Abstract
Owing to the growing elderly population, age-related problems are gaining increasing attention from the scientific community. With senescence, the intestine undergoes a spectrum of changes and infirmities that are likely the causes of overall aging. Therefore, identification of the aged intestine and the search for novel strategies to rescue it, are required. Although progress has been made in research on some components of the aged intestine, such as intestinal stem cells, the comprehensive understanding of intestinal aging is still limited, and this restricts the in-depth search for efficient strategies. In this concise review, we discuss several aspects of intestinal aging. More emphasis is placed on the appraisal of current and potential strategies to alleviate intestinal aging, as well as future targets to rejuvenate the aged intestine.
Keywords: aging, intestine, intestinal microbiota, bile acids, short-chain fatty acids
Because of the growing elderly population, age-related healthcare issues are gaining increasing interest from the scientific community. Recent advances highlight efficient strategies, such as diet restrictions[1], rapamycin[2], and nicotinamide adenine dinucleotide (NAD) replacements [3] to prevent and ameliorate the overall dysregulation in various aging processes.
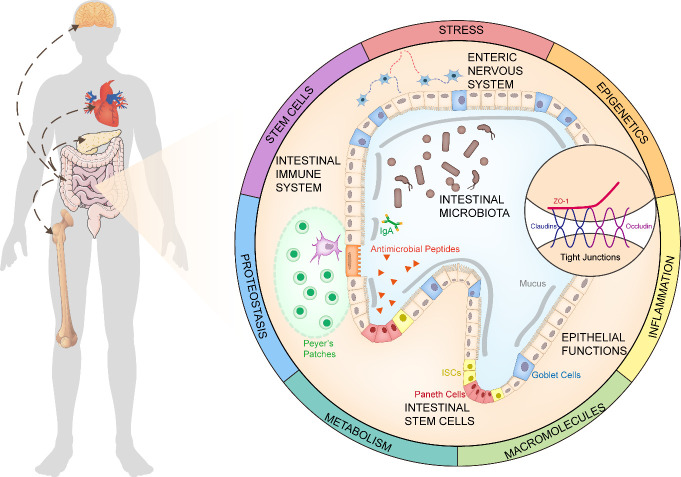
The intestine is considered a pervasive and important player involved in an array of biological events, including digestion, absorption, and immune modulation. Its wide-ranging function determines its profound impact on overall health [4,5]. Similar to other body organ systems, the intestine also undergoes senescence. Increasing age enhances intestinal disease incidence, such as malnutrition [6], chronic constipation [7], and colorectal cancer [8]. Central to understanding the underlying mechanisms, is to clarify age-related changes in commensal microbiota, the immune system, intestinal stem cell (ISC), the epithelial function, and the enteric nervous system (ENS). These changes not only account for localized gastroenterology disorders, but are associated with a decline in multiple systems throughout the body, including the nervous [9, 10], cardiovascular [11], endocrine [12-14], and skeletal [15] systems (Fig.1). Therefore, it is important to explore strategies to rescue the aged intestine. Over the last few decades, a vast number of studies have shown the capacity to delay intestinal aging through a variety of mechanisms. However, it is still far from our aspiration of strategies with high and all-round effects against intestinal aging, as well as security.
Figure 1.
The Age-related Changes in the Intestine. During senescence, the intestine gains changes in terms of the intestinal microbiota, immune system, intestinal stem cells, epithelial functions, and the enteric nervous system. These changes in the aged intestine are responsible for many overall age-related diseases, such as the brain, heart, bone, and endocrine system. The Geroscience perspective that makes it more comprehensive to understand anti-aging mechanisms, would enlighten us on the development of strategies to rejuvenate the aged intestine.
The advancement of metabolomics, in conjunction with transcriptomics and proteomics, expands our knowledge on the aged intestine. Results from a metabolomic analysis serve as a reservoir from which to identify novel targets against intestinal aging. On the contrary, an unprecedented number of intrinsic and extrinsic compounds pose an enormous challenge for accurate verification.
In this review, we describe changes occurring in the aged intestine, including dysbiosis, immune imbalance, stem cell exhaustion, barrier dysfunction, and enteric neurodegeneration. Additionally, particular focus is placed on strategies and potential molecular targets that contribute to the alleviation of intestinal aging.
1. Age-related Changes in the Intestine
Intestinal function has a profound impact on aging. In terms of longevity, for example, the intestine of Caenorhabditis elegans is the key longevity signaling center that is signaled by the brain and further propagates longevity signals to other tissues in the body[4]. As for progeroid mice, their lifespan can be prolonged through the transplantation of fecal microbiota in wild-type mice [5]; therefore, indicating that commensal microbiota inside the intestine can serve as a target against aging. Moreover, several studies have confirmed a strong association between the gastrointestinal tract and age-related complications, such as Alzheimer’s disease [9], cognitive decline [10], obesity and insulin resistance [12-14], cardiovascular disorder [11], arthritis [15], and overall frailty[16], in other organs and systems.
Besides disturbing overall health, the aged intestine also suffers from morphological and functional changes over time. These relationships have been well established between aging and the intestinal age-related changes, including alterations in the microbiota, immune system, ISCs, epithelial functions, and ENS. Further studies show interactions among these changes. Thus, fully understanding the complex crosstalk between intestinal age-related changes and aging, is essential to develop intestinal aging interventions.
1.1 Morphological and Functional Changes in the Aged Intestine
The intestine is an important digestive apparatus in digestion and absorption across species. Its morphology and function vary with age, which has been supported by studies in several models and humans.
Combining transmission electron microscopy with confocal microscopy with 3-D volumetric reconstructions, McGee et al. illustrated loss of intestinal nuclei and microvilli of aging C. elegans, in addition to increased variability of the shape and size of the intestinal lumen that age-related germline swelling was partly blamed for [17]. An abnormal intestinal structure also occurs in aging mammals. Morphological changes in aged mouse and rat models have been described as thicker muscular layers, distorted villi, more secretory Paneth and goblet cells, and impaired junctions between adjacent enterocytes [18-24]. Mice have wider and higher villi with increasing age [22], while rats have wider and shorter ones [23]. Furthermore, rats have darkly stained nuclei in their aged intestines [23]. Human studies showed no significant changes in the duodenum [25]. However, abnormal hyperproliferation and apoptosis were found in enterocytes of a normal elderly group, which resulted in impaired function of the aged intestine [26, 27].
In rodents, the intestinal functions are also affected by aging, including degenerative digestion and absorption [28], as a result of the reduced activity [29] or lower production [30] of related enzymes. In aged mice, impaired adaptive mechanisms to diet were also found [31]. However, researchers have rarely reported changes in intestinal secretions and absorption in elderly people [32]. Nevertheless, multiple studies have confirmed humans share similar age-related changes with rodents, ranging from reduced absorption [33] to lower digestive secretions[34], and declined motility [35]. Although more evidence is needed to clarify the conflicting results, the idea is still acceptable that the elderly suffer from an elevated incidence of gastrointestinal disorders such as associated cancer [8] and infections [36] (comprehensively reviewed by Dumic et al.[37]).
1.2 The Intestinal Microbiota
Bacteria, fungi, protozoa, and viruses are located in the intestine at high quantities. They play critical roles in human physiology and disease, due to their abilities to limit pathogenic growth, ferment food, as well as produce mucus and lipid metabolites[38]. As reported, gut microbes vary with age, not only in terms of composition imbalances [39], such as fewer Bacteroidetes and more Firmicutes [40], but also in terms of degraded intrinsic functions, such as evolution and mutations [41]. A study containing four age groups, covering almost the entire adult lifespan, showed the sustained reduction of particular microbiota with age [42]. Intriguingly, among the healthy aging people, aged 70-82 years, and an elderly cohort with diabetes or other age-associated disorders, no gut microbiome changes were observed, except the proportion of the genus Akkermansia [43]. Together, both indicated that the altered microbiome may account for senescence itself rather than age-related infirmities, with bacterial taxa contributing to respective disorders.
Besides expanding lifespan [5], the imbalances of intestinal microbiota can induce or reduce aging[39], and age-related illnesses [44,45]. Current studies confirmed that only in combination with dysbiosis, can the diminished intestinal barrier lead to systemic inflammation [46] This is a key hallmark and driver of senescence [47], supporting the indispensable importance of gut flora in aging. An intestinal microbiota that was reported to change with age, Akkermansia muciniphila, has been linked to colitis-associated tumorigenesis [48] and cancer therapy [49], indicating the relationship between age-associated changes in intestinal microbiota and the elevated incidence of cancer.
However, the general mechanisms by which microbiota affects the host are still unclear. It is widely accepted that the consequent changes in microbiota-derived metabolites, especially small-molecule metabolites, could be the main contribution of microbiota to host biology [50], which lays a preliminary theoretical foundation for the investigation of bacterially derived metabolites against intestinal aging.
1.3 The Intestinal Immune System
“Immunosenescence” refers to immune changes related to poor clinical outcomes in the elderly compared to that in young individuals, such as inflammageing [51,52]. Interleukin (IL)-10-producing T follicular helper cells [53] and the imbalance of immunological mediators [52] are involved in this systemic immune degeneration.
The intestinal immune system is the largest immune compartment, consisting of gut-associated lymphoid tissues (GALT) as well as effector cells. Its mucosal immune responses are important for defense against pathogens, including antigen uptake by M cells, presentation in Peyer’s patches, differentiation and migration of B immunoblasts, and production and transport of antibodies [54]. Over the years, the intestine experiences immune degeneration and aggravates systemic aging. Aging impairs the migration of IgA immunoblasts [54] to the intestinal lamina propria and lowers antibody titers [55], resulting in a diminished mucosal immune response [56]. Consequently, it is more common for the elderly to suffer from bacterial or viral gastrointestinal infections [36]. In addition, aging reshapes the gut microbiota, making it a modulator of age-related changes in the immune system. For example, the age-related decline in Firmicutes and an increase in Enterobacteriaceae exacerbate inflammageing [57]. The additional consumption of tryptophan by aged gut microbiota is speculated to enhance inflammation in centenarians [58].
1.4 Intestinal Stem Cells
For maintaining tissue homeostasis, ISCs are highly active in supporting the repair of damaged tissues, and the continuous and rapid cell turnover of intestine [59, 60]. As long as rapid replication occurs, ISCs are exposed to age-related risks. The features of aged ISCs are identified as altered numbers and declined functions. No conflicts have been reported in the weakening regenerative capacity [24, 61], but the quantitative issue is unclear. In old Drosophila, the intestinal epithelium exhibits an increased number of proliferating cells [62]. However, there are competing data in aged mice. No change in the established marker Lgr5 ISCs has been reported [24], while others had an increased population of cells expressing sub-low SOX9 [61], a marker of progenitor cells in old mice [63]. At the mercy of biomarkers selected for investigation, different studies exhibit competing changes in the quantity variance of ISCs upon aging. Some researchers have described it as a constant absolute number of ISCs with alternative expression of markers [24]. However, the overall understanding of the effect of aging on ISCs is awaiting more data, especially human studies. On the contrary, the loss of regulation of ISC proliferation for self-renewal results in disrupted organ homeostasis and impaired self-repair function after damage, even shortening lifespan[64].
Both cell-intrinsic and extrinsic factors contribute to ISC aging. Telomere dysfunction induced by ISC replication [65] triggers DNA damage response (DDR) pathways [66] and mitochondrial dysfunction [67]. DDR further activates the innate immune response as a senescence-associated secretory phenotype (SASP) [68], which spreads senescence to neighboring ISCs [65, 66] in a paracrine manner [69]. Mitochondrial dysfunction, including impaired lipid metabolism [70, 71] and mitophagy [72] produces oxidative stress to accelerate aging. Accumulation of DNA mutations by age drives the dysregulation of ISC proliferation [73]. Abnormalities in cell signaling pathways, such as target of rapamycin (TOR) and Wnt also participate in aged ISCs [74] (Fig.2).
Figure 2.
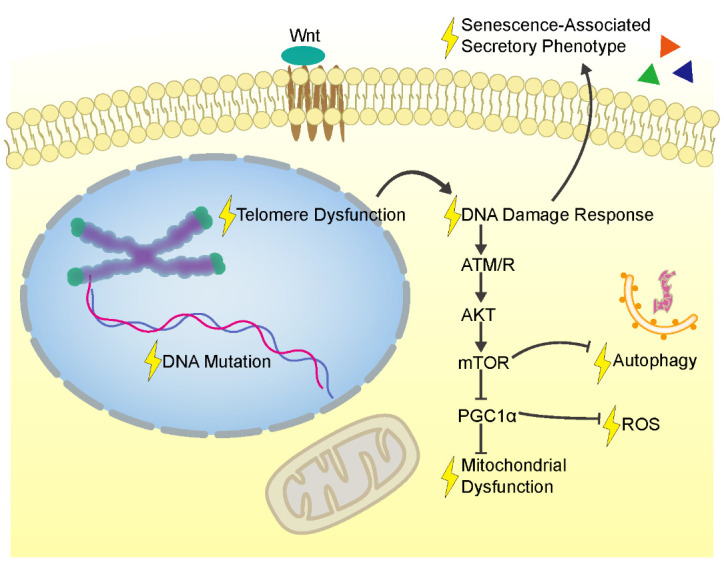
The Factor Network Contributing to the Intestinal Stem Cell Aging. An intracellular and extracellular network contributes to the senescence of ISCs, including telomere dysfunction, DNA damage response, DNA mutation, mitochondrial dysfunction, oxidation stress, autophagy dysregulation, and senescence-associated secretory phenotype. Several cellular signaling pathways are also involved, such as the Wnt and mTOR pathways. ATM/R, protein kinases ATR and ATM; AKT, protein kinase B; mTOR, mammalian target of rapamycin; ROS, reactive oxygen species; PGC1α, peroxisome proliferator activated receptor-γ co-activator 1-α.
Intestinal cancer is strongly associated with age and originates mainly from ISCs [75]. Although more work is required to elucidate the importance of age-related risks in the carcinomatous transformation of ISCs, rejuvenation of ISCs, including remodeling related signaling pathways such as Wnt signaling, could be a promising strategy to revive the aging intestine, at least reducing age-related intestinal cancer.
1.5 Intestinal Epithelial Barrier Function
The integrity of the intestinal epithelial barrier function requires a contiguous cell layer, an intracellular junctional complex of molecules [76], expression of mucus, defensin secretion from multiple cells with respective functions [77], including ISCs, immune cells, and goblet cells, which is pivotal to ensure that the intestine is a semipermeable membrane that exerts the admission of nutrients and the prevention of pathogens or toxins. Age-related intestinal barrier dysfunction in elderly organisms can be observed in species varying from rats [78] to baboons [79]. In two cohorts of healthy adults, the older group exhibited increasing levels of zonulin, an intestinal permeability biomarker [80].
The age-related increase in gut permeability accounts for chronic and systemic mild inflammatory responses [81] that accelerate aging in mammals [82]. This inflammation, as above, arises owing to dysbiosis [46]. In the context of dysbiosis, the degraded intestinal barrier permits the translocation of gut contents, such as bacteria and their products, into the circulatory system [46], which shortens the lifespan of C. elegans [76], and foreshadows the death of Drosophila [83].
1.6 Enteric Nervous System
Considered as the second brain, the ENS consists of more than 500 million neurons to form the myenteric and submucosal plexus [84]. Similar to the brain, ENS suffers from neurodegeneration during aging, which is a cause of constipation in the elderly [85]. The prevalence of constipation in patients with Parkinson’s disease [86] supports this idea. The accumulation of age witnesses a significant loss of enteric neurons [87], especially choline acetyltransferase positive ones [88]. Whether a decrease in the neuron density or the decreasing number played a more prior role [89], we could highlight the age-related changes of ENS. However, except for 5-hydroxytryptamine (5-HT) [90], more therapeutic approaches to protect ENS from aging are vague.
Collectively, age-related phenomena and mechanisms of the intestine rely on the combination of gut microbiota, immune system, ISCs, intestinal barriers, and ENS, but more efforts are needed to better understand these mechanisms. From the Geroscience perspective, aging research was conducted in seven areas: adaptation to stress, epigenetics, inflammation, macromolecular damage, metabolism, proteostasis, and stem cell exhaustion [91]. A comprehensive view about age-related intestinal changes also covers oxidative stress responses [92], genomic modifications [73], heterochromatin maintenance [93], lipid metabolism [70], mitophagy and autophagy[94], etc (Fig.1).
It should be noted that the degeneration phenomena differ among organisms. Taking intestinal architecture as an example, a quantitative histology performed on patient jejunal biopsy specimens showed no significant differences in surface to volume ratios and enterocyte height between elderly patients and the younger ones [95]. In the mouse intestine, aging causes a decreased number of crypts; however, an increase in the number of cells per crypt, in addition to an elevated villus height [24]. The same issue also arised, when the age-related trend of the ISC number across species was identified [24, 61, 62]. More data are required to delineate the complex changes in the human intestine during aging to select targeted aging models.
2. Strategies to Rejuvenate the Aged Intestine
The intestine is a key interface between the host and nutrient substances or microbiota. Several studies are emerging showing that appropriate means, such as diet control and pharmaceutical intervention, motivate people to fight against intestinal aging. Furthermore, the changes in intestinal and microbial metabolites caused by these means could be valuable to the interpretation of the underlying mechanisms. In this section, we discuss diet regimens and pharmaceutical interventions as well as metabolites derived by the host and microbiota to elucidate on the potential strategies to rescue the aged intestine.
2.1 Dietary Restriction Regimens
Dietary restriction regimens (DR) such as caloric restriction, ketogenic diet, and intermittent fasting, are strongly proven anti-aging interventions in a wide range of species [96]. Multiple mechanistic pathways are involved in its effects on expanding lifespan and alleviating age-related diseases, such as hindering oxidative damage, suppressing TOR, and the insulin/insulin-like growth factor 1 (IGF-1) pathway [97]. In the intestine, however, DR impairs the mucus in the small bowel and decreases the number of several cells in gut-associated lymphoid tissue, which was intensely reviewed by Genton [98] indicating the possible harmful effects of DR on the intestine. On the contrary, by rebalancing apoptosis with intestinal cell repair, DR enhances the intestinal barrier in Drosophila [99]. In mice, DR boosts ISC competition to drive-out fewer fit cells, with the mutation retention decreasing [100]. The effect of DR on ISCs brings hope for cancer prevention and aging postponement in the intestine. In addition, DR provides mice with alternative microbiota as well as altered fecal metabolites [101]. In short, DR plays differential roles in various aspects of intestinal aging and requires further assessment.
2.2 Resveratrol
Resveratrol (RSV), a natural non-flavonoid polyphenolic compound, is widely found in food such as wine and mulberries [102]. Since the report about RSV extending the lifespan of Saccharomyces cerevisiae as a remarkable stimulus of Sirtuin1 (SIRT1) [103], numerous studies on the anti-aging benefits of RSV have emerged over the last decade. Through adenosine 5’-monophosphate-activated protein kinase (AMPK), Sirtuins, and AKT, RSV contributes to anti-oxidant, anti-inflammation [104], anti-infection [105], calorie restriction mimetic, telomere maintenance [104], mitochondrial fission [106], and endoplasmic reticulum stress (ER stress) [107], thus preventing aging in multiple body systems [104]. At the same time, AMPK [108] and SIRT1 [109] signaling pathways are the underlying mechanisms of RSV mitigating adult stem cell aging, in addition to activating nuclear factor erythroid-2-related factor 2 (Nrf2) [110]. The scientific community pays more attention to the anti-inflammatory effects in the intestine. Dozens of drug-induced colitis studies in animals have pushed forward research on RSV and human inflammatory bowel disease [102]. RSV also confers intestinal permeability benefits by increasing tight junction protein expression. In mice fed with a high fatty diet, resveratrol co-administration was found to improve dysbiosis and the leaky gut by impairing the loss of tight junction protein, and then ameliorate systemic inflammation and endotoxemia [111]. Furthermore, the current study in the highly fatty-diet rats showed that it was the gut endocannabinoid system that mediated the maintenance of intestinal barrier function by RSV[112]. Furthermore, knockdown of Nrf2, as well as inhibition of PI3K/AKT, abolished the RSV-induced increase of tight junction protein expression against oxidative stress [113], indicating that more mechanisms remain to be explored. By demonstrating that RSV rehabilitates the debris of villus structures and goblet cells by heat-stress responses [114], a study on black-boned chickens highlighted the potential benefits of RSV on the morphological changes in the aging intestine.
2.3 Metformin
Metformin, the prescribed oral antidiabetic therapy, delays aging in C. elegans [115] and mice [116], with beneficial effects on diabetes, cognitive function, and cancer in humans, and is involved in the complex of IGF-1, mTOR, AMPK, regulation of reactive oxygen species (ROS) production, and DNA damage [117], and age-related cellular processes such as mitochondrial function, ER stress [107], inflammation, autophagy, and cellular senescence [117]. As for intestinal aging, metformin is considered sufficient to mitigate restoration-related deterioration in a variety of ways (Fig.3). Metformin remodels the metabolism of intestinal bacteria to retard aging, that is accounted for by altered microbial folate metabolism [115] and the increased yield of beneficial microbial productions by metformin [118]. Administration of metformin activates AMPK and inhibits P53, leading to less colonic pathological inflammation [119]. Moreover, improvement of superoxide leakage by increasing the expression of related mitochondrial genes is another efficient way for metformin to inhibit chronic inflammation [116]. A series of studies in the Drosophila midgut, revealed that metformin inhibits ISC aging, described as hyperproliferation, by improving DNA damage and genomic instability [120], further being accounted for by AKT/TOR signaling modulation [121] and Atg6-dependent autophagy [122]. In the mouse intestine, metformin treatment recovers the tight junction protein expression abated by a high liquid controlled diet [123], as well as lipopolysaccharide (LPS) [124], where in part an AMPK/JNK-dependent signaling pathway participates [125]. By modulating the differentiation of ISCs in older mice by suppressing Wnt signaling, metformin raises the number of goblet cells, in which metformin further increases Muc2 [118]. The combined action on tight junctions, ISCs, and goblet cells endows metformin with the ability to reinforce the intestinal barrier. The restoration of autophagy and NAD levels in senescent cells [126] contributes to a more comprehensive understanding of metformin in aging.
Figure 3.
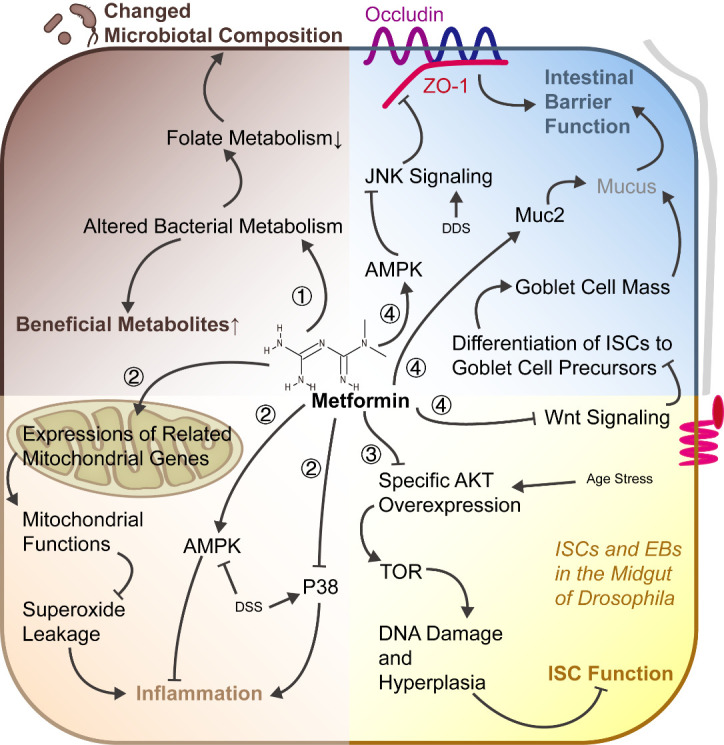
An integral view on the anti-aging effect of metformin in the intestine. Four main changes take place in the aging intestine, including the intestinal microbiota, immune system, ISCs, and epithelial functions. Metformin exerts its integral effects to mitigate age-related changes in the intestine. ?Metformin alters bacterial metabolism to improve the production of beneficial metabolites, as well as to interfere with folate metabolism, which leads to a changed microbial composition. ?The anti-inflammatory effect of metformin is mediated by the regulation of mitochondrial gene expression, activation of AMPK, and inhibition of P38. ?Through attenuating the age-related specific AKT overexpression, metformin relieves DNA damage and ISC hyperplasia in the midgut of Drosophila, favoring homeostasis of ISCs. ?Tight junctions and mucus produced by goblet cells are both important components in the maintenance of the intestinal barrier. The inhibition of JNK signaling and Wnt signaling by metformin contributes to the expression of tight junction proteins and the differentiation of ISCs to goblet cells, which, accompanied by a metformin-induced increase in Muc2 expression, reinforces the intestinal barrier. AMPK, adenosine 5’-monophosphate-activated protein kinase; DSS, dextran sulfate sodium; AKT (PKB), protein kinase B; TOR, target of rapamycin; ISC, intestinal stem cell; EB, enteroblast; JNK, c-Jun N-terminal kinase.
2.4 Bile Acids
Bile acids (BAs), small steroid molecules synthesized in the liver and modified by intestinal microbiota, are of various kinds. What distinguishes between BA types in terms of molecular structure lies in the existence, position, and conformation of the hydroxyl group, in addition to the binding of taurine or glycine, that are responsible for diverse extensions of solubility, metabolism processes, and physiological functions [127]. A recent study reported extraordinary BAs conjugated to phenylalanine and leucine, denoting a continually rising number of BA types, as more data emerge [128]. Despite individual differences in the composition of BAs in feces, unconjugated BAs account for the major components, such as deoxycholic acid (DCA) and lithocholic acid (LCA) [129]. Through activation or resistance, especially the farnesoid X receptor (FXR), transmembrane G protein-coupled receptor 5 (TGR5 or GPBAR1), pregnane X receptor (PXR), and vitamin D receptor (VDR), BAs take part in various physiological processes such as synthesis modulation of their own, lipid absorption, metabolism, and the immune system [127].
An analysis of metabolites in a Chinese cohort exhibited a higher level of total BAs in the feces of centenarians [130]. Changes take place in the reabsorption of BAs with advanced aging, rather than in biliary secretion [131]. Age-related alterations of the BA profile have been reported in rats [132] and mice [133] (Table 1), albeit in the absence of features of the elderly ones. Moreover, metabolomic analysis showed altered BA profiles accompanied by age-related dysbiosis in LmnaG609G/G609G and Zmpste24-/- mice, two typical progeroid animal models [5, 134], among which potentially anti-aging BAs would be discussed as follows.
Table 1.
Age-related Changes of Bile Acids.
| Total BA | Unconjugated BAs | Conjugated BAs | Ref | |||
|---|---|---|---|---|---|---|
| Rat Bile | INCREASED | DECREASED: αMCA, βMCA, ωMCA, CA, CDCA, DCA, UDCA |
INCREASED: T-CA, T-MCA DECREASED: G-DCA, G-LCA UNCHANGED: G-UDCA, G-CDCA, T-UDCA, T-CDCA, T-DCA, T-LCA |
[132] | ||
| Male Mouse Serum | UNCHANGED | Concentration: INCREASED: UDCA INCREASED and DECREASED therefore: βMCA, CA, HDCA UNCHANGED: CDCA, DCA |
Proportion: INCREASED: βMCA, HDCA, UDCA DECREASED: CDCA, DCA |
Concentration: UNCHANGED: T-αMCA, T-βMCA, T-CA, T-HDCA, T-UDCA |
Proportion: INCREASED: T-βMCA, T-MDCA DECREASED: T-DCA, T-HDCA |
[133] |
| Female Mouse Serum | INCREASED | Concentration: INCREASED: βMCA, CDCA, DCA UDCA UNCHANGED: CA, HDCA |
Proportion: INCREASED: βMCA DECREASED: DCA, HDCA |
Concentration: INCREASED: T-βMCA, T-ωMCA, T-UDCA UNCHANGED: T-αMCA, T-CA, T-DCA, T-HDCA, T-MDCA |
Proportion: INCREASED: T-βMCA, T-UDCA DECREASED: T-DCA, T-MDCA |
|
| Male Mouse Liver | UNCHANGED | Concentration: INCREASED and DECREASED thereafter: βMCA UNCHANGED: αMCA, CDCA, DCA, HDCA, LCA, UDCA |
Proportion: INCREASED: βMCA DECREASED: ωMCA, CA, DCA, HDCA, MDCA, LCA, UDCA |
Concentration: INCREASED: T-αMCA, T-βMCA DECREASED: T-DCA UNCHANGED: T-ωMCA, T-CA, T-CDCA, T-HDCA, T-MDCA, T-LCA, T-UDCA |
Proportion: INCREASED: T-αMCA, T-βMCA DECREASED: T-ωMCA, T-DCA, T-LCA |
|
| Female Mouse Liver | UNCHANGED | Concentration: INCREASED: CA INCREASED and DECREASED thereafter: ωMCA UNCHANGED: αMCA, CDCA, DCA, HDCA, LCA, UDCA |
Proportion: DECREASED: αMCA, DCA, HDCA, LCA, MCA, MDCA |
Concentration: INCREASED and DECREASED thereafter: T-αMCA, T-βMCA, T-CDCA, T-LCA, T-MDCA, T-UDCA UNCHANGED: T-ωMCA, T-CA, T-DCA, T-HDCA |
Proportion: INCREASED: T-αMCA, T-βMCA DECREASED: T-DCA, T-HDCA, T-LCA, T-MDCA |
BA, bile acid; MCA, Muricholic acid; MDCA, Murideoxycholic acid; CA, cholic acid; CDCA, Chenodeoxycholic acid; DCA, Deoxycholic acid; HDCA, Hyodeoxycholic acid; LCA, lithocholic acid; UDCA, Ursodeoxycholic acid; T-, Taurourso-; G-, Glyco-.
The altered BA profiles play double-edged roles during aging. On the one hand, BAs exert beneficial effects in attenuating metabolic disorders [135], cardiovascular disease [136, 137], impairment of the nervous system function [138, 139], and deterioration of cartilage [140] and bone [141] common in the elderly. Functions of the BA receptor are also involved in age-related mechanisms or signaling pathways, such as AMPK [141, 143], Nrf2 [144], and autophagy [145]. On the other hand, cytotoxicity [146] and tumor promotion [147, 148] of BAs call our attention to prudent assessment of the situation of BAs in aging. In view of different natures, alongside different age-related trends of BAs, it is imperative to identify the anti-aging effects of a special kind of BA separately.
In animal experiments, cholic acid (CA), whose activation ability to related receptors is weaker than that of others [127], is frequently administered. A diet enriched with CA extends lifespan and alleviates weight loss associated with intestinal aging in progeroid mice [134]. However, the mechanism behind this effect remains to be explored. DCA and LCA show biological toxicity due to their strong hydrophobicity. Such toxicity, on the contrary, means inhibition of both the infection [149] and growth of a tumor [148, 150], which disturbs the elderly. Previous studies have revealed the anti-aging effect of LCA in yeast and worms mediated by a mitochondria-centered mechanism, including remodeling lipid and carbohydrate metabolism, and attenuating mitochondrial network fragmentation [151, 152]. In addition, LCA has a unique effect of activation of VDR to augment tight junction proteins, preventing and ameliorating intestinal epithelial barrier injury [153]. The current study showed that incubation with bile extract or LCA promoted mouse intestinal organoid growth via activation of TGR5 in ISCs. Furthermore, elevating endogenous BAs by intraperitoneal injection of cholecystokinin contributes to intestinal cell renewal in vivo [154]. These data depict the possibility of the anti-aging effect of DCA or LCA in the mammalian or human intestine. In the liver, interestingly, neither DCA [155] nor LCA [156, 157] delayed aging, and did not promote aging, indicating that organ specificity is essential to the anti-aging effect of DCA and LCA. Ursodeoxycholic acid (UDCA) and tauroursodeoxycholic acid (TUDCA), used as a remedy for cholestasis, has attracted attention for its cytoprotective effect against ER stress [158], especially in the nervous system [138]. UDCA and TUDCA alleviate age-related changes and diseases such as Alzheimer’s disease [159], osteoarthritis [160], and cancer [161]. Notwithstanding the increase in progeroid mice [5], UDCA and TUDCA can also be regarded as anti-aging molecules worthy of further work.
Because of the risk of toxicity that certain BAs have, multiple trace BAs have captured the attention of scientists, proposed to be more effective and harmless. For example, 12-keto-chenodeoxycholic acid decreased in progeroid mice and recovered after fecal microbiota transplantation to prolong life [5]. However, vast amounts of BAs can be used to estimate anti-aging effects.
2.5 Short-Chain Fatty Acids
As key metabolites in the intestinal lumina, short-chain fatty acids (SCFAs) are fermented from resistant starch, dietary fiber, and other complex carbohydrates by a system of multiple microbes. The major SCFAs in the body, acetate, propionate, and butyrate, are mainly involved in physiological functions as follows: 1) energy metabolism, such as butyrate and propionate consumed in the intestine and liver, respectively; 2) histone deacetylases (HDAC) inhibitors; and 3) G protein-coupled receptor (GPCR) agonists, such as GPR43, GPR41, and GPR109A [162, 163]. SCFAs are implicated in a variety of neuropsychiatric disorders [164, 169], metabolic [170] and cardiovascular diseases [171], cancer [172, 173], and bone loss [174]. The fecal contents of total SCFA, consistent with acetate, propionate, and butyrate, are higher in centenarians than in those aged 80-90 years at the same area [130]. However, age-related decreases in serum acetate have been observed in Parkinson’s disease patients [175]. No age-related changes were shown by SCFA analysis of the Balb/c mouse cecal contents [176]. In progeroid mice, butyrate declines markedly [5, 134]. Further analysis to clarify the age-related tendency of SCFAs to set forth their anti-aging effects is required. It encourages studies on the anti-aging effect of SCFAs in a high-fiber diet, an efficient way to promote SCFAs, suppressed the central and peripheral inflammation caused by LPS common in the elderly [176].
The butyrate paradox that colorectal cancer is inhibited by butyrate but normal intestinal cells survive [177] is crucial to understanding the function of SCFAs in the intestinal tract. Butyrate is an energy source for colonocytes [162]. Meanwhile, SCFAs play a role as maintainers of intestinal homeostasis via regulation of autophagy [178], cell proliferation, and inflammation. For example, administration of a high-fiber diet reduces age-related colonic inflammation in mice fed with a low-fiber diet [176], which resulted partly from upregulated anti-inflammation factors such as age-related IL-10 [179] [180], as well as downregulated pro-inflammatory factors such as indoleamine 2,3-dioxygenase-1 (IDO-1) expression [181]. On the contrary, SCFAs facilitate immunological defense against pathogens by inducing antimicrobial peptide (AMP) production [182] and repairing intestinal tissue damaged by parasitic infection [183]. In addition, the age-induced breakage of intestinal permeability in mice is aggravated by the intake of SCFAs [176], resembling what was confirmed in the stressed mice because in part SCFAs contributed to rescuing the function of tight junctions diminished by stress [169]. In addition, increasing goblet cells [183] and bolstering Claudin-1, a tight junction protein [184], are involved in the protective effects of butyrate on the intestinal epithelial barrier.
The confusion comes up with deepening research on SCFAs in various organisms, besides the butyrate paradox in cancer. In contrast to a higher levels of fecal SCFAs in women with metabolic syndrome [185], active SCFA-producing bacteria are linked to lower hemoglobin A1c levels [170]. Meanwhile, SCFAs promoted exercise damage in Parkinson’s disease mice [166], however, improved clinical features in another drug-induced model mice [165]. The study also indicated a promoting nervous inflammation after treatment with SCFAs [166], in marked contrast to what is discussed above. Overall, it is noteworthy that the anti-aging effect of SCFAs varies in different species and animal models of a given species. Isobutyric acid, valeric acid, isovaleric acid, elevating in centenarians [130], also requires further study.
2.6 Tryptophan and Indoles
Tryptophan (Trp) is an essential aromatic amino acid. Dietary unabsorbed Trp follows three metabolic pathways in the intestine to kynurenine (Kyn), 5-HT, and indole derivatives [186]. Trp is prone to exert protection against aging as a kind of NAD precursor [187] although no evidence demonstrates the influence of it, to date. In the Kyn pathway, the key enzyme is IDO-1, which is thought to destroy the intestinal barrier by pro-inflammation [188, 181]. 5-HT basically acts on the ENS [186].
Produced by different bacterial strains with respective tryptophan enzymes, various indole derivatives participate in wide-ranged biological activities partly mediated by aryl hydrocarbon receptor (AhR) and PXR [186], further benefiting atherosclerosis [189], hypertension [190], fatty liver [191-194], tumor [195], and other age-related dysfunction. Indole extends the health span of C. elegans and Drosophila [196]. Moreover, dietary indole-3-carboxaldehyde (IAld) increased the survival rate of mice after total-body irradiation [196], validating the conservative protection effects of indoles. In the intestinal organoid system, IAld improves the proliferation of ISCs after damage through AhR [197]. Transcriptome analysis revealed that indole contributes to an increased expression of tight junctions in HCT-8 cells [198]. Emerging data showed indole acrylic acid (IA) and indole propionic acid (IPA) protect the intestinal epithelial barrier by enhancement of goblet cell function or moderation of inflammatory responses partly via PXR [50, 199-201]. Interestingly, both IA and IPA lie downstream of indole lactic acid (ILA), which Bifidobacterium species, a long-recognized probiotic genera [202], metabolizes tryptophan in vitro to produce only [203], denoting more benefits of those indoles that remain to be investigated.
2.7 Nicotinamide Adenine Dinucleotide and its Precursors
NAD is a vital coenzyme in all cells. As part of electron transfer, NAD participates in a vast body of internal reactions, particularly energy metabolism and sensing [187]. Three pathways guide five current, known precursors, Trp, nicotinic acid (NA), nicotinamide (NAM), nicotinamide riboside (NR), and nicotinamide mononucleotide (NMN) to NAD in cells [187]. Its nature as a substrate of poly-ADP-ribose-polymerases (PARPs) and sirtuins endows NAD with a target for aging anomalies [204]. In turn, aging witnesses a gradual depletion of cellular NAD in multiple tissues [205]. In goblet cells, in vitro, NAD treatment increases MUC2 expression, a major component of mucus [206]. A recent study showed that the provision of NR in drinking water reverses the age-related changes in the mouse intestine, such as the number of ISCs, formation of in vitro intestinal organoids, and recuperation from drug damage. The recovery of exacerbated ISCs is abrogated by the inhibitor of mTORC1 or SIRT1 [207]. This study sheds light on the benefits of the NAD/SIRT1/mTORC1 axis in the rejuvenation of the aging intestine. NAD and its replacement therapies deserve further investigation by well-designed clinical trials to validate the anti-aging value. Owing to the fact that not all of these precursors share consistent efficiency for conversion[208], the best oral NAD supplement strategy waits for our test. Besides, both inhibition of NAD consumption enzymes, such as PARP and CD38, and reinforcement of the key enzyme of NAD salvage pathways, NAM phosphoribosyltransferase (NAMPT), are theoretical options for improving NAD. Anti-CD38 antibodies are approved for use in multiple myeloma[209]. Moreover, intraperitoneal injection starting at the age of 26 months of NAMPT-containing extracellular vesicles purified from young mice is reported to restore movement activity and extend lifespan in elderly mice [210].
2.8 Urolithin A
Among the five products from the gut microbial fermentative activity of ellagitannin abundant in pomegranate as well as in nuts and berries, urolithin A (UA) is the hottest spot for its benefits in cancer and inflammation [211]. An increasing body mass index is followed by a decline in the distribution of UA, while a rising distribution of urolithin B. Similar changes occur to aging [212]. Treated with UA, C. elegans has a prolonged lifespan, and prevention or amelioration of age-related fitness decline, accompanied by activated mitophagy. However, in mev-1 (associated with mitochondrial function) mutants, improvement of UA vanishes entirely. Furthermore, in rats and mice fed with UA daily, muscle function is promoted by the induction of mitophagy in the elderly. The power UA has to refit muscle and brain aging [213, 214] is shown to be involved in diverse biological processes such as antioxidation [215], autophagy [216], and ER stress [217]. In human skin fibroblasts, an Nrf2-dependent manner mediated UA’s antioxidative response to mitigate replicative senescence [218]. Through AhR/Nrf2, postinjury intake of UA protects mouse intestines from acute or chronic drug-induced damage by upregulation of tight junction proteins [219]. Moreover, the decrease in serum inflammatory markers [219] reveals that the gut protective effect of UA is attributed to suppression of systemic inflammation. Thanks to safety assessment guaranteeing the security of UA in clinical application [220], a promising avenue for UA intervention in the aging intestine comes into being.
2.9 Spermidine
Spermidine (SPD), a natural polyamine, elicits its essential effects on cell growth, proliferation, and tissue regeneration [221]. SPD pool in mammals is contributed by dietary supply and synthesis of the intestinal microbiota [221], which suffer from an aging-related decline [222, 223] emphasizing the association between SPD and aging. Highly conserved ability of SPD is wildly reported to extend lifespan of Saccharomyces, C. elegans, Drosophila [224], mice [225], and human cells [224]. Restoration of autophagy, improvement of mitochondrial function, and reduction of ER stress are believed to be key for SPD to improve aging impairments, especially neurodegeneration [226], metabolic diseases [227], and cardiovascular and muscle-related disorders [225, 228, 229]. As a stimulus of T cell protein-tyrosine phosphatase, SPD rescues intestinal epithelial barrier dysfunction disrupted by inflammatory cytokine treatment in vitro [230]. It has been demonstrated in vitro that polyamine deprivation disturbs the synthesis and stability of a tight junction protein, occluding [231].
Discussion
Unlike obvious wrinkles in the aged skin, senescence-associated deterioration in the intestine is too inconspicuous to draw people’s attention. However, the increased incidence of intestinal disease and morphological and functional changes of significance remind us of the damage of aging on the intestine. Notaly, progressive strategies help scientists trace slight modifications, especially dysbiosis, immune imbalance, stem cell exhaustion, barrier dysfunction, and enteric neurodegeneration, and further smooth out injury in the aged intestine.
Through the cooperation of sample analysis data and experimental results, several strategies are emerging for their anti-aging effects. In this review, we have appraised several strategies that we consider as candidates to postpone or avert the aged intestine. Ranging from diet control to pharmaceuticals and compounds metabolized by both host and microbiota, we reviewed DR and 8 kinds of compounds, which are listed in Table 2, except for BAs, SCFAs, and Trp for their large amounts of different derivatives to enumerate.
Table 2.
Mechanisms of strategies underlying anti-aging effects in intestine.
| Strategy | Target | Mechanism | Ref |
|---|---|---|---|
| Diet restriction | Intestinal barrier | Enhancing gut barrier by upregulating MYC and rebalancing apoptosis | [99] |
| ISCs | Enhancing stem cell competition to reduce mutation retention | [100] | |
| Resveratrol | Immune system | Resisting inflammation by inhibiting NF-κB activation | [102] |
| Intestinal barrier | Increasing tight junction proteins expression through PI3K/AKT pathway | [111] [112] [113] | |
| Morphological changes | - | [114] | |
| Metformin | Intestinal microbiota | Altering microbial metabolism | [115] [118] |
| Immune system | Resist colonic pathological inflammation by activating AMPK, inhibiting p53 activation | [119] [116] | |
| ISCs | Improving DNA damage and genomic instability by AKT/TOR signaling | [120] [121] | |
| Retarding ISCs aging by Atg6-depend autophagy | [122] | ||
| Intestinal barrier | Increasing tight junction proteins expression through AMPK/JNK-dependent signaling | [123] [124] [125] | |
| Improving mucus by suppressing Wnt signaling to raise the number of goblet cells | [118] | ||
| NAD | Intestinal barrier | Improving mucus by increasing MUC2 expression | [206] |
| ISCs | Improving ISC function by NAD/SIRT1/mTORC1 axis | [207] | |
| Urolithin A | Intestinal barrier | Increasing tight junction proteins expression through AhR/Nrf2 pathway | [219] |
| Spermidine | Intestinal barrier | Reducing epithelial cell permeability by preserving location of tight junction proteins | [230] |
| Increasing tight junction proteins in terms of synthesis and stability | [231] |
ISC, intestinal stem cell; NF-κB, nuclear factor kappa B; PI3K, phosphatidylinositol-4,5-bisphosphate 3-kinase; AKT, protein kinase B; AMPK, adenosine-5’-monophosphate-activated protein kinase; TOR, target of rapamycin; Atg6, autophagy related 6; JNK, c-Jun N-terminal kinase; MUC2, Mucin 2; NAD, Nicotinamide adenine dinucleotide; SIRT1, Sirtuin 1; mTORC1, mammalian target of rapamycin complex 1; AhR, aryl hydrocarbon receptor; Nrf2, nuclear factor E2-related factor 2.
Among those means listed, we suggest that metformin should receive more focus as a highlighted strategy to rejuvenate the intestine under consideration of its power, covering comprehensive changes with the aged intestine. Metformin is a widely used antidiabetic drug for decades. After 2000, its pleiotropic effects beyond antidiabetic[232] have come to light. As mentioned above, we discuss the protective effect of metformin on intestine against aging. However, such anti-aging effects are mediated by multiple targets. The identification of a certain and integral action mode of metformin needs more studies. And further clinical data are also required to support metformin to be a treatment to rejuvenate the aged intestine in humans.
The search for potential strategies, especially special metabolites, to rejuvenate the aged intestine is ongoing. The fact that SCFAs amplify the function of AhR, a receptor of indoles[233], illuminates the cooperative effect of multiple dietary supplements. Hang et al.[234] selected 3-oxo-LCA and isoallo-LCA from nearly 30 types of BAs in the study, to search for the effect of BAs on the differentiation of immune cells[234], which inspired us to eliminate an integral screening process. However, it is worthwhile to consider several concepts. First, the age-related decreasing trend does not equal the anti-aging benefits. Moreover, aging in the intestine has many aspects. Confirmation in vitro does not always reappear in vivo. Concerning future directions, we highlight the convergence of deep metabolomic studies and experiments with speed and efficiency. It is anticipated to achieve a dozen well-received strategies to maintain the young intestine.
References
- [1].Dorling JL, Martin CK, Redman LM (2020). Calorie restriction for enhanced longevity: The role of novel dietary strategies in the present obesogenic environment. Ageing Res Rev, 101038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kennedy BK, Lamming DW (2016). The Mechanistic Target of Rapamycin: The Grand ConducTOR of Metabolism and Aging. Cell Metab, 23:990-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Verdin E (2015). NAD+ in aging, metabolism, and neurodegeneration. Science, 350:1208-1213. [DOI] [PubMed] [Google Scholar]
- [4].Zhang B, Gong J, Zhang W, Xiao R, Liu J, Xu XZS (2018). Brain-gut communications via distinct neuroendocrine signals bidirectionally regulate longevity in C. elegans. Genes Dev, 32:258-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bárcena C, Valdés-Mas R, Mayoral P, Garabaya C, Durand S, Rodríguez F, et al. (2019). Healthspan and lifespan extension by fecal microbiota transplantation into progeroid mice. Nat Med, 25:1234-1242. [DOI] [PubMed] [Google Scholar]
- [6].Drozdowski L, Thomson ABR (2006). Aging and the intestine. World J Gastroenterol, 12:7578-7584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Vazquez Roque M, Bouras EP (2015). Epidemiology and management of chronic constipation in elderly patients. Clin Interv Aging, 10:919-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB (2019). Colorectal cancer. The Lancet, 394:1467-1480. [DOI] [PubMed] [Google Scholar]
- [9].Hu X, Wang T, Jin F (2016). Alzheimer’s disease and gut microbiota. Sci China Life Sci, 59:1006-1023. [DOI] [PubMed] [Google Scholar]
- [10].Gao L, Li J, Zhou Y, Huang X, Qin X, Du G (2018). Effects of Baicalein on Cortical Proinflammatory Cytokines and the Intestinal Microbiome in Senescence Accelerated Mouse Prone 8. ACS Chem Neurosci, 9:1714-1724. [DOI] [PubMed] [Google Scholar]
- [11].Tang WHW, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, et al. (2013). Intestinal Microbial Metabolism of Phosphatidylcholine and Cardiovascular Risk. N Engl J Med, 368:1575-1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kallus SJ, Brandt LJ (2012). The Intestinal Microbiota and Obesity. J Clin Gastroenterol, 46:16-24. [DOI] [PubMed] [Google Scholar]
- [13].Winer DA, Luck H, Tsai S, Winer S (2016). The Intestinal Immune System in Obesity and Insulin Resistance. Cell Metab, 23:413-426. [DOI] [PubMed] [Google Scholar]
- [14].Bodogai M, O’Connell J, Kim K, Kim Y, Moritoh K, Chen C, et al. (2018). Commensal bacteria contribute to insulin resistance in aging by activating innate B1a cells. Sci Transl Med, 10:eaat4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Maeda Y, Kurakawa T, Umemoto E, Motooka D, Ito Y, Gotoh K, et al. (2016). Dysbiosis Contributes to Arthritis Development via Activation of Autoreactive T Cells in the Intestine: DYSBIOSIS CONTRIBUTES TO ARTHRITIS DEVELOPMENT. Arthritis Rheumatol, 68:2646-2661. [DOI] [PubMed] [Google Scholar]
- [16].Claesson MJ, Jeffery IB, Conde S, Power SE, O’Connor EM, Cusack S, et al. (2012). Gut microbiota composition correlates with diet and health in the elderly. Nature, 488:178-184. [DOI] [PubMed] [Google Scholar]
- [17].McGee MD, Weber D, Day N, Vitelli C, Crippen D, Herndon LA, et al. (2011). Loss of intestinal nuclei and intestinal integrity in aging C. elegans. Aging Cell, 10:699-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Holt PR, Yeh KY, Kotler DP (1988). Altered controls of proliferation in proximal small intestine of the senescent rat. Proc Natl Acad Sci U S A, 85:2771-2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Höhn P, Gabbert H, Wagner R (1978). Differentiation and aging of the rat intestinal mucosa. II. Morphological, enzyme histochemical and disc electrophoretic aspects of the aging of the small intestinal mucosa. Mech Ageing Dev, 7:217-226. [DOI] [PubMed] [Google Scholar]
- [20].Martin K, Kirkwood TBL, Potten CS (1998). Age Changes in Stem Cells of Murine Small Intestinal Crypts. Exp Cell Res, 241:316-323. [DOI] [PubMed] [Google Scholar]
- [21].Wang L, Li J, Li Q, Zhang J, Duan X-L (2003). Morphological changes of cell proliferation and apoptosis in rat jejunal mucosa at different ages. World J Gastroenterol, 9:2060-2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Rosa EF, Silva AC, Ihara SSM, Mora OA, Aboulafia J, Nouailhetas VLA (2005). Habitual exercise program protects murine intestinal, skeletal, and cardiac muscles against aging. J Appl Physiol, 99:1569-1575. [DOI] [PubMed] [Google Scholar]
- [23].Hassan ZA, Zauszkiewicz-Pawlak A, Abdelrahman SA, Algaidi S, Desouky M, Shalaby SM (2017). Morphological alterations in the jejunal mucosa of aged rats and the possible protective role of green tea. Folia Histochem Cytobiol, 55:124-139. [DOI] [PubMed] [Google Scholar]
- [24].Nalapareddy K, Nattamai KJ, Kumar RS, Karns R, Wikenheiser-Brokamp KA, Sampson LL, et al. (2017). Canonical Wnt Signaling Ameliorates Aging of Intestinal Stem Cells. Cell Rep, 18:2608-2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lipski PS, Bennett MK, Kelly PJ, James OF (1992). Ageing and duodenal morphometry. J Clin Pathol, 45:450-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Corazza GR, Ginaldi L, Quaglione G, Ponzielli F, Vecchio L, Biagi F, et al. (1998). Proliferating cell nuclear antigen expression is increased in small bowel epithelium in the elderly. Mech Ageing Dev, 104:1-9. [DOI] [PubMed] [Google Scholar]
- [27].Ciccocioppo R, Di Sabatino A, Luinetti O, Rossi M, Cifone MG, Corazza GR (2002). Small bowel enterocyte apoptosis and proliferation are increased in the elderly. Gerontology, 48:204-208. [DOI] [PubMed] [Google Scholar]
- [28].Schoffen JPF, Natali MRM (2007). Effect of age on the myosin-V immunoreactive myenteric neurons of rats ileum. Biocell Off J Soc Latinoam Microsc Electron Al, 31:33-39. [PubMed] [Google Scholar]
- [29].Varljen J, Detel D, Batičić L, Eraković V, Štrbo N, Ćuk M, et al. (2005). Age dependent activity of brush-border enzymes in BALB/c mice. Croat Chem Acta, 78:379-384. [Google Scholar]
- [30].Steegenga WT, de Wit NJ, Boekschoten MV, Ijssennagger N, Lute C, Keshtkar S, et al. (2012). Structural, functional and molecular analysis of the effects of aging in the small intestine and colon of C57BL/6J mice. BMC Med Genomics, 5:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ferraris RP, Vinnakota RR (1993). Regulation of intestinal nutrient transport is impaired in aged mice. J Nutr, 123:502-511. [DOI] [PubMed] [Google Scholar]
- [32].D’Souza AL (2007). Ageing and the gut. Postgrad Med J, 83:44-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Nagaratnam N (2019). Malnutrition and Malabsorption in the Elderly. In: Nagaratnam N, Nagaratnam K, Cheuk G, editors Adv. Age Geriatr. Care Compr. Guide. Cham: Springer International Publishing, 225-233. [Google Scholar]
- [34].Rémond D, Shahar DR, Gille D, Pinto P, Kachal J, Peyron M-A, et al. (2015). Understanding the gastrointestinal tract of the elderly to develop dietary solutions that prevent malnutrition. Oncotarget, 6:13858-13898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Bai JPF, Burckart GJ, Mulberg AE (2016). Literature Review of Gastrointestinal Physiology in the Elderly, in Pediatric Patients, and in Patients with Gastrointestinal Diseases. J Pharm Sci, 105:476-483. [DOI] [PubMed] [Google Scholar]
- [36].Mabbott NA, Kobayashi A, Sehgal A, Bradford BM, Pattison M, Donaldson DS (2015). Aging and the mucosal immune system in the intestine. Biogerontology, 16:133-145. [DOI] [PubMed] [Google Scholar]
- [37].Dumic I, Nordin T, Jecmenica M, Stojkovic Lalosevic M, Milosavljevic T, Milovanovic T (2019). Gastrointestinal Tract Disorders in Older Age. Can J Gastroenterol Hepatol, 2019:1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Cani PD (2018). Human gut microbiome: hopes, threats and promises. Gut, 67:1716-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Biragyn A, Ferrucci L (2018). Gut dysbiosis: a potential link between increased cancer risk in ageing and inflammaging. Lancet Oncol, 19:e295-e304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Shen X, Miao J, Wan Q, Wang S, Li M, Pu F, et al. (2018). Possible correlation between gut microbiota and immunity among healthy middle-aged and elderly people in southwest China. Gut Pathog, 10:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Barreto HC, Sousa A, Gordo I (2020). The Landscape of Adaptive Evolution of a Gut Commensal Bacteria in Aging Mice. Curr Biol, 30:1102-1109.e5. [DOI] [PubMed] [Google Scholar]
- [42].Biagi E, Franceschi C, Rampelli S, Severgnini M, Ostan R, Turroni S, et al. (2016). Gut Microbiota and Extreme Longevity. Curr Biol, 26:1480-1485. [DOI] [PubMed] [Google Scholar]
- [43].Singh H, Torralba MG, Moncera KJ, DiLello L, Petrini J, Nelson KE, et al. (2019). Gastro-intestinal and oral microbiome signatures associated with healthy aging. GeroScience, 41:907-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Lynch SV, Pedersen O (2016). The Human Intestinal Microbiome in Health and Disease. N Engl J Med, 375:2369-2379. [DOI] [PubMed] [Google Scholar]
- [45].Vaiserman AM, Koliada AK, Marotta F (2017). Gut microbiota: A player in aging and a target for anti-aging intervention. Ageing Res Rev, 35:36-45. [DOI] [PubMed] [Google Scholar]
- [46].Thevaranjan N, Puchta A, Schulz C, Naidoo A, Szamosi JC, Verschoor CP, et al. (2017). Age-Associated Microbial Dysbiosis Promotes Intestinal Permeability, Systemic Inflammation, and Macrophage Dysfunction. Cell Host Microbe, 21:455-466.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Franceschi C, Garagnani P, Vitale G, Capri M, Salvioli S (2017). Inflammaging and “Garb-aging”. Trends Endocrinol Metab TEM, 28:199-212. [DOI] [PubMed] [Google Scholar]
- [48].Wang L, Tang L, Feng Y, Zhao S, Han M, Zhang C, et al. (2020). A purified membrane protein from Akkermansia muciniphila or the pasteurised bacterium blunts colitis associated tumourigenesis by modulation of CD8+ T cells in mice. Gut, 69:1988-1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Zhang T, Li Q, Cheng L, Buch H, Zhang F (2019). Akkermansia muciniphila is a promising probiotic. Microb Biotechnol, 12:1109-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Dodd D, Spitzer MH, Van Treuren W, Merrill BD, Hryckowian AJ, Higginbottom SK, et al. (2017). A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites. Nature, 551:648-652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Pawelec G (2018). Age and immunity: What is “immunosenescence”? Exp Gerontol, 105:4-9. [DOI] [PubMed] [Google Scholar]
- [52].Nikolich-Žugich J (2018). The twilight of immunity: emerging concepts in aging of the immune system. Nat Immunol, 19:10-19. [DOI] [PubMed] [Google Scholar]
- [53].Almanan M, Raynor J, Ogunsulire I, Malyshkina A, Mukherjee S, Hummel SA, et al. (2020). IL-10-producing Tfh cells accumulate with age and link inflammation with age-related immune suppression. Sci Adv, 6:eabb0806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Schmucker DL, Thoreux K, Owen RL (2001). Aging impairs intestinal immunity. Mech Ageing Dev, 122:1397-1411. [DOI] [PubMed] [Google Scholar]
- [55].Thoreux K, Owen RL, Schmucker DL (2000). Intestinal lymphocyte number, migration and antibody secretion in young and old rats. Immunology, 101:161-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Stebegg M, Silva-Cayetano A, Innocentin S, Jenkins TP, Cantacessi C, Gilbert C, et al. (2019). Heterochronic faecal transplantation boosts gut germinal centres in aged mice. Nat Commun, 10:2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Biagi E, Candela M, Turroni S, Garagnani P, Franceschi C, Brigidi P (2013). Ageing and gut microbes: Perspectives for health maintenance and longevity. Pharmacol Res, 69:11-20. [DOI] [PubMed] [Google Scholar]
- [58].Rampelli S, Candela M, Turroni S, Biagi E, Collino S, Franceschi C, et al. (2013). Functional metagenomic profiling of intestinal microbiome in extreme ageing. Aging, 5:902-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Cheng H, Leblond CP (1974). Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian Theory of the origin of the four epithelial cell types. Am J Anat, 141:537-561. [DOI] [PubMed] [Google Scholar]
- [60].Potten CS, Kellett M, Roberts SA, Rew DA, Wilson GD (1992). Measurement of in vivo proliferation in human colorectal mucosa using bromodeoxyuridine. Gut, 33:71-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Moorefield EC, Andres SF, Blue RE, Van Landeghem L, Mah AT, Santoro MA, et al. (2017). Aging effects on intestinal homeostasis associated with expansion and dysfunction of intestinal epithelial stem cells. Aging, 9:1898-1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Choi N-H, Kim J-G, Yang D-J, Kim Y-S, Yoo M-A (2008). Age-related changes in Drosophila midgut are associated with PVF2, a PDGF/VEGF-like growth factor. Aging Cell, 7:318-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Wang Y, Song W, Wang J, Wang T, Xiong X, Qi Z, et al. (2019). Single-cell transcriptome analysis reveals differential nutrient absorption functions in human intestine. J Exp Med. doi: 10.1084/jem.20191130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Biteau B, Karpac J, Supoyo S, Degennaro M, Lehmann R, Jasper H (2010). Lifespan extension by preserving proliferative homeostasis in Drosophila. PLoS Genet, 6:e1001159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Jurk D, Wilson C, Passos JF, Oakley F, Correia-Melo C, Greaves L, et al. (2014). Chronic inflammation induces telomere dysfunction and accelerates ageing in mice. Nat Commun, 5:4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Kumar S, Suman S, Fornace AJ, Datta K (2019). Intestinal stem cells acquire premature senescence and senescence associated secretory phenotype concurrent with persistent DNA damage after heavy ion radiation in mice. Aging, 11:4145-4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Zhu Y, Liu X, Ding X, Wang F, Geng X (2019). Telomere and its role in the aging pathways: telomere shortening, cell senescence and mitochondria dysfunction. Biogerontology, 20:1-16. [DOI] [PubMed] [Google Scholar]
- [68].Ribezzo F, Shiloh Y, Schumacher B (2016). Systemic DNA damage responses in aging and diseases. Semin Cancer Biol, 37-38:26-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Birch J, Gil J (2020). Senescence and the SASP: many therapeutic avenues. Genes Dev, 34:1565-1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Singh SR, Zeng X, Zhao J, Liu Y, Hou G, Liu H, et al. (2016). The lipolysis pathway sustains normal and transformed stem cells in adult Drosophila. Nature, 538:109-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Mihaylova MM, Cheng C-W, Cao AQ, Tripathi S, Mana MD, Bauer-Rowe KE, et al. (2018). Fasting Activates Fatty Acid Oxidation to Enhance Intestinal Stem Cell Function during Homeostasis and Aging. Cell Stem Cell, 22:769-778.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Koehler CL, Perkins GA, Ellisman MH, Jones DL (2017). Pink1 and Parkin regulate Drosophila intestinal stem cell proliferation during stress and aging. J Cell Biol, 216:2315-2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Siudeja K, Nassari S, Gervais L, Skorski P, Lameiras S, Stolfa D, et al. (2015). Frequent Somatic Mutation in Adult Intestinal Stem Cells Drives Neoplasia and Genetic Mosaicism during Aging. Cell Stem Cell, 17:663-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Liang S, Zhou J, Wang X (2020). Signaling Network Centered on mTORC1 Dominates Mammalian Intestinal Stem Cell Ageing. Stem Cell Rev Rep. doi: 10.1007/s12015-020-10073-y. [DOI] [PubMed] [Google Scholar]
- [75].Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, et al. (2009). Crypt stem cells as the cells-of-origin of intestinal cancer. Nature, 457:608-611. [DOI] [PubMed] [Google Scholar]
- [76].Egge N, Arneaud SLB, Wales P, Mihelakis M, McClendon J, Fonseca RS, et al. (2019). Age-Onset Phosphorylation of a Minor Actin Variant Promotes Intestinal Barrier Dysfunction. Dev Cell, 51:587-601.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Cerqueira César Machado M, Pinheiro da Silva F (2016). Intestinal Barrier Dysfunction in Human Pathology and Aging. Curr Pharm Des, 22:4645-4650. [DOI] [PubMed] [Google Scholar]
- [78].Ren W, Wu K, Li X, Luo M, Liu H, Zhang S, et al. (2014). Age-related changes in small intestinal mucosa epithelium architecture and epithelial tight junction in rat models. Aging Clin Exp Res, 26:183-191. [DOI] [PubMed] [Google Scholar]
- [79].Tran L, Greenwood-Van Meerveld B (2013). Age-associated remodeling of the intestinal epithelial barrier. J Gerontol A Biol Sci Med Sci, 68:1045-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Qi Y, Goel R, Kim S, Richards EM, Carter CS, Pepine CJ, et al. (2017). Intestinal Permeability Biomarker Zonulin is Elevated in Healthy Aging. J Am Med Dir Assoc, 18:810.e1-810.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Ahmadi S, Wang S, Nagpal R, Wang B, Jain S, Razazan A, et al. (2020). A human-origin probiotic cocktail ameliorates aging-related leaky gut and inflammation via modulating the microbiota/taurine/tight junction axis. JCI Insight, 5:e132055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Massimo De Martinis, Claudio Franceschi, Daniela Monti (2005). Inflamm-ageing and lifelong antigenic load as major determinants of ageing rate and longevity. Febs Lett. doi: 10.1016/j.febslet.2005.02.055. [DOI] [PubMed] [Google Scholar]
- [83].Rera M, Clark RI, Walker DW (2012). Intestinal barrier dysfunction links metabolic and inflammatory markers of aging to death in Drosophila. Proc Natl Acad Sci U S A, 109:21528-21533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Schneider S, Wright CM, Heuckeroth RO (2019). Unexpected Roles for the Second Brain: Enteric Nervous System as Master Regulator of Bowel Function. Annu Rev Physiol, 81:235-259. [DOI] [PubMed] [Google Scholar]
- [85].Wiskur B, Greenwood-Van Meerveld B (2010). The Aging Colon: The Role of Enteric Neurodegeneration in Constipation. Curr Gastroenterol Rep, 12:507-512. [DOI] [PubMed] [Google Scholar]
- [86].Abbott RD, Petrovitch H, White LR, Masaki KH, Tanner CM, Curb JD, et al. (2001). Frequency of bowel movements and the future risk of Parkinson’s disease. Neurology, 57:456-462. [DOI] [PubMed] [Google Scholar]
- [87].Sun T, Li D, Hu S, Huang L, Sun H, Yang S, et al. (2018). Aging-dependent decrease in the numbers of enteric neurons, interstitial cells of Cajal and expression of connexin43 in various regions of gastrointestinal tract. Aging, 10:3851-3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Bernard CE, Gibbons SJ, Gomez-Pinilla PJ, Lurken MS, Schmalz PF, Roeder JL, et al. (2009). Effect of age on the enteric nervous system of the human colon. Neurogastroenterol Motil Off J Eur Gastrointest Motil Soc, 21:746-e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Peck CJ, Samsuria SD, Harrington AM, King SK, Hutson JM, Southwell BR (2009). Fall in density, but not number of myenteric neurons and circular muscle nerve fibres in guinea-pig colon with ageing. Neurogastroenterol Motil Off J Eur Gastrointest Motil Soc, 21:1075-e90. [DOI] [PubMed] [Google Scholar]
- [90].Liu M-T, Kuan Y-H, Wang J, Hen R, Gershon MD (2009). 5-HT4 receptor-mediated neuroprotection and neurogenesis in the enteric nervous system of adult mice. J Neurosci Off J Soc Neurosci, 29:9683-9699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Kennedy BK, Berger SL, Brunet A, Campisi J, Cuervo AM, Epel ES, et al. (2014). Geroscience: linking aging to chronic disease. Cell, 159:709-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Wang L, Zeng X, Ryoo HD, Jasper H (2014). Integration of UPRER and oxidative stress signaling in the control of intestinal stem cell proliferation. PLoS Genet, 10:e1004568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Sousa-Victor P, Ayyaz A, Hayashi R, Qi Y, Madden DT, Lunyak VV, et al. (2017). Piwi Is Required to Limit Exhaustion of Aging Somatic Stem Cells. Cell Rep, 20:2527-2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Funk MC, Zhou J, Boutros M (2020). Ageing, metabolism and the intestine. EMBO Rep, e50047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Corazza GR, Frazzoni M, Gatto MRA, Gasbarrini G (1986). Ageing and Small-Bowel Mucosa: A Morphometric Study. Gerontology, 32:60-65. [DOI] [PubMed] [Google Scholar]
- [96].Fontana L, Partridge L (2015). Promoting health and longevity through diet: from model organisms to humans. Cell, 161:106-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Hanjani NA, Vafa M (2018). Protein Restriction, Epigenetic Diet, Intermittent Fasting as New Approaches for Preventing Age-associated Diseases. Int J Prev Med. doi: 10.4103/ijpvm.IJPVM_397_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Genton L, Cani PD, Schrenzel J (2015). Alterations of gut barrier and gut microbiota in food restriction, food deprivation and protein-energy wasting. Clin Nutr Edinb Scotl, 34:341-349. [DOI] [PubMed] [Google Scholar]
- [99].Akagi K, Wilson KA, Katewa SD, Ortega M, Simons J, Hilsabeck TA, et al. (2018). Dietary restriction improves intestinal cellular fitness to enhance gut barrier function and lifespan in D. melanogaster. PLOS Genet, 14:e1007777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Bruens L, Ellenbroek SIJ, Suijkerbuijk SJE, Azkanaz M, Hale AJ, Toonen P, et al. (2020). Calorie Restriction Increases the Number of Competing Stem Cells and Decreases Mutation Retention in the Intestine. Cell Rep, 32:107937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Duszka K, Ellero-Simatos S, Ow GS, Defernez M, Paramalingam E, Tett A, et al. (2018). Complementary intestinal mucosa and microbiota responses to caloric restriction. Sci Rep, 8:11338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Nunes S, Danesi F, Del Rio D, Silva P (2018). Resveratrol and inflammatory bowel disease: the evidence so far. Nutr Res Rev, 31:85-97. [DOI] [PubMed] [Google Scholar]
- [103].Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, et al. (2003). Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature, 425:191-196. [DOI] [PubMed] [Google Scholar]
- [104].Li Y-R, Li S, Lin C-C (2018). Effect of resveratrol and pterostilbene on aging and longevity. BioFactors Oxf Engl, 44:69-82. [DOI] [PubMed] [Google Scholar]
- [105].Ma DSL, Tan LT-H, Chan K-G, Yap WH, Pusparajah P, Chuah L-H, et al. (2018). Resveratrol—Potential Antibacterial Agent against Foodborne Pathogens. Front Pharmacol. doi: 10.3389/fphar.2018.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Morselli E, Maiuri MC, Markaki M, Megalou E, Pasparaki A, Palikaras K, et al. (2010). Caloric restriction and resveratrol promote longevity through the Sirtuin-1-dependent induction of autophagy. Cell Death Dis, 1:e10-e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Li A, Zhang S, Li J, Liu K, Huang F, Liu B (2016). Metformin and resveratrol inhibit Drp1-mediated mitochondrial fission and prevent ER stress-associated NLRP3 inflammasome activation in the adipose tissue of diabetic mice. Mol Cell Endocrinol, 434:36-47. [DOI] [PubMed] [Google Scholar]
- [108].Zhou T, Yan Y, Zhao C, Xu Y, Wang Q, Xu N (2019). Resveratrol improves osteogenic differentiation of senescent bone mesenchymal stem cells through inhibiting endogenous reactive oxygen species production via AMPK activation. Redox Rep Commun Free Radic Res, 24:62-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Liu B, Ghosh S, Yang X, Zheng H, Liu X, Wang Z, et al. (2012). Resveratrol rescues SIRT1-dependent adult stem cell decline and alleviates progeroid features in laminopathy-based progeria. Cell Metab, 16:738-750. [DOI] [PubMed] [Google Scholar]
- [110].Wu M, Ma L, Xue L, Ye W, Lu Z, Li X, et al. (2019). Resveratrol alleviates chemotherapy-induced oogonial stem cell apoptosis and ovarian aging in mice. Aging, 11:1030-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Chen K, Zhao H, Shu L, Xing H, Wang C, Lu C, et al. (2020). Effect of resveratrol on intestinal tight junction proteins and the gut microbiome in high-fat diet-fed insulin resistant mice. Int J Food Sci Nutr, 1-14. [DOI] [PubMed] [Google Scholar]
- [112].Chen M, Hou P, Zhou M, Ren Q, Wang X, Huang L, et al. (2020). Resveratrol attenuates high-fat diet-induced non-alcoholic steatohepatitis by maintaining gut barrier integrity and inhibiting gut inflammation through regulation of the endocannabinoid system. Clin Nutr Edinb Scotl, 39:1264-1275. [DOI] [PubMed] [Google Scholar]
- [113].Zhuang Y, Wu H, Wang X, He J, He S, Yin Y (2019). Resveratrol Attenuates Oxidative Stress-Induced Intestinal Barrier Injury through PI3K/Akt-Mediated Nrf2 Signaling Pathway. Oxid Med Cell Longev, 2019:7591840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Liu L, Fu C, Yan M, Xie H, Li S, Yu Q, et al. (2016). Resveratrol modulates intestinal morphology and HSP70/90, NF-κB and EGF expression in the jejunal mucosa of black-boned chickens on exposure to circular heat stress. Food Funct, 7:1329-1338. [DOI] [PubMed] [Google Scholar]
- [115].Cabreiro F, Au C, Leung K-Y, Vergara-Irigaray N, Cochemé HM, Noori T, et al. (2013). Metformin Retards Aging in C. elegans by Altering Microbial Folate and Methionine Metabolism. Cell, 153:228-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Martin-Montalvo A, Mercken EM, Mitchell SJ, Palacios HH, Mote PL, Scheibye-Knudsen M, et al. (2013). Metformin improves healthspan and lifespan in mice. Nat Commun, 4:2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Barzilai N, Crandall JP, Kritchevsky SB, Espeland MA (2016). Metformin as a Tool to Target Aging. Cell Metab, 23:1060-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Ahmadi S, Razazan A, Nagpal R, Jain S, Wang B, Mishra SP, et al. (2020). Metformin reduces aging-related leaky gut and improves cognitive function by beneficially modulating gut microbiome/goblet cell/mucin axis. J Gerontol A Biol Sci Med Sci. doi: 10.1093/gerona/glaa056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Di Fusco D, Dinallo V, Monteleone I, Laudisi F, Marafini I, Franzè E, et al. (2018). Metformin inhibits inflammatory signals in the gut by controlling AMPK and p38 MAP kinase activation. Clin Sci Lond Engl 1979, 132:1155-1168. [DOI] [PubMed] [Google Scholar]
- [120].Na H-J, Park J-S, Pyo J-H, Lee S-H, Jeon H-J, Kim Y-S, et al. (2013). Mechanism of metformin: inhibition of DNA damage and proliferative activity in Drosophila midgut stem cell. Mech Ageing Dev, 134:381-390. [DOI] [PubMed] [Google Scholar]
- [121].Na H-J, Park J-S, Pyo J-H, Jeon H-J, Kim Y-S, Arking R, et al. (2015). Metformin inhibits age-related centrosome amplification in Drosophila midgut stem cells through AKT/TOR pathway. Mech Ageing Dev, 149:8-18. [DOI] [PubMed] [Google Scholar]
- [122].Na H-J, Pyo J-H, Jeon H-J, Park J-S, Chung H-Y, Yoo M-A (2018). Deficiency of Atg6 impairs beneficial effect of metformin on intestinal stem cell aging in Drosophila. Biochem Biophys Res Commun, 498:18-24. [DOI] [PubMed] [Google Scholar]
- [123].Brandt A, Hernández-Arriaga A, Kehm R, Sánchez V, Jin CJ, Nier A, et al. (2019). Metformin attenuates the onset of non-alcoholic fatty liver disease and affects intestinal microbiota and barrier in small intestine. Sci Rep, 9:6668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Wu W, Wang S, Liu Q, Shan T, Wang Y (2018). Metformin Protects against LPS-Induced Intestinal Barrier Dysfunction by Activating AMPK Pathway. Mol Pharm, 15:3272-3284. [DOI] [PubMed] [Google Scholar]
- [125].Deng J, Zeng L, Lai X, Li J, Liu L, Lin Q, et al. (2018). Metformin protects against intestinal barrier dysfunction via AMPKα1-dependent inhibition of JNK signalling activation. J Cell Mol Med, 22:546-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Han X, Tai H, Wang X, Wang Z, Zhou J, Wei X, et al. (2016). AMPK activation protects cells from oxidative stress-induced senescence via autophagic flux restoration and intracellular NAD(+) elevation. Aging Cell, 15:416-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Wahlström A, Sayin SI, Marschall H-U, Bäckhed F (2016). Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab, 24:41-50. [DOI] [PubMed] [Google Scholar]
- [128].Quinn RA, Melnik AV, Vrbanac A, Fu T, Patras KA, Christy MP, et al. (2020). Global chemical effects of the microbiome include new bile-acid conjugations. Nature, 579:123-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Hamilton JP, Xie G, Raufman J-P, Hogan S, Griffin TL, Packard CA, et al. (2007). Human cecal bile acids: concentration and spectrum. Am J Physiol-Gastrointest Liver Physiol, 293:G256-G263. [DOI] [PubMed] [Google Scholar]
- [130].Cai D, Zhao S, Li D, Chang F, Tian X, Huang G, et al. (2016). Nutrient Intake Is Associated with Longevity Characterization by Metabolites and Element Profiles of Healthy Centenarians. Nutrients. doi: 10.3390/nu8090564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Salemans JM, Nagengast FM, Tangerman A, van Schaik A, Hopman WP, de Haan AF, et al. (1993). Effect of ageing on postprandial conjugated and unconjugated serum bile acid levels in healthy subjects. Eur J Clin Invest, 23:192-198. [DOI] [PubMed] [Google Scholar]
- [132].Lee G, Lee H, Hong J, Lee SH, Jung BH (2016). Quantitative profiling of bile acids in rat bile using ultrahigh-performance liquid chromatography-orbitrap mass spectrometry: Alteration of the bile acid composition with aging. J Chromatogr B Analyt Technol Biomed Life Sci, 1031:37-49. [DOI] [PubMed] [Google Scholar]
- [133].Fu ZD, Csanaky IL, Klaassen CD (2012). Gender-divergent profile of bile acid homeostasis during aging of mice. PloS One, 7:e32551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Bárcena C, Quirós PM, Durand S, Mayoral P, Rodríguez F, Caravia XM, et al. (2018). Methionine Restriction Extends Lifespan in Progeroid Mice and Alters Lipid and Bile Acid Metabolism. Cell Rep, 24:2392-2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Huang F, Zheng X, Ma X, Jiang R, Zhou W, Zhou S, et al. (2019). Theabrownin from Pu-erh tea attenuates hypercholesterolemia via modulation of gut microbiota and bile acid metabolism. Nat Commun, 10:4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Pols TWH, Nomura M, Harach T, Lo Sasso G, Oosterveer MH, Thomas C, et al. (2011). TGR5 activation inhibits atherosclerosis by reducing macrophage inflammation and lipid loading. Cell Metab, 14:747-757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Bennett BJ, Vallim TQ de A, Wang Z, Shih DM, Meng Y, Gregory J, et al. (2013). Trimethylamine-N-Oxide, a Metabolite Associated with Atherosclerosis, Exhibits Complex Genetic and Dietary Regulation. Cell Metab, 17:49-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Vang S, Longley K, Steer CJ, Low WC (2014). The Unexpected Uses of Urso- and Tauroursodeoxycholic Acid in the Treatment of Non-liver Diseases. Glob Adv Health Med, 3:58-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Li C-X, Wang X-Q, Cheng F-F, Yan X, Luo J, Wang Q-G (2019). Hyodeoxycholic acid protects the neurovascular unit against oxygen-glucose deprivation and reoxygenation-induced injury in vitro. Neural Regen Res, 14:1941-1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Huang H, Lei H, Yang F, Fan X, Dang Q, Li Y (2018). Activation of the bile acid receptor GPBAR1 (TGR5) ameliorates interleukin-1β (IL-1β)- induced chondrocytes senescence. Biomed Pharmacother Biomedecine Pharmacother, 106:1713-1719. [DOI] [PubMed] [Google Scholar]
- [141].Li Z, Huang J, Wang F, Li W, Wu X, Zhao C, et al. (2019). Dual Targeting of Bile Acid Receptor-1 (TGR5) and Farnesoid X Receptor (FXR) Prevents Estrogen-Dependent Bone Loss in Mice. J Bone Miner Res Off J Am Soc Bone Miner Res, 34:765-776. [DOI] [PubMed] [Google Scholar]
- [142].Li X, Liu R, Yu L, Yuan Z, Sun R, Yang H, et al. (2016). Alpha-naphthylisothiocyanate impairs bile acid homeostasis through AMPK-FXR pathways in rat primary hepatocytes. Toxicology, 370:106-115. [DOI] [PubMed] [Google Scholar]
- [143].Li X, Liu R, Luo L, Yu L, Chen X, Sun L, et al. (2017). Role of AMP-activated protein kinase α1 in 17α-ethinylestradiol-induced cholestasis in rats. Arch Toxicol, 91:481-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Khambu B, Li T, Yan S, Yu C, Chen X, Goheen M, et al. (2019). Hepatic Autophagy Deficiency Compromises Farnesoid X Receptor Functionality and Causes Cholestatic Injury. Hepatol Baltim Md, 69:2196-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Ye H-L, Zhang J-W, Chen X-Z, Wu P-B, Chen L, Zhang G (2020). Ursodeoxycholic acid alleviates experimental liver fibrosis involving inhibition of autophagy. Life Sci, 242:117175. [DOI] [PubMed] [Google Scholar]
- [146].Hofmann AF (2004). Detoxification of Lithocholic Acid, A Toxic Bile Acid: Relevance to Drug Hepatotoxicity. Drug Metab Rev, 36:703-722. [DOI] [PubMed] [Google Scholar]
- [147].Reddy BS, Watanabe K, Weisburger JH, Wynder EL (1977). Promoting effect of bile acids in colon carcinogenesis in germ-free and conventional F344 rats. Cancer Res, 37:3238-3242. [PubMed] [Google Scholar]
- [148].Fu T, Coulter S, Yoshihara E, Oh TG, Fang S, Cayabyab F, et al. (2019). FXR Regulates Intestinal Cancer Stem Cell Proliferation. Cell, 176:1098-1112.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Tian Y, Gui W, Koo I, Smith PB, Allman EL, Nichols RG, et al. (2020). The microbiome modulating activity of bile acids. Gut Microbes, 1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Kovács P, Csonka T, Kovács T, Sári Z, Ujlaki G, Sipos A, et al. (2019). Lithocholic Acid, a Metabolite of the Microbiome, Increases Oxidative Stress in Breast Cancer. Cancers. doi: 10.3390/cancers11091255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Arlia-Ciommo A, Piano A, Svistkova V, Mohtashami S, Titorenko VI (2014). Mechanisms underlying the anti-aging and anti-tumor effects of lithocholic bile acid. Int J Mol Sci, 15:16522-16543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Arlia-Ciommo A, Leonov A, Mohammad K, Beach A, Richard VR, Bourque SD, et al. (2018). Mechanisms through which lithocholic acid delays yeast chronological aging under caloric restriction conditions. Oncotarget, 9:34945-34971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Yao B, He J, Yin X, Shi Y, Wan J, Tian Z (2019). The protective effect of lithocholic acid on the intestinal epithelial barrier is mediated by the vitamin D receptor via a SIRT1/Nrf2 and NF-κB dependent mechanism in Caco-2 cells. Toxicol Lett, 316:109-118. [DOI] [PubMed] [Google Scholar]
- [154].Sorrentino G, Perino A, Yildiz E, El Alam G, Sleiman MB, Gioiello A, et al. (2020). Bile Acids Signal via TGR5 to Activate Intestinal Stem Cells and Epithelial Regeneration. Gastroenterology. doi: 10.1053/j.gastro.2020.05.067. [DOI] [PubMed] [Google Scholar]
- [155].Yoshimoto S, Loo TM, Atarashi K, Kanda H, Sato S, Oyadomari S, et al. (2013). Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature, 499:97-101. [DOI] [PubMed] [Google Scholar]
- [156].Yang H, Li TWH, Ko KS, Xia M, Lu SC (2009). Switch from Mnt-Max to Myc-Max induces p53 and cyclin D1 expression and apoptosis during cholestasis in mouse and human hepatocytes. Hepatol Baltim Md, 49:860-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Yang H, Ko K, Xia M, Li TWH, Oh P, Li J, et al. (2010). Induction of avian musculoaponeurotic fibrosarcoma proteins by toxic bile acid inhibits expression of glutathione synthetic enzymes and contributes to cholestatic liver injury in mice. Hepatol Baltim Md, 51:1291-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Launay N, Ruiz M, Grau L, Ortega FJ, Ilieva EV, Martínez JJ, et al. (2017). Tauroursodeoxycholic bile acid arrests axonal degeneration by inhibiting the unfolded protein response in X-linked adrenoleukodystrophy. Acta Neuropathol (Berl), 133:283-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Lo AC, Callaerts-Vegh Z, Nunes AF, Rodrigues CMP, D’Hooge R (2013). Tauroursodeoxycholic acid (TUDCA) supplementation prevents cognitive impairment and amyloid deposition in APP/PS1 mice. Neurobiol Dis, 50:21-29. [DOI] [PubMed] [Google Scholar]
- [160].Arai Y, Choi B, Kim BJ, Rim W, Park S, Park H, et al. (2019). Tauroursodeoxycholic acid (TUDCA) counters osteoarthritis by regulating intracellular cholesterol levels and membrane fluidity of degenerated chondrocytes. Biomater Sci, 7:3178-3189. [DOI] [PubMed] [Google Scholar]
- [161].Kusaczuk M (2019). Tauroursodeoxycholate-Bile Acid with Chaperoning Activity: Molecular and Cellular Effects and Therapeutic Perspectives. Cells. doi: 10.3390/cells8121471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Koh A, De Vadder F, Kovatcheva-Datchary P, Bäckhed F (2016). From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell, 165:1332-1345. [DOI] [PubMed] [Google Scholar]
- [163].Morrison DJ, Preston T (2016). Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes, 7:189-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Resende WR, Valvassori SS, Réus GZ, Varela RB, Arent CO, Ribeiro KF, et al. (2013). Effects of sodium butyrate in animal models of mania and depression: implications as a new mood stabilizer. Behav Pharmacol, 24:569-579. [DOI] [PubMed] [Google Scholar]
- [165].St Laurent R, O’Brien LM, Ahmad ST (2013). Sodium butyrate improves locomotor impairment and early mortality in a rotenone-induced Drosophila model of Parkinson’s disease. Neuroscience, 246:382-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, et al. (2016). Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell, 167:1469-1480.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Ho L, Ono K, Tsuji M, Mazzola P, Singh R, Pasinetti GM (2018). Protective roles of intestinal microbiota derived short chain fatty acids in Alzheimer’s disease-type beta-amyloid neuropathological mechanisms. Expert Rev Neurother, 18:83-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Rose S, Bennuri SC, Davis JE, Wynne R, Slattery JC, Tippett M, et al. (2018). Butyrate enhances mitochondrial function during oxidative stress in cell lines from boys with autism. Transl Psychiatry, 8:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].van de Wouw M, Boehme M, Lyte JM, Wiley N, Strain C, O’Sullivan O, et al. (2018). Short-chain fatty acids: microbial metabolites that alleviate stress-induced brain-gut axis alterations. J Physiol, 596:4923-4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Zhao L, Zhang F, Ding X, Wu G, Lam YY, Wang X, et al. (2018). Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science, 359:1151-1156. [DOI] [PubMed] [Google Scholar]
- [171].Ganesh BP, Nelson JW, Eskew JR, Ganesan A, Ajami NJ, Petrosino JF, et al. (2018). Prebiotics, Probiotics, and Acetate Supplementation Prevent Hypertension in a Model of Obstructive Sleep Apnea. Hypertens Dallas Tex 1979, 72:1141-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Ryu TY, Kim K, Son M-Y, Min J-K, Kim J, Han T-S, et al. (2019). Downregulation of PRMT1, a histone arginine methyltransferase, by sodium propionate induces cell apoptosis in colon cancer. Oncol Rep, 41:1691-1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Belcheva A, Irrazabal T, Robertson SJ, Streutker C, Maughan H, Rubino S, et al. (2014). Gut Microbial Metabolism Drives Transformation of Msh2-Deficient Colon Epithelial Cells. Cell, 158:288-299. [DOI] [PubMed] [Google Scholar]
- [174].Lucas S, Omata Y, Hofmann J, Böttcher M, Iljazovic A, Sarter K, et al. (2018). Short-chain fatty acids regulate systemic bone mass and protect from pathological bone loss. Nat Commun, 9:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Shin C, Lim Y, Lim H, Ahn T-B (2020). Plasma Short-Chain Fatty Acids in Patients With Parkinson’s Disease. Mov Disord Off J Mov Disord Soc. doi: 10.1002/mds.28016. [DOI] [PubMed] [Google Scholar]
- [176].Matt SM, Allen JM, Lawson MA, Mailing LJ, Woods JA, Johnson RW (2018). Butyrate and Dietary Soluble Fiber Improve Neuroinflammation Associated With Aging in Mice. Front Immunol, 9:1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Liu H, Wang J, He T, Becker S, Zhang G, Li D, et al. (2018). Butyrate: A Double-Edged Sword for Health? Adv Nutr, 9:21-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Zhou C, Li L, Li T, Sun L, Yin J, Guan H, et al. (2020). SCFAs induce autophagy in intestinal epithelial cells and relieve colitis by stabilizing HIF-1α. J Mol Med Berl Ger. doi: 10.1007/s00109-020-01947-2. [DOI] [PubMed] [Google Scholar]
- [179].Dagdeviren S, Jung DY, Friedline RH, Noh HL, Kim JH, Patel PR, et al. (2017). IL-10 prevents aging-associated inflammation and insulin resistance in skeletal muscle. FASEB J Off Publ Fed Am Soc Exp Biol, 31:701-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Sun M, Wu W, Chen L, Yang W, Huang X, Ma C, et al. (2018). Microbiota-derived short-chain fatty acids promote Th1 cell IL-10 production to maintain intestinal homeostasis. Nat Commun, 9:3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Martin-Gallausiaux C, Larraufie P, Jarry A, Béguet-Crespel F, Marinelli L, Ledue F, et al. (2018). Butyrate Produced by Commensal Bacteria Down-Regulates Indolamine 2,3-Dioxygenase 1 (IDO-1) Expression via a Dual Mechanism in Human Intestinal Epithelial Cells. Front Immunol, 9:2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Zhao Y, Chen F, Wu W, Sun M, Bilotta AJ, Yao S, et al. (2018). GPR43 mediates microbiota metabolite SCFA regulation of antimicrobial peptide expression in intestinal epithelial cells via activation of mTOR and STAT3. Mucosal Immunol, 11:752-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Piekarska J, Miśta D, Houszka M, Króliczewska B, Zawadzki W, Gorczykowski M (2011). Trichinella spiralis: the influence of short chain fatty acids on the proliferation of lymphocytes, the goblet cell count and apoptosis in the mouse intestine. Exp Parasitol, 128:419-426. [DOI] [PubMed] [Google Scholar]
- [184].Wang H-B, Wang P-Y, Wang X, Wan Y-L, Liu Y-C (2012). Butyrate enhances intestinal epithelial barrier function via up-regulation of tight junction protein Claudin-1 transcription. Dig Dis Sci, 57:3126-3135. [DOI] [PubMed] [Google Scholar]
- [185].Teixeira TFS, Grześkowiak Ł, Franceschini SCC, Bressan J, Ferreira CLLF, Peluzio MCG (2013). Higher level of faecal SCFA in women correlates with metabolic syndrome risk factors. Br J Nutr, 109:914-919. [DOI] [PubMed] [Google Scholar]
- [186].Agus A, Planchais J, Sokol H (2018). Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe, 23:716-724. [DOI] [PubMed] [Google Scholar]
- [187].Xiao W, Wang R-S, Handy DE, Loscalzo J (2018). NAD(H) and NADP(H) Redox Couples and Cellular Energy Metabolism. Antioxid Redox Signal, 28:251-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Laurans L, Venteclef N, Haddad Y, Chajadine M, Alzaid F, Metghalchi S, et al. (2018). Genetic deficiency of indoleamine 2,3-dioxygenase promotes gut microbiota-mediated metabolic health. Nat Med, 24:1113-1120. [DOI] [PubMed] [Google Scholar]
- [189].Cason CA, Dolan KT, Sharma G, Tao M, Kulkarni R, Helenowski IB, et al. (2018). Plasma microbiome-modulated indole- and phenyl-derived metabolites associate with advanced atherosclerosis and postoperative outcomes. J Vasc Surg, 68:1552-1562.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Huć T, Nowinski A, Drapala A, Konopelski P, Ufnal M (2018). Indole and indoxyl sulfate, gut bacteria metabolites of tryptophan, change arterial blood pressure via peripheral and central mechanisms in rats. Pharmacol Res, 130:172-179. [DOI] [PubMed] [Google Scholar]
- [191].Choi Y, Abdelmegeed MA, Song B-J (2018). Preventive effects of indole-3-carbinol against alcohol-induced liver injury in mice via antioxidant, anti-inflammatory, and anti-apoptotic mechanisms: Role of gut-liver-adipose tissue axis. J Nutr Biochem, 55:12-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Krishnan S, Ding Y, Saedi N, Choi M, Sridharan GV, Sherr DH, et al. (2018). Gut Microbiota-Derived Tryptophan Metabolites Modulate Inflammatory Response in Hepatocytes and Macrophages. Cell Rep, 23:1099-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Hendrikx T, Duan Y, Wang Y, Oh J-H, Alexander LM, Huang W, et al. (2019). Bacteria engineered to produce IL-22 in intestine induce expression of REG3G to reduce ethanol-induced liver disease in mice. Gut, 68:1504-1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Ma L, Li H, Hu J, Zheng J, Zhou J, Botchlett R, et al. (2020). Indole Alleviates Diet-induced Hepatic Steatosis and Inflammation in a Manner Involving Myeloid Cell PFKFB3. Hepatology, hep. 31115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Fujioka N, Fritz V, Upadhyaya P, Kassie F, Hecht SS (2016). Research on cruciferous vegetables, indole-3-carbinol, and cancer prevention: A tribute to Lee W. Wattenberg. Mol Nutr Food Res, 60:1228-1238. [DOI] [PubMed] [Google Scholar]
- [196].Sonowal R, Swimm A, Sahoo A, Luo L, Matsunaga Y, Wu Z, et al. (2017). Indoles from commensal bacteria extend healthspan. Proc Natl Acad Sci U S A, 114:E7506-E7515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Hou Q, Ye L, Liu H, Huang L, Yang Q, Turner J, et al. (2018). Lactobacillus accelerates ISCs regeneration to protect the integrity of intestinal mucosa through activation of STAT3 signaling pathway induced by LPLs secretion of IL-22. Cell Death Differ, 25:1657-1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Bansal T, Alaniz RC, Wood TK, Jayaraman A (2010). The bacterial signal indole increases epithelial-cell tight-junction resistance and attenuates indicators of inflammation. Proc Natl Acad Sci U S A, 107:228-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Venkatesh M, Mukherjee S, Wang H, Li H, Sun K, Benechet AP, et al. (2014). Symbiotic bacterial metabolites regulate gastrointestinal barrier function via the xenobiotic sensor PXR and Toll-like receptor 4. Immunity, 41:296-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Wlodarska M, Luo C, Kolde R, d’Hennezel E, Annand JW, Heim CE, et al. (2017). Indoleacrylic Acid Produced by Commensal Peptostreptococcus Species Suppresses Inflammation. Cell Host Microbe, 22:25-37.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Jennis M, Cavanaugh CR, Leo GC, Mabus JR, Lenhard J, Hornby PJ (2018). Microbiota-derived tryptophan indoles increase after gastric bypass surgery and reduce intestinal permeability in vitro and in vivo. Neurogastroenterol Motil Off J Eur Gastrointest Motil Soc. doi: 10.1111/nmo.13178. [DOI] [PubMed] [Google Scholar]
- [202].Sanders ME, Merenstein DJ, Reid G, Gibson GR, Rastall RA (2019). Probiotics and prebiotics in intestinal health and disease: from biology to the clinic. Nat Rev Gastroenterol Hepatol, 16:605-616. [DOI] [PubMed] [Google Scholar]
- [203].Sakurai T, Odamaki T, Xiao J-Z (2019). Production of Indole-3-Lactic Acid by Bifidobacterium Strains Isolated fromHuman Infants. Microorganisms. doi: 10.3390/microorganisms7090340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Bonkowski MS, Sinclair DA (2016). Slowing ageing by design: the rise of NAD+ and sirtuin-activating compounds. Nat Rev Mol Cell Biol, 17:679-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Chini CCS, Tarragó MG, Chini EN (2017). NAD and the aging process: Role in life, death and everything in between. Mol Cell Endocrinol, 455:62-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Ma S, Yeom J, Lim Y-H (2020). Exogenous NAD+ Stimulates MUC2 Expression in LS 174T Goblet Cells via the PLC-Delta/PTGES/PKC-Delta/ERK/CREB Signaling Pathway. Biomolecules. doi: 10.3390/biom10040580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Igarashi M, Miura M, Williams E, Jaksch F, Kadowaki T, Yamauchi T, et al. (2019). NAD+ supplementation rejuvenates aged gut adult stem cells. Aging Cell, 18:e12935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Liu L, Su X, Quinn WJ, Hui S, Krukenberg K, Frederick DW, et al. (2018). Quantitative Analysis of NAD Synthesis-Breakdown Fluxes. Cell Metab, 27:1067-1080.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Chini EN, Chini CCS, Espindola Netto JM, de Oliveira GC, van Schooten W (2018). The Pharmacology of CD38/NADase: An Emerging Target in Cancer and Diseases of Aging. Trends Pharmacol Sci, 39:424-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Yoshida M, Satoh A, Lin JB, Mills KF, Sasaki Y, Rensing N, et al. (2019). Extracellular Vesicle-Contained eNAMPT Delays Aging and Extends Lifespan in Mice. Cell Metab, 30:329-342.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Espín JC, Larrosa M, García-Conesa MT, Tomás-Barberán F (2013). Biological significance of urolithins, the gut microbial ellagic Acid-derived metabolites: the evidence so far. Evid-Based Complement Altern Med ECAM, 2013:270418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Cortés-Martín A, García-Villalba R, González-Sarrías A, Romo-Vaquero M, Loria-Kohen V, Ramírez-de-Molina A, et al. (2018). The gut microbiota urolithin metabotypes revisited: the human metabolism of ellagic acid is mainly determined by aging. Food Funct, 9:4100-4106. [DOI] [PubMed] [Google Scholar]
- [213].Chen P, Chen F, Lei J, Li Q, Zhou B (2019). Activation of the miR-34a-Mediated SIRT1/mTOR Signaling Pathway by Urolithin A Attenuates D-Galactose-Induced Brain Aging in Mice. Neurother J Am Soc Exp Neurother, 16:1269-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Fang EF, Hou Y, Palikaras K, Adriaanse BA, Kerr JS, Yang B, et al. (2019). Mitophagy inhibits amyloid-β and tau pathology and reverses cognitive deficits in models of Alzheimer’s disease. Nat Neurosci, 22:401-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Kim KB, Lee S, Kim JH (2020). Neuroprotective effects of urolithin A on H2O2-induced oxidative stress-mediated apoptosis in SK-N-MC cells. Nutr Res Pract, 14:3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Zhao W, Shi F, Guo Z, Zhao J, Song X, Yang H (2018). Metabolite of ellagitannins, urolithin A induces autophagy and inhibits metastasis in human sw620 colorectal cancer cells. Mol Carcinog, 57:193-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Ahsan A, Zheng Y-R, Wu X-L, Tang W-D, Liu M-R, Ma S-J, et al. (2019). Urolithin A-activated autophagy but not mitophagy protects against ischemic neuronal injury by inhibiting ER stress in vitro and in vivo. CNS Neurosci Ther, 25:976-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Liu C-F, Li X-L, Zhang Z-L, Qiu L, Ding S-X, Xue J-X, et al. (2019). Antiaging Effects of Urolithin A on Replicative Senescent Human Skin Fibroblasts. Rejuvenation Res, 22:191-200. [DOI] [PubMed] [Google Scholar]
- [219].Singh R, Chandrashekharappa S, Bodduluri SR, Baby BV, Hegde B, Kotla NG, et al. (2019). Enhancement of the gut barrier integrity by a microbial metabolite through the Nrf2 pathway. Nat Commun, 10:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Heilman J, Andreux P, Tran N, Rinsch C, Blanco-Bose W (2017). Safety assessment of Urolithin A, a metabolite produced by the human gut microbiota upon dietary intake of plant derived ellagitannins and ellagic acid. Food Chem Toxicol, 108:289-297. [DOI] [PubMed] [Google Scholar]
- [221].Madeo F, Eisenberg T, Pietrocola F, Kroemer G (2018). Spermidine in health and disease. Science, 359(6374): eaan2788. [DOI] [PubMed] [Google Scholar]
- [222].Nishimura K, Shiina R, Kashiwagi K, Igarashi K (2006). Decrease in polyamines with aging and their ingestion from food and drink. J Biochem (Tokyo), 139:81-90. [DOI] [PubMed] [Google Scholar]
- [223].Gupta VK, Scheunemann L, Eisenberg T, Mertel S, Bhukel A, Koemans TS, et al. (2013). Restoring polyamines protects from age-induced memory impairment in an autophagy-dependent manner. Nat Neurosci, 16:1453-1460. [DOI] [PubMed] [Google Scholar]
- [224].Eisenberg T, Knauer H, Schauer A, Büttner S, Ruckenstuhl C, Carmona-Gutierrez D, et al. (2009). Induction of autophagy by spermidine promotes longevity. Nat Cell Biol, 11:1305-1314. [DOI] [PubMed] [Google Scholar]
- [225].Eisenberg T, Abdellatif M, Schroeder S, Primessnig U, Stekovic S, Pendl T, et al. (2016). Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat Med, 22:1428-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Zhu W-W, Xiao F, Tang Y-Y, Zou W, Li X, Zhang P, et al. (2018). Spermidine prevents high glucose-induced senescence in HT-22 cells by upregulation of CB1 receptor. Clin Exp Pharmacol Physiol, 45:832-840. [DOI] [PubMed] [Google Scholar]
- [227].Tirupathi Pichiah PB, Suriyakalaa U, Kamalakkannan S, Kokilavani P, Kalaiselvi S, SankarGanesh D, et al. (2011). Spermidine may decrease ER stress in pancreatic beta cells and may reduce apoptosis via activating AMPK dependent autophagy pathway. Med Hypotheses, 77:677-679. [DOI] [PubMed] [Google Scholar]
- [228].García-Prat L, Martínez-Vicente M, Perdiguero E, Ortet L, Rodríguez-Ubreva J, Rebollo E, et al. (2016). Autophagy maintains stemness by preventing senescence. Nature, 529:37-42. [DOI] [PubMed] [Google Scholar]
- [229].Wang J, Li S, Wang J, Wu F, Chen Y, Zhang H, et al. (2020). Spermidine alleviates cardiac aging by improving mitochondrial biogenesis and function. Aging, 12:650-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].Penrose HM, Marchelletta RR, Krishnan M, McCole DF (2013). Spermidine stimulates T cell protein-tyrosine phosphatase-mediated protection of intestinal epithelial barrier function. J Biol Chem, 288:32651-32662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Guo X, Rao JN, Liu L, Zou T, Keledjian KM, Boneva D, et al. (2005). Polyamines are necessary for synthesis and stability of occludin protein in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol, 288:G1159-1169. [DOI] [PubMed] [Google Scholar]
- [232].Soukas AA, Hao H, Wu L (2019). Metformin as Anti-Aging Therapy: Is It for Everyone? Trends Endocrinol Metab, 30:745-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233].Jin U-H, Cheng Y, Park H, Davidson LA, Callaway ES, Chapkin RS, et al. (2017). Short Chain Fatty Acids Enhance Aryl Hydrocarbon (Ah) Responsiveness in Mouse Colonocytes and Caco-2 Human Colon Cancer Cells. Sci Rep, 7:10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234].Hang S, Paik D, Yao L, Kim E, Trinath J, Lu J, et al. (2019). Bile acid metabolites control TH17 and Treg cell differentiation. Nature, 576:143-148. [DOI] [PMC free article] [PubMed] [Google Scholar]