Most adverse events after immunisation with adenoviral vector vaccines—such as ChAdOx1 nCoV-19 (Oxford–AstraZeneca) and Ad26.COV2.S (Janssen)—are mild; however, rare life-threatening adverse events such as thrombosis with thrombocytopenia syndrome or Guillain-Barré syndrome have been reported.1, 2 Over a 6-month period comprising January to June, 2021, we encountered three cases of post-vaccination myositis at our hospital.
A 74-year-old man presented with a 3-week history of intermittent low-grade fever and polyarthralgia. These symptoms started 48 h after his first dose of ChAdOx1 nCoV-19 vaccination. The patient was febrile (38·5°C), tachycardic, and had tenderness in both calf muscles. Elevated inflammatory parameters were noted (appendix). On day 26, 18FDG-PET-CT showed a tree-root-like uptake pattern in the lower limbs suggestive of small–medium vessel vasculitis. The patient was started on 1 mg/kg oral prednisolone with rapid resolution of his symptoms within 3 days. On day 30, whole-body short tau inversion recovery (STIR)-MRI showed diffuse ill-defined muscle hyperintensities suggestive of inflammatory myositis. Nerve conduction studies were normal, but electromyography showed fibrillations, positive sharp waves, and complex repetitive discharges in the distal leg muscles. Skin and muscle biopsy showed features of small–medium vessel vasculitis (figure ). The patient remains in remission with oral steroids after 2 months of follow-up. Detailed cases of two further patients are presented in the appendix. None of these patients had a pre-vaccination history of COVID-19, rheumatic disease, or comorbidities such as diabetes or hypertension.
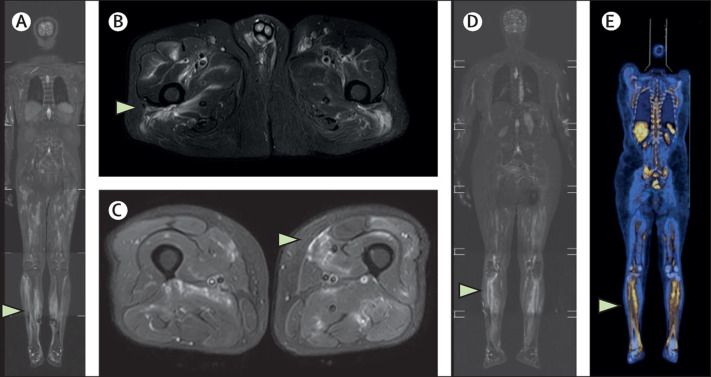
Figure.
Whole-body STIR-MRI and FDG-PET CT images
(A) Patient 1. Whole-body coronal STIR-MRI image showing lower limb predominant muscle hyperintensities (white arrows). (B, C) Patient 1. Axial STIR MRI images through the pelvis and thighs showing scattered muscle hyperintensities (white arrows). (D) Patient 2. Whole-body coronal STIR-MRI image showing muscle hyperintensities in the calf muscles bilaterally (white arrows). (E) Patient 2. Whole-body coronal PET CT images showing FDG avidity in the leg muscles bilaterally (white arrows). STIR=short tau inversion recovery.
The patients presented here developed post-vaccination inflammatory myositis within 2 days after the first dose (two patients) or second dose (one patient) of ChAdOx1 nCoV-19 vaccination. The median time to hospital admission was 33 days (range 2–65), as most patients expected that they would improve spontaneously and delayed seeking medical care. All our patients were older (>70 years) and had fever with elevated inflammatory markers. In the two cases in which PET-CT was done, the images showed features of myositis in one patient and possible vasculitis in both cases.
All three patients showed features consistent with inflammatory myositis (muscle hyperintensities) on whole-body STIR-MRI.1 One patient underwent a biopsy, which showed features of myositis and vasculitis. All patients were receiving treatment (with oral steroids with or without mycophenolate mofetil) at the time of writing.
COVID-19 produces complications, including hyperinflammation, thrombocytopathy, and endotheliopathy, leading to thrombo-inflammation. These factors can culminate in COVID-19 vasculitis or immune-mediated syndromes. Immune system alterations in patients with COVID-19 can promote a CD4-Th2 response against the SARS-CoV-2 virus instead of a CD4-Th1 response, which leads to type 3 hypersensitivity with vascular immune complex deposition, complement activation, and generalised immune cell recruitment.4 COVID-19 vaccination is associated with a much lower frequency of serious systemic immune-mediated adverse events than is COVID-19 itself, but such events can include thrombosis with thrombocytopenia syndrome, pernio or chilblains (so-called COVID toes), autoimmune hepatitis, Guillain-Barré syndrome, or transient post-COVID-19 vaccination vasculitis.1, 2, 5
Because of the high amount of antigenic similarity between the SARS-CoV-2 spike protein and human proteins, anti-SARS-Cov2 antibodies can potentially bind to human antigens, such as extractable nuclear antigens, nuclear antigen, and myelin basic proteins.2 In fact, new findings suggest that critically ill patients with COVID-19 might develop a post-infectious immune-mediated myopathy, the mechanisms of which are still unclear.7 Some patients display a type I interferon signature and a perifascicular expression of major histocompatibility complex antigens similar to dermatomyositis.
In most vaccine recipients, vaccine antigens are recognised by the immune system, and local immune cells are stimulated, followed by the recruitment of circulating immune cells to the local site. These cells produce different vasodilators and cytokines that trigger only local inflammation. Thus, adequate vaccine reactogenicity induces protective responses without substantial systemic effects. Injection site inflammation and transient axillary lymphadenopathy are classic examples of vaccine-initiated local, self-limiting immune responses. When these vasodilators and cytokines enter the bloodstream, they induce a short-lived systemic inflammatory response syndrome. A hyper-reactive or prolonged reactogenicity against host antigens can lead to more severe adverse events such as myositis, vasculitis, thrombosis with thrombocytopenia syndrome, or Guillain-Barré syndrome. Post-vaccination inflammatory myositis could possibly develop secondary to the same mechanisms that cause COVID-19-related immune myopathy.
Our patients developed post-vaccination myositis with compatible clinical features, inflammatory biomarkers, and imaging findings. As of June 22, 2021, in the Ernakulam district of Kerala, India, around 1·4 million doses of SARS-CoV-2 vaccines had been administered. Of these, more than 90% of individuals received the ChAdOx1 nCoV-19 vaccine (1·26 million people). Thus, the calculated crude incidence rate of post vaccination myositis was three cases per 1·26 million (<2·3 cases per million). These individuals required prolonged treatment for weeks to months.
By July 14, 2021, vaccinated individuals (380 [29%] million) outnumbered the number of COVID-19 cases in India (30 million, around 2% of the total population). As the target is to vaccinate at least 70% of the Indian population (950 million people), approximately 2261 cases of post-vaccination myositis could be anticipated by this endpoint. Prolonged fever, myalgia, or polyarthralgia after ChAdOx1 nCoV-19 vaccination should arouse suspicion of this complication.
We declare no competing interests. The patients gave written informed consent for the publication of this report and the use of the accompanying images.
Supplementary Material
References
- 1.Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384:2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maramattom BV, Krishnan P, Paul R, et al. Guillain-Barré syndrome following ChAdOx1-S/nCoV-19 vaccine. Ann Neurol. 2021;90:312–314. doi: 10.1002/ana.26143. [DOI] [PubMed] [Google Scholar]
- 4.Roncati L, Nasillo V, Lusenti B, Riva G. Signals of Th2 immune response from COVID-19 patients requiring intensive care. Ann Hematol. 2020;99:1419–1420. doi: 10.1007/s00277-020-04066-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guzmán-Pérez L, Puerta-Peña M, Falkenhain-López D, et al. Small vessel vasculitis following Oxford-AstraZeneca vaccination against SARS-CoV-2. J Eur Acad Dematol Venereol. 2021 doi: 10.1111/jdv.17547. published online July 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aschman T, Schneider J, Greuel S, et al. Association between SARS-CoV-2 infection and immune-mediated myopathy in patients who have died. JAMA Neurol. 2021;78:948–960. doi: 10.1001/jamaneurol.2021.2004. [DOI] [PubMed] [Google Scholar]
Uncited References
- 3.Adams EM, Chow CK, Premkumar A, Plotz PH. The idiopathic inflammatory myopathies: spectrum of MR imaging findings. Radiographics. 1995;15:563–574. doi: 10.1148/radiographics.15.3.7624563. [DOI] [PubMed] [Google Scholar]
- 6.Vojdani A, Kharrazian D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin Immunol. 2020;217 doi: 10.1016/j.clim.2020.108480. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.