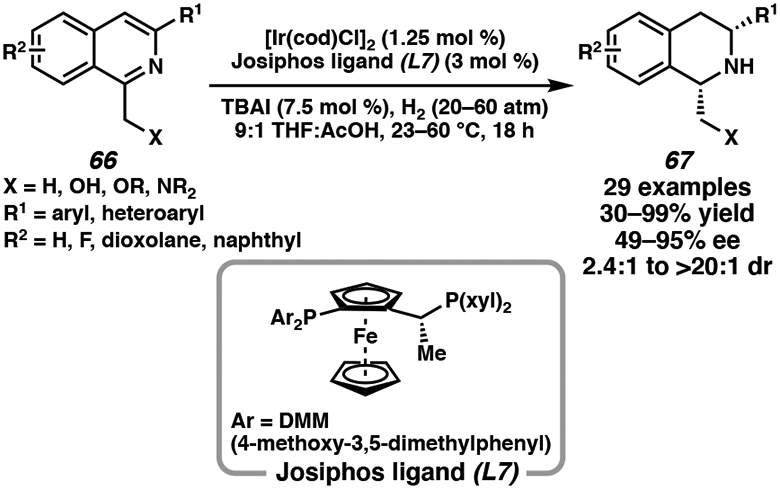
Ir-Catalyzed Asymmetric Hydrogenation of 1,3-Disubstituted Isoquinolines
Phosphine-phosphite (P–OP) ligands, an emerging class of chiral bisphosphine ligands, have been developed by Vidal-Ferran and coworkers for the iridium-catalyzed asymmetric hydrogenation of heterocycles.38 This catalyst system is particularly reactive in the enantioselective synthesis of indolines from unprotected indoles,38b as well as the asymmetric hydrogenation of benzoxazines and benzothiazinones.38c Although stoichiometric amounts of sulfonic acids are needed to promote isomerization to the iminium intermediate, the transformation can be carried out using only 0.5 mol % of [Ir(cod)Cl]2 (Scheme 11).38
Scheme 11.
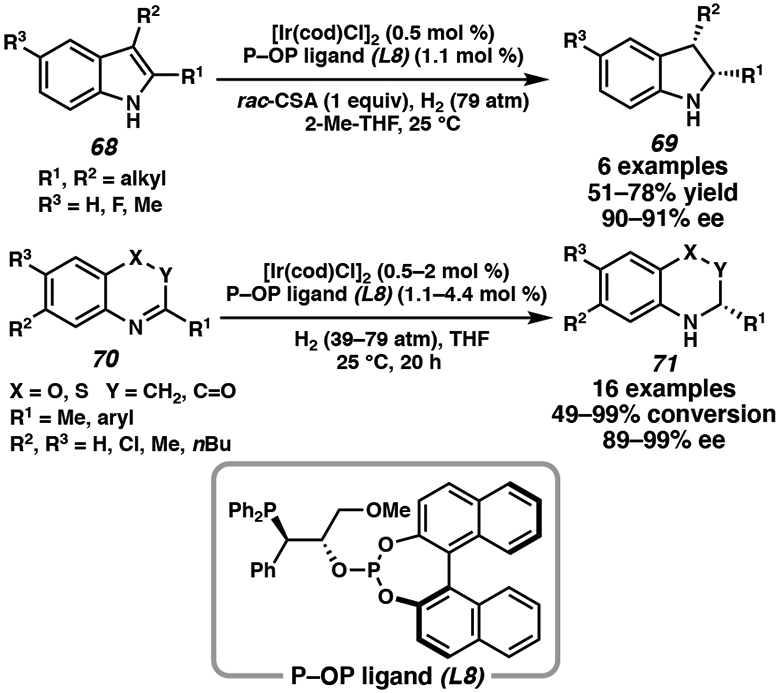
Ir-Catalyzed Asymmetric Hydrogenation of Indoles, Benzoxazines, and Benzothiazinones with P–OP Ligand L8
Although chiral bisphosphine ligands are most commonly employed for iridium-catalyzed asymmetric hydrogenation, chiral PHOX ligands with nitrogen and phosphorous chelating groups (N,P) have also been used to reduce a range of heteroarenes.39 Most recently, Ding and coworkers reported the use of a SpinPHOX (spiro[4,4]-1,6-nonadiene-based phosphinooxazoline) ligand (L9) for the enantioselective hydrogenation of indole and benzofuran derivatives (Scheme 12). This iridium catalyst system is effective for the asymmetric hydrogenation of a diverse range of indoles with high functional group tolerance, using 1 mol % of catalyst loading and mild reaction conditions.39a Although preformation of the Ir/SpinPHOX catalyst complex is necessary, up to 99% isolated yield and 99% enantiomeric excess are observed across differentially substituted indole and benzofuran substrates.
Scheme 12.
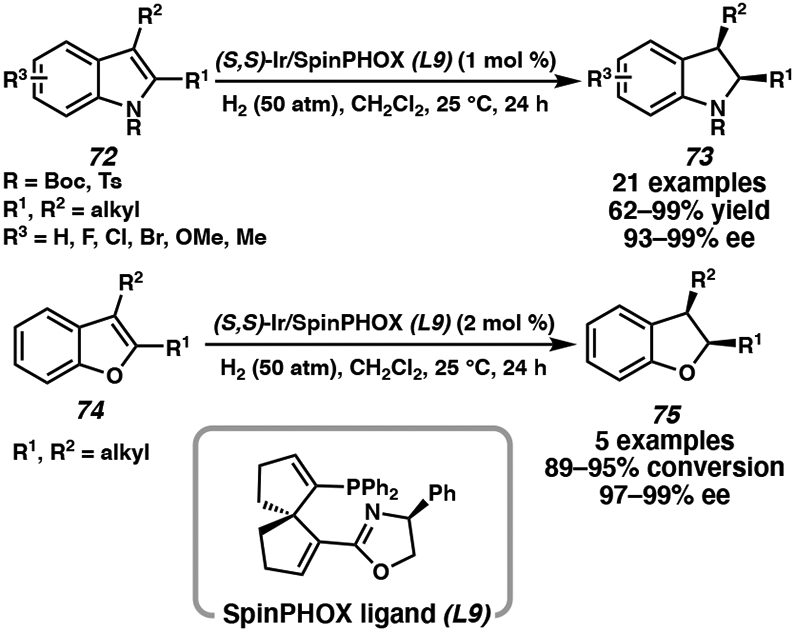
Ir-Catalyzed Asymmetric Hydrogenation of Indoles and Benzofurans with SpinPHOX Ligand L9
Unlike the ruthenium catalyst system, only a few reports employ the TsDPEN ligand for iridium-catalyzed asymmetric heteroarene hydrogenation.16c,40 In 2019, Fan and coworkers developed Ir/TsDPEN catalyst complex 78 for the enantioselective synthesis of vicinal chiral diamines. These products are synthesized through a relay sequence of intermolecular reductive amination followed by asymmetric hydrogenation of a range of 2-quinoline aldehydes and aromatic amines (Scheme 13).16c For more sterically encumbered substrates, an addition of 10 mol % TfOH is required to generate chiral diamines in high levels of selectivity.
Scheme 13.
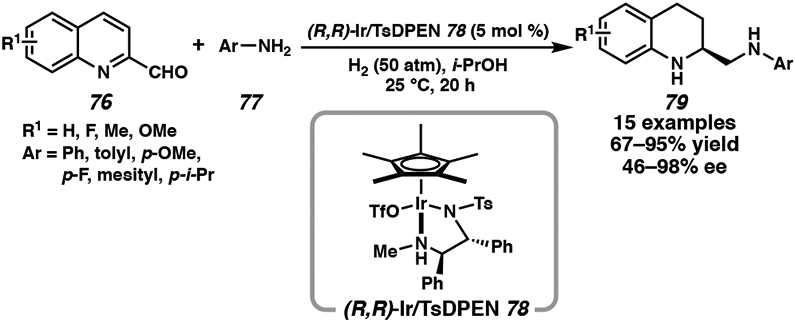
Asymmetric Hydrogenation of Quinolines with Iridium Catalyst 78
5. RHODIUM-CATALYZED ASYMMETRIC HYDROGENATION
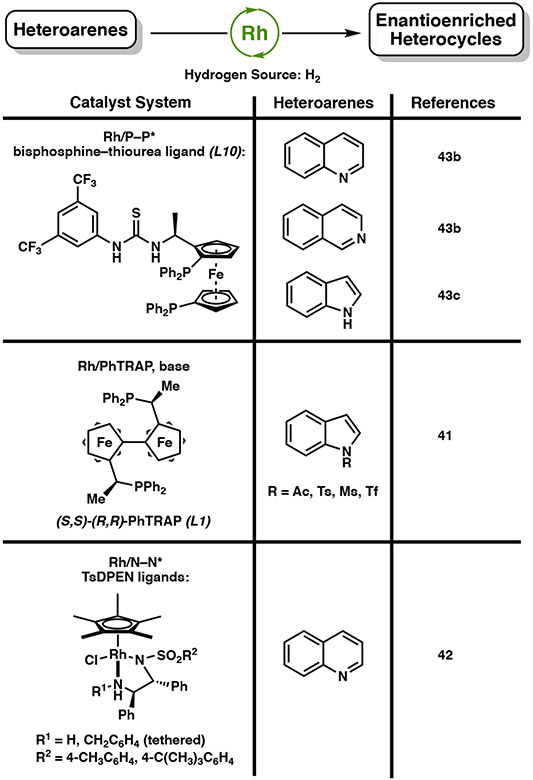
Apart from iridium and ruthenium catalyst systems in the asymmetric hydrogenation of heteroarenes, rhodium catalysts have also been effective for the reduction of quinolines, isoquinolines, and indoles (Figure 6). The combination of a rhodium(I) source and either a PhTRAP41 or TsDPEN42 ligand have been explored for the enantioselective hydrogenation of indoles and quinolines, respectively. More recently, Zhang and coworkers have developed a novel ferrocene-based bisphosphine-thiourea ligand, ZhaoPhos (L10), for the cooperative catalysis of a transition metal center for hydrogenation and anion binding of the thiourea derivative.43 This strategy allows a broader range of Brønsted acids to be tolerated through hydrogen bonding interactions with the thiourea moiety, and simple tunability with the phosphine substituents to improve selectivity. Using ligand L10 with 0.5 mol % rhodium catalyst loading, differentially substituted quinolines, isoquinolines, and unprotected indoles are hydrogenated with excellent yield and enantioselectivity (Scheme 14). The addition of HCl as a strong acid is well tolerated under this catalyst system due to the stabilizing interaction with the thiourea derivative.
Figure 6.
Common Rh-based catalyst systems for the asymmetric hydrogenation of heteroarenes (≥90% ee, minimum three substrates). Only one enantiomer of ligand shown for simplicity.
Scheme 14.
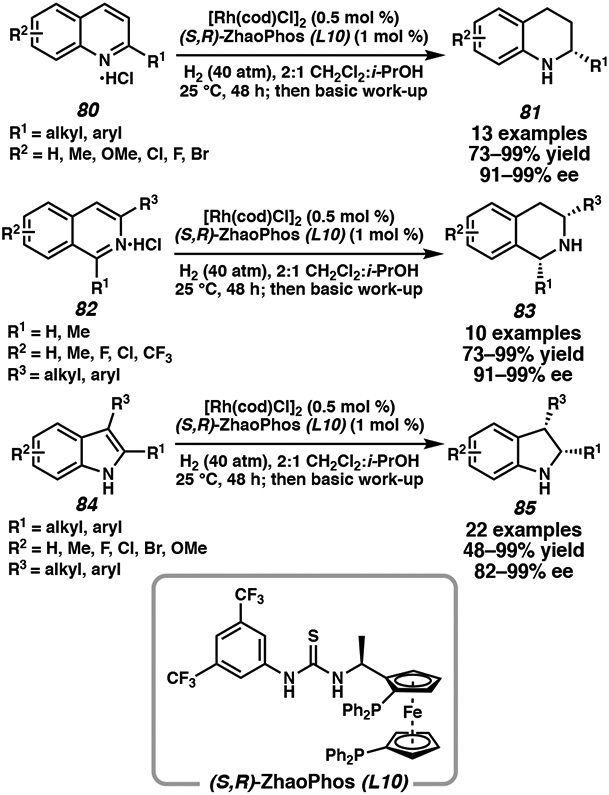
Rh-Catalyzed Asymmetric Hydrogenation of N-Heteroarenes with ZhaoPhos Ligand L10
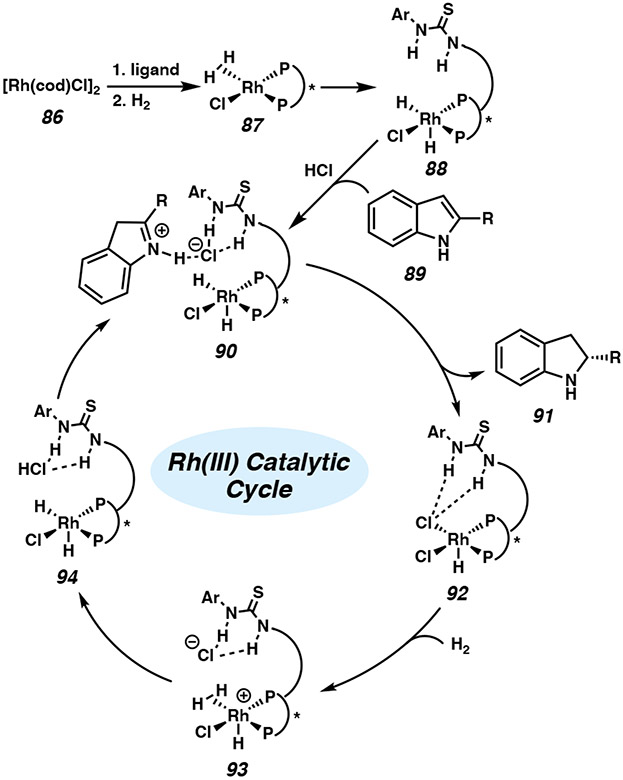
Zhang and coworkers performed DFT calculations to gain insight into the mechanism of the Rh-catalyzed transformation (Figure 7).43c Rh(I) catalyst 86 undergoes oxidative addition with H2 to generate the active Rh(III) species 88. Protonation of the indole substrate with HCl allows anion binding with the thiourea moiety to form intermediate 90, allowing hydrogen bonding interactions between the chloride ion and the indole N–H group. Hydride transfer then proceeds through an outer-sphere pathway to release the chiral indoline product 91 and complex 92. Addition of another molecule of H2 facilitates heterolytic cleavage of dihydrogen in complex 93 to generate Rh(III) catalyst 94 and HCl, in what was computed to be the rate-determining step. Overall, the thiourea–chloride anion binding proved to be crucial for inducing high enantioselectivity and reactivity in this system.43c
Figure 7.
Proposed catalytic cycle for the Rh-catalyzed asymmetric hydrogenation of indoles with ZhaoPhos L10.
6. PALLADIUM-CATALYZED ASYMMETRIC HYDROGENATION
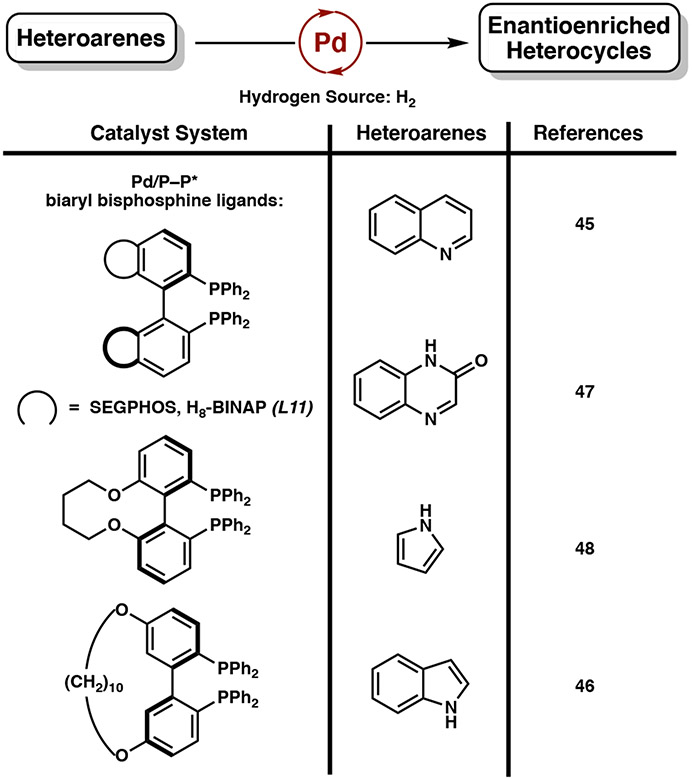
Compared to ruthenium, iridium, and rhodium catalysts, homogeneous palladium catalysts for the asymmetric hydrogenation of heteroarenes remain relatively unexplored.4 In 2010, Zhou and Zhang reported the first Pd-catalyzed asymmetric hydrogenation of N–H indoles using Pd(OCOCF3)2, a chiral H8-BINAP ligand, and (−)-camphorsulfonic acid.44 Since their seminal report, the homogeneous palladium catalyst system has been extended to accommodate a range of N-heteroarenes, including quinolines,45 indoles,46 quinoxalinones,47 and the partial hydrogenation of pyrroles (Figure 8).48 Common amongst all these catalytic systems is the use of chiral biaryl bisphosphine ligands with different steric environments of the naphthyl ring.
Figure 8.
Common Pd-based catalyst systems for the asymmetric hydrogenation of heteroarenes (≥90% ee, minimum three substrates). Only one enantiomer of ligand shown for simplicity.
Zhou and coworkers have extensively developed palladium catalyst systems for the hydrogenation of N-heteroarenes, as palladium complexes demonstrate a higher tolerance for strong Brønsted acids. Using chiral ligand (R)-H8-BINAP (L11), palladium(II) trifluoroacetate, and 1 equivalent of (−)-camphorsulfonic acid or TsOH•H2O, the asymmetric hydrogenation of 2-substituted and 2,3-disubstituted indoles is achieved to synthesize enantioenriched indolines in up to 99% yield and 98% ee (Scheme 15).46c Isotope-labeling studies demonstrate that the acid is necessary for formation of the iminium salt, which then undergoes hydrogenation by a Pd–H species.
Scheme 15.
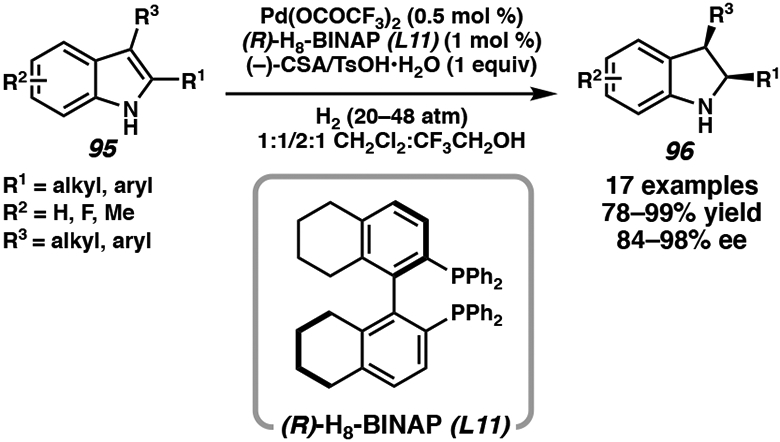
Pd-Catalyzed Asymmetric Hydrogenation of N–H Indoles
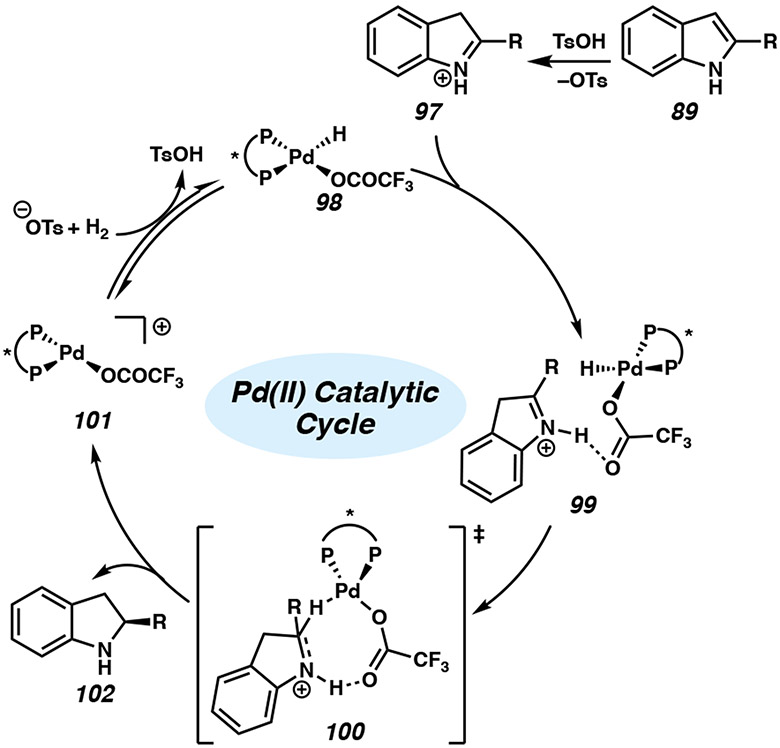
Using both experimental and theoretical methods, Zhou and coworkers analyzed several possible mechanisms and propose a stepwise outer-sphere pathway (Figure 9).46c The indole is initially protonated by TsOH to give the iminium 97, subsequently inducing hydrogen-bonding interactions with the trifluoroacetate ligand to generate intermediate 99. A hydride transfer from the Pd(II) center to the hydrogen-bound iminium group of complex 100 is proposed to be the enantiodetermining step. The addition is postulated to occur through an eight-membered transition state, in which differentiation of the enantiotopic face is achieved through steric interactions with the chiral bisphosphine ligand. After dissociation of the chiral indoline product 102, palladium hydride species 98 is regenerated with the release of TsOH from activation of H2. Additional mechanistic studies of palladium-catalyzed asymmetric hydrogenation of N-heteroarenes may provide key insights for further chiral catalyst design to improve the substrate scope of this transformation.
Figure 9.
Proposed catalytic cycle for the Pd-catalyzed asymmetric hydrogenation of indoles.
7. IRON-CATALYZED ASYMMETRIC HYDROGENATION
Despite the development of many efficient transition metal catalysts for the asymmetric hydrogenation of heteroarenes, the application of earth-abundant metals such as iron as hydrogenation catalysts are a promising avenue of further development. The first report of the homogeneous asymmetric hydrogenation of imines using first-row transition metals has only recently been published in 2011.49 Beller and coworkers developed a novel cooperative catalytic system combining an achiral iron hydrogenation catalyst with a chiral Brønsted acid that facilitates the enantioselective reduction of quinoxalines and benzoxazines.50 Using chiral phosphoric acid 105 to activate the substrate and control the enantioselectivity, an iron complex (104) reacts with the activated intermediate to deliver enantioenriched tetrahydroquinoxalines and dihydrobenzoxazines in high yields and selectivity (Scheme 16).50a Notably, both electron-donating and electron-withdrawing substituents on the C2-substituted phenyl ring, as well as meta- and para-substituted substrates, had little impact on the reactivity and enantioselectivity of the hydrogenation reaction. The levels of selectivity observed with the iron catalyst system rival those of late transition metal-based catalysts for the hydrogenation of the same heteroarenes.
Scheme 16.
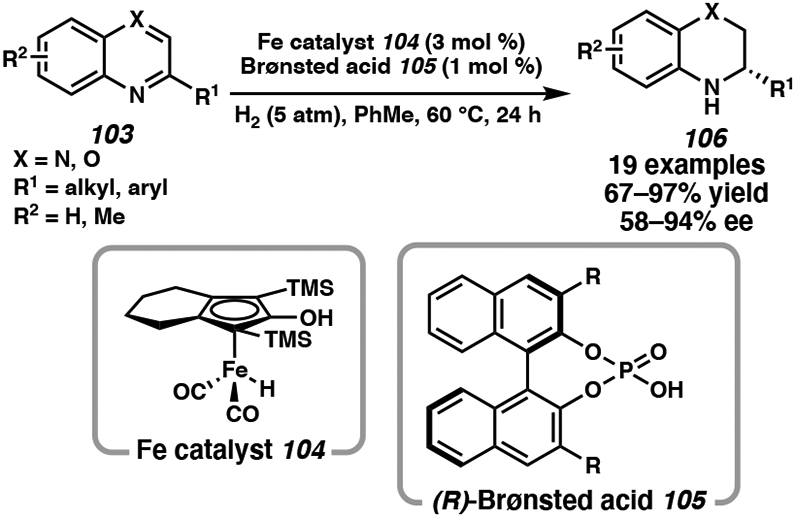
Fe-Catalyzed Asymmetric Hydrogenation of Quinoxalines and Benzoxazines with Chiral Phosphoric Acid 105
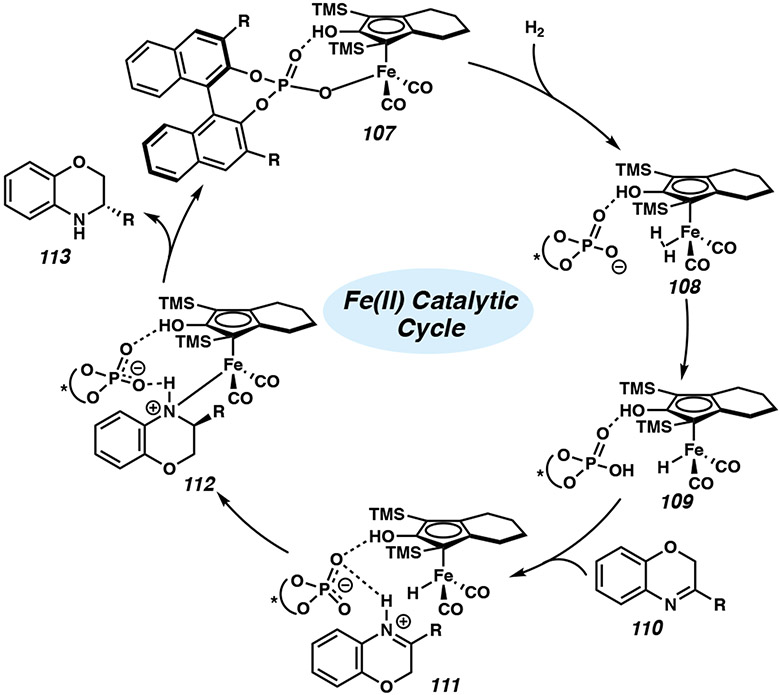
Although the mechanism for this transformation has been previously investigated computationally using acyclic imines,51 Hopmann has proposed an alternative mechanism with benzoxazine as the substrate (Figure 10).52 Calculations indicate that hydrogenation may likely occur through a stepwise mechanism, in which initially the resting state 107 involves an adduct between the deprotonated acid and the iron complex. A molecule of H2 generates dihydrogen complex 108, subsequently splitting H2 through proton abstraction by the phosphate to yield iron hydride species 109. Benzoxazine 110 then coordinates to the chiral acid through hydrogen bonding interactions, allowing hydride transfer to occur to form catalyst species 112. Finally, product 113 is released with regeneration of the catalyst complex 107.52 Computationally predicted enantioselectivities are in excellent agreement with experimental values, and the proposed mechanism is consistent with experimental observations by Beller and coworkers on possible reaction intermediates.50
Figure 10.
Proposed cooperative catalytic cycle for the Fecatalyzed asymmetric hydrogenation of benzoxazines in the presence of a chiral phosphoric acid.
8. BORANE-CATALYZED ASYMMETRIC HYDROGENATION
Avoiding the use of transition metals for catalysis, the metal-free asymmetric hydrogenation of heteroarenes is an emerging research field for further development. Recently, frustrated Lewis pair (FLP) catalysis has been explored for the hydrogenation of heteroarenes with hydrogen gas or NH3•BH3 as the hydrogen source.53 Du and coworkers reported a novel FLP catalyst system using a chiral borane catalyst (115), generated in situ from the direct hydroboration of chiral dienes 114 with HB(C6F5)2 (Scheme 17).54 The binaphthyl substituent presumably serves to control the stereoselectivity of the transformation, often requiring bulky aryl groups to achieve high selectivity. Although the mechanism for the asymmetric hydrogenation of heteroarenes using chiral borane catalysts has not been investigated thoroughly, FLP catalysts are well known to activate H2 splitting and undergo hydride transfer. 55
Scheme 17.
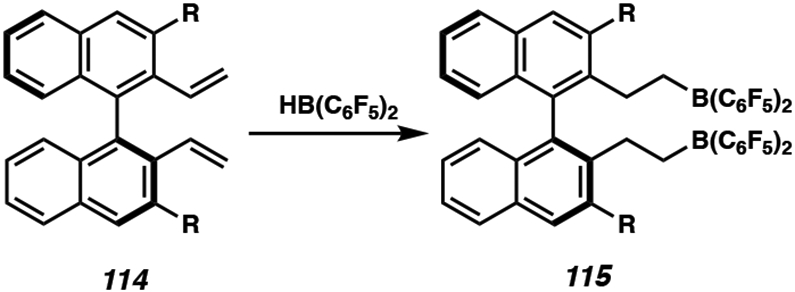
In Situ Formation of Chiral Borane Catalyst 115 from Chiral Dienes
Chiral borane catalysts such as 115 are effective for the asymmetric hydrogenation of trisubstituted quinolines and disubstituted quinoxalines.53 Du optimized the catalyst system to generate the borane catalyst in situ with 2 to 5 mol % of the chiral diene ligand 117 and HB(C6F5)2 in the presence of molecular H2 gas (Scheme 18). Under mild reaction conditions, a range of chiral tetrahydroquinolines and tetrahydroquinoxalines were synthesized in high yield and enantioselectivity.53a-d Overall, this catalyst system is a promising step toward the in situ generation of a library of chiral borane catalysts, and their application to the asymmetric hydrogenation of other heteroarenes could be explored.
Scheme 18.
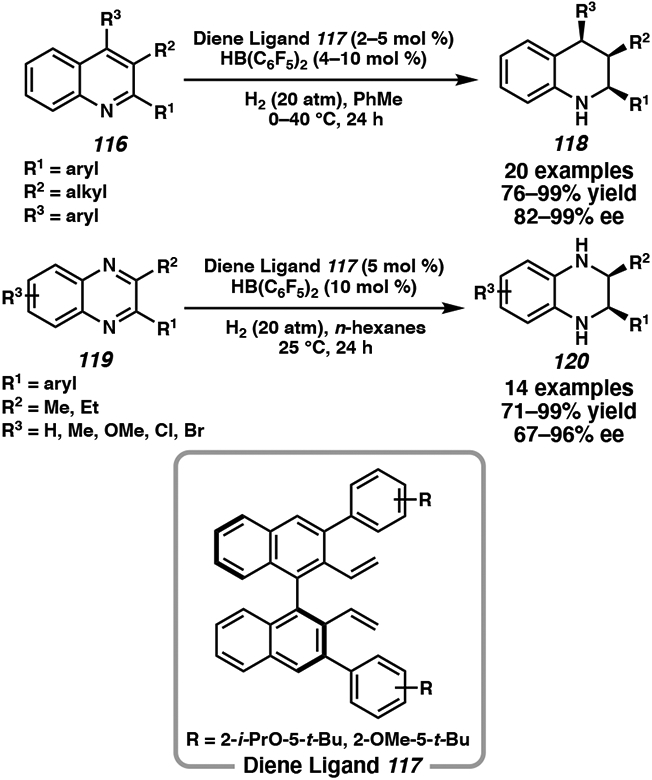
Metal-Free Hydrogenation of Heteroarenes through In Situ Formation of a Chiral Borane Catalyst
In 2019, Wang and coworkers developed a similar catalytic system, employing chiral spiro-bicyclic bisborane catalysts for the asymmetric hydrogenation of C(2)-substituted quinolines.53e The novel spiro-bicyclic bisborane catalyst 122 exhibited excellent activity and selectivity for alkyl-substituted quinolines at the C(2)-position, which previously have not been well tolerated (Scheme 19). A range of quinolines bearing alkenes, alkynes, and heterocycles were hydrogenated in excellent yield, enantioselectivity, and chemoselectivity, demonstrating the catalyst’s broad functional group tolerance.
Scheme 19.
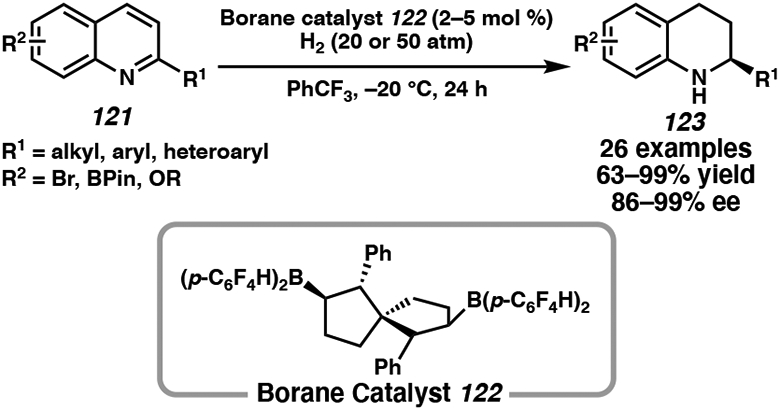
Metal-Free Hydrogenation of Quinolines Using Spiro-Bicyclic Bisborane Catalyst 122
9. CHIRAL PHOSPHORIC ACID-CATALYZED ASYMMETRIC HYDROGENATION
Since Rueping’s first report of an organic-catalyzed asymmetric reduction of heteroarenes in 2006, various chiral Brønsted acid catalysts have emerged as powerful agents for asymmetric arene hydrogenation (Scheme 20).4,56 Asymmetric transfer hydrogenation (ATH) reactions are promising alternatives to the use of transition metals or high pressure reactors, instead employing hydrogen sources like Hantzsch esters (HEH), dihydrophenanthridine (DHPD), and 4,5-dihydropyrrolo[1,2-a]quinoxalines.57 In most cases, however, an unrecyclable excess of reductant is required for hydrogenation.
Scheme 20.
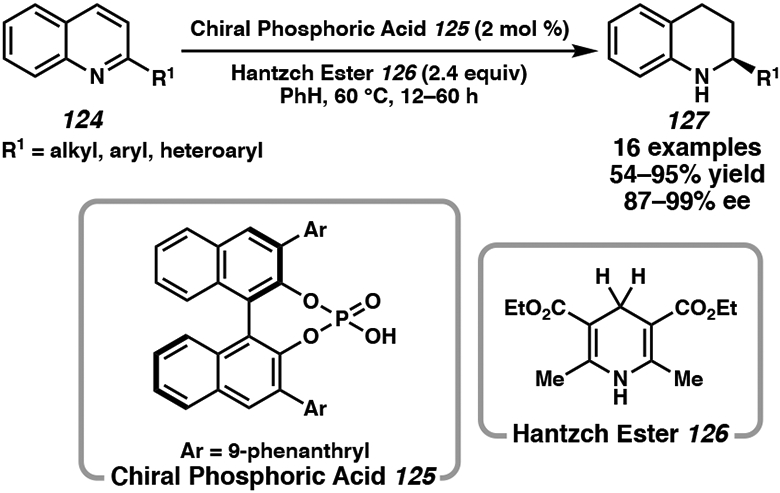
First Reported Chiral Phosphoric Acid-Catalyzed Asymmetric Hydrogenation of Quinolines
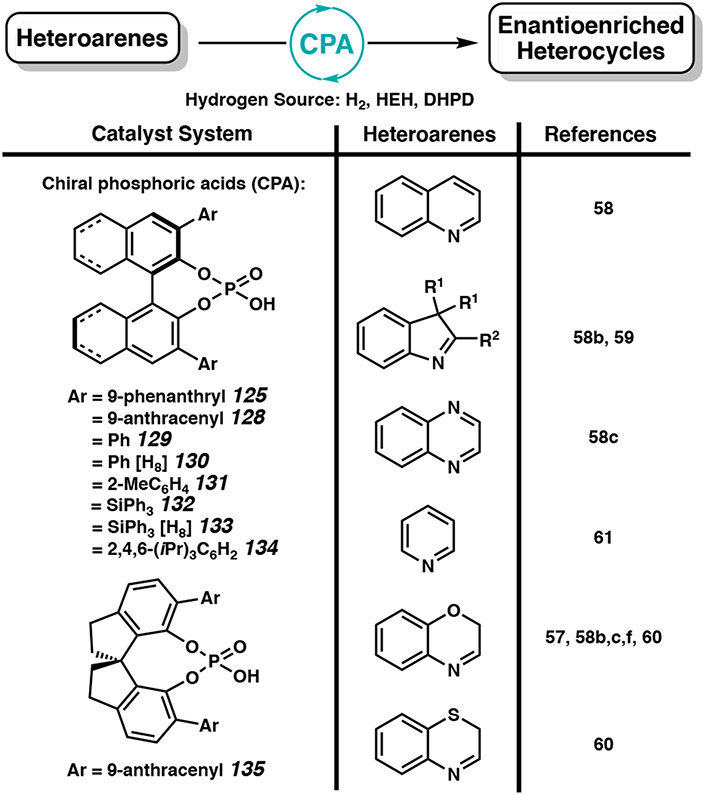
Over the past decade, several effective organic catalyst systems have been developed with chiral phosphoric acids (CPA) for the asymmetric reduction of quinolines,58 indoles,58b,59 quinoxalines,58c benzoxazines,57,58b,c,f,60 benzothiazines,60 and the partial hydrogenation of pyridines (Figure 11).61 Rueping, Zhou, Gong, and Bhanage have reported the use of BINOL-based phosphoric acid catalysts for the asymmetric hydrogenation of heteroarenes, with similar catalyst systems as shown in Scheme 20.56 More recently, Shi and coworkers have developed a new class of CPA catalysts with a 1,1’-spirobiindane-7,7’-diol (SPINOL) backbone for the enantioselective hydrogenation of a range of quinolines and benzoxazines.58f Using 1 mol % of CPA 135 and 1.25 to 2.5 equivalents of HEH (126), electron-withdrawing and electron-donating substituents at the C(2)-position are all well tolerated and afforded excellent enantioselectivities (Scheme 21).58f The metal-free and mild reaction conditions of this transformation is an appealing alternative to transition metal catalyst systems.
Figure 11.
Common chiral phosphoric acid catalyst systems for the asymmetric hydrogenation of heteroarenes (≥90% ee, minimum three substrates). Only one enantiomer of catalyst shown for simplicity.
Scheme 21.
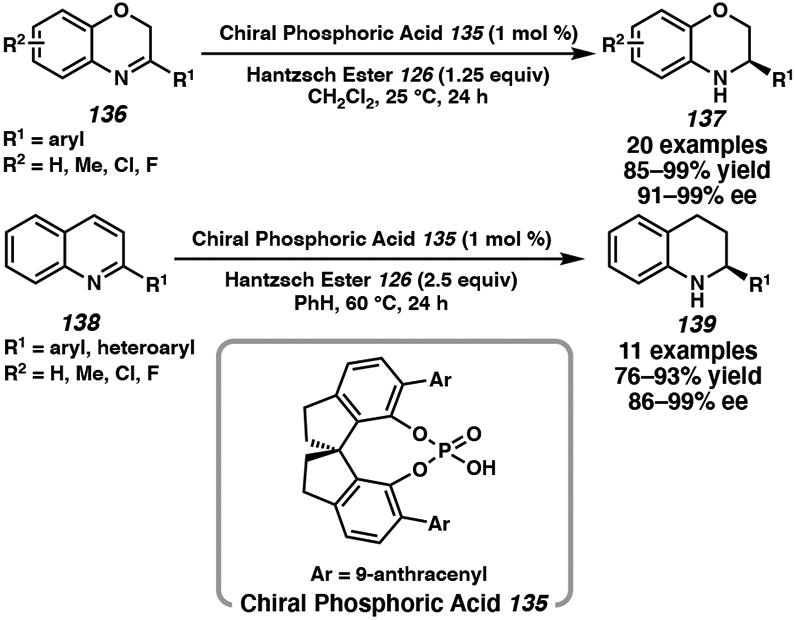
Asymmetric Transfer Hydrogenation of Quinolines with SPINOL-Derived Phosphoric Acid 135
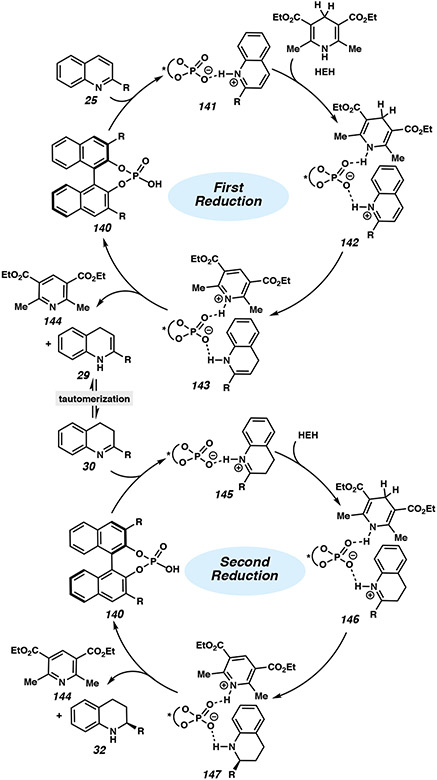
The general mechanism of the asymmetric transfer hydrogenation of quinolines by Hantzsch esters has been explored computationally by Frison and coworkers.62 It is well-precedented that two subsequent reduction cycles occur, each with one equivalent of the hydride source and a chiral phosphoric acid. Quinoline 25 is first activated through a proton transfer from the phosphoric acid to the substrate, followed by a 1,4-hydride addition using HEH. The resulting dihydroquinoline can then isomerize to the imine, entering a second reduction cycle involving a similar stepwise process (Figure 12). Although previous studies have proposed the hydride transfer from 146 to 147 to be the rate- and enantiodetermining step,63 Frison revisited these calculations and proposed that the stereocontrol is determined from the steric constraints by the aryl substituent of the CPA with HEH instead. Thus, the enantiodetermining step would not be the hydride transfer, but rather the coordination of HEH to complex 145 to generate intermediate 146, hindering access of the HEH to the catalytic site and controlling the facial approach of the hydride addition.62
Figure 12.
Proposed general catalytic cycle for the asymmetric transfer hydrogenation of quinolines in the presence of a chiral phosphoric acid and Hantzsch ester.
Using relay catalysis, Zhou and coworkers took inspiration from NAD(P)H models that enable the biomimetic asymmetric hydrogenation of heteroarenes, harnessing the in situ generation of dihydrophenanthridine (DHPD) from catalytic amounts of ruthenium, phenanthridine, and H2 as the terminal reductant.58c,64 Using 0.5 mol % of [Ru(p-cymene)I2]2, 10 mol % of DHPD, and 1 mol % of CPA 130 or 131, the asymmetric hydrogenation of 2-substituted quinolines, quinoxalines, and benzoxazines proceeded in up to 99% yield and 97% ee (Scheme 22A).
Scheme 22.
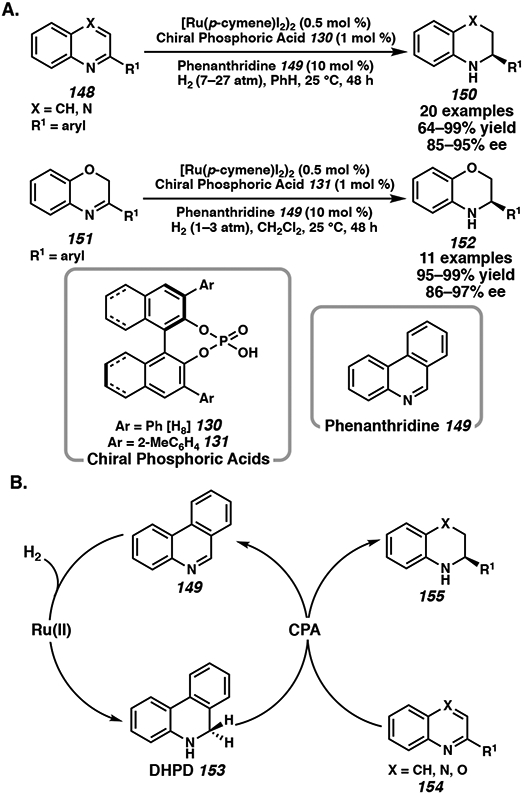
Biomimetic Asymmetric Transfer Hydrogenation of Heteroarenes
The mechanism of the biomimetic asymmetric hydrogenation reaction is proposed to involve two catalytic cycles, initially with Ru-catalyzed hydrogenation of phenanthridine 149 to generate DHPD 153. DHPD then coordinates to the chiral phosphoric acid to enantioselectively reduce the substrate 154, which is then regenerated to DHPD by the ruthenium hydride species (Scheme 22B). Interestingly, using a Hantzsch ester as the hydride source reverses the stereoselectivity due to the different steric demands of the coordination of the substrate and CPA, favoring the Re-face reduction instead.58c Although a transition metal is employed to recycle the hydride source, the biomimetic cascade hydrogenation can be performed under mild conditions with excellent activities and enantioselectivities.
10. CONCLUDING REMARKS
Over the past decade, many effective catalyst systems have been developed for the asymmetric hydrogenation or asymmetric transfer hydrogenation of heteroarenes. Although transition metal catalysts are most commonly employed for stereoselective hydrogenation with molecular hydrogen gas, a number of organic catalysts and dual catalytic systems have also been successfully applied for the synthesis of enantioenriched saturated heterocycles.
Despite the recent advances made in the field of asymmetric hydrogenation, as well as reports that have been discussed in prior reviews,4 significant challenges in this field remain. The discovery of a catalyst system with a broad substrate scope of more than four different heteroarenes has still not been achieved. Moreover, many asymmetric hydrogenation systems rely on acid activation of the substrates, which can be a limitation for more basic products. Development of novel ligand scaffolds and homogeneous catalyst systems will continue to be explored to design hydrogenation catalysts with improved selectivities. Creative activation strategies for these transformations should also be considered to extend the substrate scope, whether it be through pendant directing groups or recyclable activating reagents. Exploring these strategies will also provide insight into the challenge of developing a technology for the exhaustive asymmetric hydrogenation of simple 5- or 6-membered heteroarenes and benzene derivatives. Finally, a thorough mechanistic understanding of these asymmetric transformations is necessary to advance this field. Although experimental mechanistic studies are often limited by high pressure reactors and catalyst deactivation pathways, further investigation is needed to guide the development of the next generation of hydrogenation catalyst systems.
The asymmetric hydrogenation of heteroarenes using homogeneous catalysts continues to be a valuable technology for the direct synthesis of enantioenriched, saturated heterocycles. These small molecules are critical structural motifs in natural products,1 as well as pharmaceuticals,2 and other molecules of industrial importance.3 Thus, the development of efficient catalyst systems for the enantioselective hydrogenation of a broad range of heteroarenes in good yields, stereoselectivity, and chemoselectivity remains highly desirable. We anticipate further advances in asymmetric hydrogenation technology that will revolutionize this field of research.
ACKNOWLEDGMENT
We thank NIH-NIGMS (R01GM127972A) and Caltech for funding and the support of our research program.
ABBREVIATIONS
- ee
enantiomeric excess
- TsDPEN
N-(p-toluenesulfonyl)-1,2-diphenylethylenediamine
- PhTRAP
(2,2”-bis[1-(diphenylphosphino)-ethyl]-1,1”-biferrocene
- NHC
N-heterocyclic carbene
- TCCA
trichloroisocyanuric acid
- PHOX
phosphinooxazolines
- DFT
density functional theory
- FLP
frustrated Lewis pair
- ATH
asymmetric transfer hydrogenation
- HEH
Hantzsch ester
- DHPD
dihydrophenanthridine
- CPA
chiral phosphoric acid
- BINOL
1,1’-bi-2-naphthol
- SPINOL
1,1’-spirobiindane-7,7’-diol
REFERENCES
- (1).(a) Heterocycles in Natural Product Synthesis; Majumdar KC; Chattopadhyay SK, Eds.; John Wiley & Sons: New York, 1991; pp 1–611. [Google Scholar]; (b) Walsh CT Nature Loves Nitrogen Heterocycles. Tetrahedron Letters 2015, 56, 3075–3081. [Google Scholar]
- (2).(a) Vitaku E; Smith DT; Njardarson JT Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem 2014, 57, 10257–10274. [DOI] [PubMed] [Google Scholar]; (b) Taylor RD; MacCoss M; Lawson ADG Rings in Drugs. J. Med. Chem 2014, 57, 5845–5859. [DOI] [PubMed] [Google Scholar]; (c) Lovering F; Bikker J; Humblet C Escape from Flatland: Increasing Saturation as an Approach to Improving Clinical Success. J. Med. Chem 2009, 52, 6752–6756. [DOI] [PubMed] [Google Scholar]; (d) Lovering F Escape from Flatland 2: Complexity and Promiscuity. Med. Chem. Commun 2013, 4, 515–519. [Google Scholar]; (e) Roughley SD; Jordan AM The Medicinal Chemist’s Toolbox: An Analysis of Reactions Used in the Pursuit of Drug Candidates. J. Med. Chem 2011, 54, 3451–3479. [DOI] [PubMed] [Google Scholar]; (f) Meanwell NA A Synopsis of the Properties and Applications of Heteroaromatic Rings in Medicinal Chemistry. Advances in Heterocyclic Chemistry. 2017, 123, 245–361. [Google Scholar]
- (3).(a) Sanemitsu Y; Kawamura S Studies on the Synthetic Development for the Discovery of Novel Heterocyclic Agrochemicals. J. Pestic. Sci 2008, 33, 175–177. [Google Scholar]; (b) Katritzky AR Heterocyclic Chemistry: An Academic Subject of Immense Industrial Importance. Chemistry of Heterocyclic Compounds. 1992, 28, 241–259. [Google Scholar]
- (4).(a) For selected reviews of asymmetric hydrogenation of heteroarenes, see: Wang D-S; Chen Q-A; Lu S-M; Zhou Y-G Asymmetric Hydrogenation of Heteroarenes and Arenes. Chem. Rev 2012, 11, 2557–2590. [DOI] [PubMed] [Google Scholar]; (b) Zhou Y-G Asymmetric Hydrogenation of Heteroaromatic Compounds. Acc. Chem. Res 2007, 40, 1357–1366. [DOI] [PubMed] [Google Scholar]; (c) Zhao D; Glorius F Enantioselective Hydrogenation of Isoquinolines. Angew. Chem. Int. Ed 2013, 52, 9616–9618. [DOI] [PubMed] [Google Scholar]; (d) Weisenfeldt MP; Nairoukh Z; Dalton T; Glorius F Selective Arene Hydrogenation for Direct Access to Saturated Carbo- and Heterocycles. Angew. Chem. Int. Ed 2019, 58, 10460–10476. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Luo Y-E; He Y-M; Fan Q-H Asymmetric Hydrogenation of Quinoline Derivatives Catalyzed by Cationic Transition Metal Complexes of Chiral Diamine Ligands: Scope, Mechanism and Catalyst Recycling. Chem. Rec 2016, 16, 2697–2711. [DOI] [PubMed] [Google Scholar]; (f) Fleury-Brégeot N; de la Fuente V; Castillón S; Claver C Highlights of Transition Metal-Catalyzed Asymmetric Hydrogenation of Imines. ChemCatChem 2010, 2, 1346–1371. [Google Scholar]
- (5).Fadhel AZ; Pollet P; Liotta CL; Eckert CA Combining the Benefits of Homogeneous and Heterogeneous Catalysis with Tunable Solvents and Nearcritical Water. Molecules 2010, 15, 8400–8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Kita Y; Yamaji K; Higashida K; Sathaiah K; Iimuro A; Mashima K Enhancing Effects of Salt Formation on Catalytic Activity and Enantioselectivity for Asymmetric Hydrogenation of Isoquinolinium Salts by Dinuclear Halide-Bridged Iridium Complexes Bearing Chiral Diphosphine Ligands. Chem. Eur. J 2015, 21, 1915–1927. [DOI] [PubMed] [Google Scholar]
- (7).Huang W-X; Wu B; Gao X; Chen M-W; Wang B; Zhou Y-G Iridium-Catalyzed Selective Hydrogenation of 3-Hydroxypyridinium Salts: A Facile Synthesis of Piperidin-3-ones. Org. Lett 2015, 17, 1640–1643. [DOI] [PubMed] [Google Scholar]
- (8).Chen Q-A; Wang D-S; Zhou Y-G; Duan Y; Fan H-J; Yang Y; Zhang Z Convergent Asymmetric Disproportionation Reactions: Metal/Brønsted Acid Relay Catalysis for Enantioselective Reduction of Quinoxalines. J. Am. Chem. Soc 2011, 133, 6126–6129. [DOI] [PubMed] [Google Scholar]
- (9).(a) Cui C-X; Chen H; Li S-J; Zhang T; Qu L-B; Lan Y Mechanism of Ir-Catalyzed Hydrogenation: A Theoretical View. Coordination Chemistry Reviews. 2020, 412, 213251. [Google Scholar]; (b) Dobereiner GE; Nova A; Schley ND; Hazari N; Miller SJ; Eisenstein O; Crabtree RH Iridium-Catalyzed Hydrogenation of N-Heterocyclic Compounds under Mild Conditions by an Outer-Sphere Pathway. J. Am. Chem. Soc 2011, 133, 7547–7562. [DOI] [PubMed] [Google Scholar]; (c) Bianchini C; Meli A; Vizza F Modelling the Hydrodenitrogenation of Aromatic N-Heterocycles in the Homogeneous Phase. Eur. J. Inorg. Chem 2001, 2001, 43–68. [Google Scholar]
- (10).Dorta R; Broggini D; Stoop R; Rüegger H; Spindler F; Togni A Chiral Xyliphos Complexes for the Catalytic Imine Hydrogenation Leading to the Metolachlor Herbicide: Isolation of Catalyst-Substrate Adducts. Chem. Eur. J 2004, 10, 267–278. [DOI] [PubMed] [Google Scholar]
- (11).(a) Hashiguchi S; Fujii A; Takehara J; Ikariya T; Noyori R Asymmetric Transfer Hydrogenation of Aromatic Ketones Catalyzed by Chiral Ruthenium(II) Complexes. J. Am. Chem. Soc 1995, 117, 7562–7563. [Google Scholar]; (b) Fujii A; Hashiguchi S; Uematsu N; Ikariya T; Noyori R Ruthenium(II)-Catalyzed Asymmetric Transfer Hydrogenation of Ketones Using a Formic Acid-Triethylamine Mixture. J. Am. Chem. Soc 1996, 118, 2521–2522. [Google Scholar]
- (12).Uematsu N; Fujii A; Hashiguchi S; Ikariya T; Noyori R Asymmetric Transfer Hydrogenation of Imines. J. Am. Chem. Soc 1996, 118, 4916–4917. [Google Scholar]
- (13).(a) For selected examples of the asymmetric hydrogenation of carbocyclic aromatic compounds, see: Kuwano R; Morioka R; Kashiwabara M; Kameyama N Catalytic Asymmetric Hydrogenation of Naphthalenes. Angew. Chem. Int. Ed 2012, 51, 4136–4139. [DOI] [PubMed] [Google Scholar]; (b) Kuwano R; Ikeda R; Hirasada K Catalytic Asymmetric Hydrogenation of Quinoline Carbocycles: Unusual Chemoselectivity in the Hydrogenation of Quinolines. Chem. Commun 2015, 51, 7558–7561. [DOI] [PubMed] [Google Scholar]; (c) Yan Z; Xie H-P; Shen H-Q; Zhou Y-G Ruthenium-Catalyzed Hydrogenation of Carbocyclic Aromatic Amines: Access to Chiral Exocyclic Amines. Org. Lett 2018, 20, 1094–1097. [DOI] [PubMed] [Google Scholar]
- (14).(a) Zhou H; Li Z; Wang Z; Wang T; Xu L; He Y; Fan Q-H; Pan J; Gu L; Chan ASC Hydrogenation of Quinolines Using a Recyclable Phosphine-Free Chiral Cationic Ruthenium Catalyst: Enhancement of Catalyst Stability and Selectivity in an Ionic Liquid. Angew. Chem. Int. Ed 2008, 47, 8464–8467. [DOI] [PubMed] [Google Scholar]; (b) Wang Z-J; Zhou H-F; Wang T-L; He Y-M; Fan Q-H Highly Enantioselective Hydrogenation of Quinolines under Solvent-Free or Highly Concentrated Conditions. Green Chem. 2009, 11, 767–769. [Google Scholar]; (c) For recent reports on the asymmetric hydrogenation of quinolines and isoquinolines using a Ru/TsDPEN catalyst, see: Ding Z-Y; Wang T; He Y-M; Chen F; Zhou H-F; Fan Q-H; Guo Q; Chan ASC Highly Enantioselective Synthesis of Chiral Tetrahydroquinolines and Tetrahydroisoquinolines by Ruthenium-Catalyzed Asymmetric Hydrogenation in Ionic Liquid. Adv. Synth. Catal 2013, 355, 3727–3735. [Google Scholar]; (d) Wang T; Chen Y; Ouyang G; He Y-M; Li Z; Fan Q-H Solvent-Regulated Asymmetric Hydrogenation of Quinoline Derivatives in Oligo(Ethylene Glycol)s through Host-Guest Interactions. Chem. Asian. J 2016, 11, 2773–2777. [DOI] [PubMed] [Google Scholar]; (e) Yang Z; Chen F; He Y-M; Yang N; Fan Q-H Efficient Asymmetric Hydrogenation of Quinolines in Neat Water Catalyzed by Chiral Cationic Ru-Diamine Complexes. Catal. Sci. Technol 2014, 4, 2887–2890. [Google Scholar]
- (15).(a) Wang T; Zhuo L-G; Li Z; Chen F; Ding Z; He Y; Fan Q-H; Xiang J; Yu Z-X; Chan ASC Highly Enantioselective Hydrogenation of Quinolines Using Phosphine-Free Chiral Cationic Ruthenium Catalysts: Scope, Mechanism, and Origin of Enantioselectivity. J. Am. Chem. Soc 2011, 133, 9878–9891. [DOI] [PubMed] [Google Scholar]; (b) Wang T; Ouyang G; He Y-M; Fan Q-H Asymmetric Tandem Reduction of 2-(Arylmethyl)quinolines with Phosphine-Free Ru-TsDPEN Catalyst. Synlett 2011, 7, 0939–0942. [Google Scholar]; (c) Xu C; Feng Y; Li F; Han J; He Y-M; Fan Q-H A Synthetic Route to Chiral Benzo-Fused N-Heterocycles via Sequential Intramolecular Hydroamination and Asymmetric Hydrogenation of Anilino-Alkynes. Organometallics 2019, 38, 3979–3990. [Google Scholar]; (d) Chen Y; He Y-M; Zhang S; Miao T; Fan Q-H Rapid Construction of Structurally Diverse Quinolizidines, Indolizidines, and Their Analogues via Ruthenium-Catalyzed Asymmetric Cascade Hydrogenation/Reductive Amination. Angew. Chem. Int. Ed 2019, 58, 3809–3813. [DOI] [PubMed] [Google Scholar]; (e) Wang L-R; Chang D; Feng Y; He Y-M; Deng G-J; Fan Q-H Highly Enantioselective Ruthenium-Catalyzed Cascade Double Reduction Strategy: Construction of Structurally Diverse Julolidines and Their Analogues. Org. Lett 2020, 22, 2251–2255. [DOI] [PubMed] [Google Scholar]
- (16).(a) Ma W; Zhang J; Xu C; Chen F; He Y-M; Fan Q-H Highly Enantioselective Direct Synthesis of Endocyclic Vicinal Diamines through Chiral Ru(diamine)-Catalyzed Hydrogenation of 2,2’-Bisquinoline Derivatives. Angew. Chem. Int. Ed 2016, 55, 12891–12894. [DOI] [PubMed] [Google Scholar]; (b) Li B; Xu C; He Y-M; Deng G-J; Fan Q-H Asymmetric Hydrogenation of Bis(quinolin-2-yl)methanes: A Direct Access to Chiral 1,3-Diamines. Chin. J. Chem 2018, 36, 1169–1173. [Google Scholar]; (c) Chen Y; Pan Y; He Y-M; Fan Q-H Consecutive Intermolecular Reductive Amination/Asymmetric Hydrogenation: Facile Access to Sterically Tunable Chiral Vicinal Diamines and N-Heterocyclic Carbenes. Angew. Chem. Int. Ed 2019, 58, 16831–16834. [DOI] [PubMed] [Google Scholar]
- (17).Liu Y; Chen F; He Y-M; Li C; Fan Q-H Enantioselective Synthesis of Tunable Chiral Pyridine–Aminophosphine Ligands and their Applications in Asymmetric Hydrogenation. Org. Biomol. Chem 2019, 17, 5099–5105. [DOI] [PubMed] [Google Scholar]
- (18).(a) Touge T; Arai T Asymmetric Hydrogenation of Unprotected Indoles Catalyzed by η6-Arene/N-Me-sulfonyldiamine-Ru(II) Complexes. J. Am. Chem. Soc 2016, 138, 11299–11305. [DOI] [PubMed] [Google Scholar]; (b) Yang Z; Chen F; He Y; Yang N; Fan Q-H Highly Enantioselective Synthesis of Indolines: Asymmetric Hydrogenation at Ambient Temperature and Pressure with Cationic Ruthenium Diamine Catalysts. Angew. Chem. Int. Ed 2016, 55, 13863–13866. [DOI] [PubMed] [Google Scholar]
- (19).Qin J; Chen F; He Y-M; Fan Q-H Asymmetric Hydrogenation of 3-substituted 2H-1,4-benzoxazines with Chiral Cationic Ru-MsDPEN Catalysts: A Remarkable Counteranion Effect. Org. Chem. Front 2014, 1, 952–955. [Google Scholar]
- (20).(a) For select examples of polycyclic heteroarene hydrogenation using Ru/TsDPEN catalyst systems, see: Zhang J; Chen F; He Y-M; Fan Q-H Asymmetric Ruthenium-Catalyzed Hydrogenation of 2,6-Disubstituted 1,5-Naphthyridines: Access to Chiral 1,5-Diaza-cis-Decalins. Angew. Chem. Int. Ed 2015, 54, 4622–4625. [DOI] [PubMed] [Google Scholar]; (b) Wang T; Chen F; Qin J; He Y-M; Fan Q-H Asymmetric Ruthenium-Catalyzed Hydrogenation of 2- and 2,9-Substituted 1,10-Phenanthrolines. Angew. Chem. Int. Ed 2013, 52, 7172–7176. [DOI] [PubMed] [Google Scholar]; (c) Ma W; Chen F; Liu Y; He Y-M; Fan Q-H Ruthenium-Catalyzed Enantioselective Hydrogenation of 1,8-Naphthyridine Derivatives. Org. Lett 2016, 18, 2730–2733. [DOI] [PubMed] [Google Scholar]; (d) Yang Z; Chen F; Zhang S; He Y; Yang N; Fan Q-H Ruthenium-Catalyzed Enantioselective Hydrogenation of Phenanthridine Derivatives. Org. Lett 2017, 19, 1458–1461. [DOI] [PubMed] [Google Scholar]
- (21).(a) Yamakawa M; Ito H; Noyori R The Metal-Ligand Bifunctional Catalysis: A Theoretical Study on the Ruthenium(II)-Catalyzed Hydrogen Transfer Between Alcohols and Carbonyl Compounds. J. Am. Chem. Soc 2000, 122, 1466–1478. [Google Scholar]; (b) Yamakawa M; Yamada I; Noyori R CH/π Attraction: The Origin of Enantioselectivity in Transfer Hydrogenation of Aromatic Carbonyl Compounds Catalyzed by Chiral η6-Arene-Ruthenium(II) Complexes. Angew. Chem. Int. Ed 2001, 40, 2818–2821. [DOI] [PubMed] [Google Scholar]
- (22).Sawamura M; Hamashima H; Ito Y A Trans-Chelating Chiral Diphosphine Ligand: Synthesis of 2,2”-Bis[1-(diphenylphosphino)ethyl]-1,1”-biferrocene and Its Complexes with Platinum(II) and Palladium(II). Tetrahedron: Asymmetry, 1991, 2, 593–596. [Google Scholar]
- (23).(a) Kuwano R; Kashiwabara M Ruthenium-Catalyzed Asymmetric Hydrogenation of N-Boc-Indoles. Org. Lett 2006, 8, 2653–2655. [DOI] [PubMed] [Google Scholar]; (b) Makida Y; Saita M; Kuramoto T; Ishizuka K; Kuwano R Asymmetric Hydrogenation of Azaindoles: Chemo- and Enantioselective Reduction of Fused Aromatic Ring Systems Consisting of Two Heteroarenes. Angew. Chem. Int. Ed 2016, 55, 11859–11862. [DOI] [PubMed] [Google Scholar]; (c) Kuwano R; Kashiwabara M; Ohsumi M; Kusano H Catalytic Asymmetric Hydrogenation of 2,3,5-Trisubstituted Pyrroles. J. Am. Chem. Soc 2008, 130, 808–809. [DOI] [PubMed] [Google Scholar]; (d) Kuwano R; Kameyama N; Ikeda R Catalytic Asymmetric Hydrogenation of N-Boc-Imidazoles and Oxazoles. J. Am. Chem. Soc 2011, 133, 7312–7315. [DOI] [PubMed] [Google Scholar]
- (24).Urban S; Ortega N; Glorius F Ligand-Controlled Highly Regioselective and Asymmetric Hydrogenation of Quinoxalines Catalyzed by Ruthenium N-Heterocyclic Carbene Complexes. Angew. Chem. Int. Ed 2011, 50, 3803–3806. [DOI] [PubMed] [Google Scholar]
- (25).(a) Wysocki J; Ortega N; Glorius F Asymmetric Hydrogenation of Disubstituted Furans. Angew. Chem. Int. Ed 2014, 53, 8751–8755. [DOI] [PubMed] [Google Scholar]; (b) Ortega N; Urban S; Beiring B; Glorius F Ruthenium NHC Catalyzed Highly Asymmetric Hydrogenation of Benzofurans. Angew. Chem. Int. Ed 2012, 51, 1710–1713. [DOI] [PubMed] [Google Scholar]; (c) Ortega N; Beiring B; Urban S; Glorius F Highly Asymmetric Synthesis of (+)-Corsifuran A. Elucidation of the Electronic Requirements in the Ruthenium-NHC Catalyzed Hydrogenation of Benzofurans. Tetrahedron 2012, 68, 5185–5192. [Google Scholar]
- (26).Paul D; Beiring B; Plois M; Ortega N; Kock S; Schlüns D; Neugebauer J; Wolf R; Glorius F A Cyclometalated Ruthenium-NHC Precatalyst for the Asymmetric Hydrogenation of (Hetero)arenes and its Activation Pathway. Organometallics, 2016, 35, 3641–3646. [Google Scholar]
- (27).(a) Wang W-B; Lu S-M; Yang P-Y; Han X-W; Zhou Y-G Highly Enantioselective Iridium-Catalyzed Hydrogenation of Heteroaromatic Compounds, Quinolines. J. Am. Chem. Soc 2003, 125, 10536–10537. [DOI] [PubMed] [Google Scholar]; (b) Wang D-W; Wang X-B; Wang D-S; Lu S-M; Zhou Y-G; Li Y-X Highly Enantioselective Iridium-Catalyzed Hydrogenation of 2-Benzylquinolines and 2-Functionalized and 2,3-Disubstituted Quinolines. J. Org. Chem 2009, 74, 2780–2787. [DOI] [PubMed] [Google Scholar]
- (28).Zhang X-C; Hu Y-H; Chen C-F; Fang Q; Yang L-Y; Lu Y-B; Xie L-J; Wu J; Li S; Fang W A Supramolecularly Tunable Chiral Diphosphine Ligand: Application to Rh and Ir-Catalyzed Enantioselective Hydrogenation. Chem. Sci 2016, 7, 4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).(a) Zhang D-Y; Wang D-S; Wang M-C; Yu C-B; Gao K; Zhou Y-G Synthesis of Electronically Deficient Atropisomeric Bisphosphine Ligands and Their Application in Asymmetric Hydrogenation of Quinolines. Synthesis 2011, 17, 2796–2802. [Google Scholar]; (b) Zhang D-Y; Yu C-B; Wang M-C; Gao K; Zhou Y-G A New Electronically Deficient Atropisomeric Diphosphine Ligand (S)-CF3O-BiPhep and its Application in Asymmetric Hydrogenation. Tetrahedron Letters 2012, 53, 2556–2559. [Google Scholar]; (c) Cai X-F; Guo R-N; Chen M-W; Shi L; Zhou Y-G Synthesis of Chiral Exocyclic Amines by Asymmetric Hydrogenation of Aromatic Quinolin-3-amines. Chem. Eur. J 2014, 20, 7245–7248. [DOI] [PubMed] [Google Scholar]; (d) Maj AM; Suisse I; Hardouin C; Agbossou-Niedercorn F Synthesis of New Chiral 2-Functionalized-1,2,3,4- Tetrahydroquinoline Derivatives via Asymmetric Hydrogenation of Substituted Quinolines. Tetrahedron 2013, 69, 9322–9328. [Google Scholar]; (e) Hu S-B; Zhai X-Y; Shen H-Q; Zhou Y-G Iridium-Catalyzed Asymmetric Hydrogenation of Polycyclic Pyrrolo/Indolo[1,2-a]quinoxalines and Phenanthridines. Adv. Synth. Catal 2018, 360, 1334–1339. [Google Scholar]
- (30).(a) Guo R-N; Cai X-F; Shi L; Ye Z-S; Chen M-W; Zhou Y-G An Efficient Route to Chiral N-Heterocycles Bearing a C–F Stereogenic Center via Asymmetric Hydrogenation of Fluorinated Isoquinolines. Chem. Commun 2013, 49, 8537–8539. [DOI] [PubMed] [Google Scholar]; (b) Iimuro A; Yamaji K; Kandula S; Nagano T; Kita Y; Mashima K Asymmetric Hydrogenation of Isoquinolinium Salts Catalyzed by Chiral Iridium Complexes: Direct Synthesis for Optically Active 1,2,3,4- Tetrahydroisoquinolines. Angew. Chem. Int. Ed 2013, 52, 2046–2050. [DOI] [PubMed] [Google Scholar]; (c) Shi L; Ye Z-S; Cao L-L; Guo R-N; Hu Y; Zhou Y-G Enantioselective Iridium-Catalyzed Hydrogenation of 3,4- Disubstituted Isoquinolines. Angew. Chem. Int. Ed 2012, 51, 8286–8289. [DOI] [PubMed] [Google Scholar]; (d) Ye Z-S; Guo R-N; Cai X-F; Chen M-W; Shi L; Zhou Y-G Enantioselective Iridium-Catalyzed Hydrogenation of 1- and 3-Substituted Isoquinolinium Salts. Angew. Chem. Int. Ed 2013, 52, 3685–3689. [DOI] [PubMed] [Google Scholar]; (e) Chen M-W; Ji Y; Wang J; Chen Q-A; Shi L; Zhou Y-G Asymmetric Hydrogenation of Isoquinolines and Pyridines Using Hydrogen Halide Generated in Situ as Activator. Org. Lett 2017, 19, 4988–4991. [DOI] [PubMed] [Google Scholar]
- (31).(a) Nagano T; Iimuro A; Schwenk R; Ohshima T; Kita Y; Togni A; Mashima K Additive Effects of Amines on Asymmetric Hydrogenation of Quinoxalines Catalyzed by Chiral Iridium Complexes. Chem. Eur. J 2012, 18, 11578–11592. [DOI] [PubMed] [Google Scholar]; Cartigny D; Berhal F; Nagano T; Phansavath P; Ayad T; Genêt J-P; Ohshima T; Mashima K; Ratovelomanana-Vidal V General Asymmetric Hydrogenation of 2-Alkyl- and 2-Aryl-Substituted Quinoxaline Derivatives Catalyzed by Iridium-Difluorphos: Unusual Halide Effect and Synthetic Application. J. Org. Chem 2012, 77, 4544–4556. [DOI] [PubMed] [Google Scholar]
- (32).(a) Huang W-X; Liu L-J; Wu B; Feng G-S; Wang B; Zhou Y-G Synthesis of Chiral Piperazines via Hydrogenation of Pyrazines Activated by Alkyl Halides. Org. Lett 2016, 18, 3082–3085. [DOI] [PubMed] [Google Scholar]; (b) Huang W-X; Yu C-B; Shi L; Zhou Y-G Iridium-Catalyzed Asymmetric Hydrogenation of Pyrrolo[1,2-a]pyrazinium Salts. Org. Lett 2014, 16, 3324–3327. [DOI] [PubMed] [Google Scholar]
- (33).Gao K; Yu C-B; Wang D-S; Zhou Y-G Iridium-Catalyzed Asymmetric Hydrogenation of 3-Substituted 2H-1,4-Benzoxazines. Adv. Synth. Catal 2012, 354, 483–488. [Google Scholar]
- (34).(a) Rahman A; Lin X Development and Application of Chiral Spirocyclic Phosphoric Acids in Asymmetric Catalysis. Org. Biomol. Chem 2018, 16, 4753–4777. [DOI] [PubMed] [Google Scholar]; (b) Ding K; Han Z; Wang Z Spiro Skeletons: A Class of Privileged Structure for Chiral Ligand Design. Chem. Asian. J 2009, 4, 32–41. [DOI] [PubMed] [Google Scholar]
- (35).Sun S; Nagorny P Exploration of Chiral Diastereomeric Spiroketal (SPIROL)-Based Phosphinite Ligands in Asymmetric Hydrogenation of Heterocycles. Chem. Commun 2020, 56, 8432–8435. [DOI] [PubMed] [Google Scholar]
- (36).Hu X-H; Hu X-P Highly Diastereo- and Enantioselective Ir-Catalyzed Hydrogenation of 2,3-Disubstituted Quinolines with Structurally Fine-Tuned Phosphine-Phosphoramidite Ligands. Org. Lett 2019, 21, 10003–10006. [DOI] [PubMed] [Google Scholar]
- (37).(a) Welin ER; Ngamnithiporn A; Klatte M; Lapointe G; Pototschnig GM; McDermott MS; Conklin D; Gilmore CD; Tadross PM; Haley CK; Negoro K; Glibstrup E; Grünanger CU; Allan KM; Virgil SC; Slamon DJ; Stoltz BM Concise Total Syntheses of (−)-Jorunnamycin A and (−)-Jorumycin Enabled by Asymmetric Catalysis. Science 2019, 363, 270–275. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kim AN; Ngamnithiporn A; Welin ER; Daiger MT; Grünanger CU; Bartberger MD; Virgil SC; Stoltz BM Iridium-Catalyzed Enantioselective and Diastereoselective Hydrogenation of 1,3-Disubstituted Isoquinolines. ACS Catal. 2020, 10, 3241–3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).(a) Núñez-Rico JL; Fernández-Pérez H; Benet-Buchholz J; Vidal-Ferran A Asymmetric Hydrogenation of Heteroaromatic Compounds Mediated by Iridium–(P-OP) Complexes. Organometallics 2010, 29, 6627–6631. [Google Scholar]; (b) Núñez-Rico JL; Fernández-Pérez H; Vidal-Ferran A Asymmetric Hydrogenation of Unprotected Indoles Using Iridium Complexes Derived from P-OP Ligands and (Reusable) Brønsted Acids. Green. Chem 2014, 16, 1153–1157. [Google Scholar]; (c) Núñez-Rico JL; Vidal-Ferran A [Ir(P-OP)]-Catalyzed Asymmetric Hydrogenation of Diversely Substituted C=N-Containing Heterocycles. Org. Lett 2013, 15, 2066–2069. [DOI] [PubMed] [Google Scholar]
- (39).(a) Ge Y; Wang Z; Han Z; Ding K Iridium-Catalyzed Enantioselective Hydrogenation of Indole and Benzofuran Derivatives. Chem. Eur. J [Online early access]. DOI: 10.1002/chem.202002532. Published Online: July 2, 2020. https://chemistry-europe.onlinelibrary.wiley.com/doi/abs/10.1002/chem.202002532 (accessed Aug 20, 2020). [DOI] [PubMed] [Google Scholar]; (b) Pauli L; Tannert R; Scheil R; Pfaltz A Asymmetric Hydrogenation of Furans and Benzofurans with Iridium-Pyridine-Phosphinite Catalysts. Chem. Eur. J 2015, 21, 1482–1487. [DOI] [PubMed] [Google Scholar]; (c) Meng K; Xia J; Wang Y; Zhang X; Yang G; Zhang W Ir/BiphPHOX-Catalyzed Asymmetric Hydrogenation of 3-Substituted 2,5-Dihydropyrroles and 2,5-Dihydrothiophene 1,1-Dioxides. Org. Chem. Front 2017, 4, 1601–1605. [Google Scholar]; (d) Qu B; Mangunuru HPR; Tcyrulnikov S; Rivalti D; Zatolochnaya OV; Kurouski D; Radomkit S; Biswas S; Karyakarte S; Fandrick KR; Sieber JD; Rodriguez S; Desrosiers J-N; Haddad N; McKellop K; Pennino S; Lee H; Yee NK; Song JJ; Kozlowski MC; Senanayake CH Enantioselective Synthesis of α-(Hetero)aryl Piperidines through Asymmetric Hydrogenation of Pyridinium Salts and its Mechanistic Insights. Org. Lett 2018, 20, 1333–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Li Z-W; Wang T-L; He Y-M; Wang Z-J; Fan Q-H; Pan J; Xu L-J Air-Stable and Phosphine-Free Iridium Catalysts for Highly Enantioselective Hydrogenation of Quinoline Derivatives. Org. Lett 2008, 10, 5265–5268. [DOI] [PubMed] [Google Scholar]
- (41).(a) Kuwano R; Sato K; Kurokawa T; Karube D; Ito Y Catalytic Asymmetric Hydrogenation of Heteroaromatic Compounds, Indoles. J. Am. Chem. Soc 2000, 122, 7614–7615. [Google Scholar]; (b) Kuwano R; Kaneda K; Ito T; Sato K; Kurokawa T; Ito Y Highly Enantioselective Synthesis of Chiral 3-Substituted Indolines by Catalytic Asymmetric Hydrogenation of Indoles. Org. Lett 2004, 6, 2213–2215. [DOI] [PubMed] [Google Scholar]; (c) Kuwano R; Kashiwabara M; Sato K; Ito T; Kaneda K; Ito Y Catalytic Asymmetric Hydrogenation of Indoles Using a Rhodium Complex With a Chiral Bisphosphine Ligand PhTRAP. Tetrahedron: Asymmetry 2006, 17, 521–535. [Google Scholar]
- (42).(a) Wang C; Li C; Wu X; Pettman A; Xiao J pH-Regulated Asymmetric Transfer Hydrogenation of Quinolines in Water. Angew. Chem. Int. Ed 2009, 48, 6524–6528. [DOI] [PubMed] [Google Scholar]; (b) Parekh V; Ramsden JA; Wills M Asymmetric Transfer Hydrogenation of Quinolines Using Tethered Ru(II) Catalysts. Tetrahedron: Asymmetry 2010, 21, 1549–1556. [Google Scholar]
- (43).(a) Zhao Q; Li S; Huang K; Wang R; Zhang X A Novel Chiral Bisphosphine-Thiourea Ligand for Asymmetric Hydrogenation of β, β-Disubstituted Nitroalkenes. Org. Lett 2013, 15, 4014–4017. [DOI] [PubMed] [Google Scholar]; (b) Wen J; Tan R; Liu S; Zhao Q; Zhang X Strong Brønsted Acid Promoted Asymmetric Hydrogenation of Isoquinolines and Quinolines Catalyzed by a Rh-thiourea Chiral Phosphine Complex via Anion Binding. Chem. Sci 2016, 7, 3047–3051. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Wen J; Fan X; Tan R; Chien H-C; Zhou Q; Chung LW; Zhang X Brønsted-Acid-Promoted Rh-Catalyzed Asymmetric Hydrogenation of N-Unprotected Indoles: A Cocatalysis of Transition Metal and Anion Binding. Org. Lett 2018, 20, 2143–2147. [DOI] [PubMed] [Google Scholar]
- (44).Wang D-S; Chen Q-A; Li W; Yu C-B; Zhou Y-G; Zhang X Pd-Catalyzed Asymmetric Hydrogenation of Unprotected Indoles Activated by Brønsted Acids. J. Am. Chem. Soc 2010, 132, 8909–8911. [DOI] [PubMed] [Google Scholar]
- (45).Cai X-F; Huang W-X; Chen Z-P; Zhou Y-G Palladium-Catalyzed Asymmetric Hydrogenation of 3-Phthalimido Substituted Quinolines. Chem. Commun 2014, 50, 9588–9590. [DOI] [PubMed] [Google Scholar]
- (46).(a) Duan Y; Chen M-W; Chen Q-A; Yu C-B; Zhou Y-G Pd-Catalyzed Asymmetric Hydrogenation of 3-(toluenesulfonamidoalkyl)-Indoles. Org. Biomol. Chem 2012, 10, 1235–1238. [DOI] [PubMed] [Google Scholar]; (b) Li C; Chen J; Fu G; Liu D; Liu Y; Zhang W Highly Enantioselective Hydrogenation of N-Unprotected Indoles Using (S)-C10-BridgePHOS as the Chiral Ligand. Tetrahedron 2013, 69, 6839–6844. [Google Scholar]; (c) Duan Y; Li L; Chen M-W; Yu C-B; Fan H-J; Zhou Y-G Homogeneous Pd-Catalyzed Asymmetric Hydrogenation of Unprotected Indoles: Scope and Mechanistic Studies. J. Am. Chem. Soc 2014, 136, 7688–7700. [DOI] [PubMed] [Google Scholar]; (d) Yu C-B; Wang J; Zhou Y-G Facile Synthesis of Chiral Indolines Through Asymmetric Hydrogenation of in situ Generated Indoles. Org. Chem. Front 2018, 5, 2805–2809. [Google Scholar]; (e) Yu C-B; Li X; Zhou Y-G A Condensation/Reductive Alkylation/Hydrogenation Cascade for Facile Synthesis of Chiral 2,3-Disubstituted Indolines. Asian J. Org. Chem 2019, 8, 1118–1121. [Google Scholar]
- (47).Chen M-W; Deng Z; Yang Q; Huang J; Peng Y Enantioselective Synthesis of Trifluoromethylated Dihydroquinoxalinones via Palladium-Catalyzed Hydrogenation. Org. Chem. Front 2019, 6, 746–750. [Google Scholar]
- (48).Wang D-S; Ye Z-S; Chen Q-A; Zhou Y-G; Yu C-B; Fan H-J; Duan Y Highly Enantioselective Partial Hydrogenation of Simple Pyrroles: A Facile Access to Chiral 1-Pyrrolines. J. Am. Chem. Soc 2011, 133, 8866–8869. [DOI] [PubMed] [Google Scholar]
- (49).Zhou S; Fleischer S; Junge K; Beller M Cooperative Transition-Metal and Chiral Brønsted Acid Catalysis: Enantioselective Hydrogenation of Imines to Form Amines. Angew. Chem. Int. Ed 2011, 50, 5120–5124. [DOI] [PubMed] [Google Scholar]
- (50).(a) Fleischer S; Zhou S; Werkmeister S; Junge K; Beller M Cooperative Iron- Brønsted Acid Catalysis: Enantioselective Hydrogenation of Quinoxalines and 2H-1,4-Benzoxazines. Chem. Eur. J 2013, 19, 4997–5003. [DOI] [PubMed] [Google Scholar]; (b) Lu L-Q; Li Y; Junge K; Beller M Relay Iron/Chiral Brønsted Acid Catalysis: Enantioselective Hydrogenation of Benzoxazinones. J. Am. Chem. Soc 2015, 137, 2763–2768. [DOI] [PubMed] [Google Scholar]
- (51).(a) von der Höh A; Berkessel A Insight into the Mechanism of Dihydrogen-Heterolysis at Cyclopentadienone Iron Complexes and Subsequent C=X Hydrogenation. ChemCatChem 2011, 3, 861–867. [Google Scholar]; (b) Moulin S; Dentel H; Pagnoux-Ozherelyeva A; Gaillard S; Poater A; Cavallo L; Lohier J-F; Renaud J-L Bifunctional (Cyclopentadienone)Iron-Tricarbonyl Complexes: Synthesis, Computational Studies and Application in Reductive Amination. Chem. Eur. J 2013, 19, 17881–17890. [DOI] [PubMed] [Google Scholar]
- (52).Hopmann KH Iron/Brønsted Acid Catalyzed Asymmetric Hydrogenation: Mechanism and Selectivity-Determining Interactions. Chem. Eur. J 2015, 21, 10020–10030. [DOI] [PubMed] [Google Scholar]
- (53).(a) Zhang Z; Du H Enantioselective Metal-Free Hydrogenations of Disubstituted Quinolines. Org. Lett 2015, 17, 6266–6269. [DOI] [PubMed] [Google Scholar]; (b) Zhang Z; Du H Cis-Selective and Highly Enantioselective Hydrogenation of 2,3,4-Trisubstituted Quinolines. Org. Lett 2015, 17, 2816–2819. [DOI] [PubMed] [Google Scholar]; (c) Li S; Meng W; Du H Asymmetric Transfer Hydrogenations of 2,3-Disubstituted Quinoxalines with Ammonia Borane. Org. Lett 2017, 19, 2604–2606. [DOI] [PubMed] [Google Scholar]; (d) Zhang Z; Du H A Highly Cis-Selective and Enantioselective Metal-Free Hydrogenation of 2,3-Disubstituted Quinoxalines. Angew. Chem. Int. Ed 2015, 54, 623–626. [DOI] [PubMed] [Google Scholar]; (e) Li X; Tian J-J; Liu N; Tu X-S; Zeng N-N; Wang X-C Spiro-Bicyclic Bisborane Catalysts for Metal-Free Chemoselective and Enantioselective Hydrogenation of Quinolines. Angew. Chem. Int. Ed. 2019, 58, 4664–4668. [DOI] [PubMed] [Google Scholar]
- (54).Liu Y; Du H Chiral Dienes as “Ligands” for Borane-Catalyzed Metal-Free Asymmetric Hydrogenation of Imines. J. Am. Chem. Soc 2013, 135, 6810–6813. [DOI] [PubMed] [Google Scholar]
- (55).(a) Feng X; Du H Metal-Free Asymmetric Hydrogenation and Hydrosilylation Catalyzed by Frustrated Lewis Pairs. Tetrahedron Lett. 2014, 55, 6959–6964. [Google Scholar]; (b) Gao B; Feng X; Meng W; Du H Asymmetric Hydrogenation of Ketones and Enones with Chiral Lewis Base Derived Frustrated Lewis Pairs. Angew. Chem. Int. Ed 2020, 59, 4498–4504. [DOI] [PubMed] [Google Scholar]
- (56).Rueping M; Antonchick AP; Theissmann T A Highly Enantioselective Brønsted Acid Catalyzed Cascade Reaction: Organocatalytic Transfer Hydrogenation of Quinolines and their Application in the Synthesis of Alkaloids. Angew. Chem. Int. Ed 2006, 45, 3683–3686. [DOI] [PubMed] [Google Scholar]
- (57).Chen Z-P; Chen M-W; Guo R-N; Zhou Y-G 4,5-Dihydropyrrolo[1,2-a]quinoxalines: A Tunable and Regenerable Biomimetic Hydrogen Sources. Org. Lett 2014, 16, 1406–1409. [DOI] [PubMed] [Google Scholar]
- (58).(a) Rueping M; Theissmann T; Stoeckel M; Antonchick AP Direct Enantioselective Access to 4-Substituted Tetrahydroquinolines by Catalytic Asymmetric Transfer Hydrogenation of Quinolines. Org. Biomol. Chem 2011, 9, 6844–6850. [DOI] [PubMed] [Google Scholar]; (b) Rueping M; Bootwicha T; Sugiono E Continuous-Flow Catalytic Asymmetric Hydrogenation: Reaction Optimization Using FTIR Inline Analysis. Beilstein J. Org. Chem 2012, 8, 300–307. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Chen Q-A; Gao K; Duan Y; Ye Z-S; Shi L; Yang Y; Zhou Y-G Dihydrophenanthridine: A New and Easily Regenerable NAD(P)H Model for Biomimetic Asymmetric Hydrogenation. J. Am. Chem. Soc 2012, 134, 2442–2448. [DOI] [PubMed] [Google Scholar]; (d) Tu X-F; Gong L-Z Highly Enantioselective Transfer Hydrogenation of Quinolines Catalyzed by Gold Phosphates: Achiral Ligand Tuning and Chiral-Anion Control of Stereoselectivity. Angew. Chem. Int. Ed 2012, 51, 11346–11349. [DOI] [PubMed] [Google Scholar]; (e) More GV; Bhanage BM Chiral Phosphoric Acid Catalyzed Asymmetric Transfer Hydrogenation of Quinolines in a Sustainable Solvent. Tetrahedron: Asymmetry 2015, 26, 1174–1179. [Google Scholar]; (f) Zhang Y; Zhao R; Bao RL; Shi L Highly Enantioselective SPINOL-Derived Phosphoric Acid Catalyzed Transfer Hydrogenation of Diverse C=N-Containing Heterocycles. Eur. J. Org. Chem 2015, 3344–3351. [Google Scholar]; (g) Wang J; Chen M-W; Ji Y; Hu S-B; Zhou Y-G Kinetic Resolution of Axially Chiral 5- or 8-Substituted Quinolines via Asymmetric Transfer Hydrogenation. J. Am. Chem. Soc 2016, 138, 10413–10416. [DOI] [PubMed] [Google Scholar]
- (59).Rueping M; Brinkmann C; Antonchick AP; Atodiresei I Asymmetric Synthesis of Indolines by Catalytic Enantioselective Reduction of 3H-Indoles. Org. Lett 2010, 12, 4604–4607. [DOI] [PubMed] [Google Scholar]
- (60).Rueping M; Antonchick AP; Theissmann T Remarkably Low Catalyst Loading in Brønsted Acid Catalyzed Transfer Hydrogenations: Enantioselective Reduction of Benzoxazines, Benzothiazines, and Benzoxazinones. Angew. Chem. Int. Ed 2006, 45, 6751–6755. [DOI] [PubMed] [Google Scholar]
- (61).Rueping M; Antonchick AP Organocatalytic Enantioselective Reduction of Pyridines. Angew. Chem. Int. Ed 2007, 46, 4562–4565. [DOI] [PubMed] [Google Scholar]
- (62).Pastor J; Rezabal E; Voituriez A; Betzer J-F; Marinetti A; Frison G Revised Theoretical Model on Enantiocontrol in Phosphoric Acid Catalyzed H-Transfer Hydrogenation of Quinoline. J. Org. Chem 2018, 83, 2779–2787. [DOI] [PubMed] [Google Scholar]
- (63).(a) Marcelli T; Hammar P; Himo F Phosphoric Acid Catalyzed Enantioselective Transfer Hydrogenation of Imines: A Density Functional Theory Study of Reaction Mechanism and the Origins of Enantioselectivity. Chem. Eur. J 2008, 14, 8562–8571. [DOI] [PubMed] [Google Scholar]; (b) Simón L; Goodman JM Theoretical Study of the Mechanism of Hantzsch Ester Hydrogenation of Imines Catalyzed by Chiral BINOL-Phosphoric Acids. J. Am. Chem. Soc 2008, 130, 8741–8747. [DOI] [PubMed] [Google Scholar]
- (64).(a) Wang J; Zhu Z-H; Chen M-W; Chen Q-A; Zhou Y-G Catalytic Biomimetic Asymmetric Reduction of Alkenes and Imines Enabled by Chiral and Regenerable NAD(P)H Models. Angew. Chem. Int. Ed 2019, 58, 1813–1817. [DOI] [PubMed] [Google Scholar]; (b) Wang J; Zhao Z-B; Zhao Y; Luo G; Zhu Z-H; Luo Y; Zhou Y-G Chiral and Regenerable NAD(P)H Models Enabled Biomimetic Asymmetric Reduction: Design, Synthesis, Scope, and Mechanistic Studies. J. Org. Chem 2020, 85, 2355–2368. [DOI] [PubMed] [Google Scholar]; (c) Zhu Z-H; Ding Y-X; Wu B; Zhou Y-G Design and Synthesis of Chiral and Regenerable [2.2]paracyclophane-based NAD(P)H Models and Application in Biomimetic Reduction of Flavonoids. Chem. Sci 2020, 11, 10220–10224. [DOI] [PMC free article] [PubMed] [Google Scholar]