Abstract
Background
Postoperative readmissions not only burden the healthcare system but may also affect clinical outcomes of cancer patients. Despite this, little is known about readmissions after cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC), or their impact on survival outcomes.
Patients and Methods
A single-institution retrospective cohort study of CRS-HIPEC procedures from April 2001 and September 2019 was performed. Early readmission (ERA) was defined as hospitalization within 30 days of discharge post-CRS/HIPEC, while late readmission (LRA) was defined as hospitalization between day 31 and 90 after discharge. Patient demographic, oncological, and perioperative factors were analyzed to identify predictors of readmission, and comparison of survival outcomes was performed.
Results
Overall, 342 patients who underwent CRS-HIPEC were included in the study. The incidence of ERA and LRA was 18.5% and 7.4%, respectively. High-grade postoperative complication was the only independent predictor of ERA (HR 3.64, 95% CI 1.47–9.02), while comorbid hypertension (HR 2.71, 95% CI 1.17–6.28) and stoma creation (HR 2.83, 95% CI 1.23–6.50) were independent predictors for LRA. Patients with readmission had significantly worse disease-free survival than patients who had no readmission (NRA) (LRA 1.1 years, ERA 1.2 years, NRA 1.8 years, p = 0.002), and patients with LRA had worse median overall survival (2.1 years) than ERA patients (3.3 years) or patients without readmission (4.4 years) (p < 0.001).
Conclusions
Readmission following CRS-HIPEC is associated with adverse survival outcomes. In particular, LRA may portend worse prognosis than ERA.
Over the past two decades, cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) have gradually gained acceptance as a treatment option for selected patients with peritoneal surface-based malignancies secondary to gastrointestinal or gynecological primaries.1–3 Complete cytoreduction during CRS is one of the most important predictors of survival outcomes.4 Therefore, it is common for multivisceral resection to be performed during CRS to ensure eradication of all macroscopic disease.5 As a result, reported rates of postoperative morbidity following CRS-HIPEC range from 10 to 50%, and mortality from 1 to 6%.6,7
In addition to morbidity and mortality, another postoperative metric attracting interest in the literature is postoperative readmission (RA) rates. Hospital readmissions after cancer-related surgeries not only contribute to higher costs of care but may also be associated with poorer clinical outcomes. For colon cancer-related colectomies, 1-year mortality for patients with 30-day RA versus without RA was reported to be 16% versus 7%, respectively,8 and similar trends were reported for patients with 90-day RA after surgery for bladder, esophageal, lung, and pancreatic cancers.9 Thirty-day RA rates post CRS-HIPEC have been reported to be between 11 and 24%, indications for which include digestive complications, pain, infection, and venous thromboembolism.10–13 Late RA occurring up to postoperative day 90 has been reported to occur at rates of up to 7.8–21%.14,15 Postoperative RA in patients who have undergone CRS-HIPEC not only poses a heavy financial burden on the healthcare system, but may also have significant implications for survival outcomes, as suggested by existing data on RA after other oncological surgeries.
Despite this, there is a paucity of data on predictors for hospital RA after CRS-HIPEC among the Asian population, and even fewer studies that evaluate its association with survival rates. To address these knowledge gaps, the aims of this study are to identify risk factors associated with early and late RA post CRS-HIPEC and their impact on oncologic outcomes.
Patients and Methods
Ethical approval from the SingHealth Centralised Institutional Review Board was obtained for the conduct of this retrospective cohort study. Data were retrieved from a prospectively maintained database of patients who had undergone CRS-HIPEC at National Cancer Centre Singapore.
Patient Selection
Patients were selected for CRS-HIPEC upon review and recommendation by a multidisciplinary tumor board discussion. All patients selected had Eastern Cooperative Group (ECOG) performance status of either 0 or 1 and no distant metastases as verified by either computed tomography (CT) scan or positron emission tomography (PET)-CT scan.
Patients who underwent CRS-HIPEC at our institution between April 2001 and September 2019 and were discharge from hospital were included in the study. Repeat CRS-HIPEC procedures of patients during the study period were excluded.
CRS-HIPEC
We previously described how CRS-HIPEC was performed at our institution.16 In brief, cytoreduction was performed as described by Sugarbaker.17 An intraperitoneal chemotherapy agent appropriate for the patient’s malignancy type was prescribed by the medical oncologist and administered intraoperatively via a hyperthermia pump into a closed abdomen at 41–42 °C for 60 min. The Peritoneal Cancer Index (PCI)17 was used to document the extent of peritoneal disease, while the completeness of cytoreduction (CC) score18 was recorded to quantify the extent of cytoreduction.
Postoperative Care
Following CRS-HIPEC, patients were typically monitored in the surgical intensive care unit (SICU) or high-dependency unit as deemed necessary by the primary surgeon and anesthetist. Postoperative complications were documented according to the Clavien–Dindo classification.19 Upon discharge, outpatient follow-up appointments were given at 1 week postdischarge, followed by a 1-month appointment, and thereafter 3-monthly appointments for 1 year, and 6-monthly appointments thereafter. Adjuvant chemotherapy was offered by medical oncologist as appropriate, and recurrences were documented.
Key Definitions
Patients were categorized into three readmission categories:
Early readmission (ERA) was defined as the first unplanned (i.e., emergency, nonelective) hospitalization within 30 days (inclusive) post discharge from index CRS/HIPEC.
Late readmission (LRA) was defined as hospitalization occurring from 31 to 90 days after discharge from index CRS/HIPEC.
No readmission (NRA) was defined as no readmission within 90 days after discharge from index CRS-HIPEC.
Disease-free survival (DFS) was defined as duration between CRS-HIPEC and first recurrence or death from any cause, whichever occurred first, while overall survival (OS) was defined as duration between CRS-HIPEC and death from any cause.
Patients who did not experience the stated events for DFS and OS were censored at their last follow-up date.
Statistics
For analysis of time to ERA and time to LRA, NRA patients were censored at day 90 post discharge from index CRS-HIPEC. For analysis of time to ERA, LRA and NRA patients were censored at day 30 post discharge from index CRS-HIPEC.
Patient demographic, oncological, operative, and postoperative factors were compared between ERA, LRA, and NRA using Fisher’s exact test and Kruskal–Wallis test for categorical and continuous variables, respectively. Cumulative incidence rate of RA was derived based on one minus the Kaplan–Meier estimate of the survival function for time to RA. Univariate and multivariable Cox proportional hazard (PH) regression models were used to examine the association of various factors with time to ERA and time to LRA. Variables with univariate p < 0.05 were included in the multivariable model. PH assumption was verified based on Schoenfeld residuals.
Follow-up duration was measured from discharge from CRS-HIPEC until date of last follow-up and estimated using the inverse Kaplan–Meier method. DFS and OS were estimated using Kaplan–Meier method. Differences in DFS and OS between patients in the three RA groups were compared using log-rank test.
Two-sided p value < 0.05 was considered statistically significant. All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC).
Results
Overall, 342 patients underwent CRS-HIPEC during the study duration. The demographics of the patients included in the study are summarized in Table 1. Our patient population had median age of 55 years, with ECOG status of 0 (87%) or 1 (12%). Colorectal cancer was the most common primary, accounting for nearly 40% of the cohort, followed by appendiceal cancer (25%), ovarian cancer (21%), and primary peritoneal disease (5%). Median PCI was 9, and after a median operative time of 495 min with median estimated blood loss of 1000 mL, CC-0 was achieved in 82% of cases, while CC-1 was achieved in 11%. Median length of hospitalization for CRS-HIPEC was 11 days (Table 2).
Table 1.
Demographics, clinical, and treatment characteristics
| Total (N = 342) | NRA (n = 259) | ERA (n = 60) | LRA (n = 23) | p value | |
|---|---|---|---|---|---|
| Demographic | |||||
| Age at CRS-HIPEC (years) | 55 (14–79) | 54 (22–79) | 56 (25–76) | 57 (14–74) | 0.677 |
| Gender | |||||
| Female | 237 (69.3) | 179 (69.1) | 44 (73.3) | 14 (60.9) | 0.537 |
| Male | 105 (30.7) | 80 (30.9) | 16 (26.7) | 9 (39.1) | |
| Ethnicity | |||||
| Chinese | 256 (74.9) | 187 (72.2) | 49 (81.7) | 20 (87.0) | 0.482 |
| Malay | 23 (6.7) | 17 (6.6) | 5 (8.3) | 1 (4.3) | |
| Indian | 16 (4.7) | 15 (5.8) | 1 (1.7) | 0 (–) | |
| Other | 47 (13.7) | 40 (15.4) | 5 (8.3) | 2 (8.7) | |
| Clinical | |||||
| ECOG performance status | |||||
| 0 | 296 (86.5) | 224 (86.5) | 53 (88.3) | 19 (82.6) | 0.782 |
| 1 | 41 (12.0) | 30 (11.6) | 7 (11.7) | 4 (17.4) | |
| Missing | 5 (1.5) | 5 (1.9) | 0 (–) | 0 (–) | |
| Comorbidities | |||||
| Absent | 124 (36.3) | 97 (37.5) | 20 (33.3) | 7 (30.4) | 0.766 |
| Present | 218 (63.7) | 162 (62.5) | 40 (66.7) | 16 (69.6) | |
| Type of comorbidities | |||||
| Hypertension | 95 (27.8) | 64 (24.7) | 21 (35.0) | 10 (43.5) | 0.056 |
| Diabetes | 42 (12.3) | 30 (11.6) | 10 (16.7) | 2 (8.7) | 0.514 |
| Hyperlipidemia | 68 (19.9) | 48 (18.5) | 16 (26.7) | 4 (17.4) | 0.341 |
| Ischemic heart disease | 9 (2.6) | 7 (2.7) | 2 (3.3) | 0 (–) | 0.827 |
| COPD | 2 (0.6) | 2 (0.8) | 0 (–) | 0 (–) | 1.000 |
| Asthma | 7 (2.0) | 3 (1.2) | 3 (5.0) | 1 (4.3) | 0.065 |
| Other malignancy | 23 (6.7) | 16 (6.2) | 5 (8.3) | 2 (8.7) | 0.668 |
| Others | 132 (38.6) | 105 (40.5) | 21 (35.0) | 6 (26.1) | 0.334 |
| Primary tumor site | |||||
| Colorectala | 129 (37.7) | 99 (38.2) | 19 (31.7) | 11 (47.8) | 0.052 |
| Ovarianb | 73 (21.3) | 52 (20.1) | 17 (28.3) | 4 (17.4) | |
| Peritoneal | 18 (5.3) | 11 (4.2) | 6 (10.0) | 1 (4.3) | |
| Appendix | 87 (25.4) | 73 (28.2) | 12 (20.0) | 2 (8.7) | |
| Mesothelioma | 13 (3.8) | 7 (2.7) | 4 (6.7) | 2 (8.7) | |
| Others | 22 (6.4) | 17 (6.6) | 2 (3.3) | 3 (13.0) | |
| PCI score | 9 (0–39) | 8 (0–39) | 13 (0–36) | 14 (0–31) | 0.088 |
| No. of patients with nonmissing data | 310 | 235 | 54 | 21 | |
| Ascites | |||||
| Absent | 200 (58.5) | 161 (62.2) | 29 (48.3) | 10 (43.5) | 0.113 |
| Present | 94 (27.5) | 63 (24.3) | 21 (35.0) | 10 (43.5) | |
| Missing | 48 (14.0) | 35 (13.5) | 10 (16.7) | 3 (13.0) | |
| Treatment | |||||
| CRS procedure | |||||
| Subdiaphragmatic stripping | 125 (36.5) | 88 (34.0) | 27 (45.0) | 10 (43.5) | 0.201 |
| Gastrectomy | 19 (5.6) | 15 (5.8) | 4 (6.7) | 0 (–) | 0.626 |
| Colectomy | 110 (32.2) | 79 (30.5) | 24 (40.0) | 7 (30.4) | 0.370 |
| Small bowel resection | 62 (18.1) | 42 (16.2) | 15 (25.0) | 5 (21.7) | 0.232 |
| Splenectomy | 58 (17.0) | 42 (16.2) | 14 (23.3) | 2 (8.7) | 0.274 |
| THBSO | 49 (14.3) | 33 (12.7) | 11 (18.3) | 5 (21.7) | 0.268 |
| Cholecystectomy | 57 (16.7) | 44 (17.0) | 11 (18.3) | 2 (8.7) | 0.618 |
| Bladder resection | 9 (2.6) | 5 (1.9) | 4 (6.7) | 0 (–) | 0.131 |
| Other procedure(s) | 100 (29.2) | 77 (29.7) | 15 (25.0) | 8 (34.8) | 0.623 |
| HIPEC agent | |||||
| Cisplatin | 109 (31.9) | 74 (28.6) | 25 (41.7) | 10 (43.5) | 0.090 |
| Mitomycin C | 215 (62.9) | 172 (66.4) | 32 (53.3) | 11 (47.8) | |
| Othersc | 12 (3.5) | 7 (2.7) | 3 (5.0) | 2 (8.7) | |
| Missing | 6 (1.8) | 6 (2.3) | 0 (–) | 0 (–) | |
| Duration of CRS-HIPEC, mins | 495 (245–1070) | 475 (245–1070) | 585 (285–1020) | 500 (310–795) | 0.016 |
| No. of patients with nonmissing data: | 297 | 221 | 54 | 22 | |
| CC score | |||||
| 0 | 279 (81.6) | 212 (81.9) | 46 (76.7) | 21 (91.3) | 0.155 |
| 1 | 36 (10.5) | 26 (10.0) | 10 (16.7) | 0 (–) | |
| 2 | 4 (1.2) | 3 (1.2) | 1 (1.7) | 0 (–) | |
| 3 | 1 (0.3) | 0 (–) | 1 (1.7) | 0 (–) | |
| Missing | 22 (6.4) | 18 (6.9) | 2 (3.3) | 2 (8.7) | |
| Chest tube placement | |||||
| No | 154 (45.0) | 126 (48.6) | 20 (33.3) | 8 (34.8) | 0.096 |
| Yes | 175 (51.2) | 122 (47.1) | 39 (65.0) | 14 (60.9) | |
| Missing | 13 (3.8) | 11 (4.2) | 1 (1.7) | 1 (4.3) | |
| Stoma creation | |||||
| No | 238 (69.6) | 190 (73.4) | 36 (60.0) | 12 (52.2) | 0.020 |
| Yes | 104 (30.4) | 69 (26.6) | 24 (40.0) | 11 (47.8) | |
| Estimated blood loss (ml) | 1000 (0–11,000) | 900 (0–11,000) | 1000 (200–5100) | 800 (0–3500) | 0.439 |
| No. of patients with nonmissing data: | 327 | 248 | 58 | 21 | |
| Intraoperative blood transfusions | |||||
| No | 110 (32.2) | 90 (34.7) | 13 (21.7) | 7 (30.4) | 0.236 |
| Yes | 224 (65.5) | 163 (62.9) | 46 (76.7) | 15 (65.2) | |
| Missing | 8 (2.3) | 6 (2.3) | 1 (1.7) | 1 (4.3) | |
NRA No readmission, ERA Early readmission, LRA Late readmission, CRS Cytoreduction surgery, HIPEC Hyperthermic intraperitoneal chemotherapy, ECOG Eastern Cooperative Oncology Group, COPD Chronic obstructive pulmonary disease, PCI Peritoneal cancer index, THBSO Total abdominal hysterectomy with bilateral salpingo-oophorectomy, CC Completeness of cytoreduction
Data presented as median (range) if variable is continuous, and number (%) if variable is categorical
p value based on Kruskal–Wallis test for continuous variable and Fisher’s exact test for categorical variable
aIncluded one patient who had an additional primary tumor in endometrium
bIncluded one patient who had an additional primary gastric tumor
cIncluded doxorubicin, oxaliplatin, and fluorouracil
Table 2.
Postoperative characteristics and recurrence
| Total (N = 342) | NRA (n = 259) | ERA (n = 60) | LRA (n = 23) | p value | |
|---|---|---|---|---|---|
| Postoperative complications | |||||
| No | 145 (42.4) | 122 (47.1) | 15 (25.0) | 8 (34.8) | 0.006 |
| Yes | 197 (57.6) | 137 (52.9) | 45 (75.0) | 15 (65.2) | |
| Worst grade of postoperative complications | |||||
| No complication | 145 (42.4) | 122 (47.1) | 15 (25.0) | 8 (34.8) | < 0.001 |
| G1 | 48 (14.0) | 37 (14.3) | 7 (11.7) | 4 (17.4) | |
| G2 | 92 (26.9) | 69 (26.6) | 17 (28.3) | 6 (26.1) | |
| G3 | 44 (12.9) | 20 (7.7) | 19 (31.7) | 5 (21.7) | |
| G4 | 13 (3.8) | 11 (4.2) | 2 (3.3) | 0 (–) | |
| Length of SICU stay (days) | 0 (0–40) | 0 (0–40) | 1 (0–5) | 1 (0–3) | 0.015 |
| No. of patients with nonmissing data | 341 | 258 | 60 | 23 | |
| Length of hospital stay (days) | 11 (5–141) | 11 (5–141) | 14 (7–66) | 13 (8–86) | 0.001 |
| No. of recurred patients | 163 | 116 | 32 | 15 | – |
| Site of relapse among recurred patients: | |||||
| Peritoneum | 110 (67.5) | 82 (70.7) | 18 (56.3) | 10 (66.7) | 0.293 |
| Lymph nodes | 36 (22.1) | 22 (19.0) | 11 (34.4) | 3 (20.0) | 0.181 |
| Lung | 38 (23.3) | 29 (25.0) | 5 (15.6) | 4 (26.7) | 0.490 |
| Liver | 36 (22.1) | 22 (19.0) | 9 (28.1) | 5 (33.3) | 0.249 |
| Bone | 7 (4.3) | 4 (3.4) | 0 (–) | 3 (20.0) | 0.016 |
| Skin | 0 (–) | 0 (–) | 0 (–) | 0 (–) | – |
| Others | 40 (24.5) | 28 (24.1) | 5 (15.6) | 7 (46.7) | 0.083 |
NRA No readmission, ERA Early readmission, LRA Late readmission, SICU Surgical intensive care unit
Data presented as median (range) if variable is continuous, and number (%) if variable is categorical
p value based on Kruskal–Wallis test for continuous variable and Fisher’s exact test for categorical variable
Sixty patients had ERA, 23 had LRA, and 259 had NRA within 90 days post discharge from CRS-HIPEC. Median time to ERA and LRA was 8 and 51 days, respectively.
Median age between RA groups was comparable, as was the distribution of ECOG status. Ovarian (ERA 28% versus LRA 17%), appendiceal (ERA 20% versus LRA 9%), and peritoneal (ERA 10% versus LRA 4.3%) primaries were more common in ERA group, while colorectal primary was more common is LRA group (LRA 48% versus ERA 32%), but these differences in distribution did not reach statistical significance. Median PCI score was comparable between RA groups, and CC-0 score was achieved in 77% of LRA group and 91% of ERA group (p = 0.155). The most common cytoreductive procedure performed across both readmission groups was subdiaphragmatic stripping, followed by colectomy and small bowel resection.
Causes of Readmission Following CRS-HIPEC
The majority (46%) of RA were due to gastrointestinal complaints, such as abdominal pain, bloatedness, nausea, and vomiting (Table 3). A total of 15% of RA were a result of stoma-related complications (e.g., high stoma output), and 8% from superficial wound infections. Although there was a higher percentage of LRA patients (26%) with stoma-related readmission compared with ERA patients (10%), on balance reasons for RA were similar between these two groups (p = 0.450).
Table 3.
Readmission characteristics
| Total (N = 83) | ERA (n = 60) | LRA (n = 23) | p value | |
|---|---|---|---|---|
| Readmission reason | ||||
| GI symptoms | 38 (45.8) | 29 (48.3) | 9 (39.1) | 0.450 |
| Wound infection | 7 (8.4) | 5 (8.3) | 2 (8.7) | |
| Other infection | 4 (4.8) | 3 (5.0) | 1 (4.3) | |
| Stoma related | 12 (14.5) | 6 (10.0) | 6 (26.1) | |
| Others | 22 (26.5) | 17 (28.3) | 5 (21.7) | |
| Death within 30 days of readmission | ||||
| Excluding alive patients with < 30 days follow-up: | 82 | 60 | 22 | |
| No | 81 (98.8) | 59 (98.3) | 22 (100) | 1.000 |
| Yes | 1 (1.2) | 1 (1.7) | 0 (–) | |
ERA Early readmission, LRA Late readmission
Data presented as number (%)
p value based on Fisher’s exact test
Factors Affecting Readmissions Following CRS-HIPEC
Comparison of operative and postoperative variables showed that, compared with the NRA and LRA groups, patients with ERA had the longest duration of CRS-HIPEC [median 475 min (NRA) versus 500 min (LRA) versus 585 min (ERA); p = 0.016], highest percentage with grade III–IV postoperative complication [12% (NRA) versus 22% (LRA) versus 35% (ERA); p < 0.001] and longest index hospital admission for CRS-HIPEC [median 11 days (NRA) versus 13 days (LRA) versus 14 days (ERA); p = 0.001] (Tables 1 and 2).
There were significantly more patients who had stoma created at index CRS-HIPEC among LRA group (48%) than ERA (40%) or NRA (27%) (p = 0.020). Demographic and oncological variables showed no significant difference between the three readmission groups (Table 1).
Univariate Cox regression analysis identified eight significant predictors for ERA: PCI score, intraoperative bladder resection, intraoperative chest tube placement, duration of CRS-HIPEC, CC score, intraoperative blood transfusion, duration of hospital stay, and high-grade postoperative complication (grade III–IV). On multivariate analysis, only high-grade postoperative complication continued to be significant (HR 3.64, 95% CI 1.47–9.02; relative to no complications) (Table 4). For LRA, the variables significant on univariate analysis, which also remained significant on multivariate analysis, were presence of hypertension (HR 2.71, 95% CI 1.17–6.28) and stoma creation (HR 2.83, 95% CI 1.23–6.50) (Table 5).
Table 4.
Predictors of early readmission
| Univariate Cox | Multivariable Cox | |||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Age at CRS-HIPEC (per year increase) | 1.01 (0.98–1.03) | 0.612 | ||
| Gender: male versus female | 0.79 (0.45–1.40) | 0.424 | ||
| Ethnicity: Malay versus Chinese | 1.32 (0.53–3.31) | 0.554 | ||
| Ethnicity: Indian versus Chinese | 0.39 (0.05–2.81) | 0.350 | ||
| Ethnicity: others versus Chinese | 0.54 (0.21–1.35) | 0.184 | ||
| ECOG performance status: 1 versus 0 | 1.00 (0.46–2.20) | 0.998 | ||
| Comorbidities: absent versus present | 0.83 (0.48–1.42) | 0.489 | ||
| Hypertension: yes versus no | 1.48 (0.87–2.52) | 0.148 | ||
| Diabetes: yes versus no | 1.61 (0.82–3.17) | 0.171 | ||
| Hyperlipidemia: yes versus no | 1.47 (0.83–2.61) | 0.186 | ||
| Ischemic heart disease: yes versus no | 1.29 (0.31–5.27) | 0.726 | ||
| COPD: yes versus no | UD | 0.985 | ||
| Asthma: yes versus no | 3.07 (0.96–9.81) | 0.058 | ||
| Other malignancy: yes versus no | 1.42 (0.57–3.54) | 0.456 | ||
| Other comorbidities: yes versus no | 0.86 (0.50–1.45) | 0.563 | ||
| Tumor site: ovarian versus colorectal | 1.67 (0.87–3.21) | 0.125 | ||
| Tumor site: peritoneal versus colorectal | 2.38 (0.95–5.95) | 0.065 | ||
| Tumor site: appendix versus colorectal | 0.98 (0.48–2.02) | 0.952 | ||
| Tumor site: mesothelioma versus colorectal | 2.44 (0.83–7.19) | 0.105 | ||
| Tumor site: others versus colorectal | 0.58 (0.14–2.49) | 0.464 | ||
| PCI score (per unit increase) | 1.03 (1.00–1.06) | 0.038 | 0.98 (0.94–1.03) | 0.446 |
| Had ascites: yes versus no | 1.64 (0.93–2.87) | 0.086 | ||
| Subdiaphragmatic stripping: yes versus no | 1.49 (0.89–2.47) | 0.128 | ||
| Gastrectomy: yes versus no | 1.21 (0.44–3.34) | 0.712 | ||
| Colectomy: yes versus no | 1.52 (0.91–2.55)a | 0.111 | ||
| Small bowel resection: yes versus no | 1.56 (0.87–2.80) | 0.136 | ||
| Splenectomy: yes versus no | 1.53 (0.84–2.78) | 0.166 | ||
| THBSO: yes versus no | 1.47 (0.77–2.83) | 0.247 | ||
| Cholecystectomy: yes versus no | 1.17 (0.61–2.25) | 0.638 | ||
| Bladder resection: yes versus no | 2.99 (1.08–8.24) | 0.035 | Noteb | |
| Other CRS procedure(s): yes versus no | 0.77 (0.43–1.39) | 0.389 | ||
| HIPEC agent: mitomycin C versus cisplatin | 0.64 (0.38–1.08) | 0.093 | ||
| HIPEC agent: others versus cisplatin | 1.14 (0.34–3.77) | 0.832 | ||
| Duration of CRS-HIPEC (per 10min increase) | 1.02 (1.01–1.04) | 0.007 | 1.01 (0.98–1.04) | 0.528 |
| CC score: ≥1 versus 0 | 1.99 (1.06–3.76) | 0.034 | 1.49 (0.59–3.80) | 0.401 |
| Chest tube placement: yes versus no | 1.88 (1.10–3.22) | 0.022 | 1.12 (0.53–2.38) | 0.767 |
| Stoma creation: yes versus no | 1.65 (0.99–2.77) | 0.056 | ||
| Blood loss (per 100-ml increase) | 1.01 (0.99–1.03) | 0.425 | ||
| Intraoperative blood transfusion: yes versus no | 1.86 (1.00–3.44) | 0.049 | 1.36 (0.61–3.06) | 0.450 |
| Postoperative complication: G1–G2 versus none | 1.72 (0.90–3.29) | 0.098 | 1.86 (0.89–3.89) | 0.098 |
| Postoperative complication: G3–G4 versus none | 4.49 (2.31–8.71) | < 0.001 | 3.64 (1.47–9.02) | 0.005 |
| Length of SICU stay (per day increase) | 1.00 (0.94–1.08) | 0.917 | ||
| Length of hospital stay (per day increase) | 1.01 (1.00–1.03) | 0.028 | 1.00 (0.97–1.02) | 0.758 |
HR Hazard ratio, CI Confidence interval, CRS Cytoreduction surgery, HIPEC Hyperthermic intraperitoneal chemotherapy, ECOG Eastern Cooperative Oncology Group, COPD Chronic obstructive pulmonary disease, PCI Peritoneal cancer index, THBSO Total abdominal hysterectomy with bilateral salpingo-oophorectomy, CC Completeness of cytoreduction, SICU Surgical intensive care unit, UD No event in one of the categories
p value based on Wald’s test
aViolated PH assumption
bExcluded from model due to small sample size
Table 5.
Predictors of late readmission
| Univariate Cox | Multivariable Cox | |||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Age at CRS-HIPEC (per year increase) | 1.00 (0.97–1.04) | 0.888 | ||
| Gender: male versus female | 1.34 (0.58–3.08) | 0.499 | ||
| Ethnicity: Malay versus Chinese | 0.66 (0.09–4.92) | 0.686 | ||
| Ethnicity: Indian versus Chinese | UD | 0.990 | ||
| Ethnicity: others versus Chinese | 0.46 (0.11–1.98) | 0.299 | ||
| ECOG performance status: 1 versus 0 | 1.85 (0.63–5.44) | 0.263 | ||
| Comorbidities: absent versus present | 0.69 (0.28–1.68) | 0.415 | ||
| Hypertension: yes versus no | 2.28 (1.00–5.21) | 0.050 | 2.71 (1.17–6.28) | 0.020 |
| Diabetes: yes versus no | 0.81 (0.19–3.47) | 0.779 | ||
| Hyperlipidemia: yes versus no | 0.90 (0.31–2.64) | 0.843 | ||
| Ischemic heart disease: yes versus no | UD | 0.992 | ||
| COPD: yes versus no | UD | 0.990 | ||
| Asthma: yes versus no | 4.60 (0.62–34.12) | 0.136 | ||
| Other malignancy: yes versus no | 1.46 (0.34–6.22) | 0.610 | ||
| Other comorbidities: yes versus no | 0.53 (0.21–1.36) | 0.187 | ||
| Tumor site: ovarian versus colorectal | 0.76 (0.24–2.39) | 0.638 | ||
| Tumor site: peritoneal versus colorectal | 0.81 (0.11–6.29) | 0.841 | ||
| Tumor site: appendix versus colorectal | 0.26 (0.06–1.17) | 0.079 | ||
| Tumor site: mesothelioma versus colorectal | 2.24 (0.50–10.10) | 0.295 | ||
| Tumor site: others versus colorectal | 1.43 (0.40–5.11) | 0.587 | ||
| PCI score (per unit increase) | 1.01 (0.97–1.06) | 0.646 | ||
| Had ascites: yes versus no | 2.36 (0.98–5.66)a | 0.055 | ||
| Subdiaphragmatic stripping: yes versus no | 1.41 (0.62–3.21) | 0.419 | ||
| Gastrectomy: yes versus no | UD | 0.988 | ||
| Colectomy: yes versus no | 0.99 (0.41–2.41) | 0.988 | ||
| Small bowel resection: yes versus no | 1.38 (0.51–3.71) | 0.528 | ||
| Splenectomy: yes versus no | 0.45 (0.11–1.91) | 0.278 | ||
| THBSO: yes versus no | 1.86 (0.69–5.00) | 0.222 | ||
| Cholecystectomy: yes versus no | 0.46 (0.11–1.96) | 0.294 | ||
| Bladder resection: yes versus no | UD | 0.989 | ||
| Other CRS procedure(s): yes versus no | 1.17 (0.50–2.76) | 0.721 | ||
| HIPEC agent: mitomycin C versus cisplatin | 0.48 (0.20–1.13) | 0.093 | ||
| HIPEC agent: others versus cisplatin | 1.67 (0.37–7.62) | 0.508 | ||
| Duration of CRS-HIPEC (per 10-min increase) | 1.01 (0.98–1.04) | 0.469 | ||
| CC score: ≥ 1 versus 0 | UD | 0.990 | ||
| Chest tube placement: yes versus no | 1.81 (0.76–4.32) | 0.181 | ||
| Stoma creation: yes versus no | 2.41 (1.06–5.46) | 0.035 | 2.83 (1.23–6.50) | 0.015 |
| Blood loss (per 100-ml increase) | 0.99 (0.95–1.04) | 0.730 | ||
| Intraoperative blood transfusion: yes versus no | 1.16 (0.47–2.85) | 0.743 | ||
| Postoperative complication: G1–G2 versus none | 1.38 (0.54–3.49) | 0.500 | ||
| Postoperative complication: G3–G4 versus none | 2.42 (0.79–7.39) | 0.122 | ||
| Length of SICU stay (per day increase) | 0.96 (0.79–1.15) | 0.631 | ||
| Length of hospital stay (per day increase) | 1.01 (0.99–1.03) | 0.194 | ||
HR hazard ratio, CI confidence interval, CRS cytoreduction surgery, HIPEC hyperthermic intraperitoneal chemotherapy, ECOG Eastern Cooperative Oncology Group, COPD chronic obstructive pulmonary disease, PCI peritoneal cancer index, THBSO total abdominal hysterectomy with bilateral salpingo-oophorectomy, CC completeness of cytoreduction, SICU surgical intensive care unit, UD no event in one of the categories
p value based on Wald’s test
aViolated PH assumption
Relationship Between Readmission and Survival Outcomes
The median follow-up of NRA, ERA, and LRA groups was 2.0, 4.5, and 1.9 years, respectively.
Disease-Free Survival
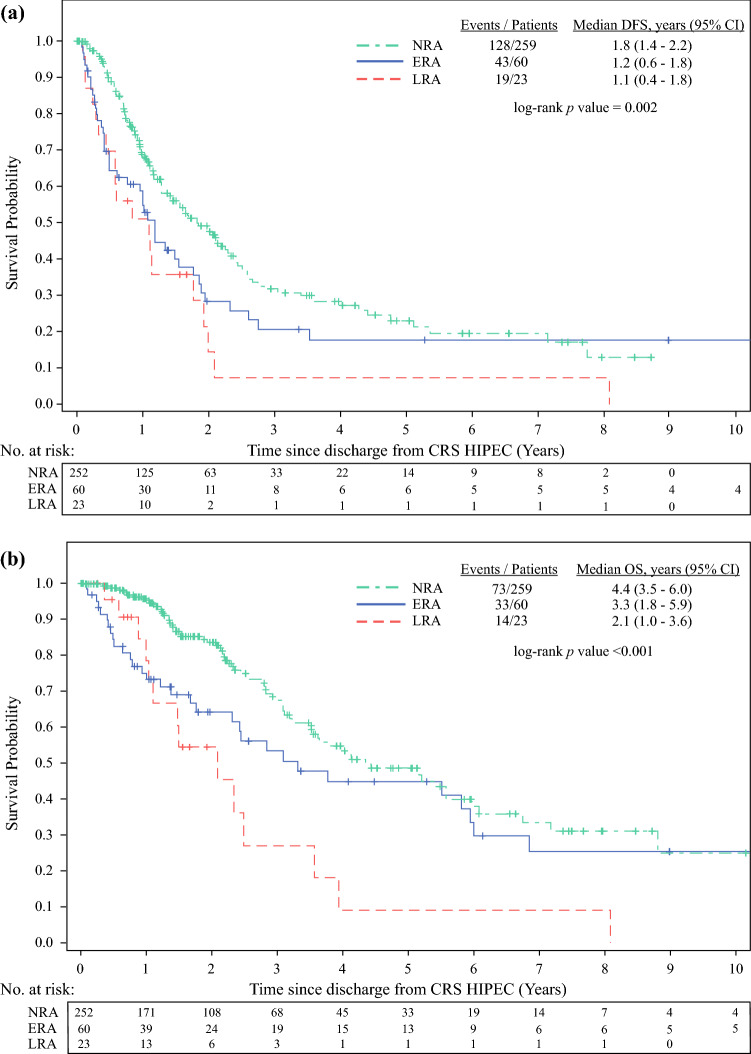
Patients with readmission had significantly worse DFS than NRA patients (Fig. 1). Median DFS was 1.1 years (95% CI 0.4–1.8 years) for LRA patients and 1.2 years (95% CI 0.6–1.8 years) for ERA patients, both being lower than the corresponding 1.8 years (95% CI 1.4–2.2 years) for NRA patients (p = 0.002; Table 6, Fig. 1a).
Fig. 1.
Kaplan–Meier curves of a DFS and b OS stratified by ERA, LRA, and NRA
Table 6.
Survival outcomes
| Total (N = 342) | NRA (n = 259) | ERA (n = 60) | LRA (n = 23) | p value* | |
|---|---|---|---|---|---|
| Follow-up duration (years) | |||||
| Median (95% CI) | 2.1 (1.7–2.3) | 2.0 (1.5–2.2) | 4.5 (1.8–9.0) | 1.9 (0.8–NE) | 0.003 |
| Disease-free survival (DFS) | |||||
| No. of recurrences/deaths | 190 | 128 | 43 | 19 | 0.002 |
| Median DFS, years (95% CI) | 1.6 (1.3–1.9) | 1.8 (1.4–2.2) | 1.2 (0.6–1.8) | 1.1 (0.4–1.8) | |
| 6-Month DFS, % (95% CI) | 82.7 (78.0–86.5) | 88.8 (83.9–92.3) | 64.0 (50.3–74.8) | 69.6 (46.6–84.2) | |
| 1-Year DFS, % (95% CI) | 65.5 (59.7–70.7) | 68.7 (61.9–74.6) | 58.4 (44.6–69.9) | 50.6 (28.6–69.0) | |
| 2-Year DFS, % (95% CI) | 41.4 (35.1–47.7) | 48.1 (40.4–55.3) | 28.0 (16.2–41.1) | 14.2 (2.6–35.1) | |
| Overall survival (OS) | |||||
| No. of deaths | 120 | 73 | 33 | 14 | < 0.001 |
| Median OS, years (95% CI) | 3.9 (3.2–5.4) | 4.4 (3.5–6.0) | 3.3 (1.8–5.9) | 2.1 (1.0–3.6) | |
| 1-Year OS, % (95% CI) | 90.7 (86.7–93.5) | 95.8 (92.0–97.8) | 75.1 (61.6–84.5) | 78.6 (52.0–91.5) | |
| 2-Year OS, % (95% CI) | 77.8 (71.9–82.6) | 83.6 (77.0–88.5) | 64.1 (49.4–75.6) | 54.4 (28.8–74.2) | |
NE not estimable
*Based on log-rank test
Numbers in bold based on small no. of patients at risk
Overall Survival
LRA patients had worst median OS (2.1 years, 95% CI 1.0–3.6 years), followed by ERA patients (3.3 years, 95% CI 1.8–5.9 years) and NRA patients (4.4 years, 95% CI 3.5–6.0 years) (p < 0.001; Table 6; Fig. 1b).
Discussion
Internationally, reported rates of postoperative readmission after CRS-HIPEC range from 14.8 to 15.9% for ERA and from 3.9 to 11% for LRA.11,15,20 Known predictors include older age, number of previous surgical procedures, postoperative complications, and length of index hospitalization.10 Lee et al. went on to the compare the differences in predictors for RA at 30 versus 31–90 days. ECOG of 3 or more, intraoperative splenectomy, low anterior resection, partial colectomy, and stoma creation were independent predictors of 30-day RA, while gastric tumor, operative time, intraoperative low anterior resection or partial colectomy, and stoma creation were predictors for 31–90-day RA.11 Beyond 90 days, age and intraoperative colonic resection have been reported as the only independent risk factors for 6-month readmission.21 In our study cohort, we found similar rates of early and late readmission of 18.5% and 7.4%, respectively, with a majority of RA occurring within 2 weeks post discharge. Only high-grade postoperative complications, stoma creation, and hypertension predicted ERA and LRA. In our study population, 57 patients (16.7%) had high-grade complications, and 21 of these patients had ERA. In contrast, 176 patients (51.5%) had risk factors for LRA, including those with both hypertension and stoma creation, hypertension only, and stoma creation only. Compared with patients with no LRA risk factors, risk of LRA was highest amongst patients with both hypertension and stoma creation (HR = 7.67, n = 23 [6.7%]), followed by patients with stoma creation only [HR = 2.83, n = 81 (23.7%)] and patients with hypertension only [HR = 2.71, n = 72 (21.1%)], with our Cox model suggesting that these two risk factors have a multiplicative effect in predisposing patients to late readmission.
Postoperative readmission has been reported to be related to adverse survival outcomes in patients who had undergone surgery for cancers of various organs, such as brain, pancreas, esophagus, and stomach.22–25 Proposed contributors to this correlation include postoperative complications, infection, and metastatic disease.24,25 Others have also reported that readmission within 30 days of surgery is associated with delay in postoperative chemotherapy, which in turn is associated with poorer DFS and OS.26 In the context of post-pancreatic-cancer surgery, Reddy et al. reported that, compared with NRA, 0–30-day readmissions had lower median OS but comparable 5-year survival. Meanwhile 30–365-day readmissions had both lower median as well as 5-year survival compared with those without 30–365-day readmission.23 These findings suggested that, if the patients who required ERA survive the first few years following the index operation, their long-term outcome is comparable to those who had no readmission, whereas patients who required LRA have worse long-term outcomes regardless. We found that ERA and LRA patients had comparable median DFS, though significantly lower than NRA. Overall survival was worst amongst LRA, followed by ERA and NRA.
To the best of the authors’ knowledge, this study is the first to compare survival outcomes in ERA, LRA, and NRA patients post CRS-HIPEC. Unfortunately, for our study, the median follow-up duration was not long enough to comment on 5-year survival outcomes. However, the survival curves at the 5-year mark seem to resonate the survival patterns reported by Reddy et al. The exact reason for these patterns of survival outcomes lies beyond the scope of this study. However, in broad conceptual terms, it may be reasonable to speculate that, post CRS-HIPEC, ERA is associated with potentially significant yet reversible causes, while LRA involves both significant and irreversible pathologies.
The adverse effect of postoperative morbidity on survival outcomes has been reported in literature for both oncological and nononcological surgeries.27–29 In the past, our center has also reported the association of high-grade complications with poor OS among post CRS-HIPEC patients; the 5-year OS rate of patients who experienced no postoperative, low-grade, and high-grade complications was found to be 52.8%, 37.0%, and 43.0%, respectively.30 As the results of the current study also show that high-grade morbidity is the sole independent risk factor for early readmission, it would be reasonable to infer that postoperative morbidity contributes to the association of ERA with poor survival outcomes.
Up to 30% of our CRS-HIPEC patients required stoma creation, a majority of which were defunctioning ileostomies for colorectal resection. Furthermore, intraoperative stoma creation was found to be a significant predictor of LRA, which was in turn associated with poorer survival outcomes. In patients with primary colorectal malignancies without peritoneal disease, post-stoma readmissions are known to be common and often occur within 30 days of discharge.31–33 Common early complications after stoma creation include skin irritation, pain, stoma retraction, and necrosis, while later in the clinical course, stoma patients may experience parastomal hernia, prolapse, stenosis, high output, and nutritional deficiencies.34,35 Our analysis of stoma formation in the setting of CRS-HIPEC found there was a greater percentage of stoma patients among the LRA than ERA group, from which we can cautiously infer that, for post CRS-HIPEC readmissions, stoma creation and its late complications may play a greater role in predisposing a patient to LRA. A small subgroup analysis of the 12 patients who were readmitted for stoma-related reasons showed that the vast majority of ERA was due to high stoma output (83%), while for LRA only 50% presented with high stoma output and complications related to stoma reversal accounted for a sizable proportion (33%). However, these figures are limited by small sample size, and a detailed analysis of stoma type, timing of reversal, related complications, and their association with readmissions and survival requires further investigation.
Based on the association of unplanned readmission with adverse survival outcomes seen in our results, future studies should investigate interventions that may reduce readmission and hence improve survival. One such intervention aimed to reduce LRA may include vigilant patient education and follow-up for those who have stoma creation during CRS-HIPEC, with special attention to known late complications such as parastomal herniations or post reversal strictures, infections, or anastomotic leak/breakdown. As our study also found that nearly half of all readmissions occurred within the first 15 days post discharge, with the majority presenting with gastrointestinal symptoms, it would be prudent to investigate whether early postoperative intervention with comprehensive discharge planning that includes appropriate discharge advice, streamlined wound care, dietician review, and close follow-up within the first month may help to off-load the high rates of ERA and provide survival benefits.
The retrospective design and relatively small number in this study may have resulted in selection bias and failure to elucidate other factors that may contribute to the differences in survival outcomes seen in the respective readmission groups. Longer follow-up may be required to further identify factors affecting long-term survival outcomes in our study population.
Conclusions
In this study conducted at the largest CRS-HIPEC center in Southeast Asia, unplanned postoperative readmission occurred at a rate of 18.5% for 30 days post discharge and 7.4% for 31–90 days. Unique sets of independent predictors were identified for these two readmission types: high-grade postoperative complication was a predictor of ERA, while stoma creation and hypertension were predictors for LRA. In addition, there were worse survival outcomes for patients with LRA as compared with ERA and NRA. Future studies may need to explore the association of poor survival with readmissions, to better identify effective measures to minimize unplanned hospitalizations post CRS-HIPEC.
Acknowledgment
This study is funded by NCCS Cancer Fund. C.-A.J.O. is supported by the National Research Council Transition Award (NMRC/TA/0061/2017).
Disclosures
None.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Huang C-Q, Min Y, Wang S-Y, et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy improves survival for peritoneal carcinomatosis from colorectal cancer: a systematic review and meta-analysis of current evidence. Oncotarget. 2017;8(33):55657–55683. doi: 10.18632/oncotarget.17497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chua TC, Moran BJ, Sugarbaker PH, et al. Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol. 2012;30(20):2449–2456. doi: 10.1200/JCO.2011.39.7166. [DOI] [PubMed] [Google Scholar]
- 3.Huo YR, Richards A, Liauw W, Morris DL. Hyperthermic intraperitoneal chemotherapy (HIPEC) and cytoreductive surgery (CRS) in ovarian cancer: a systematic review and meta-analysis. Eur J Surg Oncol. 2015;41(12):1578–1589. doi: 10.1016/j.ejso.2015.08.172. [DOI] [PubMed] [Google Scholar]
- 4.Sugarbaker PH, Jablonski KA. Prognostic features of 51 colorectal and 130 appendiceal cancer patients with peritoneal carcinomatosis treated by cytoreductive surgery and intraperitoneal chemotherapy. Ann Surg. 1995;221(2):124–132. doi: 10.1097/00000658-199502000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wagner PL, Austin F, Maduekwe U, et al. Extensive cytoreductive surgery for appendiceal carcinomatosis: morbidity, mortality, and survival. Ann Surg Oncol. 2013;20(4):1056–1062. doi: 10.1245/s10434-012-2791-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartlett EK, Meise C, Roses RE, Fraker DL, Kelz RR, Karakousis GC. Morbidity and mortality of cytoreduction with intraperitoneal chemotherapy: outcomes from the ACS NSQIP database. Ann Surg Oncol. 2014;21(5):1494–1500. doi: 10.1245/s10434-013-3223-z. [DOI] [PubMed] [Google Scholar]
- 7.Chua TC, Yan TD, Saxena A, Morris DL. Should the treatment of peritoneal carcinomatosis by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy still be regarded as a highly morbid procedure? A systematic review of morbidity and mortality. Ann Surg. 2009;249(6):900–907. doi: 10.1097/SLA.0b013e3181a45d86. [DOI] [PubMed] [Google Scholar]
- 8.Greenblatt DY, Weber SM, O'Connor ES, LoConte NK, Liou JI, Smith MA. Readmission after colectomy for cancer predicts one-year mortality. Ann Surg. 2010;251(4):659–669. doi: 10.1097/SLA.0b013e3181d3d27c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stitzenberg KB, Chang Y, Smith AB, Nielsen ME. Exploring the burden of inpatient readmissions after major cancer surgery. J Clin Oncol. 2015;33(5):455–464. doi: 10.1200/JCO.2014.55.5938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paredes AZ, Abdel-Misih S, Schmidt C, Dillhoff ME, Pawlik TM, Cloyd JM. Predictors of readmission after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Surg Res. 2019;234:103–109. doi: 10.1016/j.jss.2018.09.022. [DOI] [PubMed] [Google Scholar]
- 11.Lee TC, Wima K, Sussman JJ, et al. Readmissions after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: a US HIPEC collaborative study. J Gastroint Surg. 2020;24(1):165–176. doi: 10.1007/s11605-019-04463-y. [DOI] [PubMed] [Google Scholar]
- 12.Jafari MD, Halabi WJ, Stamos MJ, et al. Surgical outcomes of hyperthermic intraperitoneal chemotherapy: analysis of the American College of Surgeons National Surgical Quality Improvement Program. JAMA Surg. 2014;149(2):170–175. doi: 10.1001/jamasurg.2013.3640. [DOI] [PubMed] [Google Scholar]
- 13.Votanopoulos KI, Swords DS, Swett KR, et al. Obesity and peritoneal surface disease: outcomes after cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for appendiceal and colon primary tumors. Ann Surg Oncol. 2013;20(12):3899–3904. doi: 10.1245/s10434-013-3087-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spiliotis J, Argiriou EO, Vafias E, et al. Re-admissions for delayed complications after cytoreductive surgery and HIPEC. Acta Chir Belg. 2016;116(2):96–100. doi: 10.1080/00015458.2016.1165019. [DOI] [PubMed] [Google Scholar]
- 15.Martin AS, Abbott DE, Hanseman D, et al. Factors associated with readmission after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis. Ann Surg Oncol. 2016;23(6):1941–1947. doi: 10.1245/s10434-016-5109-3. [DOI] [PubMed] [Google Scholar]
- 16.Teo MC, Tan GH, Tham CK, Lim C, Soo KC. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in Asian patients: 100 consecutive patients in a single institution. Ann Surg Oncol. 2013;20(9):2968–2974. doi: 10.1245/s10434-013-2947-0. [DOI] [PubMed] [Google Scholar]
- 17.Sugarbaker PH. Peritonectomy procedures. Ann Surg. 1995;221(1):29–42. doi: 10.1097/00000658-199501000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res. 1996;82:359–374. doi: 10.1007/978-1-4613-1247-5_23. [DOI] [PubMed] [Google Scholar]
- 19.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dreznik Y, Hoffman A, Hamburger T, et al. Hospital readmission rates and risk factors for readmission following cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) for peritoneal surface malignancies. Surgeon. 2018;16(5):278–282. doi: 10.1016/j.surge.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 21.Dranichnikov P, Graf W, Cashin PH. Readmissions after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy—a national population-based study. World J Surg Oncol. 2020;18(1):67. doi: 10.1186/s12957-020-01837-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dickinson H, Carico C, Nuño M, et al. Unplanned readmissions and survival following brain tumor surgery. J Neurosurg. 2015;122(1):61–68. doi: 10.3171/2014.8.JNS1498. [DOI] [PubMed] [Google Scholar]
- 23.Fernandez FG, Khullar O, Force SD, et al. Hospital readmission is associated with poor survival after esophagectomy for esophageal cancer. Ann Thoracic Surg. 2015;99(1):292–297. doi: 10.1016/j.athoracsur.2014.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Merchant SJ, Ituarte PH, Choi A, et al. Hospital readmission following surgery for gastric cancer: frequency, timing, etiologies, and survival. J Gatrointest Surg. 2015;19(10):1769–1781. doi: 10.1007/s11605-015-2883-3. [DOI] [PubMed] [Google Scholar]
- 25.Reddy DM, Townsend CM, Jr, Kuo YF, Freeman JL, Goodwin JS, Riall TS. Readmission after pancreatectomy for pancreatic cancer in Medicare patients. J Gastrointest Surg. 2009;13(11):1963–1974. doi: 10.1007/s11605-009-1006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tevis SE, Kohlnhofer BM, Stringfield S, et al. Postoperative complications in patients with rectal cancer are associated with delays in chemotherapy that lead to worse disease-free and overall survival. Dis Colon Rectum. 2013;56(12):1339–1348. doi: 10.1097/DCR.0b013e3182a857eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou S, Feng Q, Zhang J, et al. High-grade postoperative complications affect survival outcomes of patients with colorectal cancer peritoneal metastases treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. BMC Cancer. 2021;21(1):41. doi: 10.1186/s12885-020-07756-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li J, Zhang Y, Hu DM, Gong TP, Xu R, Gao J. Impact of postoperative complications on long-term outcomes of patients following surgery for gastric cancer: a systematic review and meta-analysis of 64 follow-up studies. Asian J Surg. 2020;43(7):719–729. doi: 10.1016/j.asjsur.2019.10.007. [DOI] [PubMed] [Google Scholar]
- 29.Khuri SF, Henderson WG, DePalma RG, Mosca C, Healey NA, Kumbhani DJ. Determinants of long-term survival after major surgery and the adverse effect of postoperative complications. Ann Surg. 2005;242(3):326–341. doi: 10.1097/01.sla.0000179621.33268.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tan JW, Tan GHC, Ng WY, et al. High-grade complication is associated with poor overall survival after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Int J Clin Oncol. 2020;25(5):984–994. doi: 10.1007/s10147-019-01609-5. [DOI] [PubMed] [Google Scholar]
- 31.Messaris E, Sehgal R, Deiling S, et al. Dehydration is the most common indication for readmission after diverting ileostomy creation. Dis Colon Rectum. 2012;55(2):175–180. doi: 10.1097/DCR.0b013e31823d0ec5. [DOI] [PubMed] [Google Scholar]
- 32.Fish DR, Mancuso CA, Garcia-Aguilar JE, et al. Readmission after ileostomy creation: retrospective review of a common and significant event. Ann Surg. 2017;265(2):379–387. doi: 10.1097/SLA.0000000000001683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seo YJ, Bailey K, Aguayo E, et al. Readmissions after ileostomy creation using a nationwide database. Am Surg. 2018;84(10):1661–1664. doi: 10.1177/000313481808401025. [DOI] [PubMed] [Google Scholar]
- 34.Shabbir J, Britton DC. Stoma complications: a literature overview. Colorectal Dis. 2010;12(10):958–964. doi: 10.1111/j.1463-1318.2009.02006.x. [DOI] [PubMed] [Google Scholar]
- 35.Park JJ, Del Pino A, Orsay CP, et al. Stoma complications: the Cook County Hospital experience. Dis Colon Rectum. 1999;42(12):1575–1580. doi: 10.1007/BF02236210. [DOI] [PubMed] [Google Scholar]